- 1Laboratory of Animal Microbiology, Graduate School of Agricultural Science, Tohoku University, Sendai, Japan
- 2Department of Oral and Maxillofacial Surgery, School of Dentistry, Health Sciences University of Hokkaido, Hokkaido, Japan
- 3Animal Research Center, Sapporo Medical University, Sapporo, Japan
Antimicrobial peptides (AMPs) play a critical role in innate host defense against microbial pathogens in many organisms. The human cathelicidin, LL-37, has a net positive charge and is amphiphilic, and can eliminate pathogenic microbes directly via electrostatic attraction toward negatively charged bacterial membranes. A number of studies have shown that LL-37 participates in various host immune systems, such as inflammatory responses and tissue repair, in addition to its antibacterial properties. Moreover, recent evidence suggests that it is also involved in the regulation of cancer. Indeed, previous studies have suggested that human LL-37 is involved in carcinogenesis via multiple reporters, such as FPR2 (FPRL1), epidermal growth factor receptor, and ERBb2, although LL-37 and its fragments and analogs also show anticancer effects in various cancer cell lines. This discrepancy can be attributed to peptide-based factors, host membrane-based factors, and signal regulation. Here, we describe the association between AMPs and cancer with a focus on anticancer peptide functions and selectivity in an effort to understand potential therapeutic implications.
Introduction
Antimicrobial peptides (AMPs) are host defense molecules of the innate immune system of all life forms (1, 2). According to the AMP database, there are over 2,000 such peptides (3). They can be divided into seven groups: (I) linear peptides; (II) cyclic peptides; (III) glycopeptides; (IV) lipoglycopeptides; (V) lipopeptides; and (VII) thiopeptides and chromopeptides. AMPs typically contain fewer than 100 amino acids and occur in many cell types. They are generally cationic and amphipathic, and homologous peptides exist in vertebrates, invertebrates, and plants.
Mammalian AMPs belong to the defensin and cathelicidin families. Defensins contain six conserved cysteine residues in their sequence and exhibit characteristic β-sheet structures stabilized by intramolecular disulfide bonds (4). Cathelicidins are characterized by a highly conserved cathelin-like prosequence and variable carboxyl-terminal sequences that correspond to the mature AMPs (5, 6). Human cationic antibacterial protein of 18 kDa (hCAP18, also called LL-37 or FALL39) is the only cathelicidin in humans, and is primarily found in the secondary granules of neutrophils (6, 7); LL-37 is released as an active domain from macrophages/monocytes and various epithelial cells (8, 9).
Antimicrobial peptides were initially identified as functional antimicrobial molecules. Recently, they have been characterized as multifunctional peptides that serve a variety of biological roles, such as immune regulation, wound healing, angiogenesis, and anticancer functions. Their anticancer activity depends on cancer types. The interactions between AMPs and cancer cells influence apoptotic or other pathways and can result in cell death. Based on their multifunctional activities, there is a growing interest in the development of AMPs as anticancer agents. Magainins, cecropins, and defensins all have anticancer effects (10). An updated list of anticancer AMPs is available in the Antimicrobial Peptide Database (APD)1. The anticancer activities of human AMPs have not been widely evaluated; only six members (HNP-1, HNP-2, HNP-3, hBD-1, LL-37, and granulysin) with anticancer effects are annotated in the APD. LL-37 is overexpressed in breast, ovarian, and lung cancers, but it occasionally suppresses tumorigenesis in gastric cancer (11). Considering these reports, LL-37 can be associated with dual aspects of cancer progression via various receptors, such as epidermal growth factor receptor (EGFR), FRP2, ERBb2, P2X7, and GAPDH, or suppression via interaction with peptide-based factors and cancer membrane components. This review is described for discussion about these functional features of AMPs including LL-37.
Our research group previously found that the modified human-derived cathelicidin-related peptide FF/CAP18 has an anti-proliferative effect on the squamous cell carcinoma-derived cell line SAS-H1 (12) and the colon cancer-derived cell line HCT-116 (13), although the detailed mechanism underlying this effect is not clear. We showed that FF/CAP18 treatment inhibits the proliferation of these cancer cell lines, and results in apoptosis and cell death. The complex involvement of LL-37 and its analogs in various cancer types requires additional studies.
LL-37
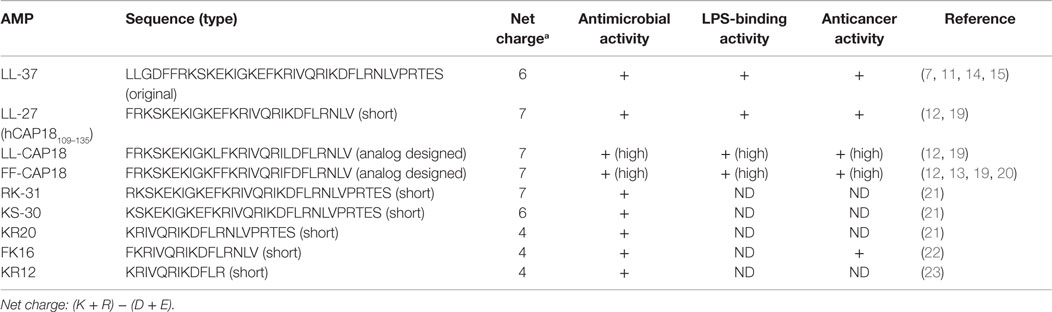
Only one cathelicidin (hCAP18/LL-37) has been found in myeloid bone marrow cDNA and isolated from neutrophils (7, 14, 15). In humans, cathelicidin exons 1–4 are located on chromosome 3p21. These are transcribed as a single gene encoding CAMP (cathelicidin antimicrobial peptide), a cationic, 18-kDa pre–pro-protein, which is also referred to as hCAP18 (14, 15). As shown Figure 1, hCAP18 is characterized by an N-terminal signal peptide (30 amino acid residues), a highly conserved pro-sequence (103 amino acid residues) called the cathelin-like domain, and a mature antimicrobial peptide referred to as LL-37 (37 amino acid residues with Leu–Leu at the N-terminus) at the C-terminal domain. LL-37 is expressed in almost all tissues and organs, such as neutrophils (15), myelocytes (16), testes (7), keratinocytes (17), and saliva (18). LL-37 is the accepted family name for mature AMPs from the C-terminal region rather than the full-length protein. FALL-39 (which differs from LL-37 by two amino acids) is analogous to PR-39 discovered in cattle (7).
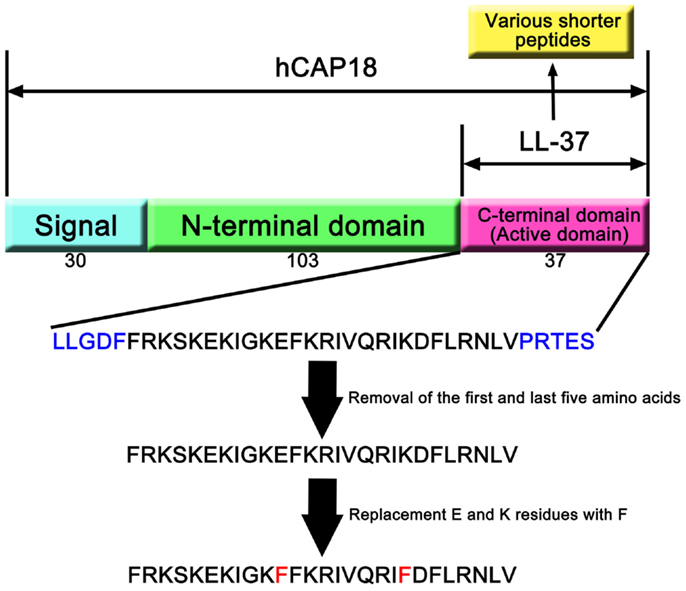
Figure 1. hCAP18 and LL-37 in cathelicidin family. The human cathelicidin hCAP18 consists of signal peptide (30 amino acids), N-terminal domain (103 amino acids), and C-terminal domain (37 amino acids). C-terminal domain shows various activities as active domain, and is called LL-37. A number of studies have revealed that shorter peptides that removed amino acids from LL-37 can show its activity. Moreover, replacement of amino acid residues can enhance its activity compared with LL-37.
LL-37 has a net positive charge of +6 at a physiological pH, a hydrophobic N-terminal domain, and an α-helical conformation that is most pronounced in the presence of negatively charged lipids (6). LL-37 is produced from the C-terminal domain of the hCAP18/LL-37 precursor protein by proteolytic cleavage. hCAP18/LL-37 from specific neutrophil granules is processed to the active peptide LL-37 following exposure to serine proteases, and particularly proteinase 3 from azurophil granules after exocytosis. Proteinase 3 cleaves hCAP18/LL-37 between the alanyl and leucyl residues (6). However, proteinase 3 is only expressed in myeloid cells and not in epithelial cells. The serine proteases, stratum corneum tryptic enzyme (SCTE, kallikrein 5) and stratum corneum chymotryptic protease (SCCE, kallikrein 7), control the activation of the precursor protein hCAP18/LL-37 on the skin surface and influence further processing to smaller peptides with alternate biological activity (5). Thus, the activity of cathelicidin is controlled by enzymatic processing of the proform to a mature peptide (LL-37) and/or various short forms, such as KR20 in humans (Table 1). In addition, the prostate-derived proteinase gastricsin (pepsin C) in the presence of vaginal fluid at low pH can also process epididymal-derived hCAP18/LL-37 in seminal plasma to functionally active ALL-38 (4). The antimicrobial activity of ALL-38 against a variety of microorganisms is equal to that of LL-37.
Induction of LL-37
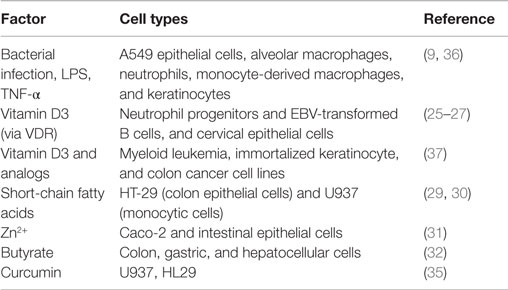
Various stimuli can induce LL-37 (Table 2). Bacterial infection is a particularly strong inducer because AMPs are functional peptides against pathogens. Mycobacterium tuberculosis infection induces the expression and production of LL-37 in a variety of cells, such as epithelial cells, alveolar macrophages, neutrophils, and monocyte-derived macrophages (9). Furthermore, LPS induces strong production of LL-37. However, some studies have reported that LPS has a minimal capacity to stimulate cathelicidin production after blood mononuclear cell activation (24). This could reflect differences among cell types. It was found to be upregulated by both 1,25-hydroxyvitamin D3 and 25-hydroxyvitamin D3, and the cathelicidin gene is regulated by the vitamin D pathway in humans (25–27). Exposure to sunlight, especially ultraviolet B photons, initiates the conversion of the provitamin D3 to previtamin D3 in the skin. The second step in vitamin activation is the formation of 1,25-dihydroxyvitamin D (active vitamin D3). LL-37 can be induced by ultraviolet B irradiation and is upregulated in infected and injured skin. Gant et al. found that ultraviolet B and vitamin D may reduce the risk of several autoimmune diseases and some cancers (28). Recently, it has been reported that LL-37 is induced by various stimuli, such as short-chain fatty acids (29, 30), Zn2+ (31), and butyrate, which is a major metabolite produced by intestinal bacteria (32), and curcumin. Curcumin has been found to have clinical therapeutic and prevention potential for various cancers (33). Karunagaran et al. showed that curcumin-induced apoptosis mainly involves the mitochondria-mediated pathway in various cancer cells and that it inhibits proliferation of cancer cells by arresting them at various phases of the cell cycle. These effects are similar to those of LL-37 and the analogs (34). Guo et al. reported that curcumin upregulated CAMP mRNA and protein levels in U937 and HT29 cells through a vitamin D receptor-independent manner. The anticancer effect of curcumin can mediate not only direct signaling pathway but also upregulation of CAMP mRNA/the protein level and vitamin D receptor expression (35).
Function of LL-37 in Cancer
Cancer is a major world health problem, and it is predicted that there will be approximately 26 million new cancer cases and 17 million cancer-related deaths annually by 2030 (38). The management of cancer currently suffers from several issues. Cancer treatment strategies include radiation therapy, chemotherapy, and a combination of these, chemoradiotherapy, all of which exert cytotoxicity on cancer cells (39, 40). In addition, specific inhibitors are available, which are used for cancer therapy, such as RTK or kinase inhibitors, in the form of monoclonal antibodies or small organic molecules (41–43). Although these treatments lead to improvements in many tumor types, they can cause severe side effects and delayed neurotoxicity owing to their non-specific mechanisms, which is the first crucial matter. The second issue is the development of resistance, which is caused by a number of factors. Many conventional anticancer reagents target factors related to cancer cell growth and show poor tumor penetration, resulting in reduced sensitivity of hypoxic cells in tumors that are in a growth-arrested state (44). Furthermore, the ABCB1 (MDR-1) gene can confer multidrug resistance in cancer cells via P-glycoprotein (P-gp), which belongs to the ATP-binding cassette family of transporters (45–47). P-gp expression may be low before chemotherapy; however, it is induced by chemotherapy, resulting in the transport of anticancer reagents from the cell before they interact with their intracellular targets (48). Therefore, to combat cancer, it is necessary to develop an innovative and unique therapeutic strategy. Several studies have indicated possible new targets of cancer treatment, such as the mitochondria (49), hybrid tubulin-targeting compounds (50), and anti-angiogenesis (51). However, it is generally accepted that the accumulation of oncogenes and tumor suppressor gene mutations promotes cancer development and cellular heterogeneity. High-throughput DNA sequencing data suggest that thousands of point mutations, translocations, amplifications, and deletions contribute to cancer development, and that the mutational range differs, even among tumors with identical histopathology (52). Therefore, any therapeutic strategy designed to target a single biological event or individual signaling molecules is limited with respect to its ability to improve current survival rates, and novel strategies are needed.
The identification and development of peptides with therapeutically useful anticancer potential can be an innovative strategy (53, 54). AMPs function in first-line defense against infections and exhibit potent cancer cell toxicity (55, 56). According to the APD1, more than 170 peptides have anticancer effects. Accumulating evidence supports the role of the human cathelicidin antimicrobial peptide LL-37 in carcinogenesis. LL-37 and its fragments and analogs show anticancer effects for various cancer cell lines. In this review, we introduce the role of AMPs, with a focus on LL-37 in human cancer in the next section.
LL-37 as a Therapeutic Target
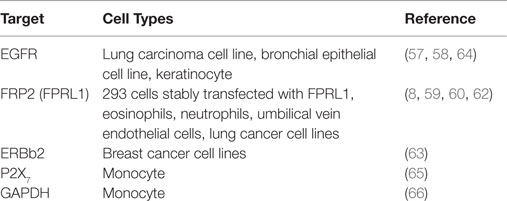
LL-37 is actively involved in physiological responses in eukaryotic cells, such as tissue repair and wound healing, although it was originally identified as an antimicrobial peptide. Previous studies have suggested that the possible molecular targets are involved in these effects (Table 3). LL-37 induces cell migration and downstream innate immunity via transactivation of EGFR (57, 58), and stimulates chemotaxis and angiogenesis via G-protein-coupled formyl-peptide receptor 2 (FRP2), also known as formyl-peptide receptor-like 1 (FPRL1) (8, 59, 60) (Figure 2). Based on these findings, it is not surprising that LL-37 is linked to cancer progression and metastasis. Indeed, hCAP18/LL-37 is expressed in breast cancer cells, and its production is most markedly higher in the breast epithelium of high-grade tumors than in normal mammary epithelia or low-grade tumors (>5 ng/mg total protein) (61); furthermore, FPRL1 is expressed in breast cancer (8, 62). Heilborn et al. also revealed that transgenic expression of LL-37 significantly increases proliferation in the human keratinocyte cell line (HaCaT) and HEK293. Furthermore, Weber et al. showed that mRNA expression of hCAP18/LL-37 is strongly correlated with that of ERBb2 and with the presence of lymph node metastasis in estrogen receptor-positive tumors from clinical samples, and LL-37 synergistically increases ErBb2 signaling (63) (Figure 2). These effects can be inhibited, suggesting the possibility of therapeutic strategies targeting LL-37. A truncated N-terminal peptide of LL-37, LL-25, inhibits LL-37 signaling and induces migration and changes in cancer cell colony morphology. Therefore, LL-37 is a putative therapeutic target to prevent progression to metastatic disease, although the detailed molecular mechanisms remain to be clarified.
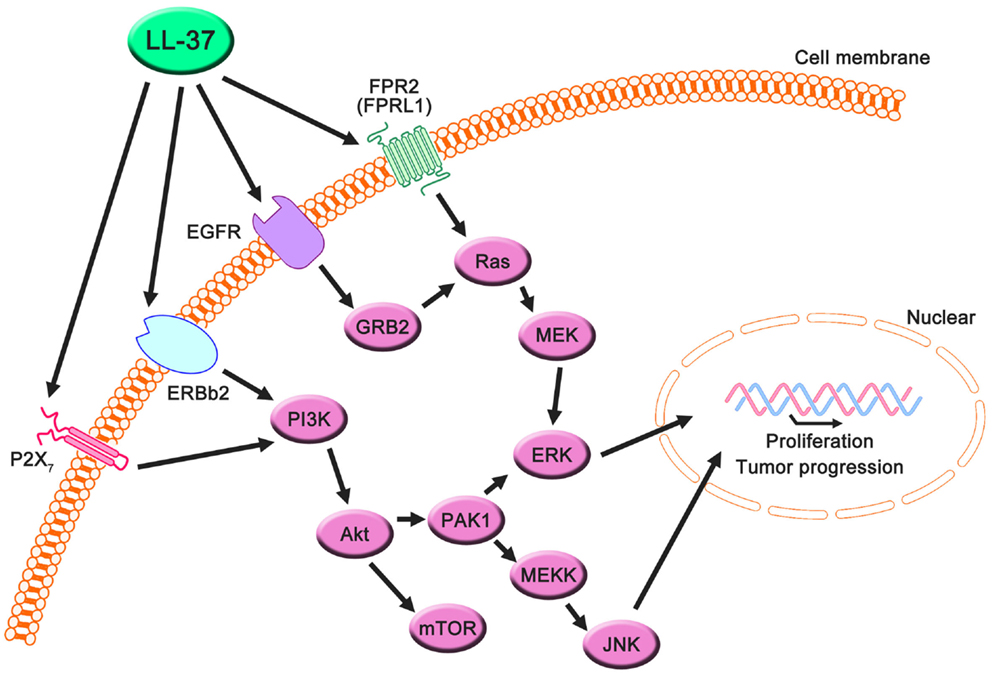
Figure 2. Proposed LL-37 signaling pathways involved in cancer cell proliferation, migration, and tumor progression. Many studies have suggested that the PI3K/Akt and MAPK/Erk signaling pathways are activated via the interaction between LL-37 and several receptors, such as FPR2 (FRPL1), EGFR, ERBb2, and P2X7. These signaling molecules can promote proliferation, migration, and tumor progression in cancer cells.
Interestingly, these reports indicate the involvement of a receptor; AMPs generally exert effects via electrostatic interactions with the cell membrane. Several studies have shown that AMPs other than LL-37, such as SK84, a glycine-rich AMP derived from the larvae of Drosophila virilis, NRC-3 and NRC-7 from Atlantic flounder species, and Temporin-1CEa isolated from skin secretions of the Chinese brown frog, show breast carcinoma cytotoxicity via membrane destruction (67–69). Accordingly, the abovementioned investigations suggest the existence of signaling pathways via an LL-37-specific receptor, despite the lack of a detailed understanding of this mechanism.
Haussen et al. reported that LL-37 is expressed in human lung cancer cells (20–30 ng/mL) and acts as a growth factor (64). In this study, the EGFR signaling inhibitor AG1478 and MEK inhibitors, PD98059 and U1260, significantly inhibited LL-37-induced proliferation. Additionally, the activation of MAP kinases was detected. Thus, the effects of LL-37 on lung cancer depend on the EGFR pathway, and its effects on breast cancer depend on the downstream activation of MEK and MAP kinases (Figure 2). It is noteworthy that the concentration of LL-37 necessary to activate lung cancer cell proliferation was on the order of nanogram per milliliter, whereas the administration of 20 μg/mL LL-37 decreased rather than increased cell numbers. The LL-37 expression level in the lungs is increased during inflammatory and infectious lung diseases (70–73), and this could promote local cancer growth. Cigarette smoke induces chronic obstructive pulmonary disease, which is an inflammatory disease, and increases the risk of lung cancer development (74, 75). Recently, it was reported that mouse homolog cathelicidin-related antimicrobial peptide (CLAMP) expressed in myeloid cells promotes cigarette smoke-induced lung tumor growth by recruiting inflammatory cells (76). Therefore, there may be a strong association between human cathelicidin antimicrobial peptide LL-37, inflammation, and cancer development, and LL-37 may have unexpected positive effects for several types of cancer in normal conditions.
In prostate cancer, LL-37 is also overexpressed. In vitro and in vivo studies have demonstrated that proliferation and invasive potential decreased as a result of the targeted downregulation of CLAMP, indicating that the targeting of LL-37 in human prostate cancers could be the basis for new therapeutic strategies (77).
Coffelt et al. reported that LL-37 is significantly overexpressed in ovarian cancers relative to normal ovarian tissue and stimulates ovarian cancer cell proliferation, migration, invasion, and matrix metalloprotease secretion (1–25 μg/mL) (78). FPR2 (FPRL1) is not only involved in LL-37-stimulated cell growth but also promotes a more aggressive phenotype in ovarian cancer cells via a number of transcription factors in LL-37-FPRL1 signaling, such as cAMP response element binding protein (CREB), which may contribute to the invasive behavior of ovarian cancer cells (79). These findings indicate that LL-37-FPRL1 interactions in ovarian cancer cells are a potential target for a novel therapeutic strategy (Figure 2). The combination of CpG oligodeoxynucleotides (CpG-ODN) and LL-37 generates significant therapeutic antitumor effects in in vivo experiments (50–100 μg/mL) (19). Chuang et al. also observed that this combination enhances the proliferation and activation of peritoneal natural killer cells, resulting in antitumor effects. LL-37 promotes DNA translocation and can significantly increase interferon-α production in plasmacytoid dendritic cells (80); thus, it potentially delivers CpG-ODN to peritoneal immune effectors, causing potent tumor cytotoxicity. Based on these reports, LL-37 can be both a target and a candidate for therapeutic strategies for ovarian cancer.
In the last decade, P2X7 receptor expression and activity have been reported in several cancers (81, 82), and LL-37 is a potential ligand (65) (Figure 2). P2X7 triggers a range of responses including cell proliferation via the PI3K/Akt pathway (83). These findings indicate that LL-37 may promote growth via the P2X7 receptor in several cancers (Figure 2).
Anticancer Effects of LL-37 as well as its Fragments and Analogs
Part of the LL-37 C-terminal domain (hCAP18109–135: FRKSKEKIGKEFKRIVQRIKDFLRNLV) shows anti-proliferative effects on human squamous cell carcinoma, SAS-H1, cells (20–40 μg/mL) (12). Specifically, hCAP18109–135 causes apoptosis via mitochondrial depolarization and DNA fragmentation but not via caspase activation. Furthermore, analog peptides with replacements of a glutamic acid residue and a lysine residue with leucine (LL/CAP18: FRKSKEKIGKLFKRIVQRILDFLRNLV) or phenylalanine (FF/CAP18: FRKSKEKIGKFFKRIVQRIFDFLRNLV) at positions 11 and 20, respectively, induce apoptotic cell death to a greater extent than did the original peptide (10–40 μg/mL). These analog peptides were designed to increase antimicrobial effects (84), which are associated with potent hydrophobic residues. This observation was based on the interactions between peptides and cancer cell membrane. These peptides, the LL-37 fragment, and its products containing amino acid substitutions can cause apoptotic cell death in cancer cells that have a more negatively charged cell membrane than in non-cancerous cells.
Several studies indicate that LL-37 and its fragments and analogs show cytotoxicity in other cancer cell types. LL-37 inhibits gastric cancer cell proliferation by the activation of bone morphogenetic protein (BMP) signaling via a proteasome-dependent mechanism (4–40 μg/mL) (22), and also induces apoptosis via the mitochondrial-associated pathway in Jurkat human T leukemia cells (25–200 μg/mL) (20). FK-16 (FKRIVQRIKDFLRNLV), which is a shorter fragment of LL-37, induces caspase-independent apoptosis and autophagy via the common p53-Bcl-2/Bax cascade in colon cancer cells (20–40 μM) (85). We have also observed that FF/CAP18 suppresses colon cancer cell proliferation via apoptotic cell death and changes metabolome levels (10–40 μg/mL) (13, 86).
In all cancer cells in which it suppressed proliferation or promoted apoptosis, autophagy, and cell cycle arrest, LL-37 expression was downregulated (87–89). In addition, cathelicidin-deficient mice exhibit increased susceptibility to azoxymethane-induced colon carcinogenesis (89). These observations suggest that LL-37 has a direct role in the suppression of tumorigenesis in several types of cancer (Figure 3), but other types of cancer may be affected by LL-37 through receptors related to proliferation or migration. These characteristics are more strongly affected by targeting the cancer membrane than by signaling induced by LL-37-receptor interactions due to the anionic cancer membrane. According to this view, the interaction between LL-37, which has a cationic charge, and the negatively charged membrane of cancer cells is extremely important with respect to the development of new therapeutic strategies, and we review the current understanding of these interactions in the next section.
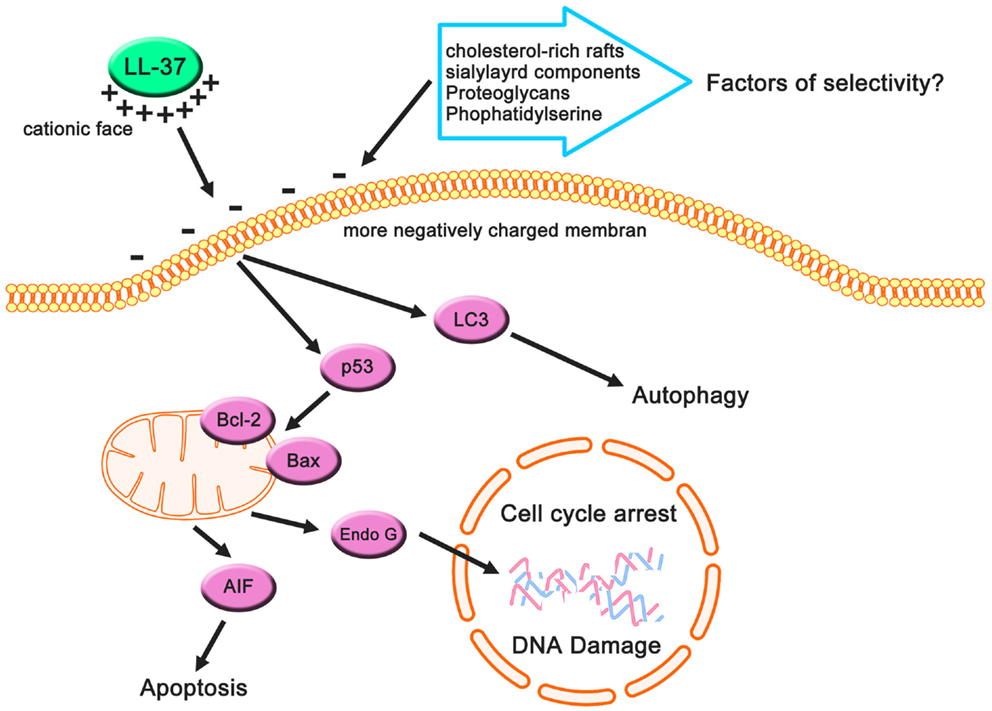
Figure 3. Cancer-suppressive effects of LL-37 based on previous studies. Cancer cells may have more negatively charged membranes compared with non-cancerous cells owing to their anionic cell components, and these components can be targets for LL-37 (which has a net positive charge). This electrostatic interaction causes apoptotic cell death, autophagy, and cell cycle arrest, resulting in the suppression of cancer cells.
Interaction between AMPs and the Cancer Membrane
When discussing the anticancer effects of LL-37 and its fragments and analogs, it is important to consider both peptide-based factors and membrane-based factors. LL-37, one of the CAMPs, has a helical structure associated with increased peptide concentration, anions, pH, detergent, and lipids (6, 90), and interacts with the membranes of eukaryotic cancer cells. Its hydrophobicity and amphiphilicity may contribute to these interactions (91). Previous studies based on designed peptides have revealed that hydrophobicity is a critical factor in interactions between peptides and target cytoplasm membranes and the associated anticancer activity (92, 93). Moreover, anticancer peptide designed using a de novo approach show high specificity, i.e., they differentiate between cancerous and non-cancerous cells. It is generally recognized that amphiphilicity is a major determinant of the ability of peptides to partition the membrane; many studies have shown that changes in the amino acid distribution that disrupt the amphiphilic structure decrease the activity against bacteria or bacteria-mimic vesicles of many α-helical AMPs (94). A net positive charge is also critical for the anticancer action of AMPs (95, 96). Lysine has strong preference for anionic membranes; however, arginine, another basic residue, exhibits a strong binding affinity to both zwitterionic and anionic membranes (97). Both arginine and lysine residues have a +1 charge in neutral buffer, indicating that lysine residues could be important in determining the selectivity of cancer cell membranes, which are more anionic, relative to those of non-cancerous cells. However, these factors alone are not sufficient to predict anticancer activities (98); thus, cancer membrane-based factors may also be important.
The widely accepted model of action of AMP interactions with cancer cell membranes is that AMPs are involved in a bilayer interaction involving a membranolytic mechanism and membrane translocation for the utilization of intracellular sites. A number of studies have revealed that cancer cell membranes have distinct features relative to non-cancerous cells, such as cholesterol and a variety of anionic components (91, 96, 99). Increased cholesterol, a major sterol in eukaryotic cell membranes (100), inhibits the lytic ability of a number of α-helical peptides toward membranes of non-cancerous cells and their lipid mimics. Therefore, cholesterol plays an important role in the general lack of anticancer action on cell membranes (91, 101–103). Interestingly, some cancer cells have increased cholesterol–lipid rafts (104), indicating that the relationship between AMPs and cholesterol-lipid rafts may decrease the cancer cell toxicity (99). The formation of cholesterol-rich lipid rafts may reduce cholesterol-depleted bulk membranes harboring phosphatidylserine that are more susceptible to peptide attacks owing to increased fluidity and hence less tightly packed lipids (96). Therefore, cholesterol-rich rafts can be a key factor in the anticancer effect of AMPS (Figure 3).
The main determinant of the selectivity and toxicity of AMPs specific to cancer cells and not non-cancerous cells appears to be the overexpression of anionic membrane components, including glycoproteins, glycolipids, proteoglycans (PGs), and phospholipids on the surface of cancer cells compared with non-cancerous cells. In cancer cells, changes in the glycosylation of glycoproteins and glycolipids, including the increased expression of their terminal sialic acids (105), contribute to the selectivity of AMPs toward cancer cells (91). Several studies have shown that anticancer effects are reduced by enzymatic digestion of sialyl residues on the surface of cancer cell membranes, strongly supporting this suggestion (106, 107). Thus, sialylated components of the cancer cell membrane play an important role in the selectivity and toxicity (Figure 2). PGs, which are negatively charged, contribute to the negatively charged glycosaminoglycan side chains (108). Several studies have suggested that the expression of PGs on cancerous cell surfaces is much higher than on the surfaces of non-cancerous cells (109–111). Zwaal et al. reported that phosphatidylserine, a negatively charged phospholipid, can be exposed on the surface of the outer membrane leaflet in cancer cells (112) (Figure 2). These factors can contribute to the anticancer effects of AMPs including LL-37.
Conclusion and Future Challenges
Despite the accumulation of scientific knowledge from a large number of studies showing that the anticancer action of LL-37 and other AMPs has potential applications for novel cancer treatment strategies, there are a few remaining challenges. In particular, its selectivity and toxicity are complicated and it will be important to consider the effects of both peptide-based and membrane-based factors. Furthermore, as we described in this review, there is a variation in the sensitivity of LL-37 among the cancer types. In breast, lung, and prostate cancers, LL-37 promotes proliferation, migration, and tumorigenesis through receptor signaling, but in other types of cancers, such as gastric cancer, colon cancer, and T-cell leukemia, it can suppress proliferation and induce apoptotic and autophagic cell death. There is no conclusive evidence to explain the opposite effects in various cancers. To resolve this issue, we may need to examine the effects of LL-37 on cancer cells from a different perspective.
It is also conceivable that LL-37 contributes to immune systems and exerts effects in combination with additional factors. Indeed, several antimicrobial agents, such as human beta defensin and LL-37, have synergistic antibacterial and anti-inflammatory activities (113–116). Therefore, LL-37 can interact with several factors to induce both positive and negative effects on cancer cells. Combination therapies with anticancer agents are a possible novel cancer treatment strategy.
Two receptors, FPR2 (55) and P2X7 (65), are thought to be involved in mediating the effects of LL-37 in various cell types. The glycolytic enzyme GAPDH has also been identified as a novel intracellular receptor, and is a direct binding partner for LL-37 in monocytes (66). However, the functions of these receptors including the intracellular effects mediated by LL-37 are not fully understood in cancer cells. For the application of AMPs as new therapeutic agents, it is necessary to clarify their receptor interactions and cellular mechanisms.
There are many well-known barriers to drug entry. Recently, new drug-delivery systems have been proposed (117). These approaches include mucoadhesives, viscous polymer vehicles, nanoparticles, and others. Local applications or missile treatments are possible for AMPs, irrespective of their toxicity. A novel nanocarrier was used to deliver the anticancer drug 5-fluorouracil to increase antitumor efficacy against breast cancer cells in vitro and in vivo (118). Eguchi et al. explained that magnetic anticancer drugs have the potential to greatly advance cancer chemotherapy for new theranostics and drug-delivery strategies (119). Anticancer therapy with AMPs could be successful when used in conjunction with new drug-delivery systems.
Conflict of Interest Statement
This research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnote
References
1. Zasloff M. Antimicrobial peptides of multicellular organisms. Nature (2002) 415(6870):389–95. doi:10.1038/415389a
2. Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol (2005) 7(2):179–96.
3. Wang G, Li X, Wang Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res (2009) 37(Database issue):D933–7. doi:10.1093/nar/gkn823
4. Sorensen OE, Gram L, Johnsen AH, Andersson E, Bangsboll S, Tjabringa GS, et al. Processing of seminal plasma hCAP-18 to ALL-38 by gastricsin: a novel mechanism of generating antimicrobial peptides in vagina. J Biol Chem (2003) 278(31):28540–6. doi:10.1074/jbc.M301608200
5. Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J (2006) 20(12):2068–80. doi:10.1096/fj.06-6075com
6. Johansson J, Gudmundsson GH, Rottenberg ME, Berndt KD, Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem (1998) 273(6):3718–24. doi:10.1074/jbc.273.6.3718
7. Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci U S A (1995) 92(1):195–9. doi:10.1073/pnas.92.1.195
8. De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med (2000) 192(7):1069–74. doi:10.1084/jem.192.7.1069
9. Rivas-Santiago B, Hernandez-Pando R, Carranza C, Juarez E, Contreras JL, Aguilar-Leon D, et al. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun (2008) 76(3):935–41. doi:10.1128/IAI.01218-07
10. Wang G, Mishra B, Epand RF, Epand RM. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim Biophys Acta (2014) 1838(9):2160–72. doi:10.1016/j.bbamem.2014.01.016
11. Wu WK, Wang G, Coffelt SB, Betancourt AM, Lee CW, Fan D, et al. Emerging roles of the host defense peptide LL-37 in human cancer and its potential therapeutic applications. Int J Cancer (2010) 127(8):1741–7. doi:10.1002/ijc.25489
12. Okumura K, Itoh A, Isogai E, Hirose K, Hosokawa Y, Abiko Y, et al. C-terminal domain of human CAP18 antimicrobial peptide induces apoptosis in oral squamous cell carcinoma SAS-H1 cells. Cancer Lett (2004) 212(2):185–94. doi:10.1016/j.canlet.2004.04.006
13. Kuroda K, Fukuda T, Yoneyama H, Katayama M, Isogai H, Okumura K, et al. Anti-proliferative effect of an analogue of the LL-37 peptide in the colon cancer derived cell line HCT116 p53+/+ and p53. Oncol Rep (2012) 28(3):829–34. doi:10.3892/or.2012.1876
14. Larrick JW, Lee J, Ma S, Li X, Francke U, Wright SC, et al. Structural, functional analysis and localization of the human CAP18 gene. FEBS Lett (1996) 398(1):74–80. doi:10.1016/S0014-5793(96)01199-4
15. Cowland JB, Johnsen AH, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett (1995) 368(1):173–6. doi:10.1016/0014-5793(95)00634-L
16. Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood (1997) 90(7):2796–803.
17. Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem (1997) 272(24):15258–63. doi:10.1074/jbc.272.24.15258
18. Murakami M, Ohtake T, Dorschner RA, Gallo RL. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J Dent Res (2002) 81(12):845–50. doi:10.1177/154405910208101210
19. Chuang CM, Monie A, Wu A, Mao CP, Hung CF. Treatment with LL-37 peptide enhances antitumor effects induced by CpG oligodeoxynucleotides against ovarian cancer. Hum Gene Ther (2009) 20(4):303–13. doi:10.1089/hum.2008.124
20. Mader JS, Mookherjee N, Hancock RE, Bleackley RC. The human host defense peptide LL-37 induces apoptosis in a calpain- and apoptosis-inducing factor-dependent manner involving Bax activity. Mol Cancer Res (2009) 7(5):689–702. doi:10.1158/1541-7786.MCR-08-0274
21. Murakami M, Lopez-Garcia B, Braff M, Dorschner RA, Gallo RL. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J Immunol (2004) 172(5):3070–7. doi:10.4049/jimmunol.172.5.3070
22. Wu WK, Sung JJ, To KF, Yu L, Li HT, Li ZJ, et al. The host defense peptide LL-37 activates the tumor-suppressing bone morphogenetic protein signaling via inhibition of proteasome in gastric cancer cells. J Cell Physiol (2010) 223(1):178–86. doi:10.1002/jcp.22026
23. Wang G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J Biol Chem (2008) 283(47):32637–43. doi:10.1074/jbc.M805533200
24. Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology (2006) 118(4):509–19. doi:10.1111/j.1365-2567.2006.02399.x
25. Frew L, Makieva S, McKinlay AT, McHugh BJ, Doust A, Norman JE, et al. Human cathelicidin production by the cervix. PLoS One (2014) 9(8):e103434. doi:10.1371/journal.pone.0103434
26. Karlsson J, Carlsson G, Larne O, Andersson M, Putsep K. Vitamin D3 induces pro-LL-37 expression in myeloid precursors from patients with severe congenital neutropenia. J Leukoc Biol (2008) 84(5):1279–86. doi:10.1189/jlb.0607437
27. Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol (2007) 178(11):7190–8. doi:10.4049/jimmunol.178.11.7190
28. Grant WB. Hypothesis – ultraviolet-B irradiance and vitamin D reduce the risk of viral infections and thus their sequelae, including autoimmune diseases and some cancers. Photochem Photobiol (2008) 84(2):356–65. doi:10.1111/j.1751-1097.2007.00266.x
29. Jiang W, Sunkara LT, Zeng X, Deng Z, Myers SM, Zhang G. Differential regulation of human cathelicidin LL-37 by free fatty acids and their analogs. Peptides (2013) 50:129–38. doi:10.1016/j.peptides.2013.10.008
30. Zeng X, Sunkara LT, Jiang W, Bible M, Carter S, Ma X, et al. Induction of porcine host defense peptide gene expression by short-chain fatty acids and their analogs. PLoS One (2013) 8(8):e72922. doi:10.1371/journal.pone.0072922
31. Talukder P, Satho T, Irie K, Sharmin T, Hamady D, Nakashima Y, et al. Trace metal zinc stimulates secretion of antimicrobial peptide LL-37 from Caco-2 cells through ERK and p38 MAP kinase. Int Immunopharmacol (2011) 11(1):141–4. doi:10.1016/j.intimp.2010.10.010
32. Schauber J, Iffland K, Frisch S, Kudlich T, Schmausser B, Eck M, et al. Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol Immunol (2004) 41(9):847–54. doi:10.1016/j.molimm.2004.05.005
33. He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules (2015) 20(5):9183–213. doi:10.3390/molecules20059183
34. Karunagaran D, Rashmi R, Kumar TR. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr Cancer Drug Targets (2005) 5(2):117–29. doi:10.2174/1568009053202081
35. Guo C, Rosoha E, Lowry MB, Borregaard N, Gombart AF. Curcumin induces human cathelicidin antimicrobial peptide gene expression through a vitamin D receptor-independent pathway. J Nutr Biochem (2013) 24(5):754–9. doi:10.1016/j.jnutbio.2012.04.002
36. Kim BJ, Rho YK, Lee HI, Jeong MS, Li K, Seo SJ, et al. The effect of calcipotriol on the expression of human beta defensin-2 and LL-37 in cultured human keratinocytes. Clin Dev Immunol (2009) 2009:645898. doi:10.1155/2009/645898
37. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J (2005) 19(9):1067–77. doi:10.1096/fj.04-3284com
38. Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis (2010) 31(1):100–10. doi:10.1093/carcin/bgp263
39. Higgins GS, O’Cathail SM, Muschel RJ, McKenna WG. Drug radiotherapy combinations: review of previous failures and reasons for future optimism. Cancer Treat Rev (2015) 41(2):105–13. doi:10.1016/j.ctrv.2014.12.012
40. Urruticoechea A, Alemany R, Balart J, Villanueva A, Vinals F, Capella G. Recent advances in cancer therapy: an overview. Curr Pharm Des (2010) 16(1):3–10. doi:10.2174/138161210789941847
41. Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev (2014) 40(7):862–71. doi:10.1016/j.ctrv.2014.03.004
42. Vincenzi B, Imperatori M, Silletta M, Marrucci E, Santini D, Tonini G. Emerging kinase inhibitors of the treatment of gastric cancer. Expert Opin Emerg Drugs (2015):1–15. doi:10.1517/14728214.2015.1051467
43. Karczmarek-Borowska B, Salek-Zan A. Hepatotoxicity of molecular targeted therapy. Contemp Oncol (Pozn) (2015) 19(2):87–92. doi:10.5114/wo.2014.43495
44. Ruan K, Song G, Ouyang G. Role of hypoxia in the hallmarks of human cancer. J Cell Biochem (2009) 107(6):1053–62. doi:10.1002/jcb.22214
45. Ozben T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett (2006) 580(12):2903–9. doi:10.1016/j.febslet.2006.02.020
46. Goda K, Bacso Z, Szabo G. Multidrug resistance through the spectacle of P-glycoprotein. Curr Cancer Drug Targets (2009) 9(3):281–97. doi:10.2174/156800909788166493
47. Liu FS. Mechanisms of chemotherapeutic drug resistance in cancer therapy – a quick review. Taiwan J Obstet Gynecol (2009) 48(3):239–44. doi:10.1016/S1028-4559(09)60296-5
48. Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control (2003) 10(2):159–65.
49. Pathania D, Millard M, Neamati N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv Drug Deliv Rev (2009) 61(14):1250–75. doi:10.1016/j.addr.2009.05.010
50. Breen EC, Walsh JJ. Tubulin-targeting agents in hybrid drugs. Curr Med Chem (2010) 17(7):609–39. doi:10.2174/092986710790416254
51. Li Y, Cozzi PJ. Angiogenesis as a strategic target for prostate cancer therapy. Med Res Rev (2010) 30(1):23–66. doi:10.1002/med.20161
52. Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science (2008) 321(5897):1807–12. doi:10.1126/science.1164382
53. Udenigwe CC, Aluko RE. Food protein-derived bioactive peptides: production, processing, and potential health benefits. J Food Sci (2012) 77(1):R11–24. doi:10.1111/j.1750-3841.2011.02455.x
54. Zheng LH, Wang YJ, Sheng J, Wang F, Zheng Y, Lin XK, et al. Antitumor peptides from marine organisms. Mar Drugs (2011) 9(10):1840–59. doi:10.3390/md9101840
55. Smolarczyk R, Cichon T, Szala S. [Peptides: a new class of anticancer drugs]. Postepy Hig Med Dosw (Online) (2009) 63:360–8.
56. Schweizer F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol (2009) 625(1–3):190–4. doi:10.1016/j.ejphar.2009.08.043
57. Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, et al. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol (2003) 171(12):6690–6. doi:10.4049/jimmunol.171.12.6690
58. Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol (2005) 175(7):4662–8. doi:10.4049/jimmunol.175.7.4662
59. Tjabringa GS, Ninaber DK, Drijfhout JW, Rabe KF, Hiemstra PS. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int Arch Allergy Immunol (2006) 140(2):103–12. doi:10.1159/000092305
60. Shaykhiev R, Beisswenger C, Kandler K, Senske J, Puchner A, Damm T, et al. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol (2005) 289(5):L842–8. doi:10.1152/ajplung.00286.2004
61. Heilborn JD, Nilsson MF, Jimenez CI, Sandstedt B, Borregaard N, Tham E, et al. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer (2005) 114(5):713–9. doi:10.1002/ijc.20795
62. Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest (2003) 111(11):1665–72. doi:10.1172/JCI17545
63. Weber G, Chamorro CI, Granath F, Liljegren A, Zreika S, Saidak Z, et al. Human antimicrobial protein hCAP18/LL-37 promotes a metastatic phenotype in breast cancer. Breast Cancer Res (2009) 11(1):R6. doi:10.1186/bcr2221
64. von Haussen J, Koczulla R, Shaykhiev R, Herr C, Pinkenburg O, Reimer D, et al. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer (2008) 59(1):12–23. doi:10.1016/j.lungcan.2007.07.014
65. Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol (2004) 172(8):4987–94. doi:10.4049/jimmunol.172.8.4987
66. Mookherjee N, Lippert DN, Hamill P, Falsafi R, Nijnik A, Kindrachuk J, et al. Intracellular receptor for human host defense peptide LL-37 in monocytes. J Immunol (2009) 183(4):2688–96. doi:10.4049/jimmunol.0802586
67. Wang C, Tian LL, Li S, Li HB, Zhou Y, Wang H, et al. Rapid cytotoxicity of antimicrobial peptide tempoprin-1CEa in breast cancer cells through membrane destruction and intracellular calcium mechanism. PLoS One (2013) 8(4):e60462. doi:10.1371/journal.pone.0060462
68. Hilchie AL, Doucette CD, Pinto DM, Patrzykat A, Douglas S, Hoskin DW. Pleurocidin-family cationic antimicrobial peptides are cytolytic for breast carcinoma cells and prevent growth of tumor xenografts. Breast Cancer Res (2011) 13(5):R102. doi:10.1186/bcr3043
69. Lu J, Chen ZW. Isolation, characterization and anti-cancer activity of SK84, a novel glycine-rich antimicrobial peptide from Drosophila virilis. Peptides (2010) 31(1):44–50. doi:10.1016/j.peptides.2009.09.028
70. Xiao W, Hsu YP, Ishizaka A, Kirikae T, Moss RB. Sputum cathelicidin, urokinase plasminogen activation system components, and cytokines discriminate cystic fibrosis, COPD, and asthma inflammation. Chest (2005) 128(4):2316–26. doi:10.1378/chest.128.4.2316
71. Soong LB, Ganz T, Ellison A, Caughey GH. Purification and characterization of defensins from cystic fibrosis sputum. Inflamm Res (1997) 46(3):98–102. doi:10.1007/s000110050114
72. Schaller-Bals S, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med (2002) 165(7):992–5. doi:10.1164/ajrccm.165.7.200110-020
73. Agerberth B, Grunewald J, Castanos-Velez E, Olsson B, Jornvall H, Wigzell H, et al. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med (1999) 160(1):283–90. doi:10.1164/ajrccm.160.1.9807041
74. Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J (2009) 34(2):380–6. doi:10.1183/09031936.00144208
75. Papi A, Casoni G, Caramori G, Guzzinati I, Boschetto P, Ravenna F, et al. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax (2004) 59(8):679–81. doi:10.1136/thx.2003.018291
76. Li D, Beisswenger C, Herr C, Schmid RM, Gallo RL, Han G, et al. Expression of the antimicrobial peptide cathelicidin in myeloid cells is required for lung tumor growth. Oncogene (2014) 33(21):2709–16. doi:10.1038/onc.2013.248
77. Hensel JA, Chanda D, Kumar S, Sawant A, Grizzle WE, Siegal GP, et al. LL-37 as a therapeutic target for late stage prostate cancer. Prostate (2011) 71(6):659–70. doi:10.1002/pros.21282
78. Coffelt SB, Waterman RS, Florez L, Honer zu Bentrup K, Zwezdaryk KJ, Tomchuck SL, et al. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int J Cancer (2008) 122(5):1030–9. doi:10.1002/ijc.23186
79. Coffelt SB, Tomchuck SL, Zwezdaryk KJ, Danka ES, Scandurro AB. Leucine leucine-37 uses formyl peptide receptor-like 1 to activate signal transduction pathways, stimulate oncogenic gene expression, and enhance the invasiveness of ovarian cancer cells. Mol Cancer Res (2009) 7(6):907–15. doi:10.1158/1541-7786.MCR-08-0326
80. Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature (2007) 449(7162):564–9. doi:10.1038/nature06116
81. Adinolfi E, Amoroso F, Giuliani AL. P2X7 receptor function in bone-related cancer. J Osteoporos (2012) 2012:637863. doi:10.1155/2012/637863
82. Adinolfi E, Capece M, Amoroso F, De Marchi E, Franceschini A. Emerging roles of P2X receptors in cancer. Curr Med Chem (2015) 22(7):878–90. doi:10.2174/0929867321666141012172913
83. Amoroso F, Capece M, Rotondo A, Cangelosi D, Ferracin M, Franceschini A, et al. The P2X7 receptor is a key modulator of the PI3K/GSK3beta/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene (2015). doi:10.1038/onc.2014.444
84. Isogai E, Isogai H, Matuo K, Hirose K, Kowashi Y, Okumuara K, et al. Sensitivity of genera Porphyromonas and Prevotella to the bactericidal action of C-terminal domain of human CAP18 and its analogues. Oral Microbiol Immunol (2003) 18(5):329–32. doi:10.1034/j.1399-302X.2003.00083.x
85. Ren SX, Shen J, Cheng AS, Lu L, Chan RL, Li ZJ, et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS One (2013) 8(5):e63641. doi:10.1371/journal.pone.0063641
86. Kuroda K, Fukuda T, Isogai H, Okumura K, Krstic-Demonacos M, Isogai E. Antimicrobial peptide FF/CAP18 induces apoptotic cell death in HCT116 colon cancer cells via changes in the metabolic profile. Int J Oncol (2015) 46(4):1516–26. doi:10.3892/ijo.2015.2887
87. Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, Ohtake T, et al. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology (2003) 125(6):1613–25. doi:10.1053/j.gastro.2003.08.028
88. Yang YH, Zheng GG, Li G, Zhang B, Song YH, Wu KF. Expression of LL-37/hCAP-18 gene in human leukemia cells. Leuk Res (2003) 27(10):947–50. doi:10.1016/S0145-2126(03)00020-1
89. Ren SX, Cheng AS, To KF, Tong JH, Li MS, Shen J, et al. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res (2012) 72(24):6512–23. doi:10.1158/0008-5472.CAN-12-2359
90. Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J (1999) 341(Pt 3):501–13. doi:10.1042/0264-6021:3410501
91. Dennison SR, Whittaker M, Harris F, Phoenix DA. Anticancer alpha-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr Protein Pept Sci (2006) 7(6):487–99. doi:10.2174/138920306779025611
92. Chen Y, Mant CT, Farmer SW, Hancock RE, Vasil ML, Hodges RS. Rational design of alpha-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J Biol Chem (2005) 280(13):12316–29. doi:10.1074/jbc.M413406200
93. Huang YB, Wang XF, Wang HY, Liu Y, Chen Y. Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol Cancer Ther (2011) 10(3):416–26. doi:10.1158/1535-7163.MCT-10-0811
94. Lorin A, Noel M, Provencher ME, Turcotte V, Masson C, Cardinal S, et al. Revisiting peptide amphiphilicity for membrane pore formation. Biochemistry (2011) 50(43):9409–20. doi:10.1021/bi201335t
95. Al-Benna S, Shai Y, Jacobsen F, Steinstraesser L. Oncolytic activities of host defense peptides. Int J Mol Sci (2011) 12(11):8027–51. doi:10.3390/ijms12118027
96. Riedl S, Zweytick D, Lohner K. Membrane-active host defense peptides – challenges and perspectives for the development of novel anticancer drugs. Chem Phys Lipids (2011) 164(8):766–81. doi:10.1016/j.chemphyslip.2011.09.004
97. Yang ST, Shin SY, Lee CW, Kim YC, Hahm KS, Kim JI. Selective cytotoxicity following Arg-to-Lys substitution in tritrpticin adopting a unique amphipathic turn structure. FEBS Lett (2003) 540(1–3):229–33. doi:10.1016/S0014-5793(03)00266-7
98. Dennison SR, Harris F, Bhatt T, Singh J, Phoenix DA. A theoretical analysis of secondary structural characteristics of anticancer peptides. Mol Cell Biochem (2010) 333(1–2):129–35. doi:10.1007/s11010-009-0213-3
99. Harris F, Dennison SR, Singh J, Phoenix DA. On the selectivity and efficacy of defense peptides with respect to cancer cells. Med Res Rev (2013) 33(1):190–234. doi:10.1002/med.20252
100. Simons K, Ikonen E. How cells handle cholesterol. Science (2000) 290(5497):1721–6. doi:10.1126/science.290.5497.1721
101. Matsuzaki K, Sugishita K, Fujii N, Miyajima K. Molecular basis for membrane selectivity of an antimicrobial peptide, magainin 2. Biochemistry (1995) 34(10):3423–9. doi:10.1021/bi00010a034
102. Wojcik C, Sawicki W, Marianowski P, Benchaib M, Czyba JC, Guerin JF. Cyclodextrin enhances spermicidal effects of magainin-2-amide. Contraception (2000) 62(2):99–103. doi:10.1016/S0010-7824(00)00143-8
103. Steiner H, Andreu D, Merrifield RB. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim Biophys Acta (1988) 939(2):260–6. doi:10.1016/0005-2736(88)90069-7
104. Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol (2006) 168(4):1107–18. doi:10.2353/ajpath.2006.050959
105. Varki A. Sialic acids in human health and disease. Trends Mol Med (2008) 14(8):351–60. doi:10.1016/j.molmed.2008.06.002
106. Fredman P, Hedberg K, Brezicka T. Gangliosides as therapeutic targets for cancer. BioDrugs (2003) 17(3):155–67. doi:10.2165/00063030-200317030-00002
107. Ohyama C. Glycosylation in bladder cancer. Int J Clin Oncol (2008) 13(4):308–13. doi:10.1007/s10147-008-0809-8
108. Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res (2010) 339(1):237–46. doi:10.1007/s00441-009-0821-y
109. Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med (2011) 15(5):1013–31. doi:10.1111/j.1582-4934.2010.01236.x
110. Asimakopoulou AP, Theocharis AD, Tzanakakis GN, Karamanos NK. The biological role of chondroitin sulfate in cancer and chondroitin-based anticancer agents. In vivo (2008) 22(3):385–9.
111. Koo CY, Sen YP, Bay BH, Yip GW. Targeting heparan sulfate proteoglycans in breast cancer treatment. Recent Pat Anticancer Drug Discov (2008) 3(3):151–8. doi:10.2174/157489208786242278
112. Zwaal RF, Comfurius P, Bevers EM. Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci (2005) 62(9):971–88. doi:10.1007/s00018-005-4527-3
113. Chen X, Niyonsaba F, Ushio H, Okuda D, Nagaoka I, Ikeda S, et al. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J Dermatol Sci (2005) 40(2):123–32. doi:10.1016/j.jdermsci.2005.03.014
114. Nagaoka I, Hirota S, Yomogida S, Ohwada A, Hirata M. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm Res (2000) 49(2):73–9. doi:10.1007/s000110050561
115. Midorikawa K, Ouhara K, Komatsuzawa H, Kawai T, Yamada S, Fujiwara T, et al. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect Immun (2003) 71(7):3730–9. doi:10.1128/IAI.71.7.3730-3739.2003
116. Maisetta G, Batoni G, Esin S, Luperini F, Pardini M, Bottai D, et al. Activity of human beta-defensin 3 alone or combined with other antimicrobial agents against oral bacteria. Antimicrob Agents Chemother (2003) 47(10):3349–51. doi:10.1128/AAC.47.10.3349-3351.2003
117. Kompella UB, Kadam RS, Lee VH. Recent advances in ophthalmic drug delivery. Ther Deliv (2010) 1(3):435–56. doi:10.4155/tde.10.40
118. Yuan Z, Qu X, Wang Y, Zhang DY, Luo JC, Jia N, et al. Enhanced antitumor efficacy of 5-fluorouracil loaded methoxy poly(ethylene glycol)-poly(lactide) nanoparticles for efficient therapy against breast cancer. Colloids Surf B Biointerfaces (2015) 128:489–97. doi:10.1016/j.colsurfb.2015.02.048
Keywords: antimicrobial peptides, anticancer, carcinogenesis, LL-37, cathelicidin
Citation: Kuroda K, Okumura K, Isogai H and Isogai E (2015) The human cathelicidin antimicrobial peptide LL-37 and mimics are potential anticancer drugs. Front. Oncol. 5:144. doi: 10.3389/fonc.2015.00144
Received: 13 April 2015; Accepted: 15 June 2015;
Published: 30 June 2015
Edited by:
Yoshihiro Suzuki-Karasaki, Nihon University School of Medicine, JapanReviewed by:
Markus A. N. Hartl, University of Innsbruck, AustriaYoshihiro Suzuki-Karasaki, Nihon University School of Medicine, Japan
Copyright: © 2015 Kuroda, Okumura, Isogai and Isogai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kengo Kuroda, Laboratory of Animal Microbiology, Graduate School of Agricultural Science, Tohoku University, 1-1 Amamiya-machi, Tsutumodori, Aoba-ku, Sendai 981-8555, Japan,a2VuZ29kb3ViaUBnbWFpbC5jb20=
 Kengo Kuroda
Kengo Kuroda Kazuhiko Okumura
Kazuhiko Okumura Hiroshi Isogai
Hiroshi Isogai Emiko Isogai
Emiko Isogai