
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 14 November 2014
Sec. Gastrointestinal Cancers
Volume 4 - 2014 | https://doi.org/10.3389/fonc.2014.00323
A commentary has been posted on this article:
Commentary: “Prom1 Function in Development, Intestinal Inflammation, and Intestinal Tumorigenesis”
Prom1/CD133 has been identified in colorectal, hepatocellular, and pancreatic cancer as a cancer stem cell marker and has been used as such to predict colon cancer recurrence in humans. Its potential molecular function as well as its role as a marker of intestinal regeneration is still not fully known. We evaluated the role of Prom1 in intestinal regeneration in inflammatory bowel disease (IBD), determined the function of Prom1, and characterized the effect of a lack of Prom1 on intestinal tumor formation in animal models. Our results suggest that Apc mutations lead to an increase in Prom1 expressing cells in the intestinal crypt stem cell compartment and in early intestinal adenomas. Also, Prom1 knockout mice are more susceptible to intestinal tumor formation. We conclude that Prom1 likely plays a role in regulating intestinal homeostasis and that these results clearly illustrate the role of Prom1 in intestinal regeneration. We further conclude that Prom1 may provide a novel therapeutic target for patients with gastrointestinal conditions such as IBD, short bowel syndrome, and colorectal cancer.
The intestinal tract is lined by mucosa, which is primarily composed of villi or the intercrypt table and epithelial-lined crypts at the mucosal base (1–4). The mucosal epithelial cells arise from intestinal stem cells located in the crypt (5). The morphological and functional characterization of intestinal stem cells could shed light on their role in intestinal regeneration. The Clever laboratory found that PROM1 is a marker for stem cells and early progenitors in the mouse small intestine and that it meets all the criteria of putative intestinal stem cells (6). However, its role as a marker of intestinal regeneration is largely unknown.
PROM1 is a cell surface membrane protein and is a homolog to the CD133 protein in human (7). PROM1 was identified as both a hematopoietic and neuroepithelial stem cell marker (8–11). The function of Prom1 is unknown, but its specific localization suggests that PROM1 is involved in the organization of plasma membrane protrusions, and suggests that PROM1 might be important in maintaining an appropriate lipid composition within the plasma membrane (12). In addition, PROM1 has been identified in the apical plasma membrane of epithelial cells of pancreas (13), liver (canals of Hering, interlobular ducts) (14), kidney (15), prostate (16), and skin (17). In the small intestine in mouse, Prom1 marks rare stem cells, as well as transit-amplifying progenitor cells (6). Moreover, PROM1 is expressed in both rod and cone photoreceptors of the eye (18, 19). Prom1 also has been used as a cancer stem cell marker alone or with other markers such as CD44 (20). Singh and Dirks found that PROM1 expressing neural tumor cells are essential for tumorigenesis (21). Tang et al. found that PROM1 positive cell populations are more tumorigenic than PROM1 negative cells in the liver (22). In colon cancer, both PROM1 positive and PROM1 negative cells are capable of being tumorigenic (23). However, PROM1 expression in colon cancer in humans was found to be predictive of increased colon cancer recurrence (24, 25).
Wnt signaling regenerative pathways control intestinal differentiation (26, 27). Upon Wnt pathway activation, β-catenin stabilizes and translocates to the nucleus where it accumulates. It then, in cooperation with the transcription factor Tcf-4, modulates expression of a variety of Tcf-4 responsive target genes such as Paneth cell alpha defensins (28–30). However, whether Prom1 expression is downstream of the Wnt and β-catenin/TCF transactivation activity is not clear.
Inflammation in the colon can play a significant role in colon cancer development (31). For example, the significantly increased risk of developing colon cancer in patients with long-standing inflammatory bowel disease (IBD) has been thought in large part to result from chronic colonic inflammation (31). The physiologic role of Prom1 in intestinal inflammation related to colon cancer development is not known. Characterization of Prom1 holds significant promise for eventual treatment of intestinal tumors and many gastrointestinal diseases such as Crohn’s Disease. We hypothesize that the Wnt signaling regenerative pathway results in dysregulation of Prom1 expression and subsequent changes in intestinal mucosal repair, and hence may be relevant to colon cancer development in IBD. To test this hypothesis, we investigated the role of Prom1 in intestinal regeneration and tumorigenesis. To gain insights into Prom1, we generated Prom1 knockout mice. Prom1 knockout mice were more susceptible to azoxymethane (AOM)/dextran sodium sulfate (DSS) induced intestinal inflammation, which promoted colorectal tumorigenesis. Also, loss of Prom1 gene contributed to higher tumorigenicity in Apc mutant mice.
Wild type (Wt), Prom1 knockout mice (Prom1-/-), and Apc mutant mice (Apc-/+) ranging from 1 day to 5 months old were used. Wt and 129-Apctm1.Δ716 mice were bred in our animal facility (27). Prom1-/- mice were provided by Dr. Kyuson Yun (un-published). To generate double mutant Apc-/+Prom1-/- mice, 129-Apc-/+ were crossed with 129-Prom1−/− mice. All animals were maintained on a 129SvEv background (>10 generations). Pups were genotyped by PCR to determine Apc and Prom1 gene status. The wild-type Prom1 allele generated a 204-bp product, and the mutant allele formed a 329-bp product. Mice were fed an AIN-76A diet and water ad libitum, exposed to 12-h-light/12-h-dark cycles, and maintained under specific pathogen free conditions without pinworms, Helicobacter spp, or Citrobacter rodentium. We used age- and sex-matched littermate controls. All animal experiments were performed in accordance with protocols approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions.
At the age of 4 months, Apc-/+Prom1-/- and Apc-/+Prom1+/+ female mice were euthanized by cervical dislocation. The intestines were opened, stained with methylene blue (Figure 7A), and the number of adenomas was counted as previously described (27).
Aberrant crypt foci (ACF) were induced using AOM and DSS. Six-week-old female Wt and Prom1-/- mice (N = 5 per group) fed an AIN-76A diet and water ad libitum were treated with a single dose of AOM (2.5, 5, 7.5, or 10 mg/kg in saline, i.p.). Mice were assessed every 12 h for 5 days. Another group of Wt and Prom1-/- mice (N = 10 per group) were treated with a single dose of AOM (5 mg/kg in saline, i.p.). Seven days after injection with AOM, the mice were given normal drinking water or drinking water containing 2% DSS for a period of 7 days followed by normal drinking water for 3 weeks. ACF in surviving mice were evaluated as previously described with minor modifications (32). Intestinal lesions were scored for the presence of inflammation, mucosal damage, and proliferation. Grade 0, normal intestine; Grade 1, mild mixed infiltration of inflammatory cells; Grade 2, moderate infiltration of mixed inflammatory cells and edema within the mucosal layer and mild epithelial crowding; Grade 3, moderate to severe inflammation, edema, mucosal disruption, and crypt proliferation (epithelial crowding, deep crypts, and thicken mucosa); and Grade 4, severe transmural inflammation, edema, mucosal disruption, and severe proliferation (thickened mucosa, elongated and branched crypts, loss of goblet cells).
At the age of 6 weeks, blood was taken from both Wt and Prom1-/- mice. Both serum and blood were sent to Antech Diagnostics for analysis. Serum was analyzed for liver, kidney, and pancreas functions as well as muscle changes. The blood exam included total red blood cells (RBC), total white blood cells (WBC), and differential WBC counts. Thrombin, partial thrombin, hemoglobin, packed cell volume, mean corpuscular hemoglobulin (MCH), and mean corpuscular hemoglobulin concentration (MCHC) were also measured.
The adult Wt, Prom1−/−, and Apc-/+ mice were euthanized by cervical dislocation. The intestines were opened longitudinally, washed in PBS, and fixed in 10% buffered formalin. Then, the intestines were rolled and submitted for embedding. Five-micrometer-thick sections were prepared, and sections were deparaffinized in xylene and rehydrated through graded alcohols. Slides were transferred to a jar containing unmasking solution (Vector Laboratories, H-3300), boiled for 10 min, and left in the same solution at room temperature for 20 min. All slides were then incubated with 10% blocking serum (Vector Laboratories), in PBS for 30 min at room temperature. The slides were incubated with primary antibody (anti-Prom1, MAB4310, or with anti-Ki67, AB9260, Millipore) diluted 1:100 for 60 min at room temperature. After three washes with 0.1% Tween 20 in PBS, sections were incubated for 30 min with fluorescence (for Prom1) or non-fluorescence (for Ki67) biotinylated secondary antibody IgG (Vector laboratories) diluted 1:500 in blocking solution. Slides were washed three times in PBS for 3 min each, and rinsed three times with distilled water. Cover slips were mounted with crystal mount (Biomeda, M02). The number of positive cells within the crypts and adenomas was quantified in 10 fields at 400× magnification for Prom1 and at 200× magnification for Ki67. Photographs of histological sections were taken using a Nikon digital camera.
Western blot analyses were performed on SDS-PAGE gels under denaturing conditions. Total proteins (30 μg/ml) from both Wt and Prom1-/- mice from intestinal crypt preparations were separated on 12 or 15% gels and electrotransferred onto nitrocellulose membranes (BioRad, 162-0115). Non-specific protein binding was blocked using 10% Blotto–0.1% Tween followed by incubation with anti-Prom1 primary antibody (Millipore, MAB4310) diluted in the blocking solution overnight at 4°C. After washing with PBS, membranes were incubated with horseradish peroxidase-conjugated anti-mouse secondary antibodies and developed using the Immobilon Western Kit (Millipore, WBKLS0100).
Real-time PCR was performed to determine Prom1 expression on the crypt cells from both Wt and Prom1-/-. Total RNA of mouse intestinal tissues were extracted using the RNAeasy Kit (Invitrogen), and cDNA was synthesized using random hexamers. The Taqman primers for the Prom1 and K19 genes were from Applied Biosystems. We calculated relative gene expression by the ΔCT method.
Data are presented as the mean and SEM or as a percentage with SD (GraphPad, PRISM software, San Diego, CA, USA). The Mann–Whitney test was used to compare body weights, and P values were determined using the Student two-tailed t-test unless otherwise indicated. Tumor data were analyzed using the Chi square (χ2) test and other differences using the Student’s t-test. Kaplan–Meier survival curves were generated, and difference in survival was determined using the log-rank test. Data were considered to be significantly relevant at p < 0.05 and are presented as mean ± SEM.
Immunofluorescence staining for the normal epithelial cell crypts in both the small and the large intestines revealed that anti-PROM1 antibody marked rare intestinal crypt cells (Figure 1A). In the Apc-/+ mice, that anti-PROM1 antibody showed intense staining in the majority of the epithelial cells within the adenomas (Figures 1B,C). The isotype matched control was negative (data not shown). Quantification showed an expansion of PROM1 positive cells in the adenomas, P < 0.001 (Figure 1D). Western blot analysis showed that mucosal cells from Apc-/+ mice have an enhanced expression of PROM1 in comparison to normal crypts from wild-type mice (Figure 2A). Real-time PCR was performed on the crypt cells using K19 or Gadph (data not shown for Gadph) as controls. Results demonstrated significant increases (four- to six-fold) in Prom1 expression in the crypt epithelial cells in the Apc-/+ mice compared to the crypts from wild-type mice (Figure 2B). These results indicate that Prom1 expression and PROM1 positive cells are increased in the mucosa of Apc-/+ mice.
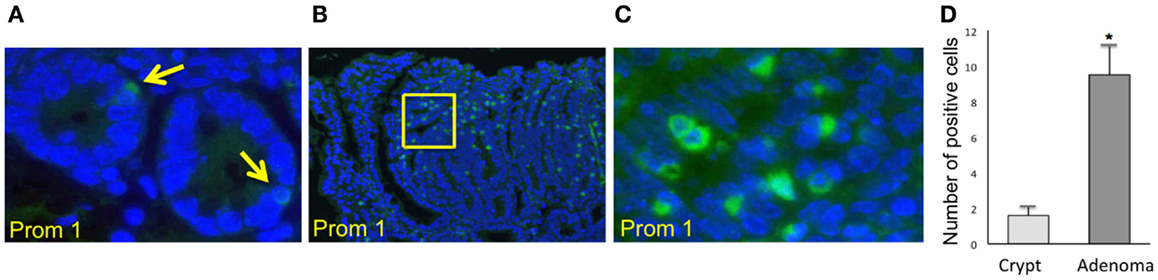
Figure 1. Immunofluorescence staining using PROM1 in antibodies for the detection of expanded positive cells from crypt cells in Wt mice and within the adenomas in Apc−/+ mice. (A) Normal crypt (magnification, ×600). (B) Adenoma (magnification, ×100). (C) Higher magnification (×600) of the image (B). (D) Quantification analysis of the positive cells within the crypt and adenomas in the Apc−/+ mice. Bar = SEM. *p < 0.01 (n = 10).
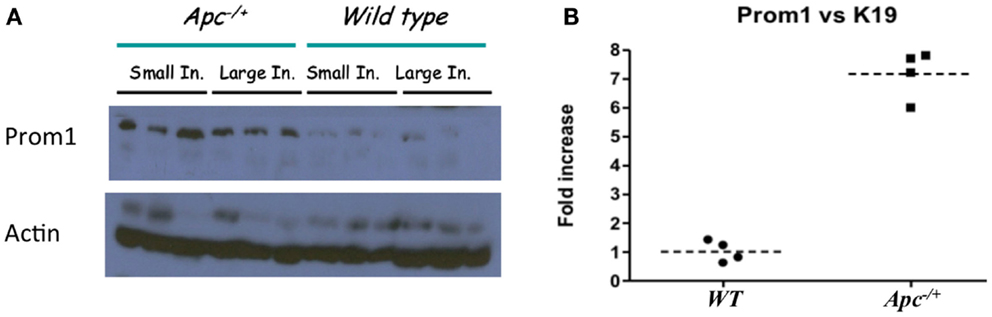
Figure 2. Affect of Apc−/+ on Prom1 expression. (A) Protein expression of PROM1 from small and large intestine in Wt mice and Apc−/+ mutant mice from three different mice. (B) mRNA expression of Prom1 from large intestine of Wt and Apc−/+ mice (n = 4).
To determine the role of Prom1 in intestinal crypt, we analyzed Prom1-/- mice. Null mice of both sexes were phenotypically normal, and crossing heterozygous male and female mice resulted in Wt, Het, and null offspring at the expected Mendelian ratios (Figure 3). Body weight analysis revealed mature obesity in Prom1-/- mice compared to Wt mice (Figure 4A). Complete histopathological analysis revealed that there is a moderate degree of germinal arrest in the testes of Prom1-/- mice compared to wild-type mice. There was multifocal seminiferous tubule atrophy and degeneration and arrest of spermatogenesis within the testes in the Prom1-/- mice at 4 months of age (Figure 4C). Serum chemistry revealed a significant increase in fasting blood glucose level in Prom1-/- mice. The mean blood glucose level was 254 mg/dl in the Prom1-/- mice compared to 158 mg/dl in the wild-type mice (Figure 4B). The knockout did not affect hematology. There were no changes in blood scores in the Prom1-/- mice compared to wild-type mice (data not shown).
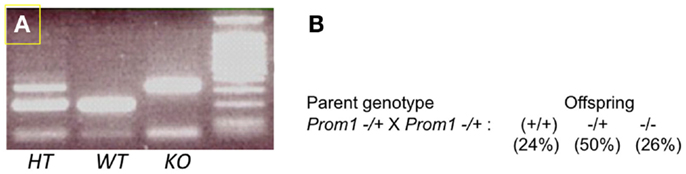
Figure 3. Genotypes of offspring obtained by cross-mating of Prom1−/+ and Prom1−/+ double heterozygous mice. (A) PCR of Prom1 heterozygous, wild type, and homozygous genomic DNA to detect mutant allele. (B) Mendelian ratios, single gene (Prom1) knockout.
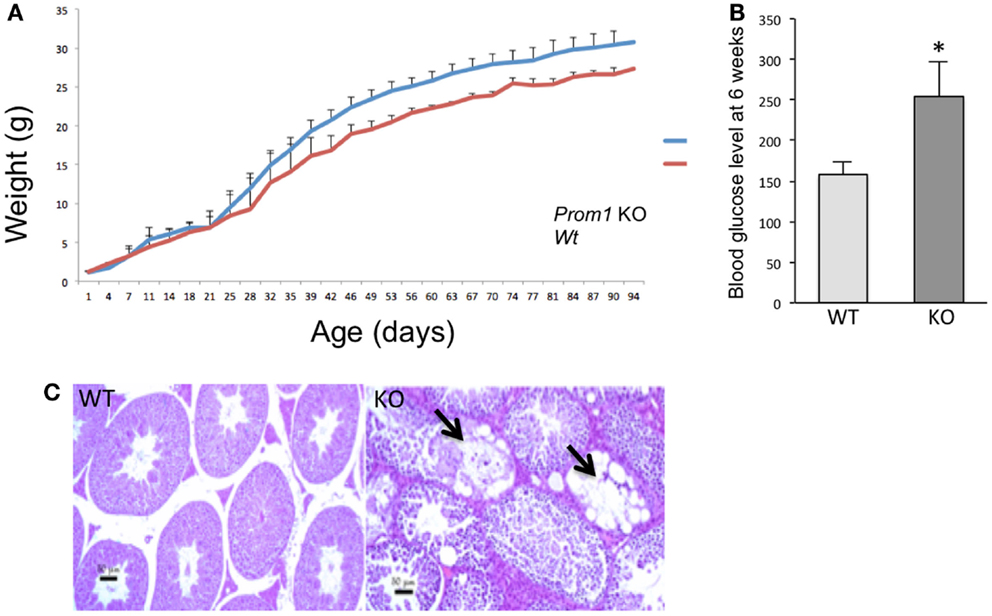
Figure 4. Phenotype of Prom1 knockout mice. (A) Body weight change from day 1 to day 94. (B) Blood glucose level at 6 weeks of age in Wt and Prom1−/− mouse (n = 10). (C) Histopathological examination of the testes at 4 months of age showing empty seminiferous tubules in the Prom1−/− mouse (arrow), H&E ×100.
To determine whether Prom1 has a role in maintaining intestinal epithelium homeostasis and injury response, we exposed control and Prom1-/- to AOM and DSS. Prom1-/- mice were more sensitive to AOM than Wt mice. After injection with a single dose of AOM (5 mg/kg), 50% of the mice died. All the Prom1-/- mice died when given a single injection of AOM at 7.5 or 10 mg/kg (Figure 5A). Remarkably, an injection of AOM followed by oral exposure to DSS resulted in increased intestinal inflammation in Prom1-/- mice, relative to Wt mice (Figures 5C,D). Inflammatory infiltrate consists predominantly of macrophages, neutrophils, and lesser numbers of lymphocytes and rare plasma cells. In addition to increase in inflammation, Prom1-/- mice showed severe dysplastic crypts and abnormal crypt proliferation. Hyperplastic crypts in the knockout mice showed markedly reduced number of goblet cells, elongated nuclei with prominent nucleoli, and abnormal crypt lumen. The wild-type mice exhibited mild crypt hyperplasia with minimal dysplasia. Peripheral complete blood counts revealed significant leukocytosis, neutrophilia, and monocytosis in Prom1 knockout mice compared with the wild-type mice (Figure 5B). The proliferation index of hyperplastic crypts measured by nuclear expression of Ki67 showed a significant positive correlation to the lack of Prom1, p < 0.01 (Figure 6).
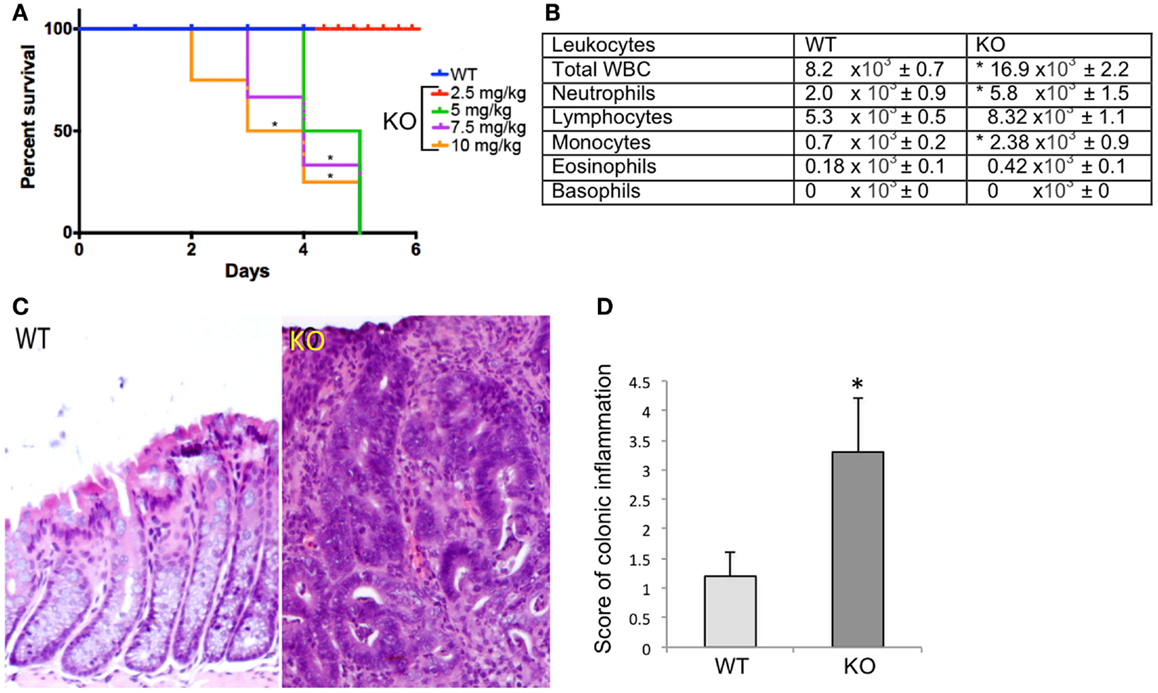
Figure 5. Significant differences in survival and inflammation in the Wt and Prom1−/− treated group. (A) Survival curve of Wt and Prom1−/− mice after AOM administration that was assessed every 12 h for 5 days. (B) Peripheral leukocyte counts of Wt and Prom1−/− mice after AOM/DSS administration. (C) H&E of large intestine in Wt and Prom1−/− mice after AOM/DSS administration. Notice: inflammation, dysplastic crypts, and abnormal proliferation in knockout mice. (D) Scores of inflammation in the inflamed colon. (*p < 0.05 compared with control, n = 6 animals/group).
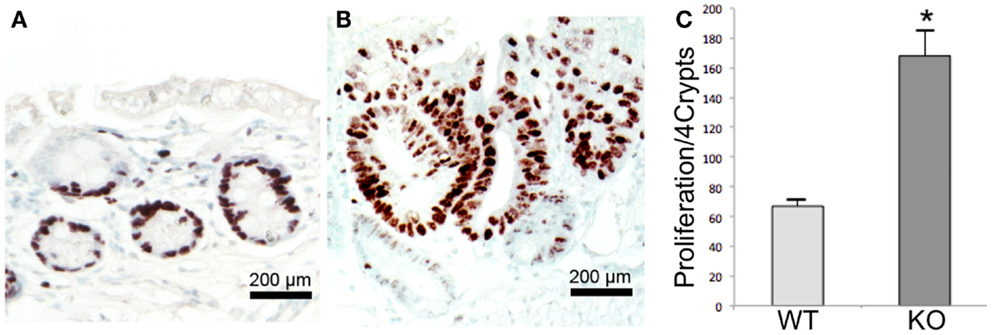
Figure 6. Proliferation assay in Wt and Prom1−/− mice after AOM/DSS administration. Immunohistochemical staining of Ki67 within the crypts in Wt(A) and Prom1−/− (B) mice. (C) Quantification analysis of Ki 67 staining. Bar = SEM. *p < 0.01 (n = 10).
To determine whether loss of Prom1 promotes intestinal tumorigenesis, we crossed Prom1-/- mice to Apc-/+ mice. All sizes of adenomas increased in the double mutant mice (Apc-/+Prom1-/-) (Figure 7A), especially small adenomas (<2 mm in diameter) (Figure 7B). Double mutant mice exhibited the highest number of adenomas (mean, 33) compared with Apc−/+ mice (mean, 27) along their entire small and large intestines at ~120 days of age. Average adenoma count was 23 in the small intestine and 10 in the large intestine of double mutant mice (Apc-/+Prom1-/-) compared to 17 and 10 in single mutant mice (Apc-/+Prom+/+), respectively. The number of adenomas increased by 18% in Apc-/+Prom1-/- mice compared to those with Apc-/+Prom+/+. This escalation was highly significant as mediated by a p value of <0.05 using a two-tailed Student’s t-test.
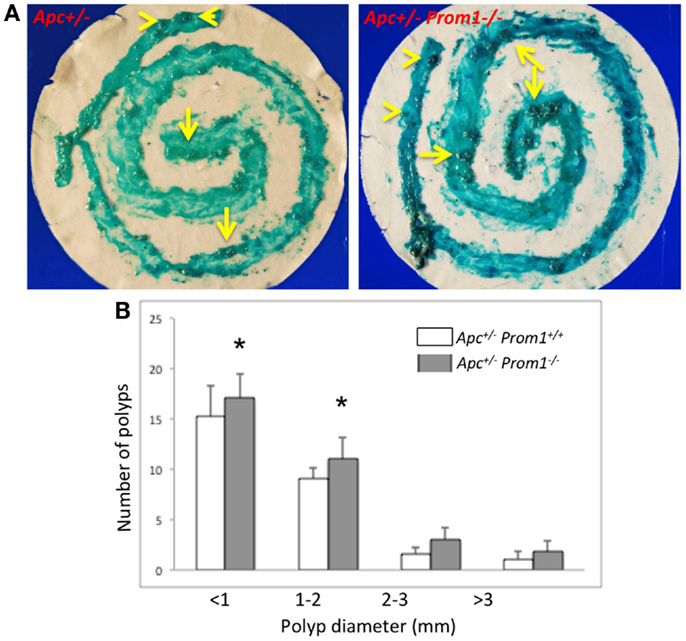
Figure 7. (A) Methylene blue staining of the entire intestine is shown. Notice various sized of adenomas within the large (arrow head) and small intestine (arrows). (B) Distribution of adenomas by size in Apc-/+prom1+/+ (white bar) and in Apc-/+Prom1-/- mice (filled bar). The percentage of control adenomas is indicated with SEM. The number of adenomas was analyzed by the Chi-square test and Student’s t-test (*p < 0.05 versus control, n = 15 animals/group).
Despite medical advances, IBD remains a significant and increasing health care burden worldwide, and the exact cause remains unknown (33). However, intestinal regeneration plays an important role in the healing of the intestinal mucosa (34). We hypothesized that PROM1 is important for intestinal homeostasis. The results reported here demonstrate that Prom1 plays an important role in intestinal healing. Knockout of Prom1 results in significantly greater intestinal inflammation, abnormal crypt proliferation, and dysplasia upon AOM/DSS administration. Inflammation in general promotes intestinal tumorigenesis (35). The use of PROM1 to mark the intestinal stem cells and colorectal cancer is controversial (36). Researchers found that both PROM1+ cells and PROM1– cells are capable of tumorigenicity and display similar differentiation capabilities. For example, Liqin Zhu found that Prom1 marks stem cells in the adult small intestine that are susceptible to transformation into tumors (37). In contrast, Meng et al. discovered that PROM1 alone could not be used as a stem cell marker because PROM1+ cells and PROM1− cells displayed similar abilities of colony formation, self-renewal, proliferation, and differentiation (38). Our results show that the loss of Prom1 combined with Apc gene heterozygosity significantly increases tumorigenesis. Together, these results suggest that Prom1 functions as a protective factor against early phase, inflammation-mediated tumorigenesis.
Several investigators found that PROM1 is a target for Wnt signaling regenerative pathways in many cell lines including malignant melanoma and glioblastoma cells (39, 40). In the absence of Wnt, Apc forms a complex in the cytoplasm that results in β-catenin phosphorylation by glycogen synthase kinase-3 (GSK-3). This results in proteolytic degradation of β-catenin. Loss of Apc function and GSK-3 phosphorylation results in stabilized β-catenin (41, 42). This in turn allows β-catenin to translocate to the nucleus and accumulate, where, in cooperation with the transcription factor Tcf-4, it modulates expression of a variety of Tcf-4 responsive target genes such as Paneth cell α-defensins (28–30). However, it was not clear whether Prom1 behaves as a positive or negative regulator in mice having Apc mutations. Our results are consistent with previous studies showing that PROM1 is expressed predominantly on the crypt stem cell compartment. We now show that PROM1 can also be detected in early premalignant lesions in Apc-/+ mice under Wnt signaling regenerative pathways and that Prom1-/- deletion stimulates adenomas in Apc-/+ mice, which is a part of Wnt signaling regenerative pathways. These data indicate that Prom1-/- is a potential target for chemoprevention treatment. The anticancer activity of PROM1 is unknown, and that is beyond the scope of this paper. Taken together, our results suggest that Prom1-/- induces inflammation and increases proliferative potential in the intestinal crypts enhanced intestinal tumor genesis.
Knockout of Prom1 did not affect early development in mice; Prom1-/- disruption did not cause any lethal embryonic defects or interfere with development or fertility, although we did observe compromised spermatogenesis in some Prom1−/− males. Histopathological analysis concluded that Prom1 is essential for maintenance of healthy testicular tissue. Also, Prom1-/- mice were significantly obese and had increased in fasting blood glucose as compared with control to Wt mice. Therefore, there appears to be a link between Prom1 and pancreatic islet cell function. Epidemiological associations of both diabetes and obesity with colon cancer risk have been well-established (43). It will be of great interest to determine if decreased Prom1 presence or activity has a role in the relationships between diabetes or obesity and risk for colon cancer in humans. Review manuscript suggests that there is a link between colon cancer progression and obese individual due to mitochondrial dysfunction (44, 45).
To our knowledge, this is the first study to provide an in-depth evaluation of the role and function of Prom1. Given the strong influence of Prom1 presence and control of progression of inflammation and development of dysplastic crypts, as well as limiting tumorigenesis in the presence of Apc loss, one may hypothesize that PROM1 may have a role in inflammation-mediated colonic dysplasia such as that which occurs not infrequently in long-standing IBD of the colon in humans. It will be of great interest to determine if PROM1 presence or activity is reduced in human IBD and particularly in IBD-associated dysplasia and colon cancer. Our findings may also imply that accommodation of the Prom1 pathway by small molecules might be a useful chemopreventive strategy in long-standing IBD. Our results also raise additional questions to be addressed in future studies regarding Prom1 function: if overexpression of Prom1 in proliferative intestinal crypts is mediated by Wnt signaling regenerative pathways and if Prom1 expression acts as a negative regulator in intestinal tumorigenesis, what role does Prom1 play in Wnt responses? Furthermore, does expression of Prom1 contribute to the maintenance of “cancer stemness”?
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research was supported by NIH K01RR021362 (Baktiar O. Karim), TEDCO grant from the state of Maryland (Baktiar O. Karim), and NIH 5 R21 NS054235-02 (Kyuson Yun).
1. Hirano S, Kataoka K. Histogenesis of the mouse jejunal mucosa, with special reference to proliferative cells and absorptive cells. Arch Histol Jpn (1986) 49(3):333–48. doi: 10.1679/aohc.49.333
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Kaur P, Potten CS. Cell migration velocities in the crypts of the small intestine after cytotoxic insult are not dependent on mitotic activity. Cell Tissue Kinet (1986) 19(6):601–10.
3. Qiu JM, Roberts SA, Potten CS. Cell migration in the small and large bowel shows a strong circadian rhythm. Epithelial Cell Biol (1994) 3(4):137–48.
4. Shaker A, Rubin DC. Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. Transl Res (2010) 156(3):180–7. doi:10.1016/j.trsl.2010.06.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Potten CS, Booth C, Hargreaves D. The small intestine as a model for evaluating adult tissue stem cell drug targets. Cell Prolif (2003) 36(3):115–29. doi:10.1046/j.1365-2184.2003.00264.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. Snippert HJ, van Es JH, van den Born M, Begthel H, Stange DE, Barker N, et al. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology (2009) 136(7):2187–2194e1. doi:10.1053/j.gastro.2009.03.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Taïeb N, Maresca M, Guo XJ, Garmy N, Fantini J, Yahi N. The first extracellular domain of the tumour stem cell marker CD133 contains an antigenic ganglioside-binding motif. Cancer Lett (2009) 278(2):164–73. doi:10.1016/j.canlet.2009.01.013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Fargeas CA, Florek M, Huttner WB, Corbeil D. Characterization of prominin-2, a new member of the prominin family of pentaspan membrane glycoproteins. J Biol Chem (2003) 278(10):8586–96. doi:10.1074/jbc.M210640200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Mehra N, Penning M, Maas J, Beerepoot LV, van Daal N, van Gils CH, et al. Progenitor marker CD133 mRNA is elevated in peripheral blood of cancer patients with bone metastases. Clin Cancer Res (2006) 12(16):4859–66. doi:10.1158/1078-0432.CCR-06-0422
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Bauer N, Fonseca AV, Florek M, Freund D, Jászai J, Bornhäuser M, et al. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133). Cells Tissues Organs (2008) 188(1–2):127–38. doi:10.1159/000112847
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Walker TL, Wierick A, Sykes AM, Waldau B, Corbeil D, Carmeliet P, et al. Prominin-1 allows prospective isolation of neural stem cells from the adult murine hippocampus. J Neurosci (2013) 33(7):3010–24. doi:10.1523/JNEUROSCI.3363-12.2013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Florek M, Bauer N, Janich P, Wilsch-Braeuninger M, Fargeas CA, Marzesco AM, et al. Prominin-2 is a cholesterol-binding protein associated with apical and basolateral plasmalemmal protrusions in polarized epithelial cells and released into urine. Cell Tissue Res (2007) 328(1):31–47. doi:10.1007/s00441-006-0324-z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology (2007) 132(2):720–32. doi:10.1053/j.gastro.2006.11.027
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Kordes C, Sawitza I, Müller-Marbach A, Ale-Agha N, Keitel V, Klonowski-Stumpe H, et al. CD133+ hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun (2007) 352(2):410–7. doi:10.1016/j.bbrc.2006.11.029
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol (2006) 17(9):2443–56. doi:10.1681/ASN.2006010089
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci (2004) 117(Pt 16):3539–45. doi:10.1242/jcs.01222
17. Nam-Cha SH, Serrano-Vargas R, Escario E, Azaña JM, Calero-Oliver R, Martín AG, et al. CD133 expression in normal skin and in epithelial cutaneous tumors. Biomed Res Int (2013) 2013:385604. doi:10.1155/2013/385604
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Khan AO, Bolz HJ. Pediatric cone-rod dystrophy with high myopia and nystagmus suggests recessive PROM1 mutations. Ophthalmic Genet (2014). doi:10.3109/13816810.2014.886266
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Pras E, Abu A, Rotenstreich Y, Avni I, Reish O, Morad Y, et al. Cone-rod dystrophy and a frameshift mutation in the PROM1 gene. Mol Vis (2009) 15:1709–16.
20. Sahlberg SH, Spiegelberg D, Glimelius B, Stenerlöw B, Nestor M. Evaluation of cancer stem cell markers CD133, CD44, CD24: association with AKT isoforms and radiation resistance in colon cancer cells. PLoS One (2014) 9(4):e94621. doi:10.1371/journal.pone.0094621
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Singh S, Dirks PB. Brain tumor stem cells: identification and concepts. Neurosurg Clin N Am (2007) 18(1):31–8. doi:10.1016/j.nec.2006.10.014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Tang KH, Ma S, Lee TK, Chan YP, Kwan PS, Tong CM, et al. CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology (2012) 55(3):807–20. doi:10.1002/hep.24739
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest (2008) 118(6):2111–20. doi:10.1172/JCI34401
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Bin Z, Guangbo Z, Yan G, Huan Z, Desheng L, Xueguang Z. Overexpression of B7-H3 in CD133+ colorectal cancer cells is associated with cancer progression and survival in human patients. J Surg Res (2014) 188(2):396–403. doi:10.1016/j.jss.2014.01.014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Chen S, Song X, Chen Z, Li X, Li M, Liu H, et al. CD133 expression and the prognosis of colorectal cancer: a systematic review and meta-analysis. PLoS One (2013) 8(2):e56380. doi:10.1371/journal.pone.0056380
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Gregorieff A, Pinto D, Begthel H, Destrée O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology (2005) 129(2):626–38. doi:10.1016/j.gastro.2005.06.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Karim BO, Rhee KJ, Liu G, Zheng D, Huso DL. Chemoprevention utility of silibinin and Cdk4 pathway inhibition in Apc(-/+) mice. BMC Cancer (2013) 13:157. doi:10.1186/1471-2407-13-157
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Wehkamp J, Stange EF. Paneth’s disease. J Crohns Colitis (2010) 4(5):523–31. doi:10.1016/j.crohns.2010.05.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Clevers H. Wnt/beta-catenin signaling in development and disease. Cell (2006) 127(3):469–80. doi:10.1016/j.cell.2006.10.018
30. Cadigan KM, Peifer M. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol (2009) 1(2):a002881. doi:10.1101/cshperspect.a002881
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol (2013) 35(2):229–44. doi:10.1007/s00281-012-0352-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Osburn WO, Karim B, Dolan PM, Liu G, Yamamoto M, Huso DL, et al. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int J Cancer (2007) 121(9):1883–91. doi:10.1002/ijc.22943
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature (2007) 448(7152):427–34. doi:10.1038/nature06005
34. Taylor KM, Irving PM. Optimization of conventional therapy in patients with IBD. Nat Rev Gastroenterol Hepatol (2011) 8(11):646–56. doi:10.1038/nrgastro.2011.172
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. De Lerma Barbaro A, Perletti G, Bonapace IM, Monti E. Inflammatory cues acting on the adult intestinal stem cells and the early onset of cancer. Int J Oncol (2014) 45(3):959–68. doi:10.3892/ijo.2014.2490
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
36. Kemper K, Sprick MR, de Bree M, Scopelliti A, Vermeulen L, Hoek M, et al. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res (2010) 70(2):719–29. doi:10.1158/0008-5472.CAN-09-1820
37. Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature (2009) 457(7229):603–7. doi:10.1038/nature07589
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
38. Meng X, Li M, Wang X, Wang Y, Ma D. Both CD133+ and CD133− subpopulations of A549 and H446 cells contain cancer-initiating cells. Cancer Sci (2009) 100(6):1040–6. doi:10.1111/j.1349-7006.2009.01144.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Rampazzo E, Persano L, Pistollato F, Moro E, Frasson C, Porazzi P, et al. Wnt activation promotes neuronal differentiation of glioblastoma. Cell Death Dis (2013) 4:e500. doi:10.1038/cddis.2013.32
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
40. Rappa G, Mercapide J, Anzanello F, Le TT, Johlfs MG, Fiscus RR, et al. Wnt interaction and extracellular release of prominin-1/CD133 in human malignant melanoma cells. Exp Cell Res (2013) 319(6):810–9. doi:10.1016/j.yexcr.2013.01.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
41. Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A. GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene (2000) 19(4):537–45. doi:10.1038/sj.onc.1203359
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
42. Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J (1998) 17(5):1371–84. doi:10.1093/emboj/17.5.1371
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
43. Rasool S, Kadla SA, Rasool V, Ganai BA. A comparative overview of general risk factors associated with the incidence of colorectal cancer. Tumour Biol (2013) 34(5):2469–76. doi:10.1007/s13277-013-0876-y
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
44. Aleksandrova K, Nimptsch K, Pischon T. Obesity and colorectal cancer. Front Biosci (Elite Ed) (2013) 5:61–77. doi:10.2741/e596
Keywords: prominin1, CD133, IBD, colitis-associated colon cancer, Apc Min mice
Citation: Karim BO, Rhee K-J, Liu G, Yun K and Brant SR (2014) Prom1 function in development, intestinal inflammation, and intestinal tumorigenesis. Front. Oncol. 4:323. doi: 10.3389/fonc.2014.00323
Received: 16 July 2014; Accepted: 28 October 2014;
Published online: 14 November 2014.
Edited by:
Giuseppe Valentino Masucci, Karolinska Institute, SwedenReviewed by:
Dan A. Dixon, University of Kansas Medical Center, USACopyright: © 2014 Karim, Rhee, Liu, Yun and Brant. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baktiar O. Karim, Department of Molecular and Comparative Pathobiology, Johns Hopkins University School of Medicine, 733 N. Broadway, MRB #849, Baltimore, MD 21205, USA e-mail:YmthcmltQGpobWkuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.