- Laboratory of Immunology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA
Mouse Mammary Tumor Viruses are beta-retroviruses that exist in both exogenous (MMTV) and endogenous (Mtv) forms. Exogenous MMTV is transmitted via the milk of lactating animals and is capable of inducing mammary gland tumors later in life. MMTV has provided a number of critical models for studying both viral infection as well as human breast cancer. In addition to the horizontally transmitted MMTV, most inbred mouse strains contain permanently integrated Mtv proviruses within their genome that are remnants of MMTV infection and vertically transmitted. Historically, Mtv have been appreciated for their role in shaping the T cell repertoire during thymic development via negative selection. In addition, more recent work has demonstrated a larger role for Mtv in modulating host immune responses due to its peripheral expression. The influence of Mtv on host response has been observed during experimental murine models of Polyomavirus- and ESb-induced lymphoma as well as Leishmania major and Plasmodium berghei ANKA infection. Decreased susceptibility to bacterial pathogens and virus-induced tumors has been observed among mice lacking all Mtv. We have also demonstrated a role for Mtv Sag in the expansion of regulatory T cells following chronic viral infection. The aim of this review is to summarize the latest research in the field regarding peripheral expression of Mtv with a particular focus on their role and influence on the immune system, infectious disease outcome, and potential involvement in tumor formation.
Introduction
The integration of viral nucleic acid sequences into the host genome is a hallmark of the retroviral life cycle. Integration in somatic cells results in infection of the host and transmission of the virus requires the production of infectious viral particles that are passed on to a new host horizontally. On the other hand, integration in the germ line results in the generation of endogenous retroviruses that become an inheritable part of the host genome. Endogenous retroviruses constitute a significant fraction of various vertebrate genomes, including both human and mouse (1–3). Two of the more widely studied endogenous retroviruses within the mouse genome are the murine leukemia virus (4) and the mouse mammary tumor virus (5), which exist in both exogenous (MMTV) and endogenous (Mtv) forms. While the exogenous MMTV has been extensively studied for its role in the establishment and transmission of mammary carcinomas (6, 7), much less remains known about the influence of the endogenous Mtv on the host. The aim of this review is to focus on recent advances in understanding the role of endogenous Mtv, particularly in relation to cancer, infection, and immunity.
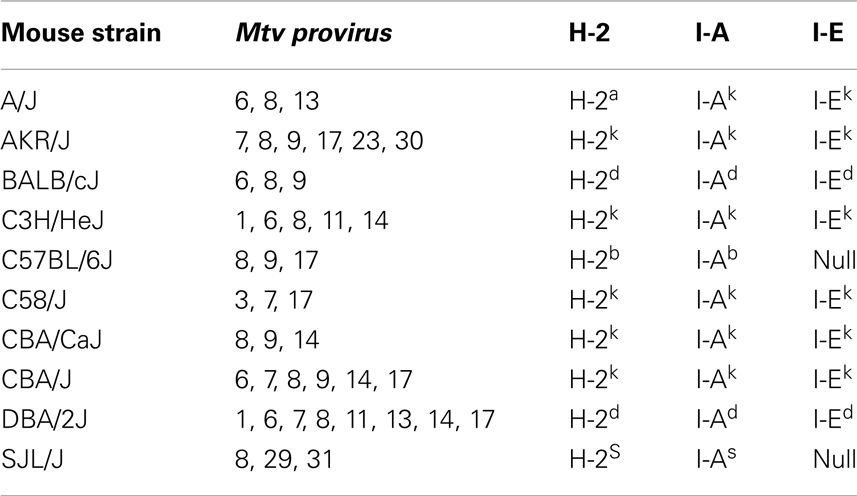
Mice inherit Mtv that have integrated into the host genome according to Mendelian inheritance patterns. Greater than 30 different endogenous Mtv have been identified (8). The most common inbred laboratory mice contain between two and eight copies of endogenous Mtv, the majority of which are shared between several different strains (Table 1) (5, 9). Certain wild-derived (feral) strains of mice, including PERA/Ei and Czech II, are completely devoid of endogenous Mtv. Only a few of the endogenous Mtv, including Mtv-1, -2, and -4 have retained the ability of forming infectious viral particles (10, 11). Some proviruses, including Mtv-2, are capable of both exogenous and endogenous transmission (12).
Endogenous Mtv maintain a genetic structure similar to their exogenous MMTV counterparts. For a detailed description of this genetic makeup see the review by Ross (13). Briefly, MMTV is a type B retrovirus of the Retroviridae family that contains a 9 kb RNA genome encoding virion capsid (Gag) proteins, reverse transcriptase and integrase enzymes necessary for viral replication (Pol), and envelope (Env) proteins used for viral entry. Like all other retroviruses, MMTV is flanked by 5′ and 3′ long terminal repeats (LTRs). The 3′ LTR of MMTV contains an open reading frame that encodes the viral accessory protein, superantigen (Sag), a type 2 transmembrane glycoprotein (14, 15). More recent data has demonstrated that the 3′ LTR of MMTV also encodes another accessory protein, regulatory of export of MMTV (Rem). Rem is required for efficient nuclear export of unspliced viral RNA via interaction with a Rem-responsive element present in MMTV RNA (16). Both Sag and Rem are encoded by alternatively spliced mRNAs. Rem is related to the human immunodeficiency virus (HIV) Rev protein, thus making MMTV a complex retrovirus (17, 18). Endogenous Mtv have accumulated various point mutations or deletions in their proviral genome (19–21). However, almost all Mtv have maintained functional Sag expression. While other viral proteins play important roles (especially in terms of viral particle assembly for MMTV), it is the expression of Sag by both MMTV and Mtv that has been the most extensively studied. Sag expression plays an important role in the biology of both forms of the virus that is best understood from a historical perspective.
Importance of Sag in Exogenous MMTV Infection
As early as 1936, it was observed that certain strains of inbred mice at Jackson Laboratories (ME, USA) displayed an inherent susceptibility to spontaneous mammary carcinomas. The incidence of tumor development ranged from high, intermediate, and low depending on the particular strain of mouse (22). It became readily apparent that the cancer-inducing agent was maternally transmitted and present in milk, and subsequent experiments demonstrated that the causative agent was a virus. Further work led to the discovery and identification of MMTV, originally known as Bittner virus, as the causative agent (23).
In order for MMTV to reach the mammary gland, a complex series of events must occur in which the virus requires and subverts cells of the immune system, particularly B and T cells, to establish infection. The initial site of milk-borne MMTV infection is the gut-associated lymphoid tissue, specifically the Peyer’s patches, where B cells represent the initial target cell (24). The initial round of B cell infection and activation occurs as a result of the interaction between MMTV Env protein and Toll-like receptor 4 (25). The ability of MMTV to disseminate from the gut to the mammary gland is dependent upon this expression of the virally encoded Sag (26). Infected B cells present Sag in conjunction with the major histocompatibility complex (MHC) class II proteins to CD4+ T cells bearing a reactive T cell receptor (TCR) Vβ chain. A polymorphic region within the carboxyl terminus of MMTV Sag determines the TCR Vβ domain specificity (27–29). Different MHC class II alleles display strikingly disparate Sag presentation capabilities, with MHC class II I-E inducing the most efficient presentation to T cells (30). Stimulation of Sag-reactive CD4+ T cells leads to their activation and production of various cytokines and chemokines. In addition, activated T cells upregulate CD40 ligand (CD40L), which binds to the CD40 receptor on B cells to further activate them (31). Such activation stimulates and recruits additional B and T cells, thereby resulting in further immune cell activation and the subsequent amplification of a reservoir of MMTV infected cells (32). Infected B cells then travel to the developing mammary gland and thereby enable the virus to infect the tumor-susceptible target organ. The critical requirement for an infected lymphocyte population during MMTV infection and dissemination is demonstrated by the resistance of nude mice which lack a functional T cell compartment (33), neonatal thymectomized mice (34), mice lacking Sag-reactive T cells (26), or B cell deficient mice (24) to milk-borne transmission of MMTV-induced tumor development. Mice with inefficient presentation of MMTV Sag due to MHC class II mutations are also resistant to viral infection (35).
Since MMTV does not encode an oncogene, mammary tumorigenesis occurs after insertion of proviral DNA near cellular proto-oncogenes and activation of transcription (36–38). Analysis of genes activated by integration of the MMTV provirus using the viral genome as a molecular tag enabled the identification of a number of MMTV-tagged genes. Of these genes, the Wnt [relating to the Drosophilia segmented polarity gene wingless (Wg)] and fibroblast growth factor (Fgf) family of genes represent major targets of mutagenic effects (36, 39–41). However, the frequency of gene activation responsible for mammary tumor development is dependent on both the strain of the mouse and the virus.
History of Endogenous Mtv
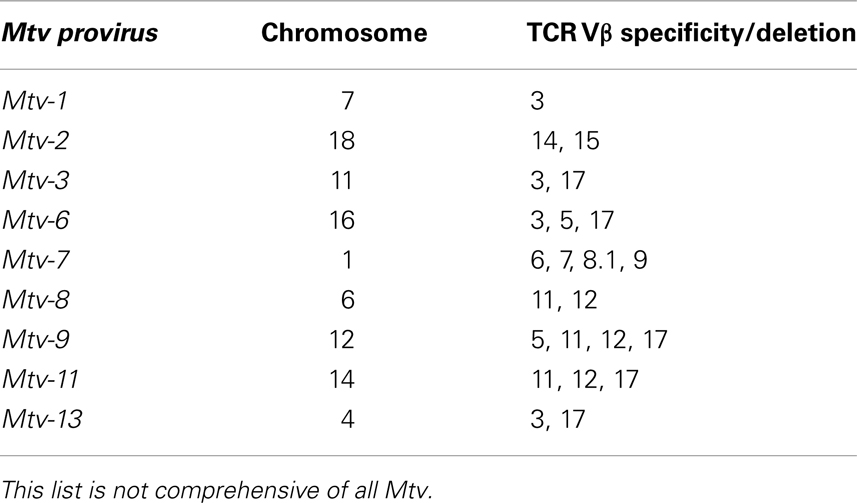
Retrospectively, evidence of Mtv Sag expression was first observed in experiments demonstrating non-reciprocal lymphocyte activation/proliferation in mixed lymphocyte cultures from MHC-identical strains of mice (42). Before it was known that Mtv Sag was the culprit, the antigens responsible for inducing lymphocyte activation/proliferation were termed minor lymphocyte stimulating (Mls) antigens. For example, Mlsa expressed by DBA/2 mice results in T cell activation of BALB/c splenocytes when co-cultured. Once monoclonal antibodies to the Vβ regions of the TCR were made available, it became apparent that Mlsa resulted in the activation of BALB/c T cells expressing Vβ6 and that Vβ6+ T cells were largely absent in the repertoire of DBA/2 mice. Subsequent genetic studies linked various Mls antigens to Mtv loci (43–46) and Sag as the element responsible for Vβ-specific T cell interaction (14, 15). Therefore, an important consequence of Mtv Sag expression is the ultimate alteration of the peripheral adaptive T cell repertoire mediated by intrathymic deletion of Sag-reactive T cells, the extent and kinetics of which vary depending on the specific Sag (47–49). For example, the Sag associated with Mtv-7 stimulates and deletes T cells bearing Vβ 6, 7, 8.1, and 9 (50, 51), while that of Mtv-9 induces complete deletion of TCR Vβ5, 11, and 12 (44, 46, 52). Table 2 provides the chromosomal location and TCR Vβ specificity/deletion for a few of the more commonly encountered endogenous Mtv among different mouse strains (53, 54).
The fact that endogenous retroviruses in general, and Mtv in specific remain present in high percentage within the host genome over a long portion of evolution would provide the opportunity for interaction with host genes and subsequent influence of cellular function. Although a large portion of such proviruses no longer encode for functional products, thereby supporting the idea that these endogenous retroviruses are simply genomic “fossils,” there do exist viral elements that have retained activity. The question is therefore what is the evolutionary role or advantage for maintaining these proviruses within the genome? One hypothesis that has been proposed is that Mtv are retained by certain mouse strains to serve as an evolutionary means of protection against exogenous milk-borne MMTV infection and MMTV-induced mammary tumors (55). This protection would result from the deletion of Mtv-encoded Sag-specific T cells and the subsequent loss of a reactive pool of T cells necessary for infection. In support of this idea, the transgenic expression of the Sag gene of the C3H strain of MMTV has been shown to protect against exogenous MMTV encoding the same Sag specificity (26). However, it is unlikely that this is the only evolutionary role for endogenous Mtv, since wild-derived mice lacking Mtv are not overwhelmed by mammary carcinomas. Therefore, the question remains, what evolutionary advantage does the maintenance of these endogenous proviral genes impart to the mouse? A number of models have demonstrated that Mtv have the capacity to modulate the immune response, thereby providing either a selective disadvantage or advantage in regards to cancer, infection, and immunity. Some of these models require Sag expression and some may require other components of the proviral genome via mechanisms that are not fully understood.
Influence of Endogenous Mtv on Cancer
Evidence exists that Mtv can influence the development of MMTV-induced mammary carcinomas via a mechanism that does not require interaction with CD4+ T cells expressing a Sag-reactive TCR Vβ region. A number of mouse strains, including GR, C3H, BR6, and R111 have been selectively inbred for a high incidence of mammary tumors due to their transmission of MMTV to offspring via milk. Two of these strains, GR and C3H, additionally contain endogenous copies of Mtv that also generate infectious viral particles. Among weanlings of GR mice, the incidence of pregnancy-independent tumors occurs at a similar frequency whether nursed on GR mothers or foster-nursed on MMTV-free mothers (56). Mtv-2 was later identified as the dominant gene responsible for tumor development as well as responsible for the expression of MMTV within the milk of GR mice. When C3H mice are freed of their corresponding exogenous C3H-MMTV via foster-nursing, mammary tumors still develop, yet with varying incidence and increased latency. This tumor development was determined to be dependent on the expression of Mtv-1 (10).
Another tumor model where Mtv has been shown to be important is infection with Polyomaviruses (PyVs) – a family of small non-enveloped, double-stranded DNA viruses with potent oncogenic capacity to induce epithelial and mesenchymal cell-derived tumors (57). Susceptibility to PyV-induced tumors has been shown among certain strains of H-2k expressing mice (58, 59). A previously identified PyV susceptibility gene (PyvS) (60) was later identified to encode the Sag from Mtv-7 among H-2k mice (C3H/BiDa mice) resulting in the intrathymic deletion of TCR Vβ6 expressing T cell populations (59). Among other mechanisms (61), preferential usage of H-2k-restricted CD8+ T cells expressing TCR Vβ6 that were specific for an immunodominant peptide derived from the viral middle T protein were demonstrated to provide the necessary antitumor immunity against PyV (62). Susceptibility to PyV is transmitted as a dominant trait due to the requirement of only a single copy of the superantigen being necessary for the deletion of T cells expressing specific Vβ TCRs. H-2k-identical C57BR/cdJ (BR mice) retain this cytotoxic CD8+ TCR Vβ6 population due to the lack of Mtv-7 and are therefore highly resistant to tumor development.
Mtv-7 has also been shown to influence the immune response among H-2d-expressing mice in a murine model of aggressive lymphoma. Although DBA/2 and B10.D2 strains of mice are syngeneic at the MHC and therefore immunologically compatible, they demonstrate varied immune response and outcome upon challenge with the highly malignant DBA/2-derived ESb cell line (63). B10.D2 mice are able to prevent metastasis of such tumors by generating and sustaining a sufficient humoral and cellular immune response. This response is evident by the increased sensitivity of B10.D2 following irradiation or depletion of CD4+ and CD8+ T cells (64). In contrast, naïve DBA/2 mice are highly susceptible to ESb-induced lymphoma (65). The generation of recombinant inbred (RI) mouse strains from the cross of B10.D2 and DBA/2 facilitated the identification of potential genes segregating with ESb-related tumor susceptibility and resistance (63). Genotyping for Mtv among several of the tumor-resistant RI strains revealed similarity to the parental DBA/2 strain except for the loss of a particular LTR that corresponded to the Mtv-7 provirus. It was further demonstrated that ESb tumor cells themselves express proviral Mtv-7 at both the mRNA and protein level (66). Instead of inducing anergy among the Sag-reactive cells, the ESb tumor-associated Mtv-7-encoded Sag was demonstrated to induce activation of Sag-specific cytotoxic T lymphocytes. TCR Vβ6 cells were demonstrated to facilitate specific killing of tumor cells expressing the endogenous Mtv-7 in vitro. Furthermore, treatment of tumor-bearing DBA/2 mice with TCR Vβ6 T cells from naïve B10.D2 mice led to a significant increase in survival and concurrent reduction in tumor growth (66). It is important to note that the reduction in tumor growth and delay in death lasted only 10 days, suggesting the potential and eventual loss of the TCR Vβ6 T cell population. Therefore, in models of both PyV and ESb-induced lymphoma, the presence of Mtv-7 affords a selective disadvantage in terms of deleting a protective population of lymphocytes that are necessary for tumor immunity.
In contrast to the deletion of Sag-reactive T cells, Mtv-encoded Sag may further modulate tumor susceptibility via activation of Sag-reactive T cells. Spontaneous reticulum cell sarcoma (RCS), a form of B cell lymphoma, has been observed to arise in >90% of SJL mice older than 12 months of age. The development of such RCS requires the presence of host T cells and specifically that of T cell-derived cytokines, such as IL-5 and IFN-γ (67, 68). The strong proliferative response of previously unsensitized CD4+ T cells to RCS cells (69) and the biased usage of such responding cells expressing TCR Vβ16 imply a role for superantigen in this model (70). CD4+ TCR Vβ16 cells have been shown to be capable of supporting RCS growth via the production of IL-2, IL-4, and IL-5. Additional analysis revealed the high expression of a novel MMTV-LTR among mRNA from RCS cells (70). It was subsequently determined that the B cell lymphomas of SJL mice contained Mtv-29, which via the Mtv-29-encoded Sag were capable of stimulating TCR Vβ16 cells that are required to support the development of spontaneous B cell lymphomagenesis (71).
Although a majority of the research examining the role of Mtv in modulation of the immune response has mainly focused on Sag-dependent processes, evidence does exist to support Mtv-associated, Sag-independent mechanisms. BALB/c mice harbor three distinct Mtv (Mtv-6, -8, and -9) all on separate chromosomes, which in the context of MHC class II I-E induce intrathymic deletion of reactive T cells expressing TCR Vβ3, 5, 11, and 12. The generation of BALB/c congenic mice lacking all endogenous Mtv (BALB/Mtv-null) enabled the unique opportunity to examine the influence of Mtv on host response to a variety of models (72). In the absence of endogenous Mtv, the incidence of mammary tumors in response to exogenous MMTV infection was reduced from 100% (in BALB/c) to 10% (in BALB/Mtv-null). This drastic reduction in tumor incidence was independent of the infection route, whether milk-borne infection or following intraperitoneal injection of a stable cell line expressing the infectious cloned MMTV provirus. Unlike some mouse strains that demonstrate resistance to exogenous MMTV infection via the production of neutralizing antibodies (73), such was not the case with the BALB/Mtv-null mice. BALB/Mtv-null mice also demonstrated resistance to infection with the MMTV variant type B leukemogenic virus (TBLV) (74), which due to a truncated Sag protein lacks the ability to induced Sag-mediated T cell deletion and thereby induces T cell lymphoma rather than MMTV-induced mammary cancers.
The C3H strain of MMTV that was shown to have reduced capacity to induce tumorigenesis in BALB/Mtv-null mice (72) encodes a weak Sag resulting in a slow and marginal deletion of CD4+ T cells expressing the C3H Sag-reactive TCR Vβ14 (48). This would suggest that endogenous Mtv-encoded Sag are necessary during the early stages of infection for exogenous Sag presentation and cognate T cell deletion. In contrast to C3H-MMTV, infection with FM-MMTV, which encodes a stronger Sag (75, 76) and therefore results in a much larger response by the cognate TCR Vβ8.2+ population, resulted in a similar magnitude and kinetics of Sag response between BALB/c and BALB/Mtv-null mice. These findings imply that the extent of deletion associated with the exogenous MMTV-encoded Sag dictates the impact of endogenous Sag during MMTV infection. While endogenous Sag appear essential during the early stages of infection with C3H-MMTV, a stronger Sag such as the FM-MMTV strain can overcome this requirement in terms of establishing the initial infection. Regardless of this early and initial infectivity, the absence of Mtv in BALB/Mtv-null mice affords them high resistance to FM-MMTV-induced tumorigenesis and such mice are further incapable of transmitting the virus via their milk (76).
Influence of Endogenous Mtv on Infection
In the experimental murine model of Leishmania major infection, differences in susceptibility have been attributed to the host’s ability to generate a sufficient Th1-biased, IFN-γ-dominated, CD4+ T cell response (77). This is most evident upon analysis of the highly susceptible and Th2/IL-4-prone BALB/c strain compared to the highly resistant C57BL/6 strain that is prototypical of a Th1/IFN-γ response. Although certainly much more resistant to disease compared to the BALB/c strain, the CBA/CaJ and CBA/J strains of mice differ in their responses to L. major infection. In contrast to CBA/CaJ mice that develop only transient lesions upon infection, persistent inflammatory lesions and 10-fold higher parasite density are observed in CBA/J mice (78). A quantitative reduction in the production of IFN-γ in the CBA/J was determined to account for the persistent lesions upon infection. This reduction in cytokine production further correlated with the presence of Mtv-7 in the CBA/J strain that is absent in the CBA/CaJ mice. However, unlike the above models of PyV and ESb-induced lymphoma, Mtv-7-specific TCR Vβ6 T cells do not constitute the predominant responding population during L. major infection. Furthermore, the overall magnitude and heterogeneity of the CD4+ T cell response was similar between the two strains (78). Such findings would suggest that T cells in general, regardless of their TCR Vβ repertoire commit somewhat poorly to a Th1-biased, IFN-γ-producing subset in the presence of Mtv-7 during leishmaniasis. This L. major-specific effect in the presence of Mtv-7 was determined to be mediated via alteration of the T cell priming ability of antigen presenting cells. Although no difference was observed in L. major-infected, bone marrow-derived dendritic cells (BMDCs) between CBA/CaJ and CBA/J in terms of their expression of various cell surface and co-stimulatory markers, CD4+ T cells from CBA/J mice did produce increased IFN-γ if primed in the presence of L. major-infected BMDCs from CBA/CaJ mice compared to their own BMDCs (78). Since impairment of the CBA/J immune response is not due to a specific TCR Vβ subset, these findings would suggest a Mtv-7-dependent, Sag-independent mechanism responsible for modulating T cell priming during the pathogenesis of leishmaniasis.
The murine model of Plasmodium berghei ANKA infection constitutes a widely used model that reflects some experimental similarities to that of human cerebral malaria (CM). The neurological symptoms of genetically susceptible mice infected with P. berghei ANKA have been shown to be immune mediated, mainly dependent on CD4+ and CD8+ T cells (79, 80). In this model, the high production of Th1-derived cytokines, including IFN-γ and TNF-α, via interferon regulatory factor-1 (IRF-1) has been implicated in malarial neuropathogenesis (81, 82). The peripheral expansion of a restricted T cell population expressing TCR Vβ8.1 and Vβ8.2 was observed in the highly susceptible B10.D2 (H-2d) strain that manifest severe neurological symptoms but not in infected mice that failed to develop CM. The occurrence of CM was significantly reduced following treatment with a monoclonal antibody against TCR Vβ8.1 specific T cells. Furthermore, the endogenous presence of Mtv-7 in congenic BALB.D2 mice, or the exogenous counterpart MMTV-SW that encodes the same Sag as Mtv-7 and is present in BALB.SW mice, results in Sag-mediated deletion or anergy of a variety of Vβ-specific populations including Vβ8.1 that has been demonstrated to provide protection against CM (83). To investigate whether the presence of Mtv-7 was sufficient to induce resistance to CM, RI strains derived from a cross between the susceptible BALB/c (negative for Mtv-7) and resistant DBA/2 (positive for Mtv-7) were examined for their response during P. berghei ANKA infection. A strong correlation was identified between the presence of Mtv-7 and protection against neuropathogenesis of CM (84). The similar levels of parasitemia observed between Mtv-7+ and Mtv-7− mice would suggest that integration of the viral genome did not have any effect on at least the initial stages of parasite growth and development (84). Overall, it was concluded that deletion of T cells expressing TCR Vβ8.1 among H-2d expressing strains of mice, whether via the exogenous or endogenous presence of Mtv-7-encoded Sag was sufficient to confer resistance to CM. Unlike the models of PyV, ESb-induced lymphoma, and L. major infection, in which the presence of Mtv confers a selective disadvantage to the host and increases susceptibility, Mtv appears to provide protection during the neuropathogenesis of P. berghei ANKA infection, by an as of yet unidentified mechanism.
In addition to modulation of the conventional population of CD8+ and CD4+ T cells, Mtv have been demonstrated to influence the immune system during viral infection via expansion of a particular subset of CD4+ T cells expressing the transcription factor Foxp3. Murine infection with the clone 13 isolate of lymphocytic choriomeningitis virus (LCMV) represents a model of chronic viral infection, in which T cell exhaustion results in infection virtually for life (85, 86). Infection of mice with LCMV clone 13 resulted in the selective expansion of a TCR Vβ specific population of Foxp3+ regulatory T cells (Treg) (87). The expansion of TCR Vβ5+ Treg was determined to be MHC class II-dependent, CD4-independent, and secondary to stimulation by the Mtv-9-encoded Sag. Treg play a key role in immune suppression and control of organ-specific autoimmune diseases via a variety of mechanisms. Whether this specific Mtv-encoded Sag-mediated expansion of Treg influences the pathogenesis of viral or other infectious agents remains to be determined. Nevertheless, it does demonstrate the wide range of influence Mtv has on the immune system, influencing both effector and suppressor lymphocyte populations.
In addition to the reduction in tumorigenicity via both a Sag-dependent and Sag-independent manner, the absence of Mtv provided further resistance to inoculation with the gram-negative bacterium Vibrio cholerae, as demonstrated by decreased bacterial replication and mortality in BALB/Mtv-null mice. However, the absence of Mtv did not provide absolute resistance to bacterial challenge, as infection with Salmonella typhimurium resulted in similar mortality between BALB/c and BALB/Mtv-null mice. The reintroduction of any single Mtv provirus into the BALB/Mtv-null mice (generating congenic mice positive for either Mtv-6, -8, or -9) was sufficient to restore susceptibility to both select viral (MMTV-induced mammary tumors) as well as bacterial (V. cholera) challenge. Interestingly, the difference in susceptibility to V. cholera was observed as early as 48 h following infection, suggesting Mtv may function to regulate processes of innate immunity. However, the exact mechanism for this increased susceptibility to disparate pathogens among mice expressing at least one or more Mtv remains to be elucidated. One clue may resolve around the unique fact that unlike Mtv-8 and -9, Mtv-6 lacks a >6 kb portion of sequence encoding most of the viral Gag, Env, and Pol proteins. Mtv-6 does encode functional Sag, which would suggest that a Sag-mediated or as of yet unidentified Mtv-encoded product may modulate an immune response that is uniquely shared between MMTV and V. cholera.
Influence of Endogenous Mtv on Immunity
In addition to the above models, Mtv-7 was implicated to modulate the immune response during the progression of graft-versus-host disease (GVHD). In this model, the transfer of either parental C57BL/6 or DBA/2 lymphoid cells into F1 offspring (B6D2F1) yields opposing disease outcome. Donor lymphoid cells from C57BL/6, which are associated with a Th1-biased, IFN-γ-mediated cytokine response, induce an acute form of GVHD. On the other hand, the Th2-prone, IL-4-mediated response characteristic of DBA/2 yields a more chronic disease. Although C57BL/6 and DBA/2 differ in MHC haplotype, such a difference between the induction of an acute and chronic response was attributed to a non-MHC-related mechanism (88). Mtv-7, or that of a closely linked locus to Mtv-7 was implicated in the relative development of acute and chronic GVDH based on data from congenic C57BL/6 mice containing DBA/2-derived alleles at the region surrounding Mtv-7 on chromosome 1 (89). When lymphoid cells from the congenic C57BL/6 (H-2d) were transferred to recipient B6D2F1 mice, the mice developed signs more consistent with that of chronic GVDH than that of the acute disease normally associated after transfer of C57BL/6 cells (89). Furthermore, transfer of the congenic C57BL/6 lymphoid cells containing the DBA/2-derived alleles around Mtv-7 resulted in decreased expression levels of IFN-γ and increased levels of IL-4 compared to the control C57BL/6 transfer. Although these data would suggest a Mtv-7-mediated influence on disease outcome, depletion of Mtv-7-encoded Sag-reactive cells from the donor population did not impact the development of acute GVHD. As such, the possibility remains that a locus near that of Mtv-7 on chromosome 1 can influence host immune response and the eventual outcome of GVHD.
Collectively, the above models demonstrate the range of influence endogenous Mtv have in their ability to modulate host susceptibility to a variety of infections and diseases in seemingly opposite manners (Figure 1). In the case of PyV infection and ESb-induced lymphoma, Mtv-encoded Sag induce deletion of tumor-reactive T cells thereby facilitating tumor development and progression. At the same time, Mtv-mediated activation/stimulation of Sag-reactive T cells appears to promote neuropathogenesis during P. berghei ANKA infection as well as induce B cell lymphoma. In addition to conventional T cells, Mtv has been shown to modulate the population of Foxp3+ regulatory T cells via a Sag-dependent manner during the course of chronic viral infection. The activation of Treg can combat and limit the extent of autoimmune disease while at the same time facilitate tumor progression by suppressing tumor immunity. The potential ability to modulate such a dynamic population of T cells can therefore have important implications on host outcome. In addition to Sag-dependent mechanisms, other as of yet unidentified genes from the Mtv provirus may be capable of influencing Sag-independent immune responses, such as during L. major infection. Furthermore, Mtv-mediated influence may not be restricted to the adaptive immune system, as evidence using BALB/Mtv-null mice may suggest modulation of potential innate immune responses.
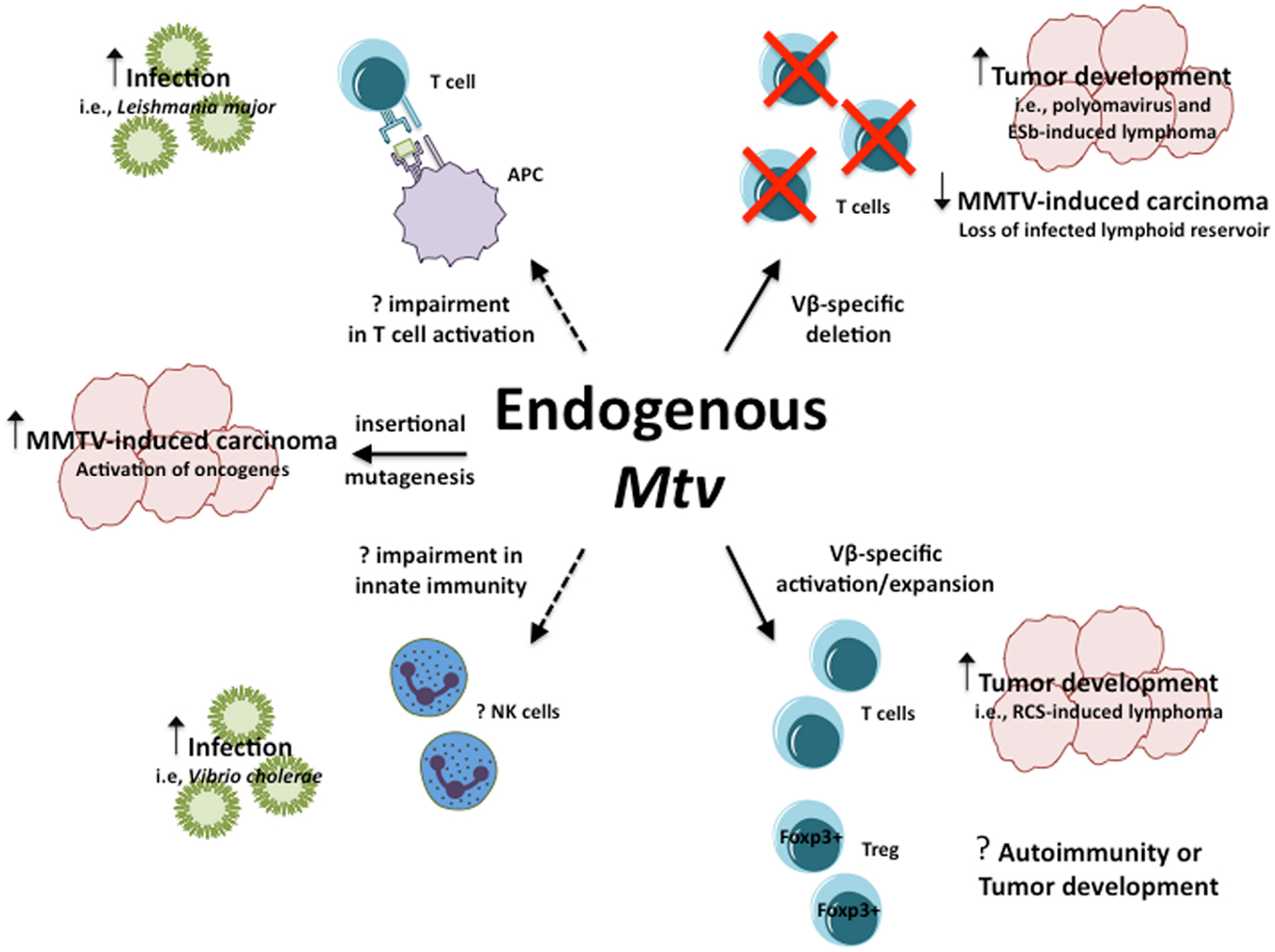
Figure 1. Influence of endogenous Mtv on host response to infection and disease. Potential mechanisms by which endogenous Mtv influence host response during infection and disease. Solid lines represent known mechanisms, dashed lines represent potential mechanisms. Question marks represent outcomes that remain to be determined.
Conclusion
The co-evolution of Mtv and its murine host has allowed ample time for the virus to develop various mechanisms to modulate immune regulatory mechanisms. While many endogenous proviral gene sequences have acquired mutations that have led them to be non-functional, Sag expression has remained intact suggesting some benefit for the host. Recent advances suggest that these advantages go beyond the initial idea that Mtv exist solely as a way to prevent exogenous MMTV-induced mammary carcinoma. While the exact mechanisms by which Mtv function in all of these various models are not fully known, further insight into how endogenous Mtv, and in particular Mtv Sag, influence the immune system can provide potential benefit. Such knowledge may prove useful for therapeutic modifications of lymphocytes using retroviral-based vectors for gene therapy (90). In addition, although there are differences in terms of the activity of viral and bacterial superantigens, the knowledge gained from endogenous retroviruses may prove potentially useful for the targeting of Sag-reactive lymphocytes to tumor cells (91). Ultimately, understanding the mechanisms underlying retroviral integration and interaction with the immune system could provide benefits to a multitude of disease processes, including infectious diseases and cancers.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Tristem M. Identification and characterization of novel human endogenous retrovirus families by phylogenetic screening of the human genome mapping project database. J Virol (2000) 74(8):3715–30. doi: 10.1128/JVI.74.8.3715-3730.2000
2. Polavarapu N, Bowen NJ, McDonald JF. Identification, characterization and comparative genomics of chimpanzee endogenous retroviruses. Genome Biol (2006) 7(6):R51. doi:10.1186/gb-2006-7-6-r51
3. Huda A, Polavarapu N, Jordan IK, McDonald JF. Endogenous retroviruses of the chicken genome. Biol Direct (2008) 3:9. doi:10.1186/1745-6150-3-9
4. Stocking C, Kozak CA. Murine endogenous retroviruses. Cell Mol Life Sci (2008) 65(21):3383–98. doi:10.1007/s00018-008-8497-0
5. Kozak C, Peters G, Pauley R, Morris V, Michalides R, Dudley J, et al. A standardized nomenclature for endogenous mouse mammary tumor viruses. J Virol (1987) 61(5):1651–4.
6. Karapetian O, Shakhov AN, Kraehenbuhl JP, Acha-Orbea H. Retroviral infection of neonatal Peyer’s patch lymphocytes: the mouse mammary tumor virus model. J Exp Med (1994) 180(4):1511–6. doi:10.1084/jem.180.4.1511
7. Hook LM, Agafonova Y, Ross SR, Turner SJ, Golovkina TV. Genetics of mouse mammary tumor virus-induced mammary tumors: linkage of tumor induction to the gag gene. J Virol (2000) 74(19):8876–83. doi:10.1128/JVI.74.19.8876-8883.2000
8. Lee BK, Eicher EM. Segregation patterns of endogenous mouse mammary tumor viruses in five recombinant inbred strain sets. J Virol (1990) 64(9):4568–72.
9. Morris VL, Medeiros E, Ringold GM, Bishop JM, Varmus HE. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol (1977) 114(1):73–91. doi:10.1016/0022-2836(77)90284-4
10. van Nie R, Verstraeten AA. Studies of genetic transmission of mammary tumour virus by C3Hf mice. Int J Cancer (1975) 16(6):922–31. doi:10.1002/ijc.2910160606
11. Michalides R, van Deemter L, Nuss RR, van Nie R. Identification of the Mtv-2 gene responsible for the early appearance of mammary tumors in the GR mouse by nucleic acid hybridization. Proc Natl Acad Sci U S A (1978) 75(5):2368–72. doi:10.1073/pnas.75.5.2368
12. Michalides R, van Nie R, Nusse R, Hynes NE, Groner B. Mammary tumor induction loci in GR and DBAf mice contain one provirus of the mouse mammary tumor virus. Cell (1981) 23(1):165–73. doi:10.1016/0092-8674(81)90281-6
13. Ross SR. MMTV infectious cycle and the contribution of virus-encoded proteins to transformation of mammary tissue. J Mammary Gland Biol Neoplasia (2008) 13(3):299–307. doi:10.1007/s10911-008-9090-8
14. Acha-Orbea H, Shakhov AN, Scarpellino L, Kolb E, Muller V, Vessaz-Shaw A, et al. Clonal deletion of V beta 14-bearing T cells in mice transgenic for mammary tumour virus. Nature (1991) 350(6315):207–11. doi:10.1038/350207a0
15. Choi Y, Kappler JW, Marrack P. A superantigen encoded in the open reading frame of the 3’ long terminal repeat of mouse mammary tumour virus. Nature (1991) 350(6315):203–7. doi:10.1038/350203a0
16. Mertz JA, Lozano MM, Dudley JP. Rev and Rex proteins of human complex retroviruses function with the MMTV Rem-responsive element. Retrovirology (2009) 6:10. doi:10.1186/1742-4690-6-10
17. Indik S, Gunzburg WH, Salmons B, Rouault F. A novel, mouse mammary tumor virus encoded protein with Rev-like properties. Virology (2005) 337(1):1–6. doi:10.1016/j.virol.2005.03.040
18. Mertz JA, Simper MS, Lozano MM, Payne SM, Dudley JP. Mouse mammary tumor virus encodes a self-regulatory RNA export protein and is a complex retrovirus. J Virol (2005) 79(23):14737–47. doi:10.1128/JVI.79.23.14737-14747.2005
19. Salmons B, Knedlitschek G, Kennedy N, Groner B, Ponta H. The endogenous mouse mammary tumour virus locus Mtv-8 contains a defective envelope gene. Virus Res (1986) 4(4):377–89. doi:10.1016/0168-1702(86)90084-5
20. Kuo WL, Vilander LR, Huang M, Peterson DO. A transcriptionally defective long terminal repeat within an endogenous copy of mouse mammary tumor virus proviral DNA. J Virol (1988) 62(7):2394–402.
21. Cho K, Ferrick DA, Morris DW. Structure and biological activity of the subgenomic Mtv-6 endogenous provirus. Virology (1995) 206(1):395–402. doi:10.1016/S0042-6822(95)80055-7
22. Bittner JJ. Some possible effects of nursing on the mammary gland tumor incidence in mice. Science (1936) 84(2172):162. doi:10.1126/science.84.2172.162
23. Bittner JJ. The milk-influence of breast tumors in mice. Science (1942) 95(2470):462–3. doi:10.1126/science.95.2470.462
24. Beutner U, Kraus E, Kitamura D, Rajewsky K, Huber BT. B cells are essential for murine mammary tumor virus transmission, but not for presentation of endogenous superantigens. J Exp Med (1994) 179(5):1457–66. doi:10.1084/jem.179.5.1457
25. Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc Natl Acad Sci U S A (2002) 99(4):2281–6. doi:10.1073/pnas.042355399
26. Golovkina TV, Chervonsky A, Dudley JP, Ross SR. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell (1992) 69(4):637–45. doi:10.1016/0092-8674(92)90227-4
27. Pullen AM, Wade T, Marrack P, Kappler JW. Identification of the region of T cell receptor beta chain that interacts with the self-superantigen MIs-1a. Cell (1990) 61(7):1365–74. doi:10.1016/0092-8674(90)90700-O
28. Acha-Orbea H, Scarpellino L, Shakhov AN, Held W, MacDonald HR. Inhibition of mouse mammary tumor virus-induced T cell responses in vivo by antibodies to an open reading frame protein. J Exp Med (1992) 176(6):1769–72. doi:10.1084/jem.176.6.1769
29. Yazdanbakhsh K, Park CG, Winslow GM, Choi Y. Direct evidence for the role of COOH terminus of mouse mammary tumor virus superantigen in determining T cell receptor V beta specificity. J Exp Med (1993) 178(2):737–41. doi:10.1084/jem.178.2.737
30. Maillard I, Erny K, Acha-Orbea H, Diggelmann H. A V beta 4-specific superantigen encoded by a new exogenous mouse mammary tumor virus. Eur J Immunol (1996) 26(5):1000–6. doi:10.1002/eji.1830260507
31. Chervonsky AV, Xu J, Barlow AK, Khery M, Flavell RA, Janeway CA Jr. Direct physical interaction involving CD40 ligand on T cells and CD40 on B cells is required to propagate MMTV. Immunity (1995) 3(1):139–46. doi:10.1016/1074-7613(95)90166-3
32. Held W, Shakhov AN, Izui S, Waanders GA, Scarpellino L, MacDonald HR, et al. Superantigen-reactive CD4+ T cells are required to stimulate B cells after infection with mouse mammary tumor virus. J Exp Med (1993) 177(2):359–66. doi:10.1084/jem.177.2.359
33. Tsubura A, Inaba M, Imai S, Murakami A, Oyaizu N, Yasumizu R, et al. Intervention of T-cells in transportation of mouse mammary tumor virus (milk factor) to mammary gland cells in vivo. Cancer Res (1988) 48(22):6555–9.
34. Squartini F, Olivi M, Bolis GB. Mouse strain and breeding stimulation as factors influencing the effect of thymectomy on mammary tumorigenesis. Cancer Res (1970) 30(7):2069–72.
35. Wrona T, Dudley JP. Major histocompatibility complex class II I-E-independent transmission of C3H mouse mammary tumor virus. J Virol (1996) 70(2):1246–9.
36. Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell (1982) 31(1):99–109. doi:10.1016/0092-8674(82)90409-3
37. Dickson C, Peters G. Potential oncogene product related to growth factors. Nature (1987) 326(6116):833. doi:10.1038/326833a0
38. Faschinger A, Rouault F, Sollner J, Lukas A, Salmons B, Gunzburg WH, et al. Mouse mammary tumor virus integration site selection in human and mouse genomes. J Virol (2008) 82(3):1360–7. doi:10.1128/JVI.02098-07
39. Dickson C, Smith R, Brookes S, Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell (1984) 37(2):529–36. doi:10.1016/0092-8674(84)90383-0
40. Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature (1984) 307(5947):131–6. doi:10.1038/307131a0
41. Peters G, Brookes S, Smith R, Placzek M, Dickson C. The mouse homolog of the hst/k-FGF gene is adjacent to int-2 and is activated by proviral insertion in some virally induced mammary tumors. Proc Natl Acad Sci U S A (1989) 86(15):5678–82. doi:10.1073/pnas.86.15.5678
42. Festenstein H. Immunogenetic and biological aspects of in vitro lymphocyte allotransformation (MLR) in the mouse. Transplant Rev (1973) 15:62–88.
43. Woodland D, Happ MP, Bill J, Palmer E. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive T cells. Science (1990) 247(4945):964–7. doi:10.1126/science.1968289
44. Dyson PJ, Knight AM, Fairchild S, Simpson E, Tomonari K. Genes encoding ligands for deletion of V beta 11 T cells cosegregate with mammary tumour virus genomes. Nature (1991) 349(6309):531–2. doi:10.1038/349531a0
45. Frankel WN, Rudy C, Coffin JM, Huber BT. Linkage of Mls genes to endogenous mammary tumour viruses of inbred mice. Nature (1991) 349(6309):526–8. doi:10.1038/349526a0
46. Woodland DL, Happ MP, Gollob KJ, Palmer E. An endogenous retrovirus mediating deletion of alpha beta T cells? Nature (1991) 349(6309):529–30. doi:10.1038/349529a0
47. Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell (1987) 49(2):273–80. doi:10.1016/0092-8674(87)90568-X
48. Marrack P, Kushnir E, Kappler J. A maternally inherited superantigen encoded by a mammary tumour virus. Nature (1991) 349(6309):524–6. doi:10.1038/349524a0
49. Kappler JW, Wade T, White J, Kushnir E, Blackman M, Bill J, et al. A T cell receptor V beta segment that imparts reactivity to a class II major histocompatibility complex product. Cell (1987) 49(2):263–71. doi:10.1016/0092-8674(87)90567-8
50. Beutner U, Frankel WN, Cote MS, Coffin JM, Huber BT. Mls-1 is encoded by the long terminal repeat open reading frame of the mouse mammary tumor provirus Mtv-7. Proc Natl Acad Sci U S A (1992) 89(12):5432–6. doi:10.1073/pnas.89.12.5432
51. Winslow GM, Scherer MT, Kappler JW, Marrack P. Detection and biochemical characterization of the mouse mammary tumor virus 7 superantigen (Mls-1a). Cell (1992) 71(5):719–30. doi:10.1016/0092-8674(92)90549-R
52. Scherer MT, Ignatowicz L, Pullen A, Kappler J, Marrack P. The use of mammary tumor virus (Mtv)-negative and single-Mtv mice to evaluate the effects of endogenous viral superantigens on the T cell repertoire. J Exp Med (1995) 182(5):1493–504. doi:10.1084/jem.182.5.1493
53. Acha-Orbea H, Held W, Waanders GA, Shakhov AN, Scarpellino L, Lees RK, et al. Exogenous and endogenous mouse mammary tumor virus superantigens. Immunol Rev (1993) 131:5–25. doi:10.1111/j.1600-065X.1993.tb01527.x
54. Scherer MT, Ignatowicz L, Winslow GM, Kappler JW, Marrack P. Superantigens: bacterial and viral proteins that manipulate the immune system. Annu Rev Cell Biol (1993) 9:101–28. doi:10.1146/annurev.cb.09.110193.000533
55. Golovkina TV, Prescott JA, Ross SR. Mouse mammary tumor virus-induced tumorigenesis in sag transgenic mice: a laboratory model of natural selection. J Virol (1993) 67(12):7690–4.
56. van Nie R, de Moes J. Development of a congeneic line of the GR mouse strain without early mammary tumours. Int J Cancer (1977) 20(4):588–94. doi:10.1002/ijc.2910200417
57. Dawe CJ, Freund R, Mandel G, Ballmer-Hofer K, Talmage DA, Benjamin TL. Variations in polyoma virus genotype in relation to tumor induction in mice. Characterization of wild type strains with widely differing tumor profiles. Am J Pathol (1987) 127(2):243–61.
58. Freund R, Dubensky T, Bronson R, Sotnikov A, Carroll J, Benjamin T. Polyoma tumorigenesis in mice: evidence for dominant resistance and dominant susceptibility genes of the host. Virology (1992) 191(2):724–31. doi:10.1016/0042-6822(92)90248-N
59. Lukacher AE, Ma Y, Carroll JP, Abromson-Leeman SR, Laning JC, Dorf ME, et al. Susceptibility to tumors induced by polyoma virus is conferred by an endogenous mouse mammary tumor virus superantigen. J Exp Med (1995) 181(5):1683–92. doi:10.1084/jem.181.5.1683
60. Lukacher AE, Freund R, Carroll JP, Bronson RT, Benjamin TL. Pyvs: a dominantly acting gene in C3H/BiDa mice conferring susceptibility to tumor induction by polyoma virus. Virology (1993) 196(1):241–8. doi:10.1006/viro.1993.1472
61. Velupillai P, Sung CK, Andrews E, Moran J, Beier D, Kagan J, et al. Polymorphisms in toll-like receptor 4 underlie susceptibility to tumor induction by the mouse polyomavirus. J Virol (2012) 86(21):11541–7. doi:10.1128/JVI.01614-12
62. Lukacher AE, Wilson CS. Resistance to polyoma virus-induced tumors correlates with CTL recognition of an immunodominant H-2Dk-restricted epitope in the middle T protein. J Immunol (1998) 160(4):1724–34.
63. Schirrmacher V, Beutner U, Bucur M, Umansky V, Rocha M, von Hoegen P. Loss of endogenous mouse mammary tumor virus superantigen increases tumor resistance. J Immunol (1998) 161(2):563–70.
64. Schirrmacher V, Zangemeisterwittke U. Gamma-irradiation suppresses T-cell mediated protective immunity against a metastatic tumor in the afferent phase of the immune-response but enhances it in the efferent phase when given before immune cell transfer. Int J Oncol (1994) 4(2):335–46.
65. Bosslet K, Schirrmacher V. Escape of metastasizing clonal tumor cell variants from tumor-specific cytolytic T lymphocytes. J Exp Med (1981) 154(2):557–62. doi:10.1084/jem.154.2.557
66. Schirrmacher V, Muerkoster S, Bucur M, Umansky V, Rocha M. Breaking tolerance to a tumor-associated viral superantigen as a basis for graft-versus-leukemia reactivity. Int J Cancer (2000) 87(5):695–706. doi:10.1002/1097-0215(20000901)87:5<695::AID-IJC12>3.0.CO;2-B
67. Lasky JL, Ponzio NM, Thorbecke GJ. Characterization and growth factor requirements of SJL lymphomas. I. Development of a B cell growth factor-dependent in vitro cell line, cRCS-X. J Immunol (1988) 140(2):679–87.
68. Lasky JL, Thorbecke GJ. Characterization and growth factor requirements of SJL lymphomas. II. Interleukin 5 dependence of the in vitro cell line, cRCS-X, and influence of other cytokines. Eur J Immunol (1989) 19(2):365–71. doi:10.1002/eji.1830190222
69. Ponzio NM, Chapman-Alexander J, Thorbecke GJ. Properties of reticulum cell sarcomas in SJL/J mice VI. Characterization of lymphoid cells that proliferate in response to RCS cells. Cell Immunol (1978) 41(1):157–71. doi:10.1016/S0008-8749(78)80035-5
70. Tsiagbe VK, Asakawa J, Miranda A, Sutherland RM, Paterson Y, Thorbecke GJ. Syngeneic response to SJL follicular center B cell lymphoma (reticular cell sarcoma) cells is primarily in V beta 16+ CD4+ T cells. J Immunol (1993) 150(12):5519–28.
71. Sen N, Simmons WJ, Thomas RM, Erianne G, Zhang DJ, Jaeggli NS, et al. META-controlled env-initiated transcripts encoding superantigens of murine Mtv29 and Mtv7 and their possible role in B cell lymphomagenesis. J Immunol (2001) 166(9):5422–9.
72. Bhadra S, Lozano MM, Payne SM, Dudley JP. Endogenous MMTV proviruses induce susceptibility to both viral and bacterial pathogens. PLoS Pathog (2006) 2(12):e128. doi:10.1371/journal.ppat.0020128
73. Purdy A, Case L, Duvall M, Overstrom-Coleman M, Monnier N, Chervonsky A, et al. Unique resistance of I/LnJ mice to a retrovirus is due to sustained interferon gamma-dependent production of virus-neutralizing antibodies. J Exp Med (2003) 197(2):233–43. doi:10.1084/jem.20021499
74. Mustafa F, Bhadra S, Johnston D, Lozano M, Dudley JP. The type B leukemogenic virus truncated superantigen is dispensable for T-cell lymphomagenesis. J Virol (2003) 77(6):3866–70. doi:10.1128/JVI.77.6.3866-3870.2003
75. Held W, Shakhov AN, Waanders G, Scarpellino L, Luethy R, Kraehenbuhl JP, et al. An exogenous mouse mammary tumor virus with properties of Mls-1a (Mtv-7). J Exp Med (1992) 175(6):1623–33. doi:10.1084/jem.175.6.1623
76. Bhadra S, Lozano MM, Dudley JP. BALB/Mtv-null mice responding to strong mouse mammary tumor virus superantigens restrict mammary tumorigenesis. J Virol (2009) 83(1):484–8. doi:10.1128/JVI.01374-08
77. Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol (1995) 13:151–77. doi:10.1146/annurev.iy.13.040195.001055
78. Varanasi V, Mattoo H, Tupperwar NC, Thyagarajan K, Das A, Kumar R, et al. A superantigen interacts with leishmanial infection in antigen-presenting cells to regulate cytokine commitment of responding CD4 T cells. J Infect Dis (2010) 202(8):1234–45. doi:10.1086/656366
79. Grau GE, Piguet PF, Engers HD, Louis JA, Vassalli P, Lambert PH. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol (1986) 137(7):2348–54.
80. Hermsen C, van de Wiel T, Mommers E, Sauerwein R, Eling W. Depletion of CD4+ or CD8+ T-cells prevents Plasmodium berghei induced cerebral malaria in end-stage disease. Parasitology (1997) 114(Pt 1):7–12. doi:10.1017/S0031182096008293
81. Grau GE, Fajardo LF, Piguet PF, Allet B, Lambert PH, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science (1987) 237(4819):1210–2. doi:10.1126/science.3306918
82. Grau GE, Heremans H, Piguet PF, Pointaire P, Lambert PH, Billiau A, et al. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci U S A (1989) 86(14):5572–4. doi:10.1073/pnas.86.14.5572
83. Boubou MI, Collette A, Voegtle D, Mazier D, Cazenave PA, Pied S. T cell response in malaria pathogenesis: selective increase in T cells carrying the TCR V(beta)8 during experimental cerebral malaria. Int Immunol (1999) 11(9):1553–62. doi:10.1093/intimm/11.9.1553
84. Gorgette O, Existe A, Boubou MI, Bagot S, Guenet JL, Mazier D, et al. Deletion of T cells bearing the V beta8.1 T-cell receptor following mouse mammary tumor virus 7 integration confers resistance to murine cerebral malaria. Infect Immun (2002) 70(7):3701–6. doi:10.1128/IAI.70.7.3701-3706.2002
85. Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol (1994) 68(12):8056–63.
86. Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol (2003) 77(8):4911–27. doi:10.1128/JVI.77.8.4911-4927.2003
87. Punkosdy GA, Blain M, Glass DD, Lozano MM, O’Mara L, Dudley JP, et al. Regulatory T-cell expansion during chronic viral infection is dependent on endogenous retroviral superantigens. Proc Natl Acad Sci U S A (2011) 108(9):3677–82. doi:10.1073/pnas.1100213108
88. Van Elven EH, Rolink AG, Veen FV, Gleichmann E. Capacity of genetically different T lymphocytes to induce lethal graft-versus-host disease correlates with their capacity to generate suppression but not with their capacity to generate anti-F1 killer cells. A non-H-2 locus determines the inability to induce lethal graft-versus-host disease. J Exp Med (1981) 153(6):1474–88.
89. Allen RD, Slayback DL, Harper JM, Aguirre TL, Dobkins JA. A locus closely linked to Mtv7 on mouse chromosome 1 influences development of acute versus chronic graft-versus-host disease in a murine model. Clin Immunol (2000) 95(1 Pt 1):9–19. doi:10.1006/clim.2000.4841
90. Suerth JD, Schambach A, Baum C. Genetic modification of lymphocytes by retrovirus-based vectors. Curr Opin Immunol (2012) 24(5):598–608. doi:10.1016/j.coi.2012.08.007
Keywords: endogenous retrovirus, mouse mammary tumor virus, immune system, infection, cancer
Citation: Holt MP, Shevach EM and Punkosdy GA (2013) Endogenous mouse mammary tumor viruses (Mtv): new roles for an old virus in cancer, infection, and immunity. Front. Oncol. 3:287. doi: 10.3389/fonc.2013.00287
Received: 01 July 2013; Paper pending published: 10 September 2013;
Accepted: 10 November 2013; Published online: 26 November 2013.
Edited by:
Iyoko Katoh, University of Yamanashi, JapanReviewed by:
Shridar Ganesan, University of Medicine and Dentistry of New Jersey, USAXiaoping Zhang, Mount Sinai School of Medicine, USA
Copyright: © 2013 Holt, Shevach and Punkosdy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: George A. Punkosdy, Department of Microbiology and Immunology, Emory University, 1510 Clifton Road, Room G-211, Atlanta, GA 30322, USA e-mail:Z2VvcmdlLnB1bmtvc2R5QGVtb3J5LmVkdQ==
†Present address: George A. Punkosdy, Department of Microbiology and Immunology, Emory University, Atlanta, GA, USA
 Michael P. Holt
Michael P. Holt Ethan M. Shevach
Ethan M. Shevach George A. Punkosdy
George A. Punkosdy