- 1Department of Medicine, Division of Hematology and Medical Oncology, Weill Cornell Medical College, New York, NY, USA
- 2Department of Urology, Weill Cornell Medical College, New York, NY, USA
- 3Department of Radiology, Division of Nuclear Medicine, Weill Cornell Medical College, New York, NY, USA
- 4Department of Pathology, Weill Cornell Medical College, New York, NY, USA
Radioimmunotherapy (RIT) has demonstrated efficacy with acceptable toxicity leading to approval in non-Hodgkin’s lymphoma, but has been slower to develop for the treatment of advanced solid tumors. Prostate cancer (PC) represents a good candidate for RIT based upon high exposure to circulating antibodies at common disease sites with a specific, highly expressed cell-surface antigen of prostate-specific membrane antigen. Four phase I and II trials utilizing 177Lu- or 90Y-J591 have been reported. Long-term toxicity and chemotherapy administration was analyzed. As expected, the only serious toxicity observed was myelosuppression. Grade 4 thrombocytopenia occurred in 33.3% without significant hemorrhage and grade 4 neutropenia occurred in 17.3% with 0.07% febrile neutropenia. Nearly all subjects (97.3%) recovered to grade 0 or 1 platelets and all had complete neutrophil recovery. The majority (81.3%) received chemotherapy at any time, with 61.3% receiving chemotherapy following RIT. Ten subjects underwent bone marrow biopsies at some point in their disease course following RIT for low counts; all had diffuse PC infiltration without evidence of myelodysplasia or leukemia. As expected, myelosuppression occurs following therapeutic doses of RIT for men with metastatic castration-resistant PC. However, toxicity is predictable and self-limited, with the majority of patients who do not refuse able to receive cytotoxic chemotherapy following RIT.
Introduction
It is estimated that in year 2013, approximately 238,590 men will be diagnosed and 29,720 will die due to prostate cancer (PC) in the United States (1). Despite the effectiveness of hormone therapy, every patient with metastatic disease is currently incurable and those who live long enough eventually progress to castration-resistant prostate cancer (CRPC) (2).
Radioimmunotherapy (RIT), using specific monoclonal antibodies (mAbs) or fragments which are radiolabeled (most typically with beta-emitting particles), has proven quite effective in the treatment of non-Hodgkin lymphoma (NHL) alone or in combination with chemotherapy (3–5). RIT for solid tumors has posed a more difficult challenge for a number of biologic, technical, and practical reasons (6). In the last decade, significant progress has been made in the development of RIT for solid tumors. As with any therapy, emphasis is laid on balancing toxicity and therapeutic effects.
Metastatic PC is a good candidate for RIT, because it is radio-responsive and typically develops as small-volume metastatic sites of disease in marrow and lymph nodes that receive high levels of circulating antibody. Several clinical trials have focused on or included subjects with PC (7–16). Importantly, unlike some other solid tumors, a well-established, specific cell-surface antigen has been identified: prostate-specific membrane antigen (PSMA) (17–19). PSMA is an ideal target as it is expressed by nearly all PCs and is not secreted (17, 19). The expression levels progressively increase in more poorly differentiated, metastatic, and castration-resistant cancers (17, 20).
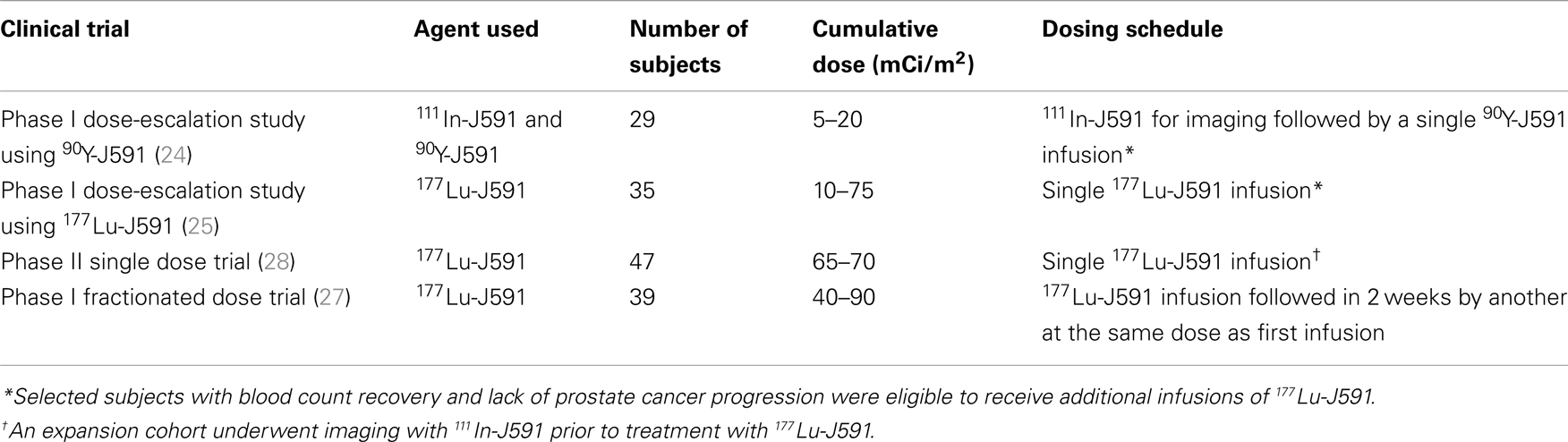
J591 is a deimmunized mAb which specifically binds with high affinity to the extracellular domain of PSMA (21, 22). In addition, the PSMA-J591 antibody complex is internalized thereby delivering any radionuclide or drug conjugated to the antibody to the interior of the targeted cancer cells (23). We have performed and reported 4 clinical trials using 177Lu and 90Y labeled J591 (24–28) (Table 1). Based on imaging studies, we have shown that J591 is able to sensitively and specifically target sites of metastatic PC in both bone and soft tissue (28). As often seen with RIT, radiolabeled-J591 was well-tolerated and serious toxicity was confined to predictable, reversible myelosuppression (24, 25, 27, 28). The preliminary efficacy data from initial phase I studies has been confirmed in a phase II clinical trial of single-dose 177Lu-J591 (28).
Several RIT studies have demonstrated that, in the absence of bone marrow or hematopoietic stem cell support, radiation-induced myelotoxicity is the dose-limiting toxicity (DLT) (29, 30). Hematologic toxicity is the biggest challenge faced by all radioimmunotherapeutic agents. The manifestations of myelotoxicity may be related to the pretreatment peripheral blood cell counts and bone marrow reserve, which may have been compromised by prior therapies (29, 31). While the vast majority of patients have spontaneous recovery of blood counts, some worry about the ability to deliver subsequent cytotoxic chemotherapy if needed clinically. The development of secondary myelodysplastic syndrome (MDS) or acute myelogenous leukemia (AML) has been reported (32) and has been estimated to be approximately 2–5% (33). Two studies have attempted to comprehensively evaluate the risk for MDS and AML following RIT with either 90Y-ibritumomab or 131I-tositumomab. These retrospective analyses of large numbers of patients both showed that RIT did not demonstrate a higher risk for MDS in comparison with similar patient populations treated with multiple chemotherapies alone (34, 35).
Long-term outcomes have rarely been reported with solid tumor RIT. Here, we report the long-term toxicity data in patients treated with radiolabeled-J591, including bone marrow recovery and ability to deliver cytotoxic chemotherapy.
Materials and Methods
Patient Population
For each study, eligible patients had a prior histologic diagnosis of PC with evidence progressive metastatic disease as defined by a serum PSA and/or radiologic studies including bone scan, computed axial tomography, and/or magnetic resonance imaging despite castrate levels of serum testosterone (i.e., progressive metastatic CRPC) (24, 25, 27, 28). Patients were required to have platelet count of ≥150,000/mm3, hemoglobin ≥ 10, and neutrophil count of ≥2,000/mm3. Prior radiation to>25% of the skeleton and systemic beta-emitting radioisotope therapy (e.g., 89Sr or 153Sm) was exclusionary. All studies were approved by the Institutional Review Boards of participating institutions and registered on clinicaltrials.gov; all subjects provided written informed consent.
Radiolabeled Antibodies
Clinical-grade J591 mAb was covalently linked to the chelating agent, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) (21). The DOTA-J591 mAb was then radiolabeled with 90Y or 177Lu by incubation at 45°C (±2°C) for 45 min in the presence of an ammonium acetate buffer (pH 7.0). The final radiolabeled drug product was purified (when necessary) and filter sterilized before administration into patients as previously described (24, 25). Radiochemical purity was ≥97% at all cases as confirmed by instant thin layer chromatography. The immunoreactive fraction was always found>0.7 when tested in PSMA+-LNCaP cells.
Treatment and Follow-Up
Subjects received single or multiple doses of radiolabeled-J591 intravenously at a rate not to exceed 5 mg/min per the particular protocol on which they were enrolled. Subjects were observed for a minimum of 12 weeks after their last dose of radiolabeled-J591 and those patients with stable or responding disease were observed until disease progression. Routine clinical and laboratory assessments (including metabolic profile, PSA, and testosterone) were performed at defined intervals. Complete blood count and platelet counts were monitored at least weekly, with more frequent monitoring with the onset of grade 3 thrombocytopenia or neutropenia. For most studies, white blood cell growth factors were allowed at the discretion of the investigator (not allowed in the fractionated dose-escalation study), and transfusions of platelets were also given at the discretion of the investigator. Following IRB approval, the number of previous therapies, the toxicity seen with radiolabeled-J591, the nature of subsequent therapies, and overall survival (OS) data were obtained by physician interview, medical record review, and post-treatment long-term follow-up with patients, families, or other physicians.
Results
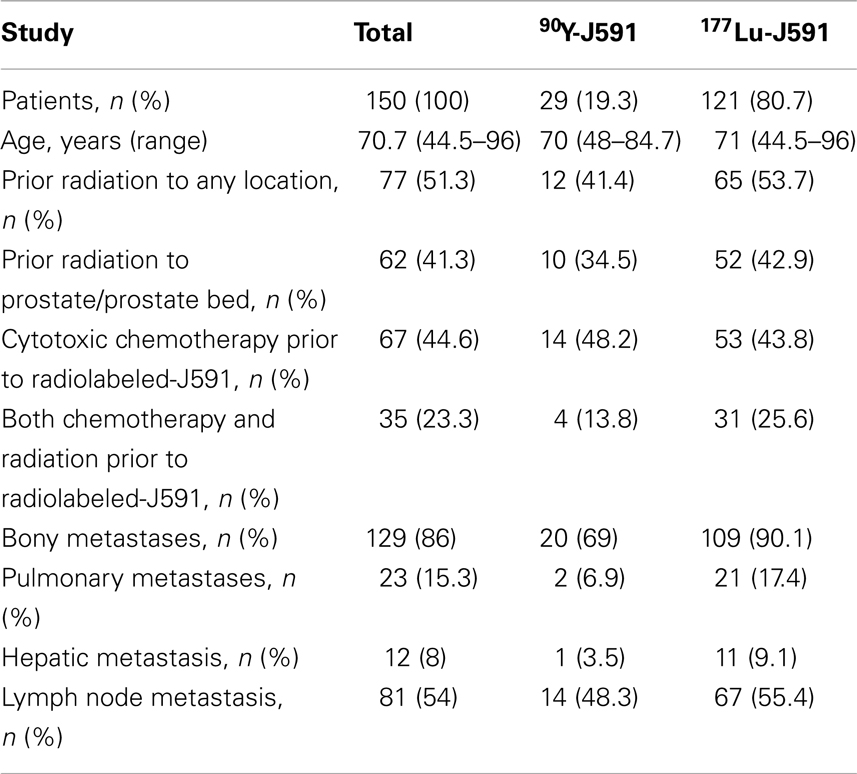
Between October 2000 and August 2012, 150 subjects with metastatic CRPC received radiolabeled-J591. One hundred and twenty-one patients received 177Lu-J591 at total doses of 20–90 mCi/m2 and 29 patients received 90Y-J591 at 5–20 mCi/m2. Patient baseline demographics are displayed in Table 2.
Acute Toxicity
Acute toxicity has previously been reported separately for each study (24, 25, 27, 28). Briefly, without pre-medication, 20.7% experienced transient grade 1 infusion reactions. Fourteen percent experienced transient low-grade transaminitis which returned to baseline in 100%. Platelet count decline was generally seen 2–3 weeks post infusion with platelet nadir occurring at 4–5 weeks after administration followed by a recovery phase. Grade 4 thrombocytopenia occurred in 33.3% patients without any significant hemorrhage. Thirty-five patients (23.3%) received platelet transfusion, 5 (17.2%) following 90Y-J591 and 30 (24.8%) following 177Lu-J591. Neutrophil decline typically occurred in parallel with thrombocytopenia, with nadir similarly 4–5 weeks following treatment. 17.3% experienced grade 4 neutropenia. One subject experienced grade 3 febrile neutropenia. Seventeen (11.3%) patients received granulocyte growth factor.
Hematologic Recovery
All subjects experienced improvement in blood counts following RIT induced nadir. Ninety-two percent had complete recovery of platelet counts to grade 0 (i.e., platelet count of at least 150,000/mcL) and 100% experienced recovery of neutrophil count to Gr 0 (i.e., ANC of at least 2000/mm3). Eight subjects (5.3%) recovered to grade 1 thrombocytopenia (platelet counts ranging from 99,000 to 140,000). Four subjects died of progressive PC prior to platelet recovery from nadir.
Some patients experienced full or partial platelet count recovery followed by subsequent decline. All were associated with evidence of simultaneous PC progression. Ten underwent bone marrow aspiration and biopsy confirming PC infiltration overtaking bone marrow (Figure 1). No evidence of MDS or leukemia was discovered.
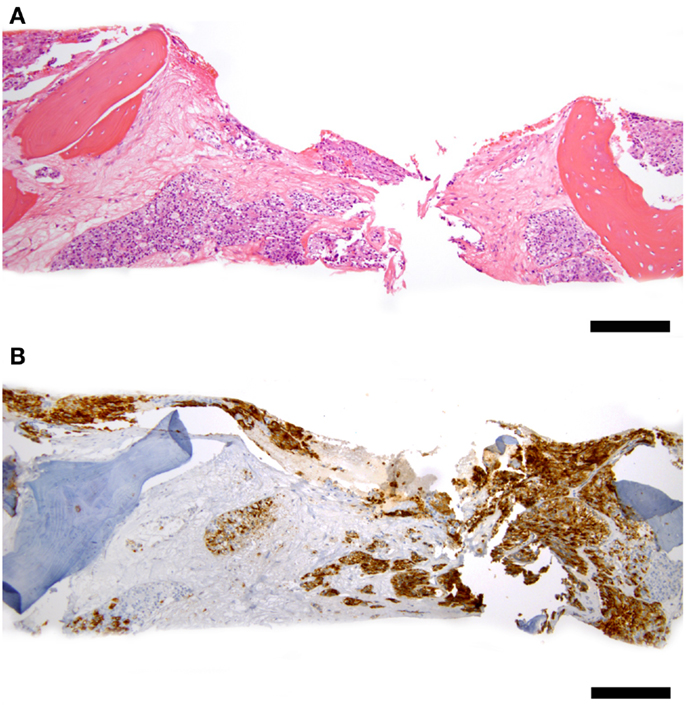
Figure 1. Representative bone marrow biopsy of a patient with progressive prostate cancer and decreasing blood counts 3 years after 177Lu-J591 radioimmunotherapy, count recovery, and several subsequent therapies including chemotherapy. (A) Hematoxylin and eosin stain at 100× total magnification low power view of bone marrow with intertrabecular marrow space entirely replaced by metastatic prostate cancer cells. (B) PSMA – 100× total magnification low power view of same field of bone marrow replaced by tumor showing PSMA-positivity in tumor cells (brown staining); scale bars = 200 microns. Cytogenetic studies revealed normal 46, XY male karyotype; normal bone marrow biopsy control not shown.
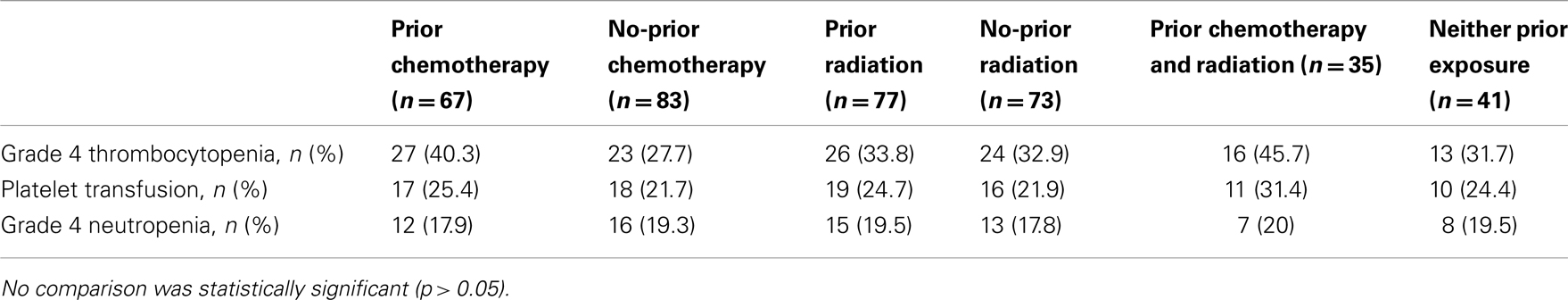
The hematologic toxicity seen with radiolabeled-J591 is a known consequence of RIT. However, patient disease and previous treatment status can also contribute to amplification of such effects. 51.3% of patients treated with radiolabeled-J591 had history of previous radiation treatment, 44.6% had prior cytotoxic chemotherapy, and 23.3% had both prior chemotherapy and radiation (Table 2).
Grade 4 thrombocytopenia occurred in 40.3% (27/67) of patients with prior chemotherapy as compared to 27.7% (23/83) in the no-prior chemotherapy group (p = 0.10); 25.4% (17/67) with prior chemotherapy received platelet transfusions versus 21.7% (18/83) in the remaining patients (p = 0.59). Grade 4 neutropenia occurred in 17.9% (12/67) with chemotherapy prior to radiolabeled-J591 treatment versus 19.3% (16/83) in those who had no previous chemotherapy (p = 0.83) (Table 3).
In those who had received prior radiation, grade 4 thrombocytopenia occurred in 33.8% (26/77) as compared to 32.9% (24/73) in the rest of patients (p = 0.90); 24.7% (19/77) with prior radiation treatment had platelet transfusions versus 21.9% (16/73) in no previous radiation therapy group (p = 0.69). Grade 4 neutropenia occurred in 19.5% (15/77) with pre radiolabeled-J591 radiation treatment versus 17.8% (13/73) in those who had no previous radiation therapy (p = 0.79).
In those who had received both prior chemotherapy and radiation, 45.7% (16/35) had Grade 4 thrombocytopenia versus 31.7% (13/41) with neither prior exposure (p = 0.21); 31.4% (11/35) received platelet transfusion with prior chemotherapy and radiation versus 24.4% (10/41) with neither prior exposure (p = 0.49) (Table 3).
Therapies Administered after Radiolabeled-J591
81.3% of patients (122/150) received cytotoxic chemotherapy either prior to or after radiolabeled-J591. In review of long-term follow-up data, 92 (61.3%) received cytotoxic chemotherapy post radiolabeled-J591 infusion. Sixty-one percent (56/92) patients who received chemotherapy post RIT were chemo-naive prior to treatment and 39% (36/92) received chemotherapy both before and after RIT. 13.3% (20/150) of patients received investigational treatment following RIT, 24.6% (37/150) had no active treatment, 13.3% (20/150) were deemed chemotherapy eligible by their physicians, but declined subsequent chemotherapy and 12% (18/150) enrolled in hospice or died before starting a new treatment.
Discussion
Prostate cancer offers a model for the investigation and development of RIT in solid tumors, given the restricted, high-level of expression of PSMA and the availability of a specific, well-tolerated mAb (J591). As myelosuppression and subsequent bone marrow recovery as well as the theoretical inability to tolerate subsequent cytotoxic chemotherapy are potential issues with RIT, we analyzed our long-term results with radiolabeled-J591. It should be noted that this analysis included radiolabeled-J591 administered across a number of different cumulative doses in phase I and II studies, including a prospective fractionated dose schedule (Table 1).
The vast majority of subjects who received radiolabeled-J591 had complete (i.e., grade 0) or near-complete (grade 1) recovery of neutrophil (100%) and platelet (97.3%) counts. Those that did not experience complete recovery or who experienced subsequent decline in blood counts had concomitant progression of PC. Those with bone marrow biopsies at subsequent count decline had diffuse PC marrow infiltration and none had evidence of MDS or leukemia. As some have proposed that prior treatment might influence subsequent bone marrow reserve and the ability to tolerate RIT, (31) we analyzed by previous exposure to chemotherapy and/or radiation. There was a trend for more grade 4 thrombocytopenia in those with both prior chemotherapy and radiation, but no clear differences in those who had received either previous chemotherapy or radiation.
Because of the myelosuppression observed in patients treated with radiolabeled-J591, a further concern is whether patients can tolerate subsequent therapeutic interventions if needed for disease progression after RIT. The available data suggest that patients treated with radiolabeled-J591 can tolerate subsequent therapies. The majority (92 of 150, 61.3%) of patients received cytotoxic chemotherapy after radiolabeled-J591, including docetaxel and cabazitaxel. Although two of the four studies were completed prior to the approval of docetaxel for metastatic CRPC, 95 of 150 (63.3%) received docetaxel at any time, a favorable number compared to the estimated third of men with metastatic CRPC ever receiving docetaxel according to a survey of urologists and oncologists in the era prior to the approval of newer agents such as Sipuleucel-T, Abiraterone, and Enzalutamide. Another concern might be a theoretic possibility of poorer chemotherapy tolerance in the setting of prior RIT. While this cannot be completely addressed in a retrospective study, patients who received docetaxel at any time following radiolabeled-J591 received a median of 23 weeks of chemotherapy (range 10–68) (i.e., 7–8 cycles) and those who received cabazitaxel received a median of 18 weeks (range 17–36) (i.e., 5–6 cycles), both within range of general community standards.
One of the most serious concerns about RIT is its potential to lead to secondary malignancies, particularly MDS/AML. The long-term data following RIT of solid tumors is limited, in part for the practical reason of populations that have been studied (generally late stage, refractory cancers). In this study, subjects were treated over a 12-year period with median time from treatment of 7.8 years (range 0.7–12.7); censored for loss to follow-up or death, median follow-up for this analysis was 16.6 months (range 0.5–133.9). The estimated median survival for the population based upon nomogram analysis is 15 months (median Halabi score 145, range 62–196) (36). As solid tumor RIT moves earlier in the disease process and as median survival for advanced solid tumors increases with more successful therapy, long-term toxicity follow-up is needed. More data exist following RIT for NHL. With a median 10-year follow-up, Kaminski et al. reported a single case of MDS out of 76 previously untreated patients who received a single treatment with 131I-tositumomab (37). Czuczman et al. reviewed the records of 746 patients treated with 90Y-ibritumomab tiuxetan with a median follow-up of 4.5 years and found a total of 2.5% of patients developed MDS at a median of nearly 2 years following RIT (35). This corresponded to an annualized MDS rate of 0.7% per year following RIT. The expected annual rate of treatment-related MDS for NHL patients receiving systemic therapy alone or in combination with rituximab is approximately 1% per year (38, 39). Higher doses of therapy may pose greater risk. Recently, Guidetti et al. published prospective data on development of MDS/AML in NHL patients receiving myeloablative doses of 90Y-Ibritumomab tiuxetan. Among 52 patients with a median follow-up of 49 months, the 5-year cumulative incidence of MDS/AML was 8.29% (40). This incidence is significantly higher than previously reported with lower doses, but the studied population consisted of subjects receiving significantly higher doses of RIT than used for solid tumors and a matched-pair analysis of patients receiving myeloablative chemotherapy conditioning instead of RIT revealed a similar 8.05% 5-year cumulative incidence of MDS/AML.
Taken together, although there have been few prospective studies designed specifically to assess the risk of development of MDS/AML following RIT for NHL, with significant follow-up, the use of RIT does not appear to significantly increase the risk of secondary MDS above the risk of chemotherapy alone in patients with NHL. Should cases of MDS or leukemia be discovered with radiolabeled-J591 or other RIT for PC, it will be important to understand that as in NHL, additional therapies may be associated with MDS/AML and there is a de novo incidence in an elderly male population (35, 41, 42).
Conclusion
Systemic targeted radiation with RIT has therapeutic promise in advanced solid tumors, in particular for radiosensitive tumors such as PC with a selective and specific cell-surface antigen such as PSMA and an available antigen-specific, non-immunogenic mAb such as J591. Using beta-emitting radionuclides at therapeutic doses, myelosuppression is expected. However, toxicity is predictable and usually self-limited, with the majority of patients who do not refuse able to receive chemotherapy. As the use of RIT becomes more attractive across diseases with the development of newer, more specific mAbs or peptides, studies examining long-term toxicity are warranted.
Conflict of Interest Statement
Neil H. Bander is an inventor on patents that are assigned to Cornell Research Foundation (“CRF”) for the J591 antibody described in this article. Dr. Bander is a paid consultant to and owns stock in BZL Biologics, the company to which the patents were licensed by CRF for further research and development. The other co-authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Sources of support: National Institutes of Health (ULI RR024996, 1-KL2-RR024997-01, PTBF5405), Department of Defense (PC040566), Prostate Cancer Foundation.
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin (2013) 63:11–30. doi:10.3322/caac.21166
2. Zelefsky MJ, Eastham JA, Sartor O. Cancer of the prostate. 9th ed. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Devita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. Philadelphia, USA: Lippincott Williams and Wilkins (2011). p. 1220–62.
3. Zinzani PL, Rossi G, Franceschetti S, Botto B, Di Rocco A, Cabras MG, et al. Phase II trial of short-course R-CHOP followed by 90Y-ibritumomab tiuxetan in previously untreated high-risk elderly diffuse large B-cell lymphoma patients. Clin Cancer Res (2010) 16:3998–4004. doi:10.1158/1078-0432.CCR-10-0162
4. Zinzani PL, Tani M, Pulsoni A, De Renzo A, Stefoni V, Broccoli A, et al. A phase II trial of short course fludarabine, mitoxantrone, rituximab followed by (90)Y-ibritumomab tiuxetan in untreated intermediate/high-risk follicular lymphoma. Ann Oncol (2012) 23:415–20. doi:10.1093/annonc/mdr145
5. Press OW, Unger JM, Rimsza LM, Friedberg JW, LeBlanc M, Czuczman MS, et al. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J Clin Oncol (2013) 31:314–20. doi:10.1200/JCO.2012.42.4101
6. Divgi C. Editorial: what ails solid tumor radioimmunotherapy? Cancer Biother Radiopharm (2006) 21:81–4. doi:10.1089/cbr.2006.21.81
7. Abdel-Nabi H, Wright GL, Gulfo JV, Petrylak DP, Neal CE, Texter JE, et al. Monoclonal antibodies and radioimmunoconjugates in the diagnosis and treatment of prostate cancer. Semin Urol (1992) 10:45–54.
8. Meredith RF, Bueschen AJ, Khazaeli MB, Plott WE, Grizzle WE, Wheeler RH, et al. Treatment of metastatic prostate carcinoma with radiolabeled antibody CC49. J Nucl Med (1994) 35:1017–22.
9. Deb N, Goris M, Trisler K, Fowler S, Saal J, Ning S, et al. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin Cancer Res (1996) 2:1289–97.
10. Meredith RF, Khazaeli MB, Macey DJ, Grizzle WE, Mayo M, Schlom J, et al. Phase II study of interferon-enhanced 131I-labeled high affinity CC49 monoclonal antibody therapy in patients with metastatic prostate cancer. Clin Cancer Res (1999) 5:3254–8.
11. Kahn D, Austin JC, Maguire RT, Miller SJ, Gerstbrein J, Williams RD. A phase II study of [90Y] yttrium-capromab pendetide in the treatment of men with prostate cancer recurrence following radical prostatectomy. Cancer Biother Radiopharm (1999) 14:99–111. doi:10.1089/cbr.1999.14.99
12. O’Donnell RT, DeNardo SJ, Yuan A, Shen S, Richman CM, Lara PN, et al. Radioimmunotherapy with (111)In/(90)Y-2IT-BAD-m170 for metastatic prostate cancer. Clin Cancer Res (2001) 7:1561–8.
13. O’Donnell RT, DeNardo SJ, Miers LA, Lamborn KR, Kukis DL, DeNardo GL, et al. Combined modality radioimmunotherapy for human prostate cancer xenografts with taxanes and 90yttrium-DOTA-peptide-ChL6. Prostate (2002) 50:27–37. doi:10.1002/pros.10029
14. DeNardo SJ, Richman CM, Albrecht H, Burke PA, Natarajan A, Yuan A, et al. Enhancement of the therapeutic index: from nonmyeloablative and myeloablative toward pretargeted radioimmunotherapy for metastatic prostate cancer. Clin Cancer Res (2005) 11:7187–94. doi:10.1158/1078-0432.CCR-1004-0013
15. Richman CM, Denardo SJ, O’Donnell RT, Yuan A, Shen S, Goldstein DS, et al. High-dose radioimmunotherapy combined with fixed, low-dose paclitaxel in metastatic prostate and breast cancer by using a MUC-1 monoclonal antibody, m170, linked to indium-111/yttrium-90 via a cathepsin cleavable linker with cyclosporine to prevent human anti-mouse antibody. Clin Cancer Res (2005) 11:5920–7.
16. Kelly MP, Lee FT, Tahtis K, Smyth FE, Brechbiel MW, Scott AM. Radioimmunotherapy with alpha-particle emitting 213Bi-C-functionalized trans-cyclohexyl-diethylenetriaminepentaacetic acid-humanized 3S193 is enhanced by combination with paclitaxel chemotherapy. Clin Cancer Res (2007) 13:5604–12. doi:10.1158/1078-0432.CCR-07-1071
17. Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Res (1994) 54:1807–11.
18. Wright GL Jr, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology (1996) 48:326–34. doi:10.1016/S0090-4295(96)00184-7
19. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res (1997) 3:81–5.
20. Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res (1987) 7(5B):927–35.
21. Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res (1997) 57:3629–34.
22. Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, Navarro V, Hunter CJ, Bastidas D, et al. In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res (2000) 60:5237–43.
23. Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V, et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res (1998) 58:4055–60.
24. Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol (2004) 22:2522–31. doi:10.1200/JCO.2004.09.154
25. Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol (2005) 23:4591–601. doi:10.1200/JCO.2005.05.160
26. Tagawa ST, Beltran H, Vallabhajosula S, Goldsmith SJ, Osborne J, Matulich D, et al. Anti-prostate-specific membrane antigen-based radioimmunotherapy for prostate cancer. Cancer (2010) 116:1075–83. doi:10.1002/cncr.24795
27. Tagawa ST, Vallabahajosula S, Osborne J, Goldsmith SJ, Petrillo K, Tyrell L, et al. Phase I trial of fractionated-dose 177lutetium radiolabeled anti-prostate-specific membrane antigen (PSMA) monoclonal antibody J591 (177Lu-J591) in patients (pts) with metastatic castration-resistant prostate cancer (metCRPC) [abstract 4667]. J Clin Oncol (2010) 28(Suppl):S15.
28. Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, Akhtar NH, et al. Phase II study of lutetium-177 labeled anti-prostate-specific membrane antigen (PSMA) monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res (2013). doi:10.1158/1078-0432.CCR-13-0231. [Epub ahead of print].
29. DeNardo GL, Siantar CL, DeNardo SJ. Radiation dosimetry for radionuclide therapy in a nonmyeloablative strategy. Cancer Biother Radiopharm (2002) 17:107–18. doi:10.1089/10849780252824127
30. Behr TM, Behe M, Sgouros G. Correlation of red marrow radiation dosimetry with myelotoxicity: empirical factors influencing the radiation-induced myelotoxicity of radiolabeled antibodies, fragments and peptides in pre-clinical and clinical settings. Cancer Biother Radiopharm (2002) 17:445–64. doi:10.1089/108497802760363231
31. Aksentijevich I, Flinn I. Chemotherapy and bone marrow reserve: lessons learned from autologous stem cell transplantation. Cancer Biother Radiopharm (2002) 17:399–403. doi:10.1089/108497802760363196
32. Roboz GJ, Bennett JM, Coleman M, Ritchie EK, Furman RR, Rossi A, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following initial treatment with chemotherapy plus radioimmunotherapy for indolent non-Hodgkin lymphoma. Leuk Res (2007) 31:1141–4. doi:10.1016/j.leukres.2006.11.011
34. Bennett JM, Kaminski MS, Leonard JP, Vose JM, Zelenetz AD, Knox SJ, et al. Assessment of treatment-related myelodysplastic syndromes and acute myeloid leukemia in patients with non-Hodgkin lymphoma treated with tositumomab and iodine I131 tositumomab. Blood (2005) 105:4576–82. doi:10.1182/blood-2004-12-4690
35. Czuczman MS, Emmanouilides C, Darif M, Witzig TE, Gordon LI, Revell S, et al. Treatment-related myelodysplastic syndrome and acute myelogenous leukemia in patients treated with ibritumomab tiuxetan radioimmunotherapy. J Clin Oncol (2007) 25:4285–92. doi:10.1200/JCO.2006.09.2882
36. Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol (2003) 21(7):1232–7. doi:10.1200/JCO.2003.06.100
37. Kaminski MS, Tuck M, Estes J. Tositumomab and iodine I-131 tositumomab for previously untreated, advanced stage, follicular lymphoma: median 10 year follow-up results. Blood (2009) 114:3759.
38. Pedersen-Bjergaard J, Ersboll J, Sorensen HM, Keiding N, Larsen SO, Philip P, et al. Risk of acute nonlymphocytic leukemia and preleukemia in patients treated with cyclophosphamide for non-Hodgkin’s lymphomas. Comparison with results obtained in patients treated for Hodgkin’s disease and ovarian carcinoma with other alkylating agents. Ann Intern Med (1985) 103:195–200. doi:10.7326/0003-4819-103-2-195
39. Morton LM, Curtis RE, Linet MS, Bluhm EC, Tucker MA, Caporaso N, et al. Second malignancy risks after non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol (2010) 28:4935–44. doi:10.1200/JCO.2010.29.1112
40. Guidetti A, Carlo-Stella C, Ruella M, Miceli R, Devizzi L, Locatelli SL, et al. Myeloablative doses of yttrium-90-ibritumomab tiuxetan and the risk of secondary myelodysplasia/acute myelogenous leukemia. Cancer (2011) 117:5074–84. doi:10.1002/cncr.26182
41. Flaig TW, Tangen CM, Hussain MH, Stadler WM, Raghavan D, Crawford ED, et al. Randomization reveals unexpected acute leukemias in Southwest Oncology Group prostate cancer trial. J Clin Oncol (2008) 26:1532–6. doi:10.1200/JCO.2007.13.4197
Keywords: prostate cancer, radioimmunotherapy, myelotoxicity, prostate-specific membrane antigen, monoclonal antibody
Citation: Tagawa ST, Akhtar NH, Nikolopoulou A, Kaur G, Robinson B, Kahn R, Vallabhajosula S, Goldsmith SJ, Nanus DM and Bander NH (2013) Bone marrow recovery and subsequent chemotherapy following radiolabeled anti-prostate-specific membrane antigen monoclonal antibody J591 in men with metastatic castration-resistant prostate cancer. Front. Oncol. 3:214. doi: 10.3389/fonc.2013.00214
Received: 02 May 2013; Accepted: 05 August 2013;
Published online: 26 August 2013.
Edited by:
Jean-Pierre Pouget, INSERM, FranceReviewed by:
Adam Paul Dicker, Thomas Jefferson University, USAArnab Chakravarti, Ohio State University, USA
Copyright: © 2013 Tagawa, Akhtar, Nikolopoulou, Kaur, Robinson, Kahn, Vallabhajosula, Goldsmith, Nanus and Bander. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Scott T. Tagawa, Department of Medicine, Division of Hematology and Medical Oncology, Weill Cornell Medical College, 525 E. 68th Street, Box 403, New York, NY 10065, USA e-mail:c3R0MjAwN0BtZWQuY29ybmVsbC5lZHU=