
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 31 May 2012
Sec. Radiation Oncology
Volume 2 - 2012 | https://doi.org/10.3389/fonc.2012.00055
 Sana D. Karam1*
Sana D. Karam1* James W. Snider1
James W. Snider1 Hongkun Wang2
Hongkun Wang2 Margaux Wooster1
Margaux Wooster1 Christopher Lominska3
Christopher Lominska3 John Deeken4
John Deeken4 Kenneth Newkirk5
Kenneth Newkirk5 Bruce Davidson5
Bruce Davidson5 K. William Harter1
K. William Harter1
Background: to review a single-institution experience with the management of parotid malignancies treated by fractionated stereotactic body radiosurgery (SBRT). Findings: Between 2003 and 2011, 13 patients diagnosed with parotid malignancies were treated with adjuvant or definitive SBRT to a median dose of 33 Gy (range 25–40 Gy). There were 11 male and two female patients with a median age of 80. Ten patients declined conventional radiation treatment and three patients had received prior unrelated radiation therapy to neighboring structures with unavailable radiation records. Six patients were treated with definitive intent while seven patients were treated adjuvantly for adverse surgical or pathologic features. Five patients had clinical or pathologic evidence of lymph node disease. Conclusion: at a median follow-up of 14 months only one patient failed locally, and four failed distantly. The actuarial 2-year overall survival, progression-free survival, and local-regional control rates were 46, 84, and 47%, respectively. Statistical analysis revealed surgery as a positive predictor of overall survival while presence of gross disease was a negatively correlated factor (p < 0.05).
Salivary gland cancers are rare, comprising only 3–5% of all head and neck cancers, with the majority localized to the parotid gland (Guzzo et al., 2010). Histologically, these tumors are very diverse with varying natural history (Guzzo et al., 2010). Surgery has historically been the mainstay of treatment for parotid malignancies (Spiro et al., 1973). In high grade tumors, advanced stage (T3/4), and/or adverse pathologic features, adjuvant postoperative radiation treatment (RT) has been shown to provide superior outcomes to surgery alone (Garden et al., 1994; Sakata et al., 1994; Mendenhall et al., 2005; Chen et al., 2007). In patients whom are considered surgically inoperable, who present with incompletely resected tumors, or who refuse surgery out of personal preference, primary RT alone is the treatment modality of choice, with reported 5-year local control rates of 55–70% (Mendenhall et al., 2005; Chen et al., 2006). However, the protracted course of conventional RT is a deterrent to some patients, especially the elderly, who have other medical co-morbidities. Additionally, in patients with prior unrelated irradiation that has exposed adjacent critical structures to previous radiation, concern about exceeding tolerance to those areas presents a major challenge to delivering adequate dosage to the salivary glands using the conventional RT approach.
Stereotactic radiosurgery (SBRT) represents an appealing option for the management of salivary gland tumors either as a means of dose escalation or for avoiding adjacent critical structures that have been previously exposed to RT. The convenience of administering the treatment over a course of 5 days is also very appealing to those who perceive the prolonged treatment course of the conventional approach as a major challenge to treatment delivery. SBRT uses multiple convergent beams with various targeting techniques to deliver highly conformal therapy in an accurate manner. Gamma Knife-based SBRT technologies, which require external frame-based fixation devices, have been previously used in the treatment of salivary malignancies with skull base invasion, with local control rates at 40 months of 82% (Douglas et al., 2008). Successful use of SBRT has also been reported for recurrent pleomorphic adenoma with skull base invasion (Kamida et al., 2005). The CyberKnife SBRT system (Accuray, Inc., Sunnyvale, CA, USA) allows conformal treatment of sites throughout the head and neck region. This system uses real-time image guidance for targeting without rigid external fixation (Dieterich and Pawlicki, 2008; Dieterich and Gibbs, 2011). Multiple treatment sessions can be used, potentially reducing late normal tissue toxicity via dose fractionation. We report our institutional experience with fractionated SBRT irradiation of salivary gland tumors, addressing feasibility, safety, and outcomes.
All patients diagnosed with parotid gland tumors and treated with SBRT either in the definitive or adjuvant setting at our institution between September 2003 and March 2011 were included in this analysis. There were no pre-existing eligibility criteria or exclusion criteria. The group consisted of 13 patients diagnosed with parotid gland tumors who either were not candidates for definitive resection or who had undergone limited procedures with gross or pathologically adverse features. Patients who refused surgery and/or the more protracted course with conventional RT were also included. Three patients had received prior unrelated radiation therapy elsewhere, and their previous radiation records were unavailable. The data were reviewed under an institutional review board-approved retrospective protocol. Prior to treatment, patients’ cases were discussed at the institutional, multidisciplinary head and neck tumor board. Only one patient received radiosensitizing chemotherapy, using carboplatin, which was given in three cycles over a course of 3 weeks, before, during, and following SBRT.
The CyberKnife SBRT system (Accuray, Inc., Sunnyvale, CA) uses a 6-MV X-band linear accelerator mounted on a fully articulated robotic arm. During treatment, two orthogonally positioned x-ray detectors provide real-time imaging of bony anatomy, allowing for intrafraction movement correction. Treatment was generally administered on an outpatient basis with each treatment lasting approximately 45–90 min. Most of the patients received their treatments over the course of 7 days, consecutive with the exception of holidays or weekends. Patients were immobilized in the supine position with an Aquaplast facemask (WRF/Aquaplast Corp., Wyckoff, NJ, USA). All patients underwent a treatment planning computed tomography (CT) scan, fused with a fluorodeoxyglucose-positron emission tomography (FDGPET/CT) scan with 1.0 mm-thick slices. In all cases, magnetic resonance imaging (MRI) scans with volumetric interpolated breath-hold examination (VIBE) sequence and 1 mm slices were also used in planning. The MRI and PET images were then fused with that of the simulation CT scan for treatment planning. The gross tumor volume (GTV) was reconstructed based on the information obtained from both the PET and the MRI together. The PET-GTV was usually contoured to the halo, which is observed around areas of maximal SUV uptake, as described by Ashamalla et al. (2007). The sum of both the PET-contoured volume and the MRI-contoured volume defined the GTV. Once the GTV was contoured, an expansion was done ranging from 2 to 10 mm depending on the pathologic margins status and proximity to critical structures to define the clinical tumor volume (CTV). Since the threshold for treatment interruption by the Cyberknife delivery system is on the order of 1 mm, no additional margin was added for the planning target volume (PTV). In postoperative cases the preoperative MRI was also fused with the postoperative PET and MRI, and the sum of all volumes defined the postoperative PTV. In patients who underwent surgical resection, the PTV encompassed the entire surgical bed when feasible. The skull base was electively included in the CTV for treatment of the patient with adenoid cystic histology. In six cases, adjacent soft tissue and immediate draining lymph nodes were targeted as a separate PTV. For delineation of lymph node volumes, the same anatomic landmarks as standard neck levels treated with conventional fractionation were used. For benign tumors or for low-grade malignancies that were pathologically or radiographically node negative, lymph nodes were not deliberately included. For high grade malignancies, ipsilateral draining LN stations at risk for microscopic disease were included. The median radiation dose was 33 Gy, but varied between 25 and 40 Gy. Median dose per fraction was 6 Gy and varied between 5 and 8 Gy. For neck irradiation, ipsilateral neck irradiation was given as 5 fractions, 7 Gy each for a total dose of 35 Gy. Inverse planning was used to determine the dose to the target volume while minimizing the dose to normal tissue. All planning was completed within 1 week of imaging and typically patients initiated treatment within 2–3 weeks after imaging depending on chemotherapy coordination. Treatment was generally completed within 7 days of initiation, consecutive with the exception of holidays or weekends.
Patients typically underwent a post-treatment surveillance with an MRI scan 3 months after the completion of SBRT and then every 6 months thereafter (with an FDG PET/CT scan and MRI). Radiographic imaging was done on follow-up to monitor disease recurrence locally, regionally, or distantly. For those with gross disease, PET and MRI were used to monitor “response” to treatment as well as regional and distant metastases. For those without evidence of gross disease on treatment initiation, radiographic evaluation was performed to monitor for disease recurrence locally, regionally, or distantly. Clinical examination was conducted at the same interval, with biopsy as indicated. Acute and late toxicity were graded using the Radiation Therapy Oncology Group (RTOG) scoring criteria.
Progression-free survival (PFS) was defined as the time from the first day of SBRT treatment to local/distant failure or last follow-up in living patients without evidence of recurrence/progression. Locoregional control (LRC) was defined similarly except that death and distant failure were not considered events. Patients were censored at the time of death. Overall survival (OS) was the time from SBRT treatment until death or last follow-up. Interpretation of available FDGPET/CT, MRI, and CT scans with correlative clinical examinations were used to assess for response of the treated lesion 3 months after SBRT. Complete response was defined as no evidence of disease in the treatment volume by both radiographic and direct clinical examination. No response was defined as absence of marked change or increase in the treated lesion. Partial response was defined as not meeting the criteria for complete response or no response. Log rank tests were used to evaluate the association between clinical factors and survival outcomes. The independent variables considered were surgery (yes, no), nodal status (yes, no), presence or absence of gross disease (yes, no), SBRT dose (<35 Gy, ≥35 Gy), grade (low, medium, high), nodal stage per the AJCC staging system, age in years, size in centimeters, and SBRT dose in Gy. The presence of positive margins was coded as absence of gross disease, since most patients had one or the other. Kaplan–Meier plots are presented for selected significant factors. For long-term toxicity analyses, dysphagia (present, absent), fibrosis (present absent), and soft tissue or bone radionecrosis (present, absent) were the dependent variables. Analyses were performed in SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Patient characteristics and treatment results are shown in Table 1. The median age was 80 years (range 34–99 years). Eleven patients were male and patients presented with variable histology (Table 1). Five patients had nodal involvement. A total of seven patients had undergone maximal surgical resection prior to starting SBRT, while six were treated with definitive SBRT. All patients that received adjuvant SBRT had adverse features requiring the addition of postoperative radiation including positive margins, perineural invasion, and extracapsular extension (Table 1). The reasons for choosing SBRT over conventional external beam radiation therapy (EBRT) included patient preference in 10 cases and prior irradiation to adjacent critical structures in the other 3 (Table 1). None of the patients had received previous radiation to the tumor bed. The median dose was 33 Gy but varied between 25 and 40 Gy. Similarly, the median dose per fraction varied between 5 and 8 Gy. Only one patient received chemotherapy.
The median follow-up was 14 months (range 0–59 months) for all patients and 24 months (range 3–59) for surviving patients. Patterns of failure in relation to disease presentation for each patient are summarized in Table 1. Only one patient failed locally, while four failed distantly. One of the patients (#4, Table 1) had initially presented with solitary metastatic adenocarcinoma to the C7 vertebral body of unknown primary that was treated with conventional EBRT prior to presentation to our clinic. At the time of treatment, he had no evidence of other metastatic disease and had declined surgical treatment to the parotid bed. Three months following SBRT treatment, he was diagnosed with widespread metastatic disease to the thoracic vertebrae, rib cage, and liver. He was treated with systemic chemotherapy but succumbed to the disease 5 months following treatment. However, he remained without evidence of locoregional recurrence on imaging studies performed 2 weeks prior to his death.
Clinical outcomes are summarized in Table 2 and Figures 1–3. Correlations with patient presentation can be seen in Table 1. The median OS and PFS were 20 and18 months, respectively (Figures 1–3; Table 2). Since only one patient failed locally, median local failure was not reportable in this series. Two-year actuarial OS, LRC, and PFS were 46, 84, and 47%, respectively. A total of seven patients died, but only four patients died of disease progression, locally or distantly. One patient died on treatment from complications related to aspiration pneumonia. The others died of complications unrelated to their disease. On univariate analysis, gross disease was adversely correlated with OS (p = 0.01). Surgery, on the other hand, was a positive predictor of OS (p = 0.01). There were no other statistically significant correlates of OS or PFS. Since there was only one local failure, local control was excluded from these analyses.
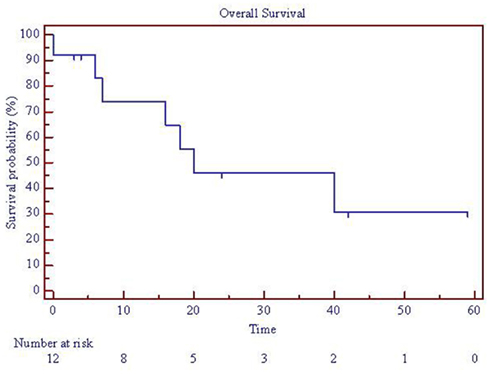
Figure 1. Overall survival for patients with parotid tumors treated with SBRT. One patient died on treatment at time 0, so 12 patients are represented. SBRT = stereotactic body radiation treatment.
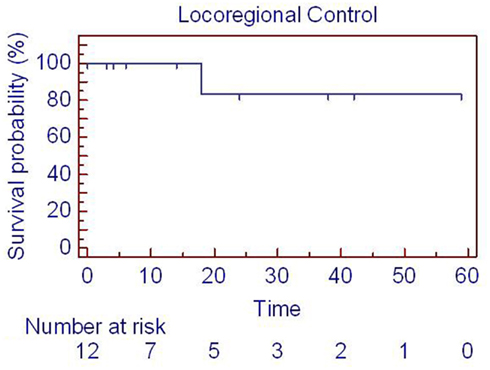
Figure 2. Locoregional control for patients with parotid tumors treated with SBRT. One patient died on treatment at time 0, so 12 patients are represented. SBRT = stereotactic body radiation treatment.
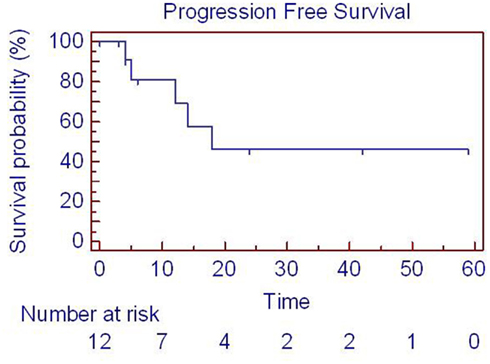
Figure 3. Progression-free survival for patients with parotid tumors with SBRT. One patient died on treatment at time 0, so 12 patients are represented. SBRT = stereotactic body radiation treatment.
Generally, the treatment was well tolerated. Six patients experienced low-grade dysphagia 1 week following the administration of the treatment. The dysphagia was usually palliated by the use of prescribed mouthwash or narcotics, but in no case did it require the placement of a feeding tube. Fibrosis and trismus was noted in one patient (#6, Table 1) and was treated with physical therapy. One patient died on treatment (#11, Table 1) 6 days after the initiation of SBRT, having received a total of 3 fractions of 7 Gy each. The cause of death was attributed to sepsis that developed secondary to aspiration pneumonia. He did report dysphagia during the treatment, but it was unclear if that led to the aspiration pneumonia. One patient developed chronic ipsilateral otalgia without evidence of pathology on clinical exam. None of the patients developed soft tissue or bone necrosis.
The present study described our recent experience with the treatment of parotid gland tumors using fractionated SBRT. The standard of care for salivary gland malignancies is surgery alone or resection followed by postoperative RT for patients with advanced pathological features (Fu et al., 1977). RT alone has been used, with relatively good response rates, for select patients with unfavorable prognosis who are poor surgical candidates. Conventionally, adjuvant fractionated RT is most commonly delivered to a dose of 66–74 Gy in 2 Gy fractions. Reported LRC rates for such treatment is on the order of 55–70% at 5 years (Mendenhall et al., 2005; Chen et al., 2006). Advanced T stage disease and radiation doses lower than 66 Gy serve as predictors of local recurrence (Mendenhall et al., 2005; Chen et al., 2006), contrary to the notion that salivary tumors represent a radioresistant group of tumors. However, the duration of conventionally fractionated RT (42–62 days) to represents a significant burden for many patients, especially the elderly. Even for patients willing to undergo conventionally fractionated RT, previous radiation to adjacent structures may prohibit the delivery of adequate doses required to control gross disease. Finally, conventional irradiation in the setting of gross disease has demonstrated poor disease control rates, with long-term LRC rates ranging from 20 to 30% in most reported studies (Laramore et al., 1993). To our knowledge, this is the first report of using SBRT for the primary (definitive or adjuvant) treatment of parotid tumors not involving the skull base.
Although these data represent a limited follow-up period, they demonstrate favorable treatment response rates for SBRT that are comparable to those reported in the literature using various RT approaches. Five-year local control rates have been reported as 100, 59, and 55–70%, using hyperfractionated photon RT (Wang and Goodman, 1991), neutron RT (Douglas et al., 2003), and conventionally fractionated photon RT (Mendenhall et al., 2005; Chen et al., 2006). With a median follow-up of 14 months (24 for surviving patients), our local control rate is 92% with an actuarial rate of 84% at 2 years. Differences in selection criteria, histological subtypes, and treatment modality most likely account for the discrepancies between observed outcomes across institutions. A trial with direct comparison between the modalities is unlikely to accrue given the relative rarity of this disease. The only randomized trial for salivary gland carcinomas compared primary conventional RT with photons to primary neutron therapy (Laramore et al., 1993). Performed by the RTOG and the Medical Research Council (MRC), this study demonstrated the superiority of neutron therapy (Laramore et al., 1993). The trial was, however, biased by the uneven small number of patients, and the disproportionate distribution of important prognosticators such as histology, tumors size, nodal involvement, presence of disease recurrence, and subtherapeutic doses of photon therapy (Laramore et al., 1993).
The OS rate reported in the current study of 46% at 2 years is lower than what has been reported in the literature, but the cancer-specific mortality of 36% is comparable to published reports (Mendenhall et al., 2005). This is likely attributable to the sample’s bias toward older patients with multiple cormorbid medical conditions. Our series also demonstrated higher distant failure rates than those previously reported in the primary setting (Mendenhall et al., 2005; Chen et al., 2006, 2007). Recent reports have demonstrated the benefit of administering concurrent and/or adjuvant chemotherapy in patients with salivary gland malignancies (Argiris et al., 2003; Salama et al., 2006; Tanvetyanon et al., 2009; Pederson et al., 2011; Schoenfeld et al., 2012). Only one patient in our series who had presented with solitary metastases received chemotherapy, but other eligible candidates for chemotherapy refused treatment.
Consistent with the published data, our results show a positive predictive correlation between surgery and OS (North et al., 1990; Garden et al., 1994; Mendenhall et al., 2005; Chen et al., 2006, 2007). Similarly the presence of gross disease represented a negative predictor of OS. Our results are limited by the small sample size. The observed differences could be due to selection biases, given that patients referred for primary treatment with RT are considered inoperable, have worse performance status, are less rigorously staged, and are of more advanced age with multiple medical co-morbidities.
Although there was no correlation between dose and survival outcomes in our current series, we have generally adopted doses of 30–35 Gy to control microscopic disease and 35–40 Gy for gross disease based on previous experience (Unger et al., 2010). The toxicity from the treatment appears minimal, and the therapy is generally well tolerated. However, one patient died of aspiration pneumonia having experienced treatment-related dysphagia. It is conceivable that this could have resulted from treatment. Additionally, three out of the four patients who died did so within 5 months from the completion of therapy. As such, there may have been insufficient time to develop complications.
This study has demonstrated the possibility of using fractionated SBRT for the treatment of parotid tumors in both the definitive or adjuvant setting, for patients who refuse or are poor candidates for conventional RT. Although at early follow-up, treatment was generally well tolerated and without significant toxicity, long-term toxicity could not be evaluated in this population given the small sample size of the study with an actuarial 2 year survival of 45%. Our retrospective review is limited by potential selection bias, small sample size, a heterogeneous population, varied tumor characteristics, and contrasting treatment parameters. Future studies with increased sample size, longer follow-up, and less histological variability are warranted.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Drs. Guiseppe Esposito and Abdul Rashid for assistance in data collection.
Argiris, A., Haraf, D. J., Kies, M. S., and Vokes, E. E. (2003). Intensive concurrent chemoradiotherapy for head and neck cancer with 5-Fluorouracil- and hydroxyurea-based regimens: reversing a pattern of failure. Oncologist 8, 350–360.
Ashamalla, H., Guirgius, A., Bieniek, E., Rafla, S., Evola, A., Goswami, G., Oldroyd, R., Mokhtar, B., and Parikh, K. (2007). The impact of positron emission tomography/computed tomography in edge delineation of gross tumor volume for head and neck cancers. Int. J. Radiat. Oncol. Biol. Phys. 68, 388–395.
Chen, A. M., Bucci, M. K., Quivey, J. M., Garcia, J., Eisele, D. W., and Fu, K. K. (2006). Long-term outcome of patients treated by radiation therapy alone for salivary gland carcinomas. Int. J. Radiat. Oncol. Biol. Phys. 66, 1044–1050.
Chen, A. M., Granchi, P. J., Garcia, J., Bucci, M. K., Fu, K. K., and Eisele, D. W. (2007). Local-regional recurrence after surgery without postoperative irradiation for carcinomas of the major salivary glands: implications for adjuvant therapy. Int. J. Radiat. Oncol. Biol. Phys. 67, 982–987.
Dieterich, S., and Gibbs, I. C. (2011). The CyberKnife in clinical use: current roles, future expectations. Front. Radiat. Ther. Oncol. 43, 181–194.
Dieterich, S., and Pawlicki, T. (2008). Cyberknife image-guided delivery and quality assurance. Int. J. Radiat. Oncol. Biol. Phys. 71(1 Suppl), S126–S130.
Douglas, J. G., Goodkin, R., and Laramore, G. E. (2008). Gamma knife stereotactic radiosurgery for salivary gland neoplasms with base of skull invasion following neutron radiotherapy. Head Neck. 30, 492–496.
Douglas, J. G., Koh, W. J., Austin-Seymour, M., and Laramore, G. E. (2003). Treatment of salivary gland neoplasms with fast neutron radiotherapy. Arch. Otolaryngol. Head Neck Surg. 129, 944–948.
Fu, K. K., Leibel, S. A., Levine, M. L., Friedlander, L. M., Boles, R., and Phillips, T. L. (1977). Carcinoma of the major and minor salivary glands: analysis of treatment results and sites and causes of failures. Cancer 40, 2882–2890.
Garden, A. S., Weber, R. S., Ang, K. K., Morrison, W. H., Matre, J., and Peters, L. J. (1994). Postoperative radiation therapy for malignant tumors of minor salivary glands. Outcome and patterns of failure. Cancer 73, 2563–2569.
Guzzo, M., Locati, L. D., Prott, F. J., Gatta, G., McGurk, M., and Licitra, L. (2010). Major and minor salivary gland tumors. Crit. Rev. Oncol. Hematol. 74, 134–148.
Kamida, T., Abe, T., Inoue, R., Kobayashi, H., Suzuki, M., and Matsumoto, A. (2005). Stereotactic radiosurgery for recurrent pleomorphic adenoma invading the skull base – case report. Neurol. Med. Chir. (Tokyo) 45, 161–163.
Laramore, G. E., Krall, J. M., Griffin, T. W., Duncan, W., Richter, M. P., Saroja, K. R., Maor, M. H., and Davis, L. W. (1993). Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG-MRC randomized clinical trial. Radiation Therapy Oncology Group. Medical Research Council. Int. J. Radiat. Oncol. Biol. Phys. 27, 235–240.
Mendenhall, W. M., Morris, C. G., Amdur, R. J., Werning, J. W., and Villaret, D. B. (2005). Radiotherapy alone or combined with surgery for salivary gland carcinoma. Cancer 103, 2544–2550.
North, C. A., Lee, D. J., Piantadosi, S., Zahurak, M., and Johns, M. E. (1990). Carcinoma of the major salivary glands treated by surgery or surgery plus postoperative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 18, 1319–1326.
Pederson, A. W., Salama, J. K., Haraf, D. J., Witt, M. E., Stenson, K. M., Portugal, L., Seiwert, T., Villaflor, V. M., Cohen, E. E., Vokes, E. E., and Blair, E. A. (2011). Adjuvant chemoradiotherapy for locoregionally advanced and high-risk salivary gland malignancies. Head Neck Oncol. 3, 31.
Sakata, K., Aoki, Y., Karasawa, K., Nakagawa, K., Hasezawa, K., Muta, N., Terahara, A., Onogi, Y., Sasaki, Y., and Akanuma, A. (1994). Radiation therapy for patients of malignant salivary gland tumors with positive surgical margins. Strahlenther. Onkol. 170, 342–346.
Salama, J. K., Vokes, E. E., Chmura, S. J., Milano, M. T., Kao, J., Stenson, K. M., Witt, M. E., and Haraf, D. J. (2006). Long-term outcome of concurrent chemotherapy and reirradiation for recurrent and second primary head-and-neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 64, 382–391.
Schoenfeld, J. D., Sher, D. J., Norris, C. M. Jr., Haddad, R. I., Posner, M. R., Balboni, T. A., and Tishler, R. B. (2012). Salivary gland tumors treated with adjuvant intensity-modulated radiotherapy with or without concurrent chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 82, 308–314.
Spiro, R. H., Koss, L. G., Hajdu, S. I., and Strong, E. W. (1973). Tumors of minor salivary origin. A clinicopathologic study of 492 cases. Cancer 31, 117–129.
Tanvetyanon, T., Qin, D., Padhya, T., McCaffrey, J., Zhu, W., Boulware, D., DeConti, R., and Trotti, A. (2009). Outcomes of postoperative concurrent chemoradiotherapy for locally advanced major salivary gland carcinoma. Arch. Otolaryngol. Head Neck Surg. 135, 687–692.
Unger, K. R., Lominska, C. E., Deeken, J. F., Davidson, B. J., Newkirk, K. A., Gagnon, G. J., Hwang, J., Slack, R. S., Noone, A. M., and Harter, K. W. (2010). Fractionated stereotactic radiosurgery for reirradiation of head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 77, 1411–1419.
Keywords: parotid malignancies, stereotactic radiosurgery, toxicity
Citation: Karam SD, Snider JW, Wang H Wooster M Lominska C Deeken J Newkirk K Davidson B and Harter KW (2012) Survival outcomes of patients treated with hypofractionated stereotactic body radiation therapy for parotid gland tumors: a retrospective analysis. Front. Oncol. 2:55. doi: 10.3389/fonc.2012.00055
Received: 08 March 2012; Paper pending published: 20 April 2012;
Accepted: 10 May 2012; Published online: 31 May 2012.
Edited by:
Silvia C. Formenti, New York University Langone Medical Center, USAReviewed by:
Peter B. Schiff, NYU School of Medicine, USACopyright: © 2012 Karam, Snider, Wang, Wooster, Lominska, Deeken, Newkirk, Davidson and Harter. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Sana D. Karam, Department of Radiation Medicine, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007, USA. e-mail:c2FuYS5kLmthcmFtQGd1bmV0Lmdlb3JnZXRvd24uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.