
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Oncol. Rev., 26 November 2024
Sec. Oncology Reviews: Reviews
Volume 18 - 2024 | https://doi.org/10.3389/or.2024.1496141
Peritoneal metastases (PM) are the spread of tumor forms into the peritoneum as metastases from another organ. PM is a frequent condition in metastatic gastrointestinal cancer (colorectal, gastric, pancreatic, appendiceal, and cholangiocarcinoma); their presence confers a poor prognosis, reducing patient survival. The standard treatment consists of systemic chemotherapy according to current guidelines. In recent years, scientific evidence has shown how combined cytoreductive surgery (CRS) techniques followed by hyperthermic intraperitoneal chemotherapy (HIPEC) can improve survival in this patient population. Despite the results still obtained, using this combined technique is still under discussion. This review aims to highlight the benefits and limitations of this combined procedure, which is already widely used to treat peritoneal metastases in gynecological tumors.
Peritoneal metastases (PM) is a common condition of gastrointestinal cancer (GI), and it is often associated with poor prognosis (1). PM has been regarded as a less common pattern of cancer metastases, particularly from the perspective of each discrete primary cancer but also collectively across the full spectrum of malignancies that can develop carcinomatosis (2). PM can occur through different mechanisms, including intraperitoneal spread by contiguity, diffusion hematogenous, lymphatic dissemination, and iatrogenic spread during surgery or bioptic procedure (3).
Today, the optimal approach with curative intent can be represented by CRS plus HIPEC. Hyperthermic intraperitoneal chemotherapy (HIPEC) is a localized chemotherapeutic treatment of PM performed after tumor cytoreduction surgery (CRS) that combines the concept of direct delivery of the chemotherapeutic agent to the peritoneum, enabling the application of higher local doses with low systemic toxicity, and the enhancement of its cytotoxic effects using hyperthermia (4). Therefore, this treatment involves a complex surgical intervention, to obtain the maximum benefit, an optimal selection of patients based on performance status and an accurate study of the extent of the disease is needed (5).
Patient selection is a crucial aspect of planning for treating PM from gastrointestinal cancer (6). Thanks to the work of the high volume centers from around the world 8, clinical and radiographic variables associated with increased chances of achieving a complete cytoreduction have been listed: Eastern Cooperative Oncology Group (ECOG) performance status <1; no evidence of extra-abdominal disease; up to three small, resectable parenchymal hepatic metastases; no evidence of biliary obstruction; no evidence of ureteral obstruction; no evidence of intestinal obstruction at more than one site; small bowel involvement: no evidence of gross disease in the mesentery with several segmental sites of partial obstruction; small volume disease in the gastro-hepatic ligament (7). Table 1 summarizes the principle clinical criteria for selecting the major possibility of treatment of PM.
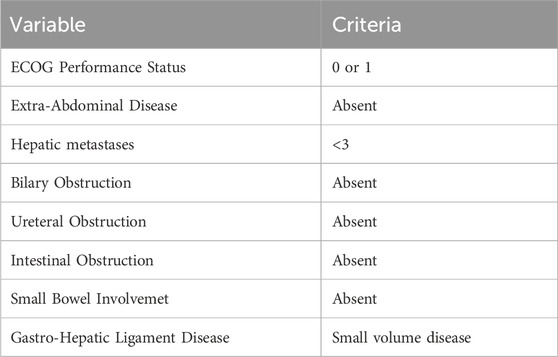
Table 1. Clinical and radiographic variables associated with increased chances of achieving complete cytoreduction in patients with peritoneal metastases (PM) from gastrointestinal cancer.
Clinical and laboratory factors influence the prognosis of good response to HIPEC and CRS. For patients undergoing CRS, the peritoneal cancer index (PCI) is one of the most important prognostic factors used in selecting patients for surgery that has an impact on treatment outcomes (8). PCI is a tool to evaluate the preoperative and intraoperative extent of the disease. The peritoneal cavity is divided into 13 regions, and a score from 1 to 3, depending on lesion size, is recorded for each. A final score from 1 to 39 can be used as a prognostic indicator for the disease course (9). Even if a higher PCI is associated with worse survival, it was never defined a cut-off for complete CRS and HIPEC failure (10).
Several studies have evaluated the timing of the onset of peritoneal metastases, but the results showed no differences in survival between the synchronous or metachronous presentation of peritoneal metastases. Grade III/IV morbidity was independently associated with worse overall survival. Cytoreduction (CC) completeness evaluates the most extensive residual tumor nodules. Patients with no visible residual tumor after surgical de-bulking are given a score of CC-0, while those with the most significant residual tumor nodules <2.5 mm are given CC-1 scores. CC-2 is designated for the most significant tumor deposits between 2.5 mm and 2.5 cm in size, and CC-3 is for tumors greater than 2.5 cm or confluence of multiple smaller nodules. Ideally, surgery with therapeutic intent is aimed at achieving a CC of 1 or less (11).
In addition to HIPEC there are several other approaches for treating PM. These techniques include pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal cancer. PIPAC delivers chemotherapy as a pressurized aerosol into the peritoneal cavity, offering minimally invasive and targeted treatment. Initial studies show promising results in terms of drug delivery and efficacy. However, further research is needed to confirm long-term benefits, safety, and optimal patient selection (12). Intraperitoneal pre-targeted radioimmunotherapy is another therapeutic approach that combines pretargeting techniques with radioimmunotherapy to treat peritoneal carcinomatosis caused by colorectal cancer. This method involves the administration of a targeting agent that binds specifically to cancer cells, followed by a radioactive agent that attaches to the targeting agent, increasing the localized radiation exposure of cancer cells while sparing healthy tissues. Among the advantages of this approach are increased efficacy and reduced systemic toxicity (13). PRIT system currently shows promise for the treatment of patients with peritoneal epithelial ovarian carcinomatosis (EOC). Ongoing studies aim to optimize targeting and improve the therapeutic index for clinical application (14).
Park SY et al. in 2016 conducted a case-control study from a single center about early postoperative intraperitoneal chemotherapy (EPIC) for colorectal cancer patients after complete cytoreductive surgery. 30 patients undergoing EPIC showed significant improvement in 3-year overall survival (74.3% vs. 34.7%) and disease-free survival (53.0% vs. 7.5%) compared with 15 controls. Multivariate analysis identified EPIC as an independent prognostic factor for overall and disease-free survival, suggesting that EPIC is a safe and effective method to prevent peritoneal recurrence and improve outcomes in these patients (15).
In light of the above, this literature review aims to highlight the potential benefits of this combined procedure in upper and lower gastrointestinal tract tumors.
Patients with peritoneal cancer, both primary and secondary, often present with a range of non-specific symptoms, including abdominal bloating, distension, nausea, indigestion, anorexia, weight loss, fatigue, constipation, and abdominal or back pain. Among these, abdominal distension and pain are the most commonly reported symptoms, while palpable abdominal masses and ascites are frequent clinical signs (16). The lack of specific symptoms can lead a difficult differential diagnosed in terms of primary or metastatic of peritoneum involvement and primary site origin of cancer.
HIPEC perfusion lasts for 30–120 min within the peritoneal cavity, but the overall median duration in the underlying publications was 90 min. The median temperature used is between 42° and 43°. There are two fulfillment methodology categories: open abdomen and closed abdomen. The open, or “Coliseum,” technique is performed during laparotomic surgery in which the patient’s abdominal skin edges are suspended by a retractor apparatus alongside the integration of a silicon sheet to establish an open space for perfusion of the hyperthermic chemotherapy solution. This technique allows for a better distribution of the drugs across the peritoneal compartment and can determine the intractable heat loss from the chemotherapeutic solution during the procedure. The closed or laparoscopic technique is done laparoscopically at the end of CRS with subsequent infusion of the hyperthermic chemotherapy solution in the sealed abdominal compartment. Unlike the laparoscopic technique, it provides superior heat loss prevention despite the inefficient distribution of perfusion fluid. Fujimura described a semi-open technique that combines the benefits of both methodologies by minimizing heat loss and allowing a homogeneous distribution of drugs. The device used in this technique is the peritoneal cavity expander (PCE) (17). It is a complex surgical procedure with the potential for high morbidity. In HIPEC, frequently used chemotherapy agents include mitomycin C, oxaliplatin, cisplatin, and doxorubicin (18). These drugs are selected for their effectiveness in treating peritoneal carcinosis and enhancing patient outcomes.
The morbidity after the procedure may be classified into two major types: surgery-related and chemotherapy-related ones. Surgical-related morbidities include postoperative ileus, wound infection and sepsis, bleeding, thrombosis, and lung embolism. Chemotherapy may slow the wound healing process, leading to anastomotic time wasters and increase the risk of post-operative infections (19).
Cytostatic agents used for HIPEC may lead to systemic toxicities: bone marrow toxicity (leucopenia, anemia, thrombopenia,) heart, liver, or renal toxicity are more frequent ones (20). Treatment-related adverse events are classified into two groups: minor complications (grade 0–2) and major complications (grade 3–5) according to the classification system Common Terminology Criteria for Adverse Events (CTCAE version 5.0). Grade 3 are serious events, Grade 4 are life-threatening or disabling events, and Grade 5 are death-related events (21). In conclusion, it can be argued that morbidity rates after CRS and HIPEC are relatively high but comparable to other major gastrointestinal surgeries. However, in existing studies, the assessment of morbidity is not standardized and, therefore, often not comparable (9).
At the state-of-the-art HIPEC and CRS, a combined technique is indicated for the treatment of peritoneal metastases in upper and lower gastrointestinal cancer.
This review lists the indications in various gastrointestinal tumors with current studies and results.
Gastric cancer (GC) is the fifth most common cancer and the third most common cause of cancer death globally (20). Clinicopathological studies reveal that the overall incidence of metastases of GC on peritoneal surfaces is 30%–50%; in addition, the presence of PM is characteristic of diffuse type (45%–75% vs. 10%–30% for intestinal type) (22). For potentially resectable stage IB-III gastric cancers, with node involvement and signet/mucinous histotype, laparoscopy and peritoneal washings for malignant cells are recommended by ESMO guidelines to detect any peritoneal metastatic disease that may not be visible on imaging or macroscopically, with an overall sensitivity of 84.6% and specificity of 100% for identifying peritoneal metastases (23). The NCCN gastric cancer guidelines classify positive peritoneal cytology (CY+) as metastatic (M1) disease and suggest palliative treatments (24).
Literature data is favoring using CRS plus HIPEC to improve the outcome of patients with PM from GC (25). A multicenter, randomized controlled trial (RCT) (GASTRIPEC-I-trial, NCT02158988) exploring the impact of HIPEC after CRS on survival, showed a similar median survival (14.9 vs. 14.9 months, p = 0.16) when compared to the CRS-only arm; however, both the progression-free survival (7.1 vs. 3.5 months P = 0.047) and metastases-free survival (10.2 vs. 9.2 months; P= 0.0286) were significantly improved in the combination arm (26). At the 2024 Fata update, the study showed no OS difference between the CRS + HIPEC and CRS-only arms. PFS and MFS were significantly better in the CRS + H group, which needs further exploration. HIPEC did not increase the frequency of grade ≥3 AEs (27). No solid evidence shows improved survival in gastric cancer patients after CRS-HIPEC treatment (18). Nevertheless, an improvement in survival outcomes has been observed with CRS-HIPEC over CRS alone (11.2 months vs. 5.6 months) in a phase III randomized trial [PMID: 21431408] (25).
Colorectal cancer (CRC) is the third most common malignancy and the second leading cause of cancer-related mortality in the world (28). The peritoneum is one of the most common metastatic sites in CRC, along with the liver and lung, and compared to other sites of disease, peritoneal metastases present a worse prognosis (29). It is estimated that the peritoneum is the only site of metastases in 25% of patients with CRC (30). PM in CRC patients are linked to significantly poor prognostic outcomes (31). With modern systemic chemotherapy combinations, including oxaliplatin and irinotecan along with 5-FU and targeted agents, the median OS for patients with stage IV colorectal cancer has improved significantly, now ranging from approximately 7 to over 24 months (32). In particular, in patients with PM alone, a median survival of 9 months was reported when treated with modern systemic chemotherapy alone (6). Peritoneal treatments can have positive effects on survival, offering significant benefits in selected patients, however, it is essential to emphasize that in the context of colon cancer, systemic chemotherapy (CT) remains crucial for disease control and improving long-term outcomes (33).
Regarding the potential beneficial effect on the survival of HIPEC, until now, only one randomized study has been conducted, a multicentric French trial (PRODIGE 7 trial, NCT00769405), which compared CRS plus HIPEC to CRS without HIPEC. The PRODIGE 7 trial showed that adjuvant HIPEC did not improve recurrence-free and overall survival in this relatively small cohort of patients. The trial established the value of high-quality CRS in both arms of the trial, with a higher-than-expected median overall survival of 41 months in both groups (34).
A multicenter study published by Fisher O.M. in 2024 assessed the efficacy of HIPEC in patients with colorectal cancer and peritoneal metastases (pmCRC) using a large international dataset. It involved 2,093 patients from 39 centers who underwent cytoreductive surgery with HIPEC between 1991 and 2018, comparing two HIPEC protocols: oxaliplatin-HIPEC and mitomycin-HIPEC. The oxaliplatin-HIPEC group had a significantly longer OS (47 months) than the mitomycin-HIPEC group (39 months). Notably, the combination of oxaliplatin and irinotecan in HIPEC yielded the best OS (61 months). The study concluded that oxaliplatin-based HIPEC provides superior outcomes compared to mitomycin-based HIPEC, with lower 90-day mortality rates observed in the oxaliplatin group and a trend toward a dose-response relationship (35).
In Table 2 are listed ongoing trials on HIPEC in CRC and GC in the metastatic setting.
Appendiceal tumors are rare and constitute less than 0.5% of all neoplasms of gastrointestinal origin (36). Appendiceal cancer can potentially disseminate into the peritoneal cavity, causing PM in approximately 20% of patients (37). Glehen et al., in their retrospective multicenter study of >500 patients, suggested clearly that the therapeutic approach combining cytoreductive surgery with peri-operative HIPEC had significantly better long-term survival than patients who did not. Patients in whom cytoreductive surgery was complete had a median survival of 32.4 months, compared with 8.4 months for patients in whom complete cytoreductive surgery was impossible (p < .001). The outcomes results are comparable to colorectal cancer but not significantly better as might have been expected in appendiceal cancer (38).
Pancreatic cancer (PC) is a relatively rare and lethal disease with incidences ranging from 6.4 to 7.7 cases per 100,000 people per year in Western countries. It is the 7th most common cause of cancer-related mortality (39). However, many patients are diagnosed at an advanced stage, and the peritoneum is the second most common site of metastases in the case of disseminated pancreatic disease (40). The presence of PM worsens its prognosis. There is currently no broad consensus for the use of intraperitoneal infusion of chemotherapeutic substances in the treatment of pancreatic cancer. HIPEC could be considered a promising technique for improving survival rate without additional morbidity in case of borderline resectable and locally advanced disease when surgical resection and CRS are possible after neoadjuvant treatment (41). There is a phase II study currently enrolling patients (NCT04858009) (42); the trial studies the effects of HIPEC in the treatment of patients with pancreatic cancer with peritoneal metastases. This study may help doctors determine the safety and effectiveness of HIPEC in treating pancreatic cancer patients. The primary objectives of the trial are OS and DFS.
Cholangiocarcinoma is an epithelial cell malignancy arising from varying locations within the biliary tree, and it is divided intrahepatic, perihilar, and distal (43). Tumors of the biliary tract account for 1% of all cancers and approximately 10%–15% of all primary cancers originating in the liver (44). The peritoneum is the most frequent site of metastases in cholangiocarcinoma (45) 10%–20% of patients have peritoneal involvement at presentation (46). In 2021, Feng et al. performed a retrospective study to compare the prognosis of patients with advanced intrahepatic cholangiocarcinoma (ICC) undergoing CRS + HIPEC versus CRS alone (47). However, prospective studies are required to validate these findings further and support the implementation of this technique in clinical practice. The median OS was longer in the CRS + HIPEC group than in the CRS group (25.53 vs. 11.17 months, p < 0.001). CRS + HIPEC could be a treatment option for patients with advanced ICC, with improved OS and similar complications and adverse events compared with CRS alone.
Several clinical trials are underway to evaluate the effectiveness of HIPEC in reducing peritoneal recurrence and improving survival outcomes when combined with CRS.
The GASTRICHIP study is a prospective, open-label, randomized multicenter phase III clinical trial designed to evaluate the effects of HIPEC with oxaliplatin in patients with GC that involves the serosa and/or lymph node metastases, or positive cytology in peritoneal washing. The trial will include patients scheduled for D1-D2 curative gastrectomy. Primary outcome is OS measured from the date of surgery to the date of death or the end of the 5-year follow-up period; secondary outcomes are efficacy assessed through 3-year and 5-year recurrence-free survival rates, localization of recurrence, morbidity, and quality of life (48). The PERISCOPE II trial assesses the efficacy of combining gastrectomy, CRS, and HIPEC for patients with gastric cancer that has limited peritoneal dissemination. This multicenter randomized controlled trial aims to determine if this treatment approach provides a survival benefit compared to standard palliative systemic chemotherapy. The primary outcome measured will be overall survival, with secondary outcomes focusing on complications and quality of life. The study began in October 2017 and is expected to complete by 2029 (49). Regarding the adjuvant setting in CRC, the COLOPEC trial investigated the efficacy of adjuvant HIPEC in patients with locally advanced CRC after cytoreductive surgery. Participants with clinical or pathological T4 N0-2 M0 or perforated colon cancer were randomly assigned to receive either adjuvant systemic chemotherapy with HIPEC or adjuvant systemic chemotherapy alone. After a median follow-up of 59 months, the results showed no significant differences in the 5-year overall survival rate (69.6% vs. 70.9%), peritoneal metastases rates (63.9% vs. 63.2%), or disease-free survival (55.7% vs. 52.3%) between the two groups. Additionally, quality-of-life outcomes were similar. The findings suggest that adjuvant HIPEC should be reserved for clinical trials rather than standard practice (50). The PROPHYLOCHIP trial examined the benefit of systematic second-look surgery combined with HIPEC in colorectal cancer patients at high risk for PM. Patients who remained recurrence-free post-surgery and chemotherapy were randomized to surveillance or a treatment group receiving HIPEC with oxaliplatin. Results showed no significant difference in 3-year disease-free survival, peritoneal recurrence-free survival, or overall survival. High postoperative complications and potential oxaliplatin resistance were noted, raising concerns about HIPEC’s effectiveness in this setting (51).
The HIPECT4 trial was a phase 3 randomized controlled trial conducted in 17 centers across Spain from 2015 to 2021. It aimed to evaluate the efficacy of HIPEC with mitomycin C in patients with T4 colon cancer, focusing on locoregional disease control. Patients were randomized to receive either CRS with HIPEC followed by systemic chemotherapy or CRS alone with systemic chemotherapy. After 3 years, the locoregional disease control rate was significantly higher in the HIPEC group (97.6%) compared to the control (87.6%), with no differences in disease-free or overall survival (51). Table 3 lists ongoing studies in the adjuvant setting.
Peritoneal metastases (PM) occur when tumor cells spread to the peritoneum from another organ, frequently arising from gastrointestinal cancers. The presence of PM generally suggests a poor prognosis and significantly reduces patient survival, leading to exclusive systemic chemotherapy. However, recent studies suggest that combining CRS with HIPEC and systemic chemotherapy may improve survival outcomes for patients with PM.
Research on the application of CRS combined with HIPEC is evolving, particularly concerning its integration with emerging cancer therapies.
Fisher O.M. et al showed that oxaliplatin-HIPEC significantly improved overall survival in pmCRC. In addition, Feng et al. in 2021 found that SRC + HIPEC improved median overall survival in patients with advanced intrahepatic cholangiocarcinoma (ICC) compared with SRC alone (25.53 vs. 11.17 months), although further prospective studies are needed.
An innovative field of research in PM management is represented by the application of nanoparticles to improve the efficacy of HIPEC. Nanoparticles possess unique properties that make them suitable for drug delivery, including a high surface-to-volume ratio, the ability to encapsulate therapeutic agents, and the potential for targeted delivery to tumor sites (52). Indeed, Tang L et al. in 2021, highlighted the unique properties of nanoparticles that allow for improved drug solubility, stability, and targeted delivery to tumor sites. Integrating nanoparticles in these therapies can enhance treatment efficacy while minimizing systemic side effects. However, toxicity, safety, and regulatory hurdles must be addressed (53).
Another intriguing field to enhance the HIPEC efficacy, is represented by the microenvironment effect on response to treatment of PM. While HIPEC enhances immune responses by promoting T- and NK-cell infiltration and increasing antigen presentation, it can also induce immunosuppressive changes, such as increased regulatory T cells and myeloid-derived suppressor cells. This complexity requires an individualized immune profile and suggests that combining HIPEC with immunotherapies may optimize treatment outcomes for patients with peritoneal carcinosis. The balance between pro- and anti-tumor immunity is crucial to improve prognosis (54). In particular, catumaxomab, a trifunctional antibody targeting EpCAM, has been shown to reduce malignant ascites. Intraperitoneal immunotherapy aims to enhance T-cell responses and includes treatments such as CAR-T cells that can enhance immune activity against tumors expressing carcinoembryonic antigen (55). The ImmunoPeCa trial (NCT02219893) was a phase 1 study assessing the safety of the MOC31PE immunotoxin in 21 colorectal cancer patients with peritoneal metastases following CRS/HIPEC. It was conducted in 2017 and found MOC31PE to be safe and well-tolerated, with cytotoxic levels in peritoneal fluid despite limited systemic absorption. Neutralizing antibodies were produced in all patients, leading to a 3-year overall survival estimate of 78% and a median progression-free survival of 21 months, indicating the need for further evaluation of MOC31PE’s effectiveness (56).
Continued exploration in this field may enhance treatment outcomes for patients with peritoneal carcinosis.
The management of PM in gastrointestinal cancers presents a significant challenge in oncology due to the historically poor prognosis associated with this condition. While systemic chemotherapy has been the conventional treatment until nowadays, recent advancements in surgical techniques and localized therapies as HIPEC, offer promising alternatives to achieve the survival outcomes. Although randomized trial evidence remains non-homogeneous, the integration of CRS with HIPEC seems to provide progression-free and metastases-free survival advantages, especially among highly select patient populations.
Nevertheless, the complexity of the procedure underscores the importance of careful patient selection and a comprehensive understanding of the disease’s extent to optimize outcomes. As research progresses, particularly with the investigation of innovative drug delivery systems, there is a significant opportunity to enhance the efficacy of HIPEC. Considering the dual influence of HIPEC on tumor’s immune environment, the recognition of immune profile of microenvironment could represent a possibility to personalize the therapeutic approach of PM. Future studies should concentrate on refining treatment protocols, standardizing assessments of morbidity, and exploring combination therapies to ensure that patients with peritoneal metastases receive the most effective and individualized care possible.
DD: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing–original draft, Writing–review and editing. FS: Supervision, Validation, Visualization, Writing–review and editing. GA: Conceptualization, Methodology, Resources, Supervision, Writing–review and editing. FM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Coccolini, F, Gheza, F, Lotti, M, Virzì, S, Iusco, D, Ghermandi, C, et al. Peritoneal carcinomatosis. World J Gastroenterol (2013) 19(41):6979–94. doi:10.3748/wjg.v19.i41.6979
2. Foster, JM, Zhang, C, Rehman, S, Sharma, P, and Alexander, HR. The contemporary management of peritoneal metastasis: a journey from the cold past of treatment futility to a warm present and a bright future. CA: A Cancer J Clinicians (2023) 73:49–71. doi:10.3322/caac.21749
3. Associazione Italiana Oncologia Medica (AIOM). Linee guida tumori peritoneali primitivi e secondari. AIOM (2021). Available at: https://www.iss.it/documents/20126/8403839/LG-457-AIOM_Peritoneo (Accessed September 15, 2024).
4. Gronau, F, Feldbruegge, L, Oberwittler, F, Gonzalez-Moreno, S, Villeneuve, L, Eveno, C, et al. HIPEC in peritoneal metastasis of gastric origin: a systematic review of regimens and techniques. J Clin Med (2022) 11(5):1456. Published online 2022 Mar 7. doi:10.3390/jcm11051456
5. Sánchez-Hidalgo, JM, Rodríguez-Ortiz, L, Arjona-Sánchez, Á, Rufián-Peña, S, Casado-Adam, Á, Cosano-Álvarez, A, et al. Colorectal peritoneal metastases: optimal management review. World J Gastroenterol (2019) 25(27):3484–502. PMID: 31367152; PMCID: PMC6658395. doi:10.3748/wjg.v25.i27.3484
6. Chua, TC, Morris, DL, Saxena, A, Esquivel, J, Liauw, W, Doerfer, J, et al. Influence of modern systemic therapies as adjunct to cytoreduction and perioperative intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis: a multicenter study. Ann Surg Oncol (2011) 18:1560–7. doi:10.1245/s10434-010-1522-1
7. Ihemelandu, CU, Shen, P, Stewart, JH, Votanopoulos, K, and Levine, EA. Management of peritoneal carcinomatosis from colorectal cancer. Semin Oncol (2011) 38(4):568–75. PMID: 21810516; PMCID: PMC3768128. doi:10.1053/j.seminoncol.2011.05.011
8. Sugarbaker, PH. Prevention and treatment of peritoneal metastases: a comprehensive review. Indian J Surg Oncol (2019) 10:3–23. doi:10.1007/s13193-018-0856-1
9. Glockzin, G, Schlitt, HJ, and Piso, P. Peritoneal carcinomatosis: patients selection, perioperative complications and quality of life related to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol (2009) 7:5. PMID: 19133112; PMCID: PMC2639355. doi:10.1186/1477-7819-7-5
10. Votanopoulos, KI, Bartlett, D, Moran, B, Haroon, CM, Russell, G, Pingpank, JF, et al. PCI is not predictive of survival after complete CRS/HIPEC in peritoneal dissemination from high-grade appendiceal primaries. Ann Surg Oncol (2018) 25(3):674–8. Epub 2017 Dec 29. PMID: 29288288; PMCID: PMC5890297. doi:10.1245/s10434-017-6315-3
11. Neuwirth, MG, Alexander, HR, and Karakousis, GC. Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol (2016) 7(1):18–28. PMID: 26941981; PMCID: PMC4754315. doi:10.3978/j.issn.2078-6891.2015.106
12. Tate, SJ, and Torkington, J. Pressurized intraperitoneal aerosol chemotherapy: a review of the introduction of a new surgical technology using the IDEAL framework. BJS Open (2020) 4(2):206–15. Epub 2020 Jan 19. PMID: 31957257; PMCID: PMC7093779. doi:10.1002/bjs5.50257
13. Chandler, CS, Bell, MM, Chung, SK, Veach, DR, Fung, EK, Punzalan, B, et al. Intraperitoneal pretargeted radioimmunotherapy for colorectal peritoneal carcinomatosis. Mol Cancer Ther (2022) 21(1):125–37. Epub 2021 Oct 19. PMID: 34667111; PMCID: PMC9157533. doi:10.1158/1535-7163.MCT-21-0353
14. Chung, SK, Vargas, DB, Chandler, CS, Katugampola, S, Veach, DR, McDevitt, MR, et al. Efficacy of HER2-targeted intraperitoneal 225Ac α-pretargeted radioimmunotherapy for small-volume ovarian peritoneal carcinomatosis. J Nucl Med (2023) 64(9):1439–45. Epub 2023 Jun 22. PMID: 37348919; PMCID: PMC10478816. doi:10.2967/jnumed.122.265095
15. Park, SY, Choi, GS, Park, JS, Kim, HJ, Yang, CS, Kim, JG, et al. Efficacy of early postoperative intraperitoneal chemotherapy after complete surgical resection of peritoneal metastasis from colorectal cancer: a case-control study from a single center. Ann Surg Oncol (2016) 23:2266–73. doi:10.1245/s10434-016-5148-9
16. Anwar, A, and Kasi, A. Peritoneal cancer. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2024). Available from: https://www.ncbi.nlm.nih.gov/books/NBK562138/ (Accessed April 30, 2024).
17. Yap, DRY, Wong, JSM, Tan, QX, Tan, JW, Chia, CS, and Ong, CAJ. Effect of HIPEC on peritoneal recurrence in peritoneal metastasis treated with cytoreductive surgery: a systematic review. Front Oncol (2021) 11:795390. doi:10.3389/fonc.2021.795390
18. Shaligram, A. Management of peritoneal surface malignancies in laparoscopic era: a concise review. Int J Surg Oncol (2016) 1(2):e05. Epub 2016 Nov 11. PMID: 29177208; PMCID: PMC5673112. doi:10.1097/IJ9.0000000000000005
19. Wu, Z, Li, Z, and Ji, J. Morbidity and mortality of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in advanced gastric cancer. Transl Gastroenterol Hepatol (2016) 1:63. PMID: 28138629; PMCID: PMC5244745. doi:10.21037/tgh.2016.07.03
20. Smyth, EC, Nilsson, M, Grabsch, HI, van Grieken, NC, and Lordick, F. Gastric cancer. The Lancet (2020) 396(10251):635–48. PMID: 32861308. doi:10.1016/S0140-6736(20)31288-5
21. Hansson, J, Graf, W, Påhlman, L, Nygren, P, and Mahteme, H. Postoperative adverse events and long-term survival after cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol (Ejso) (2009) 35(2):202–8. doi:10.1016/j.ejso.2008.04.002
22. Averbach, AM, and Jacquet, P. Strategies to decrease the incidence of intra-abdominal recurrence in resectable gastric cancer. J Br Surg (1996) 83(6):726–33. PMID: 8696727. doi:10.1002/bjs.1800830605
23. Ramos, RF, Scalon, FM, Scalon, MM, and Dias, DI. Staging laparoscopy in gastric cancer to detect peritoneal metastases: a systematic review and meta-analysis. Eur J Surg Oncol (Ejso) (2016) 42(9):1315–21. Epub 2016 Jul 9. PMID: 27432515. doi:10.1016/j.ejso.2016.06.401
24. National Comprehensive Cancer Network. Clinical practice guidelines in oncology (NCCN Guidelines®). Version 3. Gastric Cancer (2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (Accessed September 15, 2024).
25. Yang, XJ, Huang, CQ, Suo, T, Mei, LJ, Yang, GL, Cheng, FL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol (2011) 18(6):1575–81. Epub 2011 Mar 23. PMID: 21431408; PMCID: PMC3087875. doi:10.1245/s10434-011-1631-5
26. Khan, H, and Johnston, FM. Current role for cytoreduction and HIPEC for gastric cancer with peritoneal disease. J Surg Oncol (2022) 125(7):1176–82. PMID: 35481913; PMCID: PMC9322542. doi:10.1002/jso.26894
27. Rau, B, Lang, H, Koenigsrainer, A, Gockel, I, Rau, HG, Seeliger, H, et al. Effect of hyperthermic intraperitoneal chemotherapy on cytoreductive surgery in gastric cancer with synchronous peritoneal metastases: the phase III GASTRIPEC-I trial. J Clin Oncol (2024) 42(2):146–56. Epub 2023 Oct 31. PMID: 37906724; PMCID: PMC10824373. doi:10.1200/JCO.22.02867
28. Baidoun, F, Elshiwy, K, Elkeraie, Y, Merjaneh, Z, Khoudari, G, Sarmini, MT, et al. Colorectal cancer epidemiology: recent trends and impact on outcomes. Curr Drug Targets (2021) 22(9):998–1009. doi:10.2174/1389450121999201117115717
29. Wang, H, and Qin, XS. Strategies and challenges in the diagnosis and treatment of colorectal cancer peritoneal metastasis. Zhonghua Wei Chang Wai Ke Za Zhi (2021) 24(3):208–13. PMID: 34645163. doi:10.3760/cma.j.cn.441530-20201105-00592
30. Koppe, MJ, Boerman, OC, Oyen, WJ, and Bleichrodt, RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg (2006) 243(2):212–22. PMID: 16432354; PMCID: PMC1448921. doi:10.1097/01.sla.0000197702.46394.16
31. Kranenburg, O, van der Speeten, K, and de Hingh, I. Peritoneal metastases from colorectal cancer: defining and addressing the challenges. Front Oncol (2021) 11:650098. PMID: 33816304; PMCID: PMC8010649. doi:10.3389/fonc.2021.650098
32. Bhatt, A, and Goéré, D. Cytoreductive surgery plus HIPEC for peritoneal metastases from colorectal cancer. Indian J Surg Oncol (2016) 7(2):177–87. Epub 2016 Feb 4. PMID: 27065708; PMCID: PMC4818622. doi:10.1007/s13193-016-0499-z
33. Best, L, Simmonds, P, Baughan, C, Buchanan, R, Davis, C, Fentiman, I, et al. Palliative chemotherapy for advanced or metastatic colorectal cancer. Colorectal Meta-analysis Collaboration. Cochrane Database Syst Rev (2000) 2000(2):CD001545. PMID: 10796809; PMCID: PMC7025779. doi:10.1002/14651858.CD001545
34. van de Vlasakker, VCJ, Lurvink, RJ, Cashin, PH, Ceelen, W, Deraco, M, Goéré, D, et al. The impact of PRODIGE 7 on the current worldwide practice of CRS-HIPEC for colorectal peritoneal metastases: a web-based survey and 2021 statement by Peritoneal Surface Oncology Group International (PSOGI). Eur J Surg Oncol (2021) 47(11):2888–92. Epub 2021 May 13. PMID: 34020808. doi:10.1016/j.ejso.2021.05.023
35. Rau, B, Gül-Klein, S, Esquivel, J, Larsen, SG, Liauw, W, Alzahrani, NA, et al. Hyperthermic intraperitoneal chemotherapy in colorectal cancer. BJS Open (2024) 8:zrae017. doi:10.1093/bjsopen/zrae049
36. Benedix, F, Reimer, A, Gastinger, I, Mroczkowski, P, Lippert, H, Kube, R, et al. Primary appendiceal carcinoma--epidemiology, surgery and survival: results of a German multi-center study. Eur J Surg Oncol (Ejso) (2010) 36(8):763–71. Epub 2010 Jun 18. PMID: 20561765. doi:10.1016/j.ejso.2010.05.025
37. Kyang, LS, Alzahrani, NA, Alshahrani, MS, Rahman, MK, Liauw, W, and Morris, DL. Early recurrence in peritoneal metastasis of appendiceal neoplasm: survival and prognostic factors. Eur J Surg Oncol (2019) 45(12):2392–7. Epub 2019 Jun 22. PMID: 31253546. doi:10.1016/j.ejso.2019.06.015
38. Glehen, O, Kwiatkowski, F, Sugarbaker, PH, Elias, D, Levine, EA, De Simone, M, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol (2004) 22(16):3284–92. PMID: 15310771. doi:10.1200/JCO.2004.10.012
39. Gentiluomo, M, Canzian, F, Nicolini, A, Gemignani, F, Landi, S, and Campa, D. Germline genetic variability in pancreatic cancer risk and prognosis. Semin Cancer Biol (2022) 79:105–31. doi:10.1016/j.semcancer.2020.08.003
40. Takeda, T, Sasaki, T, Mie, T, Furukawa, T, Yamada, Y, Kasuga, A, et al. Improved prognosis of pancreatic cancer patients with peritoneal metastasis. Pancreatology (2021) 21(5):903–11. Epub 2021 Mar 18. PMID: 33766484. doi:10.1016/j.pan.2021.03.006
41. Tentes, AK. Hyperthermic intra-operative intraperitoneal chemotherapy as an adjuvant to pancreatic cancer resection. J Gastrointest Oncol (2021) 12(Suppl. 1):S91–S98. PMID: 33968429; PMCID: PMC8100705. doi:10.21037/jgo-20-46
42. Grotz, TE, Yonkus, JA, Thiels, CA, Warner, SG, McWilliams, RR, Mahipal, A, et al. Cytoreduction with hyperthermic intraperitoneal chemoperfusion for pancreatic cancer with low-volume peritoneal metastasis: results from a prospective pilot study. Ann Surg Oncol (2023) 30(1):395–403. Epub 2022 Aug 16. PMID: 35972667. doi:10.1245/s10434-022-12328-z
43. Razumilava, N, and Gores, GJ. Cholangiocarcinoma. Lancet (2014) 383:2168–79. doi:10.1016/S0140-6736(13)61903-0
44. Valle, JW, Borbath, I, Khan, SA, Huguet, F, Gruenberger, T, and Arnold, D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2016) 27:v28–v37. doi:10.1093/annonc/mdw324
45. Hernandez, DL, Restrepo, J, and Garcia Mora, M. Peritoneal Metastasis of Cholangiocarcinoma Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy at the Instituto Nacional de Cancerología, Colombia. Cureus (2020) 12(1):e6697. PMID: 32117649; PMCID: PMC7029824. doi:10.7759/cureus.6697
46. Khan, SA, Davidson, BR, Goldin, R, Pereira, SP, Rosenberg, WMC, Taylor-Robinson, SD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut (2002) 51:1–9. doi:10.1136/gut.51.suppl_6.vi1
47. Feng, F, Gao, Q, Wu, Y, Liu, C, Yu, Y, Li, B, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy vs. cytoreductive surgery alone for intrahepatic cholangiocarcinoma with peritoneal metastases: a retrospective cohort study. Eur J Surg Oncol (2021) 47(9):2363–8. Epub 2021 May 14. PMID: 34119376. doi:10.1016/j.ejso.2021.05.014
48. Glehen, O, Passot, G, Villeneuve, L, Vaudoyer, D, Bin-Dorel, S, Boschetti, G, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer (2014) 14:183. doi:10.1186/1471-2407-14-183
49. Sarvestani, AL, Gregory, SN, Akmal, SR, Hernandez, JM, van der Sluis, K, and van Sandick, JW. Gastrectomy + cytoreductive surgery + HIPEC for gastric cancer with peritoneal dissemination (PERISCOPE II). Ann Surg Oncol (2024) 31(1):28–30. Epub 2023 Nov 10. PMID: 37947975. doi:10.1245/s10434-023-14415-1
50. Zwanenburg, ES, El Klaver, C, Wisselink, DD, Punt, CJA, Snaebjornsson, P, Crezee, J, et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): 5-year results of a randomized multicenter trial. J Clin Oncol (2024) 42(2):140–5. doi:10.1200/JCO.22.02644
51. Sun, BJ, Daniel, SK, and Lee, B. The role of prophylactic and adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in prevention of peritoneal metastases in advanced colorectal cancer. J Clin Med (2023) 12(20):6443. PMID: 37892582; PMCID: PMC10607874. doi:10.3390/jcm12206443
52. Hsu, CY, Rheima, AM, Kadhim, MM, Ahmed, NN, Mohammed, SH, Abbas, FH, et al. An overview of nanoparticles in drug delivery: properties and applications. South Afr J Chem Eng (2023) 46:233–70. doi:10.1016/j.sajce.2023.08.009
53. Tang, L, Li, J, Zhao, Q, Pan, T, Zhong, H, and Wang, W. Advanced and innovative nano-systems for anticancer targeted drug delivery. Pharmaceutics (2021) 13(8):1151. PMID: 34452113; PMCID: PMC8398618. doi:10.3390/pharmaceutics13081151
54. Chia, DKA, Demuytere, J, Ernst, S, Salavati, H, and Ceelen, W. Effects of hyperthermia and hyperthermic intraperitoneal chemoperfusion on the peritoneal and tumor immune contexture. Cancers (2023) 15:4314. doi:10.3390/cancers15174314
55. Ornella, MSC, Badrinath, N, Kim, KA, Kim, JH, Cho, E, Hwang, TH, et al. Immunotherapy for peritoneal carcinomatosis: challenges and prospective outcomes. Cancers (Basel) (2023) 15(8):2383. PMID: 37190310; PMCID: PMC10137063. doi:10.3390/cancers15082383
56. Frøysnes, IS, Andersson, Y, Larsen, SG, Davidson, B, Øien, JMT, Julsrud, L, et al. ImmunoPeCa trial: long-term outcome following intraperitoneal MOC31PE immunotoxin treatment in colorectal peritoneal metastasis. Eur J Surg Oncol (2021) 47(1):134–8. Epub 2019 Apr 22. PMID: 31036394. doi:10.1016/j.ejso.2019.04.014
Keywords: peritoneal metastases (PM), cytoreductive surgery (CRS), hyperthermic intraperitoneal chemotherapy (hipec), gastrointestinal cancer (GI), colorectal cancer (CRC), gastric cancer (GC), appendiceal cancer, pancreatic cancer (PC)
Citation: Drittone D, Schipilliti FM, Arrivi G and Mazzuca F (2024) Cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy applications in upper and lower gastrointestinal cancer, a review. Oncol. Rev. 18:1496141. doi: 10.3389/or.2024.1496141
Received: 13 September 2024; Accepted: 14 November 2024;
Published: 26 November 2024.
Edited by:
Mauro Cives, University of Bari Aldo Moro, ItalyReviewed by:
Gaetano Pezzicoli, University of Bari Aldo Moro, ItalyCopyright © 2024 Drittone, Schipilliti, Arrivi and Mazzuca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Denise Drittone, ZGVuaXNlLmRyaXR0b25lQHVuaXJvbWExLml0; Federica Mazzuca, ZmVkZXJpY2EubWF6enVjYUB1bmlyb21hMS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.