- 1Department of Radiation Oncology, The Breast Cancer Institute, Nanchang People’s Hospital, Nanchang, Jiangxi, China
- 2Department of Breast Surgery, The Breast Cancer Institute, Nanchang People’s Hospital, Nanchang, Jiangxi, China
- 3Department of Radiation Oncology, Affiliated Rehabilitation Hospital of Nanchang University, Nanchang, Jiangxi, China
Background: Sentinel lymph node biopsy (SLNB) has become standard procedure for early breast cancer patients with clinically node negative disease. The patients with SLN metastasis normally underwent axillary lymph node dissection (ALND). However, the metastatic status of non-sentinel Lymph nodes (non-SLNs) varied significantly in different reports. Here, we evaluated the prevalence of non-SLNs metastasis among breast cancer patients with sentinel lymph node metastasis and its impact on clinical decision-making.
Materials and Methods: We identified 892 female patients with operable cT1-3N0 invasive breast cancer who underwent ALND in our center due to SLN metastasis from 2017 to 2023, retrospectively. The prevalence of non-SLN metastasis among different clinicopathological traits and its correlation with the number of positive SLNs were analyzed. The optimal clinical decision-making was generalized.
Results: The median number of SLN+, SLN, non-SLN+ and non-SLN was 2, 4, 1 and 14 among the enrolled 892 female patients, respectively. 504 (56.50%) patients with SLN + had at least one metastatic lymph node in the harvested non-SLNs. Among the enrolled 892 female patients, 435 (48.77%) patients with 1 positive SLN, of which 180 (41.38%) had at least one additional metastatic non-SLNs. 242 (27.13%) patients with 2 positive SLNs, of which 146 (60.33%) had at least one metastatic non-SLNs. For the rest 215 (24.10%) patients with at least 3 metastatic SLNs, 178 (82.79%) had at least one metastatic non-SLNs. In the univariate analysis, the non-SLNs metastatic status was correlated with the number of SLNs+, tumor size, tumor grade, lymphovascular invasion (LVI) and molecular subtypes, but not histopathologic type. In the multivariate analysis, the risk of additional non-SLNs metastasis correlated with the number of SLNs+, SLNs, non-SLNs and LVI.
Conclusion: Omiting ALND in patients with higher non-SLNs + rate outside the American College of Surgeons Oncology Group (ACSOG) Z0011 and the European Organization for Research and Treatment of Cancer (EORTC) 10,981–22023 AMAROS criteria should be considered with caution in clinical decision-making. To evaluate whether axillary radiotherapy and ALND provides equivalent regional control in breast cancer patients with obvious residual metastatic lymph nodes undesected in the axilla, a well-matched prospective randomized controlled trial is an urgent need.
Introduction
The breast cancer is one of the most frequently diagnosed cancers and the fifth most common cause of cancer related death among women worldwide (1, 2). Its prognosis has improved due to the tremendous efforts made in the early detection and the development of treatment modalities including surgical techniques and adjuvant treatments post-surgery, such as endocrine therapy, chemotherapy, human epidermal growth factor receptor type 2 (HER2) targeted therapy and radiotherapy (3, 4, 5, 6). Patient quality of life (QoL) is increasingly being highly valued. Axillary lymph node metastasis is an important factor in determining the stage of breast cancer and deciding postoperative treatment, which is an independent prognostic factor associated with local or distant metastatic recurrence (7). Therefore, to precisely evaluate axillary lymph node status is essential for the standardized diagnosis and treatment of breast cancer, and axillary lymph node dissection (ALND) has been applied in surgical treatment of breast cancer for several decades. However, postoperative lymphedema of the upper limb often puzzles patients with breast cancer after ALND (8). Lymphedema is a chronic disease characterized by an accumulation of lymphatic fluid, resulting in skin and tissue changes (9). Breast cancer-related lymphedema develops as a result of damage or dysfunction of the normally functioning lymphatic system mainly due to ALND, which significantly impacts patient QoL (8, 10, 11). Therefore, it is very important to preserve the armpit for improving the QoL of breast cancer patients, especially for early breast cancer patients with clinically negative axillary lymph nodes. Over 2 decades ago, the sentinel lymph node biopsy (SLNB) was introduced into axillary staging in operable early breast cancer (12). SLNB was originally used for cT1-2 disease but was also widely used for cT3 recently. For patients with no SLN tumor cell involvement, ALND could be omitted (13). The incidence of lymphedema of the upper limb has declined dramatically and the patient QoL has improved. SLNB has also become a standard surgical procedure for clinically negative axillary lymph nodes breast cancer patients in our institute since early 2010s. Recent years, many scholars believed that ALND for patients with low burden positive SLNs might be omitted either, since non-sentinel lymph node (non-SLN) metastasis was very low for these patients (14, 15, 16, 17). However, the universal management of axillary lymph nodes in patients with positive SLNs was still unknown. Several clinical trials including American College of Surgeons Oncology Group (ACSOG) Z0011 and European Organisation for the Research and Treatment of Cancer (EORTC) 10,981–22023 AMAROS had demonstrated that axillary radiotherapy and ALND had comparable locoregional control and much lower morbidity among patients with limited SLN metastasis. However, most patients enrolled in these two trials had no additional positive axillary lymph nodes (non-SLNs) in the ALND group. Only 97 of 355 (27.3%) patients had additional non-SLNs metastasis, and 13.7% of patients undergoing ALND had 4 or more involved nodes in Z0011. 220 of 672 (33%) patients with additional positive non-SLNs nodes in AMAROS. Thus, more than two-thirds of the patients underwent ALND without a therapeutic benefit from this surgical procedure, and preserving fossa axillaris should be the optimal choice for these patients, which were adopted by many prestigious breast cancer center and guidelines. Nevertheless, the management and outcomes of these trials have also been being questioned by many medical colleagues, because the rate of non-SLN involvement among the patients with SLNs metastasis varied from 30% to 80% in different publications, which were much higher than Z0011 and AMAROS (18, 19, 20, 21). Therefore, it may be irrational to extend the conclusion of avoiding ALND based on Z0011 and AMAROS to different clinical scenarios without predicting the residual metastatic lymph nodes burdens. To more accurately make clinical decision, several studies have suggested using scoring systems or nomograms to predict the probability of non-SLN involvement in patients with at least one SLN metastasis (12, 22, 23). However, none of such findings has been adopted by international breast cancer guidelines. Consequently, for breast cancer patients with positive SLNs, ALND, to do or to omit remained a dilemma of choice (13). Here in this study, we reviewed female patients with operable cT1-3N0 invasive breast cancer who underwent ALND in our breast cancer center due to SLN metastasis and tried to evaluate the prevalence of non-SLNs metastasis among breast cancer patients with SLN metastasis in multiple clinical scenarios and its impact on clinical decision-making.
Material and methods
We identified 892 female patients with operable cT1-3N0 invasive breast cancer who underwent ALND in our breast cancer center due to SLN metastasis from 2017 to 2023. Patients who received neoadjuvant chemotherapy were excluded. All patients underwent breast-conserving surgery (BCS) or modified radical mastectomy (MRM) in the light of the tumor characteristics, current breast cancer guidelines and the patient’s preferences. The SLNB was performed using the nano-carbon dye injection in the periareolar/intradermal location. About 10 min after the injection, all visible stained and nonstained lymph nodes were resected as SLNs by surgeons trained for SLN biopsy. All SLNs were assessed immediately via frozen section examination and subsequently paraffin-embedded for further pathological diagnosis. SLN metastases were defined as macro-metastasis (pN1, metastasis size >2 mm), micro-metastasis (pN1mi, metastasis size between >0.2 mm and ≤2 mm), or isolated tumor cells (ITCs) (pN0 [i+], metastasis size ≤0.2 mm) according to Eighth Edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (24). The patients with SLN metastasis including micrometastases but not ITCs (either frozen or paraffin-embedded sections identified) further underwent ALND. The number of metastatic and nonmetastatic lymph nodes in the SLNs and non-SLNs calculated separately. We extracted clinicopathological features of patients from the medical records. The tumor histopathologic type, tumor size, histological and nuclear grade, lymphovascular invasion (LVI), estrogen receptor (ER) status, progesterone receptor (PR) status, HER2 status and Ki67 index were recorded. Molecular subtype of breast cancer was defined based on the status of ER, PR, HER2 and Ki67 index. Tumors were classified hormone receptor (HR) positivity if either ER (+≥1%) or PR (+≥20%) was positive (25, 26). HER2 positivity was determined if immunohistochemistry (IHC) yielded 3+ or the in situ hybridization (FISH) amplification test was positive (27). TNM stage was categorized according to the AJCC Cancer Staging Manual, 8th Edition. The prevalence of non-SLN metastasis among different clinicopathological traits and its correlation with the number of positive SLNs were analyzed. The optimal clinical decision-making was generalized. This study was approved by the institutional review board ethics committee of our hospital.
Statistical analysis
D’Agostino and Pearson normality test was applied to check the normal distribution when indicated. Unpaired t-Test was applied to evaluate the difference between the means of two groups. Variable distribution was evaluated using the Chisquare Test and followup Tukey’s Multiple Comparison Test among different groups and between every other group, respectively. Multivariate analysis was performed by multiple logistic regression. All statistical analyses were performed using Graphpad Prism version 9.0. The number of lymph nodes was presented in mean ± SEM (Standard Error of Mean). All tests were two sided, and p < 0.05 was considered statistically significant. In order to assess the predictive value of the multivariate logistic regression model, the ROC was plotted to calculate the AUC and evaluate the predictive power of the nomogram model. The area under the ROC curve (AUC) was used to measure model discrimination.
Results
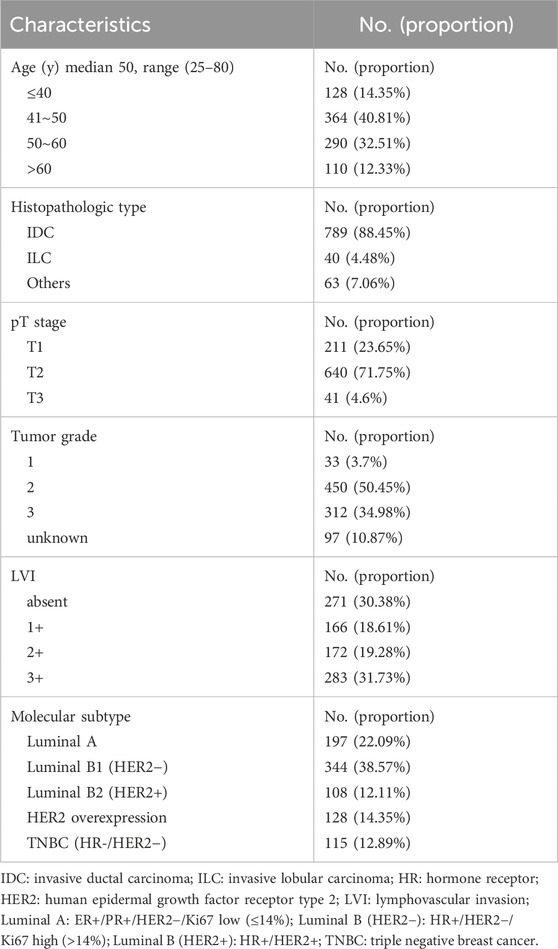
The patients’ median age was 50 years old, ranging from 25 to 80 years old. Most cases were invasive ductal carcinoma (IDC), accounting for 88.45%. Invasive lobular carcinoma (ILC) accounted for 4.49%, and other special infiltrative breast cancer accounted for 7.06%. The median tumor size was 2.6 cm, ranging from 0.5 to 6.8 cm. 211 (23.65%) of the patients had pT1 disease, 41 (4.60%) of the patients had pT3 disease, and the rest 640 (71.75%) had pT2 disease. 33 (3.7%) had a grade 1 tumor, 450 (50.45%) had a grade 2 tumor, 312 (34.98%) had a grade 3 tumor and the rest 97 (10.87%) had no tumor grade data. The prevalence of breast cancer molecular subtype was also analyzed. The proportions of luminal A (ER+/PR+/HER2-/Ki-67 low (≤14%)), luminal B1 (HR+/HER2−/Ki-67 high (>14%)), luminal B2 (HR+/HER2+), HER2 overexpression, triple negative breast cancer (TNBC) (HR-/HER2-) subtype were 22.09%, 38.57%, 12.11%, 14.35% and 12.89%, respectively. LVI was presented in 621 (69.62%) patients. The summary of patients’ clinicopathological characteristics was shown in Table 1.
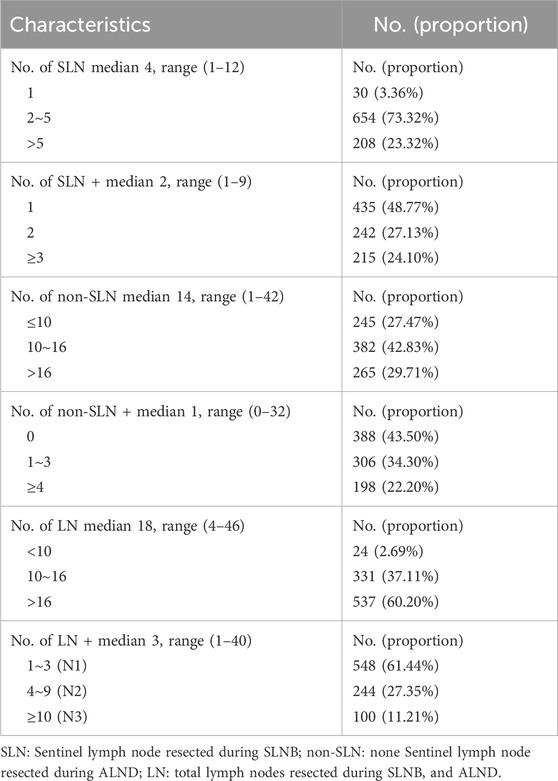
The median number of harvested SLN was 4, ranging from 1 to 12, and the median number of SLN+ was 2, ranging from 1 to 9. In general, over three-fourths of the patients had only one or two SLNs with cancer cell metastasis in all patients enrolled. The median number of additionally dissected lymph nodes (non-SLNs) during ALND was 14, ranging from 1 to 42, and the median number of non-SLN+ was only 1, ranging from 0 to 32. 388 (43.50%) patients had no extra metastatic non-SLNs. 306 (34.30%) had only 1 to 3 metastatic non-SLNs. 198 (22.20%) patients had over 3 metastatic non-SLNs. In summary, the median number of total LNs resected during SLNB and ALND was 18, ranging from 4 to 46, and the median number of LN+ was 3, ranging from 1 to 40. 548 (61.44%) patients were N1, 244 (27.35%) patients were N2, and 100 (11.21%) patients were N3, according to AJCC breast cancer staging manual, Eighth edition. The summary of the number of lymph nodes distribution during different surgical procedures was shown in Table 2.
To clarify the main factors affecting non-SLNs metastasis, both univariate analysis and multivariate analysis were performed. We evaluated the number of non-SLN + among different clinicopathological traits, including patients’ age, the number of positive SLNs, tumor size, histologic grade, LVI and molecular subtypes. In univariate analysis, patients’ age may be a potential factor, patients older than 60 years old showed a trend of relatively larger number (3.282 ± 0.541) of non-SLN + than that of younger than 60 years old (2.368 ± 0.144), p = 0.0359, using unpaired t-test (Figure 1). The probability of non-SLN + among patients with one, two and three or more SLNs+ was 41.38%, 60.33% and 82.79%, respectively, 56.5% patients had positive non-SLN (Figure 2). The number of SLNs+ was positively correlated with the number of SLN and non-SLN+, but not the number of non-SLN. The number of SLN and non-SLN + among patients with 3 or more SLNs+ was 5.284 ± 0.1197 and 5.191 ± 0.3924, more than that of patients with less than 3 SLNs+ (Figure 3). Tumor size also affected the number of SLNs+ and non-SLNs+, but not the number of SLNs and non-SLNs. The mean number of SLNs+ was 1.801 ± 0.076, 2.032 ± 0.058 and 2.366 ± 0.256 in T1, T2 and T3 patients respectively. The mean number of non-SLNs+ was 1.645 ± 0.192, 2.792 ± 0.213 and 5.732 ± 1.067 in T1, T2 and T3 patients respectively, using Tukey’s Multiple Comparison between every other group (Figure 4). Only the number of non-SLNs+ was larger in histologic grade III patients than histologic grade I patients (Figure 5). Both the number of SLNs+ and non-SLNs+ were larger in LVI 3+ patients than absent of LVI or 1+ patients (Figure 6). The difference of non-SLNs + among different molecular subtypes showed statistical significance (Figure 7). None of the number of SLN, SLN+, non-SLN and non-SLN+ was related with histopathologic type (Figure 8).
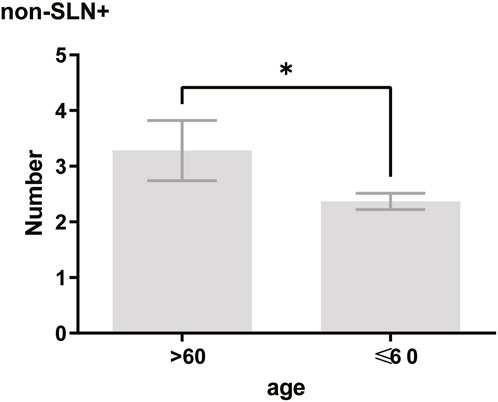
Figure 1. In univariate analysis, patients older than 60 years old showed a trend of relatively larger number (3.282 ± 0.541) of non-SLN + than that of younger than 60 years old (2.368 ± 0.144). Unpaired t-test, p = 0.0359.
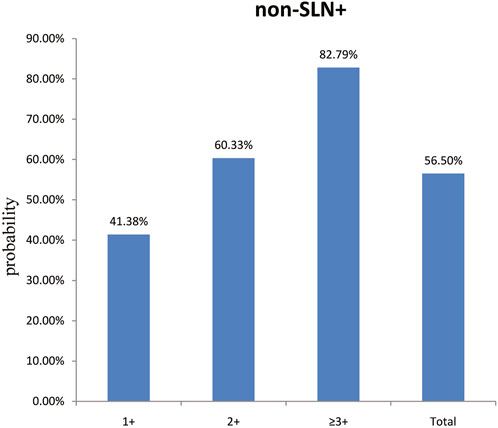
Figure 2. The probability of non-SLN + among patients with one, two and three or more SLNs+ was 41.38%, 60.33% and 82.79%, respectively. Overall, 56.50% of the patients had extra non-SLN metastasis.
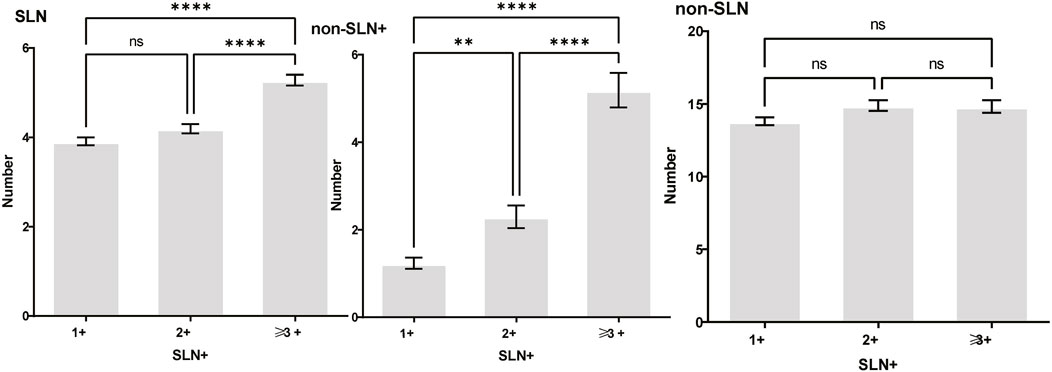
Figure 3. The number of SLNs+ was positively correlated with the number of SLN and non-SLN+, but not the number of non-SLN. The number of SLN and non-SLN + among patients with 3 or more SLNs+ was 5.284 ± 0.1197 and 5.191 ± 0.3924, more than that of patients with less than 3 SLNs+. Tukey’s multiple comparisons test, p < 0.05 was considered statistically significant.
Figure 4. Tumor size also affected the number of SLNs+ and non-SLNs+, but not the number of SLNs and non-SLNs. The mean number of SLNs+ was 1.801 ± 0.076, 2.032 ± 0.058 and 2.366 ± 0.256 in T1, T2 and T3 patients respectively. The mean number of non-SLNs+ was 1.645 ± 0.192, 2.792 ± 0.213 and 5.732 ± 1.067 in T1, T2 and T3 patients respectively. Tukey’s Multiple Comparison between every other group.
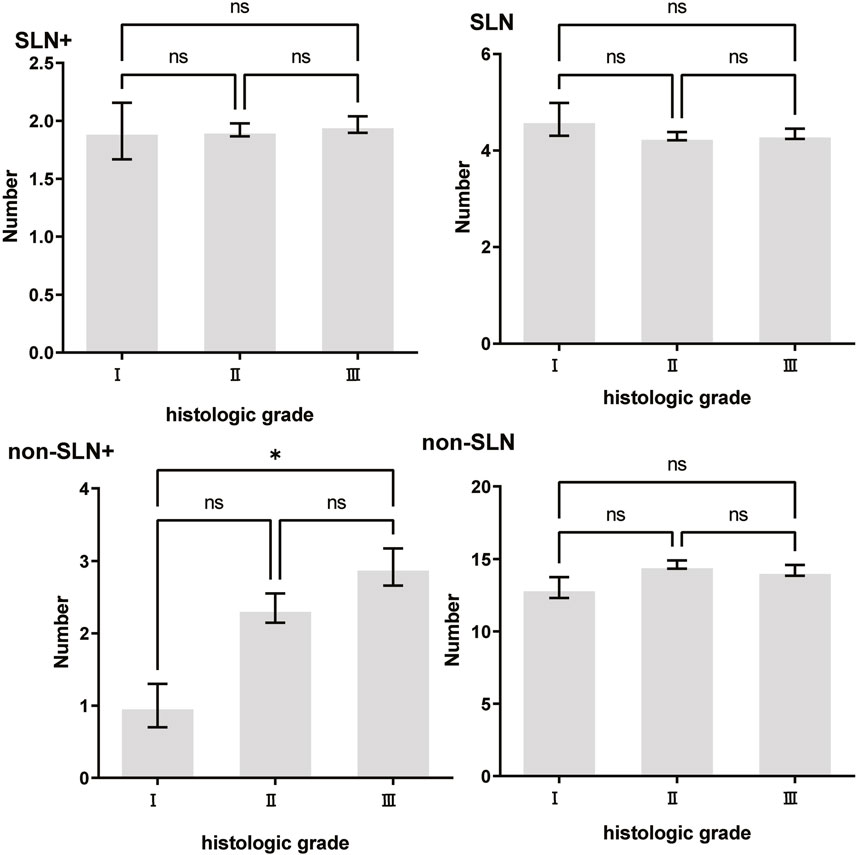
Figure 5. The impact of histologic grade on lymph node metastasis. Only the number of non-SLNs+ was largerin histologic grade III (2.927 ± 0.256) patients than histologic grade I (1.000 ± 0.302) patients, p = 0.038.
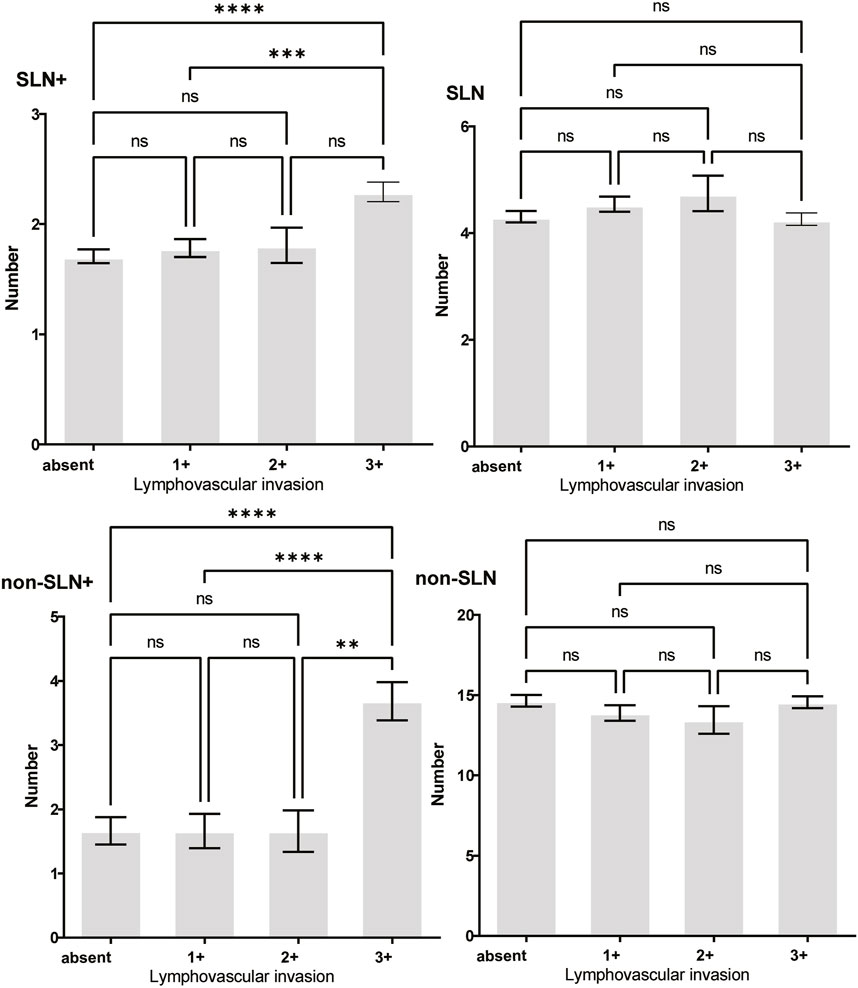
Figure 6. The impact of lymphovascular invasion (LVI) on lymph node metastasis. Both the number of SLNs+ and non-SLNs+ were larger in LVI 3+ patients than absent of LVI or 1+ patients.
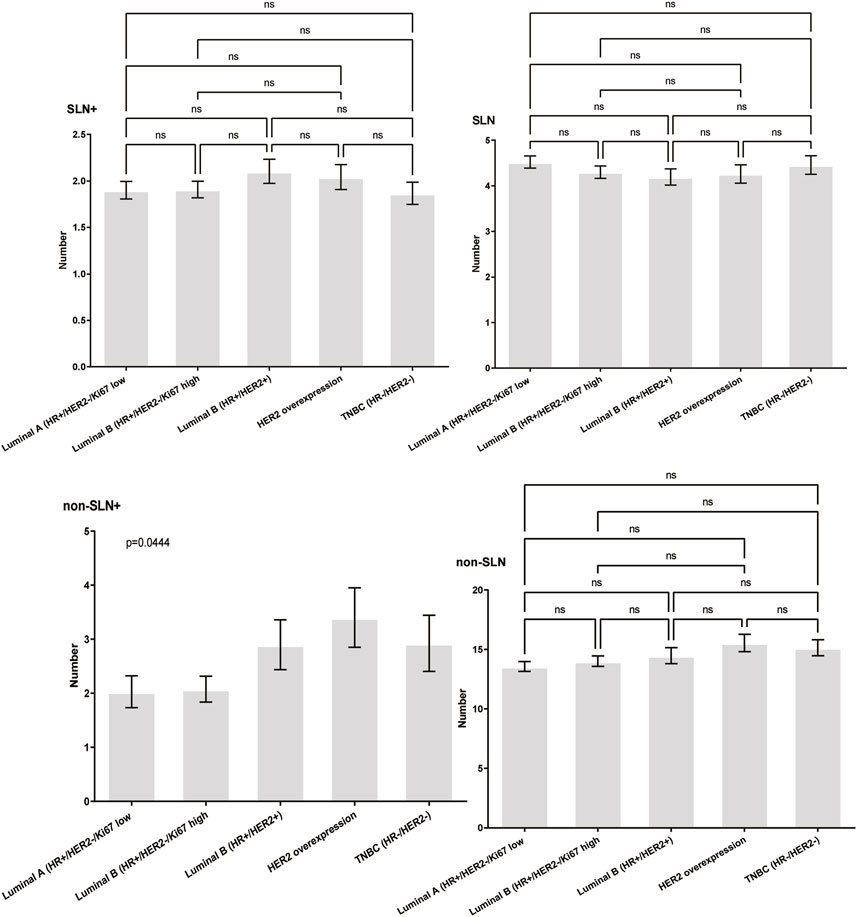
Figure 7. The impact of molecular subtypes on lymph node metastasis. The difference of non-SLNs + among different molecular subtypes showed statistical significance trend, using the Chisquare Test, p = 0.044.
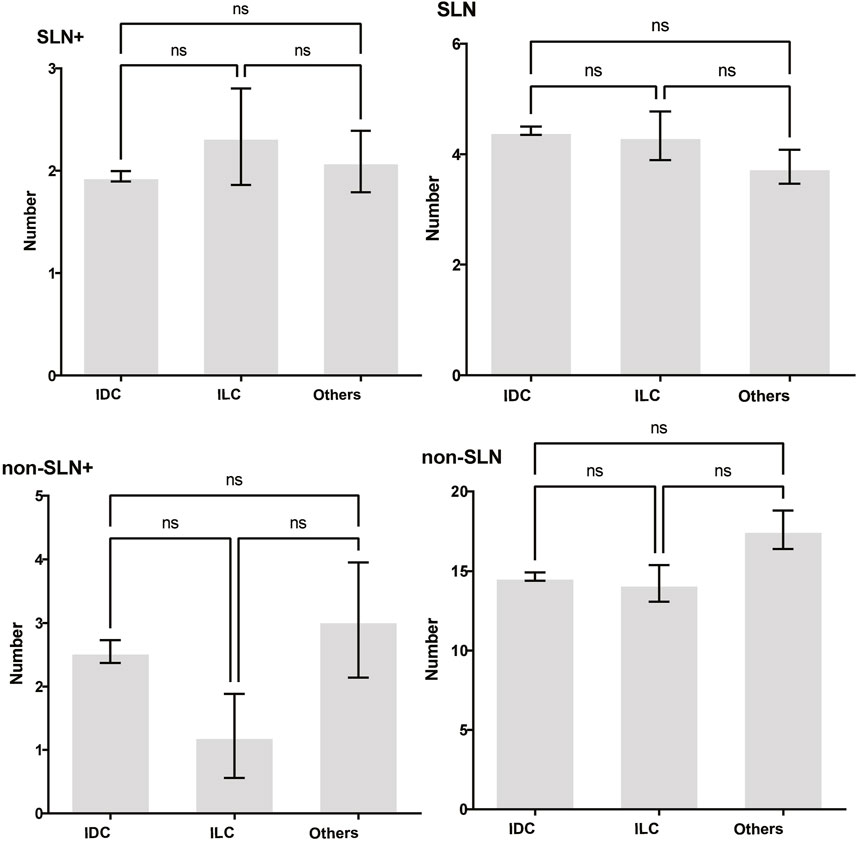
Figure 8. The impact of histopathologic type on lymph node metastasis. None of the number of SLN, SLN+, non-SLN and non-SLN+ was related with histopathologic type. Tukey’s Multiple Comparison between every other group, p > 0.05.
To evaluate the risk factors influencing non-SLN spread among different clinicopathological traits by multivariate analysis, the patients’ age, tumor size, histologic grade, LVI, ER, PR, HER2 and Ki67 index, and the number of SLNs+, SLNs and non-SLNs were used. The number of SLNs+, SLNs and non-SLNs and LVI were identified as the independent factors for non-SLNs metastasis in the multivariate analysis (Table 3).
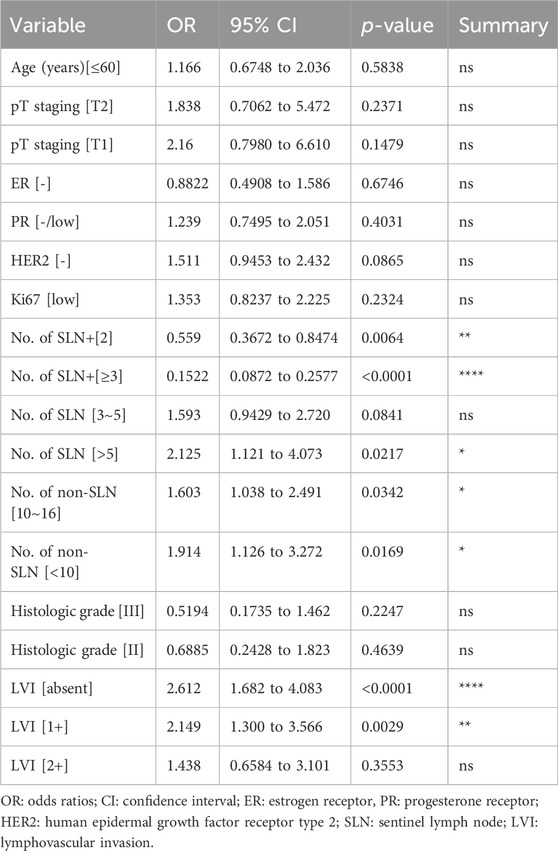
Table 3. Multivariate analysis for clinicopathological risk factors of non-sentinel lymph node metastasis.
The predictive nomogram found that the area under the ROC (receiver operating characteristic) curve was 0.7666 (95% confidence interval 0.7293–0.8040, p < 0.0001). Negative predictive power was 74.73%, while positive predictive power was only 66.25% (Figure 9). What’s more, the number of SLN metastases was the most significant predictive factor in both univariate and multivariate analysis.
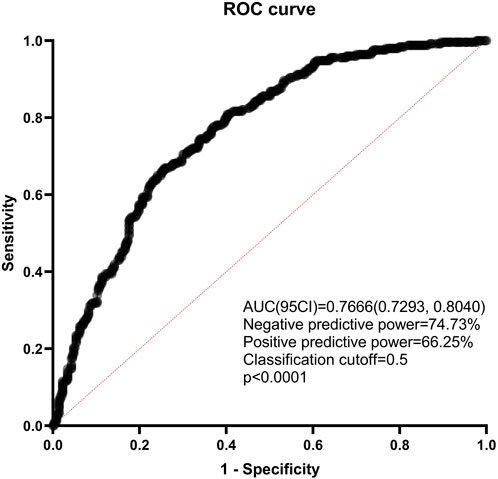
Figure 9. ROC curve of the combined with the estimated risk factors. ROC: receiver operating characteristics; AUC: areas under the ROC curve; CI: confidence interval.
Discussion
Lymph node staging is essential for breast cancer treatment and prognosis. In recent two decades, this goal could be achieved through SLNB in clinically node negative breast cancer. The SLN has been considered as the first station of breast cancer metastasis, so SLNB may be a reliable procedure to evaluate lymph nodes spread, though there is a relatively high false negative rate even for the well-trained breast surgeons. For patients with no positive SLNs, ALND may be unnecessary. However, in the case of less than 3 positive SLNs, the properly clinical decision making is full of challenging (28, 29).
More and more publications in the last few years implied that ALND did not improve patients’ outcome, because the vast majority of the patients underwent ALND showed no additionally positive non-SLNs (30, 31, 32, 33, 34, 35). Consequently, how to precisely predict the probability of non-SLNs spread is essential. There are several different nomograms available to predict the risk of non-SLNs involvement in the presence of SLNs metastasis. Those tools commonly integrated patients’ clinicopathological traits including patient’s age, tumor size, histologic grade, LVI, ER, PR, HER2, Ki67 index and the number of SLNs + to evaluate the likelihood of non-SLNs metastasis, but they used a limited number of cases and lacked external validation (22, 36). The concept of total tumor load may accurately predict the status of non-SLNs and is another important tool for clinical decisions on early breast cancer patients. Nomogram to predict non-sentinel lymph node status using total tumor load determined by one-step nucleic acid amplification was first report from Thailand (37). Nevertheless, the optimal indication for ALND among the patients with a positive SLN still remains unclear.
In this study, 504 of 892 (56.50%) patients had extra metastasis in the non-SLN, the probability of non-SLN metastasis is roughly consistent with that in previous studies, but much higher than that of Z0011 and AMAROS trials. The number of SLNs+, SLNs and non-SLNs and LVI were predictive factors for non-SLN metastasis by multivariate analysis. Though the results of the Z0011 and AMAROS showed that the survival outcomes of SLNB plus radiotherapy were not inferior to those of ALND in breast cancer patients with limited SLNs metastasis and were cited by the American Society of Clinical Oncology Clinical Practice Guidelines, it is still too early to promote large-scale application among different breast cancer centers and ethnic groups since the non-SLN metastasis status varied significantly (from less than 30% to over 80%) and the regimen of radiotherapy including sites, dose and daily fraction was undefined. Whether axillary radiotherapy and ALND provides equivalent regional control in breast cancer patients with obvious residual metastatic lymph nodes in the axilla is still unknown.
In this context, there are massive studies conducted to predict non-SLN metastasis using scoring systems and nomograms. Because both preventive ALND and radiotherapy do not improve survival outcome but instead cause complications, accurately predicting the risk of non-SLN spread could be beneficial as it will help determine clinical decision making. Several nomograms integrating clinicopathologic factors such as tumor size, LVI, and positive and negative SLN metastases have been developed (38, 39). These nomograms found that the area under the ROC (receiver operating characteristic) curve was approximately 0.7 (40). What’s more, the number of SLN metastases was the most significant predictive factor in both univariate and multivariate analysis.
In the present study, we focused on the number of positive and total SLNs, the number of non-SLNs dissected and the LVI. Our study found that the negative predictive power of the nanogram was 74.73%, while positive predictive power was only 66.25%. Thus, the accurate prediction of non-SLN metastasis remains challenging to date. Our result suggests that clinicians should consider the risk of underestimating axillary lymph node metastases in patients who omitted ALND because even only 1 positive SLN did not ensure negative non-SLNs (41.38% probability with metastasis). Confirming negative non-SLNs in cases where the Z0011 criteria applied may help to avoid underestimating non-SLNs metastasis in certain clinical scenarios, but please do not assume that non-SLNs have no metastasis and omit ALND in patients with less than 3 positive SLNs. Whatever, ALND not only removed the potential metastatic lymph nodes but also provided decision-making basis for adjuvant CDK4/6 inhibitors treatment for luminal breast cancer. Since adjuvant CDK4/6 inhibitors (eg, abemaciclib) improve survival of luminal breast cancer at high risk, without ALND makes revealing four or more lymph nodes metastases impossible, which results in these patients not meeting the criteria of adjuvant CDK4/6 inhibitors therapy (41).
Conclusion
To omit ALND in patients with higher tumor burden outside the Z0011 and AMAROS criteria should be considered with caution in clinical decision-making. ALND not only removed the potential metastatic lymph nodes but also provided more detailed lymph node staging for clinical adjuvant therapy, especially for CDK4/6 inhibitors usage in luminal breast cancer. To evaluate whether axillary radiotherapy and ALND provides equivalent regional control in breast cancer patients with obvious residual metastatic lymph nodes in the axilla, a well-matched prospective randomized controlled trial is an urgent need.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Review Board of Nanchang People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective study.
Author contributions
JD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. XJ: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. ZH: Data curation, Investigation, Methodology, Writing–original draft, Writing–review and editing. QJ: Data curation, Methodology, Writing–original draft, Writing–review and editing. JL: Data curation, Methodology, Writing–original draft, Writing–review and editing. YC: Funding acquisition, Project administration, Supervision, Writing–original draft, Writing–review and editing. YG: Project administration, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (82060482), and the Natural Science Foundation of Jiangxi Province (20171BAB205057). Funding sources were not involved in the study design, data collection, analysis and interpretation, writing of the report or decision to submit the article for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wu, J, Fan, D, Shao, Z, Xu, B, Ren, G, Jiang, Z, et al. Caca guidelines for holistic integrative management of breast cancer. Holist Integr Oncol (2022) 1:7. doi:10.1007/s44178-022-00007-8
2. Siegel, RL, Giaquinto, AN, and Jemal, A. Cancer statistics, 2024. CA: A Cancer J Clinicians (2024) 74:12–49. doi:10.3322/caac.21820
3. Mathur, A, Arya, N, Pasupa, K, Saha, S, Roy Dey, S, and Saha, S. Breast cancer prognosis through the use of multi-modal classifiers: current state of the art and the way forward. Brief Funct Genomics (2024) 23:561–9. doi:10.1093/bfgp/elae015
4. Li, X, Li, X, Yang, B, Sun, S, Wang, S, Yu, F, et al. Deciphering breast cancer prognosis: a novel machine learning-driven model for vascular mimicry signature prediction. Front Immunol (2024) 15:1414450. doi:10.3389/fimmu.2024.1414450
5. Li, J, Liu, H, Han, M, Jiang, H, He, J, Miao, J, et al. Tumour mutation burden and infiltrating immune cell subtypes influenced the breast cancer prognosis. Transl Cancer Res (2024) 13:2208–21. doi:10.21037/tcr-23-2195
6. Lei, C, Li, Y, Yang, H, Zhang, K, Lu, W, Wang, N, et al. Unraveling breast cancer prognosis: a novel model based on coagulation-related genes. Front Mol Biosci (2024) 11:1394585. doi:10.3389/fmolb.2024.1394585
7. Deberti, M, Goupille, C, Arbion, F, Vilde, A, Body, G, and Ouldamer, L. Prognostic value of axillary lymph node metastases in invasive lobular breast carcinoma. J Gynecol Obstet Hum Reprod (2023) 52:102665. doi:10.1016/j.jogoh.2023.102665
8. Sharifi, N, and Ahmad, S. Breast cancer-related lymphedema: a critical review on recent progress. Surg Oncol (2024) 56:102124. doi:10.1016/j.suronc.2024.102124
9. Ahn, HR, Jeong, HE, Jeong, C, Kang, SY, Jung, SH, Youn, HJ, et al. Incidence and risk factors of breast cancer-related lymphedema in korea: a nationwide retrospective cohort study. Int J Surg (2024) 110:3518–26. doi:10.1097/JS9.0000000000001278
10. Li, H, Li, WB, Sun, ZX, Yu, J, Lv, PY, Li, CX, et al. Analysis of the risk factors of breast cancer-related lymphedema and construction and evaluation of a prediction model. Lymphatic Res Biol (2023) 21:565–73. doi:10.1089/lrb.2022.0058
11. Siotos, C, Arnold, SH, Seu, M, Lunt, L, Ferraro, J, Najafali, D, et al. Breast cancer-related lymphedema: a comprehensive analysis of risk factors. J Surg Oncol (2024). doi:10.1002/jso.27841
12. Yang, L, Zhao, X, Yang, L, Chang, Y, Cao, C, Li, X, et al. A new prediction nomogram of non-sentinel lymph node metastasis in ct1-2 breast cancer patients with positive sentinel lymph nodes. Sci Rep (2024) 14:9596. doi:10.1038/s41598-024-60198-0
13. Liu, Y, Fan, Y, Jin, Z, Cui, M, Yu, X, Jin, F, et al. Axillary management for early invasive breast cancer patients: who will truly benefit? Front Oncol (2022) 12:989975. doi:10.3389/fonc.2022.989975
14. Jo, H, Lee, EG, Song, E, Han, JH, Jung, SY, Kang, HS, et al. Comparison of clinical outcomes between sentinel lymph node biopsy and axillary lymph node dissection in a single-center z0011-eligible breast cancer cohort. Korean J Clin Oncol (2020) 16:18–24. doi:10.14216/kjco.20004
15. Bartels, S, Donker, M, Poncet, C, Sauve, N, Straver, ME, van de Velde, C, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-year results of the randomized controlled eortc 10981-22023 amaros trial. J Clin Oncol (2023) 41:2159–65. doi:10.1200/JCO.22.01565
16. Tinterri, C, Gentile, D, Gatzemeier, W, Sagona, A, Barbieri, E, Testori, A, et al. Preservation of axillary lymph nodes compared with complete dissection in t1-2 breast cancer patients presenting one or two metastatic sentinel lymph nodes: the sinodar-one multicenter randomized clinical trial. Ann Surg Oncol (2022) 29:5732–44. doi:10.1245/s10434-022-11866-w
17. Giuliano, AE, Ballman, K, McCall, L, Beitsch, P, Whitworth, PW, Blumencranz, P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the american college of surgeons oncology group (alliance) acosog z0011 randomized trial. Ann Surg (2016) 264:413–20. doi:10.1097/SLA.0000000000001863
18. Mikami, Y, Yamada, A, Suzuki, C, Adachi, S, Harada, F, Yamamoto, S, et al. Predicting nonsentinel lymph node metastasis in breast cancer: a multicenter retrospective study. J Surg Res (2021) 264:45–50. doi:10.1016/j.jss.2021.01.047
19. Petousis, S, Christidis, P, Margioula-Siarkou, C, Liberis, A, Vavoulidis, E, Margioula-Siarkou, G, et al. Axillary lymph node dissection vs. Sentinel node biopsy for early-stage clinically node-negative breast cancer: a systematic review and meta-analysis. Arch Gynecol Obstet (2022) 306:1221–34. doi:10.1007/s00404-022-06458-8
20. Galimberti, V, Cole, BF, Zurrida, S, Viale, G, Luini, A, Veronesi, P, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (ibcsg 23-01): a phase 3 randomised controlled trial. Lancet Oncol (2013) 14:297–305. doi:10.1016/S1470-2045(13)70035-4
21. Cai, SL, Wei, RM, Han, L, Chen, XG, Gong, GX, Lin, XQ, et al. Risk factors of non-sentinel lymph node metastasis in 443 breast cancer patients with sentinel lymph node-positive. Medicine (Baltimore) (2022) 101:e29286. doi:10.1097/MD.0000000000029286
22. Wang, XF, Zhang, GC, Zuo, ZC, Zhu, QL, Liu, ZZ, Wu, SF, et al. A novel nomogram for the preoperative prediction of sentinel lymph node metastasis in breast cancer. Cancer Med (2023) 12:7039–50. doi:10.1002/cam4.5503
23. Katz, A, Smith, BL, Golshan, M, Niemierko, A, Kobayashi, W, Raad, RA, et al. Nomogram for the prediction of having four or more involved nodes for sentinel lymph node-positive breast cancer. J Clin Oncol (2008) 26:2093–8. doi:10.1200/JCO.2007.11.9479
24. Giuliano, AE, Edge, SB, and Hortobagyi, GN. Eighth edition of the ajcc cancer staging manual: breast cancer. Ann Surg Oncol (2018) 25:1783–5. doi:10.1245/s10434-018-6486-6
25. Prat, A, Cheang, MC, Martin, M, Parker, JS, Carrasco, E, Caballero, R, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal a breast cancer. J Clin Oncol (2013) 31:203–9. doi:10.1200/JCO.2012.43.4134
26. Goldhirsch, A, Winer, EP, Coates, AS, Gelber, RD, Piccart-Gebhart, M, Thurlimann, B, et al. Personalizing the treatment of women with early breast cancer: highlights of the st gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol (2013) 24:2206–23. doi:10.1093/annonc/mdt303
27. Wolff, AC, Hammond, M, Allison, KH, Harvey, BE, Mangu, PB, Bartlett, J, et al. Human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of american pathologists clinical practice guideline focused update. J Clin Oncol (2018) 36:2105–22. doi:10.1200/JCO.2018.77.8738
28. Beck, AC, and Morrow, M. Axillary lymph node dissection: dead or still alive? The Breast (2023) 69:469–75. doi:10.1016/j.breast.2023.01.009
29. Fisher, CS. To perform an axillary lymph node dissection or not? That is (still) the question. Ann Surg Oncol (2020) 27:3565–6. doi:10.1245/s10434-020-08692-3
30. Owusu-Brackett, N, and Oppong, BA. Can targeted axillary dissection reliably advise de-escalation of completion axillary lymph node dissection? Gland Surg (2023) 12:730–2. doi:10.21037/gs-23-106
31. You, JY, Lee, ES, Lim, SK, Kwon, Y, and Jung, SY. Could axillary lymph node dissection be omitted in the mastectomy patient with tumor positive sentinel node? Front Oncol (2023) 13:1181069. doi:10.3389/fonc.2023.1181069
32. Andersson, Y, Bergkvist, L, Frisell, J, and de Boniface, J. Omitting completion axillary lymph node dissection after detection of sentinel node micrometastases in breast cancer: first results from the prospective senomic trial. Br J Surg (2021) 108:1105–11. doi:10.1093/bjs/znab141
33. Bukovszky, B, Fodor, J, Matrai, Z, Dorogi, B, Zongor, Z, Mihaly, D, et al. Radiotherapy instead of axillary lymph node dissection: evaluation of axillary lymph node dose coverage with whole breast radiotherapy. Rep Pract Oncol Radiother (2022) 27:458–66. doi:10.5603/RPOR.a2022.0043
34. Mokbel, K, and Almoodi, M. Redefining axillary lymph node dissection in mastectomy: insights from the sinodar-one study. Br J Surg (2023) 110:1903. doi:10.1093/bjs/znad327
35. Li, P, Yang, C, Zhang, J, Chen, Y, Zhang, X, Liang, M, et al. Survival after sentinel lymph node biopsy compared with axillary lymph node dissection for female patients with t3-4c breast cancer. The Oncologist (2023) 28:e591–9. doi:10.1093/oncolo/oyad038
36. Xiang, K, Chen, J, Min, Y, Chen, H, Yang, J, Hu, D, et al. A multi-dimensional nomogram to predict non-sentinel lymph node metastases in t1-2hr+ breast cancer. Front Endocrinol (Lausanne) (2023) 14:1121394. doi:10.3389/fendo.2023.1121394
37. Sa-Nguanraksa, D, O-Charoenrat, E, Kulprom, A, Samarnthai, N, Lohsiriwat, V, Nimpoonsri, K, et al. Nomogram to predict non-sentinel lymph node status using total tumor load determined by one-step nucleic acid amplification: first report from Thailand. Breast Cancer (2019) 26:471–7. doi:10.1007/s12282-019-00945-8
38. Wang, XY, Wang, JT, Guo, T, Kong, XY, Chen, L, Zhai, J, et al. Risk factors and a predictive nomogram for non-sentinel lymph node metastases in Chinese breast cancer patients with one or two sentinel lymph node macrometastases and mastectomy. Curr Oncol (2019) 26:e210–5. doi:10.3747/co.26.4295
39. Zhu, L, Jin, L, Li, S, Chen, K, Jia, W, Shan, Q, et al. Which nomogram is best for predicting non-sentinel lymph node metastasis in breast cancer patients? A meta-analysis. Breast Cancer Res Treat (2013) 137:783–95. doi:10.1007/s10549-012-2360-6
40. Orsaria, P, Caredda, E, Genova, F, Materazzo, M, Capuano, I, Vanni, G, et al. Additional nodal disease prediction in breast cancer with sentinel lymph node metastasis based on clinicopathological features. Anticancer Res (2018) 38:2109–17. doi:10.21873/anticanres.12451
41. de Boniface, J, Appelgren, M, Szulkin, R, Alkner, S, Andersson, Y, Bergkvist, L, et al. Completion axillary lymph node dissection for the identification of pn2-3 status as an indication for adjuvant cdk4/6 inhibitor treatment: a post-hoc analysis of the randomised, phase 3 senomac trial. Lancet Oncol (2024) 25:1222–30. doi:10.1016/S1470-2045(24)00350-4
Keywords: breast cancer, lymph node metastasis, sentinel lymph node biopsy, axillary lymph node dissection, radiotherapy
Citation: Ding J, Jiang X, Huang Z, Ji Q, Long J, Cao Y and Guo Y (2024) The prevalence of non-sentinel lymph node metastasis among breast cancer patients with sentinel lymph node involvement and its impact on clinical decision-making: a single-centred retrospective study. Oncol. Rev. 18:1495133. doi: 10.3389/or.2024.1495133
Received: 12 September 2024; Accepted: 24 October 2024;
Published: 31 October 2024.
Edited by:
Stella D’Oronzo, University of Bari Aldo Moro, ItalyReviewed by:
Yun Ling, The Second Affiliated Hospital of Guangzhou Medical University, ChinaGeorgios Androutsopoulos, University of Patras, Greece
Copyright © 2024 Ding, Jiang, Huang, Ji, Long, Cao and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingxian Ding, amluZ3hpYW5kaW5nMTBAZnVkYW4uZWR1LmNu; Yonghong Guo, NDA0NzE3NDM0QHFxLmNvbQ==
†These authors have contributed equally to this work
 Jingxian Ding
Jingxian Ding Xiaoliu Jiang1†
Xiaoliu Jiang1†