
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Oncol. Rev., 06 January 2025
Sec. Oncology Reviews: Reviews
Volume 18 - 2024 | https://doi.org/10.3389/or.2024.1445826
Non-small-cell lung cancer (NSCLC) is the poster child of personalized medicine. With increased knowledge about biomarkers and the consequent improvement in survival rates, NSCLC has changed from being a therapeutic nihilistic disease to that characterized by therapeutic enthusiasm. The routine biomarkers tested in NSCLC are EGFR, ALK, and ROS1. However, several additional biomarkers have been added to the diagnostic landscape. Current guidelines recommend testing at least seven biomarkers upfront at the time of NSCLC diagnosis—emphasizing the wide range of targets and corresponding therapies that can be leveraged for disease management. Sequential single-gene testing is not only time-consuming but also leads to tissue exhaustion. Multigene panel testing using next-generation sequencing (NGS) offers an attractive diagnostic substitute that aligns with the evolving dynamics of precision medicine. NGS enables the identification of point mutations, insertions, deletions, copy number alterations, fusion genes, and microsatellite instability information needed to guide the potential use of targeted therapy. This article reviews the existing guidelines, proposed recommendations for NGS in non-squamous NSCLC, real-world data on its use, and the advantages of adopting broader panel-based NGS testing over single-gene testing.
At the beginning of this century, the survival rate of a stage 4 lung cancer patient was a dismal 1 year (1). However, with increasing knowledge about the biomarkers in lung cancer, there has been a paradigm shift in the prognosis and survival rates of these patients.
Currently, polymerase chain reaction (PCR)-based methods and conventional direct sequencing methods like Sanger sequencing and pyrosequencing are employed to identify these biomarkers. However, these approaches allow sequencing of a few genes per run, resulting in a technically cumbersome, time-consuming, and expensive diagnostic test. Multigene sequencing using next-generation sequencing (NGS), also known as massively parallel sequencing, avoids performing multiple sequential single tests for all these biomarkers. It has advantages such as sparing tissue samples, avoiding delays for patients, and helping match the patient to the most appropriate clinical trial. Although cost, slow turnaround time, and the enormity of data returned are certain issues, the pieces of information and advantages outweigh the same.
This article reviews the concepts of precision oncology in non-small-cell lung cancer (NSCLC), with an in-depth description of canonical biomarkers, advantages and disadvantages of NGS-based testing for the biomarkers, and current recommendations.
The NGS process involves three main phases (2) (Figure 1):
1. Sample preparation: DNA/RNA extraction, target region capture/fragmentation, and library preparation.
2. Sequencing process: Each library fragment is read multiple times from either one end (single end) or from both ends (paired end).
3. Bioinformatics: This is the most critical phase. This involves the alignment of the reads to the reference genome using specific algorithms; filtering low-quality data; coverage, which reports the number of reads for each region that is sequenced; variant calling, which reports all the genetic variants using specific software applications like GATK best practices; and, finally, the annotation, which defines and links the variants to the disease in context.

Figure 1. Flow diagram depicting the steps involved in NGS. Step 1: extraction, Step 2: library preparation, Step 3: bioinformatics, and Step 4: final clinical report. DNA, deoxyribonucleic acid; RNA, ribonucleic acid; PCR, polymerase chain reaction; HGVS, Human Genome Variation Society; ACMG/AMP, American College of Medical Genetics and Genomics/Association of Molecular Pathology; VUS, variant of uncertain significance.
The 2018 CAP/AMP/IASLC (College of American Pathologists/Association of Molecular Pathology/International Association for the Study of Lung Cancer) guidelines (3) advocated testing for EGFR, ALK, ROS1, and BRAF upfront. Currently, NGS is offered only when sequential single-gene testing yields negative results. However, with the advent of newer drugs, the Spanish Society of Medical Oncology (4) has advocated for upfront panel-based testing. ACMG/AMP (5, 6) (American College of Medical Genetics and Genomics) guidelines are employed to ascertain the pathogenicity of the called variant and are detailed in Table 1.

Table 1. Classification of somatic variants in cancer based on pathogenicity as per ACMG/AMP guidelines and FDA approval of drugs.
Presently, clinical biomarker testing in NSCLC involves investigating any potential druggable alterations (7). The comprehensive genomic profiling of DNA and RNA using NGS panels allows almost complete detection of all these alterations. The various biomarkers, their corresponding drugs, and testing modalities are listed in Table 2.
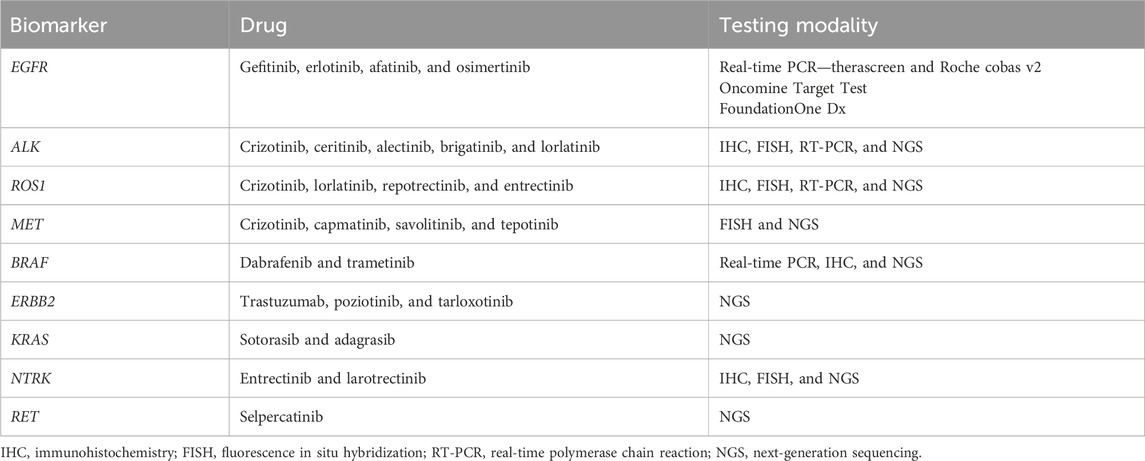
Table 2. Biomarkers in non-small cell lung cancer with corresponding targeted drugs and testing modalities.
Epidermal growth factor receptor (EGFR)-mutated NSCLC is a distinct molecularly refined subgroup, first described in 2004 (8). These mutations are known to occur in 33%–35% of Asians (9) and 8%–10% in the West (9). Affected patients are usually female individuals, non-smokers, and have adenocarcinoma histology, although the absence of these characteristics should not preclude testing for EGFR mutations. Almost all EGFR mutations span across exons 18–21 (10). Approximately 90% of the activating mutations detected in EGFR are p. L858R and in-frame deletions in exon 19 of the gene (11). The other mutations detected in exons 18, 20, and 21 of the gene are grouped as “uncommon mutations,” some of which are sensitive to tyrosine kinase inhibitors (TKIs), whereas exon 20 insertions are not sensitive to the abovementioned TKIs (12). Drugs like amivantamab are being used for these mutations (13).
Detection methods include real-time PCR-based testing of tumor tissue using FDA-approved CDx like therascreen and Roche cobas v2 tests. However, these are limited by the spectrum of mutations covered by the primer–probe sets, and hence, any complex indels and other rare mutations may be missed. Sanger-based sequencing, although still considered the gold standard, is limited by its sensitivity and user-dependent variability. In our experience, approximately 10% of cases are missed with single-gene testing, which were subsequently detected with broader panel-based NGS testing.
The sensitizing mutations can be targeted using EGFR TKIs like gefitinib, erlotinib, afatinib, and osimertinib. Many randomized controlled trials have demonstrated the superior efficacy and safety of these over platinum-based chemotherapy. Phase III clinical trials, such as IPASS (14), WJTOG (15), NEJ002 (16), EURTAC (17), LUX-Lung3 (18), and LUX-Lung6 (18, 19), have demonstrated a median progression-free survival (PFS) rate of 9–13 months and overall survival times that exceed 24–30 months. Despite all the advantages, resistance mutation p. T790M (exon 20) develops in these patients, which can be targeted using osimertinib (a third-generation TKI). This was investigated in AURA 3 (20) and demonstrated a benefit over pemetrexed and platinum-based therapy with a median PFS rate of approximately 10.4 months in the osimertinib subgroup.
With the results of the FLAURA trial (21), osimertinib (a third-generation TKI) treatment, in the first line, has been shown to have a longer PFS than other comparator EGFR TKIs (median duration, 18.9 months vs 10.2 months; hazard ratio for disease progression or death, 0.46; P< 0.001) with a better safety profile and overall survival (OS) of 38.1 months vs 32 months in the comparator arm. Resistance to osimertinib in the form of p. C797S mutation has also been described (22). Other resistance mutations rarely described include p. L692V, p. E709K, p. L718E/V, p. L792F/H/V, p.G796D/S/R, p. C797G, and p. L798I (22). Apart from these EGFR-dependent mechanisms, EGFR-independent mechanisms, including MET, PIK3CA, BRAF, and KRAS mutations, can also cause resistance (23). With all this evidence, NGS testing becomes relevant in order to detect any potential resistance mechanisms upfront and avoid over/under treatment.
Activating mutations in BRAF have been reported in 2%–8% NSCLC cases (24), with almost 50% of them being p. V600E mutations in exon 15 of the gene. Other activating mutations include p. G469X, p. L597R, and p. K601E, as well as impaired mutations like p. G466V, p. D594X, and p. G596C, which are found across exons 11–15 of the gene (25). The single-gene PCR-based technique is currently approved as a companion diagnostic for the treatment of melanoma. The emergence of BRAF mutation has also been reported as a resistance mechanism to other targeted TKIs like EGFR TKIs in NSCLC. In NSCLC, two platforms, namely, the Oncomine Dx Target Test and FoundationOne Liquid, are approved for the initiation of targeted therapy.
Targeted therapy in the form of dabrafenib and trametinib in NSCLC is currently recommended only for p. V600E mutations and not for the other rare variants (26). The ESMO recommendations advocate dabrafenib combined with trametinib for BRAF-inhibitor-naive patients with BRAF p. V600-mutated NSCLC (stage IV) (27). A trial investigating encorafenib and binimetinib in BRAF p. V600E-mutated NSCLC is still under study (NCT3915951).
MET is a receptor tyrosine kinase, and its dysregulation may involve gene amplifications, MET exon 14 splice site alterations, MET exon 14 skipping mutations, and missense variants in the TK domain (28). MET exon 14 alterations are detected in 3%–4% of cases of NSCLC, and amplifications are found in approximately 1%–5% of cases (28). Cases of pulmonary sarcomatoid carcinoma almost have recurrent MET exon 14 alterations. MET alterations can be both primary drivers of the oncogenic process and secondary resistance mechanisms to EGFR TKIs in EGFR-mutated NSCLC. Hence, testing for MET in both these situations is quintessential.
Currently, recommended testing for amplification includes FISH. For exon 14 skipping mutation, capmatinib is the only FDA-approved drug, and FoundationOne CDx is the only approved companion diagnostic for the same. Additionally, direct Sanger sequencing may also be used to detect tyrosine kinase domain alterations. However, various targeted NGS panels, including DNA and RNA, can easily detect MET exon 14 alterations and, hence, can be employed.
Clinical trials studying the efficacy of MET TKIs in the treatment of patients with MET exon 14 mutant NSCLC include studies of crizotinib (NCT00585195), capmatinib (Geometry Mono 1 (29)) (NCT02414139), tepotinib (NCT02864992), and savolitinib (NCT02897479). They have shown a response rate to type I TKIs ranging from 32% to 68%. Early results show a median (PFS) ranging from 5.4 months to 12.2 months depending on the drug and the line of therapy.
Gain-of-function mutations in the KRAS gene encompassing exons 2–4 occur in almost 30% of cases of NSCLC (10), alone or in combination with other drivers. These patients are usually female individuals and young; however, no race/histology-specific associations have been described. Transversion p. G12C and p. G12V mutations are known to occur in smokers, whereas transition p. G12D mutations are known to occur in non-smokers (30). Concurrent TP53 and STK11 alterations are known to occur with a high mutation burden in smokers (31).
However, recently, the directly targeting mutant KRAS has been studied, and new drugs targeting KRAS p. G12C are in the pipeline for approval. G12C occurs in 14% of cases of lung adenocarcinoma, and the KRYSTAL-1 (NCT03785249) phase I/II trial tested the agent (32) adagrasib (MRTX849). Another drug sotorasib (AMG-10) has been tested in the CodeBreak 100 trial (33) (NCT03600883) with an ORR of 32.2%. The FDA has approved sotorasib as a new drug after testing for p. G12C using an FDA-approved test, following at least 1 prior line of systemic therapy. Testing for this mutation, hence, is clinically relevant, and DNA-based NGS panels incorporate it.
ERBB2 (Her2/neu) exon 20 insertions and a few point mutations have been reported in 4% (34) of cases of lung adenocarcinoma in The Cancer Genome Atlas (TCGA) database and 3% of cases in the Lung Cancer Mutation Consortium project (35). In our experience with 145 cases of NSCLC that underwent NGS-based testing, 6.2% of cases showed exon 20 ERBB2 alterations (36). One-third of these cases are also known to harbor EGFR mutations. Hence, incorporating next-generation sequencing is critical to effectively capture uncommon mutations and amplifications in ERBB2 so that patients may be offered therapy directly targeted to their genomic alterations. Among the targeted agents available, tarloxotinib (NCT03805841), trastuzumab deruxtecan (NCT03505710), pyrotinib (NCT02500199), and poziotinib (NCT03318939) are just a few of the novel ERBB2 inhibitors available in clinical trials. DESTINY Lung01 (37) is an ongoing multicenter trial investigating the role of trastuzumab deruxtecan in non-squamous NSCLC with activating ERBB2 mutation/overexpression. The initial results show an ORR of 61.9% and a disease control rate (DCR) of 90.5% with a median PFS of 14 months.
ALK-rearranged NSCLC has been reported in ∼7% of cases, and EML4-ALK was the first fusion identified in 2007 (38). These patients are usually young non-smokers with adenocarcinoma histology. Mutations and amplifications are also known to occur in the kinase domain of the ALK gene, which develop as resistance mechanisms to ALK TKIs. Crizotinib was the first ALK TKI approved for these cases, followed by the development of second- and third-generation TKIs (39).
Second-generation ALK inhibitors, ceritinib and alectinib, have now been both approved as first line treatments of ALK-rearranged NSCLC. The ALEX trial (40) demonstrated significantly improved PFS (34.8 months vs 10.9 months) and OS (NR vs 57.4 months; 5 years OS rate: 62.5% for alectinib vs 45.5% for crizotinib) with alectinib compared to crizotinib in treatment-naive ALK-positive NSCLC. Recent studies have addressed the impact of ALK-fusion variants, depending on breakpoints, on the response to ALK inhibitors. V1 and V3 EML4-ALK variants have been reported to be the most frequent. The traditionally used screening tool is the D5F3 monoclonal antibody by IHC, and trials like ALEX and PROFILE 1014 (41) have used the same for the detection of ALK rearrangement. Break-apart FISH was once considered confirmatory for the same; however, it suffers from signal intensity issues and observer-dependent variabilities. Owing to various breakpoints and fusion partners described, RT-PCR and Sanger sequencing are not optimal, owing to limited coverage and sensitivities. NGS using targeted RNA-based panels offers a one-stop solution not only to detect and characterize the fusion partners but also for response monitoring and the detection of additional resistance mutations (p.G1202R and p. L1196M) and other co-mutations like TP53 (25), which are known to affect responses and prognosis. However, there are discordance instances reported; hence, IHC still forms the first step for detection.
Analogous to ALK, ROS1 rearrangements have been described as drivers of NSCLC and have been reported in 1%–2% of cases (42). The patients are young and non-smokers, with a higher prevalence in Asians, similar to the profile of ALK-positive cases. Testing for ROS1 has been recommended using break-part FISH, although IHC using the D4D6 rabbit monoclonal antibody has also been described with almost 100% sensitivity, which is optimal for screening. Similar to ALK, ROS1 also has multiple fusion partners, which can be characterized using NGS, RT-PCR, and even NanoString technology. However, the combined sensitivity and specificity of IHC, followed by FISH, are higher than any of the other abovementioned technologies.
In two independent phase II prospective studies, the efficacy of crizotinib in ROS1-positive cases was evaluated, which depicted an ORR of 72% and 70%, respectively, with a median PFS of 19.2 months and 15.9 months (42). Analogous to ALK, resistance mutations are also described in the ROS1 kinase domain, namely, p. G2032R and p. D2033N, which are resistant to crizotinib but have shown some sensitivity to lorlatinib. In a single-arm phase 1–2 trial (NCT01970865) investigating the efficacy of lorlatinib in advanced NSCLC, 62% of patients who were TKI-naive showed an objective response. However, another substitution at 2032: p. G2032K has also shown lorlatinib resistance.
Co-mutations with ROS1 are rare; however, performing NGS in this context is relevant, owing to solvent front resistance mutations, which develop during the course of the disease.
RET fusions have been reported in 1%–2% of cases of NSCLC (10). The recent approval of selpercatinib, following the results from LIBRETTO-001 (43) for RET-rearranged NSCLC cases, has sparked keen interest in the detailed characterization of clinicopathologic features and response outcomes of this disease. The current practice of performing single-gene testing does not incorporate RET fusion detection, and hence, NGS-based panel testing may prove promising.
Testing for RET traditionally has been using break-apart FISH and was not recommended upfront. With newer drugs making their way into the clinic, it is important to test the same upfront. Panel-based NGS testing using RNA-based panels like the Oncomine fusion panel, anchored multiplex PCR, Illumina TruSight, and the FDA-approved FoundationOne incorporate RET gene alterations. Recently, resistance mutation RET p. S904F has been identified, which is resistant to vandetanib. Hence, NGS with both RNA and DNA are now mandatory in the first line.
NTRK fusions are detected across multiple pediatric and adult malignancies. The frequency of these fusions varies from <1% in malignancies like lung, colorectal, pancreatic, breast cancers, melanoma, and other solid or hematological cancers (44). They have gained importance as tissue-agnostic markers, owing to the development of specific inhibitors like entrectinib and larotrectinib, following the results from trials like START-TRK. In February 2015, entrectinib was granted FDA Orphan Drug Designation for NTRK-positive NSCLC and colorectal neoplasms. Larotrectinib activity has been evaluated in three trials: a phase I trial in adults (NCT02122913), a phase I/II trial in pediatric patients (SCOUT, NCT02637687), and a phase II trial involving adults and adolescents (NAVIGATE, NCT02576431) (45).
The current ESMO recommendations (46) incorporate the use of IHC for NTRK testing both for screening in the case of tissue unavailability in common cancers and confirmatory in NTRK-enriched tumors, after NGS-based testing. IHC, although less sensitive and variable for each of the three NTRK1, 2, and 3 with variable staining patterns, is still appealing, owing to its low cost and good sensitivity, making it a good screening tool.
NRG1 fusions have emerged as uncommon biomarkers, especially in invasive mucinous adenocarcinoma (47). They activate Her2/Her3 signaling, and anecdotal case reports have shown durable responses to afatinib (48). However, this has yet to be validated in controlled trials. Hence, owing to the paucity of the literature on this gene, it is not yet included in upfront diagnostic tumor profiling, and no NGS panels currently incorporate it. However, future RNA sequencing and fusion panels will have to eventually include it.
The proposed panel of genes and biomarkers to be tested in NSCLC is depicted in Figure 2. The ongoing clinical trials for the biomarkers are detailed in Table 3.
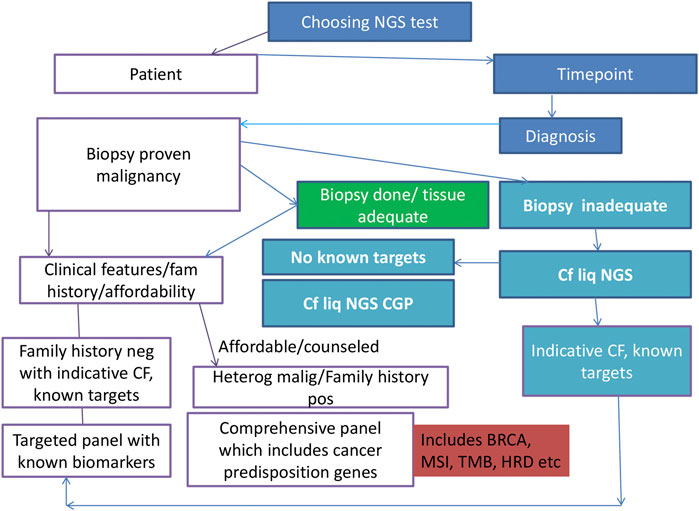
Figure 2. Algorithm/flow diagram depicting the best-proposed panel for molecular profiling in lung carcinoma. NSCLC, non-small cell lung carcinoma; DNA, deoxyribonucleic acid; RNA, ribonucleic acid; NGS: next-generation sequencing.
Targeted therapies and precision medicine have paved the way for broader molecular testing, offering an insight into disease biology and evolution. However, clinicians need to be aware that sequential single-gene testing results in tissue exhaustion with higher false negative rates. Additionally, whole exome/whole genome approaches are not suitable for all samples. Targeted NGS panels may prove to be more promising than sequential single-gene testing. Test performances may vary due to differences in sensitivities, specificities, depth, and coverage, and hence, the test performance should be a part of the final molecular report rendered to the clinician in order to deliver appropriate therapy. Clinical interpretation should be made with utmost care and involve tumor board discussions, prior to the initiation of therapy.
From the data presented on various biomarkers in NSCLC, it is clearly evident that NGS-based testing forms the foundation in this constantly evolving field of precision medicine. Testing strategies must advance to take into account the ever-expanding list of new biomarkers, new drugs, and the need to not only diagnose but also to monitor disease responses.
UB: conceptualization, supervision, and writing–review and editing. SN: data curation, formal analysis, methodology, and writing–original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. National Comprehensive Cancer Network. Non-small cell lung cancer (Version3.2020) (2020). Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (Accessed April 10, 2020).
2. Strom, SP. Current practices and guidelines for clinical next-generation sequencing oncology testing. Cancer Biol Med (2016) 13(1):3–11. doi:10.28092/j.issn.2095-3941.2016.0004
3. Lindeman, N, Cagle, P, Aisner, DL, Arcila, ME, Beasley, MB, Bernicker, EH, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the international association for the study of lung cancer, and the association for molecular Pathology. Arch Pathol and Lab Med (2018) 142:321–46. doi:10.5858/arpa.2017-0388-CP
4. Garrido, P, Conde, E, de Castro, J, Gómez-Román, JJ, Felip, E, Pijuan, L, et al. Updated guidelines for predictive biomarker testing in advanced non - small - cell lung cancer: a National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin Transl Oncol (2020) 22(7):989–1003. doi:10.1007/s12094-019-02218-4
5. Richards, S, Aziz, N, Bale, S, Bick, D, Das, S, Gastier-Foster, J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and genomics and the association for molecular Pathology. Genet Med (2015) 17(5):405–24. doi:10.1038/gim.2015.30
6. Froyen, G, Le Mercier, M, Lierman, E, Vandepoele, K, Nollet, F, Boone, E, et al. Standardization of somatic variant classifications in solid and haematological tumours by a two-level approach of biological and clinical classes: an initiative of the Belgian ComPerMed expert panel. Cancers (Basel) (2019) 11:2030. doi:10.3390/cancers11122030
7. Garinet, S, Laurent-puig, P, Blons, H, and Oudart, JB. Current and future molecular testing in NSCLC, what can we expect from new sequencing technologies. J Clin Med (2018) 7:144–23. doi:10.3390/jcm7060144
8. Paez, JG, Ja, PA, Tracy, S, Greulich, H, Gabriel, S, Herman, P, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (2004) 304(June):1497–501.
9. Yatabe, Y, Kerr, KM, Rajadurai, P, Tran, VK, Du, X, Chou, TY, et al. EGFR mutation testing practices within the asia pacific region results of a multicenter diagnostic survey participating sites and study design. J Thorac Oncol (2015) 10(3):438–45. doi:10.1097/JTO.0000000000000422
10. Gaughan, EM, and Costa, DB. Genotype-driven therapies for non-small cell lung cancer: focus on EGFR, KRAS and ALK gene abnormalities. Ther Adv Med Oncol (2011) 3(3):113–25. doi:10.1177/1758834010397569
11. Chakravarty, D, Gao, J, Phillips, S, Kundra, R, Zhang, H, Wang, J, et al. OncoKB: a precision oncology knowledge base. JCO Precision Oncol (2017) 2017(1):1–16. doi:10.1200/po.17.00011
12. Wu, JY, Yu, CJ, Chang, YC, Yang, CH, Shih, JY, and Yang, PC. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res (2011) 17(11):3812–21. doi:10.1158/1078-0432.CCR-10-3408
13. Collisson, E, Campbell, JD, Brooks, AN, Berger, A, Lee, W, Chmielecki, J, et al. Comprehensive molecular profiling of lung adenocarcinoma: the cancer genome atlas research network. Nature (2014) 511(7511):543–50. doi:10.1038/nature13385
14. Mok, TS, Wu, Y, Thongprasert, S, Yang, CH, Chu, DT, Saijo, N, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med (2009) 361(10):947–57. doi:10.1056/nejmoa0810699
15. Yoshioka, H, Shimokawa, M, Seto, T, Morita, S, Yatabe, Y, Okamoto, I, et al. Final overall survival results of WJTOG3405, a randomized phase III trial comparing gefitinib versus cisplatin with docetaxel as the first-line treatment for patients with stage IIIB/IV or postoperative recurrent EGFR mutation-positive non-small-cell lung cancer. Ann Oncol (2019) 30(12):1978–84. doi:10.1093/annonc/mdz399
16. Inoue, A, Kobayashi, K, Maemondo, M, Sugawara, S, Oizumi, S, Isobe, H, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol (2013) 24(1):54–9. doi:10.1093/annonc/mds214
17. Rosell, R, Carcereny, E, Gervais, R, Vergnenegre, A, Massuti, B, Felip, E, et al. Erlotinib versus standard chemotherapy as fi rst-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol (2012) 13(3):239–46. doi:10.1016/S1470-2045(11)70393-X
18. Sharma, N, and Graziano, S. Overview of the LUX-Lung clinical trial program of afatinib for non-small cell lung cancer. Cancer Treat Rev (2018) 69(June):143–51. doi:10.1016/j.ctrv.2018.06.018
19. Yang, JC, Wu, Y, Schuler, M, Sebastian, M, Popat, S, Yamamoto, N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol (2015) 16(14):141–51. doi:10.1016/S1470-2045(14)71173-8
20. Wu, YL, Mok, T, Han, JY, Ahn, MJ, Delmonte, A, Ramalingam, S, et al. Overall survival (OS) from the AURA3 phase III study: osimertinib vs platinum-pemetrexed (plt-pem) in patients (pts) with EGFR T790M advanced non-small cell lung cancer (NSCLC) and progression on a prior EGFR-tyrosine kinase inhibitor (TKI). Ann Oncol (2019) 30(November):ix158. doi:10.1093/annonc/mdz437.001
21. Ramalingam, SS, Vansteenkiste, J, Planchard, D, Cho, BC, Gray, JE, Ohe, Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med (2020) 382(1):41–50. doi:10.1056/NEJMoa1913662
22. Lazzari, C, Gregorc, V, Karachaliou, N, Rosell, R, and Santarpia, M. Mechanisms of resistance to osimertinib. J Thorac Dis (2020) 12(5):2851–8. doi:10.21037/jtd.2019.08.30
23. Sequist, LV, Waltman, BA, Dias-santagata, D, Digumarthy, S, Turke, AB, Fidias, P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med (2011) 3(75):75ra26. doi:10.1126/scitranslmed.3002003
24. Luk, PP, Yu, B, Ng, CC, Mercorella, B, Selinger, C, Lum, T, et al. BRAF mutations in non-small cell lung cancer. Transl Lung Cancer Res (2015) 4(7):142–8. doi:10.3978/j.issn.2218-6751.2014.08.08
25. Farago, AF, and Azzoli, CG. Beyond ALK and ROS1: RET, NTRK, EGFR and BRAF gene rearrangements in non-small cell lung cancer. Transl Lung Cancer Res (2017) 6(5):550–9. doi:10.21037/tlcr.2017.08.02
26. Anguera, G, and Majem, M. BRAF inhibitors in metastatic non-small cell lung cancer. J Thorac Dis (2018) 10(2):589–92. doi:10.21037/jtd.2018.01.129
27. Schil, PEV, Hellmann, MD, Peters, S, and Guidelines, E. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2019) 29(September).
28. Recondo, G, Che, J, Jänne, PA, and Awad, MM. TargetingMETDysregulation in cancer. Cancer Discov (2020) 10(july):922–34. doi:10.1158/2159-8290.CD-19-1446
29. Wolf, J, Han, J, Nishio, M, Souquet, P, Paz-Ares, L, De Marinis, F, et al. PS04.06 GEOMETRY mono-1: phase II, multicenter study of MET inhibitor capmatinib (INC280) in EGFR wt, MET-dysregulated advanced NSCLC. J Thorac Oncol (2017) 12(11):S1578–S1579. doi:10.1016/j.jtho.2017.09.075
30. Liu, P, Wang, Y, and Li, X. Targeting the untargetable KRAS in cancer therapy. Acta Pharmaceutica Sinica B (2019) 9(5):871–9. doi:10.1016/j.apsb.2019.03.002
31. Cserepes, M, Ostoros, G, Lohinai, Z, Raso, E, Barbai, T, Timar, J, et al. Subtype-specific KRAS mutations in advanced lung adenocarcinoma: a retrospective study of patients treated with platinum-based chemotherapy. Eur J Cancer (2014) 50:1819–28. doi:10.1016/j.ejca.2014.04.001
32. Jänne, PA, Rybkin, I, Spira, AI, et al. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in advanced/metastatic non–small-cell lung cancer (NSCLC) harboring KRAS G12C mutation. Eur J Cancer (2020) 2020(138S2):S1–S62. doi:10.1016/S0959-8049(20)31076-5
33. Fakih, M, Desai, J, Kuboki, Y, Strickler, JH, Price, TJ, Durm, GA, et al. CodeBreak 100: activity of AMG 510, a novel small molecule inhibitor of KRASG12C, in patients with advanced colorectal cancer. J Clin Oncol (2020) 38(15):4018. doi:10.1200/jco.2020.38.15_suppl.4018
34. Arcila, ME, Chaft, JE, Nafa, K, Roy-Chowdhuri, S, Lau, C, Zaidinski, M, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res (2012) 18(18):4910–8. doi:10.1158/1078-0432.CCR-12-0912
35. Pillai, RN, Behera, M, Berry, LD, Rossi, MR, Kris, MG, Johnson, BE, et al. HER2 mutations in lung adenocarcinomas: a report from the Lung Cancer Mutation Consortium. Cancer (2017) 123(21):4099–105. doi:10.1002/cncr.30869
36. Mehta, A, Nathany, S, Tripathi, R, Sharma, SK, Saifi, M, and Batra, U. Non-amplification genetic alterations of HER2 gene in non- - small cell lung carcinoma. J Clin Pathol (2020) 74:106–10. doi:10.1136/jclinpath-2020-206730
37. Peters, S, and Zimmermann, S. Targeted therapy in NSCLC driven by HER2 insertions. Transl Lung Cancer Res (2014) 3(2):84–8. doi:10.3978/j.issn.2218-6751.2014.02.06
38. Lin, Y, Liu, Y, and Shih, J. The impact of clinical factors, ALK fusion variants, and BIM polymorphism on crizotinib-treated advanced EML4 – ALK rearranged non-small cell lung cancer. Front Oncol (2019) 9(September):880. doi:10.3389/fonc.2019.00880
39. Mcleer-florin, A, Duruisseaux, M, Pinsolle, J, Dubourd, S, Mondet, J, Phillips Houlbracq, M, et al. ALK fusion variants detection by targeted RNA-next generation sequencing and clinical responses to crizotinib in ALK-positive non-small cell lung cancer. Lung Cancer (2018) 116(December 2017):15–24. doi:10.1016/j.lungcan.2017.12.004
40. Mok, T, Peters, S, Camidge, DR, Noé, J, Gadgeel, S, Ignatius Ou, SH, et al. Outcomes according to ALK status determined by central IHC or FISH in patients with ALK-positive NSCLC enrolled in the phase III ALEX study. J Thorac Oncol (2020) S1556-0864(20):30815–7. doi:10.1016/j.jtho.2020.10.007
41. Shaw, AT, Janne, PA, Besse, B, Solomon, BJ, Blackhall, FH, Camidge, DR, et al. Crizotinib vs chemotherapy in ALK+ advanced non-small cell lung cancer (NSCLC): final survival results from PROFILE 1007. J Clin Oncol (2016) 34:9066. doi:10.1200/jco.2016.34.15_suppl.9066
42. Bubendorf, L, Büttner, R, Al-dayel, F, Dietel, M, Elmberger, G, Kerr, K, et al. Testing for ROS1 in non-small cell lung cancer: a review with recommendations. Virchows Arch (2016) 469:489–503. doi:10.1007/s00428-016-2000-3
43. Drilon, A, Oxnard, G, Wirth, L, Besse, B, Gautschi, O, Tan, S, et al. PL02.08 registrational results of LIBRETTO-001: a phase 1/2 trial of LOXO-292 in patients with RET fusion-positive lung cancers. J Thorac Oncol (2019) 14(10):S6–S7. doi:10.1016/j.jtho.2019.08.059
44. Haratake, N, and Seto, T. NTRK fusion-positive non-small-cell lung cancer -The diagnosis and targeted therapy. Clin Lung Cancer (2021) 22:1–5. doi:10.1016/j.cllc.2020.10.013
45. Amatu, A, Sartore-Bianchi, A, Bencardino, K, Pizzutilo, EG, Tosi, F, and Siena, S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann Oncol (2019) 30(Suppl. 8):5–15. doi:10.1093/annonc/mdz383
46. Marchiò, C, Scaltriti, M, Ladanyi, M, Iafrate, A, Bibeau, F, Dietel, M, et al. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann Oncol (2019) 30:1417–27. doi:10.1093/annonc/mdz204
47. Fernandez-cuesta, L, and Thomas, RK. Molecular pathways: targeting NRG1 fusions in lung cancer. Clin Cancer Res (2015) 21:1989–94. doi:10.1158/1078-0432.CCR-14-0854
Keywords: biomarker, next-generation sequencing, targeted therapy, lung cancer, precision oncology
Citation: Batra U and Nathany S (2025) Biomarker testing in lung cancer: from bench to bedside. Oncol. Rev. 18:1445826. doi: 10.3389/or.2024.1445826
Received: 08 June 2024; Accepted: 12 December 2024;
Published: 06 January 2025.
Edited by:
Deepa Kushwaha, Rare Genomics Institute, United StatesReviewed by:
Mihai Vasile Marinca, Grigore T. Popa University of Medicine and Pharmacy, RomaniaCopyright © 2025 Batra and Nathany. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ullas Batra, dWxsYXNiYXRyYUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.