- 1Department of Physiology, Mbeya College of Health and Allied Sciences, University of Dar es Salaam, Mbeya, Tanzania
- 2Department of Obstetrics and Gynecology, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
- 3Department of Surgical Oncology, Ocean Road Cancer Institute, Dar es Salaam, Tanzania
Survivin belongs to the inhibitor of apoptosis protein (IAP) family and is encoded by the baculoviral inhibitor of apoptosis repeat-containing, or BIRC5, gene. It is preferentially expressed in cancers with functional complexity in cell signaling cascades such as extracellular signal-regulated kinases (ERK), mitogen-activated protein kinases (MAPK), heat shock protein-90 (HSP90), epidermal growth factor receptor (EGFR), phosphoinositide 3-kinase (PI3K), signal transducer and activator of transcription (STAT), hypoxia-inducible factor-1 alpha (HIF-1α), vascular endothelial growth factor (VEGF), and others. Survivin plays a role in cell division and cell death, properties that have attracted a large body of research to decipher its therapeutic and prognostic significance in cancer. Survivin has tumor-promoting effects in endometrial (EC) and ovarian (OC) cancers, and its upregulation in endometrial cancer has been associated with poor overall survival (OS). While survivin protein is abundantly expressed in OC, it is barely detectable in normal ovarian tissue or benign ovarian tumors. Survivin expression is also a marker for cervical intraepithelial neoplasia (CIN) and high-risk human papillomavirus, and a predictor of viral clearance and prognosis in uterine cervical cancer (UCC). Furthermore, nuclear survivin expression is very low in normal vulvar squamous epithelium and increases to become abundant in vulvar invasive squamous cell carcinoma (ISCC), conferring resistance to apoptosis in vulvar carcinogenesis. In this review, we discuss in detail the impact of survivin signaling on gynecological cancers and provide insight on its therapeutic and diagnostic potential, existing research gaps, and areas for future research.
1 Introduction
Survivin, also known as BIRC5, is the smallest member of the inhibitor of apoptosis proteins (IAPs) family, which plays a role in mitotic regulation and inhibition of apoptosis as well as carcinogenesis (1). Eight members of the IAPs have been described namely, X-linked inhibitor of apoptosis (XIAP), NLR family apoptosis inhibitory protein (NAIP), cellular inhibitor of apoptosis proteins-1 and -2 (cIAP1, cIAP2), IAP-like protein-2 (ILP2), melanoma inhibitor of apoptosis (MLIAP) also known as livin’, survivin and baculovirus inhibitor of apoptosis repeat-containing ubiquitin conjugating enzyme (BRUCE). Human survivin has 142 amino acids and is a 16.5 kDa protein. The survivin gene was first cloned by Ambrosini et al (2). The BIRC5 gene spans 147 kb and consists of three introns and four exons. The survivin gene has two functional domains; a BIR (baculoviral IAP repeat-containing) domain in the N-terminus and an α-helix in the C-terminus (Figure 1).
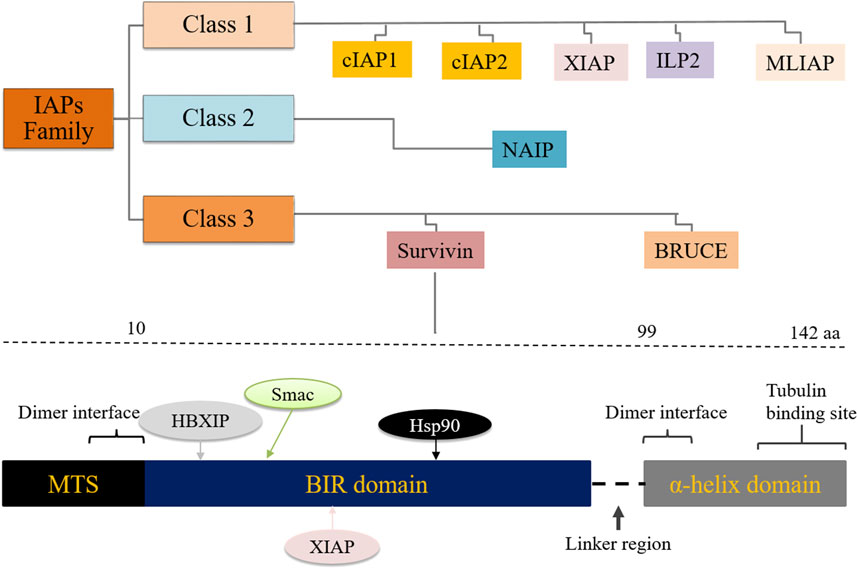
Figure 1. Classification of the inhibitor of apoptosis (IAP) protein family and the survivin gene BIRC5 showing interacting partner proteins/molecules. MTS: mitochondrial targeting sequence, HBXIP: hepatitis B X-interacting protein.
Both domains control the regulation of cell mitosis, but only the BIR domain is involved in the regulation of apoptosis. BIRC5 lacks the caspase activation and recruitment domain (CARD) motif. This feature prevents it from directly binding to and inhibiting caspases. It also lacks the IAP C-terminal really interesting new gene (RING) finger domain, which would bind zinc ions with the BIR domain (3). While the helical domain of the BIRC5 gene interacts mainly with tubulin structures, (Figure 1), the BIR domain plays a central role in the control of cell division and cell death (4, 5). Several genetic survivin splice variants (isoforms) with unique functions have been documented, including survivin-wt, survivin-2α, survivin-2B, survivin-3B and survivin-ΔEx-3 (6, 7). Survivin is expressed in proliferating cells, including cancer stem cells (CSCs), and is barely detectable in well-differentiated somatic cells. It can be detected in virtually all cellular compartments, including the extracellular matrix, the outer surface of the cell membrane, the cytoplasm, mitochondria, exosomes and the nucleus. Survivin plays a central role in several cellular pathways including cell division, angiogenesis, apoptosis and carcinogenesis (8). Survivin functions are influenced by its extensive post-translational modification, reversible dimerization and subcellular localization (9). Overexpression of survivin protein has been documented in a variety of human cancers, including pancreatic, breast, gastric, lung, uterine, ovarian, esophageal and cervical cancers (10, 11, 12, 13).
2 Survivin signaling and gynecological cancer
2.1 Survivin signaling in endometrial cancer
Among gynecological cancers, survivin signaling has been best studied in endometrial cancer. Although survivin expression has been reported in non-malignant endometrium, the levels are comparatively low. Information on the precise function of survivin in non-malignant endometrial tissue is limited; however, it is overexpressed in cycling endometrium, early pregnancy placenta, endometriosis and endometrial hyperplasia (14, 15, 16, 17, 18, 19, 20). There are conflicting reports on the role of survivin in endometrial physiology. Garcia et al. studied normal/low abortion (CBA/J x BALB/c) and abortion-prone (CBA/J x DBA/2J) mouse models and showed that high levels of survivin expression may be associated with pregnancy loss in mice (21). In contrast, work by Fest et al. using the same mouse models showed decreased levels of survivin mRNA and protein expression in the abortion-prone mice compared to the normal group, suggesting that survivin plays a role in promoting trophoblast cell survival and proliferation, thus maintaining pregnancy (22). It has also been shown that BIRC5 gene expression is significantly repressed in the deciduae of interleukin-11 receptor alpha null (IL-11Rα) mice, which are infertile due to defective decasualization and impaired trophoblast invasion (23). Interestingly, treatment of cells with IL-11 stimulated survivin expression, suggesting that survivin may be a target of IL-11 in the decidua and may play a role in maintaining fertility. IL-11Rα has been implicated in doxorubicin chemoresistance in high-grade endometrioid adenocarcinoma, the most common type of endometrial cancer (24). Overexpression of survivin in endometrial cancer has been documented and correlates with both tumor stage and grade; some of the reports are shown in Table 1.

Table 1. Survivin protein expression expression and its correlated effects in gynecological cancers.
Stavropoulos A. et al. examined survivin expression in primary endometrial cancer tissue samples and found that 88% of the samples (n = 99) were immunopositive. Of the samples examined, 60% showed co-expression of survivin, phosphatase and tension homolog (PTEN) and p53 (25). The sum of survivin staining intensity and scores was significantly associated with patient age, histological grade, clinical stage and metastasis. In our previous work published in Gynecologic Oncology, we also showed that survivin was expressed in 87.5% of endometrial cancer cell lines and that high expression of BIRC5 gene was significantly associated with poor progression-free survival (p = 0.006), and was an independent prognostic factor (HR = 1.97, 95% CI = 1.29–4.5, p = 0.045) (26). Other reports have also shown a positive correlation between high levels of survivin expression and advanced clinical stage, histological grade, invasiveness and prognosis of endometrial cancer (20), (32, 33, 34). Survivin is involved in several cellular signaling pathways in endometrial cancer. The survivin gene BIRC5, among other hub genes (GTSE1, AURKA and KNSTRN), has been associated with AKT1, one of three closely related serine/threonine protein kinases that regulate various cellular processes including metabolism, growth, proliferation, angiogenesis and cell survival (35).
PTEN is an established tumor suppressor gene encoding a phosphatase; a key regulatory enzyme in signaling via the PI3K-AKT-mTOR pathway which controls cell growth, mitosis, metabolism and apoptosis. PTEN functions by antagonizing the PI3K/AKT pathway through dephosphorylation of PIP3, which results in decreased translocation of AKT to cell membranes, subsequently downregulating its phosphorylation and activation (36). In endometrioid endometrial cancer (which accounts for 80% of endometrial cancers), molecular aberrations in this pathway occur in approximately 80%–90% of cases (37, 38, 39). Guha M. et al. investigated the association between survivin expression, PTEN and p53 and confirmed that PTEN-mediated endogenous tumor suppression involves silencing of the survivin gene, BIRC5 (40). This association may be a potential therapeutic target, as PTEN has been confirmed as a major target gene in endometrial carcinogenesis and a predictive marker for the development of high-grade endometrial cancer (41, 42). Furthermore, hypermethylation of the BIRC5 promoter in endometrial cancer is known to block the binding of TP53 to its promoter region, thereby increasing survivin expression (43). The BIRC5 promoter is unmethylated in normal endometrial tissue, but methylation levels increase from low to high grade endometrial cancer and correlate with increased survivin protein expression (44).
The tumor suppressor gene TP53 and the transcription factor Egr1 are known repressors of the survivin gene promoter. The putative p53-mediated inhibition of the survivin gene BIRC5 has been demonstrated (45). In addition, p53 suppresses tumor development by activating apoptosis signaling genes such as PUMA, BAX and Noxa to induce the process of programmed cell death (apoptosis) in the event of irreparable DNA damage. The frequency of TP53 mutations is 10%–40% and approximately 90% in type I and type II endometrial cancer, respectively. Several studies have investigated p53 protein expression levels and their significance in endometrial cancer (46, 47, 48, 49, 50, 51). Nuclear accumulation of p53 protein resulting from a missense mutation in the TP53 gene is observed as overexpression by immunohistochemistry (IHC). High p53-positive expression was observed in 10%–44% of type I endometrial cancer and 30%–86% of type II endometrial cancer. Thus, the association between survivin and the two tumor suppressor genes, PTEN and TP53, is likely to be an attractive molecular therapeutic target, as mutation of these two genes is the most common gene mutation documented in endometrial cancer (52, 53, 54). In addition, co-expression of survivin and vascular endothelial growth factor (VEGF), a highly specific vascular endothelial cell mitogen that promotes angiogenesis in tumor tissue, has recently been found to be associated with recurrence-free progression and overall survival in endometrial cancer patients (55, 56, 57). Such co-overexpression is associated with the International Federation of Gynecology and Obstetrics (FIGO) stage, deep myometrial invasion, lymph node metastasis and survival status, and was an independent predictor of poor prognosis (58).
Progestin therapy is a conservative treatment often used in younger endometrial cancer patients who are not candidates for surgery or who wish to preserve fertility, but about 30% of patients do not respond to progestin therapy. Survivin signaling has been strongly implicated in hormone therapy resistance in endometrial cancer. Overexpression of survivin and nuclear factor erythroid 2-related factor-2 (Nrf2) has been reported in partially progestin-responsive and progestin-resistant EC tissue samples, and negative expression of these biomarkers in responsive tissue samples (59). Survivin-induced progestin resistance was indeed confirmed by pre- and post-treatment assays showing a 20-fold decrease in survivin expression in progestin responders and a non-significant change in survivin expression in non-responders (20). We have also previously reported that treatment of endometrial cancer cells with estradiol significantly induced co-expression of nuclear ERα and survivin proteins (p < 0.001), whereas inhibition of survivin caused apoptotic cell death (60). Endometrial cancer tends to progress slowly and is often diagnosed at an early stage due to noticeable symptoms such as abnormal vaginal bleeding. This provides an opportunity for early intervention. In contrast, ovarian cancer is often called the ‘silent killer’ because it tends to progress quickly and is often diagnosed at an advanced stage due to subtle and non-specific symptoms such as bloating, pelvic pain or gastrointestinal problems.
2.2 Survivin signaling in ovarian cancer
Ovarian cancer is associated with the highest mortality of all gynecological tumors. Among malignant ovarian tumors, epithelial ovarian carcinoma (EOC) is the most common and is associated with the highest mortality, largely due to late detection, aggressiveness and chemoresistance (61, 62, 63). Survivin overexpression has also been observed in human ovarian cancer. Cohen C. et al. reported survivin overexpression in 74% (n = 49) of tissue sections examined, which correlated with poor prognostic parameters, i.e., high grade, histological type and p53 mutation (63). It has also been described that mean survivin mRNA expression levels are higher in ovarian carcinoma than in benign and borderline ovarian tumors and correlate with clinical stage, degree of differentiation and lymph node metastasis (64, 65). The study examined 111 ovarian tissue samples for survivin expression using reverse transcription-polymerase chain reaction, quantified the expression using quantitative real-time PCR and found that survivin was highly expressed in ovarian carcinoma (73%) compared to benign (47%) and borderline (19%) ovarian tumors (p < 0.001).
Survivin expression, among other biomarkers, has also been associated with poorer tumor cell differentiation and has been suggested to help assess the aggressiveness of ovarian mucinous, serous and clear cell adenocarcinomas (66). Similarly, studies conducted elsewhere on EOC tissue samples have found a significant correlation between survivin overexpression and increased cell proliferation, malignant potential and poor prognosis (64, 67, 68, 69). As in EC, survivin is involved in several cellular signaling pathways in EOC. It has previously been shown that genetic deletion of survivin gene by a gene-editing technology system using clustered regularly interspaced palindromic repeats and the Cas9 enzyme (CRISPR/Cas9) in orthotopic ovarian cancer mouse models prevented tumor metastasis (70). The underlying mechanisms were further confirmed in another study where ovarian cancer cells resistant to standard chemotherapy were treated with the selective survivin inhibitor MX106. This inhibitor blocked epithelial-to-mesenchymal transition (EMT) by dampening the transforming growth factor-β (TGF-β) signaling pathway, thereby suppressing tumor growth and metastasis (71). EMT is a physiological process in which epithelial cells adopt the migratory and invasive characteristics of mesenchymal cells. When this process is inappropriately activated, often due to changes in the microenvironment or cellular signaling disruptions, it contributes to cancer progression. The three known isoforms of TGF-β in mammals; TGF-β1, β2, and β3 are involved in key physiological processes such as embryonic development, immune responses, proliferation, differentiation, and apoptosis (28).
PTEN alterations in tumors are often due to mutations or deletions. About 20%–30% of ovarian cancers contain mutations in the PTEN gene. While a key step in the development of high-grade serous ovarian cancer (HGSOC) - the most common and lethal subtype - is thought to be TP53 gene mutations that cause protein stabilization in the fallopian tube, accumulating evidence suggests that alterations in PTEN expression may be involved in the early stages of serous ovarian cancer development, similar to endometrioid endometrial cancer (72, 73). Investigation of PTEN expression patterns as a biomarker in 5,400 EOC tissue samples concluded that PTEN loss is a common driver in the development of HGSOC (73). Furthermore, Pradeep S. et al. used mouse models to investigate the role of PTEN in ovarian cancer and observed that simultaneous deletion of PTEN and the liver kinase-B1 (LKB1) induced the development of HGSOC with full penetrance (74). It has also been reported that among ovarian cancer patients, those with PTEN (−)/survivin (+) expression have the worst prognosis (75). The interaction between survivin, PTEN and TP53 in cancer is well described. In addition, the tumor-suppressing effect of caspase-2, a death effector, has been shown to be associated with the silencing of survivin gene transcription (76). Survivin associates with specificity protein-1 (Sp1) to induce chemoresistance in OC patients treated with cisplatin (77). Survivin has also been shown to interact with class III β-tubulin and Sox2 to induce taxane resistance in EOC (78, 79). Several survivin-targeted therapeutic strategies are currently being evaluated in various stages of clinical trials for potential translation into the clinic (Table 2). Among the gynecological cancers, ovarian cancer is relatively less common than endometrial and cervical cancer, but it is still one of the leading causes of gynecological cancer-related mortality.
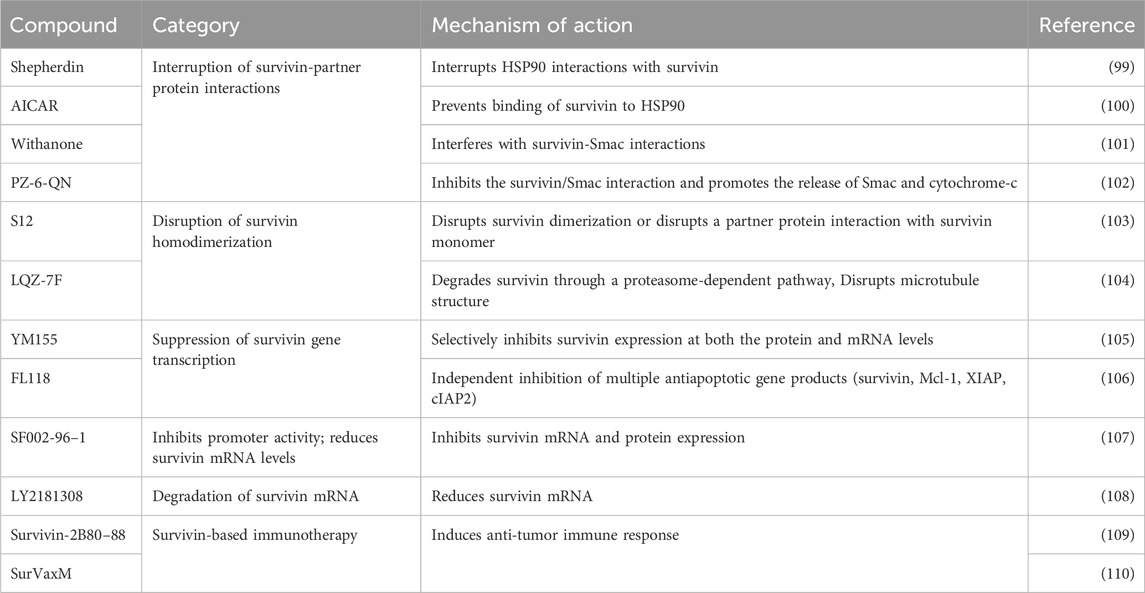
Table 2. Survivin-based therapies that have been investigated against various human cancers over the past 2 decades, their mechanisms of action, and their effects on cancer cells.
2.3 Survivin signaling in cervical cancer
Research has shown that survivin expression in uterine cervical cancer (UCC) is associated with invasiveness, clinical stage, metastasis, chemoresistance and poor prognosis (31, 80, 81, 82). In UCC, survivin has been shown to interact with several molecules involved not only in apoptosis but also in other cell signaling pathways. Overexpression of survivin was significantly associated with poor survival in cervical cancer compared to cancer tissues negative for survivin expression (83). Mechanisms involved in the development of cervical cancer include abnormal cell proliferation and cell cycle deregulation. The p16INK4a, Ki-67, survivin and cyclooxygenase-2 (COX-2) have been described as potential biomarkers involved in the progression of UCC. It has been postulated that survivin may control apoptosis in UCC through interactions with p16INK4a, COX-2 and Ki-67. Indeed, survivin was found to competitively interact with CDK4/p16INK4a upon initiation of cell cycle entry and the correlation of survivin expression levels with p16INK4a, COX-2 and Ki-67 in UCC was significant (84, 85). Furthermore, treatment response in patients with cervical squamous cell carcinoma (CSCC) treated with paclitaxel and carboplatin was independently correlated with grade, Ki67 and survivin expression (86). This suggests that survivin expression may be a marker of prognosis following neoadjuvant therapy in patients with CSCC. This is supported by studies showing that higher levels of survivin expression are associated with treatment resistance in UCC patients treated with cisplatin, paclitaxel and radiotherapy (87, 88, 89). In addition, erythropoietin has been shown to promote survivin expression through activation of STAT3, which negatively affects the sensitivity of cisplatin to cervical cancer cells (90).
Resistance to apoptosis in UCC is often due to loss of TP53 function caused by HPV infection. It has been shown that the use of survivin inhibitors such as sepantronium bromide (YM155) or resveratrol (RSV), a polyphenol with the potential to suppress survivin expression, enhances tumor necrosis factor-related apoptosis-inducing (TRAIL) ligand induced apoptosis and overcome cisplatin resistance in UCC treatment (87). TRAIL induces apoptosis in a p53-independent manner. Survivin has also been suggested as a marker for CIN and high-risk human papillomavirus (HR-HPV) and as a predictor of HPV clearance and disease outcome in cervical cancer (91). Other survivin-related interactions reported in UCC include positive regulation of survivin promoter activity by Sp1 and Sp3, interaction with STAT3 and MDM2, stabilization by HSP90 through physical association, Bcl-2 and KAI1/CD82 proteins, and survivin phosphorylation at Thr34 (92, 93, 94). While extensive work is underway to explore the therapeutic potential of survivin in cancer, attempts are also being made to unravel its diagnostic value. Xue Yan et al. designed survivin-specific molecular beacons (MBs) linked to fluorescein isothiocyanate (FITC) and cyanine-3 (Cy3) dyes and used molecular beacon imaging to investigate the diagnostic potential of survivin. Molecular beacons are single-stranded oligonucleotide probes that form stem-and-loop structures. They found a sensitivity of 61.4% and a specificity of 72.8% (n = 44) for survivin MBs (95). Such findings suggest that survivin MBs may be specific and sensitive probes for the detection of cervical cancer cells. In addition to endometrial, ovarian and cervical cancers, survivin has also been implicated in other gynecological cancers.
2.4 Survivin signaling in other gynecological cancers
Data on survivin expression and signaling in vulvar squamous cell carcinoma (VSCC) are scarce, largely due to the rarity of the tumors. Most vulvar cancers are squamous cell carcinomas (90%) and a small percentage (10%) are vulvar melanomas. Squamous cell carcinomas are associated with higher morbidity and mortality because they are highly invasive to adjacent tissues and can metastasize to distant organs (96). There are currently only three published articles on the involvement of survivin in the development of vulvar cancer (29, 30, 97). Gene expression profiling of VSCC by Zhang T et al. found that among the 18 genes associated with the biological characteristics of VSCC, BIRC5 expression was positively and significantly associated with the clinical stage of the tumors (29). In their results, VEGF and MMP1 were also overexpressed, but did not correlate with any of the clinicopathological characteristics examined. Similarly, two other studies found an increase in nuclear survivin immuno-expression from normal epithelium and lichen sclerosus to high grade classic vulvar intraepithelial neoplasia, differentiated vulvar intraepithelial neoplasia and invasive keratinizing squamous cell carcinoma (p = 0.0001), suggesting its diagnostic and prognostic potential in VSCC (30, 97). Survivin has been proposed as a molecular target for therapeutic intervention in squamous cell carcinomas (96, 98). Survivin-targeted strategies currently under investigation in gynecological and other tumors include inhibitors of survivin gene transcription, homodimerization, survivin-partner protein interaction, mRNA inhibitors and survivin immunotherapy (Table 2).
Some of the more promising emerging survivin-targeted therapies in cancer include PZ-6-QN, brexpiprazole and SurVaxM. The small molecule PZ-6-QN has been tested in several cancer cell lines, including MCF-7 (breast cancer) and HCT-116 (colon cancer), and has been shown to inhibit the interaction between survivin and the second mitochondrial-derived activator of caspase (Smac) (102). This disruption increases the release of Smac and cytochrome-c from the mitochondria. Smac, a mitochondrial protein, binds to IAPs, neutralizing their inhibitory effect and promoting the activation of caspases, particularly caspase-9 in the cytochrome-c/Apaf-1/caspase-9 pathway, ultimately leading to cell death. Brexpiprazole and SurVaxM were evaluated for their effects on brain tumors cells. Brexpiprazole, a well-established atypical antipsychotic agent, has been shown to increase the sensitivity of glioma cells to the small molecule osimertinib by reducing survivin expression (111). In contrast, SurVaxM is an immunotherapeutic vaccine specifically designed to target survivin-expressing cancer cells. A phase IIa clinical trial demonstrated its significant efficacy in promoting anti-tumors immune responses against brain tumors by generating cytotoxic CD8+ and CD4+ T-cell responses, resulting in tumors regression and prevention of relapse (112). While these newer survivin-targeted strategies may also prove beneficial for patients with gynecological cancers, there are currently few clinical trials focusing on survivin-based therapies in gynecological cancers (Table 3).
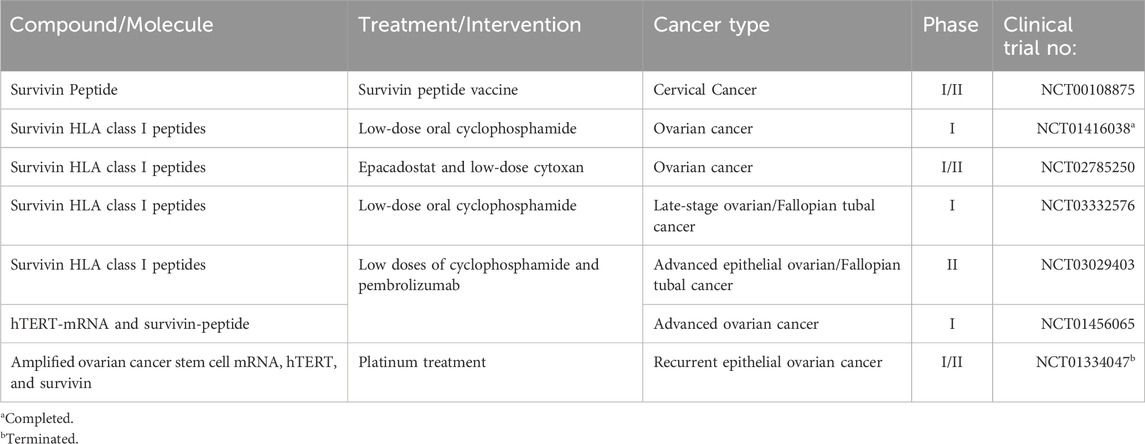
Table 3. Suvrivin-targeted therapies that are currently in clinical trials to evaluate their anti-tumor activity in the treatment of gynecological cancers.
3 Discussion
Survivin is an important multifaceted oncoprotein with tumor-promoting effects in several human cancers. Importantly, survivin plays a major role in cellular proliferation and progression of gynecological tumors. The tumor-promoting effects of survivin in EC and OC are largely dependent on spontaneous mutation of the PTEN and TP53 genes. In UCC, the tumorigenic effects depend on loss of TP53 function due to HPV infection and association with STAT3, MDM2, HSP90 and Sp1/Sp3. Survivin is also associated with resistance to chemoradiation and hormonal therapy in patients with gynecological tumors. Although survivin-based therapies have yet to reach the clinic, survivin inhibitors hold promise for patients with EC, OC, UCC and VSCC, even when they become resistant to standard therapy.
In conclusion, survivin exerts multiple effects in different types of gynecological cancers. Evidence suggests that various survivin-targeted therapeutic strategies such as inhibitors of survivin gene transcription, inhibitors of survivin homodimerization, drugs that disrupt survivin-partner protein interactions, survivin mRNA inhibitors and survivin-based immunotherapy, may be effective either alone or in combination with standard chemotherapy and/or radiotherapy. These approaches have the potential to promote tumor regression, enhance chemo- or radiosensitivity and prevent metastasis. Targeting survivin signaling may therefore improve outcomes for patients with gynecological cancers. However, further research is needed to better understand the role of survivin in the development and metastasis of these cancers, its involvement in resistance to standard therapies, and strategies to overcome such resistance. In particular, clinical trials targeting survivin signaling in gynecological cancer patients are urgently needed.
Author contributions
AC: Conceptualization, Writing–original draft. DM: Validation, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Altieri, DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene (2003) 22(53):8581–9. doi:10.1038/sj.onc.1207113
2. Ambrosini, GAC, Adida, C, and Altieri, DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med (1997) 3(8):917–21. doi:10.1038/nm0897-917
3. Mittal, R, Jaiswal, P, and Goel, A. Survivin: a molecular biomarker in cancer. Indian J Med Res (2015) 141(4):389–97. doi:10.4103/0971-5916.159250
4. Wheatley, SPMIA, and Mcneish, I. Survivin: a protein with dual roles in mitosis and apoptosis. Int Rev Cytol (2005) 247:35–88. doi:10.1016/s0074-7696(05)47002-3
5. Fant, XRS, and Ruchaud, S. An emerging function of survivin’s BIR domain phosphorylation in the control of cell division and cell death. Cell Cycle (2011) 10(6):879–8. doi:10.4161/cc.10.6.15156
6. Yamada, YKT, Kuroiwa, T, Nakagawa, T, Kajimoto, Y, Dohi, T, Azuma, H, et al. Transcriptional expression of survivin and its splice variants in brain tumors in humans. J Neurosurg (2003) 99:738–45. doi:10.3171/jns.2003.99.4.0738
7. Caldas, HHLE, Honsey, LE, and Altura, RA. Survivin 2α: a novel Survivin splice variant expressed in human malignancies. Mol Cancer (2005) 4(11):11. doi:10.1186/1476-4598-4-11
8. Warrier, NM, Agarwal, P, and Kumar, P. Emerging importance of survivin in stem cells and cancer: the development of new cancer therapeutics. Stem Cel Rev Rep (2020) 16(5):828–52. doi:10.1007/s12015-020-09995-4
9. Li, YLW, Lu, W, Yang, J, Edwards, M, and Jiang, S. Survivin as a biological biomarker for diagnosis and therapy. Expert Opin Biol Ther (2021) 21(11):1429–41. doi:10.1080/14712598.2021.1918672
10. Nasu, SYA, Yagihashi, A, Izawa, A, Saito, K, Asanuma, K, Nakamura, M, et al. Survivin mRNA expression in patients with breast cancer. Anticancer Res (2002) 22:1839–43.
11. Grabowski, PKT, Kühnel, T, Mühr-Wilkenshoff, F, Heine, B, Stein, H, Höpfner, M, et al. Prognostic value of nuclear survivin expression in oesophageal squamous cell carcinoma. Br J Cancer (2003) 88:115–9. doi:10.1038/sj.bjc.6600696
12. Yu, JLWK, Leung, WK, Ebert, MPA, Ng, EKW, Go, MYY, Wang, HB, et al. Increased expression of survivin in gastric cancer patients and in first-degree relatives. Br J Cancer (2002) 87:91–7. doi:10.1038/sj.bjc.6600421
13. Liu, HQWYH, Wang, YH, Wang, LL, and Hao, M. P16INK4A, and survivin: diagnostic and prognostic markers in cervical intraepithelial neoplasia and cervical squamous cell carcinoma. Exp Mol Pathol (2015) 99(1):44–9. doi:10.1016/j.yexmp.2015.04.004
14. Vaskivuo, TESF, Karhumaa, P, Risteli, J, Dunkel, L, and Tapanainen, JS. Apoptosis and apoptosis-related proteins in human endometrium. Mol Cell Endocrinol (2000) 165:75–83. doi:10.1016/s0303-7207(00)00261-6
15. Fukuda, SPLM, and Pelus, LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther (2006) 5:1087–98. doi:10.1158/1535-7163.mct-05-0375
16. Hongfa, L, Jing, Y, and Yongyu, S. Expression of survivin in early villus and decidua and its implication. Curr Med Sci (2002) 22(170):118–20. doi:10.1007/bf02857670
17. Ueda, MYY, Takehara, M, Terai, Y, Kumagai, K, Ueki, K, Kanda, K, et al. Survivin gene expression in endometriosis. J Clin Endocrinol Metab (2002) 87(7):3452–9. doi:10.1210/jcem.87.7.8682
18. Goteri, GLG, Lucarini, G, Pieramici, T, Filosa, A, Pugnaloni, A, Montik, N, et al. Endothelial cell survivin is involved in the growth of ovarian endometriotic cysts. Anticancer Res (2005) 25(6B):4313–8.
19. Chen, XZZ, Zhang, Z, Feng, Y, Fadare, O, Wang, J, Ai, Z, et al. Aberrant survivin expression in endometrial hyperplasia: another mechanism of progestin resistance. Mod Pathol (2009) 22:699–708. doi:10.1038/modpathol.2009.25
20. Lehner, R, Enomoto, T, McGregor, JA, Shroyer, L, Haugen, BR, Pugazhenthi, U, et al. Correlation of survivin mRNA detection with histologic diagnosis in normal endometrium and endometrial carcinoma. Acta Obstetrica Gynecologica Scand (2002) 81(2):162–7. doi:10.1034/j.1600-0412.2002.810213.x
21. Garcia, MGT-GI, Tirado-Gonzalez, I, Handjiski, B, Tometten, M, Orsal, AS, Hajos, SE, et al. High expression of survivin and down-regulation of Stat-3 characterize the feto-maternal interface in failing murine pregnancies during the implantation period. Placenta (2007) 28(7):650–7. doi:10.1016/j.placenta.2006.09.010
22. Fest, SBN, Brachwitz, N, Schumacher, A, Zenclussen, ML, Khan, F, Wafula, PO, et al. Supporting the hypothesis of pregnancy as a tumor: survivin is upregulated in normal pregnant mice and participates in human trophoblast proliferation. Am J Reprod Immunol (2008) 59(1):75–83. doi:10.1111/j.1600-0897.2007.00557.x
23. Li, F, Devi, YS, Bao, L, Mao, J, and Gibori, G. Involvement of cyclin D3, CDKN1A (P21), and BIRC5 (survivin) in interleukin 11 stimulation of decidualization in mice. Biol Reprod (2008) 78(1):127–33. doi:10.1095/biolreprod.107.063313
24. Winship, A, Van Sinderen, M, Rainczuk, K, and Dimitriadis, E. Therapeutically blocking interleukin-11 receptor-α enhances doxorubicin cytotoxicity in high grade type I endometrioid tumours. Oncotarget (2017) 8(14):22716–29. doi:10.18632/oncotarget.15187
25. Stavropoulos, AVM, Varras, M, Vasilakaki, T, Varra, V, Varra, F, Tsavari, A, et al. Expression of anti-apoptotic protein survivin in human endometrial carcinoma: clinical and pathological associations as a separate factor and in combination with concomitant PTEN and p53 expression. Oncol Lett (2020) 20(2):1033–54. doi:10.3892/ol.2020.11690
26. Chuwa, AHKS, Sone, K, Oda, K, Ikeda, Y, Fukuda, T, Wada-Hiraike, O, et al. Significance of survivin as a prognostic factor and a therapeutic target in endometrial cancer. Gynecol Oncol (2016) 141(3):564–9. doi:10.1016/j.ygyno.2016.04.003
27. Cortez, AJTP, Tudrej, P, Kujawa, KA, and Lisowska, KM. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol (2018) 81(1):17–38. doi:10.1007/s00280-017-3501-8
28. Batlle, EMJ, and Massagué, J. Transforming growth factor-β signaling in immunity and cancer. Immunity (2019) 50(4):924–40. doi:10.1016/j.immuni.2019.03.024
29. Zhang, TLQ, Liu, Q, Yu, M, Lan, Y, and Zhou, J. Expression profiles reveal involvement of VEGF, IGF1, BIRC5, and MMP1 in vulvar carcinogenesis. Technology Cancer Res and Treat (2021) 20:15330338211004922. doi:10.1177/15330338211004922
30. Brustmann, HHS, and Brunner, A. Immunohistochemical expression of survivin and γ-H2AX in vulvar intraepithelial neoplasia and low-stage squamous cell carcinoma. Int J Gynecol patholog (2011) 30(6):583–90. doi:10.1097/PGP.0b013e31821e18fd
31. Zhou, JGX, Guo, X, Chen, W, Wang, L, and Jin, Y. Targeting survivin sensitizes cervical cancer cells to radiation treatment. Bioengineered (2020) 11(1):130–40. doi:10.1080/21655979.2020.1717297
32. Liu, YHT, Hua, T, Chi, S, and Wang, H. Identification of key pathways and genes in endometrial cancer using bioinformatics analyses. Oncol Lett (2019) 17(1):897–906. doi:10.3892/ol.2018.9667
33. Erkanli, SBF, Bolat, F, Kayaselcuk, F, Demirhan, B, and Kuscu, E. COX-2 and survivin are overexpressed and positively correlated in endometrial carcinoma. Gynecol Oncol (2007) 104(2):320–5. doi:10.1016/j.ygyno.2006.08.044
34. Liu, LLJ, Lin, J, and He, H. Identification of potential crucial genes associated with the pathogenesis and prognosis of endometrial cancer. Front Genet (2019) 10(373):373. doi:10.3389/fgene.2019.00373
35. Huo, XSH, Sun, H, Liu, Q, Ma, X, Peng, P, Yu, M, et al. Clinical and expression significance of AKT1 by Co-expression network analysis in endometrial cancer. Front Oncol (2019) 9(1147):1147. doi:10.3389/fonc.2019.01147
36. Downes, CPBD, Bennett, D, McConnachie, G, Leslie, N, Pass, I, MacPhee, C, et al. Antagonism of PI 3-kinase-dependent signalling pathways by the tumour suppressor protein, PTEN. Biochem Soc Trans (2001) 29(Pt 6):846–51. doi:10.1042/0300-5127:0290846
37. Maehama, TDJE. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biology (1999) 9(4):125–8. doi:10.1016/s0962-8924(99)01519-6
38. Cheung, LWHBT, Li, J, Yu, S, Myers, AP, Djordjevic, B, Lu, Y, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer discover (2011) 1(2):170–85. doi:10.1158/2159-8290.CD-11-0039
39. Erkanli, SKF, Kuscu, E, Bagis, T, Bolat, F, Haberal, A, and Demirhan, B. Expression of survivin, PTEN and p27 in normal, hyperplastic, and carcinomatous endometrium. Int J Gynecol cance (2006) 16(3):1412–8. doi:10.1111/j.1525-1438.2006.00541.x
40. Guha, MPJ, Plescia, J, Leav, I, Li, J, Languino, LR, and Altieri, DC. Endogenous tumor suppression mediated by PTEN involves survivin gene silencing. Cancer Res (2009) 69(12):4954–8. doi:10.1158/0008-5472.can-09-0584
41. Bussaglia, E, Delrio, E, Matiasguiu, X, and Prat, J. PTEN mutations in endometrial carcinomas: a molecular and clinicopathologic analysis of 38 cases. Hum Pathol (2000) 31(3):312–7. doi:10.1016/s0046-8177(00)80244-0
42. Gbelcová, HGL, Gergely, L, Šišovský, V, Straka, Ľ, Böhmer, D, Pastoráková, A, et al. PTEN mutations as predictive marker for the high-grade endometrial cancer development in Slovak women. Physiol Res (2022) 71(Suppl. 1):S125–S135. doi:10.33549/physiolres.935030
43. Nabilsi, NHBRR, Broaddus, RR, and Loose, DS. DNA methylation inhibits p53-mediated survivin repression. Oncogene (2009) 28(19):2046–50. doi:10.1038/onc.2009.62
44. Zahedi, P, Aminimoghaddam, S, Sayahpour, FA, Haghpanah, V, Amiri, P, Fereidoni, F, et al. Association of survivin gene polymorphism with endometrial cancer. Int J Gynecol Cancer (2012) 22(1):35–7. doi:10.1097/igc.0b013e318229902c
45. Fischer, M, Quaas, M, Nickel, A, and Engeland, K. Indirect p53-dependent transcriptional repression of Survivin, CDC25C, and PLK1 genes requires the cyclin-dependent kinase inhibitor p21/CDKN1A and CDE/CHR promoter sites binding the DREAM complex. Oncotarget (2015) 6(39):41402–17. doi:10.18632/oncotarget.6356
46. Chang, HL, Chen, CY, Hsu, YF, Kuo, WS, Ou, G, Chiu, PT, et al. Simvastatin induced HCT116 colorectal cancer cell apoptosis through p38MAPK-p53-survivin signaling cascade. Biochim Biophys Acta (BBA) - Gen Subjects (2013) 1830(8):4053–64. doi:10.1016/j.bbagen.2013.04.011
47. Zheng, W, Xiang, L, Fadare, O, and Kong, B. A proposed model for endometrial serous carcinogenesis. Am J Surg Pathol (2011) 35(1):e1–e14. doi:10.1097/pas.0b013e318202772e
48. Chang, YWKH, Kuo, HL, Chen, TC, Chen, J, Lim, L, Wang, KL, et al. Abnormal p53 expression is associated with poor outcomes in grade I or II, stage I, endometrioid carcinoma: a retrospective single-institute study. J Gynecol Oncol (2024) 35(6):e78. doi:10.3802/jgo.2024.35.e78
49. Oprić, D, Suskic, A, Šuškić, SH, Nikolić, G, and Filipović, I. Value of p53 and estrogen receptors immunohistochemical staining in endometrial carcinoma. Int J Reprod contraception, Obstet Gynecol (2019) 8(12):4885–5. doi:10.18203/2320-1770.ijrcog20195339
50. Ballester, MCG, Canlorbe, G, Cortez, A, Gonin, J, Laas, E, Bendifallah, S, et al. Histological and immunohistochemical profiles predict lymph node status in women with low-intermediate risk endometrial cancer. Gynecol Oncol (2013) 130(3):457–62. doi:10.1016/j.ygyno.2013.06.001
51. Urabe, R, Hachisuga, T, Kurita, T, Kagami, S, Kawagoe, T, Matsuura, Y, et al. Prognostic significance of overexpression of p53 in uterine endometrioid adenocarcinomas with an analysis of nuclear grade. J Obstet Gynaecol Res (2014) 40(3):812–9. doi:10.1111/jog.12215
52. Stavropoulos, A, Varras, M, Vasilakaki, T, Varra, VK, Tsavari, A, Varra, FN, et al. Expression of p53 and PTEN in human primary endometrial carcinomas: clinicopathological and immunohistochemical analysis and study of their concomitant expression. Oncol Lett (2019) 17(5):4575–89. doi:10.3892/ol.2019.10093
53. Hameed J S, F, Devarajan, A, Devu Priya, MS, Bhattacharyya, A, Shirude, MB, Dutta, D, et al. PTEN-negative endometrial cancer cells protect their genome through enhanced DDB2 expression associated with augmented nucleotide excision repair. BMC Cancer (2023) 23(1):399. doi:10.1186/s12885-023-10892-5
54. Yang, YSFW, and Bao, W. Molecular subtypes of endometrial cancer: implications for adjuvant treatment strategies. Int J Gynaecol Obstet (2023) 162(2):436–59. doi:10.1002/ijgo.14969
55. Gu, JJZ, Ji, Z, Li, D, and Dong, Q. Proliferation inhibition and apoptosis promotion by dual silencing of VEGF and Survivin in human osteosarcoma. Acta Biochim Biophys Sinica (2018) 51(1):59–67. doi:10.1093/abbs/gmy146
56. Garrido, MPTI, Vega, M, and Romero, C. Angiogenesis in gynecological cancers: role of neurotrophins. Front Oncol (2019) 19(9). doi:10.3389/fonc.2019.00913
57. Dong, JHL, He, L, Wang, Y, Yu, F, Yu, S, Liu, L, et al. A highly sensitive colorimetric aptasensor for the detection of the vascular endothelial growth factor in human serum. Spectrochimica Acta A: Mol Biomol Spectrosc (2020) 226:117622. doi:10.1016/j.saa.2019.117622
58. Yao, YZX, Liu, L, Chen, H, and He, W. Coexpression of Survivin+/VEGF+ indicates poor prognosis of endometrial cancer. Curr Trends Intern Med (2020) 4:141. doi:10.29011/2638-003x.100041
59. Fan, R, Wang, Y, Wang, Y, Wei, L, and Zheng, W. Mechanism of progestin resistance in endometrial precancer/cancer through Nrf2-survivin pathway. Am J translational Res (2017) 9(3):1483–91.
60. Chuwa, AH, Sone, K, Oda, K, Tanikawa, M, Kukita, A, Kojima, M, et al. Kaempferol, a natural dietary flavonoid, suppresses 17β-estradiol-induced survivin expression and causes apoptotic cell death in endometrial cancer. Oncol Lett (2018) 16(5):6195–201. doi:10.3892/ol.2018.9340
61. Ramalingam, P. Morphologic, immunophenotypic, and molecular features of epithelial ovarian cancer. Oncology (Williston Park, NY) (2016) 30(2):166–76.
62. Shazly, SA, Laughlin-Tommaso, SK, Dowdy, SC, and Famuyide, AO. Staging for low malignant potential ovarian tumors: a global perspective. Am J Obstet Gynecol (2016) 215(2):153–68.e2. doi:10.1016/j.ajog.2016.04.035
63. Cohen, C, Lohmann, CM, Cotsonis, G, Lawson, D, and Santoianni, R. Survivin expression in ovarian carcinoma: correlation with apoptotic markers and prognosis. Mod Pathol (2003) 16(6):574–83. doi:10.1097/01.mp.0000073868.31297.b0
64. Gąsowska-Bajger, B, Gąsowska-Bodnar, A, Knapp, P, and Bodnar, L. Prognostic significance of survivin expression in patients with ovarian carcinoma: a meta-analysis. J Clin Med (2021) 10(4):879. doi:10.3390/jcm10040879
65. Liguang, ZPL, Peishu, L, Hongluan, M, Hong, J, Rong, W, Wachtel, M, et al. Survivin expression in ovarian cancer. Exp Oncol (2007) 29(2):121–5.
66. Lin, CK, Chao, TK, Yu, CP, Yu, MH, and Jin, JS. The expression of six biomarkers in the four most common ovarian cancers: correlation with clinicopathological parameters. APMIS (2009) 117(3):162–75. doi:10.1111/j.1600-0463.2008.00003.x
67. He, X, Yang, K, Wang, H, Chen, X, Wu, H, Yao, L, et al. Expression and clinical significance of survivin in ovarian cancer: a meta-analysis. PloS one (2018) 13(5):e0194463. doi:10.1371/journal.pone.0194463
68. Dewi, IGASM, and Sriwidyani, NP. Survivin expression in ovarian carcinoma and its role as apoptotic inhibitor and prognostic predictor. Indonesia J Biomed Sci (2024) 18(1):70–6. doi:10.15562/ijbs.v18i1.532
69. Nan, XW, Gong, LH, Chen, X, Zhou, HH, Ye, PP, Yang, Y, et al. Survivin promotes piperlongumine resistance in ovarian cancer. Front Oncol (2019) 9(9):1345. doi:10.3389/fonc.2019.01345
70. Zhao, G, Wang, Q, Wu, Z, Tian, X, Yan, H, Wang, B, et al. Ovarian primary and metastatic tumors suppressed by survivin knockout or a novel survivin inhibitor. Mol Cancer Ther (2019) 18(12):2233–45. doi:10.1158/1535-7163.mct-19-0118
71. Zhao, G, Wang, Q, Gu, Q, Qiang, W, Wei, JJ, Dong, P, et al. Lentiviral CRISPR/Cas9 nickase vector mediated BIRC5 editing inhibits epithelial to mesenchymal transition in ovarian cancer cells. Oncotarget (2017) 8(55):94666–80. doi:10.18632/oncotarget.21863
72. Roh, MH, Yassin, Y, Miron, A, Mehra, KK, Mehrad, M, Monte, NM, et al. High-grade fimbrial-ovarian carcinomas are unified by altered p53, PTEN and PAX2 expression. Mod Pathol (2010) 23(10):1316–24. doi:10.1038/modpathol.2010.119
73. Martins, FCCD, Couturier, DL, Paterson, A, Karnezis, AN, Chow, C, Nazeran, TM, et al. Clinical and pathological associations of PTEN expression in ovarian cancer: a multicentre study from the Ovarian Tumour Tissue Analysis Consortium. Br J Cancer (2020) 123(5):793–802. doi:10.1038/s41416-020-0900-0
74. Tanwar, PS, Mohapatra, G, Chiang, S, Engler, DA, Zhang, LH, Kaneko-Tarui, T, et al. Loss of LKB1 and PTEN tumor suppressor genes in the ovarian surface epithelium induces papillary serous ovarian cancer. Carcinogenesis (2014) 35(3):546–53. doi:10.1093/carcin/bgt357
75. Sui, LDY, Dong, Y, Watanabe, Y, Yamaguchi, F, Sugimoto, K, and Tokuda, M. Alteration and clinical relevance of PTEN expression and its correlation with survivin expression in epithelial ovarian tumors. Oncol Rep (2006) 15(4):773–8. doi:10.3892/or.15.4.773
76. Guha, MXF, Xia, F, Raskett, CM, and Altieri, DC. Caspase 2-mediated tumor suppression involves survivin gene silencing. Oncogene (2010) 29(9):1280–92. doi:10.1038/onc.2009.428
77. Sankpal, UT, Ingersoll, SB, Ahmad, S, Holloway, RW, Bhat, VB, Simecka, JW, et al. Association of Sp1 and survivin in epithelial ovarian cancer: Sp1 inhibitor and cisplatin, a novel combination for inhibiting epithelial ovarian cancer cell proliferation. Tumor Biol (2016) 37(10):14259–69. doi:10.1007/s13277-016-5290-9
78. Du, J, Li, B, Fang, Y, Liu, Y, Wang, Y, Li, J, et al. Overexpression of Class III β-tubulin, Sox2, and nuclear Survivin is predictive of taxane resistance in patients with stage III ovarian epithelial cancer. BMC cancer (2015) 15:536. doi:10.1186/s12885-015-1553-x
79. Vivas-Mejia, PE, Rodriguez-Aguayo, C, Han, HD, Shahzad, MM, Valiyeva, F, Shibayama, M, et al. Silencing survivin splice variant 2B leads to antitumor activity in taxane--resistant ovarian cancer. Clin Cancer Res (2011) 17(11):3716–26. doi:10.1158/1078-0432.ccr-11-0233
80. Zhou, XL, and Wang, M. Expression levels of survivin, Bcl-2, and KAI1 proteins in cervical cancer and their correlation with metastasis. Genet Mol Res (2015) 14(4):17059–67. doi:10.4238/2015.december.16.6
81. Fan, Y, and Chen, J. Clinicopathological significance of survivin expression in patients with cervical cancer: a systematic meta-analysis. Bioengineered (2017) 8(5):511–23. doi:10.1080/21655979.2016.1252879
82. Xu, H, Liang, T, Yang, Y, Dong, Y, and Zhu, L. Antisense of survivin inhibits cervical cancer growth in mice. Arch Med Sci (2019) 15(5):1345–51. doi:10.5114/aoms.2017.71069
83. Cheng, KY, Wang, ZL, Gu, QY, and Hao, M. Survivin overexpression is associated with aggressive clinicopathological features in cervical carcinoma: a meta-analysis. PloS one (2016) 11(10):e0165117. doi:10.1371/journal.pone.0165117
84. Zhou, WQ, Sheng, QY, Sheng, YH, Hou, WJ, Xu, GX, Wu, YM, et al. Expressions of survivin, P16INK4a, COX-2, and Ki-67 in cervical cancer progression reveal the potential clinical application. Eur J Gynaecol Oncol (2015) 36(1):62–8.
85. Portari, EA, Russomano, FB, de Camargo, MJ, Machado Gayer, CR, da Rocha Guillobel, HC, Santos-Rebouças, CB, et al. Immunohistochemical expression of cyclin D1, p16Ink4a, p21WAF1, and Ki-67 correlates with the severity of cervical neoplasia. Int J Gynecol Pathol (2013) 32(5):501–8. doi:10.1097/pgp.0b013e31826f5cf6
86. Zhang, Y, Yan, H, Li, R, Guo, Y, and Zheng, R. High expression of survivin predicts poor prognosis in cervical squamous cell carcinoma treated with paclitaxel and carboplatin. Medicine (2019) 98(20):e15607. doi:10.1097/md.0000000000015607
87. Nakamura, H, Taguchi, A, Kawana, K, Baba, S, Kawata, A, Yoshida, M, et al. Therapeutic significance of targeting survivin in cervical cancer and possibility of combination therapy with TRAIL. Oncotarget (2018) 9(17):13451–61. doi:10.18632/oncotarget.24413
88. Yu, M, Xu, B, Yang, H, Xue, S, Zhang, R, Zhang, H, et al. MicroRNA-218 regulates the chemo-sensitivity of cervical cancer cells through targeting survivin. Cancer Manag Res (2019) 11:6511–9. doi:10.2147/cmar.s199659
89. Gu, F, Li, L, Yuan, QF, Li, C, and Li, ZH. Down-regulation of survivin enhances paclitaxel-induced Hela cell apoptosis. Eur Rev Med Pharmacol Sci (2017) 21(15):3504–9.
90. Vázquez-Mellado, MJ, Cortés-Ballinas, LG, Blanco-Flores, IC, Aguilar, C, Vázquez-Gómez, G, and Rocha-Zavaleta, L. Erythropoietin promotes expression of survivin via STAT3 activation and reduces sensitivity to cisplatin in cervical cancer cells. Oncol Rep (2019) 41(2):1333–41. doi:10.3892/or.2018.6890
91. Demir, F, Kimiloglu, E, Igdem, AA, Ayanoglu, YT, and Erdogan, N. High risk HPV in situ hybridization, p16 INK 4A, and survivin expressions in cervical carcinomas and intraepithelial neoplasms: evaluation of prognostic factors. Eur J Gynaecol Oncol (2014) 35(6):708–17.
92. Xu, R, Zhang, P, Huang, J, Ge, S, Lu, J, and Qian, G. Sp1 and Sp3 regulate basal transcription of the survivin gene. Biochem biophysical Res Commun (2007) 356(1):286–92. doi:10.1016/j.bbrc.2007.02.140
93. Chen, X, Duan, N, Zhang, C, and Zhang, W. Survivin and tumorigenesis: molecular mechanisms and therapeutic strategies. J Cancer (2016) 7(3):314–23. doi:10.7150/jca.13332
94. O'Connor, DS, Grossman, D, Plescia, J, Li, F, Zhang, H, Villa, A, et al. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA (2000) 97(24):13103–7. doi:10.1073/pnas.240390697
95. Xue, Y, An, R, Zhang, D, Zhao, J, Wang, X, Yang, L, et al. Detection of survivin expression in cervical cancer cells using molecular beacon imaging: new strategy for the diagnosis of cervical cancer. Eur J Obstet Gynecol Reprod Biol (2011) 159(1):204–8. doi:10.1016/j.ejogrb.2011.06.038
96. Khan, Z, Khan, AA, Yadav, H, Prasad, GBKS, and Bisen, PS. Survivin, a molecular target for therapeutic interventions in squamous cell carcinoma. Cell and Mol Biol Lett (2017) 22(1):8. doi:10.1186/s11658-017-0038-0
97. Wellenhofer, A, and Brustmann, H. Expression of human telomerase reverse transcriptase in vulvar intraepithelial neoplasia and squamous cell carcinoma: an immunohistochemical study with survivin and p53. Arch Pathol and Lab Med (2012) 136(11):1359–65. doi:10.5858/arpa.2011-0440-oa
98. Santarelli, AMM, Mascitti, M, Lo Russo, L, Sartini, D, Troiano, G, Emanuelli, M, et al. Survivin-based treatment strategies for squamous cell carcinoma. Int J Mol Sci (2018) 19(4):971. doi:10.3390/ijms19040971
99. Plescia, J, Salz, W, Xia, F, Pennati, M, Zaffaroni, N, Daidone, MG, et al. Rational design of shepherdin, a novel anticancer agent. Cancer cell (2005) 7(5):457–68. doi:10.1016/j.ccr.2005.03.035
100. Meli, M, Pennati, M, Curto, M, Daidone, MG, Plescia, J, Toba, S, et al. Small-molecule targeting of heat shock protein 90 chaperone function: rational identification of a new anticancer lead. J Med Chem (2006) 49(26):7721–30. doi:10.1021/jm060836y
101. Wadegaonkar, VP, and Wadegaonkar, PA. Withanone as an inhibitor of survivin: a potential drug candidate for cancer therapy. J Biotechnol (2013) 168(2):229–33. doi:10.1016/j.jbiotec.2013.08.028
102. Park, SH, Shin, I, Park, SH, Kim, ND, and Shin, I. An inhibitor of the interaction of survivin with smac in mitochondria promotes apoptosis. Chem Asian J (2019) 14(22):4035–41. doi:10.1002/asia.201900587
103. Berezov, A, Cai, Z, Freudenberg, JA, Zhang, H, Cheng, X, Thompson, T, et al. Disabling the mitotic spindle and tumor growth by targeting a cavity-induced allosteric site of survivin. Oncogene (2012) 31(15):1938–48. doi:10.1038/onc.2011.377
104. Qi, J, Dong, Z, Liu, J, Peery, RC, Zhang, S, Liu, JY, et al. Effective targeting of the survivin dimerization interface with small-molecule inhibitors. Cancer Res (2016) 76(2):453–62. doi:10.1158/0008-5472.can-15-1874
105. Nakahara, T, Takeuchi, M, Kinoyama, I, Minematsu, T, Shirasuna, K, Matsuhisa, A, et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res (2007) 67(17):8014–21. doi:10.1158/0008-5472.can-07-1343
106. Li, F. Anticancer drug FL118 is more than a survivin inhibitor: where is the Achilles’ heel of cancer? Am J Cancer Res (2014) 4(3):30411.
107. Felix, S, Sandjo, LP, Opatz, T, and Erkel, G. SF002-96-1, a new drimane sesquiterpene lactone from an Aspergillus species, inhibits survivin expression. Beilstein J Org Chem (2013) 9:2866–76. doi:10.3762/bjoc.9.323
108. Carrasco, RA, Stamm, NB, Marcusson, E, Sandusky, G, Iversen, P, and Patel, BK. Antisense inhibition of survivin expression as a cancer therapeutic. Mol Cancer Ther (2011) 10(2):221–32. doi:10.1158/1535-7163.mct-10-0756
109. Miyazaki, A, Kobayashi, J, Torigoe, T, Hirohashi, Y, Yamamoto, T, Yamaguchi, A, et al. Phase I clinical trial of survivin-derived peptide vaccine therapy for patients with advanced or recurrent oral cancer. Cancer Sci (2011) 102(2):324–9. doi:10.1111/j.1349-7006.2010.01789.x
110. Fenstermaker, RA, Ciesielski, MJ, Qiu, J, Yang, N, Frank, CL, Lee, KP, et al. Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immunol Immunother (2016) 65(11):1339–52. doi:10.1007/s00262-016-1890-x
111. Suzuki, S, Yamamoto, M, Sanomachi, T, Togashi, K, Sugai, A, Seino, S, et al. Brexpiprazole, a serotonin-dopamine activity modulator, can sensitize glioma stem cells to osimertinib, a third-generation EGFR-TKI, via survivin reduction. Cancers. (2019) 11(7):947. doi:10.3390/cancers11070947
Keywords: survivin, BIRC5, gynecological cancer, chemoresistance, targeted therapy
Citation: Chuwa AH and Mvunta DH (2024) Prognostic and clinicopathological significance of survivin in gynecological cancer. Oncol. Rev. 18:1444008. doi: 10.3389/or.2024.1444008
Received: 04 June 2024; Accepted: 20 November 2024;
Published: 02 December 2024.
Edited by:
Ram N. Ganapathi, Cleveland Clinic, United StatesReviewed by:
Lara Hilal, American University of Beirut Medical Center, LebanonLing Ding, University of Pittsburgh Medical Center, United States
Copyright © 2024 Chuwa and Mvunta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Agapiti H. Chuwa, Y2h1d2EuYWdhcGl0aUB1ZHNtLmFjLnR6
†ORCID: Agapiti H. Chuwa, orcid.org/0000-0001-7325-1167; David H. Mvunta, orcid.org/0000-0003-1729-9523
 Agapiti H. Chuwa
Agapiti H. Chuwa David H. Mvunta2,3†
David H. Mvunta2,3†