- 1Faculty of Nursing, College of Health Sciences, Third Floor Edmonton, Clinic Health Academy, University of Alberta, Edmonton, AB, Canada
- 2Cancer Strategic Clinical Network, Cancer Care Alberta, Alberta Health Services, Foothills Medical Centre, South Tower, Calgary, AB, Canada
- 3School of Public Health, Edmonton Clinic Health Academy, University of Alberta, Edmonton, AB, Canada
- 4Cancer Strategic Clinical Network, Cancer Care Alberta, Alberta Health Services, Edmonton, AB, Canada
- 5Cancer Research and Analytics, Cancer Care Alberta, Alberta Health Services, Edmonton, AB, Canada
- 6Geoffrey and Robyn Sperber Health Sciences Library, 1-150M Edmonton Clinic Health Academy, University of Alberta, Edmonton, AB, Canada
Background: Underserved populations both globally and in Canada face serious cancer inequities that result from systemic economic, environmental, and social conditions. These pose barriers in access to cancer care and lead to suboptimal cancer care experiences and outcomes. Knowledge of effective interventions to improve access to cancer care is needed to inform the design of tailored interventions for these populations.
Aim: To identify interventions and programs to improve access to cancer care for underserved populations in high income countries with universal health coverage (UHC) and the United States (US) throughout the cancer care continuum.
Methods: We conducted a systematic review following the PRISMA standards. We searched Medline, EMBASE, PsycINFO, CINAHL, Scopus, and the Cochrane Library. Inclusion criteria: quantitative and qualitative studies published in English in the last 10 years (2013–2023), describing interventions/programs to improve access to cancer care for underserved populations (18 years and over). We included studies in the US given the body of scholarship on equity in cancer care in that country. Screening, data extraction and analysis were undertaken by two independent reviewers.
Results: Our search yielded 7,549 articles, and 74 met the inclusion criteria. Of these, 56 were conducted in the US, 8 in Australia, 6 in Canada, and 4 in the United Kingdom. Most (90.5%) were quantitative studies and 47.3% were published between 2020–2023. Seven types of interventions were identified: patient navigation, education and counselling, virtual health, service redesign, financial support, improving geographical accessibility and multicomponent interventions. Interventions were mainly designed to mitigate language, distance, financial, lack of knowledge and cultural barriers. Most interventions focused on access to cancer screening, targeted rural populations, racialized groups and people with low socioeconomic status, and were conducted in community-based settings. The majority of interventions or programs significantly improved access to cancer care.
Conclusion: Our systematic review findings suggest that interventions designed to remove specific barriers faced by underserved populations can improve access to cancer care. Few studies came from countries with UHC. Research is required to understand tailored interventions for underserved populations in countries with UHC.
Introduction
Cancer is among the leading causes of death globally (1). In 2020, there were 19.3 million new cancer cases and 10 million cancer deaths in the world (2). In Canada, cancer is the leading cause of death representing 28.2% of all deaths in 2022 (3). In 2023, 239,100 Canadians were expected to have a cancer diagnosis while 86,700 were expected to die of cancer (4). Two in five Canadians are likely to develop cancer in their lifetime and one in four Canadians are likely to die of cancer (5). Advances in cancer care in Canada have resulted in prolonged survival for some cancers (6). Increases in cancer survival rates are reported for many cancers and across Canadian provinces (4). The 5-year cancer survival index (CSI), an indicator of cancer survival for all cancers, grew 8.4 percentage points from 1992 to 1994 to the 2015–2017 period, nearing 64% (4). Similarly, although the number of cancer deaths has increased, mostly due to population growth and aging, cancer mortality rates have decreased 39% in males and 26% in females since 1988 (4).
Disparities in cancer care represent a significant global challenge (1). In the United States (US), disparities in cancer survival and mortality affecting Blacks and Latino populations are reported (7–9). The lack of universal health coverage (UHC) is associated with inequalities in access to healthcare (10). However, health inequalities in countries with UHC exist (11, 12). In Canada, a country with UHC, progress in cancer care has not been equal for all Canadians. Late cancer diagnoses (13) and lower survival rates are among cancer disparities affecting underserved populations (14, 15). These outcomes are associated with inequalities in access to screening, diagnosis, curative treatment, survivorship care, and palliative care (14, 16, 17). Underserved populations may be overrepresented among those with a late cancer diagnosis (18, 19). For example, incidence rates of lung cancer, the main cause of cancer deaths in Canada, are 1.7 times higher in Canadians living in low-income areas than those living in high-income areas (20). Canadians living with low income also are less likely to receive curative treatment even when diagnosed at an earlier stage (21, 22). The highest rates of lung and colorectal cancer prevalence—the first and second leading causes of cancer deaths in Canada, respectively–were found among people from the lowest income quintiles (6) Cancer disparities affecting rural (13), remote (23), and immigrant populations also exist (13, 24–26). Studies report disparities in breast cancer screening and diagnosis for immigrant women (24–26). Higher rates of mastectomies were reported for women living in rural and remote areas and those with longer travel distances to radiation treatment centres than women living in urban areas and those living closer to radiation treatment centres (14).
Universal health coverage entails the provision of high quality health services, access to high quality health services, and financial risk protection for people who need to use these services (27). The commitment of world leaders to achieve UHC is seen in target 3.8 of the United Nations Sustainable Development Goal #3 “Ensure healthy lives and promote wellbeing for all at all ages” (28). Canada ensures UHC for all Canadians for medically essential hospital, physician, and diagnostic services (29). This entails the provision of care at no charge for these services (29). However, systemic barriers associated with income, race and ethnicity, Indigenous identity, immigration status, geographical location, gender identity, and language, among others, contribute to inequities in access to healthcare (29).
In 2023, the Canadian Cancer Society issued a report identifying 10 underserved communities experiencing systematic disadvantage and barriers in access to cancer information and services as a result of their racial background, gender identity, sexual orientation, geographical location, socioeconomic status, or language, among others (30). In Alberta, the home province of the study authors, low uptake of cancer screening services was identified for low income, rural and remote, gender diverse, and Indigenous populations in this province (31). Studies report successful interventions to support access to cancer care in underserved groups such as comprehensive interprofessional care, intersectoral collaboration, community engagement, empowerment, consultation services, and patient navigation (32–36). However, evidence concerning the types of interventions needed to improve access to cancer care in underserved populations in high income countries with UHC is limited.
To our knowledge, no systematic review of interventions in countries with UHC has been conducted. Increasing our understanding of these interventions and their impact could help inform strategies to improve cancer equity and outcomes in underserved populations in Alberta and Canada (37). In collaboration with Cancer Care Alberta stakeholders (APB, PR), we designed a systematic review of interventions to improve access to cancer care throughout the cancer care continuum in underserved populations in high income countries with UHC, with a view to inform the design of tailored interventions in our province. We included studies from the US considering scholarship in the area in this country and policy changes resulting in increased access to healthcare for underserved communities (10).
Methods
Population, interventions, comparison group and outcome
The population for this study was adult patients (18 years and older) diagnosed with cancer at any stage from underserved populations in high income countries with UHC and the US. Studies with Indigenous peoples were excluded.
The interventions of interest for this review were any intervention with the goal to increase access to cancer care along the cancer care continuum (38, 39). We included formal evaluations of programs that had the same goal. Interventions that addressed access to healthcare dimensions (40), such as availability and accommodation, approachability, accessibility, acceptability, and affordability of services were included.
Data sources and search strategy
Our review is reported in adherence to the PRISMA statement, the PRISMA for Searching (PRISMA-S) extension (41). Methodological guidance was taken from the Cochrane Handbook for Systematic Reviews of Interventions (42).
In order to identify all relevant published studies, a comprehensive, systematic search was conducted by a health sciences librarian (MK) familiar with systematic review methodology. Searches were conducted using the following bibliographic databases on 10 May 2023: Medline, EMBASE, and PsycINFO via OVID; Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost; Scopus; and the Cochrane Library via Wiley. All databases were searched from inception to present. The search strategy was derived from three main concepts: 1) Vulnerable populations including people living in rural communities, people with intellectual disabilities, people with physical disabilities or mobility problems, people with lower socioeconomic status, and people from racially marginalized groups; 2) Cancer care including treatment, management, and surgical care; 3) Access to healthcare services or health services accessibility. The search strategies for each database were constructed using a combination of natural language keywords and subject headings, such as MeSH, wherever they were available. Results were focused geographically on Canada, Australia, New Zealand, the United Kingdom, Scandinavian nations, and the United States. Limits of English language and publication date 2013–2023 were applied. We limited the search to the last 10 years to ensure review feasibility. Randomized controlled trials, controlled trials, before-and-after studies and interrupted time series (with or without control) and observational, qualitative, or mixed methods were included. Additionally, publication types of case reports, comments, letters, editorials, conference materials, and news items were removed from the results. See Supplementary Appendix 1 for full search strategies for each database.
Results were exported in complete batches from the databases on 10 May 2023. The synthesis review management software, Covidence©, was used to remove duplicate records and manage the title/abstract and full-text screening phases of the review. The reference list of all included articles was searched for additional studies. We also conducted a Scopus citation chaining to identify other potentially eligible articles and a focused grey literature search on PubMed and Google to identify additional Canadian studies.
Study selection process
The review team was trained by the senior team members (NB, AS) to ensure consistency with review processes. First, titles and abstracts were screened by two independent reviewers. Secondly, full-texts of potentially eligible studies were assessed for eligibility by two independent reviewers. Disagreements were resolved through discussion and by a third senior team member (AS, NB).
Quality assessment and risk of bias
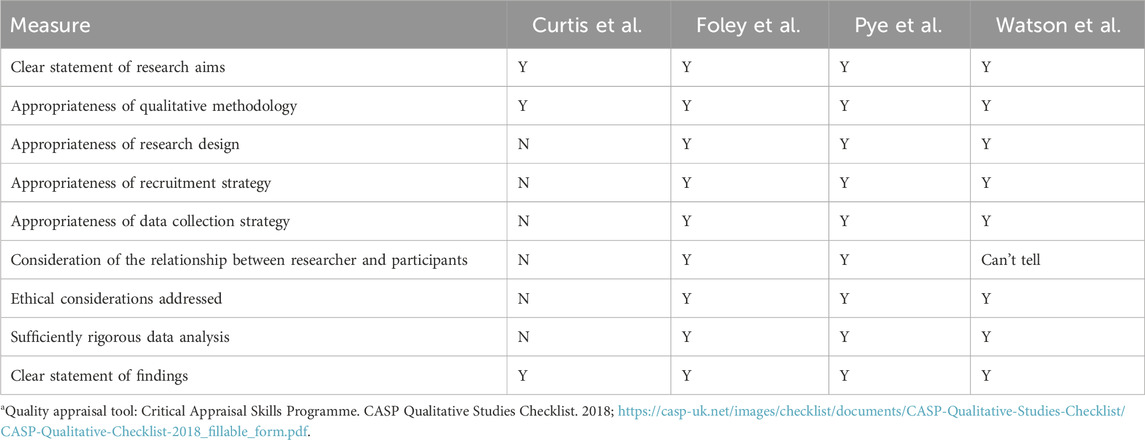
Four reviewers (NB, JB, IqI, HJ) were involved in quality appraisal of included articles, with two independent reviewers assessing the methodological quality of each included study. We employed the Quality Assessment Tool for Quantitative Studies (43), and the Critical Appraisal Skills Programme (CASP) Qualitative Studies Checklist (44).
Data synthesis and analysis
Data were extracted using a structured form developed based on review objectives. Categories included authors and year of publication, country, aim, design, cancer care setting, population characteristics, type of intervention, intervention characteristics, and outcomes. We undertook thematic analysis (45) of the findings and produced a narrative synthesis of the themes. Interventions and programs were grouped according to shared characteristics and were organized under a primary theme. We undertook a separate analysis of Canadian studies.
Results
Study characteristics
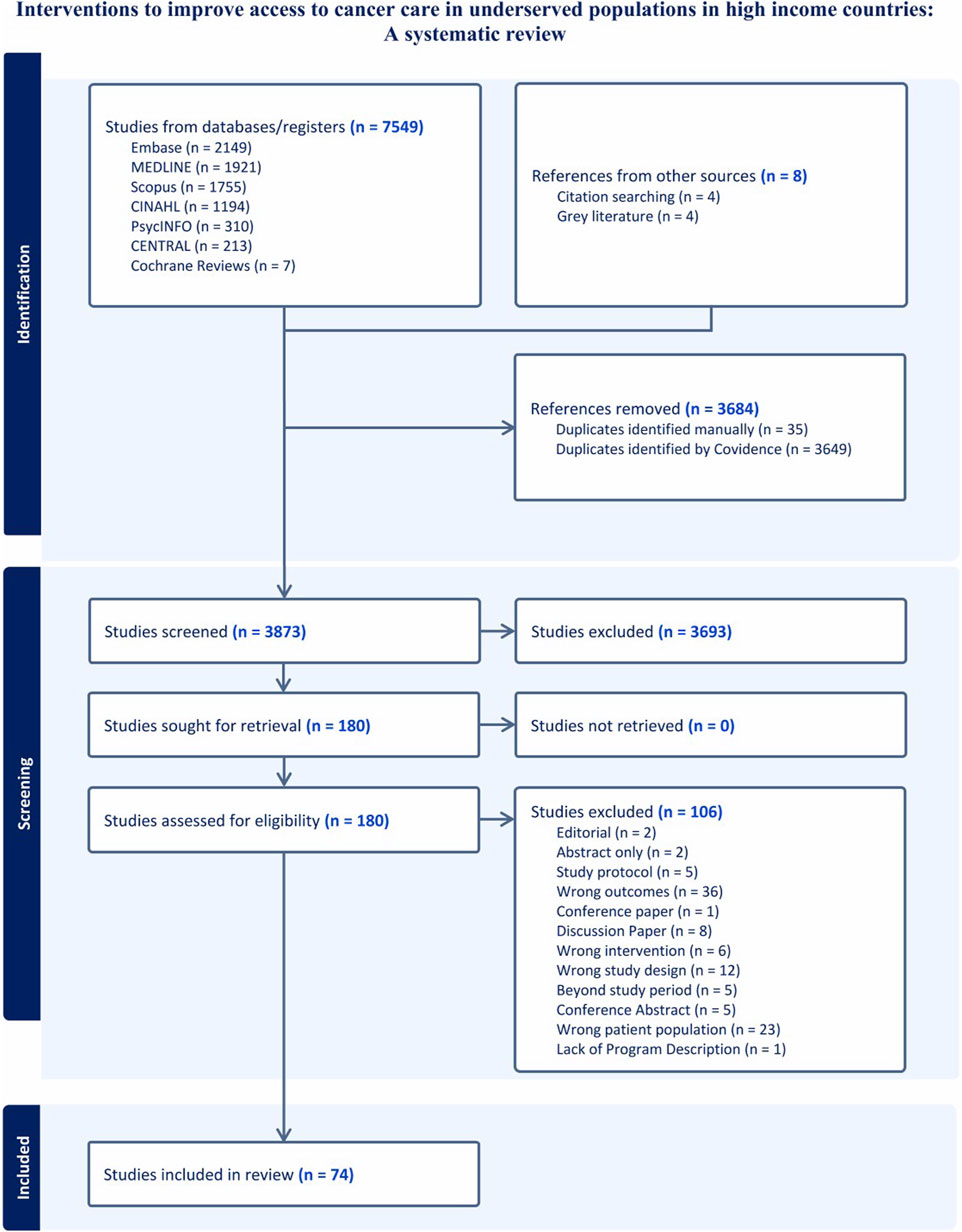
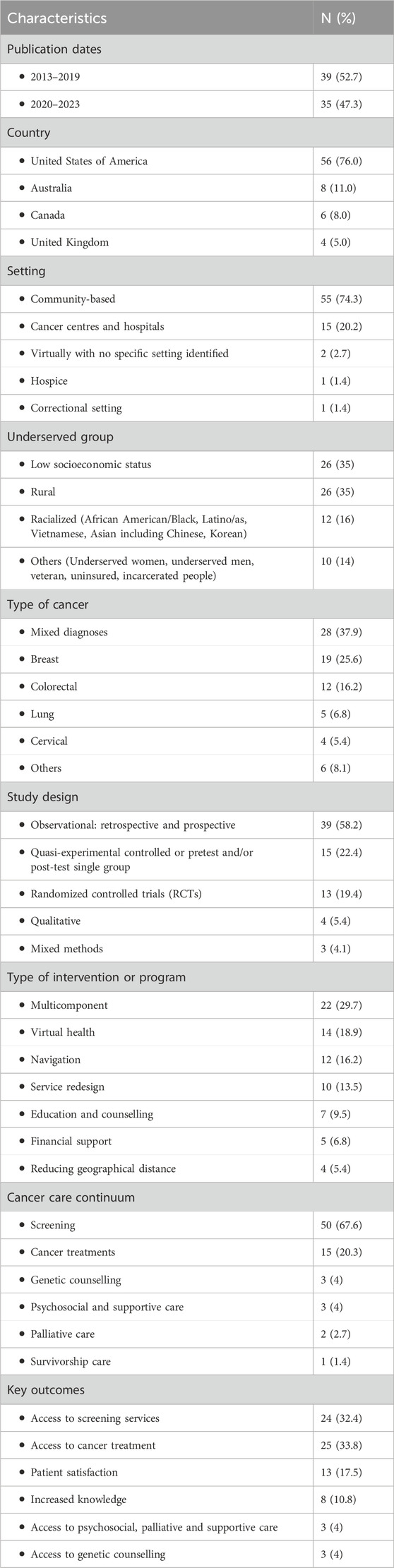
We obtained 7,549 articles from database searches and 8 from citation chaining and grey literature. A total of 3,684 duplicates were removed. We screened the titles and abstracts of 3,873 articles and 3,693 did not meet our inclusion criteria. A total of 180 full texts were screened and 74 met the inclusion criteria (Figure 1). Of the 74 studies, 56 were conducted in the US, 8 in Australia, 6 in Canada, and 4 in the United Kingdom. They were published between 2013 and 2023. There was an increase in studies describing access to cancer care interventions for underserved populations in the literature, with 47% published in the last 3 years (2020–2023) (Table 1).
Sixty-seven studies (90.5%) were quantitative, 4 (5.4%) were qualitative (46–49) and 3 (4.1%) were mixed methods (50–52). Among the quantitative studies, 13 (19.4%) were randomized controlled trials (RCTs) (53–65), 15 (22.4%) were quasi-experimental controlled or pretest and/or post-test single group studies (66–80), and 39 (58.2%) were observational including natural experiments, and retrospective and/or prospective analyses of service data.
Sample sizes were above 100 participants for most studies, ranging from 3 to 190,284 participants, with the largest samples registered in retrospective studies. In most studies, participants were 50 years and older (Table 2).
Most studies were community-based (74.3%) while others took place in cancer centres and hospitals (47, 71, 75, 81–92), hospice (93), correctional service (70), and with no specific setting identified (51, 67) (Tables 1, 2).
Access to cancer care was the primary outcome in most studies, but 8.1% of included studies reported access as a secondary outcome (73, 74, 76, 82, 94, 95) Measures of access included access to cancer screening (screening rates, no show rates, appointment rates); access to cancer treatment (time from diagnosis to treatment initiation, adherence to treatment and follow ups, proportion of patients accessing cancer treatment or supportive care); out-of-pocket and time savings; and travel distance to cancer care services. Knowledge and patient satisfaction were reported as secondary outcomes. Supplementary Table 1 provides an overview of study outcomes by intervention category and individual studies.
Characteristics of underserved populations
The most common underserved groups targeted by included studies were rural populations and people experiencing socioeconomic deprivation. Other studies were focused on Blacks and African Americans (65, 73, 95–97), Latino/Hispanics (52, 69, 79, 98), Asian women including Chinese (72) Vietnamese (99), and Korean women (100), underserved women (82, 101, 102), underserved men (103), veterans (54, 81), incarcerated people (70) and uninsured patients (92, 104, 105) (Tables 1, 3). In many cases, study populations had mixed characteristics such as low income and racialized background.
Types of cancers
The majority of included studies either included people with any type of cancer (mixed) or were focused on breast (69, 72, 75, 79, 80, 87, 95, 97, 98, 99, 101, 104, 106–111), cervical (86, 88, 100, 102) , and colorectal cancers (53–55, 58, 60, 61, 65, 70, 76, 77, 78, 105), or a combination of these three cancers (62, 64, 73, 82, 112). The rest of the studies were focused on lung (56, 68, 74, 113, 114) thoracic (115), ovarian (116), skin (59), oral (71), head and neck (48), and prostate cancers (103) (Tables 1, 2).
Targeted stage across the cancer care continuum
Cancer screening was the stage of focus for most studies, with a few focusing on genetic counselling (66, 94, 96), psychosocial and supportive care (51, 75, 81), survivorship care (98), and palliative care (85, 93). There were 15 studies that aimed to improve access to cancer treatments specifically with respect to: treatment initiation (57, 89), post acute care (48), chemotherapy and medical oncology (46, 47, 84, 117), thoracic surgery (115), gynaecologic oncology (50, 116), exercise oncology (67), treatment adherence and toxicity management (99), lymphedema management (59), breast reconstruction (108), and oral cancer care (71) Supplementary Table 1.
Quality of included studies
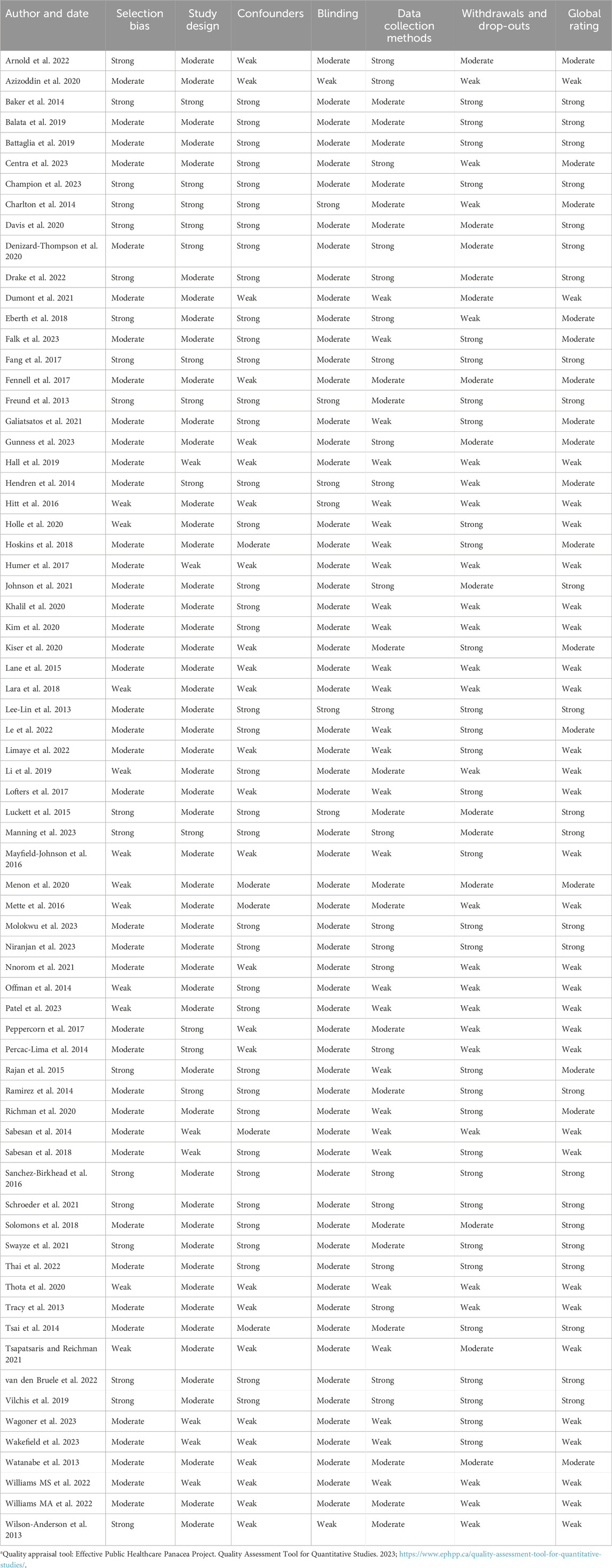
Among the quantitative studies, 24 were appraised to have an overall strong quality, 16 were of moderate quality and 27 of weak quality (Table 3). The study designs used in most studies were of strong to moderate quality, and included participants who were representative of the target populations. Some methodological limitations found were the poor reporting of numbers and reason for dropouts (77, 105, 108, 118), lack of information about reliability and validity of data collection tools, (71, 104, 113) and poor reporting about control of confounders (67, 82, 93). Regarding the qualitative studies, two studies met all the quality criteria (46, 48, 49) one met 8/9 criteria, while one did not meet the majority of the quality criteria (47) (Table 4).
Interventions to improve access to cancer care
We found seven main types of interventions that were used to mitigate barriers in access to cancer care experienced by underserved populations. These included lack of knowledge and health literacy, health system navigation issues, financial constraints, language and cultural barriers, and distance to cancer services. These tailored interventions included patient navigation, education and counselling, virtual health, service-redesign, financial support, improving geographical accessibility and multicomponent interventions. These are described below.
Core features of interventions
Salient characteristics of interventions included: 1) Most interventions/programs used a combination of languages and offered translation services to enhance communication and understanding. The most common languages used were English, Spanish, and French; 2) Participant recruitment was via community organizations, churches, local bulletin boards, community centres, health fairs, community events, food banks and social medial platforms, using flyers and leaflets which sometimes featured racialized groups (60, 72, 73, 91, 119); 3) There was a predominant use of the community health approach to program development and delivery with significant involvement of community health workers and peers for education and navigation (80, 98); 4) Several interventions and programs included reminder phone calls, mailed letters, and electronic medical record alerts, setting reminders to both patients and clinicians, invitations to patients overdue for screening and/or those who did not attend their appointments as well as to request fecal immunochemical test (FIT) patient samples (61, 64, 65, 78–80, 98, 100, 103, 119); 5) Most programs were delivered to participants at no charge or low cost (79, 80, 98, 100, 114); 6) Some studies included all participants who met the inclusion criteria irrespective of legal/immigration status, arranged travel services and/or offered food at no cost, (98, 114, 119) and employed racialized healthcare staff for program delivery (119). The majority of interventions were in publicly funded healthcare settings regardless of country.
Patient navigation
Eleven studies reported use of navigation to assist underserved women to obtain a mammogram and navigate the health system when an abnormal finding was detected (52, 69, 75, 99), assist women who self-identified as having barriers to care to navigate the health system after receiving an abnormal cervical cancer screening result (88), assist patients to obtain colorectal cancer screening (55, 58), and help patients following cancer diagnosis to gain access to care (56, 57, 29). Navigators were either nurses (75), people with high school diploma (57, 69), or patients who had received training through national and local navigation programs. These interventions/programs employed bilingual navigators or translators and were designed to be culturally sensitive, especially when serving specific racialized groups like Latinos/as and Spanish speaking individuals (52, 58, 69, 89) and Vietnamese American women (99). Study findings suggested that patients were generally satisfied with navigators and services provided (52, 56, 75, 89, 99). They reported that navigation increased the rate of mammography and colorectal cancer screening (52, 55, 58), had a positive impact on the communication between patients, navigators and healthcare providers (58, 69, 88, 89, 99) decreased time from diagnosis to treatment (92, 57, 69, 89), and resulted in decreased numbers of missed appointments (88, 99). Notwithstanding, cultural beliefs about breast cancer and difficulties with reaching patients by phone for initial navigation appointments and follow-ups were identified as barriers in the navigation process (57, 58, 88).
Education and counselling
Education and counselling interventions were reported in 7 studies. They were used to increase awareness and therefore uptake of breast, cervical and oral cancer screening services for Chinese American (72), African American (95), Black women (73), and people experiencing precarious socioeconomic conditions or those who were uninsured (74, 91). Delivery was either by healthcare providers (91, 120) community health educators, faith-based group leaders, or women’s social organizations (73, 74). These interventions/programs were designed to be flexible, supportive, culturally appropriate and interactive (91, 120, 72, 73, 95). These characteristics were reflected in the availability of pre-booking and/or walk-ins options, onsite screening services, onsite childcare services, transportation cost (72, 73, 91), posters, flyers and videos with culturally appropriate philosophy, content, graphics and language (72, 73), and the provision of opportunities for questions, discussions and hands on demonstration of skills (72, 73, 91, 95). In order to enhance participant’s learning, teaching resources were developed at a low reading level, such as grade 5 (120) and teaching was done in the first language of participants (72). The effectiveness of education and counselling was assessed through retrospective program data (72–74, 95, 120) and pre- and post-intervention mammogram completion questionnaires (72, 74, 91, 95). Studies reported: increase in participants’ cancer awareness and cancer screening for various types of cancer (73, 91), increase in mammography screening rates (72, 73, 95) and feasibility, acceptability and satisfaction with educational programs (73, 91).
Virtual health interventions/programs
Virtual health interventions were reported in 14 studies and generally targeted rural populations to improve access to specialized care. Mobile phone applications were used to assist patients to self-order screening tests and receive follow-up messages (53) and to send cancer screening reminders (107), while video conferencing employed interactions for genetic counselling (66, 94), specialized cancer care provision (86, 115, 118), chemotherapy supervision (46, 83, 84), exercise-oncology (67), and palliative care (85). They were used to reach as many as 6–63 remote sites (83, 84, 115), 193–254 km away (46, 118).
Video conferencing strategies involved collaboration and supervision between specialised staff in cancer centres and primary healthcare professionals. For example, there were reports of rural generalist nurses administering chemotherapy and biologic therapy agents under the direct supervision of chemotherapy nurses from larger primary centres, using a telenursing platform (46, 83, 84). The uptake of virtual health tools was enhanced by sending messages to patients to remind them of screening schedules (107), sending prescriptions electronically to remote sites or via mail directly to the patient (83, 84), enhancing competencies of remote site staff (67, 84), and providing outreach clinics for patients to see their oncologist in-person (46, 115).
These interventions were assessed using data from retrospective chart reviews (83, 84, 115), and surveys (50, 53, 66). Key outcomes were increased access to cancer care (86, 115), with patients seen and managed locally, within 24 h of referral (83) and patient satisfaction (50, 66, 85, 94). There were significant improvements in screening uptake and adherence (53, 94) and reduction in travel time and cost of care (66, 67, 86, 115). One study reported that patients saved up to 4 h and 40 min on average, 534 km round trip and about $333,074 from lost wages and mileage reimbursement (118). There were also reports of feelings of independence from patients as they received care in their hometown which reduced their reliance on family, friends and government services for transportation (51).
Service redesign
Service redesign was reported in 10 studies, and entailed modifying or creating innovative healthcare service delivery models to address workforce and geographical accessibility issues. Redesign served to accommodate the cancer care needs of underserved populations, especially in primary healthcare settings. Supervision, education and government support were vital to service redesign (59, 96). Services were redesigned in three main ways: developing capacity of primary healthcare staff to provide specialized services (59, 101), embedding specialized professional or services into primary healthcare packages (71, 76, 93, 96, 108, 113, 116, 117) and developing intersectoral or interdisciplinary collaborations to facilitate care provision (70). Some examples were a nurse-managed health centre that provided breast health services (101), a long-term palliative care unit designed as a hybrid between a hospice and a nursing home (93), and developing a cross-agency collaboration between public health and corrections to provide annual colorectal cancer screening service in a state prison using FIT (70).
Successful service redesign was dependent on capacity building for primary healthcare providers and provision of financial resources by government (59, 96). These services were mainly evaluated via retrospective analyses of routinely gathered data (93, 96, 101, 116, 117). Some reported outcomes were: significant improvement in overall survival and access to care (116, 70, 113, 59, 108), reduction in driving time to medical oncology care, from 51.6 to 19.2 min, with the use of visiting consultant clinics (117), and reduction in treatment delays and increased adherence to treatment and toxicity management (71).
Financial support
This strategy involved no-cost access to cancer services for people living in poverty or uninsured. They included elimination of copayment, coinsurance, and deductible fees for screening mammography for all women 40 years or older (92, 106), providing free colonoscopy screening to uninsured, asymptomatic patients aged 50–64 years and living below the poverty line (92, 105), and developing a student-run free clinic to provide breast cancer screening opportunities for uninsured patients (104).
These were evaluated using retrospective analyses of service data by either assessing uptake during the intervention period or by comparing trends in screening before and after intervention for periods ranging from 1 to 13 years. Reported outcomes were significant increase in mammography screening rates (106), with 84% of intervention group participants having a mammography post intervention (104), significant increase in colorectal cancer screening, with 79% of participants completing a colonoscopy (105), increased access to timely treatment and improved survival for breast cancer patients (82, 92).
Reducing geographical barriers
Four studies were specifically aimed at reducing geographical barriers to cancer care in three ways: mobile services, satellite services, and mailouts. Satellite services were designed to provide a similar quality of service as would be found at the main centre (47), while mailouts provided free postal services with paid self-addressed envelopes for return of samples as well as assistance, across the continuum for patients with a positive test (54). Some examples of these interventions were: a mobile, no-cost breast cancer screening program that provided a free screening service to medically underserved women (109); a satellite chemotherapy infusion centre in rural communities where visiting oncology nurse practitioners and/or oncologists from a large urban cancer centre offered chemotherapy services to rural communities (47), and a mailout service where a FIT was mailed to asymptomatic, average-risk veterans overdue for colorectal screening (54). Nurses played a key role in the delivery of the interventions (68, 109). This included performing symptom assessment (68), clinical breast exam (109), and offering chemotherapy services (47).
Intervention evaluation data were collected using questionnaires administered to both patients (54, 68) and healthcare providers (47) or retrospective program data (109). They evaluated participants’ uptake of screening services post intervention (54, 109) and influence of geographical location on screening and screening adherence (68). Studies reported up to 90% screening rates (54) and adherence to screening (68) and participants’ satisfaction with services.
Multicomponent interventions/programs
Twenty-two studies reported use of a combination of the six categories described above to improve access to cancer care. This ranged from a combination of 2–4 interventions, with education, navigation and financial support being core components of most multicomponent interventions/programs. An example included using a combination of education, financial and geographical accessibility strategies where members of the healthcare team were educated on colorectal cancer screening and ways to improve access, patients had assistance obtaining insurance approvals, and patients who were homebound or unhoused were supplied test kits via a mobile unit (77).
These interventions reported increased access to screening mammography (64, 80, 121), with more than 90% of study participants undertaking screening post intervention (79, 97) and increased access to colorectal cancer screening (60, 61, 65, 77), cervical cancer screening (100, 102) and screening for other types of cancers (103, 114). There were also reports of increased access to psychosocial care, (81) survivorship (98), and patient satisfaction (48).
Interventions in Canada
Three main interventions were employed by the 6 Canadian studies: virtual health, education and navigation. They were designed specifically for rural populations and Blacks and conducted in Alberta (49, 85), Ontario (73, 119) and British Columbia (115). One study used multiple sites in Alberta, Nova Scotia, and Ontario (67). Virtual health interventions were used to provide palliative care consults (85) and thoracic surgical care (115) to rural and remote patients, and videoconferencing was employed to deliver an exercise oncology program (67). These interventions were found to reduce travel distance and cost and expand access to specialized care for many rural and remote patients as well as improve patient satisfaction. Educational interventions (73), also used in combination with virtual health (119), focused on increasing awareness about breast, cervical and colorectal cancer screening among Blacks and immigrant populations. These studies reported significant increase in cancer screening participation for eligible patients as well as increased awareness of cancer susceptibility and screening guidelines, and improvements in screening self-efficacy. In Alberta, a province-wide standard navigation program was designed for rural Albertans (49). Participants reported a positive impact on their experiences of cancer care, and that accessing a navigator made a difference (49). There was a decrease in visits to the emergency rooms or hospital admissions for cancer-related symptoms, improvements in continuity of care, patient’s ability to access cancer information and meaningful support as well as improved satisfaction with care.
Discussion
This review explored interventions and programs to improve access to cancer care in underserved populations in high income countries with UHC and the US. Most studies were published between 2018 and 2023 and conducted in the US. Interventions targeted specific access barriers resulting from geographical distance, finances, culture and language, knowledge and health literacy, and health system navigation. Most studies were conducted in community-based settings and recruited participants from faith- and community-based organizations. Participants included underserved women or men, rural populations, and people experiencing low socioeconomic status, incarceration, or multiple social disadvantages. Over two-thirds of interventions and programs focused on access to cancer screening and diagnosis.
Our findings suggest tailored interventions or programs can improve access to cancer care in underserved populations, especially at the screening and diagnosis stage. However, interventions to improve access to cancer treatment, survivorship and palliative/end-of-life care with underserved populations are needed. Most interventions or programs integrated supports to mitigate language, financial, cultural, and geographical barriers. Addressing barriers that prevent underserved populations from timely accessing cancer care is an essential consideration in the delivery of tailored interventions.
Our review findings suggest virtual health interventions and those reducing geographical distance can improve access to screening and diagnosis, oncology treatments (e.g., chemotherapy), supportive care, patient satisfaction as well as reduce travel distance and cost. There were 14 studies that employed virtual health while four focused on reducing geographical distance targeting rural and remote populations and people from low socioeconomic status. Telehealth was the most common intervention (videoconferencing, phone calls) while text or email messages and websites were also utilized. Positive effects of telehealth in cancer care during the COVID-19 pandemic were reported (122). Telehealth may improve access to care for head and neck cancer patients, symptom control and quality of life, and be cost-efficient (123). Findings suggest a need to consider barriers to technology adoption and improve internet access and health literacy (124–126).
Interventions reducing geographical distance included mailing FIT kits, satellite centres, and mobile clinics. A study in Alberta, Canada found that individuals living further from diagnostic facilities had higher odds of no record of colorectal cancer screening (CRC) and people from rural and remote areas had higher odds of being overdue for CRC (127). The study also found that people with higher levels of material deprivation tended to have lower rates of CRC compared to those with lower material deprivation (127). Another Canadian study found that living more than 1-h driving time from a cancer centre was associated with worse overall survival and disease-free survival (128). In geographically large countries such as Canada, interventions aimed at reducing geographical distance and increasing accessibility may contribute to improve cancer outcomes in populations living farther away from cancer centres and those from low income areas.
Interventions that improve affordability of cancer care can have a significant impact on access to cancer screening and treatment. There were five studies that implemented some form of financial support by offering services at no cost, covering the cost of screening, eliminating co-payments, or by expanding Medicaid eligibility. The financial burden of cancer care includes costs related to hospital and physician services, diagnostics, medications, caregiving, employment, travel, and inability to save, among others (129). It affects all dimensions of access to healthcare and will lead underserved populations such as racialized and rural people to delay or decline care (129). In Canada, provincial disparities in public coverage of take-home cancer drugs exist with patient co-payments nearing 20% (130). These out-of-pocket expenses can negatively affect patient’s cancer care decisions and access (130). With over two-thirds of Canadians disclosing financial distress when facing a cancer diagnosis, the need for financial distress mitigation interventions as well as federal and provincial policies is urgent (131).
There were 12 studies that reported patient navigation to improve access to screening and diagnosis, treatment, and patient satisfaction in rural, racialized, uninsured, or low socioeconomic status populations. Navigation interventions in this review incorporated education and counselling, coordination of care, addressing barriers to care, psychosocial care, and financial navigation. The majority of studies showed significant improvements in care outcomes such as access to screening and reduced times from diagnosis to treatment. Patient navigators can play a significant role in the cancer care of patients by improving access to care, patient experience, and care coordination (132). Patient navigators in Canada were described as agents of change who improved patients’ health literacy, built partnerships with agencies to address care inequities, built trust with underserved communities and patient’s trust in the healthcare system (133). The success that patient navigation has shown in improving access to cancer care (36), highlights the key role they can play in improving cancer care outcomes in underserved groups.
Education and service redesign interventions had significant impacts on access to screening, cancer treatment and palliative care, and patient satisfaction in racialized, rural, or people from low socioeconomic status. There were seven studies that reported educational interventions and 10 studies focused on service redesign. Education was provided in the language best understood by participants and used sociocultural adapted resources. Education can be an excellent strategy to increase cancer and cancer service awareness among racialized and immigrant populations (134, 135). Service redesign interventions resulted in cost savings and shorter travel distances. The reorganization of oncology multidisciplinary teams resulted in improved access to and quality of care for lung cancer patients in the United Kingdom (136). A lung cancer service redesign initiative in Australia had impacts on the proportion of new referrals seen within 14 days by specialists and documentation of patients presented at multidisciplinary meetings (137). Studies to evaluate the impact of service redesign on access to cancer care are needed. Considering the rapid growth of immigrant populations in Canada and other countries, promoting access to screening and early cancer diagnoses through education and service redesign may contribute to improve cancer care outcomes.
We identified 22 multi-component interventions with education, navigation, and financial support being the most common core components. The interventions identified in this review showed positive effects on access to cancer screening, psychosocial care, survivorship care, and patient satisfaction. Complex health interventions involve multiple components and are designed to address complex health challenges (138, 139). Our review findings suggest multi-component interventions are suitable to tackle disparities in access to cancer care. Understanding the mechanisms underlying change in complex interventions is important to inform decision makers (139). Further research is needed to understand the interactive effects of intervention components as well as those between the intervention and the context in which it takes place.
The contributions of underserved populations as study participants in included studies reflects their interest in participating in initiatives to improve access to cancer care. Our findings suggest that their successful accrual can be achieved via community organizations, places of worship, and local bulletin boards, social media, flyers and leaflets. Patient navigators and translators, culturally tailored recruitment materials, and covering travel and parking costs can be effective ways of recruiting underserved populations in oncology (140, 141). Most interventions in this review were integrated within community settings. This approach has potential to improve coordination and delivery of cancer prevention, diagnostic, treatment and supportive care services for these populations (142, 143). Establishing partnerships with community members can increase participation and acceptability of interventions for racialized groups (144, 145).
The underserved populations most frequently targeted were those of low socioeconomic status, rural populations, and racialized people. In Canada, populations of low socioeconomic status experience significant cancer disparities (13, 21). In contrast, our review yielded no Canadian studies focused on this population group. This finding may reflect a gap in collecting and reporting study population sociodemographic characteristics. We identified four Canadian studies focusing on rural and remote populations that employed education (73), telehealth (67, 85) or navigation (49) to improve access to prevention and screening services, surgery, rehabilitation, and palliative care. Although evidence is limited, this research can inform the design of services for this population group. Lastly, review findings suggest a need for further research to improve access to cancer care among racialized communities. Although there are calls to improve access to cancer care for underserved populations in Canada (37), review findings suggest minimal Canadian evidence in this area.
Review findings point to the need to increase health equity research in access to cancer care in Canada. The US had the largest number of articles. This likely reflects research funding to address cancer disparities as well as requirements to include underrepresented populations in research. We acknowledge that different healthcare systems might influence the applicability of findings to Canada. This calls for national, provincial, and intersectoral efforts to determine priorities in access to cancer care for underserved populations, advocate before government stakeholders, funding bodies and influence the Canadian research agenda in cancer care.
Implications
Review findings can inform research, practice and education in the area of access to cancer care for underserved populations in Canada and Alberta. There is a need to accelerate health equity research in cancer care to a) generate evidence of barriers in access to cancer care and determine the magnitude of inequities in cancer care; and b) design, implement and evaluate interventions to improve access to cancer care. There is a need to increase cancer-related health equity research funding to achieve these goals. While this review highlights gaps, research questions to address those gaps need to be informed by affected patients, families, and communities as well as those who provide treatment and care. Engagement of these stakeholders supports integration of research and clinical practice and has potential to accelerate the research to outcome/impact pathway. Involvement of clinical and operational teams in research design and execution is likely to increase uptake of research findings in the cancer care realm. Similarly, research is likely to have greater impact if we engage patients, families, and community members throughout the research cycle.
Clinical practice implications include identifying services and programs currently in place that support underserved populations. Incorporating strategies such as education, service redesign, virtual health, navigation, and the provision of financial, transportation, cultural, and language supports may increase awareness of as well as access to cancer care services. Review findings also show the need to incorporate health equity knowledge in the curricula of health professions, increase both health equity and cultural competency of healthcare professionals, and advance knowledge of educational models to work with underserved populations. The work of critical educator Paulo Freire can inform initiatives aimed at fostering social transformation, empowerment, emancipation, and critical awareness of conditions leading to inequities in access to cancer care (146).
Limitations
We limited our search to the last 10 years and only included articles published in the English language. Although this period likely reflects time where the majority of studies were published, we may have missed important works published prior to the review period. We also focused on countries with healthcare systems similar to the Canadian universal healthcare system with the exception of the United States. Our exclusion criteria may have resulted in leaving out studies in other countries reporting health equity interventions directed at our populations of interest. Underserved populations comprise a large and diverse group. Our review focused on selected underserved groups. Studies yielding evidence concerning other underserved groups may exist. Estimating the effectiveness of included interventions and their comparative impacts was beyond the scope of this review. We are confident that the breath and recent nature of the studies included provide a current and comprehensive list of interventions tailored to address specific barriers in access to cancer care. This knowledge may inform cancer system stakeholders in the design of programs to support underserved populations facing specific obstacles in access to cancer care such as distance, lack of cancer awareness and health literacy, language and cultural barriers, financial constraints, or health system navigation challenges.
Conclusion
This systematic review yielded evidence of a wide range of interventions and programs to improve access to cancer care for underserved populations. Utilizing diverse strategies to reach underserved populations and increase intervention uptake is necessary. Review findings suggest these interventions can have a significant impact on patient experiences, satisfaction, and cancer outcomes. Although the majority of interventions were conducted in the US, there is potential to incorporate knowledge from those studies into the Alberta and Canadian cancer care systems. The interventions and programs identified in this review reveal a collective and committed effort to tackle cancer inequities. This is a critical step towards achieving equity in cancer care.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
AS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. NB: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. AP: Conceptualization, Investigation, Resources, Validation, Visualization, Writing–review and editing. PR: Conceptualization, Investigation, Resources, Validation, Visualization, Writing–review and editing. JB: Data curation, Formal Analysis, Investigation, Validation, Visualization, Writing–review and editing. II: Data curation, Formal Analysis, Investigation, Validation, Visualization, Writing–review and editing. MK: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Alberta Health Services Cancer Strategic Clinical Network (Grant No RES0063552). This funding support did not influence the development of the study concept, designing the research, collecting and analysing the data, or writing the manuscript.
Acknowledgments
We thank the Alberta Health Services Strategic Clinical Network for the funding support. We thank research program coordinator Dr. Harkeert Judge for her helpful assistance with quality appraisal of qualitative studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/or.2024.1427441/full#supplementary-material
References
1. Vaccarella, S, Lortet-Tieulent, J, Saracci, R, Conway, DI, Straif, K, and Wild, CP. Reducing social inequalities in cancer: evidence and priorities for research. IARC Scientific Publication (2019). No 168.
2. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clinicians (2021) 71(3):209–49. doi:10.3322/caac.21660
3. Canadian Cancer Society. Cancer statistics at a glance (2024). Available at: https://cancer.ca/en/research/cancer-statistics/cancer-statistics-at-a-glance#:∼:text=Researchers%20estimated%20that%20there%20would,for%2028.2%25%20of%20all%20deaths (Accessed July 15, 2024).
4. Canadian Cancer Statistics. Canadian cancer statistics advisory, in collaboration with the Canadian cancer society, statistics Canada, the public health agency of Canada. Toronto, ON: Canadian Cancer Statistics (2023).
5. Canadian Cancer Statistics. Canadian cancer statistics advisory committee, Canadian cancer society, statistics Canada, public health agency of Canada. Toronto, ON: Canadian cancer statistics (2021).
6. Canadian Cancer Statistics. Canadian Cancer Statistics: a 2022 special report on cancer prevalence. Health Promot chronic Dis Prev Can (2023) 43(1):49. doi:10.24095/hpcdp.43.1.05
7. Islami, F, Guerra, CE, Minihan, A, Yabroff, KR, Fedewa, SA, Sloan, K, et al. American Cancer Society's report on the status of cancer disparities in the United States, 2021. CA: A Cancer J Clinicians (2022) 72(2):112–43. doi:10.3322/caac.21703
8. Miller, KD, Ortiz, AP, Pinheiro, PS, Bandi, P, Minihan, A, Fuchs, HE, et al. Cancer statistics for the US Hispanic/Latino population, 2021. CA: A Cancer J Clinicians (2021) 71(6):466–87. doi:10.3322/caac.21695
9. Williams, PA, Zaidi, SK, and Sengupta, R. AACR cancer disparities progress report 2022. Cancer Epidemiol Biomarkers and Prev (2022) 31(7):1249–50. doi:10.1158/1055-9965.epi-22-0542
10. Crowley, R, Daniel, H, Cooney, TG, and Engel, LS. Envisioning a better U.S. Health care system for all: coverage and cost of care. Ann Intern Med (2020) 172(2 Suppl. l):S7–s32. doi:10.7326/m19-2415
11. Asaria, M, Ali, S, Doran, T, Ferguson, B, Fleetcroft, R, Goddard, M, et al. How a universal health system reduces inequalities: lessons from England. J Epidemiol Community Health (2016) 70(7):637–43. doi:10.1136/jech-2015-206742
12. Veugelers, PJ, and Yip, AM. Socioeconomic disparities in health care use: does universal coverage reduce inequalities in health? J Epidemiol Community Health (2003) 57(6):424–8. doi:10.1136/jech.57.6.424
13. Canadian Partnership Against Cancer. Examining disparities in cancer control: a system performance special focus report. Toronto, ON: The Canadian Partnership Against Cancer (2014). p. 83.
14. Canadian Partnership Against Cancer. Cancer system performance: 2017 report. Toronto, ON: The Canadian Partnership Against Cancer (2017).
15. Dabbikeh, A, Peng, Y, Mackillop, WJ, Booth, CM, and Zhang-Salomons, J. Temporal trends in the association between socioeconomic status and cancer survival in Ontario: a population-based retrospective study. CMAJ open (2017) 5(3):E682–E689. doi:10.9778/cmajo.20170025
16. Truant, TLO, Lambert, LK, and Thorne, S. Barriers to equity in cancer survivorship care: perspectives of cancer survivors and system stakeholders. Glob Qual Nurs Res (2021) 8:233339362110067. doi:10.1177/23333936211006703
17. Canadian Partnership Against Cancer. The 2018 cancer system performance report. May 6, 2019 (2018). p. 1–63.
18. Erickson, B, Biron, VL, Zhang, H, Seikaly, H, and Côté, DWJ. Survival outcomes of First Nations patients with oral cavity squamous cell carcinoma (Poliquin 2014). Article. J Otolaryngol - Head Neck Surg (2015) 44:2. doi:10.1186/s40463-015-0056-8
19. Canadian Partnership Against Cancer. The 2016 cancer system performance report. Toronto, ON: Canadian Partnership Against Cancer (2016). p. 128. Available at: http://www.systemperformance.ca/report/2016-cancer-system-performance-report/ (Accessed May 11, 2019).
20. Public Health Agency of Canada. Key health inequalities in Canada: a national portrait. Ottawa, ON: Pan Canadian Health Inequalities Reporting Initiative (2018).
21. Canadian Partnership Against Cancer. Lung cancer and equity report (2020). Available at: https://www.partnershipagainstcancer.ca/topics/lung-cancer-equity/ (Accessed July 18, 2022).
22. Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics: a 2020 special report on lung cancer (2020). Available at: https://cancer.ca/canadian-cancer-ctatistics-2020-EN (Accessed July 14, 2021).
23. Simkin, J, Woods, R, and Elliott, C. Cancer mortality in Yukon 1999-2013: elevated mortality rates and a unique cancer profile. Int J Circumpolar Health (2017) 76(1):1324231. doi:10.1080/22423982.2017.1324231
24. Iqbal, J, Ginsburg, O, Fischer, HD, Austin, PC, Creatore, MI, Narod, SA, et al. A population-based cross-sectional study comparing breast cancer stage at diagnosis between immigrant and canadian-born women in Ontario. Breast J (2017) 23(5):525–36. doi:10.1111/tbj.12785
25. Lofters, AK, McBride, ML, Li, D, Whitehead, M, Moineddin, R, Jiang, L, et al. Disparities in breast cancer diagnosis for immigrant women in Ontario and BC: results from the CanIMPACT study. journal article. BMC Cancer (2019) 19(1):42. doi:10.1186/s12885-018-5201-0
26. Vahabi, M, Lofters, A, Kumar, M, and Glazier, RH. Breast cancer screening disparities among immigrant women by world region of origin: a population-based study in Ontario, Canada. Cancer Med (2016) 5(7):1670–86. doi:10.1002/cam4.700
27. World Health Organization. Research for universal health coverage (2013). Available at: https://www.afro.who.int/publications/world-health-report-2013-research-universal-health-coverage (Accessed May 29, 2023).
28. United Nations Department of Economic and Social Affairs Sustainable Development. Goal 3 Ensure healthy lives and promote well-being for all at all ages (2024). Available at: https://sdgs.un.org/goals/goal3 (Accessed May 14, 2024).
29. Martin, D, Miller, AP, Quesnel-Vallée, A, Caron, NR, Vissandjée, B, and Marchildon, GP. Canada's universal health-care system: achieving its potential. The Lancet (2018) 391(10131):1718–35. doi:10.1016/s0140-6736(18)30181-8
30. Canadian Cancer Society. Advancing health equity through cancer information and support services (2023). Report on communities that are underserved.
31. Alberta Cancer Strategic Clinical Network. Future of cancer impact in Alberta. Alberta, Canada (2023).
32. Patel, M, Andrea, N, Jay, B, and Coker, TR. A community-partnered, evidence-based approach to improving cancer care delivery for low-income and minority patients with cancer. J Community Health (2019) 44(5):912–20. doi:10.1007/s10900-019-00632-x
33. Maliski, SL, Clerkin, B, and Litwin, MS. Describing a nurse case manager intervention to empower low-income men with prostate cancer. Oncol Nurs Forum (2004) 31(1):57–64. doi:10.1188/04.onf.57-64
34. Bergman, J, Chi, AC, and Litwin, MS. Quality of end-of-life care in low-income, uninsured men dying of prostate cancer. Cancer (2010) 116(9):2126–31. doi:10.1002/cncr.25039
35. Simon, MA, Nonzee, NJ, McKoy, JM, Liu, D, Luu, TH, Byer, P, et al. Navigating veterans with an abnormal prostate cancer screening test: a quasi-experimental study. BMC Health Serv Res (2013) 13:314. doi:10.1186/1472-6963-13-314
36. Chan, RJ, Milch, VE, Crawford-Williams, F, Agbejule, OA, Joseph, R, Johal, J, et al. Patient navigation across the cancer care continuum: an overview of systematic reviews and emerging literature. CA: A Cancer J Clinicians (2023) 73(6):565–89. doi:10.3322/caac.21788
38. Rajaguru, V, Jang, J, Kwon, JA, Kim, JH, Shin, J, and Chun, M. A scoping review on population-centered indicators for cancer care continuum. Front Public Health (2022) 10:912946. doi:10.3389/fpubh.2022.912946
39. Scanlon, B, Brough, M, Wyld, D, and Durham, J. Equity across the cancer care continuum for culturally and linguistically diverse migrants living in Australia: a scoping review. Glob Health (2021) 17(1):87. doi:10.1186/s12992-021-00737-w
40. Levesque, JF, Harris, MF, and Russell, G. Patient-centred access to health care: conceptualising access at the interface of health systems and populations. Int J Equity Health (2013) 12:18. doi:10.1186/1475-9276-12-18
41. Page, MJ, Moher, D, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (2021) 372:n160. doi:10.1136/bmj.n160
42. Cochrane Handbook. Cochrane Handbook (2024). Available at: https://training.cochrane.org/handbook/current (Accessed February 12, 2024).
43. Effective Public Healthcare Panacea Project. Quality assessment tool for quantitative studies (2023). Available at: https://www.ephpp.ca/quality-assessment-tool-for-quantitative-studies/(Accessed May 4, 2023).
44. Critical Appraisal Skills Programme. CASP qualitative studies checklist (2023). Available at: https://casp-uk.net/images/checklist/documents/CASP-Qualitative-Studies-Checklist/CASP-Qualitative-Checklist-2018_fillable_form.pdf (Accessed May 4, 2023).
45. Kiger, ME, and Varpio, L. Thematic analysis of qualitative data: AMEE Guide No. 131. Med Teach (2020) 42(8):846–54. doi:10.1080/0142159x.2020.1755030
46. Pye, S, Webster, E, Zielinski, R, and Honeyball, F. The best thing since sliced bread': patient experiences of teleoncology in western NSW. Aust J Rural Health (2023) 31(1):90–7. doi:10.1111/ajr.12921
47. Curtis, ML, and Eschiti, VS. Geographic health disparities: satellite clinics for cancer care in rural communities. Clin J Oncol Nurs (2018) 22(5):500–6. doi:10.1188/18.Cjon.500-506
48. Foley, J, Ward, EC, Burns, CL, Nund, RL, Wishart, LR, Graham, N, et al. Enhancing speech-language pathology head and neck cancer service provision in rural Australia: using a plan, do, study, act approach. Int J Speech-Language Pathol (2023) 25(2):292–305. doi:10.1080/17549507.2022.2050300
49. Watson, LC, Vimy, K, Anderson, J, Champ, S, and DeIure, A. Developing a provincial cancer patient navigation program utilizing a quality improvement approach. Part three: evaluation and outcomes. Can Oncol Nurs J (2016) 26(4):276–85. doi:10.5737/23688076264276285
50. Arnold, JL, Anderson, E, and Roeder, L. Rural patients highly satisfied with gynaecological oncology care via telehealth. Aust New Zealand J Obstet Gynaecol (2022) 62(2):280–5. doi:10.1111/ajo.13452
51. Fennell, KM, Turnbull, DA, Bidargaddi, N, McWha, JL, Davies, M, and Olver, I. The consumer-driven development and acceptability testing of a website designed to connect rural cancer patients and their families, carers and health professionals with appropriate information and psychosocial support. Eur J Cancer Care (Engl) (2017) 26(5):e12533. doi:10.1111/ecc.12533
52. Li, Y, Carlson, E, Hernández, DA, Green, B, Calle, T, Kumaresan, T, et al. Patient perception and cost-effectiveness of a patient navigation program to improve breast cancer screening for hispanic women. Health Equity (2019) 3(1):280–6. doi:10.1089/heq.2018.0089
53. Denizard-Thompson, NM, Miller, DP, Snavely, AC, Spangler, JG, Case, LD, and Weaver, KE. Effect of a digital health intervention on decreasing barriers and increasing facilitators for colorectal cancer screening in vulnerable patients. Cancer Epidemiol Biomarkers and Prev (2020) 29(8):1564–9. doi:10.1158/1055-9965.Epi-19-1199
54. Charlton, ME, Mengeling, MA, Halfdanarson, TR, Makki, NM, Malhotra, A, Klutts, JS, et al. Evaluation of a home-based colorectal cancer screening intervention in a rural state. The J Rural Health (2014) 30(3):322–32. doi:10.1111/jrh.12052
55. Menon, U, Szalacha, LA, Kue, J, Herman, PM, Bucho-Gonzalez, J, Lance, P, et al. Effects of a community-to-clinic navigation intervention on colorectal cancer screening among underserved people. Ann Behav Med (2020) 54(5):308–19. doi:10.1093/abm/kaz049
56. Battaglia, TA, Gunn, CM, Bak, SM, Flacks, J, Nelson, KP, Wang, N, et al. Patient navigation to address sociolegal barriers for patients with cancer: a comparative-effectiveness study. Cancer (2022) 128(Suppl. 13):2623–35. doi:10.1002/cncr.33965
57. Freund, KM, Battaglia, TA, Calhoun, E, Darnell, JS, Dudley, DJ, Fiscella, K, et al. Impact of patient navigation on timely cancer care: the Patient Navigation Research Program. J Natl Cancer Inst (2014) 106(6):dju115. doi:10.1093/jnci/dju115
58. Percac-Lima, S, López, L, Ashburner, JM, Green, AR, and Atlas, SJ. The longitudinal impact of patient navigation on equity in colorectal cancer screening in a large primary care network. Cancer (2014) 120(13):2025–31. doi:10.1002/cncr.28682
59. Hall, F, Gordon, S, Hulcombe, J, and Stephens, C. Compression garment service model: facilitating access to compression garments through workforce and service redesign. Aust J Rural Health (2019) 27(3):257–61. doi:10.1111/ajr.12509
60. Baker, DW, Brown, T, Buchanan, DR, Weil, J, Balsley, K, Ranalli, L, et al. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med (2014) 174(8):1235–41. doi:10.1001/jamainternmed.2014.2352
61. Davis, TC, Rademaker, A, Morris, J, Ferguson, LA, Wiltz, G, and Arnold, CL. Repeat annual colorectal cancer screening in rural community clinics: a randomized clinical trial to evaluate outreach strategies to sustain screening. The J Rural Health (2020) 36(3):307–15. doi:10.1111/jrh.12399
62. Champion, VL, Paskett, ED, Stump, TE, Biederman, EB, Vachon, E, Katz, ML, et al. Comparative effectiveness of 2 interventions to increase breast, cervical, and colorectal cancer screening among women in the rural US: a randomized clinical trial. JAMA Netw Open (2023) 6(4):e2311004. doi:10.1001/jamanetworkopen.2023.11004
63. Fang, ML, Sixsmith, J, Sinclair, S, and Horst, G. A knowledge synthesis of culturally- and spiritually-sensitive end-of-life care: findings from a scoping review. BMC Geriatr (2016) 16:107. doi:10.1186/s12877-016-0282-6
64. Hendren, S, Winters, P, Humiston, S, Idris, A, Li, SXL, Ford, P, et al. Randomized, controlled trial of a multimodal intervention to improve cancer screening rates in a safety-net primary care practice. J Gen Intern Med (2014) 29(1):41–9. doi:10.1007/s11606-013-2506-1
65. Manning, M, Lucas, T, Thompson, H, and Penner, L. Results of an African American-targeted norm-based colorectal cancer screening intervention: a pilot study. J Behav Med (2023) 46(3):391–404. doi:10.1007/s10865-022-00367-6
66. Solomons, NM, Lamb, AE, Lucas, FL, McDonald, EF, and Miesfeldt, S. Examination of the patient-focused impact of cancer telegenetics among a rural population: comparison with traditional in-person services. Telemed e-Health (2018) 24(2):130–8. doi:10.1089/tmj.2017.0073
67. Wagoner, CW, Dreger, J, Keats, MR, Santa Mina, D, McNeely, ML, Cuthbert, C, et al. First-year implementation of the EXercise for cancer to enhance living well (EXCEL) study: building networks to support rural and remote community access to exercise oncology resources. Int J Environ Res Public Health (2023) 20(3):1930. doi:10.3390/ijerph20031930
68. Balata, H, Tonge, J, Barber, PV, Colligan, D, Elton, P, Evison, M, et al. Attendees of Manchester's Lung Health Check pilot express a preference for community-based lung cancer screening. Thorax (2019) 74(12):1176–8. doi:10.1136/thoraxjnl-2018-212601
69. Ramirez, A, Perez-Stable, E, Penedo, F, Talavera, G, Carrillo, JE, Fernández, M, et al. Reducing time-to-treatment in underserved latinas with breast cancer: the six cities study. Cancer (2014) 120(5):752–60. doi:10.1002/cncr.28450
70. Dumont, DM, Davis, D, Sadacharan, R, Lamy, E, and Clarke, JG. A correctional-public health collaboration for colorectal cancer screening in a state prison system. Public Health Rep (2021) 136(5):548–53. doi:10.1177/0033354920974668
71. Patel, JV, Hughes, DM, and Ko, NY. OPTIMAL breast cancer care: effect of an outpatient pharmacy team to improve management and adherence to oral cancer treatment. JCO Oncol Pract (2023) 19(3):e306–e314. doi:10.1200/op.22.00135
72. Lee-Lin, F, Menon, U, Leo, MC, and Pedhiwala, N. Feasibility of a targeted breast health education intervention for Chinese American immigrant women. Oncol Nurs Forum (2013) 40(4):361–72. doi:10.1188/13.Onf.361-372
73. Lofters, A, Jain, A, Siu, W, Kyte, M, Lee-Foon, N, Scott, F, et al. Ko-Pamoja: the feasibility of a lay health educator-led breast and cervical screening program for Black women in Ontario, Canada (short report). Cancer Causes Control (2017) 28(11):1207–18. doi:10.1007/s10552-017-0920-0
74. Niranjan, SJ, Opoku-Agyeman, W, Hardy, CM, Bowman, T, Vedre-Kyanam, A, Hearld, KR, et al. Using community health advisors to increase lung cancer screening awareness in the Black belt: a pilot study. J Cancer Education (2023) 38(4):1286–95. doi:10.1007/s13187-022-02261-w
75. Williams, MA, Nielsen, DR, Dayao, Z, Brown-Glaberman, U, and Tawfik, B. Patient-reported measures of a breast cancer nurse navigator program in an underserved, rural, and economically disadvantaged patient population. Oncol Nurs Forum (2022) 49(6):532–9. doi:10.1188/22.Onf.532-539
76. Holle, LM, Levine, J, Buckley, T, White, CM, White, C, and Hadfield, MJ. Pharmacist intervention in colorectal cancer screening initiative. J Am Pharm Assoc (2003) (2020) 60(4):e109–e116. doi:10.1016/j.japh.2020.02.014
77. Centra, T, and Fogg, C. Addressing barriers to colorectal cancer screening in a federally qualified health center. J Am Assoc Nurse Pract (2023) 35(7):415–24. doi:10.1097/jxx.0000000000000828
78. Kim, KE, Tangka, FKL, Jayaprakash, M, Randal, FT, Lam, H, Freedman, D, et al. Effectiveness and cost of implementing evidence-based interventions to increase colorectal cancer screening among an underserved population in chicago. Health Promotion Pract (2020) 21(6):884–90. doi:10.1177/1524839920954162
79. Molokwu, JC, Dwivedi, A, Alomari, A, and Shokar, N. Effectiveness of a breast cancer education screening and NavigaTion (BEST) intervention among hispanic women. Health Promotion Pract (2023):15248399221135762. doi:10.1177/15248399221135762
80. Richman, AR, Torres, E, Wu, Q, and Kampschroeder, AP. Evaluating a community-based breast cancer prevention program for rural underserved latina and Black women. J Community Health (2020) 45(6):1205–10. doi:10.1007/s10900-020-00856-2
81. Azizoddin, DR, Lakin, JR, Hauser, J, Rynar, LZ, Weldon, C, Molokie, R, et al. Meeting the guidelines: implementing a distress screening intervention for veterans with cancer. Psychooncology (2020) 29(12):2067–74. doi:10.1002/pon.5565
82. Rajan, SS, Begley, CE, Highfield, LD, and Kim, B. Survival benefits of treatment access among underserved breast cancer patients diagnosed through the Texas breast and cervical cancer services program. J Public Health Management Pract (2015) 21(5):477–86. doi:10.1097/phh.0000000000000255
83. Sabesan, S, Roberts, LJ, Aiken, P, Joshi, A, and Larkins, S. Timely access to specialist medical oncology services closer to home for rural patients: experience from the Townsville Teleoncology Model. Aust J Rural Health (2014) 22(4):156–9. doi:10.1111/ajr.12101
84. Sabesan, S, Senko, C, Schmidt, A, Joshi, A, Pandey, R, Ryan, CA, et al. Enhancing chemotherapy capabilities in rural hospitals: implementation of a telechemotherapy model (QReCS) in north queensland, Australia. J Oncol Pract (2018) 14(7):e429–e437. doi:10.1200/jop.18.00110
85. Watanabe, SM, Fairchild, A, Pituskin, E, Borgersen, P, Hanson, J, and Fassbender, K. Improving access to specialist multidisciplinary palliative care consultation for rural cancer patients by videoconferencing: report of a pilot project. Support Care Cancer (2013) 21(4):1201–7. doi:10.1007/s00520-012-1649-7
86. Hitt, WC, Low, GM, Lynch, CE, Gauss, CH, Magann, EF, Lowery, CL, et al. Application of a telecolposcopy program in rural settings. Telemed e-Health (2016) 22(10):816–20. doi:10.1089/tmj.2015.0260
87. Drake, B, James, A, Miller, H, Anandarajah, A, Davis, KL, Jackson, S, et al. Strategies to achieve breast health equity in the St. Louis region and beyond over 15+ years. Cancers (Basel) (2022) 14(10):2550. doi:10.3390/cancers14102550
88. Luckett, R, Pena, N, Vitonis, A, Bernstein, MR, and Feldman, S. Effect of patient navigator program on no-show rates at an academic referral colposcopy clinic. J Women's Health (2015) 24(7):608–15. doi:10.1089/jwh.2014.5111
89. Vilchis, H, Onstad, LE, Benavidez, R, Castillo, R, Bush, N, Sanchez, J, et al. Una mano amiga: pilot test of a patient navigator program for southwest New Mexico. J Cancer Education (2019) 34(1):173–9. doi:10.1007/s13187-017-1283-7
90. Lara, CL, Means, KL, Morwood, KD, Lighthall, WR, Hoover, S, Tangka, FK, et al. Colorectal cancer screening interventions in 2 health care systems serving disadvantaged populations: screening uptake and cost-effectiveness. Cancer (2018) 124(21):4130–6. doi:10.1002/cncr.31691
91. Williams, MS, Wells, J, Duhé, RJ, Shirley, T, Lampkin, A, Murphy, M, et al. The college of American pathologists foundation’s see, test and treat Program®: an evaluation of a one-day cancer screening program implemented in Mississippi. J Cancer Education (2022) 37(6):1912–7. doi:10.1007/s13187-021-02060-9
92. Johnson, CJ, Morawski, BM, Hobbs, L, Lewis, D, Cariou, C, and Rycroft, RK. Time from breast cancer diagnosis to treatment among Idaho's national breast and cervical cancer early detection program population, 2011-2017. Cancer Causes Control (2021) 32(6):667–73. doi:10.1007/s10552-021-01407-3
93. Wakefield, D If not home, where? Implementing an innovative model of care as an alternative place of care and death for patients living in an area of high socio-economic deprivation. Short-report on opening a long-term palliative care unit. Palliat Med (2023) 37(4):652–6. doi:10.1177/02692163221133984
94. Mette, LA, Saldívar, AM, Poullard, NE, Torres, I, Seth, S, Pollock, B, et al. Reaching high-risk underserved individuals for cancer genetic counseling by video-teleconferencing. J Community Support Oncol (2016) 14(4):162–8. doi:10.12788/jcso.0247
95. Wilson-Anderson, K, Williams, PR, Beacham, T, and McDonald, N. Breast health teaching in predominantly African American rural Mississippi Delta. The ABNF J (2013) 24(1):28–33.
96. Hoskins, KF, Tejeda, S, Vijayasiri, G, Chukwudozie, IB, Remo, MH, Shah, HA, et al. A feasibility study of breast cancer genetic risk assessment in a federally qualified health center. Cancer (2018) 124(18):3733–41. doi:10.1002/cncr.31635
97. Mayfield-Johnson, S, Fastring, D, Fortune, M, and White-Johnson, F. Addressing breast cancer health disparities in the Mississippi delta through an innovative partnership for education, detection, and screening. J Community Health (2016) 41(3):494–501. doi:10.1007/s10900-015-0121-2
98. Sanchez-Birkhead, AC, Carbajal-Salisbury, S, Arce Larreta, J, Hendricks, H, and Beck, SL. Addressing disparities: the alliance breast cancer community-based program for hispanic women. Clin J Oncol Nurs (2016) 20(5):481–6. doi:10.1188/16.Cjon.20-05ap
99. Thai, CL, Ong, G, Tran, T, and Le, Y. Assessing the impact of a patient navigator intervention program for Vietnamese-American women with abnormal mammograms. J Cancer Education (2022) 37(3):621–30. doi:10.1007/s13187-020-01856-5
100. Fang, CY, Ma, GX, Handorf, EA, Feng, Z, Tan, Y, Rhee, J, et al. Addressing multilevel barriers to cervical cancer screening in Korean American women: a randomized trial of a community-based intervention. Cancer (2017) 123(6):1018–26. doi:10.1002/cncr.30391
101. Tsai, PY, Peterman, B, Baisch, MJ, Ji, ES, and Zwiers, K. Providing and funding breast health services in urban nurse-managed health centers. Nurs Outlook (2014) 62(3):204–11. doi:10.1016/j.outlook.2014.01.001
102. Kiser, LH, and Butler, J. Improving equitable access to cervical cancer screening and management. AJN, Am J Nurs (2020) 120(11):58–67. doi:10.1097/01.Naj.0000721944.67166.17
103. Limaye, N, Zorzato, D, Nadarajasundaram, A, and Ong, SBY. Increasing the uptake in a general practice prostate cancer screening programme. BMJ Open Qual (2022) 11(1):e001701. doi:10.1136/bmjoq-2021-001701
104. Khalil, S, Hatch, L, Price, CR, Palakurty, SH, Simoneit, E, Radisic, A, et al. Addressing breast cancer screening disparities among uninsured and insured patients: a student-run free clinic initiative. J Community Health (2020) 45(3):501–5. doi:10.1007/s10900-019-00767-x
105. Eberth, JM, Thibault, A, Caldwell, R, Josey, MJ, Qiang, B, Peña, E, et al. A statewide program providing colorectal cancer screening to the uninsured of South Carolina. Cancer (2018) 124(9):1912–20. doi:10.1002/cncr.31250
106. Peppercorn, J, Horick, N, Houck, K, Rabin, J, Villagra, V, Lyman, GH, et al. Impact of the elimination of cost sharing for mammographic breast cancer screening among rural US women: a natural experiment. Cancer (2017) 123(13):2506–15. doi:10.1002/cncr.30629
107. Offman, J, Myles, J, Ariyanayagam, S, Colorado, Z, Sharp, M, Cruice, M, et al. A telephone reminder intervention to improve breast screening information and access. Public Health (2014) 128(11):1017–22. doi:10.1016/j.puhe.2014.09.007
108. Gunness, P, Hamilton, S, Capstick, R, Masters, J, and Toma, R. The development of a rural breast reconstruction service: patient reported outcomes and benefits. ANZ J Surg (2023) 93(7-8):1935–7. doi:10.1111/ans.18389
109. van den Bruele, AB, Sevilimedu, V, Jochelson, M, Formenti, S, Norton, L, and Sacchini, V. Mobile mammography in New York City: analysis of 32,350 women utilizing a screening mammogram program. NPJ Breast Cancer (2022) 8(1):14. doi:10.1038/s41523-022-00381-6
110. Gabitova, G, and Burke, NJ. Improving healthcare empowerment through breast cancer patient navigation: a mixed methods evaluation in a safety-net setting. BMC Health Serv Res (2014) 14:407. doi:10.1186/1472-6963-14-407
111. Li, J, Cornacchi, SD, Farrokhyar, F, Johnston, N, Forbes, S, Reid, S, et al. Relation between socioeconomic variables and surgical, systemic and radiation treatment in a cohort of patients with breast cancer in an urban Canadian centre. Can J Surg (2019) 62(2):83–92. doi:10.1503/cjs.009217
112. Mema, SC, Yang, H, Elnitsky, S, Jiang, Z, Vaska, M, and Xu, L. Enhancing access to cervical and colorectal cancer screening for women in rural and remote northern Alberta: a pilot study. CMAJ Open (2017) 5(4):E740–e745. doi:10.9778/cmajo.20170055
113. Galiatsatos, P, Schreiber, R, Green, K, Shah, R, Lee, H, Feller-Kopman, D, et al. Improving lung cancer screening: an equitable strategy through a tobacco treatment clinic. Prev Med Rep (2021) 24:101558. doi:10.1016/j.pmedr.2021.101558
114. Le, T, Miller, S, Berry, E, Zamarripa, S, Rodriguez, A, Barkley, B, et al. Implementation and uptake of rural lung cancer screening. J Am Coll Radiol (2022) 19(3):480–7. doi:10.1016/j.jacr.2021.12.003
115. Humer, MF, and Campling, BG. The role of telemedicine in providing thoracic oncology care to remote areas of British Columbia. Curr Oncol Rep (2017) 19(8):52. doi:10.1007/s11912-017-0612-7
116. Swayze, EJ, Strzyzewski, L, Avula, P, Zebolsky, AL, and Hoekstra, AV. The impact of expanding gynecologic oncology care to ovarian cancer patients in small cities and rural communities. Gynecol Oncol (2021) 161(3):852–7. doi:10.1016/j.ygyno.2021.04.021
117. Tracy, R, Nam, I, and Gruca, TS. The influence of visiting consultant clinics on measures of access to cancer care: evidence from the state of Iowa. Health Serv Res (2013) 48(5):1719–29. doi:10.1111/1475-6773.12050
118. Thota, R, Gill, DM, Brant, JL, Yeatman, TJ, and Haslem, DS. Telehealth is a sustainable population health strategy to lower costs and increase quality of health care in rural Utah. JCO Oncol Pract (2020) 16(7):e557–e562. doi:10.1200/jop.19.00764
119. Nnorom, O, Sappong-Kumankumah, A, Olaiya, OR, Burnett, M, Akor, N, Shi, N, et al. Afrocentric screening program for breast, colorectal, and cervical cancer among immigrant patients in Ontario. Can Fam Physician (2021) 67(11):843–9. doi:10.46747/cfp.6711843
120. Schroeder, K, Panny, A, and Shimpi, N. Community awareness and oral cancer screening in rural Wisconsin. J Dent Hyg (2021) 95(4):51–8.
121. Lane, AJ, and Martin, MT. Ten year profile of a best practice program aimed at rural women. Online J Rural Nurs Health Care (2015) 15(2). doi:10.14574/ojrnhc.v15i2.363
122. Mostafaei, A, Sadeghi-Ghyassi, F, Kabiri, N, and Hajebrahimi, S. Experiences of patients and providers while using telemedicine in cancer care during COVID-19 pandemic: a systematic review and meta-synthesis of qualitative literature. Support Care Cancer (2022) 30(12):10483–94. doi:10.1007/s00520-022-07415-6
123. Caputo, MP, Rodriguez, CS, Padhya, TA, and Mifsud, MJ. Telehealth interventions in head and neck cancer patients: a systematic review. Cancer Nurs (2023) 46(5):E320–e327. doi:10.1097/ncc.0000000000001130
124. Chesser, A, Burke, A, Reyes, J, and Rohrberg, T. Navigating the digital divide: a systematic review of eHealth literacy in underserved populations in the United States. Inform Health Social Care (2016) 41(1):1–19. doi:10.3109/17538157.2014.948171
125. Lee, EW, McCloud, RF, and Viswanath, K. Designing effective eHealth interventions for underserved groups: five lessons from a decade of eHealth intervention design and deployment. J Med Internet Res (2022) 24(1):e25419. doi:10.2196/25419
126. Nagler, RH, Ramanadhan, S, Minsky, S, and Viswanath, K. Recruitment and retention for community-based eHealth interventions with populations of low socioeconomic position: strategies and challenges. J Commun (2013) 63(1):201–20. doi:10.1111/jcom.12008
127. Jessiman-Perreault, G, Law, J, Adhikari, K, Machado, AA, Moysey, B, Xu, L, et al. Geospatial analysis and participant characteristics associated with colorectal cancer screening participation in Alberta, Canada: a population-based cross-sectional study. BMC Health Serv Res (2023) 23(1):1454. doi:10.1186/s12913-023-10486-8
128. Gotfrit, J, Thangarasa, T, Dudani, S, Goodwin, R, Tang, PA, Monzon, J, et al. The impact of driving time, distance, and socioeconomic factors on outcomes of patients with locally advanced rectal cancer. Public Health Pract (Oxford, England) (2020) 1:100012. doi:10.1016/j.puhip.2020.100012
129. Abrams, HR, Durbin, S, Huang, CX, Johnson, SF, Nayak, RK, Zahner, GJ, et al. Financial toxicity in cancer care: origins, impact, and solutions. Translational Behav Med (2021) 11(11):2043–54. doi:10.1093/tbm/ibab091
130. MacPhail, C, and Snow, S. Not all Canadian cancer patients are equal-disparities in public cancer drug funding across Canada. Curr Oncol (2022) 29(3):2064–72. doi:10.3390/curroncol29030166
131. Wood, TF, and Murphy, RA. Tackling financial toxicity related to cancer care in Canada. CMAJ (2024) 196(9):E297–e298. doi:10.1503/cmaj.230677
132. Canadian Association of Nurses in Oncology. Patient Navigator in cancer care-A specialized oncology nurse role that contributes to high-quality, person-centred care experiences and clinical efficiencies. Can Oncol Nurs J Summer (2020) 30(3):227–8.
133. Katerenchuk, J, and Santos Salas, A. An integrative review on the oncology nurse navigator role in the Canadian context. Can Oncol Nurs J (2023) 33(4):385–99. doi:10.5737/23688076334385
134. Ghahari, S, Burnett, S, and Alexander, L. Development and pilot testing of a health education program to improve immigrants' access to Canadian health services. BMC Health Serv Res (2020) 20(1):321. doi:10.1186/s12913-020-05180-y
135. Dunn, SF, Lofters, AK, Ginsburg, OM, Meaney, CA, Ahmad, F, Moravac, MC, et al. Cervical and breast cancer screening after cares: a community program for immigrant and marginalized women. Am J Prev Med (2017) 52(5):589–97. doi:10.1016/j.amepre.2016.11.023
136. Salem, A, and Bayman, N. Multidisciplinary team service redesign: a step to improved quality of care for lung cancer patients. Clin Oncol (2016) 28(12):800–1. doi:10.1016/j.clon.2016.06.012
137. Largey, G, Briggs, P, Davies, H, Underhill, C, Ross, C, Harvey, K, et al. Victorian Lung Cancer Service Redesign Project: impacts of a quality improvement collaborative on timeliness and management in lung cancer. Intern Med J (2021) 51(12):2061–8. doi:10.1111/imj.15043
138. O'Cathain, A, Croot, L, Duncan, E, Rousseau, N, Sworn, K, Turner, KM, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ open (2019) 9(8):e029954. doi:10.1136/bmjopen-2019-029954
139. Skivington, K, Matthews, L, Simpson, SA, Craig, P, Baird, J, Blazeby, JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ (2021) 374:n2061. doi:10.1136/bmj.n2061
140. Cook, ED, Yeager, KA, Cecchini, RS, Boparai, J, Brown, CL, Duncan, M, et al. Recruitment practices for U.S. minority and underserved populations in NRG oncology: results of an online survey. Contemp Clin trials Commun (2018) 10:100–4. doi:10.1016/j.conctc.2018.03.003
141. Rogers, CR, Matthews, P, Brooks, E, Le Duc, N, Washington, C, McKoy, A, et al. Barriers to and facilitators of recruitment of adult african American men for colorectal cancer research: an instrumental exploratory case study. JCO Oncol Pract (2021) 17(5):e686–e694. doi:10.1200/op.21.00008
142. Stockman, LS, Gundersen, DA, Gikandi, A, Akindele, RN, Svoboda, L, Pohl, S, et al. The colocation model in community cancer care: a description of patient clinical and demographic attributes and referral pathways. JCO Oncol Pract (2023) 19(6):e916–e926. doi:10.1200/op.22.00487
143. Winkfield, KM, Regnante, JM, Miller-Sonet, E, González, ET, Freund, KM, and Doykos, PM. Development of an actionable framework to address cancer care disparities in medically underserved populations in the United States: expert roundtable recommendations. JCO Oncol Pract (2021) 17(3):e278–e293. doi:10.1200/op.20.00630
144. Matsuda, Y, Brooks, JL, and Beeber, LS. Guidelines for research recruitment of underserved populations (EERC). Appl Nurs Res (2016) 32:164–70. doi:10.1016/j.apnr.2016.07.009
145. Huang, B, De Vore, D, Chirinos, C, Wolf, J, Low, D, Willard-Grace, R, et al. Strategies for recruitment and retention of underrepresented populations with chronic obstructive pulmonary disease for a clinical trial. BMC Med Res Methodol (2019) 19(1):39. doi:10.1186/s12874-019-0679-y
Keywords: systematic review, health equity, neoplasms, underserved populations, healthcare access, high income countries, universal healthcare
Citation: Santos Salas A, Bassah N, Pujadas Botey A, Robson P, Beranek J, Iyiola I and Kennedy M (2024) Interventions to improve access to cancer care in underserved populations in high income countries: a systematic review. Oncol. Rev. 18:1427441. doi: 10.3389/or.2024.1427441
Received: 03 May 2024; Accepted: 30 September 2024;
Published: 05 November 2024.
Edited by:
Jozsef Dudas, Innsbruck Medical University, AustriaReviewed by:
Samvel Bardakhchyan, Yerevan State Medical University, ArmeniaThomas Rosenthal, University at Buffalo, United States
Copyright © 2024 Santos Salas, Bassah, Pujadas Botey, Robson, Beranek, Iyiola and Kennedy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Santos Salas, YXZzQHVhbGJlcnRhLmNh
 Anna Santos Salas
Anna Santos Salas Nahyeni Bassah
Nahyeni Bassah Anna Pujadas Botey2,3
Anna Pujadas Botey2,3