- 1Department of Nuclear Medicine, Red Cross Children’s Hospital and Groote Schuur Hospital, University of Cape Town, Cape Town, South Africa
- 2Clinical Theranostics, Department of Nuclear Medicine, Mallinckrodt Institute of Radiology, Washington University, St Louis, MO, United States
Neuroendocrine tumors (NETs) are not commonly diagnosed in children. Metastatic NETs tend to have poor outcomes, and this is seen in adult and pediatric populations. The role of somatostatin receptor imaging using [68Ga]Ga-DOTA-TATE for imaging and peptide receptor radionuclide therapy (PRRT) with [177Lu]Lu-DOTA-TATE in children is currently not well established. The guidelines for treating pediatric neuroendocrine tumors are still lacking. Extensive trials have been conducted in adult patients and have demonstrated improved survival in metastatic NETs with PRRT using [177Lu]Lu-DOTA-TATE. We present two pediatric patients with metastatic NETs who were imaged with [68Ga]Ga-DOTA-TATE PET/CT and treated with [177Lu]Lu-DOTA-TATE therapy.
1. Introduction
Neuroendocrine tumors (NETs) in the pediatric population are not common in occurrence; however, their incidence and prevalence have increased over the last few decades (1). The incidence of NETs in children has been documented as 2.8 per 1 million children according to the SEER registries of 1976–2006, which looked at the incidence, survival, and prevalence of neuroendocrine tumors in children (2). NETs are commonly seen in the midgut in the pediatric population (1, 2). Several treatment options are available according to different NET guidelines; however, none of these are directly aimed at pediatric populations (3–6). The majority of NETs express somatostatin receptors (SSTR), which are targeted by somatostatin analogs (SSA). Novel radiopeptides labeled with SSA are now the method of choice to fully stage and localize the extent of disease in patients with NETs via [68Ga]Ga-DOTA-TATE positron emission tomography/computed tomography (PET/CT). The same SSA analog can be coupled in the form of DOTA with [177Lu]Lu-DOTA-TATE to provide peptide receptor radionuclide therapy (PRRT) in patients with metastatic well-differentiated NETs (3, 6, 7).
In metastatic non-resectable disease, NETs are managed sequentially with SSA, novel targeted drugs (Everolimus/Sunitinib), chemotherapy, and PRRT (6). Radioligand therapy in pediatrics has been demonstrated with [90Y]Y-DOTA-TOC and [177Lu]Lu-DOTA-TATE in solid tumors, including NETs; however, no prospective trials have been carried out and evidence is limited (8–10). In this communication, we present and report our experience in treating two pediatric patients with extra-appendiceal gastroenteropancreatic (GEP) NETs. The two patients are both approximately the same age, with high tumor burden, hepatic metastasis, and molecular imaging and therapy with SSTR ligands.
2. Patient A
2.1. Clinical history and investigations (before PRRT)
A 9-year-old boy presented to the hospital in December 2017 after fainting at school preceded by shortness of breath on minimal exertion a few weeks prior. At presentation, he was noted to be severely anemic, with a hemoglobin of 4.0 g/dL, and in heart failure. He was resuscitated with transfusions. Further evaluation revealed severe iron deficiency and the patient was started on iron supplementation. At the time, chronic infections, gastrointestinal bleeding, and parasite infestation were excluded and the cause was considered to be dietary.
Six months later, there was a second fainting spell, now with a hemoglobin of 3 g/dL at presentation, and more frequent relapses of approximately four more episodes over the next year, each requiring blood transfusion. Bone marrow biopsy showed hyperplastic erythropoiesis secondary to anemia. The iron deficiency was also not corrected despite therapeutic iron supplementation.
An upper gastrointestinal tract endoscopy that was subsequently performed to exclude peptic ulceration revealed a macroscopic gastric ulcer, which was biopsied. The histology showed numerous Helicobacter pylori and florid active chronic Helicobacter pylori gastritis. A repeat scope 2 months later showed no interval decrease in the lesion macroscopically, despite proton pump inhibitors and H. pylori eradication therapy, and numerous H. pylori on histology. Zollinger-Ellison syndrome was excluded based on normal serum gastrin levels. Biopsies from endoscopy showed neuroendocrine cells, on account of which a partial gastrectomy was carried out, involving the greater curvature of the stomach and the primary tumor site. Histology demonstrated a transmural infiltrating tumor with a nested growth pattern, with lymphovascular as well as perigastric soft-tissue invasion. There was no discohesive or tubular morphology. A grade 2 neuroendocrine tumor with regional lymph node involvement was diagnosed with the following characteristics: mitotic count=2 per 10 high-power fields; immunohistochemistry=CK7 – patchy, chromogranin A-positive, HEPAR 1-positive, synaptophysin-positive, and Ki-67 5%–8%; staging=pT4a, N1b; and Mx= Stage IIIb.
The patient had no family history of malignancies and genetic screening was not acquired for this patient.
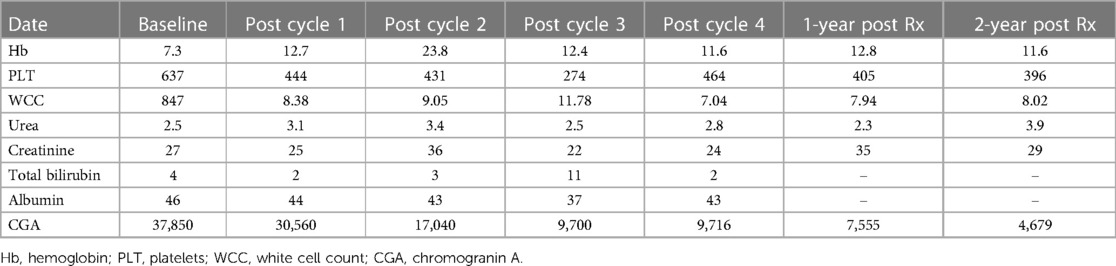
The patient had multiple investigations. Abdominal ultrasound showed multiple hypoechoic lesions in the liver that were also seen on CT. A staging [68Ga]Ga-DOTA-NOC PET/CT demonstrated multiple [68Ga]Ga-DOTA-NOC avid liver and intra-abdominal lymph node metastases (Figure 1). The initial chromogranin A was 37 850 ng/ml (normal: <109); urine 5-hydroxyindoleacetic acid (HIAA): creatinine ratio=10.2 (normal: 0–8.3). Hematological, renal, and liver functions were within normal limits for therapy. Due to the non-availability of somatostatin analogs at his referring hospital, the patient was referred for PRRT with [177Lu]Lu-DOTA-TATE therapy.
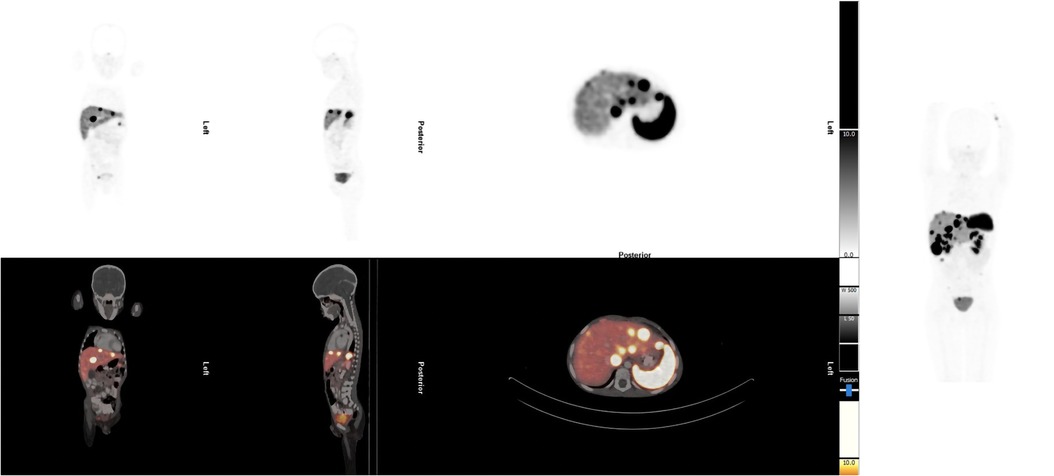
Figure 1. Staging [68Ga]Ga-DOTA-NOC PET/CT demonstrated multiple [68Ga]Ga-DOTA-NOC avid liver and intra-abdominal lymph node metastases.
The patient received four cycles of PRRT (2.5 Giga-becquerels per cycle) (100 MBq/kg) (11), with [177Lu]Lu-DOTA-TATE, and had no adverse effects after therapy. The treatment resulted in a biochemical response and stable disease. The patient is still well to date, is growing well, and has not experienced any relapses. The overall survival since the start of therapy is 37 months (Figure 2A demonstrating post-therapy uptake in whole body imaging after each cycle) (12). The uptake in whole-body imaging and Figure 2B show post-therapy tumor markers. The patient’s follow-up radiological imaging demonstrated stable disease. RECIST 1.1 (12) The biochemistry following each cycle has been included in Table 1.
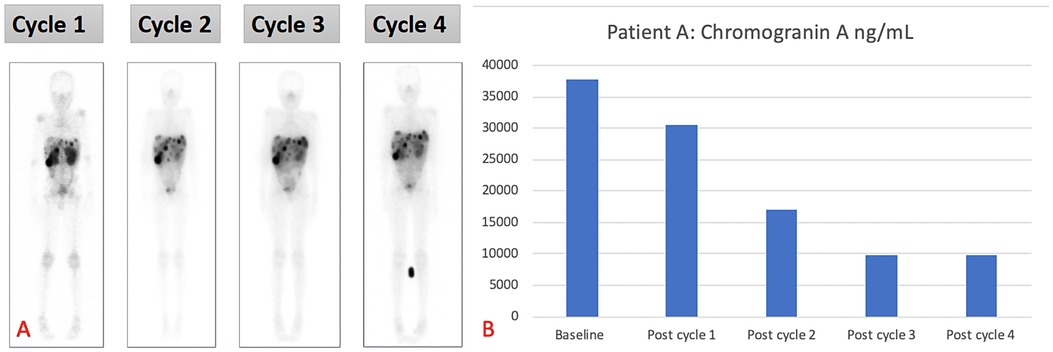
Figure 2. (A) Demonstrating post-therapy uptake in whole-body imaging and (B) post-therapy tumor markers for patient A.
3. Patient B
3.1. Clinical history and investigations (before PRRT)
A 9-year-old boy presented with a 5-month history of intermittent chronic abdominal pain, 1 month of intermittent vomiting with occasional hemoptysis, and associated weight loss. He had no other significant medical history except for preterm birth at 34 weeks of gestation. His surgical history included a right inguinal herniotomy in March 2019. On clinical examination, there was hepatomegaly 3 cm below the costal margin and non-significant lymphadenopathy. A liver biopsy confirmed the diagnosis of NET. Histology confirmed a metastatic NET, the primary neoplasm could not be demonstrated on the liver biopsy, the comment being that primary neoplasm in these cases may be inconspicuous or microscopic. The tumor demonstrated a Ki67 of 15% and a mitotic count of 6 per 10 high-power fields. The immunohistochemistry demonstrated the following positive markers: synaptophysin, chromogranin A, CAM5.2, S100, and CD99. The following markers were negative: gastrin, glucagon, insulin, CDX2, HEPAR1, TTF1, and Glypican3.. The patient had no family history of malignancies, and genetic screening was not acquired for this patient.
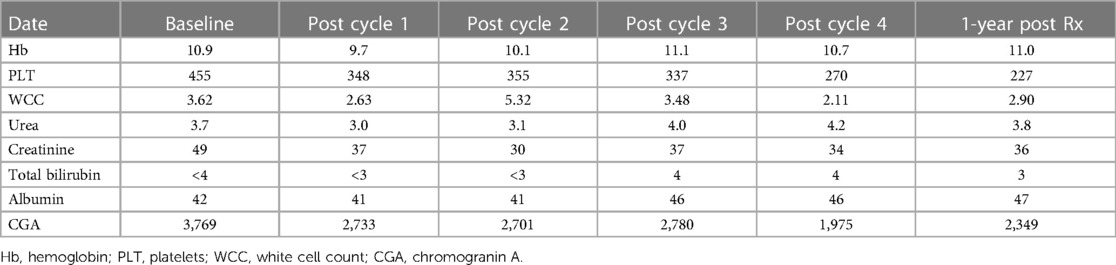
Abdominal ultrasound showed one hypoechoic lesion of 8 mm × 6 mm in both lobes of the liver with no other abnormal finding. A CT scan of the abdomen demonstrated hepatomegaly with multiple heterogeneous round lesions scattered in the right and left lobes. An underlying non-benign process was suggested for consideration, including metastatic deposits, lymphoma, or multifocal hepatoblastoma. The CT scan of the chest was normal. The liver biopsy demonstrated features in keeping with metastatic NET, Ki-67 of approximately 15%, and a mitotic count of 6 per 10 high-power fields. The baseline chromogranin A was 2,748 ng/mL. Hematological, renal, and liver functions were within the normal limits for therapy. A staging [68Ga]Ga-DOTATATE PET/CT demonstrated multiple [68Ga]Ga-DOTA-TATE avid liver lesions with no other sites of abnormal uptake (Figure 3). Due to the non-availability of somatostatin analogs at his referring hospital, the patient was referred for PRRT with [177Lu]Lu-DOTA-TATE therapy.
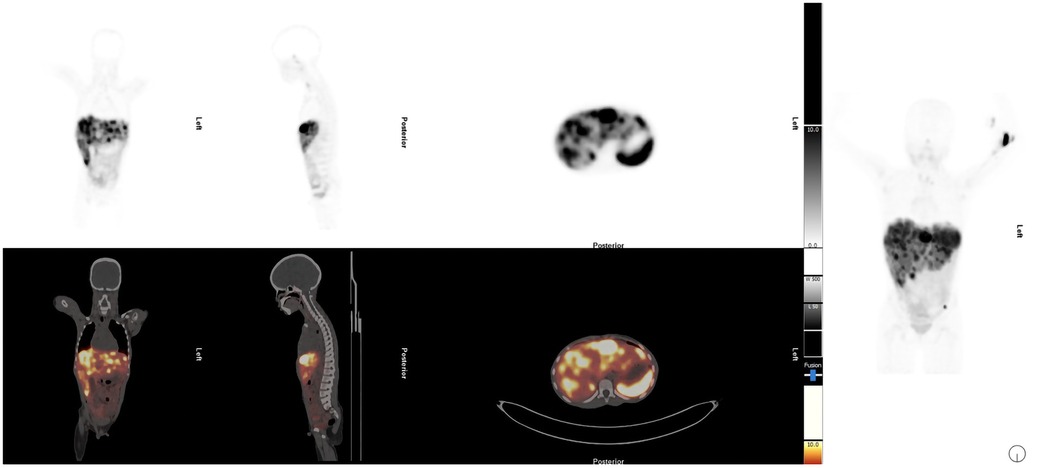
Figure 3. Staging [68Ga]Ga-DOTATATE PET/CT demonstrated multiple [68Ga]Ga-DOTA-TATE avid liver lesions with no other sites of abnormal uptake.
The patient received four cycles of PRRT (2.5 GBq/cycle) with [177Lu]Lu-DOTA-TATE and had no adverse effects after therapy. The treatment resulted in a biochemical response (Figure 4A shows the post-therapy uptake in whole-body images and Figure 4B shows the post-therapy tumor markers). The patient relapsed 6 months after completion of the four cycles of PRRT and was treated with somatostatin analogs. Somatostatin analogs were available in the state hospital sector by the time of relapse but were not initially available on presentation. He remains stable, is growing well, and is alive to date with an overall survival of 21 months after the initial therapy. His follow-up radiological imaging demonstrated stable disease (RECIST 1.1) (12). The biochemistry results after each treatment cycle are shown in Table 2.
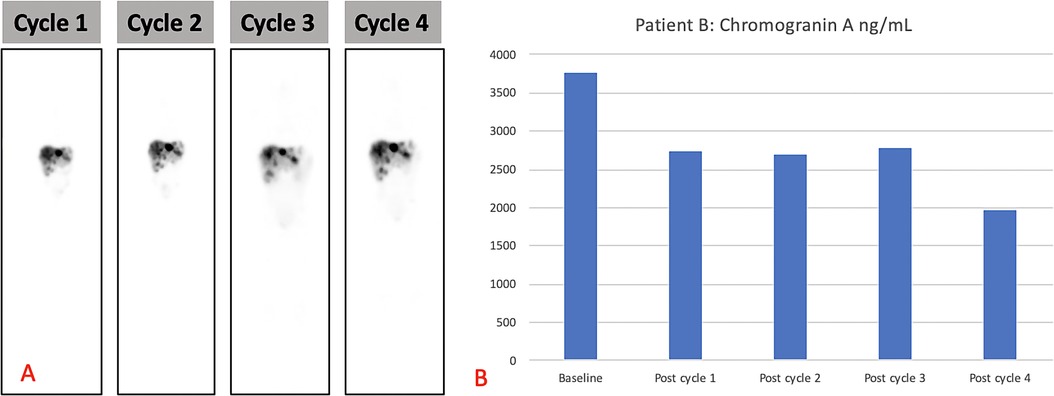
Figure 4. (A) Demonstrating post-therapy uptake in whole-body images and (B) post-therapy tumor markers for patient B.
4. Discussion
We report our initial experience with [68Ga]Ga-DOTA-TATE/[177Lu]Lu-DOTA-TATE theragnostic combination in two children with metastatic NETs. In this report, we have demonstrated a good biochemical response to [177Lu]Lu-DOTA-TATE with evidence of stable disease on post-therapy imaging. Our findings demonstrate the feasibility of utilizing [177Lu]Lu-DOTA-TATE in pediatric patients with metastatic NET and achieving a durable treatment response. These results are concordant with the case report by Foster et al., who treated two patients of a similar age and demonstrated minimal side effects after therapy, a biochemical response, and stable disease after therapy for one patient (13).
We also demonstrate the utility of the [68Ga]Ga-DOTA-TATE/[177Lu]Lu-DOTA-TATE theragnostic pair in NETs. The pair has also been used in pediatric patients with refractory metastatic neuroblastoma. The therapy was found to be feasible and well tolerated with limited toxicity (14). Pretherapy imaging was beneficial in that it demonstrated additional disease that was not visualized on standard radiological imaging (14).
Our report underscores that the two children had unique and rather rare presentations for pediatric NETs. Presenting symptoms in pediatric NETs more commonly include features of acute appendicitis, non-specific abdominal pain, and, less commonly, features of carcinoid syndrome (1). No report was found in the literature with a presentation of syncope and anemia as presented in patient A. There is also a low incidence of hepatic metastasis on pediatric NETs demonstrated in the literature, and a high tumor burden at presentation is not common. The more common presentation is midgut NET, even though patients are diagnosed late and often with metastatic disease (1). Both patients A and B, unlike the predominant pattern, had hepatic metastasis and high tumor burden findings that were clearly demonstrated on [68Ga]Ga-DOTA-TATE imaging. This important imaging modality also serves as the bedrock for planning PRRT for NETs and has been clearly demonstrated to have significant benefits. PRRT has been demonstrated as a safe procedure with low toxicity to the marrow and kidneys (15). Similar to diagnostic imaging with SSTR, there is also a paucity of literature on PRRT in the pediatric population.
Clinical trials in adults with midgut NETs preliminarily demonstrated improved progression-free survival and evidence of overall survival when using [177Lu]Lu-DOTA-TATE (16). However, the final analysis did not reach statistical significance regarding overall survival despite remaining clinically relevant with minimal side effects and toxicity in the long-term follow-up (17). There are various other phase-III clinical trials that aim to establish the role of PRRT in low- and high-grade NETS, namely, the COMPETE trial, which evaluates the safety of [177Lu]Lu-Edotreotide in comparison with Everolimus in patients with G1 and G2 pancreatic NETs and GE NETs. Another trial using a similar radionuclide is COMPOSE, which compares [177Lu]Lu-Edotreotide as the first- or second-line treatment with the best standard of care in patients with well-differentiated G2 and G3 GEPNETs. Lastly, NETTER-2 compares [177Lu]Lu-DOTA-TATE as first-line therapy compared to Octerotide in patients with well-differential G2 or G3 GEP-NETs (18, 19). Prospective trials in pediatric patients with metastatic NET are yet to be established, but the advantage of therapy has been demonstrated in solid tumors, neuroblastoma, and in limited NET (8–10). The limitation of this case series is the absence of dosimetry and the standardization of therapy in pediatric populations. Nevertheless, these cases highlight the benefit of using PRRT in children with metastatic NETs and its potential advantages. Large-scale prospective data will be helpful in order to establish the use of PRRT in the pediatric population.
Conclusion
The findings of this case series demonstrate that PRRT is a safe and effective option in children with metastatic GEP NETs. Clinical trials in pediatrics need to be established to have a standard of care for this patient population.
Data availability statement
The data analyzed in this study are subject to the following licenses/restrictions: The data presented in this article are not readily available as they include information pertaining to minors. Requests to access the datasets should be directed to Red Cross Children's Hospital/Groote Schuur Hospital Department of Nuclear Medicine. Requests to access these datasets should be directed toc3R1YXJ0Lm1vcmVAdWN0LmFjLnph.
Ethics statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minors’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
KH, OK, AA, RS, AB, VP, and SM contributed to the conception and design of the case report. KH and OK wrote the first draft of the manuscript. KH, OK, AA, RS, AB, VP, and SM wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to acknowledge all the staff in the nuclear medicine department who assisted in treating our patients and the multidisciplinary team for trusting us with their patients.
Conflict of interest
The author VP declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Farooqui ZA, Chauhan A. Neuroendocrine tumors in pediatrics. Glob Pediatr Health. (2019) 6:2333794X19862712. doi: 10.1177/2333794X19862712
2. Navalkele P, O’Dorisio MS, O’Dorisio TM, Zamba GKD, Lynch CF. Incidence, survival, and prevalence of neuroendocrine tumors versus neuroblastoma in children and young adults: nine standard SEER registries, 1975–2006. Pediatr Blood Cancer. (2011) 56:50–7. doi: 10.1002/pbc.22559
3. Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. ENETS Consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. (2016) 103:153–71. doi: 10.1159/000443171
4. Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2020) 31:844–60. doi: 10.1016/j.annonc.2020.03.304
5. Strosberg JR, Halfdanarson TR, Bellizzi AM, Chan JA, Dillon JS, Heaney AP, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. (2017) 46:707–14. doi: 10.1097/MPA.0000000000000850
6. Pavel M, O'Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. (2016) 103:172–85. doi: 10.1159/000443167
7. Zaknun JJ, Bodei L, Mueller-Brand J, Pavel ME, Baum RP, Hörsch D, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. (2013) 40:800–16. doi: 10.1007/s00259-012-2330-6
8. Menda Y, Dorisio MS, Kao S, Khanna G, Michael S, Connolly M, et al. Phase I trial of 90Y-DOTATOC therapy in children and young adults with refractory solid tumors that express somatostatin receptors. J Nucl Med. (2010) 51:1524. doi: 10.2967/jnumed.110.075226
9. Gains JE, Bomanji JB, Fersht NL, Sullivan T, Souza D, Sullivan KP, et al. 177Lu-DOTATATE molecular radiotherapy for childhood neuroblastoma. J Nucl Med. (2011) 52:1041. doi: 10.2967/jnumed.110.085100
10. Chen S, Cheung W, Leung YL, Cheng K, Wong KN, Wong YH, et al. 177Lu-DOTATATE radionuclide therapy for pediatric patients with relapsed high-risk neuroblastoma negative on 131I-MIBG imaging—a pilot study. J Nucl Med. (2018) 59:307. doi: 10.2967/jnumed.117.204685
11. Gains JE, Moroz V, Aldridge MD, Wan S, Wheatley K, Laidler J, et al. A phase IIa trial of molecular radiotherapy with 177-Lutetium DOTATATE in children with primary refractory or relapsed high-risk neuroblastoma. Eur J Nucl Med Mol Imaging. (2020) 47:2348–57. doi: 10.1007/s00259-020-04741-x
12. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
13. Foster JH, Sher A, Seghers V, Poston J, Wells D, Delpassand ES, et al. Peptide receptor radionuclide therapy for treatment of metastatic neuroendocrine tumors in children. Pediatr Blood Cancer. (2021) 68:e29056. doi: 10.1002/pbc.29056
14. Kong G, Hofman M, Murray WK, Wilson S, Wood P, Downie P, et al. Initial experience with gallium-68 DOTA-octreotate PET/CT and peptide receptor radionuclide therapy for pediatric patients with refractory metastatic neuroblastoma. J Pediatr Hematol Oncol. (2016) 38:87–96. doi: 10.1097/MPH.0000000000000411
15. Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. (2015) 42:5–19. doi: 10.1007/s00259-014-2893-5
16. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-DOTATATE for midgut neuroendocrine tumors. N Engl J Med. (2017) 376:125–35. doi: 10.1056/NEJMoa1607427
17. Strosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar A, et al. 177Lu-DOTATATE plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. (2021) 22:1752–63. doi: 10.1016/S1470-2045(21)00572-6
18. Harris PE, Zhernosekov K. The evolution of PRRT for the treatment of neuroendocrine tumors; what comes next? Front Endocrinol. (2022) 13. doi: 10.3389/fendo.2022.941832
Keywords: PRRT, neuroendocrine tumor, pediatrics, DOTA-TATE, theranostics (combined therapeutic and diagnostic technology)
Citation: Hlongwa K, Kolade O, Alnabulsi A, Steyn R, Brink A, Prasad V and More S (2023) Case report: Peptide receptor radioligand therapy in metastatic pediatric neuroendocrine tumors. Front. Nucl. Med. 3:1193880. doi: 10.3389/fnume.2023.1193880
Received: 25 March 2023; Accepted: 10 July 2023;
Published: 3 August 2023.
Edited by:
Egesta Lopci, University of Milan, ItalyReviewed by:
Chiara Maria Grana, European Institute of Oncology (IEO), ItalyDan Granberg, Karolinska Institutet (KI), Sweden
© 2023 Hlongwa, Kolade, Alnabulsi, Steyn, Brink, Prasad and More. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khanyisile Hlongwa a2hhbnlpc2lsZS5obG9uZ3dhQHVjdC5hYy56YQ==
 Khanyisile Hlongwa
Khanyisile Hlongwa Olumayowa Kolade1
Olumayowa Kolade1 Rachelle Steyn
Rachelle Steyn Anita Brink
Anita Brink Vikas Prasad
Vikas Prasad Stuart More
Stuart More