
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nucl. Eng. , 17 October 2023
Sec. Radioactive Waste Management
Volume 2 - 2023 | https://doi.org/10.3389/fnuen.2023.1192463
This article is part of the Research Topic Solubility Phenomena in The Context of Nuclear Waste Disposal View all 10 articles
The solubility and hydrolysis of Be(II) was investigated from undersaturation conditions in alkaline, dilute to concentrated CaCl2 solutions (0.05–3.5 M). Experiments were performed with α-Be(OH)2(cr) under Ar atmosphere at T = (22 ± 2)°C. Aqueous Be speciation was further investigated by means of molecular dynamics (MD) calculations. For the most diluted CaCl2 systems (0.05 and 0.25 M), a solubility minimum is observed at pHm ≈ 9.5 {with [Be(II)] ≈ 10−7 M}, consistent with solubility data previously reported in NaCl and KCl solutions. Above this pHm, and at higher CaCl2 concentrations, a steep increase in the solubility with a slope of ∼ +2 is observed, hinting towards the predominance of the moiety [Be(OH)42–] in the aqueous phase. In NaCl and KCl systems, this hydrolysis species prevails only above pHm ∼ 13, thus supporting the formation of ternary complex/es Ca–Be(II)–OH(aq) in CaCl2 solutions. The analysis of solubility data in combination with MD calculations underpin the key role of the complex Ca2[Be(OH)4]2+ in alkaline to hyperalkaline systems containing Ca. In combination with our previous work in NaCl–NaOH and KCl–KOH systems, complete chemical, thermodynamic and (SIT) activity models are derived for the first time for the system Be2+–Ca2+–Na+–K+–H+–Cl––OH––H2O(l). This model provides an accurate and robust tool for the evaluation of Be(II) solubility and speciation in a diversity of geochemical conditions, including source term calculations of beryllium in the context of repositories for nuclear waste disposal with a high cement inventory.
Beryllium is a widely used metal in test and research fission reactors as neutron reflector/moderator. It is characterized by a relatively low thermal neutron absorption cross section and unique chemical and structural properties (Beeston, 1970; Chandler et al., 2009). During the reactor operation, 9Be undergoes (n, 2n) and (n, α) nuclear reactions resulting in the generation of large quantities of 4He, 3He and 3H. This leads to the swelling of beryllium components with the corresponding alteration of their mechanical properties. The disposal of these components results in specific streams of nuclear waste containing a significant inventory of chemotoxic beryllium (Beeston, 1970; Longhurst et al., 2003; Chandler et al., 2009).
In contrast to the rest of the alkali-earth element series and due to its very small ionic radii (rBe2+ = 0.27 Å, for a coordination number (CN) of 4) (Shannon, 1976; Alderighi et al., 2000), beryllium is characterized by very strong hydrolysis. This results in an amphoteric behaviour involving the formation of positively- and negatively-charged hydrolysis species. Several solubility and potentiometric studies previously investigated the solubility and hydrolysis of beryllium in acidic to near-neutral pH conditions, deriving thermodynamic data for the hydrolysis species Ben(OH)m2n–m, with (n,m) = (1,1), (1,2), (2,1), (3,3), (5,6) and (6,8) (Mattock, 1954; Gilbert and Garrett, 1956; Kakihana and Sillen, 1956; Schindler and Garett, 1960; Carell and Olin, 1961; Schwarzenbach, 1962; Hietanen and Sillen, 1964; Bertin et al., 1967; Mesmer and Baes, 1967; Ohtaki, 1967; Ohtaki and Kato, 1967; Lanza and Carpéni, 1968; Pâris and Gregoire, 1968; Schwarzenbach and Wenger, 1969; Kakihana and Maeda, 1970; Tsukuda et al., 1975; Vanni et al., 1975; Baes and Mesmer, 1976; Bruno et al., 1987b; Bruno, 1987; Chinea et al., 1997; Brown and Ekberg, 2016). A significantly scarcer number of studies investigated the hydrolysis of Be(II) in alkaline to hyperalkaline conditions (Gilbert and Garrett, 1956; Green and Alexander, 1965; Bruno et al., 1987a), where the hydrolysis species Be(OH)3– and Be(OH)42– are expected to prevail. In our recent solubility and 9Be-NMR study, we systematically investigated the solubility and hydrolysis of Be(II) in dilute to concentrated NaCl–NaOH and KCl–KOH systems. Experiments were conducted with a well-defined solid phase, α-Be(OH)2(cr), and extended from acidic to hyperalkaline conditions (Çevirim-Papaioannou et al., 2020). In combination with previous studies available in the literature, chemical, thermodynamic and (SIT) activity models for the system Be2+–Na+–K+–H+–Cl––OH––H2O(l) were derived.
Cementitious materials are widely used in underground repositories for nuclear waste disposal for the stabilization of the waste and for construction purposes. Upon contact with groundwater, cement materials undergo degradation through a sequence of dissolution reactions. In the degradation stage I, the dissolution of K- and Na-oxides/hydroxides imposes hyperalkaline conditions (pH ≈ 13.3) and high alkali content in the pore water. The degradation stage II is characterized by a pore water composition buffered by portlandite at pH ≈ 12.5 and [Ca]tot ≈ 2∙10−2 M. After the complete dissolution of portlandite, the degradation stage III is dominated by the incongruent dissolution of calcium silicate hydrate (C-S-H) phases, with Ca:Si ratios of ≈1.6 to ≈0.6 and 12.5 ≤ pH ≤ 10. In the context of waste disposal in rock salt formations, the corrosion of cementitious waste forms has been reported to potentially generate CaCl2-rich brines (up to 4 M) in combination with very high pH values (pHm ≈ 12, with pHm = –log [H+] in molal units) (Neck et al., 2009; Bube et al., 2013). Intermediate ionic strength conditions (I = 2–3 M) are also found in Cretaceous argillites in Northern Germany (Brewitz, 1980), with pore waters mostly dominated by NaCl and CaCl2.
Previous studies investigating the solubility and hydrolysis of transition metals, lanthanides and actinides in alkaline, concentrated CaCl2 systems have shown the formation and predominance of highly hydrolysed moieties stabilized by the coordination with Ca2+ ions. Using a combination of solubility and TRLFS studies, Neck and co-workers reported the formation of the ternary complexes Ca[M(III)(OH)3]2+, Ca2[M(III)(OH)4]3+ and Ca3[M(III)(OH)6]3+ (with M = Nd, Am, Cm and Pu) in concentrated CaCl2 solutions (Rabung et al., 2008; Neck et al., 2009). The formation of analogous ternary complexes of tetravalent metal ions, e.g., Ca4[An(IV)(OH)8]4+ (An(IV) = Th(IV), Pu(IV) and Np(IV)), Ca3[Zr(IV)(OH)6]4+ and Ca3[Tc(IV)O(OH)5]3+, has been reported to increase the solubility of the corresponding hydrous oxides by several orders of magnitude in alkaline CaCl2 solutions (Brendebach et al., 2007; Altmaier et al., 2008; Fellhauer et al., 2010; Fellhauer, 2013; Yalcintas et al., 2016). In spite of the weaker hydrolysis of the pentavalent actinides, Fellhauer and co-workers observed the formation of the ternary complexes Ca[Np(V)O2(OH)2]+ and Ca3[Np(V)O2(OH)5]2+ in alkaline, concentrated CaCl2 solutions (Fellhauer et al., 2016a; Fellhauer et al., 2016b). Spectroscopic evidence for the participation of Ca2+ in the formation of inner-sphere complexes was provided for Cm(III), Zr(IV), Th(IV) and Np(V), whereas DFT calculations underpin the stability of such complexes in the case of Tc(IV). All investigations above emphasize the key role of Ca2+ in the stabilization of highly hydrolyzed metal ions in concentrated CaCl2 systems with high pH, regardless of the oxidation state of the metal ion. In the case of Zr(IV) and possibly due to the stronger hydrolysis of this metal ion, the ternary complex/es Ca–Zr(IV)–OH become predominant at [CaCl2] > 10−2 M, which emphasizes their relevance also in dilute cementitious systems.
The behaviour of beryllium in aqueous solutions at the molecular level has been investigated in a number of computational studies. Beryllium preferentially coordinates ligands in tetrahedral geometry, has strong hydrolysing and polarising abilities, and shows complex pH-dependent solution chemistry (Marx et al., 1997; Alderighi et al., 2000; Jin et al., 2015; Perera et al., 2017). In our previous studies, it was shown that in cementitious environment Ca2+ ions play a key role in the uptake of Be(II) by C-S-H phases (Çevirim-Papaioannou et al., 2021a; Çevirim-Papaioannou et al., 2021b). Together with evidences gained for M(III), M(IV) and M(V) systems, this anticipates the possible formation of previously unreported ternary complexes Ca–Be(II)–OH in alkaline CaCl2 solutions. Through a combination of experimental solubility studies, molecular dynamics calculations and thermodynamic modelling, this work aims at providing a comprehensive understanding of the solution chemistry of beryllium in Ca-containing alkaline systems representative of cementitious environments with low to elevated ionic strength conditions.
All samples were prepared, stored and handled in an Ar-glove box (O2 < 1 ppm) at T = (22 ± 2)°C. All solutions were prepared with purified water (Milli-Q academic, Millipore, 18.2 MΩ) purged with Ar for at least 1 h to remove CO2(g). Beryllium sulfate tetrahydrate (BeSO4∙H2O, 99.99% purity) and calcium chloride (CaCl2∙H2O) EMSURE® were purchased from Merck. Calcium hydroxide (Ca(OH)2) was purchased from Sigma-Aldrich.
A combination pH electrode (ROSS Orion with 3.0 M KCl as filling solution) was used to determine the proton concentration ([H+] in mol∙kg–1, with pHm = –log [H+]). The pH electrode was calibrated using commercial pH buffers (pH = 1–12, Merck). In salt solutions of ionic strength I ≥ 0.1 mol kg–1, the measured pH value (pHexp) is an operational apparent value related to [H+] by pHm = pHexp + Am. The values Am entail both the activity coefficient of H+ and the liquid junction potential of the electrode at a given background electrolyte concentration and temperature. Am values reported by Altmaier and co-workers for CaCl2 systems were used for the determination of pHm (Altmaier et al., 2008).
The solid phase used for undersaturation solubility experiments, α-Be(OH)2(cr), was precipitated by slow addition of a 0.35 M BeSO4 solution to a 2.0 M carbonate-free NaOH solution with a final pH ≈ 10.5. After an ageing time of ≈2 months, the solid phase was washed thoroughly with slightly alkaline water (pH ≈ 9.5) to remove the residues of the matrix solution (Na2SO4 and NaOH), and characterized by X-ray diffraction (XRD). Details on the preparation and characterization of the solid phase used in this work were provided in our previous study (Çevirim-Papaioannou et al., 2020).
A total of 30 independent batch samples were prepared by contacting 0.5–5 mg of α-Be(OH)2(cr) to alkaline CaCl2 solutions (0.05, 0.25, 1.0 and 3.5 M) with 8.9 ≤ pHm ≤ 11.8. Be(II) concentrations and pHm values were monitored regularly from 7 to 337 days. In each sampling, aliquots (100–500 µL) of the supernatant of each sample were pipetted into 10 kD filters (2–3 nm cut-off Nanosep®, Pall Life Sciences), centrifuged for 10 min at 6000 g, and diluted (1:100 and 1:4000) with 2% ultrapure HNO3 before the quantification by inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer ELAN 610). The detection limit of the technique ranged between 10−6.5 and 10−8 M, depending upon the dilution factors applied at different CaCl2 concentrations. Equilibrium conditions were assumed after repeated measurements over time with constant [Be] and pHm values, typically after 372 days. After attaining equilibrium conditions, the solid phase of a selected sample (1.0 M CaCl2, pHm = 11.1) was characterized by XRD. For this purpose, an aliquot of the solid phase (≈1–2 mg) was washed 7 times with ethanol (0.5–1 mL) under Ar atmosphere in order to remove the background electrolyte, i.e., CaCl2. After the last cleaning step, the washed solid phase was re-suspended in ethanol, deposited on the XRD sample holder and left drying for a few minutes inside the glovebox. The XRD diffractogram was collected using a Bruker D8 Advance X-Ray powder diffractometer (Cu anode) within the range 2θ = 2–80⁰, incremental steps of 0.02 and a measurement time of 0.4 s per step.
The simulation model was built in a box with dimensions of 43 Å × 43 Å × 49 Å, which contained 3,120 water molecules with 3 Be(OH)42–, 50 Ca2+, and 84 Cl–randomly distributed ions. This approximately corresponds to 1 M CaCl2 and 0.05 M beryllium ion concentration. The high concentration of Be(II) is required in the simulations for better statistical sampling, and is in line with experimental observations in concentrated CaCl2 solutions. The total electroneutrality of the simulation cell was ensured by balancing the number of counterions. Periodic boundary conditions were applied in three dimensions. Water was described using the extended simple point charge (SPC/E) model (Berendsen et al., 1987). The 12-6-4 Lennard-Jones-type nonbonded parameters for Ca2+ and Be2+ for SPC/E water, which include the contribution from the ion-induced dipole interaction, were taken from Li and Merz (Li and Merz, 2014; Li et al., 2020). Standard Lorenz-Berthelot mixing rules (Allen and Tildesley, 2017) were applied to calculate short-range Lennard-Jones interactions between the unlike atoms (with a cut-off distance of 1.4 nm). Long-range electrostatic forces were evaluated using the Ewald summation method. All molecular dynamics (MD) simulations were performed using the LAMMPS software package (3 March 2020 version) (Plimpton, 1995). Model equilibration was carefully monitored by assessing the temperature, pressure, kinetic and potential energy of the system, and dimensions of the simulation box, in order to confirm that these parameters reach their equilibrium steady state values on average (Braun et al., 2019). The Newtonian equations of the atomic motions were numerically integrated with a time step of 1 fs, and the model systems were initially equilibrated for 5 ns in the isobaric–isothermal statistical ensemble (NPT), then for 5 ns in the canonical ensemble (NVT). Temperature and pressure were constrained using the Nose-Hoover thermostat and barostat (Braun et al., 2019) at ambient conditions (T = 295 K, p = 0.1 MPa). The production run was performed in the NVT ensemble for 5 ns, and the trajectory was recorded every 500 fs. To describe local structural properties, radial distribution functions for pairs of atoms, and running coordination numbers were calculated from the recorded trajectory. The VMD software package (version 1.9.3) has been used for visualisation (Humphrey et al., 1996).
XRD patterns collected for the beryllium solid phase equilibrated in 1.0 M CaCl2 at pHm = 11.1 and 3.5 M CaCl2 at pHm = 10.6 are shown in Figure 1, together with the diffractograms previously reported for α-Be(OH)2(cr) in Bear and Turnbull (1965) and in our previous study (Çevirim-Papaioannou et al., 2020). The main features of this sample are in excellent agreement with the patterns previously reported for α-Be(OH)2(cr). This observation confirms that the starting material used in the experiments, α-Be(OH)2(cr), is the solid phase controlling the solubility of beryllium also in CaCl2 systems.
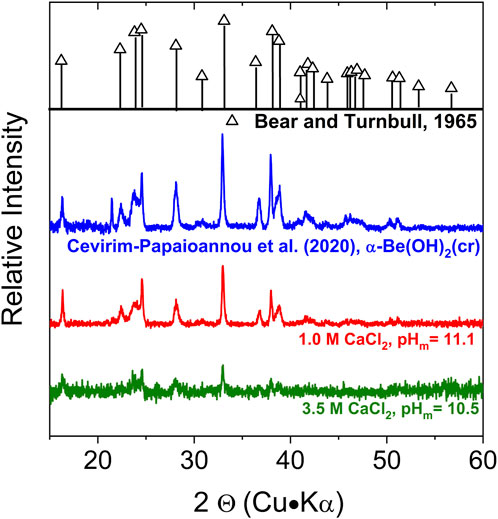
FIGURE 1. X-Ray diffraction patterns of the solid phase recovered from the solubility sample equilibrated in 1.0 M CaCl2 at pHm = 11.1, together with reference patterns reported for α-Be(OH)2(cr) (Bear and Turnbull, 1965) and patterns obtained in our previous study (Çevirim-Papaioannou et al., 2020).
Figure 2 shows the experimental solubility data determined in alkaline CaCl2 solutions with 8.9 ≤ pHm ≤ 11.8, together with the solubility curve of α-Be(OH)2(cr) at I = 0 calculated using the thermodynamic model reported in (Çevirim-Papaioannou et al., 2020) (see Supplementary Tables SI-S1, S2 in the Supplementary Material). The solubility of Be(II) in 0.05 M CaCl2 shows a pH-independent behaviour within 9 ≤ pHm ≤ 10.5, which is in good agreement with the calculated solubility line and can be assigned to the solubility reaction α-Be(OH)2(cr) ⇔ Be(OH)2(aq). Above pH ≈ 10.5, the solubility in 0.05 M CaCl2 increases steadily with a slope of ≈ +2 (as log [Be(II] vs. pHm), in disagreement with the slope of +1 observed for NaCl/KCl systems within the same pH-range and also predicted by the calculated solubility line. This observation reflects that in CaCl2 solutions the solubility of the α-Be(OH)2(cr) phase is dominated by an equilibrium reaction involving the release of 2 H+. A well-defined slope of ≈ +2 is also observed for all other CaCl2 systems investigated in this work, with the highest solubility being achieved in 3.5 M CaCl2 solutions. Our experimental observations in CaCl2 systems and the direct comparison with solubility data in NaCl/KCl solutions support the earlier predominance of the [Be(OH)4]2– moiety in the former systems, expectedly as a result of its stabilization by Ca2+ ions. This is in line with previous observations reported in the literature for strongly hydrolyzing metal ions, e.g., M(III), M(IV) and M(V) (Altmaier et al., 2008; Neck et al., 2009; Fellhauer et al., 2010; Fellhauer, 2013; Yalcintas et al., 2016).
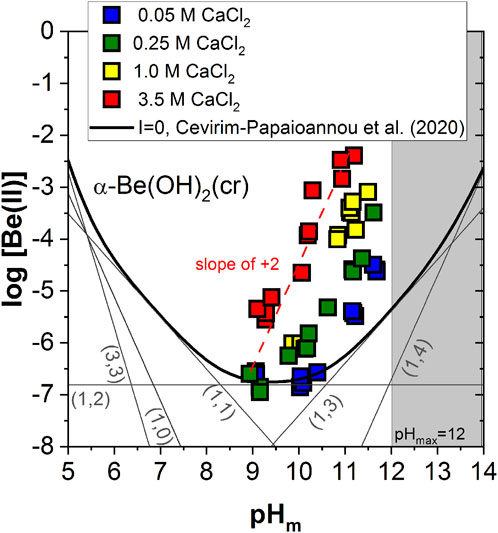
FIGURE 2. Experimental Be(II) solubility data obtained in the present work in 0.05, 0.25, 1.0 and 3.5 M CaCl2 solutions with 8.9 ≤ pHm ≤ 11.8. Solid thick line corresponds to the solubility curve of α-Be(OH)2(cr) at I = 0 calculated with thermodynamic data reported in Çevirim-Papaioannou et al. (2020). Dashed red line indicates the slope analysis performed in this study. Shadowed area represents the precipitation of Ca(OH)2(s) [at (CaCl2) ≤ 2 M] or Ca–OH–Cl(s) solid phases. Uncertainties of individual measurements are within the size of the symbols.
The local structure of a solution can be described by means of the radial distribution functions of the ion-ion and ion-water pairs. The RDF shows the probability of finding atoms at curtain distance during simulation time, and allows to calculate time-averaged coordination numbers. Figure 3 presents calculation results for Be2+ ions in CaCl2 aqueous solution.
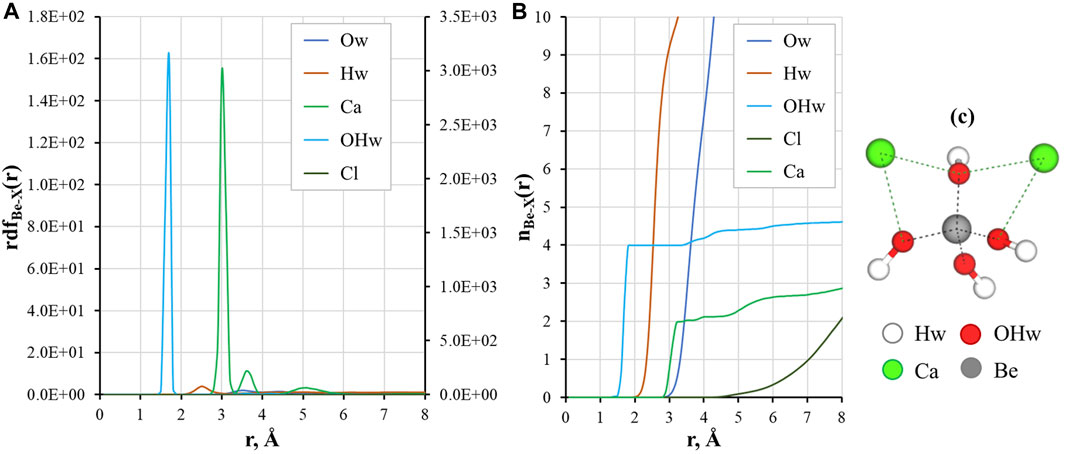
FIGURE 3. Local structural properties of Be(OH)42– in a CaCl2 solution: (A) radial distribution functions (the secondary axes show rdf(r) for the Be-OHw pair); (B) running coordination numbers for Be-X atom pairs, where X is O, H, Cl, and Ca; (C) Simulation snapshot of the complex, water molecules omitted for clarity.
Figures 3A, B shows that the interaction of Be2+ with hydroxyl ions is strong and was stable during all the simulation time. There are 4 oxygen atoms of the aqueous hydroxyl ions in the first coordination sphere of Be2+ at r ≈ 1.7 Å. This result agrees well with the reported distances between Be2+ and hydroxyl ions calculated by DFT (Jin et al., 2015). The oxygen atoms of the first shell are coordinated with other solution molecules through hydrogen bonding (Hw peak at distances around 2–3 Å) to the second solvation shell (small Ow peak at r ≈ 3–4 Å). As it can be seen from Figures 3A, B, there is a low probability of binding between Be2+ and Cl−ions: no distinct peak of chloride ions was recorded, and Cl−presence was only found at r > 5 Å.
Three peaks for Ca2+ are found in the outer coordination sphere of Be2+: a high-intensity peak at r ≈ 3.1 Å, a small peak between 3 Å and 4 Å, and very broad peak of very low intensity around 5 Å. The first peak corresponds to binding of two calcium cations with oxygens of the hydroxyl ions in bidentate coordination, as can be seen in the snapshot from simulations (Figure 3C). The peak between 3 and 4 Å is the coordination of Ca2+ with a single hydroxyl group. The broad peak after 5 Å stands for Ca2+ ions in the solution that are not bound to the complex. We have previously shown that Ca2+ plays a crucial role in the sorption of Be(II) in cementitious systems (Çevirim-Papaioannou et al., 2021a; Çevirim-Papaioannou et al., 2021b). The surface of C-S-H provides a more structured environment, where both ions and water molecules are coordinated with the surface in the sorption layer increasing the probability for Be hydroxyl species to form complex with multiple Ca2+ ions (up to three). From the results of this study, it can be concluded that in the solution complexes of Be(OH)42– with two Ca2+ are dominant, while binding of the third calcium cation remains possible, which is reflected in the slightly higher value of running coordination for the Be-Ca pair (n ≈ 2.2 at a distance of 4 Å).
The specific ion interaction theory (SIT) (Ciavatta, 1980) based on the Brønsted-Guggenheim-Scatchard model is used in this study to account for ion interactions in systems at I > 0. This is also the method adopted for ion strength corrections within the NEA-TDB project (Grenthe et al., 2020). In SIT, the activity coefficient of a given ion j (γj) is calculated according to:
where zj is the charge of the ion j, D is the Debye-Hückel term (
Based on the slope analysis discussed in Section 3.1 and considering that α-Be(OH)2(cr) is the solid phase controlling the solubility in all investigated systems, the equilibrium reaction (2) is proposed to describe the solubility of Be(II) in CaCl2 solutions above pHm ≈ 9–10 (depending upon salt concentration). As reflected in reaction (2), the number of Ca-atoms participating in the complexation reaction (n) is unknown and must be included in the optimization process.
with
Conditional solubility constants, log *K′s,CanBe(OH)4(2n−2), were determined from the experimental solubility data obtained in 0.05, 0.25, 1.0 and 3.5 M CaCl2 using the minimization function ∑((log [Be]exp–log [Be]calc)2)1/2. Three different chemical models were considered in the calculations, assuming the predominance of the ternary complexes CanBe(OH)4(2n−2) with n = 1–3 in the aqueous phase. The SIT-plot was used to derive the solubility constant at the reference state, log *K°s,CanBe(OH)4(2n-2), and corresponding SIT ion interaction coefficient/s based on the linear regression (log *K′s,CanBe(OH)4(2n-2) + Δz2D—2 log aw) vs. [Cl−] (in molal units). The SIT ion interaction coefficients of the ternary complexes CanBe(OH)4(2n−2) were calculated from the slope of the linear regression (–Δε) in combination with ε(H+, Cl−) = (0.12 ± 0.01) kg∙mol–1, ε(Ca2+, Cl−) = (0.14 ± 0.01) kg∙mol–1 and aw values as reported in the NEA-TDB (Grenthe et al., 2020). The criteria considered for the selection of the chemical model describing the solubility of Be(II) in CaCl2 was based on:
(i) minimization of the quality parameter (Δ) calculated as
(ii) reasonable values of the SIT interaction coefficients, considering charge analogies described in Hummel (2009), and
(iii) smooth shape of the plot log *K′s,CanBe(OH)4(2n-2) vs. [Cl−]
Table 1 shows the conditional solubility constants, log *K′s,CanBe(OH)4(2n-2), determined for each chemical model and background electrolyte concentration, together with log *K°s,CanBe(OH)4(2n-2), ε(CanBe(OH)4(2n−2), Ca2+/Cl−) and the quality parameter (Δ). The SIT-plot as well as the representation of log *K′s,CanBe(OH)4(2n-2) vs. [Cl−] for the three chemical models evaluated in this work are shown in Supplementary Figures SI-S1, S2 of the Supporting Information.

TABLE 1. Values of log *K′s,CanBe(OH)4(2n-2) determined from solubility data of Be(II) in 0.05–3.5 M CaCl2 solutions for the chemical models assuming the formation of the ternary complexes Ca[Be(OH)4](aq), Ca2[Be(OH)4]2+ or Ca3[Be(OH)4]4+. Equilibrium constants in the reference state, log *K’s,CanBe(OH)4(2n-2) and SIT coefficients as determined from the corresponding SIT-plots (see Supplementary Figures SI-S1 in the Supporting Information). Quality parameter (Δ) calculated for each chemical model as described in the text.
Table 1 shows very large values of the quality parameter (Δ) for the chemical models including the aqueous complexes CaBe(OH)4(aq) and Ca3Be(OH)44+, which reflect the significant deviations between experimental and calculated log *K′s,CanBe(OH)4(2n-2) for these systems (see also Supplementary Figures SI-S1 in the Supplementary Material). Note that although values of ε(i, j) = 0 for neutral species are considered per definition in SIT, a number of neutral species with ε(i, j) ≠ 0 are reported in the NEA-TDB books (see for instance Grenthe et al., 2020), and this cannot be considered as criteria to reject a specific chemical model. The chemical model including the complex Ca2[Be(OH)4]2+ shows the lowest value of Δ, a reasonable SIT coefficient, as well as a smooth shape of the plot log *K′s,CanBe(OH)4(2n-2) vs. [Cl−] (see Supplementary Figures SI-S2). This outcome is in agreement with the MD calculations summarized in Section 3.3, which predict the predominance of an aqueous complex with two Ca-atoms. The same model calculations described above but excluding solubility data in 3.5 M CaCl2 were also performed in order to remain within the range of ionic strength normally considered for SIT. The best fit was obtained again for the chemical model including the complex Ca2[Be(OH)4]2+ (see Supplementary Figures SI-S3; Supplementary Tables SI-S4). Consistent values of log *K°s,Ca2[Be(OH)4]2+ and ε(Ca2[Be(OH)4]2+, Cl−) were obtained using both datasets, and thus the model derived including also solubility data in 3.5 M CaCl2 was finally favored.
The chemical reaction (6) and corresponding thermodynamic functions are thus considered for the extension of the thermodynamic and activity models reported in Çevirim-Papaioannou et al. (2020) to the system Be2+–Ca2+–Na+–K+–H+–Cl––OH––H2O(l).
Figures 4A–D shows the excellent agreement between the experimental solubility data determined in 0.05–3.5 M CaCl2 solutions with solubility calculations conducted using the thermodynamic and activity models derived in Çevirim-Papaioannou et al. (2020) and extended in this work. Future work will target the development of a Pitzer activity model for this system, which is clearly favored for the description of brine systems.
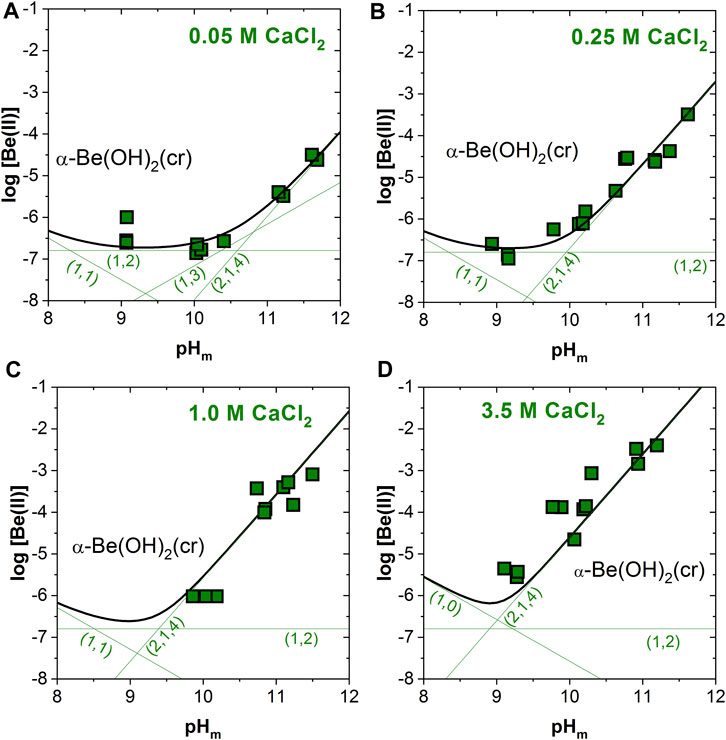
FIGURE 4. Experimental and calculated solubility of α-Be(OH)2(cr) in (A) 0.05, (B) 0.25, (C) 1.0 and (D) 3.5 M CaCl2 solutions. Calculations conducted using the thermodynamic and activity models derived in Çevirim-Papaioannou et al. (2020) and extended in this work. Uncertainties of individual measurements are within the size of the symbols.
C-S-H phases of various compositions determine the most relevant properties of cement pastes in ordinary Portland and blended systems. The non-ideal multisite CASH + solid solution model (Miron et al., 2022a; Miron et al., 2022b; Kulik et al., 2022) can accurately model the C-S-H solubility, water content and elemental uptake accounting for the continuous change of solid and pore solution compositions as a function of pH, alkali concentration, and Ca/Si ratio in the system. In the present work, the CASH + model is used to compute the evolution of a pore solution composition assuming the degradation of a Portland cement in a repository setting defined by low ionic strength conditions. This is done by removing alkalis and of Ca from the system (Ca-Si-Na-K-H2O) in equilibrium with C-S-H to produce the change of the pore solution composition (Figures 5A, B) as a function of [H+], present at different cement degradation stages. At the starting point the pore solution composition in equilibrium with a hydrated Portland cement is taken from (Vollpracht et al., 2016) and has a pH = 13.40 and [NaOH + KOH] = 0.555 M (with K/Na = 2). Alkalis are then removed and the pH decreases to a value of 12.4, when the system is in equilibrium with high Ca/Si C-S-H and portlandite. In natural systems, the pore solution composition is buffered at this condition until all portlandite is dissolved. By removing Ca from the system, after the full dissolution of portlandite, the composition of C-S-H goes towards lower Ca/Si ratios and the pH of the solution decreases to 9.8 at which point the system is in equilibrium with low Ca/Si C-S-H and amorphous silica. The evolution of the pore water composition obtained from calculations with the CASH + solid solution model in combination with the thermodynamic and activity models derived in this work and reported in Çevirim-Papaioannou et al. (2020) have been used to calculate the aqueous speciation of beryllium throughout the complete degradation process of cement (see Figure 5C).

FIGURE 5. (A) Evolution of the Ca/Si ratio in C-S-H phases and (B) simulated pore solution composition as a function of pH during the process of cement degradation. (C) Fraction diagram of Be(II) within 9.8 ≤ pHm ≤ 13.5 for the pore water composition shown in (B), as calculated using the thermodynamic and activity models derived in Çevirim-Papaioannou et al. (2020) and extended in this work.
Figure 5C shows that the hydrolysis species Be(OH)3− and (to less extent) Be(OH)42− prevail within the degradation stage I of cement, characterized by very high pH and low Ca concentration. Due to the enhanced Ca concentration defined by the equilibrium with portlandite, the ternary complex Ca2[Be(OH)4]2+ dominates the aqueous speciation of Be(II) within the degradation stage II, as well as in the early steps of the degradation stage III at pH > 12.1. With the decrease in Ca concentration, the hydrolysis species Be(OH)3− becomes predominant again at 10.6 ≤ pH ≤ 12.1, in systems controlled by C-S-H phases with Ca:Si = 0.80–1.27. Below pH ≈ 10.6, the neutral species Be(OH)2(aq) becomes predominant until the full degradation of cement.
Note that CASH + has not been yet verified for calculating systems with concentrated CaCl2 solutions. In particular, the evolution of C-S-H and other cement phases at high Ca concentrations remains ill-defined. Although this contribution has focused on the SIT activity model, the development of the corresponding Pitzer activity model for the system Be2+–Ca2+–Na+–K+–H+–Cl––OH––H2O(l) is foreseen in the next phase of this study. Pitzer formalism is clearly favored for the description of high-saline systems. Efforts dedicated to the thermodynamic modelling of cementitious systems in high saline conditions are also on-going in the context of the THEREDA Reference Database project (Moog et al., 2015).
The solubility and hydrolysis of Be(II) in dilute to concentrated CaCl2 solutions (0.05–3.5 M) was investigated using a combination of undersaturation solubility experiments with α-Be(OH)2(cr) and molecular dynamics (MD) calculations. The solubility of Be(II) in 0.05 M CaCl2 shows a pH-independent behaviour in weakly alkaline systems (9 ≤ pHm ≤ 10.5), which corresponds to the solubility equilibrium α-Be(OH)2(cr) ⇔ Be(OH)2(aq). These observations are in excellent agreement with previous solubility experiments in NaCl and KCl solutions. A steep increase of the solubility with a slope of ≈ +2 (as log [Be(II)] vs. pHm) is observed above pHm ≈ 10.5 and at higher CaCl2 concentrations. At pHm ≈ 12, this results in solubility values significantly higher (1–4 orders of magnitude, depending upon CaCl2 concentration) than those reported previously for analogous NaCl and KCl systems (Çevirim-Papaioannou et al., 2020). In line with previous studies in the literature on M(III), M(IV) and M(V) metal ions in CaCl2 systems, these observations unequivocally point towards the formation and predominance of ternary complexes of the type CanBe(OH)4(2n−2) in alkaline to hyperalkaline systems containing Ca. MD calculations show the coordination of two Ca-ions in the second coordination sphere of Be2+ at r ≈ 3.1 Å. These Ca-ions are coordinated in bi-dentate mode to the hydroxyl groups of the [Be(OH)4]2– moiety. Consistently with MD calculations, the fit of solubility data indicates the predominance of the complex Ca2[Be(OH)4]2+ in the investigated CaCl2 systems.
In combination with data previously reported in (Çevirim-Papaioannou et al., 2020), chemical, thermodynamic and (SIT) activity models are derived in this work for the system Be2+–Ca2+–Na+–K+–H+–Cl––OH––H2O(l). These new Be(II) models allow the precise characterization of the solubility and speciation of beryllium throughout all degradation stages of cement, as analysed in this paper for low ionic strengths systems. This can be used for source term estimations of beryllium in the context of nuclear waste disposal, as well as input parameter for advanced surface complexation or solid-solution sublattice models for the retention of beryllium in cementitious systems.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
NÇ-P: Methodology, Investigation, Writing–Original Draft. IA: Simulation, Investigation, Writing–Original Draft. GM: Investigation, Writing–Original Draft. XG: Conceptualization, Writing–Review and Editing, Supervision, Project administration. MA: Writing–Review and Editing, Project administration, Funding acquisition. All authors contributed to the article and approved the submitted version.
The research leading to these results has received funding from the European Union’s European Atomic Energy Community’s (Euratom) Horizon 2020 Programme (NFRP-2014/2015) under grant agreement, 662147—Cebama.
Frank Geyer, Annika Fried and Cornelia Beiser (all KIT–INE) are gratefully acknowledged for the ICP–MS and ICP–OES measurements. We thank Bianca Schacherl for her support on sample preparation. We acknowledge support by the KIT-Publication Fund of the Karlsruhe Institute of Technology. The authors acknowledge support by the state of Baden-Wurttemberg through bwHPC and the German Research Foundation (DFG) through grant no INST 40/575-1 FUGG (JUSTUS 2 cluster).
The author XG declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnuen.2023.1192463/full#supplementary-material
Alderighi, L., Gans, P., Midollini, S., and Vacca, A. (2000). “Aqueous solution chemistry of beryllium,” in Main chemistry group, advances in inorganic chemistry, vol 50. Editors A. G. Sykes, and A. Cowley (San Diego: Academic Press), 110–172.
Allen, R., and Tildesley, D. J. (2017). Computer simulation of liquids. 2nd edition. New York: Oxford University Press, 626.
Altmaier, M., Neck, V., and Fanghanel, T. (2008). Solubility of Zr(IV), Th(IV) and Pu(IV) hydrous oxides in CaCl2 solutions and the formation of ternary Ca-M(IV)-OH complexes. Radiochim. Acta 96, 541–550. doi:10.1524/ract.2008.1535
Altmaier, M., Yalcintas, E., Gaona, X., Neck, V., Müller, R., Schlieker, M., et al. (2017). Solubility of U(VI) in chloride solutions. I. The stable oxides/hydroxides in NaCl systems, solubility products, hydrolysis constants and SIT coefficients. J. Chem. Thermodyn. 114, 2–13. doi:10.1016/j.jct.2017.05.039
Bear, I. J., and Turnbull, A. G. (1965). The heats of formation of beryllium compounds. I. Beryllium hydroxides. J. Phys. Chem. 69, 2828–2833. doi:10.1021/j100893a004
Beeston, J. M. (1970). Beryllium metal as a neutron moderator and reflector material. Nucl. Eng. Des. 14, 445–474. doi:10.1016/0029-5493(70)90161-5
Berendsen, H. J. C., Grigera, J. R., and Straatsma, T. P. (1987). The missing term in effective pair potentials. J. Phys. Chem. 91, 6269–6271. doi:10.1021/j100308a038
Bertin, F., Thomas, G., and Merlin, J. C. (1967). Studies on beryllium complexes. I. Solvolysis of Be2+ ions in aqueous media. J. C. Bull. Soc. Chim. Fr. 2393.
Braun, E., Gilmer, J., Mayes, H. B., Mobley, D. L., Monroe, J. I., Prasad, S., et al. (2019). Best practices for foundations in molecular simulations [article v1.0]. Living J. Comp. Mol. Sci. 1, 5957. doi:10.33011/livecoms.1.1.5957
Brendebach, B., Altmaier, M., Rothe, J., Neck, V., and Denecke, M. A. (2007). EXAFS study of aqueous ZrIV and ThIV complexes in alkaline CaCl2 solutions: ca3[Zr(OH)6]4+ and Ca4[Th(OH)8]4+. Inorg. Chem. 46, 6804–6810. doi:10.1021/ic070318t
Brewitz, W. (1980). Zusammenfassender zwischenbericht, GSF T 114. Braunschweig: Ges. f. Strahlen-und Umweltforschung.
Bruno, J. (1987). Beryllium(II) hydrolysis in 3.0 mol·dm-3 perchlorate. J. Chem. Soc. Dalton Trans. 1987, 2431–2437. doi:10.1039/dt9870002431
Bruno, J., Grenthe, I., and Munoz, M. (1987a). Studies of metal carbonate equilibria. Part 16. The beryllium(II) - water-carbon dioxide(g) system in neutral-to-alkaline 3.0 mol dm-3 perchlorate media at 25oC. J. Chem. Soc. Dalton Trans. 1987, 2445–2449. doi:10.1039/dt9870002445
Bruno, J., Grenthe, I., Sandstrom, M., and Ferri, D. (1987b). Studies of metal carbonate equilibria. 15. The beryllium(II)- water-carbon dioxide(g) system in acidic 3.0 mol dm-3 perchlorate media. J. Chem. Soc. Dalton Trans. 1987, 2439–2444. doi:10.1039/dt9870002439
Bube, C., Metz, V., Bohnert, E., Garbev, K., Schild, D., and Kienzler, B. (2013). Long-term cement corrosion in chloride-rich solutions relevant to radioactive waste disposal in rock salt - leaching experiments and thermodynamic simulations. Phys. Chem. Earth 64, 87–94. doi:10.1016/j.pce.2012.11.001
Carell, B., Olin, A., and Valen, K. (1961). Studies on the hydrolysis of metal ions. 37. Application of the self-medium method to the hydrolysis of beryllium perchlorate. Acta Chem. Scand. 15, 1875–1884. doi:10.3891/acta.chem.scand.15-1875
Çevirim-Papaioannou, N., Androniuk, I., Han, S., Mouheb, N. A., Gaboreau, S., Um, W., et al. (2021a). Sorption of beryllium in cementitious systems relevant for nuclear waste disposal: quantitative description and mechanistic understanding. Chemosphere 282, 131094. doi:10.1016/j.chemosphere.2021.131094
Çevirim-Papaioannou, N., Gaona, X., Böttle, M., Bethune Yalcintas, E., Schild, D., Adam, C., et al. (2020). Thermodynamic description of Be(II) solubility and hydrolysis in acidic to hyperalkaline NaCl and KCl solutions. Appl. Geochem. 117, 104601–104613. doi:10.1016/j.apgeochem.2020.104601
Çevirim-Papaioannou, N., Han, S., Androniuk, I., Um, W., Altmaier, M., and Gaona, X. (2021b). Uptake of Be(II) by cement in degradation stage I: wet-chemistry and molecular dynamics studies. Minerals 11, 1149. doi:10.3390/min11101149
Chandler, D., Primm, R. T., and Maldonado, G. I. (2009). Reactivity accountability attributed to beryllium reflector poisons in the high flux isotope reactor. Oak Ridge, USA: ORNL Report. TM-2009/188.
Chinea, E., Dominguez, S., Mederos, A., Brito, F., Sanchez, A., Ienco, A., et al. (1997). Hydrolysis of beryllium(II) in DMSO:H2O. Main. Group Mater. Chem. 20, 11–17. doi:10.1515/mgmc.1997.20.1.11
Ciavatta, L. (1980). The specific interaction theory in evaluating ionic equilibria. Ann. Chim-Rome 70, 551–567.
Fellhauer, D., Altmaier, M., Gaona, X., Lutzenkirchen, J., and Fanghanel, T. (2016a). Np(V) solubility, speciation and solid phase formation in alkaline CaCl2 solutions. Part II: thermodynamics and implications for source term estimations of nuclear waste disposal. Radiochim. Acta 104, 381–397. doi:10.1515/ract-2015-2490
Fellhauer, D., Neck, V., Altmaier, M., Lutzenkirchen, J., and Fanghanel, T. (2010). Solubility of tetravalent actinides in alkaline CaCl2 solutions and formation of Ca4[an(OH)8]4+ complexes: A study of Np(IV) and Pu(IV) under reducing conditions and the systematic trend in the an(IV) series. Radiochim. Acta 98, 541–548. doi:10.1524/ract.2010.1751
Fellhauer, D., Rothe, J., Altmaier, M., Neck, V., Runke, J., Wiss, T., et al. (2016b). Np(V) solubility, speciation and solid phase formation in alkaline CaCl2 solutions. Part I: experimental results. Radiochim. Acta 104, 355–379. doi:10.1515/ract-2015-2489
Fellhauer, D. (2013). Untersuchungen zur Redoxchemie und Löslichkeit von Neptunium und Plutonium. PhD thesis. Germany: University of Heidelberg.
Gilbert, R. A., and Garrett, A. B. (1956). The equilibria of the metastable crystalline form of beryllium hydroxide - Be(OH)2 in hydrochloric acid, perchloric acid and sodium hydroxide solutions at 25°C. J. Am. Chem. Soc. 78, 5501–5505. doi:10.1021/ja01602a012
Green, R. W., and Alexander, P. W. (1965). Schiff base equilibria .2. Beryllium complexes of N-N-butylsalicylideneimine and hydrolysis of Be2+ ion. Aust. J. Chem. 18, 651–659. doi:10.1071/ch9650651
Grenthe, I., Gaona, X., Plyasunov, A. V., Rao, L., Run-de, W. H., Grambow, B., et al. (2020). Second update on the chemical thermodynamics of uranium, neptunium, plutonium, americium and technetium. Paris, France 2020: OECD Publications. OECD Nuclear Energy Agency Paris.
Hietanen, S., Sillen, L. G., Toplin, I., Melera, A., and Nilsson, L. (1964). Studies on the hydrolysis of metal ions. 52. A recalculation of emf data on beryllium hydrolysis. Acta Chem. Scand. 18, 1015–1016. doi:10.3891/acta.chem.scand.18-1015
Humphrey, W., Dalke, A., and Schulten, K. (1996). VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38. doi:10.1016/0263-7855(96)00018-5
Jin, X. Y., Liao, R. B., Wu, H., Huang, Z. J., and Zhang, H. (2015). Structures and formation mechanisms of aquo/hydroxo oligomeric beryllium in aqueous solution: A density functional theory study. J. Mol. Model. 21, 232. doi:10.1007/s00894-015-2779-x
Kakihana, H., and Maeda, M. (1970). The hydrolysis of the beryllium ion in heavy water. Bull. Chem. Soc. Jpn. 43, 109–113. doi:10.1246/bcsj.43.109
Kakihana, H., Sillen, L. G., Ormerod, J. G., Stenhagen, E., and Thorell, B. (1956). Studies on the hydrolysis of metal ions. XVI. The hydrolysis of the beryllium ion, Be2+. Acta Chem. Scand. 10, 985–1005. doi:10.3891/acta.chem.scand.10-0985
Kulik, D. A., Miron, G. D., and Lothenbach, B. (2022). A structurally-consistent CASH+ sublattice solid solution model for fully hydrated C-S-H phases: thermodynamic basis, methods, and Ca-Si-H2O core sub-model. Cem. Conc. Res. 151, 106585. doi:10.1016/j.cemconres.2021.106585
Lanza, E., and Carpéni, G. (1968). Recherches sur le point isohydruque et les equilibres de condensation ou association-XXV. Etude electromerique de l'hydroxoxomplexation du cation (Be. aq)2+ Electrochim. Acta 13, 519–533. doi:10.1016/0013-4686(68)87023-9
Li, P. F., and Merz, K. M. (2014). Taking into account the ion-induced dipole interaction in the nonbonded model of ions. J. Chem. Theory Comput. 10, 289–297. doi:10.1021/ct400751u
Li, Z., Song, L. F., Li, P. F., and Merz, K. M. (2020). Systematic parametrization of divalent metal ions for the OPC3, OPC, TIP3P-FB, and TIP4P-FB water models. J. Chem. Theory Comput. 16, 4429–4442. doi:10.1021/acs.jctc.0c00194
Longhurst, G. R., Carboneau, M. I., and Mullen, C. K. (2003). “Challenges for disposal of irradiated beryllium,” in Proceedings of the 6th IEA Workshop on Beryllium Technology, Mizayaki, Japan, Dec. 2-5.
Marx, D., Sprik, M., and Parrinello, M. (1997). Ab initio molecular dynamics of ion solvation. The case of Be2+ in water. Chem. Phys. Lett. 273, 360–366. doi:10.1016/s0009-2614(97)00618-0
Mattock, G. (1954). The hydrolysis and aggregation of the beryllium ion. J. Am. Chem. Soc. 76, 4835–4838. doi:10.1021/ja01648a019
Mesmer, R. E., and Baes, C. F. (1967). Hydrolysis of beryllium(II) in 1 m NaCl. Chem: Inorganic Chemistry Inorg.
Miron, G. D., Kulik, D. A., and Lothenbach, B. (2022a). Porewater compositions of Portland cement with and without silica fume calculated using the fine-tuned CASH+NK solid solution model. Mat. Struct. 55, 212. doi:10.1617/s11527-022-02045-0
Miron, G. D., Kulik, D. A., Yan, Y., Tits, J., and Lothenbach, B. (2022b). Extensions of CASH+ thermodynamic solid solution model for the uptake of alkali metals and alkaline earth metals in C-S-H. Cem. Conc. Res. 152, 106667. doi:10.1016/j.cemconres.2021.106667
Moog, H. C., Bok, F., Marquardt, C. M., and Brendler, V. (2015). Disposal of nuclear waste in host rock formations featuring high-saline solutions - implementation of a thermodynamic reference database (THEREDA). Appl. Geochem. 55, 72–84. doi:10.1016/j.apgeochem.2014.12.016
Neck, V., Altmaier, M., Rabung, T., Lutzenkirchen, J., and Fanghanel, T. (2009). Thermodynamics of trivalent actinides and neodymium in NaCl, MgCl2, and CaCl2 solutions: solubility, hydrolysis, and ternary Ca-M(III)-OH complexes. Pure Appl. Chem. 81, 1555–1568. doi:10.1351/pac-con-08-09-05
Ohtaki, H. (1967). Ionic equilibria in mixed solvents. I. Hydrolysis of beryllium ion in a 0.2 mole fraction dioxane-water mixture containing 3 M LiClO4 as an ionic medium. Inorg. Chem. 6, 808–813. doi:10.1021/ic50050a033
Ohtaki, H., and Kato, H. (1967). Ionic equilibria in mixed solvents. 2. Hydrolysis of beryllium ion in a 0.1 mole fraction dioxane-water mixture and in aqueous solution containing 3 M LiClO4 as an ionic medium. Inorg. Chem. 6, 1935–1937. doi:10.1021/ic50056a045
Pâris, M. R., and Gregoire, C. (1968). Application de la coulometrie à l'etude des complexes. I. Appareillage et application à l'hydrolyse de l'ion beryllium(II). Anal. Chim. Acta 42, 431–437. doi:10.1016/s0003-2670(01)80335-4
Perera, L. C., Raymond, O., Henderson, W., Brothers, P. J., and Plieger, P. G. (2017). Advances in beryllium coordination chemistry. Coord. Chem. Rev. 352, 264–290. doi:10.1016/j.ccr.2017.09.009
Plimpton, S. (1995). Fast parallel algorithms for short-range molecular-dynamics. J. Comput. Phys. 117, 1–19. doi:10.1006/jcph.1995.1039
Rabung, T., Altmaier, M., Neck, V., and Fanghanel, T. (2008). A TRLFS study of Cm(III) hydroxide complexes in alkaline CaCl2 solutions. Radiochim. Acta 96, 551–560. doi:10.1524/ract.2008.1536
Schwarzenbach, G. (1962). Metastabile Protonierungs-und Deprotonierungsprodukte anorganischer Molekeln und Ionen. Pure Appl. Chem. 5, 377–402. doi:10.1351/pac196205030377
Schindler, P., and Garett, A. B. (1960). Löslichkeitsprodukte Von Metalloxiden Und -Hydroxiden .5. Die Loslichkeit von -Be(OH)2 in Verdunnten Säuren. Helv. Chim. Acta 43, 2176–2178. doi:10.1002/hlca.19600430740
Schwarzenbach, G., and Wenger, H. (1969). Die protonierung von metall-aquaionen I.: be∙aq2+ solvatations-isomerie. Helv. Chim. Acta 52, 644–665. doi:10.1002/hlca.19690520313
Shannon, R. D. (1976). Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A32, 751–767. doi:10.1107/s0567739476001551
Tsukuda, H., Kawai, T., Maeda, M., and Ohtaki, H. (1975). Ionic equilibria in mixed solvents. XI. A critical survey of hydroxo complexes of beryllium in aqueous and aqueous mixed solvents. Bull. Chem. Soc. Jpn. 48, 691–695. doi:10.1246/bcsj.48.691
Vanni, A., Gennaro, M. C., and Ostacoli, G. (1975). Equilibrium studies of beryllium complexes. J. Inorg. Nucl. Chem. 37, 1443–1451. doi:10.1016/0022-1902(75)80788-3
Vollpracht, A., Lothenbach, B., Snellings, R., and Haufe, J. (2016). The pore solution of blended cements: A review. Mater. Struct. 49, 3341–3367. doi:10.1617/s11527-015-0724-1
Keywords: beryllium, calcium, solubility, hydrolysis, thermodynamics, SIT, cement
Citation: Çevirim-Papaioannou N, Androniuk I, Miron GD, Altmaier M and Gaona X (2023) Beryllium solubility and hydrolysis in dilute to concentrated CaCl2 solutions: thermodynamic description in cementitious systems. Front. Nucl. Eng. 2:1192463. doi: 10.3389/fnuen.2023.1192463
Received: 23 March 2023; Accepted: 26 September 2023;
Published: 17 October 2023.
Edited by:
Johan Andersson, JABIA AB, SwedenReviewed by:
Vladimir Petrov, Lomonosov Moscow State University, RussiaCopyright © 2023 Çevirim-Papaioannou, Androniuk, Miron, Altmaier and Gaona. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nese Çevirim-Papaioannou, bmVzZS5jZXZpcmltQGtpdC5lZHU=; Xavier Gaona, eGF2aWVyLmdhb25hQGtpdC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.