
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Nucl. Eng. , 04 January 2023
Sec. Radioactive Waste Management
Volume 1 - 2022 | https://doi.org/10.3389/fnuen.2022.1112080
This article is part of the Research Topic Solubility Phenomena in The Context of Nuclear Waste Disposal View all 10 articles
Technetium-99 (99Tc), a radionuclide generated from nuclear industry is a great environmental concern because of its long half-life (2.13 × 105 years) and high mobility in environment. Therefore, apposite management of 99Tc is imperative to control its hazardous radiological impact on humans and other livings. So far, the major strategy implementation has been the solidification and immobilization of 99Tc radioactive waste in various matrices as waste forms and disposal in deep geological repository. However, by passing the time, 99Tc may leach out/solubilize from the waste forms under different geochemical/environmental conditions. In this minireview, we discuss some key contributions towards the solubility of 99Tc and rhenium (Re; a well-known surrogate of 99Tc) from different waste forms. Specifically, we review the solubility of 99Tc from glass, cement, ceramic, and geopolymer waste forms. The final section (conclusion) presents a short summary and future challenges need be addressed to impede the solubility of 99Tc from the designed waste forms. We believe this minireview will be beneficial to provide a significant insight on the solubility of 99Tc from aforementioned waste forms and in the design of robust matrices to minimize/prevent 99Tc migration in various environments.
Radioactive wastes generated from nuclear power plants’ operation and decommissioning of nuclear reactors are serious environmental threat due to their long half-lives and impact of extremely hazardous radiations on geosphere as well as humans (Singh et al., 2021). The proper management of these radioactive wastes including high-level waste (HLW), low- and intermediate-level waste (LILW), and low-level waste (LLW)) is necessary to control their radiological effect on the environment. Among several radionuclides present in radioactive wastes, 99Tc is one of the significant environmental risk contributors due to it long half-life and highly mobile behavior in oxic environment (Santikari et al., 2022). Therefore, it is essential to control/prevent the mobility of 99Tc into the environment by immobilizing it in suitable and durable waste forms. In several studies, rhenium (Re) has been often used as a preferred non-radioactive surrogate for 99Tc due to their close proximities in terms of speciation, ionic size, and hydration energy (Kim and Kruger, 2018; Duckworth et al., 2021). Since decades, various waste forms including glass, cements, geopolymer, and ceramics have been tested and used for solidification, immobilization, and retention of 99Tc by disposing these waste forms in the deep geological repository (Um, 2018).
Over the time, 99Tc immobilized in different waste forms (glass, cement, geopolymer, ceramics, etc.) under deep geological repository can be released/leached out from the solid matrices and migrate into environment under different environmental and geochemical conditions (Klein et al., 2021). Once released, 99Tc can exist as Tc(VII) in oxic environment (pertechnetate (TcO4−); most common chemical species of Tc in the environment) or it can be reduced to Tc(IV) in the presence of electron donors (Meena et al., 2017). In order to prevent and control the solubility and leaching of 99Tc from immobilized solid matrices and to design durable waste forms, it is necessary to understand the solubility behavior of 99Tc from waste forms under different geochemical conditions. In this minireview, we summaries and discuss some of the key studies on solubility of 99Tc and/or Re from the glass, cement, geopolymer, and ceramic waste forms. Additionally, we highlight the future challenges concerning the solubility of 99Tc from waste forms and the design and formulation of durable waste form for efficient 99Tc immobilization and retention capacity.
The design and formulation of different solid matrices can significantly affect the retention capacity and solubility of 99Tc in the immobilized waste forms. This section presents the selected studies on glass, cement, and other waste forms (geopolymer and ceramics) used for immobilization of 99Tc and/or Re and their leaching/solubility behavior under different experimental conditions.
Glass waste form has been preferably applied for immobilization of various radionuclides, including 99Tc globally (Danilov et al., 2021; Donald et al., 1997; Jantzen, 2011). Therefore, the leaching behavior of Tc/Re from glass waste forms under various experimental conditions is necessary to address the durability of the designed glass matrices and long-term mobility of 99Tc. Bibler and Jurgensen determined the effects of the redox conditions on the release of 99Tc from borosilicate glass (Bibler and Jurgensen, 1987). The authors prepared two batches of glass waste forms under ambient oxidizing and reducing conditions. The leaching tests were performed according to the materials characterization center (MCC-1) test at 90°C and surface area to volume ratio (SA/V) 100 m−1. The obtained leaching test results revealed that 99Tc leached no faster than the glass-forming elements at ambient oxidizing conditions. Additionally, under high pH and reducing conditions, the normalized 99Tc mass loss was observed as 0.02 g/m2 only, even at significant dissolution of the glass.
A few studies performed the static leaching experiments to examine the leaching behavior of 99Tc in 99Tc doped borosilicate glass in disposal environment (Lemmens & Wang, 1992; Pirlet et al., 2002, 2004). A mixture of clay with synthetic pore water was used as the leachant to simulate the actual interstitial pore water. All tests were conducted in the glove box at SA/V 100 m−1. The authors suggested that 99Tc mass losses enhanced linearly with the time, and was observed to be faster at 90°C than at 40°C. Moreover, 99Tc concentrations were higher in the oxidizing medium than in reducing medium at both 40 and 90°C.
Xu et al. evaluated Re retention and its stability in iron phosphate glass (Xu et al., 2013). The authors observed ∼1.1 wt% retention of Re in iron phosphate glass. Moreover, when 2 wt% or more Re added, a white spherical inclusion (Na, K)ReO4 was detected by XRD and EDX mapping. The chemical durability tests were performed according to American Society for Testing and Materials (ASTM) C 1285-02. In this method, 1.5 g of glass powder was mixed with 15 ml of deionized water (DIW) in a Teflon vessel and kept inside an oven at 90 °C. The normalized Re-released amount after 7-day Product Consistency Test (PCT) was found to be < 10-2 g/m2. Normalized Re- released along with other elements are shown in Figure 1.
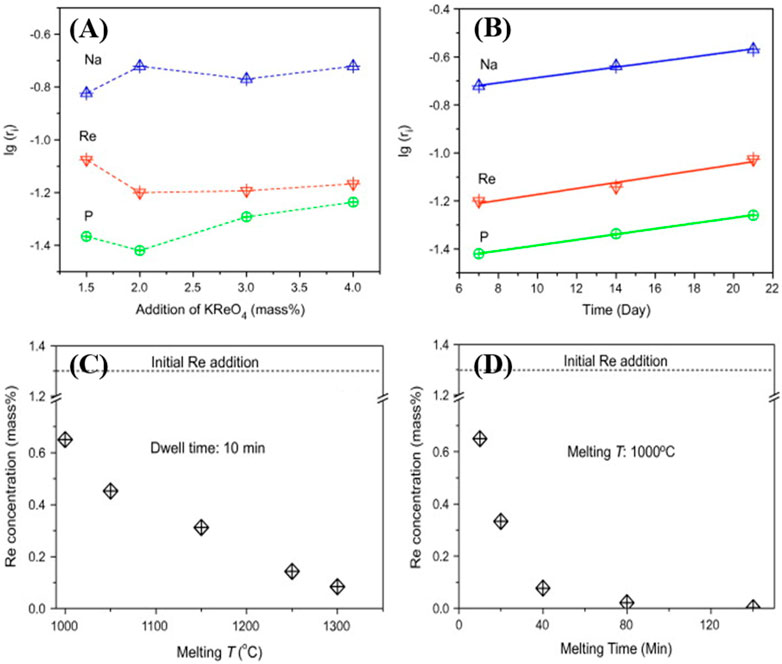
FIGURE 1. Normalized elemental release after 7 days product consistency test (PCT) at different mass% of KReO4 (A). Elemental release at 2-mass% of KReO4 vs. PCT duration (B). Concentrations of Re in 2-mass% of KReO4 samples at different melting temperatures (C) and times (D). Reproduced with permission from the reference Xu et al., 2013. Copyright from Elsevier 2013.
Stefanovsky et al. developed sodium aluminum phosphate (SAP) glass and sodium aluminum iron phosphate (SAIP) glass and determined Re-released rate in both glasses (Stefanovsky et al., 2019). Re- released rate was evaluated via PCT test performed at 90°C for 7 days (d). The normalized Re leaching rates were calculated as 2.55 × 10-6 and 9.12 × 10-9 for SAP glass and 3.90 × 10-8 and 7.90 × 10-8 for SIAP glass in the oxidizing and reducing conditions, respectively. Both SAP and SAIP exhibited lower leach rates than the regulatory values (∼10–5 g/cm2d) specified for HLW glasses. Especially, a sharp decrease in the Re leach rate in SAP glass synthesized under reducing conditions as compared to the same glass produced under oxidizing conditions, was attributed to the enhanced fraction of Re in the reduced form as Re (0).
Pyo et al. developed a new alkali-alumino tellurite glass to incorporate highly volatile Tc/Re (Pyo et al., 2017) and tested leaching behavior of Re via PCT method for 7 d at 90°C. The developed tellurite glass incorporated 7 wt% of Re as Re(VII), in which ReO4− tetrahedra were linked to the glass network by bonding between non-bridging oxygens and Na+ or Ca2+ ions (Figure 2A). The normalized elemental release data for Re and Te were observed as ∼ 1 g/m2 and 0.5 g/m2, respectively, which is lower than the safety standard (<2 g/m2) for the immobilized low activity waste requirement (Figure 2B).
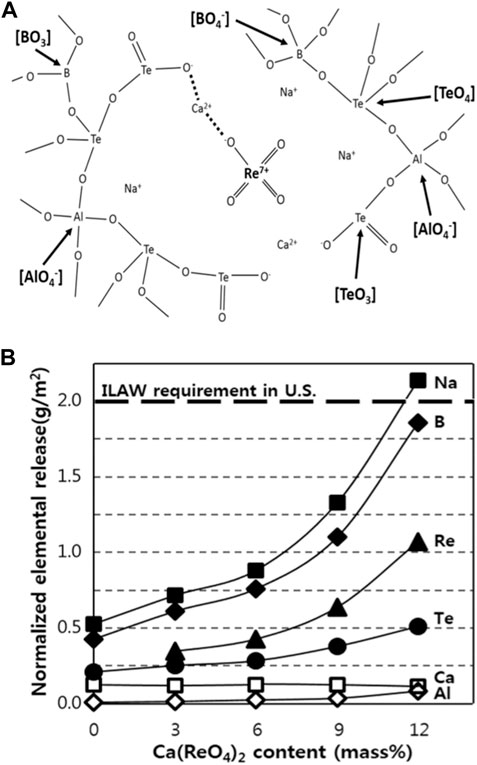
FIGURE 2. Illustration of the tellurite glass structure containing ReO4− ion units (A). Normalized release of elements from glasses after 7-day PCT at different Ca(ReO4)2 contents (B). Reproduced with permission from the reference Pyo et al., 2017. Copyright from Elsevier 2017.
Cement is one of the known matrices used for efficient immobilization of various radioactive wastes due to its durable properties; chemical and thermal stability as well as corrosion resistivity (El-Kamash et al., 2006; Faiz et al., 2017; Goo et al., 2021). In this section, we discuss the solubility/leaching of Tc/Re from cementitious waste forms in different testing conditions. Simner et al. reported a Dynamic Leaching Method (DLM) to investigate 99Tc leaching behavior from the saltstone monolith (cementitious material) samples at the Savannah River Site (SRS), in the United States of America (USA) (Simner et al., 2017). In order to simulate the transport of groundwater through saltstone, DLM applied a flexible-wall permeameter for 99Tc leaching under the elevated hydraulic gradient. This method enabled a continuous flow of permeant through the monolith and analysis of the leachate to determine 99Tc leaching as a function of pore volumes exchanged within the saltstone monolith. Approximately six pore volumes were passed through the monolith. 99Tc concentrations in the leachate were found to be ∼1 × 10-8 mol/L, which indicated that 99Tc leaching in saltstone was controlled by the solubility of TcO2. xH2O compounds.
Santikari et al. investigated the leaching and transport of 99Tc from cementitious waste forms in field lysimeters (Santikari et al., 2022). The authors tested two cementitious waste forms; a) slag-grout of 45%:45%:10% mixture of fly ash, blast furnace slag, and cement, respectively, and b) cement of 55%:45% mixture of cement and fly ash, respectively. The study used a duplicate source of each waste form buried in four lysimeters for approximately 10 months to compare the leaching behavior of 99Tc under natural meteorological conditions in South Carolina, United States. Cumulative 99Tc activity in the effluent was low (2 × 105 Bq) until ∼300 ml of effluent produced from each lysimeter, and then it was enhanced at a rate of ∼3,000 Bq/mL for all the lysimeters.
Cantrell et al. studied the solubility control of Tc from saltstone by TcO2?xH2O and revealed that the Tc-release in water from saltstone under anoxic conditions can be considerably altered by the solubility of a TcO2?xH2O phase (Cantrell et al., 2013). The authors prepared three reactors and a control with different saltstone sample compositions. All three reactors were composed of 45%, 45%, and 10% of blast furnace slag (BFS), fly ash (FA), and Portland cement (PC), respectively. However, 99Tc content was altered in reactor first (0 μCi/kg), and kept similar in the reactors second and third (170 μCi/kg). The obtained data suggested that the solubility of TcO2.1.6H2O acquired an equilibrium within 2 weeks with ∼1.5 × 10–6 M99Tc concentration. The starting redox potential (Eh) value was slightly below −100 mV, which lowered considerably between 0–14 d and almost stabilized below −400 mV by 20 d. The authors proposed that the concentrations of 99Tc at equilibrium are expected to vary with TcO2.1.6H2O, as the saltstone pore fluid evolved over a time. Moreover, the solubility of TcO2·xH2O can be dropped considerably due to lowered pH of the samples via carbonation over the times in an actual disposal scenario.
Geopolymers and ceramic matrices have also been largely used for the incorporation and immobilization of various radionuclides. This section presents key contributions on the solubility/release of Tc and Re from geopolymer and ceramic waste forms. Pierce et al. reported an alkali alumino-silicate geopolymer, DuraLith geopolymer to encapsulate 99Tc liquid radioactive waste (Pierce et al., 2010). The secondary waste simulant of the Hanford Tank Waste Treatment and Immobilization Plant was used to prepare the geopolymer monoliths and three methods developed for the U.S. Environmental Protection Agency (EPA) were applied to assess the stabilization of the DuraLith geopolymer. Diffusivity of 99Tc was found to be as 3.76 × 10-12 cm2/s and 9.93 × 10-9 cm2/s, respectively, for 63 and 2 d cumulative leaching time in batch 1 batch 2 geopolymers. In addition, Xu et al. investigated the effect of blast furnace slag grades on fly ash based geopolymer waste forms (Xu et al., 2014). Ground granulated blast furnace slag (GGBFS) was used to improve the disadvantage of the fly ash based geopolymer and was classified into three grades, 80, 100, and 120. The authors prepared geopolymer mixed with different grades of GGBFSs using the Hanford secondary waste (HSW) simulants spiked by Re. The toxicity characteristic leaching procedure (TCLP) was also applied for the leaching test on fly ash geopolymer waste forms after 28 d of curing at room temperature. Based on the results, the authors suggested that all heavy metals and toxic elements present in the HSW simulant can be more efficiently immobilized by the geopolymer waste forms than Re.
Neeway et al. investigated 99Tc (Re used as surrogate) and I solubility from a sodalite-bearing ceramic waste form using single-pass-flow-through (SPFT) tests (Neeway et al., 2016). The granular fluidized bed steam reforming (FBSR) materials were derived from non-radioactive Hanford low-activity waste (LAW) simulant, radioactive LAW simulant, and the actual radioactive Hanford waste from Tank SX-105. The release rate of Re was determined in the range between 16 × 10-4 g/m2d and 24 × 10-4 g/m2d, which is up to 3 times larger than that of the network-forming elements (Na, Al, and Si). Moreover, the release of Re, I, and 99Tc from the FBSR samples exhibited similar behavior. Based on results, the authors presumed that the solubility of Re and 99Tc was controlled by the mineral phase (sodalite), which was produced during the FBSR process and incorporated Re into the cage structure of the mineral.
Hartmann et al. investigated the crystallographic and hydrodynamic data of 99Tc-based ceramic waste form (Hartmann et al., 2014). Pyrochlore (Nd2Tc2O7), perovskite (SrTcO3), and layered perovskite (Sr2TcO4) were synthesized using anhydrous crystalline TcO2. Chemical durability of Nd2Tc2O7 pyrochlore was measured and compared with 99Tc containing borosilicate glass in close compliance with ASTM C1220. Pyrochlore was insufficiently sintered and did not produce ceramic, however, the specific weight loss of the porous Nd2Tc2O7 was determined as 1.48 × 10-7 g/mm2d; lower than that of the 99Tc-containing borosilicate glass (6.43 × 10-7 g/mm2d). Moreover, the relative 99Tc-release was evaluated as 0.67% for Nd2Tc2O7 pyrochlore, compared to 1.026 for the 99Tc- containing borosilicate glass.
Alekseeva et al. reported the chemical stability (the leaching tests) of the phosphate-based ceramic (NaRe2(PO4)3) produced by spark plasma sintering (Alekseeva et al., 2021). In this study, the authors used Re as a surrogate of 99Tc. The leaching experiments were conducted for 28 d in distilled water at room temperature under the static mode. The Re-leaching rate was evaluated as 1.3 × 10-5 g/cm2d after 28 d. In another approach, Singh et al. demonstrated the immobilization and leaching behavior of 99Tc from magnesium potassium phosphate (MKP) ceramics using SnCl2 as a reducing agent (Singh et al., 2006). The designed MKP ceramic waste forms were characterized by XRD and SEM-EDX. Based on results, the authors proposed that 99Tc could be immobilized in a tetravalent state (Tc(IV)) in MKP ceramics because of reducing environment and microencapsulation in the ceramic matrix. The leaching tests were performed using PCT method for 36% elution-loaded waste forms. 99Tc-leaching rate was evaluated between 1.1 × 10-3 g/m2d and 8.5 × 10-3 g/m2d at ambient temperature (25°C), in agreement with ASTM C 1285-94.
In summary, this mini review highlights and summarizes some of the key developments on solubility and release of 99Tc and Re (surrogate of 99Tc) from glass, cement, geopolymer, and ceramic waste forms using different leaching test methods and experimental conditions. In the section two (2) of this mini review, we emphasize on the leaching/release and retention capacity of 99Tc and Re immobilized in these waste forms under various physicochemical testing parameters and geochemical conditions. In section 2.1, we presents solubility and leaching of Re and Tc from glass waste forms. Section 2.2. Discusses the solubility of Tc and Re from cementitious waste forms, whereas, section 2.3. Describes a few (key) contributions on solubility of Tc and Re from geopolymer and ceramic waste forms. In order to impede the solubility and release of immobilized 99Tc from various waste forms disposed in underground repository and to minimize the mobility of 99Tc in environment/geosphere, future studies and researches (both experimental and theoretical approaches) should be focused on to acquire a wide range of datasets (at different testing conditions) necessary for the design of robust and durable waste forms. This may include more fundamental structural information of candidate waste forms using a various advanced techniques, such as HRTEM, EXAFS, etc. By establishing a comprehensive database for relevant waste forms, it will be easier to design and select most suitable candidates for Re/Tc immobilization. We believe that this mini review will be beneficial to the readers to understand the solubility and release behavior of 99Tc from aforementioned waste forms and to correlate its migration in environment under various geochemical conditions.
BKS, JK, DP, and KK contributed in writing and WU supervised this mini review. All authors discussed, finalized the contents to the article, and approved the submitted version.
This work was supported by the Institute for Korea Spent Nuclear Fuel (iKSNF) and National Research Foundation (NRF) of Korea grant funded by the Korea government (Ministry of Science and ICT, MSIT) (No. 2021M2E1A1085202).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alekseeva, L., Nokhrin, A., Orlova, A., Boldin, M., Lantcev, E., Murashov, A., et al. (2021). NaRe2(PO4)3 phosphate-based ceramic with kosnarite structure as a matrix for technetium immobilization. arXiv preprint arXiv:2111.12973 https://arxiv.org/abs/2111.12973 (Accessed November 25, 2021).doi:10.48550/arXiv.2111.12973
Bibler, N., and Jurgensen, A. (1987). Leaching Tc-99 from SRP glass in simulated tuff and salt groundwaters. MRS Online Proc. Libr. Opl. 112, 585. doi:10.1557/PROC-112-585
Cantrell, K. J., and Williams, B. D. (2013). Solubility control of technetium release from Saltstone by TcO2· xH2O. J. Nucl. Mat. 437 (1-3), 424–431. doi:10.1016/j.jnucmat.2013.02.049
Danilov, S. S., Frolova, A. V., Kulikova, S. A., Vinokurov, S. E., Maslakov, K. I., Teterin, A. Y., et al. (2021). Immobilization of rhenium as a technetium surrogate in aluminum iron phosphate glass. Radiochemistry 63 (1), 99–106. doi:10.1134/s106636222101015x
Donald, I., Metcalfe, B., and Taylor, R. (1997). The immobilization of high level radioactive wastes using ceramics and glasses. J. Mat. Sci. 32 (22), 5851–5887. doi:10.1023/A:1018646507438
Duckworth, S., Gaona, X., Castaño, D., Park, S., Altmaier, M., and Geckeis, H. (2021). Redox chemistry, solubility and hydrolysis of Re in reducing aquatic systems. Thermodynamic description and comparison with Tc. Appl. Geochem. 132, 105037. doi:10.1016/j.apgeochem.2021.105037
El-Kamash, A. M., El-Naggar, M. R., and El-Dessouky, M. I. (2006). Immobilization of cesium and strontium radionuclides in zeolite-cement blends. J. Hazard. Mat. 136, 310–316. doi:10.1016/j.jhazmat.2005.12.020
Faiz, Z., Fakhi, S., Bouih, A., Outayad, R., Benkdad, A., and Hannache, H. (2017). Leaching study of cesium from spent ion-exchange resins and Portland cement package. Int. J. Environ. Sci. Technol. 14, 1019–1026. doi:10.1007/s13762-016-1203-0
Goo, J. Y., Kim, B. J., Kang, M., Jeong, J., Jo, H. Y., and Kwon, J. S. (2021). Leaching behavior of cesium, strontium, cobalt, and europium from immobilized cement matrix. Appl. Sci. 11 (18), 8418. doi:10.3390/app11188418
Hartmann, T., and Alaniz-Ortez, I. J. (2014)., .Fabrication and chemical durability of ceramic technetium-based pyrochlores and perovskites as potential waste forms, Adv. Sci. Technol., 94. Trans Tech Publications Ltd, 85–92. doi:10.4028/www.scientific.net/AST.94.85
Jantzen, C. (2011). “Development of glass matrices for high level radioactive wastes,” in Handbook of advanced radioactive waste conditioning technologies (Elsevier), 230–292. Chennai, Tamil Nadu. doi:10.1533/9780857090959.2.230
Kim, D., and Kruger, A. A. (2018). Volatile species of technetium and rhenium during waste vitrification. J. Non-Cryst. Solids 481, 41–50. doi:10.1016/j.jnoncrysol.2017.10.013
Klein, E., Hardie, S. M., Kickmaier, W., and McKinley, I. G. (2021). Testing repository safety assessment models for deep geological disposal using legacy contaminated sites. Sci. Total Environ. 776, 145949. doi:10.1016/j.scitotenv.2021.145949
Lemmens, K., and Wang, L. (1992). The leaching of Pu, Am, Np and Tc from high-level waste glasses in clay media. MRS Online Proc. Libr. Opl. 294, 147. doi:10.1557/PROC-294-147
Meena, A. H., and Arai, Y. (2017). Environmental geochemistry of technetium. Environ. Chem. Lett. 15 (2), 241–263. doi:10.1007/s10311-017-0605-7
Neeway, J. J., Qafoku, N. P., Williams, B. D., Snyder, M. M., Brown, C. F., and Pierce, E. M. (2016). Evidence of technetium and iodine release from a sodalite-bearing ceramic waste form. Appl. Geochem. 66, 210–218. doi:10.1016/j.apgeochem.2015.12.017
Pierce, E. M., Cantrell, K. J., Westsik, J. H., Parker, K. E., Um, W., Valenta, M. M., et al. (2010). Secondary waste form screening test results—cast stone and alkali alumino-silicate geopolymer (No. PNNL-19505). Richland, WA, US: Pacific Northwest National Lab.PNNL. doi:10.2172/989447
Pirlet, V., Lemmens, K., and Van Iseghem, P. (2004). Influence of the near-field conditions on the mobile concentrations of Np and Tc leached from vitrified HLW. MRS Online Proc. Libr. Opl. 824. doi:10.1557/PROC-824-CC7.5
Pirlet, V., Lemmens, K., and Van Iseghem, P. (2002). Leaching of Np and Tc from doped nuclear waste glasses in clay media: The effects of redox conditions. MRS Online Proc. Libr. Opl. 713, JJ13.6. doi:10.1557/PROC-713-JJ13.6
Pyo, J.-Y., Lee, C. W., Park, H.-S., Yang, J. H., Um, W., and Heo, J. (2017). Tellurite glasses for vitrification of technetium-99 from pyrochemical processing. J. Nucl. Mat. 493, 1–5. doi:10.1016/j.jnucmat.2017.05.052
Santikari, V. P., Witmer, M., Murdoch, L. C., Kaplan, D. I., and Powell, B. A. (2022). Leaching and transport of technetium from reducing cementitious waste forms in field lysimeters. Sci. Total Environ. 841 , 156596. doi:10.1016/j.scitotenv.2022.156596
Simner, S., Coutelot, F., Chang, H., and Seaman, J. (2017). Technetium leaching from cementitious materials. MRS Adv. 2 (13), 717–722. doi:10.1557/adv.2017.35
Singh, B. K., Hafeez, M. A., Kim, H., Hong, S., Kang, J., and Um, W. (2021). Inorganic waste forms for efficient immobilization of radionuclides. ACS ES&T Eng. 1 (8), 1149–1170. doi:10.1021/acsestengg.1c00184
Singh, D., Mandalika, V. R., Parulekar, S. J., and Wagh, A. S. (2006). Magnesium potassium phosphate ceramic for 99Tc immobilization. J. Nucl. Mat. 348 (3), 272–282. doi:10.1016/j.jnucmat.2005.09.026
Stefanovsky, S. V., Prusakov, I. L., Stefanovsky, O. I., Kadyko, M. I., Averin, A. A., Makarenkov, V. I., et al. (2019). The structure of rhenium containing sodium alumino (iron) phosphate glasses. Int. J. Appl. Glass Sci. 10 (4), 479–487. doi:10.1111/ijag.13476
Um, W. (2018). “99Tc immobilization in various waste forms,” in Proceedings of the 10th International Symposium on Technetium and Rhenium-Science and Utilization, 185–207.
Xu, H., Gong, W., Syltebo, L., Izzo, K., Lutze, W., and Pegg, I. L. (2014). Effect of blast furnace slag grades on fly ash based geopolymer waste forms. Fuel 133, 332–340. doi:10.1016/j.fuel.2014.05.018
Keywords: TC, solubility, leaching, waste form, Re
Citation: Singh BK, Kim J, Pak D, Kim K and Um W (2023) Technetium (Tc)/Rhenium (Re) solubility and leaching behavior from waste forms: An overview. Front. Nucl. Eng. 1:1112080. doi: 10.3389/fnuen.2022.1112080
Received: 30 November 2022; Accepted: 14 December 2022;
Published: 04 January 2023.
Edited by:
Taishi Kobayashi, Kyoto University, JapanReviewed by:
Shingo Kimuro, Japan Atomic Energy Agency, JapanCopyright © 2023 Singh, Kim, Pak, Kim and Um. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wooyong Um, d29veW9uZ3VtQHBvc3RlY2guYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.