- 1Jan and Dan Duncan Neurological Research Institute and Texas Children's Hospital, Houston, TX, United States
- 2Department of Neurology, Baylor College of Medicine, Houston, TX, United States
- 3MRC Mitochondrial Biology Unit, University of Cambridge, Cambridge, United Kingdom
- 4MitoPhenomics Lab, Biological Research Center Margarita Salas-CSIC, Madrid, Spain
- 5INCLIVA Biomedical Research Institute, Unversitat de València, Valencia, Spain
- 6Andalusian Center for Developmental Biology (CABD), CSIC/Universidad Pablo de Olavide/Junta de Andalucía, and CIBERER (ISCIII), Seville, Spain
Editorial on the Research Topic
Molecular and cellular pathways leading to mitochondrial dysfunction and neurodegeneration: lessons from in vivo models, volume II
The second volume of our editorial is a continuation of the first (Hannan et al., 2022) and features two reviews and three research articles that highlight how mitochondrial dysfunction can cause neurological diseases and suggest potential therapeutic targets for Parkinson's disease (PD) and neurological effects of SARS-CoV-2 infection. The research discussed in this special edition highlights the multifaceted nature of mitochondrial biology, with reviews on mitochondrial protein synthesis, ATAD3 mutations and their developmental impact, as well as research on sleep dysregulation, PD, and the neurological effects of SARS-CoV-2.
Brügel et al. provide a comprehensive review of studies investigating the role of mutations in ATAD3, which encodes a mitochondrial membrane protein associated with the inner mitochondrial membrane structure, nucleoid organization, cholesterol trafficking, and lipid metabolism, but whose primary function is unclear. Mutations in ATAD3 result in several human diseases, including developmental delay, optic atrophy, muscular atrophy, peripheral neuropathy, and congenital cataracts. This review brings together findings from Drosophila and mice model studies exploring the effects of several pathogenic mutations, including muscle- and neuron-specific ablation of ATAD3 function. In mice, ATAD3 dysfunction leads to progressive myopathy and premature death, highlighting the central role of this protein in cellular survival. In Drosophila, some variants are associated with embryonic lethality and increased mitophagy, while others show partial or failed lethality rescue in loss-of-function models. These variants caused locomotion defects, small mitochondria with cristae abnormalities, accumulation of autophagic intermediates, and cholesterol aggregates. The authors offer an in-depth overview of the current state of ATAD3 research, providing a useful resource for investigators interested in its function.
For the second review article in this special research edition, Antolinez-Fernández et al. explore the critical role of mitochondrial protein synthesis in neurodegenerative and muscular diseases. Despite its bacterial origin, mitochondrial protein synthesis is a highly specialized process that differs significantly from prokaryotic translation. Impairment in this process has been implicated in a wide array of diseases. The authors provide a detailed molecular overview of mitoribosome structure and different stages of mitochondrial protein translation. Although only 13 of the ~1,100 mitochondrial proteins are encoded by the mtDNA, several diseases are directly linked to mutations in mitochondrial protein synthesis. The review emphasizes the importance of understanding the molecular mechanisms underlying mitochondrial protein translation to develop therapeutic strategies for disorders caused by defects in this process, which are multisystemic and often associated with neurological symptoms. Future advancements in gene editing techniques may offer deeper insights into the tissue-specific impact of mitochondrial translation dysfunction that will probably help to understand the clinically heterogeneous consequence of defects in this process.
A research article by Favaro et al. explores the potential therapeutic benefits of downregulating Usp14 function, a deubiquitinating enzyme, in a Pink1 knockout (KO) fly model of PD. PD is a progressive neurodegenerative disorder primarily characterized by the loss of dopaminergic neurons in the brain, leading to motor dysfunction and other neuropsychiatric symptoms. One of the hallmark features of PD is mitochondrial dysfunction, which plays a critical role in the progression of the disease. Favaro et al. demonstrated that downregulation of Usp14 in Pink1 KO flies rescued both circadian rhythms and sleep disturbances. While the mechanisms behind the improved functions remain unclear, Usp14 inhibition may function by promoting proteostasis and reducing the buildup of dysfunctional proteins and mitochondria that contribute to neurodegeneration. This study suggests that targeting Usp14 could provide a novel therapeutic approach for early intervention in PD, potentially improving circadian rhythm disturbances and sleep defects before the onset of motor symptoms. Moreover, the availability of Usp14 inhibitors that are effective in mice and can cross the blood-brain barrier makes this a promising avenue for PD treatment.
Ay et al. found that Mito-Met, a metformin analog, exerts neuroprotective effects in cell and mouse models of PD by activating PKD1, Akt, and AMPK pathways. Mito-Met protects against neurotoxicity, mitochondrial fragmentation, and dopamine depletion, indicating its potential as a therapeutic agent for PD that warrants further evaluation.
Lastly, Guo et al. examine the effects of SARS-CoV-2 infection in neuronal vulnerability. The study focuses on Nsp7, a non-structural protein encoded by the viral genome. Both in vivo and in vitro studies suggest that expression of Nsp7 results in reduced levels of synaptic proteins. Furthermore, expression of Nsp7 leads to oxidative stress and impaired ATP production. Interestingly, treatment with antioxidant N-acetylcysteine in vitro rescued the deleterious effect of Nsp7 expression on synaptic proteins PSD95 and synaptophysin. This research points to the role of mitochondria in COVID-19-related neurological disorders, offering a potential target for therapeutic intervention. The findings suggest that mitochondrial damage may be an important factor in the neurological manifestations of COVID-19, including memory deficits and other cognitive impairments.
In summary, these articles collectively underscore the diverse and critical roles of mitochondria in various neurological diseases, from neurodegenerative and neurodevelopmental disorders to the neurological effects of viral infections like SARS-CoV-2 (see Figure 1). The research underscores the importance of mitochondrial function in cellular health and highlights the promising potential of targeting mitochondrial pathways for disease treatment.
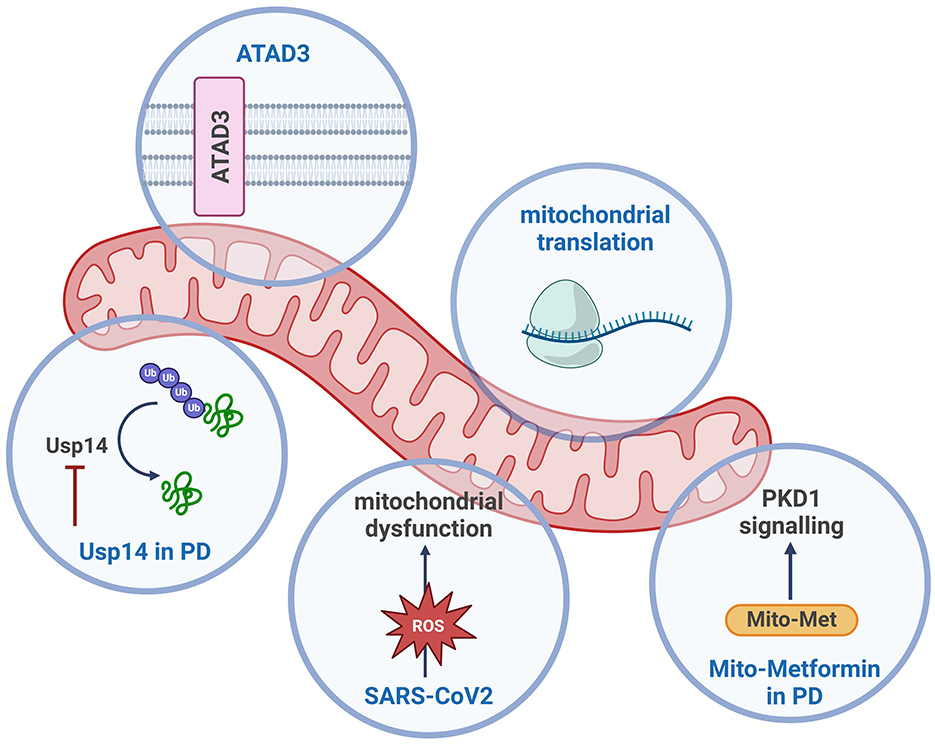
Figure 1. Schematic overview of the mitochondrial processes discussed in this Research Topic. Created with BioRender.com.
Author contributions
SH: Writing – original draft, Writing – review & editing, Conceptualization. FDL: Writing – original draft, Writing – review & editing, Conceptualization. AG-D: Writing – review & editing. JN: Writing – review & editing. GB-C: Writing – original draft, Writing – review & editing, Conceptualization. AS-M: Writing – original draft, Writing – review & editing, Conceptualization, Supervision.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Keywords: mitochondria, Drosophila, Parkinson's disease, ATAD3, SARS-CoV-2, Usp14, metformin, Pink1
Citation: Hannan SB, De Lazzari F, Gomez-Duran A, Navarro JA, Brea-Calvo G and Sanchez-Martinez A (2025) Editorial: Molecular and cellular pathways leading to mitochondrial dysfunction and neurodegeneration: lessons from in vivo models, volume II. Front. Neurosci. 19:1557013. doi: 10.3389/fnins.2025.1557013
Received: 07 January 2025; Accepted: 14 January 2025;
Published: 29 January 2025.
Edited and reviewed by: Wendy Noble, University of Exeter, United Kingdom
Copyright © 2025 Hannan, De Lazzari, Gomez-Duran, Navarro, Brea-Calvo and Sanchez-Martinez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alvaro Sanchez-Martinez, YXNtODBAY2FtLmFjLnVr
 Shabab B. Hannan
Shabab B. Hannan Federica De Lazzari
Federica De Lazzari Aurora Gomez-Duran
Aurora Gomez-Duran Juan A. Navarro
Juan A. Navarro Gloria Brea-Calvo
Gloria Brea-Calvo Alvaro Sanchez-Martinez
Alvaro Sanchez-Martinez