
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 09 April 2025
Sec. Translational Neuroscience
Volume 19 - 2025 | https://doi.org/10.3389/fnins.2025.1549295
This article is part of the Research TopicNeurobiological Basis of Substance Use Disorders: New Findings and PerspectivesView all 3 articles
 Elizabeth T. C. Lippard1,2,3,4,5*
Elizabeth T. C. Lippard1,2,3,4,5* Dylan E. Kirsch1,3,4,6
Dylan E. Kirsch1,3,4,6 Vanessa Le1,7
Vanessa Le1,7 Skyler Lee1
Skyler Lee1 Nadia Bibb1
Nadia Bibb1 Kaitlyn Meek1
Kaitlyn Meek1 Raquel Kosted1
Raquel Kosted1 Ansley Huffman1
Ansley Huffman1 J. R. C. Almeida1
J. R. C. Almeida1 Kim Fromme2,3
Kim Fromme2,3 Stephen M. Strakowski1,3,8
Stephen M. Strakowski1,3,8Introduction: Alcohol use disorder (AUD) occurs at higher rates in individuals with bipolar disorder compared to the general population. A paucity of data are available on specific mechanisms that may contribute to bipolar and AUD co-occurrence. We recently reported differences in alcohol expectancies and placebo response during alcohol administration in early-stage bipolar disorder, compared to healthy young adults. This current report investigated subjective and neural response following placebo beverage consumption in young adults with bipolar disorder.
Methods: As part of a within-subject placebo-controlled alcohol administration study, 54 young adults (53% with bipolar disorder type I, agemean + SD = 23 + 2 years, 64% female) completed resting state functional MRI (rsfMRI) scans at baseline (pre-beverage) and following placebo and alcohol consumption (counter-balanced). Participants completed subjective response measures during placebo and alcohol beverage conditions. Between-group differences in subjective response and placebo-related changes in functional connectivity of the Nucleus Accumbens (NAc) with other brain regions, compared to a pre-beverage rsfMRI baseline condition, were investigated. Fisher-transformed correlation coefficients between ROIs and seed-to-clusters showing a significant group-by-condition (placebo, pre-beverage rsfMRI) interaction were calculated. Associations with prospective alcohol use and problems were explored in a subgroup with longitudinal data.
Results: Young adults with bipolar disorder reported greater intoxication during the placebo condition, compared to healthy young adults (main effects of group: p < 0.05). Compared to pre-beverage rsfMRI, the placebo condition related to increased connectivity between bilateral NAc and regions within the sensorimotor network in bipolar disorder. Comparison participants showed the opposite pattern of placebo-related changes in connectivity (group-by-condition, p-FDR < 0.05). Greater anxiolytic effects endorsed during placebo and associated increases in NAc functional connectivity related to greater alcohol use and alcohol problems at follow-up in bipolar disorder (p < 0.05).
Discussion: Results suggest differences in placebo response in bipolar disorder, including distinct neural correlates, that may relate to prospective alcohol use/problems. Given the theoretical association between placebo response and self-reported alcohol expectancies, findings could open the door to interventions aimed at changing expectancies.
A diagnosis of bipolar disorder coincides with one of the highest prevalence rates of alcohol use disorder (AUD) of any psychiatric diagnosis (Conway et al., 2006). While a 20%–40% prevalence of AUD in bipolar disorder is suggested (Di Florio et al., 2014; Hunt et al., 2016; Nery et al., 2014; Simhandl et al., 2016), estimates as high as 60% prevalence of lifetime AUD in bipolar disorder have been reported (Regier et al., 1990). This co-occurrence is associated with a more pernicious illness course than when either condition is presented on their own, ranging from worse mood episodes to greater cognitive deficits and a higher risk of suicide (Cardoso et al., 2016; Finseth et al., 2012; Goldberg, 2001; Goldstein et al., 2006; McGrady et al., 2017; Messer et al., 2017; Nery et al., 2014; Oquendo et al., 2010; Sanchez-Moreno et al., 2009; Strakowski et al., 2005; Strakowski et al., 2000). Despite the widely recognized association between these conditions and associated clinical sequelae, few developmental studies exist investigating behavioral and neural mechanisms that may underlie risk and serve as early intervention targets. However, emerging work suggests youth with bipolar disorder may show differences in sensitivity to alcohol that may contribute to alcohol use and risk for development of AUD (Kirsch et al., 2023a; Lippard et al., 2023; Tretyak et al., 2021; Yip et al., 2012).
Variability in how individuals respond to alcohol is suggested to increase risk for developing AUD (King et al., 2021; King et al., 2011; King et al., 2014; Quinn and Fromme, 2011; Ray et al., 2010)—however, prior studies in this area typically excluded individuals with bipolar disorder. We recently completed a within-subject placebo-controlled alcohol administration study in young adults with bipolar disorder and healthy comparison young adults. Preliminary data suggested increased sensitivity to alcohol in young adults with bipolar disorder, compared to healthy comparison young adults (Lippard et al., 2023), with greater positive stimulating effects of alcohol and greater past month alcohol use associated with changes in nucleus accumbens (NAc) functional connectivity during the alcohol compared to placebo condition (Kirsch et al., 2023a). Moreover, the most robust differences observed in subjective response to alcohol were main effects of group, with young adults with bipolar disorder reporting greater subjective experience of intoxication during both the placebo and alcohol beverage conditions (Lippard et al., 2023), suggesting variability in alcohol expectations in bipolar disorder.
Placebo response is driven in part by alcohol expectancies, i.e., a person’s belief about what happens when they consume alcohol. Alcohol expectancies correlate with alcohol use in healthy populations (Fromme et al., 1993; Hasking et al., 2011; Kilbey et al., 1998) and recent work suggested greater neural response to a placebo beverage condition predicts greater alcohol problems in healthy young adults over a 5 years period (McKenna et al., 2022). However, this prior study did not include a non-placebo fMRI condition to distinguish if neural responses are specific to placebo manipulation or a baseline trait. We previously reported NAc functional connectivity changes during resting state fMRI (rsfMRI) that were associated with subjective level of intoxication during placebo beverage consumption, compared to a pre-beverage rsfMRI scan, in healthy young adults (Kirsch et al., 2023b), suggesting striatal reward systems may underlie alcohol expectancies. This extends prior work suggesting a neural basis for alcohol expectancies, i.e., alcohol expectancies positively relate to resting limbic and striatal functional connectivity (Le et al., 2022; Le et al., 2020; Zhornitsky et al., 2018), to suggest placebo-related change in striatal connectivity can be distinguished from trait neural phenotypes that may indirectly relate to alcohol expectancies as previously discussed (Kirsch et al., 2023b). It is unknown whether similar placebo-related changes in NAc functional connectivity are observed in bipolar disorder and associated with elevated placebo response.
This study is a secondary data analysis from a placebo-controlled alcohol administration study in bipolar disorder (NCT04063384). As part of the study design, a rsfMRI scan was collected prior to and following both alcohol and placebo administration. This study is hypothesis driven. We hypothesized that young adults with bipolar disorder, compared with healthy comparison young adults, would report greater subjective effects of intoxication during both alcohol and placebo conditions with increased perceived intoxication during placebo, compared to a pre-beverage fMRI scan, relating to greater increases in NAc functional connectivity with ventral prefrontal regions of interest (ROIs) consistent with prior work in healthy young adults (Kirsch et al., 2023b). Additionally, we conducted an exploratory analysis of placebo-associated changes in NAc functional connectivity that may extend outside of a priori ROIs. By focusing on the placebo manipulation, we aimed to investigate the neural underpinnings of expectancy-related effects that occur following alcohol consumption (in the absence of alcohol pharmacokinetics). This study extends our prior work to investigate neural underpinnings of alcohol expectancies in young adults with bipolar disorder. As it is unknown if placebo response—and underlying neural systems—relates to prospective alcohol use/problems in bipolar disorder, a subset of individuals provided longitudinal data on alcohol outcomes and associations between placebo response and alcohol-related outcomes were also explored. We hypothesized greater sensitivity to placebo manipulation—and associated changes in NAc functional connectivity—in bipolar disorder would relate to greater alcohol use/problems over time. A better understanding of factors that contribute to alcohol misuse and problems over time could support novel interventions for individuals with bipolar disorder (e.g., alcohol expectancy challenges or neuromodulation targets).
Participants were recruited from Central Texas between July 2019 and February 2024 as previously described (Lippard et al., 2023). Participants completed a fMRI session prior to beverage sessions and another fMRI session following placebo and alcohol beverage consumption. Sixty participants enrolled into the parent study (50% with bipolar disorder type I). A power analysis prior to the start of study suggested 30 individuals per group would provide adequate power for testing our hypothesis. Specifically, at an alpha = 0.05, 30 subjects per group will provide > 80% statistical power to detect a within-subject ES d = 0.5 and a between group ES d = 0.7. Five participants were excluded from this secondary analysis (two with bipolar disorder and three healthy comparison young adults) owing to placebo manipulation issues. Additionally, one healthy comparison subject was excluded because they later revealed a history of a major depressive episode. The final dataset for this secondary analysis therefore included 28 young adults with bipolar I disorder and 26 healthy comparison young adults. We also excluded one additional healthy comparison young adult from the rsfMRI analysis as the image acquisition protocol changed after the first enrolled participant’s MRI scan. Our final resting state fMRI analysis included 28 young adults with bipolar disorder and 25 healthy comparison young adults. Participants with bipolar disorder were euthymic and stable on medication at the time of fMRI and beverage sessions. Individuals in the healthy comparison subgroup had no history of psychiatric hospitalizations; neurodevelopmental, affective, or psychotic disorder; or > 1 month of lifetime psychotropic medication. Exclusion criteria for all participants included: full scale intelligence quotient (IQ) < 85; contraindication to MRI scanning; significant head trauma (loss of consciousness for greater than 5 min); positive pregnancy test; history of severe AUD; a current SUD (other than alcohol, cannabis, or nicotine). Additional exclusion criteria (all participants) included heart trouble; high blood pressure; history of heart attack; diabetes; liver disease; ever being in an abstinence-oriented treatment program for alcohol use; reporting wanting to quit drinking but not being able to; any medical, religious, or other reasons for not drinking alcohol; an adverse reaction to alcoholic beverages (e.g., severe flushing; seizure); an Alcohol Use Disorder Identification Test (AUDIT) score > 15 (assessed at time of phone screen), and unwillingness to have friend/family member drive them home after beverage sessions. All participants had to report consuming four (men) or three (women) or more drinks on a drinking occasion in the past year. Inclusion/exclusion criteria were defined to minimize risk associated with an alcohol administration study in accordance with the NIAAA Guidance for Conducting Alcohol Administration Studies with Human Participants. Specifically, individuals needed to drink to the point of intoxication they would be dosed to in the lab (0.08g%) to minimize individuals having a negative reaction to alcohol, but not drink too much or endorse alcohol-related problems where it would be an ethical concern to provide alcohol in the lab (e.g., someone reporting wanting to quit drinking but not being able to). Study procedures were approved by the Institutional Review Board at the University of Texas at Austin.
The Structured Clinical Interview for DSM 5, Research Version (SCID-5-RV) was used to confirm presence of a prior manic episode in individuals with bipolar disorder. The Wechsler Abbreviated Scale of Intelligence matrix reasoning and vocabulary subtests were used as a measure of IQ. The Timeline Follow-Back [TLFB (Sobell and Sobell, 1992)] was used to measure recent alcohol, tobacco, and marijuana use over the past 30 days. Total drinks, number of days drinking, and average number of drinks per drinking day was calculated. We also calculated maximum number of drinks consumed on a drinking day and number of heavy episodic drinking episodes (> 5 drinks for men and > 4 drinks for women). Participants completed the Barratt Impulsiveness Scale-11 [BIS-11 (Patton et al., 1995)].
Following enrollment and the pre-beverage fMRI scan, participants completed an alcohol and placebo beverage condition on two separate days (counter-balanced). Beverage consumption occurred in a private room in the Biomedical Imaging Center with research staff present, as previously described (Lippard et al., 2023). The order of beverage session was randomized. Participants were informed they would be drinking alcohol on both beverage days and could receive different amounts of alcohol on each respective day, but they would not be dosed to exceed a breath alcohol concentration (BrAC) of 0.08 g%. Participants were blinded to the beverage condition they would receive on each respective day, as described in previous studies (Quinn and Fromme, 2016). The Beck Depression and Anxiety Inventories (BDI and BAI, respectively) were completed as soon as individuals arrived on each drinking day. Participants were asked to fast from food for 4 h prior to drinking and then consumed a weight-adjusted snack of pretzels prior to beverage consumption. This approach delays the rate of alcohol absorption, increasing the duration of the ascending limb of the blood alcohol concentration curve (Jones and Jonsson, 1994). On the alcohol condition day, participants were dosed to a 0.08 g% target BrAC based on the participants’ age, sex, height, and weight (Cofresí et al., 2020; Corbin et al., 2021). Participants were given 20 min to consume two beverages (1:3 mixture of 80 proof vodka to mixer, 10 min per beverage). The mixer contained cranberry juice, diet seven-up, and lime juice. After 20 min of drinking and a 10 min absorption period (during which participants rinsed their mouth with alcohol free mouth wash), BrAC, heart rate, and measures of subjective response to alcohol was collected. The placebo and alcohol conditions were identical per standardized protocols (Cofresí et al., 2020; Corbin et al., 2021; Quinn and Fromme, 2016) with decarbonated tonic water stored in Absolute vodka bottles used in place of vodka for the placebo condition. For both beverage sessions, participants remained seated during the beverage session but saw vodka (or tonic) being poured out of an Absolute bottle (visual cue), the table was wiped down with tequila before the participant entered the room (olfactory cue), and an alcohol floater was added to beverages (gustatory cues). There were minimal environmental (i.e., alcohol-related) cues during the beverage session with the exception of participants seeing their drinks being made. Post-beverage BrAC assessment during the placebo condition was yoked to timing of BrAC collection during the alcohol session.
Subjective response to alcohol (or placebo) was collected on each beverage day with the Subjective Effects of Alcohol Scale [SEAS (Morean et al., 2013a)]. Four SEAS continuous subscale scores were calculated: positive valence/positive arousal (“lively, talkative, fun, funny”); positive valence/negative arousal (“mellow, relaxed, secure, calm”); negative valence/positive arousal (“aggressive, rude, demanding”); and negative valence/negative arousal (“woozy, dizzy, wobbly”). BrAC, heart rate, and the SEAS was collected before each beverage session began (BrAC was 0.0 g% for all participants prior to beverage consumption) and after beverage consumption/absorption [immediately prior to the fMRI scan (defined as pre-scan BrAC, heart rate, and subjective response)]. BrAC, heart rate, and subjective response were collected again immediately after participants exited the scanner (defined as post-scan BrAC, heart rate, and subjective response). Changes in SEAS at pre-scan and post-scan BrAC, compared to pre-beverage baseline SEAS scores on each respective day, was calculated for both beverage conditions. We collected a modified version of the Drug Effects Questionnaire [DEQ (Morean et al., 2013b)] at pre- and post-scan BrAC which assessed the extent to which participants (1) felt drunk, (2) felt effects of alcohol, (3) liked how they were feeling, and (4) wanted more of what they had consumed. At pre-scan BrAC, we asked participants to estimate the number of standard alcohol drinks they were served during the experiment which served as a placebo manipulation check. This study excluded anyone that did not report having consumed at least one alcohol beverage during their placebo manipulation. Following post-scan BrAC data collection, BrAC readings were continued every 30 min until participants were below a 0.04 g% BrAC during the alcohol condition.
All imaging occurred with a 3-Tesla Siemens VIDA MR scanner (Siemens, Erlangen, Germany) using a 64-channel head coil at the University of Texas at Austin Biomedical Imaging Center as previously described (Kirsch et al., 2023b). Briefly, a sagittal three-dimensional MPRAGE T1-weighted sequence was acquired with parameters: repetition time (TR) = 2,400 ms, echo time (TE) = 2.18 ms, matrix = 208 × 300 × 320, flip angle = 8°; field of view = 167 mm × 240 mm × 256 mm, 0.8 mm slices and one average with isotropic voxel geometry (0.8 mm × 0.8 mm × 0.8 mm). A rsfMRI scan was collected prior to beverage consumption and following both alcohol and placebo beverage conditions. For this analysis, we only examined pre-beverage and post-placebo rsfMRI data to focus on group differences in placebo response. FMRI data were collected with a single-shot echo-planar imaging sequence aligned with the anterior-posterior commissure plane with multiband factor: 6, TR = 778 ms, TE = 30 ms, matrix = 86 × 86, flip angle = 52°, field of view = 215 mm × 215 mm, and 60 2.5 mm thick slices without gap (voxel size = 2.5 mm × 2.5 mm × 2.5 mm). During the rsfMRI scan, participants viewed the Headspace Studios’ Inscapes video (6 min). The video contains an inanimate object changing shape and has been used previously in rsfMRI studies, including those involving clinical populations (Chung et al., 2023; Plewnia et al., 2021). While some differences in viewing the Inscapes video, compared to traditional rsfMRI paradigms, are reported (Vanderwal et al., 2015), we utilized the video to minimize motion, improve signal to noise (Vanderwal et al., 2017; Vandewouw et al., 2021), and reduce concerns that alcohol or placebo beverage consumption may alter an individual’s thoughts enough, which could limit our ability to interpret differences across conditions.
The CONN toolbox1 (Whitfield-Gabrieli and Nieto-Castanon, 2012) was used for rsfMRI data preprocessing as previously described (Kirsch et al., 2023b). Denoising included aCompCor (anatomical component analysis correction) regression (Behzadi et al., 2007) followed by quadratic detrending and band-pass filtering (0.008–1 Hz). Cerebrospinal fluid, white matter, six motion parameters and their first derivatives, scrubbing parameters, and condition effects were included as nuisance regressors. We utilized both ROI-to-ROI and seed-to-voxel based approaches to investigate group differences in NAc changes underlying placebo response (further detailed below).
Between group differences in continuous variables were assessed with t-tests and Mann-Whitney-Wilcoxon tests, as appropriate. Categorical variables were assessed with Chi-square or Fisher’s exact tests, as appropriate. Group differences and group-by-time interactions on BrAC during the alcohol condition were investigated with BrAC (pre- and post-scan) as repeated within-subject variables. Parallel models were used to investigate changes in heart rate, with heart rate on the alcohol condition day (baseline, pre-scan, and post-scan) as repeated within-subject variables. We repeated this model with heart rate collected on the placebo day to explore if the placebo manipulation related to changes in heart rate across the study session. Mixed models were used to investigate group-by-beverage condition interactions in time to BrAC collection and number of standard drinks estimated to have consumed at pre- and post-scan time points, as well as BAI and BDI total scores.
Group (bipolar, healthy)-by-condition (alcohol, placebo)-by-time of subjective response interactions on subjective response to alcohol were modeled, covarying beverage condition order, biological sex, and age as previously described (Lippard et al., 2023), with SEAS and DEQ subscale scores as the dependent variables. Time of subjective response (pre- and post-scan) and beverage condition (alcohol, placebo) were within-subject factors and group was an independent between-subject factor. Following no group-by-condition-by-time of subjective response interaction, the three-way interaction term was dropped and group-by-condition interactions were investigated. Following no two-way interaction, the two-way interaction term was dropped and main effects of group and condition were assessed. Findings for these planned analyses were considered significant at p < 0.05.
A ROI-to-ROI bivariate correlation first-level analysis was performed using the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012) to calculate and extract fisher transformed correlation coefficients between a priori ROI-to-ROI pairs during the placebo and pre-beverage rsfMRI scans. ROIs were defined with the FSL Harvard Oxford Atlas and included ventromedial prefrontal cortex (vmPFC), subcallosal cingulate cortex (SCC), and bilateral NAc. We focused on these ROIs as previous data suggested placebo response in healthy young adults relate to increases in functional connectivity of the NAc with the vmPFC and SCC when viewing the Inscapes video (Kirsch et al., 2023b). We used mixed models to examine group-by-condition (placebo, pre-beverage rsfMRI)-by-NAc hemisphere (left, right) interactions on ROI-to-ROI functional connectivity (SCC and vmPFC modeled separately). RsfMRI condition (placebo, pre-beverage) and NAc hemisphere were within-subject factors, and ROI-to-ROI functional connectivity between the NAc with vmPFC and SCC were the dependent variables. Following no three-way interaction with hemisphere, the three-way interaction term was dropped, and group-by-condition interactions were investigated. Following no two-way interaction, the two-way interaction term was dropped and main effects of group and condition were investigated. Results of primary models were considered significant at p < 0.05. All models included biological sex, session order (if placebo came before or after the alcohol session), and age as covariates.
The CONN toolbox was also used for a seed-to-voxel functional connectivity analysis of the NAc (pre-defined seed region) to investigate functional connectivity changes that may relate to placebo response outside of our a priori ROIs. The bilateral NAc seed was defined with the FSL Harvard Oxford Atlas as above. The mean time series for left and right NAc was used as a predictor in the multiple regression general linear model for each voxel during first-level modeling. Between group differences in placebo-related changes in NAc functional connectivity (placebo rsfMRI minus pre-beverage rsfMRI) were modeled at the second level. Cluster-extent based thresholding was used with results considered significant if they survived a primary threshold of voxel-wise p < 0.005 and a cluster-level extent threshold of p < 0.05 using the false discovery rate correction (p-FDR). Following a group difference, for the placebo and pre-beverage rsfMRI, fisher transformed correlation coefficients between NAc and cluster pairs were calculated and extracted. Data was extracted for both the left and right NAc seed region. We conducted a post hoc analysis with extracted data modeling group-by-condition-by-NAc hemisphere interactions. Age, biological sex, and order of the placebo condition (if it came before or after the alcohol condition) were included as covariates. If there were no interactions with hemisphere, the three-way interaction term was dropped, and group-by-condition interactions investigated. If a group-by-condition interaction was observed, the effects of condition (placebo, pre-beverage rsfMRI) were modeled (stratified by group). Main effects of group and condition were investigated if there was no two-way interaction. If a group-by-condition-by-hemisphere interaction was observed, the models were stratified by hemisphere, and group-by-condition interactions in each hemisphere were investigated as above.
Post hoc sensitivity analyses were conducted to confirm if group-by-condition interactions or main effects of group identified in above models remained significant after (1) covarying history of AUD (current or past) and (2) covarying history of cannabis use disorder (CUD; current or past). We also repeated analyses when controlling for anxiety symptoms (BAI total score) and when covarying total impulsivity (BIS total score). Anxiety symptoms and total BIS scores were included as covariates in post hoc sensitivity analyses since prior work suggests anxiety and impulsivity relates to variation in subjective response to alcohol (Chutuape and de Wit, 1995; Leeman et al., 2014) and greater anxiety and impulsivity is often reported in bipolar disorder (Lippard et al., 2023; Najt et al., 2007) and could represent a confound. Additionally, we completed a sensitivity analysis for models incorporating fMRI data above after removing one participant with bipolar disorder because of technical issues with the Inscapes video on their placebo beverage day.
We also explored effects of medication in individuals with bipolar disorder (any subclass of medication with more than five individuals on and off a respective medication subclass). Specifically, we investigated main effects of medication (on/off: antipsychotic, anticonvulsant, antidepressant/SSRI, or sedative/antihistamine) on subjective response variables that showed a between group difference in analyses above. Beverage condition and time of subjective response was considered repeated within-subject factors and medication-by-condition interactions were modeled covarying age, sex, and order of beverage session. If the interaction term was not significant, the two-way interaction term was dropped and main effects of a medication subclass investigated. We similarly investigated medication effects on placebo-associated change in ROI-to-ROI and seed-to-voxel functional connectivity that showed group differences above, with condition (pre-beverage and post-placebo consumption rsfMRI) as a repeated within-subject factor. Normality of data was investigated and for measures not normally distributed (e.g., SEAS positive valence/positive arousal), data was transformed and models repeated.
Placebo-adjusted changes in NAc seed-to-voxel based functional connectivity (placebo session minus pre-beverage rsfMRI session) were calculated for each participant for seed-to-cluster pairs that showed a significant group-by-condition interaction in the seed-to-voxel analysis above. Group-by-placebo-adjusted changes in functional connectivity interactions were modeled with subjective response subscales during placebo beverage condition (that showed a group difference in the subjective response analysis) as the dependent variables. Only pre-scan subjective response variables were used in this analysis to avoid effects of participants laying in the scanner and so results would be more comparable to other placebo-controlled studies. Age, sex, and order of placebo session were included as covariates. Following no significant group-by-placebo-adjusted change in NAc functional connectivity, the two-way interaction term was dropped and main effect of placebo-adjusted change in NAc functional connectivity was investigated. NAc hemisphere was considered a repeated within-subject variable unless interactions with NAc hemisphere were identified above. If group differences localized to a specific hemisphere, analysis was conducted only within the hemisphere showing group differences. Results were considered significant at p < 0.05.
A subset of participants (n = 20 with bipolar disorder and 16 healthy comparison young adults) to date have completed a follow-up clinical evaluation (on average 2 years after their baseline assessment/beverage sessions), which included reassessment of AUD symptoms on the SCID-5-RV and TLFB to assess past month alcohol use. Between group differences in number of AUD symptoms reaching a three on the SCID-5-RV and past month total drinking days, total drinks, maximum drinks on a drinking day, number of heavy episodic drinking days, and average drinks per drinking day (measured with the TLFB) over time were investigated. Time from baseline enrollment to clinical follow-up was included as a covariate with AUD symptoms at baseline and follow-up (or TLFB measures) considered repeated dependent variables. Additionally, we explored relations between subjective response to placebo (on any subjective response variable that differed by group) and alcohol use outcomes. Three healthy comparison participants were excluded from the exploratory analysis on subjective response to placebo since three of the healthy comparison individuals with longitudinal data did not believe the placebo manipulation. All individuals in the bipolar disorder subgroup with longitudinal data believed the placebo manipulation. Specifically, group-by-subjective response interactions were modeled with average drinks per drinking day at follow-up as the dependent variable, controlling for baseline average drinks per drinking day and time between baseline and follow-up assessment. Models were repeated for other TLFB variables. We also repeated these models to investigate relations between baseline subjective response during placebo with prospective symptoms of AUD on the SCID (controlling for baseline AUD symptoms), however, we only modeled this relation in the subgroup with bipolar disorder as there was a lack of variability in number of symptoms meeting threshold on the SCID at follow-up in the healthy comparison group. Parallel models described above were used to explore effects of placebo-related change in NAc functional connectivity (for any seed-to-cluster pair that showed a significant change in functional connectivity during the placebo session in bipolar disorder in above analyses). Functional connectivity of the left and right NAc was modeled separately. Results for these exploratory analyses were considered significant at p < 0.05.
Young adults with bipolar I disorder had greater depression and anxiety symptoms at enrollment, and higher impulsivity than healthy comparison young adults (p < 0.001; Table 1). No between group differences were observed in BrAC or group-by-time interactions on BrAC during the alcohol condition. No between group differences (or group-by-beverage condition interactions) were observed in time to pre-scan or post-scan BrAC collection. However, a main effect of beverage condition in time to post-scan BrAC collection (p = 0.02) was noted, with time to post-scan BrAC being longer during the alcohol, compared to placebo, condition day (94 min vs. 91 min, respectively). A main effect of time on heart rate was also observed during the alcohol condition (p = 0.01), with both groups showing an increase in heart rate over the course of the alcohol condition. No effect of time on heart rate was seen during the placebo session. Also, no group-by-time interactions in heart rate were noted. Young adults with bipolar disorder endorsed greater symptoms of depression and anxiety on their beverage days (main effect of group on BAI: p = 0.002, BDI: p < 0.001) compared to healthy young adults, but there were no differences in depression or anxiety symptoms between alcohol and placebo condition days. A main effect of condition was seen on number of standard drinks estimated to have consumed at pre-scan BrAC (p < 0.0001) but there was no group-by-condition interaction or main effect of group on number of standard drinks estimated to have consumed (placebo manipulation check).
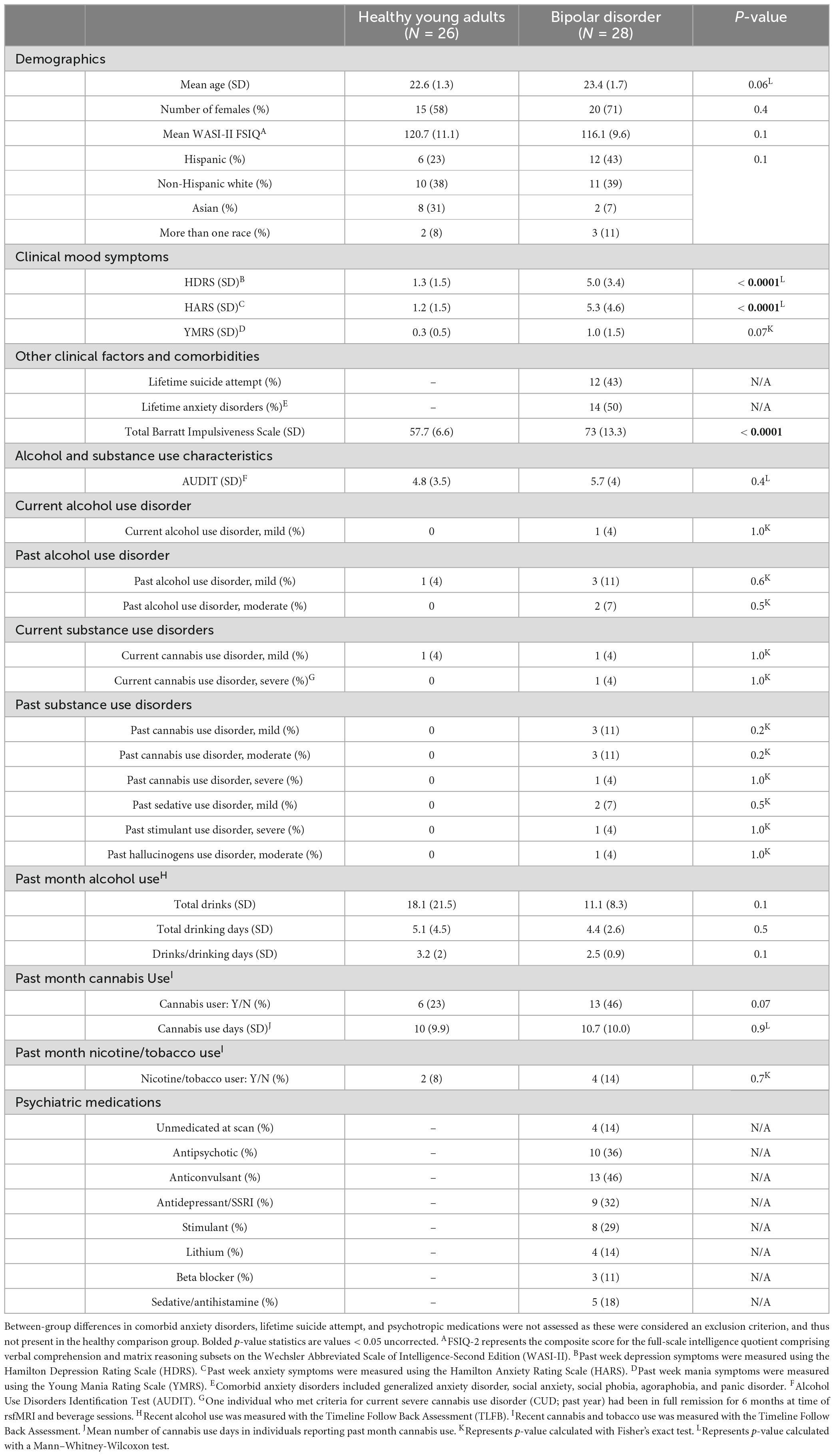
Table 1. Between-group (bipolar disorder vs. healthy comparison young adults) differences in demographics, clinical mood symptoms, alcohol and substance use characteristics, and past month alcohol/cannabis/nicotine use were assessed using two-sample t-tests, Mann–Whitney-Wilcoxon tests, chi-square, or Fisher’s exact as appropriate.
A significant main effect of group was seen for SEAS positive valence/positive arousal [F = 7.6, p = 0.007, 95% confidence interval (CI) (1.1, 6.5)], SEAS positive valence/negative arousal [F = 6.1, p = 0.02, 95% CI (0.6, 5.5)], feeling drunk [F = 28.0, p < 0.0001, 95% CI (4.6, 10.1)], and feeling alcohol effects [F = 18.4, p0.00, 95% CI (3.6, 9.7)], with young adults with bipolar disorder feeling more effects across all subscale scores for both alcohol and placebo conditions (see Figure 1). Main effects of condition were observed for SEAS positive valence/positive arousal [F = 11.9, p = 0.0009, 95% CI (1.9, 7.0)], SEAS negative valence/negative arousal [F = 60.7, p < 0.0001, 95% CI (5.2, 8.7)], feeling drunk [F = 91.7, p < 0.0001, 95% CI (9.8, 15)], and feeling alcohol effects [F = 103.8, p < 0.0001, 95% CI (11.4, 16.9)] with greater effects reported during the alcohol, compared to the placebo, condition for both groups. While there was a trend for a group-by-condition interaction on SEAS positive valence/positive arousal (F = 3.0, p = 0.09), there were no significant group-by-condition interactions across any subjective response variable. There were no group interactions with time of subjective response (pre- to post-scan subjective response collection). There were no other main effects of group. When covarying total BIS scores, the main effects of group on SEAS positive valence/positive arousal [F = 2.3, p = 0.1, 95% CI (−0.8, 5.8)] and SEAS positive valence/negative arousal [F = 1.9, p = 0.2, 95% CI (−0.9, 5.0)] were no longer significant. However, significant main effects of group in feeling “drunk” and feeling “effects of alcohol” persisted. All results remained significant when covarying if individuals had a history of AUD or CUD (current or past) and when covarying BAI scores. When exploring effects of medication, we observed individuals on an anticonvulsant reported feeling more drunk [F = 6.3, p = 0.02, 95% CI (1, 8.8)] and more effects of alcohol [F = 9.4, p = 0.003, 95% CI (2, 9.7)] compared to individuals not on an anticonvulsant. There were no other effects of medication. The SEAS positive valence/negative arousal subscale score was normally distributed and related residuals from models were normally distributed. Feeling drunk was not normally distributed (Shapiro-Wilk p < 0.05), however, when testing normality of residuals from models investigating “feeling drunk,” residuals were normally distributed. Other subjective response variables (and their model residuals) were not normally distributed (Shapiro-Wilk p < 0.05), however, Q-Q plots suggest data is approaching univariate normality. We log transformed subjective response variables that were not normally distributed, and results reported above remained the same.
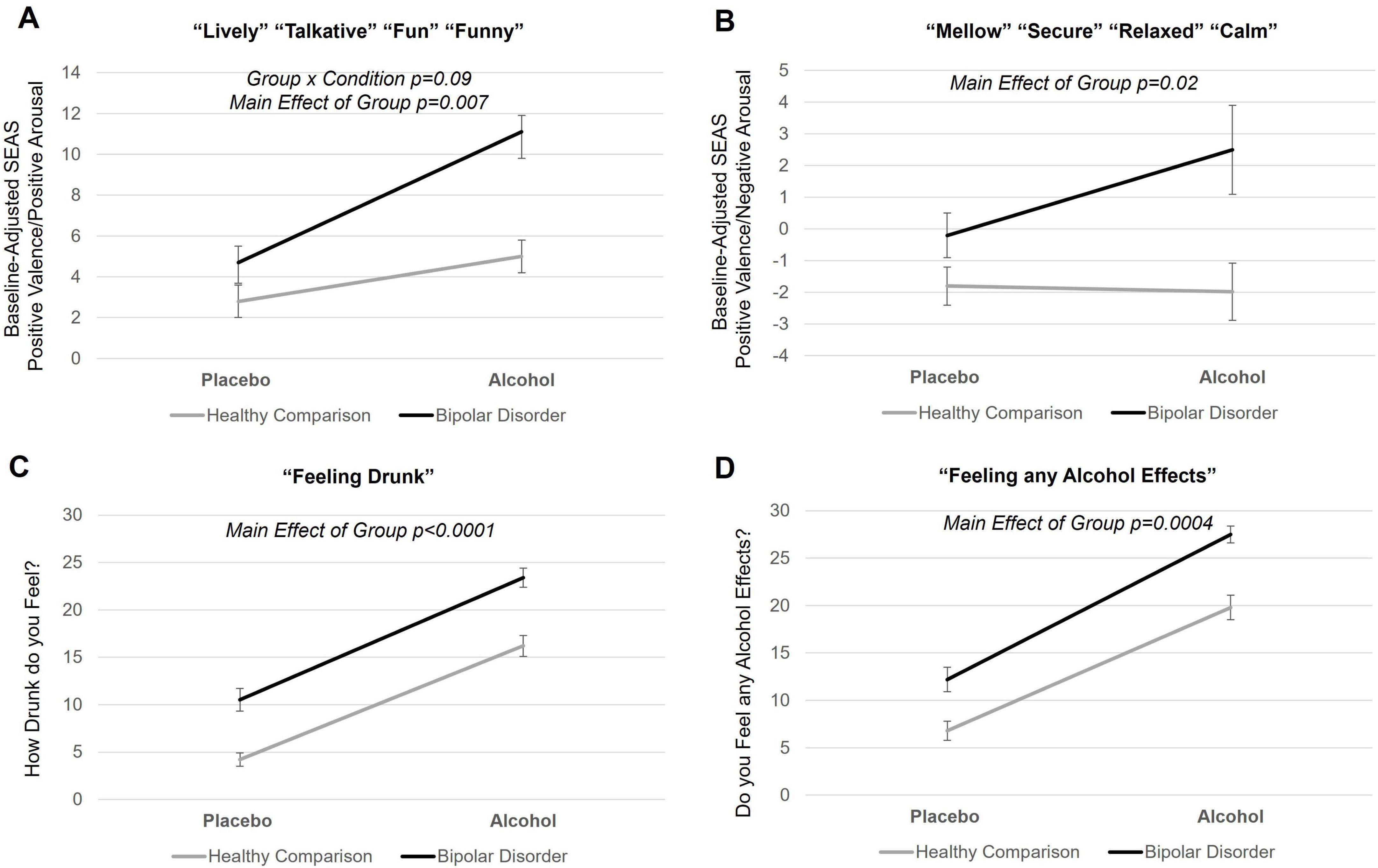
Figure 1. Subjective response to alcohol in young adults with bipolar disorder and healthy comparison young adults. (A,B) Mean self-report of subjective response to alcohol scores for young adults with bipolar disorder and healthy young adults during placebo and alcohol beverage conditions. Self-report of subjective response to alcohol measures included the Subjective Effects of Alcohol Scale (SEAS) and Drug Effects Questionnaire (DEQ). Each SEAS subscale score was baseline adjusted. (C,D) There was main effect of group on SEAS positive valence/positive arousal subscale (p = 0.007), SEAS positive valence/negative arousal subscale (p = 0.02), DEQ “feeling drunk” (p < 0.0001), and DEQ “feeling alcohol effects” (p = 0.0004), with young adults with bipolar disorder reporting greater scores across all these subjective response variables during both the alcohol and placebo condition. Means include pre- and post-scan subjective response as there was no time of subjective response interactions. Error bars represent standard errors. Black line indicates young adults with bipolar disorder; gray line indicates healthy comparison young adults.
There was a significant group-by-condition interaction [F = 4.2, p = 0.04, 95% CI (0.003, 0.2)] when investigating functional connectivity between the NAc and SCC (see Figure 2). When stratifying by group, the healthy comparison group showed an increase in NAc functional connectivity with SCC during the placebo, compared to the pre-beverage, rsfMRI scan [main effect of condition: F = 13.8, p = 0.0006, 95% CI (0.05, 0.2)], while young adults with bipolar disorder did not show a change in NAc-to-SCC functional connectivity [F = 0.06, p = 0.8, 95% CI (−0.07, 0.1)]. There were no significant interactions with hemisphere. Functional connectivity between the NAc and vmPFC ROI did not differ by group or show a group-by-condition interaction. Results remained significant in sensitivity analyses. There were no effects of medication. Data and residuals from models were normally distributed.
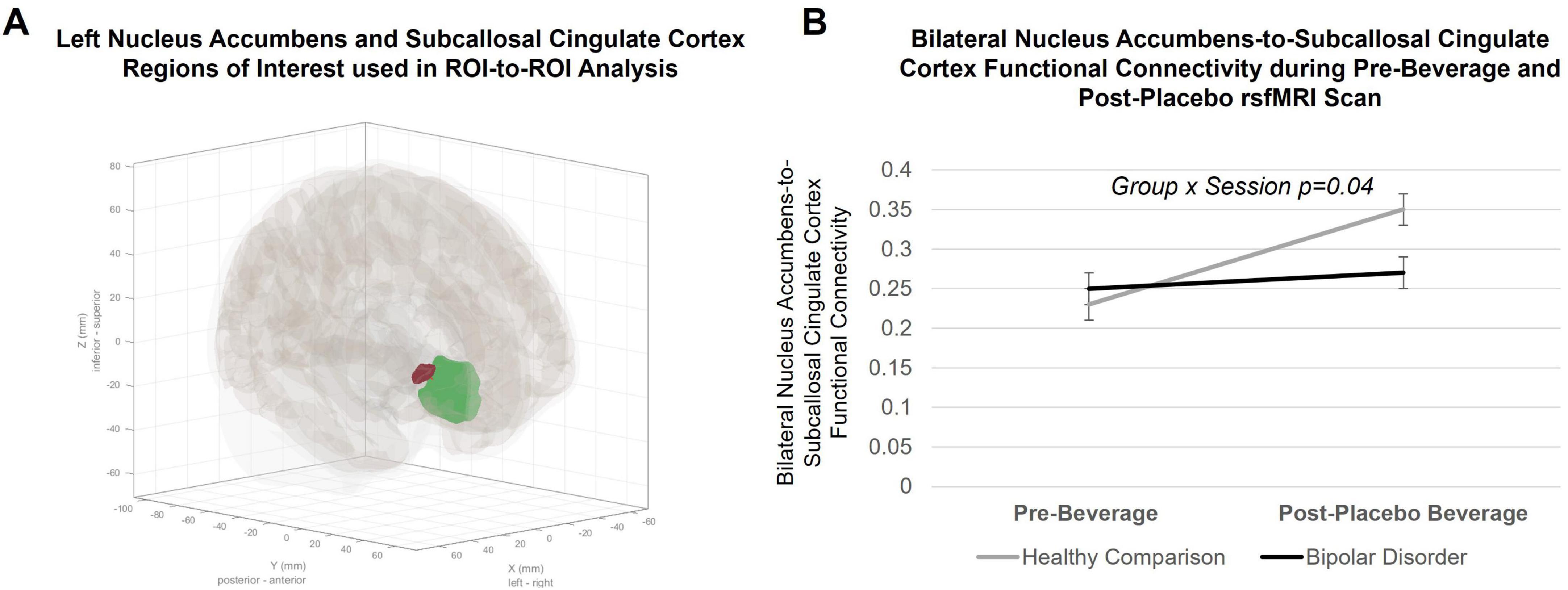
Figure 2. Region of interest (ROI)-to-ROI group-level analysis in the CONN toolbox. (A) Anatomical ROIs for the left Nucleus Accumbens (NAc; red ROI) and the Subcallosal Cingulate Cortex (SCC; green ROI) defined in CONN. (B) Within-subject changes in bilateral Nucleus Accumbens-to-Subcallosal Cingulate Cortex resting state functional connectivity from pre- to post-placebo beverage consumption. Lines indicate within-subject changes from pre- to post-beverage consumption. Black line indicates young adults with bipolar disorder; gray line indicates healthy comparison young adults. A significant group-by-condition interaction (p = 0.04) was observed. Post hoc connection-level comparisons indicated a significant increase in NAc-to-SCC functional connectivity during the placebo condition in healthy comparison young adults (F = 13.8, p = 0.0006), but not changes in functional connectivity between ROIs in bipolar disorder (F = 0.06, p = 0.8).
There was a significant group difference in change in functional connectivity of the NAC during the placebo, compared to pre-beverage rsfMRI scan, with a cluster within the left postcentral gyrus extending into the central and parietal operculum cortex and supramarginal gyrus (NAc-to-left postcentral/SMG cluster: x = −58 mm, y = −30 mm, z = +24 mm; 323 voxels; p-FDR = 0.000009, see Figure 3A) and with a cluster in the right postcentral gyrus extending into the parietal operculum cortex and supramarginal gyrus (NAc-to-right postcentral/SMG cluster: x = +66 mm, y = −16 mm, z = +24 mm; 117 voxels; p-FDR = 0.02). When modeling extracted data, there were no interactions with NAc hemisphere. As seen in Figures 3B, C, when stratifying by group to interpret the group-by-condition interaction on NAc-to-left postcentral gyrus/SMG [F = 24.7, p < 0.0001, 95% CI (0.1, 0.25)] and NAc-to-right postcentral gyrus/SMG [F = 21.9, p < 0.0001, 95% CI (0.09, 0.23)] functional connectivity, young adults with bipolar disorder showed a significant increase in NAc functional connectivity during the placebo, compared to pre-beverage rsfMRI scan, with the left postcentral/SMG [β = 0.09, p = 0.0004, 95% CI (0.04, 0.14)] and the right postcentral/SMG [β = 0.08, p = 0.001, 95% CI (0.03, 0.12)], while healthy comparison young adults showed a decrease in NAc-to-left postcentral/SMG [β = −0.08, p = 0.003, 95% CI (−0.03, −0.14)] as well as the right postcentral/SMG functional connectivity [β = −0.08, p = 0.004, 95% CI (−0.03, −0.14)]. There were no effects of medication. Data and residuals from models were normally distributed.
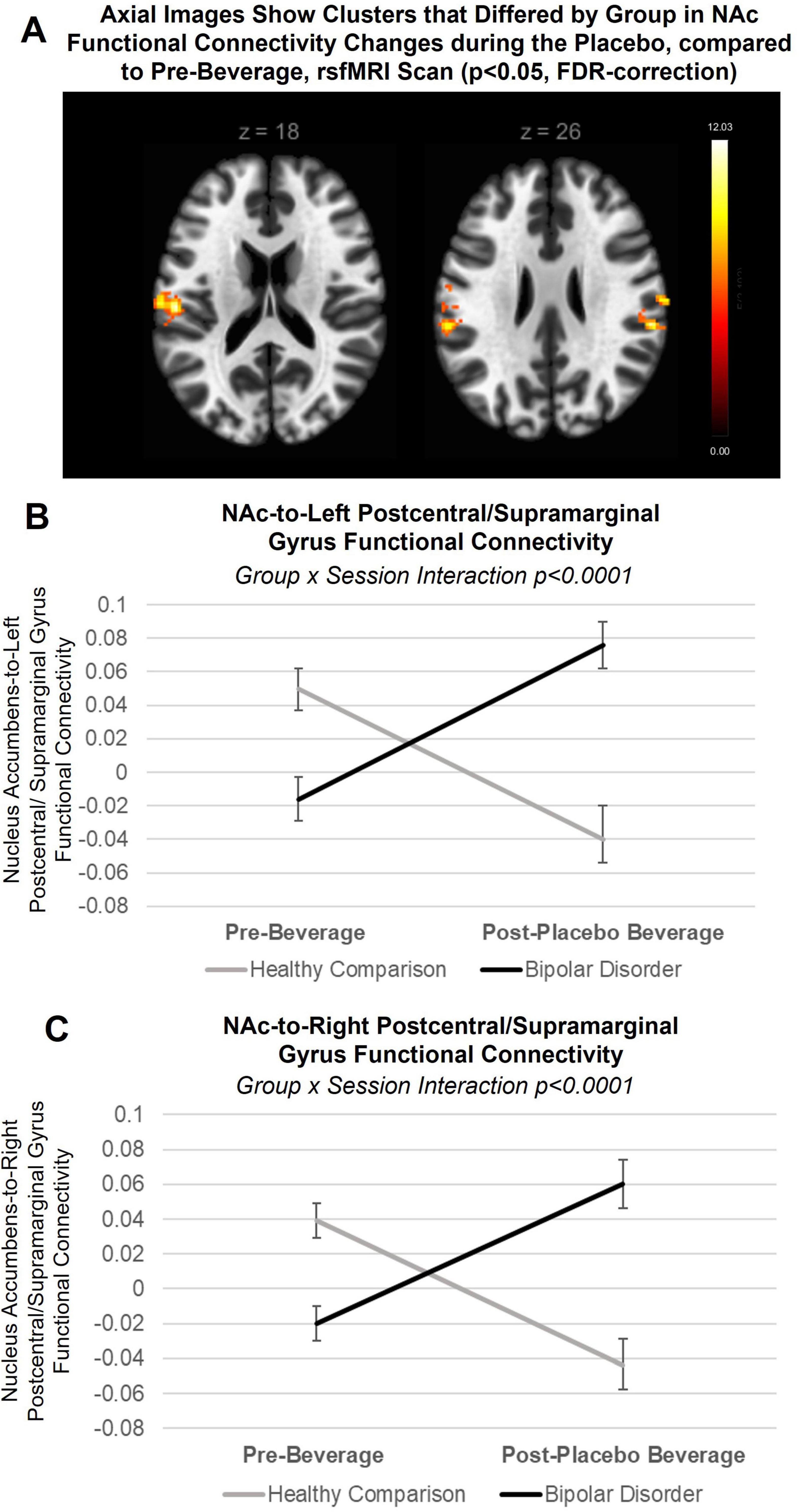
Figure 3. Between group differences in placebo response: nucleus accumbens (NAc) seed-to-voxel approach. (A) Axial slices showing clusters that differed by group in NAc functional connectivity changes during the placebo condition, compared to pre-beverage rsfMRI scan (pFDR < 0.05). Post hoc analysis revealed group-by-condition (placebo, pre-beverage rsfMRI) interactions (but not interaction with NAc hemisphere) in functional connectivity with both the (B) left postcentral/supramarginal gyrus (F = 24.7, p < 0.0001) and (C) right postcentral/supramarginal gyrus clusters (F = 21.9, p < 0.0001). When stratifying by group to interpret the group-by-condition interactions, young adults with bipolar disorder showed a significant increase in NAc functional connectivity during the placebo, compared to pre-beverage rsfMRI scan, with the left postcentral/supramarginal gyrus (β = 0.09, p = 0.0004) and the right postcentral/supramarginal gyrus (β = 0.08, p = 0.001), while healthy comparison young adults showed a decrease in NAc-to-left postcentral/supramarginal gyrus (β = −0.08, p = 0.003) as well as the right postcentral/supramarginal gyrus functional connectivity (β = −0.08, p = 0.004). Lines indicate within-subject changes from pre- to post-beverage consumption. Black line indicates young adults with bipolar disorder; gray line indicates healthy comparison young adults.
There was a significant group-by-placebo-adjusted change in functional connectivity between NAc-to-left postcentral/SMG [F = 4.7, p = 0.03, 95% CI (1.2, 26.5)] on SEAS positive valence/negative arousal (see Figure 4A). When stratifying by group, a positive relation between placebo-adjusted NAc-to-postcentral/SMG functional connectivity and SEAS positive valence/negative arousal score reported during the placebo session was observed in bipolar disorder [β = 11.8, p = 0.02, 95% CI (1.6, 22.0)] but not in the healthy comparison group [β = −4.7, p = 0.3, 95% CI (−12.9, 3.5)]. A main effect of placebo-adjusted functional connectivity change in NAc-to-left postcentral/SMG was also observed on SEAS positive valence/positive arousal [F = 6.2, p = 0.01, 95% CI (−22.2, −2.5)], with a decrease in functional connectivity associated with greater stimulating effects reported in both groups. Functional connectivity changes between NAc-to-right postcentral/SMG did not relate to subjective response reported during the placebo condition.
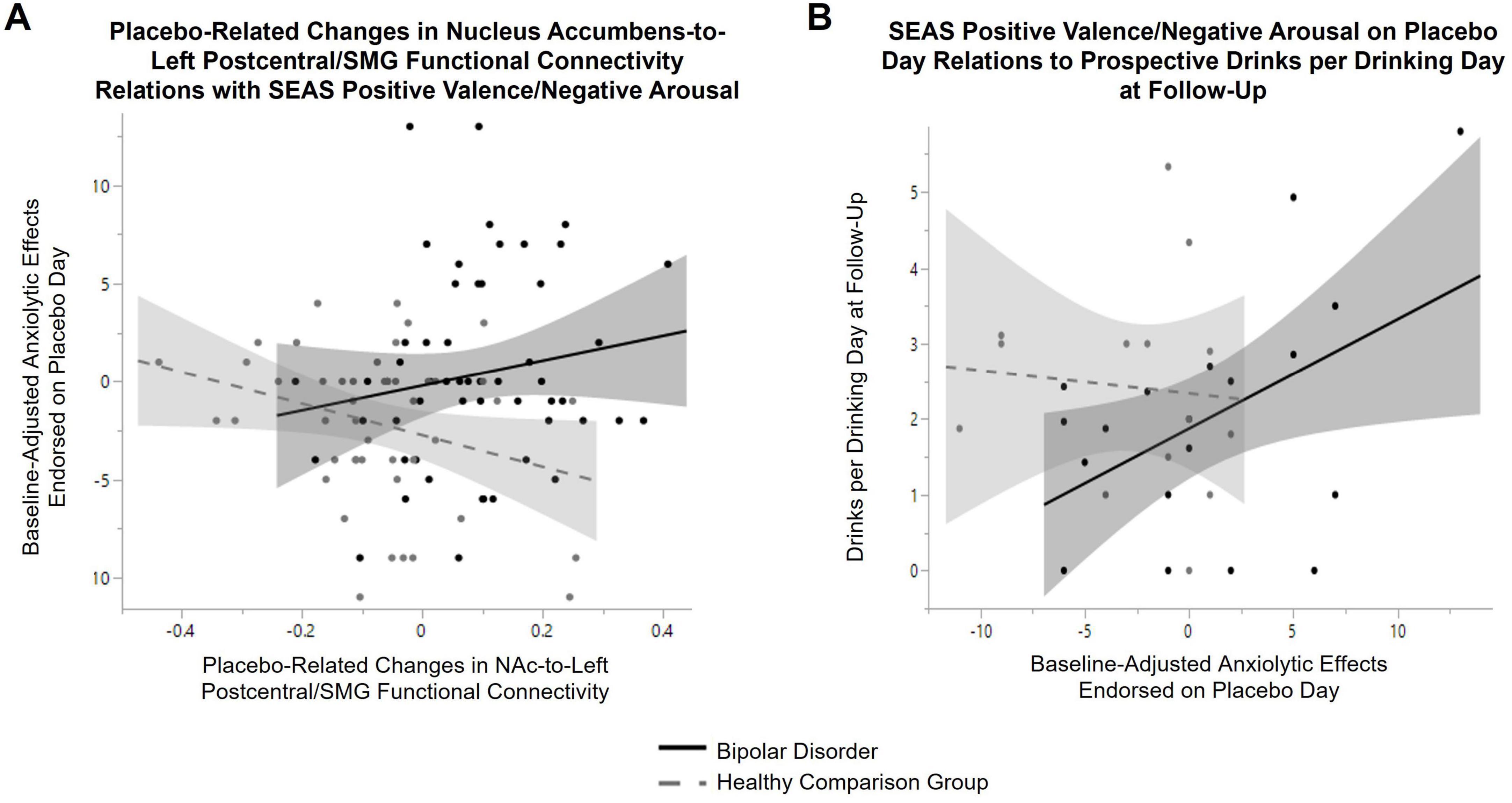
Figure 4. Relations between subjective response to placebo, placebo-related changes in Nucleus Accumbens (NAc) functional connectivity and prospective alcohol use in bipolar disorder. (A) There was a significant group-by-placebo-adjusted change in functional connectivity between NAc-to-left postcentral/supramarginal gyrus (F = 4.7, p = 0.03) on SEAS positive valence/negative arousal. When stratifying by group, a positive relation between placebo-adjusted NAc-to-postcentral/supramarginal gyrus functional connectivity and anxiolytic effects endorsed during the placebo condition was observed in bipolar disorder (β = 11.8, p = 0.02, black line), but not in the healthy comparison group (β = −4.7, p = 0.3, gray dashed line). (B) A group-by-baseline positive valence/negative arousal score on the SEAS during placebo condition interaction on average drinks per drinking day at follow-up (controlling for baseline drinks per drinking day and time between baseline and follow-up) was observed (F = 5.9, p = 0.02). When stratifying by group, a positive relation between anxiolytic effects endorsed during the placebo condition and prospective alcohol use was observed in bipolar disorder (β = 0.2, p = 0.01, black line), while there was no significant relation in the healthy comparison group (β = −0.04, p = 0.6, gray dashed line).
Groups did not differ in time between baseline and follow-up (on average 2.1 years in bipolar disorder and 2.3 years in healthy comparison young adults, t-test: p = 0.4). There was a main effect of time on symptoms of AUD (F = 5.9, p = 0.02), with greater symptoms of AUD reported at follow-up across all participants. There was not a significant group-by-time interaction (F = 2.3, p = 0.14). Three participants with bipolar disorder converted to meeting threshold for AUD for the first time over the follow-up period (two developed mild and one developed a moderate AUD). No healthy comparison young adult converted to meeting threshold for onset of AUD over the follow-up period. There was no main effect of time (or group-by-time interactions) on prospective alcohol use (measured with the TLFB at baseline and follow-up assessment). A group-by-baseline positive valence/negative arousal score on the SEAS during placebo condition interaction on average drinks per drinking day at follow-up (F = 5.9, p = 0.02) and maximum number of drinks per drinking day (F = 4.1, p = 0.05) was observed. As seen in Figure 4B, when stratifying by group, a positive relation between SEAS positive valence/negative arousal reported during the placebo session and prospective average drinks per drinking day (β = 0.2, p = 0.01) was observed in bipolar disorder. A similar relation in bipolar disorder was observed for maximum drinks per drinking day (β = 0.26, p = 0.03). These relations with SEAS positive valence/negative arousal was not observed in the healthy comparison group (average drinks: β = −0.04, p = 0.6; maximum drinks: β = −0.11, p = 0.5). A positive relation between SEAS positive valence/negative arousal and number of heavy episodic drinking days at follow-up was also observed across both groups (main effect: β = 0.14, p = 0.03). A significant positive relation between placebo-related changes in left NAc-to-left postcentral/SMG functional connectivity with AUD symptoms meeting threshold on the SCID at follow-up (β = 5.7, p = 0.008) was also observed in bipolar disorder. No other significant relations between subjective response reported during the placebo condition or placebo-related changes in NAc functional connectivity with alcohol-related outcomes in bipolar disorder were observed.
Our results suggest that youth with bipolar disorder show differences in placebo response compared to healthy comparison young adults, with stronger alcohol expectancies (greater stimulating, anxiolytic, and feeling “drunk/effects of alcohol”) during the placebo beverage condition in addition to the alcohol beverage condition observed in young adults with bipolar disorder. Youth with bipolar disorder also showed differences, compared to healthy young adults, in NAc functional connectivity changes during the placebo condition rsfMRI scan compared to a pre-beverage rsfMRI. Specifically, while healthy comparison young adults showed increases in NAc-to-SCC functional connectivity, young adults with bipolar disorder did not. Young adults with bipolar disorder demonstrated increases in NAc-to-postcentral/SMG functional connectivity during the placebo condition, compared to pre-beverage rsfMRI session, which was not observed in healthy comparison young adults. Findings suggest distinct neural correlates of placebo response in bipolar disorder, compared to healthy comparison young adults. Increased NAc-to-postcentral/SMG functional connectivity during the placebo condition related to greater anxiolytic effects reported during placebo in young adults with bipolar disorder (but not healthy comparison young adults). Differences in expectations of anxiolytic effects after consuming alcohol has previously been reported to relate to alcohol use in bipolar disorder (Lippard et al., 2023). While preliminary, longitudinal data reported here suggest a positive association between anxiolytic alcohol expectancies (observed during the placebo condition) and quantity of alcohol use at follow-up in bipolar disorder. A positive association between changes in left NAc-to-postcentral/SMG functional connectivity during the placebo condition and prospective symptoms of AUD was also observed in young adults with bipolar disorder. These findings extend prior work in healthy adults suggesting neural responses to a placebo beverage condition increases risk for future alcohol problems (McKenna et al., 2022). While longitudinal data should be interpreted with caution, preliminary longitudinal results serve as proof of concept for future studies of placebo response in bipolar disorder. Findings highlight the young adult period as a critical time in risk for, and development of, alcohol problems in bipolar disorder and that variability in placebo response may relate to prospective alcohol use.
The postcentral and supramarginal gyrus are key nodes in the sensorimotor network, exhibit high subcortical inputs (for bottom-up processing of sensory information), and are involved in interoceptive and exteroceptive awareness (Salvato et al., 2020; Stern et al., 2017; Wang et al., 2019). Variability in functional connectivity of regions within the sensorimotor network (Bi et al., 2022) and differences in NAc function during rewards anticipation are reported in bipolar disorder (Caseras et al., 2013; Nusslock et al., 2012). Variability in function within the sensorimotor network is also suggested to relate to reward and loss anticipation in bipolar disorder (Manelis et al., 2016). Additionally, a role of the postcentral and supramarginal gyrus is also suggested in AUD (Florence et al., 2022; Gowin et al., 2013; Syan et al., 2023). Findings converge to suggest a role of the sensorimotor network—and subcortical connections—in alcohol expectancies (and possibly rewarding properties of alcohol that may underlie alcohol seeking behavior) in bipolar disorder and could serve as targets for intervention. Interestingly, variability in function within the sensorimotor network, i.e., supramarginal gyrus, is also suggested to relate to placebo analgesia (Kong et al., 2006; Nemoto et al., 2007; Zunhammer et al., 2021). While speculative, future research might investigate if placebo response relates to pain relief—in addition to reward anticipation—in bipolar disorder and whether it could underlie greater coping drinking motives previously reported (Tretyak et al., 2022). As variability in NAc-to-postcentral gyrus functional connectivity is observed in youth with family history of AUD (Cservenka et al., 2014), future research investigating familial and psychosocial factors that contribute to alcohol expectancy development and maintenance in bipolar disorder is needed.
Variability in alcohol expectancies could relate to familial risk factors. Bipolar disorder and AUD often co-aggregate in studies on familial risk for bipolar disorder (Wilens et al., 2014). Alcohol related norms and alcohol expectancies emerge during childhood and are influenced by family and sociocultural factors (Schor, 1996; Skylstad et al., 2022; Smit et al., 2018). Familial factors are also supported as contributing to variability in alcohol cue reactivity even in alcohol naïve adolescents (Nguyen-Louie et al., 2018). Additionally, early life stress is suggested to relate to variability in alcohol expectancies and interact with familial risk to contribute to subjective response to alcohol and alcohol use (Kirsch et al., 2021; Kosted et al., 2023). Interactions between alcohol and stress that affect behavioral and neural plasticity (Brancato et al., 2023) could also impact alcohol-related outcomes, including alcohol expectancies, and early interventions focused on family support may foster resiliency and improved outcomes (Castelli et al., 2020). Collectively these results point to psychosocial factors (for example, familial risk for alcohol use problems, environmental stress, parental attitudes toward alcohol, alcohol availability, and parental support and monitoring) that may interact with genetic vulnerability to contribute to alcohol use in bipolar disorder. While these psychosocial factors may generalize across diagnostic boundaries, they may also be distinct from those underlying risk in healthy young adults—as previously discussed (Tretyak et al., 2022; Tretyak et al., 2021).
Several limitations of this study must be noted to frame interpretation. We cannot rule out type I errors; future study with larger sample size is needed to confirm and extend these findings. Many confounding factors, including medication effects, mood state variability, or comorbid conditions could also influence alcohol response. While youth with bipolar disorder were euthymic at the time of study they varied in subthreshold mood symptoms. When covarying subthreshold anxiety symptoms, results remain the same. When covarying impulsivity scores, the main effect of group on anxiolytic and stimulating effects of alcohol was no longer significant. It remains unclear whether impulsivity is a confounding variable, a mechanistic factor, or a potential mediator/moderator. Future research on the role(s) of impulsivity on subjective response to alcohol (and placebo/alcohol expectancies) is needed. There is existing literature regarding subjective responses to drugs, e.g., amphetamine, suggesting relations with personality traits including impulsivity (Kirkpatrick et al., 2013; Weafer and de Wit, 2013). However, distinct relations between subjective response to alcohol, compared to other drugs, are also reported (Wardle et al., 2015). We cannot say if findings would generalize to other substances and might contribute to other substance use comorbidities in bipolar disorder. While we observed that anticonvulsant use was associated with young adults with bipolar disorder reporting feeling more drunk/effects of alcohol, this study was not powered to investigate the impact of medication on subjective response to alcohol. Effects of medication should be interpreted with caution. Future research investigating effects of medication on development of alcohol misuse over time is needed to inform treatment decision-making in bipolar disorder, especially in individuals who are starting to show signs of alcohol use problems. Medication did not relate to placebo-associated changes in NAc functional connectivity observed in bipolar disorder. This study focused on individuals with bipolar disorder type I. While this decreased heterogeneity in the sample, it does limit our ability to generalize findings to other subtypes.
This manuscript is intended to focus more on placebo response (a measure of alcohol expectancy). BrAC varied within each group across their alcohol condition and could have contributed to differences in subjective response to alcohol; this effect is less of a concern when investigating placebo response. Other methods of alcohol administration (i.e., IV administration) could control variability in BrAC during the alcohol condition but the oral consumption used in this study strengthens the placebo manipulation and increases ecological validity. The fMRI data used in this study was during a pre-beverage session and after individuals had consumed the placebo beverage (not the beverage containing alcohol). The resting state findings reported are therefore not a direct effect of alcohol pharmacokinetics. It is possible differences observed following the placebo beverage consumption (compared to pre-beverage rsfMRI scan) could relate to alcohol cue exposure. This proof-of-concept study highlights the need for future work on alcohol expectancies/placebo response in bipolar disorder and risk for future alcohol problems. Future work should include counter-balanced “Told No Alcohol/Get No Alcohol” (no alcohol expectancy) and “Told Alcohol/Get No Alcohol” (alcohol expectancy) conditions. Both groups had more women than men. Risk for AUD in bipolar disorder is suggested to differ between men and women (Lippard et al., 2017), and gender differences in subjective response to alcohol and alcohol expectancies are reported (Ide et al., 2017; Satre and Knight, 2001; Schuckit et al., 2012; Schultz et al., 2021). Additionally, studies suggest in general males may respond more strongly to placebo effects (Vambheim and Flaten, 2017) although greater placebo response in women to some drugs are reported (Singha et al., 2000). Collectively, these results emphasize a need for research investigating gender differences; we were not powered to investigate gender differences in the current study. Older adults, compared to younger adults, report differences in alcohol expectancies (Satre and Knight, 2001). While the homogeneous age range (21–26 years of age) decreased heterogeneity and focused on a period of risk for alcohol misuse, findings may not generalize to older samples. Increased left SMG activity has been reported following expectancy violation and to correlate with decreased placebo response (Colloca et al., 2019). However, we excluded individuals from these analyses that reported not believing the placebo manipulation and no group difference in estimated number of drinks consumed was noted during the placebo manipulation check. While there was no difference in recent alcohol or cannabis use between groups at enrollment, we cannot rule out groups may have differed in past alcohol or cannabis use which could have contributed to variability in placebo response. We cannot discern the molecular mechanisms that may underly variability in placebo response. The rewarding/activating and sedating effects of alcohol may be mediated by the dopaminergic and GABAergic systems, respectively. Dopamine and GABA dynamics may contribute to variability in alcohol expectancies, suggesting a biological influence on some cognitions underlying alcohol use (Young et al., 2004). While we did not observe group differences in the sedative effects reported following beverage consumption (measured with the SEAS), more research on dopamine and GABA dynamics and interactions with alcohol/substance use that may drive development of expectancies in bipolar disorder is needed. Our group with bipolar disorder had more individuals with a history of AUD as well as CUD. We did not exclude individuals with a mild/moderate AUD or a history of CUD to avoid limiting generalizability. It is possible that this difference contributed to variability in alcohol expectancies. However, when covarying for individuals with a history of AUD or CUD in sensitivity analyses, the results remained significant, suggesting differences in alcohol expectancies may predate the development of AUD or CUD in individuals with bipolar disorder. Additionally, co-use of alcohol and cannabis is suggested to interact to alter subjective response to alcohol and contribute to drinking behavior (Waddell et al., 2023, 2024) and we cannot rule out the possibility that differences in patterns of co-use might have contributed to placebo findings. Findings may not generalize to heavier drinkers and those with AUD. We also cannot rule out effects of medication as most individuals with bipolar disorder were medicated at the time of this study. Post hoc analysis of NAc-to-SGM may suffer from sequential testing issues, i.e., variables used to identify the effect being statistically related to the variables tested post hoc. We recently reported acute alcohol-related changes (compared to placebo condition) in NAc-to-prefrontal cortex and amygdala-to-prefrontal cortex functional connectivity in bipolar disorder that related to subjective response to alcohol (Kirsch et al., 2023a). We did not observe NAc-to-prefrontal cortex functional connectivity changes that related specifically to placebo response in bipolar disorder in this analysis. As prior work suggests interactions between anticipated effects and subjective response to alcohol on alcohol-related outcomes in healthy adults (Morean et al., 2015; Waddell et al., 2022), future work with the power to investigate interactions between placebo response and acute alcohol effects on alcohol-related outcomes in bipolar disorder is needed. Participants drank in a controlled non-bar setting. Alcohol cue exposure in a bar context is suggested to enhance alcohol expectancies and craving compared to a non-bar context (Chen et al., 2021; Corbin et al., 2015; Kuerbis et al., 2020). Additionally, in this study the participant was the only one drinking (although study personnel were with the participant). While the use of oral alcohol administration improves ecological validity, the controlled setting (where participants were drinking alone in a non-bar setting) differs from real-world social drinking experiences. Variability in subjective response to alcohol is observed in different social contexts [group vs. solitary drinking (Corbin et al., 2021)] and modifying the drinking context and the rewarding value of drinking (Corbin et al., 2008; Sayette et al., 2012) might alter differences in subjective response than those reported here. Increases in sensitivity to alcohol is suggested to relate to maintenance/escalation of AUD (King et al., 2021). Variability in sensitivity to alcohol may emerge over time and relate to AUD onset/maintenance in bipolar disorder. We are not able to determine the temporal associations between placebo response and alcohol use. It is possible that variability in subjective experience of alcohol (at time of alcohol initiation) contributes to variability in alcohol expectancies in bipolar disorder. It is premature to disentangle if findings reported here relate to risk or resiliency for future alcohol problems in bipolar disorder. It is possible young adults with bipolar disorder recruited for this study are a biased “healthier” cohort that may be more resilient to development of AUD. We can only speculate the level of risk for AUD in this sample. If the sample underrepresents individuals at the highest risk for AUD, results could represent an underestimation of the true relationship between placebo response and alcohol use in bipolar disorder. While when looking at preliminary longitudinal data, placebo response related to prospective alcohol use and problems being reported in bipolar disorder, more longitudinal data with larger sample sizes and longer periods of follow-up are needed.
In summary, this analysis suggests that young adults with bipolar disorder show differences in alcohol expectancies (suggested by elevated placebo response), with variability in alcohol expectancies relating to changes in NAc-to-sensorimotor network functional connectivity and future alcohol use. As emerging data suggest alcohol expectancies can be targeted for interventions to improve alcohol-related outcomes (Dunn et al., 2020; Dunn et al., 2022; Lau et al., 2022; Schick et al., 2022), future research in this area is needed. Specifically, longitudinal studies powered to capture the emergence of alcohol problems are necessary to investigate unique alcohol effects that may interact with alcohol expectancies to contribute to risk for future alcohol use problems in bipolar disorder. Ultimately, this line of research might inform interventions and prevention efforts that are more effective in youth with bipolar disorder than existing treatments.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Institutional Review Board at the University of Texas at Austin. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
EL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. DK: Conceptualization, Investigation, Project administration, Writing – review and editing. VL: Investigation, Writing – review and editing, Project administration. SL: Data curation, Investigation, Project administration, Writing – review and editing. NB: Data curation, Investigation, Project administration, Writing – review and editing. KM: Data curation, Investigation, Project administration, Writing – review and editing. RK: Investigation, Writing – review and editing, Project administration. AH: Data curation, Investigation, Writing – review and editing, Project administration. JA: Investigation, Supervision, Writing – review and editing, Resources. KF: Methodology, Resources, Supervision, Writing – review and editing. SS: Resources, Writing – review and editing, Supervision.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) K01AA027573 and R01AA030038. Authors were supported by research grants from the NIAAA: K01AA027573 (EL, RK, KM, NB, SL); R01AA030038 (EL, KM, NB, SL, AH, JA); R21AA027884 (EL, RK, VL, KF, SS, JA); F31AA029005 (DK); and the Jones/Bruce Fellowship from the Waggoner Center for Alcohol and Addiction Research (DK).
We thank our participants for volunteering their time and supporting our research. This work was performed with the support of the Biomedical Imaging Center (RRID:SCR_021898), a core facility within the Center for Biomedical Research Support at the University of Texas at Austin. This clinical trial is registered at ClinicalTrials.gov under the title “Acute Alcohol Response In Bipolar Disorder: a fMRI Study” (Identifier: NCT04063384) and can be found at: https://clinicaltrials.gov/ct2/show/NCT04063384. Longitudinal data is from an ongoing clinical trial that is registered under the title “Acute Alcohol Response In Bipolar Disorder: a Longitudinal Alcohol Administration/fMRI Study” (Identifier: NCT05838274).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Behzadi, Y., Restom, K., Liau, J., and Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. doi: 10.1016/j.neuroimage.2007.04.042
Bi, B., Che, D., and Bai, Y. (2022). Neural network of bipolar disorder: Toward integration of neuroimaging and neurocircuit-based treatment strategies. Transl. Psychiatry 12:143. doi: 10.1038/s41398-022-01917-x
Brancato, A., Castelli, V., Lavanco, G., D’Amico, C., Feo, S., Pizzolanti, G., et al. (2023). Social stress under binge-like alcohol withdrawal in adolescence: Evidence of cannabidiol effect on maladaptive plasticity in rats. Psychol. Med. 53, 5538–5550. doi: 10.1017/S0033291722002744
Cardoso, T. A., Bauer, I. E., Jansen, K., Suchting, R., Zunta-Soares, G., Quevedo, J., et al. (2016). Effect of alcohol and illicit substance use on verbal memory among individuals with bipolar disorder. Psychiatry Res. 243, 225–231. doi: 10.1016/j.psychres.2016.06.044
Caseras, X., Lawrence, N. S., Murphy, K., Wise, R. G., and Phillips, M. L. (2013). Ventral striatum activity in response to reward: Differences between bipolar I and II disorders. Am. J. Psychiatry 170, 533–541. doi: 10.1176/appi.ajp.2012.12020169
Castelli, V., Lavanco, G., Brancato, A., and Plescia, F. (2020). Targeting the stress system during gestation: Is early handling a protective strategy for the offspring? Front. Behav. Neurosci. 14:9. doi: 10.3389/fnbeh.2020.00009
Chen, A. J., Chen, W. T., Wang, I. A., Wang, N., Chen, W. J., and Chen, C. Y. (2021). Association between childhood negative life events with alcohol expectancies in early adolescence: Cumulative risk and latent class approaches. Drug Alcohol. Depend. 226:108853. doi: 10.1016/j.drugalcdep.2021.108853
Chung, Y. I., White, R., Geier, C. F., Johnston, S. J., Smyth, J. M., Delgado, M. R., et al. (2023). Testing the efficacy of real-time fMRI neurofeedback for training people who smoke daily to upregulate neural responses to nondrug rewards. Cogn. Affect. Behav. Neurosci. 23, 440–456. doi: 10.3758/s13415-023-01070-y
Chutuape, M. A., and de Wit, H. (1995). Preferences for ethanol and diazepam in anxious individuals: An evaluation of the self-medication hypothesis. Psychopharmacology (Berl) 121, 91–103. doi: 10.1007/BF02245595
Cofresí, R. U., Bartholow, B. D., and Fromme, K. (2020). Female drinkers are more sensitive than male drinkers to alcohol-induced heart rate increase. Exp. Clin. Psychopharmacol. 28, 540–552. doi: 10.1037/pha0000338
Colloca, L., Schenk, L. A., Nathan, D. E., Robinson, O. J., and Grillon, C. (2019). When expectancies are violated: A functional magnetic resonance imaging study. Clin. Pharmacol. Ther. 106, 1246–1252. doi: 10.1002/cpt.1587
Conway, K. P., Compton, W., Stinson, F. S., and Grant, B. F. (2006). Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: Results from the national epidemiologic survey on alcohol and related conditions. J. Clin. Psychiatry 67, 247–257. doi: 10.4088/JCP.v67n0211
Corbin, W. R., Gearhardt, A., and Fromme, K. (2008). Stimulant alcohol effects prime within session drinking behavior. Psychopharmacology (Berl) 197, 327–337. doi: 10.1007/s00213-007-1039-x
Corbin, W. R., Hartman, J. D., Bruening, A. B., and Fromme, K. (2021). Contextual influences on subjective alcohol response. Exp. Clin. Psychopharmacol. 29, 48–58. doi: 10.1037/pha0000415
Corbin, W. R., Scott, C., Boyd, S. J., Menary, K. R., and Enders, C. K. (2015). Contextual influences on subjective and behavioral responses to alcohol. Exp. Clin. Psychopharmacol. 23, 59–70. doi: 10.1037/a0038760
Cservenka, A., Casimo, K., Fair, D. A., and Nagel, B. J. (2014). Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res. 221, 210–219. doi: 10.1016/j.pscychresns.2013.12.004
Di Florio, A., Craddock, N., and van den Bree, M. (2014). Alcohol misuse in bipolar disorder. A systematic review and meta-analysis of comorbidity rates. Eur. Psychiatry 29, 117–124. doi: 10.1016/j.eurpsy.2013.07.004
Dunn, M. E., Fried-Somerstein, A., Flori, J. N., Hall, T. V., and Dvorak, R. D. (2020). Reducing alcohol use in mandated college students: A comparison of a brief motivational intervention (BMI) and the expectancy challenge alcohol literacy curriculum (ECALC). Exp. Clin. Psychopharmacol. 28, 87–98. doi: 10.1037/pha0000290
Dunn, M. E., Schreiner, A. M., Flori, J. N., Crisafulli, M. J., Willis, E. A., Lynch, G. T., et al. (2022). Effective prevention programming for reducing alcohol-related harms experienced by first year college students: Evaluation of the expectancy challenge alcohol literacy curriculum (ECALC). Addict. Behav. 131:107338. doi: 10.1016/j.addbeh.2022.107338
Finseth, P. I., Morken, G., Andreassen, O. A., Malt, U. F., and Vaaler, A. E. (2012). Risk factors related to lifetime suicide attempts in acutely admitted bipolar disorder inpatients. Bipolar Disord. 14, 727–734. doi: 10.1111/bdi.12004
Florence, L., Lassi, D. L. S., Kortas, G. T., Lima, D. R., de Azevedo-Marques Périco, C., Andrade, A. G., et al. (2022). Brain correlates of the alcohol use disorder pharmacotherapy response: A systematic review of neuroimaging studies. Brain Sci. 12:386. doi: 10.3390/brainsci12030386
Fromme, K., Stroot, E. A., and Kaplan, D. (1993). Comprehensive effects of alcohol: Development and psychometric assessment of a new expectancy questionnaire. Psychol. Assess. 5, 19–26. doi: 10.1037/1040-3590.5.1.19
Goldberg, J. F. (2001). Bipolar disorder with comorbid substance abuse: Diagnosis, prognosis, and treatment. J. Psychiatr Pract. 7, 109–122. doi: 10.1097/00131746-200103000-00004
Goldstein, B. I., Velyvis, V. P., and Parikh, S. V. (2006). The association between moderate alcohol use and illness severity in bipolar disorder: A preliminary report. J. Clin. Psychiatry 67, 102–106. doi: 10.4088/JCP.v67n0114
Gowin, J. L., Mackey, S., and Paulus, M. P. (2013). Altered risk-related processing in substance users: Imbalance of pain and gain. Drug Alcohol. Depend. 132, 13–21. doi: 10.1016/j.drugalcdep.2013.03.019
Hasking, P., Lyvers, M., Carlopio, C., and Raber, A. (2011). The relationship between coping strategies, alcohol expectancies, drinking motives and drinking behaviour. Addict. Behav. 36, 479–487. doi: 10.1016/j.addbeh.2011.01.014
Hunt, G. E., Malhi, G. S., Cleary, M., Lai, H. M., and Sitharthan, T. (2016). Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: Systematic review and meta-analysis. J. Affect. Disord. 206, 331–349. doi: 10.1016/j.jad.2016.07.011
Ide, J. S., Zhornitsky, S., Hu, S., Zhang, S., Krystal, J. H., and Li, C. R. (2017). Sex differences in the interacting roles of impulsivity and positive alcohol expectancy in problem drinking: A structural brain imaging study. Neuroimage Clin. 14, 750–759. doi: 10.1016/j.nicl.2017.03.015
Jones, A. W., and Jonsson, K. A. (1994). Food-induced lowering of blood-ethanol profiles and increased rate of elimination immediately after a meal. J. Forensic Sci. 39, 1084–1093. doi: 10.1520/JFS13687J
Kilbey, M. M., Downey, K., and Breslau, N. (1998). Predicting the emergence and persistence of alcohol dependence in young adults: The role of expectancy and other risk factors. Exp. Clin. Psychopharmacol. 6, 149–156. doi: 10.1037/1064-1297.6.2.149
King, A. C., de Wit, H., McNamara, P. J., and Cao, D. (2011). Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch. Gen. Psychiatry 68, 389–399. doi: 10.1001/archgenpsychiatry.2011.26
King, A. C., McNamara, P. J., Hasin, D. S., and Cao, D. (2014). Alcohol challenge responses predict future alcohol use disorder symptoms: A 6-year prospective study. Biol. Psychiatry 75, 798–806. doi: 10.1016/j.biopsych.2013.08.001
King, A., Vena, A., Hasin, D. S., deWit, H., O’Connor, S. J., and Cao, D. (2021). Subjective responses to alcohol in the development and maintenance of alcohol use disorder. Am. J. Psychiatry 178, 560–571. doi: 10.1176/appi.ajp.2020.20030247
Kirkpatrick, M. G., Johanson, C. E., and de Wit, H. (2013). Personality and the acute subjective effects of d-amphetamine in humans. J. Psychopharmacol. 27, 256–264. doi: 10.1177/0269881112472564
Kirsch, D. E., Kosted, R., Le, V., Almeida, J. R. C., Fromme, K., Strakowski, S. M., et al. (2023a). Ventral prefrontal network response to alcohol in young adults with bipolar disorder: A within-subject randomized placebo-controlled alcohol administration study. Neuropsychopharmacology 48, 1910–1919. doi: 10.1038/s41386-023-01657-6
Kirsch, D. E., Le, V., Kosted, R., Fromme, K., and Lippard, E. T. C. (2023b). Neural underpinnings of expecting alcohol: Placebo alcohol administration alters nucleus accumbens resting state functional connectivity. Behav. Brain Res. 437:114148. doi: 10.1016/j.bbr.2022.114148
Kirsch, D. E., Tretyak, V., Radpour, S., Weber, W. A., Nemeroff, C. B., Fromme, K., et al. (2021). Childhood maltreatment, prefrontal-paralimbic gray matter volume, and substance use in young adults and interactions with risk for bipolar disorder. Sci. Rep. 11:123. doi: 10.1038/s41598-020-80407-w
Kong, J., Gollub, R. L., Rosman, I. S., Webb, J. M., Vangel, M. G., Kirsch, I., et al. (2006). Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J. Neurosci. 26, 381–388. doi: 10.1523/JNEUROSCI.3556-05.2006
Kosted, R., Kirsch, D. E., Le, V., Fromme, K., and Lippard, E. T. C. (2023). Subjective response to alcohol: Interactive effects of early life stress, parental risk for mood and substance use disorders, and drinking context. Pharmacol. Biochem. Behav. 229:173591. doi: 10.1016/j.pbb.2023.173591
Kuerbis, A. N., Shao, S., Treloar Padovano, H., Jadanova, A., Selva Kumar, D., Vitale, R., et al. (2020). Context and craving among individuals with alcohol use disorder attempting to moderate their drinking. Exp. Clin. Psychopharmacol. 28, 677–687. doi: 10.1037/pha0000349
Lau, Y. C. Y., Bryant, S. J., and Gullo, M. J. (2022). Development of an individualized procedure to induce reward-related impulsivity and evaluating its impact on drinking control. Addict. Behav. 133:107378. doi: 10.1016/j.addbeh.2022.107378
Le, T. M., Malone, T., and Li, C. R. (2022). Positive alcohol expectancy and resting-state functional connectivity of the insula in problem drinking. Drug Alcohol. Depend. 231:109248. doi: 10.1016/j.drugalcdep.2021.109248
Le, T. M., Zhornitsky, S., Zhang, S., and Li, C. R. (2020). Pain and reward circuits antagonistically modulate alcohol expectancy to regulate drinking. Transl. Psychiatry 10:220. doi: 10.1038/s41398-020-00909-z
Leeman, R. F., Ralevski, E., Limoncelli, D., Pittman, B., O’Malley, S. S., and Petrakis, I. L. (2014). Relationships between impulsivity and subjective response in an IV ethanol paradigm. Psychopharmacology 231, 2867–2876. doi: 10.1007/s00213-014-3458-9
Lippard, E. T. C., Kirsch, D. E., Kosted, R., Le, V., Almeida, J. R. C., Fromme, K., et al. (2023). Subjective response to alcohol in young adults with bipolar disorder and recent alcohol use: A within-subject randomized placebo-controlled alcohol administration study. Psychopharmacology (Berl) 240, 739–753. doi: 10.1007/s00213-023-06315-9
Lippard, E. T., Mazure, C. M., Johnston, J. A., Spencer, L., Weathers, J., Pittman, B., et al. (2017). Brain circuitry associated with the development of substance use in bipolar disorder and preliminary evidence for sexual dimorphism in adolescents. J. Neurosci. Res. 95, 777–791. doi: 10.1002/jnr.23901
Manelis, A., Almeida, J. R., Stiffler, R., Lockovich, J. C., Aslam, H. A., and Phillips, M. L. (2016). Anticipation-related brain connectivity in bipolar and unipolar depression: A graph theory approach. Brain 139, 2554–2566. doi: 10.1093/brain/aww157
McGrady, A., Lynch, D., and Rapport, D. (2017). Psychosocial factors and comorbidity associated with suicide attempts: Findings in patients with bipolar disorder. Psychopathology 50, 171–174. doi: 10.1159/000453272
McKenna, B. S., Anthenelli, R. M., Smith, T. L., and Schuckit, M. A. (2022). Low versus high level of response to alcohol affects amygdala functional connectivity during processing of emotional stimuli. Alcohol. Clin. Exp. Res. 46, 66–76. doi: 10.1111/acer.14744
Messer, T., Lammers, G., Muller-Siecheneder, F., Schmidt, R. F., and Latifi, S. (2017). Substance abuse in patients with bipolar disorder: A systematic review and meta-analysis. Psychiatry Res. 253, 338–350. doi: 10.1016/j.psychres.2017.02.067
Morean, M. E., Corbin, W. R., and Treat, T. A. (2013a). The subjective effects of alcohol scale: Development and psychometric evaluation of a novel assessment tool for measuring subjective response to alcohol. Psychol. Assess. 25, 780–795. doi: 10.1037/a0032542
Morean, M. E., Corbin, W. R., and Treat, T. A. (2015). Evaluating the accuracy of alcohol expectancies relative to subjective response to alcohol. Addict. Behav. 51, 197–203. doi: 10.1016/j.addbeh.2015.07.027
Morean, M. E., de Wit, H., King, A. C., Sofuoglu, M., Rueger, S. Y., and O’Malley, S. S. (2013b). The drug effects questionnaire: Psychometric support across three drug types. Psychopharmacology (Berl) 227, 177–192. doi: 10.1007/s00213-012-2954-z
Najt, P., Perez, J., Sanches, M., Peluso, M., Glahn, D., and Soares, J. C. (2007). Impulsivity and bipolar disorder. Eur. Neuropsychopharmacol. 17, 313–320. doi: 10.1016/j.euroneuro.2006.10.002
Nemoto, H., Nemoto, Y., Toda, H., Mikuni, M., and Fukuyama, H. (2007). Placebo analgesia: A PET study. Exp. Brain Res. 179, 655–664. doi: 10.1007/s00221-006-0821-z
Nery, F. G., Miranda-Scippa, A., Nery-Fernandes, F., Kapczinski, F., and Lafer, B. (2014). Prevalence and clinical correlates of alcohol use disorders among bipolar disorder patients: Results from the Brazilian Bipolar Research Network. Compr. Psychiatry 55, 1116–1121. doi: 10.1016/j.comppsych.2014.02.006
Nguyen-Louie, T. T., Courtney, K. E., Squeglia, L. M., Bagot, K., Eberson, S., Migliorini, R., et al. (2018). Prospective changes in neural alcohol cue reactivity in at-risk adolescents. Brain Imaging Behav. 12, 931–941. doi: 10.1007/s11682-017-9757-0
Nusslock, R., Almeida, J. R., Forbes, E. E., Versace, A., Frank, E., Labarbara, E. J., et al. (2012). Waiting to win: Elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar. Disord. 14, 249–260. doi: 10.1111/j.1399-5618.2012.01012.x
Oquendo, M. A., Currier, D., Liu, S. M., Hasin, D. S., Grant, B. F., and Blanco, C. (2010). Increased risk for suicidal behavior in comorbid bipolar disorder and alcohol use disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). J. Clin. Psychiatry 71, 902–909. doi: 10.4088/JCP.09m05198gry
Patton, J. H., Stanford, M. S., and Barratt, E. S. (1995). Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 51, 768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1
Plewnia, C., Brendel, B., Schwippel, T., Nieratschker, V., Ethofer, T., Kammer, T., et al. (2021). Treatment of major depressive disorder with bilateral theta burst stimulation: Study protocol for a randomized, double-blind, placebo-controlled multicenter trial (TBS-D). Eur. Arch. Psychiatry Clin. Neurosci. 271, 1231–1243. doi: 10.1007/s00406-021-01280-w
Quinn, P. D., and Fromme, K. (2011). Subjective response to alcohol challenge: A quantitative review. Alcohol. Clin. Exp. Res. 35, 1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x
Quinn, P. D., and Fromme, K. (2016). Individual differences in subjective alcohol responses and alcohol-related disinhibition. Exp. Clin. Psychopharmacol. 24, 90–99. doi: 10.1037/pha0000065
Ray, L. A., Mackillop, J., and Monti, P. M. (2010). Subjective responses to alcohol consumption as endophenotypes: Advancing behavioral genetics in etiological and treatment models of alcoholism. Subst Use Misuse 45, 1742–1765. doi: 10.3109/10826084.2010.482427
Regier, D. A., Farmer, M. E., Rae, D. S., Locke, B. Z., Keith, S. J., Judd, L. L., et al. (1990). Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA 264, 2511–2518. doi: 10.1001/jama.1990.03450190043026
Salvato, G., Richter, F., Sedeño, L., Bottini, G., and Paulesu, E. (2020). Building the bodily self-awareness: Evidence for the convergence between interoceptive and exteroceptive information in a multilevel kernel density analysis study. Hum. Brain Mapp. 41, 401–418. doi: 10.1002/hbm.24810
Sanchez-Moreno, J., Martinez-Aran, A., Colom, F., Scott, J., Tabares-Seisdedos, R., Sugranyes, G., et al. (2009). Neurocognitive dysfunctions in euthymic bipolar patients with and without prior history of alcohol use. J. Clin. Psychiatry 70, 1120–1127. doi: 10.4088/JCP.08m04302
Satre, D. D., and Knight, B. G. (2001). Alcohol expectancies and their relationship to alcohol use: Age and sex differences. Aging Ment. Health 5, 73–83. doi: 10.1080/13607860020020672
Sayette, M. A., Creswell, K. G., Dimoff, J. D., Fairbairn, C. E., Cohn, J. F., Heckman, B. W., et al. (2012). Alcohol and group formation: A multimodal investigation of the effects of alcohol on emotion and social bonding. Psychol. Sci. 23, 869–878. doi: 10.1177/0956797611435134
Schick, M. R., Todi, A. A., and Spillane, N. S. (2022). Subjective happiness interrupts the association between alcohol expectancies and alcohol consumption among reserve-dwelling first nation adolescents. Am. J. Orthopsychiatry 92, 497–504. doi: 10.1037/ort0000607
Schor, E. L. (1996). Adolescent alcohol use: Social determinants and the case for early family-centered prevention. Family-focused prevention of adolescent drinking. Bull. N. Y. Acad. Med. 73, 335–356.
Schuckit, M. A., Smith, T. L., Trim, R. S., Kuperman, S., Kramer, J., Hesselbrock, V., et al. (2012). Sex differences in how a low sensitivity to alcohol relates to later heavy drinking. Drug Alcohol. Rev. 31, 871–880. doi: 10.1111/j.1465-3362.2012.00469.x
Schultz, N. R., Graupensperger, S., and Lostutter, T. W. (2021). Effects of within- and between-person assessments of alcohol expectancies and valuations on use and consequences moderated by sex. Alcohol. Clin. Exp. Res. 45, 1888–1900. doi: 10.1111/acer.14677
Simhandl, C., Radua, J., König, B., and Amann, B. L. (2016). Prevalence and impact of comorbid alcohol use disorder in bipolar disorder: A prospective follow-up study. Aust. N. Z. J. Psychiatry 50, 345–351. doi: 10.1177/0004867415585855
Singha, A. K., McCance-Katz, E. F., Petrakis, I., Kosten, T. R., and Oliveto, A. (2000). Sex differences in self-reported and physiological response to oral cocaine and placebo in humans. Am. J. Drug Alcohol. Abuse 26, 643–657. doi: 10.1081/ADA-100101900
Skylstad, V., Babirye, J. N., Kiguli, J., Skar, A. S., Kühl, M. J., Nalugya, J. S., et al. (2022). Are we overlooking alcohol use by younger children? BMJ Paediatr Open 6:e001242. doi: 10.1136/bmjpo-2021-001242
Smit, K., Voogt, C., Hiemstra, M., Kleinjan, M., Otten, R., and Kuntsche, E. (2018). Development of alcohol expectancies and early alcohol use in children and adolescents: A systematic review. Clin. Psychol. Rev. 60, 136–146. doi: 10.1016/j.cpr.2018.02.002
Sobell, L. C. S., and Sobell, M. B. (1992). “Timeline follow-back: A technique for assessing selfreported alcohol consumption,” in Measuring Alcohol Consumption: Psychosocial and Biological Methods, ed. R. Z. L. J. Allen (New Jersey: Humana Press), 41–72. doi: 10.1007/978-1-4612-0357-5_3
Stern, E. R., Grimaldi, S. J., Muratore, A., Murrough, J., Leibu, E., Fleysher, L., et al. (2017). Neural correlates of interoception: Effects of interoceptive focus and relationship to dimensional measures of body awareness. Hum. Brain Mapp. 38, 6068–6082. doi: 10.1002/hbm.23811
Strakowski, S. M., DelBello, M. P., Fleck, D. E., Adler, C. M., Anthenelli, R. M., and Keck, P. E. Jr., et al. (2005). Effects of co-occurring alcohol abuse on the course of bipolar disorder following a first hospitalization for mania. Arch. Gen. Psychiatry 62, 851–858. doi: 10.1001/archpsyc.62.8.851
Strakowski, S. M., DelBello, M. P., Fleck, D. E., and Arndt, S. (2000). The impact of substance abuse on the course of bipolar disorder. Biol. Psychiatry 48, 477–485. doi: 10.1016/S0006-3223(00)00900-8
Syan, S. K., McIntyre-Wood, C., Vandehei, E., Vidal, M. L., Hargreaves, T., Levitt, E. E., et al. (2023). Resting state functional connectivity as a predictor of brief intervention response in adults with alcohol use disorder: A preliminary study. Alcohol. Clin. Exp. Res. 47, 1590–1602. doi: 10.1111/acer.15123
Tretyak, V., Kirsch, D. E., Le, V., Fromme, K., Strakowski, S. M., and Lippard, E. T. C. (2022). Coping drinking motives, neural functional coupling during emotion processing, and alcohol use in young adults with bipolar disorder. Alcohol. Clin. Exp. Res. 46, 1482–1496. doi: 10.1111/acer.14885
Tretyak, V., Kirsch, D. E., Radpour, S., Weber, W. A., Fromme, K., Strakowski, S. M., et al. (2021). Subjective response to alcohol: Associated alcohol use and orbitofrontal gray matter volume in bipolar disorder. J. Affect. Disord. 279, 671–679. doi: 10.1016/j.jad.2020.10.046
Vambheim, S. M., and Flaten, M. A. (2017). A systematic review of sex differences in the placebo and the nocebo effect. J. Pain Res. 10, 1831–1839. doi: 10.2147/JPR.S134745
Vanderwal, T., Eilbott, J., Finn, E. S., Craddock, R. C., Turnbull, A., and Castellanos, F. X. (2017). Individual differences in functional connectivity during naturalistic viewing conditions. Neuroimage 157, 521–530. doi: 10.1016/j.neuroimage.2017.06.027
Vanderwal, T., Kelly, C., Eilbott, J., Mayes, L. C., and Castellanos, F. X. (2015). Inscapes: A movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage 122, 222–232. doi: 10.1016/j.neuroimage.2015.07.069
Vandewouw, M. M., Dunkley, B. T., Lerch, J. P., Anagnostou, E., and Taylor, M. J. (2021). Characterizing Inscapes and resting-state in MEG: Effects in typical and atypical development. Neuroimage 225:117524. doi: 10.1016/j.neuroimage.2020.117524
Waddell, J. T., Corbin, W. R., Chassin, L., and Anderson, S. F. (2022). The prospective interactive effects of alcohol expectancies and subjective response on future drinking behavior. Exp. Clin. Psychopharmacol. 30, 300–312. doi: 10.1037/pha0000430
Waddell, J. T., Corbin, W. R., Grimm, K. J., Metrik, J., Lee, C. M., and Trull, T. J. (2023). Dynamic relations among simultaneous alcohol and cannabis use, subjective responses, and problem drinking during naturally occurring drinking episodes. Drug Alcohol. Depend. 249:110837. doi: 10.1016/j.drugalcdep.2023.110837
Waddell, J. T., Corbin, W. R., Grimm, K. J., Metrik, J., Lee, C. M., and Trull, T. J. (2024). Within-episode relations among simultaneous alcohol and cannabis use and continued drinking: The role of momentary subjective responses, craving, and drinking context. Alcohol. Clin. Exp. Res. 48, 2175–2187. doi: 10.1111/acer.15451
Wang, P., Kong, R., Kong, X., Liégeois, R., Orban, C., Deco, G., et al. (2019). Inversion of a large-scale circuit model reveals a cortical hierarchy in the dynamic resting human brain. Sci. Adv. 5:eaat7854. doi: 10.1126/sciadv.aat7854
Wardle, M. C., Marcus, B. A., and de Wit, H. (2015). A preliminary investigation of individual differences in subjective responses to D-Amphetamine, alcohol, and delta-9-tetrahydrocannabinol using a within-subjects randomized trial. PLoS One 10:e0140501. doi: 10.1371/journal.pone.0140501
Weafer, J., and de Wit, H. (2013). Inattention, impulsive action, and subjective response to D-amphetamine. Drug Alcohol. Depend. 133, 127–133. doi: 10.1016/j.drugalcdep.2013.05.021
Whitfield-Gabrieli, S., and Nieto-Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141. doi: 10.1089/brain.2012.0073
Wilens, T. E., Yule, A., Martelon, M., Zulauf, C., and Faraone, S. V. (2014). Parental history of substance use disorders (SUD). and SUD in offspring: A controlled family study of bipolar disorder. Am. J. Addict. 23, 440–446. doi: 10.1111/j.1521-0391.2014.12125.x
Yip, S. W., Doherty, J., Wakeley, J., Saunders, K., Tzagarakis, C., de Wit, H., et al. (2012). Reduced subjective response to acute ethanol administration among young men with a broad bipolar phenotype. Neuropsychopharmacology 37, 1808–1815. doi: 10.1038/npp.2012.45
Young, R. M., Lawford, B. R., Feeney, G. F., Ritchie, T., and Noble, E. P. (2004). Alcohol-related expectancies are associated with the D2 dopamine receptor and GABAA receptor beta3 subunit genes. Psychiatry Res. 127, 171–183. doi: 10.1016/j.psychres.2003.11.004
Zhornitsky, S., Ide, J. S., Wang, W., Chao, H. H., Zhang, S., Hu, S., et al. (2018). Problem drinking, alcohol expectancy, and thalamic resting-state functional connectivity in nondependent adult drinkers. Brain Connect. 8, 487–502. doi: 10.1089/brain.2018.0633
Keywords: bipolar disorder, placebo, alcohol expectancies, fMRI, drinking, prospective
Citation: Lippard ETC, Kirsch DE, Le V, Lee S, Bibb N, Meek M, Kosted R, Huffman A, Almeida JRC, Fromme K and Strakowski SM (2025) Nucleus accumbens functional connectivity changes underlying alcohol expectancies in bipolar disorder and prospective alcohol outcomes: a within-subject randomized placebo-controlled alcohol administration fMRI study. Front. Neurosci. 19:1549295. doi: 10.3389/fnins.2025.1549295
Received: 20 December 2024; Accepted: 05 March 2025;
Published: 09 April 2025.
Edited by:
Cristiane Aparecida Favoretto, The Scripps Research Institute, United StatesReviewed by:
Carla Cannizzaro, University of Palermo, ItalyCopyright © 2025 Lippard, Kirsch, Le, Lee, Bibb, Meek, Kosted, Huffman, Almeida, Fromme and Strakowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth T. C. Lippard, ZWxpemFiZXRoLmxpcHBhcmRAYXVzdGluLnV0ZXhhcy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.