- Behavioural Neuroscience Laboratory, Department of Animal Science, Bharathidasan University, Tiruchirappalli, India
Maternal stress exposure during pregnancy is known to affect offspring behavior, including learning and memory. We hypothesized that maternal stress-induced changes transmit this effect through maternal line mediated transgenerational epigenetic inheritance. To test our hypothesis, pregnant rats (F0) were undisturbed (Control, Ctrl)/exposed to social stress during gestational days (GD) 16–18 (PMS)/exposed to social stress and treated with oxytocin during GD-16 to 18 (PMS+OXT). Subsequently, F1 female offspring from Ctrl, PMS, and PMS+OXT were mated with Ctrl F1 males to examine maternal line mediated transgenerational impacts. Female animals (F1 and F2) were subjected to behavioral test and the levels of global H3K4me2/H3K4me3 methylation, methylation in the CRH promoter, expression of Crh, Crh receptors (Crhr1, Crhr2), and BDNF were determined. It was found that prenatal maternal stress (PMS) reduced reference and working memory in F1 and F2 offspring, increased global and specific H3K4me2, H3K4me3 methylation in the CRH promoter, expression of Crh, Crh receptors, and corticosterone (CORT), and down-regulated the expression of pro-and mature BDNF by differentially regulating Bdnf transcripts III, IV and VI in the amygdala. Oxytocin exposure reduced PMS-induced global and specific H3K4me2/3 changes, which repressed the expression of Crh, Crh receptors, reduced CORT levels, up-regulated the expression of pro-BDNF and mature BDNF, and improved memory in F1 and F2 offspring. Collectively, our study revealed that PMS reduced reference and working memory performance in F1 and F2 offspring through maternal line transgenerational inheritance of H3K4me2, H3K4me3 methylation, and associated mechanisms that regulate BDNF expression and synaptic plasticity.
1 Introduction
Clinical and animal studies have demonstrated that physical and social stressors during gestation can generate developmental behavioral disorders in offspring (Coussons-Read, 2013; Dieckmann and Czamara, 2024; Dong and Pandey, 2021; McDonald et al., 2023; Blaze and Roth, 2015). Maternal stress is known to increase corticosterone (CORT) and adrenocorticotrophin (ACTH) levels in maternal plasma (Herman and Cullinan, 1997) as a result of the stress response of hypothalamic—pituitary—adrenal (HPA) axis (Bagley et al., 2019). The maternal placental—fetal (MPF) pathway may not completely attenuate CORT transfer (Kassotaki et al., 2021), which generates a shift in offspring HPA axis homeostasis and alters the developmental trajectory and behavioral phenotype (Babenko et al., 2015). In the HPA axis, corticotrophin-releasing hormone (CRH) acts as the chief mediator of the stress-responsive mechanism to facilitate the release of CORT (Chaves et al., 2021).
CRH exerts its action through its high-affinity G-protein-coupled receptor (GPCR), Crhr1 and Crhr2 (Holsboer and Ising, 2008; Inda et al., 2017). Elevated levels of CRH activate its receptors, which alter the transmission of serotonin (5-Hydroxytriptomain, 5-HT), dopamine (DA), and oxytocin (OXT) through a feedback mechanism, and may lead to developmental behavioral changes (Smith and Wang, 2014; Roque et al., 2022; Alcántara-Alonso et al., 2024). Oxytocin (OXT), a peptide (9 aa) hormone synthesized in hypothalamus (paraventricular nuclei, PVN; supraoptic nuclei, SON) (Buijs, 1978), is delivered through the posterior pituitary to the central nervous system (CNS), and other structure in the brain including hippocampus, amygdala, bed nucleus of the stria terminalis (BNST) and nucleus accumbens (NAc) (Knobloch et al., 2012). OXT exerts its function through typical class I G-protein receptor (GPCR) (Gimpl and Fahrenholz, 2001) and activates neuron throughout the brain, including in central nucleus of amygdala (CeA) (Lee et al., 2005). OXT acts on the HPA axis via negative feedback regulation of CRH and the interaction between CRH and central OXT critically regulates the neuroendocrine response to stress (Winter and Jurek, 2019). CRH inhibits OXT action, and OXT suppresses the stress-mediated induction of CRH expression to maintain homeostasis (Nomura et al., 2003; Janeček and Dabrowska, 2019). Activation of the oxytocin system regulates emotion, stress response in virgin, pregnant and lactating rats (Neumann et al., 2000), social behavior (Neumann, 2008), and fear response (Viviani et al., 2011), and protects memory in stressed rats (Lee et al., 2015). Earlier studies have demonstrated that stressful experiences alter the epigenetic signatures embedded in the genome, specifically genes associated with the HPA axis and stress response (Cánepa and Berardino, 2024; Ohi et al., 2024).
Evidence indicates that maternal stress can alter fetal epigenetic signatures, including methylation of genes implicated in the stress response (Darnaudéry and Maccari, 2008; Dion et al., 2022), and may have a long-lasting impact by potentially inheriting to the following generations (Wiley et al., 2023; Moelling, 2024). Stress has been known to alter Histon-3 Lysine4 (H3K4me2/me3) methylation in the CRH promoter, which facilitates the transcriptional activation of downstream signaling molecules (Hunter et al., 2009). Transcriptional activation of the CRH promoter is often a core factor in the regulation of stress response/resilience, synaptic plasticity, learning, and memory (Fuge et al., 2014). Elevated levels of CRH negatively influence the expression of brain-derived neurotrophic factor (BDNF), and vice versa (Jeanneteau et al., 2012; Hu et al., 2020). BDNF is encoded by multiple bipartite transcripts (exon I-XI), which tightly regulate the expression of BDNF in response to stress response (Colliva and Tongiorgi, 2021) and synaptic plasticity, learning, and memory (Bruijniks et al., 2020; Kim et al., 2024).
A large number of animal and clinical studies have reported the underlying mechanism of the parental effect on transgenerational inheritance based on paternal lineage (reviewed in Dion et al., 2022). We previously reported that prenatal maternal stress (PMS) alters neurobehavior in offspring through stress hormones, neurotransmitters, oxidative stress, and other signaling molecules (Sivasangari and Rajan, 2020; Sivasangari et al., 2023). Several animal models have reported that maternal stress induces long – term effects on offspring, likely through epigenetic changes (Blaze and Roth, 2015; Santilli and Boskovic, 2023), however, there are differences in the types of stressors and markers tested. Therefore, we predicted that maternal line-mediated transgenerational epigenetic inheritance might transmit alterations and behavioral phenotype from F1 to F2 offspring. To test our hypothesis, we designed the following experiment : (i) pregnant rats (F0) were undisturbed (Control, Ctrl), (ii) pregnant rats (F0) were exposed to social stress during gestational day (GD) 16-18 (PMS), and (iii) pregnant rats (F0) were exposed to social stress during GD 16-18 and treated with oxytocin (PMS+OXT) on GD-18. Further F1 female offspring (CtrlF1, PMSF1, PMS+OXTF1) were mated with Ctrl F1males to examine maternal line transgenerational effects.
2 Materials and methods
2.1 Animal and experimental design
The estrous cycle of female Wistar rats (Rattus norvegicus) was continuously monitored, and selected females were housed individually (43 × 27 × 15 cm) with a male for mating. Rats were maintained under standard laboratory conditions (12 h light/ dark cycle; 22–25°C) with ad libitum access to feed (chow pellets) and water. Paddy husks were provided as bedding, and a portion of husk was removed every day to clean and maintain the home ’se odor to avoid stress (Carbone et al., 2016). The day of presence of sperm in the vaginal lavage was noted as gestational day (GD-0; n = 19), and pregnant female rats were housed individually. Pregnant female rats were randomly assigned to three groups: (i) control (Ctrl), (ii) prenatal maternal stress (PMS), and (iii) prenatal maternal stress + drug (oxytocin) treatment (PMS + OXT). Pregnant rats in the PMS and PMS+OXT groups were subjected to social stress during GD-16 to 18, and the PMS+OXT group received Oxytocin [0.5 IU (Cat # 103H05241, Sigma Aldrich) in 50μl of PBS intraperitoneally (i.p)]. The dosage of oxytocin was established based on earlier reports, which showed that oxytocin reaches the brain and elicit substantial effects, i.e., memory (Balmus et al., 2018; Pãdurariu et al., 2018). Animals received oxytocin during light-phase (between 9.00 and 11.00) of GD-16-18, 30 min after social stress (Gulevich et al., 2019). F1 female offspring Ctrl F1 (n = 13), PMS F1 (n = 15), and PMS+OXT F1 (n = 17) were subjected to behavioral test during their early adolescent postnatal day (PND) 32–36, and samples were processed and retained for the F2 experiment. In the second set of experiments, sexually matured adult F1 females from the CtrlF1 (n = 7), PMS F1 (n = 8), and PMS+OXT F1 (n = 8) groups were selected randomly, housed individually, and allowed to mate with CtrlF1 male (never exposed to stress). F2 offspring from control (CtrlF2; n = 8), stressed (PMS F2, n = 11), stressed, and oxytocin treated (PMS+OXT F2; n = 8) groups were subjected to behavioral testing and samples were processed for molecular analysis (Figure 1). Animals were treated following guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA, India) and all the protocols are approved (BDU/IAEC/P23/2022 dated 25.08.2022) by Institutional Animal Ethical Committee (IAEC), Bharathidasan University.
Figure 1. Experimental timeline showing the complete sequence of experiments performed. In this study, two experiments were performed; Control (Ctrl) pregnant rats were housed in standard laboratory condition without any disturbance. Prenatal maternal stress (PMS) provided during gestational day (GD-16 to 18), to stressed mother (F0) –St and stressed mother treated with oxytocin (OXT) during GD-16 to 18, 30 min after stress. F1 offspring (CtrlF1, PMS F1, PMS+OXT F1) were subjected to behavioral test during their early adolescent age (postnatal day -33 to 37). In the second experiment, female F1 offspring (CtrlF1, PMS F1, PMS+OXT F1) were mated with CtrlF1 male, and F2 offspring (CtrlF2, PMSF2, PMS+OXT F2) were subjected to behavioral test.
2.2 Prenatal maternal stress
Prenatal maternal stress (PMS) was induced individually by allowing the pregnant rat to interact with a resident stranger (senescent male; 24 months old) from GD-16 to GD-18 (10 min/day), as previously reported earlier (Sivasangari and Rajan, 2020; Sivasangari et al., 2023). A three chambers social defeat apparatus [30 × 30 × 30 cm each, resident chamber (RC), intruder chamber (IC), and observer chamber (OC)] was connected to a housing laboratory cage (43 × 27 × 15 cm) for resident stranger (Supplementary Figure S1). Pregnant rats were introduced into the OC, and after five minutes the interaction was facilitated by opening the sliding wire mesh door, the pregnant rats were allowed to interact with the stranger (10 min/day). The control rats were allowed to explore the clean apparatus in the absence of a stranger.
2.3 Behavioral test
2.3.1 Hole—board test
Hole-board test (HBT) apparatus (44.5 × 44.5 × 30.5 cm) was originally designed with 16 holes, i.e., four identical rows each containing four holes at equidistant (Boissier et al., 1964), and offers to measure various aspects of behavior such as exploration and anxiety, habituation to a novel environment, spatial learning and memory (working and reference memory) (Lundberg et al., 2019, 2020). Therefore, HBT was used to measure the working and reference memory performance. On postnatal day (PND)–33, animals were transferred to the experimental room, after 1 h allowed exploring the apparatus for 10 min. PND-34 animals were food deprived for 8 h and habituated to the apparatus with all holes baited. On PND-35, 36 animals were trained individually; four holes were baited in a fixed pattern, and the other holes were closed. On PND-37, the animals were tested in the HBT by placing baits in four holes in a fixed pattern, and the remaining holes were kept open. The apparatus was wiped with 75% ethanol after each training/testing session to avoid olfactory cues, and all activities were recorded. Ratio of reference memory = no. of visits + revisits to baited holes/Total No. of holes visits. Ratio of working memory = no. of food-rewarded visitors/no. of visits and revisits to baited holes were calculated (Kuc et al., 2006). The time spent freezing in the hole-board apparatus was scored as the absence of all movement except respiration and considered anxiety-like behavior (Treit and Fundytus, 1988).
2.4 Sample preparation
Immediately after the behavioral test, female rats [CtrlF1 (n = 6), PMS F1 (n = 6), PMS+OXT F1 (n = 6), CtrlF2 (n = 6), PMS F2 (n = 6), PMS+OXT F2 (n = 6)] were sacrificed, the whole brain was carefully removed and placed on an ice-cold Petri dish, and the amygdala was dissected (Zapala et al., 2005). Tissue (left and right amygdala) samples were divided for preparation of the corticosterone analysis, genomic DNA, total RNA, and total and histone proteins.
2.4.1 Corticosterone analysis
Amygdala tissues (25 mg) were washed in buffer, homogenized (50 μL), and centrifuged (10,000 × g, 4°C, 10 min). Supernatants were collected, and the level of corticosterone (CORT) was estimated using Enzyme-Linked Immunosorbent Assay (ELISA) (ALPCO Diagnostics, Salem, NH) according to the manufacturer’s instructions. The CORT level were calculated in ng/mg for wet tissues.
2.4.2 Western blot analysis
Three samples from each experimental group were pooled together into two groups, and then total protein and histone protein were isolated from each group. Total protein was prepared by homogenize the tissue sample in ice-cold lysis buffer containing protease inhibitor (4 μL/mL) (Sigma-Aldrich, India). The homogenate was incubated on ice (30 min) followed by centrifugation at 4°C (10,000 × g for 30 min at 4). The supernatant was collected and centrifuged again at 4°C (12,000 × g, 15 min), and aliquots were stored at −80°C.
2.4.2.1 Histone protein
Tissue was homogenized with TX buffer (Tris—HCL, NaCl, EDTA, Triton 100, protease inhibitor cocktail). The homogenates were incubated on ice for 15 min and then centrifuged at 4°C (10 min). The pellet was dissolved in HCL—TX buffer (0.2M) and incubated on ice for 30 min, followed by centrifugation at 4°C (10 min). The supernatant was collected and stored at −80°C.
Samples were quantified (Bio-Rad Protein Assay Kit, Bio-Rad Laboratories Inc., United States) using a Biophotometer Plus (Eppendorf Inc., Germany), and equal concentrations of total protein (50μg) were separated on a polyacrylamide gel (10%). The separated proteins were transferred onto a membrane using a Trans-Blot Turbo Transfer System (Bio-Rad Laboratories, Inc., United States). The membrane was incubated for 2 h in a blocking solution (Tris-buffered saline, Tween-20; TBS-T, 0.1%; non-fat milk, 5%). Membranes were gently washed with TBS-T (5 min/wash) and then incubated in any one of the following primary antibodies [anti-histone H3 rabbit monoclonal antibody (Cat# 4499, 1:5,000; Cell Signalling Technology, Inc.); anti-H3K4me2 rabbit monoclonal antibody (Cat# 9725, 1:5,000; Cell Signalling Technology, Inc.); antiH3K4me3 rabbit monoclonal antibody (Cat# 9725, 1:5,000; Cell Signalling Technology, Inc.); anti-pro-BDNF rabbit polyclonal antibody (Cat# SC-546, 1:2,000; Santa Cruz Biotechnology), mature BDNF rabbit polyclonal antibody (Cat# PA5-85730, 1: 2,000; Invitrogen), and antiß-actin rabbit polyclonal antibody (Cat# SC-47778, 1:2,000; Santa Cruz Biotechnology, Inc.) for about 12–16 h at 4°C. The membrane was washed three times (5 min) in 1XTBS-T and specific protein-bound antibodies were detected by goat anti-rabbit (GeNei™, 1:5,000; Cat# 621100180011730)/anti-mouse (GeNei™, 1:5,000; Cat# 105215) IgG secondary antibody conjugated with alkaline phosphatase (ALP) by incubating for 4 h at room temperature. Subsequently, the membrane was washed with 1XTBS-T (2 × 5 min), and membrane-bound ALP activity was determined using an alkaline phosphatase substrate (AP Detection Reagent Kit, Merck). After standardizing the western blot using combined linear range detection, the experiment was performed separately for each group. The values from each blot were measured (before saturation) three times in a linear range during development. Images were obtained and the specific band intensity was calculated using a molecular imager (ChemiDoc XRS; Image Lab-2, Bio-Rad Laboratories, Inc., United States). Data showing normalized values with Total H3 / beta-actin, and all uncropped western blot images are shown in Supplementary Information.
2.4.3 Chromatin immunoprecipitation assay
Genomic DNA was isolated from amygdala tissue following the manufacturer’s instructions (Cat# FATGEM-001B; Tissue Genomic DNA extraction kit, Favorgen). The concentration of the DNA sample was estimated using a Biophotometer Plus (Eppendorf Inc., Germany) and stored at −80°C. In the Chromatin Immunoprecipitation (ChIP) assay, immunoprecipitation (IP) was facilitated by incubating genomic DNA (10 μg) with specific antibodies [anti-H3K4me2 rabbit monoclonal antibody/ anti-H3K4me3 rabbit monoclonal antibody/ anti-histone H3 rabbit monoclonal antibody)]. For 12 h at 4°C, the procedure described by the manufacturer (SimpleChIP® Enzymatic Chromatin IP Kit (Magnetic Beads) Cat # 9003. Cell Signalling Technology). Protein G magnetic beads (30 μL) were added to each IP reaction mixture and incubated for 2 h at 4°C in a rotating shaker. The DNA-protein complex was washed with a graded washing solution, and DNA was eluted from the antibody/ protein G magnetic beads using ChIP Elution Buffer. Chromatin was reverse cross-linked by incubating NaCl (5M) and Proteinase K at 65°C for 2 h. DNA was eluted using a DNA spin column following a series of procedures, and the column was washed with DNA binding buffer (centrifuged at 18,500 × g for 30 s), transferred to the sample (450 μL), centrifuged at 18,500 × g for 30 s, and washed with wash buffer by centrifugation at 18,500 × g for 30 s. Finally, the DNA was eluted with DNA elution buffer by centrifugation at 18,500 × g for 30 s, quantified, and stored at -20°C.
Methylation of the CRH promoter was quantified by quantitative real—time chain reaction (qRT–PCR). The total reaction volume (20 μL) contained real-time mixture (SYBR green super mix, Bio-Rad Laboratories Inc.) and specific primers (For 5′CTGTCAAGAGAGCGTCAGC TTATTA-3′ and Rev 5′-CTCTTCAGTTTCTCAAGGTAC TTGGC-3′ each 100 pm) (Singh-Taylor et al., 2018) with the following reaction conditions [denaturation (92°C, 3 min), 40 cycles of denaturation (92°C, 5 s), annealing (63.6°C, 5 s) extension (72°C, 5 s), and final extension (72°C, 10 min). Specific amplification was confirmed by dissociation and melting curve analysis (CFX-96 Touch Real-Time PCR detection system; CFX Manager version 2 software; Bio-Rad Laboratories Inc., United States). The data were normalized to the internal control and presented as the mean fold change.
2.4.4 Quantitative real—time PCR
RNA was isolated using PureZOL (cat. # 732-6880; Bio-Rad Laboratories Inc., United States) and stored at −80°C. Total RNA (2 μg) was used to synthesize cDNA (cat# 170-8891; Iscript™ cDNA synthesis kit, Bio-Rad Laboratories Inc., United States). cDNA (0.2 μg) and specific primers [Crh: For 5′-GAAACTCAGAGCCCAAGTACGTTGAG-3′; Rev: 5′-GTTG TTCTGCGAGGTACCTCTCTCAG3′; Crhr1: For 5′-GTCCCT GACCAGCAATGTTT-3′; Rev 5′-CGGAGTTTGGTCATGAGG AT-3′; Crhr2: For 5′-AAGGTCCTAGGAGTGATCCGATT-3′; Rev 5′GGAGCCCACCAGAGAGTGCAG-3′; β –actin: For 5′-AACAT CATCCCTGCATCCAC-3′; Rev 5′-AGGAACACGGAAGGCCA TGC-3′; Brain- derived neurotrophic factor (Bdnf) For 5′-GGCCCAACGAAGAAAACCAT-3′; Rev 5′-AGCATCACC CGGGAAGTGT-3′; exon- III For 5′-TTGGAGGGCTCCTGC TTTCT-3′; Rev 5′-CTGGGCTCAATGAAGCAT CCAG3′; exon-IV For 5′-ACTGAAGGCGTGCGAGTATT-3′; Rev 5′-TGG TGGCCGATATGT ACTCC-3′; exon-VI For 5′-GATGAGA CCGGGTTCCCTCA-3′; Rev 5′-TTGTTGTCACGCTCCTG GTC-3′; (100 pm/each)] (Nair and Wong-Riley, 2016) in separate reaction (10 μL) containing real-time mixture (SYBR green super mix, Bio-Rad laboratories Inc.) was used to estimate expression with the following reaction cycle: 92°C initial denaturation (3 min), subsequent denaturation at 92°C (5 s), annealing [(5 s:Crh (63.4°C), Crhr1 (62.0°C), Crhr2 (63.6°C), B-actin (60.0°C), total Bdnf (59.0°C); exon-III (63.0°C); exon-IV (59.8°C); exon-VI (59.0°C)], and extension at 72°C (5 s) for 40 cycles and final extension at 72°C (10 min). Specific amplification was confirmed by dissociation and melting curve analysis (CFX-96 Touch Real-Time PCR detection system; CFX Manager version 2 software; Bio-Rad Laboratories Inc., United States). The data were normalized to the internal control and presented as the mean fold change.
2.5 Statistical analysis
GraphPad prism (ver 8.0) was used to plot the values (mean ± standard error of the mean (SEM)) as a graphical representation. The significant difference among the experimental groups (Ctrl, PMS, PMS+OXT) were tested with One–way Analysis of Variance (ANOVA), Two–way ANOVA for Bdnf Transcript variant followed by post hoc (Bonferroni test) analysis and Regression analysis (Sigma Stat version 11.0). Significant difference noted as (*p < 0.05, **p < 0.01, ***p < 0.001 and NS—indicate not significantly different.
3 Results
3.1 Transgenerational inheritance of PMS induced deficit in reference and working memory
First, we examined the PMS-induced transgenerational effect on learning and memory in F1 and F2 offspring. We found a significant difference in reference (F2,44 = 145.36; P < 0.001) and working memory (F2,44 = 174.82; P < 0.001) in the F1 offspring. Post hoc analysis indicated greater effects of PMS-induced deficit in reference and working memory in PMS F1 offspring. The PMSF1 offspring number of errors was higher (P < 0.001) than that of Ctrl and PMS+OXT; however, PMS+OXTF1 offspring made more errors than CtrlF1 offspring (P < 0.001) (Figures 2A,B). Further analysis showed the impact of PMS in F2 offspring, and the calculated reference (F2, 26 = 145.36; P < 0.001) and working memory (F2,26 = 60.625; P < 0.001) were significantly different among F2 offspring from Ctrl, PMS, PMS+OXT. Post hoc analysis indicated that PMS F2 offspring displayed more errors than Ctrl F2 and PMS+OXT F2 offspring (P < 0.001). In contrast, the performance of PMS+OXT F2 offspring was significantly lower than that of Ctrl F2 (Figures 2C,D). The behavioral profile of the F1 and F2 offspring in the hole board test are shown in Supplementary Figures S2, S3. In addition, anxiety-like behaviors were observed. The freezing duration of F1 offspring was significantly different (F2,44 = 4.783; P = 0.013). Post hoc comparisons indicated that PMS F1 offspring freezing duration was significantly higher than that of Ctrl F1 and PMS+OXT F1 (P < 0.01) offspring, and no significant difference was observed between PMS F1 and PMS+OXT F1 (P > 0.05) and Ctrl F1 vs. PMS+OXT F1 (P > 0.05) (Figure 3A). Notably, there was no significant difference among the F2 offspring (F2,26 = 0.595; P = 0.559), and also in all comparison between F2 offspring (P > 0.05) (Figure 3B). The observed behavioral data suggest that PMS induced memory deficit in F1 offspring, and was inherited to F2 offspring at some extent, which was confirmed by partial recovery by administration of oxytocin.
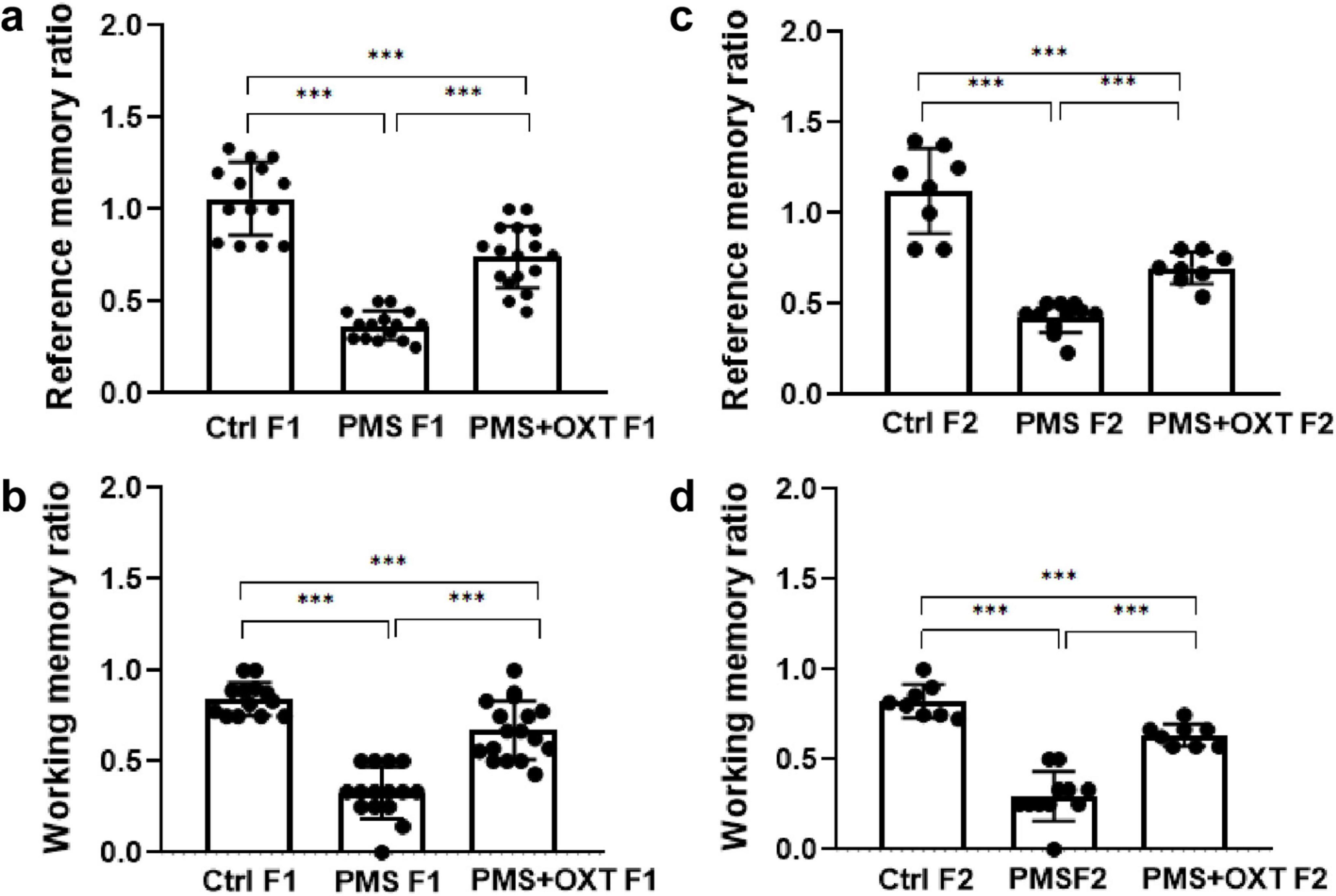
Figure 2. Effect of prenatal maternal stress (PMS) on F1 and F2 offspring reference and working memory. Behavioral profile in the hole board test showing that PMS reduced the reference and working memory of the F1 generation (A,B) of stressed offspring (PMS F1) and F2 generation (C,D) from the stressed maternal line (PMS F2). Exposure to oxytocin relieved the PMS-induced effect and improved the reference and working memory of F1 generation (A,B) of offspring (PMS+OXT F1) and F2 generation (C,D) from the maternal line (PMS+OXT F2). Data are represented as mean ± SE, and statistical significance is indicated by ***p < 0.001; NS, not significant.
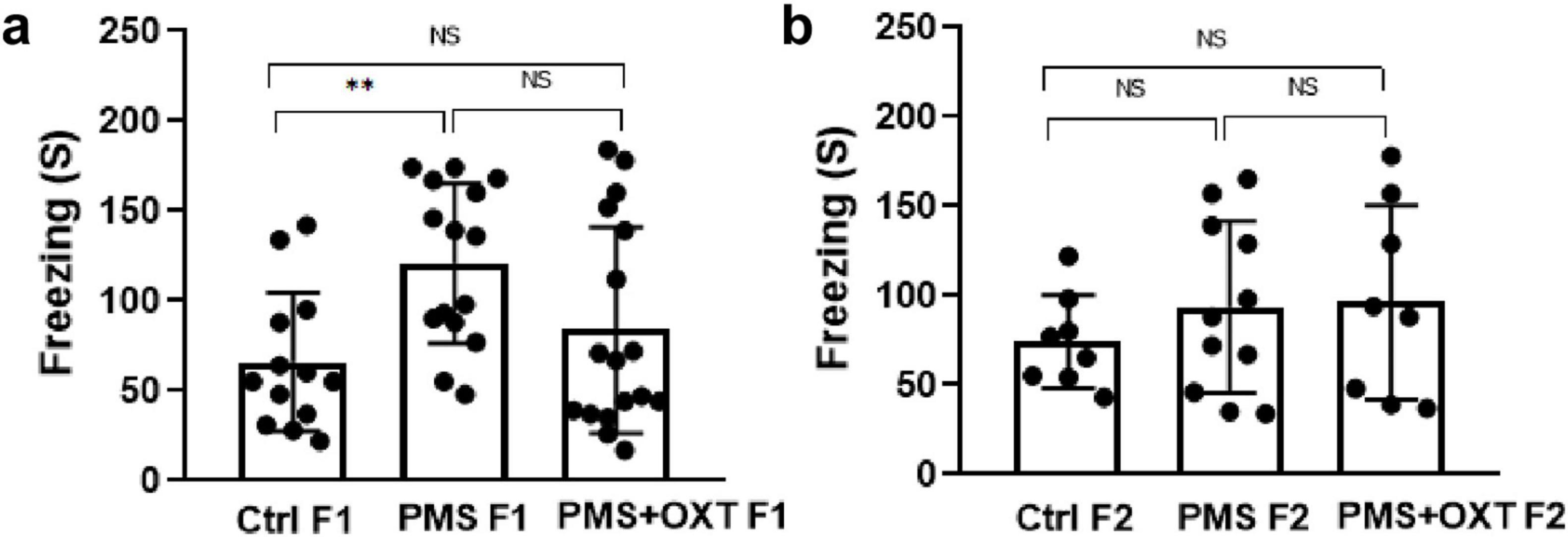
Figure 3. Effect of prenatal maternal stress (PMS) on anxiety-like behavior in F1 and F2 offspring Freezing behavior recorded for F1 generation (A) of stressed offspring (PMSF1) and F2 offspring (B) from the stressed maternal line (PMSF2) in the hole board arena. Exposure to oxytocin relieved PMS-induced stress in F1 offspring (PMS+OXT F1) and F2 offspring from the maternal line (PMS+OXT F2). Data are represented as mean ± SE, and statistical significance is indicated by **p < 0.01; NS, not significant.
3.2 Transgenerational inheritance of H3K4me2 and H3K4me3 methylation
We examined the levels of H3K4me2 and H3K4me3 to determine whether PMS induced methylation. We found that the levels of H3K4me2 [F(2,17) = 146.259; P < 0.001] and H3K4me3 [F(2,17) = 48.160, P < 0.001] were significantly different among F1 offspring. Further analysis showed that PMS significantly increased H3K4me2 and H3K4me3 methylation, which was significantly higher than that in the Ctrl F1 (P < 0.001) and PMS+OXT F1 (P < 0.001) offspring, and there was a significant difference between CtrlF1 and PMS+OXT F1 (P < 0.05) (Figures 4A–C). We observed a similar pattern of H3K4me2 [F(2,17) = 13.023; P < 0.01] and H3K4me3 [F(2,17) = 38.908, P < 0.001] methylation status in F2 offspring. The observed changes in the levels of H3K4me2 and H3K4me3 were significantly higher in the PMS F2 offspring than in CtrlF2 and PMS+OXT F2 offspring (P < 0.001). However, no significant difference was detected between CtrlF2 and PMS+OXT F2 (P > 0.05) in H3K4me2 or H3K4me3 levels (Figures 4A,D,E). The observed methylation status suggests that PMS induced changes possibly inherited from the F1 to F2 offspring.

Figure 4. Effect of prenatal maternal stress (PMS) on H3K4me2 and H3K4me3 methylation levels in F1 and F2 offspring. Western blot analysis showed H3K4me2 and H3K4me3 methylation patterns in the F1 and F2 offspring. (A) PMS increases the level of methylation in F1 (PMS F1) offspring [H3K4me2 (B), H3K4me3 (C)] and F2 (PMS F2) offspring [H3K4me2 (D), H3K4me3 (E)]. Exposure to oxytocin reduced the PMS-induced effect and decreased the levels of H3K4me2 and H3K4me3 in F1 (PMS+OXT F1) and F2 (PMS+OXT F2) offspring. Data are represented as mean ± SE, and statistical significance is indicated by *P < 0.05; ***P < 0.001 NS – not significant.
3.3 Transgenerational inheritance of H3K4me2/me3 methylation in CRH promoter
Subsequently, we examined whether PMS induced H3K4me2/ H3K4me3 methylation of the CRH promoter. In F1 offspring, we found that the levels of H3K4me2 [F2, 17 = 56.099; P < 0.001) and H3K4me3 (F2, 17 = 28.809, P < 0.001) were significantly different in the CRH promoter. Furthermore, PMS-induced H3K4me2 and H3K4me3 methylation in the CRH promoter was significantly higher in PMS offspring than in Ctrl F1 (P < 0.001) and PMS+OXT F1 (P < 0.001) offspring, but no significant difference was detected between CtrlF1 and PMS+OXT F1 (P > 0.05) (Supplementary Figures S4A,B). A similar pattern of methylation status was observed in the CRH promoter of F2 offspring. The level of H3K4me2 [F(2,17) = 11.160; P < 0.001] and H3K4me3 [F(2,17) = 38.521, P < 0.001] were significantly different among F2 offspring. H3K4me2 and H3K4me3 levels were significantly higher in the PMS F2 offspring than in the CtrlF2 and PMS+OXT F2 offspring (P < 0.001). However, no significant difference was detected between CtrlF2 and PMS+OXT F2 (P > 0.05) in H3K4me2 levels, but not in H3K4me3 levels (P < 0.05) (Supplementary Figures S4C,D). Furthermore, we have observed negative correlation in the level of methylation with reference/ working memory in F1, and F2 offspring. We found that elevated level of H3K4me2, H3K4me3 methylation significantly reduced reference (Figures 5A,C) and working memory (Figures 5B,D) in F1 offspring. Similar effect was observed in F2 offspring reference (Figures 6A,C) and working memory (Figures 6B,D). The detected methylation level demonstrates that PMS induced H3K4me2 and H3K4me3 methylation status of the CRH promoter inherited from F1 to F2 offspring.
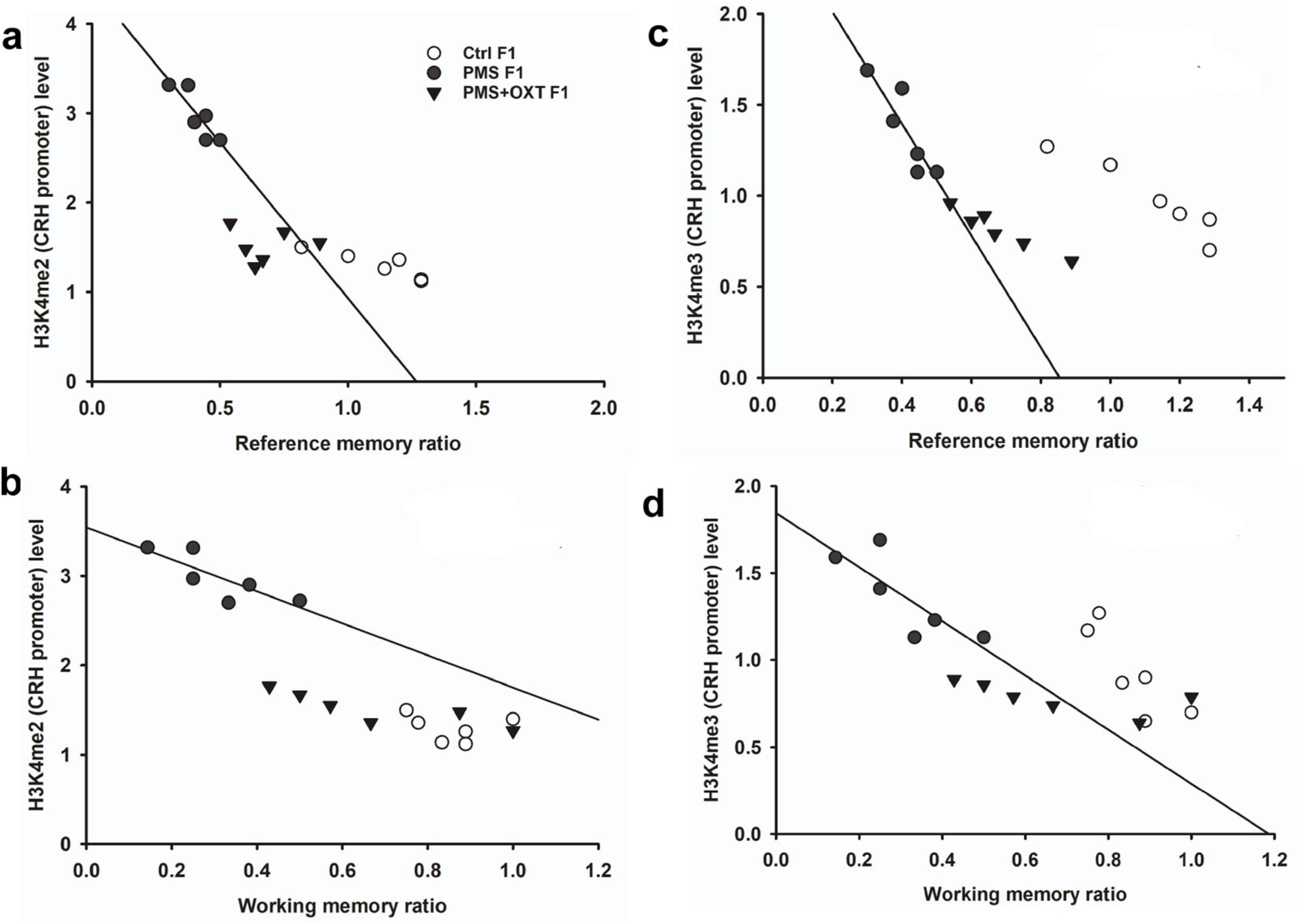
Figure 5. Prenatal maternal stresses (PMS) alters the levels of H3K4me2 and H3k4me3 methylation in the CRH promoter. A chromatin immunoprecipitation (ChIP) assay followed by quantitative real-time PCR analysis showed H3K4me2 and H3K4me3 methylation status in the CRH promoter. Further analysis showed that level of H3K4me2 (A,B) and H3K4me3 (C,D) significantly influenced reference memory working memory, respectively, in F1 offspring. PMS increased the level of methylation in the CRH promoter of PMSF1 and oxytocin exposure decreased methylation in PMS+OXT F1 offspring.
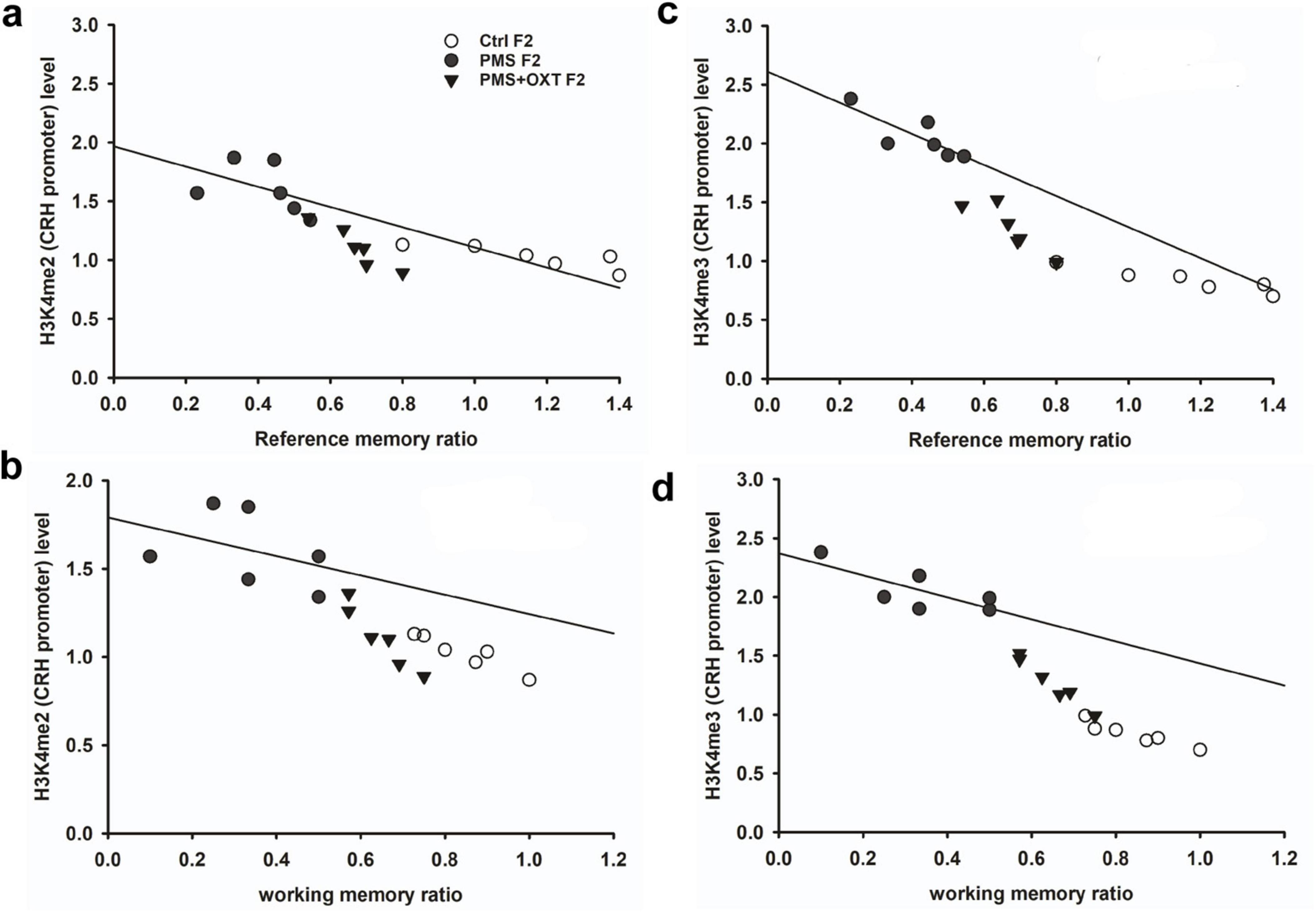
Figure 6. Prenatal maternal stresses (PMS) alters the levels of H3K4me2 and H3k4me3 methylation in the CRH promoter. A chromatin immunoprecipitation (ChIP) assay followed by quantitative real-time PCR analysis showed H3K4me2 and H3K4me3 methylation status in the CRH promoter. Further analysis showed that level of H3K4me2 (A,B) and H3K4me3 (C,D) significantly influenced reference memory working memory, respectively, in F2 offspring. PMS increased the level of methylation in the CRH promoter of PMSF2 offspring, and oxytocin exposure decreased methylation in PMS+OXT F2 offspring.
3.4 Transgenerational inheritance of regulation of Crh expression
We examined the effects of CRH promoter methylation on Crh mRNA levels. We found a significant difference in the level of Crh mRNA between the F1 offspring [F(2,17) = 321.516, P < 0.001]. Post-hoc analysis suggested that Crh mRNA levels were significantly higher in PMS F1 offspring than in CtrlF1 and PMS+OXT F1 offspring (P < 0.001). However, the Crh mRNA levels were significantly higher in the PMS+OXT F1 group than in the CtrlF1 group (P < 0.05) (Supplementary Figure S5A). A similar pattern was observed in F2 offspring [F(2,17) = 175.331, P < 0.001]. Accordingly, the level of Crh mRNA was significantly higher in PMS F2 offspring than in CtrlF2 and PMS+OXT F2 offspring (P < 0.001), however, the level was significantly higher in PMS+OXT F2 offspring than in Ctrl F2 offspring (P < 0.001) (Supplementary Figure S5D). The observed data demonstrate that exposure to oxytocin may counteract PMS induced transcriptional activation of Crh.
Crh facilitates regulation possibly through Crhr1 and Crhr2; therefore, we examined the levels of Crh receptors. The estimated level of Crhr1 in F1 offspring was significantly different between the F1 offspring [F(2,17) = 30.249, P < 0.001]. Post hoc analysis showed that PMS influenced the expression of Crhr1, which was significantly higher in PMS than in CtrlF1, PMS+OXT F1 (P < 0.001); however, the level of Crhr1 in PMS+OXT was not different from that in Ctrl F1 (P > 0.05) (Supplementary Figure S5B). The effect of PMS was observed in the F2 offspring; thus, we observed a significant difference between the experimental groups [F(2,17) = 32.920, P < 0.001]. The estimated levels in PMS F2 offspring were significantly higher than those in Ctrl F2 and PMS+OXT F2 (P < 0.001), but there was no significant difference between the Ctrl F2 and PMS+OXT F2 offspring (P > 0.05) (Supplementary Figure S5E).
Subsequently, we found that the expression of Crhr2 was significantly different among the experimental groups in F1 offspring [F(2,17) = 46.008, P < 0.001]. PMS significantly induced the expression of Crhr2; the estimated level in the PMS F1 offspring was higher than that in Ctrl F1 and PMS+OXT F1 offspring (P < 0.001), but the level was significantly higher in the PMS+OXT F1 offspring (P < 0.01) (Supplementary Figure S5C). A similar pattern of Crhr2 expression in F2 offspring was observed in F2 offspring [F(2,17) = 16.736, P < 0.001]. Expression in PMS F2 offspring was higher than in Ctrl F2 and PMS+OXT F2 offspring (P < 0.001), whereas a higher level of Crhr2 was observed in PMSF2 offspring than in Ctrl F2 offspring (P < 0.001) (Supplementary Figure S5F). Interestingly, we found that elevated level of Crh, Crh R1 and Crh R2 expression significantly reduced the reference and working memory in F1 and F2 generation. We have observed a strong significant negative association between reference memory [Crh (r = -0.71, P < 0.001), Crh R1 (r = -0.23, P > 0.05), Crh R2 (r = -0.417, P < 0.01)] and working memory [Crh (r = -0.23, P > 0.05), Crh R1 (r = -0.42, P < 0.05), Crh R2 (r = -0.817, P < 0.001)] in F1 offspring (Figure 7). Similar pattern of relationship was observed in F2 offspring’s reference memory [Crh (r = -0.89, P < 0.001), Crh R1 (r = -0.67, P < 0.001), Crh R2 (r = -0.84, P < 0.01)] and working memory [Crh (r = -0.78, P < 0.001), Crh R1 (r = -0.86, P < 0.001), Crh R2 (r = -0.88, P < 0.001)] (Figure 8).
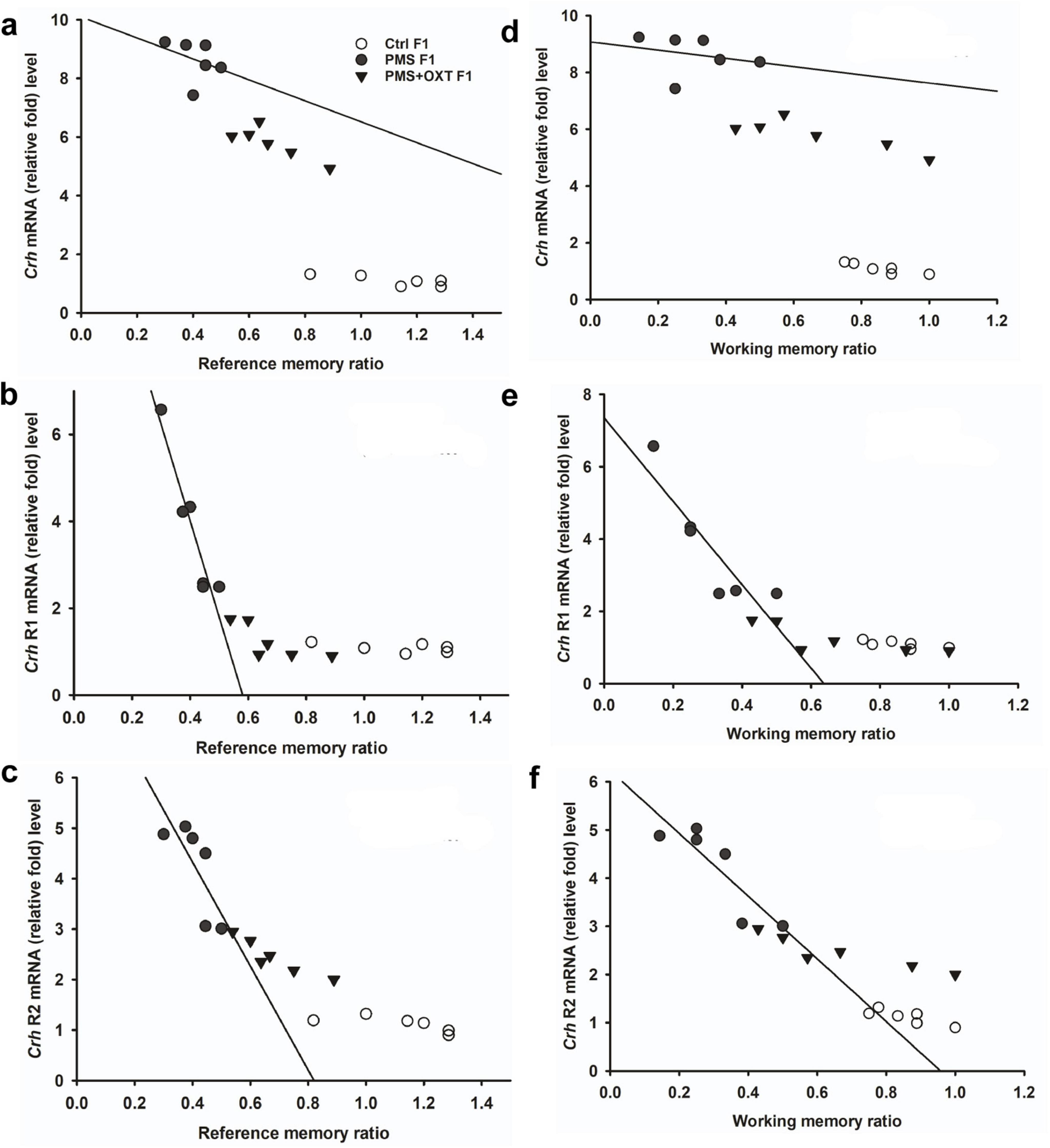
Figure 7. Prenatal maternal stress (PMS) alters the expression of corticotrophin-releasing hormone (Crh mRNA) and its receptors (Crhr1 and Crhr2). Quantitative real– time PCR analysis showing the expression pattern of Crh, Crhr1 Crhr2 in F1 offspring. The analysis showed that PMS significantly increased the levels of Crh, Crhr1 Crhr2 and decreased the reference (A–C) and working memory (D–F) in PMSF1 offspring, and oxytocin exposure decreased the levels and increased memory in PMS+OXT F1 offspring.
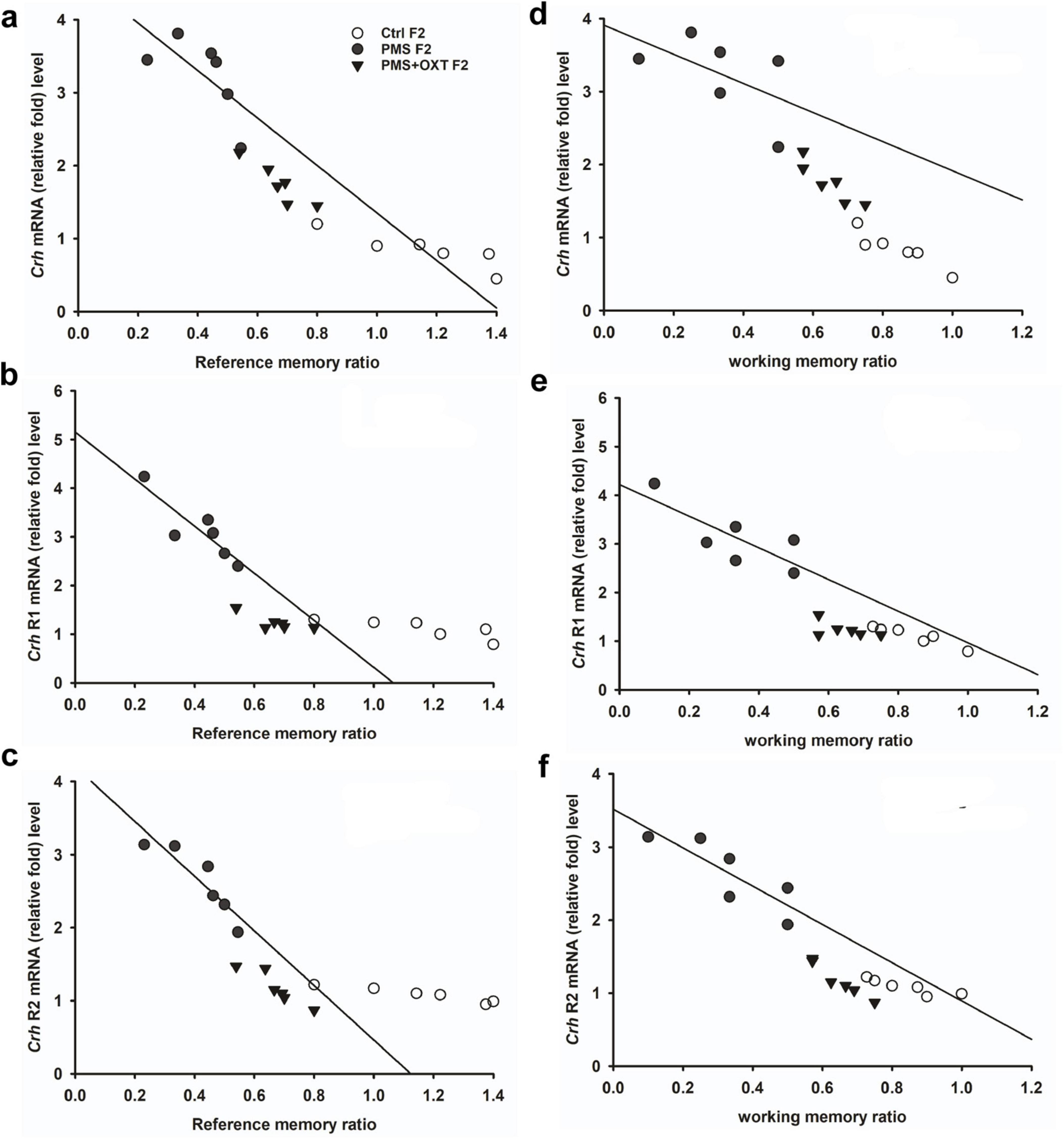
Figure 8. Prenatal maternal stress (PMS) alters the expression of corticotrophin-releasing hormone (Crh mRNA) and its receptors (Crhr1 and Crhr2). Quantitative real–time PCR analysis showing the expression pattern of Crh, Crhr1 Crhr2 in F2 offspring. The analysis showed that PMS significantly increased the levels of Crh, Crhr1 Crhr2 expression, and decreased the reference (A–C), working memory (D–F) in PMSF2 offspring, and oxytocin exposure decreased the levels and increased memory in PMS+OXT F2 offspring.
3.5 Transgenerational inheritance of regulation of corticosterone
To investigate the effect of PMS induction on stress hormone levels, we estimated CORT levels in the amygdala. We found that PMS significantly increased CORT level in F1 and F2 offspring. The level of CORT was significantly altered in F1 [F(2,17) = 303.106, P < 0.001] and F2 [F(2,17) = 183.7, P < 0.001] offspring. In comparison, the level of CORT was significantly higher in PMS F1 and PMS F2 offspring (P < 0.001) than in Crtl F1, CrtlF2, PMS+OXT F1, and PMS+OXT F2 offspring, but significant differences were not detected between CrtlF1, CrtlF2, PMS+OXT F1, and PMS+OXTF2 (P > 0.05) offspring (Supplementary Figure S6). Further analysis revealed that level of methylation of H3K4me2/ H3K4me3 associated with level of Crh mRNA and CORT. The regression analysis showed significant positive association between H3K4me2/ H3K4me3 methylation with Crh mRNA (r = 0.67, P < 0.001; r = 0.53, P < 0.01) and CORT (r = 0.52, P < 0.01; r = 0.51, P < 0.01) (Figure 9) in F1 offspring. Similarly, significant positive association detected between H3K4me2/ H3K4me3 methylation with Crh mRNA (r = 0.86, P < 0.001; r = 0.85, P < 0.001) and CORT (r = 0.83, P < 0.001; r = 0.89, P < 0.001) in F2 offspring (Figure 10).
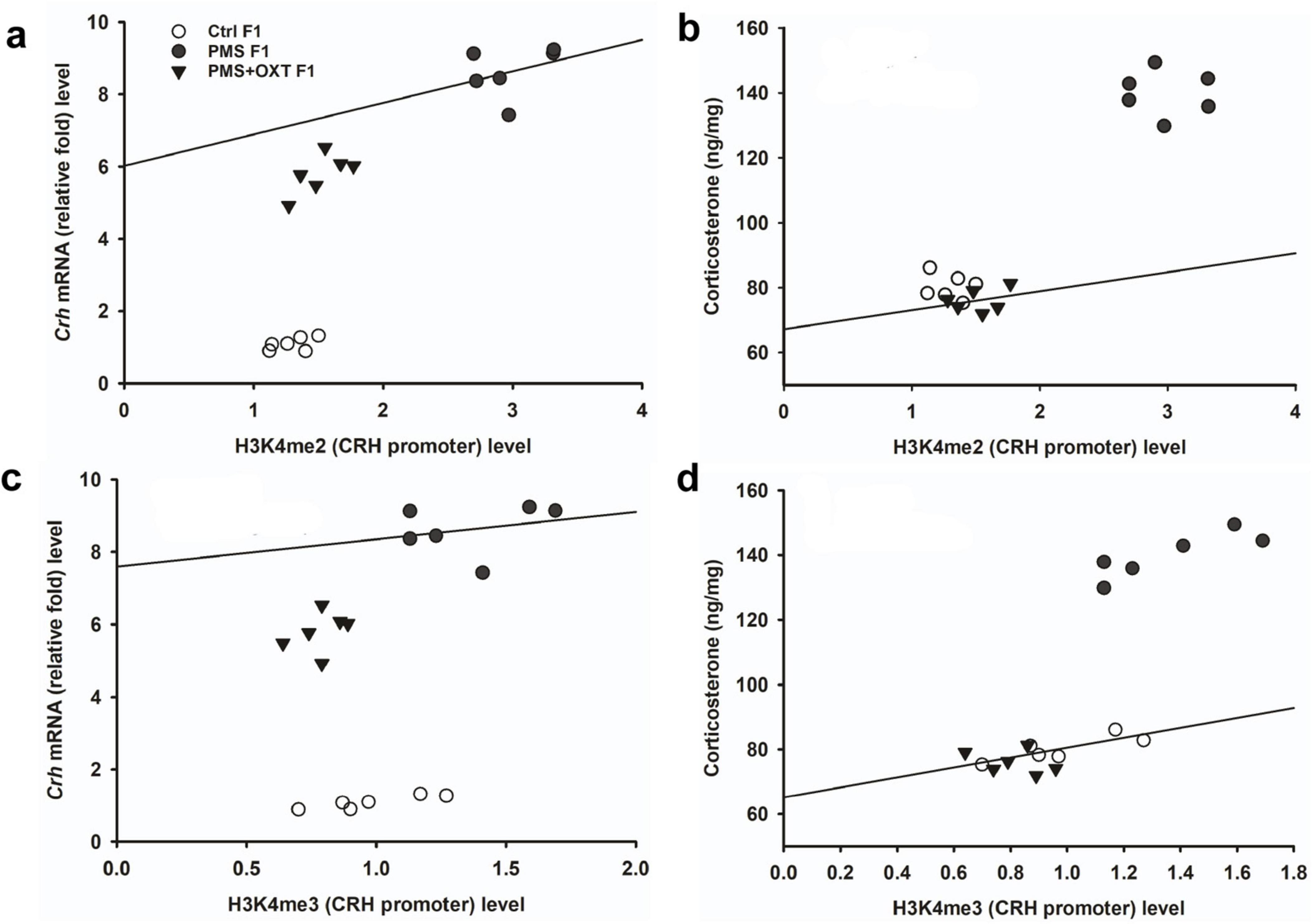
Figure 9. Effect of prenatal maternal stress (PMS)-associated H3K4me2, H3 K4me3 methylation CRH promoter significantly changes Crh mRNA and corticosterone (CORT) level in F1 generations. The level of H3K4me2, H3 K4me3 methylation significantly influences the level of Crh mRNA (A,C) and CORT (B,D) in F1 generation was significantly increased by PMS F1 offspring, but exposure to oxytocin attenuated the PMS induced effect and reduced the Crh mRNA, CORT level in PMS+OXT F1 offspring.
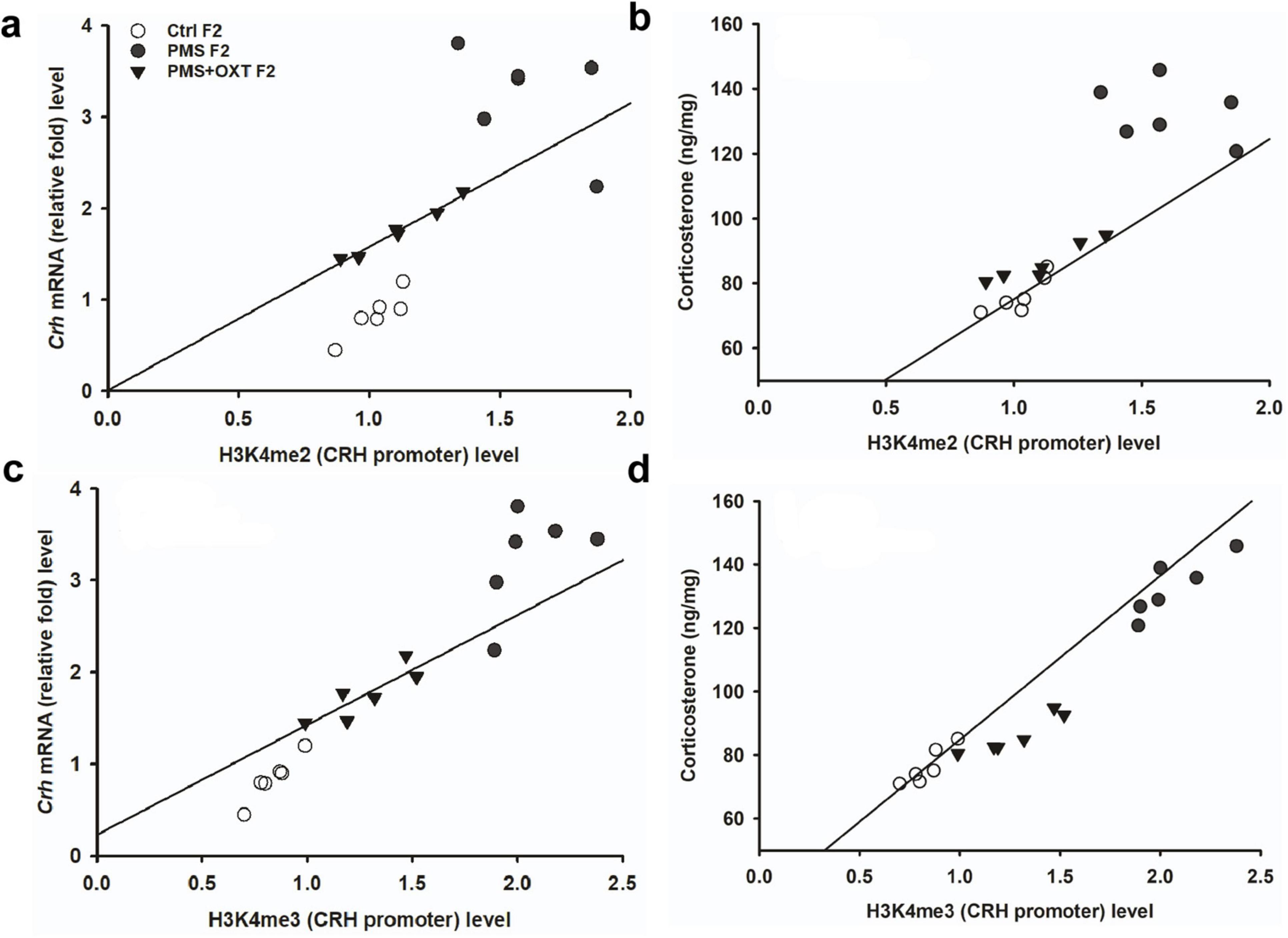
Figure 10. Effect of prenatal maternal stress (PMS)-associated H3K4me2, H3 K4me3 methylation CRH promoter significantly changes Crh mRNA and corticosterone (CORT) level in F2 generations. The level of H3K4me2, H3 K4me3 methylation significantly influences the level of Crh mRNA (A,C) and CORT (B,D) in F2 generation was significantly increased by PMS F2 offspring, but exposure to oxytocin attenuated the PMS induced effect and reduced the Crh mRNA, CORT level in PMS+OXT F2 offspring.
3.6 Transgenerational inheritance of regulation of Bdnf splices variant expression
We examined the influence of CRH on BDNF by examining the levels of the Bdnf variants. We detected contrasting patterns in the mRNA levels of exon-III and exon-IV. A two-way ANOVA revealed that there was a significant difference between the experimental groups with exon III, IV and VI [F(2,53) = 4.024, P < 0.05], within the group between exon-III IV and VI [F(2,53) = 103.08, P < 0.010], and there was a significant interaction between group and exon-III/IV/VI [F(2,53) = 31.77, P < 0.01]. Post hoc analysis showed that PMS significantly (P < 0.001) up-regulated the expression of exon-III and down regulated exon-IV and VI in PMS F1 (P < 0.001), but no significant difference was observed in the levels of exon-IV and VI (P > 0.05). In contrast, the level of exon-IV was restored in PMS+OXT F1. Thus, the level of exon-III, IV were significantly higher than those of exon-VI (P < 0.001), but there was no significant difference between exon-III and IV (P > 0.05). Note to mention that OXT treatment significantly restore the level of exon-IV, which was higher than that of PMS F1 (P < 0.001) and Ctrl F1(P < 0.001) (Figure 11A). Differential expression of splice variants significantly alter the level of Bdnf total mRNA [F(2,17) = 41.21, P < 0.001]. PMS significantly reduced the level (P < 0.001) compared to than CtrlF1 and PMS+OXT F1, however, there was significant difference between Ctrl F1 and PMS+OXT F1 (P < 0.001), and PMS F1 and PMS+OXT F1 (P < 0.01). Further analysis showed that Bdnf mRNA level significantly influenced the behavior, we found significant positive correlation between Bdnf mRNA and reference memory (r = 0.48, P < 0.05), and working memory (r = 0.43, P < 0.05) in F1 generation (Figures 11B,C).
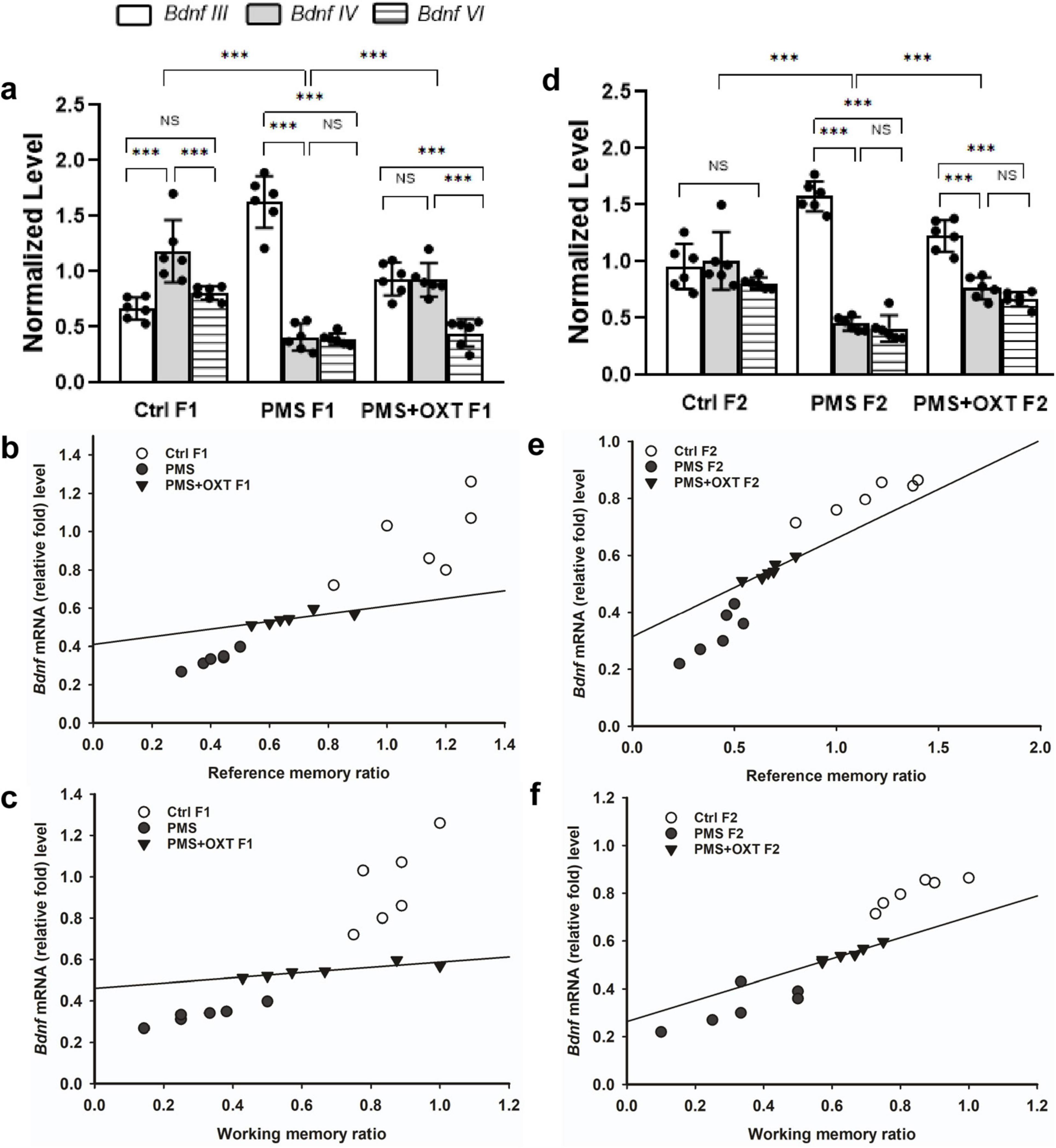
Figure 11. Effect of prenatal maternal stress (PMS) regulation of Bdnf splice variant and total Bdnf expression in the F1 and F2 generations. Quantitative real–time PCR analysis showed that PMS/oxytocin mediated alterations in the expression of Bdnf exon—III, IV and exon—VI transcripts. The analysis showed that PMS differentially altered the expression, level of exon-III increased, and exon-IV and VI decreased in (A) F1 (PMS) and (D) F2 (PMS) offspring. Administration of oxytocin minimized the PMS-induced effect, decreased exon-III, and increased exon-IV levels in (A) F1 (PMS+OXT) and (D) F2 (PMS+OXT) offspring. Furthermore, the level of total Bdnf was decreased by PMS in the PMS F1 and PMS F2 offspring, and oxytocin administration reverse the PMS induced effect. Correlation analysis showed that the level of total Bdnf mRNA significantly associated reference (B,E), working memory (C,F) in F1 and F2 offspring. Data are represented as mean ± SE, and statistical significance is indicated by **p < 0.01; ***p < 0.001; NS, not significant.
A similar pattern was observed in F2 offspring, and a significant difference was detected between experimental groups with exon III, IV and exon-VI [F(2,53) = 4.45, P < 0.05]. Significant differences were observed between exon-III, IV and VI within the group [F(2,53) = 49.99, P < 0.001], and there was a significant interaction between group and exon-III/ IV/VI [F(2,53) = 52.81, P < 0.001]. Furthermore, we found that the level of exon-III was increased and that of exon-IV, and VI was decreased in PMS F2 offspring, but no significant difference was detected between exon-IV and VI in CtrlF2 or PMS F2, PMS+OXT F2 offspring (Figure 11D). However, a significant difference in total Bdnf was observed [F(2,17) = 88.56, P < 0.001]. We found that level of total Bdnf was significantly reduced PMS F2 (P < 0.001) compared to CtrlF2 and PMS+OXT F2, and there was significant difference between Ctrl F2 and PMS+OXT F2 (P < 0.001), and PMS F2 and PMS+OXT F2 (P < 0.001). Correlation analysis demonstrated a significant positive association between Bdnf level to reference memory (r = 0.638, p = 0.025) and working memory (r = 0.757, p = 0.004) in F2 offspring (Figures 11E,F).
Further, we examined whether differential levels of exon-III, IV and exon-VI- mRNA influence the translation of BDNF protein. Significant differences were found between the experimental groups in the F1 [F(2,17) = 127.144, P < 0.001] and F2 [F(2,17) = 42.556, P < 0.001] offspring. PMS significantly influenced pro-BDNF levels in PMS F1 and PMS F2 offspring, which were lower than those in CtrlF1, CtrlF2, PMS+OXT F1, and PMS+OXTF2 (P < 0.001) offspring; however, the difference between CtrlF1 vs. PMS+OXT F1 and Ctrl2 vs. PMS+OXT F2 was not significant (P > 0.05) (Supplementary Figures S7B,C). In addition, we found positive correlation between pro-BDNF with reference and working memory. The analysis showed that elevated level of pro-BDNF positively associated with reference memory (F1: r = 0.742, P < 0.001; F2: r = 0.88, P < 0.001), working memory (F1: r = 0.674, P < 0.001; F2: r = 0.84, P < 0.001) in F1 and F2 generation (Figures 12A–C, 13A,B). Similar impact was observed in mature BDNF, significant difference was observed between the experimental groups in F1 [F(2,17) = 252.53, P < 0.001] and F2 [F(2,17) = 60.57, P < 0.001]. Level of mature BDNF in PMS F1 and PMS F2 offspring was significantly lower than CtrlF1, CtrlF2, PMS+OXT F1, and PMS+OXTF2 (P < 0.001) offspring, and there was a difference between Ctrl F1 vs. PMS+OXT F1 (P < 0.001), but not in F2 offspring Ctrl F2 vs. PMS+OXT F2 was not significant (P > 0.05) (Supplementary Figures S7D,E). Similar to pro-BDNF, level of mature BDNF positively associated with reference memory (F1: r = 0.312, P < 0.05; F2: r = 0.86, P < 0.001), working memory (F1: r = 0.28, P > 0.05; F2: r = 0.81, P < 0.001) in F1 and F2 generation (Figures 12D,E, 13C,D). The results suggest that PMS influences the expression of exon-III exon-IV, exon-VI variations, which alter the level of pro-and mature BDNF protein. Observed reference, working memory could be correlated with the level of pro-and mature BDNF.
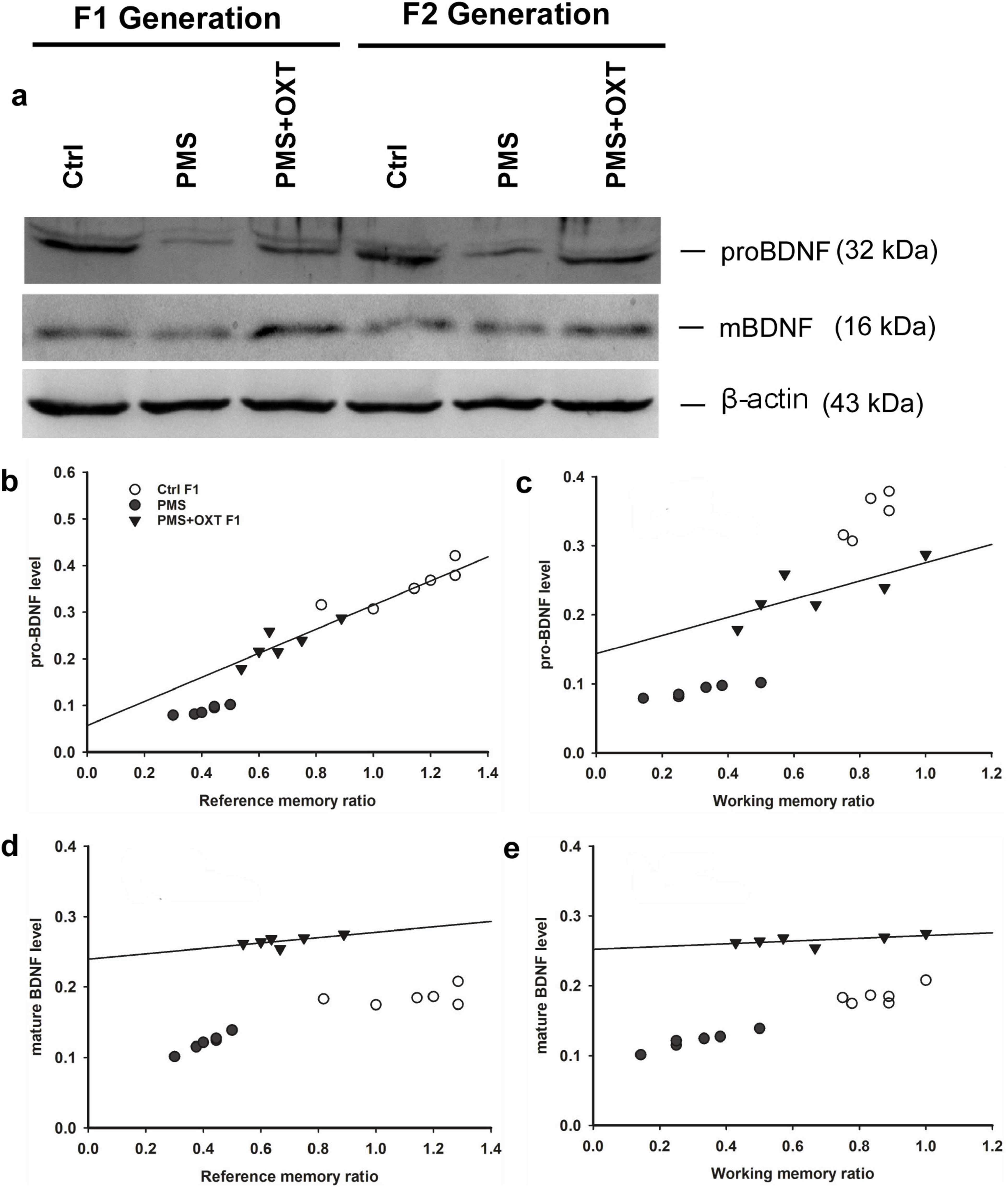
Figure 12. Effect of prenatal maternal stress (PMS) alter the level of pro-BDNF and mature BDNF in the F1 and F2 generations. (A) Representative western blots showing the expression levels of pro-and mature BDNF in the F1 and F2. The analysis showed that PMS significantly reduced the level of pro-and mature BDNF in F1 offspring, which is positively correlated with reference (B,D), working memory (C,E) in F1 offspring. Administration of oxytocin minimized the PMS-induced effect, restored the level of pro-and mature BDNF in F1 (PMS+OXT) and F2 (PMS+OXT) offspring.
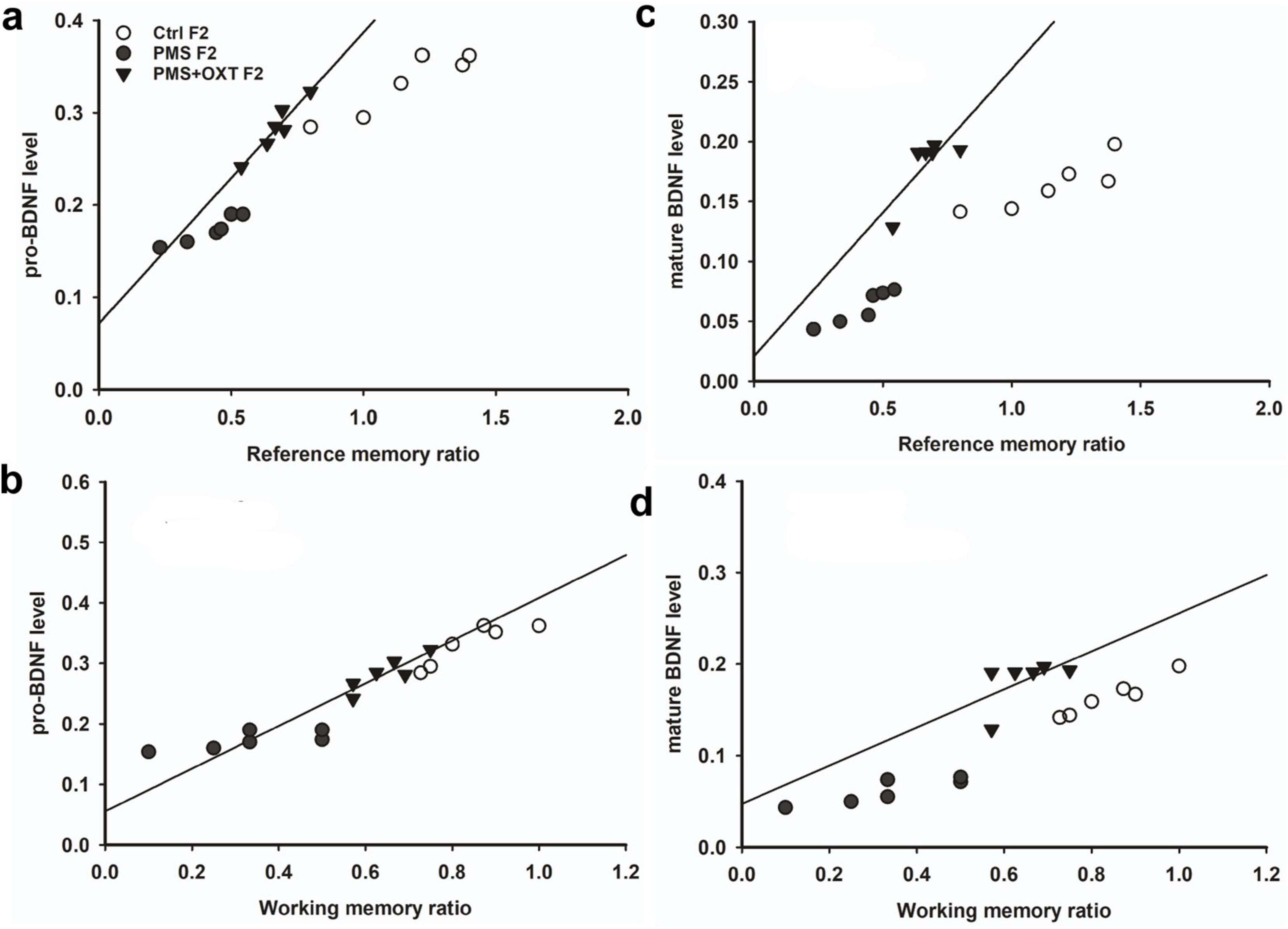
Figure 13. Effect of prenatal maternal stress (PMS) alter the level of pro-BDNF and mature BDNF in the F1 and F2 generations. (A) The analysis showed that PMS significantly reduced the level of pro-and mature BDNF in F2 offspring, which is positively correlated with reference (B,D), working memory (C,E) in F2 offspring. Administration of oxytocin minimized the PMS-induced effect, restored the level of pro-and mature BDNF in F1 (PMS+OXT) and F2 (PMS+OXT) offspring.
4 Discussion
Recent studies have shown that many environmental, social and metabolic stressors affect the next generation(s) physiology and behavior. The long-term effects of maternal stress are not well established, particularly in offspring (McDonald et al., 2023). Therefore, the present study aimed to examine maternal line-mediated transgenerational epigenetic inheritance in an animal model of prenatal maternal stress. Indeed, we found that reference and working memory were reduced in PMSF1 and PMSF2 offspring, which may be associated with the effect of maternal stress (F0) on F1 offspring (Grundwald and Brunton, 2015; McDonald et al., 2023). Oxytocin treatment rescued reference and working memory deficit in PMS+OXT F1 and PMS+OXT F2 offspring. Oxytocin exposure possibly facilitate the interaction between oxytocin and CORT possibly regulate the stress response (Matsushita et al., 2019; Young et al., 2021), inhibition of maternal CORT response to stressors possibly attenuates or reduces several maternal stress induced effects in offspring (Ma et al., 2020; Thirtamara Rajamani et al., 2024), thus, the oxytocin exposed offspring showed improved memory. Furthermore, the observed memory deficits in the PMS F1/PMS F2 offspring could be linked to freezing. Our observations are consistent with earlier reports that documented that exposure to oxytocin exerts antidepressant and anxiolytic effects (Naja and Aoun, 2017; Yoon and Kim, 2022). Early life stress has been known to induce functional and developmental changes in the amygdala, i.e., dynamic changes in morphology, volume, cell proliferation and other physiology (Kraszpulski et al., 2006; Fareri and Tottenham, 2016). Extensive studies in the amygdala suggest that the activation of oxytocin exerts inhibitory effects within the amygdala through GABAergic interneuron to decrease anxiety, stress and facilitate social behavior (Labuschage et al., 2010). Furthermore, activation of the oxytocin receptor specifically in amygdala facilitate a range of social behavior (Fam et al., 2018, 2024) and regulates both bottom-up and top-down emotion (Xin et al., 2020). Therefore, we examined PMS/oxytocin treatment mediated epigenetic changes in the amygdala in this study. The induction of CORT by PMS may be a critical factor in the observed freezing and memory deficit (Conrad et al., 2024). We found that CORT levels were higher in PMS F1, PMS F2, than CtrlF1, CtrlF2, PMS+OXT F1, and PMS+OXT F2. The amygdala is a key integrating region that connects the emotional, endocrine and autonomic responses to stress. In addition, daily circulating levels of CORT in the amygdala in response to stress induce anxiety-like behavior and delivery of corticosterone to the amygdala after stress prevents stress induced effects (Venkova et al., 2010; Chakraborty et al., 2020). Thus, the observed higher level of CORT in PMS F1 offspring could be associated with in utero transmission of stress from the mother (F0) (Erisman et al., 1990; Bingham et al., 2013) and then from PMS F1 to PMS F2 offspring. The interplay between OXT and CRH reveals that OXT treatment elicits stress resilience and may minimize long-term effects in individuals (Li et al., 2019; Zhang et al., 2023). Therefore, the CORT level was reduced in the PMS+OXT F1 and PMS+OXT F2 groups, which showed less freezing and improved memory. Maternal stress is known to alter developmental programs, including epigenetic memory, and generate transgenerational stress lineages and behavioral phenotypes (Darnaudéry and Maccari, 2008; Ambeskovic et al., 2019). Epigenetic modification is a critical molecular mechanism that regulates developmental, cellular, and biological functions by tightly controlling gene expression (Moisiadis et al., 2017). At this point, global H3K4me2 and H3K4me3 methylation status is sensitive to social and environmental stressors (Lindeman et al., 2010). Specifically, maternal stress has been proposed to alter the epigenome, and induced changes can be transmitted across generations (Dion et al., 2022). Similarly, we found that PMS increased the levels of H3K4me2 and H3K4me3 in PMS F1 and PMS F2 offspring. Our observations provide additional support to recent clinical (Cardenas et al., 2019; Wiley et al., 2023; Dieckmann and Czamara, 2024), animal studies (Babenko et al., 2015; Grundwald and Brunton, 2015; Legoff et al., 2019), and suggest that observed memory deficit could be linked with PMS-induced methylation in F1 and transgenerational inheritance in F2 offspring. Oxytocin exposure reduced the levels of H3K4me2 and H3K4me3 in PMS+OXT F1 and PMS+OXT F2 offspring, and augmented oxytocin treatment to relieve the PMS-induced effect (Ma et al., 2020; Matsushita et al., 2019; Janeček and Dabrowska, 2019) and improve memory (Flanagan et al., 2018; Thirtamara Rajamani et al., 2024; Walia et al., 2024).
Oxytocin is known to regulate corticotropin-releasing hormone (CRH) and orchestrate feedback mechanisms of the stress response, neuronal circuitry, and synaptic plasticity (Janeček and Dabrowska, 2019). Stress-induced methylation of the CRH promoter is considered a key factor in the regulation of the HPA axis and is associated with transcriptional regulation of CORT (Zhou and Fang, 2018). We observed increased levels of H3K4me2 and H3K4me3 in the CRH promoter of PMS F1 and PMS F2 offspring compared to CtrlF1, CtrlF2/ PMS+OXT F1, and PMS+OXT F2 offspring. Elliott et al. (2010) reported that higher levels of CRH mRNA significantly associated with decreased promoter CpG methylation. However, recent studies have documented that DNA methylation not always silence the expression of genes, depending on the exact methylation site in the genome and the associated downstream transcriptional regulations (Zhu et al., 2016; Chatzittofis et al., 2021). In this study, we observed that PMS increased H3K4me2, H3K4me3 and the expression of Crh mRNA. The observed methylation pattern and Crh mRNA level possibly by two different mechanism reported earlier, possibly hypermethylation in the CRH promoter at CpGs outside the cyclicAMP response element (CRE) site (Sterrenburg et al., 2011; Chen et al., 2012) or an undetectable site for methyl-CpG-binding protein (MeCP2) at the CRH promoter lead to increased Crh mRNA level (McGill et al., 2006; Bhave and Uht, 2017). PMS increases the level of H3K4me2 and H3K4me3 methylation in the CRH promoter through intergenerational inheritance in offspring PMS F1 and transgenerational inheritance in their PMS F2 offspring; thus, they display deficit in reference and working memory (Blaze and Roth, 2015; Fransquet et al., 2022; Alyamani et al., 2022). Furthermore, oxytocin treatment suppresses the release of CORT and activate the stress response system (Smith and Wang, 2014; Stevenson et al., 2023), which in turn reduces the methylation level in PMS+OXT F1 offspring and is transgenerationally inherited by PMS+OXT F2 offspring. Stressors are rapidly activate CRH neurons to release CRH as a stress response and regulate the release of CORT, which in turn regulates CRH through a feedback mechanism (Kim et al., 2019) involving H3K4me2 and H3K4me3. We found a significant correlation between the levels of H3K4me2 and H3K4me3 in the CRH promoter and the level of Crh mRNA in F1 and F2 offspring, which could be linked to their behavioral phenotype (Zhou and Fang, 2018; Kassotaki et al., 2021; Alyamani et al., 2022). Previous studies have documented that Crh predominantly acts through Crh receptors (Crhr1/ Crhr2) to produce anxiety/depression-like behaviors (Bakshi et al., 2002; Paretkar and Dimitrov, 2018) and its antagonist resilience stress (Holsboer and Ising, 2008; Menke, 2024). The roles of Crhr1 and Crhr2 in regulating anxiety/anxiolytic behavior are unclear; Crhr1 mediates anxiety—like behavior (Müller et al., 2003; Wang et al., 2012), and its knockdown reduces anxiety—like behavior (Smith et al., 1998).
In line with this, we found that the levels of Crhr1 and Crhr2 mRNA were higher in PMS F1 and PMS F2 offspring, and activated Crhr1 and Crhr2 in PMS F1; PMS F2 offspring might exert stress and reduce reference and working memory (Greetfeld et al., 2009; Pournajafi-Nazarloo et al., 2009). CRH regulates positive/negative feedback mechanisms through its receptors and mediates the stress response/adaptive plasticity, in which BDNF is also involved (Hu et al., 2020; Kussainova et al., 2022). Notably, stress can induce long-lasting alterations through different programs, including the induction of splice variants (St-Cyr and McGowan, 2015), and alter Bdnf splice variants, synaptic plasticity, and behavior. Similar to other animal models (Chen et al., 2019; Begni et al., 2020; Kamaladevi and Rajan, 2023), PMS differentially alters Bdnf variants. HPA axis activation is known to regulate the transcription of Bdnf (Yuluğ et al., 2009), level of stress and CORT can selectively regulate the transcription of Bdnf exons (Murgatroyd et al., 2009; Hansson et al., 2006). The level of Bdnf exon-III mRNA was increased, but exon-IV and VI mRNA were reduced in PMS F1 and PMS F2 offspring compared to those in CtrlF1, CtrlF2/ PMS+OXT F1, and PMS+OXT F2 offspring Exon-III/IV variation is known to critically control the Bdnf total transcript and subsequently the level of protein (Tsankova et al., 2006; Nair et al., 2007). In contrast, exon-VI transcripts are transported to the distal dendritic compartments and released in response to stress/stimuli to regulate neuronal activity, while exon transcripts III and IV are localized in the soma and proximal region (Chiaruttini et al., 2009). A recent study demonstrate that stress induced epigenetic changes in the BDNF promoter reduced the transcripts IV and VI. This has been recognized as a key transcript in maintaining Bdnf levels and secretion at synapses, which may be correlated with plasticity and behavior (Mallei et al., 2015). In line with this, the levels of both pro-BDNF and mature BDNF were significantly lower in the PMS F1 and PMS F2 offspring than in the CtrlF1, CtrlF2/ PMS+OXT F1 and PMS+OXT F2 offspring. BDNF regulates a wide range of functions. Previous studies have documented that the intracellular conversion of pro-BDNF to mature BDNF is controversial and differs with developmental time points (Matsumoto et al., 2008; Yang et al., 2014). However, upon secretion into the extracellular matrix to mediate their effects, pro-and mature BDNF interact with their cognate receptors tropomyosin, related kinase receptor B (TrkB) and p75 neurotrophin receptor (p75NTR), respectively (Thomas and Davies, 2005). Their interaction effectively regulate long-term potentiation (LTP) (Pang et al., 2016), inhibits of GABAergic transmission (Riffault et al., 2014) and supports synaptic plasticity, learning and memory (Sakata et al., 2010; Orefice et al., 2013; Zheng et al., 2016). Thus, the observed changes in BDNF levels in the F1 and F2 offspring could be linked to their behavioral phenotypes (Lu et al., 2008; Jaehne et al., 2023; Persaud and Cates, 2023).
5 Conclusion
The present study summarizes that PMS induces heritable changes in global H3K4me2 and H3K4me3 methylation, which affect the level of methylation in the CRH promoter. Upregulation of Crh and its receptor was accompanied by methylation of the CRH promoter, whereas upregulation of CRH caused a decrease in BDNF protein expression by differentially regulating Bdnf exon-III, IV and VI transcripts. Furthermore, oxytocin exposure inhibits PMS induced effects that regulate synaptic plasticity, learning, and memory. Our data suggest that F0 stress alters the methylation status of H3K4me2 and H3K4me3 in F1 offspring, and altered epigenetic changes are transgenerationally inherited from F1 to F2 offspring through the maternal line. We did not examine maternal (F0) stress response or behavior, and did not compare it with male offspring, which is a limitation of our study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA, India) and all the protocols are approved (BDU/IAEC/P23/2022 dated 25.08.2022) by Institutional Animal Ethical Committee (IAEC), Bharathidasan University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
PDAD: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. KER: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was partially supported by the Department of Science and Technology, Government of India, through a major project (EMR/2016/005217 March-2018), and RUSA 2.0 to KER. PDAD received research support from Savitribai Jyotirao Phule Fellowship for PhD (File No. 09/475(0204)/2021-EMR-I) from the Government of India, India.
Acknowledgments
We acknowledge the RUSA 2.0 Biological Sciences and the Department of Science and Technology (DST)-Fund for Improvement of S&T Infrastructure (FIST) for supporting the Department of Animal Science, Bharathidasan University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2025.1518046/full#supplementary-material
References
Alcántara-Alonso, V., García-Luna, C., Soberanes-Chávez, P., Estrada-Camarena, E., and de Gortari, P. (2024). Two Adverse early life events induce differential changes in brain CRH and serotonin systems in rats along with Hyperphagia and depression. J. Integr. Neurosci. 23:41. doi: 10.31083/j.jin2302041
Alyamani, R., Nephew, B., and Murgatroyd, C. (2022). Intergenerational changes in hippocampal transcription in an animal model of maternal depression. Eur. J. Neurosci. 55, 2242–2252. doi: 10.1111/ejn.15180
Ambeskovic, M., Babenko, O., Ilnytskyy, Y., Kovalchuk, I., Kolb, B., and Metz, G. A. S. (2019). Ancestral stress alters lifetime mental health trajectories and cortical neuromorphology via epigenetic regulation. Sci. Rep. 9:6389. doi: 10.1038/s41598-019-42691-z
Babenko, O., Kovalchuk, I., and Metz, G. A. (2015). Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci. Biobehav. Rev. 48, 70–91. doi: 10.1016/j.neubiorev.2014.11.013
Bagley, J. R., Adams, J., Bozadjian, R. V., Bubalo, L., and Kippin, T. E. (2019). Strain differences in maternal neuroendocrine and behavioral responses to stress and the relation to offspring cocaine responsiveness. Int. J. Dev. Neurosci. 78, 130–138. doi: 10.1016/j.ijdevneu.2019.06.009
Bakshi, V. P., Smith-Roe, S., Newman, S. M., Grigoriadis, D. E., and Kalin, N. H. (2002). Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J. Neurosci. 22, 2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002
Balmus, I. M., Lefter, R., Ciobica, A., Antioch, I., Ababei, D., and Dobrin, R. (2018). Preliminary data on some behavioral changes induced by short-term intraperitoneal oxytocin administration in aged rats. Psychiatria Danubina 30, 91–98. doi: 10.24869/psyd.2018.91
Begni, V., Sanson, A., Pfeiffer, N., Brandwein, C., Inta, D., Talbot, S. R., et al. (2020). Social isolation in rats: Effects on animal welfare and molecular markers for neuroplasticity. PLoS One 15:e0240439. doi: 10.1371/journal.pone.0240439
Bhave, S. A., and Uht, R. M. (2017). CpG methylation and the methyl CpG binding protein 2 (MeCP2) are required for restraining corticotropin releasing hormone (CRH) gene expression. Mol. Cell. Endocrinol. 454, 158–164. doi: 10.1016/j.mce.2017.06.024
Bingham, B. C., Sheela Rani, C. S., Frazer, A., Strong, R., and Morilak, D. A. (2013). Exogenous prenatal corticosterone exposure mimics the effects of prenatal stress on adult brain stress response systems and fear extinction behavior. Psychoneuroendocrinology 38, 2746–2757. doi: 10.1016/j.psyneuen.2013.07.003
Blaze, J., and Roth, T. L. (2015). Evidence from clinical and animal model studies of the long-term and transgenerational impact of stress on DNA methylation. Semin. Cell Dev. Biol. 43, 76–84. doi: 10.1016/j.semcdb.2015.04.004
Boissier, J. R., Simon, P., and lwoff, J. M. (1964). Use of a particular mouse reaction (Hole Board method) for the study of psychotropic drugs. Therapie 19, 571–583.
Bruijniks, S. J. E., van Grootheest, G., Cuijpers, P., de Kluiver, H., Vinkers, C. H., Peeters, F., et al. (2020). Working memory moderates the relation between the brain-derived neurotropic factor (BDNF) and psychotherapy outcome for depression. J. Psychiatric Res. 130, 424–432. doi: 10.1016/j.jpsychires.2020.07.045
Buijs, R. M. (1978). Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell. Tissue. Res. 192, 423–435. doi: 10.1007/BF00212323
Cánepa, E. T., and Berardino, B. G. (2024). Epigenetic mechanisms linking early-life adversities and mental health. Biochem. J. 481, 615–642. doi: 10.1042/BCJ20230306
Carbone, E. T., Kass, P. H., and Evans, K. D. (2016). Feasibility of using rice hulls as bedding for laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 55, 268–276.
Cardenas, A., Faleschini, S., Cortes Hidalgo, A., Rifas-Shiman, S. L., Baccarelli, A. A., DeMeo, D. L., et al. (2019). Prenatal maternal antidepressants, anxiety, and depression and offspring DNA methylation: Epigenome-wide associations at birth and persistence into early childhood. Clin. Epigenet. 11:56. doi: 10.1186/s13148-019-0653-x
Chakraborty, P., Datta, S., McEwen, B., and Chattarji, S. (2020). Corticosterone after acute stress prevents the delayed effects on the amygdala. Neuropsychopharmacology 45, 2139–2146. doi: 10.1038/s41386-020-0758-0
Chatzittofis, A., Boström, A. D. E., Ciuculete, D. M., Öberg, K. G., Arver, S., Schiöth, H. B., et al. (2021). HPA axis dysregulation is associated with differential methylation of CpG-sites in related genes. Sci. Rep. 11:20134. doi: 10.1038/s41598-021-99714-x
Chaves, T., Fazekas, C. L., Horváth, K., Correia, P., Szabó, A., Török, B., et al. (2021). Stress adaptation and the brainstem with focus on corticotropin releasing hormone. Int. J. Mol. Sci. 22:9090. doi: 10.3390/ijms22169090
Chen, H., Amazit, L., Lombès, M., and Le Menuet, D. (2019). Crosstalk between glucocorticoid receptor and early-growth response Protein 1 accounts for repression of brain-derived neurotrophic factor transcript 4Expression. Neuroscience 399, 12–27. doi: 10.1016/j.neuroscience.2018.12.012
Chen, J., Evans, A. N., Liu, Y., Honda, M., Saavedra, J. M., and Aguilera, G. (2012). Maternal deprivation in rats is associated with corticotrophin-releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood. J. Neuroendocrinol. 24, 1055–1064. doi: 10.1111/j.1365-2826.2012.02306.x
Chiaruttini, C., Vicario, A., Li, Z., Baj, G., Braiuca, P., Wu, Y., et al. (2009). Dendritic trafficking of BDNF mRNA is mediated by translin and blocked by the G196A (Val66Met) mutation. Proc. Natl. Acad. Sci. U S Am. 106, 16481–16486. doi: 10.1073/pnas.0902833106
Colliva, A., and Tongiorgi, E. (2021). Distinct role of 5′UTR sequences in dendritic trafficking of BDNF mRNA: Additional mechanisms for the BDNF splice variants spatial code. Mol. Brain 14:10. doi: 10.1186/s13041-020-00680-8
Conrad, C. D., Peay, D. N., Acuña, A. M., Whittaker, K., and Donnay, M. E. (2024). Corticosterone disrupts spatial working memory during retention testing when highly taxed, which positively correlates with depressive-like behavior in middle-aged, ovariectomized female rats. Hormones Behav. 164:105600. doi: 10.1016/j.yhbeh.2024.105600
Coussons-Read, M. E. (2013). Effects of prenatal stress on pregnancy and human development: Mechanisms and pathways. Obstetric Med. 6, 52–57. doi: 10.1177/1753495X12473751
Darnaudéry, M., and Maccari, S. (2008). Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res. Rev. 57, 571–585. doi: 10.1016/j.brainresrev.2007.11.004
Dieckmann, L., and Czamara, D. (2024). Epigenetics of prenatal stress in humans: The current research landscape. Clin. Epigenet. 16:20. doi: 10.1186/s13148-024-01635-9
Dion, A., Muñoz, P. T., and Franklin, T. B. (2022). Epigenetic mechanisms impacted by chronic stress across the rodent lifespan. Neurobiol. Stress 17:100434. doi: 10.1016/j.ynstr.2022.100434
Dong, E., and Pandey, S. C. (2021). Prenatal stress induced chromatin remodeling and risk of psychopathology in adulthood. Int. Rev. Neurobiol. 156, 185–215. doi: 10.1016/bs.irn.2020.08.004
Elliott, E., Ezra-Nevo, G., Regev, L., Neufeld-Cohen, A., and Chen, A. (2010). Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat. Neurosci. 13, 1351–1353. doi: 10.1038/nn.2642
Erisman, S., Carnes, M., Takahashi, L. K., and Lent, S. J. (1990). The effects of stress on plasma ACTH and corticosterone in young and aging pregnant rats and their fetuses. Life Sci. 47, 1527–1533. doi: 10.1016/0024-3205(90)90181-p
Fam, J., Holmes, N., and Westbrook, R. F. (2024). Stimulating oxytocin receptors in the basolateral amygdala enhances stimulus processing: Differential and consistent effects for stimuli paired with fear versus sucrose in extinction and reversal learning. Psychoneuroendocrinology 160:106917. doi: 10.1016/j.psyneuen.2023.106917
Fam, J., Holmes, N., Delaney, A., Crane, J., and Westbrook, R. F. (2018). Oxytocin receptor activation in the basolateral complex of the amygdala enhances discrimination between discrete cues and promotes configural processing of cues. Psychoneuroendocrinology 96, 84–92. doi: 10.1016/j.psyneuen.2018.06.006
Fareri, D. S., and Tottenham, N. (2016). Effects of early life stress on amygdala and striatal development. Developmental Cogn. Neurosci. 19, 233–247. doi: 10.1016/j.dcn.2016.04.005
Flanagan, J. C., Hand, A., Jarnecke, A. M., Moran-Santa Maria, M. M., Brady, K. T., and Joseph, J. E. (2018). Effects of oxytocin on working memory and executive control system connectivity in posttraumatic stress disorder. Exp. Clin. Psychopharmacol. 26, 391–402. doi: 10.1037/pha0000197
Fransquet, P. D., Hjort, L., Rushiti, F., Wang, S. J., Krasniqi, S. P., Çarkaxhiu, S. I., et al. (2022). DNA methylation in blood cells is associated with cortisol levels in offspring of mothers who had prenatal posttraumatic stress disorder. Stress Health J. Int. Soc. Investigation Stress 38, 755–766. doi: 10.1002/smi.3131
Fuge, P., Aust, S., Fan, Y., Weigand, A., Gärtner, M., Feeser, M., et al. (2014). Interaction of early life stress and corticotropin-releasing hormone receptor gene: Effects on working memory. Biol. Psychiatry 76, 888–894. doi: 10.1016/j.biopsych.2014.04.016
Gimpl, G., and Fahrenholz, F. (2001). The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 81, 629–683. doi: 10.1152/physrev.2001.81.2.629
Greetfeld, M., Schmidt, M. V., Ganea, K., Sterlemann, V., Liebl, C., and Müller, M. B. (2009). A single episode of restraint stress regulates central corticotrophin- releasing hormone receptor expression and binding in specific areas of the mouse brain. J. Neuroendocrinol. 21, 473–480. doi: 10.1111/j.1365-2826.2009.01865.x
Grundwald, N. J., and Brunton, P. J. (2015). Prenatal stress programs neuroendocrine stress responses and affective behaviors in second generation rats in a sex-dependent manner. Psychoneuroendocrinology 62, 204–216. doi: 10.1016/j.psyneuen.2015.08.010
Gulevich, R., Kozhemyakina, R., Shikhevich, S., Konoshenko, M., and Herbeck, Y. (2019). Aggressive behavior and stress response after oxytocin administration in male Norway rats selected for different attitudes to humans. Physiol. Behav. 199, 210–218. doi: 10.1016/j.physbeh.2018.11.030
Hansson, A. C., Sommer, W. H., Metsis, M., Strömberg, I., Agnati, L. F., and Fuxe, K. (2006). Corticosterone actions on the hippocampal brain-derived neurotrophic factor expression are mediated by exon IV promoter. J. Neuroendocrinol. 18, 104–114. doi: 10.1111/j.1365-2826.2005.01390.x
Herman, J. P., and Cullinan, W. E. (1997). Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 20, 78–84. doi: 10.1016/s0166-2236(96)10069-2
Holsboer, F., and Ising, M. (2008). Central CRH system in depression and anxiety-evidence from clinical studies with CRH1 receptor antagonists. Eur. J. Pharmacol. 583, 350–357. doi: 10.1016/j.ejphar.2007.12.032
Hu, P., Maita, I., Phan, M. L., Gu, E., Kwok, C., Dieterich, A., et al. (2020). Early-life stress alters affective behaviors in adult mice through persistent activation of CRH-BDNF signaling in the oval bed nucleus of the stria terminalis. Transl. Psychiatry 10:396. doi: 10.1038/s41398-020-01070-3
Hunter, R. G., McCarthy, K. J., Milne, T. A., Pfaff, D. W., and McEwen, B. S. (2009). Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc. Natl. Acad. Sci. U S Am. 106, 20912–20917. doi: 10.1073/pnas.0911143106
Inda, C., Armando, N. G., Dos Santos, Claro, P. A., and Silberstein, S. (2017). Endocrinology and the brain: Corticotropin-releasing hormone signaling. Endocrine Connect. 6, R99–R120. doi: 10.1530/EC-17-0111
Jaehne, E. J., Kent, J. N., Lam, N., Schonfeld, L., Spiers, J. G., Begni, V., et al. (2023). Chronic running-wheel exercise from adolescence leads to increased anxiety and depression-like phenotypes in adulthood in rats: Effects on stress markers and interaction with BDNF Val66Met genotype. Developmental Psychobiol. 65:e22347. doi: 10.1002/dev.22347
Janeček, M., and Dabrowska, J. (2019). Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies-potential interaction with the corticotropin-releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST). Cell Tissue Res. 375, 143–172. doi: 10.1007/s00441-0182889-8
Jeanneteau, F. D., Lambert, W. M., Ismaili, N., Bath, K. G., Lee, F. S., Garabedian, M. J., et al. (2012). BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc. Natl. Acad. Sci. U S Am. 109, 1305–1310. doi: 10.1073/pnas.1114122109
Kamaladevi, A., and Rajan, K. E. (2023). Antibiotic treatment during post-natal reverses behavioural and molecular alterations in experimental meningitis survivor rat model. Neurotoxicol. Teratol. 97:107178. doi: 10.1016/j.ntt.2023.107178
Kassotaki, I., Valsamakis, G., Mastorakos, G., and Grammatopoulos, D. K. (2021). Placental CRH as a signal of pregnancy adversity and impact on fetal neurodevelopment. Front. Endocrinol. 12:714214. doi: 10.3389/fendo.2021.714214
Kim, H. J., Ko, E. A., Kwon, O. B., and Jung, S. C. (2024). Prenatal treatment with corticosterone via maternal injection induces learning and memory impairments via delaying postsynaptic development in hippocampal CA1 neurons of rats. J. Neurosci. Res. 102:e25323. doi: 10.1002/jnr.25323
Kim, J. S., Han, S. Y., and Iremonger, K. J. (2019). Stress experience and hormone feedback tune distinct components of hypothalamic CRH neuron activity. Nat. Commun. 10:5696. doi: 10.1038/s41467-019-13639-8
Knobloch, H. S., Charlet, A., Hoffmann, L. C., Eliava, M., Khrulev, S., Cetin, A. H., et al. (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553–566. doi: 10.1016/j.neuron.2011.11.030
Kraszpulski, M., Dickerson, P. A., and Salm, A. K. (2006). Prenatal stress affects the developmental trajectory of the rat amygdala. Stress (Amsterdam, Netherlands) 9, 85–95. doi: 10.1080/10253890600798109
Kuc, K. A., Gregersen, B. M., Gannon, K. S., and Dodart, J. C. (2006). Holeboard discrimination learning in mice. Genes Brain Behav. 5, 355–363. doi: 10.1111/j.1601183X.2005.00168.x
Kussainova, A., Kassym, L., Akhmetova, A., Dvoryankova, E., Glushkova, N., Khismetova, Z., et al. (2022). Associations between serum levels of brain-derived neurotrophic factor, corticotropin releasing hormone and mental distress in vitiligo patients. Sci. Rep. 12:7260. doi: 10.1038/s41598-022-11028-8
Labuschagne, I., Phan, K. L., Wood, A., Angstadt, M., Chua, P., Heinrichs, M., et al. (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 35, 2403–2413. doi: 10.1038/npp.2010.12
Lee, P. R., Brady, D. L., Shapiro, R. A., Dorsa, D. M., and Koenig, J. I. (2005). Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology 30, 1883–1894. doi: 10.1038/sj.npp.1300722
Lee, S. Y., Park, S. H., Chung, C., Kim, J. J., Choi, S. Y., and Han, J. S. (2015). Oxytocin protects hippocampal memory and plasticity from uncontrollable stress. Sci. Rep. 5:18540. doi: 10.1038/srep18540
Legoff, L., D’Cruz, S. C., Tevosian, S., Primig, M., and Smagulova, F. (2019). Transgenerational inheritance of environmentally induced epigenetic alterations during mammalian development. Cells 8:1559. doi: 10.3390/cells8121559
Li, Y., Hassett, A. L., and Seng, J. S. (2019). Exploring the mutual regulation between oxytocin and cortisol as a marker of resilience. Arch. Psychiatric Nurs. 33, 164–173. doi: 10.1016/j.apnu.2018.11.008
Lindeman, L. C., Winata, C. L., Aanes, H., Mathavan, S., Alestrom, P., and Collas, P. (2010). Chromatin states of developmentally-regulated genes revealed by DNA and histone methylation patterns in zebrafish embryos. Int. J. Developmental Biol. 54, 803–813. doi: 10.1387/ijdb.103081ll
Lu, Y., Christian, K., and Lu, B. (2008). BDNF: A key regulator for protein synthesisdependent LTP and long-term memory? Neurobiol. Learn. Mem. 89, 312–323. doi: 10.1016/j.nlm.2007.08.018
Lundberg, S., Högman, C., and Roman, E. (2019). Adolescent exploratory strategies and behavioral types in the multivariate concentric square Field™ test. Front. Behav. Neurosci. 13:41. doi: 10.3389/fnbeh.2019.00041
Lundberg, S., Nylander, I., and Roman, E. (2020). Behavioral profiling in early adolescence and early adulthood of male wistar rats after short and prolonged maternal separation. Front. Behav. Neurosci. 14:37. doi: 10.3389/fnbeh.2020.00037
Ma, X., Wei, Q., Jiang, Z., Shi, Y., Zhang, Y., and Shi, H. (2020). The role of serum oxytocin levels in the second trimester in regulating prenatal anxiety and depression: A sample from Shanghai Maternal-Child Pairs Cohort study. J. Affect. Disord. 264, 150–156. doi: 10.1016/j.jad.2019.12.019
Mallei, A., Baj, G., Ieraci, A., Corna, S., Musazzi, L., Lee, F. S., et al. (2015). Expression and dendritic trafficking of BDNF-6 splice variant are impaired in knock-in mice carrying human BDNF Val66met polymorphism. Int. J. Neuropsychopharmacology. 18:pyv069. doi: 10.1093/ijnp/pyv069
Matsumoto, T., Rauskolb, S., Polack, M., Klose, J., Kolbeck, R., Korte, M., et al. (2008). Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat. Neurosci. 11, 131–133. doi: 10.1038/nn2038
Matsushita, H., Latt, H. M., Koga, Y., Nishiki, T., and Matsui, H. (2019). Oxytocin and stress: Neural mechanisms, stress-related disorders, and therapeutic approaches. Neuroscience 417, 1–10. doi: 10.1016/j.neuroscience.2019.07.046
McDonald, R. J., Hong, N. S., Trow, J. S., Kaupp, C., Balog, R. J., Gokarn, L., et al. (2023). Effects of maternal social isolation on adult rodent offspring cognition. Sci. Rep. 13:7748. doi: 10.1038/s41598-023-34834-0
McGill, B. E., Bundle, S. F., Yaylaoglu, M. B., Carson, J. P., Thaller, C., and Zoghbi, H. Y. (2006). Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U S Am. 103, 18267–18272. doi: 10.1073/pnas.0608702103
Menke, A. (2024). The HPA axis as target for depression. Curr. Neuropharmacol. 22, 904–915. doi: 10.2174/1570159X21666230811141557
Moelling, K. (2024). Epigenetics and transgenerational inheritance. J. Physiol. 602, 2537–2545. doi: 10.1113/JP284424
Moisiadis, V. G., Constantinof, A., Kostaki, A., Szyf, M., and Matthews, S. G. (2017). Prenatal glucocorticoid exposure modifies endocrine function and behaviour for 3 generations following maternal and paternal transmission. Sci. Rep. 7:11814. doi: 10.1038/s41598-017-11635-w
Müller, M. B., Zimmermann, S., Sillaber, I., Hagemeyer, T. P., Deussing, J. M., Timpl, P., et al. (2003). Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat. Neurosci. 6, 1100–1107. doi: 10.1038/nn1123
Murgatroyd, C., Patchev, A. V., Wu, Y., Micale, V., Bockmühl, Y., Fischer, D., et al. (2009). Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 12, 1559–1566. doi: 10.1038/nn.2436
Nair, A., Vadodaria, K. C., Banerjee, S. B., Benekareddy, M., Dias, B. G., Duman, R. S., et al. (2007). Stressor-specific regulation of distinct brain-derived neurotrophic factor transcripts and cyclic AMP response element-binding protein expression in the postnatal and adult rat hippocampus. Neuropsychopharmacology 32, 1504–1519. doi: 10.1038/sj.npp.1301276
Nair, B., and Wong-Riley, M. T. (2016). Transcriptional regulation of brain-derived neurotrophic factor coding exon IX: Role of nuclear respiratory factor 2. J. Biol. Chem. 291, 22583–22593. doi: 10.1074/jbc.M116.742304
Naja, W. J., and Aoun, M. P. (2017). Oxytocin and anxiety disorders: Translational and therapeutic aspects. Curr. Psychiatry Rep. 19:67. doi: 10.1007/s11920-017-0819-1
Neumann, I. D. (2008). Brain oxytocin: A key regulator of emotional and social behaviours in both females and males. J. Neuroendocrinol. 20, 858–865. doi: 10.1111/j.1365-2826.2008.01726.x
Neumann, I. D., Torner, L., and Wigger, A. (2000). Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience 95, 567–575. doi: 10.1016/s0306-4522(99)00433-9
Nomura, M., Saito, J., Ueta, Y., Muglia, L. J., Pfaff, D. W., and Ogawa, S. (2003). Enhanced up-regulation of corticotropin-releasing hormone gene expression in response to restraint stress in the hypothalamic paraventricular nucleus of oxytocin gene-deficient male mice. J. Neuroendocrinol. 15, 1054–1061. doi: 10.1046/j.1365-2826.2003.01095.x
Ohi, K., Fujikane, D., Takai, K., Kuramitsu, A., Muto, Y., Sugiyama, S., et al. (2024). Epigenetic signatures of social anxiety, panic disorders and stress experiences: Insights from genome-wide DNA methylation risk scores. Psychiatry Res. 337:115984. doi: 10.1016/j.psychres.2024.115984
Orefice, L. L., Waterhouse, E. G., Partridge, J. G., Lalchandani, R. R., Vicini, S., and Xu, B. (2013). Distinct roles for somatically and dendritically synthesized brain-derived neurotrophic factor in morphogenesis of dendritic spines. J. Neurosci. 33, 11618–11632. doi: 10.1523/JNEUROSCI.0012-13.2013
Pãdurariu, M., Balmus, M., Ciobica, A., Lefter, R., Cojocaru, S., Antioch, I., et al. (2018). Oxytocin administration improves memory, anxiety and some oxidative stress parameters in a methionine – induced rat model of schizophrenia. Farmacia 66, 421–431. doi: 10.31925/farmacia.2018.3.6
Pang, P. T., Nagappan, G., Guo, W., and Lu, B. (2016). Extracellular and intracellular cleavages of proBDNF required at two distinct stages of late-phase LTP. NPJ Sci. Learn. 1:16003. doi: 10.1038/npjscilearn.2016.3
Paretkar, T., and Dimitrov, E. (2018). The central amygdala corticotropin-releasing hormone (CRH) Neurons modulation of anxiety-like behavior and hippocampus-dependent memory in mice. Neuroscience 390, 187–197. doi: 10.1016/j.neuroscience.2018.08.019
Persaud, N. S., and Cates, H. M. (2023). The epigenetics of anxiety pathophysiology: A DNA Methylation and histone modification focused review. eNeuro 10:ENEURO.109–ENEURO.121. doi: 10.1523/ENEURO.0109-21.2021
Pournajafi-Nazarloo, H., Partoo, L., Sanzenbacher, L., Paredes, J., Hashimoto, K., Azizi, F., et al. (2009). Stress differentially modulates mRNA expression for corticotrophin-releasing hormone receptors in hypothalamus, hippocampus and pituitary of prairie voles. Neuropeptides 43, 113–112. doi: 10.1016/j.npep.2008.12.002
Riffault, B., Medina, I., Dumon, C., Thalman, C., Ferrand, N., Friedel, P., et al. (2014). Pro-brain-derived neurotrophic factor inhibits GABAergic neurotransmission by activating endocytosis and repression of GABAA receptors. J. Neurosci. 34, 13516–13534. doi: 10.1523/JNEUROSCI.2069-14.2014
Roque, A., Valles Méndez, K. M., Ruiz, R., Pineda, E., and Lajud, N. (2022). Early life stress induces a transient increase in hippocampal corticotropin-releasing hormone in rat neonates that precedes the effects on hypothalamic neuropeptides. Eur. J. Neurosci. 55, 2108–2121. doi: 10.1111/ejn.15193
Sakata, K., Jin, L., and Jha, S. (2010). Lack of promoter IV-driven BDNF transcription results in depression-like behavior. Genes Brain Behav. 9, 712–721. doi: 10.1111/j.1601-183X.2010.00605.x
Santilli, F., and Boskovic, A. (2023). Mechanisms of transgenerational epigenetic inheritance: Lessons from animal model organisms. Curr. Opin. Genet. Development 79:102024. doi: 10.1016/j.gde.2023.102024
Singh-Taylor, A., Molet, J., Jiang, S., Korosi, A., Bolton, J. L., Noam, Y., et al. (2018). NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Mol. Psychiatry 23, 648–657. doi: 10.1038/mp.2016.240
Sivasangari, K., and Rajan, K. E. (2020). Standardized Bacopa monnieri extract ameliorates learning and memory impairments through synaptic protein, neurogranin, pro-and mature BDNF Signaling, and HPA axis in prenatally stressed rat offspring. Antioxidants 9:1229. doi: 10.3390/antiox9121229
Sivasangari, K., Sivamaruthi, B. S., Chaiyasut, C., and Rajan, K. E. (2023). Maternal stress-induced changes in adolescent and adult offspring: Neurobehavioural improvement and telomere maintenance. Heliyon 9:e20385. doi: 10.1016/j.heliyon.2023.e20385
Smith, A. S., and Wang, Z. (2014). Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry 76, 281–288. doi: 10.1016/j.biopsych.2013.09.017
Smith, G. W., Aubry, J. M., Dellu, F., Contarino, A., Bilezikjian, L. M., Gold, L. H., et al. (1998). Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 20, 1093–1102. doi: 10.1016/s0896-6273(00)80491-2
St-Cyr, S., and McGowan, P. O. (2015). Programming of stress-related behavior and epigenetic neural gene regulation in mice offspring through maternal exposure to predator odor. Front. Behav. Neurosci. 9:145. doi: 10.3389/fnbeh.2015.00145
Sterrenburg, L., Gaszner, B., Boerrigter, J., Santbergen, L., Bramini, M., Elliott, E., et al. (2011). Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS One 6:e28128. doi: 10.1371/journal.pone.0028128
Stevenson, J. R., McMahon, E. K., McNeely, T. L., and Haussmann, M. F. (2023). Oxytocin prevents dysregulation of the acute stress response and glucocorticoid-induced oxidative stress in chronically isolated prairie voles. Psychoneuroendocrinology 153:106121. doi: 10.1016/j.psyneuen.2023.106121
Thirtamara Rajamani, K., Barbier, M., Lefevre, A., Niblo, K., Cordero, N., Netser, S., et al. (2024). Oxytocin activity in the paraventricular and supramammillary nuclei of the hypothalamus is essential for social recognition memory in rats. Mol. Psychiatry 29, 412–424. doi: 10.1038/s41380-023-02336-0
Thomas, K., and Davies, A. (2005). Neurotrophins: A ticket to ride for BDNF. Curr. Biol. 15, R262–R264. doi: 10.1016/j.cub.2005.03.023
Treit, D., and Fundytus, M. (1988). Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol. Biochem. Behav. 31, 959–962.
Tsankova, N. M., Berton, O., Renthal, W., Kumar, A., Neve, R. L., and Nestler, E. J. (2006). Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 9, 519–525. doi: 10.1038/nn1659
Venkova, K., Johnson, A., Myers, B., and Greenwood-Van Meerveld, B. (2010). Exposure of the amygdala to elevated levels of corticosterone alters colonic motility in response to acute psychological stress. Neuropharmacology 58, 1161–1167. doi: 10.1016/j.neuropharm.2010.02.012
Viviani, D., Charlet, A., van den Burg, E., Robinet, C., Hurni, N., Abatis, M., et al. (2011). Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333, 104–107. doi: 10.1126/science.1201043
Walia, V., Wal, P., Mishra, S., Agrawal, A., Kosey, S., and Dilipkumar Patil, A. (2024). Potential role of oxytocin in the regulation of memories and treatment of memory disorders. Peptides 177:171222. doi: 10.1016/j.peptides.2024.171222
Wang, X. D., Labermaier, C., Holsboer, F., Wurst, W., Deussing, J. M., Müller, M. B., et al. (2012). Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1. Eur. J. Neurosci. 36, 2360–2367. doi: 10.1111/j.1460-9568.2012.08148.x
Wiley, K. S., Camilo, C., Gouveia, G., Euclydes, V., Panter-Brick, C., Matijasevich, A., et al. (2023). Maternal distress, DNA methylation, and fetal programing of stress physiology in Brazilian mother-infant pairs. Developmental Psychobiol. 65:e22352. doi: 10.1002/dev.22352
Winter, J., and Jurek, B. (2019). The interplay between oxytocin and the CRF system: Regulation of the stress response. Cell Tissue Res. 375, 85–91. doi: 10.1007/s00441-018-2866-2
Xin, F., Zhou, X., Dong, D., Zhao, Z., Yang, X., Wang, Q., et al. (2020). Oxytocin differentially modulates amygdala responses during top-down and bottom-up aversive anticipation. Adv. Sci. 7:2001077. doi: 10.1002/advs.202001077
Yang, J., Harte-Hargrove, L. C., Siao, C. J., Marinic, T., Clarke, R., Ma, Q., et al. (2014). proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep. 7, 796–806. doi: 10.1016/j.celrep.2014.03.040
Yoon, S., and Kim, Y. K. (2022). Possible oxytocin-related biomarkers in anxiety and mood disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 116:110531. doi: 10.1016/j.pnpbp.2022.110531
Young, K. S., Pressman, S. D., Celniker, J., Grewen, K. M., Sumida, K. D., Jonathan, N., et al. (2021). Oxytocin, cortisol, and cognitive control during acute and naturalistic stress. Stress 24, 370–383. doi: 10.1080/10253890.2021.1876658
Yuluğ, B., Ozan, E., Gönül, A. S., and Kilic, E. (2009). Brain-derived neurotrophic factor, stress and depression: A minireview. Brain Res. Bull. 78, 267–269. doi: 10.1016/j.brainresbull.2008.12.002
Zapala, M. A., Hovatta, I., Ellison, J. A., Wodicka, L., Del Rio, J. A., Tennant, R., et al. (2005). Adult mouse brain gene expression patterns bear an embryologic imprint. Proc. Natl. Acad. Sci. U S Am. 102, 10357–10362. doi: 10.1073/pnas.0503357102
Zhang, S., Zhang, Y. D., Shi, D. D., and Wang, Z. (2023). Therapeutic uses of oxytocin in stress-related neuropsychiatric disorders. Cell Biosci. 13:216. doi: 10.1186/s13578-023-01173-6
Zheng, Y., Fan, W., Zhang, X., and Dong, E. (2016). Gestational stress induces depressive-like and anxiety-like phenotypes through epigenetic regulation of BDNF expression in offspring hippocampus. Epigenetics 11, 150–162. doi: 10.1080/15592294.2016.1146850
Zhou, J. N., and Fang, H. (2018). Transcriptional regulation of corticotropin-releasing hormone gene in stress response. IBRO Rep. 5, 137–146. doi: 10.1016/j.ibror.2018.08.003
Keywords: prenatal maternal stress, working memory, DNA methylation, corticosterone, oxytocin, brain-derived neurotrophic factor, transgenerational inheritance, epigenetic mechanism
Citation: Adeline Dorothy PD and Rajan KE (2025) Prenatal maternal life adversity impacts on learning and memory in offspring: implication to transgenerational epigenetic inheritance. Front. Neurosci. 19:1518046. doi: 10.3389/fnins.2025.1518046
Received: 27 October 2024; Accepted: 22 January 2025;
Published: 13 February 2025.
Edited by:
Lucia Carboni, University of Bologna, ItalyReviewed by:
Laura Musazzi, University of Milano Bicocca, ItalyVeronica Begni, University of Milan, Italy
Copyright © 2025 Adeline Dorothy and Rajan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Koilmani Emmanuvel Rajan, ZW1tYW51dmVsMTk3MkB5YWhvby5jb20=
 Prince David Adeline Dorothy
Prince David Adeline Dorothy Koilmani Emmanuvel Rajan
Koilmani Emmanuvel Rajan