- 1Brain Health Imaging Centre, Centre for Addiction and Mental Health, Toronto, ON, Canada
- 2Institute of Medical Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 3Translation and Computational Neurosciences Unit (TCNU), Faculty of Health and Education, Manchester Metropolitan University, Manchester, United Kingdom
- 4Edmond J. Safra Parkinson Disease Program, Neurology Division, Toronto Western Hospital and Krembil Brain Institute, University Health Network, University of Toronto, Toronto, ON, Canada
Cognitive impairment is a prevalent non-motor symptom of Parkinson’s disease (PD), increasing the risk of dementia as the disease progresses. Despite its clinical significance, the etiology of cognitive impairment in PD remains unclear. Apolipoprotein E4 (APOE4), a well-known genetic risk factor of Alzheimer’s disease, has been studied for its potential role in PD-related cognitive impairment. However, findings have been conflicting and thus inconclusive, highlighting a need to critically evaluate the current research. Several studies using neuroimaging modalities have explored the brains of individuals with PD and atypical parkinsonian disorders who have APOE4. Some of these studies have identified distinct neuropathological changes that have been previously reported to be associated with cognitive impairments in those with Parkinsonisms. Here, we review the role of APOE4 on cognitive impairment in PD and atypical Parkinsonisms using neuroimaging evidence. We will examine how APOE4 may contribute to pathological changes within the brain and its association with cognitive impairment.
1 Introduction
Parkinson’s disease (PD) is a progressive, neurodegenerative disorder classically characterized by its motor symptoms of resting tremor, muscle rigidity, bradykinesia, and postural instability (Aarsland et al., 2017). However, non-motor symptoms also play a role toward the disease’s burden, with some appearing before clinical diagnosis and others emerging decades after motor deficits (Kalia and Lang, 2015; Aarsland et al., 2017). Cognitive impairment is among the most prominent non-motor symptoms. Over 30% of individuals newly diagnosed with PD have cognitive impairment (PD-MCI) and around 40% with PD-MCI may develop dementia (PDD) within 5 years of their diagnosis (Santangelo et al., 2015; Pedersen et al., 2017; Monastero et al., 2018). Additionally, a multi-center study found that approximately 80% of their newly diagnosed patients with PD who survived had dementia at a 20 year follow up (Hely et al., 2008). Due to this prevalence, previous studies established several risk factors of cognitive impairment in PD, with age being a notable predictor (Aarsland et al., 2017, 2021). Older PD patients have a higher risk in experiencing cognitive impairment, with the incidence of PDD increasing as they age (Hely et al., 2008; Santangelo et al., 2015; Aarsland et al., 2017, 2021; Monastero et al., 2018). As a result, aging is considered a key predictive marker for the development of PDD (Aarsland et al., 2017, 2021). Other established risk factors of PD-related cognitive impairment include visual hallucinations, lower education levels, presence of depression, as well as motor symptom severity (Aarsland et al., 2017, 2021). Despite these findings however, the etiology of cognitive impairment in PD remains poorly understood and inadequately researched in comparison to motor symptoms (Chaudhuri et al., 2006; Marinus et al., 2018). Therefore, determining the factors that contribute to the manifestation of cognitive impairment in PD is of great interest.
Genetic factors may contribute to cognitive impairment in PD (Collins and Williams-Gray, 2016). At present, this includes Apolipoprotein E4 (APOE4), a well-established gene polymorphism that increases risk for Alzheimer’s Disease (AD) and has been associated with cognitive impairments (Reitz and Mayeux, 2010; Collins and Williams-Gray, 2016; Fernández et al., 2021; Fernández-Calle et al., 2022). The relationship between APOE4 and cognitive function in subjects with PD has been examined, however, studies have produced contradictory conclusions. While several studies have linked APOE4 to cognitive impairment in PD individuals (Pankratz et al., 2006; Monsell et al., 2014; Samat et al., 2017; Tropea et al., 2018; Szwedo et al., 2022), others found no association (Ezquerra et al., 2008; Kurz et al., 2009; Williams-Gray et al., 2009; Federoff et al., 2012).
Interestingly, the association of APOE4 has also been studied in Dementia with Lewy Bodies (DLB), an atypical parkinsonian disorder that shares significant overlapping characteristics with both AD and PDD (Tsuang et al., 2013; van der Lee et al., 2018; Shiner et al., 2021; Nasri et al., 2022; Bousiges et al., 2023; Carceles-Cordon et al., 2023). DLB is the second most common dementia followed by AD, exhibiting similar neuropathological and clinical features to both disorders (Donaghy and McKeith, 2014; Jellinger and Korczyn, 2018; Carceles-Cordon et al., 2023). Notably, DLB and PDD share key similarities, as both are characterized as Lewy body diseases (LBD) due to the presence of Lewy body pathology, primarily composed of alpha-synuclein proteins (Donaghy and McKeith, 2014; Jellinger and Korczyn, 2018; Carceles-Cordon et al., 2023). However, the distinction between the two is controversially dependent on the timing of cognitive impairment; DLB is diagnosed when cognitive impairment occurs before parkinsonian motor symptoms arise, and PDD is diagnosed when cognitive impairment occurs after (Donaghy and McKeith, 2014; Jellinger and Korczyn, 2018; Carceles-Cordon et al., 2023). Though some studies have also identified that there is an association between APOE4 with DLB (Tsuang et al., 2013; van der Lee et al., 2018; Shiner et al., 2021; Nasri et al., 2022; Bousiges et al., 2023), the role of APOE4 toward DLB pathogenesis and its associated cognitive impairment also still remains unclear. As a result, preclinical studies have investigated APOE4’s role on potential mechanisms that may be linked with this non-motor symptom in PD and DLB (Dickson et al., 2018; Davis et al., 2020; Zhao et al., 2020). However, these studies are currently still limited, leading to significant gaps in our understanding of APOE4-dependent mechanisms underlying cognitive impairment in these parkinsonian disorders.
Neuroimaging studies have revealed that cognitive impairment in PD and DLB is associated with distinct neuropathological changes in the brain (Galvin et al., 2011; Garcia-Garcia et al., 2012; Irwin et al., 2012; Gomperts et al., 2013; Noh et al., 2014; Peraza et al., 2014; Segura et al., 2014; Blanc et al., 2016; Xu et al., 2016; O’Callaghan and Lewis, 2017; van der Zande et al., 2018; Chung et al., 2019; Chabran et al., 2020; Kantarci et al., 2020; Liu et al., 2021). Yet, analysis of these changes and their association with APOE4 in PD is inconsistent, and are limited (Apostolova et al., 2012; Gomperts et al., 2012, 2013; Beyer et al., 2013; Campbell et al., 2013; Nicoletti et al., 2016; Mashima et al., 2017; Rane et al., 2018; Sampedro et al., 2019). APOE4 may serve as a potential genetic biomarker for a higher risk of cognitive impairment in parkinsonian individuals, and therefore may have a significant clinical impact to allow early identification of at-risk individuals. As such, this review aims to enhance clarity regarding the potential contribution of APOE4 in cognitive impairment and the associated neuropathological changes in PD and atypical Parkinsonisms, specifically DLB, using human neuroimaging studies.
2 Apolipoprotein E4 (APOE4)
APOE4 is one of the 3 major isoforms (APOE2, APOE3, APOE4) of the Apolipoprotein E (APOE) gene in humans, which are encoded by the E2, E3, and E4 alleles, respectively (Fernández-Calle et al., 2022). Considering APOE is polymorphic in humans, the three alleles form six major genotypes (APOE2/2, APOE3/3, APOE4/4, APOE2/3, APOE2/4, and APOE3/4). The APOE gene is then translated to the APOE protein, which is primarily produced by astrocytes, microglia, oligodendrocytes, pericytes, the choroid plexus, and neurons in the central nervous system (CNS) (Husain et al., 2021; Fernández-Calle et al., 2022). The APOE protein plays various functions in the brain, including synaptic plasticity, neural signaling, modulation of the immune response, neuronal repair, and lipid transport (Husain et al., 2021; Fernández-Calle et al., 2022).
An association between cognitive function and APOE4 has been numerously reported in the literature. Several studies discovered that APOE4 carriers, whether healthy or diagnosed with AD, demonstrate an accelerated rate of cognitive impairment after follow-up when compared to non-carriers (Cosentino et al., 2008; Rawle et al., 2018; Emrani et al., 2020; Gharbi-Meliani et al., 2021). These individuals also exhibited a greater decline in specific cognitive domains, including executive function and language, as well as have an increased dementia risk (Slooter et al., 1998; Rawle et al., 2018; Gharbi-Meliani et al., 2021). Therefore, APOE4 is distinguished to be one of the several factors that contribute to cognitive impairment (Qian et al., 2017; Gharbi-Meliani et al., 2021).
APOE4 has also been extensively studied for its association with AD, revealing that individuals carrying at least one APOE4 allele have an increased risk of developing the disease (Liu et al., 2013; Fernández-Calle et al., 2022). Over the years, its role toward AD pathogenesis has been reviewed to involve amyloid-beta protein dependent mechanisms (Theendakara et al., 2018; Hunsberger et al., 2019; Yamazaki et al., 2019; Husain et al., 2021; Serrano-Pozo et al., 2021; Martens et al., 2022; Raulin et al., 2022; Pires and Rego, 2023). Notably, enhanced aggregations of amyloid-beta proteins, one of the key hallmarks of AD protein pathology, has been widely examined to be associated with cognitive impairment in AD research (Westerman et al., 2002; Zhu et al., 2017; Pang et al., 2022). In preclinical studies, amyloid-beta precursor (APP) transgenic mice, an established animal model for amyloid-beta deposition and AD, exhibit changes in cognitive domains greatly impaired in AD patients, including learning and memory dysfunction (Westerman et al., 2002; Jahn, 2013; Webster et al., 2013; Zhu et al., 2017; Pang et al., 2022). In a corresponding manner, AD patients positive for amyloid-beta proteins not only exhibit memory impairments, but also deficits in executive and visuospatial function, language, attention, and overall cognitive performance (Lim et al., 2014; Meng et al., 2019). Thus, these findings suggest a direct association between enhanced amyloid-beta proteins in AD pathogenesis that may adversely affect cognitive function. Remarkably, numerous studies proposed potential mechanisms by which APOE4 may contribute to the aggregation and deposition of amyloid-beta proteins to understand why it is a major risk factor of AD. This includes its role in increasing amyloid-beta production and formation, such as accelerating the development of amyloid-beta fibrils and oligomers (Liu et al., 2017; Yamazaki et al., 2019; Serrano-Pozo et al., 2021; Pires and Rego, 2023; Zhang et al., 2023). For instance, APOE4 has been examined to accelerate early seeding of amyloid-beta pathology in mice models, resulting in a significant enhancement of amyloid-beta production and oligomerization (Liu et al., 2017). Inhibition of APOE4 using anti-sense oligonucleotides during the seeding stage of amyloid-beta proteins reduced this pathology (Huynh et al., 2017). Other studies found that APOE4 may increase amyloid-beta synthesis by enhancing APP transcription (Huang et al., 2017; Theendakara et al., 2018). Further, impaired amyloid-beta clearance has been linked to APOE4 as well (Castellano et al., 2011; Fitz et al., 2012; Verghese et al., 2013; Wildsmith et al., 2013; Theendakara et al., 2018; Hunsberger et al., 2019; Liu et al., 2019; Serrano-Pozo et al., 2021; Raulin et al., 2022; Pires and Rego, 2023). APOE4 has been shown to be less effective in modulating receptor-mediated clearance of amyloid-beta by disrupting the binding between clearance receptors with the proteins, likely due to APOE4’s competitive binding of these receptors on astrocytes and microglia (Verghese et al., 2013; Theendakara et al., 2018; Hunsberger et al., 2019; Yamazaki et al., 2019; Serrano-Pozo et al., 2021; Raulin et al., 2022; Pires and Rego, 2023). It also prevents proteolytic clearance of amyloid by blocking the activity of enzymes crucial for the process (Cook et al., 2003; Wildsmith et al., 2013).
APOE4 has also been linked to AD risk, pathogenesis, and its associated cognitive impairment through mechanisms independent of amyloid-beta pathology. This includes enhancing the presence of neurofibrillary tangles, which is primarily composed of hyperphosphorylated tau protein and is one of the other hallmarks of AD protein pathology (Theendakara et al., 2018; Hunsberger et al., 2019; Husain et al., 2021; Raulin et al., 2022; Pires and Rego, 2023). Preclinical studies using AD mouse models have highlighted that hyperphosphorylated tau is associated with cognitive impairment (Rowe et al., 2007; Zhang et al., 2020; Chen and Yu, 2023). Notably, APOE4 has also been found to influence tau-related processes, including enhancing phosphorylation, deposition and aggregation when compared to APOE2 and APOE3, as observed using post-mortem brains and transgenic mice (Tesseur et al., 2000; Brecht et al., 2004; Andrews-Zwilling et al., 2010). Thus, it is possible that APOE4 may play a role in exacerbating tau-related neurodegeneration to overall contribute to cognitive impairment in AD. This speculation is further supported by a study conducted by Emrani et al. (2020), reporting that AD APOE4 carriers exhibit greater tau accumulation when compared to non-carriers, which was associated with greater memory impairments (Emrani et al., 2020). Another suggested mechanism of APOE4’s relation with AD pathogenesis is its association with lipid transport, especially involving cholesterol (Yamazaki et al., 2019; Husain et al., 2021). APOE serves as a major lipid transporter in the CNS by delivering cholesterol to neurons (Liu et al., 2013; Yamazaki et al., 2019; Husain et al., 2021). Notably, APOE4 has been associated with deficient cholesterol transport, as it is less effective in transporting brain cholesterol when compared to APOE3, as well as linked to cholesterol accumulation (Rapp et al., 2006; Husain et al., 2021; Piccarducci et al., 2023). Since cholesterol is crucial for synaptic formation, axonal growth, and neuronal health, all of which are essential for learning and memory, APOE4’s association with impaired cholesterol transport has been inferred to contribute to AD risk and its associated cognitive impairment as well (Liu et al., 2013). Other mechanisms thought to be associated with APOE4 in AD are neuroinflammation, mitochondrial deregulation, synaptic deficits, reduced vascular integrity as well as impaired glucose metabolism and autophagy (Theendakara et al., 2018; Hunsberger et al., 2019; Yamazaki et al., 2019; Husain et al., 2021; Serrano-Pozo et al., 2021; Martens et al., 2022; Raulin et al., 2022; Pires and Rego, 2023). These processes are also associated with cognitive impairment as observed in AD patients and transgenic mice, exacerbating neurodegeneration (Dragicevic et al., 2010; Sharma et al., 2015; Kuang et al., 2019; Loera-Valencia et al., 2019; Nicastro et al., 2020; Barisano et al., 2022; Kumar et al., 2022; Lecca et al., 2022; Eisenmenger et al., 2023; Yin, 2023; del Campo et al., 2024).
Because of APOE4’s well-established prevalence in AD, its impact toward other CNS diseases has been investigated, including Amyotrophic Lateral Sclerosis (ALS), Multiple Sclerosis (MS), Traumatic Brain Injury (TBI), and PD (Fernández-Calle et al., 2022). Figure 1 illustrates mechanisms with APOE4-related neurodegeneration linked to the diseases, adapted from Fernández-Calle et al. (2022). Similarly in AD, APOE4 may play a role in altering microglia activation to enhance neuroinflammation, disrupting the blood–brain barrier, influencing synaptic function, dysregulating protein clearance, increasing protein aggregations, and impairing autophagic processes in other neurodegenerative conditions as well (Krasemann et al., 2017; Main et al., 2018; Davis et al., 2020; Montagne et al., 2020; Fernández-Calle et al., 2022; Jin et al., 2022; Nechushtai et al., 2023). Given these shared mechanisms, further research onto how APOE4 plays a role in these diseases may provide essential insight into its potential as both a clinical biomarker and a therapeutic target for them, especially for PD.
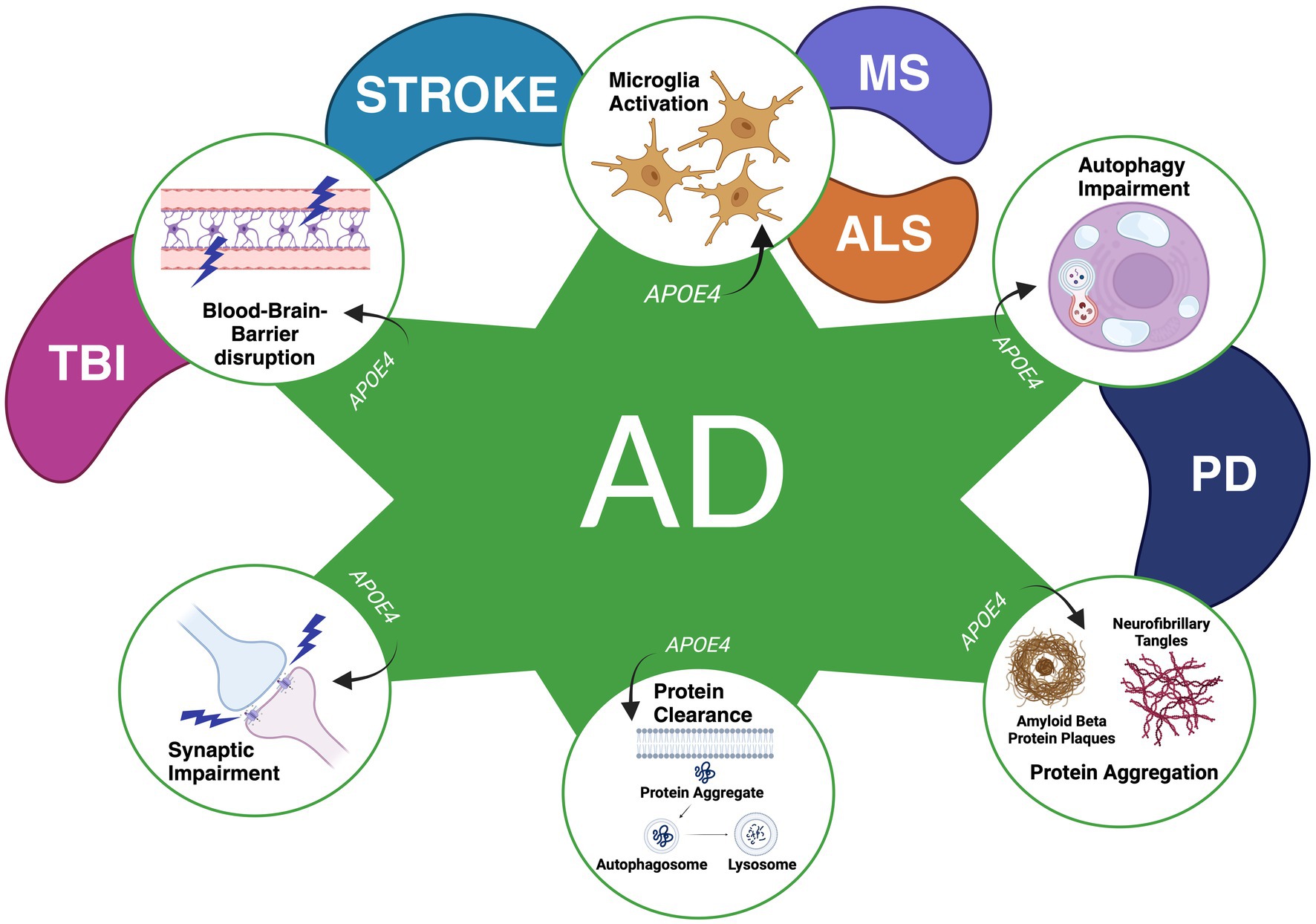
Figure 1. Mechanisms found to be influenced by the APOE4 genotype in various CNS diseases thus far. A variety of mechanisms have been found to be influenced by APOE4 in Alzheimer’s Disease (AD), Traumatic Brain Injury (TBI), Stroke, Multiple Sclerosis (MS), Amyotrophic Lateral Sclerosis (ALS), and Parkinson’s Disease (PD). APOE4 has been found to contribute to enhanced synaptic impairment (AD), deficits in the clearance of proteins (AD), protein aggregations (PD and AD), autophagy impairment (AD and PD), disruptions in the blood–brain barrier (AD, TBI, Stroke) and the activation of microglia to promote neuroinflammation and alterations in microglial reactivity (AD, Stroke, MS, and ALS). Figure adapted from Fernández-Calle et al. 2022. Licensed under a Creative Commons Attribution 4.0 International License, http://creativecommons.org/licenses/by/4.0/. Figure was changed to focus on APOE4’s effect on specific neurodegenerative mechanisms as observed in previous literature.
2.1 APOE4 and Parkinsonism
Notably, clinically and pathologically, there is significant overlap between AD and PD, suggesting that they may have common underlying mechanisms. Both diseases contain similar pathological features, particularly protein aggregations: amyloid-beta plaques as well as neurofibrillary tangles in AD, as previously outlined, and Lewy bodies composed of alpha-synuclein protein in PD. Post-mortem analyses have revealed that these protein aggregations can co-occur in both diseases as well (Kalaitzakis et al., 2008; Burack et al., 2010; Irwin et al., 2012; Twohig and Nielsen, 2019; Visanji et al., 2019; Fernández-Calle et al., 2022). Approximately 50% of AD patients exhibit alpha-synuclein pathology, while up to 50% of PD patients with dementia have amyloid-beta proteins and neurofibrillary tangles that are sufficient enough for secondary AD diagnosis (Irwin et al., 2013; Lim et al., 2019). Moreover, both AD and PD also exhibit overlapping clinical symptoms, such as changes in cognitive function and extrapyramidal features (Morra and Donovick, 2014; Collins and Williams-Gray, 2016; Foguem and Manckoundia, 2018; Pierzchlińska et al., 2018; Wakasugi and Hanakawa, 2021). Considering these commonalities, the association between APOE4 with PD and PD-related cognitive impairment has been debated over the years, with discussion that APOE4 may potentially influence AD-type changes that contribute to the development of cognitive changes in PD subjects (Fernández-Calle et al., 2022; Haikal et al., 2024).
In the context of Parkinsonisms, the APOE4 allele is prominently found in individuals with PD-MCI, PDD, and DLB, when compared to cognitively unimpaired healthy controls (HCs) and/or PD patients with normal cognition (PD-NC) (Parsian et al., 2002; Lashley et al., 2008; Tsuang et al., 2013; Outeiro et al., 2019). In PD, APOE4 carriers are significantly more prevalent among those with PDD, with a 20–35% higher prevalence than in HCs and PD-NC subjects (Parsian et al., 2002; Williams-Gray et al., 2009; Tsuang et al., 2013). This association between PDD and APOE4 is further supported by multiple studies demonstrating that those with PD and at least one APOE4 allele have a greater likelihood in developing PDD when compared to those without the allele (Pankratz et al., 2006; Monsell et al., 2014; Huertas et al., 2017; Chung et al., 2021). Monsell et al. (2014) specifically reported a five-times higher risk for dementia within 5 years of diagnosis among PD APOE4 carriers (Monsell et al., 2014). Interestingly, PD patients with APOE4 also progress to PDD much earlier in comparison to non-carriers (Huertas et al., 2017; Tunold et al., 2021; Szwedo et al., 2022). Hence, APOE4 may be a significant risk factor for dementia in PD and serve as a potential clinical biomarker for PDD. To note, individuals with PD who carry APOE4 are diagnosed at a younger age compared to non-carriers as well, indicating that APOE4 may also be a significant predictor for early onset PD (Pankratz et al., 2006; Szwedo et al., 2022).
In terms of PD-related cognitive impairment, PD patients who are APOE4 carriers show greater cognitive impairment (Pavlova et al., 2014; Paul et al., 2016; Chung et al., 2021). Tropea et al. (2018) report a 3.6 fold increased risk of cognitive impairment in PD APOE4 carriers when compared to non-carriers (Tropea et al., 2018). Additionally, Szwedo et al. (2022) observed that PD APOE4 carriers demonstrate an accelerated rate of cognitive impairment over a 10-year-follow up (Szwedo et al., 2022). Thus, this indicates that APOE4 may play an essential role in facilitating an enhanced likelihood and faster progression of cognitive impairment in those with PD. Further, PD patients with APOE4 also exhibit a decline in specific cognitive processes. This includes executive and visuospatial function, impaired short-term memory, language, and attention, contrasting to patients without the polymorphism and/or HCs (Mata et al., 2014; Pavlova et al., 2014; Paul et al., 2016; Samat et al., 2017; Myers et al., 2022). Studies utilizing the Mattis Dementia Rating Scale-2 (DRS) for neuropsychological evaluation also demonstrate that PD APOE4 carriers have a greater decline in distinct cognitive abilities, including conceptualization, construction, initiation, and overall memory performance when compared to those without the allele (Morley et al., 2012; Tropea et al., 2018). Therefore, this may also suggest that APOE4 adversely impacts certain cognitive processes in individuals with PD.
Despite this evidence, some studies have failed to find a link between APOE4 and cognitive impairment in PD (Ezquerra et al., 2008; Kurz et al., 2009; Williams-Gray et al., 2009; Federoff et al., 2012). For instance, Federoff et al. (2012) found no relationship between memory loss at PD diagnosis and APOE4 amongst a large PD cohort (Federoff et al., 2012). Similarly, an observational cohort study by Mengel et al. (2016) found that APOE4 was not associated with the diagnosis of PD-MCI and PDD, nor with declines in specific neuropsychological domains in PD subjects (Mengel et al., 2016). Several studies also reported no association between APOE4 carrier status and PDD (Ezquerra et al., 2008; Kurz et al., 2009; Williams-Gray et al., 2009). Some findings have shown that possessing the polymorphism does not affect the risk, development, and progression to dementia, as well as the overall rate of cognitive impairment in PD instead (Ezquerra et al., 2008; Kurz et al., 2009; Williams-Gray et al., 2009). Discrepancies in the literature have been attributed to several factors. For example, small sample sizes resulting in low statistical power have been noted to weaken findings on the association between APOE4 and cognitive impairment in PD (Kurz et al., 2009; Federoff et al., 2012). In addition, results from cross-sectional studies have been discussed to potentially be misleading, as the effect of APOE4 may be time-dependent in PD by emerging later in disease progression like in AD (Collins and Williams-Gray, 2016). Studies that did not find an association between APOE4 and cognitive impairment in their cohorts have emphasized the need for longitudinal studies to further evaluate the effect of APOE4 and cognitive function in PD, which are currently limited (Ezquerra et al., 2008; Collins and Williams-Gray, 2016; Mengel et al., 2016; Zhu et al., 2024). It should be noted however that these studies are outdated, and over the past 5 years, an increasing number of research demonstrate that APOE4 in PD patients is indeed linked to a greater risk in cognitive impairment, a decline in specific cognitive processes, and serves as a predictor for PDD, with an earlier and more rapid onset (Rawle et al., 2018; D’Souza and Rajkumar, 2020; Tan et al., 2021; Tipton et al., 2021; Tunold et al., 2021; Myers et al., 2022; Okubadejo et al., 2022; Szwedo et al., 2022; Umeh et al., 2022; Chen et al., 2023; Liu et al., 2023; Real et al., 2023; Zenuni et al., 2023). Thus, understanding the role of APOE4 toward PD-related cognitive impairment may be worthwhile due to growing evidence showing its association with such non-motor symptom.
In the context of DLB, it is also rationalized that APOE4 may play a role in its pathogenesis, and a strong risk factor of the disease (Tsuang et al., 2013; Rongve et al., 2019; Kaivola et al., 2021). Genome-wide association studies (GWAS) examined APOE4 to be a significant loci linked to DLB (Bras et al., 2014; Guerreiro et al., 2018; Rongve et al., 2019; Chia et al., 2021; Kaivola et al., 2021; Bousiges et al., 2023). A GWAS study of 828 clinically diagnosed DLB subjects showed that APOE4 alongside the GBA gene was significantly associated with higher DLB risk (Rongve et al., 2019). Another GWAS study including 495 DLB cases showed that APOE4 was significantly associated in DLB subjects with AD-co-pathology when compared to those with no-co-pathology, potentially highlighting the relevance of APOE4 and its relationship to AD-like changes in DLB (Kaivola et al., 2021). Similar to PDD and AD, neuropathological studies show that DLB subjects also exhibit amyloid-beta and tau pathology co-occurring with alpha-synuclein pathology, where approximately 40–70% harbor amyloid-beta plaques across studies (Vrillon et al., 2024). DLB subjects also show decreases in Cerebrospinal (CSF) biofluid markers that measure amyloid-beta and tau proteins, indicating that amyloid and tau metabolism may be disrupted in DLB as well (van Steenoven et al., 2019; Bolsewig et al., 2024; Vrillon et al., 2024). Thus, this may suggest that APOE4 is also critical in DLB pathogenesis, especially in contributing to AD-like pathology to exacerbate cognitive impairment. Furthermore, more pronounced cognitive impairment is observed in DLB APOE4 carriers when compared to non-carriers, including in attention, learning, memory, and executive function (Ballard et al., 2001; Mirza et al., 2019; Nasri et al., 2022). Therefore, further reinforcing APOE4’s potential role in cognitive impairment in those with DLB.
Overall, in the current literature, mechanisms involving APOE4 that may be associated with cognitive impairment in PD and DLB is less understood. However, a possible mechanism may include enhancing the accumulation of alpha-synuclein aggregations in Lewy bodies (McKeith et al., 2005; Davis et al., 2020; Zhao et al., 2020; Attems et al., 2021; Haikal et al., 2024). This is supported with preclinical studies using alpha-synuclein mice models, which examined that those expressing the allele developed the most burden of alpha-synuclein pathology, as well as greater and faster cognitive impairment, including memory dysfunction, when compared to those with APOE2 and APOE3 (Davis et al., 2020; Zhao et al., 2020). It has been proposed that this relation between APOE4 and enhanced alpha-synuclein pathology may be due to various factors. APOE4’s role in the disruption of cholesterol homeostasis has been discussed, considering that one of the preclinical studies by Zhao et al. (2020) observed in their APOE4 cerebral organoids to have both increases in alpha-synuclein and lipid droplet accumulation (Zhao et al., 2020). Another alternative explanation may involve alpha-synuclein directly interacting and co-aggregating with APOE, as observed in post-mortem studies, suggested to be accelerated at the presence of APOE4 (Rohn and Mack, 2018; Paslawski et al., 2019; Davis et al., 2020; Zhao et al., 2020). However, further research is needed to characterize the cellular and molecular processes involving APOE4 that may exacerbate alpha-synuclein pathology (Davis et al., 2020). Other mechanisms may include APOE4’s role in enhancing amyloid-beta pathology, as established in AD, due to the significant prevalence in amyloid-beta in the brains of both DLB and PDD subjects. However, a study by Tsuang et al. (2013) examined a high proportion of individuals with APOE4 among those with PDD and pure DLB, a type of DLB characterized by the presence of Lewy bodies without AD-like pathology, but they exhibited low amyloid-beta plaques. This is consistent with other studies analyzing no association between APOE4 and pure DLB (Dickson et al., 2018; Kaivola et al., 2021). Therefore, APOE4 may also play a role in amyloid-independent mechanisms in PD and DLB to induce neurodegeneration and cognitive changes, just like in AD.
3 Imaging APOE4’s effect in Parkinsonism
Neuroimaging techniques aid the understanding of how changes in the brain may be associated with mental, behavioral, and cognitive processes (Yen et al., 2023). Herein, we will review neuroimaging studies conducted in humans to determine the effect of APOE4 on brain changes that may influence cognitive function in people with PD, PD-MCI, and PDD. Considering that APOE4 is associated with DLB risk, and that DLB APOE4 carriers also exhibit greater cognitive impairment than non-carriers, we also review studies that investigate the effect of APOE4 toward the brains of DLB subjects (Ballard et al., 2001; Boot et al., 2013; Tsuang et al., 2013; Guerreiro et al., 2018; Mirza et al., 2019; Rongve et al., 2019; Kaivola et al., 2021; Nasri et al., 2022). Imaging metrics, cognitive function, and genetic status will be evaluated and discussed to determine potential relationships.
3.1 APOE4 and amyloid-beta protein pathology
Abnormal accumulations of amyloid-beta proteins have been observed in the brains of subjects with PD-related cognitive impairment (Gomperts et al., 2013; O’Callaghan and Lewis, 2017). Several studies have reported higher levels of amyloid-beta proteins in individuals with PD-MCI and PDD when compared to HCs and PD-NC subjects (Irwin et al., 2012; Gomperts et al., 2013; Hepp et al., 2016). These increases in amyloid-beta in PD subjects with cognitive impairment is also observed in specific brain areas, including the temporal, parietal, and frontal regions (Maetzler et al., 2008; Hepp et al., 2016; Mihaescu et al., 2022).
Pre-clinical and clinical research have shown that APOE4 carriers have higher amyloid-beta protein accumulation, as observed in AD subjects and transgenic mice (Liu et al., 2013; Kanekiyo et al., 2014). Similarly, this association between APOE4 and enhanced amyloid-beta accumulation is also observed in PD. Various Positron Emission Tomography (PET) studies using radiotracers that measure these proteins in the brain, including [11C]-Pittsburgh Compound B (PiB), [18F]-Florbetaben (FBB), or [18F]-florbetapir ([18F]-AV-45), revealed significant increased retention and standardized uptake value ratios (SUVR) in PD APOE4 carriers than non-carriers (See Table 1 for more details) (Maetzler et al., 2009; Gomperts et al., 2012; Vijayaraghavan et al., 2014; Akhtar et al., 2017; Jung et al., 2021). Notably, these studies showed that higher retention and SUVR values for amyloid-beta proteins in specific brain regions was associated with APOE4 in PD subjects with mild cognitive impairment and/or dementia, highlighting APOE4’s potential role in influencing enhanced regional amyloid-beta pathology. For instance, Gomperts et al. (2012) discovered that the number of APOE4 alleles (0, 1, 2) was correlated with amyloid-beta protein within the precuneus (via [11C]-PiB PET) across their PD-MCI, PDD, DLB, and HC groups (Gomperts et al., 2012). This brain region has been associated with memory deficits when containing higher amyloid-beta protein depositions (Perrotin et al., 2012; Farrell et al., 2018; Fang et al., 2020). However, the authors did not find a significant correlation between precuneus amyloid-beta and cognitive function, as measured by the Mini-Mental State Examination (MMSE), in their PD-MCI (mean age = 69.4) and PDD groups (mean age = 71.7) (Gomperts et al., 2012). It was discussed that this may be due to the possibility that other pathological mechanisms could underlie the progression of PDD, rather than amyloid-beta burden (Gomperts et al., 2012). Such rationale may align with findings that amyloid-beta deposition is not well-correlated with cognitive impairment in older adults, and that other abnormalities known to be more strongly correlated with cognitive changes may be the reason for cognitive impairment instead, such as tau pathologies (Villemagne et al., 2013; Jack et al., 2014; Jansen et al., 2015; Bejanin et al., 2017; Brookmeyer and Abdalla, 2018; Ramanan et al., 2021). Low-levels of amyloid-beta burden in their PDD group was also discussed to potentially affect their ability to robustly detect an association between amyloid levels and cognitive function (Gomperts et al., 2012). Correspondingly, Maetzler et al. (2009) identified that all their subjects positive for amyloid-beta (via [11C]-PiB PET), measured in their frontal brain regions, posterior cingulate, as well as in superior parietal, lateral temporal and occipital lobe, had dementia (PDD and DLB) and a higher prevalence of APOE4 when compared to non-demented and demented subjects negative for [11C]-PiB (Maetzler et al., 2009). This may support APOE4’s association with dementia in PD and DLB, as previously discussed, which may be explained by enhanced amyloid-beta pathology in specific brain regions. Similarly, Akhtar et al. (2017) also found that higher amyloid-beta (via [18F]-AV-45 PET SUVR) in memory-related brain areas, including the anterior and posterior cingulate, precuneus, as well as temporal, frontal and parietal cortices, was associated with APOE4 in their PD-MCI and PD-NC groups when controlling for age (Akhtar et al., 2017). This may indicate that APOE4 enhances amyloid-beta burden in regions essential for cognitive domains affected in those with PD. Moreover, Vijayaraghavan et al. (2014) observed in their combined sample of APOE4 carriers (PD-NC, PD-MCI, PDD, and DLB) to have higher amyloid-beta (assessed via [11C]-PiB retention) in their striatal and cortical regions, as well as a lower MMSE score when compared to non-carriers (Vijayaraghavan et al., 2014). Jung et al. (2021) also found using PET with [18F]-FBB that typical LBD was linked to increased occipital beta-amyloid burden though its interaction with APOE4, as analyzed in their combined group including subjects with DLB with amyloid and pure LBD (PD and DLB). However, it should be noted that the heterogeneity of the samples in these two studies since they combined subjects with distinct Parkinsonisms and different stages of cognitive impairment may complicate interpretations. Combining PD subjects with varying cognitive levels may make it challenging to interpret the relationships between APOE4, cognition, and amyloid-beta accumulation in PD, since a previous systematic review of 11 PiB studies analyzed that each cognitive stage differs in amyloid-beta burden (Petrou et al., 2015). Including DLB subjects alongside PD subjects may further introduce complexity, given the distinct cognitive profiles and trajectories of DLB and PD (Aarsland et al., 2021). Overall, all these studies suggests that APOE4 may influence cognitive impairment in those with PD by contributing to region-specific amyloid-beta protein burden (Maetzler et al., 2008; Hepp et al., 2016; Mihaescu et al., 2022). Though the brain regions differ across studies, it is apparent that higher retention and SUVR values are found in areas known to play a role in cognitive processes impaired in PD subjects.
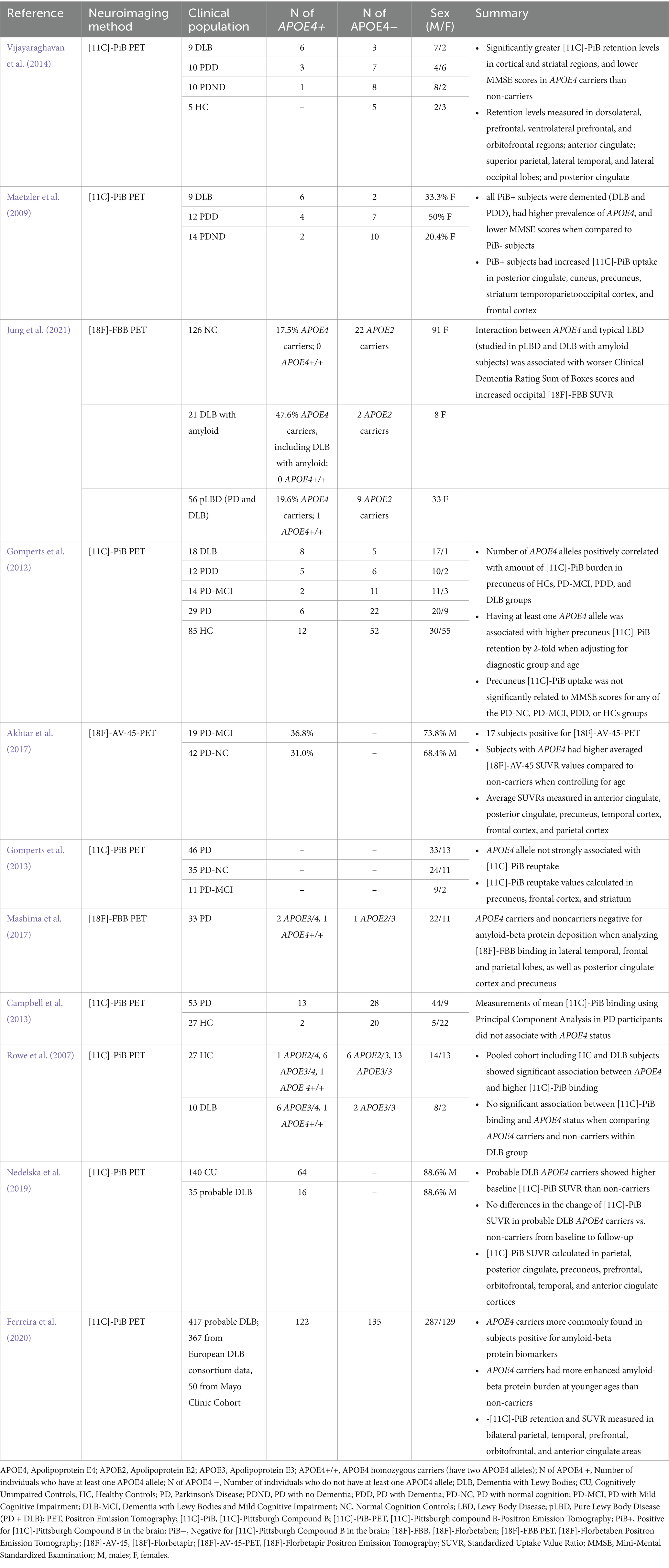
Table 1. Details of studies evaluating amyloid-beta protein burden including parkinsonian subjects (PD and DLB) with APOE4 genotype.
In contrast, several studies have reported no association between APOE4 and amyloid-beta protein burden instead. A study by Campbell et al. (2013) using principal component analysis provided evidence that APOE4 status did not affect amyloid-beta burden (assessed via mean [11C]-PiB binding) in 13 PD subjects experiencing cognitive impairment (Campbell et al., 2013). Mashima et al. (2017) also found that PD APOE4 carriers and non-carriers were both negative for amyloid-beta protein pathology using PET with [18F]-FBB (Mashima et al., 2017). However, the analysis of only 3 carriers and 1 non-carrier in a total of 33 subjects may lack statistical power, in contrast to larger studies showing a higher prevalence of amyloid-beta protein pathology in PD individuals with APOE4 as previously discussed (Maetzler et al., 2008; Gomperts et al., 2012; Vijayaraghavan et al., 2014; Akhtar et al., 2017; Mashima et al., 2017). Similarly, Gomperts et al. (2013) study claimed that APOE4 was not strongly associated with amyloid-beta burden (via [11C]-PiB) in their PD group, including those with PD-MCI, but proposed that such observations may be due to their sample sizes (n = 46). Observed discrepancies amongst PiB studies may be attributed to the types of groups analyzed and their levels of cognitive severity. Some studies identifying a significant association between APOE4 and amyloid-beta burden included cohorts with more severe cognitive impairment (i.e., classified to have DLB and PDD), whereas all the studies finding no association included participants with PD-MCI or PD without dementia only (Maetzler et al., 2009; Gomperts et al., 2012, 2013; Campbell et al., 2013; Vijayaraghavan et al., 2014; Mashima et al., 2017). This indicates that the severity of cognitive impairment may potentially play a critical role in the relationship between APOE4 status and amyloid-beta accumulation in PD. Discrepancies may also be attributed to the presence of co-morbidities of AD and PD protein pathology, as well as their co-localization with neuroinflammation, which may complicate the accurate measurement of amyloid-beta proteins. Individuals with advanced PD are more likely to exhibit overlapping protein pathologies, as both AD and PD pathologies commonly occur at later stages of the disease (Visanji et al., 2019). Similarly, enhanced neuroinflammation, measured through reactive astrocytes, has been shown to increase with age in DLB and PDD subjects, highlighting the prevalence of neuroinflammation in later stages of the disease also (van den Berge et al., 2012). Thus, since some significant studies included individuals with advance stages of the disease (PDD and DLB) while studies finding no significant association included none, it is also possible that prevalence of heightened co-pathology and neuroinflammation may potentially influence radiotracer uptake. This is further supported with evidence showing that PiB can bind to Lewy bodies and neurofibrillary tangles in vivo (Fodero-Tavoletti et al., 2007; Lockhart et al., 2007).
Furthermore, higher amyloid-beta protein pathology is potentially associated with greater impaired cognition in DLB subjects (Nelson et al., 2009; Gomperts et al., 2012). However, several studies reported that DLB APOE4 carriers had higher amyloid burden (via [11C]-PiB) than non-carriers, while others did not (See Table 1) (Rowe et al., 2007; Maetzler et al., 2009; Gomperts et al., 2012; Vijayaraghavan et al., 2014; Nedelska et al., 2019; Ferreira et al., 2020; Jung et al., 2021). Thus, further research is warranted to mitigate these inconsistencies to make robust conclusions on how APOE4 influences DLB-related cognitive impairment via increasing amyloid-beta proteins also.
3.2 APOE4 and structural brain changes in Parkinsonian subjects
Numerous studies, summarized in Table 2, have investigated the relationship between APOE4 and structural brain measures, including those that quantify gray and white matter, as well as ventricular size, in those with PD and DLB.
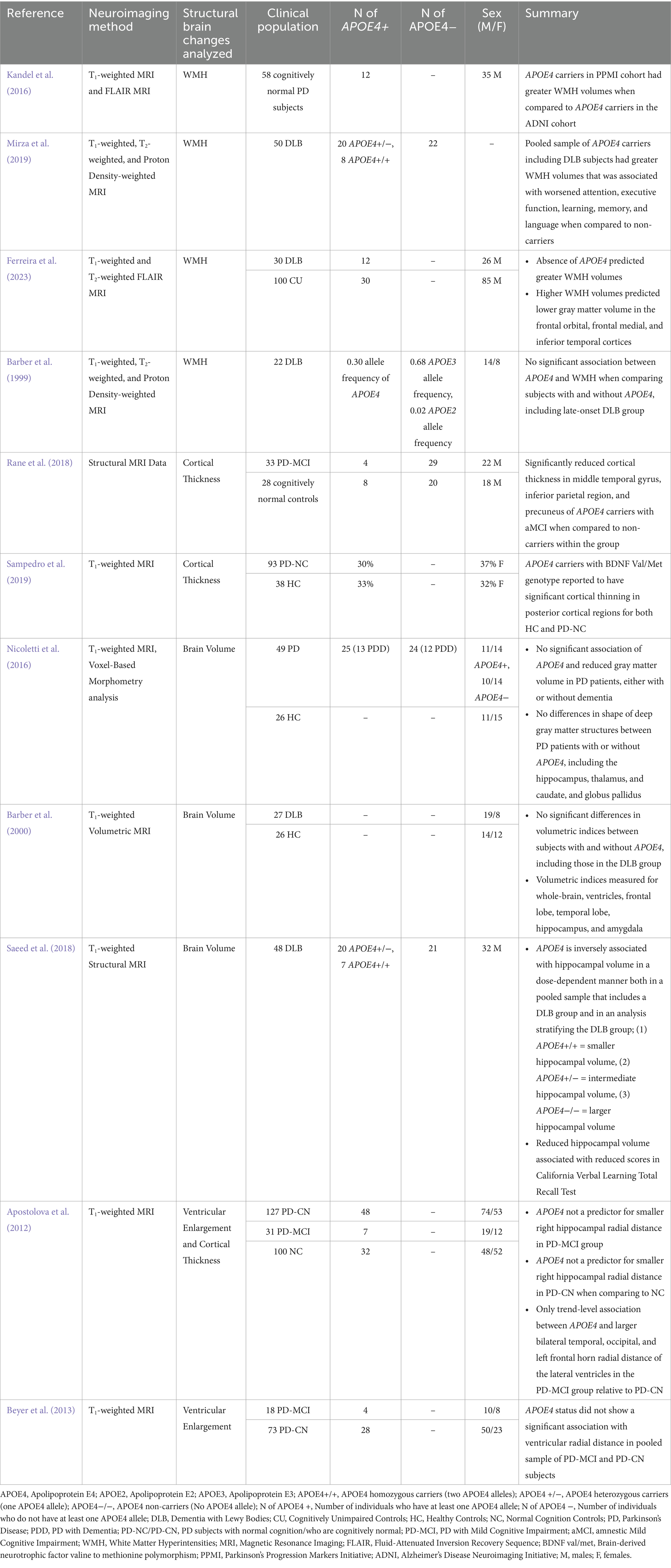
Table 2. Details of studies evaluating structural brain changes including parkinsonian subjects (PD and DLB) with APOE4 genotype.
Measurements known to help assess gray matter changes have been explored to be associated with PD-related cognitive impairment. This includes cortical thickness, where its reduction has been examined in PD-MCI and PDD subjects using Magnetic Resonance Imaging (MRI) (Segura et al., 2014; Koshimori et al., 2015; O’Callaghan and Lewis, 2017; Gasca-Salas et al., 2019), and is proposed to be a potential marker for the conversion from PD-MCI to PDD (Segura et al., 2014; Chung et al., 2019). Two studies identified a relationship between APOE4 and decreases in cortical thickness of specific regions of PD patients, especially within posterior cortical areas, including in the temporal and parietal regions (Rane et al., 2018; Sampedro et al., 2019). Rane et al. (2018) showed that 28 APOE4 carriers (24 AD and 4 PD) who had amnestic MCI (aMCI) showed significant decreases in the cortical thickness of the middle temporal gyrus when compared to non-carriers, a brain region essential for language, visual perception, and episodic memory processing (Onitsuka et al., 2004; Rane et al., 2018). This is consistent with previous literature showing that the middle temporal gyrus is atrophied and thinner in those with PD-MCI when compared to PD subjects without cognitive impairments or HCs (Zhang et al., 2015; Hanganu and Monchi, 2016). Rane et al. (2018) also examined cortical thinning in the parietal and precuneus regions in their APOE4 carriers, areas previously found to be affected in PD individuals experiencing cognitive impairment and are also essential for memory function (Perrotin et al., 2012; O’Callaghan and Lewis, 2017; Farrell et al., 2018; Rane et al., 2018; Jawabri and Sharma, 2023). The results of Rane et al.’s (2018) study may be challenging to generalize however, as only a small sample size of PD subjects within their APOE4 group was observed (Rane et al., 2018). Furthermore, Sampedro et al. (2019) reported that an interaction between APOE4 and a specific genotype for the Brain Derived Neurotrophic Factor (BDNF) was associated with greater cortical thinning in posterior cortical regions in both their PD-NC and HC groups (Sampedro et al., 2019). These results may also suggest that a combination of genetic factors, including APOE4, may contribute to reduced cortical thickness of specific regions in PD that may potentially predict cognitive impairment. This is supported by evidence examining that posterior-cortical degeneration in PD is associated with more severe cognitive impairment in PD and is strongly associated with PDD (Pagonabarraga and Kulisevsky, 2012; Uribe et al., 2018). Collectively, we infer that both studies indicate that APOE4 influences reduced cortical thickness in regions essential for cognitive function, potentially contributing to future cognitive changes in those with PD. However, findings from Apostolova et al. (2012) may contradict this, as the thickness of the hippocampus, a region well-known for memory function, was not significantly affected by APOE4 status in PD patients instead, despite strong positive correlations with MMSE score and cognitive status (PD vs. PD-MCI) (Apostolova et al., 2012). Yet, methodological differences in thickness analysis, such as the use of radial distance measured by Apostolova et al. (2012) versus the FreeSurfer pipeline in the two studies, may have impacted sensitivity in examining APOE4-related thickness changes to contribute to these discrepancies (Apostolova et al., 2012; Rane et al., 2018; Sampedro et al., 2019).
Decreases in gray matter volume (GMV) have also been a widely examined gray matter change to be associated with PD-related cognitive impairment (Xia et al., 2013; Noh et al., 2014; O’Callaghan and Lewis, 2017; Hou and Shang, 2022). Reduced GMV was detected in PD patients with cognitive deficits, and associated with worsened performance in the Montreal Cognitive Assessment (MoCA) and MMSE (Xia et al., 2013; Noh et al., 2014; O’Callaghan and Lewis, 2017; Li et al., 2022). A meta-analysis by Xu et al. (2016) also found that individuals with PD-MCI and PDD had greater decreases in GMV when compared to PD patients without cognitive impairments, including in the hippocampus, insula, left superior and inferior temporal lobe, and left superior frontal lobe (Xu et al., 2016). There is limited data however with regards to the association of APOE4 and GMV alterations in PD. One study by Nicoletti et al. (2016) reported no significant differences in whole brain GMV when comparing PD patients with and without APOE4, regardless of dementia status (Nicoletti et al., 2016). They also found no significant differences in the shape of deep gray matter structures between PD APOE4 carriers and non-carriers, including the hippocampus, thalamus, globus pallidus, and caudate nucleus (Nicoletti et al., 2016). This may indicate that APOE4 may not influence GMV changes relevant in PD individuals with cognitive impairment. However, caution is necessary as these conclusions are derived from a single study. Since there are existing studies that do observe a relationship between APOE4 and GMV alterations in regions relevant for cognitive function, it may be worthwhile to conduct more research in PD to further evaluate APOE4’s association with GMV (Spampinato et al., 2011; Haller et al., 2017; Cacciaglia et al., 2018). In terms of DLB, there is conflicting evidence with regards to the association of APOE4 and hippocampal GMV, a brain region moderately atrophied in those with the disease (Chow et al., 2012). Saeed et al. (2018) studied in their combined group of APOE4 carriers with DLB and AD, as well as after stratifying the two groups that APOE4 was linked to hippocampal volume loss, with two alleles causing greater atrophy than one or none, and that this reduction in volume was associated with impaired learning performance (Saeed et al., 2018). However, Barber et al. (2000) found no differences in volumetric measurements, including the hippocampus, of DLB patients when comparing those with and without APOE4 (Barber et al., 2000). Variations in analyzing volume measurements may influence the observed differences between studies, particularly given that each employed different imaging software (Barber et al., 2000; Saeed et al., 2018). Saeed et al. (2018) also offered a thorough characterization of their sample, detailing the characteristics of both APOE4 carriers and non-carriers, which may further strengthen their findings (Saeed et al., 2018). Contrastingly, Barber et al. (2000) lacked demographic information for their APOE4 carriers and non-carriers, such as the number of subjects in each group, which may limit the interpretability of their results and lead to weaker conclusions (Barber et al., 2000).
White matter changes are also found in subjects with PD-related cognitive impairment and DLB (Joki et al., 2018; Yang et al., 2023). Commonly seen are White Matter Hyperintensities (WMH), which are white matter lesions analyzed as bright regions in T2-weighted, fluid attenuated inversion recovery (FLAIR) and proton density-weighted MRI images, while they appear darker in T1-weighted MRI scans (Wardlaw et al., 2015; Yang et al., 2023). A meta-analysis identified that enhanced WMH volumes are more prevalent in PDD subjects when compared to those with PD-MCI and PD-NC, indicating it may be a structural marker for PDD (Liu et al., 2021). Previous studies also observed that more WMH volumes in PD and DLB patients predict greater cognitive impairments, especially regarding memory function and processing speed (Dadar et al., 2018; Linortner et al., 2020; Ferreira et al., 2021). APOE4 has also been associated with increased WMH volumes in PD and DLB subjects in some studies, suggesting it may also contribute to white matter abnormalities known to be linked to cognitive impairment in these conditions (Kandel et al., 2016; Saeed et al., 2018). For example, one study found that PD APOE4 carriers showed significantly higher WMH volumes when compared to AD APOE4 carriers (Kandel et al., 2016). This may indicate that PD APOE4 carriers show pronounced white matter changes, which could potentially contribute to an increase risk of cognitive impairment. However, interpretations must be made with caution, as it is based from a single study. For DLB, Mirza et al. (2019) found their pooled cohort of AD and DLB subjects with APOE4 to have higher levels of WMH volumes that were linked to worsened learning, language processing, memory, and executive functioning (Mirza et al., 2019). Yet, due to sample imbalances in the cohort (239 AD vs. 50 DLB subjects), interpreting if these observed relationships occurs solely in DLB subjects may be challenging (Mirza et al., 2019). In addition, other studies also did not find an association between APOE4 and WMH volumes in DLB instead, further complicating conclusions. Ferreira et al. (2023) observed higher WMH volumes in DLB patients without APOE4, indicating a lack of association between APOE4 and increased WMH volumes in DLB subjects (Ferreira et al., 2023). However, since the study focused on those with mild to moderate DLB, the influence of APOE4 on enhanced WMH volumes could be more apparent in individuals with severe DLB who were ineligible for their investigations (Ferreira et al., 2023). Barber et al. (1999) also found no association between the presence of APOE4 and white matter lesions including WMH in their APOE4 cohort that had DLB subjects (Barber et al., 1999). But, the allele frequency of APOE4 in their DLB cohort was small, specifically 0.30 amongst 22 patients, and should be considered (Barber et al., 1999). Further research is needed to robustly understand the effect of APOE4 on WMH that may be associated with cognitive impairment in PD and DLB.
Lastly, there is some evidence that changes in ventricular size may be associated with PD-related cognitive impairment. PD-MCI patients showed significantly larger ventricles than those with PD-NC, and that this brain change is associated with cognitive changes including a decline in memory performance (Dalaker et al., 2011). However, two studies found no significant association between enlarged ventricles in their pooled sample of APOE4 carriers that included those with PD-NC and PD-MCI (Apostolova et al., 2012; Beyer et al., 2013). Therefore, we theorize that the effect of APOE4 toward PD-related cognitive impairment may not involve ventricle enlargement.
3.3 APOE4 and functional brain changes in Parkinsonian subjects
The relationship between APOE4 and functional brain measures have been examined in the brains of PD and DLB subjects with APOE4 in various studies, as detailed in Table 3. However, most reviewed studies involved PD subjects, where there are limited studies in DLB subjects exploring these relationships.
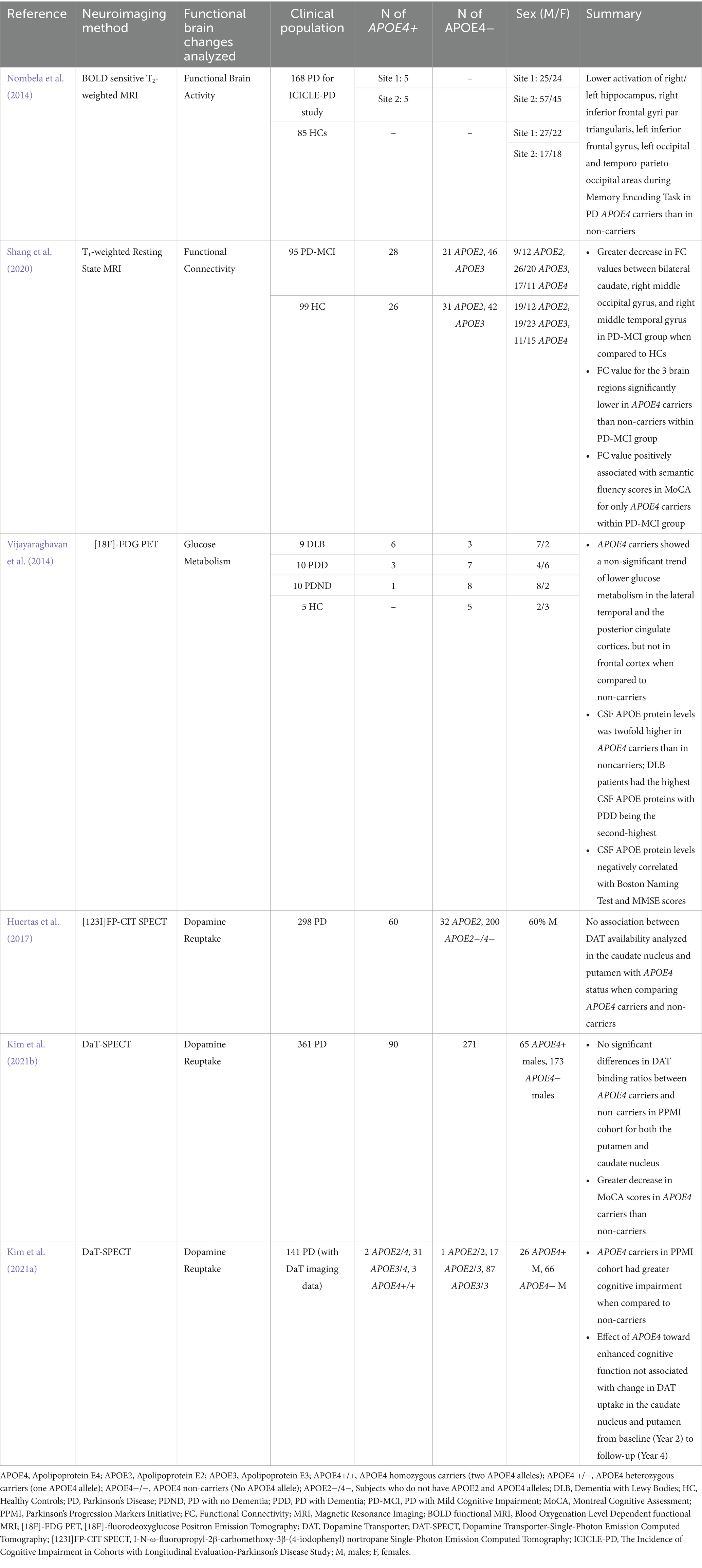
Table 3. Details of studies evaluating the functional brain changes including parkinsonian subjects (PD and DLB) with APOE4 genotype.
Functional abnormalities as measured using functional MRI (fMRI), a non-invasive technique that quantifies neural activity by assessing blood oxygen levels, have been found to be relevant in subjects with PD-related cognitive impairment (Sasikumar and Strafella, 2020; Yen et al., 2023). This includes changes in functional activity in specific brain regions, such as reductions in the prefrontal cortex, caudate nucleus, and fronto-striatal regions when doing cognitive tasks (Sasikumar and Strafella, 2020). It also includes altered functional connectivity as measured using resting fMRI, which quantifies the communication between brain regions through distinct patterns of brain activity amongst them (van den Heuvel and Hulshoff Pol, 2010; Baggio and Junqué, 2019). For example, van Eimeren et al. (2009) determined disruptions in the functional connectivity of the Default Mode Network when investigating PD patients, a network crucial for executive function and greatly defective in those experiencing PD-related cognitive impairment (van Eimeren et al., 2009). Limited fMRI evidence is available on the relation between APOE4 and functional activity as well as connectivity in the brains of those with PD. Of the studies reviewed, reductions in functional activity and connectivity were overall found within specific subcortical regions, including the caudate and hippocampus, as well as posterior cortical regions, which was then associated with cognitive changes. In particular, Nombela et al. (2014) reported significant reductions of activity during a memory encoding task in 10 subjects with early PD and APOE4 when compared to APOE2 and APOE3 carriers (Nombela et al., 2014). These reductions were observed in their right and left hippocampus, and posterior cortical areas, specifically the right inferior frontal gyri pars triangularis, left inferior frontal gyrus, left occipital, and temporo-parieto-occipital areas (Nombela et al., 2014). However, Nombela et al. (2014) acknowledged that the sample size for their APOE4 carriers is small, which is inferred to potentially lead to biased findings (Nombela et al., 2014). To correspond, Shang et al. (2020) examined in 28 Chinese individuals with PD-MCI and APOE4 that significantly lower functional connectivity values between the bilateral caudate nucleus and posterior cortical regions, including the right superior occipital and middle temporal gyrus, was significantly associated with reduced semantic fluency scores when compared to PD-MCI APOE2 and APOE3 carriers (Shang et al., 2020). Notably, the cognitive domains analyzed in both studies have been previous analyzed to be associated with those with PD-related cognitive impairment. Memory encoding has been evaluated to be adversely impacted in PD-MCI patients when compared to those with normal cognition (Weintraub et al., 2004; Gratwicke et al., 2015; Devignes et al., 2021) Worsened semantic fluency predicts progression to PDD, and is more disrupted in those with PD-MCI than those with PD-NC (Bohnen et al., 2011; Ye et al., 2017; Yang et al., 2022). Thus, both studies suggest that APOE4 may play a role in PD-related cognitive impairment by affecting the activity of and communication between specific brain regions essential for cognitive processes deficient in PD, especially posterior cortical areas.
Changes in glucose metabolism as measured by [18F]-Fluodeoxyglucose ([18F]-FDG) is also prevalent in PD subjects with cognitive impairment. Both PDD and PD-MCI subjects exhibit distinct brain patterns of glucose hypometabolism associated with changes in memory, attention, executive function, and visuospatial processing (Garcia-Garcia et al., 2012; Meyer et al., 2017). However, only one study exhibited the relationship between APOE4, cognitive status, and altered glucose metabolism in those with PD and DLB (Vijayaraghavan et al., 2014). Vijayaraghavan et al. (2014) observed a trend of reduced glucose metabolism in the posterior cingulate and lateral temporal cortex in their combined cohort of APOE4 carriers (PD-NC, PDD, and DLB subjects) when compared to non-carriers (Vijayaraghavan et al., 2014). Interestingly, CSF APOE protein levels were also found to be significantly higher in their DLB and PDD APOE4 carriers compared to non-carriers, and that these levels were negatively correlated with glucose metabolism in the lateral temporal cortex and posterior cingulate, as well as with cognitive scores on the Boston Naming Test and MMSE. These results are consistent with previous literature that examined the prevalence of reduced glucose metabolism in these regions in PD patients who converted to dementia, and in PD-MCI and PDD patients when compared to cognitively unimpaired individuals (Vander Borght et al., 1997; Bohnen et al., 2011). In addition, the posterior cingulate is important for attention, and the lateral temporal cortex is essential for language comprehension, facial recognition, and visual processing, all of which are adversely affected in PD individuals with cognitive impairment (Vander Borght et al., 1997; Watson and Leverenz, 2010; Goldstein et al., 2017; Vogt, 2019; Fang et al., 2020; Vijayaraghavan et al., 2014). Thus, we infer that reduced glucose metabolism in specific brain areas important for cognitive function in PD and DLB is linked to APOE4, leading to adverse cognitive changes. However, such conclusions are based on limited evidence, and further research is warranted to explore these associations more comprehensively.
Lastly, reduced dopamine neurotransmission has been a key characteristic of PD, occurring from the degeneration of dopaminergic neurons in the substantia nigra and midbrain (Triarhou, 2013). Though its effects are associated with its motor features, some studies report that it may also be linked to cognitive impairment in PD patients (Triarhou, 2013; Fang et al., 2020). Dopamine-Transporter (DAT)-Single Photon Emission Tomography (SPECT) imaging measures dopamine neurotransmission by visualizing the quantity of DAT, a protein found in the endings of presynaptic dopaminergic neurons that facilitate dopamine reuptake from synapses (Akdemir et al., 2021). Specific to APOE4 and PD, two studies found no significant differences in DAT binding between patients with and without polymorphism in the caudate nucleus and putamen using DaT-SPECT (Huertas et al., 2017; Kim et al., 2021b). Similarly, Kim et al. (2021a) observed that individuals with APOE4 experience greater cognitive impairment than those without it. Yet, this relationship was not linked with significant reductions in DAT uptake in the caudate and putamen as well between baseline and follow-up (Kim et al., 2021a). Thus, we infer that APOE4’s impact toward PD-related cognitive impairment may not include dopaminergic processes.
4 Discussion
This review utilized neuroimaging evidence from multiple human studies to assess the potential impact of APOE4 on brain changes in individuals with Parkinsonisms and cognitive status. It is recognizable that specific brain regions with an essential role in cognitive function consistently showed structural, functional, and/or amyloid-beta protein pathologies in PD individuals with APOE4 in some studies identified. As discussed and outlined in the tables provided, this includes posterior cortical regions, including parietal, occipital, and temporal cortices; frontal regions, including the frontal and prefrontal cortex; and subcortical regions such as the anterior and posterior cingulate, precuneus, caudate nucleus, and middle temporal gyrus (Maetzler et al., 2009; Gomperts et al., 2012; Nombela et al., 2014; Vijayaraghavan et al., 2014; Akhtar et al., 2017; Rane et al., 2018; Shang et al., 2020; Jung et al., 2021). Specific brain regions crucial for cognition also showed more significant structural and functional alterations in DLB APOE4 carriers between some of the studies reviewed (Maetzler et al., 2008; Gomperts et al., 2012; Vijayaraghavan et al., 2014; Ferreira et al., 2020; Jung et al., 2021; Diaz-Galvan et al., 2023). Given this, APOE4 may play a role in the development and progression of PD- and DLB-related cognitive impairment by influencing adverse changes in particular brain areas known to contribute to cognitive function.
Notably, APOE4 has been found in some studies to be associated with increased amyloid-beta proteins in the brains of PD patients with cognitive impairment and DLB. This strengthens the idea that APOE4 may play a role in contributing to AD-like pathology in these Parkinsonisms. Thus, it is inferred that its role on amyloid-dependent mechanisms in AD pathogenesis to facilitate enhance amyloid-beta protein pathology may also be similar in subjects with PD-related cognitive impairment and DLB, considering the commonalities between AD, PDD, and DLB, as previously discussed. We note however that no imaging studies were found that examined the relationship between APOE4 and tau in those with PD, PD-related cognitive impairment, and DLB. Over the years, various PET radiotracers have been developed to image tau proteins (Groot et al., 2022; Mena and Strafella, 2022). Yet, it is possible that no tau imaging studies were found due to the known challenges for tau radiotracers, including off-target binding, and difficulties of radiotracers binding to tau aggregates since they form intracellularly (Marquié et al., 2017; Groot et al., 2022; Mena and Strafella, 2022). Due to this gap, it may be worthwhile in the future to analyze APOE4’s impact on tau pathology in PD-related cognitive impairment and DLB using neuroimaging, given the prevalence of tau aggregation in these disorders, and APOE4’s known association with tau in AD.
Furthermore, the mechanisms by which APOE4 specifically contributes to the structural and functional changes observed in the reviewed studies remains poorly understood in individuals with PD and DLB. However, considering that such changes have also been found in diagnosed and at-risk AD patients with APOE4 (Drzezga et al., 2005; Lind et al., 2006; Trivedi et al., 2006; Xu et al., 2009; Liu et al., 2010; Spampinato et al., 2011; Canuet et al., 2012; Ossenkoppele et al., 2013; Wesnes et al., 2014; Wang et al., 2015; Mirza et al., 2019; Abushakra et al., 2020), it is plausible to hypothesize that APOE4 contributes to these brain pathologies in PD and DLB through mechanisms implicated in AD pathogenesis, overall leading to cognitive impairment.
For instance, the association between gray matter changes, including cortical thickness and GMV, with APOE4 in PD and DLB may be attributed to APOE4’s impact on impaired cholesterol transport, given cholesterol’s critical role in maintaining neuronal health and synaptogenesis, as previously outlined. However, this association may also be driven by APOE4’s contributions toward mechanisms discussed in AD research known to be associated with neurotoxicity, neuronal cell death and synaptic loss, including enhanced amyloid-beta and tau pathology, neuroinflammation, as well as mitochondrial dysfunction (Goel et al., 2022). In addition to APOE4’s effect toward AD-like pathology as outlined earlier, which have been found to be associated with neuronal damage, APOE4 is linked with heightening neuroinflammation in response to these pathologies, and overall neurodegeneration (Yamazaki et al., 2019; Goel et al., 2022). For example, APOE4 has been found to be associated with driving a pro-inflammatory environment in the brain, such as by enhancing the activation of pro-inflammatory microglia, as well as increasing pro-inflammatory cytokine levels as observed in mouse models (Vitek et al., 2009; Shi et al., 2017; Yamazaki et al., 2019). APOE4 also affects microglial and astrocytic function when compared to APOE3, such as reducing their ability to phagocytose and clear amyloid-beta plaques, respectively (Krasemann et al., 2017; Yamazaki et al., 2019; Parhizkar and Holtzman, 2022). These pathological events contribute to sustained chronic inflammation, exacerbating neuronal cell death and synaptic loss as observed in AD (Krasemann et al., 2017; Yamazaki et al., 2019; Parhizkar and Holtzman, 2022). Correspondingly, APOE4 has been examined to impair mitochondrial function such as by increasing levels of ROS production, leading to aberrant oxidative stress, as well as disrupting calcium homeostasis by enhancing the influx of calcium ions into mitochondria, all of which contribute to neurotoxicity and neuronal damage (Goel et al., 2022; Mahley, 2023; Pires and Rego, 2023). Therefore, we also infer that APOE4 contributes to the found gray matter changes in PD and DLB, potentially influencing risk and development to cognitive impairment, through pathological pathways that increase neuroinflammation and impact mitochondrial function as well.
Moreover, the association of higher WMH volumes observed in PD and DLB subjects with APOE4 may potentially be due to APOE4’s role toward vascular function as observed in AD. WMH are often presumed to be vascular in origin, representing cerebrovascular lesions as a result of ischemic changes in the brain (Alber et al., 2019). Notably, APOE4 has been associated with impairments in cerebrovascular function in AD, such as contributing to reduced cerebral blood flow, vascular density, and disrupting the integrity of the blood brain barrier (Yamazaki et al., 2019). Therefore, there is a possibility that APOE4 enhances the accumulation of WMH in PD and DLB subjects to facilitate cognitive impairment by contributing to adverse cerebrovascular changes.
Further, the association between APOE4 and reductions in functional activity and connectivity examined in PD subjects may reflect APOE4’s role toward synaptic processes, as also mentioned by Nombela et al. (2014). As observed in AD brains and APOE4 transgenic mice, APOE4 has been linked to synaptic degeneration, reductions in dendritic spine density, as well as impaired synaptic transmission (Yamazaki et al., 2019; Fernández-Calle et al., 2022). Thus, it is also possible that synaptic dysfunction linked to APOE4 may impair neuronal signaling both within and between regions, impacting functional activity and connectivity in PD subjects that may exacerbate cognitive impairment.
Lastly, reduced glucose metabolism found in specific brain regions of APOE4 subjects with PD and DLB may likely be due to APOE4’s association with glucose hypometabolism (Yamazaki et al., 2019). APOE4 has been found to reduce glucose uptake by downregulating signaling pathways as well as decreasing proteins critical for the regulation of such process as examined in preclinical studies (Keeney et al., 2015; Yamazaki et al., 2019). APOE4 has also been examined to impair insulin signaling and glycolysis when compared to APOE3 as seen in transgenic mice, such as by impairing the trafficking of insulin receptors (Zhao et al., 2017). Thus, it is inferred that APOE4 may influence decreases in glucose metabolism in those with PD and DLB that may contribute to cognitive impairment by adversely affecting mechanisms that may primarily affect glucose uptake. Overall though, all proposed mechanisms are speculative due to limited exploration within PD and DLB cohorts, with most of the current understanding derived from AD research. It is also possible that APOE4’s effect in enhancing alpha-synuclein pathology is associated with the brain changes examined, considering it a major protein pathology in PD and DLB, as well as its role in neurodegeneration independent of AD-like changes (Davis et al., 2020; Zhao et al., 2021; Calabresi et al., 2023). Therefore, more research is needed to validate what APOE4-dependent mechanisms are specifically involved in the brain changes found in those with PD and DLB that may potentially be correlated to adverse changes in cognitive function. This may also provide further insight on whether such mechanisms are similar or distinct to AD.
It should be noted also that some studies provided evidence that APOE4 may not play a role in PD-related cognitive impairment instead. Negative findings may indicate that APOE4 is not the sole factor driving cognitive impairment in PD and DLB. For example, differences in cognitive outcomes in Lewy body diseases (including PD, PDD and DLB), such as timing, severity and pace of decline, have been discussed to not only be explained by the effects of APOE4, but also by other individual factors (Carceles-Cordon et al., 2023). This includes the impact of other genes, such as GBA, SNCA, and BIN1, and environmental factors, such as traumatic injuries and pesticide exposure (Carceles-Cordon et al., 2023). Thus, this indicates that PD-related cognitive impairment and DLB cannot be fully explained by APOE status alone. In addition, factors such as family history, sex, smoking status, and cholesterol levels are discussed to explain why some APOE4 carriers do not develop AD, and why not all AD subjects carry the allele (Pires and Rego, 2023). Thus, the role of APOE4 in PD and DLB related cognitive impairment is likely complex, where a combination of environmental and genetic factors contribute to cognitive impairment. Further research is needed to clarify if APOE4 directly influences cognitive impairment in PD and DLB, or whether other factors interact with APOE4 to contribute to cognitive changes.
There are several limitations when making conclusions regarding the articles reviewed. Numerous studies have small sample sizes, which may challenge the ability to draw accurate and reliable conclusions. Future research should attempt to use larger sample sizes to better assess the association between brain changes with APOE4 in PD and DLB that may be linked to cognitive impairment. In addition, the limited availability of neuroimaging findings investigating the association of APOE4 and brain changes in PD restricts a robust understanding on how such relationship can be linked to PD-related cognitive impairment (See Figure 2). This is evident in studies analyzing changes in glucose metabolism, brain volume and activity, functional connectivity, and WMH volumes in PD APOE4 carriers, where only one study was used to evaluate potential relationships between APOE4 status, brain alterations, and cognitive function. Thus, more neuroimaging studies are needed to advance knowledge on the impact of APOE4 toward the brain that may facilitate PD-related cognitive impairment. Inconsistent results between studies are also noted, especially regarding the possible effect of APOE4 toward amyloid-beta burden for both PD and DLB, reduced cortical thickness in PD patients, and alterations in brain volumes and WMH in DLB patients (See Figure 2). Therefore, more studies are needed to clarify these conflicting findings and strengthen interpretations of APOE4’s effects on cognitive changes in PD and DLB.

Figure 2. Influences of APOE4 on brain changes in PD and DLB that may be associated with cognitive impairment. APOE4 has been found to be associated with brain changes in PD and DLB. These brain changes in PD and DLB APOE4 carriers have also been observed in diagnosed and at-risk AD subjects carrying the APOE4 allele. In PD, APOE4 may play a role toward cognitive impairment by influencing enhanced amyloid-beta protein burden and reduced cortical thickness in brain regions important for cognitive function, similarly found in the brains of those with AD. To note however, there are inconsistent findings within the literature, as other studies found no effect of APOE4 toward such brain changes. APOE4 may also be associated with increased WMH volumes, as well as decreased glucose metabolism, brain activity, and functional connectivity in those with PD, potentially leading to cognitive impairment, just like in AD as well. Yet, such inferences are based on limited data, with only one study to make conclusions. In DLB, APOE4 may play a role in cognitive impairment by influencing enhanced WMH volumes and amyloid-beta protein pathology, as well as reduced brain volume and glucose metabolism in regions essential for cognition also. However, there are limited as well as inconsistent results in the literature, with some studies reporting no association of APOE4 toward these brain changes in DLB patients. To evaluate the strength of the evidence, findings of APOE4’s effect toward the brain changes were based on consistency and the number of studies found. Brain changes with inconsistent findings, where studies reported both significant and non-significant associations with APOE4, were categorized as having “moderate” evidence for APOE4’s influence on brain changes that may lead to cognitive impairment in PD or DLB. Brain changes associated with APOE4 but only supported with a single study were classified as having “weak” evidence due to the limited data available. One study was deemed to provide “inconclusive” evidence toward the effect of APOE4 toward amyloid-beta burden in DLB, as they found significant differences in amyloid-beta during baseline but not during follow-up when comparing DLB APOE4 carriers and non-carriers.
Nevertheless, identifying the potential role of APOE4 toward the brain that may influence cognitive impairment in parkinsonian individuals can provide crucial insight on the importance of genetic factors as clinical biomarkers for this non-motor symptom. This may aid early detection of those susceptible to cognitive impairment in PD and DLB patients, overall facilitating personalized treatments to better improve patient outcomes.
Author contributions
AR: Writing – original draft, Writing – review & editing. SM: Writing – review & editing. AS: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Canadian Institute of Health Research (CIHR) (PJT-173540).
Acknowledgments
APS is supported by the Krembil-Rossy Chair program. Figures 1, 2 are created with Biorender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarsland, D., Batzu, L., Halliday, G. M., Geurtsen, G. J., Ballard, C., Ray Chaudhuri, K., et al. (2021). Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 7, 1–21. doi: 10.1038/s41572-021-00280-3
Aarsland, D., Creese, B., Politis, M., Chaudhuri, K. R., ffytche, D., Weintraub, D., et al. (2017). Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 13, 217–231. doi: 10.1038/nrneurol.2017.27
Abushakra, S., Porsteinsson, A. P., Sabbagh, M., Bracoud, L., Schaerer, J., Power, A., et al. (2020). APOE ε4/ε4 homozygotes with early Alzheimer’s disease show accelerated hippocampal atrophy and cortical thinning that correlates with cognitive decline. A&D Transl. Res. Clin. Interv. 6:e12117. doi: 10.1002/trc2.12117
Akdemir, Ü. Ö., Bora Tokçaer, A., and Atay, L. Ö. (2021). Dopamine transporter SPECT imaging in Parkinson’s disease and parkinsonian disorders. Turk. J. Med. Sci. 51, 400–410. doi: 10.3906/sag-2008-253
Akhtar, R. S., Xie, S. X., Chen, Y. J., Rick, J., Gross, R. G., Nasrallah, I. M., et al. (2017). Regional brain amyloid-β accumulation associates with domain-specific cognitive performance in Parkinson disease without dementia. PLoS One 12:e0177924. doi: 10.1371/journal.pone.0177924
Alber, J., Alladi, S., Bae, H.-J., Barton, D. A., Beckett, L. A., Bell, J. M., et al. (2019). White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): knowledge gaps and opportunities. Alzheimers Dement. (N Y) 5, 107–117. doi: 10.1016/j.trci.2019.02.001
Andrews-Zwilling, Y., Bien-Ly, N., Xu, Q., Li, G., Bernardo, A., Yoon, S. Y., et al. (2010). Apolipoprotein E4 causes age- and tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci. 30, 13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010
Apostolova, L. G., Alves, G., Hwang, K. S., Babakchanian, S., Bronnick, K., Larsen, J. P., et al. (2012). Hippocampal and ventricular changes in Parkinson’s disease mild cognitive impairment. Neurobiol. Aging 33, 2113–2124. doi: 10.1016/j.neurobiolaging.2011.06.014
Attems, J., Toledo, J. B., Walker, L., Gelpi, E., Gentleman, S., Halliday, G., et al. (2021). Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: a multi-centre study. Acta Neuropathol. 141, 159–172. doi: 10.1007/s00401-020-02255-2
Baggio, H. C., and Junqué, C. (2019). Functional MRI in Parkinson’s disease cognitive impairment. Int. Rev. Neurobiol. 144, 29–58. doi: 10.1016/bs.irn.2018.09.010
Ballard, C., O’Brien, J., Morris, C. M., Barber, R., Swann, A., Neill, D., et al. (2001). The progression of cognitive impairment in dementia with Lewy bodies, vascular dementia and Alzheimer’s disease. Int. J. Geriatr. Psychiatry 16, 499–503. doi: 10.1002/gps.381
Barber, R., Ballard, C., McKeith, I. G., Gholkar, A., and O’Brien, J. T. (2000). MRI volumetric study of dementia with Lewy bodies: a comparison with AD and vascular dementia. Neurology 54, 1304–1309. doi: 10.1212/WNL.54.6.1304
Barber, R., Gholkar, A., Scheltens, P., Ballard, C., McKeith, I. G., Morris, C. M., et al. (1999). Apolipoprotein E ⑀4 allele, temporal lobe atrophy, and white matter lesions in late-life dementias. Arch. Neurol. 56:961. doi: 10.1001/archneur.56.8.961
Barisano, G., Kisler, K., Wilkinson, B., Nikolakopoulou, A. M., Sagare, A. P., Wang, Y., et al. (2022). A “multi-omics” analysis of blood–brain barrier and synaptic dysfunction in APOE4 mice. J. Exp. Med. 219:e20221137. doi: 10.1084/jem.20221137
Bejanin, A., Schonhaut, D. R., la, R., Kramer, J. H., Baker, S. L., Sosa, N., et al. (2017). Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 140, 3286–3300. doi: 10.1093/brain/awx243
Beyer, M. K., Alves, G., Hwang, K. S., Babakchanian, S., Bronnick, K. S., Chou, Y.-Y., et al. (2013). CSF Aβ levels correlate with structural brain changes in Parkinson’s disease. Mov. Disord. 28, 302–310. doi: 10.1002/mds.25282
Blanc, F., Colloby, S. J., Cretin, B., de Sousa, P. L., Demuynck, C., O’Brien, J. T., et al. (2016). Grey matter atrophy in prodromal stage of dementia with Lewy bodies and Alzheimer’s disease. Alzheimers Res. Ther. 8:31. doi: 10.1186/s13195-016-0198-6
Bohnen, N. I., Koeppe, R. A., Minoshima, S., Giordani, B., Albin, R. L., Frey, K. A., et al. (2011). Cerebral glucose metabolic features of Parkinson disease and incident dementia: longitudinal study. J. Nucl. Med. 52, 848–855. doi: 10.2967/jnumed.111.089946
Bolsewig, K., van Unnik, A. A. J. M., Blujdea, E. R., Gonzalez, M. C., Ashton, N. J., Aarsland, D., et al. (2024). Association of plasma amyloid, P-tau, GFAP, and NfL with CSF, clinical, and cognitive features in patients with dementia with Lewy bodies. Neurology 102:e209418. doi: 10.1212/WNL.0000000000209418
Boot, B. P., Orr, C. F., Ahlskog, J. E., Ferman, T. J., Roberts, R., Pankratz, V. S., et al. (2013). Risk factors for dementia with Lewy bodies: a case-control study. Neurology 81, 833–840. doi: 10.1212/WNL.0b013e3182a2cbd1
Bousiges, O., Cretin, B., Muller, C., Botzung, A., Sanna, L., Anthony, P., et al. (2023). Involvement of ApoE4 in dementia with Lewy bodies in the prodromal and demented stages: evaluation of the Strasbourg cohort. GeroScience 46, 1527–1542. doi: 10.1007/s11357-023-00883-6
Bras, J., Guerreiro, R., Darwent, L., Parkkinen, L., Ansorge, O., Escott-Price, V., et al. (2014). Genetic analysis implicates APOE, SNCA and suggests lysosomal dysfunction in the etiology of dementia with Lewy bodies. Hum. Mol. Genet. 23, 6139–6146. doi: 10.1093/hmg/ddu334
Brecht, W. J., Harris, F. M., Chang, S., Tesseur, I., Yu, G.-Q., Xu, Q., et al. (2004). Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J. Neurosci. 24, 2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004
Brookmeyer, R., and Abdalla, N. (2018). Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimers Dement. 14, 981–988. doi: 10.1016/j.jalz.2018.03.005
Burack, M. A., Hartlein, J., Flores, H. P., Taylor-Reinwald, L., Perlmutter, J. S., and Cairns, N. J. (2010). In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology 74, 77–84. doi: 10.1212/WNL.0b013e3181c7da8e
Cacciaglia, R., Molinuevo, J. L., Falcón, C., Brugulat-Serrat, A., Sánchez-Benavides, G., Gramunt, N., et al. (2018). Effects of APOE-ε4 allele load on brain morphology in a cohort of middle-aged healthy individuals with enriched genetic risk for Alzheimer’s disease. Alzheimers Dement. 14, 902–912. doi: 10.1016/j.jalz.2018.01.016
Calabresi, P., Mechelli, A., Natale, G., Volpicelli-Daley, L., Di Lazzaro, G., and Ghiglieri, V. (2023). Alpha-synuclein in Parkinson’s disease and other synucleinopathies: from overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 14, 1–16. doi: 10.1038/s41419-023-05672-9
Campbell, M. C., Markham, J., Flores, H., Hartlein, J. M., Goate, A. M., Cairns, N. J., et al. (2013). Principal component analysis of PiB distribution in Parkinson and Alzheimer diseases. Neurology 81, 520–527. doi: 10.1212/WNL.0b013e31829e6f94
Canuet, L., Tellado, I., Couceiro, V., Fraile, C., Fernandez-Novoa, L., Ishii, R., et al. (2012). Resting-state network disruption and APOE genotype in Alzheimer’s disease: a lagged functional connectivity study. PLoS One 7:e46289. doi: 10.1371/journal.pone.0046289
Carceles-Cordon, M., Weintraub, D., and Chen-Plotkin, A. S. (2023). Cognitive heterogeneity in Parkinson’s disease: a mechanistic view. Neuron 111, 1531–1546. doi: 10.1016/j.neuron.2023.03.021
Castellano, J. M., Kim, J., Stewart, F. R., Jiang, H., DeMattos, R. B., Patterson, B. W., et al. (2011). Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 3:89ra57. doi: 10.1126/scitranslmed.3002156
Chabran, E., Noblet, V., Loureiro de Sousa, P., Demuynck, C., Philippi, N., Mutter, C., et al. (2020). Changes in gray matter volume and functional connectivity in dementia with Lewy bodies compared to Alzheimer’s disease and normal aging: implications for fluctuations. Alzheimers Res. Ther. 12:9. doi: 10.1186/s13195-019-0575-z
Chaudhuri, K. R., Healy, D. G., and Schapira, A. H. V.National Institute for Clinical Excellence (2006). Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 5, 235–245. doi: 10.1016/S1474-4422(06)70373-8
Chen, Y., and Yu, Y. (2023). Tau and neuroinflammation in Alzheimer’s disease: interplay mechanisms and clinical translation. J. Neuroinflammation 20:165. doi: 10.1186/s12974-023-02853-3
Chen, J., Zhao, D., Wang, Q., Chen, J., Bai, C., Li, Y., et al. (2023). Predictors of cognitive impairment in newly diagnosed Parkinson’s disease with normal cognition at baseline: a 5-year cohort study. Front. Aging Neurosci. 15:1142558. doi: 10.3389/fnagi.2023.1142558
Chia, R., Sabir, M. S., Bandres-Ciga, S., Saez-Atienzar, S., Reynolds, R. H., Gustavsson, E., et al. (2021). Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat. Genet. 53, 294–303. doi: 10.1038/s41588-021-00785-3
Chow, N., Aarsland, D., Honarpisheh, H., Beyer, M. K., Somme, J. H., Elashoff, D., et al. (2012). Comparing hippocampal atrophy in Alzheimer’s dementia and dementia with Lewy bodies. Dement. Geriatr. Cogn. Disord. 34, 44–50. doi: 10.1159/000339727
Chung, J., Ushakova, A., Doitsidou, M., Tzoulis, C., Tysnes, O.-B., Dalen, I., et al. (2021). The impact of common genetic variants in cognitive decline in the first seven years of Parkinson’s disease: a longitudinal observational study. Neurosci. Lett. 764:136243. doi: 10.1016/j.neulet.2021.136243
Chung, S. J., Yoo, H. S., Lee, Y. H., Lee, H. S., Ye, B. S., Sohn, Y. H., et al. (2019). Frontal atrophy as a marker for dementia conversion in Parkinson’s disease with mild cognitive impairment. Hum. Brain Mapp. 40, 3784–3794. doi: 10.1002/hbm.24631
Collins, L. M., and Williams-Gray, C. H. (2016). The genetic basis of cognitive impairment and dementia in Parkinson’s disease. Front. Psych. 7:89. doi: 10.3389/fpsyt.2016.00089
Cook, D. G., Leverenz, J. B., McMillan, P. J., Kulstad, J. J., Ericksen, S., Roth, R. A., et al. (2003). Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-epsilon4 allele. Am. J. Pathol. 162, 313–319. doi: 10.1016/s0002-9440(10)63822-9
Cosentino, S., Scarmeas, N., Helzner, E., Glymour, M. M., Brandt, J., Albert, M., et al. (2008). APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology 70, 1842–1849. doi: 10.1212/01.wnl.0000304038.37421.cc
D’Souza, T., and Rajkumar, A. P. (2020). Systematic review of genetic variants associated with cognitive impairment and depressive symptoms in Parkinson’s disease. Acta Neuropsychiatr. 32, 10–22. doi: 10.1017/neu.2019.28
Dadar, M., Zeighami, Y., Yau, Y., Fereshtehnejad, S.-M., Maranzano, J., Postuma, R. B., et al. (2018). White matter hyperintensities are linked to future cognitive decline in de novo Parkinson’s disease patients. Neuroimage Clin. 20, 892–900. doi: 10.1016/j.nicl.2018.09.025
Dalaker, T. O., Zivadinov, R., Ramasamy, D. P., Beyer, M. K., Alves, G., Bronnick, K. S., et al. (2011). Ventricular enlargement and mild cognitive impairment in early Parkinson’s disease. Mov. Disord. 26, 297–301. doi: 10.1002/mds.23443
Davis, A. A., Inman, C. E., Wargel, Z. M., Dube, U., Freeberg, B. M., Galluppi, A., et al. (2020). APOE genotype regulates pathology and disease progression in synucleinopathy. Sci. Transl. Med. 12:eaay3069. doi: 10.1126/scitranslmed.aay3069
del Campo, M., Quesada, C., Vermunt, L., Peeters, C. F. W., Hok-A-Hin, Y. S., Trieu, C., et al. (2024). CSF proteins of inflammation, proteolysis and lipid transport define preclinical AD and progression to AD dementia in cognitively unimpaired individuals. Mol. Neurodegener. 19:82. doi: 10.1186/s13024-024-00767-z
Devignes, Q., Viard, R., Betrouni, N., Carey, G., Kuchcinski, G., Defebvre, L., et al. (2021). Posterior cortical cognitive deficits are associated with structural brain alterations in mild cognitive impairment in Parkinson’s disease. Front. Aging Neurosci. 13:668559. doi: 10.3389/fnagi.2021.668559
Diaz-Galvan, P., Przybelski, S. A., Lesnick, T. G., Schwarz, C. G., Senjem, M. L., Gunter, J. L., et al. (2023). β-Amyloid load on PET along the continuum of dementia with Lewy bodies. Neurology 101, e178–e188. doi: 10.1212/WNL.0000000000207393
Dickson, D. W., Heckman, M. G., Murray, M. E., Soto, A. I., Walton, R. L., Diehl, N. N., et al. (2018). APOE ε4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology 91, e1182–e1195. doi: 10.1212/WNL.0000000000006212
Donaghy, P. C., and McKeith, I. G. (2014). The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res. Ther. 6:46. doi: 10.1186/alzrt274
Dragicevic, N., Mamcarz, M., Zhu, Y., Buzzeo, R., Tan, J., Arendash, G. W., et al. (2010). Mitochondrial amyloid-beta levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer’s transgenic mice. J. Alzheimers Dis. 20, S535–S550. doi: 10.3233/JAD-2010-100342
Drzezga, A., Riemenschneider, M., Strassner, B., Grimmer, T., Peller, M., Knoll, A., et al. (2005). Cerebral glucose metabolism in patients with AD and different APOE genotypes. Neurology 64, 102–107. doi: 10.1212/01.WNL.0000148478.39691.D3
Eisenmenger, L. B., Peret, A., Famakin, B. M., Spahic, A., Roberts, G. S., Bockholt, J. H., et al. (2023). Vascular contributions to Alzheimer’s disease. Transl. Res. 254, 41–53. doi: 10.1016/j.trsl.2022.12.003
Emrani, S., Arain, H. A., DeMarshall, C., and Nuriel, T. (2020). APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer’s disease: a systematic review. Alzheimers Res. Ther. 12:141. doi: 10.1186/s13195-020-00712-4
Ezquerra, M., Campdelacreu, J., Gaig, C., Compta, Y., Muñoz, E., Martí, M. J., et al. (2008). Lack of association of APOE and tau polymorphisms with dementia in Parkinson’s disease. Neurosci. Lett. 448, 20–23. doi: 10.1016/j.neulet.2008.10.018
Fang, C., Lv, L., Mao, S., Dong, H., and Liu, B. (2020). Cognition deficits in Parkinson’s disease: mechanisms and treatment. Parkinsons Dis. 2020, 1–11. doi: 10.1155/2020/2076942
Farrell, M. E., Chen, X., Rundle, M. M., Chan, M. Y., Wig, G. S., and Park, D. C. (2018). Regional amyloid accumulation and cognitive decline in initially amyloid-negative adults. Neurology 91, e1809–e1821. doi: 10.1212/WNL.0000000000006469
Federoff, M., Jimenez-Rolando, B., Nalls, M. A., and Singleton, A. B. (2012). A large study reveals no association between APOE and Parkinson’s disease. Neurobiol. Dis. 46, 389–392. doi: 10.1016/j.nbd.2012.02.002
Fernández, A., Vaquero, L., Bajo, R., and Zuluaga, P. (2021). Apolipoprotein E ɛ4–related effects on cognition are limited to the Alzheimer’s disease spectrum. GeroScience 44, 195–209. doi: 10.1007/s11357-021-00450-x
Fernández-Calle, R., Konings, S. C., Frontiñán-Rubio, J., García-Revilla, J., Camprubí-Ferrer, L., Svensson, M., et al. (2022). APOE in the Bullseye of neurodegenerative diseases: impact of the APOE genotype in Alzheimer’s disease pathology and brain diseases. Mol. Neurodegener. 17:62. doi: 10.1186/s13024-022-00566-4
Ferreira, D., Nedelska, Z., Graff-Radford, J., Przybelski, S. A., Lesnick, T. G., Schwarz, C. G., et al. (2021). Cerebrovascular disease, neurodegeneration, and clinical phenotype in dementia with Lewy bodies. Neurobiol. Aging 105, 252–261. doi: 10.1016/j.neurobiolaging.2021.04.029
Ferreira, D., Przybelski, S. A., Lesnick, T. G., Lemstra, A. W., Londos, E., Blanc, F., et al. (2020). β-Amyloid and tau biomarkers and clinical phenotype in dementia with Lewy bodies. Neurology 95, e3257–e3268. doi: 10.1212/WNL.0000000000010943
Ferreira, D., Przybelski, S. A., Lesnick, T. G., Schwarz, C. G., Diaz-Galvan, P., Graff-Radford, J., et al. (2023). Cross-sectional associations of β-amyloid, tau, and cerebrovascular biomarkers with neurodegeneration in probable dementia with Lewy bodies. Neurology 100, e846–e859. doi: 10.1212/WNL.0000000000201579
Fitz, N. F., Cronican, A. A., Saleem, M., Fauq, A. H., Chapman, R., Lefterov, I., et al. (2012). Abca1 deficiency affects Alzheimer’s disease-like phenotype in human ApoE4 but not in ApoE3-targeted replacement mice. J. Neurosci. 32, 13125–13136. doi: 10.1523/JNEUROSCI.1937-12.2012
Fodero-Tavoletti, M. T., Smith, D. P., McLean, C. A., Adlard, P. A., Barnham, K. J., Foster, L. E., et al. (2007). In vitro characterization of Pittsburgh compound-B binding to Lewy bodies. J. Neurosci. 27, 10365–10371. doi: 10.1523/JNEUROSCI.0630-07.2007
Foguem, C., and Manckoundia, P. (2018). Lewy body disease: clinical and pathological “overlap syndrome” between synucleinopathies (Parkinson disease) and tauopathies (Alzheimer disease). Curr. Neurol. Neurosci. Rep. 18:24. doi: 10.1007/s11910-018-0835-5
Galvin, J. E., Price, J. L., Yan, Z., Morris, J. C., and Sheline, Y. I. (2011). Resting bold fMRI differentiates dementia with Lewy bodies vs Alzheimer disease. Neurology 76, 1797–1803. doi: 10.1212/WNL.0b013e31821ccc83
Garcia-Garcia, D., Clavero, P., Gasca Salas, C., Lamet, I., Arbizu, J., Gonzalez-Redondo, R., et al. (2012). Posterior parietooccipital hypometabolism may differentiate mild cognitive impairment from dementia in Parkinson’s disease. Eur. J. Nucl. Med. Mol. Imaging 39, 1767–1777. doi: 10.1007/s00259-012-2198-5
Gasca-Salas, C., García-Lorenzo, D., Garcia-Garcia, D., Clavero, P., Obeso, J. A., Lehericy, S., et al. (2019). Parkinson’s disease with mild cognitive impairment: severe cortical thinning antedates dementia. Brain Imaging Behav. 13, 180–188. doi: 10.1007/s11682-017-9751-6
Gharbi-Meliani, A., Dugravot, A., Sabia, S., Regy, M., Fayosse, A., Schnitzler, A., et al. (2021). The association of APOE ε4 with cognitive function over the adult life course and incidence of dementia: 20 years follow-up of the Whitehall II study. Alzheimers Res. Ther. 13:5. doi: 10.1186/s13195-020-00740-0
Goel, P., Chakrabarti, S., Goel, K., Bhutani, K., Chopra, T., and Bali, S. (2022). Neuronal cell death mechanisms in Alzheimer’s disease: an insight. Front. Mol. Neurosci. 15:937133. doi: 10.3389/fnmol.2022.937133
Goldstein, I. S., Erickson, D. J., Sleeper, L. A., Haynes, R. L., and Kinney, H. C. (2017). The lateral temporal lobe in early human life. J. Neuropathol. Exp. Neurol. 76, 424–438. doi: 10.1093/jnen/nlx026
Gomperts, S. N., Locascio, J. J., Marquie, M., Santarlasci, A. L., Rentz, D. M., Maye, J., et al. (2012). Brain amyloid and cognition in Lewy body diseases. Mov. Disord. 27, 965–973. doi: 10.1002/mds.25048
Gomperts, S. N., Locascio, J. J., Rentz, D., Santarlasci, A., Marquie, M., Johnson, K. A., et al. (2013). Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology 80, 85–91. doi: 10.1212/WNL.0b013e31827b1a07
Gratwicke, J., Jahanshahi, M., and Foltynie, T. (2015). Parkinson’s disease dementia: a neural networks perspective. Brain 138, 1454–1476. doi: 10.1093/brain/awv104
Groot, C., Villeneuve, S., Smith, R., Hansson, O., and Ossenkoppele, R. (2022). Tau PET imaging in neurodegenerative disorders. J. Nucl. Med. 63, 20S–26S. doi: 10.2967/jnumed.121.263196
Guerreiro, R., Ross, O. A., Kun-Rodrigues, C., Hernandez, D. G., Orme, T., Eicher, J. D., et al. (2018). Investigating the genetic architecture of dementia with Lewy bodies: a two-stage genome-wide association study. Lancet Neurol. 17, 64–74. doi: 10.1016/S1474-4422(17)30400-3
Haikal, C., Winston, G. M., and Kaplitt, M. G. (2024). Cognitive dysfunction in animal models of human lewy-body dementia. Front. Aging Neurosci. 16:1369733. doi: 10.3389/fnagi.2024.1369733
Haller, S., Montandon, M.-L., Rodriguez, C., Ackermann, M., Herrmann, F. R., and Giannakopoulos, P. (2017). APOE*E4 is associated with Gray matter loss in the posterior cingulate cortex in healthy elderly controls subsequently developing subtle cognitive decline. Am. J. Neuroradiol. 38, 1335–1342. doi: 10.3174/ajnr.A5184
Hanganu, A., and Monchi, O. (2016). Structural neuroimaging markers of cognitive decline in Parkinson’s disease. Parkinsons Dis. 2016, 1–8. doi: 10.1155/2016/3217960
Hely, M. A., Reid, W. G. J., Adena, M. A., Halliday, G. M., and Morris, J. G. L. (2008). The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov. Disord. 23, 837–844. doi: 10.1002/mds.21956
Hepp, D. H., Vergoossen, D. L. E., Huisman, E., Lemstra, A. W., Netherlands Brain BankBerendse, H. W., et al. (2016). Distribution and load of amyloid-β pathology in Parkinson disease and dementia with Lewy bodies. J. Neuropathol. Exp. Neurol. 75, 936–945. doi: 10.1093/jnen/nlw070
Hou, Y., and Shang, H. (2022). Magnetic resonance imaging markers for cognitive impairment in Parkinson’s disease: current view. Front. Aging Neurosci. 14:788846. doi: 10.3389/fnagi.2022.788846
Huang, Y.-W. A., Zhou, B., Wernig, M., and Südhof, T. C. (2017). ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Aβ secretion. Cell 168, 427–441.e21. doi: 10.1016/j.cell.2016.12.044
Huertas, I., Jesús, S., García-Gómez, F. J., Lojo, J. A., Bernal-Bernal, I., Bonilla-Toribio, M., et al. (2017). Genetic factors influencing frontostriatal dysfunction and the development of dementia in Parkinson’s disease. PLoS One 12:e0175560. doi: 10.1371/journal.pone.0175560
Hunsberger, H. C., Pinky, P. D., Smith, W., Suppiramaniam, V., and Reed, M. N. (2019). The role of APOE4 in Alzheimer’s disease: strategies for future therapeutic interventions. Neuronal Signal. 3:NS20180203. doi: 10.1042/NS20180203
Husain, M. A., Laurent, B., and Plourde, M. (2021). APOE and Alzheimer’s disease: from lipid transport to physiopathology and therapeutics. Front. Neurosci. 15:630502. doi: 10.3389/fnins.2021.630502
Huynh, T.-P. V., Liao, F., Francis, C. M., Robinson, G. O., Serrano, J. R., Jiang, H., et al. (2017). Age-dependent effects of apoE reduction using antisense oligonucleotides in a model of β-amyloidosis. Neuron 96, 1013–1023.e4. doi: 10.1016/j.neuron.2017.11.014
Irwin, D. J., Lee, V. M.-Y., and Trojanowski, J. Q. (2013). Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci. 14, 626–636. doi: 10.1038/nrn3549
Irwin, D. J., White, M. T., Toledo, J. B., Xie, S. X., Robinson, J. L., van, V., et al. (2012). Neuropathologic substrates of Parkinson disease dementia. Ann. Neurol. 72, 587–598. doi: 10.1002/ana.23659
Jack, C. R., Wiste, H. J., Weigand, S. D., Rocca, W. A., Knopman, D. S., Mielke, M. M., et al. (2014). Age-specific population frequencies of cerebral β-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: a cross-sectional study. Lancet Neurol. 13, 997–1005. doi: 10.1016/S1474-4422(14)70194-2
Jahn, H. (2013). Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 15, 445–454. doi: 10.31887/DCNS.2013.15.4/hjahn
Jansen, W. J., Ossenkoppele, R., Knol, D. L., Tijms, B. M., Scheltens, P., Verhey, F. R. J., et al. (2015). Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313, 1924–1938. doi: 10.1001/jama.2015.4668
Jawabri, K. H., and Sharma, S. (2023). “Physiology, cerebral cortex functions” in StatPearls ((Treasure Island (FL): StatPearls Publishing).
Jellinger, K. A., and Korczyn, A. D. (2018). Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med. 16:34. doi: 10.1186/s12916-018-1016-8
Jin, Y., Li, F., Sonoustoun, B., Kondru, N. C., Martens, Y. A., Qiao, W., et al. (2022). APOE4 exacerbates α-synuclein seeding activity and contributes to neurotoxicity in Alzheimer’s disease with Lewy body pathology. Acta Neuropathol. 143, 641–662. doi: 10.1007/s00401-022-02421-8
Joki, H., Higashiyama, Y., Nakae, Y., Kugimoto, C., Doi, H., Kimura, K., et al. (2018). White matter hyperintensities on MRI in dementia with Lewy bodies, Parkinson’s disease with dementia, and Alzheimer’s disease. J. Neurol. Sci. 385, 99–104. doi: 10.1016/j.jns.2017.12.018
Jung, J. H., Jeon, S., Baik, K., Lee, Y. H., Chung, S. J., Yoo, H. S., et al. (2021). Apolipoprotein E4, amyloid, and cognition in Alzheimer’s and Lewy body disease. Neurobiol. Aging 106, 45–54. doi: 10.1016/j.neurobiolaging.2021.06.004
Kaivola, K., Shah, Z., Chia, R., and Scholz, S. W. (2021). Genetic evaluation of dementia with Lewy bodies implicates distinct disease subgroups. Brain 145, 1757–1762. doi: 10.1093/brain/awab402
Kalaitzakis, M. E., Graeber, M. B., Gentleman, S. M., and Pearce, R. K. B. (2008). Striatal β-amyloid deposition in Parkinson disease with dementia. J. Neuropathol. Exp. Neurol. 67, 155–161. doi: 10.1097/NEN.0b013e31816362aa
Kalia, L. V., and Lang, A. E. (2015). Parkinson’s disease. Lancet 386, 896–912. doi: 10.1016/S0140-6736(14)61393-3
Kandel, B. M., Avants, B. B., Gee, J. C., McMillan, C. T., Erus, G., Doshi, J., et al. (2016). White matter hyperintensities are more highly associated with preclinical Alzheimer’s disease than imaging and cognitive markers of neurodegeneration. Alzheimers Dement. (Amst) 4, 18–27. doi: 10.1016/j.dadm.2016.03.001
Kanekiyo, T., Xu, H., and Bu, G. (2014). ApoE and Aβ in Alzheimer’s disease: accidental encounters or partners? Neuron 81, 740–754. doi: 10.1016/j.neuron.2014.01.045
Kantarci, K., Lowe, V. J., Chen, Q., Przybelski, S. A., Lesnick, T. G., Schwarz, C. G., et al. (2020). β-Amyloid PET and neuropathology in dementia with Lewy bodies. Neurology 94, e282–e291. doi: 10.1212/WNL.0000000000008818
Keeney, J. T.-R., Ibrahimi, S., and Zhao, L. (2015). Human ApoE isoforms differentially modulate glucose and amyloid metabolic pathways in female brain: evidence of the mechanism of neuroprotection by ApoE2 and implications for Alzheimer’s disease prevention and early intervention. J. Alzheimers Dis. 48, 411–424. doi: 10.3233/JAD-150348
Kim, R., Park, S., Yoo, D., Jun, J.-S., and Jeon, B. (2021a). Association of physical activity and APOE genotype with longitudinal cognitive change in early Parkinson disease. Neurology 96, e2429–e2437. doi: 10.1212/WNL.0000000000011852
Kim, R., Park, S., Yoo, D., Jun, J.-S., and Jeon, B. (2021b). Impact of the apolipoprotein E ε4 allele on early Parkinson’s disease progression. Parkinsonism Relat. Disord. 83, 66–70. doi: 10.1016/j.parkreldis.2021.01.004
Koshimori, Y., Segura, B., Christopher, L., Lobaugh, N., Duff-Canning, S., Mizrahi, R., et al. (2015). Imaging changes associated with cognitive abnormalities in Parkinson’s disease. Brain Struct. Funct. 220, 2249–2261. doi: 10.1007/s00429-014-0785-x
Krasemann, S., Madore, C., Cialic, R., Baufeld, C., Calcagno, N., el, R., et al. (2017). The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581.e9. doi: 10.1016/j.immuni.2017.08.008
Kuang, H., Tan, C., Tian, H., Liu, L., Yang, M., Hong, F., et al. (2019). Exploring the bi-directional relationship between autophagy and Alzheimer’s disease. CNS Neurosci. Ther. 26, 155–166. doi: 10.1111/cns.13216
Kumar, V., Kim, S.-H., and Bishayee, K. (2022). Dysfunctional glucose metabolism in Alzheimer’s disease onset and potential pharmacological interventions. Int. J. Mol. Sci. 23:9540. doi: 10.3390/ijms23179540
Kurz, M. W., Dekomien, G., Nilsen, O. B., Larsen, J. P., Aarsland, D., and Alves, G. (2009). APOE alleles in Parkinson disease and their relationship to cognitive decline: a population-based, longitudinal study. J. Geriatr. Psychiatry Neurol. 22, 166–170. doi: 10.1177/0891988709332945
Lashley, T., Holton, J. L., Gray, E., Kirkham, K., O’Sullivan, S. S., Hilbig, A., et al. (2008). Cortical α-synuclein load is associated with amyloid-β plaque burden in a subset of Parkinson’s disease patients. Acta Neuropathol. 115, 417–425. doi: 10.1007/s00401-007-0336-0
Lecca, D., Jung, Y. J., Scerba, M. T., Hwang, I., Kim, Y. K., Kim, S., et al. (2022). Role of chronic neuroinflammation in neuroplasticity and cognitive function: a hypothesis. Alzheimers Dement. 18, 2327–2340. doi: 10.1002/alz.12610
Li, L., Ji, B., Zhao, T., Cui, X., Chen, J., and Wang, Z. (2022). The structural changes of gray matter in Parkinson disease patients with mild cognitive impairments. PLoS One 17:e0269787. doi: 10.1371/journal.pone.0269787
Lim, E. W., Aarsland, D., Ffytche, D., Taddei, R. N., van Wamelen, D. J., Wan, Y.-M., et al. (2019). Amyloid-β and Parkinson’s disease. J. Neurol. 266, 2605–2619. doi: 10.1007/s00415-018-9100-8
Lim, Y. Y., Maruff, P., Pietrzak, R. H., Ames, D., Ellis, K. A., Harrington, K., et al. (2014). Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain 137, 221–231. doi: 10.1093/brain/awt286
Lind, J., Persson, J., Ingvar, M., Larsson, A., Cruts, M., van, C., et al. (2006). Reduced functional brain activity response in cognitively intact apolipoprotein E ε4 carriers. Brain 129, 1240–1248. doi: 10.1093/brain/awl054
Linortner, P., McDaniel, C., Shahid, M., Levine, T. F., Tian, L., Cholerton, B., et al. (2020). White matter hyperintensities related to Parkinson’s disease executive function. Mov. Disord. Clin. Pract. 7, 629–638. doi: 10.1002/mdc3.12956
Liu, H., Deng, B., Xie, F., Yang, X., Xie, Z., Chen, Y., et al. (2021). The influence of white matter hyperintensity on cognitive impairment in Parkinson’s disease. Ann. Clin. Transl. Neurol. 8, 1917–1934. doi: 10.1002/acn3.51429
Liu, C.-C., Kanekiyo, T., Xu, H., and Bu, G. (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nat. Rev. Neurol. 9, 106–118. doi: 10.1038/nrneurol.2012.263
Liu, J.-Y., Ma, L.-Z., Wang, J., Cui, X.-J., Sheng, Z.-H., Fu, Y., et al. (2023). Age-related association between APOE ɛ4 and cognitive progression in de novo Parkinson’s disease. J. Alzheimers Dis. 91, 1121–1132. doi: 10.3233/JAD-220976
Liu, Y., Paajanen, T., Westman, E., Wahlund, L.-O., Simmons, A., Tunnard, C., et al. (2010). Effect of APOE ε4 allele on cortical thicknesses and volumes: the AddNeuroMed study. J. Alzheimers Dis. 21, 947–966. doi: 10.3233/JAD-2010-100201
Liu, S., Suzuki, H., Ito, H., Korenaga, T., Akatsu, H., Meno, K., et al. (2019). Serum levels of proteins involved in amyloid-β clearance are related to cognitive decline and neuroimaging changes in mild cognitive impairment. Alzheimers Dement. (Amst) 11, 85–97. doi: 10.1016/j.dadm.2018.11.003
Liu, C.-C., Zhao, N., Fu, Y., Wang, N., Linares, C., Tsai, C.-W., et al. (2017). ApoE4 accelerates early seeding of amyloid pathology. Neuron 96, 1024–1032.e3. doi: 10.1016/j.neuron.2017.11.013
Lockhart, A., Lamb, J. R., Osredkar, T., Sue, L. I., Joyce, J. N., Ye, L., et al. (2007). PIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosis. Brain 130, 2607–2615. doi: 10.1093/brain/awm191
Loera-Valencia, R., Goikolea, J., Parrado-Fernandez, C., Merino-Serrais, P., and Maioli, S. (2019). Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: potential novel targets for treatment. J. Steroid Biochem. Mol. Biol. 190, 104–114. doi: 10.1016/j.jsbmb.2019.03.003
Maetzler, W., Liepelt, I., Reimold, M., Reischl, G., Solbach, C., Becker, C., et al. (2009). Cortical PIB binding in Lewy body disease is associated with Alzheimer-like characteristics. Neurobiol. Dis. 34, 107–112. doi: 10.1016/j.nbd.2008.12.008
Maetzler, W., Reimold, M., Liepelt, I., Solbach, C., Leyhe, T., Schweitzer, K., et al. (2008). [11C]PIB binding in Parkinson’s disease dementia. NeuroImage 39, 1027–1033. doi: 10.1016/j.neuroimage.2007.09.072
Mahley, R. W. (2023). Apolipoprotein E4 targets mitochondria and the mitochondria-associated membrane complex in neuropathology, including Alzheimer’s disease. Curr. Opin. Neurobiol. 79:102684. doi: 10.1016/j.conb.2023.102684
Main, B. S., Villapol, S., Sloley, S. S., Barton, D. J., Parsadanian, M., Agbaegbu, C., et al. (2018). Apolipoprotein E4 impairs spontaneous blood brain barrier repair following traumatic brain injury. Mol. Neurodegener. 13:17. doi: 10.1186/s13024-018-0249-5
Marinus, J., Zhu, K., Marras, C., Aarsland, D., and van Hilten, J. J. (2018). Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol. 17, 559–568. doi: 10.1016/S1474-4422(18)30127-3
Marquié, M., Verwer, E. E., Meltzer, A. C., Kim, S. J. W., Agüero, C., Gonzalez, J., et al. (2017). Lessons learned about [F-18]-AV-1451 off-target binding from an autopsy-confirmed Parkinson’s case. Acta Neuropathol. Commun. 5:75. doi: 10.1186/s40478-017-0482-0
Martens, Y. A., Zhao, N., Liu, C.-C., Kanekiyo, T., Yang, A. J., Goate, A. M., et al. (2022). ApoE Cascade hypothesis in the pathogenesis of Alzheimer’s disease and related dementias. Neuron 110, 1304–1317. doi: 10.1016/j.neuron.2022.03.004
Mashima, K., Ito, D., Kameyama, M., Osada, T., Tabuchi, H., Nihei, Y., et al. (2017). Extremely low prevalence of amyloid positron emission tomography positivity in Parkinson’s disease without dementia. Eur. Neurol. 77, 231–237. doi: 10.1159/000464322
Mata, I. F., Leverenz, J. B., Weintraub, D., Trojanowski, J. Q., Hurtig, H. I., van, V., et al. (2014). APOE, MAPT, and SNCA genes and cognitive performance in Parkinson disease. JAMA Neurol. 71, 1405–1412. doi: 10.1001/jamaneurol.2014.1455
McKeith, I. G., Dickson, D. W., Lowe, J., Emre, M., O’Brien, J. T., Feldman, H., et al. (2005). Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65, 1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1
Mena, A. M., and Strafella, A. P. (2022). Imaging pathological tau in atypical Parkinsonisms: a review. Clin. Park. Relat. Disord. 7:100155. doi: 10.1016/j.prdoa.2022.100155
Meng, X., Li, T., Wang, X., Lv, X., Sun, Z., Zhang, J., et al. (2019). Association between increased levels of amyloid-β oligomers in plasma and episodic memory loss in Alzheimer’s disease. Alzheimers Res. Ther. 11:89. doi: 10.1186/s13195-019-0535-7
Mengel, D., Dams, J., Ziemek, J., Becker, J., Balzer-Geldsetzer, M., Hilker, R., et al. (2016). Apolipoprotein E ε4 does not affect cognitive performance in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 29, 112–116. doi: 10.1016/j.parkreldis.2016.04.013
Meyer, P. T., Frings, L., Rücker, G., and Hellwig, S. (2017). 18F-FDG PET in parkinsonism: differential diagnosis and evaluation of cognitive impairment. J. Nucl. Med. 58, 1888–1898. doi: 10.2967/jnumed.116.186403
Mihaescu, A. S., Valli, M., Uribe, C., Diez-Cirarda, M., Masellis, M., Graff-Guerrero, A., et al. (2022). Beta amyloid deposition and cognitive decline in Parkinson’s disease: a study of the PPMI cohort. Mol. Brain 15:79. doi: 10.1186/s13041-022-00964-1
Mirza, S. S., Saeed, U., Knight, J., Ramirez, J., Stuss, D. T., Keith, J., et al. (2019). APOE ε4, white matter hyperintensities, and cognition in Alzheimer and Lewy body dementia. Neurology 93, e1807–e1819. doi: 10.1212/WNL.0000000000008377
Monastero, R., Cicero, C. E., Baschi, R., Davì, M., Luca, A., Restivo, V., et al. (2018). Mild cognitive impairment in Parkinson’s disease: the Parkinson’s disease cognitive study (PACOS). J. Neurol. 265, 1050–1058. doi: 10.1007/s00415-018-8800-4
Monsell, S. E., Besser, L. M., Heller, K. B., Checkoway, H., Litvan, I., and Kukull, W. A. (2014). Clinical and pathologic presentation in Parkinson’s disease by apolipoprotein e4 allele status. Parkinsonism Relat. Disord. 20, 503–507. doi: 10.1016/j.parkreldis.2014.02.001
Montagne, A., Nation, D. A., and Zlokovic, B. V. (2020). APOE4 accelerates development of dementia after stroke: is there a role for cerebrovascular dysfunction? Stroke 51, 699–700. doi: 10.1161/STROKEAHA.119.028814
Morley, J. F., Xie, S. X., Hurtig, H. I., Stern, M. B., Colcher, A., Horn, S., et al. (2012). Genetic influences on cognitive decline in Parkinson’s disease. Mov. Disord. 27, 512–518. doi: 10.1002/mds.24946
Morra, L. F., and Donovick, P. J. (2014). Clinical presentation and differential diagnosis of dementia with Lewy bodies: a review. Int. J. Geriatr. Psychiatry 29, 569–576. doi: 10.1002/gps.4039
Myers, P. S., O’Donnell, J. L., Jackson, J. J., Lessov-Schlaggar, C. N., Miller, R. L., Foster, E. R., et al. (2022). Proteinopathy and longitudinal cognitive decline in Parkinson disease. Neurology 99, e66–e76. doi: 10.1212/WNL.0000000000200344
Nasri, A., Sghaier, I., Gharbi, A., Mrabet, S., Ben Djebara, M., Gargouri, A., et al. (2022). Role of Apolipoprotein E in the clinical profile of atypical parkinsonian syndromes. Alzheimer Dis. Assoc. Disord. 36, 36–43. doi: 10.1097/WAD.0000000000000479
Nechushtai, L., Frenkel, D., and Pinkas-Kramarski, R. (2023). Autophagy in Parkinson’s disease. Biomol. Ther. 13:1435. doi: 10.3390/biom13101435
Nedelska, Z., Schwarz, C. G., Lesnick, T. G., Boeve, B. F., Przybelski, S. A., Lowe, V. J., et al. (2019). Association of longitudinal β-amyloid accumulation determined by positron emission tomography with clinical and cognitive decline in adults with probable Lewy body dementia. JAMA Netw. Open 2:e1916439. doi: 10.1001/jamanetworkopen.2019.16439
Nelson, P. T., Kryscio, R. J., Jicha, G. A., Abner, E. L., Schmitt, F. A., Xu, L. O., et al. (2009). Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology 73, 1127–1133. doi: 10.1212/WNL.0b013e3181bacf9e
Nicastro, N., Malpetti, M., Mak, E., Williams, G. B., Bevan-Jones, W. R., Carter, S. F., et al. (2020). Gray matter changes related to microglial activation in Alzheimer’s disease. Neurobiol. Aging 94, 236–242. doi: 10.1016/j.neurobiolaging.2020.06.010
Nicoletti, G., Manners, D. N., Novellino, F., Testa, C., Gagliardi, M., Tonon, C., et al. (2016). Voxel-based morphometry to detect effect of APOE on brain gray matter changes in Parkinson’s disease. Psychiatry Res. Neuroimaging 254, 177–179. doi: 10.1016/j.pscychresns.2016.07.006
Noh, S. W., Han, Y. H., Mun, C. W., Chung, E. J., Kim, E. G., Ji, K. H., et al. (2014). Analysis among cognitive profiles and gray matter volume in newly diagnosed Parkinson’s disease with mild cognitive impairment. J. Neurol. Sci. 347, 210–213. doi: 10.1016/j.jns.2014.09.049
Nombela, C., Rowe, J. B., Winder-Rhodes, S. E., Hampshire, A., Owen, A. M., Breen, D. P., et al. (2014). Genetic impact on cognition and brain function in newly diagnosed Parkinson’s disease: ICICLE-PD study. Brain 137, 2743–2758. doi: 10.1093/brain/awu201
O’Callaghan, C., and Lewis, S. J. G. (2017). Cognition in Parkinson’s disease. Int. Rev. Neurobiol. 133, 557–583. doi: 10.1016/bs.irn.2017.05.002
Okubadejo, N. U., Okunoye, O., Ojo, O. O., Arabambi, B., Akinyemi, R. O., Osaigbovo, G. O., et al. (2022). APOE E4 is associated with impaired self-declared cognition but not disease risk or age of onset in Nigerians with Parkinson’s disease. NPJ Parkinsons Dis. 8:155. doi: 10.1038/s41531-022-00411-x
Onitsuka, T., Shenton, M. E., Dickey, C. C., Kasai, K., Toner, S. K., Frumin, M., et al. (2004). Middle and inferior temporal gyrus Gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am. J. Psychiatry 161, 1603–1611. doi: 10.1176/appi.ajp.161.9.1603
Ossenkoppele, R., van der Flier, W. M., Zwan, M. D., Adriaanse, S. F., Boellaard, R., Windhorst, A. D., et al. (2013). Differential effect of APOE genotype on amyloid load and glucose metabolism in AD dementia. Neurology 80, 359–365. doi: 10.1212/WNL.0b013e31827f0889
Outeiro, T. F., Koss, D. J., Erskine, D., Walker, L., Kurzawa-Akanbi, M., Burn, D., et al. (2019). Dementia with Lewy bodies: an update and outlook. Mol. Neurodegener. 14:5. doi: 10.1186/s13024-019-0306-8
Pagonabarraga, J., and Kulisevsky, J. (2012). Cognitive impairment and dementia in Parkinson’s disease. Neurobiol. Dis. 46, 590–596. doi: 10.1016/j.nbd.2012.03.029
Pang, K., Jiang, R., Zhang, W., Yang, Z., Li, L.-L., Shimozawa, M., et al. (2022). An app knock-in rat model for Alzheimer’s disease exhibiting Aβ and tau pathologies, neuronal death and cognitive impairments. Cell Res. 32, 157–175. doi: 10.1038/s41422-021-00582-x
Pankratz, N., Byder, L., Halter, C., Rudolph, A., Shults, C. W., Conneally, P. M., et al. (2006). Presence of an APOE4 allele results in significantly earlier onset of Parkinson’s disease and a higher risk with dementia. Mov. Disord. 21, 45–49. doi: 10.1002/mds.20663
Parhizkar, S., and Holtzman, D. M. (2022). APOE mediated neuroinflammation and neurodegeneration in Alzheimer’s disease. Semin. Immunol. 59:101594. doi: 10.1016/j.smim.2022.101594
Parsian, A., Racette, B., Goldsmith, L. J., and Perlmutter, J. S. (2002). Parkinson’s disease and Apolipoprotein E: possible association with dementia but not age at onset. Genomics 79, 458–461. doi: 10.1006/geno.2002.6707
Paslawski, W., Zareba-Paslawska, J., Zhang, X., Hölzl, K., Wadensten, H., Shariatgorji, M., et al. (2019). α-Synuclein−lipoprotein interactions and elevated ApoE level in cerebrospinal fluid from Parkinson’s disease patients. Proc. Natl. Acad. Sci. USA 116, 15226–15235. doi: 10.1073/pnas.1821409116
Paul, K. C., Rausch, R., Creek, M. M., Sinsheimer, J. S., Bronstein, J. M., Bordelon, Y., et al. (2016). APOE, MAPT, and COMT and Parkinson’s disease susceptibility and cognitive symptom progression. J. Parkinsons Dis. 6, 349–359. doi: 10.3233/JPD-150762
Pavlova, R., Mehrabian, S., Petrova, M., Skelina, S., Mihova, K., Jordanova, A., et al. (2014). Cognitive, neuropsychiatric, and motor features associated with Apolipoprotein E ∊4 allele in a sample of Bulgarian patients with late-onset Parkinson’s disease. Am. J. Alzheimers Dis. Other Dement. 29, 614–619. doi: 10.1177/1533317514525655
Pedersen, K. F., Larsen, J. P., Tysnes, O.-B., and Alves, G. (2017). Natural course of mild cognitive impairment in Parkinson disease: a 5-year population-based study. Neurology 88, 767–774. doi: 10.1212/WNL.0000000000003634
Peraza, L. R., Kaiser, M., Firbank, M., Graziadio, S., Bonanni, L., Onofrj, M., et al. (2014). fMRI resting state networks and their association with cognitive fluctuations in dementia with Lewy bodies. NeuroImage Clin. 4, 558–565. doi: 10.1016/j.nicl.2014.03.013
Perrotin, A., Mormino, E. C., Madison, C. M., Hayenga, A. O., and Jagust, W. J. (2012). Subjective cognition and amyloid deposition imaging: a Pittsburgh compound B positron emission tomography study in normal elderly individuals. Arch. Neurol. 69, 223–229. doi: 10.1001/archneurol.2011.666
Petrou, M., Dwamena, B. A., Foerster, B. R., MacEachern, M. P., Bohnen, N. I., Müller, M. L., et al. (2015). Amyloid deposition in Parkinson’s disease and cognitive impairment: a systematic review. Mov. Disord. 30, 928–935. doi: 10.1002/mds.26191
Piccarducci, R., Giacomelli, C., Bertilacchi, M. S., Benito-Martinez, A., Di Giorgi, N., Daniele, S., et al. (2023). Apolipoprotein E ε4 triggers neurotoxicity via cholesterol accumulation, acetylcholine dyshomeostasis, and PKCε mislocalization in cholinergic neuronal cells. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 1869:166793. doi: 10.1016/j.bbadis.2023.166793
Pierzchlińska, A., Białecka, M., Kurzawski, M., and Sławek, J. (2018). The impact of Apolipoprotein E alleles on cognitive performance in patients with Parkinson’s disease. Neurol. Neurochir. Pol. 52, 477–482. doi: 10.1016/j.pjnns.2018.04.003
Pires, M., and Rego, A. C. (2023). Apoe4 and Alzheimer’s disease pathogenesis—mitochondrial deregulation and targeted therapeutic strategies. Int. J. Mol. Sci. 24:778. doi: 10.3390/ijms24010778
Qian, J., Wolters, F. J., Beiser, A., Haan, M., Ikram, M. A., Karlawish, J., et al. (2017). APOE-related risk of mild cognitive impairment and dementia for prevention trials: An analysis of four cohorts. PLoS Med. 14:e1002254. doi: 10.1371/journal.pmed.1002254
Ramanan, V. K., Lesnick, T. G., Przybelski, S. A., Heckman, M. G., Knopman, D. S., Graff-Radford, J., et al. (2021). Coping with brain amyloid: genetic heterogeneity and cognitive resilience to Alzheimer’s pathophysiology. Acta Neuropathol. Commun. 9:48. doi: 10.1186/s40478-021-01154-1
Rane, S., Donahue, M. J., and Claassen, D. O. (2018). Amnestic mild cognitive impairment individuals with dissimilar pathologic origins show common regional vulnerability in the default mode network. Alzheimers Dement. (Amst) 10, 717–725. doi: 10.1016/j.dadm.2018.08.004
Rapp, A., Gmeiner, B., and Hüttinger, M. (2006). Implication of apoE isoforms in cholesterol metabolism by primary rat hippocampal neurons and astrocytes. Biochimie 88, 473–483. doi: 10.1016/j.biochi.2005.10.007
Raulin, A.-C., Doss, S. V., Trottier, Z. A., Ikezu, T. C., Bu, G., and Liu, C.-C. (2022). ApoE in Alzheimer’s disease: pathophysiology and therapeutic strategies. Mol. Neurodegener. 17:72. doi: 10.1186/s13024-022-00574-4
Rawle, M. J., Davis, D., Bendayan, R., Wong, A., Kuh, D., and Richards, M. (2018). Apolipoprotein-E (Apoe) ε4 and cognitive decline over the adult life course. Transl. Psychiatry 8, 1–8. doi: 10.1038/s41398-017-0064-8
Real, R., Martinez-Carrasco, A., Reynolds, R. H., Lawton, M. A., Tan, M. M. X., Shoai, M., et al. (2023). Association between the LRP1B and APOE loci and the development of Parkinson’s disease dementia. Brain 146, 1873–1887. doi: 10.1093/brain/awac414
Reitz, C., and Mayeux, R. (2010). Use of genetic variation as biomarkers for mild cognitive impairment and progression of mild cognitive impairment to dementia. J. Alzheimers Dis. 19, 229–251. doi: 10.3233/JAD-2010-1255
Rohn, T. T., and Mack, J. M. (2018). Apolipoprotein E fragmentation within Lewy bodies of the human Parkinson’s disease brain. Int. J. Neurodegener. Dis. 1:002. doi: 10.23937/IJND-2017/1710002
Rongve, A., Witoelar, A., Ruiz, A., Athanasiu, L., Abdelnour, C., Clarimon, J., et al. (2019). GBA and APOE ε4 associate with sporadic dementia with Lewy bodies in European genome wide association study. Sci. Rep. 9:7013. doi: 10.1038/s41598-019-43458-2
Rowe, C. C., Ng, S., Ackermann, U., Gong, S. J., Pike, K., Savage, G., et al. (2007). Imaging beta-amyloid burden in aging and dementia. Neurology 68, 1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea
Saeed, U., Mirza, S. S., MacIntosh, B. J., Herrmann, N., Keith, J., Ramirez, J., et al. (2018). APOE -ε4 associates with hippocampal volume, learning, and memory across the spectrum of Alzheimer’s disease and dementia with Lewy bodies. Alzheimers Dement. 14, 1137–1147. doi: 10.1016/j.jalz.2018.04.005
Samat, N. A., Abdul Murad, N. A., Mohamad, K., Abdul Razak, M. R., and Mohamed Ibrahim, N. (2017). Apolipoprotein Eε4: a biomarker for executive dysfunction among Parkinson’s disease patients with mild cognitive impairment. Front. Neurosci. 11:712. doi: 10.3389/fnins.2017.00712
Sampedro, F., Marín-Lahoz, J., Martínez-Horta, S., Pagonabarraga, J., and Kulisevsky, J. (2019). Pattern of cortical thinning associated with the BDNF Val66Met polymorphism in Parkinson’s disease. Behav. Brain Res. 372:112039. doi: 10.1016/j.bbr.2019.112039
Santangelo, G., Vitale, C., Picillo, M., Moccia, M., Cuoco, S., Longo, K., et al. (2015). Mild cognitive impairment in newly diagnosed Parkinson’s disease: a longitudinal prospective study. Parkinsonism Relat. Disord. 21, 1219–1226. doi: 10.1016/j.parkreldis.2015.08.024
Sasikumar, S., and Strafella, A. P. (2020). Imaging mild cognitive impairment and dementia in Parkinson’s disease. Front. Neurol. 11:47. doi: 10.3389/fneur.2020.00047
Segura, B., Baggio, H. C., Marti, M. J., Valldeoriola, F., Compta, Y., Garcia-Diaz, A. I., et al. (2014). Cortical thinning associated with mild cognitive impairment in Parkinson’s disease. Mov. Disord. 29, 1495–1503. doi: 10.1002/mds.25982
Serrano-Pozo, A., Das, S., and Hyman, B. T. (2021). APOE and Alzheimer’s disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 20, 68–80. doi: 10.1016/S1474-4422(20)30412-9
Shang, S., Chen, Y.-C., Zhang, H., Dou, W., Qian, L., Yin, X., et al. (2020). Mapping the interactive effects of ApoE gene polymorphism on caudate functional connectivity in mild cognitive impairment associated with Parkinson’s disease. Front. Neurosci. 14:857. doi: 10.3389/fnins.2020.00857
Sharma, N. K., Robbins, K., Wagner, K., and Colgrove, Y. M. (2015). A randomized controlled pilot study of the therapeutic effects of yoga in people with Parkinson’s disease. Int. J. Yoga 8, 74–79. doi: 10.4103/0973-6131.146070
Shi, Y., Yamada, K., Liddelow, S. A., Smith, S. T., Zhao, L., Luo, W., et al. (2017). ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527. doi: 10.1038/nature24016
Shiner, T., Mirelman, A., Rosenblum, Y., Kavé, G., Weisz, M. G., Bar-Shira, A., et al. (2021). The effect of GBA mutations and APOE polymorphisms on dementia with Lewy bodies in Ashkenazi Jews. J. Alzheimers Dis. 80, 1221–1229. doi: 10.3233/JAD-201295
Slooter, A. J. C., Cruts, M., Kalmijn, S., Hofman, A., Breteler, M. M. B., van, C., et al. (1998). Risk estimates of dementia by Apolipoprotein E genotypes from a population-based incidence study: the Rotterdam study. Arch. Neurol. 55, 964–968. doi: 10.1001/archneur.55.7.964
Spampinato, M. V., Rumboldt, Z., Hosker, R. J., and Mintzer, J. E. (2011). Apolipoprotein E and Gray matter volume loss in patients with mild cognitive impairment and Alzheimer disease. Radiology 258, 843–852. doi: 10.1148/radiol.10100307
Szwedo, A. A., Dalen, I., Pedersen, K. F., Camacho, M., Bäckström, D., Forsgren, L., et al. (2022). GBA and APOE impact cognitive decline in Parkinson’s disease: a 10-year population-based study. Mov. Disord. 37, 1016–1027. doi: 10.1002/mds.28932
Tan, M. M. X., Lawton, M. A., Jabbari, E., Reynolds, R. H., Iwaki, H., Blauwendraat, C., et al. (2021). Genome-wide association studies of cognitive and motor progression in Parkinson’s disease. Mov. Disord. 36, 424–433. doi: 10.1002/mds.28342
Tesseur, I., Van Dorpe, J., Spittaels, K., Van den Haute, C., Moechars, D., and Van Leuven, F. (2000). Expression of human apolipoprotein E4 in neurons causes hyperphosphorylation of protein tau in the brains of transgenic mice. Am. J. Pathol. 156, 951–964. doi: 10.1016/S0002-9440(10)64963-2
Theendakara, V., Peters-Libeu, C. A., Bredesen, D. E., and Rao, R. V. (2018). Transcriptional effects of ApoE4: relevance to Alzheimer’s disease. Mol. Neurobiol. 55, 5243–5254. doi: 10.1007/s12035-017-0757-2
Tipton, P. W., Bülbül, N., Crook, J., Quicksall, Z., Ross, O. A., Uitti, R. J., et al. (2021). Effects of sex and APOE on Parkinson’s disease-related cognitive decline. Neurol. Neurochir. Pol. 55, 559–566. doi: 10.5603/PJNNS.a2021.0071
Triarhou, L. C. (2013). “Dopamine and Parkinson’s disease” in Madame curie bioscience database [internet], (Austin, TX: Landes Bioscience). Available at: https://www.ncbi.nlm.nih.gov/books/NBK6271/ (Accessed November 28, 2023).
Trivedi, M. A., Schmitz, T. W., Ries, M. L., Torgerson, B. M., Sager, M. A., Hermann, B. P., et al. (2006). Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer’s disease: a cross-sectional study. BMC Med. 4:1. doi: 10.1186/1741-7015-4-1
Tropea, T. F., Xie, S. X., Rick, J., Chahine, L. M., Dahodwala, N., Doshi, J., et al. (2018). APOE, thought disorder, and SPARE-AD predict cognitive decline in established Parkinson’s disease. Mov. Disord. 33, 289–297. doi: 10.1002/mds.27204
Tsuang, D., Leverenz, J. B., Lopez, O. L., Hamilton, R. L., Bennett, D. A., Schneider, J. A., et al. (2013). APOE ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 70, 223–228. doi: 10.1001/jamaneurol.2013.600
Tunold, J.-A., Geut, H., Rozemuller, J. M. A., Henriksen, S. P., Toft, M., van de Berg, W. D. J., et al. (2021). APOE and MAPT are associated with dementia in neuropathologically confirmed Parkinson’s disease. Front. Neurol. 12:631145. doi: 10.3389/fneur.2021.631145
Twohig, D., and Nielsen, H. M. (2019). α-Synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 14:23. doi: 10.1186/s13024-019-0320-x
Umeh, C. C., Mahajan, A., Mihailovic, A., and Pontone, G. M. (2022). APOE4 allele, sex, and dementia risk in Parkinson’s disease: lessons from a longitudinal cohort. J. Geriatr. Psychiatry Neurol. 35, 810–815. doi: 10.1177/08919887211060019
Uribe, C., Segura, B., Baggio, H. C., Abos, A., Garcia-Diaz, A. I., Campabadal, A., et al. (2018). Cortical atrophy patterns in early Parkinson’s disease patients using hierarchical cluster analysis. Parkinsonism Relat. Disord. 50, 3–9. doi: 10.1016/j.parkreldis.2018.02.006
van den Berge, S. A., Kevenaar, J. T., Sluijs, J. A., and Hol, E. M. (2012). Dementia in Parkinson’s disease correlates with α-synuclein pathology but not with cortical astrogliosis. Parkinsons Dis. 2012:420957, 1–13. doi: 10.1155/2012/420957
van den Heuvel, M. P., and Hulshoff Pol, H. E. (2010). Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 20, 519–534. doi: 10.1016/j.euroneuro.2010.03.008
van der Lee, S. J., Wolters, F. J., Ikram, M. K., Hofman, A., Ikram, M. A., Amin, N., et al. (2018). The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease and dementia: a community-based cohort study. Lancet Neurol. 17, 434–444. doi: 10.1016/S1474-4422(18)30053-X
van der Zande, J. J., Steenwijk, M. D., ten Kate, M., Wattjes, M. P., Scheltens, P., and Lemstra, A. W. (2018). Gray matter atrophy in dementia with Lewy bodies with and without concomitant Alzheimer’s disease pathology. Neurobiol. Aging 71, 171–178. doi: 10.1016/j.neurobiolaging.2018.07.005
van Eimeren, T., Monchi, O., Ballanger, B., and Strafella, A. P. (2009). Dysfunction of the default mode network in Parkinson disease. Arch. Neurol. 66, 877–883. doi: 10.1001/archneurol.2009.97
van Steenoven, I., van der Flier, W. M., Scheltens, P., Teunissen, C. E., and Lemstra, A. W. (2019). Amyloid-β peptides in cerebrospinal fluid of patients with dementia with Lewy bodies. Alzheimers Res. Ther. 11:83. doi: 10.1186/s13195-019-0537-5
Vander Borght, T., Minoshima, S., Giordani, B., Foster, N. L., Frey, K. A., Berent, S., et al. (1997). Cerebral metabolic differences in Parkinson’s and Alzheimer’s diseases matched for dementia severity. J. Nucl. Med. 38, 797–802.
Verghese, P. B., Castellano, J. M., Garai, K., Wang, Y., Jiang, H., Shah, A., et al. (2013). ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc. Natl. Acad. Sci. USA 110, E1807–E1816. doi: 10.1073/pnas.1220484110
Vijayaraghavan, S., Maetzler, W., Reimold, M., Lithner, C. U., Liepelt-Scarfone, I., Berg, D., et al. (2014). High apolipoprotein E in cerebrospinal fluid of patients with Lewy body disorders is associated with dementia. Alzheimers Dement. 10, 530–540.e1. doi: 10.1016/j.jalz.2013.03.010
Villemagne, V. L., Burnham, S., Bourgeat, P., Brown, B., Ellis, K. A., Salvado, O., et al. (2013). Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 12, 357–367. doi: 10.1016/S1474-4422(13)70044-9
Visanji, N. P., Lang, A. E., and Kovacs, G. G. (2019). Beyond the synucleinopathies: alpha synuclein as a driving force in neurodegenerative comorbidities. Transl. Neurodegener. 8:28. doi: 10.1186/s40035-019-0172-x
Vitek, M. P., Brown, C. M., and Colton, C. A. (2009). APOE genotype-specific differences in the innate immune response. Neurobiol. Aging 30, 1350–1360. doi: 10.1016/j.neurobiolaging.2007.11.014
Vogt, B. A. (2019). Cingulate cortex in Parkinson’s disease. Handb. Clin. Neurol. 166, 253–266. doi: 10.1016/B978-0-444-64196-0.00013-3
Vrillon, A., Bousiges, O., Götze, K., Demuynck, C., Muller, C., Ravier, A., et al. (2024). Plasma biomarkers of amyloid, tau, axonal, and neuroinflammation pathologies in dementia with Lewy bodies. Alzheimers Res. Ther. 16:146. doi: 10.1186/s13195-024-01502-y
Wakasugi, N., and Hanakawa, T. (2021). It is time to study overlapping molecular and circuit Pathophysiologies in Alzheimer’s and Lewy body disease spectra. Front. Syst. Neurosci. 15:777706. doi: 10.3389/fnsys.2021.777706
Wang, X., Wang, J., He, Y., Li, H., Yuan, H., Evans, A., et al. (2015). Apolipoprotein E ε4 modulates cognitive profiles, hippocampal volume, and resting-state functional connectivity in Alzheimer’s disease. JAD 45, 781–795. doi: 10.3233/JAD-142556
Wardlaw, J. M., Valdés Hernández, M. C., and Muñoz-Maniega, S. (2015). What are white matter hyperintensities made of? J. Am. Heart Assoc. 4:e001140. doi: 10.1161/JAHA.114.001140
Watson, G. S., and Leverenz, J. B. (2010). Profile of cognitive impairment in Parkinson’s disease. Brain Pathol. 20, 640–645. doi: 10.1111/j.1750-3639.2010.00373.x
Webster, S. J., Bachstetter, A. D., and Van Eldik, L. J. (2013). Comprehensive behavioral characterization of an APP/PS-1 double knock-in mouse model of Alzheimer’s disease. Alzheimers Res. Ther. 5:28. doi: 10.1186/alzrt182
Weintraub, D., Moberg, P. J., Culbertson, W. C., Duda, J. E., and Stern, M. B. (2004). Evidence for impaired encoding and retrieval memory profiles in Parkinson disease. Cogn. Behav. Neurol. 17, 195–200.
Wesnes, K. A., Annas, P., Basun, H., Edgar, C., and Blennow, K. (2014). Performance on a pattern separation task by Alzheimer’s patients shows possible links between disrupted dentate gyrus activity and apolipoprotein E ∈4 status and cerebrospinal fluid amyloid-β42 levels. Alzheimers Res. Ther. 6:20. doi: 10.1186/alzrt250
Westerman, M. A., Cooper-Blacketer, D., Mariash, A., Kotilinek, L., Kawarabayashi, T., Younkin, L. H., et al. (2002). The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer’s disease. J. Neurosci. 22, 1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002
Wildsmith, K. R., Holley, M., Savage, J. C., Skerrett, R., and Landreth, G. E. (2013). Evidence for impaired amyloid β clearance in Alzheimer’s disease. Alzheimers Res. Ther. 5:33. doi: 10.1186/alzrt187
Williams-Gray, C. H., Goris, A., Saiki, M., Foltynie, T., Compston, D. A. S., Sawcer, S. J., et al. (2009). Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson’s disease. J. Neurol. 256, 493–498. doi: 10.1007/s00415-009-0119-8
Xia, J., Miu, J., Ding, H., Wang, X., Chen, H., Wang, J., et al. (2013). Changes of brain gray matter structure in Parkinson’s disease patients with dementia. Neural Regen. Res. 8, 1276–1285. doi: 10.3969/j.issn.1673-5374.2013.14.004
Xu, G., Mclaren, D. G., Ries, M. L., Fitzgerald, M. E., Bendlin, B. B., Rowley, H. A., et al. (2009). The influence of parental history of Alzheimer’s disease and apolipoprotein E ε4 on the BOLD signal during recognition memory. Brain 132, 383–391. doi: 10.1093/brain/awn254
Xu, Y., Yang, J., Hu, X., and Shang, H. (2016). Voxel-based meta-analysis of gray matter volume reductions associated with cognitive impairment in Parkinson’s disease. J. Neurol. 263, 1178–1187. doi: 10.1007/s00415-016-8122-3
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C.-C., and Bu, G. (2019). Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 15, 501–518. doi: 10.1038/s41582-019-0228-7
Yang, J., McMahon, K. L., Copland, D. A., Pourzinal, D., Byrne, G. J., Angwin, A. J., et al. (2022). Semantic fluency deficits and associated brain activity in Parkinson’s disease with mild cognitive impairment. Brain Imaging Behav. 16, 2445–2456. doi: 10.1007/s11682-022-00698-7
Yang, K., Wu, Z., Long, J., Li, W., Wang, X., Hu, N., et al. (2023). White matter changes in Parkinson’s disease. npj Parkinsons Dis. 9, 1–10. doi: 10.1038/s41531-023-00592-z
Ye, B. S., Jeon, S., Ham, J. H., Lee, J. J., Lee, J. M., Lee, H. S., et al. (2017). Dementia-predicting cognitive risk score and its correlation with cortical thickness in Parkinson disease. Dement. Geriatr. Cogn. Disord. 44, 203–212. doi: 10.1159/000479057
Yen, C., Lin, C.-L., and Chiang, M.-C. (2023). Exploring the Frontiers of neuroimaging: a review of recent advances in understanding brain functioning and disorders. Life (Basel) 13:1472. doi: 10.3390/life13071472
Yin, F. (2023). Lipid metabolism and Alzheimer’s disease: clinical evidence, mechanistic link and therapeutic promise. FEBS J. 290, 1420–1453. doi: 10.1111/febs.16344
Zenuni, H., Bovenzi, R., Bissacco, J., Grillo, P., Simonetta, C., Mascioli, D., et al. (2023). Clinical and neurochemical correlates of the APOE genotype in early-stage Parkinson’s disease. Neurobiol. Aging 131, 24–28. doi: 10.1016/j.neurobiolaging.2023.07.011
Zhang, Y., Chen, H., Li, R., Sterling, K., and Song, W. (2023). Amyloid β-based therapy for Alzheimer’s disease: challenges, successes and future. Sig. Transduct. Target Ther. 8, 1–26. doi: 10.1038/s41392-023-01484-7
Zhang, H., Lin, A., Gong, P., Chen, Y., Ye, R. D., Qian, F., et al. (2020). The chemokine-like receptor 1 deficiency improves cognitive deficits of AD mice and attenuates tau hyperphosphorylation via regulating tau seeding. J. Neurosci. 40, 6991–7007. doi: 10.1523/JNEUROSCI.0455-20.2020
Zhang, J., Zhang, Y.-T., Hu, W.-D., Li, L., Liu, G.-Y., and Bai, Y.-P. (2015). Gray matter atrophy in patients with Parkinson’s disease and those with mild cognitive impairment: a voxel-based morphometry study. Int. J. Clin. Exp. Med. 8, 15383–15392.
Zhao, N., Attrebi, O. N., Ren, Y., Qiao, W., Sonustun, B., Martens, Y. A., et al. (2020). APOE4 exacerbates α-synuclein pathology and related toxicity independent of amyloid. Sci. Transl. Med. 12:eaay1809. doi: 10.1126/scitranslmed.aay1809
Zhao, N., Liu, C.-C., van, A., Martens, Y. A., Linares, C., Knight, J. A., et al. (2017). Apolipoprotein E4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron 96, 115–129.e5. doi: 10.1016/j.neuron.2017.09.003
Zhao, J., Lu, W., Ren, Y., Fu, Y., Martens, Y. A., Shue, F., et al. (2021). Apolipoprotein E regulates lipid metabolism and α-synuclein pathology in human iPSC-derived cerebral organoids. Acta Neuropathol. 142, 807–825. doi: 10.1007/s00401-021-02361-9
Zhu, S.-G., Chen, Z.-L., Xiao, K., Wang, Z.-W., Lu, W.-B., Liu, R.-P., et al. (2024). Association analyses of apolipoprotein E genotypes and cognitive performance in patients with Parkinson’s disease. Eur. J. Med. Res. 29:334. doi: 10.1186/s40001-024-01924-2
Keywords: Apolipoprotein E4, Parkinsonism, DLB, cognitive impairment, neuroimaging, Parkinson’s disease
Citation: Rosal AE, Martin SL and Strafella AP (2025) The role of Apolipoprotein E4 on cognitive impairment in Parkinson’s disease and Parkinsonisms. Front. Neurosci. 19:1515374. doi: 10.3389/fnins.2025.1515374
Edited by:
Matilde Otero-Losada, National Scientific and Technical Research Council (CONICET), ArgentinaReviewed by:
Michael Henderson, Van Andel Institute, United StatesMinyoung Oh, Asan Medical Center, Republic of Korea
Lenora Higginbotham, Emory University, United States
Copyright © 2025 Rosal, Martin and Strafella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angenelle Eve Rosal, YW5nZW5lbGxlLnJvc2FsQG1haWwudXRvcm9udG8uY2E=; Antonio P. Strafella, YW50b25pby5zdHJhZmVsbGFAdXRvcm9udG8uY2E=
 Angenelle Eve Rosal
Angenelle Eve Rosal Sarah L. Martin
Sarah L. Martin Antonio P. Strafella
Antonio P. Strafella