
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 04 April 2025
Sec. Autonomic Neuroscience
Volume 19 - 2025 | https://doi.org/10.3389/fnins.2025.1514513
This article is part of the Research TopicThe correlation between neurological diseases and cardiovascular diseasesView all articles
 Zhenhuan Chen1†
Zhenhuan Chen1† Ying Li1,2†
Ying Li1,2† Yuan Liu1
Yuan Liu1 Guowang Shen1
Guowang Shen1 Ganwei Xiong1
Ganwei Xiong1 Bo Wu1
Bo Wu1 Yanfeng Liu1
Yanfeng Liu1 Xiantao Huang1
Xiantao Huang1 Hongyan Li1
Hongyan Li1 Haiwen Zhou1
Haiwen Zhou1 Zhicheng Xu1
Zhicheng Xu1 Gulao Zhang1
Gulao Zhang1 Yu Tao1
Yu Tao1 Fanzhi Zhang1
Fanzhi Zhang1 Hengli Lai1*
Hengli Lai1*Objective: Cardioneuroablation (CNA) is effective for cardiac inhibitory and mixed vasovagal syncope (VVS) but not for vasodepressor VVS. This study aimed to assess the therapeutic benefits of CNA in vasodepressor VVS.
Methods: VVS patients hospitalized in the Department of Cardiology of Jiangxi Provincial People’s Hospital were retrospectively reviewed. Holter monitoring was performed before, during, and 3 months after CNA. Changes in heart rate and atrioventricular conduction before and after ablation were compared.
Results: Thirty-five patients (18 M/17F, 47.48 ± 16.49 years) were included. Median duration of syncope was 24.0 months (range, 2.5–66.0). Median number of syncope episodes before treatment was two (range, 2–4). The time domain indexes of heart rate variability, mean heart rate, maximum heart rate, and minimum heart rate were significantly higher 3 months after CNA. Mean follow-up was 11 ± 4.67 months. Recurrent syncope occurred in two patients with vasodepressor VVS, one of them with presyncope symptoms in vasodepressor type; and one patient occurred with mixed VVS, without presyncope symptoms. The syncope free survival is 76.92%. No serious complications occurred. CNA is safe and effective in the treatment of vasodepressor VVS.
Conclusion: CNA is effective for treating vasodepressor VVS. Our study provides a theoretical basis for individualization of treatment in patients with vasodepressor VVS.
Syncope is transient loss of consciousness due to impaired autonomic regulation of the heart and consequent brain hypoperfusion (Freeman et al., 2011). Vasovagal syncope (VVS) is the most common type of syncope of all age without obvious cardiac or neurostructural disease. Patients who experience VVS usually experience prodromal symptoms such as nausea, dizziness, fatigue, sweating, and/or amaurosis. The pathogenesis of VVS has not been fully elucidated yet. The possible mechanisms include Bezold-Jarisch reflex, autonomic nervous dysfunction, neurohumoral factors, fluid-mediated vasodilatation, decreased baroreceptor sensitivity, and genetic factors (Marquez et al., 2016). VVS is diagnosed and classified based on the patient’s blood pressure and heart rate at the time of syncope at the head-up tilt table test (HUTT). Based on HUTT, VVS is divided into cardioinhibitory type, vasodepressor type, and mixed type (Stavrakis and Po, 2017), Recurrent VVS affect quality of life and may result in patient injury. It is reported that recurrence rates of VVS are as high as 61% (Pachon et al., 2005; Gonzalez-Hermosillo, 2007; Brignole et al., 2002; Brignole et al., 2018). Therefore, the main goal of treatment is prevention of recurrent events.
Non-pharmacological treatments such as maintenance of adequate fluid and salt intake, avoidance of syncope triggers, and tilt training have no significant effect in many patients. Cardiac postganglionic parasympathetic neurons, which are mainly located in the epicardial fat pad, regulate cardiac rhythm and conduction (Pachon et al., 2005; Writing Committee Members et al., 2017). In preliminary studies, cardioneuroablation (CNA) was effective in treating some VVS and achieved good long-term outcomes (Brignole et al., 2000; Xia et al., 2011). Preliminary data did not demonstrate efficacy for vasodepressor syncope.
Here, we evaluated the safety and effectiveness of CNA for VVS using heart rate variability (HRV) indexes and recurrent syncope as outcome measures. We also compared the effectiveness of CNA between patients with vasodepressor and mixed types of VVS.
We retrospectively reviewed consecutive patients with VVS who were hospitalized in Jiangxi Provincial People’s Hospital from July 2020 to July 2022 and underwent CNA. Before the CNA, we need to performed the preoperative examination, including the exclusion of thyroid dysfunction, blood routine, biochemical, cardiac structural abnormalities, including other arrhythmias, and patients with other serious diseases such as myocardial infarction, cerebral infarction or other operations within 3 months are excluded. Head-up tilt table testing was positive in all. VVS was diagnosed using the 2018 European Society of Cardiology Guidelines. In our institution, patients with recurrent syncope that seriously affects quality of life or causes injury who do not respond to conventional treatment are considered for CNA. Patients diagnosed with causes of syncope other than VVS were excluded; all underwent examinations to exclude structural heart disease, nervous system disease, and other causes. We also excluded patients with atrial thrombus on transesophageal echocardiography. Informed consent was obtained from all subjects and/or their legal guardians. The retrospective study was approved by the Institutional Review Board of Jiangxi Provincial People’s Hospital (Batch number: Kekuai 2024 (49)) and written informed consent for all patients were confirmation.
1. Fasting for 4 h, establish venous access, keep the examination room environment quiet, soft light, and appropriate temperature (20 ~ 25°C). Lie flat for at least 10 min before the tilt begins. Tilt Angle 70°.
2. The vertical tilt duration of the foundation stops at any time with the positive reaction, if no positive reaction occurs, it should be continued for a maximum of 45 min.
3. Sublingual administration of nitroglycerin, fixed dose 300 ~ 400 μg (domestic nitroglycerin 0.5 mg, 3/4 tablets), the longest duration of 20 min.
4. When isoproterenol was administered, it was increased by 1 μg/min every 5 min from 1 μg/min to 3 μg/min, so that the average heart rate exceeded 20 to 25% of the baseline level, the fastest heart rate should not exceed 150 beats /min, and the longest duration was 20 min.
The head-up tilt table test was considered positive in patients who experienced syncope, near syncope, or prodromal symptoms such as pallor, sweating, palpitations, nausea and vomiting, dizziness, or dyspnea in conjunction with a decrease in blood pressure and/or heart rate. The positive reactions were classified into the following types according to changes in blood pressure and heart rate:
1. type 1 (mixed type)—heart rate decreased during syncope but was ≥40 beats/min or < 40 beats/min for less than 10 s, with or without sinus pause<3 s; blood pressure also decreased
2. type 2a (cardioinhibitory type)—heart rate < 40 beats/min for more than 10 s, no sinus pause; blood pressure decreased before heart rate
3. type 2b (cardiac depression type)—sinus pause >3 s; heart rate and blood pressure decreased at the same time or heart rate decreased before blood pressure did
4. type 3 (vasodepressor type)—systolic blood pressure < 80 mm Hg or mean blood pressure decrease >30 mm Hg; heart rate decrease not more than 10% during syncope
Before the test, electrocardiographic monitoring and venous access were established. After remaining supine for 10 min, the patient’s heart rate and blood pressure were recorded; then, the bed was tilted 70° to begin the test phase.
All patients underwent the same ablation protocol. High-frequency stimulation (HFS; 20 Hz, 10–20 V, pulse width 5 ms; MicroPace EPS320; Micropace EP) was applied to identify the GPs in the LA. Four GP sites were the particular focus of a previous report (Sun et al., 2016). Three-dimensional electroanatomic guidance was highly recommended in this protocol because it provided detailed spatial information and different projection views to help identify the GP locations.
There are five autonomic GPs in the left atrium. The left superior GP (LSGP) is located at the anterolateral junction of the left superior pulmonary vein and left atrium. The left inferior GP (LIGP) is located in the posterior inferior region of the junction between the left inferior pulmonary vein and the left atrium. The posteromedial left GP (PMLGP) is in the posterior atrial septum, between the left atrial posterior wall, inferior vena cava, and coronary sinus opening. The right anterior GP (RAGP) is in the anterior region of the junction between the right superior pulmonary vein and the left atrium. The right inferior GP (RIGP) is in the anterior region of the junction between the right inferior pulmonary vein and the left atrium. After anatomical localization, high-frequency stimulation (HFS) was used to determine the location of the left atrial vagal ganglion. When performing HFS near the mitral annulus, attention was paid to prevent malignant arrhythmias such as ventricular tachycardia or ventricular fibrillation. Vagal reflexes during HFS manifested as transient ventricular arrest, atrioventricular block, or 50% prolongation of the mean RR interval. Sites with positive vagal responses were recorded as GP targets.
To perform CNA, the patient was positioned flat on the operating table and the skin of the head, neck, chest, and both inguinal regions was sterilized and draped. After injection of 2% lidocaine as a local anesthetic, the left and right femoral veins were puncturing using the Seldinger technique. The right ventricular and coronary electrodes were placed to induce an intracavitary electrocardiogram. Then, an 8.5\u00B0F SL1 SWARTZ sheath (Abbott Laboratories, Chicago, IL, United States) was inserted into the right atrium through the right femoral vein. Interatrial septal puncture was performed under fluoroscopy. After successful puncture, the patient was heparinized to maintain an activated clotting time between 200 and 300 ms. Then, the sheath was advanced into the left atrium and a three-dimensional anatomical model of the left atrium was constructed using the ablation electrodes under the guidance of the CARTO3 system (Biosense Webster, Irvine, CA, United States). The location of common cardiac ganglia were marked and HFS performed at each location. When a vagal response and hypotension were observed within a few seconds after ablation, the appropriate ablation site was determined. Power and temperature were set at 35-45 W and 43°C, respectively. The ablation endpoint was no vagal response induced by repeated HFS.
Statistical analyses were performed using SPSS software version 17.0 (IBM Corp., Armonk, NY, United States). Shapiro–Wilk test to the method section to verify whether the data conforms to the normal distribution. Continuous data are expressed as means with standard deviation and were compared using one-way analysis of variance or the paired sample t test as appropriate. Categorical variables are expressed as numbers with percentage and were compared using the chi-square test. p < 0.05 was considered significant.
Of 35 patients in all type of VVS, 13 were vasodepressor type, including seven men and six women. Mean age all type VVS patient was 47.48 ± 16.49 years. Median duration of syncope was 24.0 months (range, 2.5–66.0). Median number of syncope episodes before treatment was two (range, 2–4). Patient characteristics are shown in Table 1.
The ablation electrode was used to perform HFS at five common GP sites in the left atrium. Figures 1, 2 show the GP distribution in the three-dimensional reconstruction model of two patients. Figure 3 shows a schematic diagram of one patient during ablation.
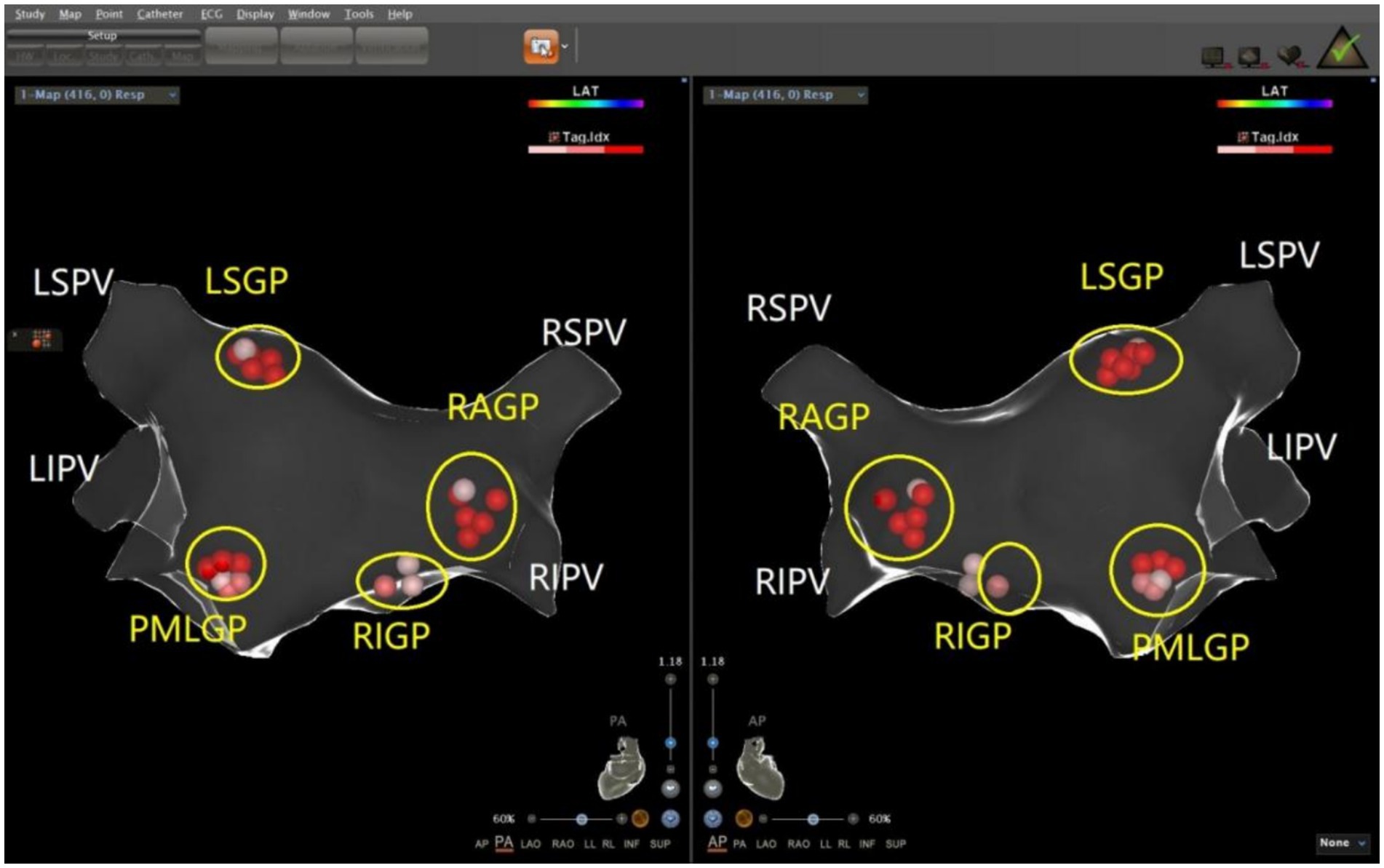
Figure 1. Schematic representation of the distribution of the autonomic ganglionated plexus in the left atrium of a patient with mixed VVS. The left superior GP (LSGP) is located at the anterolateral junction of the left superior pulmonary vein and left atrium. The left inferior GP (LIGP) is located in the posterior inferior region of the junction between the left inferior pulmonary vein and the left atrium. The posteromedial left GP (PMLGP) is in the posterior atrial septum, between the left atrial posterior wall, inferior vena cava, and coronary sinus opening. The right anterior GP (RAGP) is in the anterior region of the junction between the right superior pulmonary vein and the left atrium. The right inferior GP (RIGP) is in the anterior region of the junction between the right inferior pulmonary vein and the left atrium (Left, posterior anterior position of heart; Right, anteroposterior position of heart).
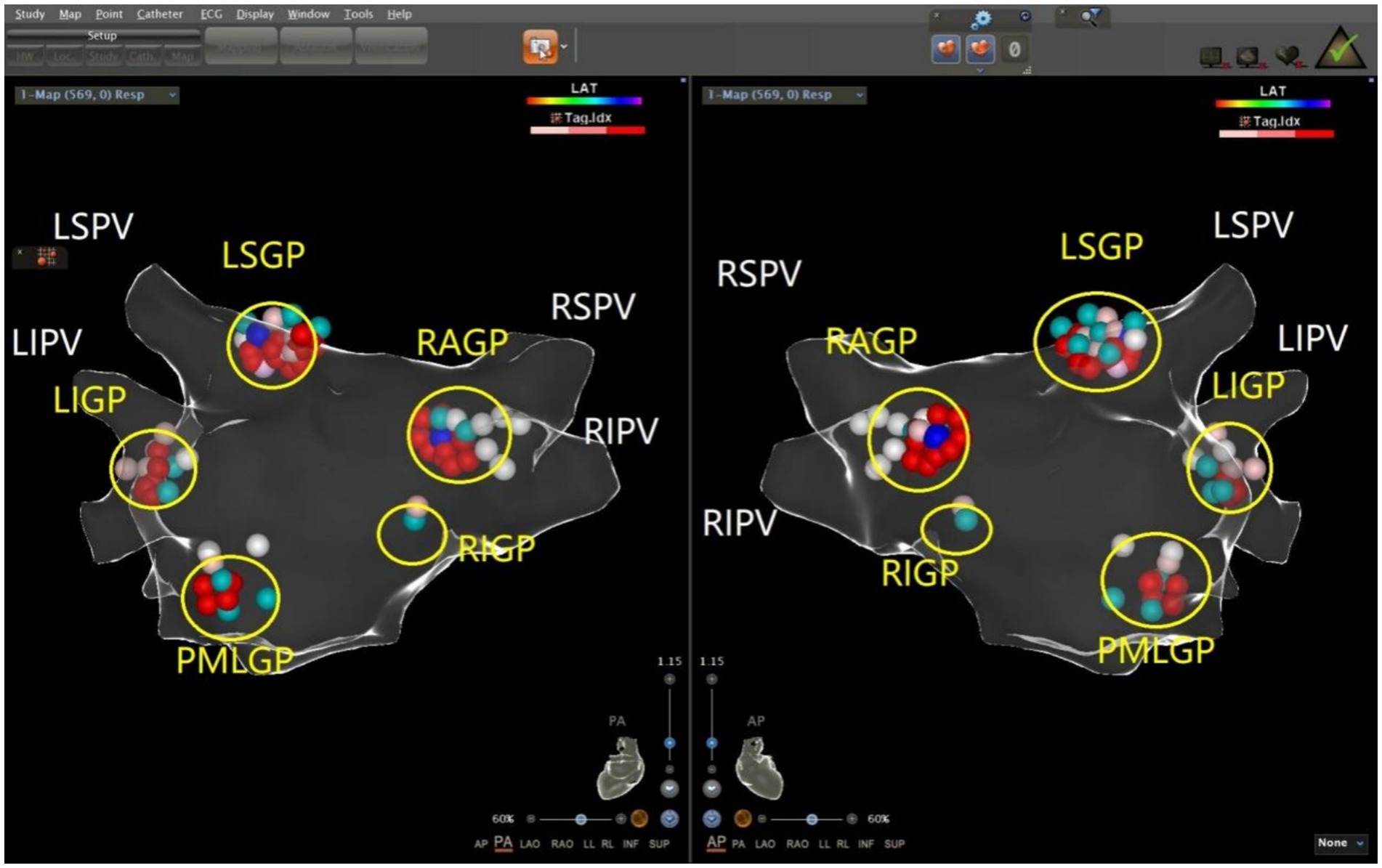
Figure 2. Schematic representation of the distribution of the autonomic ganglionated plexus in the left atrium of a patient with vasodepressor VVS. The left superior GP (LSGP) is located at the anterolateral junction of the left superior pulmonary vein and left atrium. The left inferior GP (LIGP) is located in the posterior inferior region of the junction between the left inferior pulmonary vein and the left atrium. The posteromedial left GP (PMLGP) is in the posterior atrial septum, between the left atrial posterior wall, inferior vena cava, and coronary sinus opening. The right anterior GP (RAGP) is in the anterior region of the junction between the right superior pulmonary vein and the left atrium. The right inferior GP (RIGP) is in the anterior region of the junction between the right inferior pulmonary vein and the left atrium (Left, posterior anterior position of heart; Right, anteroposterior position of heart).
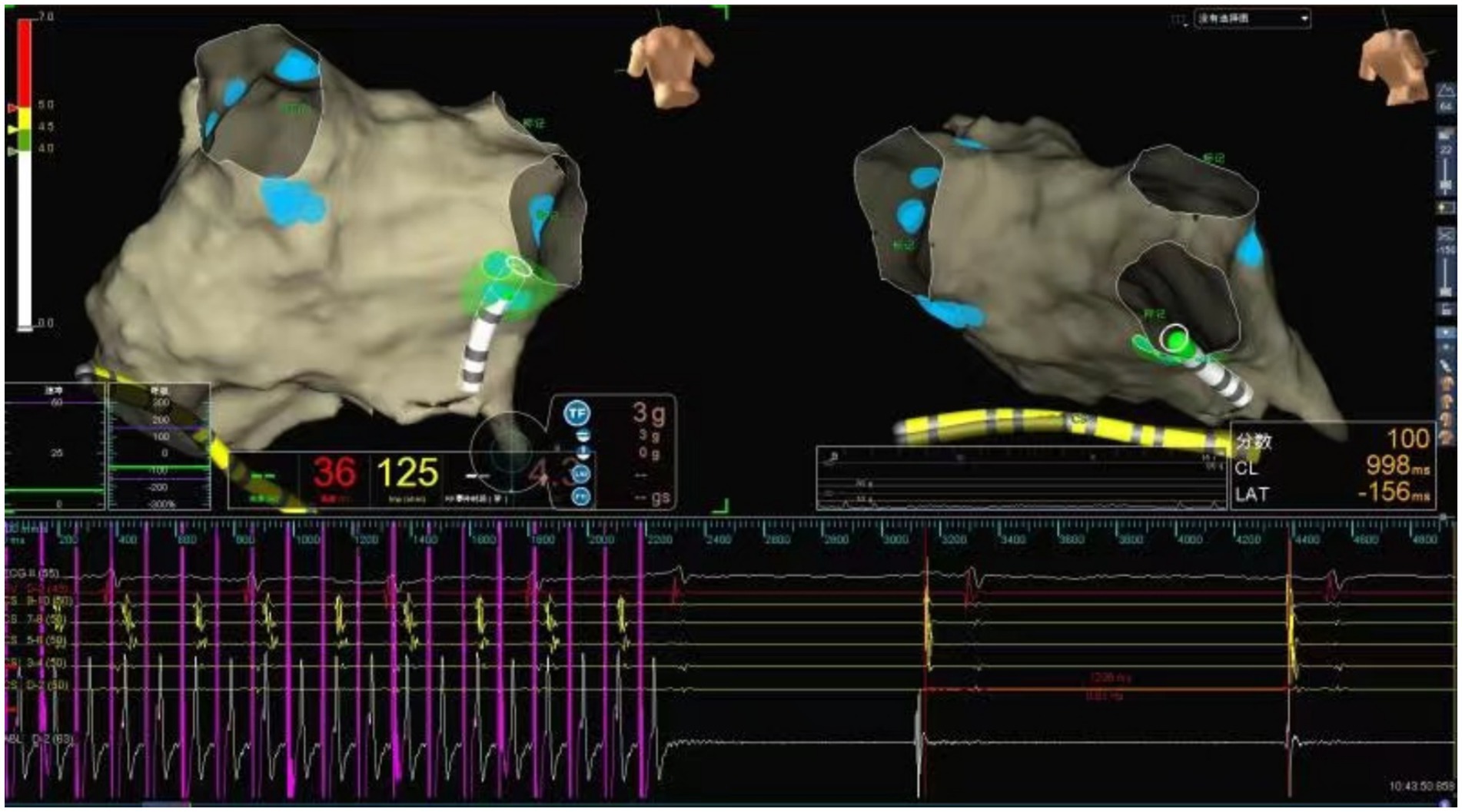
Figure 3. Schematic representation of cardiac ganglionated plexus ablation in a patient with VVS. Illustration of the vagal response induced by radiofrequency energy delivery at the RIGP. The geometry of the left atrium was constructed using the Ensite Navx mapping system. The blue balls represent the ablated lesions with a positive vagal response at each GP site. The green dots represent the real-time shadow of the catheter tip on the left atrial geometry. Radiofrequency ablation at the LSGP induced sinus arrest lasting 2,400 ms.
24-h ECG monitoring to all patients included in the study were performed before and after CNA. Heart rate and electrophysiological parameters of GP ablation targets before and after ablation in patients with vasodepressor VVS are shown in Table 2 and frequency of positive vagal reaction during GPs ablation in patients with vasodepressor and mixed are shown in Figure 4. In both mixed and vasodepressor VVS, vagal responses were most frequently observed at the LSGP and RAGP. Responses were observed at LSGP, LIGP, PMLGP, RAGP, and RIGP in 19 (100%), 17 (89.47%), 8 (42.11%), 16 (84.21%) and 18 (94.74%) patients, respectively. Among the 13 patients with vasodepressor VVS, vagal responses were observed at the LSGP, LIGP, PMLGP, RAGP, and RIGP in 12 (92.30%), 12 (92.30%), 5 (38.46%), 13 (100%), and 13 (92.30%), respectively. The number of ablation targets did not significantly differ between the vasodepressor and the mixed types of VVS. LSGP was the site where vagal responses were most commonly observed during HFS. No serious complications such as cardiac perforation or pericardial effusion occurred.
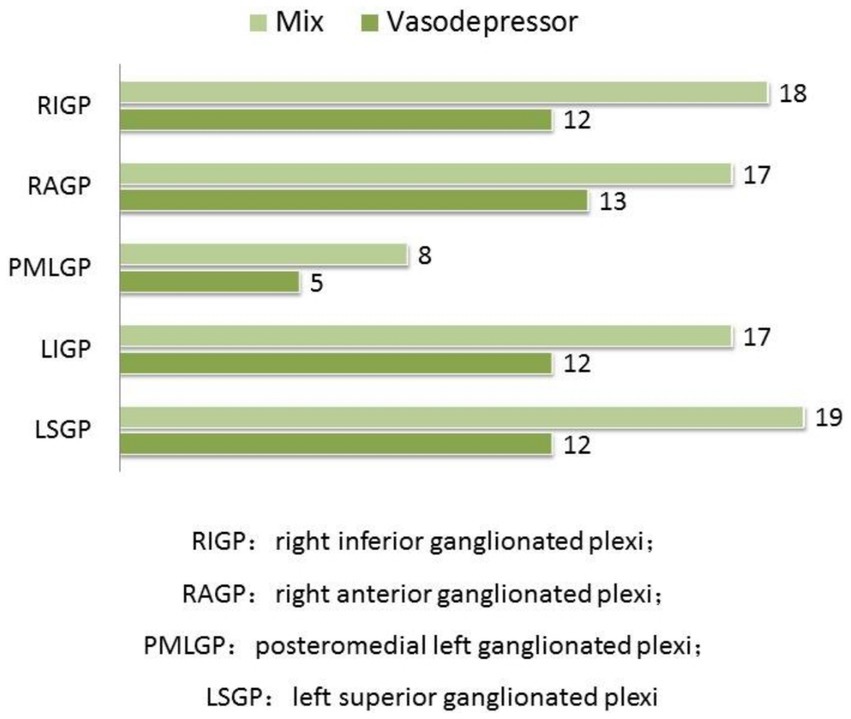
Figure 4. Comparison of the frequency of positive vagal reaction during GPs ablation. In both mixed and vasodepressor VVS, vagal responses were most frequently observed at the LSGP and RAGP.
In the vasodepressor VVS group, mean preoperative heart rate was 67.80 ± 12.22 beats/min and the Wenckebach point of atrioventricular conduction was 446.25 ± 115.73 ms. After ablation, the mean preoperative heart rate was significantly higher (84.17 ± 15.91 beats/min) and the Wenckebach point was significantly lower (350.00 ± 75.19 ms), as shown in Table 3.
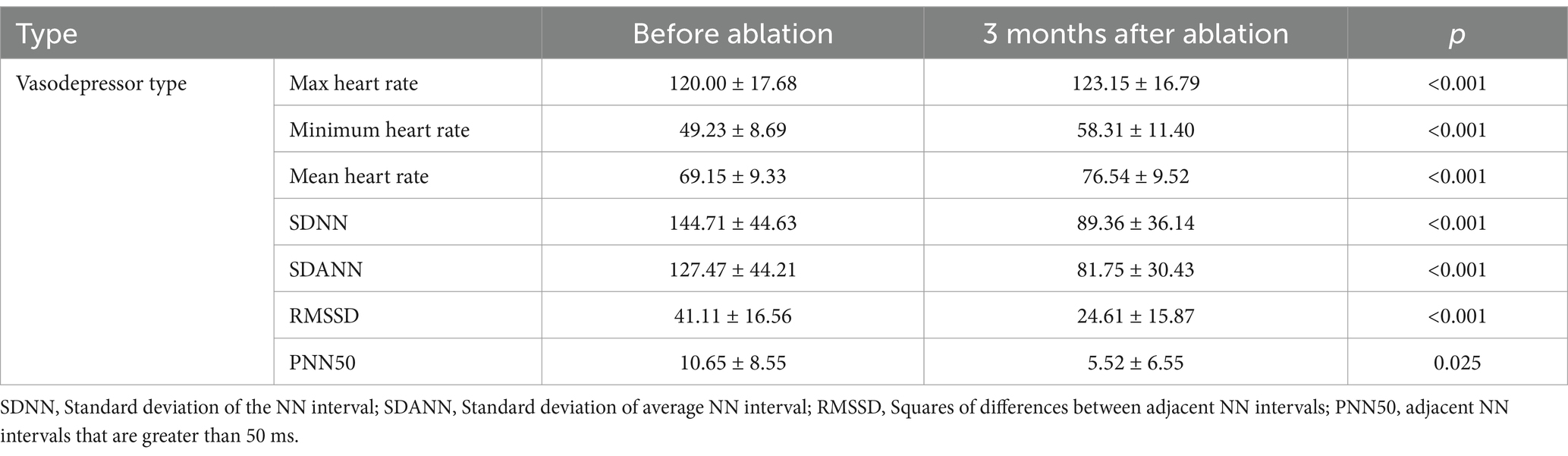
Table 3. Heart rate and heart rate variability parameters on holter monitoring before and after ablation.
Mean follow-up was 11 ± 4.67 months. Syncope recurred in two of 13 patients with vasodepressor VVS after treatment, one at 3 months and the other at 8 months, one of them had prodromal symptoms after ablation, and the symptoms were relieved compared with those before ablation. The remaining patients did not experience any syncope or prodromal symptoms (Table 4). In patients with vasodepressor VVS, mean heart rate on 24-h Holter monitoring increased from 67.80 ± 12.22 beats/min to 84.17 ± 15.91 beats/min after ablation. Among the HRV indexes, standard deviation of the NN interval (SDNN), standard deviation of average NN interval (SDANN), standard deviation of the NN interval index (SDNNi), squares of differences between adjacent NN intervals (rMSSD), and adjacent NN intervals that are greater than 50 ms (pNN50) were significantly lower 3 months after ablation (p < 0.001), as shown in Table 5. No serious adverse reactions such as cardiac tamponade, pulmonary embolism, or arrhythmia occurred after ablation. One patient developed sinus tachycardia 20 days after ablation, which was successfully treated with oral metoprolol. No significant difference in syncope recurrence rate between patients with mixed and vasodepressor VVS as shown in Figure 5.

Table 4. Follow-up results of syncope symptoms in two groups of patients with different types of VVS.
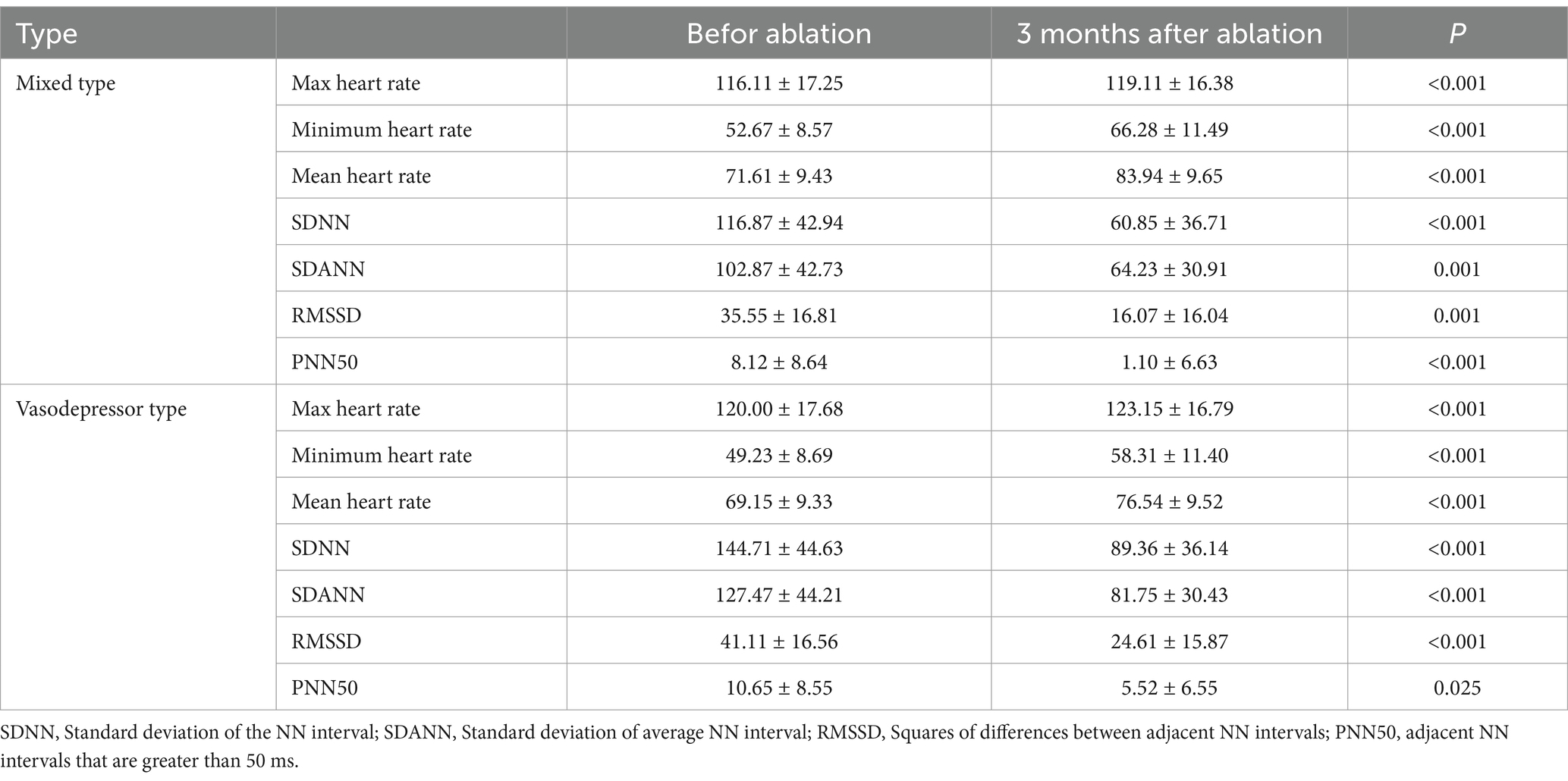
Table 5. Heart rate and HRV time domain parameters of holter were recorded before and after ganglionated plexus ablation.
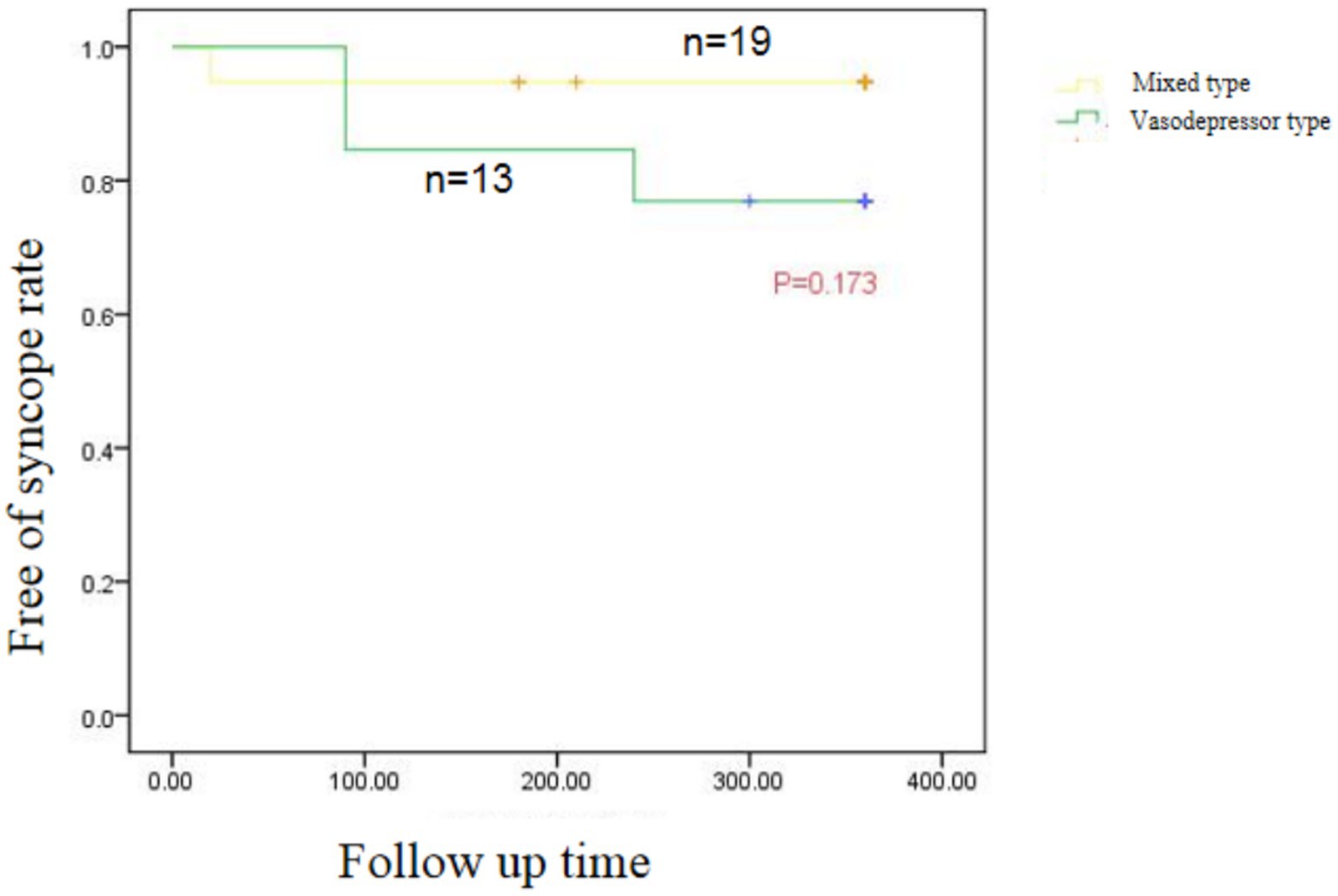
Figure 5. Kaplan–Meier curve of recurrent syncope in patients with Vascular inhibitory and mixed VVS after CNA.
This study suggests that CNA of the left atrial GPs is safe and effective in patients with VVS. Data were consistent across study patients, suggesting good reproducibility. All patients had symptoms of syncope before treatment and experienced a significant increase in minimum and mean heart rate and improvement in symptoms after CNA.
Radiofrequency ablation of the vagal plexus has become a new strategy for treating VVS. Through the continuous research of various medical teams and advances in technique and technology, the procedure has shown promise in VVS patients. In this retrospective study, 13 patients with vasodepressor VVS were treated with radiofrequency ablation using HFS combined with anatomical localization. During the procedure, radiofrequency ablation was performed on five common vagal ganglia. Dynamic electrocardiography showed that HRV indexes were significantly lower at the 3-month follow-up than before the procedure. At the 12-month follow-up, only two patients had experienced recurrent syncope and one had experienced prodromal symptoms. The others reported no recurrence or physical injury. Our results show that radiofrequency ablation of the vagal plexus is effective and safe in patients with VVS.
The autonomic nervous system affects the function of the cardiovascular system by regulating the balance between sympathetic and parasympathetic tone. VVS is caused by the imbalance of sympathetic and parasympathetic input and the negative effect of pathological vagal nerve tone on cardiac conduction and vascular tone. In two studies, Pachon et al. (2005, 2011) showed that permanent endocardial denervation can be achieved to treat VVS by catheter ablation of epicardial ganglia in the atrial wall from the endocardial surface. Clinical research has demonstrated that catheter ablation can significantly improve symptoms in patients with VVS (Xia et al., 2011).
In the atrium, parasympathetic nerves are more densely distributed than sympathetic nerves and mainly located throughout the subendocardium. The GPs in the left atrium are mainly located around the roots of the pulmonary veins. Sun et al. (2016) performed GP ablation only in the left atrium, which had good long-term clinical results: postoperative head-up tilt testing changed from positive to negative in 81.5% of patients and 79.1% changed from mixed inhibition to negative; 11.9% changed from mixed inhibition to vascular inhibition, suggesting that catheter ablation is more effective for mixed inhibition of VVS and can effectively relieve the inhibition of heart rate. The vagal response frequency at LSGP and RIGP was significantly higher in patients with mixed and vasodepressor VVS during ablation, suggesting that these should be the main ablation targets, which is consistent with the results of our study.
It is also crucial to accurately determine the end point of radiofrequency ablation of the vagal plexus. In our study, HFS was used to directly stimulate the vagus nerve when determining the ablation end point. A negative vagal response proved that the ablation end point was reached.
VVS occurs due to an imbalance in the autonomic nervous system, where pathological vagal tone impairs cardiac conduction and vascular tone. The dense distribution of parasympathetic nerves in the atrium, particularly around the roots of the pulmonary veins, makes the GPs in the left atrium crucial targets for ablation. Previous studies have shown that catheter ablation can significantly improve symptoms in patients with VVS, particularly by targeting the vagal plexus. Our results align with these findings, with significant improvements observed in patients following CNA. In particular, the left superior ganglionated plexus (LSGP) was the most commonly involved site of vagal responses, highlighting its central role in autonomic regulation and the potential for targeted interventions.
Our study supports the short-term effectiveness of CNA in treating VVS. After the procedure, most patients experienced a significant improvement in symptoms, with a marked increase in heart rate and a reduction in syncope episodes. These results were observed within the first few months following ablation, suggesting that the procedure provides prompt relief from the debilitating effects of VVS. This aligns with findings from other clinical trials that have reported significant improvement in symptom control and heart rate variability (HRV) after vagal plexus ablation. The positive short-term results suggest that CNA is a viable option for patients with refractory VVS who have not responded to conventional treatments. The recurrence of syncope in 35 patients with VVS after cardiac radiofrequency ablation was compared. We found no significant difference in long-term effectiveness between the mixed and vasodepressor types after ablation, indicating that cardiac GP radiofrequency ablation is equally suitable for both. We divided VVS patients into cardioinhibitory, vasodepressor, and mixed types according to head-up tilt testing results. The reason for the hemodynamic differences in VVS patients is still unclear, but may be related to different sensitivities of neuromodulation of the heart and blood vessels. Our study showed that during head-up tilt table testing, there was no difference in baseline blood pressure, heart rate, mean vascular resistance, or cardiac index between the mixed and vasodepressor types. However, when syncope occurred, mean vascular resistance in the vasodepressor type group significantly decreased and the heart rate and cardiac index significantly increased. In contrast, mean vascular resistance, heart rate, and cardiac index decreased in the mixed type group. The balance of sympathetic and vagal tone can be reflected by heart rate and vascular resistance is mainly controlled by sympathetic input (Pachon et al., 2020). Folino et al. (2007) found in a study of the regulation of autonomic nervous activity in head-up tilt-induced syncope that HRV did not significantly differ between VVS patients and controls in the basal state (Calo et al., 2021). Before syncope, sympathetic activity was increased in patients with cardioinhibitory and mixed types of VVS; however, heart rate decreased because of stronger vagal activity. Patients with vasodepressor type VVS have decreased vascular tone because of insufficient sympathetic activation. Radiofrequency ablation of the vagal plexus has a therapeutic effect on patients with VVS by inhibiting vagus nerve function. Therefore, it has a better effect on patients with cardioinhibitory and mixed VVS, which are caused by excessive activation of vagus nerve function. Xia et al. (2011) found significant improvement in symptoms of cardiac depression but not vascular depression when the vagal reflex was repeatedly induced after ablation of the epicardial fat pad in dogs. Their analysis was driven by the fact that radiofrequency ablation of the cardiac GP significantly attenuated the cardiac vagal component but had no effect on the vasodepressor component of the Bezold-Jarisch reflex. Studies have suggested that although CNA can significantly improve the symptoms related to cardiac depression, the improvement of vagus depression symptoms has not reached the ideal effect. Therefore, VVS patients should be evaluated and classified to formulate an individualized treatment plan before CNA is performed (Xu et al., 2022). These results suggest that radiofrequency GP ablation is more effective for cardioinhibitory and mixed VVS and less effective for vasodepressor VVS. This differs from our results, as we found that CNA has the same effectiveness in patients with vasodepressor VVS. There may have been differences in GP localization and ablation accuracy between different operators in our study. In addition, the sequence of ablation targets in our study was not necessarily the same between operators. Studies have shown that different sequences may affect efficacy of ablation. Finally, because the patients did not have an implantable electrocardiogram recorder, the cause of their syncope at the time of recurrence is not known. Future randomized large-scale trials are needed to study the effectiveness of vagal plexus radiofrequency ablation for treating the different types of VVS.
In this study, the LSGP was the most common ganglia with vagal responses. Previous studies have found that the left atrial GPs are distributed primarily around the pulmonary vein orifice, especially the junction between the left superior pulmonary vein and left atrium. In an autopsy study, Chevalier et al. (2005) found that GPs in the left atrium were mainly distributed at the proximal end of the left and right pulmonary veins and the junction of the atrium; in addition, the left atrium was more highly innervated than the right (Piotrowski et al., 2023). Therefore, radiofrequency ablation of the GPs of the left atrium is usually used in clinical practice. However, right atrial ablation has also been reported. Armour et al. (1997) found that LSGP and RAGP had a significantly greater number of ganglia distributed than LIGP and RIGP. An updated systematic review and meta-analysis performed by Armani Prata et al. (2025) included 27 observational studies and 1 randomized controlled trial encompassing 1,153 patients with refractory VVS who underwent CNA to evaluate CNA efficacy and safety in patients with refractory VVS showed that using the GP targeting method and GP ablation location resulted in a higher prevalence of syncope recurrence in the electroanatomic mapping subgroup and in the right atrium approach. Pachon (2019) found that the LSGP is the GP with the highest distribution density in the left atrium and plays a key role in autonomic regulation of the atrioventricular node (Pokushalov et al., 2009). Stimulation of the LSGP can cause negative time and conduction effects on the heart, which can be significantly reduced after LSGP ablation. In an ablation study of patients with atrial fibrillation, the vagal response was more common on the left atrial side of the pulmonary vein around the ostium, and the vast majority of the vagal responses occurred in the LSGP. The results of our study were similar. Ablation points during CNA are mainly concentrated in the GP on the atrial septal side, while the degree of ablation around the pulmonary vein ostium, especially the LSGP, was less or not ablated. This incomplete denervation may be the reason for syncope recurrence after VVS (Pachon et al., 2020; Rebecchi et al., 2012). In our study, three patients experienced recurrence of syncope or prodromal symptoms after ablation; however, the prodromal symptoms were improved. In some patients who underwent head-up tilt testing, the syncope induction time was longer than before, because vascular depression could not be completely eliminated by cardiac ablation; however, the late vascular depression was significantly delayed and weakened compared with before ablation. Because of interoperator differences, the degree of vagal plexus ablation and vagal denervation may have been different or incomplete. Several studies have shown that a similar situation may occur in patients who have undergone pacemaker implantation. In these patients, vascular depression-induced syncope may occur despite the prevention of sinus pause by the pacemaker (Candemir et al., 2022). Even if residual vasodepressor syncope is present, it can be cured by radiofrequency ablation of the cardiac GP to increase the threshold of symptoms.
Despite the promising findings, this study has several important limitations that should be taken into consideration. First, the study was retrospective in design, which inherently introduces biases related to the selection of participants and the interpretation of historical data. As a result, our conclusions are preliminary, and our findings need to be confirmed in a large, prospective, randomized controlled trial to establish their validity and generalizability.
Second, short follow-up duration presents another limitation. Given the chronic nature of vasodepressor vasovagal syncope (VVS), a longer follow-up period would be crucial to assess the long-term effectiveness and durability of the treatment. A short follow-up may not capture late recurrences or delayed adverse effects of therapy that could emerge over time. Therefore, extended monitoring is required to fully understand the treatment’s sustained impact.
Third, implantable loop recorder (ILR) monitoring was not used post-treatment in this study. While symptoms of syncope were recorded and analyzed, the absence of continuous electrocardiogram (ECG) data means we cannot precisely determine the underlying mechanisms of syncope recurrence. ILR monitoring would have allowed for more detailed insights into the arrhythmic or neurogenic causes of syncope, enabling us to better understand whether the recurrence was related to vagal overactivity, bradycardia, or other potential factors.
Moreover, while this study assessed clinical outcomes, there was no objective analysis of autonomic function (e.g., heart rate variability or baroreceptor sensitivity) following treatment. This limits our understanding of how the treatment affects the underlying autonomic dysfunction that characterizes VVS. Further studies incorporating these measures would be valuable to assess whether the treatment is directly modulating the autonomic pathways involved.
Additionally, the lack of a control group in the study limits our ability to draw definitive conclusions about the treatment’s efficacy. Without a matched comparison group, we cannot fully rule out the possibility that the observed improvements were due to natural disease progression or other unaccounted-for factors.
Finally, this study did not include a broad enough demographic to explore potential differences in treatment outcomes based on factors such as age, sex, comorbid conditions, or other patient-specific characteristics. A more diverse population would be needed to understand how these variables might influence the effectiveness of treatment for VVS.
This study confirmed the effectiveness of radiofrequency GP ablation for preventing recurrence of vasodepressor VVS, providing a theoretical basis for individualized treatment of VVS patients. HRV indexes after ablation were significantly lower, indicating that CNA can achieve good results for vasodepressor type VVS. However, further large-scale prospective randomized controlled studies are needed.
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
The studies involving humans were approved by Medical Ethics Committee of Jiangxi Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
ZC: Writing – review & editing, Conceptualization. YiL: Writing – original draft, Conceptualization. YuL: Formal analysis, Writing – review & editing. GS: Validation, Writing – review & editing. GX: Methodology, Writing – review & editing. BW: Methodology, Writing – review & editing. YaL: Data curation, Writing – review & editing. XH: Software, Writing – review & editing. HoL: Resources, Writing – review & editing. HZ: Visualization, Writing – review & editing. ZX: Supervision, Writing – review & editing. GZ: Supervision, Writing – review & editing. YT: Project administration, Writing – review & editing. FZ: Project administration, Writing – review & editing. HeL: Conceptualization, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (82000276), Supported by the Natural Science Foundation of Jiangxi Province (20242BAB20341), Supported by Open project of State Key Laboratory of Organ Failure Research, Southern Medical University (202001), Grant for Science and Technology Project of Jiangxi Provincial Health Commission (202310005).
We thank Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Armani Prata, A., Katsuyama, E., Scardini, P., Antunes, V., Granja, J., Coan, A. C., et al. (2025). Cardioneuroablation in patients with vasovagal syncope: an updated systematic review and meta-analysis. Heart Rhythm. 22, 526–535. doi: 10.1016/j.hrthm.2024.07.103
Armour, J. A., Murphy, D. A., Yuan, B. X., Macdonald, S., and Hopkins, D. A. (1997). Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat. Rec. 247, 289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L
Brignole, M., Croci, F., Menozzi, C., Solano, A., Donateo, P., Oddone, D., et al. (2002). Isometric arm counter-pressure maneuvers to abort impending vasovagal syncope. J. Am. Coll. Cardiol. 40, 2053–2059. doi: 10.1016/S0735-1097(02)02683-9
Brignole, M., Menozzi, C., Del Rosso, A., Costa, S., Gaggioli, G., Bottoni, N., et al. (2000). New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal syncope international study. Europace 2, 66–76. doi: 10.1053/eupc.1999.0064
Brignole, M., Moya, A., de Lange, F. J., Deharo, J. C., Elliott, P. M., Fanciulli, A., et al. (2018). ESC guidelines for the diagnosis and management of syncope. Eur. Heart J. 39, 1883–1948. doi: 10.1093/eurheartj/ehy037
Calo, L., Rebecchi, M., Sette, A., Sciarra, L., Borrelli, A., Scara, A., et al. (2021). Catheter ablation of right atrial ganglionated plexi to treat cardioinhibitory neurocardiogenic syncope: a long-term follow-up prospective study. J. Interv. Card. Electrophysiol. 61, 499–510. doi: 10.1007/s10840-020-00840-9
Candemir, B., Baskovski, E., Beton, O., Shanableh, N., Akbulut, I. M., Kozluca, V., et al. (2022). Procedural characteristics, safety, and follow-up of modified right-sided approach for cardioneuroablation. Anatol. J. Cardiol. 26, 629–636. doi: 10.5152/AnatolJCardiol.2022.217
Chevalier, P., Tabib, A., Meyronnet, D., Chalabreysse, L., Restier, L., Ludman, V., et al. (2005). Quantitative study of nerves of the human left atrium. Heart Rhythm. 2, 518–522. doi: 10.1016/j.hrthm.2005.01.022
Folino, A. F., Russo, G., Porta, A., Buja, G., Cerutti, S., and Iliceto, S. (2007). Modulations of autonomic activity leading to tilt-mediated syncope. Int. J. Cardiol. 120, 102–107. doi: 10.1016/j.ijcard.2006.03.093
Freeman, R., Wieling, W., Axelrod, F. B., Benditt, D. G., Benarroch, E., Biaggioni, I., et al. (2011). Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton. Neurosci. 161, 46–48. doi: 10.1016/j.autneu.2011.02.004
Gonzalez-Hermosillo, J. A. (2007). Vasovagal syncope: from genetic to bedside. Arch. Cardiol. Mex. 77 Suppl 2, S2-32–S32-36.
Marquez, M. F., Gomez-Flores, J. R., Gonzalez-Hermosillo, J. A., Ruiz-Siller, T. J., and Cardenas, M. (2016). Role of the sympathetic nervous system in vasovagal syncope and rationale for beta-blockers and norepinephrine transporter inhibitors. Medwave 16:e6824. doi: 10.5867/medwave.2016.6824
Pachon, M. J. (2019). Cardioneuroablation for neurocardiogenic syncope. Heart Rhythm. 16, 1552–1553. doi: 10.1016/j.hrthm.2019.08.008
Pachon, J. C., Pachon, E. I., Cunha Pachon, M. Z., Lobo, T. J., Pachon, J. C., and Santillana, T. G. (2011). Catheter ablation of severe neurally meditated reflex (neurocardiogenic or vasovagal) syncope: cardioneuroablation long-term results. Europace 13, 1231–1242. doi: 10.1093/europace/eur163
Pachon, J. C., Pachon, E. I., Pachon, J. C., Lobo, T. J., Pachon, M. Z., Vargas, R. N., et al. (2005). “Cardioneuroablation” – new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace 7, 1–13. doi: 10.1016/j.eupc.2004.10.003
Pachon, M. J., Pachon, M. E., Pachon, C. T. C., Santillana, P. T., Lobo, T. J., Pachon, M. J., et al. (2020). Long-term evaluation of the vagal denervation by cardioneuroablation using holter and heart rate variability. Circ. Arrhythm. Electrophysiol. 13:e008703. doi: 10.1161/CIRCEP.120.008703
Piotrowski, R., Baran, J., Sikorska, A., Krynski, T., and Kulakowski, P. (2023). Cardioneuroablation for reflex syncope: efficacy and effects on autonomic cardiac regulation-a prospective randomized trial. JACC. Clin. Electrophysiol. 9, 85–95. doi: 10.1016/j.jacep.2022.08.011
Pokushalov, E., Romanov, A., Shugayev, P., Artyomenko, S., Shirokova, N., Turov, A., et al. (2009). Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm. 6, 1257–1264. doi: 10.1016/j.hrthm.2009.05.018
Rebecchi, M., de Ruvo, E., Strano, S., Sciarra, L., Golia, P., Martino, A., et al. (2012). Ganglionated plexi ablation in right atrium to treat cardioinhibitory neurocardiogenic syncope. J. Interv. Card. Electrophysiol. 34, 231–235. doi: 10.1007/s10840-012-9666-5
Stavrakis, S., and Po, S. (2017). Ganglionated plexi ablation: physiology and clinical applications. Arrhythmia Electrophysiol. Rev. 6, 186–190. doi: 10.15420/aer2017.26.1
Sun, W., Zheng, L., Qiao, Y., Shi, R., Hou, B., Wu, L., et al. (2016). Catheter ablation as a treatment for vasovagal syncope: long-term outcome of endocardial autonomic modification of the left atrium. J. Am. Heart Assoc. 5:5. doi: 10.1161/JAHA.116.003471
Writing Committee MembersShen, W. K., Sheldon, R. S., Benditt, D. G., Cohen, M. I., Forman, D. E., et al. (2017). 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. Heart Rhythm. 14, e155–e217. doi: 10.1016/j.hrthm.2017.03.004
Xia, Y., Zhao, W., Yang, Z. J., Zhang, J. Y., Zhao, L., Gu, X. J., et al. (2011). Catheter ablation of cardiac fat pads attenuates Bezold-Jarisch reflex in dogs. J. Cardiovasc. Electrophysiol. 22, 573–578. doi: 10.1111/j.1540-8167.2010.01922.x
Keywords: vasovagal syncope, left atrial vagal plexus, high frequency stimulation, radiofrequency ablation, heart rate variability
Citation: Chen Z, Li Y, Liu Y, Shen G, Xiong G, Wu B, Liu Y, Huang X, Li H, Zhou H, Xu Z, Zhang G, Tao Y, Zhang F and Lai H (2025) Efficacy of cardioneuroablation for vasodepressor vasovagal syncope. Front. Neurosci. 19:1514513. doi: 10.3389/fnins.2025.1514513
Received: 21 October 2024; Accepted: 10 March 2025;
Published: 04 April 2025.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Runmei Zou, Central South University, ChinaCopyright © 2025 Chen, Li, Liu, Shen, Xiong, Wu, Liu, Huang, Li, Zhou, Xu, Zhang, Tao, Zhang and Lai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hengli Lai, bGFpaGVuZ2xpQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.