- Department of Psychiatry, Huzhou Third Municipal Hospital, the Affiliated Hospital of Huzhou University, Huzhou, China
Introduction: With the global rise in life expectancy, the incidence of dementia is increasing, often accompanied by depressive symptoms. Understanding the interplay between dementia and depression is crucial, as depression may not only co-occur with but also potentially exacerbate the progression of dementia. This study employs bibliometric analysis to map the global research landscape, identify prevailing themes, and discern future research directions.
Methods: We analyzed reviews and original research articles on dementia and depression extracted from the Web of Science Core Collection spanning from 2005 to 2024. Utilizing tools such as CiteSpace, VOSviewer, and an R-based bibliometric analysis package, we assessed trends in publication volume, citation frequency, contributing countries, leading institutions, predominant journals, influential authors, and emergent keywords.
Results: A total of 1972 publications were obtained, revealing a consistent increase in both the number of publications and their citation impact over the study period. The United States is the country with the most publications and the most extensive collaborations. The University of Toronto and the Journal of Alzheimer’s Disease were identified as key contributors to this field. This research area is currently focused on cognitive impairments, the role of gut microbiota, and non-drug interventions. Future directions emphasize the importance of early detection and intervention, a deeper understanding of the gut-brain axis, and the integration of technology in treatment strategies. Additionally, there is a growing interest in the physiological and psychological interplays such as oxidative stress and its implications.
Conclusion: This study underscores pathogenesis, comorbid conditions, and non-drug interventions as primary research focal points, suggesting these areas as potential pathways for therapeutic innovation. These insights are intended to deepen our understanding, enhance diagnostics, and improve the management of dementia and depression, providing guidance for future research aimed at addressing these escalating global health challenges.
1 Introduction
Dementia is a chronic and progressive disease that severely affects memory, intelligence, thinking ability, and social skills (Weiner and Lipton, 2009). The primary types are Alzheimer’s disease (AD) and vascular dementia (Goodman et al., 2017). The global prevalence of dementia is expected to rise from approximately 57.4 million cases in 2019 to 152.8 million by 2050 (GBD 2019 Dementia Forecasting Collaborators, 2022). Study indicates a trend toward earlier onset of dementia (Ryan et al., 2022). Depression, an affective disorder, is characterized by low mood, decreased interest, and slowed thinking (Smith, 2014), affecting over 300 million individuals worldwide (World Health Organization, 2017). Depression is debilitating and results in a diminished quality of life (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018; Bromet et al., 2011). Both dementia and depression not only profoundly affect cognitive and emotional state but also impose significant burdens on social and economic systems. It is estimated that dementia was costing the global economy US$ 818 billion in 2015, which is equivalent to 1.1% of the world’s GDP (Alzheimer's Disease International, n.d.). Meanwhile, depression is ranked by the World Health Organization as the third leading cause of the global burden of disease (World Health Organization, 2021).
Research indicates that a significant proportion of individuals with dementia also experience depressive symptoms; approximately 38–40% present with these symptoms, while 16% are diagnosed with major depressive disorder (MDD) (Leung et al., 2021; Goodarzi et al., 2017). Concurrently, chronic depressive states may increase the likelihood of dementia (Cantón-Habas et al., 2020). The connection between dementia and depression is evident at the molecular and cellular levels, where neurodegeneration, neuroinflammation, and neurotransmitter imbalances serve as common pathological substrates. A notable finding is that amyloid-β (Aβ) deposition—a hallmark of dementia—is associated with an increased prevalence of depressive symptoms among cognitively normal older adults, suggesting that such symptoms could be early indicators of dementia (Donovan et al., 2018). Depression is further characterized by a dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, which leads to elevated glucocorticoid secretion (Hermida et al., 2012). This increase can cause structural and functional impairments in the hippocampus, crucial for memory and cognition (Frodl and O’Keane, 2013; Sudheimer et al., 2014), thereby providing biological evidence that depression may accelerate the progression of dementia.
Bibliometrics is widely used in the medical field as a common research methodology (Kokol et al., 2021). By analyzing the literature, bibliometrics provides a visual, comprehensive, and systematic elucidation of the development of a particular field, identifying current research hotspots and future trends (Chen et al., 2012). A bibliometric study of behavioral and psychological symptoms of dementia, published in 2023, found that depression was ranked in the top 10 keywords (Cai et al., 2024). Furthermore, analysis from a bibliometric study on late-life depression predicts that dementia will be a hot topic for future research (Du et al., 2024). Studying of the interaction between dementia and depression will not only elucidate the mechanisms of comorbidity in these diseases but also assist physicians in more effectively managing these common and complex conditions in their clinical practice.
This study employs bibliometrics to analyze the information of countries, regions, authors, and journals, aiding researchers in identifying potential collaborators and selecting appropriate journals for submissions. Additionally, by analyzing keywords and highly cited papers, the study investigates current research trends and key focus areas in the fields of dementia and depression. This comprehensive analysis provides invaluable information, serving as a crucial guide for future research directions and the development of effective treatment strategies.
2 Methods
2.1 Data collection
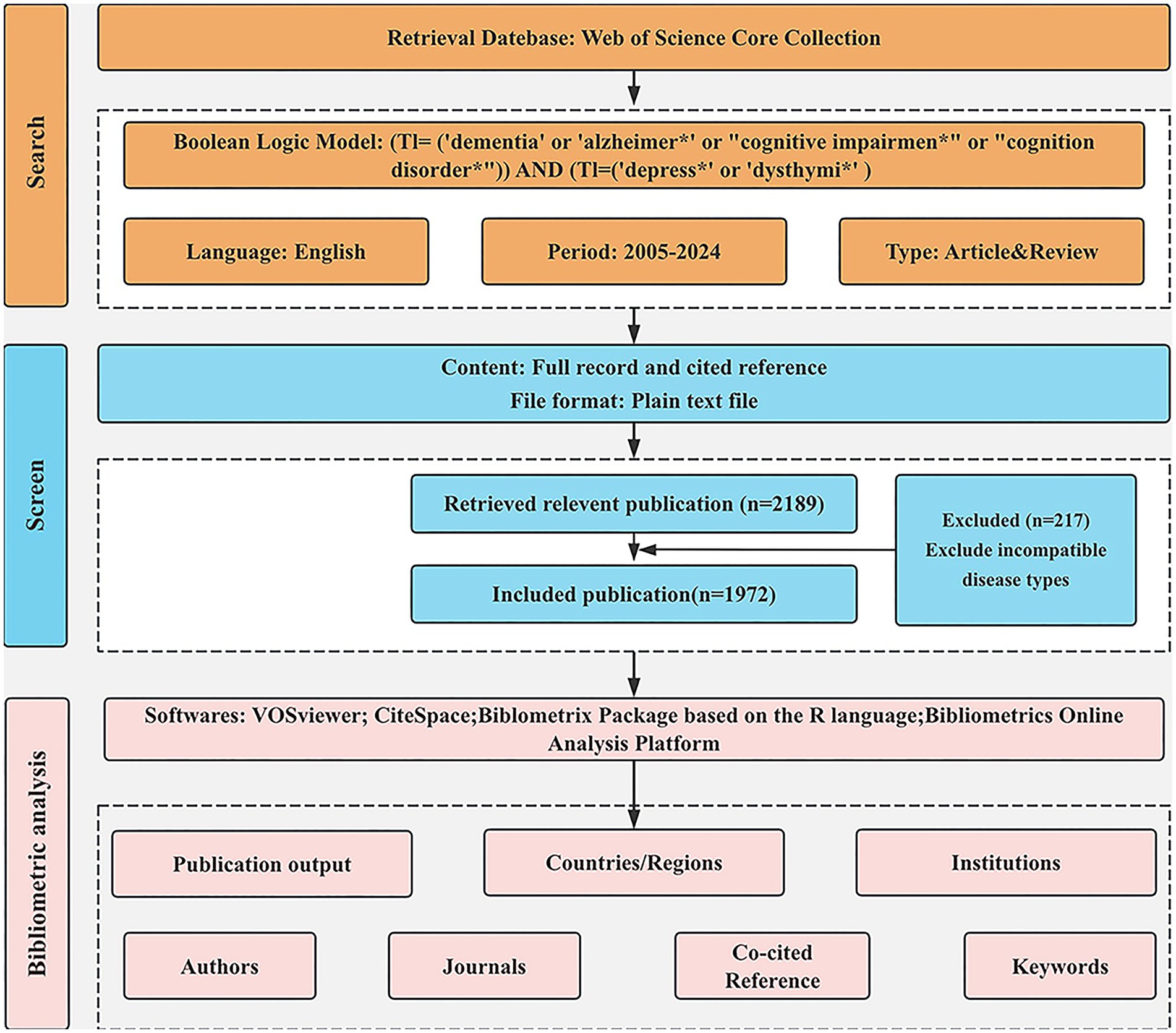
The data relevant to dementia and depression for this study were obtained from the Web of Science Core Collection (WoSCC). WoSCC is the best known and most influential scientific citation database used for bibliometric analysis. The search strategy is as follows: TI = [(‘dementia’ OR ‘Alzheimer∗’ OR ‘cognition disorder*’ OR ‘cognitive impairment*’) AND (‘depress∗’ OR ‘dysthymi*’)]. The search was conducted over a period from June 19, 2005, to June 19, 2024. A total of 3,390 documents were obtained. We restricted our focus to English-language publications categorized as “reviews” or “articles.” 1,201 articles of other types were excluded, including Proceeding Paper (n = 51), Retracted Publication (n = 2), Letter (n = 66), Meeting Abstract (n = 794), Editorial Material (n = 99), Correction (n = 32), News Item (n = 21), Early Access (n = 11), Book Chapters (n = 36), Book (n = 1), Book Review (n = 1), Retraction (n = 1), Non-English (n = 86). A total of 2,189 publications were initially retrieved. Papers that were irrelevant to the research topic were systematically excluded after reviewing titles, abstracts, and, in some cases, the full texts. We excluded articles where the subjects were spouses or caregivers of people with dementia. Finally, 1,972 papers were deemed relevant and included in the analysis. There are 1,697 articles and 275 reviews. The search process is shown in Figure 1.
This study did not involve any animal or human subjects; therefore, no ethical approval was required.
2.2 Data analysis and tools
VOSviewer 1.6.20 and CiteSpace 6.3.R1 were used for bibliometric analysis in this study. VOSviewer is one of the most widely used tools for quantitative analysis in bibliometrics. It provides a systematic understanding of the structure and dynamic development of scientific research (Van Eck and Waltman, 2010). The VOSviewer tool is employed to visualize highly productive countries, institutions, journals, and authors, as well as highly cited journals and authors, thereby enhancing the understanding of relevant research.
CiteSpace, a Java-based visual bibliometric analysis software developed by Chen and Song (2019) and Chen (2006), visualizes research field hotspots and evolution, predicting developments to some extent. Co-occurrence analysis can reveal the focal areas of research. Additionally, CiteSpace uses cluster analysis to group co-cited references and keywords, further unveiling the relationships and structures between different research topics. Its burst detection feature is able to identify keywords that experience a sharp increase in citations within a specific time window, which aids in predicting research trends and identifying emerging research hotspots. And Microsoft Office Excel 16.84 is used to analyze annual publication numbers and related citation trends.
Bibliometric online analysis platform1 to analyze international cooperation between countries. Bibliometrix is an online visualization software package based on R language, which can perform statistical analysis on literature, calculate indexes, and present research hotspots.
3 Results
3.1 Annual outputs and citation trends
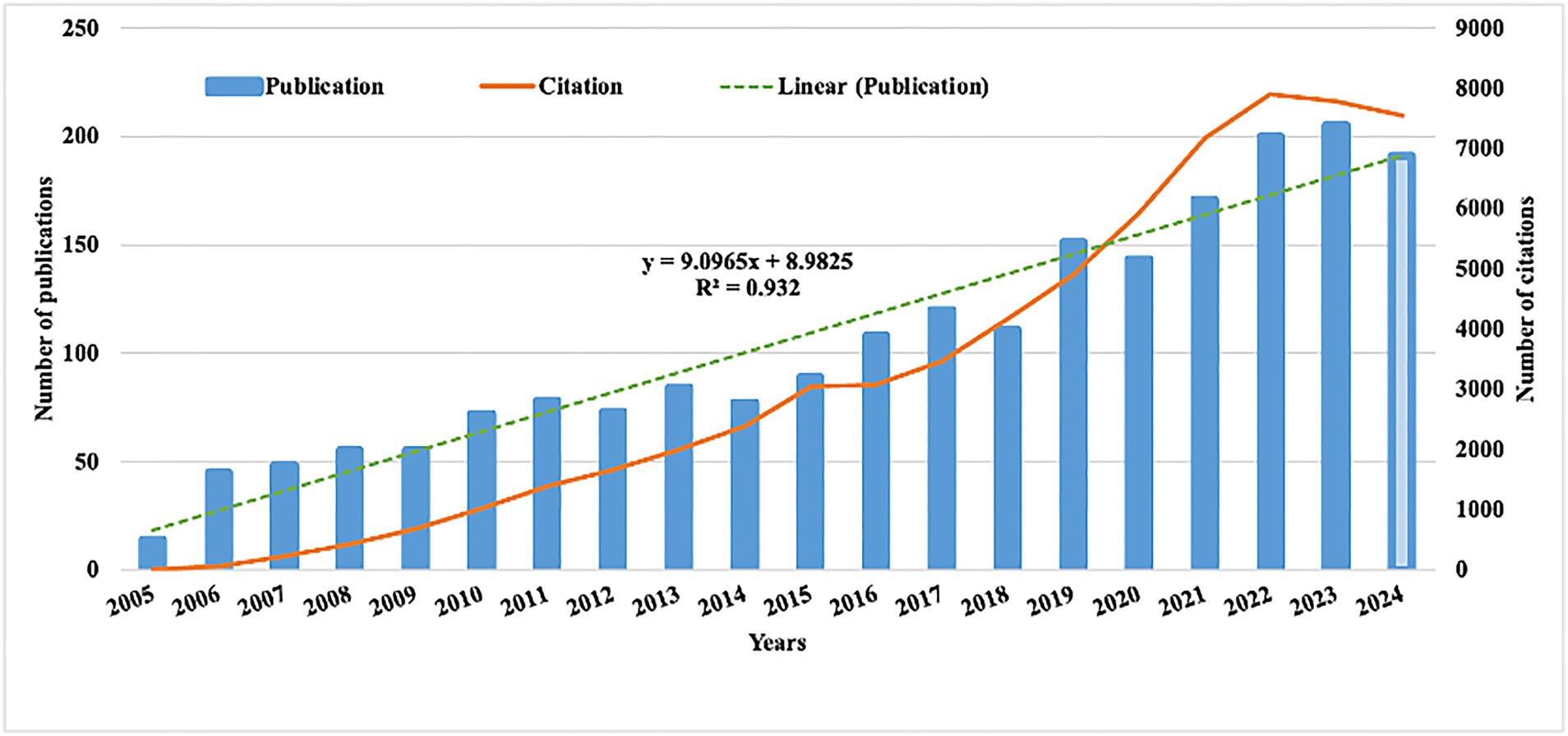
The number of publications and their related citations serve as key indicators to visualize research trends. Over the past 20 years, the volume of publications concerning dementia and depression has steadily increased (Figure 2). As of June 19, 2024, a total of 1,972 articles and reviews have been published, underscoring the continuing significance of this research area. This trend is mirrored in the increasing number of citations, reflecting the growing influence and ongoing relevance of this research area. Using Excel software, a predictive growth model was developed. By the year 2030, it is projected that 236 new articles will be published in this field.
3.2 Most contributing countries/regions and institutions
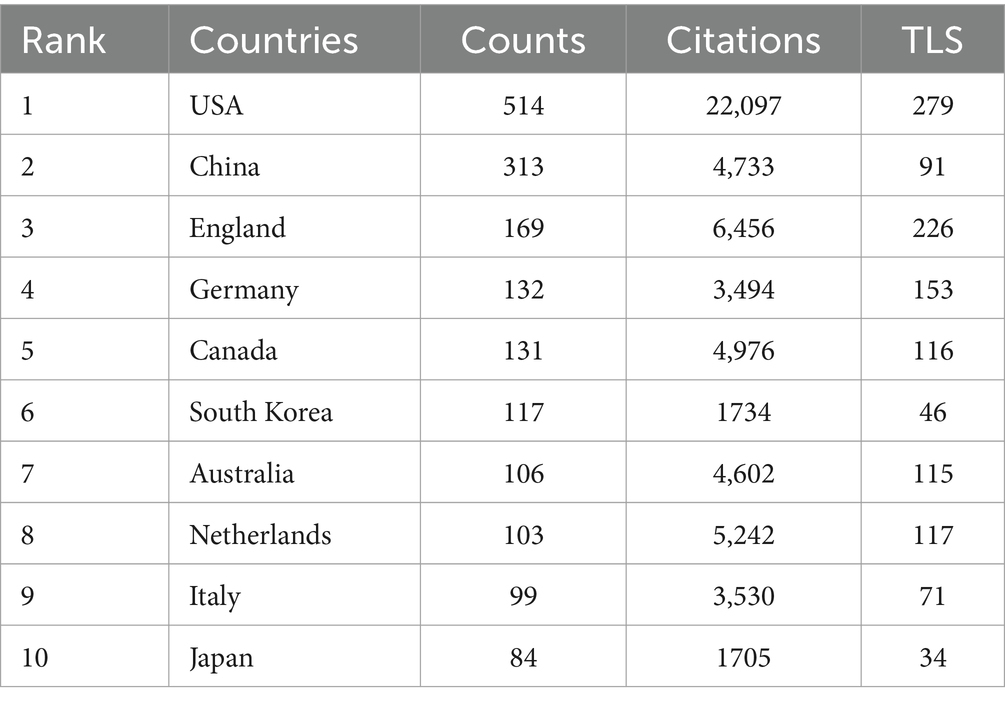
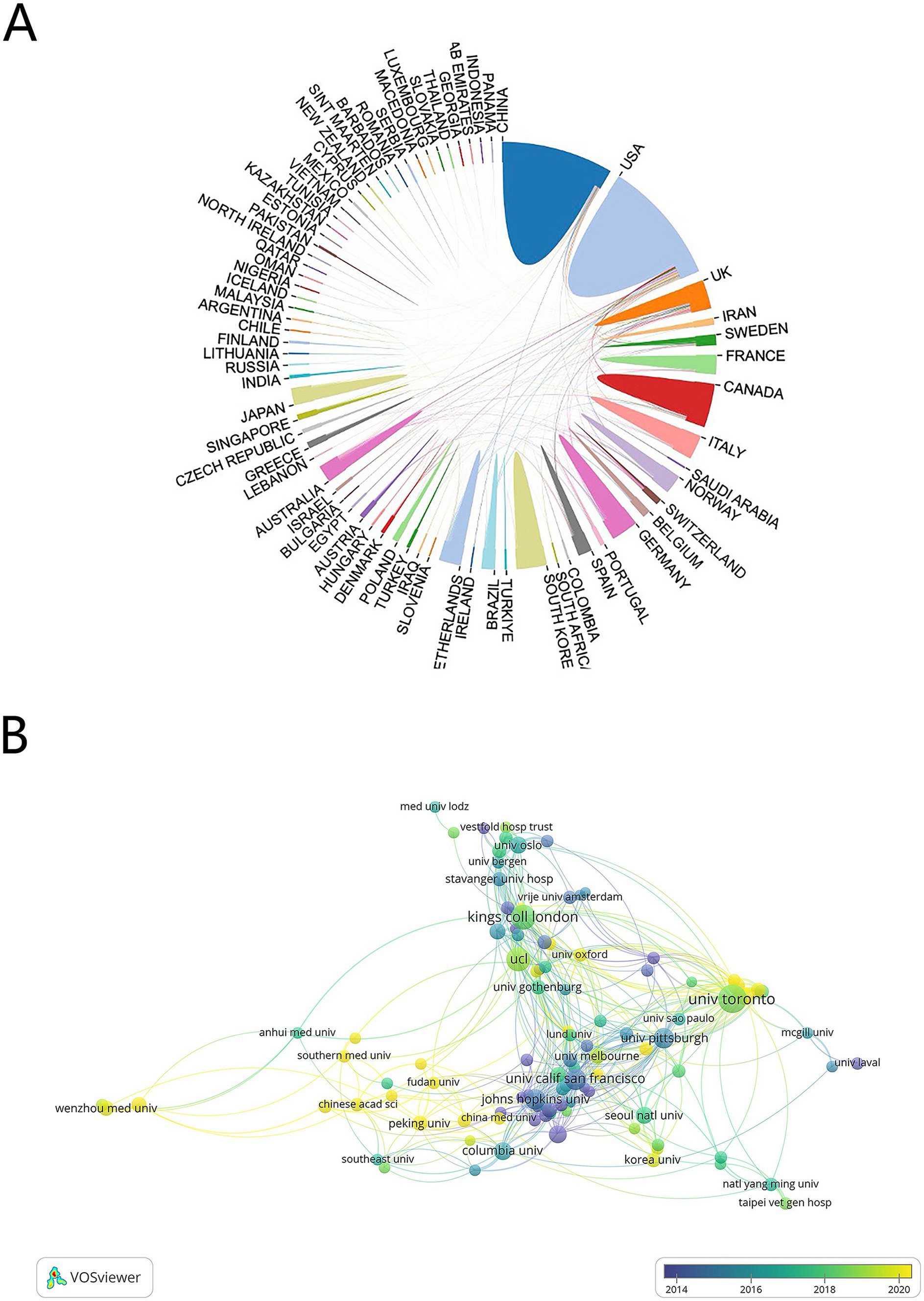
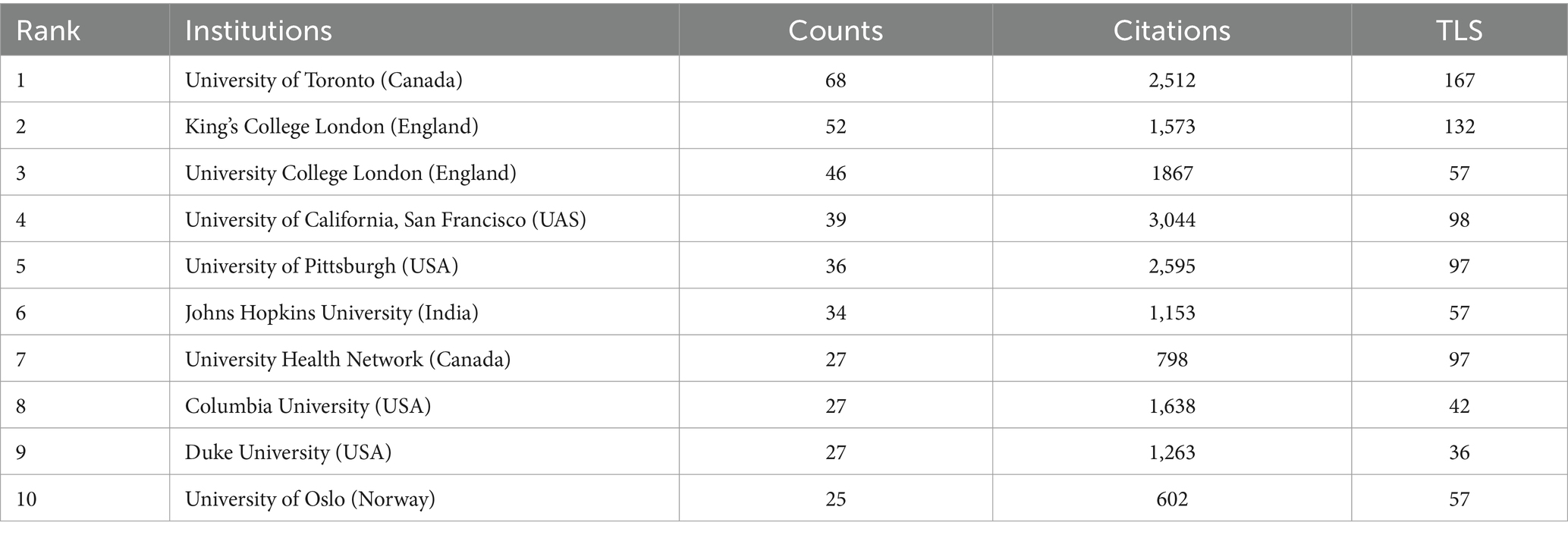
A total of 81 countries and regions participated in this area, with 27 of these contributing 20 or more publications each. The USA leads with 514 publications (22,097 citations), followed by China with 313 publications (4,733 citations) (Table 1). An international cooperation network map (Figure 3A) reveals collaborative relationships among 70 of the 81 countries, with different colors marking each on the map. The USA is the most frequent collaborator, followed by the United Kingdom and Germany, though China, despite its high publication count, shows fewer international collaborations. On the institutional level, 2,658 organizations engage in this research, with 104 having published 10 or more works. The University of Toronto, with 68 publications, leads, followed by King’s College London and University College London with 52 and 46 publications, respectively. Four of the top 10 institutions are in the USA, collectively publishing 129 papers (Table 2). Figure 3B shows the temporal distribution of publications, highlighting the University of Toronto’s significant output and extensive collaborations from 2018 to 2020, with yellow indicating recent activity.
3.3 Analysis of author
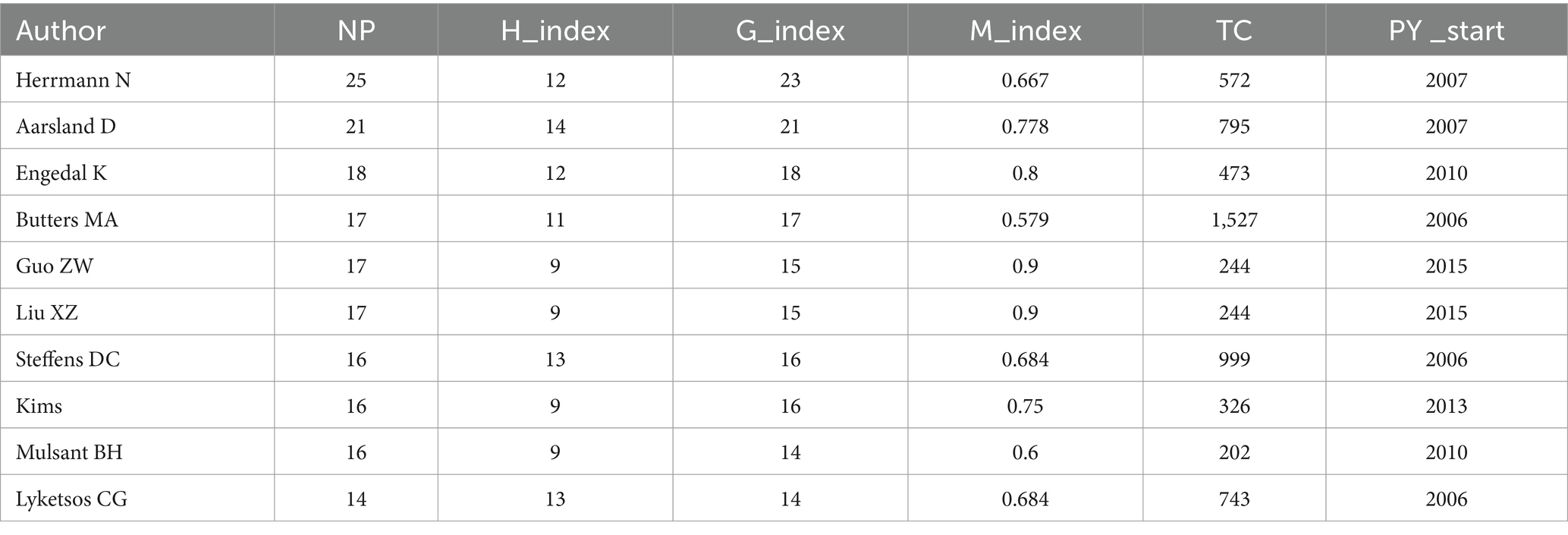
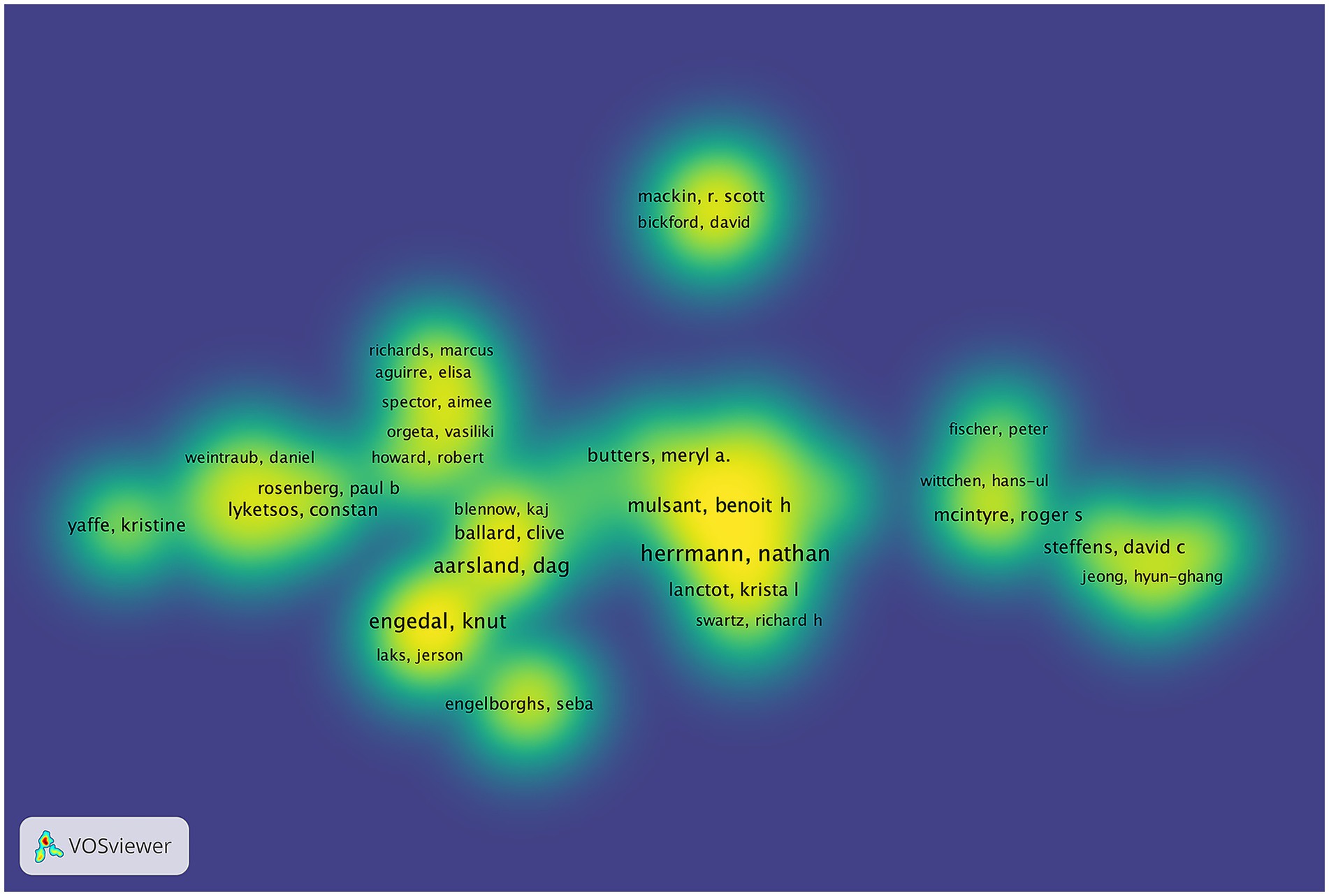
The study identified 9,570 authors publishing on dementia and depression, with Herrmann N (Hirsch, 2005), Aarsland D (Du et al., 2024), and Engedal K (Kokol et al., 2021) leading (Table 3). Aarsland D has the highest H-index, indicating significant citation impact (Hirsch, 2005; Hirsch, 2007). The G-index and M-index further reflect the authors’ scholarly impact (Ali, 2021). A central cooperative network among these top contributors is evident in the author density visualization (Figure 4). Notably, Engedal K has ceased publishing since 2022, while Herrmann N remains highly active and cited (Figure 5).
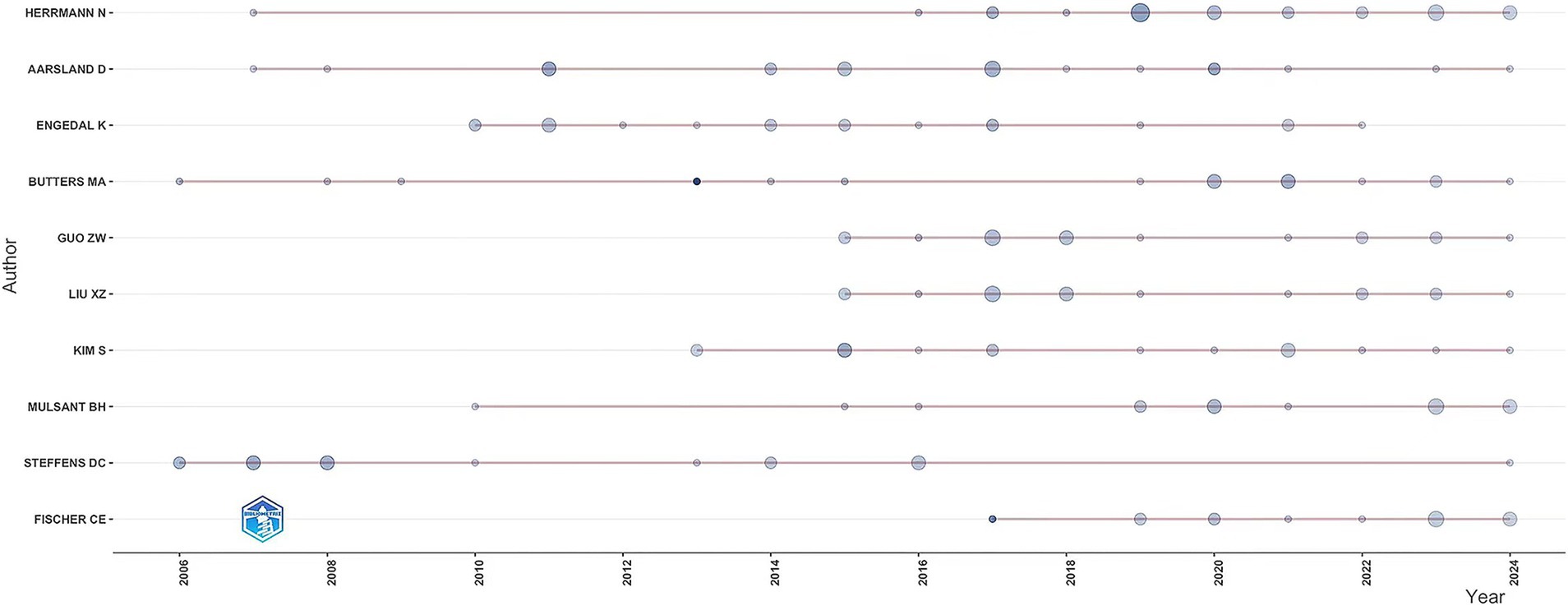
Figure 5. The line represents an author’s timeline. The bubble size is proportional to the n (n is year) of documents. The color intensity is proportional to the total citation per year.
3.4 Analysis of journals and co-cited journals
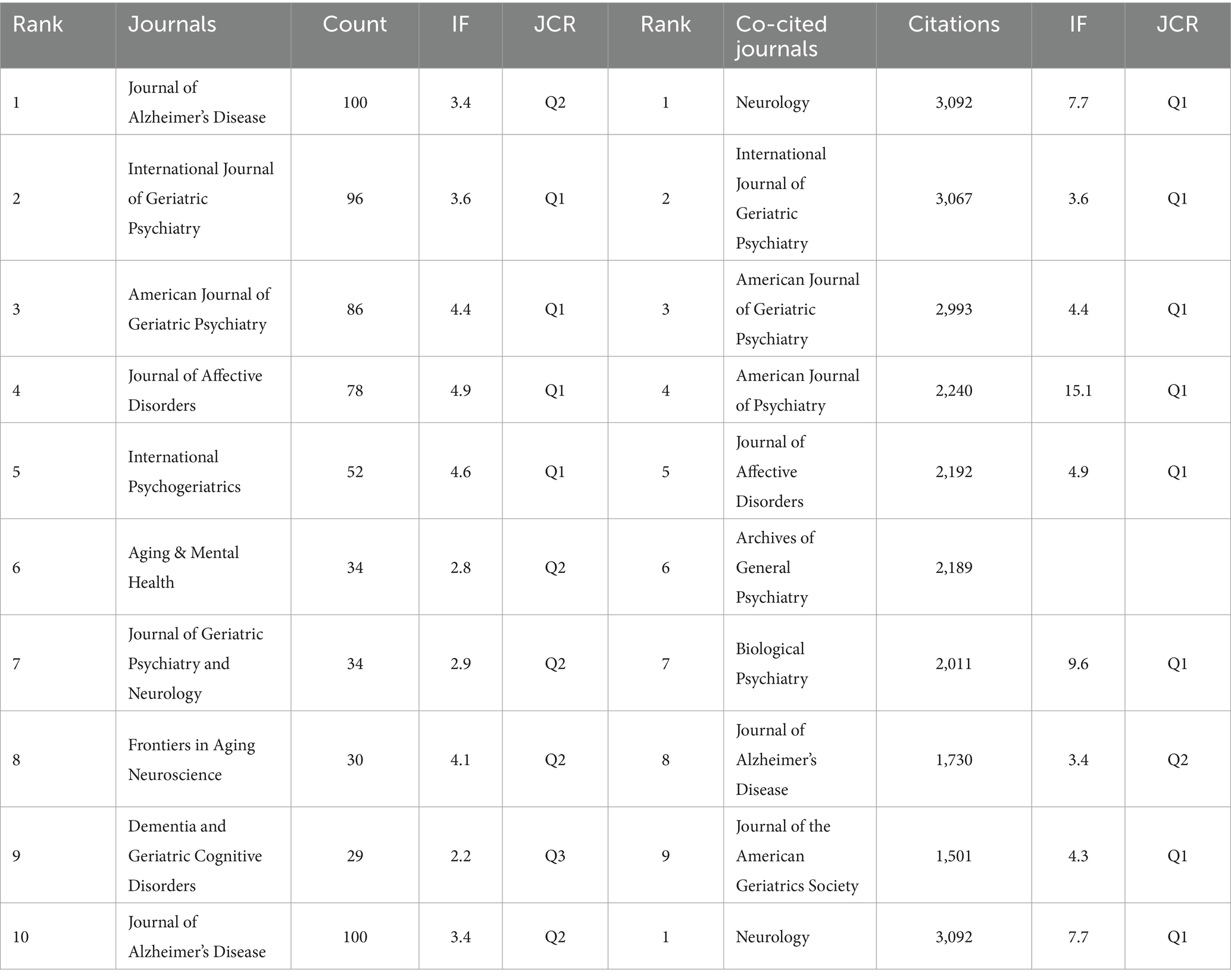
A total of 605 journals have published papers on dementia and depression. Table 4 shows that the top 10 journals, primarily in the fields of geriatric psychiatry and neurology, include the Journal of Alzheimer’s Disease, which leads with 100 publications. It is closely followed by the International Journal of Geriatric Psychiatry and the American Journal of Geriatric Psychiatry, with 96 and 86 publications, respectively. The influence of each journal is gauged by the number of co-citations it receives. The more co-citations, the greater the journal’s impact. Neurology ranks as the most cited journal, with 3,092 citations, highlighting its significant academic value in neurology. The International Journal of Geriatric Psychiatry and American Journal of Geriatric Psychiatry are also highly cited, with 3,067 and 2,993 citations, respectively.
3.5 Analysis of keywords
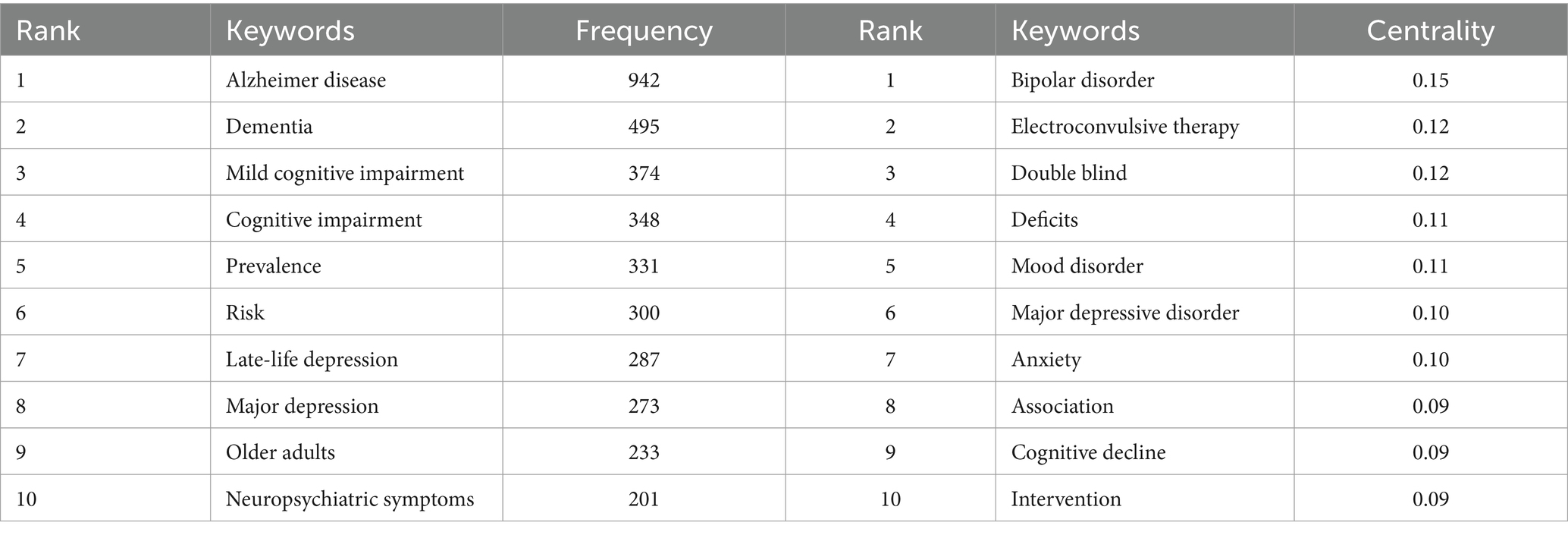
3.5.1 Keyword co-occurrence
We deleted and merged keywords. The merged keywords are synonyms, such as merging Alzheimer disease into Alzheimer’s disease and late-life depression into late life depression. The deleted keywords are meaningless keywords that cannot provide key information, such as patient and life. Table 5 highlights the top 10 keywords ranked by frequency in this field. A visual map of the keywords was shown in Figure 6A. The frequent keywords Alzheimer’s disease, dementia, and mild cognitive impairment (MCI) emphasize the research focus on the link between depression and dementia. This highlights ongoing efforts to understand how depressive disorders interact with cognitive declines, aiming to identify diagnostic markers and therapeutic targets for these coexisting conditions. Centrality highlights key concepts in the research network. The top three keywords—bipolar disorder (0.15), electroconvulsive therapy (0.12), and double blind (0.12)—underscore the importance of rigorous study design, the link between bipolar disorder and dementia, and treatment of patients with dementia and depression.
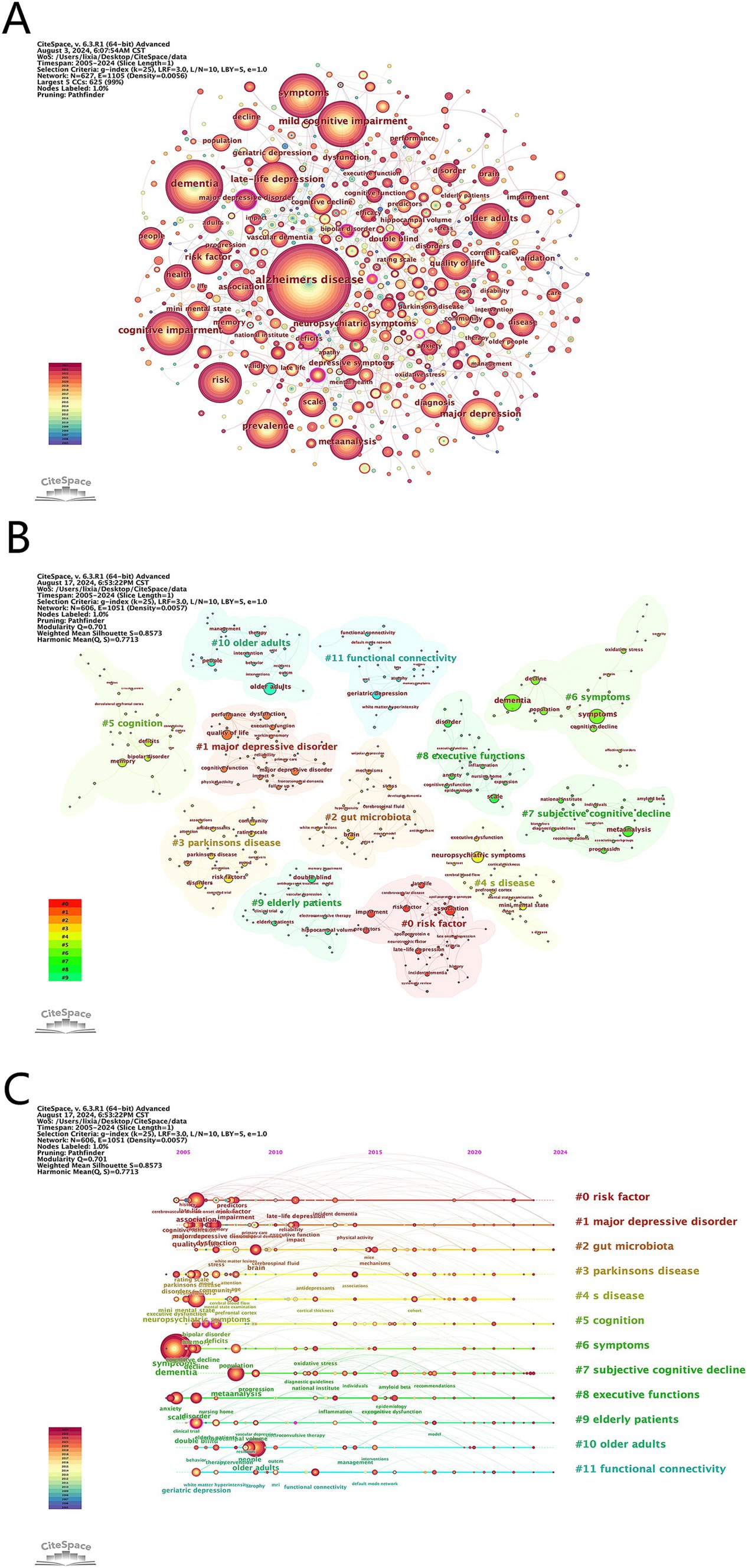
Figure 6. (A) Visualization of keywords. (B) Cluster analysis of keywords. (C) Timeline graph of cluster analysis.
3.5.2 Keyword clustering timeline
We performed a cluster analysis of the keywords (Figure 6B) to identify trending topics in the field. Modularity Q and Mean Silhouette (S) are crucial indicators for evaluating the validity of a clustered map. A Q value greater than 0.3 indicates a significant clustering structure, while an S value greater than 0.7 suggests convincing clustering. In this study, the S value is 0.86 and the Q value is 0.71, indicating reliable clustering results. Figure 6C presents a timeline of the keywords, highlighting the research progress of keyword nodes in each cluster. The clustering results reveal that #2 gut microbiota, #8 executive functions and #11 functional connectivity are current research hotspots. These clusters reflect an interest in how gut health affects neurological conditions, the cognitive deficits common in both disorders, and the brain network changes linking depression and cognitive impairments.
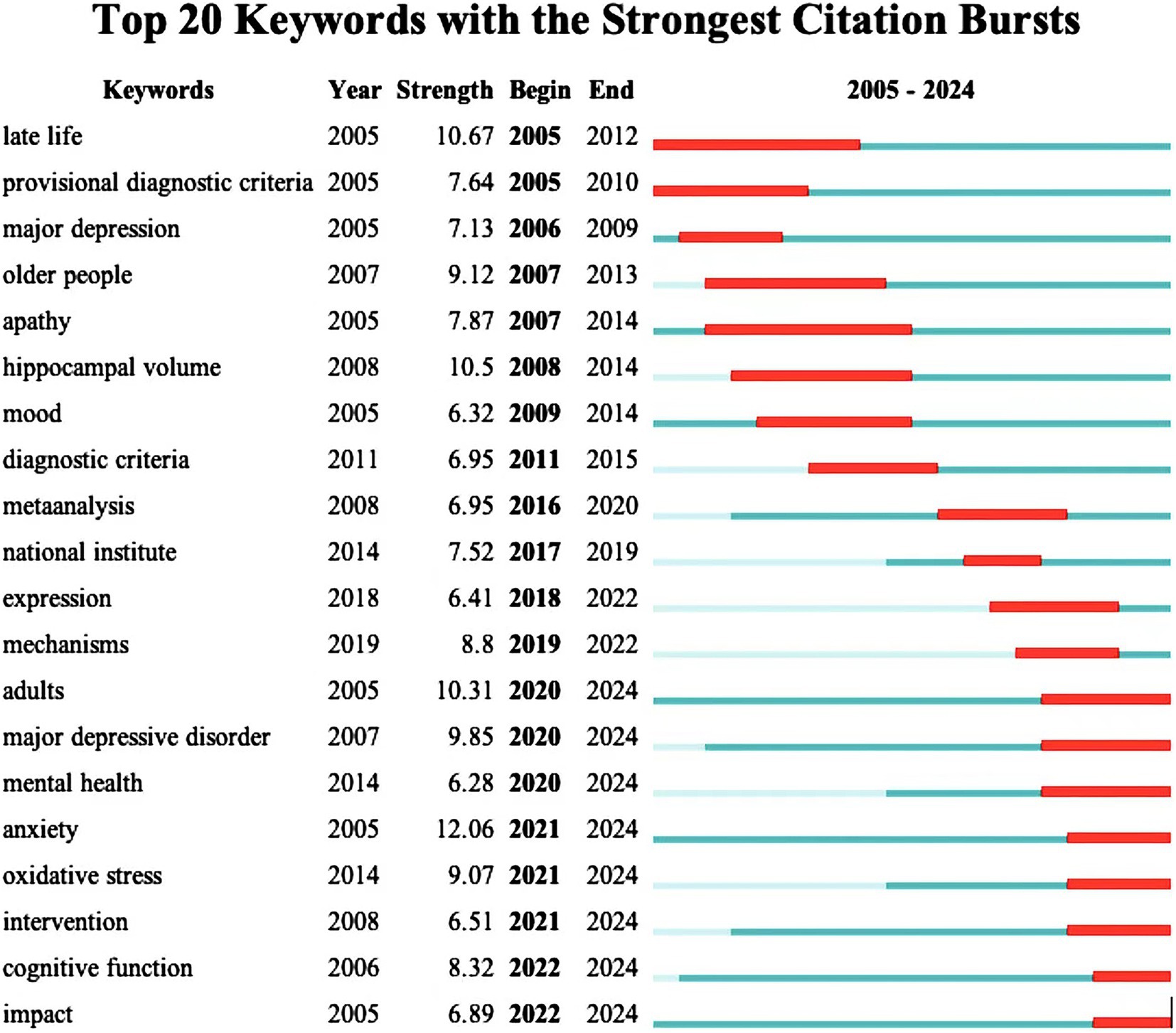
The citation burst analysis reveals evolving trends in dementia and depression research (Figure 7). Early (2005–2011) focus areas included “late life,” “major depression” and “hippocampus volume,” while mid-period (2012–2019) research emphasized “diagnostic criteria,”” meta-analysis “and “mechanisms.” Recent (2020–2024) hotspots highlight “oxidative stress,” “anxiety,” “cognitive function,” and “intervention,” indicating current cutting-edge interests. Overall, the study highlights a shift towards understanding oxidative stress, anxiety, and cognitive aspects in dementia and depression.
The keyword quadrant map (Figure 8) highlights Motor Themes such as “nursing home,” “randomized controlled,” “home residents,” “music therapy” and “reminiscence therapy,” showing they are central and well-developed areas focusing on elderly care interventions. Basic Themes like “cognitive impairment” and “depressive symptoms” are crucial but need further development. Niche and Emerging/Declining Themes indicate specialized and less-attended areas. In conclusion, trends point to early detection of cognitive decline, the impact of gut health on cognition, and managing dementia and depression in elderly care.
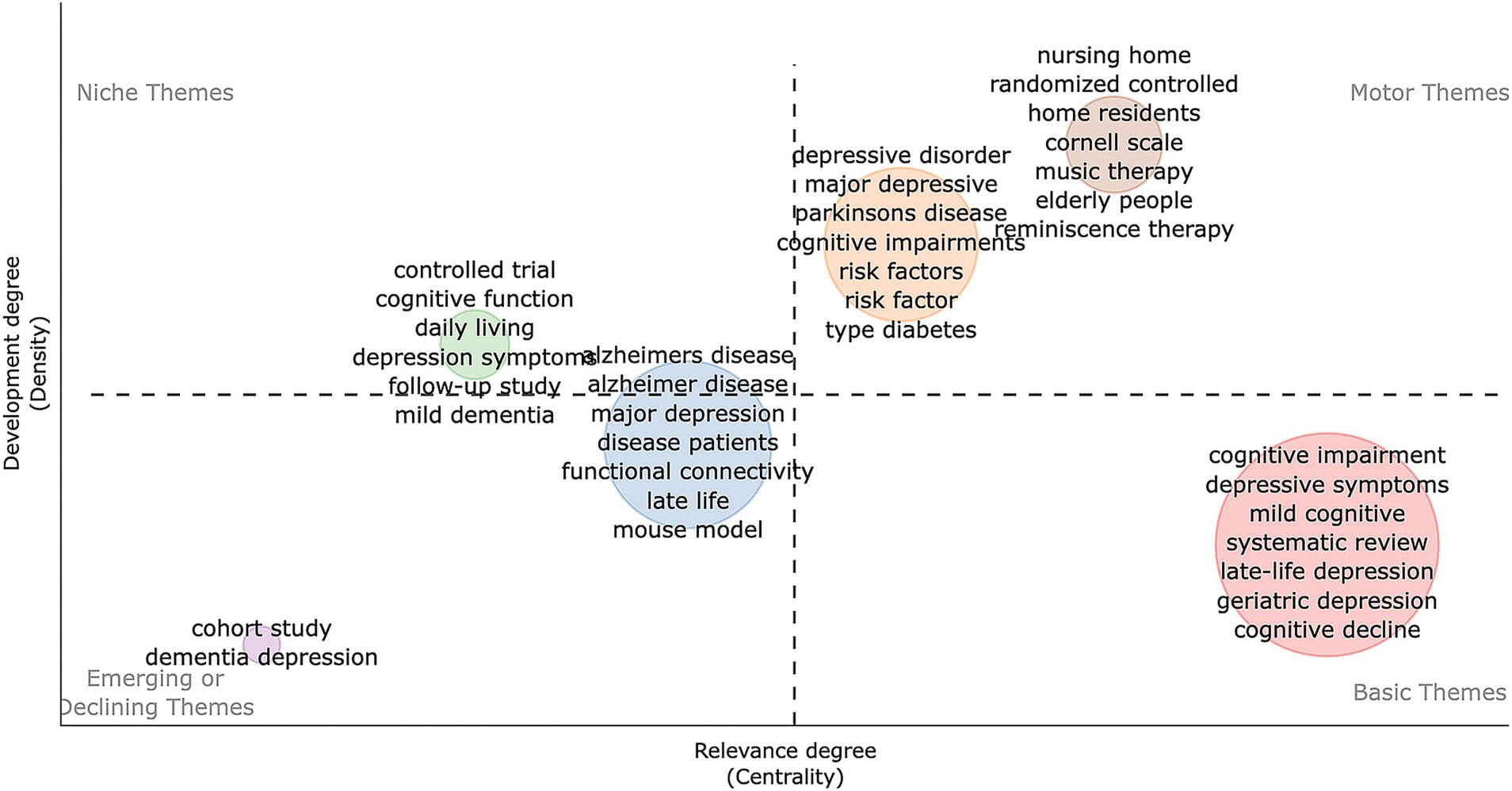
Figure 8. Development trend and importance of hot spots. The X-axis represents the centrality indicating the importance of a theme; The Y-axis symbolizes the density indicating the development of a theme.
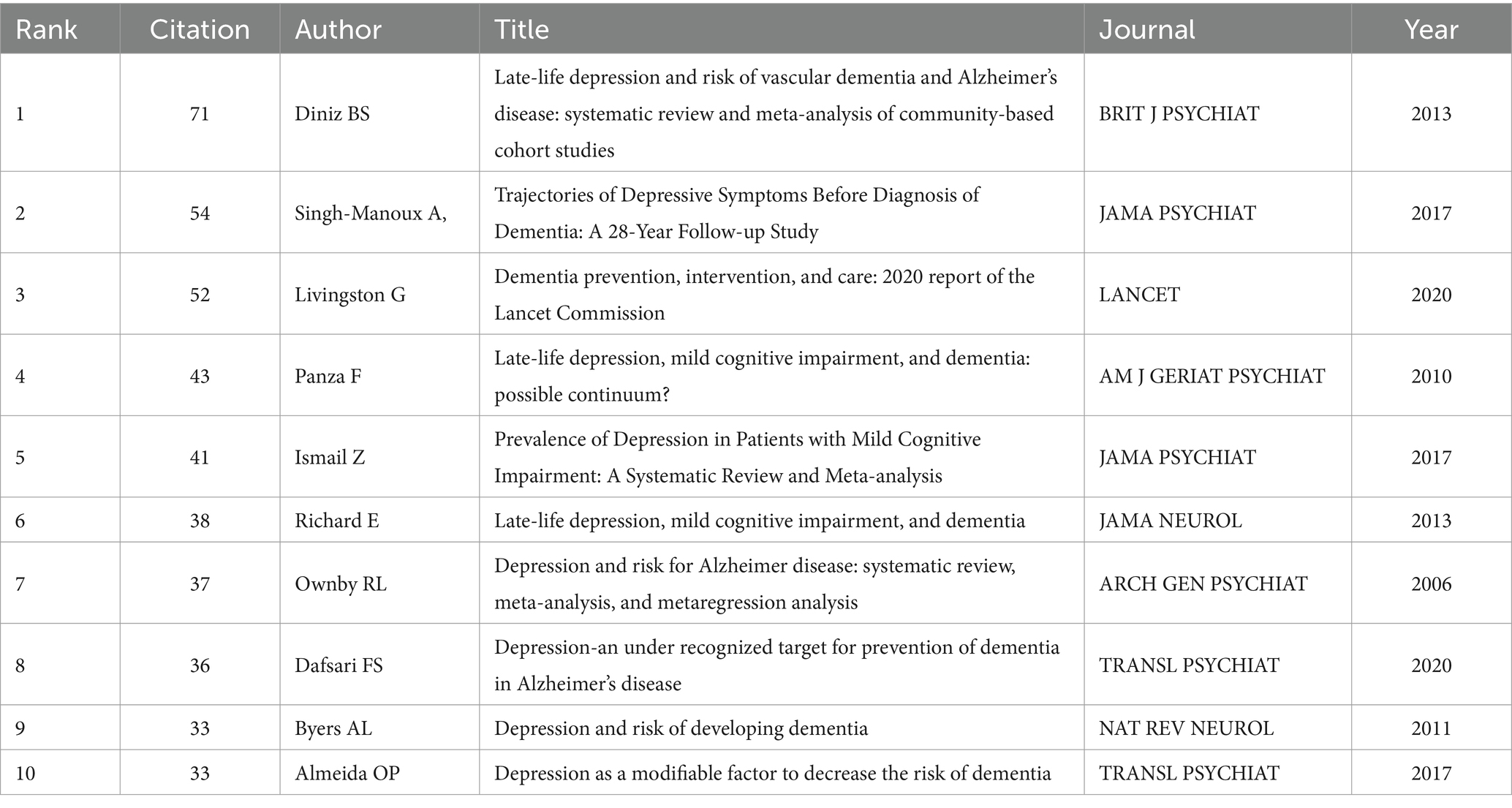
3.6 Analysis of co-cited references
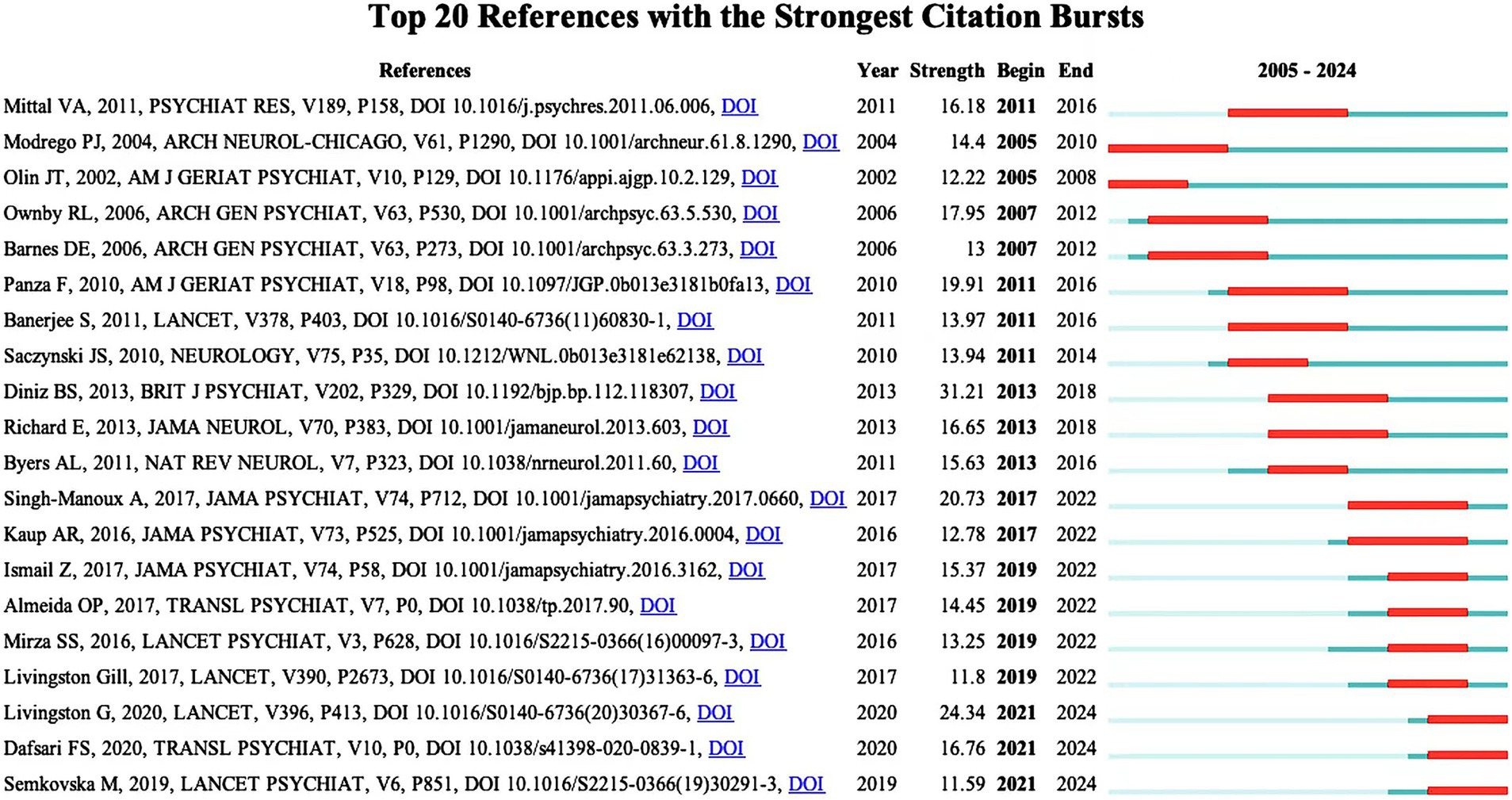
Table 6 lists the top 10 most frequently co-cited literature. They have high academic value and influence. It can be used by new researchers to get a quick overview of the field. Figure 9 shows the top 20 References with the Strongest Citation Bursts. Recent citation bursts between 2021 and 2024 highlight three key publications in The Lancet and Translational Psychiatry. Livingston G’s 2020 study discusses management strategies for depression and dementia. Dafsari FS explores links between antidepressant treatment and prevention of dementia in 2020. Semkovska M’s 2019 paper focuses on effects of depression remission on cognition. These studies, significant in the fields of depression and dementia, have garnered substantial attention for advancing understanding and treatment approaches.
4 Discussion
4.1 General information
In this study, we conducted a comprehensive bibliometric analysis of 1972 publications related to dementia and depression published on WoSCC from 2005 to 2024. From 2005 to 2024, the growing number of publications on dementia and depression underscores an expanding research focus and increasing awareness of these critical public health issues. The growing number of publications on dementia and depression can be attributed to several key factors. First, the aging of the global population has exacerbated the prevalence of dementia and depressive diseases, thereby increasing research interest among researchers. The United Nations Population Division released data in July 2024. By the end of the 2070s, the global population aged 65 and over is expected to reach 2.2 billion, exceeding the number of children under the age of 18, which will exacerbate social and economic impacts. Second, advances in diagnostic techniques such as neuroimaging and biomarkers enable early detection of dementia and its early depressive symptoms. Aggrephay-related gense (ARGs) can also be used to identify new diagnostic markers for AD through machine learning methods (Xu et al., 2023). This has stimulated research into their common pathologies and potential treatments. Third, increasing awareness of the critical role of mental health has fueled research into depression and dementia. In addition, there has been a significant increase in research into non-pharmacological interventions, including cognitive training and physical activity, which are becoming complementary to traditional pharmacological treatments. The field demonstrates robust global engagement, with the USA leading in publication volume followed by China. The USA also leads in research impact, evidenced by high citation and collaboration rates. China needs to improve the quality of its publications and international collaborations. The close collaborations, especially between the USA and China, emphasize the field’s global scope and the importance of international partnerships in fostering advancements. In recent years, some countries have made outstanding contributions in the field of dementia and depression. In 2023, a Norwegian scholar published an article in Lancet Diabetes Endocrino that systematically sorted out the effects of steroid hormones, especially estrogen, on the female brain and its impact on the risk of depression and dementia. This furthered our understanding of the complex interactions of risk and protective factors on female brain health and provided a neurological framework for vulnerability and resilience (Barth et al., 2023). In addition, a randomized clinical trial study published in JAMA Psychiatry by Canadian researchers in 2024 found that cognitive remediation and transcranial direct current stimulation, both targeting the prefrontal cortex, can effectively slow the risk of cognitive decline in those with remitted MDD (with or without MCI) and those with a low genetic risk of Alzheimer’s disease (Rajji et al., 2024). Analyzing each international contribution to dementia and depression research will help identify key research strengths and potential international partnerships.
The University of Toronto stands out among the top ten research institutions, with Herrmann N as its leading author. The 2023 publication by Herrmann N in Biological Psychiatry investigates the integrity of gray and white matter in cases of remitted major depressive disorder (rMDD), MCI, and concurrent rMDD and MCI. The study indicates that early-onset, treated MDD might not induce structural changes linked to cognitive decline (Marawi et al., 2023). Herrmann’s highly cited 2018 study of 1,965 participants with depression and MCI found that the amnestic MCI subtype and active depression within the past 2 years independently increased the risk of AD dementia (Gallagher et al., 2018). Another influential study reported that MCI patients with apathy alone or both apathy and depression had a higher risk of progressing to AD compared to those without neuropsychiatric symptoms (Ruthirakuhan et al., 2019). Furthermore, Aarsland D, who boasts the highest H-index, along with his colleagues, discovered an association between late-life depression and abnormal amyloid protein metabolism by comparing synaptic function markers and AD pathology in patients with geriatric depression, pre-dementia AD, and a control group (Siafarikas et al., 2021). In a separate study in 2022, Engedal K used magnetic resonance imaging to compare patients with dementia against those with both dementia and depressive symptoms, noting distinct differences in the morphology of the temporal lobe—specifically, reduced sizes in the left temporal pole and right transverse temporal cortex in the latter group (Barca et al., 2022). Research on dementia and depression is predominantly published in Q1 and Q2 geriatric psychiatry and neurology journals, underscoring the high quality and credibility of the studies. The Journal of Alzheimer’s Disease leads in publications on Alzheimer’s etiology, pathogenesis, and treatment, while Neurology, sponsored by the American Academy of Neurology, is the most cited, reflecting its prestige and quality.
4.2 Research basic
High-frequency keywords in co-occurrence analyses reflect the research hotspots within a field. In this study, the top three keywords are AD, dementia, and MCI. AD is the main type of dementia, accounting for approximately 60–80% of all dementia (Yepes, 2021). According to the latest clinical staging guidelines for AD, the development of dementia is divided into 7 stages. Stage 0 refers to carrying the familial AD pathogenic gene; stage 1 is the discovery of positive core markers of AD; stage 2 is characterized by subjective cognitive decline; stage 3 is mild cognitive impairment; stage 4 is mild dementia; stage 5 is moderate dementia; stage 6 is severe dementia (Jack et al., 2024).
Early detection and intervention of MCI are crucial to delaying the progression of dementia (Ganguli et al., 2004). Depression is a common psychiatric symptom in patients with MCI and may signal the transition from MCI to dementia (Asmer et al., 2018; Cooper et al., 2015). Seoyoung Yoon demonstrated that the cognitive impairment in MCI patients with active depression was more pronounced than in those without depression, based on a one-year follow-up (Yoon et al., 2017). Meanwhile, Brooks et al. (2024) found that theta phase-gamma amplitude coupling (TGC) can distinguish individuals with MCI or MCI with rMDD (MCI + rMDD). In contrast, another study reported no significant in general cognition, as measured by Mini-Mental State Examination (MMSE), between MCI + rMDD and MCI patients (Duffy et al., 2014). The increased risk of cognitive decline in MCI patients with concurrent major depression compared to those with MCI alone remains uncertain and warrants further investigation.
The complex interplay between depression and dementia has been extensively studied, with particular attention to the temporal relationship between these conditions. The review published in 2013 from The British Journal of Psychiatry by Diniz et al. (2013) holds the highest citation count, revealing that late-life depression notably increases the risk of dementia, particularly vascular dementia and AD, through a comprehensive meta-analysis of 23 studies. Generally speaking, patients with depression in their later years have a poorer overall health condition, especially with a higher risk of cardiovascular and cerebrovascular diseases (Charlson and Peterson, 2002; Sheline et al., 2010). In addition, the co-occurrence of vascular diseases and AD suggests that many diagnoses of AD may actually represent mixed dementia cases (Laukka et al., 2010; Gold et al., 2007).
Further exploring the relationship between depression and dementia, a 28-year follow-up study involving 10,308 individuals by Singh-Manoux et al. (2017) a revealed that while depression in middle age does not increase the risk of dementia, but depressive symptoms in late life do. This finding suggests that these symptoms could be early indicators of dementia or have a common underlying cause, challenging the hypothesis that depression directly increases dementia risk (Singh-Manoux et al., 2017).
Enhancing the current understanding, the 2020 Lancet Commission report accentuates depression’s critical role in dementia progression (Livingston et al., 2020). The report refers to a pivotal study showing that the administration of selective serotonin-reuptake inhibitors (SSRIs) for over 4 years can delay the clinical manifestation of AD symptoms, which invites further exploration into whether antidepressants could lower the risk of dementia (Bartels et al., 2018). Together, these findings highlight the necessity of preventing, diagnosing early, and actively managing late-life depression not only to improve overall health but also to potentially decrease the incidence of dementia. The compelling evidence calls for additional research to determine how effective management of depression could delay or modify the course of dementia.
In conclusion, while late-life depression is consistently associated with an increased risk of dementia, its role as a precursor, comorbidity, or risk factor remains complex and multifaceted. The current evidence underscores the importance of early identification and effective management of late-life depression, not only to enhance overall health outcomes but also to potentially delay or modify the trajectory of dementia. Future research should prioritize disentangling the temporal and causal relationships between depression and dementia to inform targeted preventive and therapeutic strategies.
Recent studies have highlighted the significant impact of demographic factors, such as age, gender, and race, on the prevalence of dementia and depression. For instance, a Danish study investigating female nurses with depression at different life stages found that those who developed depression in late life exhibited a higher risk of progressing to dementia compared to those who experienced depression in middle age (Hickey et al., 2023). Gender differences also play a pivotal role; women have a 55% higher risk of developing dementia than men starting at the age of 65 (Shaw et al., 2021). Similarly, depression disproportionately affects women compared to men, with these gender disparities becoming evident as early as adolescence (Kuehner, 2003). Racial disparities further complicate the clinical landscape. Among patients with comorbid dementia and depression, notable differences were observed in the utilization of antidepressant treatments (Bhattacharjee et al., 2017). Additionally, Asian patients within this cohort demonstrated a lower mortality rate compared to white British patients (Lewis et al., 2018). These findings underscore the importance of considering demographic variables in understanding the complex interplay between dementia and depression.
4.3 Research hotspots and development trends
4.3.1 Neuropsychiatric manifestations in dementia and depression
Neuropsychiatric symptoms associated with dementia and depression present a complex challenge, as they significantly impact cognitive functions and emotional regulation in affected individuals. These symptoms can manifest as part of several underlying conditions but are particularly prominent in disorders like MDD and dementia.
4.3.1.1 Bipolar disorder
Bipolar disorder (BD) is a chronic condition that often leads to cognitive impairments affecting memory, attention, and executive function, even during remission phases (Li et al., 2020; Martínez-Arán et al., 2004). These impairments significantly reduce patients’ quality of life and daily functioning (O’Donnell et al., 2017; Sanchez-Moreno et al., 2018). The prevalence of dementia in BD patients ranges from 20 to 25%, notably higher than the 7% observed in the general population over 60 (Almeida et al., 2016; Nunes et al., 2007; Prince et al., 2013). Factors such as antipsychotic treatment, age, and polypharmacy have been identified as predictors of cognitive decline in these patients (Tsapekos et al., 2021). Additionally, cerebral white matter deficits are associated with these cognitive challenges (Macoveanu et al., 2021). Meta-analyses indicate that BD patients have an elevated risk of developing dementia, particularly AD (Velosa et al., 2020; Aprahamian et al., 2013), with each BD episode increasing dementia risk by 6% (Kessing and Andersen, 2004). Research by Knorr et al. (2022) suggests that variations in cerebrospinal fluid markers during emotional relapses, specifically increased a Aβ42 and decreased hyperphosphorylated tau, could contribute to cognitive deterioration and a higher AD risk in BD patients AD related biomarkers in BD—A longitudinal one-year case–control study. Moreover, a study by Xiao Ming revealed that BD patients using valproate have a 59% higher risk of dementia compared to those not using the drug (Moon et al., 2022).
4.3.1.2 Anxiety
Anxiety is commonly observed in dementia and MCI, often coexisting with depression (Hwang et al., 2004; Seignourel et al., 2008). A history of anxiety is linked to a higher risk of all-cause dementia (Kuring et al., 2020). Meta-analysis shows anxiety prevalence in dementia at 38, 41, and 37% across mild, moderate, and severe stages, respectively (Leung et al., 2021), and between 9.9 to 52% in MCI patients (Chan et al., 2011; Gallagher et al., 2011; Lyketsos et al., 2002; Palmer et al., 2007). Studies suggest that anxiety and Aβ interactions accelerate cognitive decline, with anxiety predicting cognitive deterioration in both cognitively unimpaired individuals and MCI patients (Johansson et al., 2020; Pietrzak et al., 2015). Anxiety contributes to the progression from MCI to AD, either directly or through depression (Palmer et al., 2007; Potvin et al., 2011). Biringer et al. (2005) noted that high anxiety impacts cognitive function only alongside depressive symptoms. Contrarily, Devier et al. (2009) argue that state anxiety is not a significant predictor of AD, while higher trait anxiety may lower AD risk. Further long-term studies are necessary to clarify anxiety’s role in dementia progression.
4.3.1.3 Major depressive disorder
Numerous studies indicate that major depression significantly increases the risk of dementia, particularly AD (Diniz et al., 2013). Elderly individuals with MDD, whether in the onset or remission phase, often experience MCI, further elevating their dementia risk (Barnes and Yaffe, 2011; Koenig et al., 2014). The potential connection between major depression and dementia may involve abnormalities in the frontal executive circuit of the executive control network and the corticolimbic circuit of the default mode network, which are thought to diminish cognitive reserve and increase the risk of dementia or AD (Butters et al., 2008). The use of antidepressants in treating MDD has garnered attention regarding its implications for dementia risk. Conflicting results have emerged from various research designs. For example, an Israeli prospective cohort study associated antidepressant use in middle and late life with an increased dementia risk after adjusting for confounders (Kodesh et al., 2019). Conversely, a study involving U.S. veterans found no correlation between antidepressant exposure and long-term dementia risk, regardless of whether antidepressants were used or not (Ramos-Cejudo et al., 2024). The role of MDD in influencing these divergent findings warrants further investigation.
Emotional disorders such as depression, mania, and anxiety are common in prodromal dementia syndrome and often precede cognitive decline. Anxiety is characterized by feelings of nervousness or unease, often without a clear objective cause. MDD primarily presents with persistent low mood, loss of interest, pessimism, cognitive slowing, lack of motivation, poor appetite, and sleep disturbances, with severe cases involving suicidal ideation or behaviors. Bipolar disorder is defined by episodes of extreme mood swings, ranging from depressive lows to hypomanic or manic highs. A survey of 1,399 elderly psychiatric patients with dementia found that 8.3% had bipolar disorder, 10.5% had anxiety disorders, and 12.2% had recurrent depression (Kershenbaum et al., 2021), indicating a higher dementia risk in depression compared to anxiety or bipolar disorder. Additionally, a study on severe mental illness and dementia risk reported that depression independently predicted dementia onset between ages 65–79, while bipolar disorder and anxiety disorders were associated with onset between ages 65–84 (Zilkens et al., 2014).
4.3.2 Mechanisms of dementia and depression
The mechanisms underlying dementia and depression are complex and multifaceted, involving biological, environmental, and psychological factors. Among the biological aspects, recent research has highlighted three critical pathways: the role of the gut microbiome, oxidative stress and functional connectivity.
4.3.2.1 Gut flora
Research over the past 20 years highlights the bidirectional communication of the “gut-brain axis,” through which the intestinal flora regulates the immune, metabolic, and nervous systems. Dysregulation of this axis is implicated in neuropsychiatric diseases, including dementia (Morais et al., 2021; Fan and Pedersen, 2021). Communication occurs via neural pathways, immune regulators, and chemical transmitters (Morais et al., 2021). The intestinal flora connects the gut to organs like the brain, influencing health by altering neuronal circuits, structure, and function through signaling and neurotrophic substances, thereby affecting brain development and behavior (Szabo, 2015; Diaz Heijtz et al., 2011). Recent studies have highlighted the significant role of the gut microbiome in brain function and behavior, particularly concerning dementia and depression. There is compelling evidence that these conditions’ pathogenesis is closely linked to changes in the gut microbiota, where dysbiosis plays a crucial regulatory role (Diaz Heijtz et al., 2011; Goyal et al., 2021; Luna and Foster, 2015). For example, Mosaferi et al. (2021) demonstrated that depleting the gut microbiota in early adolescent mice predisposed to AD could prevent associated anxiety and depressive behaviors. Research also suggests that SSRIs like escitalopram not only improve depressive symptoms in AD patients but may also slow disease progression and reduce the progression from MCI to dementia (Bartels et al., 2018; Dafsari and Jessen, 2020). Notably, escitalopram treatment has been shown to decrease Aβ42 levels in the cerebrospinal fluid of healthy elderly subjects and restore gut flora composition to normal levels (Sheline et al., 2020; Shen et al., 2021). These effects suggest that SSRIs may influence AD and cognitive deterioration partly through their impact on the gut microbiota. Intestinal microbial disturbances promote endotoxins, prostaglandins, and inflammatory cytokines, contributing to neuroinflammation and activating peripheral immune cells (Singh et al., 2016). Immune dysregulation is a key link between depression and cognitive decline (Hayley et al., 2021). Chronic stress accelerates dementia onset in depression via cytokines, with elevated interleukin-6 and tumor necrosis factor-α levels observed in the serum, cerebrospinal fluid, and prefrontal cortices of depressed patients (Pandey et al., 2018; Tonelli et al., 2008). Proinflammatory cytokines increase blood–brain barrier permeability, impair serotonin metabolism, and elevate oxidative stress byproducts like quinolinic acid, damaging neurons and glia and leading to cognitive decline (Hayley et al., 2021; Myint and Kim, 2014). Chronic neuroinflammation disrupts synaptic plasticity and neurogenesis, linking serotonin dysregulation to AD pathogenesis (Allison and Ditor, 2014; Caraci et al., 2010). These findings highlight depression as both a risk factor and prodromal symptom of AD (Elsworthy and Aldred, 2019; Caraci et al., 2010).
Additionally, both depression and AD have been linked to an increase in gut bacteria such as Actinobacteria and Ruminococcus, with a notable decrease in Akkermansia Muciniphila (Zhao et al., 2022; Zhuang et al., 2018). Enhancing the presence of Akkermansia Muciniphila and its secreted extracellular vesicles has shown promise in boosting serotonin signaling and synthesis in the brain and gut, suggesting a therapeutic potential (Yaghoubfar et al., 2020; Yaghoubfar et al., 2021). This understanding of the relationship between serotonin signaling and gut microbiota alterations could be crucial for improving diagnostics and interventions for AD and depression comorbidities. The serotonergic system, notably impaired in early-stage AD, plays a critical role in maintaining cerebral vascular health (Kepe et al., 2006). Serotonergic innervation of brain blood vessels has been shown to regulate cerebral blood flow, which is essential for understanding AD pathophysiology (Colaianna et al., 2010). Studies demonstrate that soluble Aβ injection in rat brains significantly decreases serotonin (5-HT) levels in the prefrontal cortex, a region crucial for mood regulation and highly susceptible to Aβ toxicity. This reduction in 5-HT contributes to depressive symptoms and neurodegeneration in AD (Krishnan and Nestler, 2008; Mattson, 2004; Egashira et al., 2005). Moreover, positron emission tomography (PET) imaging in cognitively normal individuals reveals that enhanced 5-HT signaling reduces Aβ accumulation (Sheline et al., 2014). Pharmacological interventions further highlight the interplay between serotonin and Aβ. Selective COX-2 inhibitors, such as celecoxib, prevent Aβ-induced reductions in 5-HT levels, ameliorating depressive-like behaviors and normalizing Aβ plasma levels (Morgese et al., 2018). In transgenic AD models, SSRIs increase 5-HT signaling, activate extracellular-regulated kinase and α-secretase pathways, and decrease Aβ production in vivo (Cirrito et al., 2011; Fisher et al., 2016). These findings underscore the potential of targeting serotonergic pathways to modulate cerebral blood flow and Aβ dynamics, offering insights into therapeutic approaches for AD and comorbid depression.
Future research should prioritize targeted interventions modulating the gut-brain axis to address depressive symptoms and cognitive decline in dementia. While SSRIs, probiotics, and dietary modifications show promise in restoring microbiota balance and reducing inflammation, large-scale clinical trials are essential to confirm their efficacy and safety. Developing therapies like Akkermansia muciniphila based treatments or fecal microbiota transplantation could offer novel strategies for slowing Alzheimer’s disease progression. Longitudinal studies integrating metagenomics and neuroimaging are needed to clarify the temporal links between microbiota dysbiosis, serotonin signaling, and neurodegeneration, paving the way for personalized treatments.
4.3.2.2 Oxidative stress
Oxidative stress refers to the imbalance between oxidants and antioxidants. When the oxidants are in favor, the redox conduction and control are destroyed or damaged (Sies, 2020). Oxidative stress is a pathogenic mechanism in most neurodegenerative diseases, and neurons are highly sensitive to free radical-mediated damage (Musiek et al., 2013). Research suggests that accumulated oxidative stress is a key mechanism affecting brain aging and the progression of AD (Ionescu-Tucker and Cotman, 2021). The association between oxidative stress and aging has also been found in patients with MDD (Luca et al., 2013) oxidative stress is implicated in disorders such as depression and AD and may serve as a connecting mechanism between them (Black et al., 2015; Butterfield and Mattson, 2020). Notably, depression is often associated with hypercortisolism, which, along with oxidative stress, reduces brain-derived neurotrophic factor expression, leading to hippocampal atrophy (Gillespie and Nemeroff, 2005; O’Brien et al., 2004). This atrophy disrupts the hippocampus’s ability to regulate the HPA axis, exacerbating glucocorticoid levels and further contributing to cognitive decline (Boku et al., 2018; Rubin et al., 2014). Additionally, oxidative stress impairs energy metabolism, particularly in the energy-sensitive hippocampus (Sies, 2015), and promotes inflammation, as evidenced by elevated levels of chemokine motif chemokine ligand 2 (CCL-2) in depression, linked to cognitive deficits and abnormal Aβ metabolism in AD (Romo-Araiza and Ibarra, 2020; Stuart and Baune, 2014; Westin et al., 2012). Moreover, emerging research by Gao et al. (2021) suggests that the α-Klotho gene could link depression and dementia by modulating oxidative stress and inflammation, underscoring its potential role in the neurobiological interplay between these conditions. This highlights the multifaceted impact of oxidative stress in linking depression to dementia, emphasizing the need for further research into its underlying mechanisms and therapeutic targeting.
4.3.2.3 Functional connectivity
Functional connectivity (FC) studies have delineated the impact of neuronal activation patterns and their communication across brain regions, essential for understanding brain function and behavior (Kelly and Castellanos, 2014). Recent research has shown that dementia patients with depressive symptoms (ADD) display reduced FC in the bilateral amygdala and inferior frontal gyrus compared to both patients without depression and healthy controls, indicating a crucial role of the amygdala-frontal circuit in emotional regulation (Du et al., 2024). Furthermore, abnormal locus coeruleus function has been associated with increased FC in the left locus coeruleus and right superior frontal gyrus in ADD patients, underscoring its relationship with depression (Du et al., 2020; Dai et al., 2023). These findings are supported by Ghanbari et al. (2022) analysis, which revealed similar subnetwork changes in AD and MDD, suggesting shared neural substrates.
In parallel, neuroimaging techniques like florbetaben positron emission tomography have provided insights into the brain structural changes due to depression and amyloid deposition, highlighting the significance of early detection in elderly patients with cognitive impairments (Hyung et al., 2021). Studies by Liu et al. further explore how depressive symptoms in MCI patients correlate with specific brain network dysfunctions, particularly noting the role of the left alpha binding cerebral hemisphere and the therapeutic potential of citalopram in improving both cognitive functions and emotional symptoms through distinct neural network interactions (Liu et al., 2022; Diaconescu et al., 2011). Lastly, reduced connectivity between the hypothalamus and temporal regions in AD patients with depression, as identified in a 2018 study, emphasizes the pathophysiological importance of these network disruptions in the manifestation of depressive symptoms within this group (Liu et al., 2018).
The kynurenine pathway, the primary route of tryptophan metabolism in humans, accounts for approximately 95% of total tryptophan metabolism and plays a crucial role in balancing neurotoxicity and neuroprotection (Allison and Ditor, 2014). Tryptophan is converted into kynurenine by indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO) (Oxenkrug, 2010). Disruptions in this pathway are implicated in both AD and major depression. In AD, an imbalance in plasma and cerebrospinal fluid kynurenine metabolites promotes disease progression (Cespedes et al., 2022). In depression, elevated glucocorticoids activate TDO, increasing kynurenine levels and its downstream neurotoxic metabolite, quinolinic acid, which damages hippocampal neurons, overactivates NMDA receptors, and leads to hippocampal atrophy, a hallmark of AD (Allison and Ditor, 2014; Rubin, 1967; Wolkowitz et al., 2009). These findings suggest that dysregulation of the kynurenine pathway is a shared pathogenic mechanism contributing to the progression of both disorders. Recent studies reveal a link between Aβ pathology and depressive symptoms in dementia. Aβ oligomers induce depressive-like behaviors in mice, which are alleviated by antidepressants, indicating a direct impact on mood regulation (Kosel et al., 2020; dos Santos et al., 2013; Ledo et al., 2013). During the COVID-19 lockdown, amyloid-positive individuals experienced more severe depressive symptoms under stress, suggesting AD pathology exacerbates depressive responses (Lewis et al., 2022). Patients with mild depressive symptoms also show elevated cerebrospinal fluid amyloid markers and an 83% higher risk of developing AD (Xu et al., 2021). Tau protein, a key factor in neurofibrillary tangles, further connects depression and AD. AD patients with depression show increased tangles due to tau hyperphosphorylation (Rapp et al., 2008). However, a longitudinal study of nearly 2,000 participants did not find a consistent association, reflecting the complexity of tau’s role in depression and AD progression (Wilson et al., 2016).
4.3.3 Management of dementia and depression
4.3.3.1 Non-drug treatment
In the context of the side effects and limited efficacy of psychotropic drugs, there has been a rising interest in non-pharmacological interventions for managing dementia and depression. Music therapy, recognized as a potentially cost-effective non-drug treatment, has demonstrated efficacy in reducing depressive symptoms among dementia patients. Notably, a randomized controlled trial in Australia reported that group music therapy and recreational choir singing significantly alleviated depressive symptoms over a six-month period (Baker et al., 2022). Further, a specific intervention involving reminiscence music therapy sessions twice a week for 4 weeks markedly reduced depressive symptoms, although it showed no significant impact on cognitive and behavioral symptoms (Tz-Han et al., 2023). A recent meta-analysis found that active music therapy combined with singing significantly improves depressive symptoms in dementia patients, outperforming music listening alone (Ting et al., 2024). Group music therapy, by enhancing social interaction and reducing loneliness, is more effective than individual therapy. Additionally, repetitive transcranial magnetic stimulation (rTMS) using intermittent theta burst stimulation (iTBS) has shown promise in improving mood and cognition in patients with dementia and depression (Jin et al., 2024). Xu et al. (2024) study further supported these findings, showing that group music therapy not only lowered depression and salivary cortisol levels but also maintained these reduced levels during a 3-month follow-up. A trial in Australia found that receptive music therapy (RCS) was more effective than group music therapy (GMT) in reducing depressive symptoms in dementia patients and showed benefits for advanced dementia (Baker et al., 2022). Another study demonstrated that music activities designed by music therapists and led by trained nursing assistants reduced depressive symptoms and improved well-being in dementia patients (Ray and Götell, 2018). These findings highlight the potential of continuous music therapy and music-based interventions.
On another front, reminiscence therapy, which uses cues like photos and music to trigger memory recall, has also been proven effective. A 2015 meta-analysis indicated that reminiscence therapy modestly improves cognitive function and significantly ameliorates depression in dementia patients (Huang et al., 2015). Li et al. (2020). found that group reminiscence therapy effectively improved depressive symptoms and neuropsychiatric symptoms in patients with mild to moderate AD. However, a 2021 meta-analysis suggested that while reminiscence therapy shows some potential benefits for enhancing quality of life, its effectiveness in reducing depressive symptoms still requires further investigation (Thomas and Sezgin, 2021). Cognitive psychotherapy is widely used in clinical practice and has shown effectiveness in treating dementia with depressive symptoms. A meta-analysis found that adding cognitive behavioral therapy (CBT) to usual care reduces depressive symptoms and increases remission rates in dementia patients (Orgeta et al., 2022). Mindfulness-based cognitive therapy has also been shown to alleviate depression and anxiety in dementia patients (Douglas et al., 2022), though a randomized controlled trial reported no significant improvement in cognition, anxiety, or depression following mindfulness interventions in mild dementia (Noone et al., 2023). Psychotherapy holds potential for enriching treatment options for dementia with depression, though further evidence is needed. Additionally, traditional Chinese medicine, such as acupuncture, has been shown to improve insomnia and depression by stimulating specific points to regulate brain function (Zhang et al., 2024). Its potential benefits on mood and cognition in dementia patients with depression warrant further exploration, offering a novel direction for future treatments.
4.3.3.2 Drug treatment
In clinical practice, while non-drug treatments can help alleviate depression in dementia patients, pharmacotherapy is essential, particularly for severe cases. Antidepressant therapy is the first-line treatment for dementia patients with depressive symptoms. A meta-analysis of 25 studies indicated that mirtazapine and sertraline are slightly more effective than placebo in managing depressive symptoms in these patients, although clomipramine was associated with an increased risk of adverse events (He et al., 2021). Observational studies have shown that vortioxetine is effective and well-tolerated in patients with prodromal and mild to moderate AD who also suffer from depression, significantly improving cognitive function in those with prodromal AD (Padovani et al., 2024). However, a recent meta-analysis involving eight studies on SSRIs, SSNRIs, tricyclic antidepressants, and atypical antidepressants found no strong evidence that antidepressant treatment improves depression in dementia patients (SMD −0.10 [−0.26, 0.07]) (Lenouvel et al., 2024). These discrepancies may stem from differences in the severity of dementia among study subjects. More accurate screening tools and diagnostic criteria are needed to further clarify the efficacy of antidepressants in this patient population. SSRIs such as sertraline, fluoxetine, paroxetine, citalopram, and escitalopram are widely used to treat depression in AD patients. Long-term SSRI use in individuals with MCI and a history of depression has been shown to delay AD progression by approximately 3 years (Bartels et al., 2018). Escitalopram reduces cerebrospinal fluid Aβ42 levels in cognitively normal older adults (Sheline et al., 2020), and fluoxetine improves spatial learning deficits in AD models by preventing neuronal damage (Ma et al., 2017). These findings suggest that SSRIs may benefit both depressive symptoms and AD progression. However, further studies are needed to clarify the differential effects of specific SSRIs.
Early intervention plays a critical role in both non-pharmacological and pharmacological approaches to managing dementia and depression. Non-drug treatments, such as music therapy and cognitive psychotherapy, have shown potential in early stages to improve mood, enhance social interaction, and possibly delay cognitive decline. For example, group music therapy and reminiscence therapy can be particularly effective in MCI or early AD, providing a non-invasive way to mitigate depressive symptoms and support cognitive health. On the pharmacological front, long-term use of SSRIs in patients with MCI and a history of depression has been shown to delay the progression to AD by up to 3 years. These findings underscore the importance of identifying at-risk individuals early and implementing timely interventions to improve clinical outcomes and slow disease progression.
5 Limitations
Although this bibliometric analysis adheres to standard methods and utilizes a robust dataset from the WoSCC database, it has several limitations that may affect the comprehensiveness of the findings. Firstly, the reliance on a single database excludes potentially relevant studies indexed in other platforms like PubMed, Scopus, or Google Scholar. Secondly, bibliometric methods primarily evaluate research impact based on citation counts, which may overlook high-quality but less cited studies. Additionally, there is also an inherent time lag in bibliometrics, which can delay the recognition of emerging research trends.
6 Conclusion
This bibliometric analysis of publications on dementia and depression from 2005 to 2024 highlights the leading role of the United States in both research volume and impact, with significant contributions from China. The University of Toronto and notable researchers like Herrmann N and Aarsland D have driven advancements in understanding the neurobiological underpinnings of these conditions. Current research hotspots include the comorbid mechanisms between dementia and depression, such as gut microbiota, oxidative stress, and FC. Additionally, prevalent neuropsychiatric symptoms like anxiety and BD are prominently featured in the literature. The study also anticipates that non-pharmacological interventions will become a crucial area of future research. These insights provide a solid foundation for advancing the understanding and treatment of dementia and depression.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.webofscience.com/wos/woscc/basic-search.
Author contributions
XL: Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. WS: Supervision, Validation, Writing – review & editing. LC: Data curation, Investigation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank all participants in this study and WOS for providing data support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Ali, M. J. (2021). Understanding the “g-index” and the “e-index.”. Semin. Ophthalmol. 36:18. doi: 10.1080/08820538.2021.1922975
Allison, D. J., and Ditor, D. S. (2014). The common inflammatory etiology of depression and cognitive impairment: a therapeutic target. J. Neuroinflammation 11:151. doi: 10.1186/s12974-014-0151-1
Almeida, O. P., McCaul, K., Hankey, G. J., Yeap, B. B., Golledge, J., and Flicker, L. (2016). Risk of dementia and death in community-dwelling older men with bipolar disorder. Br. J. Psychiatry J. Ment. Sci. 209, 121–126. doi: 10.1192/bjp.bp.115.180059
Alzheimer's Disease International. (n.d.). Dementia statistics. Retrieved August 11, 2024, from Alzheimer’s Disease International (ADI) website: Available at: https://www.alzint.org/about/dementia-facts-figures/dementia-statistics/
Aprahamian, I., Nunes, P. V., and Forlenza, O. V. (2013). Cognitive impairment and dementia in late-life bipolar disorder. Curr. Opin. Psychiatry 26, 120–123. doi: 10.1097/YCO.0b013e32835ac5f6
Asmer, M. S., Kirkham, J., Newton, H., Ismail, Z., Elbayoumi, H., Leung, R. H., et al. (2018). Meta-analysis of the prevalence of major depressive disorder among older adults with dementia. J. Clin. Psychiatry 79:17r11772. doi: 10.4088/JCP.17r11772
Baker, F. A., Lee, Y. E. C., Sousa, T. V., Stretton-Smith, P. A., Tamplin, J., Sveinsdottir, V., et al. (2022). Clinical effectiveness of music interventions for dementia and depression in elderly care (MIDDEL): Australian cohort of an international pragmatic cluster-randomised controlled trial. Lancet Healthy Longev. 3, e153–e165. doi: 10.1016/S2666-7568(22)00027-7
Barca, M. L., Alnæs, D., Engedal, K., Persson, K., Eldholm, R. S., Siafarikas, N., et al. (2022). Brain morphometric correlates of depressive symptoms among patients with and without dementia. Dement. Geriatr. Cogn. Disord. Extra 12, 107–114. doi: 10.1159/000521114
Barnes, D. E., and Yaffe, K. (2011). The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10, 819–828. doi: 10.1016/S1474-4422(11)70072-2
Bartels, C., Wagner, M., Wolfsgruber, S., Ehrenreich, H., and Schneider, A. (2018). Alzheimer’s Disease Neuroimaging Initiative. Impact of SSRI therapy on risk of conversion from mild cognitive impairment to Alzheimer’s dementia in individuals with previous depression. Am. J. Psychiatry 175, 232–241. doi: 10.1176/appi.ajp.2017.17040404
Barth, C., Crestol, A., de Lange, A. M. G., and Galea, L. A. M. (2023). Sex steroids and the female brain across the lifespan: insights into risk of depression and alzheimer’s disease. Lancet Diabetes Endocrinol. 11, 926–941. doi: 10.1016/S2213-8587(23)00224-3
Bhattacharjee, S., Oh, Y. M., Reiman, E. M., and Burke, W. J. (2017). Prevalence, patterns, and predictors of depression treatment among community-dwelling elderly individuals with dementia in the United States. Am. J. Geriatr. Psychiatry Off. 25, 803–813. doi: 10.1016/j.jagp.2017.03.003
Biringer, E., Mykletun, A., Dahl, A. A., Smith, A. D., Engedal, K., Nygaard, H. A., et al. (2005). The association between depression, anxiety, and cognitive function in the elderly general population—the Hordaland health study. Int. J. Geriatr. Psychiatry 20, 989–997. doi: 10.1002/gps.1390
Black, C. N., Bot, M., Scheffer, P. G., Cuijpers, P., and Penninx, B. W. J. H. (2015). Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 51, 164–175. doi: 10.1016/j.psyneuen.2014.09.025
Boku, S., Nakagawa, S., Toda, H., and Hishimoto, A. (2018). Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 72, 3–12. doi: 10.1111/pcn.12604
Bromet, E., Andrade, L. H., Hwang, I., Sampson, N. A., Alonso, J., de Girolamo, G., et al. (2011). Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 9:90. doi: 10.1186/1741-7015-9-90
Brooks, H., Wang, W., Zomorrodi, R., Blumberger, D. M., Bowie, C. R., Daskalakis, Z. J., et al. (2024). Cognitive function based on theta-gamma coupling vs. clinical diagnosis in older adults with mild cognitive impairment with or without major depressive disorder. Transl. Psychiatry 19:153. doi: 10.1002/alz.076553
Butterfield, D. A., and Mattson, M. P. (2020). Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer’s disease. Neurobiol. Dis. 138:104795. doi: 10.1016/j.nbd.2020.104795
Butters, M. A., Young, J. B., Lopez, O., Aizenstein, H. J., Mulsant, B. H., Reynolds, C. F., et al. (2008). Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin. Neurosci. 10, 345–357. doi: 10.31887/DCNS.2008.10.3/mabutters
Cai, H., Du, R., Yang, K., Li, W., and Wang, Z. (2024). Bibliometric analysis of the research status and global trends in behavioral and psychological symptoms of dementia in Alzheimer’s disease from 2002 to 2022. Curr. Neuropharmacol. 22, 1720–1732. doi: 10.2174/1570159X21666230807144750
Cantón-Habas, V., Rich-Ruiz, M., Romero-Saldaña, M., and MDP, C.-G. (2020). Depression as a risk factor for dementia and Alzheimer’s disease. Biomedicines 8:457. doi: 10.3390/biomedicines8110457
Caraci, F., Copani, A., Nicoletti, F., and Drago, F. (2010). Depression and alzheimer’s disease: neurobiological links and common pharmacological targets. Eur. J. Pharmacol. 626, 64–71. doi: 10.1016/j.ejphar.2009.10.022
Cespedes, M., Jacobs, K. R., Maruff, P., Rembach, A., Fowler, C. J., Trounson, B., et al. (2022). Systemic perturbations of the kynurenine pathway precede progression to dementia independently of amyloid-β. Neurobiol. Dis. 171:105783. doi: 10.1016/j.nbd.2022.105783
Chan, W. C., Lam, L. C. W., Tam, C. W. C., Lui, V. W. C., Leung, G. T. Y., Lee, A. T. C., et al. (2011). Neuropsychiatric symptoms are associated with increased risks of progression to dementia: a 2-year prospective study of 321 Chinese older persons with mild cognitive impairment. Age Ageing 40, 30–35. doi: 10.1093/ageing/afq151
Charlson, M., and Peterson, J. C. (2002). Medical comorbidity and late life depression: what is known and what are the unmet needs? Biol. Psychiatry 52, 226–235. doi: 10.1016/S0006-3223(02)01422-1
Chen, C. M. (2006). CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 57, 359–377. doi: 10.1002/asi.20317
Chen, C., Hu, Z., Liu, S., and Tseng, H. (2012). Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther [Internet]. 12, 593–608. doi: 10.1517/14712598.2012.674507
Chen, C., and Song, M. (2019). Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One 14:e0223994. doi: 10.1371/journal.pone.0223994
Cirrito, J. R., Disabato, B. M., Restivo, J. L., Verges, D. K., Goebel, W. D., Sathyan, A., et al. (2011). Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc. Natl. Acad. Sci. USA 108, 14968–14973. doi: 10.1073/pnas.1107411108
Colaianna, M., Tucci, P., Zotti, M., Morgese, M. G., Schiavone, S., Govoni, S., et al. (2010). Soluble beta amyloid (1-42): a critical player in producing behavioural and biochemical changes evoking depressive-related state? Br. J. Pharmacol. 159, 1704–1715. doi: 10.1111/j.1476-5381.2010.00669.x
Cooper, C., Sommerlad, A., Lyketsos, C. G., and Livingston, G. (2015). Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am. J. Psychiatry 172, 323–334. doi: 10.1176/appi.ajp.2014.14070878
Dafsari, F. S., and Jessen, F. (2020). Depression-an underrecognized target for prevention of dementia in Alzheimer’s disease. Transl. Psychiatry 10:160. doi: 10.1038/s41398-020-0839-1
Dai, M., Guo, Z., Chen, J., Liu, H., Li, J., Zhu, M., et al. (2023). Altered functional connectivity of the locus coeruleus in Alzheimer’s disease patients with depression symptoms. Exp. Gerontol. 179:112252. doi: 10.1016/j.exger.2023.112252
Devier, D. J., Pelton, G. H., Tabert, M. H., Liu, X., Cuasay, K., Eisenstadt, R., et al. (2009). The impact of anxiety on conversion from mild cognitive impairment to Alzheimer’s disease. Int. J. Geriatr. Psychiatry 24, 1335–1342. doi: 10.1002/gps.2263
Diaconescu, A. O., Kramer, E., Hermann, C., Ma, Y., Dhawan, V., Chaly, T., et al. (2011). Distinct functional networks associated with improvement of affective symptoms and cognitive function during citalopram treatment in geriatric depression. Hum. Brain Mapp. 32, 1677–1691. doi: 10.1002/hbm.21135
Diaz Heijtz, R., Wang, S., Anuar, F., Qian, Y., Björkholm, B., Samuelsson, A., et al. (2011). Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad Sci USA 108, 3047–3052. doi: 10.1073/pnas.1010529108
Diniz, B. S., Butters, M. A., Albert, S. M., Dew, M. A., and Reynolds, C. F. (2013). Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry J. Ment. Sci. 202, 329–335. doi: 10.1192/bjp.bp.112.118307
Donovan, N. J., Locascio, J. J., Marshall, G. A., Gatchel, J., Hanseeuw, B. J., Rentz, D. M., et al. (2018). Longitudinal Association of Amyloid Beta and Anxious-Depressive Symptoms in cognitively Normal older adults. Am. J. Psychiatry 175, 530–537. doi: 10.1176/appi.ajp.2017.17040442
dos Santos, V. V., Santos, D. B., Lach, G., Rodrigues, A. L. S., Farina, M., De Lima, T. C. M., et al. (2013). Neuropeptide Y (NPY) prevents depressive-like behavior, spatial memory deficits and oxidative stress following amyloid-β (aβ (1-40)) administration in mice. Behav. Brain Res. 244, 107–115. doi: 10.1016/j.bbr.2013.01.039
Douglas, S., Stott, J., Spector, A., Brede, J., Hanratty, É., Charlesworth, G., et al. (2022). Mindfulness-based cognitive therapy for depression in people with dementia: a qualitative study on participant, carer and facilitator experiences. Dement Lond Engl. 21, 457–476. doi: 10.1177/14713012211046150
Du, R., Yang, K., Li, W., Wang, Z., and Cai, H. (2024). Research status and global trends of late-life depression from 2004 to 2023: bibliometric analysis. Front. Aging Neurosci. 16:1393110. doi: 10.3389/fnagi.2024.1393110
Du, X., Yin, M., Yuan, L., Zhang, G., Fan, Y., Li, Z., et al. (2020). Reduction of depression-like behavior in rat model induced by ShRNA targeting norepinephrine transporter in locus coeruleus. Transl. Psychiatry 10:130. doi: 10.1038/s41398-020-0808-8
Du, Y., Zhang, S., Qiu, Q., Fang, Y., Zhao, L., Yue, L., et al. (2024). The mediating effect of the amygdala-frontal circuit on the association between depressive symptoms and cognitive function in Alzheimer’s disease. Transl. Psychiatry 14:301. doi: 10.1038/s41398-024-03026-3
Duffy, S. L., Paradise, M., Hickie, I. B., Lewis, S. J. G., Naismith, S. L., and Lagopoulos, J. (2014). Cognitive impairment with and without depression history: an analysis of white matter microstructure. J. Psychiatry Neurosci. 39, 135–143
Egashira, N., Iwasaki, K., Takashima, A., Watanabe, T., Kawabe, H., Matsuda, T., et al. (2005). Altered depression-related behavior and neurochemical changes in serotonergic neurons in mutant R406W human tau transgenic mice. Brain Res. 1059, 7–12. doi: 10.1016/j.brainres.2005.08.004
Elsworthy, R. J., and Aldred, S. (2019). Depression in alzheimer’s disease: an alternative role for selective serotonin reuptake inhibitors? J Alzheimers Dis. 69, 651–661. doi: 10.3233/JAD-180780
Fan, Y., and Pedersen, O. (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71. doi: 10.1038/s41579-020-0433-9
Fisher, J. R., Wallace, C. E., Tripoli, D. L., Sheline, Y. I., and Cirrito, J. R. (2016). Redundant gs-coupled serotonin receptors regulate amyloid-β metabolism in vivo. Mol. Neurodegener. 11:45. doi: 10.1186/s13024-016-0112-5
Frodl, T., and O’Keane, V. (2013). How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol. Dis. 52, 24–37. doi: 10.1016/j.nbd.2012.03.012
Gallagher, D., Coen, R., Kilroy, D., Belinski, K., Bruce, I., Coakley, D., et al. (2011). Anxiety and behavioural disturbance as markers of prodromal Alzheimer’s disease in patients with mild cognitive impairment. Int. J. Geriatr. Psychiatry 26, 166–172. doi: 10.1002/gps.2509
Gallagher, D., Kiss, A., Lanctot, K., and Herrmann, N. (2018). Depression and risk of alzheimer dementia: a longitudinal analysis to determine predictors of increased risk among older adults with depression. Am. J. Geriatr. Psychiatry Off. 26, 819–827. doi: 10.1016/j.jagp.2018.05.002
Ganguli, M., Dodge, H. H., Shen, C., and DeKosky, S. T. (2004). Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology 63, 115–121. doi: 10.1212/01.WNL.0000132523.27540.81
Gao, X., Li, Y., Sun, Z., Xu, H., Ma, G., Deng, Q., et al. (2021). Could α-klotho unlock the key between depression and dementia in the elderly: from animal to human studies. Mol. Neurobiol. 58, 2874–2885. doi: 10.1007/s12035-021-02313-0
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Lond Engl 392, 1789–1858.
GBD 2019 Dementia Forecasting Collaborators (2022). Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health 7, e105–e125. doi: 10.1016/S2468-2667(21)00249-8
Ghanbari, M., Soussia, M., Jiang, W., Wei, D., Yap, P. T., Shen, D., et al. (2022). Alterations of dynamic redundancy of functional brain subnetworks in Alzheimer’s disease and major depression disorders. Neuro Image Clin. 33:102917. doi: 10.1016/j.nicl.2021.102917
Gillespie, C. F., and Nemeroff, C. B. (2005). Hypercortisolemia and depression. Psychosom. Med. 67, S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2
Gold, G., Giannakopoulos, P., Herrmann, F. R., Bouras, C., and Kövari, E. (2007). Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain J. Neurol. 130, 2830–2836. doi: 10.1093/brain/awm228
Goodarzi, Z. S., Mele, B. S., Roberts, D. J., and Holroyd-Leduc, J. (2017). Depression case finding in individuals with dementia: a systematic review and Meta-analysis. J. Am. Geriatr. Soc. 65, 937–948. doi: 10.1111/jgs.14713
Goodman, R. A., Lochner, K. A., Thambisetty, M., Wingo, T. S., Posner, S. F., and Ling, S. M. (2017). Prevalence of dementia subtypes in United States medicare fee-for-service beneficiaries, 2011-2013. Alzheimers Dement J Alzheimers Assoc. 13, 28–37. doi: 10.1016/j.jalz.2016.04.002
Goyal, D., Ali, S. A., and Singh, R. K. (2021). Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 106:110112. doi: 10.1016/j.pnpbp.2020.110112
Hayley, S., Hakim, A. M., and Albert, P. R. (2021). Depression, dementia and immune dysregulation. Brain J. Neurol. 144, 746–760. doi: 10.1093/brain/awaa405
He, Y., Li, H., Huang, J., Huang, S., Bai, Y., Li, Y., et al. (2021). Efficacy of antidepressant drugs in the treatment of depression in Alzheimer disease patients: a systematic review and network meta-analysis. J. Psychopharmacol. Oxf. Engl. 35, 901–909. doi: 10.1177/02698811211030181
Hermida, A. P., McDonald, W. M., Steenland, K., and Levey, A. (2012). The association between late-life depression, mild cognitive impairment and dementia: is inflammation the missing link? Expert. Rev. Neurother. 12, 1339–1350. doi: 10.1586/ern.12.127
Hickey, M., Hueg, T. K., Priskorn, L., Uldbjerg, C. S., Beck, A. L., Anstey, K. J., et al. (2023). Depression in mid-and later-life and risk of dementia in women: a prospective study within the danish nurses cohort. J Alzheimers Dis. 93, 779–789. doi: 10.3233/JAD-230091
Hirsch, J. E. (2005). An index to quantify an individual’s scientific research output. Proc. Natl. Acad. Sci. USA 102, 16569–16572. doi: 10.1073/pnas.0507655102
Hirsch, J. E. (2007). Does the H index have predictive power? Proc. Natl. Acad. Sci. USA 104, 19193–19198. doi: 10.1073/pnas.0707962104
Huang, H. C., Chen, Y. T., Chen, P. Y., Huey-Lan Hu, S., Liu, F., Kuo, Y. L., et al. (2015). Reminiscence therapy improves cognitive functions and reduces depressive symptoms in elderly people with dementia: a meta-analysis of randomized controlled trials. J. Am. Med. Dir. Assoc. 16, 1087–1094. doi: 10.1016/j.jamda.2015.07.010
Hwang, T. J., Masterman, D. L., Ortiz, F., Fairbanks, L. A., and Cummings, J. L. (2004). Mild cognitive impairment is associated with characteristic neuropsychiatric symptoms. Alzheimer Dis. Assoc. Disord. 18, 17–21. doi: 10.1097/00002093-200401000-00004
Hyung, W. S. W., Kang, J., Kim, J., Lee, S., Youn, H., Ham, B. J., et al. (2021). Cerebral amyloid accumulation is associated with distinct structural and functional alterations in the brain of depressed elders with mild cognitive impairment. J. Affect. Disord. 281, 459–466. doi: 10.1016/j.jad.2020.12.049
Ionescu-Tucker, A., and Cotman, C. W. (2021). Emerging roles of oxidative stress in brain aging and alzheimer’s disease. Neurobiol. Aging 107, 86–95. doi: 10.1016/j.neurobiolaging.2021.07.014
Jack, C. R., Andrews, J. S., Beach, T. G., Buracchio, T., Dunn, B., Graf, A., et al. (2024). Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s association workgroup. Alzheimers Dement. J. Alzheimers Assoc. 20, 5143–5169. doi: 10.1002/alz.13859
Jin, X., Xu, C. Y., Fei, J. F., Fang, Y., and Sun, C. H. (2024). Alzheimer’s disease with depressive symptoms: clinical effect of intermittent theta burst stimulation repetitive transcranial magnetic stimulation. World J. Psychiatry 14, 1216–1223. doi: 10.5498/wjp.v14.i8.1216
Johansson, M., Stomrud, E., Lindberg, O., Westman, E., Johansson, P. M., van Westen, D., et al. (2020). Apathy and anxiety are early markers of Alzheimer’s disease. Neurobiol. Aging 85, 74–82. doi: 10.1016/j.neurobiolaging.2019.10.008
Kelly, C., and Castellanos, F. X. (2014). Strengthening connections: functional connectivity and brain plasticity. Neuropsychol. Rev. 24, 63–76. doi: 10.1007/s11065-014-9252-y
Kepe, V., Barrio, J. R., Huang, S. C., Ercoli, L., Siddarth, P., Shoghi-Jadid, K., et al. (2006). Serotonin 1A receptors in the living brain of alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA 103, 702–707. doi: 10.1073/pnas.0510237103
Kershenbaum, A., Cardinal, R. N., Chen, S., Underwood, B. R., Seyedsalehi, A., Lewis, J., et al. (2021). Investigation of risk of dementia diagnosis and death in patients in older people’s secondary care mental health services. Int. J. Geriatr. Psychiatry 36, 573–582. doi: 10.1002/gps.5455
Kessing, L. V., and Andersen, P. K. (2004). Does the risk of developing dementia increase with the number of episodes in patients with depressive disorder and in patients with bipolar disorder? J. Neurol. Neurosurg. Psychiatry 75, 1662–1666. doi: 10.1136/jnnp.2003.031773
Knorr, U., Simonsen, A. H., Jensen, C. S., Zetterberg, H., Blennow, K., Akhøj, M., et al. (2022). Alzheimer’s disease related biomarkers in bipolar disorder - a longitudinal one-year case-control study. J. Affect. Disord. 297, 623–633. doi: 10.1016/j.jad.2021.10.074
Kodesh, A., Sandin, S., Reichenberg, A., Rotstein, A., Pedersen, N. L., Ericsson, M., et al. (2019). Exposure to antidepressant medication and the risk of incident dementia. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 27, 1177–1188. doi: 10.1016/j.jagp.2019.05.019
Koenig, A. M., Bhalla, R. K., and Butters, M. A. (2014). Cognitive functioning and late-life depression. J. Int. Neuropsychol. Soc. 20, 461–467. doi: 10.1017/S1355617714000198
Kokol, P., Blažun Vošner, H., and Završnik, J. (2021). Application of bibliometrics in medicine: a historical bibliometrics analysis. Health Inf. Libr. J. 38, 125–138. doi: 10.1111/hir.12295
Kosel, F., Pelley, J. M. S., and Franklin, T. B. (2020). Behavioural and psychological symptoms of dementia in mouse models of alzheimer’s disease-related pathology. Neurosci. Biobehav. Rev. 112, 634–647. doi: 10.1016/j.neubiorev.2020.02.012
Krishnan, V., and Nestler, E. J. (2008). The molecular neurobiology of depression. Nature 455, 894–902. doi: 10.1038/nature07455
Kuehner, C. (2003). Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr. Scand. 108, 163–174. doi: 10.1034/j.1600-0447.2003.00204.x
Kuring, J. K., Mathias, J. L., and Ward, L. (2020). Risk of dementia in persons who have previously experienced clinically-significant depression, anxiety, or PTSD: a systematic review and Meta-analysis. J. Affect. Disord. 274, 247–261. doi: 10.1016/j.jad.2020.05.020
Laukka, E. J., Fratiglioni, L., and Bäckman, L. (2010). The influence of vascular disease on cognitive performance in the preclinical and early phases of Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 29, 498–503. doi: 10.1159/000313978
Ledo, J. H., Azevedo, E. P., Clarke, J. R., Ribeiro, F. C., Figueiredo, C. P., Foguel, D., et al. (2013). Amyloid-β oligomers link depressive-like behavior and cognitive deficits in mice. Mol. Psychiatry 18, 1053–1054. doi: 10.1038/mp.2012.168
Lenouvel, E., Tobias, S., Mühlbauer, V., Dallmeier, D., Denkinger, M., Klöppel, S., et al. (2024). Antidepressants for treating depression among older adults with dementia: a systematic review and meta-analysis. Psychiatry Res. 340:116114. doi: 10.1016/j.psychres.2024.116114
Leung, D. K. Y., Chan, W. C., Spector, A., and Wong, G. H. Y. (2021). Prevalence of depression, anxiety, and apathy symptoms across dementia stages: a systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 36, 1330–1344. doi: 10.1002/gps.5556
Lewis, C. K., Bernstein, O. M., Grill, J. D., Gillen, D. L., and Sultzer, D. L. (2022). Anxiety and depressive symptoms and cortical amyloid-β burden in cognitively unimpaired older adults. J. Prev Alzheimers Dis. 9, 286–296. doi: 10.14283/jpad.2022.13
Lewis, G., Werbeloff, N., Hayes, J. F., Howard, R., and Osborn, D. P. J. (2018). Diagnosed depression and sociodemographic factors as predictors of mortality in patients with dementia. Br. J. Psychiatry J. Ment. Sci. 213, 471–476. doi: 10.1192/bjp.2018.86
Li, M., Lyu, J. H., Zhang, Y., Gao, M. L., Li, R., Mao, P. X., et al. (2020). Efficacy of group reminiscence therapy on cognition, depression, neuropsychiatric symptoms, and activities of daily living for patients with Alzheimer disease. J. Geriatr. Psychiatry Neurol. 33, 272–281. doi: 10.1177/0891988719882099
Li, W., Zhou, F. C., Zhang, L., Ng, C. H., Ungvari, G. S., Li, J., et al. (2020). Comparison of cognitive dysfunction between schizophrenia and bipolar disorder patients: a meta-analysis of comparative studies. J. Affect. Disord. 274, 652–661. doi: 10.1016/j.jad.2020.04.051
Liu, X., Chen, W., Tu, Y., Hou, H., Huang, X., Chen, X., et al. (2018). The Abnormal Functional Connectivity between the Hypothalamus and the Temporal Gyrus Underlying Depression in Alzheimer’s Disease Patients. Front. Aging Neurosci. 10:37. doi: 10.3389/fnagi.2018.00037
Liu, M., Ma, J., Fu, C. Y., Yeo, J., Xiao, S. S., Xiao, W. X., et al. (2022). Dysfunction of emotion regulation in mild cognitive impairment individuals combined with depressive disorder: a neural mechanism study. Front. Aging Neurosci. 14:884741. doi: 10.3389/fnagi.2022.884741
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Lancet Lond. Engl. 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
Luca, M., Luca, A., and Calandra, C. (2013). Accelerated aging in major depression: the role of nitro-oxidative stress. Oxidative Med. Cell. Longev. 2013:230797, 1–6. doi: 10.1155/2013/230797
Luna, R. A., and Foster, J. A. (2015). Gut brain axis: diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 32, 35–41. doi: 10.1016/j.copbio.2014.10.007
Lyketsos, C. G., Lopez, O., Jones, B., Fitzpatrick, A. L., and Breitner, J. (2002). Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 288:1475. doi: 10.1001/jama.288.12.1475
Ma, J., Gao, Y., Jiang, L., Chao, F. L., Huang, W., Zhou, C. N., et al. (2017). Fluoxetine attenuates the impairment of spatial learning ability and prevents neuron loss in middle-aged APPswe/PSEN1dE9 double transgenic alzheimer’s disease mice. Oncotarget 8, 27676–27692. doi: 10.18632/oncotarget.15398
Macoveanu, J., Freeman, K. O., Kjaerstad, H. L., Knudsen, G. M., Kessing, L. V., and Miskowiak, K. W. (2021). Structural brain abnormalities associated with cognitive impairments in bipolar disorder. Acta Psychiatr. Scand. 144, 379–391. doi: 10.1111/acps.13349
Marawi, T., Zhukovsky, P., Rashidi-Ranjbar, N., Bowie, C. R., Brooks, H., Fischer, C. E., et al. (2023). Brain-cognition associations in older patients with remitted major depressive disorder or mild cognitive impairment: a multivariate analysis of gray and white matter integrity. Biol. Psychiatry 94, 913–923. doi: 10.1016/j.biopsych.2023.05.018
Martínez-Arán, A., Vieta, E., Reinares, M., Colom, F., Torrent, C., Sánchez-Moreno, J., et al. (2004). Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am. J. Psychiatry 161, 262–270. doi: 10.1176/appi.ajp.161.2.262
Mattson, M. P. (2004). Pathways towards and away from alzheimer’s disease. Nature 430, 631–639. doi: 10.1038/nature02621
Moon, W., Ji, E., Shin, J., Kwon, J. S., and Kim, K. W. (2022). Effect of valproate and lithium on dementia onset risk in bipolar disorder patients. Sci. Rep. 12:14142. doi: 10.1038/s41598-022-18350-1
Morais, L. H., Schreiber, H. L., and Mazmanian, S. K. (2021). The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255. doi: 10.1038/s41579-020-00460-0
Morgese, M. G., Schiavone, S., Bove, M., Mhillaj, E., Tucci, P., and Trabace, L. (2018). Sub-chronic celecoxib prevents soluble beta amyloid-induced depressive-like behaviour in rats. J. Affect. Disord. 238, 118–121. doi: 10.1016/j.jad.2018.05.030
Mosaferi, B., Jand, Y., and Salari, A. A. (2021). Gut microbiota depletion from early adolescence alters anxiety and depression-related behaviours in male mice with Alzheimer-like disease. Sci. Rep. 11:22941. doi: 10.1038/s41598-021-02231-0
Musiek, E. S., Lim, M. M., Yang, G., Bauer, A. Q., Qi, L., Lee, Y., et al. (2013). Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Invest. 123, 5389–5400. doi: 10.1172/JCI70317
Myint, A. M., and Kim, Y. K. (2014). Network beyond IDO in psychiatric disorders: revisiting neurodegeneration hypothesis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 48, 304–313. doi: 10.1016/j.pnpbp.2013.08.008
Noone, D., Payne, J., Stott, J., Aguirre, E., Patel-Palfreman, M. M., Stoner, C., et al. (2023). The feasibility of a mindfulness intervention for depression in people with mild dementia: a pilot randomized controlled trial. Clin. Gerontol. 46, 346–358. doi: 10.1080/07317115.2022.2094741
Nunes, P. V., Forlenza, O. V., and Gattaz, W. F. (2007). Lithium and risk for Alzheimer’s disease in elderly patients with bipolar disorder. Br. J. Psychiatry J. Ment. Sci. 190, 359–360. doi: 10.1192/bjp.bp.106.029868
O’Brien, J. T., Lloyd, A., McKeith, I., Gholkar, A., and Ferrier, N. (2004). A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am. J. Psychiatry 161, 2081–2090. doi: 10.1176/appi.ajp.161.11.2081
O’Donnell, L. A., Deldin, P. J., Grogan-Kaylor, A., McInnis, M. G., Weintraub, J., Ryan, K. A., et al. (2017). Depression and executive functioning deficits predict poor occupational functioning in a large longitudinal sample with bipolar disorder. J. Affect. Disord. 215, 135–142. doi: 10.1016/j.jad.2017.03.015
Orgeta, V., Leung, P., Del-Pino-Casado, R., Qazi, A., Orrell, M., Spector, A. E., et al. (2022). Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Syst. Rev. 4:CD009125. doi: 10.1002/14651858.CD009125.pub3
Oxenkrug, G. F. (2010). Tryptophan kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: the serotonin hypothesis revisited 40 years later. Isr. J. Psychiatry Relat. Sci. 47, 56–63
Padovani, A., Caratozzolo, S., Benussi, A., Galli, A., Rozzini, L., Cosseddu, M., et al. (2024). Vortioxetine treatment for depression in patients with prodromal vs mild Alzheimer’s disease: a six-month, open-label observational study. J Prev Alzheimers Dis. 11, 375–381. doi: 10.14283/jpad.2023.132
Palmer, K., Berger, A. K., Monastero, R., Winblad, B., Bäckman, L., and Fratiglioni, L. (2007). Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology 68, 1596–1602. doi: 10.1212/01.wnl.0000260968.92345.3f
Pandey, G. N., Rizavi, H. S., Zhang, H., Bhaumik, R., and Ren, X. (2018). Abnormal protein and mRNA expression of inflammatory cytokines in the prefrontal cortex of depressed individuals who died by suicide. J. Psychiatry Neurosci. 43, 376–385. doi: 10.1503/jpn.170192
Pietrzak, R. H., Lim, Y. Y., Neumeister, A., Ames, D., Ellis, K. A., Harrington, K., et al. (2015). Amyloid-β, anxiety, and cognitive decline in preclinical Alzheimer disease: a multicenter, prospective cohort study. JAMA Psychiatry 72, 284–291. doi: 10.1001/jamapsychiatry.2014.2476
Potvin, O., Forget, H., Grenier, S., Préville, M., and Hudon, C. (2011). Anxiety, depression, and 1-year incident cognitive impairment in community-dwelling older adults. J. Am. Geriatr. Soc. 59, 1421–1428. doi: 10.1111/j.1532-5415.2011.03521.x
Prince, M., Bryce, R., Albanese, E., Wimo, A., Ribeiro, W., and Ferri, C. P. (2013). The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. J. Alzheimers Assoc. 9, 63–75.e2. doi: 10.1016/j.jalz.2012.11.007
Rajji, T. K., Bowie, C. R., Herrmann, N., Pollock, B. G., Lanctôt, K. L., Kumar, S., et al. (2024). Slowing cognitive decline in major depressive disorder and mild cognitive impairment: a randomized clinical trial. JAMA Psychiatry :e243241.
Ramos-Cejudo, J., Corrigan, J. K., Zheng, C., Swinnerton, K. N., Jacobson, S. R., La, J., et al. (2024). Antidepressant exposure and long-term dementia risk in a nationwide retrospective study on US veterans with midlife major depressive disorder. Alzheimers Dement. J. Alzheimers Assoc. 20, 4106–4114. doi: 10.1002/alz.13853
Rapp, M. A., Schnaider-Beeri, M., Purohit, D. P., Perl, D. P., Haroutunian, V., and Sano, M. (2008). Increased neurofibrillary tangles in patients with alzheimer disease with comorbid depression. Am. J. Geriatr. Psychiatry 16, 168–174. doi: 10.1097/JGP.0b013e31816029ec
Ray, K. D., and Götell, E. (2018). The use of music and music therapy in ameliorating depression symptoms and improving well-being in nursing home residents with dementia. Front. Med. 5:287. doi: 10.3389/fmed.2018.00287
Romo-Araiza, A., and Ibarra, A. (2020). Prebiotics and probiotics as potential therapy for cognitive impairment. Med. Hypotheses 134:109410. doi: 10.1016/j.mehy.2019.109410
Rubin, R. T. (1967). Adrenal cortical activity changes in manic-depressive illness. Influence on intermediary metabolism of tryptophan. Arch. Gen. Psychiatry 17, 671–679. doi: 10.1001/archpsyc.1967.01730300031006
Rubin, R. D., Watson, P. D., Duff, M. C., and Cohen, N. J. (2014). The role of the hippocampus in flexible cognition and social behavior. Front. Hum. Neurosci. 8:742. doi: 10.3389/fnhum.2014.00742
Ruthirakuhan, M., Herrmann, N., Vieira, D., Gallagher, D., and Lanctôt, K. L. (2019). The roles of apathy and depression in predicting alzheimer disease: a longitudinal analysis in older adults with mild cognitive impairment. Am. J. Geriatr. Psychiatry 27, 873–882. doi: 10.1016/j.jagp.2019.02.003
Ryan, B., To EMa’u, E., AHY, C., Rivera-Rodriguez, C., Curtis, M. A., et al. (2022). Prevalence of young-onset dementia: nationwide analysis of routinely collected data. J. Neurol. Neurosurg. Psychiatry 93, 1066–1073. doi: 10.1136/jnnp-2022-329126
Sanchez-Moreno, J., Bonnin, C. M., González-Pinto, A., Amann, B. L., Solé, B., Balanzá-Martinez, V., et al. (2018). Factors associated with poor functional outcome in bipolar disorder: sociodemographic, clinical, and neurocognitive variables. Acta Psychiatr Scand [Internet]. 138, 145–154. doi: 10.1111/acps.12894
Seignourel, P. J., Kunik, M. E., Snow, L., Wilson, N., and Stanley, M. (2008). Anxiety in dementia: a critical review. Clin. Psychol. Rev. 28, 1071–1082. doi: 10.1016/j.cpr.2008.02.008
Shaw, C., Hayes-Larson, E., Glymour, M. M., Dufouil, C., Hohman, T. J., Whitmer, R. A., et al. (2021). Evaluation of selective survival and sex/gender differences in dementia incidence using a simulation model. JAMA Netw. Open 4:e211001. doi: 10.1001/jamanetworkopen.2021.1001
Sheline, Y. I., Pieper, C. F., Barch, D. M., Welsh-Bohmer, K., McKinstry, R. C., Mac Fall, J. R., et al. (2010). Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch. Gen. Psychiatry 67, 277–285. doi: 10.1001/archgenpsychiatry.2009.204
Sheline, Y. I., Snider, B. J., Beer, J. C., Seok, D., Fagan, A. M., Suckow, R. F., et al. (2020). Effect of escitalopram dose and treatment duration on CSF Aβ levels in healthy older adults: a controlled clinical trial. Neurology 95, e2658–e2665. doi: 10.1212/WNL.0000000000010725
Sheline, Y. I., West, T., Yarasheski, K., Swarm, R., Jasielec, M. S., Fisher, J. R., et al. (2014). An antidepressant decreases CSF aβ production in healthy individuals and in transgenic AD mice. Sci. Transl. Med. 6:236re4. doi: 10.1126/scitranslmed.3008169
Shen, Y., Yang, X., Li, G., Gao, J., and Liang, Y. (2021). The change of gut microbiota in MDD patients under SSRIs treatment. Sci. Rep. 11:14918. doi: 10.1038/s41598-021-94481-1
Siafarikas, N., Kirsebom, B. E., Srivastava, D. P., Eriksson, C. M., Auning, E., Hessen, E., et al. (2021). Cerebrospinal fluid markers for synaptic function and Alzheimer type changes in late life depression. Sci. Rep. 11:20375. doi: 10.1038/s41598-021-99794-9
Sies, H. (2015). Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4, 180–183. doi: 10.1016/j.redox.2015.01.002
Sies, H. (2020). Oxidative stress: concept and some practical aspects. Antioxidants 9:852. doi: 10.3390/antiox9090852
Singh, V., Roth, S., Llovera, G., Sadler, R., Garzetti, D., Stecher, B., et al. (2016). Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 36, 7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016
Singh-Manoux, A., Dugravot, A., Fournier, A., Abell, J., Ebmeier, K., Kivimäki, M., et al. (2017). Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiatry 74, 712–718. doi: 10.1001/jamapsychiatry.2017.0660
Stuart, M. J., and Baune, B. T. (2014). Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci. Biobehav. Rev. 42, 93–115. doi: 10.1016/j.neubiorev.2014.02.001
Sudheimer, K. D., O’Hara, R., Spiegel, D., Powers, B., Kraemer, H. C., Neri, E., et al. (2014). Cortisol, cytokines, and hippocampal volume interactions in the elderly. Front. Aging Neurosci. 6:153. doi: 10.3389/fnagi.2014.00153
Szabo, G. (2015). Gut-liver axis in alcoholic liver disease. Gastroenterology 148, 30–36. doi: 10.1053/j.gastro.2014.10.042
Thomas, J. M., and Sezgin, D. (2021). Effectiveness of reminiscence therapy in reducing agitation and depression and improving quality of life and cognition in long-term care residents with dementia: a systematic review and meta-analysis. Geriatr. Nurs. 42, 1497–1506. doi: 10.1016/j.gerinurse.2021.10.014
Ting, B., Chen, D. T. L., Hsu, W. T., Tsai, C. L., Malau, I. A., Lee, S. L., et al. (2024). Multifaceted music therapy for depression in dementia: a network Meta-analysis of randomized controlled trials. Eur. J. Investig. Health Psychol. Educ. 14, 351–367. doi: 10.3390/ejihpe14020024
Tonelli, L. H., Stiller, J., Rujescu, D., Giegling, I., Schneider, B., Maurer, K., et al. (2008). Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr. Scand. 117, 198–206. doi: 10.1111/j.1600-0447.2007.01128.x
Tsapekos, D., Strawbridge, R., Cella, M., Wykes, T., and Young, A. H. (2021). Cognitive impairment in euthymic patients with bipolar disorder: prevalence estimation and model selection for predictors of cognitive performance. J. Affect. Disord. 294, 497–504. doi: 10.1016/j.jad.2021.07.036
Tz-Han, L., Wan-Ru, W., I-Hui, C., and Hui-Chuan, H. (2023). Reminiscence music intervention on cognitive, depressive, and behavioral symptoms in older adults with dementia. Geriatr. Nurs. 49, 127–132. doi: 10.1016/j.gerinurse.2022.11.014
Van Eck, N., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. doi: 10.1007/s11192-009-0146-3
Velosa, J., Delgado, A., Finger, E., Berk, M., Kapczinski, F., and de Azevedo, C. T. (2020). Risk of dementia in bipolar disorder and the interplay of lithium: a systematic review and meta-analyses. Acta Psychiatr. Scand. 141, 510–521. doi: 10.1111/acps.13153
Weiner, M., and Lipton, A. F. (2009). Alzheimer's disease and other dementias. Arlington VA: American Psychiatric Publishing.
Westin, K., Buchhave, P., Nielsen, H., Minthon, L., Janciauskiene, S., and Hansson, O. (2012). CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PLoS One 7:525. doi: 10.1371/journal.pone.0030525
Wilson, R. S., Boyle, P. A., Capuano, A. W., Shah, R. C., Hoganson, G. M., Nag, S., et al. (2016). Late-life depression is not associated with dementia-related pathology. Neuropsychology 30, 135–142. doi: 10.1037/neu0000223
Wolkowitz, O. M., Burke, H., Epel, E. S., and Reus, V. I. (2009). Glucocorticoids. Mood, memory, and mechanisms. Ann. N. Y. Acad. Sci. 1179, 19–40. doi: 10.1111/j.1749-6632.2009.04980.x
World Health Organization. (2017). Depression and other common mental disorders: Global health estimates. Available at: https://www.who.int/publications/i/item/depression-global-health-estimates (Accessed 15 Oct 2021).
World Health Organization (2021). Suicide worldwide in 2019: Global Health estimates. Geneva: World Health Organization.
Xu, W., Feng, W., Shen, X. N., Bi, Y. L., Ma, Y. H., Li, J. Q., et al. (2021). Amyloid pathologies modulate the associations of minimal depressive symptoms with cognitive impairments in older adults without dementia. Biol. Psychiatry 89, 766–775. doi: 10.1016/j.biopsych.2020.07.004
Xu, J., Gou, S., Huang, X., Zhang, J., Zhou, X., Gong, X., et al. (2023). Uncovering the impact of aggrephagy in the development of alzheimer’s disease: insights into diagnostic and therapeutic approaches from machine learning analysis. Curr. Alzheimer Res. 20, 618–635. doi: 10.2174/0115672050280894231214063023
Xu, H., Li, A., and Apuke, O. D. (2024). The impact of group music therapy in ameliorating the depression among patients with dementia in care homes: a randomized control trial. Geriatr. Nurs. 56, 304–311. doi: 10.1016/j.gerinurse.2024.02.021
Yaghoubfar, R., Behrouzi, A., Ashrafian, F., Shahryari, A., Moradi, H. R., Choopani, S., et al. (2020). Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci. Rep. 10:22119. doi: 10.1038/s41598-020-79171-8
Yaghoubfar, R., Behrouzi, A., Zare Banadkoki, E., Ashrafian, F., Lari, A., Vaziri, F., et al. (2021). Effect of Akkermansia muciniphila, Faecalibacterium prausnitzii, and their extracellular vesicles on the serotonin system in intestinal epithelial cells. Probiotics Antimicrob. Proteins 13, 1546–1556. doi: 10.1007/s12602-021-09786-4
Yepes, M. (2021). The plasminogen activating system in the pathogenesis of Alzheimer’s disease. Neural Regen. Res. 16, 1973–1977. doi: 10.4103/1673-5374.308076
Yoon, S., Shin, C., and Han, C. (2017). Depression and cognitive function in mild cognitive impairment: a 1-year follow-up study. J. Geriatr. Psychiatry Neurol. 30, 280–288. doi: 10.1177/0891988717723741
Zhang, J., Zhou, X., Jiang, H., Zhu, W., Chi, H., Jiang, L., et al. (2024). Acupuncture for insomnia symptoms in hypertensive patients: a systematic review and meta-analysis. Front. Neurol. 15:1329132. doi: 10.3389/fneur.2024.1329132
Zhao, H., Jin, K., Jiang, C., Pan, F., Wu, J., Luan, H., et al. (2022). A pilot exploration of multi-omics research of gut microbiome in major depressive disorders. Transl. Psychiatry 12:8. doi: 10.1038/s41398-021-01769-x
Zhuang, Z. Q., Shen, L. L., Li, W. W., Fu, X., Zeng, F., Gui, L., et al. (2018). Gut microbiota is altered in patients with Alzheimer’s disease. J. Alzheimers Dis. 63, 1337–1346. doi: 10.3233/JAD-180176
Keywords: dementia, depression, bibliometric, global trends, mechanism
Citation: Li X, Su W and Cai L (2025) A bibliometric analysis of research on dementia comorbid with depression from 2005 to 2024. Front. Neurosci. 19:1508662. doi: 10.3389/fnins.2025.1508662
Edited by:
Thomas Müller, Alexianer St. Joseph Hospital, GermanyReviewed by:
Harry Wilhelm Steinbusch, Maastricht University, NetherlandsJieying Zhang, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, China
Copyright © 2025 Li, Su and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Su, YW5hbjMwOTlAMTYzLmNvbQ==
 Xia Li
Xia Li Wei Su*
Wei Su*