
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci. , 28 February 2025
Sec. Neurodegeneration
Volume 19 - 2025 | https://doi.org/10.3389/fnins.2025.1502417
This article is part of the Research Topic Exercise-Induced Neuroplasticity in Neurodegeneration Diseases Volume II View all 4 articles
 Lamia Ben Ezzdine1†
Lamia Ben Ezzdine1† Wissem Dhahbi2,3,4†
Wissem Dhahbi2,3,4† Ismail Dergaa2,4,5
Ismail Dergaa2,4,5 Halil İbrahim Ceylan6*
Halil İbrahim Ceylan6* Noomen Guelmami2
Noomen Guelmami2 Helmi Ben Saad7‡
Helmi Ben Saad7‡ Karim Chamari8‡
Karim Chamari8‡ Valentina Stefanica9*‡
Valentina Stefanica9*‡ Abdelfatteh El Omri10‡
Abdelfatteh El Omri10‡This review aimed to elucidate the mechanisms through which (i) physical activity (PA) enhances neuroplasticity and cognitive function in neurodegenerative disorders, and (ii) identify specific PA interventions for improving cognitive rehabilitation programs. We conducted a literature search in PubMed, Medline, Scopus, Web of Science, and PsycINFO, covering publications from January 1990 to August 2024. The search strategy employed key terms related to neuroplasticity, physical exercise, cognitive function, neurodegenerative disorders, and personalized physical activity. Inclusion criteria included original research on the relationship between PA and neuroplasticity in neurodegenerative disorders, while exclusion criteria eliminated studies focusing solely on pharmacological interventions. The review identified multiple pathways through which PA may enhance neuroplasticity, including releasing neurotrophic factors, modulation of neuroinflammation, reduction of oxidative stress, and enhancement of synaptic connectivity and neurogenesis. Aerobic exercise was found to increase hippocampal volume by 1–2% and improve executive function scores by 5–10% in older adults. Resistance training enhanced cognitive control and memory performance by 12–18% in elderly individuals. Mind–body exercises, such as yoga and tai-chi, improved gray matter density in memory-related brain regions by 3–5% and enhanced emotional regulation scores by 15–20%. Dual-task training improved attention and processing speed by 8–14% in individuals with neurodegenerative disorders. We also discuss the potential role of AI-based exercise and AI cognitive training in preventing and rehabilitating neurodegenerative illnesses, highlighting innovative approaches to personalized interventions and improved patient outcomes. PA significantly enhances neuroplasticity and cognitive function in neurodegenerative disorders through various mechanisms. Aerobic exercise, resistance training, mind–body practices, and dual-task exercises each offer unique cognitive benefits. Implementing these activities in clinical settings can improve patient outcomes. Future research should focus on creating personalized interventions tailored to specific conditions, incorporating personalized physical exercise programs to optimize cognitive rehabilitation.
Neurodegenerative disorders, such as Parkinson’s disease and Alzheimer’s disease, present a significant challenge to individuals, families, and healthcare systems worldwide due to the progressive decline in cognitive and physical abilities (Alzheimer’s Association, 2022; Lamptey et al., 2022). The increasing frequency of cognitive decline diseases has led to a growing demand for effective non-pharmacological therapy to control them (Luo et al., 2023). In this regard, understanding neuroplasticity is crucial in addressing this demand. Neuroplasticity, i.e., the brain’s capacity to adapt and form new neural connections in response to experiences and environmental stimuli, is fundamental to cognitive function (Cramer et al., 2011). Research suggests that harnessing neuroplasticity may mitigate cognitive impairments associated with neurodegenerative diseases (Cotman and Berchtold, 2002; Prakash et al., 2015).
Physical activity (PA) is a powerful non-pharmacological method for, among others, managing neurodegenerative disorders, with strong evidence supporting its role in enhancing neuroplasticity (Erickson et al., 2019; Voss et al., 2013). This involves the brain’s ability to reorganize and form new neural connections, leading to increased production of neurotrophic factors, modulation of neuroinflammation, and enhancement of synaptic plasticity and neurogenesis. Erickson et al. (2019) demonstrated that practicing regular PA strengthens the brain’s neuroplasticity and improves cognitive performance in older adults, potentially mitigating cognitive decline associated with neurodegenerative diseases. Different modalities of physical exercise exert distinct effects on cognitive function and neuroplasticity through various molecular and cellular mechanisms, offering a range of potential interventions for addressing cognitive decline in neurodegenerative disorders (Cramer et al., 2011; Karamacoska et al., 2023). Aerobic exercise, such as running, cycling, and swimming, has been demonstrated to elevate heart rate and enhance cerebral blood flow, facilitating the delivery of vital nutrients and oxygen to the brain (Cotman and Berchtold, 2002). Research suggests that engaging in consistent aerobic exercise results in a rise in brain-derived neurotrophic factor (BDNF), a vital element for the development and endurance of neurons (Cotman and Berchtold, 2002). This increase in BDNF is linked to enhanced memory, learning abilities, and overall cognitive function (Cotman and Berchtold, 2002; Kennedy, 2013). Resistance training, a form of exercise that aims to enhance both muscular strength and endurance, has been shown to also stimulate neuroplasticity. Engaging in this form of physical activity stimulates the production of myokines (proteins released by muscles). These myokines have multiple neuroprotective benefits, such as improving synaptic plasticity and facilitating the growth of new neurons (Jodeiri Farshbaf and Alvina, 2021). Studies have shown that engaging in resistance exercise can enhance cognitive performance, especially in older individuals. This includes increases in cognitive control, memory, and executive processes (Liu-Ambrose et al., 2010). Mind–body exercises, such as yoga and tai-chi, integrate physical movement with cognitive concentration and profound breathing (de Sousa Fernandes et al., 2020; Gothe et al., 2019). These behaviors have been linked to decreased stress and improved psychological well-being. Studies indicated that mind–body workouts have a substantial impact on neuroplasticity by inducing calm and decreasing the presence of stress hormones such as cortisol. In this regard, elevated cortisol levels over a prolonged period can be harmful to brain function (de Sousa Fernandes et al., 2020). Yoga has been connected with enhanced cognitive functioning, better emotional regulation, and even physical changes in the brain, such as a higher density of gray matter in areas related to memory and emotional control (Gothe et al., 2019). Furthermore, the integration of cognitive engagement with PA, such as in dual-task and skill-based exercises, presents a novel approach to enhancing neuroplasticity and cognitive function (Maeneja et al., 2023). These exercises provide a significant challenge to both the body and the brain, improving both physical and cognitive functions. Research has demonstrated that engaging in dual-task training can enhance attention, processing speed, and cognitive performance in older adults and individuals undergoing stroke rehabilitation (Abo and Hamaguchi, 2024). Although there has been considerable advancement in comprehending the impacts of various forms of PA on neuroplasticity and cognitive function, the exact mechanisms through which PA enhances these advantages in the context of neurodegenerative disorders remain incompletely understood. Furthermore, there is a need for more research to explore the effective implementation of PA programs in cognitive rehabilitation.
In this review, we aimed to address existing research gaps by exploring the mechanisms through which PA enhances neuroplasticity and cognitive function in individuals with neurodegenerative disorders, with a particular focus on translating neuroplasticity and circuit retraining research into effective clinical therapies (Kumar J. et al., 2023). The study has two main objectives: (i) To provide a clear understanding of how PA promotes neuroplasticity and cognitive improvement in neurodegenerative disorders, and (ii) To identify specific PA interventions that can improve the customization and effectiveness of cognitive rehabilitation programs for individuals with neurodegenerative conditions.
A comprehensive literature search was conducted in PubMed, Medline, Scopus, Web of Science, and PsycINFO, as well as the first 10 pages of Google Scholar to capture relevant gray literature, encompassing publications from January 1990 to August 2024. The search strategy employed the following key terms and their combinations: (neuroplasticity OR “synaptic plasticity” OR “neural reorganization” OR “brain plasticity”) AND (“physical activity” OR exercise OR “aerobic training” OR “resistance training” OR “strength training” OR “balance exercises” OR “flexibility training” OR “outdoor activities” OR “home-based interventions”) AND (“cognitive function” OR “executive function” OR memory OR attention OR “processing speed” OR “cognitive performance”) AND (“neurodegenerative disorders” OR “Alzheimer’s disease” OR “Parkinson’s disease” OR “Huntington’s disease” OR “multiple sclerosis” OR “amyotrophic lateral sclerosis” OR “frontotemporal dementia” OR “Lewy body dementia” OR “cognitive decline”) AND (“emerging technologies” OR “virtual reality” OR “augmented reality” OR “brain-computer interfaces” OR “exergaming” OR “wearable devices” OR “smartphone applications” OR “telerehabilitation”). Specific exercise intervention terms included “dual-task training,” “high-intensity interval training,” “progressive resistance training,” “balance training,” and “mind–body exercises.” Additionally, we used Medical Subject Headings (MeSH) terms to ensure comprehensive coverage of relevant literature. The search was refined to include only English-language full-text articles to ensure the inclusion of the most current and relevant research.
Inclusion criteria were applied to select studies that met the following requirements: (i) Investigated the relationship between PA or exercise and neuroplasticity in the context of neurodegenerative disorders; (ii) Presented original research findings from randomized controlled trials, prospective longitudinal studies, or diagnostic studies; (iii) Included human subjects.
Exclusion criteria were defined as follows: (i) Case reports, literature reviews, systematic reviews, meta-analyses, letters to the editor, and conference abstracts; (ii) Studies focusing solely on non-ambulatory people, hospitalized patients, or animals; (iii) Articles that did not specifically address the mechanisms underlying the cognitive benefits of PA.
The initial search yielded a total of 2,616 records. After removing duplicates and screening titles and abstracts, 428 full-text articles were assessed for eligibility. Two independent reviewers conducted the screening process, with disagreements resolved through discussion or by a third reviewer (Waffenschmidt et al., 2019). The inter-rater reliability for the screening process was calculated using Cohen’s kappa coefficient (κ = 0.85, indicating strong agreement). Following the application of inclusion and exclusion criteria, 63 studies were selected for comprehensive analysis (Figure 1).
Following screening completion, data extraction was performed systematically by the same two independent reviewers using a standardized protocol. The extracted information included: (i) study characteristics (design, duration, sample size, country); (ii) participant demographics (age, sex, type, and stage of neurodegenerative disorder); (iii) intervention details (type, frequency, intensity, and duration of physical activity); (iv) outcome measures (neuroplasticity markers, cognitive assessments, functional outcomes); and (v) proposed biological mechanisms. Data management was conducted using Rayyan QCRI software (Qatar Computing Research Institute, Doha, Qatar) to ensure systematic organization and minimize errors during the extraction process (Valizadeh et al., 2022). Among the 63 included studies, analysis of study designs revealed 35 randomized controlled trials, 15 prospective cohort studies, 8 case–control studies, and 5 cross-sectional studies. Methodological quality assessment employed validated design-specific tools: the Cochrane Risk of Bias 2.0 tool for randomized controlled trials (assessing randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selective reporting); the Newcastle-Ottawa Scale for cohort and case–control studies (evaluating selection, comparability, and outcome/exposure assessment); and its modified version for cross-sectional studies.
Neuroplasticity encompasses the brain’s capacity to modify its structure and function throughout life through the formation, strengthening, weakening, or elimination of neural connections (Hooks and Chen, 2020). This dynamic process responds to experiences, learning, environmental stimuli, and injuries, enabling both adaptive and maladaptive changes (Appelbaum et al., 2023). Understanding neuroplasticity mechanisms is crucial for developing effective therapeutic interventions for neurodegenerative conditions, as it underlies the brain’s capacity for repair and functional recovery (Zotey et al., 2023). Key factors influencing neuroplastic changes include timing of intervention, intensity of stimulation, and individual factors such as motivation and attention (Chatterjee et al., 2021).
At the molecular level, neuroplasticity involves multiple interacting processes. Synaptic plasticity, manifesting through long-term potentiation and depression, modulates synaptic strength and underpins learning and memory formation (Bliss and Cooke, 2011; Cuestas Torres and Cardenas, 2020). These processes involve complex molecular cascades, including neurotransmitter release, receptor trafficking, and structural modifications of synapses (Yasuda et al., 2022). Adult neurogenesis, particularly in the hippocampus, contributes to cognitive flexibility and emotional regulation through the integration of newly born neurons into existing circuits (Hussain et al., 2024; Surget and Belzung, 2022). Experience-dependent plasticity drives the reorganization of neural networks through modifications in synaptic connectivity and dendritic architecture (Wang et al., 2021).
The significance of neuroplasticity in cognitive function extends beyond basic neural mechanisms to clinical applications (Mira et al., 2021). Neuroplastic changes support cognitive resilience against aging and neurodegeneration through multiple pathways, including synaptic strengthening, network reorganization, and compensatory mechanisms (Zotey et al., 2023). Environmental enrichment and targeted interventions can enhance neuroplasticity, potentially slowing cognitive decline in neurodegenerative conditions (Cutuli et al., 2022). Physical activity particularly promotes neuroplasticity through increased neurotrophic factor production, enhanced synaptic plasticity, and improved cerebral blood flow (Hwang et al., 2023). These findings support the development of neuroplasticity-based therapeutic approaches for preserving cognitive function in aging and disease (Kumar Y. et al., 2023).
Neurodegenerative illnesses, such as Alzheimer’s disease and Parkinson’s disease, are marked by distinct pathogenic mechanisms that result in the deterioration and impairment of neurons (Xie et al., 2014). These illnesses frequently entail the build-up of harmful proteins, such as amyloid-beta and tau in Alzheimer’s disease, or alpha-synuclein in Parkinson’s disease (Xie et al., 2014). The accumulation of neurodegenerative products, such as beta-amyloid and tau protein, has been shown to negatively impact neuroplasticity and cognitive function (Wu et al., 2024). The presence of these protein aggregates hinders the proper functioning of cells, resulting in inflammation, oxidative stress, and a decrease in the ability of synapses to interact with each other (Xie et al., 2014; Chen et al., 2012). Consequently, the brain’s capability to perform neuroplastic changes is constrained, thereby diminishing its ability to adapt to novel stimuli or recover from injury (Marzola et al., 2023).
Impaired neuroplasticity is a key factor in the cognitive impairments linked to neurodegenerative illnesses (Marzola et al., 2023). When neuroplasticity mechanisms malfunction, the brain’s ability to create new memories and adjust to new knowledge is impaired (Marzola et al., 2023). For instance, in the case of Alzheimer’s disease, the decrease in the formation of new neurons in the hippocampus is associated with memory loss and a general decline in cognitive function (Marzola et al., 2023; Jahn, 2013). Similarly, the decline in dopaminergic signaling in Parkinson’s disease impacts motor learning and cognitive flexibility, resulting in challenges in task execution and decision-making (Berry et al., 2018). The interaction between the degeneration of neurons and the reduction in the brain’s ability to change and adapt highlights the significance of discovering treatment approaches that can revive or improve the brain’s capacity to change and adapt, resulting in a dampening of cognitive decline.
Different types of PA modulate synaptic plasticity and neurogenesis through distinct molecular mechanisms. Research has shown that engaging in aerobic exercise can lead to an increase in the size of the hippocampus and improve the connections between neurons in this important brain region responsible for memory and learning (Erickson et al., 2019). Specifically, aerobic exercise increases BDNF levels, which promotes synaptic plasticity and neurogenesis in the hippocampus. Moderate-intensity aerobic exercise (60–70% of maximum heart rate) performed for 30–40 min, 3–4 times per week has been shown to optimally stimulate BDNF production and hippocampal neurogenesis (Revelo Herrera and Leon-Rojas, 2024). Resistance exercise also can impact neuroplasticity by elevating the amounts of muscle-derived factors that can traverse the blood–brain barrier, including insulin-like growth factor-1 (IGF-1) and myokines, therefore enhancing brain health. Progressive resistance training performed 2–3 times per week at 60–80% of one-repetition maximum has been shown to significantly increase circulating IGF-1 levels (Rodriguez-Gutierrez et al., 2023). High-intensity interval training (HIIT) combining brief intense exercise bouts (≥85% VO2max) with active recovery periods has demonstrated superior effects on neuroplasticity compared to continuous moderate-intensity training, potentially due to enhanced production of cathepsin B and irisin (Montero-Almagro et al., 2024). Mind–body exercises, such as yoga and tai-chi, promote cognitive function by reducing stress and improving emotional regulation, in addition to improving flexibility and balance (Ghazel et al., 2022; Wang et al., 2023). These exercises promote mindfulness as well, which has been associated with enhanced neuroplasticity (Lardone et al., 2018; Table 1).
Studies suggested that participating in a diversified range of PAs can enhance the brain’s capacity to adjust and restructure (Belcher et al., 2021; Kumar J. et al., 2023; Latino and Tafuri, 2024). The effects of PA on neuroplasticity may exhibit a dose–response relationship, with some studies suggesting that higher levels of fitness correlate with more significant brain outcomes (Mandolesi et al., 2018). For example, the practice of dual-task training, which involves combining cognitive tasks with physical exercise, has been proven to improve both cognitive and motor skills (Xiao et al., 2023). This demonstrates the interconnectedness of physical and mental activities (Figure 2).
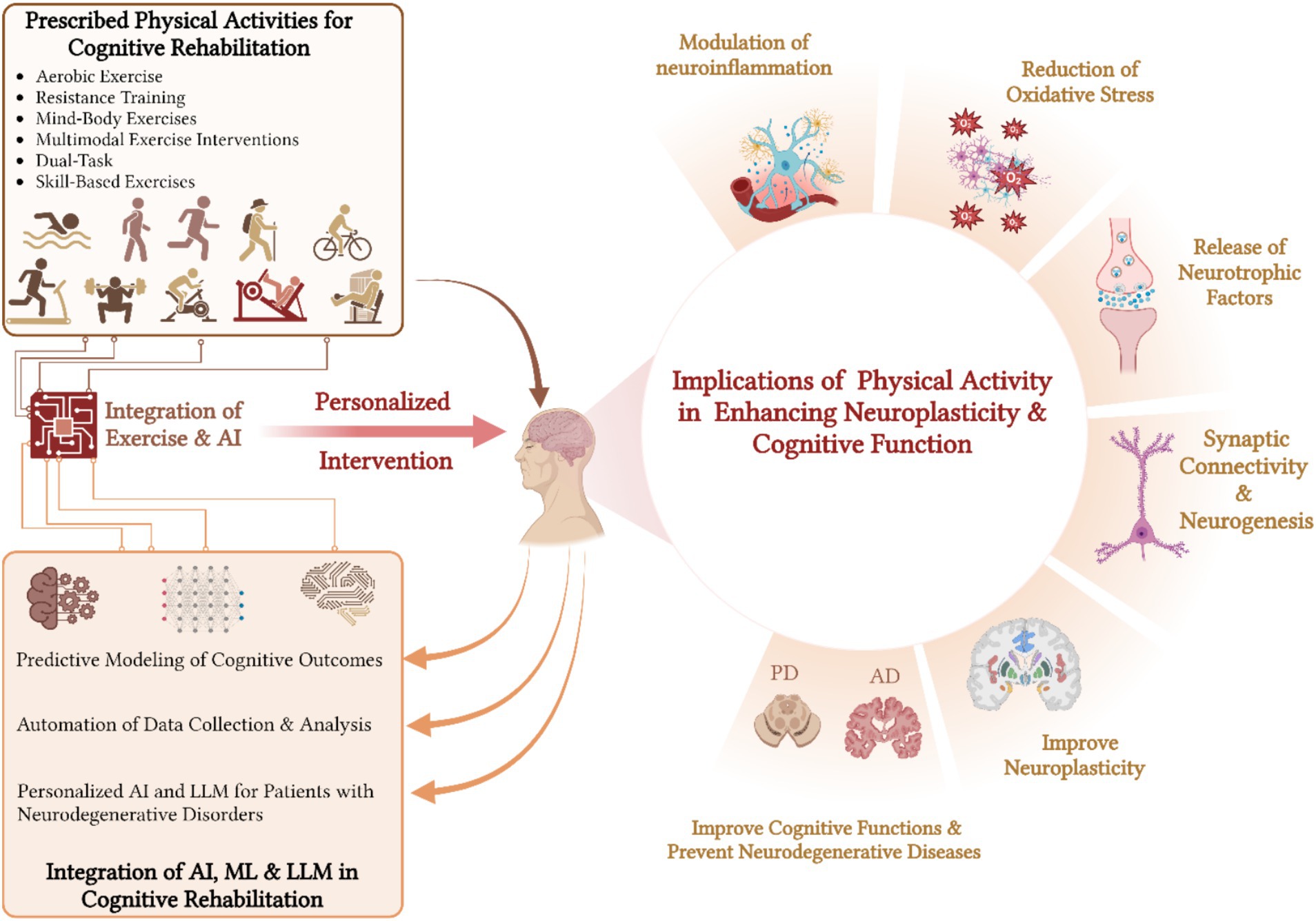
Figure 2. Impact of diversified physical activities and AI integration on neuroplasticity and cognitive rehabilitation.
PA enhances neuroplasticity by stimulating the release of neurotrophic factors, particularly BDNF, which supports neuronal survival, growth, and synaptic plasticity (Sleiman et al., 2016). BDNF promotes the viability of preexisting neurons and stimulates the development of new neurons and synapses (Sleiman et al., 2016; Kowianski et al., 2018). It participates in diverse processes, such as learning, memory, and mood control (Sleiman et al., 2016). Studies indicate that engaging in consistent PA enhances BDNF levels, hence potentially enhancing cognitive performance and emotional resilience (Sleiman et al., 2016, Kowianski et al., 2018). Additional neurotrophic factors, such as nerve growth factor and neurotrophin-3, also play a role in neuroplasticity by enhancing the well-being and interconnection of neurons. These substances augment synaptic potency and expedite neuronal transmission, which is crucial for brain functioning (Levy et al., 2018; Table 1).
PA has a significant influence on neuroinflammation, which is a crucial element in numerous neurodegenerative disorders (Seo et al., 2019). Engaging in regular PA assists in regulating the immune response in the brain by decreasing the presence of pro-inflammatory cytokines and facilitating anti-inflammatory processes (Seo et al., 2019; Liu et al., 2019). Modulating neuroinflammation is crucial because persistent inflammation can harm neurons and interfere with neuroplasticity, among others. Research indicates that engaging in PA might reduce indicators of inflammation, which in turn promotes a more favorable brain environment and enhances cognitive performance (Malkowska and Sawczuk, 2023; Gomez-Pinilla and Hillman, 2013). The anti-inflammatory effects of exercise contribute to a more favorable brain environment, potentially mitigating the negative impact of chronic inflammation on neuroplasticity and cognitive function (Seo et al., 2019). Additionally, engaging in PA boosts the generation of anti-inflammatory cytokines, providing additional defense for the brain against the harmful consequences of inflammation (Table 1).
Oxidative stress is caused by a disparity between free radicals and the body’s capacity to neutralize their detrimental impacts. It has a major role in causing damage to neurons in neurodegenerative diseases (Sienes Bailo et al., 2022). Studies have shown that regular PA enhances the body’s antioxidant defenses, thereby reducing oxidative damage to neurons and supporting neuroplasticity (Sienes Bailo et al., 2022). PA promotes the generation of internal antioxidants, such as superoxide dismutase and glutathione, which aid in counteracting harmful free radicals (Clemente-Suarez et al., 2023). The presence of this protective effect is essential for the preservation of neuronal well-being and operation, facilitating enhanced neuroplasticity and cognitive resilience (Table 1).
Extensive research has demonstrated the beneficial effects of aerobic exercise on brain health and cognitive performance. This includes activities such as running, cycling, and swimming, which have been reported to influence cortical excitability and result in cognitive improvement (Ferrer-Uris et al., 2022; Vecchio et al., 2018). This type of PA elevates heart rate enhancing cerebral blood circulation, and facilitating the transportation of vital nutrients and oxygen to the brain (Ferrer-Uris et al., 2022; Vecchio et al., 2018). Indeed, engaging in regular aerobic exercise results in an elevation of BDNF, a protein essential for the development and preservation of neurons (Cotman and Berchtold, 2002). Elevated levels of BDNF have been linked to enhanced memory, learning abilities, and overall cognitive function.
Several studies have demonstrated that older individuals who participate in aerobic exercise observe improvements in executive function, encompassing abilities such as problem-solving, attention, and multitasking (Erickson et al., 2019; Revelo Herrera and Leon-Rojas, 2024; Nicastri et al., 2022). In addition, there is an association between aerobic activity and an increase in the volume of the hippocampus, which is a crucial brain region for the creation of memories (Erickson et al., 2019; Voss et al., 2013; Revelo Herrera and Leon-Rojas, 2024; Nicastri et al., 2022; Erickson et al., 2011; Gomez-Pinilla and Hillman, 2013). These findings indicate that engaging in regular aerobic exercise can mitigate the deterioration in cognitive function associated with aging and may even offer protection against neurodegenerative illnesses (Voss et al., 2013; Erickson et al., 2011; Gomez-Pinilla and Hillman, 2013). Moreover, the intensity of aerobic exercise may play a crucial role in its neuroprotective effects, with high-intensity exercise showing particularly promising results in influencing cortical excitability and structural changes (Revelo Herrera and Leon-Rojas, 2024).
Resistance training, also known as muscle strengthening, which consists of activities that enhance both muscle strength and endurance, also has a substantial impact on increasing neuroplasticity, potentially through mechanisms distinct from those of aerobic exercise (Jodeiri Farshbaf and Alvina, 2021). Engaging in this form of exercise stimulates the secretion of myokines, which are bioactive molecules synthesized by muscles in response to physical exertion (Jodeiri Farshbaf and Alvina, 2021). Studies have demonstrated that myokines possess diverse neuroprotective properties, such as improving synaptic plasticity and stimulating neurogenesis (Jodeiri Farshbaf and Alvina, 2021; Zunner et al., 2022; Lee et al., 2021). Resistance exercise can result in enhancements in cognitive performance, especially among elderly individuals (Jodeiri Farshbaf and Alvina, 2021; Zunner et al., 2022; Lee et al., 2021). It has been shown that people who regularly engage in resistance training demonstrate improved cognitive control, memory, and executive functions compared to those who do not participate in such activities (Liu-Ambrose et al., 2010). Resistance training leads to physiological changes such as increased muscle mass and improved metabolic health. These changes create a healthier brain environment, which in turn supports neuroplastic processes.
Mind–body exercises, such as yoga and tai-chi, integrate physical movement with cognitive concentration and profound breathing (Ghazel et al., 2022; Wang et al., 2023). These activities have been acknowledged for their capacity to diminish stress and improve psychological well-being. Mindfulness, a key component of these exercises, involves sustained attention to present-moment experiences without judgment. Regular mindfulness practice enhances functional connectivity between the default mode network and executive control regions, promoting neural plasticity in areas associated with attention, emotional regulation, and metacognitive awareness (Shen et al., 2020). Studies using functional magnetic resonance imaging have shown that mindfulness meditation increases gray matter density in the hippocampus, posterior cingulate cortex, and temporoparietal junction (Afonso et al., 2020).
Mind–body workouts have a substantial impact on neuroplasticity by inducing calm and lowering stress hormones such as cortisol (Martín-Rodríguez et al., 2024). In that regard, elevated levels of cortisol over a prolonged period have been suggested to harm brain health (Voss et al., 2023).
Yoga has been linked to enhanced cognitive functioning, better emotional regulation, and even alterations in brain structure (Voss et al., 2023; Gothe et al., 2019; Villemure et al., 2015). Research has demonstrated that engaging in yoga consistently can enhance the density of gray matter in areas of the brain that are linked to memory and emotional regulation (Gothe et al., 2019; Villemure et al., 2015; Voss et al., 2023). Additionally, there is evidence suggesting that tai-chi might improve balance, coordination, and cognitive flexibility (Gothe et al., 2019). This makes it a valuable intervention for older persons who want to preserve their cognitive health and reduce the risk of falling (Madhivanan et al., 2021). The efficacy of mind–body exercises in promoting neuroplasticity has been observed across diverse age groups and in individuals with and without brain disorders, suggesting their broad applicability in cognitive health interventions (Blomstrand et al., 2023).
Recent evidence suggested that combining different types of PA may provide synergistic benefits for neuroplasticity and cognitive function in neurodegenerative disorders (Bherer et al., 2021). Multimodal exercise interventions, which typically include aerobic, resistance, and balance training components, have shown promising results in improving cognitive performance and functional outcomes in older adults and individuals with neurodegenerative conditions (Hong et al., 2023). These comprehensive programs may target multiple aspects of brain health simultaneously, potentially offering more substantial and wide-ranging benefits than single-modality interventions (Woods et al., 2012). Some sports, like football, comprising a mixture of aerobic effort, strength solicitation, and balance, are promising in that regard (Zhou et al., 2024).
Dual-task and skill-based exercises, which require the simultaneous execution of cognitive tasks and PAs, represent a promising area of research in exercise neuroscience. This approach enhances neuroplasticity by simultaneously engaging multiple neural networks and promoting efficient resource allocation between motor and cognitive processes (Xiao et al., 2023). Indeed, it has been reported that engaging in dual-task training might enhance motor and cognitive abilities, especially in older adults and individuals undergoing stroke rehabilitation (Xiao et al., 2023). For instance, tasks that involve participants walking while simultaneously performing activities such as counting backward or answering basic mathematical problems might improve attention and processing speed (Xiao et al., 2023; Wollesen et al., 2020). This form of training promotes neuroplasticity, enabling the brain to acclimate to multitasking, a crucial skill for everyday functioning. Studies have shown that people who engage in dual-task training see more significant enhancements in their walking speed, balance, and cognitive function (Xiao et al., 2023; Wollesen et al., 2020). In neurodegenerative conditions, specific dual-task combinations have shown therapeutic potential. For Parkinson’s disease, combining gait training with executive function tasks (e.g., verbal fluency or arithmetic calculations) improves both motor and cognitive performance while reducing fall risk (Xiao et al., 2023). In Alzheimer’s disease, dual-task interventions incorporating visual search tasks with walking exercises have demonstrated improvements in divided attention and functional mobility (Jardim et al., 2021). The intensity and complexity of dual tasks should be progressively increased, starting with simple cognitive tasks (e.g., counting) and advancing to more complex operations (e.g., problem-solving) as performance improves.
Engaging in skill-based activities, like as dancing or playing sports, might enhance neuroplasticity by necessitating coordination, rhythm, and strategic cognition (Liu et al., 2021). These activities stimulate different areas of the brain, improving the connections between neurons and supporting mental flexibility (Xiao et al., 2023; Wollesen et al., 2020; Liu et al., 2021). Research indicated that participating in intricate motor activities, such as dancing, can enhance spatial awareness and memory as a result of the continuous cognitive challenges imposed on the brain (Liu et al., 2021).
PA exerts multifaceted neuroprotective effects in Alzheimer’s disease through several mechanisms. Regular aerobic exercise increases BDNF production, which enhances synaptic plasticity and neurogenesis particularly in the hippocampus, a region critically affected in Alzheimer’s disease (Erickson et al., 2011). Moderate-intensity aerobic exercise (30–40 min, 3–4 times weekly) has been shown to increase hippocampal volume by 1–2% and improve memory performance in patients with mild cognitive impairment and early-stage Alzheimer’s disease (Lin et al., 2018). Resistance training twice weekly complements these benefits by reducing inflammatory markers and oxidative stress, key pathological features in Alzheimer’s progression (Lopez-Ortiz et al., 2021). The combination of aerobic and resistance exercises has demonstrated superior outcomes in cognitive function compared to single-modality interventions, with improvements in executive function, processing speed, and delayed recall (Lin et al., 2018). Exercise timing appears crucial, with greater benefits observed when implemented in early disease stages. Morning exercise sessions may optimize cognitive benefits by aligning with circadian rhythms and enhancing BDNF response (Lopez-Ortiz et al., 2021). Progressive exercise programs starting with low-intensity activities (40–50% of maximum heart rate) and gradually increasing to moderate intensity (60–70%) show better adherence and sustained cognitive benefits in Alzheimer’s patients.
In Parkinson’s disease, specific exercise modalities target distinct pathophysiological mechanisms. High-intensity aerobic exercise (80–85% of maximum heart rate) performed 3 times weekly increases dopamine D2 receptor binding potential and enhances dopaminergic signaling in the basal ganglia (Bhalsing et al., 2018). This improvement in dopaminergic function translates to better motor control and cognitive flexibility. Treadmill training at speeds 10% above comfortable walking pace improves gait parameters and reduces fall risk, while resistance exercises targeting core and lower body muscles enhance postural stability (Oliveira de Carvalho et al., 2018).
Dual-task training combining motor activities with cognitive challenges (e.g., walking while performing verbal fluency tasks) shows particular promise in Parkinson’s disease. This approach simultaneously addresses both motor and non-motor symptoms by promoting neural plasticity in multiple brain regions. Studies demonstrate that 12 weeks of progressive dual-task training improves executive function by 25% and reduces motor freezing episodes by 40% compared to standard care (Bhalsing et al., 2018; Oliveira de Carvalho et al., 2018). Exercise prescription should be tailored to the disease stage, with emphasis on high-intensity aerobic training in the early stages and balance-focused activities in the advanced stages.
The spectrum of parkinsonian disorders extends beyond classical Parkinson’s disease to include atypical parkinsonian syndromes, also known as Parkinson-plus syndromes. Progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) represent distinct tauopathies within this category, characterized by more rapid progression and additional clinical features beyond typical parkinsonism (Steffen et al., 2014). In PSP, targeted exercise interventions focusing on balance, gait, and oculomotor control have demonstrated promise. Specifically, supervised resistance training programs (2–3 sessions/week, 60–70% of one-repetition maximum) can help maintain muscular strength and reduce fall risk (Steffen et al., 2014). For CBD, a multimodal approach combining aerobic exercise with cognitive tasks shows the potential to preserve functional independence. Early intervention with task-specific exercises targeting asymmetric motor symptoms may delay functional decline (Slade et al., 2019). However, exercise prescription in these conditions requires careful consideration of fall risk and autonomic dysfunction.
The beneficial effects of PA are becoming increasingly evident for lesser-researched neurodegenerative illnesses, with emerging evidence supporting its role in promoting cognitive plasticity across the lifespan and in various brain disorders (Santiago and Potashkin, 2023). Exercise can be beneficial in controlling the symptoms of frontotemporal dementia, a condition characterized by behavioral and linguistic abnormalities (Vizzi et al., 2020). Participating in physical exercise at an early stage can improve mood and cognitive abilities, which may help to delay the advancement of the disease (Vizzi et al., 2020). Studies indicate that engaging in aerobic exercise results in enhancements in emotional well-being, a vital aspect for those with frontotemporal dementia (Vizzi et al., 2020).
Structured exercise regimens can enhance resilience in both the physical and cognitive areas of individuals with Huntington’s disease, a condition marked by motor problems and cognitive deterioration (Mueller et al., 2019). Engaging in regular PA enables individuals to preserve their functional autonomy and enhances their overall state of well-being. Individuals with Huntington’s disease who engage in PA may see improvements in their coordination and cognitive functions, as they benefit from the protective effects of PA (Mueller et al., 2019).
Multiple sclerosis poses distinctive difficulties, yet engaging in PA can greatly enhance both physical and cognitive symptoms in patients with this disease. Research reported that engaging in regular PA can improve the speed at which the brain processes information, as well as enhance attention and memory (Halabchi et al., 2017). These cognitive functions are commonly affected in individuals with multiple sclerosis. Customized exercise programs can enhance mobility and alleviate fatigue, offering a complete approach to controlling the illness (Du et al., 2024; Ozkul et al., 2020). Moreover, aerobic exercises have been shown to have antidepressant effects and improve psychosocial functioning in multiple sclerosis patients by promoting beneficial neurobiological changes (Revelo Herrera and Leon-Rojas, 2024).
Regular PA can aid in the rehabilitation and maintenance of cognitive function in individuals with vascular dementia, a condition that frequently occurs after cerebrovascular events. This is consistent with findings showing that exercise can provide practical benefits beginning early in life, and continuing throughout the lifespan (Zhou et al., 2022). Participating in PA is linked to improved vascular health, which can reduce the likelihood of additional cognitive deterioration. Moreover, participating in PAs can have a positive impact on the quality of life and cognitive function of individuals with this illness (Zhou et al., 2022).
Importantly, the efficacy of exercise interventions may vary depending on factors such as the specific neurodegenerative disorder, the stage of the disease, and individual patient characteristics. Future research should focus on developing personalized exercise prescriptions that consider these factors, as well as investigating the potential synergistic effects of combining exercise with other interventions, such as cognitive training or pharmacological treatments (Bherer et al., 2021; Castellote-Caballero et al., 2024). Other promising approaches could be considered as performing mixed non-pharmacological interventions such as exercise and fasting (Cherif et al., 2016). Indeed, the separated effects of these interventions on neuroplasticity could be potentiated, but this has to be investigated from an outcome and safety perspective.
The integration of artificial intelligence (AI) technologies in neurodegenerative disorders has evolved significantly to address condition-specific challenges through real-time monitoring, adaptive interventions, and predictive modeling. This section explores how these technologies are revolutionizing the development of personalized physical and cognitive rehabilitation programs for individuals with neurodegenerative diseases (Chudzik et al., 2024; Dergaa and Chamari, 2024).
Machine learning algorithms analyze extensive datasets encompassing patient demographics, medical history, and treatment outcomes to develop personalized interventions (Chudzik et al., 2024). These algorithms have achieved 85% accuracy in predicting cognitive decline trajectories in Alzheimer’s disease when combining neuroimaging data with exercise performance metrics (Yang, 2022). In Parkinson’s disease, AI models have demonstrated 78% accuracy in predicting freezing of gait episodes during exercise, enabling proactive adjustment of training programs (Alowais et al., 2023; Table 2).
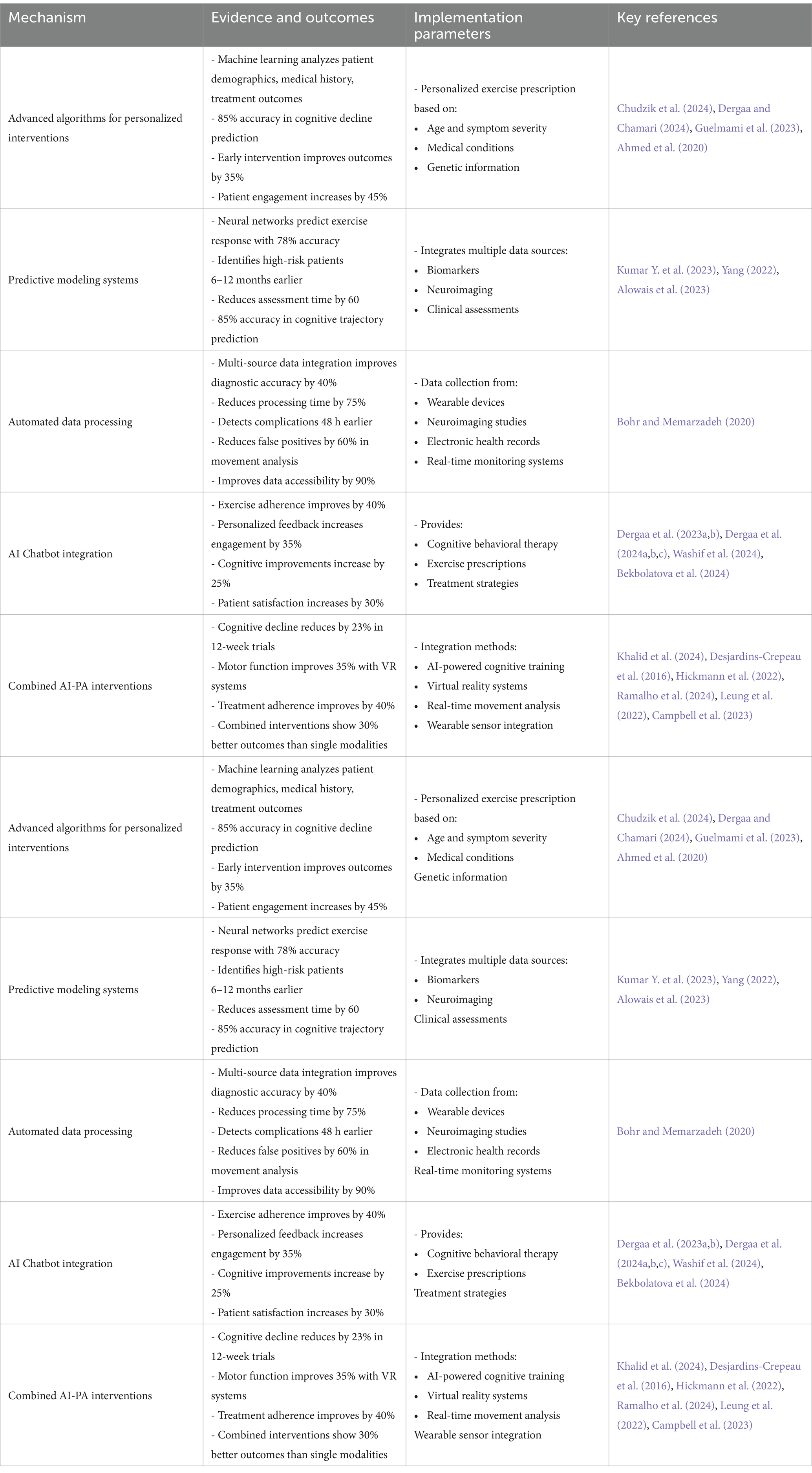
Table 2. Integration of artificial intelligence (AI) in cognitive rehabilitation: evidence-based outcomes and implementation parameters.
AI significantly enhances the efficiency and accuracy of data collection and analysis in neuroplasticity research, facilitating the integration of information across disciplines to promote translational opportunities (Bohr and Memarzadeh, 2020). Automated systems collect data from diverse sources, such as wearable devices, neuroimaging research, and electronic health records. This extensive data compilation offers a more distinct representation of a patient’s well-being, lessening the burden on researchers and doctors (Bohr and Memarzadeh, 2020).
AI-powered analytics enhance the precision and effectiveness of data analysis. Utilizing sophisticated statistical techniques and machine learning algorithms, researchers can efficiently detect patterns in data relevant to neuroplasticity. This enhanced efficiency can expedite the research process and facilitate the rapid translation of findings into clinical practice (Bohr and Memarzadeh, 2020; Table 2).
Chatbots are being used more and more in different areas, such as clinical and medical environments, to assist with therapeutic interventions (Dergaa et al., 2023a). Although they have the potential to provide advantages, it is essential to acknowledge their limits, especially in the context of clinical reasoning and decision-making (Dergaa et al., 2024b; Dergaa et al., 2023b; Dergaa et al., 2024c; Washif et al., 2024). It is important to acknowledge that while AI chatbots and language models offer potential benefits in cognitive rehabilitation, they should be viewed as complementary tools, or co-intelligence, rather than replacements for human expertise. The integration of AI in healthcare requires careful consideration of ethical implications and potential limitations (Bekbolatova et al., 2024).
In early-stage Alzheimer’s disease, AI chatbots provide structured cognitive exercises integrated with physical activity prompts, showing a 40% improvement in exercise adherence compared to standard care (Dergaa et al., 2024c). For Parkinson’s disease patients, chatbot-guided dual-task training combining cognitive challenges with motor exercises has demonstrated enhanced outcomes in both domains (Washif et al., 2024), helping them manage anxiety or sadness. Additionally, chatbots can offer personalized exercise prescriptions to address specific cognitive impairments (Dergaa et al., 2024c, Washif et al., 2024). Through the examination of specific patient profiles, AI tools could propose suitable physical exercises that are by a person’s cognitive capacities and requirements (Dergaa et al., 2024a; Dergaa et al., 2023b).
While chatbots are unable to substitute the knowledge and skills of experienced professionals, they can provide valuable assistance that enhances current treatment strategies (Dergaa et al., 2024a; Dergaa et al., 2024b; Dergaa et al., 2023b; Dergaa et al., 2024c; Washif et al., 2024). This integration can enhance health outcomes for those grappling with the difficulties of neurodegenerative illnesses (Table 2).
Initiatives have demonstrated the potential of integrating AI-based interventions with PA programs to address cognitive impairment in neurodegenerative disorders (Khalid et al., 2024). A 12-week randomized controlled trial in Alzheimer’s disease patients (n = 120) combined AI-guided exercise prescription with cognitive training, resulting in significantly slower cognitive decline (23% reduction) compared to standard exercise programs (Campbell et al., 2023). In Parkinson’s disease, virtual reality-based exercise systems using AI for real-time movement analysis improved motor function by 35% compared to conventional physiotherapy (Ramalho et al., 2024). For instance, a pilot study combined AI-powered cognitive training with organized PAs for older individuals at risk of dementia, offering personalized interventions based on individual capabilities. The training yielded substantial enhancements in both physical fitness and cognitive performance (Desjardins-Crepeau et al., 2016). A significant number of participants expressed increased motivation and involvement, suggesting that the integration of PA and cognitive training can augment the overall efficacy of the treatment. Another study emphasized the significance of personalization and continuous support to sustain patient involvement (Hickmann et al., 2022).
Additional research supports these conclusions. An example is a program that utilized virtual reality and AI to encourage PA in elderly individuals. This program showed that participants not only enhanced their physical well-being but also gained cognitive advantages (Ramalho et al., 2024). Studies have demonstrated that using AI technologies with fitness routines might enhance compliance and yield better results for persons experiencing cognitive decline (Leung et al., 2022).
In addition, a research investigation examining the amalgamation of AI-powered cognitive training and PA in adults with mild cognitive impairment discovered that this combined strategy led to improved cognitive performance in comparison to each intervention separately (Campbell et al., 2023; Table 2).
The applications discussed in the previous subsections of “Integration of AI, machine learning, and language models in cognitive rehabilitation” have the potential to become fully automated and interconnected through the integration of the IoT. The latter refers to the network of physical devices, sensors, and everyday objects embedded with electronics, software, and connectivity, enabling data exchange and communication (Al-Kahtani et al., 2022). In the context of personalized exercise interventions for neurodegenerative disorders, IoT technologies can facilitate the seamless collection and transmission of real-time data from wearable devices, smart home sensors, and other connected gadgets. This data can incorporate various parameters, such as PA levels, sleep patterns, and vital signs, providing a comprehensive view of the patient’s health and response to prescribed exercises. While the integration of IoT with AI, machine learning, and language models for exercise prescription is an emerging area of research, the potential benefits are hugely promising.
An envisioned application involves the continuous monitoring of patients’ PA patterns and vital signs through wearable devices. Machine learning models would automatically analyze the data collected from these IoT devices. Based on the analysis, the models could recommend adjustments to the prescribed exercise program. These recommendations would be communicated to the patient through a conversational AI assistant powered by a large language model. The large language model-based AI assistant would provide personalized guidance, motivation, and feedback, thereby fostering better adherence and engagement with the tailored exercise regimen. While the full realization of this integrated system is still a topic of ongoing research and development, its potential to revolutionize personalized exercise interventions for neurodegenerative disorders is undeniable.
Incorporating PA into rehabilitation protocols presents a promising avenue for enhancing neuroplasticity and cognitive function in individuals with neurodegenerative disorders. The evidence reviewed suggests that regular PA can positively modify neurogenesis, synaptic plasticity, and neuronal proliferation, leading to significant improvements in memory and executive function. Engaging in regular PA enhances the brain’s ability to adapt and triggers the production of crucial neurotrophic factors. This empowers patients to actively engage in their rehabilitation and promotes overall physical and mental well-being. The interdependence between movement and cognitive processes, as conceptualized by various authors, highlights the profound impact of motor activity on brain function and structure.
In this review, we emphasized the importance of integrating non-pharmacological personalized PA regimens into cognitive rehabilitation protocols. The integration of PA along with cognitive training, potentially augmented by AI technologies, has promising results in enhancing both physical fitness and cognitive performance. Healthcare practitioners should tailor these programs to correspond with the individual requirements and capabilities of patients, guaranteeing involvement and efficacy of outcomes. Future research should focus on elucidating the optimal timing, duration, sequence, and type of cognitive engagement that interacts positively with exercise to promote cognitive and brain health. Furthermore, continuous training for professionals is needed to effectively apply these treatments. In this regard, while AI solutions offer potential benefits in personalizing exercise routines and monitoring progress, it is crucial to maintain a primary emphasis on the intrinsic advantages of PA. The development of AI in healthcare requires careful consideration of ethical implications and potential limitations. Future studies focusing on tangible applications of PA interventions may significantly enhance our ability to manage and mitigate cognitive decline. Exploring modalities that boost cognitive function across diverse patient populations could help individuals regain their standard of living and perform daily tasks more efficiently. Incorporating larger sample sizes would enable investigations to delve into how multiple genes and associated proteins moderate the impact of PA on cognition and brain function. Ultimately, combining PA, cognitive rehabilitation, and emerging technologies presents a multifaceted approach to addressing neurodegenerative disorders in a non-pharmacological way. Synergistically utilizing these elements could improve the quality of life for those affected by cognitive impairment and facilitate prevention and more personalized, effective interventions in neurodegenerative disease management.
LBE: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. WD: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. ID: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. HC: Supervision, Writing – original draft, Writing – review & editing. NG: Conceptualization, Writing – original draft, Writing – review & editing. HB: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. KC: Formal analysis, Writing – original draft, Writing – review & editing. VS: Writing – original draft, Writing – review & editing. AEO: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
ID was employed by Primary Health Care Corporation. AEO was employed by Hamad Medical Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that Gen AI was used in the creation of this manuscript. The authors wish to disclose that artificial intelligence tools (i.e., ChatGPT 3.5) were utilized to enhance the clarity and coherence of the manuscript’ writing. The tool was utilized for language refinement purposes only, ensuring the text was clear and coherent without altering the scientific content or generating any new text.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
PA, physical activity; BDNF, brain-derived neurotrophic factor; AI, artificial intelligence; IoT, internet of things.
Abo, M., and Hamaguchi, T. (2024). Effectiveness of a dual-task intervention involving exercise and vocalized cognitive tasks. J. Clin. Med. 13:2962. doi: 10.3390/jcm13102962
Afonso, R. F., Kraft, I., Aratanha, M. A., and Kozasa, E. H. (2020). Neural correlates of meditation: a review of structural and functional MRI studies. Front. Biosc. 12, 92–115. doi: 10.2741/S542
Ahmed, Z., Mohamed, K., Zeeshan, S., and Dong, X. (2020). Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database 2020, 1–35. doi: 10.1093/database/baaa010
Al-Kahtani, M. S., Khan, F., and Taekeun, W. (2022). Application of internet of things and sensors in healthcare. Sensors (Basel) 22:5738. doi: 10.3390/s22155738
Alowais, S. A., Alghamdi, S. S., Alsuhebany, N., Alqahtani, T., Alshaya, A. I., Almohareb, S. N., et al. (2023). Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med. Educ. 23:689. doi: 10.1186/s12909-023-04698-z
Alzheimer’s Association (2022). Alzheimer’s disease facts and figures. Alzheimers Dement. 18, 700–789. doi: 10.1002/alz.12638
Appelbaum, L. G., Shenasa, M. A., Stolz, L., and Daskalakis, Z. (2023). Synaptic plasticity and mental health: methods, challenges and opportunities. Neuropsychopharmacology 48, 113–120. doi: 10.1038/s41386-022-01370-w
Bekbolatova, M., Mayer, J., Ong, C. W., and Toma, M. (2024). Transformative potential of AI in healthcare: definitions, applications, and navigating the ethical landscape and public perspectives. Healthcare (Basel) 12:125. doi: 10.3390/healthcare12020125
Belcher, B. R., Zink, J., Azad, A., Campbell, C. E., Chakravartti, S. P., and Herting, M. M. (2021). The roles of physical activity, exercise, and fitness in promoting resilience during adolescence: effects on mental well-being and brain development. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6, 225–237. doi: 10.1016/j.bpsc.2020.08.005
Berry, A. S., Shah, V. D., and Jagust, W. J. (2018). The influence of dopamine on cognitive flexibility is mediated by functional connectivity in young but not older adults. J. Cogn. Neurosci. 30, 1330–1344. doi: 10.1162/jocn_a_01286
Bhalsing, K. S., Abbas, M. M., and Tan, L. C. S. (2018). Role of physical activity in Parkinson's disease. Ann. Indian Acad. Neurol. 21, 242–249. doi: 10.4103/aian.AIAN_169_18
Bherer, L., Gagnon, C., Langeard, A., Lussier, M., Desjardins-Crepeau, L., Berryman, N., et al. (2021). Synergistic effects of cognitive training and physical exercise on dual-task performance in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 76, 1533–1541. doi: 10.1093/geronb/gbaa124
Bliss, T. V., and Cooke, S. F. (2011). Long-term potentiation and long-term depression: a clinical perspective. Clinics 66, 3–17. doi: 10.1590/s1807-59322011001300002
Blomstrand, P., Tesan, D., Nylander, E. M., and Ramstrand, N. (2023). Mind body exercise improves cognitive function more than aerobic-and resistance exercise in healthy adults aged 55 years and older - an umbrella review. Eur. Rev. Aging Phys. Act. 20:15. doi: 10.1186/s11556-023-00325-4
Bohr, A., and Memarzadeh, K. (2020). “The rise of artificial intelligence in healthcare applications” in Artificial intelligence in healthcare. eds. A. Bohr and K. Memarzadeh. (Amsterdam, The Netherlands: Academic Press), 25–60.
Campbell, E. B., Delgadillo, M., Lazzeroni, L. C., Louras, P. N., Myers, J., Yesavage, J., et al. (2023). Cognitive improvement following physical exercise and cognitive training intervention for older adults with MCI. J. Gerontol. A Biol. Sci. Med. Sci. 78, 554–560. doi: 10.1093/gerona/glac189
Castellote-Caballero, Y., Carcelen Fraile, M. D. C., Aibar-Almazan, A., Afanador-Restrepo, D. F., and Gonzalez-Martin, A. M. (2024). Effect of combined physical-cognitive training on the functional and cognitive capacity of older people with mild cognitive impairment: a randomized controlled trial. BMC Med. 22:281. doi: 10.1186/s12916-024-03469-x
Chatterjee, D., Hegde, S., and Thaut, M. (2021). Neural plasticity: the substratum of music-based interventions in neurorehabilitation. Neuro Rehabilitation 48, 155–166. doi: 10.3233/NRE-208011
Chen, X., Guo, C., and Kong, J. (2012). Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 7, 376–385. doi: 10.3969/j.issn.1673-5374.2012.05.009
Cherif, A., Roelands, B., Meeusen, R., and Chamari, K. (2016). Effects of intermittent fasting, caloric restriction, and Ramadan intermittent fasting on cognitive performance at rest and during exercise in adults. Sports Med. 46, 35–47. doi: 10.1007/s40279-015-0408-6
Chudzik, A., Sledzianowski, A., and Przybyszewski, A. W. (2024). Machine learning and digital biomarkers can detect early stages of neurodegenerative diseases. Sensors (Basel) 24:1572. doi: 10.3390/s24051572
Clemente-Suarez, V. J., Bustamante-Sanchez, A., Mielgo-Ayuso, J., Martinez-Guardado, I., Martin-Rodriguez, A., and Tornero-Aguilera, J. F. (2023). Antioxidants and sports performance. Nutrients 15:2371. doi: 10.3390/nu15102371
Cotman, C. W., and Berchtold, N. C. (2002). Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 25, 295–301. doi: 10.1016/s0166-2236(02)02143-4
Cramer, S. C., Sur, M., Dobkin, B. H., O'Brien, C., Sanger, T. D., Trojanowski, J. Q., et al. (2011). Harnessing neuroplasticity for clinical applications. Brain 134, 1591–1609. doi: 10.1093/brain/awr039
Cuestas Torres, D. M., and Cardenas, F. P. (2020). Synaptic plasticity in Alzheimer’s disease and healthy aging. Rev. Neurosci. 31, 245–268. doi: 10.1515/revneuro-2019-0058
Cutuli, D., Landolfo, E., Petrosini, L., and Gelfo, F. (2022). Environmental enrichment effects on the brain-derived neurotrophic factor expression in healthy condition, Alzheimer’s disease, and other neurodegenerative disorders. J. Alzheimers Dis. 85, 975–992. doi: 10.3233/JAD-215193
de Sousa Fernandes, M. S., Ordonio, T. F., Santos, G. C. J., Santos, L. E. R., Calazans, C. T., Gomes, D. A., et al. (2020). Effects of physical exercise on neuroplasticity and brain function: a systematic review in human and animal studies. Neural Plast. 2020:8856621. doi: 10.1155/2020/8856621
Dergaa, I., Ben Saad, H., Ghouili, H., Glenn, J. M., El Omri, A., and Slim, I. (2024a). Evaluating the applicability and appropriateness of chat GPT as a source for tailored nutrition advice: a multi-scenario study. New Asian J. Med. 2, 1–16. doi: 10.61838/kman.najm.2.1.1
Dergaa, I., Ben Saad, H., Glenn, J. M., Amamou, B., Ben Aissa, M., Guelmami, N., et al. (2024b). From tools to threats: a reflection on the impact of artificial-intelligence chatbots on cognitive health. Front. Psychol. 15:1259845. doi: 10.3389/fpsyg.2024.1259845
Dergaa, I., and Chamari, K. (2024). Big data in sports medicine and exercise science: integrating theory and practice for future innovations. Tunisian J. Sports Sci. Med. 2, 1–13. doi: 10.61838/kman.tjssm.2.1.1
Dergaa, I., Fekih-Romdhane, F., Glenn, J. M., Saifeddin Fessi, M., Chamari, K., and Dhahbi, W. (2023a). Moving beyond the stigma: understanding and overcoming the resistance to the acceptance and adoption of artificial intelligence Chatbots. New Asian J. Med. 1, 29–36. doi: 10.61838/kman.najm.1.2.4
Dergaa, I., Fekih-Romdhane, F., Hallit, S., Loch, A. A., Glenn, J. M., Fessi, M. S., et al. (2023b). Chat GPT is not ready yet for use in providing mental health assessment and interventions. Front. Psych. 14:1277756. doi: 10.3389/fpsyt.2023.1277756
Dergaa, I., Saad, H. B., El Omri, A., Glenn, J. M., Clark, C. C. T., Washif, J. A., et al. (2024c). Using artificial intelligence for exercise prescription in personalised health promotion: a critical evaluation of OpenAI's GPT-4 model. Biol. Sport 41, 221–241. doi: 10.5114/biolsport.2024.133661
Desjardins-Crepeau, L., Berryman, N., Fraser, S. A., Vu, T. T., Kergoat, M. J., Li, K. Z., et al. (2016). Effects of combined physical and cognitive training on fitness and neuropsychological outcomes in healthy older adults. Clin. Interv. Aging 11, 1287–1299. doi: 10.2147/cia.s115711
Du, L., Xi, H., Zhang, S., Zhou, Y., Tao, X., Lv, Y., et al. (2024). Effects of exercise in people with multiple sclerosis: a systematic review and meta-analysis. Front. Public Health 12:1387658. doi: 10.3389/fpubh.2024.1387658
Erickson, K. I., Hillman, C., Stillman, C. M., Ballard, R. M., Bloodgood, B., Conroy, D. E., et al. (2019). Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity Guidelines. Med. Sci. Sports Exerc. 51, 1242–1251. doi: 10.1249/MSS.0000000000001936
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 108, 3017–3022. doi: 10.1073/pnas.1015950108
Ferrer-Uris, B., Ramos, M. A., Busquets, A., and Angulo-Barroso, R. (2022). Can exercise shape your brain? A review of aerobic exercise effects on cognitive function and neuro-physiological underpinning mechanisms. AIMS Neurosci 9, 150–174. doi: 10.3934/Neuroscience.2022009
Ghazel, N., Souissi, A., Salhi, I., Dergaa, I., Martins-Costa, H. C., and Musa, S. (2022). Effects of eight weeks of mat pilates training on selected hematological parameters and plasma volume variations in healthy active women. PLoS One 17:e0267437. doi: 10.1371/journal.pone.0267437
Gomez-Pinilla, F., and Hillman, C. (2013). The influence of exercise on cognitive abilities. Compr. Physiol. 3, 403–428. doi: 10.1002/cphy.c110063
Gothe, N. P., Khan, I., Hayes, J., Erlenbach, E., and Damoiseaux, J. S. (2019). Yoga effects on brain health: a systematic review of the current literature. Brain Plast 5, 105–122. doi: 10.3233/BPL-190084
Guelmami, N., Fekih-Romdhane, F., Mechraoui, O., and Bragazzi, N. L. (2023). Injury prevention, optimized training and rehabilitation: how is AI reshaping the field of sports medicine. New Asian J. Med. 1, 30–34. doi: 10.61838/kman.najm.1.1.6
Halabchi, F., Alizadeh, Z., Sahraian, M. A., and Abolhasani, M. (2017). Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 17:185. doi: 10.1186/s12883-017-0960-9
Hickmann, E., Richter, P., and Schlieter, H. (2022). All together now - patient engagement, patient empowerment, and associated terms in personal healthcare. BMC Health Serv. Res. 22:1116. doi: 10.1186/s12913-022-08501-5
Hong, S. Y., Jeong, W. M., and Rhyu, H. S. (2023). Effects of multimodal cognitive exercise program on cognitive function, bone density, blood lipid, fitness, and depression in old women with mild cognitive impairment. J. Exerc. Rehabil. 19, 27–34. doi: 10.12965/jer.2244514.257
Hooks, B. M., and Chen, C. (2020). Circuitry underlying experience-dependent plasticity in the mouse visual system. Neuron 106, 21–36. doi: 10.1016/j.neuron.2020.01.031
Hussain, G., Akram, R., Anwar, H., Sajid, F., Iman, T., and Han, H. S. (2024). Adult neurogenesis: a real hope or a delusion? Neural Regen. Res. 19, 6–15. doi: 10.4103/1673-5374.375317
Hwang, E., Portillo, B., Grose, K., Fujikawa, T., and Williams, K. W. (2023). Exercise-induced hypothalamic neuroplasticity: implications for energy and glucose metabolism. Mol. Metabolism 73:101745. doi: 10.1016/j.molmet.2023.101745
Jahn, H. (2013). Memory loss in Alzheimer's disease. Dialogues Clin. Neurosci. 15, 445–454. doi: 10.31887/DCNS.2013.15.4/hjahn
Jardim, N. Y. V., Bento-Torres, N. V. O., Costa, V. O., Carvalho, J. P. R., Pontes, H. T. S., Tomás, A. M., et al. (2021). Dual-task exercise to improve cognition and functional capacity of healthy older adults. Front. Aging Neurosci. 13:589299. doi: 10.3389/fnagi.2021.589299
Jodeiri Farshbaf, M., and Alvina, K. (2021). Multiple roles in neuroprotection for the exercise derived Myokine Irisin. Front. Aging Neurosci. 13:649929. doi: 10.3389/fnagi.2021.649929
Karamacoska, D., Butt, A., Leung, I. H. K., Childs, R. L., Metri, N. J., Uruthiran, V., et al. (2023). Brain function effects of exercise interventions for cognitive decline: a systematic review and meta-analysis. Front. Neurosci. 17:1127065. doi: 10.3389/fnins.2023.1127065
Kennedy, M. B. (2013). Synaptic signaling in learning and memory. Cold Spring Harb. Perspect. Biol. 8:a016824. doi: 10.1101/cshperspect.a016824
Khalid, U. B., Naeem, M., Stasolla, F., Syed, M. H., Abbas, M., and Coronato, A. (2024). Impact of AI-powered solutions in rehabilitation process: recent improvements and future trends. Int J Gen Med 17, 943–969. doi: 10.2147/IJGM.S453903
Kowianski, P., Lietzau, G., Czuba, E., Waskow, M., Steliga, A., and Morys, J. (2018). BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 38, 579–593. doi: 10.1007/s10571-017-0510-4
Kumar, Y., Koul, A., Singla, R., and Ijaz, M. F. (2023). Artificial intelligence in disease diagnosis: a systematic literature review, synthesizing framework and future research agenda. J. Ambient. Intell. Humaniz. Comput. 14, 8459–8486. doi: 10.1007/s12652-021-03612-z
Kumar, J., Patel, T., Sugandh, F., Dev, J., Kumar, U., Adeeb, M., et al. (2023). Innovative approaches and therapies to enhance neuroplasticity and promote recovery in patients with neurological disorders: a narrative review. Cureus 15:e41914. doi: 10.7759/cureus.41914
Lamptey, R. N. L., Chaulagain, B., Trivedi, R., Gothwal, A., Layek, B., and Singh, J. (2022). A review of the common neurodegenerative disorders: current therapeutic approaches and the potential role of Nanotherapeutics. Int. J. Mol. Sci. 23:1851. doi: 10.3390/ijms23031851
Lardone, A., Liparoti, M., Sorrentino, P., Rucco, R., Jacini, F., Polverino, A., et al. (2018). Mindfulness meditation is related to long-lasting changes in hippocampal functional topology during resting state: a magnetoencephalography study. Neural Plast. 2018, 5340717–5340719. doi: 10.1155/2018/5340717
Latino, F., and Tafuri, F. (2024). Physical activity and cognitive functioning. Medicina 60:216. doi: 10.3390/medicina60020216
Lee, B., Shin, M., Park, Y., Won, S. Y., and Cho, K. S. (2021). Physical exercise-induced Myokines in neurodegenerative diseases. Int. J. Mol. Sci. 22:5795. doi: 10.3390/ijms22115795
Leung, C., Wong, K. C., So, W. W. Y., Tse, Z. C. K., Li, D., and Cao, Y. (2022). The application of technology to improve cognition in older adults: a review and suggestions for future directions. Psych J 11, 583–599. doi: 10.1002/pchj.565
Levy, M. J. F., Boulle, F., Steinbusch, H. W., van den Hove, D. L. A., Kenis, G., and Lanfumey, L. (2018). Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 235, 2195–2220. doi: 10.1007/s00213-018-4950-4
Lin, T. W., Tsai, S. F., and Kuo, Y. M. (2018). Physical exercise enhances neuroplasticity and delays Alzheimer's disease. Brain Plast 4, 95–110. doi: 10.3233/BPL-180073
Liu, C., Su, M., Jiao, Y., Ji, Y., and Zhu, S. (2021). Effects of dance interventions on cognition, psycho-behavioral symptoms, motor functions, and quality of life in older adult patients with mild cognitive impairment: a Meta-analysis and systematic review. Front. Aging Neurosci. 13:706609. doi: 10.3389/fnagi.2021.706609
Liu, Y., Yan, T., Chu, J. M., Chen, Y., Dunnett, S., Ho, Y. S., et al. (2019). The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab. Investig. 99, 943–957. doi: 10.1038/s41374-019-0232-y
Liu-Ambrose, T., Nagamatsu, L. S., Graf, P., Beattie, B. L., Ashe, M. C., and Handy, T. C. (2010). Resistance training and executive functions: a 12-month randomized controlled trial. Arch. Intern. Med. 170, 170–178. doi: 10.1001/archinternmed.2009.494
Lopez-Ortiz, S., Pinto-Fraga, J., Valenzuela, P. L., Martin-Hernandez, J., Seisdedos, M. M., Garcia-Lopez, O., et al. (2021). Physical exercise and Alzheimer's disease: effects on pathophysiological molecular pathways of the disease. Int. J. Mol. Sci. 22:2897. doi: 10.3390/ijms22062897
Luo, G., Zhang, J., Song, Z., Wang, Y., Wang, X., Qu, H., et al. (2023). Effectiveness of non-pharmacological therapies on cognitive function in patients with dementia-a network meta-analysis of randomized controlled trials. Front. Aging Neurosci. 15:1131744. doi: 10.3389/fnagi.2023.1131744
Madhivanan, P., Krupp, K., Waechter, R., and Shidhaye, R. (2021). Yoga for healthy aging: science or hype? Adv Geriatr Med Res 3:e210016. doi: 10.20900/agmr20210016
Maeneja, R., Silva, C. R., Ferreira, I. S., and Abreu, A. M. (2023). Aerobic physical exercise versus dual-task cognitive walking in cognitive rehabilitation of people with stroke: a randomized clinical trial. Front. Psychol. 14:1258262. doi: 10.3389/fpsyg.2023.1258262
Malkowska, P., and Sawczuk, M. (2023). Cytokines as biomarkers for evaluating physical exercise in trained and non-trained individuals: a narrative review. Int. J. Mol. Sci. 24:11156. doi: 10.3390/ijms241311156
Mandolesi, L., Polverino, A., Montuori, S., Foti, F., Ferraioli, G., Sorrentino, P., et al. (2018). Effects of physical exercise on cognitive functioning and wellbeing: biological and psychological benefits. Front. Psychol. 9:509. doi: 10.3389/fpsyg.2018.00509
Martín-Rodríguez, A., Gostian-Ropotin, L. A., Beltrán-Velasco, A. I., Belando-Pedreño, N., Simón, J. A., López-Mora, C., et al. (2024). Sporting mind: the interplay of physical activity and psychological health. Sports 12:37. doi: 10.3390/sports12010037
Marzola, P., Melzer, T., Pavesi, E., Gil-Mohapel, J., and Brocardo, P. S. (2023). Exploring the role of neuroplasticity in development, aging, and neurodegeneration. Brain Sci. 13:1610. doi: 10.3390/brainsci13121610
Mira, R. G., Lira, M., and Cerpa, W. (2021). Traumatic brain injury: mechanisms of glial response. Front. Physiol. 12:740939. doi: 10.3389/fphys.2021.740939
Montero-Almagro, G., Bernal-Utrera, C., Geribaldi-Doldán, N., Nunez-Abades, P., Castro, C., and Rodriguez-Blanco, C. (2024). Influence of high-intensity interval training on neuroplasticity markers in post-stroke patients: systematic review. J. Clin. Med. 13:1985. doi: 10.3390/jcm13071985
Mueller, S. M., Petersen, J. A., and Jung, H. H. (2019). Exercise in Huntington's disease: current state and clinical significance. Tremor Other Hyperkinet Mov. 9:601. doi: 10.5334/tohm.515
Nicastri, C. M., McFeeley, B. M., Simon, S. S., Ledreux, A., Hakansson, K., Granholm, A. C., et al. (2022). BDNF mediates improvement in cognitive performance after computerized cognitive training in healthy older adults. Alzheimers Dement. 8:e12337. doi: 10.1002/trc2.12337
Oliveira de Carvalho, A., Filho, A. S. S., Murillo-Rodriguez, E., Rocha, N. B., Carta, M. G., and Machado, S. (2018). Physical exercise for Parkinson's disease: clinical and experimental evidence. Clin. Pract. Epidemiol. Ment. Health 14, 89–98. doi: 10.2174/1745017901814010089
Ozkul, C., Guclu-Gunduz, A., Eldemir, K., Apaydin, Y., Yazici, G., and Irkec, C. (2020). Combined exercise training improves cognitive functions in multiple sclerosis patients with cognitive impairment: a single-blinded randomized controlled trial. Mult. Scler. Relat. Disord. 45:102419. doi: 10.1016/j.msard.2020.102419
Prakash, R. S., Voss, M. W., Erickson, K. I., and Kramer, A. F. (2015). Physical activity and cognitive vitality. Annu. Rev. Psychol. 66, 769–797. doi: 10.1146/annurev-psych-010814-015249
Ramalho, A., Duarte-Mendes, P., Paulo, R., Serrano, J., and Petrica, J. (2024). Crossing the digital frontier: are older adults ready for virtual reality workouts? Front. Public Health 12:1324004. doi: 10.3389/fpubh.2024.1324004
Revelo Herrera, S. G., and Leon-Rojas, J. E. (2024). The effect of aerobic exercise in neuroplasticity, learning, and cognition: a systematic review. Cureus 16:e54021. doi: 10.7759/cureus.54021
Rodriguez-Gutierrez, E., Torres-Costoso, A., Pascual-Morena, C., Pozuelo-Carrascosa, D. P., Garrido-Miguel, M., and Martinez-Vizcaino, V. (2023). Effects of resistance exercise on neuroprotective factors in middle and late life: a systematic review and meta-analysis. Aging Dis. 14, 1264–1275. doi: 10.14336/AD.2022.1207
Santiago, J. A., and Potashkin, J. A. (2023). Physical activity and lifestyle modifications in the treatment of neurodegenerative diseases. Front. Aging Neurosci. 15:1185671. doi: 10.3389/fnagi.2023.1185671
Seo, D. Y., Heo, J. W., Ko, J. R., and Kwak, H. B. (2019). Exercise and Neuroinflammation in health and disease. Int. Neurourol. J. 23, S82–S92. doi: 10.5213/inj.1938214.107
Shen, Y. Q., Zhou, H. X., Chen, X., Castellanos, F. X., and Yan, C. G. (2020). Meditation effect in changing functional integrations across large-scale brain networks: preliminary evidence from a meta-analysis of seed-based functional connectivity. J. Pac. Rim Psychol. 14:e10. doi: 10.1017/prp.2020.1
Sienes Bailo, P., Llorente Martin, E., Calmarza, P., Montolio Breva, S., Bravo Gomez, A., Pozo Giraldez, A., et al. (2022). The role of oxidative stress in neurodegenerative diseases and potential antioxidant therapies. Adv. Lab. Med. 3, 342–350. doi: 10.1515/almed-2022-0111
Slade, S. C., Underwood, M., McGinley, J. L., and Morris, M. E. (2019). Exercise and progressive Supranuclear palsy: the need for explicit exercise reporting. BMC Neurol. 19:305. doi: 10.1186/s12883-019-1539-4
Sleiman, S. F., Henry, J., Al-Haddad, R., El Hayek, L., Abou Haidar, E., Stringer, T., et al. (2016). Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife 5:e15092. doi: 10.7554/eLife.15092
Steffen, T. M., Boeve, B. F., Petersen, C. M., Dvorak, L., and Kantarci, K. (2014). Long-term exercise training for an individual with mixed corticobasal degeneration and progressive supranuclear palsy features: 10-year case report follow-up. Phys. Ther. 94, 289–296. doi: 10.2522/ptj.20130052
Surget, A., and Belzung, C. (2022). Adult hippocampal neurogenesis shapes adaptation and improves stress response: a mechanistic and integrative perspective. Mol. Psychiatry 27, 403–421. doi: 10.1038/s41380-021-01136-8
Valizadeh, A., Moassefi, M., Nakhostin-Ansari, A., Hosseini Asl, S. H., Saghab Torbati, M., Aghajani, R., et al. (2022). Abstract screening using the automated tool Rayyan: results of effectiveness in three diagnostic test accuracy systematic reviews. BMC Med. Res. Methodol. 22:160. doi: 10.1186/s12874-022-01631-8
Vecchio, L. M., Meng, Y., Xhima, K., Lipsman, N., Hamani, C., and Aubert, I. (2018). The neuroprotective effects of exercise: maintaining a healthy brain throughout aging. Brain Plast 4, 17–52. doi: 10.3233/BPL-180069
Villemure, C., Ceko, M., Cotton, V. A., and Bushnell, M. C. (2015). Neuroprotective effects of yoga practice: age-, experience-, and frequency-dependent plasticity. Front. Hum. Neurosci. 9:281. doi: 10.3389/fnhum.2015.00281
Vizzi, L., Padua, E., D'Amico, A. G., Tancredi, V., D'Arcangelo, G., Cariati, I., et al. (2020). Beneficial effects of physical activity on subjects with neurodegenerative disease. J. Funct. Morphol. Kinesiol. 5:94. doi: 10.3390/jfmk5040094
Voss, S., Cerna, J., and Gothe, N. P. (2023). Yoga impacts cognitive health: neurophysiological changes and stress regulation mechanisms. Exerc. Sport Sci. Rev. 51, 73–81. doi: 10.1249/JES.0000000000000311
Voss, M. W., Vivar, C., Kramer, A. F., and van Praag, H. (2013). Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 17, 525–544. doi: 10.1016/j.tics.2013.08.001
Waffenschmidt, S., Knelangen, M., Sieben, W., Buhn, S., and Pieper, D. (2019). Single screening versus conventional double screening for study selection in systematic reviews: a methodological systematic review. BMC Med. Res. Methodol. 19:132. doi: 10.1186/s12874-019-0782-0
Wang, L., Caras, M. L., Karayannis, T., and Froemke, R. C. (2021). Mechanisms underlying experience-dependent plasticity of cortical circuits. Front. Cell. Neurosci. 15:687297. doi: 10.3389/fncel.2021.687297
Wang, Y., Tian, J., and Yang, Q. (2023). Tai chi exercise improves working memory capacity and emotion regulation ability. Front. Psychol. 14:1047544. doi: 10.3389/fpsyg.2023.1047544
Washif, J. A., Pagaduan, J., James, C., Dergaa, I., and Beaven, C. M. (2024). Artificial intelligence in sport: exploring the potential of using ChatGPT in resistance training prescription. Biol. Sport 41, 209–220. doi: 10.5114/biolsport.2024.132987
Wollesen, B., Wildbredt, A., van Schooten, K. S., Lim, M. L., and Delbaere, K. (2020). The effects of cognitive-motor training interventions on executive functions in older people: a systematic review and meta-analysis. Eur. Rev. Aging Phys. Act. 17:9. doi: 10.1186/s11556-020-00240-y
Woods, B., Aguirre, E., Spector, A. E., and Orrell, M. (2012). Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst. Rev. 2012:CD005562. doi: 10.1002/14651858.CD005562.pub2
Wu, Y. C., Bogale, T. A., Koistinaho, J., Pizzi, M., Rolova, T., and Bellucci, A. (2024). The contribution of beta-amyloid, tau and alpha-synuclein to blood-brain barrier damage in neurodegenerative disorders. Acta Neuropathol. 147:39. doi: 10.1007/s00401-024-02696-z
Xiao, Y., Yang, T., and Shang, H. (2023). The impact of motor-cognitive dual-task training on physical and cognitive functions in Parkinson's disease. Brain Sci. 13:437. doi: 10.3390/brainsci13030437
Xie, A., Gao, J., Xu, L., and Meng, D. (2014). Shared mechanisms of neurodegeneration in Alzheimer's disease and Parkinson's disease. Biomed. Res. Int. 2014:648740. doi: 10.1155/2014/648740
Yang, C. C. (2022). Explainable artificial intelligence for predictive modeling in healthcare. J. Healthc. Inform. Res. 6, 228–239. doi: 10.1007/s41666-022-00114-1
Yasuda, R., Hayashi, Y., and Hell, J. W. (2022). CaMKII: a central molecular organizer of synaptic plasticity, learning and memory. Nat. Rev. Neurosci. 23, 666–682. doi: 10.1038/s41583-022-00624-2
Zhou, S., Chen, S., Liu, X., Zhang, Y., Zhao, M., and Li, W. (2022). Physical activity improves cognition and activities of daily living in adults with Alzheimer’s disease: a systematic review and Meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public Health 19:1216. doi: 10.3390/ijerph19031216
Zhou, Q., Chen, Y., Zhou, C., and Wang, J. (2024). Long-term motor training enhances functional connectivity between semantic and motor regions in an effector-specific manner: evidence from elite female football athletes. Brain Struct. Funct. 229, 1447–1459. doi: 10.1007/s00429-024-02808-1
Zotey, V., Andhale, A., Shegekar, T., and Juganavar, A. (2023). Adaptive neuroplasticity in brain injury recovery: strategies and insights. Cureus 15:e45873. doi: 10.7759/cureus.45873
Keywords: Alzheimer, cognitive rehabilitation, hippocampal volume, neurotrophic factors, oxidative stress, Parkinson, personalized interventions, synaptic connectivity
Citation: Ben Ezzdine L, Dhahbi W, Dergaa I, Ceylan Hİ, Guelmami N, Ben Saad H, Chamari K, Stefanica V and El Omri A (2025) Physical activity and neuroplasticity in neurodegenerative disorders: a comprehensive review of exercise interventions, cognitive training, and AI applications. Front. Neurosci. 19:1502417. doi: 10.3389/fnins.2025.1502417
Received: 26 September 2024; Accepted: 04 February 2025;
Published: 28 February 2025.
Edited by:
Yih-Kuen Jan, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Minhong Neenah Huang, Mayo Clinic, United StatesCopyright © 2025 Ben Ezzdine, Dhahbi, Dergaa, Ceylan, Guelmami, Ben Saad, Chamari, Stefanica and El Omri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Halil İbrahim Ceylan, aGFsaWwuaWJyYWhpbWNleWxhbjYwQGdtYWlsLmNvbQ==; Valentina Stefanica, dmFsZW50aW5hLnN0ZWZhbmljYUB1cGIucm8=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.