
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci., 07 January 2025
Sec. Neurodegeneration
Volume 18 - 2024 | https://doi.org/10.3389/fnins.2024.1513095
 Seyyed Sam Mehdi Hosseininasab1
Seyyed Sam Mehdi Hosseininasab1 Rasoul Ebrahimi2
Rasoul Ebrahimi2 Shirin Yaghoobpoor2
Shirin Yaghoobpoor2 Kiarash Kazemi3
Kiarash Kazemi3 Yaser Khakpour2
Yaser Khakpour2 Ramtin Hajibeygi3
Ramtin Hajibeygi3 Ashraf Mohamadkhani4
Ashraf Mohamadkhani4 Mobina Fathi2
Mobina Fathi2 Kimia Vakili2
Kimia Vakili2 Arian Tavasol2
Arian Tavasol2 Zohreh Tutunchian2
Zohreh Tutunchian2 Tara Fazel5
Tara Fazel5 Mohammad Fathi6*
Mohammad Fathi6* Mohammadreza Hajiesmaeili1*
Mohammadreza Hajiesmaeili1*Alzheimer’s Disease (AD) is the most prevalent type of dementia and is characterized by the presence of senile plaques and neurofibrillary tangles. There are various theories concerning the causes of AD, but the connection between viral and bacterial infections and their potential role in the pathogenesis of AD has become a fascinating area of research for the field. Various viruses such as Herpes simplex virus 1 (HSV-1), Epstein–Barr virus (EBV), Cytomegalovirus (CMV), influenza viruses, and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), as well as bacteria such as Chlamydia pneumoniae (CP), Helicobacter pylori (HP), Porphyromonas gingivalis (P. gingivalis), Spirochetes and eukaryotic unicellular parasites (e.g., Toxoplasma gondii), have been linked to AD due to their ability to activate the immune system, induce inflammation and increase oxidative stress, thereby leading to cognitive decline and AD. In addition, microRNAs (miRNAs) might play a crucial role in the pathogenesis mechanisms of these pathogens since they are utilized to target various protein-coding genes, allowing for immune evasion, maintaining latency, and suppressing cellular signaling molecules. Also, they can regulate gene expression in human cells. This article provides an overview of the association between AD and various infectious agents, with a focus on the mechanisms by which these pathogens may be related to the pathogenesis of AD. These findings suggest important areas for further research to be explored in future studies.
Alzheimer’s disease (AD), the most common type of dementia, especially among the elderly, is recognized as an inflammatory, chronic and progressive neurodegenerative disease (Agostini et al., 2019; Agostini et al., 2017; Bourgade et al., 2016). It is the most prevalent neurodegenerative disease globally, with current estimates of around 24 million affected individuals and projections indicating that this number may increase fourfold by 2050 (dos Santos Picanco et al., 2018). There is ample evidence that AD has been associated with various risk factors, including aging, genetic factors, infectious agents, and environmental factors. However, the underlying etiology of pathological alterations in AD is still not known (Breijyeh and Karaman, 2020).
Senile plaques composed of insoluble amyloid-β (Aβ) peptide and intraneuronal neurofibrillary tangles (NFTs) compopsed of tau protein are the two main pathological characteristics observed in AD brains (Kumar et al., 2015). Notably, Aβ plaques and NFTs are not exclusive to AD; other central nervous system (CNS) disorders, such as chronic infections, also exhibit these specific histopathological markers (Mawanda and Wallace, 2013). The antimicrobial activity of Aβ has been indicated, suggesting that infections may induce the production and deposition of Aβ in the brain (Bourgade et al., 2016; White et al., 2014). Immune response and inflammation are critical components of AD pathogenesis, and an inappropriate immune response in the brain can lead to neurodegenerative processes (Heneka et al., 2014). Increasing Aβ deposits activate glial cells, lymphocytes, and macrophages, which release inflammatory mediators and reactive oxygen species (ROS) (Li et al., 2014). Reactive microglia and astrocytes induce neuronal apoptosis and blood–brain barrier (BBB) dysfunction, leading to the recruitment of peripheral blood leukocytes and exacerbating other AD pathologies (Heneka et al., 2014; Lim et al., 2015; Jacobs and Tavitian, 2012).
The exact mechanism that initiates the upregulation of Aβ aggregation remains mostly unknown. However, the presence of microbial pathogens in brain samples from AD patients indicates that Aβ aggregation may act as an innate immune response to microbial infections (Prosswimmer et al., 2024). Because of their structural similarities, it is proposed that Aβ peptides function as antimicrobial peptides within the innate immune system. Under certain conditions, both antimicrobial peptides and Aβ peptides form α-helical structures in the membranes of pathogens, creating ion channels that disrupt cellular homeostasis and lead to cell death (Spitzer et al., 2016). Aβ peptides can self-assemble into Aβ structures, a characteristic often seen in misfolded pathological proteins. These peptides can form channel-like structures in cellular plasma membranes, similar to channel-forming toxins. As a result, the creation of these leaky channels or pores causes the lysis of the targeted organism, ultimately leading to cell death (Bourgade et al., 2016). Moreover, innate immunity against virus infection is impaired in AD, and even in healthy young persons, the immune system cannot completely eradicate pathogens. Repeated activation and latency cycles with infective agent persistence may accelerate immune system senescence. Regulatory mechanisms of innate immunity genes in response to amyloid-Aβ peptide expression have been poorly explored, but it may function as an emergency defense mechanism to compensate for other immune defensive gene inefficiencies in the aging brain (Romagnoli et al., 2020).
The interaction of various infectious agents with environmental, inflammatory, and genetic factors may work as triggers initiating the processes causing Aβ formation, abnormal tau phosphorylation, and consequent neuronal loss (Agostini et al., 2017). Consequently, some possible associations between AD and some infectious agents were investigated, such as Herpes simplex virus 1 (HSV-1) (Bourgade et al., 2015), Epstein Barr virus (EBV) (Ou et al., 2020), Cytomegalovirus (CMV) (Lövheim et al., 2018), Influenza viruses (Jang et al., 2009), Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (Ciaccio et al., 2021), Chlamydia pneumoniae (CP) (Chacko et al., 2022), Porphyromonas gingivalis (P. gingivalis) (Gaur and Agnihotri, 2015), Spirochetes (Miklossy, 2008), and Toxoplasma gondii (T. gondii) (Nayeri Chegeni et al., 2019).
Mechanisms by which these pathogens may cause AD include the induction of Aβ accumulation (Bourgade et al., 2022; Yirün et al., 2023), tau phosphorylation (Dezfulian, 2018), inflammation (Carbone et al., 2014; Shim et al., 2017), DNA damage, neuronal cell death (Sait et al., 2021), microglial overactivation, reduced brain plasticity (Sadasivan et al., 2015), and impaired Aβ clearance (Liu et al., 2017). Pathogens can penetrate the CNS and stimulate the production of Aβ plaques, neurofibrillary tangles, and tau pathology (Dominy et al., 2019). They can also activate the processing of amyloid precursor proteins (APPs), leading to the progression of AD (Griffin and Barger, 2010). Additionally, inflammatory cytokines and neuroinflammatory markers may contribute to AD pathogenesis (Dezfulian, 2018).
Regarding the role of microRNAs (miRNAs) in the pathogenesis mechanisms, EBV uses miRNAs to target various protein-coding genes, allowing for immune evasion (Lung et al., 2009) and maintaining latency in EBV-associated tumors (Jung et al., 2014). On the other hand, the impact of SARS-CoV-2 on host miRNA populations is relatively minimal (Pawlica et al., 2021); however, the virus does express an miRNA-like small RNA, CoV2-miR-O7a, which is functional in repressing human mRNAs to evade the host immune response (Pawlica et al., 2021). Also, the pathogenesis of Chlamydia psittaci and P. gingivalis involve the regulation of miRNAs in human bronchial epithelial cells and human periodontal ligament cells, respectively (Chen et al., 2023; Fan et al., 2023), highlighting the importance of miRNA synthesis in understanding viral and bacterial pathogenesis.
In this review article, we aim to give an overview of the association between AD and the infectious agents SARS-CoV-2, HSV-1, CMV, EBV, Influenza viruses, T. gondii, HP, Spirochetes, CP, and P. gingivalis, focusing on the mechanisms by which these pathogens are related to the AD pathogenesis.
It is a well-established fact that pathogens are involved in AD development, along with HSV-1 gaining some intense attention as a possible risk factor. HSV-1 infection can accelerate the development of AD and similar neurodegeneration by promoting amyloid buildup and neuroinflammation (Berger and Houff, 2008; Kristen et al., 2015; Zhang et al., 2021). Also, the presence of anti-HSV IgM antibodies in the serum, a sign of HSV reactivation, has been associated with an elevated risk of developing AD (Letenneur et al., 2008; Santana et al., 2013). Neuroinflammation plays a critical role in the pathogenesis of AD. The term “neuroinflammation” was introduced to describe an inflammatory response that occurs in the CNS following an injury or infection (Morales et al., 2014). It is important to note that the immune and inflammatory reactions in the CNS differ from those in the rest of the body because of the BBB, which restricts the entry of leukocytes into the brain tissue. Moreover, most of the immune and inflammatory responses in the CNS are driven by the interactions of microglia and astrocytes (Ransohoff et al., 2015). Despite the protective role of neuroinflammation in response to CNS injury or infection, an inappropriate response can lead to neurodegenerative diseases such as AD (Ransohoff, 2016). Pathogens like HSV-1 can activate microglia and astrocytes, leading to the production of inflammatory mediators such as cytokines and chemokines (Hong et al., 2018). This chronic inflammation can damage neurons and promote the accumulation of Aβ plaques, a hallmark of AD (Hong et al., 2018). Studies have shown that HSV-1 infection can induce the recruitment of microglia to the viral core, triggering microglial phagocytosis of HSV-green fluorescent protein (GFP)-positive neuronal cells. This process activates the Nod-like receptor protein 3 (NLRP3) inflammasome pathway, which plays a crucial role in Aβ deposition and the progression of AD (Wang et al., 2024).
HSV-1 typically starts by infecting epithelial cells at mucosal surfaces, such as the mouth or nose. The virus can travel along peripheral nerves to reach the CNS. It often uses the trigeminal nerve or the olfactory nerve to gain access to the brain (Bello-Morales et al., 2020). HSV-1 moves through neurons via retrograde axonal transport, a process where the virus travels backward along the nerve fibers to reach the neuronal cell bodies in the brain (Bello-Morales et al., 2020). Moreover, HSV-1 infection can alter the integrity and permeability of the BBB, allowing other infectious agents to enter the brain parenchyma and exacerbate infection and inflammation. It can lead to the downregulation of tight junction proteins (like occludin and claudin-5) that maintain the tight seal of BBB. This disruption increases the permeability of the BBB, allowing the virus and immune cells to enter the brain (He et al., 2020; Liu et al., 2019). This can lead to a cascade of events that accelerate neurodegeneration and cognitive decline (Feng et al., 2023).
In 1982, Ball suggested a correlation between HSV-1 and AD by observing that herpes simplex encephalitis and AD both impact identical brain regions, and individuals who recovered from herpes simplex encephalitis showed symptoms such as cognitive impairment and memory loss, which are also visible in AD (Ball et al., 1982). AD predominantly affects individuals aged 60 and older, while infections like Herpes and COVID-19 can occur at any age. Despite the difference in age distribution, infections and AD may still be related, as infections can contribute to the development and progression of AD. Many infectious agents, including Herpes viruses, can remain latent in the body and reactivate later in life, particularly under conditions of weakened immunity which are more common in older adults (Oh et al., 2019). This reactivation may then contribute to the pathophysiology of AD. Morover, even if infections occur earlier in life, the long-term inflammatory and immune responses they trigger can have lasting effects on brain health, potentially contributing to neurodegenerative diseases like AD later in life (Sekino et al., 2022). Moreover, about two thirds of persons diagnosed with AD dementia are women (Mielke, 2018). The higher prevalence of AD in women could be influenced by several factors, including the role of pathogens in the pathogenesis of AD. Women generally have a stronger immune response compared to men, which can be a double-edged sword. While a robust immune system can help fight off infections, it can also lead to chronic inflammation, which is a known risk factor for AD (Jung and Mook-Jung, 2024). Additionally, hormonal changes, particularly during menopause, can affect brain health. Estrogen has been shown to have a protective effect on the brain, and its decline during menopause may increase vulnerability to infections and neurodegenerative diseases (Jung and Mook-Jung, 2024). Furthermore, women may experience reactivation of latent infections more frequently due to hormonal fluctuations, which can contribute to chronic inflammation and neurodegeneration (Jung and Mook-Jung, 2024; Filon et al., 2016). Also, it has been noted that there is a high prevalence of certain infectious agents, such as HSV-1, yet a relatively lower incidence of AD. However, it is important to note that not all individuals exposed to these pathogens will develop AD. This may be due to genetic factors, such as the APOE ε4 allele, which is a well-known genetic risk factor for AD. Individuals carrying this allele may be more susceptible to the neurodegenerative effects of infectious agents (Corder et al., 1993). The timing and duration of infection can also play a significant role. Early-life infections or chronic, persistent infections may have a more substantial impact on brain health compared to acute, short-term infections. Moreover, factors such as diet, exercise, exposure to toxins, smoking, and alcohol consumption can influence the risk of developing AD. These factors can interact with infectious agents, either exacerbating or mitigating their effects on brain health (Livingston et al., 2017).
From the overall perspective, the mesial temporal and sub-frontal regions injured through acute Herpes encephalitis are among the regions innervated by trigeminal ganglia-derived fibers. These limbic areas play a crucial role in the recall and memory processes. HSV resides in the trigeminal ganglia of humans and can cause a long-term infiltration of lymphocytes without inducing pathological alterations in those neurons. As a result, these lymphocytes are considered a histological sign of latent Herpes infection whose reactivation can lead to degenerative lesions seen in AD and normal aged brain tissue (Ball et al., 2001).
Table 1 presents an overview of the underlying mechanisms mechanisms that by which HSV-1 is involved in the pathogenesis of AD. There are two pathways through which HSV-1 causes impairments leading to acute neurodegeneration, the APP proteolysis and the autophagy process. An abortive autophagic reaction is promoted by HSV-1, which helps in the accumulation of autophagosomes (Santana et al., 2012). Failure in the degradation of Aβ through autophagy, as well as inhibited secretion of Aβ, can justify the effects of HSV-1 infection on Aβ deposition in autophagic compartments within cells (Santana et al., 2012). It is evident that human herpesvirus 6 (HHV6A) and HSV1, among the neurotropic herpesviruses can affect many kinds of cells existing in the CNS and impair the mechanism of autophagy, which is needed for cellular homeostasis, particularly that of neurons (Tallóczy et al., 2006). To be precise, autophagosome accumulation, which demonstrates an imbalance between autophagosome formation and destruction, has been witnessed in AD patients, and this accumulation may contribute to the extracellular deposition of Aβ and intracellular alterations of tau protein.
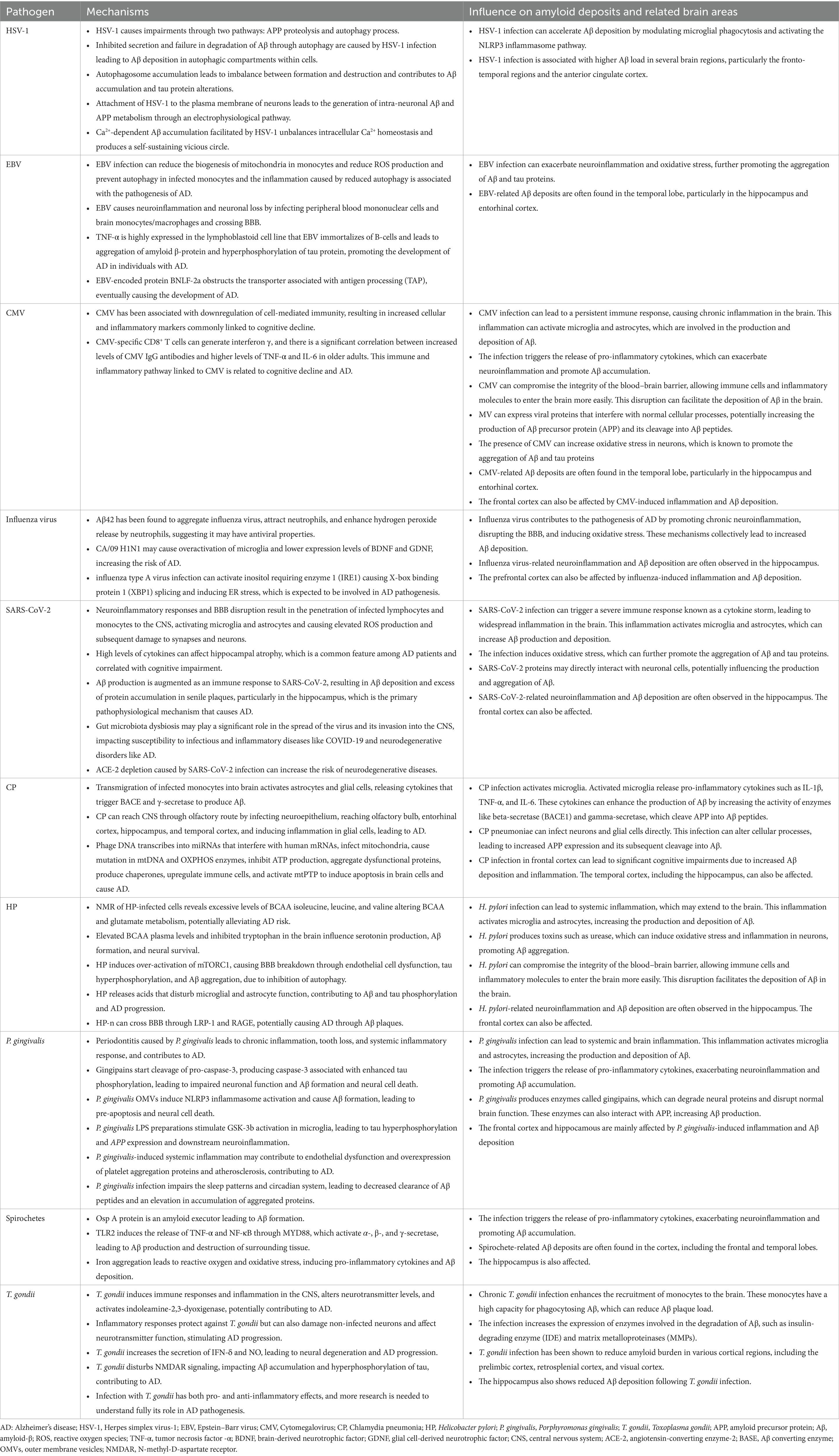
Table 1. A summary of the mechanisms underlying pathogens involvement in the pathogenesis of Alzheimer’s disease.
Furthermore, herpesviral infection of microglial and other glial cells can elevate the generation of ROS by promoting inflammation and mitochondrial dynamic changes, which can be considered as another AD sign. HSV-1 has also been demonstrated to cause the accumulation of Aβ42 in neuronal cells and human induced pluripotent stem cells from healthy individuals, even at low infection levels (Abrahamson et al., 2021; Wozniak et al., 2011). In addition, studies have revealed that rat cortical neurons infected with HSV-1 showed higher levels of intracellular Ca2+, which triggered the Ca2+-dependent phosphorylation of APP and the subsequent intracellular accumulation of Aβ42 (Piacentini et al., 2011). So, a significant alteration will occur in the homeostasis and intracellular Ca2+ ([Ca2+]i) signaling of the neural cells infected with HSV-1. Also, HSV-1 infection induces intracellular Ca2+ transients that generate a perceivable elevation in basal [Ca2+]i within a few minutes. It is worth mentioning that HSV-1-induced elevation in [Ca2+]i has also been detected in cervical cancer and renal epithelial cells. They are mostly caused by inositol 1,4,5-trisphosphate receptor (IP3R) activation, which leads to Ca2+ discharge from the endoplasmic reticulum (ER). The Ca2+ signaling in these cells is conceivably assigned to the relationship between heparan sulfate proteoglycans existing on the cell membrane and viral glycoproteins gC and gB, which prompt G-protein-dependent stimulation of the phospholipase C γ that hydrolyzes phosphatidylinositol 4,5-bisphosphate to IP3. Attachment of HSV-1 to specific receptors, such as nectin-1 (HveC, CD111) and heparan sulfate (Karasneh and Shukla, 2011), on the plasma membrane of neurons activates a pathway of electrophysiological reactions, leading to the generation of intra-neuronal Aβ and altered APP metabolism. The alteration of ion channels is an initial event leading to subsequent incidents, allowing the virus to trigger APP phosphorylation. These ion channels function following neuronal firing or at the resting membrane potential. Subsequently, crucial tasks assisted by intracellular Ca2+ signals, which are mainly linked to Ca2+ influx through VGCCs, discharge calcium from intracellular stores. Besides, the Ca2+-dependent Aβ accumulation facilitated by HSV-1 may later unbalance the intracellular Ca2+ homeostasis and hence produce a self-sustaining vicious circle (Piacentini et al., 2011).
One of the most prevalent herpesviruses, recognized for its asymptomatic infection in most adults, is human herpesvirus 4 (HHV4), also known as EBV (Jha et al., 2015). This double-stranded DNA virus mainly infects B lymphocytes (Jha et al., 2015). Based on the previous studies, EBV may play a role in the pathogenesis of AD (Shim et al., 2017; Zhang et al., 2022). Below is a brief outline of some of the mechanisms which can some mechanisms potentially associated with AD. To begin with, Talwar et al. investigated the interaction between Hepatitis C virus (HCV), EBV, Human Herpes Virus 8 (HHV8), and HPV and AD candidate genes, including AKT1, GSK3B, APP, APOE, EGFR, PIN1, CASP8, and synuclein alpha (SNCA) (Talwar et al., 2019). In their study, the involvement of EBV with epidermal growth factor receptor (EGFR), which affects cell proliferation, growth, and survival, has been shown (Talwar et al., 2019; Shafi, 2016). Moreover, epidermal growth factor (EGF) is a peptide that regulates neural stem cells and plays a role in neurogenesis in the hippocampus and the improvement of cognitive functions (Shafi, 2016). Furthermore, Thomas et al. demonstrated that EGF prevents impairment of cognitive function and cerebrovascular defects (Thomas et al., 2017). So EGFR levels are one of the parameters that can be used to distinguish AD patients from controls (Talwar et al., 2019). Also, some therapeutic options (e.g., angiogenic growth factors [AGF]-like drugs) may be provided to reduce AD risk (Thomas et al., 2016).
It is widely accepted that EBV utilizes autophagic machinery to increase viral production. EBV can inhibit the last phases of the autophagy process, through the interaction of its protein TRS1 with Beclin1 (Romeo et al., 2020). Reduction of autophagy in infected monocytes leads to the accumulation of p62/SQSTM1 and Nuclear factor erythroid 2-Related Factor 2 (NRF2) up-regulation which prevents escalation in reactive oxygen species (ROS) levels induced by Interleukin-4 (IL-4) and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF). On the other hand, EBV infection can reduce the biogenesis of mitochondria in monocytes and by this mechanism, it can reduce ROS production and prevent autophagy in infected monocytes. Reduction of ROS can strongly impair the formation of dendritic cells from monocytes. Also, increased ER stress and the provocation of the unfolded protein response (UPR) play an important role in the inflammation, because of the reduction of autophagy (Romeo et al., 2020). Ultimately, it can be said that this inflammatory process is associated with the pathogenesis of AD (Talwar et al., 2019; Carbone et al., 2014).
Having considered the association of EBV and serological markers, a previous study has pointed out that anti-EBV IgG levels were considerably higher in patients with amnestic mild cognitive impairment (aMCI) in comparison with the control group over the 2-year follow-up period in Korean elderly people (Shim et al., 2017). In addition, EBV causes neuroinflammation and neuronal loss in the brain by infecting peripheral blood mononuclear cells and brain monocytes/macrophages and crossing the BBB (Kanakry et al., 2016). Decreased levels of the cytokine TNF-α can reduce the hyperphosphorylation of tau protein (Dezfulian, 2018). In individuals with AD, TNF-α is highly expressed in the lymphoblastoid cell line that EBV immortalizes of B-cells, leading to aggregation of amyloid β-protein and hyperphosphorylation of tau protein, ultimately promoting the development of AD (Dezfulian, 2018). Gate et al. discovered that EBNA3A and BZLF-1 antigens trigger an immune response mediated by CD8+TEMRA cells associated with immune memory, which are negatively correlated with cognitive performance. In individuals with AD, CD8+TEMRA cells release pro-inflammatory cytokines, such as IFNγ, TNF-α, and cytotoxic factors (NKG7, GZMA, and B2M), which lead to a decline in cognitive function and exacerbate the symptoms of AD (Carbone et al., 2014; Gate et al., 2020; Kang and Liu, 2020; Tiwari et al., 2021). Moreover, EBV-encoded protein BNLF-2a obstructs transporter associated protein (TAP), thus triggering the downregulation of MHC-I and II expression. By doing so, neuronal cells accumulate viral polypeptides in the environment, eventually causing the development of AD (Tiwari et al., 2021). EBV can also trigger a stress immune response that causes inflammation and cognitive decline during aging, both in its latency and reactivation phases (Carbone et al., 2014; Shim et al., 2017). However, there are few studies on how EBV contributes to AD, and more studies are necessary to fully understand the pathogenesis.
miRNAs also play a significant role in the relationship between EBV and AD by regulating gene expression and influencing various cellular processes. They are crucial in regulating inflammatory responses. Dysregulation of specific miRNAs can lead to chronic inflammation, which is a known factor in AD pathogenesis (Sequeira and Godad, 2024). Certain miRNAs are involved in the metabolism of Aβ and tau proteins, both of which are central to AD pathology. For instance, miRNAs can influence the expression of enzymes like BACE1, which is involved in Aβ production (Sequeira and Godad, 2024). Moreover, miRNAs play roles in maintaining synaptic health and plasticity. Dysregulation of these miRNAs can contribute to synaptic dysfunction, a hallmark of AD (Abidin et al., 2023). They are also involved in cellular stress responses, including oxidative stress and autophagy, which are critical in the context of neurodegenerative diseases (Abidin et al., 2023). Furthermore, factors such as diet, pollutants, stress, and lifestyle choices can lead to epigenetic modifications. For example, exposure to pollutants or a poor diet can alter DNA methylation patterns, which may affect genes involved in immune response and inflammation (Klibaner-Schiff et al., 2024; Migliore and Coppedè, 2022). These environmental exposures can induce changes in the epigenome, such as DNA methylation and histone modifications, which can influence the expression of genes related to both EBV and AD. For instance, altered DNA methylation patterns can affect the expression of genes involved in amyloid-β production and tau phosphorylation (Liu et al., 2018; Xiao et al., 2020). Epigenetic changes can mediate the effects of environmental factors on gene expression, potentially exacerbating the impact of EBV on neuroinflammation and neurodegeneration. For example, chronic stress can lead to epigenetic changes that enhance the inflammatory response, which is a known factor in both EBV infection and AD (Migliore and Coppedè, 2022; De Plano et al., 2024). Environmental factors can also affect the expression of miRNAs, which play a crucial role in regulating gene expression. Dysregulated miRNAs can impact the expression of genes involved in immune response, inflammation, and neuronal health, thereby influencing the relationship between EBV and AD (Xiao et al., 2020; De Plano et al., 2024).
Several studies have identified different EBV-encoded miRNAs and their targets. For example, miR-BART21 and miR-BART22 are highly expressed in NPC and modulate the expression of the immunogenic viral antigen LMP2A, allowing escape of EBV-infected cells from host immune surveillance (Lung et al., 2009). Moreover, miR-BART20-5p helps maintain latency in EBV-associated tumors by directly targeting immediate early genes BZLF1 and BRLF1 (Jung et al., 2014). Furthermore, EBV encodes miRNAs (e.g., BART 18-5p) that suppress the cellular signaling molecule MAP kinase kinase kinase 2 (MAP3K2) at exactly the same site as the oncogenic cellular miRNA mir–26a-5p, thus blocking viral replication and maintaining latency in memory B cells (Qiu and Thorley-Lawson, 2014). Additionally, EBV-miR-BART2 targets the viral DNA polymerase BALF5, which inhibits the transition from latent to lytic viral replication (Barth et al., 2008). Finally, miR-BART6 of EBV is edited in latently infected cells, suppressing processing of miR-BART6 RNAs and silencing Dicer through multiple target sites located in the 3′-UTR (untranslated region) of Dicer mRNA (Iizasa et al., 2010).
Cytomegalovirus (CMV), similar to EBV, is from the Herpesviridae group (Lövheim et al., 2018). CMV can be transmitted from one person to another through contact with bodily fluids of persons who have symptomatic or asymptomatic infection (Barnes et al., 2015). Investigations have linked CMV to an increased risk of AD and cognitive decline associated with aging. CMV may also contribute to cognitive decline in elderly individuals and dementia in patients with Down syndrome (Licastro et al., 2011). A study found that high CMV antibody levels were linked to faster cognitive decline over four years (Aiello et al., 2006). Both AD patients and healthy elderly individuals tested positive for CMV, but there was no significant difference between the groups. Moreover, CMV was more frequently found in patients with vascular dementia, suggesting a potential role for the virus in this condition (Carbone et al., 2014). Although some findings suggest that there is no direct correlation between CMV and AD pathogenesis (Ji et al., 2023), several studies revealed an association between CMV infection and AD (Lövheim et al., 2018). Numerous studies have reported that there is a relationship between CMV serological markers and AD. A clear example is the study by Barnes et al., in which the authors showed the association of CMV seropositivity with enhanced the risk of AD development (relative risk, 2.15; 95% confidence interval [CI], 1.42–3.27) in a cohort study with 849 participants (Barnes et al., 2015). In another study, a significant correlation between the CMV seropositivity and AD has been reported by Bu et al. (adjusted odds ratio, 2.33; 95%CI, 1.14–4.77) (Bu et al., 2015). Furthermore, a study carried out by Lurain et al. showed that there is an association between increased levels of anti-CMV IgG and density of NFTs (Lurain et al., 2013). In sum, given above-mentioned studies, there might be a relationship between CMV and AD.
However, the exact mechanisms linking CMV to the risk of AD are unclear. Despite often being undiagnosed due to its asymptomatic nature, CMV remains in a latent state within the immune system, with a higher likelihood of reactivation in older age (Koch et al., 2006). Several factors suggest CMV might be linked to AD risk through its impact on the aging immune system. Firstly, older adults exhibit higher levels of IgG antibodies to CMV compared to younger individuals (Barnes et al., 2015), and aging-related changes in cell-mediated immune parameters can lead to subclinical CMV reactivation (Stowe et al., 2007). Secondly, CMV has been associated with the downregulation of cell-mediated immunity, resulting in increased cellular and inflammatory markers commonly linked to cognitive decline (Koch et al., 2006; Almanzar et al., 2005). CMV-specific CD8+ T cells can generate interferon γ (Almanzar et al., 2005), and there is a significant correlation between increased levels of CMV IgG antibodies and higher levels of tumor necrosis factor α and IL-6 in older adults (Roberts et al., 2010; Schmaltz et al., 2005). This immune and inflammatory pathway that is linked to CMV is also related to cognitive decline and AD (Zhao et al., 2024).
Influenza viruses are single-stranded RNA viruses belonging to the Orthomyxoviridae family (Luo, 2012). Influenza and pneumonia has been significantly linked to five out of six neurodegenerative diseases (AD, ALS, dementia, Parkinson’s disease [PD], and vascular dementia). These associations has been confirmed using cross-sectional data from the UK Biobank (Levine et al., 2023).
However, there is some argument over the role of these viruses in AD. The virus can change through antigenic drift and shift. The latter can cause epidemics like avian flu (H5N1) and swine flu (H1N1), named after the variant proteins that allow the virus to enter host cells and facilitate lysis after replication (Piekut et al., 2022). H5N1 has been associated with the phosphorylation and aggregation of alpha-synuclein, which is known to play a significant role in PD neurodegeneration (Jang et al., 2009). Although this pathology is more evident in Lewy body dementia and PD, the structural resemblance between Aβ and influenza hemagglutinin indicates that H5N1 might also contribute to AD (Piekut et al., 2022). The similarities are particularly notable in the C-termini of both proteins, where hemagglutinin contains a domain responsible for cell membrane binding. This aligns with the hypothesis that influenza virus-induced membrane-poration could lead to neurotoxicity (Piekut et al., 2022). Aβ42 is a significant component of Aβ plaques found in AD, and recent studies suggest that it may have antiviral properties. In one study, Aβ42 was found to aggregate influenza virus, attract neutrophils, and enhance hydrogen peroxide release by neutrophils (White et al., 2018). The ability to aggregate is thought to be caused by a C-terminal loop in Aβ42 that includes residues Met35 to Ala42, which is also involved in Aβ42 oligomerization (Ahmed et al., 2010; Wagoner et al., 2014). Also, H5N1 can penetrate the CNS through the peripheral nervous system and cause innate immune system activation and subsequently dopaminergic neuronal degeneration in the substantia nigra pars compacta (SNpc) (Jang et al., 2009). Although this dopaminergic neurons loss can be restored after about 90 days of infection, a long-term inflammation and activation of microglia can cause some impairments in the neural cells (Limphaibool et al., 2019). So, some disorders that are related to the aggregation of proteins and neurodegeneration can take place due to the activation of microglial cells (Limphaibool et al., 2019; Sadasivan et al., 2015).
CA/09 H1N1 is another strain of influenza virus that may be associated with neurological changes. Although it is not believed to actively cross the BBB and is considered non-neurotropic (Sadasivan et al., 2015), one study showed that it causes overactivation of microglia, which persisted for up to 90 days post-infection (Sadasivan et al., 2015). Infection with this virus also results in a decrease in brain-derived neurotrophic factor (BDNF) and glial cell-derived neurotrophic factor (GDNF), which encode key factors essential for maintaining neural plasticity. BDNF and GDNF are also responsible for regulating microglial activation, and lower expression levels may lead to inflammation in the CNS that, coupled with reduced brain plasticity, increases the risk of AD (Sadasivan et al., 2015).
Ultimately, Hassan et al. revealed that there is a correlation between unfolded protein response (UPR), ER stress, and the pathogenesis of influenza type A virus (IAV) infection. In this study, it has been indicated that inositol requiring enzyme 1 (IRE1) can be activated by IAV infection. Subsequently, activated IRE1 can cause X-box binding protein 1 (XBP1) splicing resulting in modulation of pro-survival responses (Mehrbod et al., 2019). Therefore, it is expected that this mechanism is involved in AD pathogenesis, as chronic ER stress and prolonged UPR activation can lead to neuronal dysfunction and death. Specifically, the persistent activation of IRE1 and XBP1 splicing may contribute to the accumulation of misfolded proteins, such as Aβ and tau, which exacerbate neuroinflammation and oxidative stress, further promoting neurodegeneration (Marques et al., 2024; Marques et al., 2023).
Vaccines can help reduce the risk of various neurodegenerative diseases including AD and PD. Studies have shown that getting vaccinated for influenza and pneumonia can lower the risk of AD, especially if the pneumonia vaccine is given between ages 65–75. Protection from bacterial and viral infections can be helpful for the brain because they may activate dormant viruses such as HSV-1 and HZV that could contribute to AD. The Zostavax vaccine for shingles has also been found to reduce the risk of AD and PD, supporting the idea that viruses may play a role in neurodegeneration (Lehrer and Rheinstein, 2022). Observational studies and a meta-analysis have shown evidence that influenza vaccination may be linked to a lower risk of dementia (Veronese et al., 2022; Liu et al., 2016; Luo et al., 2020). A large cohort study involving over 2 million participants also reported a 40% reduced risk of AD among vaccinated elderly individuals (Bukhbinder et al., 2022). Experiments in mice have shown that flu vaccination affects microglial activity and Aβ clearance (Yang et al., 2020), supporting the theory that the protective impact of flu vaccination on dementia may be due to nonspecific effects on the immune system (Hjelholt et al., 2023).
In brief, foregoing discussions indicates that mechanisms mentioned above have profound impact in the AD pathogenesis by activating immune system, aggregating proteins, and inducing ER stress.
In December 2019, a coronavirus, SARS-CoV-2, emerged in China, which can cause severe acute respiratory syndrome (SARS). COVID-19 pandemic dramatically impacted people’s lives worldwide in various ways, including health, economic, political, and social (Nicola et al., 2020). Although currently, to minimize the mortality rate, it is mainly focused on the relief of cardiovascular and pulmonary consequences of COVID-19, there are also reports of neurological presentations in the cases (Mao et al., 2020; Vakili et al., 2021). This virus, like other human coronaviruses, is considered to be an opportunistic microorganism of the CNS (Desforges et al., 2020). According to post-mortem investigations, the SARS-CoV-2 antigen and RNA were found in the brains of the patients (Matschke et al., 2020). Therefore, the assumption has been raised that SARS-CoV-2 infection may lead to long-term neurological consequences in particular cognitive decline and dementia, which draw our attention to AD (Mao et al., 2020). The findings of Baranova et al. suggest that COVID-19 infection may contribute to the development of clinical AD. The study reveals a positive genetic correlation between hospitalized COVID-19 and AD, with genetic liabilities to severe COVID-19 associated with an increased risk for the latter (Baranova et al., 2023). Moreover, it has been shown that Covid-19 infection is linked to an increase in the development of new onset clinical AD. Wang et al. that those who had contracted Covid-19 were at a significantly higher risk of being newly diagnosed with AD within a 360-day period after their initial diagnosis of Covid-19 (with a hazard ratio of 1.69 and a 95% confidence interval ranging from 1.53 to 1.72) (Wang et al., 2022). Interestingly, despite the difference in age distribution between Covid-19 and AD, they may still be associated. While Covid-19 has the potential to affect individuals of all ages, older adults are more susceptible to severe infections and complications that can have neurological impacts. Also, the processes by which Covid-19 contribute to AD may interact with aging mechanisms. For example, age-related decline in immune function (immunosenescence) could worsen the effects of infections on the brain.
It has been demonstrated that it is the interaction between the S1 spike protein of SARS-CoV-2 and angiotensin-converting enzyme-2 (ACE-2) which is responsible for the invasion of this virus to the cells and ACE-2 is highly represented in brain tissue as well (Alberti et al., 2009; Dolatshahi et al., 2021). In brain, ACE-2 is expressed on neurons, glial cells, endothelial cells, smooth muscle cells of arteries, and also hippocampus and temporal lobe involved in AD pathogenesis (Dong et al., 2020).
A number of interrelated pathways are usually observed which led to SARS-CoV-2 entrance into CNS. first and foremost, due to the virus interaction with ACE-2 receptors on the endothelium, BBB is disrupted. Thus, the infected lymphocytes can cross the barrier (Varga et al., 2020; Hascup and Hascup, 2020). Another route is the axonal transport of the virus through olfactory neurons (Mao et al., 2020). Transmembrane serine protease 2 (TRPMSS2), and ACE-2 are abundantly expressed on the olfactory epithelium. After the infection, TRPMSS2 expression is elevated in olfactory neurons and facilitates virus entrance. Also, changes in olfaction are a primary symptom of neurodegenerative diseases including AD (Bagasra et al., n.d.). On the other hand, lymphatics or hematogenous dissemination is another probable pathway (Bostancıklıoğlu, 2020). Virus receptors, including ACE-2, TMPRSS2, and FURIN, are expressed by dorsal root ganglion (DRG) and sensory neurons (Shiers et al., 2020). Therefore, free nerve terminals of skin or lumens epithelium are another potential route that contributes to the virus’s entry into the brain. Furthermore, one of the determining pathways which could account for the transmission of virus into the brain is the vagus nerve and enteric nervous system (Esposito et al., 2020). P2X7 receptors are known as ion channels expressed in the CNS which can be triggered by ATP originating from distressed cells (Ribeiro et al., 2021; Di Virgilio et al., 2017; Sluyter, 2017). SARS-CoV-2 augments extracellular ATP, which can stimulate P2X7 receptors hyperactivation. This, in turn, triggers NOD-like receptor protein 3 (NLRP3) inflammasome (Ribeiro et al., 2021). Other factors responsible for NLRP3 activation are the open reading frame 3a (ORF3a) protein of the virus, systemic inflammation, and acute lung injury. Subsequently, NLRP3 activation upregulates cytokines such as interleukin-1beta (IL-1β), increases pathogenic fibrils by increasing aggregation of peptides, causes mitochondrial failure, apoptosis, and thus neurodegeneration, which is a hallmark of AD (Siu et al., 2019; Heneka et al., 2013). Inflammatory mediators such as IL-1β, 2 and 6 and TNF-α are elevated in COVID-19 cases and assist in this CNS alterations. Moreover, Stain et al. found that SARS-CoV-2 is present in various tissues, including the brain, during early stages of infection, especially in severe COVID-19 patients who died. Their study also demonstrated that SARS-CoV-2 RNA can persist in multiple sites, even in the brain up to 230 days after the onset of symptoms in one case (Stein et al., 2022).
Above all, high levels of cytokines and chemokines can cross BBB, resulting in neuroinflammatory responses and BBB disruption. Thus, lymphocytes and monocytes infected by the virus, penetrate to CNS and lead to activation of microglia and astrocytes and thus neuroinflammation (Chaves Filho et al., 2021; Jakhmola et al., 2020; Yarlagadda et al., 2009). It is the activated microglia by which nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) enzyme is triggered and therefore elevates ROS production significantly. ROS impact, not only neuronal oxidative disruption, but also the development or aggravation of neurodegenerative diseases (Cahill-Smith and Li, 2014; Geng et al., 2020). It should be stated that NOX2 plays a role in the pathogenesis of neurodegenerative disorders, including AD (Kumar et al., 2016). Recently Violi et al. have attempted to assess the correlation between COVID-19 clinical exacerbation and NOX2 levels (Violi et al., 2020). It is proven that the continuous activation of microglia contributes to the activation of other microglia and causes elevated tau hyperphosphorylation, mitochondrial failure, and apoptosis. This causes damage to both synapses and neurons, which ultimately contributes to neurodegeneration (Miksys and Tyndale, 2006; Domingues et al., 2017). Ultimately, It has been proven that systemic inflammation, specifically high levels of cytokines, similar to sepsis, can affect hippocampal atrophy (Lindlau et al., 2015; Heneka et al., 2020), which is a common feature among AD patients, and correlated with cognitive impairment (Lu et al., 2020) (Figure 1).
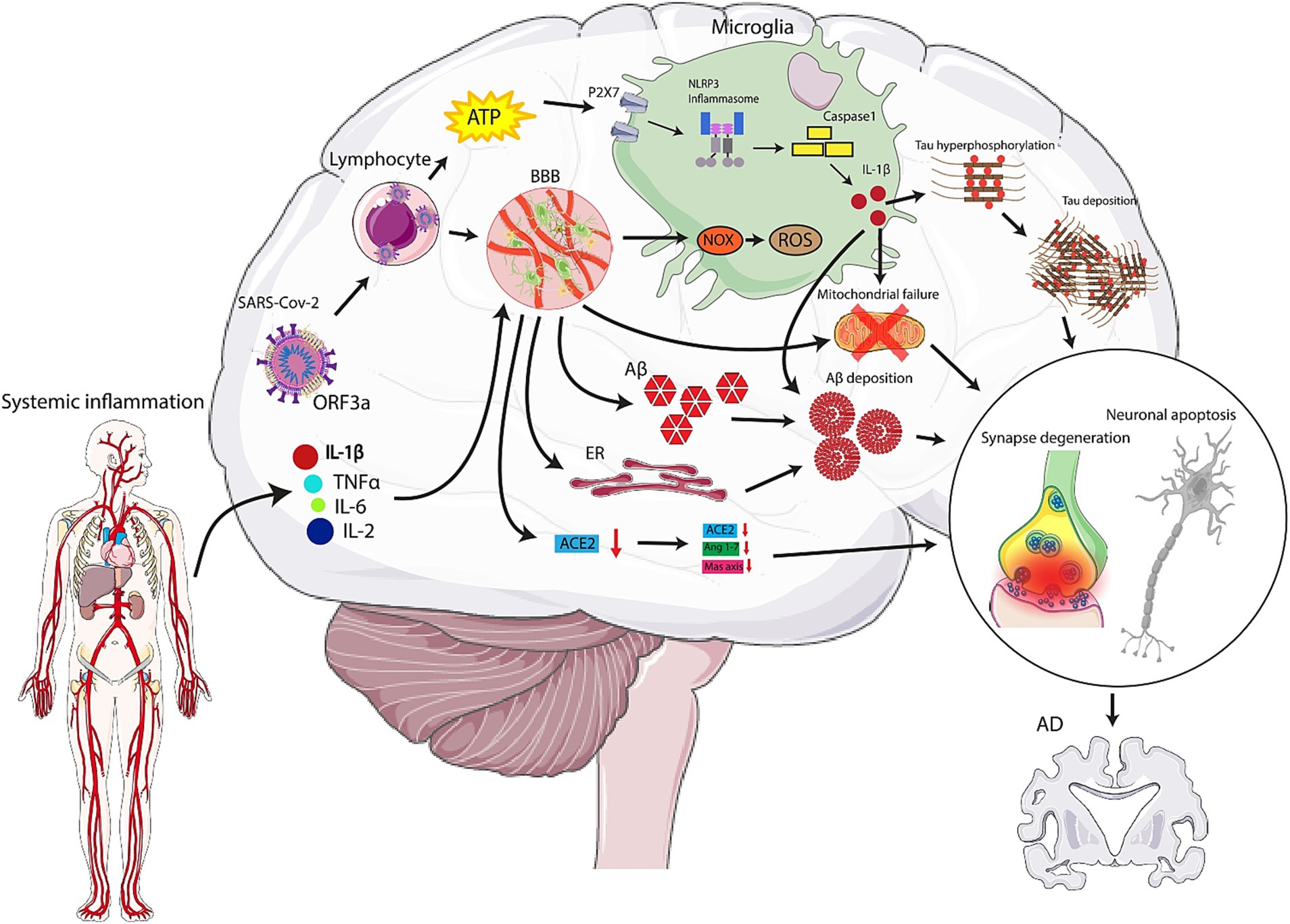
Figure 1. Mechanisms by which SARS-CoV-2 can play role in the pathogenesis of Alzheimer’s Disease (AD). High levels of cytokines and chemokines can cross the blood brain barrier (BBB), causing neuroinflammatory responses and disruption. Virus-infected lymphocytes and monocytes penetrate the central nervous system (CNS), leading to activation of microglia and astrocytes and resulting in neuroinflammation. Activated microglia trigger NADPH oxidase (NOX) enzyme, significantly increasing reactive oxygen species (ROS) production, which impacts neuronal oxidative disruption and can aggravate neurodegenerative diseases, including AD. Also, the virus can enter the nasal epithelium and travel along the olfactory nerve fibers. It uses the ACE2 receptor, present in the nasal epithelium, to enter cells. From there, it hijacks the cellular machinery to replicate and spread along the olfactory nerve. Once the virus reaches the olfactory bulb, it can potentially spread to other parts of the brain. The proximity of the olfactory bulb to the brain allows the virus to access deeper brain structures.
Another mechanism explaining the intensified risk of AD among COVID-19 patients is associated with Aβ which has potential anti-microbial functions. As an immune response to the SARS-CoV-2 invasion into CNS, Aβ production and its cascade are augmented, resulting in Aβ deposition (Soscia et al., 2010). Moreover, when pericytes are lost and endothelial function is impaired, the clearance of cerebral metabolites, including Aβ peptides, is decreased leading to an excess of Aβ protein accumulation in senile plaques, particularly in the hippocampus (Ciaccio et al., 2021), which is the primary pathophysiological mechanism that causes AD. Additionally, hijack of the protein machinery by the virus and thus impairment of ER and mitochondrial functions can be another possible negative mechanism. This can propagate the aggregation of the misfolded proteins, which in turn, set out apoptosis and neurodegeneration (Scheper and Hoozemans, 2015; Dhungana and Jankovic, 2013; Wang et al., 2019).
One more variable which can be considered is that in critically severe COVID-19 patients, acute respiratory distress syndrome (ARDS) is accompanied by a prevalent long-term cognitive decline.
The standard therapy for this condition is mechanical ventilation, which leads to long-term cognitive impairment as well. It has been suggested that short-term mechanical ventilation may induce Aβ peptide accumulation in the brain, BBB impairment, neurologic and systemic inflammation, although the exact mechanisms are unknown (Zlokovic, 2004; Sasannejad et al., 2019; Montagne et al., 2017; van den Boogaard et al., 2011; Sharshar et al., 2004).
It is possible that pathogens can enter the CNS through peripheral nerve endings and lead to neurodegeneration, while gut microbiota dysbiosis may play a significant role in the spread of the virus and its invasion into the CNS (Dolatshahi et al., 2021). Studies have shown that some changes in the gastrointestinal tract (GI), such as GI lesions and increased permeability, occur decades before the onset of neurodegenerative diseases and may contribute to their development (Ambrosini et al., 2019). The alterations caused by gut microbiota dysbiosis can increase GI permeability, alter neurotransmission and lead to the activation of the immune system through mechanisms such as molecular mimicry and oxidative stress. These processes can then contribute to neurodegenerative disorders. Therefore, there is a possibility that SARS-CoV-2 infection, by modifying gut microbiota increases the risk of developing neurodegenerative diseases (Ambrosini et al., 2019). The diversity of gut microbiota is crucial for maintaining immunological balance and may impact susceptibility to infectious and inflammatory diseases such as COVID-19 (van der Lelie and Taghavi, 2020; Dhar and Mohanty, 2020). Older people, who are at greater risk of severe COVID-19 infection and neurodegenerative diseases, typically have less diverse gut microbiota (Dhar and Mohanty, 2020). Therefore, individuals who survive COVID-19 may have a higher risk of developing neurodegenerative conditions due to the common risk factor of reduced gut microbiota diversity (Dolatshahi et al., 2021).
The other influential mechanisms linking dysbiosis to neurodegenerative processes can be addressed by promoted intestine and BBB permeability, molecular mimicry, and oxidative stress which hyper-activate the immune system. These mechanisms disturb the neurotransmission balance and can cause neurodegenerative processes such as AD (Ambrosini et al., 2019). Intestinal bacteria produce short chain fatty acids (SCFAs) such as butyrate, folate, and thiamine, which are vital for maintaining the function of the epithelial barrier (Tan et al., 2014; Scheperjans et al., 2015). Long-term exposure to these SCFAs has been linked to clinical improvement in patients with PD, possibly due to ketogenesis (Luong and Nguyễn, 2013; Liu et al., 2017). Additionally, the similarity in structure between bacterial amyloid proteins and human Aβ can result in an increased inflammatory response to cerebral Aβ as a consequence of changes in the gut microbiota (Delzenne et al., 2011; Muegge et al., 2011; Rosenfeld, 2015). When lipopolysaccharide (LPS) aggregates, it can form structures that interact with various cellular components, leading to oxidative stress. This oxidative stress can, in turn, promote further protein aggregation, including amyloid species. The interaction between LPS and amyloid proteins can exacerbate the formation of insoluble aggregates, which are implicated in various neurodegenerative diseases (Schromm and Brandenburg, 2021).
Besides, one of the other determining factors which are common among severe COVID-19 patients can be disseminated intravascular coagulation (DIC) and hypercoagulability. These conditions can reduce perfusion, leading to ischemic white matter lesions. Admittedly, ischemic white matter damage is an early phase in AD patients and plays a role in AD progression and cognitive impairment. Cerebral hypoperfusion, in turn, can augment tau phosphorylation rate as well.
APOE4 polymorphism is a proven predisposing factor for AD. It is worthy to mention that the growth of COVID-19 infection prevalence among people with homozygous APOE e4 alleles has been reported. This genotype is mainly associated with an increased risk of severe COVID-19, independent of other comorbidities, as well. Thus, APOE4 can be considered as a common risk factor for AD development and SARS-CoV-2 infection. Consequently, it is assumed that among genetically susceptible individuals, COVID-19 infection can be regarded as a factor accelerating neurodegeneration (Ciaccio et al., 2021; Kloske and Wilcock, 2020).
The last but not least probable mechanism linking AD to COVID-19 is that when SARS-CoV-2 binds to ACE-2, it can cause ACE-2 downregulation. Since ACE-2/ angiotensin (Agostini et al., 2019; Agostini et al., 2017; Bourgade et al., 2016; dos Santos Picanco et al., 2018; Breijyeh and Karaman, 2020; Kumar et al., 2015; Mawanda and Wallace, 2013)/Mas axis has neuroprotective functions, ACE-2 depletion can increase the risk of NDs development including AD (Ni et al., 2020). Therefore, according to the mentioned evidences, SARS-CoV-2 infection may worsen the AD development.
miRNAs can influence the expression of genes involved in both SARS-CoV-2 infection and AD. Research has identified common transcriptional signatures and pathways between COVID-19 and AD. miRNAs can target these pathways, potentially affecting the progression of both diseases. For example, miRNAs might modulate the PI3K-AKT, Neurotrophin, and JAK–STAT signaling pathways, which are implicated in both conditions (Premkumar and Sajitha, 2023). miRNAs can regulate neuroinflammatory responses, which are a key feature of both COVID-19 and AD. Moreover, exposure to pollutants, toxic metals, and endocrine-disrupting chemicals can alter the epigenetic regulation of key immune pathways (Bulka et al., 2022). This can increase susceptibility to SARS-CoV-2 and potentially exacerbate neurodegenerative processes associated with AD. Environmental factors can lead to changes in DNA methylation, histone modification, and miRNA expression (AbdelHamid et al., 2021). These epigenetic modifications can influence gene expression patterns, affecting both the immune response to SARS-CoV-2 and the progression of AD.
Chlamydia pneumoniae (CP) is an intracellular respiratory pathogen that can participate in the pathogenesis of pneumonia, multiple sclerosis (MS) and AD (Woods et al., 2020). Intravascular and olfactory pathways are the two means by which CP infects CNS. CP contaminates monocytes and human brain microvascular endothelial cells (HBMECs) in order to cross the BBB (Subedi et al., 2024). The infection of the HBMECs induces overexpression of surface adhesion molecules, intracellular adhesion molecule-1 (ICAM-1), vascular cellular adhesion molecule-1 (VCAM-1), VE-cadherin, N-cadherin, and β-catenin, and downregulates Occludin which is a tight junction membrane protein. These alterations increase the BBB permeability. Also, infection of the THP-1 monocytes upregulates integrin LFA-1 and MAC-1, the ligand of ICAM-1, and VLA-4, the ligand of VCAM-1, which promotes the transmigration of monocytes across the BBB (Itzhaki et al., 2004).
Transmigration of infected monocytes into the brain leads to the activation of astrocytes and glial cells, releasing pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α, which trigger β-secretase and γ-secretase. Followingly, APP cleavage produces Aβ (Woods et al., 2020; Shima et al., 2010).
The exact mechanism by which CP infects the CNS is still unknown. Research suggests that C. pneumoniae can infect lung macrophages that move through the mucosal barrier and enter the bloodstream. The bacteria can then enter the vasculature by surviving intracellularly in blood monocytes, which can cross the BBB and spread to the CNS (Gieffers et al., 2004). However, other studies have proposed alternative routes of infection, such as the olfactory and trigeminal nerves that connect the nasal cavity to the brain. These structures are known to be a gateway for CNS infection by various pathogens. Interestingly, the structures of the CNS that show the earliest signs of pathology in AD (both familial and late-onset) are the olfactory bulb, entorhinal cortex, hippocampal formation, and brainstem, all of which are olfactory structures (Chacko et al., 2022).
Finally, there are five chlamydial phages identified from Microvicridae family viruses. They can enter the cells accompanied by CP. These phage’s DNA enters the mitochondria through transporter proteins and natural competence mechanism (Schmitz-Esser et al., 2004; Koulintchenko et al., 2006). Having entered the mitochondria, the DNA starts to transcribe as miRNAs. The phage’s miRNAs can interfere with human mRNAs and block their pathways (Dezfulian et al., 2008). They also infect the mitochondria and cause mutation in mitochondrial DNA and defects in mitochondrial oxidative phosphorylation enzymes (OXPHOS), including cytochrome C oxidase (Torrens-Mas et al., 2020). These mechanisms can result in the prohibition of ATP production, aggregation of dysfunctional proteins, production of chaperones, upregulation of the immune cells, and also the activation of the mitochondrial permeability transition pore (mtPTP) by escalating the production of ROS which contribute to apoptosis in the brain cells and cause AD (Dezfulian et al., 2008).
The pathogenesis of Chlamydia psittaci via miRNAs in human bronchial epithelial cells (HBE cells) has been investigated. Chen et al. found that C. psittaci induces oxidative stress in HBE cells and regulates the expression of miR-184 and FOXO1. MiR-184 was found to be significantly upregulated, and FOXO1 was confirmed as one of the target genes of miR-184 (Chen et al., 2023). The study also found that miR-184 can promote C. psittaci-induced oxidative stress in HBE cells by promoting the activity of the Wnt/β-catenin signaling pathway. Inhibition of the Wnt/β-catenin signaling pathway was found to reduce oxidative stress in C. psittaci-infected HBE cells. These findings highlight the importance of miRNA synthesis from viral structures in understanding the pathogenesis of C. psittaci (Chen et al., 2023).
HP is classified as a gram-negative bacterium (Kountouras et al., 2007). It has an oral-fecal transition (Cuomo et al., 2020), colonizing the gastric mucosa and causing digestive disease and increasing the risk for vascular disorders and neurodegenerative diseases, such as AD (Kountouras et al., 2007). Douros et al. examined whether clinically apparent HP infection (CAHPI) is linked to AD. The population-based cohort included all UK Clinical Practice Research Datalink dementia-free subjects aged over 50 years, and matched each AD case with 40 controls. Results indicated that CAHPI was moderately associated with AD (11% increased risk) (Douros et al., 2024). Moreover, the nuclear magnetic resonance (NMR) of HP-infected cells has revealed excessive levels of branch chain amino acids (BCAA) isoleucine, leucine, and valine (Lynch and Adams, 2014). BCAA and glutamate metabolism have been found altered in AD patients (Lynch and Adams, 2014). The researches implicate that excessive levels of isoleucine and valine could play a role in the alleviation of AD risk (Tynkkynen et al., 2018). BCAA transmits across the BBB through the large neutral amino acid transporter (LAT 1) (Singh and Ecker, 2018). Although the chronically elevated BCAA plasma levels are the prime driving force for rising the BCAA uptakes of the brain, tryptophan, as an inhibitor for the production of serotonin, is prohibitive in the brain. Serotonin is a substance that alleviates the formation of Aβ and promotes neural survival. On the other hand, the branched-chain amino acid transaminase (BCAT) enzyme turns BCAA into glutamate. High levels of glutamate can induce neuronal death due to excitotoxicity. Furthermore, HP induces over-activation of mammalian target of rapamycin complex 1 (mTORC1) (Lynch and Adams, 2014). This activation could cause the breakdown of the BBB through endothelial cell dysfunction, as well as lead to tau hyperphosphorylation and the formation and aggregation of amyloid plaques in the brain. This is due to the inhibition of autophagy, which helps prevent the buildup of Aβ. When autophagy is inhibited, the aggregation of Aβ occurs (Mueed et al., 2018).
Moreover, HP induces AD by BBB disruption. The immune system produces TNF-α against HP and this is followed by over-activation of matrix metalloproteinase, leading to BBB disruption. However, HP itself produces vacuolating cytotoxin (VacA) which exerts bone marrow-derived mast cells (BMDMCs) and provokes them to produce pro-inflammatory cytokines such as vascular endothelial growth factor (VEGF), IL-8, chymase, and tryptase, therefore disturbing the BBB (Kountouras et al., 2012; Kountouras et al., 2007; Kountouras, 2009). HP infection has a likely effect on α-synuclein accumulation which can be transferred to the brain via blood or vagus nerve and exert microglial cells to release IL-1β. This process damages the BBB and provokes oxidative stress, causing degeneration of the neurons (Fu et al., 2020). Disruption of the BBB induces entrance of immune cells, including CD4+ and CD8+ T cells, and promotes their infiltration which results in brain tissue degeneration (Kountouras et al., 2012). Furthermore, HP releases acids such as (iso)valeric, (iso)butyric, propionic, acetic, and formic acid that can disturb the function of microglia and astrocytes, contributing to aggregation of Aβ and tau phosphorylation and thus the progression of AD (Kountouras et al., 2007). HP-n, a histidine-rich protein found abundant in HP, which plays a crucial role in the formation of amyloid-like oligomers, can cross the BBB through the main LRP-1 and RAGE. These are both transporters for Aβ in BBB, and have a potential role in causing AD through Aβ plaques mechanism (Kountouras et al., 2012; Zavos et al., 2012). Finally, an effective way to delay the AD progression could be the eradication of HP in the initial stages of AD (Kountouras et al., 2010). To clarify this point, Kountouras et al. have investigated 46 patients with AD, all tested by upper GI colonoscopy and bereaved from taking H2 receptor antagonists and proton pump inhibitors. During the 5-year investigation period, all participants received the same ChEI. At the end, AD patients with successful HP eradication survived 10.62 months longer than those patients whose eradication was unsuccessful (Kountouras et al., 2010).
Periodontitis is a chronic inflammation leading to tooth loss and arousing the systemic inflammatory response. Various Gram-negative bacteria contribute to this inflammation, including P. gingivalis, and different viruses. P. gingivalis contains variety of virulence factors such as gingipains, outer membrane vesicles (OMVs) and cathepsin B (cat B) (Zhang et al., 2020). P. gingivalis has been detected in the brains of individuals with AD, alongside its harmful proteases, gingipains. This discovery correlates these levels with the existence of tau and ubiquitin pathology. Oral infection of mice with P. gingivalis has led to heightened production of Aβ1-42 and studies indicate that gingipains are detrimental to tau and possess neurotoxicity in vivo and vitro (Dominy et al., 2019). In vivo research has demonstrated that small molecule inhibitors of P. gingivalis gingipains can prevent neurodegeneration induced by gingipains and reduce P. gingivalis presence in the brain, whilst decreasing host Aβ1-42 response to P. gingivalis brain infection (Dominy et al., 2019).
P. gingivalis infects the oral cavity that is involved in the production of various pro-inflammatory molecules, including IL-1β, IL-6, and TNF-α. Besides, P. gingivalis accesses the brain through different mechanisms such as increasing the permeability of the BBB, pervading via cranial nerves like olfactory or trigeminal nerves and infecting the monocytes which are engaged by the brain (Dominy et al., 2019; Sadrameli et al., 2020). When P. gingivalis reaches the brain, gingipains, including arg-gingipain (Rgp) and lys-gingipain (Kgp), start the cleavage of pro-caspase-3 to produce activated caspase-3, a caspase which was associated with enhanced tau phosphorylation. This process impairs the neuronal function (Dominy et al., 2019; Costa et al., 2021). Furthermore, recent researches have shown that P. gingivalis OMVs carry out NLRP3 inflammasome activation and ASC speck accumulation and cause Aβ formation, inducing pre-apoptosis and neural cell death (Dominy et al., 2019).
LPS is a crucial part of the structure of OMVs in Gram-negative bacteria. When P. gingivalis LPS/lipoprotein interacts with pattern recognition receptors on innate immune cells, it can trigger immune responses. This can also lead to neuroinflammation in microglia-dependent manner. There is some debate, but evidence suggests that TLR2 and/or TLR4 on microglia may be the targets for P. gingivalis LPS/lipoprotein preparations. From here, downstream NF-κB and STAT3 pathways are activated, leading to increased expression and secretion of pro-inflammatory cytokines, including TNF-a, IL-1β, IL-6, IL-17 and IL-23 (Nativel et al., 2017; Qiu et al., 2018). P. gingivalis LPS preparations induce cathepsin B in microglial cells through the NF-κB pathway, leading to IL-1β production by microglia. When released, IL-1β acts on neuronal IL-1 receptors which promotes APP expression and tau phosphorylation (Villemagne et al., 2013). This further activates the NF-κB pathway and promotes microglia-mediated neuroinflammation (Wu et al., 2017). Moreover, P. gingivalis LPS preparations stimulate GSK-3b activation in microglia leading to the expression and secretion of TNF-a that acts on neurons and promotes AKT-GSK-3b-mediated tau hyperphosphorylation (Jiang et al., 2021). Conversely, P. gingivalis LPS treatment has been shown to upregulate the inactive form of protein phosphatase 2, a principal phosphatase for tau de-phosphorylation, rather than directly change GSK-3b activity in an APP-over-expressing neuroblastoma cell line (Zeng et al., 2021). A recent transcriptome study found that P. gingivalis LPS treatment of human neuroblastoma cells affects an array of interconnected pathways involving cellular oxidative stress, inflammation, and metabolism (Bahar and Singhrao, 2021). All these findings suggest a complex role for P. gingivalis LPS in AD pathophysiology, much like its role in the pathology of periodontitis.
As mentioned before, chronic periodontitis causes tooth loss in adults. Having fewer teeth leads to a reduction in chewing and also decreases acetylcholine level in the hippocampus through degeneration of pyramidal cells, resulting in memory loss (Gaur and Agnihotri, 2015). It should be noted that periodontitis caused by P. gingivalis can leads to the overexpression of platelet aggregation proteins and atherosclerosis. Moreover, a systemic inflammation caused by this pathogen may be contributed in endothelial dysfunction. So, considering the accumulating evidence suggesting the role of vascular dysfunction in the pathogenesis of AD, the periodontitis caused by P. gingivalis may have a contribution role in the development of AD (Uppoor et al., 2013). P. gingivalis infection induces changes in the molecular clock function of the microglial cells. Followingly, this impairs the sleep patterns and circadian system which is known as an important factor in the phagocytosis activity of microglial cells. Therefore, this process can lead to decreased clearance of Aβ peptides and an elevation in accumulation of aggregated proteins (Harding et al., 2017). The glymphatic system, which uses glial water channels to clear interstitial solutes and Aβ plaques from the brain (Xie et al., 2013), is “turned on” during normal sleep and reduced during the awake state (Xie et al., 2013). With aging and mixed pathological factors, intrinsic stress due to infections, which affects sleep quality and duration, impairments to the glymphatic system may interact to influence AD development and progression (Slats et al., 2013; Ju et al., 2013). P. gingivalis can disturb the microglial cell phagocytic activity by disrupting the circadian system that controls sleep–wake cycles (Takayama et al., 2016). Consequently, the glymphatic system appears less efficient, and this may lead to aggregated protein build-up. This connection has crucial relevance to sleep pattern disturbances in AD and supports how poor oral hygiene and rising levels of intrinsic and extrinsic sources of cytokines may act as crucial early modifiers of neurodegeneration and disease severity leading to deteriorating memory, sleep, and ultimately the development of pathology (Harding et al., 2017).
The pathogenesis of P. gingivalis via miRNAs in human periodontal ligament cells (hPDLCs) has been explored. Fan et al. found that P. gingivalis OMVs promote alveolar bone resorption in vivo and decrease cell viability in hPDLCs by inducing apoptosis and inflammation (Fan et al., 2023). Transcriptome sequencing results showed that P. gingivalis OMVs were involved in gene regulation, mRNA processing, endocytosis, ubiquitination, and the cell cycle process, and several small RNAs secreted via P. gingivalis OMVs were identified, including sRNA45033. This small RNA directly bound to the 3′ UTR of the downstream target gene CBX5, resulting in decreased levels of CBX5 in P. gingivalis OMV-stimulated hPDLCs, which regulated apoptosis through p53 and H3K9me3 methylation (Fan et al., 2023).
Spirochetes, Gram-negative, helical bacteria have been found in various human tissues such as mouth, genital mucosa, and GI tract. The spirochete Borrelia burgdorferi is a tick-borne agent that causes Lyme disease. The symptoms include influenza-like illness and neurological indications. Many studies have indicated that there is an association between spirochetes and their induced illnesses, especially Lyme disease with AD (Miklossy, 2008). This relationship develops through various mechanisms. Studies demonstrate that the outer surface protein (Osp A) of spirochetes is an amyloid executor that leads to Aβ formation and thus AD (Miklossy, 2008). Furthermore, B.burgdorferi evades the immune system through binding to the complement inhibitor factor H (FH) and factor H like protein-1 (FHL_1) (Dulipati et al., 2020). Through this mechanism the bacterium is protected against phagocytosis and complement lysis and thus continues to survive and reach the brain via blood circulation. It can cross the BBB and proliferate in the infected tissue (Miklossy, 2008). When they reach a quorum, they generate a biofilm. Production of the biofilm protects the microorganism against the immune system (Rutherford and Bassler, 2012). The first factor which responds to this organism is TLR2. TLR2 induces the release of TNF-α and NF-κB through myeloid differentiation pathway D88 (MYD88) (Allen et al., 2016). TNF-α, in combination with TNF-α converting enzyme (TACE), forms the α-secretase to produce amyloid alpha. Thus, NF-κB and Aβ converting enzyme (BACE) activate β- and γ-secretase. This process finally leads to the production of Aβ which attacks the biofilms but is not able to annihilate them, instead cause the destruction of the surrounding tissue (Allen, 2016). Besides, iron is a necessary growth factor for the bacterium. The aggregation of the iron in the brain accelerates the production of reactive oxygen which leads to lipid peroxidation and induces oxidative stress (Carocci et al., 2018). Iron also induces T cells to produce pro-inflammatory cytokines (Griffiths, 1991; Weinberg, 1992). Both of these processes lead to Aβ deposition and the pathogenesis of AD (Miklossy, 2008).
Generally, B.burgdorferi may cause AD through its various surface antigens such as Osp A, producing a biofilm to protect them against the immune system and aggregating iron for its division.
T. gondii is a protozoan parasite belonging to the phylum Apicomplexa. Research has indicated that the parasite Toxoplasma gondii (T. gondii) impairs learning and memory functions. However, interestingly, the same studies have also reported that this infection enhances synaptic plasticity in the dorsal hippocampus. This dual effect suggests a complex interaction between the parasite and the host’s neural mechanisms. On one hand, the cognitive deficits highlight the detrimental impact of T. gondii on overall brain function. On the other, the increase in synaptic plasticity points to a paradoxical enhancement in the adaptability and connectivity of neurons within the dorsal hippocampus, which is critical for memory formation and spatial navigation (Choopani et al., 2023). Possible involvement of T. gondii in the progression of AD could be due to various mechanisms, including its ability to induce the host immune responses, cause inflammation in the central nervous system, alter neurotransmitter levels, and activate indoleamine-2,3-dyoxigenase (Nayeri et al., 2021). The development of AD is influenced by neuroinflammation, oxidative stress, and vascular factors (Aliev et al., 2014). Inflammation has been identified as a factor in AD for almost 20 years (Kusbeci et al., 2011). Taking nonsteroidal anti-inflammatory drugs can reduce the risk of developing AD (Vlad et al., 2008). Inflammatory responses are also the innate defense against T. gondii infection (Guerreiro et al., 2013; Lambert et al., 2010). T. gondii triggers the immune system to release cytokines such as IFN-γ, IL-12, IL-1, IL-6, and TNF (Kusbeci et al., 2011; Glass et al., 2010). While these cytokines can protect against T. gondii, they can also damage non-infected neurons and affect neurotransmitter function and synaptic transmission (Dunn, 2006; McCusker and Kelley, 2013). Inflammatory mediators can stimulate the progression of AD by activating the processing of APPs (Griffin and Barger, 2010). During toxoplasmic encephalitis, the activity of neurotransmitters is affected by cytokines and inflammatory mediators through various mechanisms including activation of indoleamine-2,3-dioxygenase enzyme, activation of mitogen-activated protein kinase pathways, changes in tetrahydrobiopterin enzyme activity, excitotoxicity, and oxidative stress (Haroon et al., 2012). Moreover, patients with AD exhibit high levels of the transcription factor NF-κB (Camandola and Mattson, 2007), which modulates immune and inflammatory responses and is activated during T. gondii infection (Gupta et al., 2010; Blader and Saeij, 2009). The activation of NF-κB can accelerate neuroinflammation to neurodegeneration in AD (Srinivasan and Lahiri, 2015), but it also prevents the apoptosis of infected cells (Molestina and Sinai, 2005). Inhibition of NF-κB target genes involved in inflammation can disrupt the immune response to T. gondii, allowing the parasite to replicate (Molestina and Sinai, 2005).
In addition, infection with T. gondii increases the INF-δ secretion and leads to NO production which causes neural degeneration resulting in AD (Nayeri Chegeni et al., 2019; Mahami-Oskouei et al., 2016). Luisa Torres et al. infected wild-type mice with T. gondii and tested their anatomical and behavioral impressions via immunohistochemistry, western blotting and immunofluorescence. They claimed that T. gondii disturbs the N-methyl-D-aspartate receptor (NMDAR) signaling which plays a vital role in synaptic plasticity. This destruction impacts Aβ accumulation and hyperphosphorylation of tau which consequently leads to AD (Torres et al., 2018).
On the other hand, some studies stated that Toxoplasmosis cannot be considered as a risk factor for progression of AD (Nayeri Chegeni et al., 2019). A meta-analysis study conducted by Mahami-Oskouei et al. (2016) reported that T. gondii cannot be considered as a risk factor for AD progression (Mahami-Oskouei et al., 2016). Accordingly, a number of researches have suggested that AD could have an adverse effect on progression as a result of T. gondii infection. Toxoplasmosis promotes expression of anti-inflammatory responses such as suppressor of cytokine signaling 1(SOCS1), Arg1, TGF-β and IL-10, and also decreases the generation of inflammatory mediators including NO (Jung et al., 2012; Rozenfeld et al., 2005; Cabral et al., 2017). Furthermore, Jung et al. (2012) indicated that T. gondii infection causes immunosuppression in its hots and inhibits AD progression. They infected mice with T. gondii and assayed anti-inflammatory cytokines, including IL-10 and TGF-β, and also Aβ accumulation in mice’s brain and used water maze and Y-maze behavioral tests. The results showed that anti-inflammatory cytokines levels were remarkably higher, while Aβ plaques deposition was significantly lower, in both the hippocampus and cortex of T. gondii infected mices (Jung et al., 2012). Furthermore, T. gondii prohibits apoptosis by upregulation of anti-apoptotic genes or by disturbing the apoptotic signaling pathway. Exposure to T. gondii promotes M1 polarization of microglia and microglial proliferation. The accelerated proliferation of microglial cells leads to Aβ phagocytosis and clearance. These processes prevent neural degeneration and AD (Shin et al., 2021).
Generally, infection with T. gondii may lead to AD through increasing inflammatory mediators, NO production, disrupting synapses and Aβ plaque deposition. On the other hand, due to the augment of anti-inflammatory cytokines, it presumably inhibits the neuronal degeneration. At last, more studies are needed in this area.
Given the literature, infectious agents are likely to play a profound role in the pathogenesis of some neurodegenerative diseases like AD. In this review, the effect of various pathogens in AD pathogenesis have been reviewed. Several studies have suggested that infectious agents can foster the Aβ cascade. Additionally, brain infection can trigger tau protein hyperphosphorylation, which results in neuronal degeneration and loss of synapses. Furthermore, both local and systemic infections can induce microglial and astrocytic activation and the release of pro-inflammatory cytokines by over-stimulating the immune system. This process has been consistently associated with excessive oxidative stress, which has emerged as a significant factor in AD pathogenesis. Genetic predispositions, immune response, lifestyle factors, and microbiome balance can all play a role in the development of AD. Aging and other health conditions can also increase vulnerability to the disease. However, not every infected person develops AD, as individual factors may influence outcomes. A robust immune system and healthy lifestyle choices can help mitigate the impact of infections on brain health. Understanding the specific mechanisms by which these pathogens influence Aβ deposition and neuroinflammation can open new avenues for therapeutic interventions. Further research is essential to elucidate the precise roles of these pathogens in AD and to develop effective treatments that address the multifaceted nature of this devastating disease.
SH: Writing – original draft. RE: Writing – original draft, Writing – review & editing. SY: Writing – original draft. KK: Writing – review & editing. YK: Writing – original draft. RH: Writing – review & editing. AM: Writing – review & editing. MobF: Writing – review & editing. KV: Writing – review & editing. AT: Writing – review & editing. ZT: Writing – review & editing. TF: Writing – review & editing. MohF: Conceptualization, Supervision, Writing – review & editing. MH: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to thank the Critical Care Quality Improvement Research Center, Shahid Modarres Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran for their support, cooperation, and assistance throughout the study. The ethical approve number for this study of Critical Care Quality Improvement Research Center, Shahid Modarres Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran is IR.SBMU.RETECH.REC.1403.568.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbdelHamid, S. G., Refaat, A. A., Benjamin, A. M., Elmawardy, L. A., Elgendy, L. A., Manolly, M. M., et al. (2021). Deciphering epigenetic(s) role in modulating susceptibility to and severity of COVID-19 infection and/or outcome: a systematic rapid review. Environ. Sci. Pollut. Res. 28, 54209–54221. doi: 10.1007/s11356-021-15588-6
Abidin, S. Z., Mat Pauzi, N. A., Mansor, N. I., Mohd Isa, N. I., and Hamid, A. A. (2023). A new perspective on Alzheimer’s disease: microRNAs and circular RNAs. Front. Genet. 14:1486. doi: 10.3389/fgene.2023.1231486
Abrahamson, E. E., Zheng, W., Muralidaran, V., Ikonomovic, M. D., Bloom, D. C., Nimgaonkar, V. L., et al. (2021). Modeling Aβ42 accumulation in response to herpes simplex virus 1 infection: two dimensional or three dimensional? J. Virol. 95, e02219–e02220. doi: 10.1128/JVI.02219-20
Agostini, S., Costa, A. S., Mancuso, R., Guerini, F. R., Nemni, R., and Clerici, M. (2019). The PILRA G78R variant correlates with higher HSV-1-specific IgG titers in Alzheimer's disease. Cell. Mol. Neurobiol. 39, 1217–1221. doi: 10.1007/s10571-019-00712-5
Agostini, S., Mancuso, R., Baglio, F., and Clerici, M. (2017). A protective role for herpes simplex virus type-1-specific humoral immunity in Alzheimer's disease. Expert Rev. Anti-Infect. Ther. 15, 89–91. doi: 10.1080/14787210.2017.1264271
Ahmed, M., Davis, J., Aucoin, D., Sato, T., Ahuja, S., Aimoto, S., et al. (2010). Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nat. Struct. Mol. Biol. 17, 561–567. doi: 10.1038/nsmb.1799
Aiello, A. E., Haan, M., Blythe, L., Moore, K., Gonzalez, J. M., and Jagust, W. (2006). The influence of latent viral infection on rate of cognitive decline over 4 years. J. Am. Geriatr. Soc. 54, 1046–1054. doi: 10.1111/j.1532-5415.2006.00796.x
Alberti, K., Eckel, R. H., Grundy, S. M., Zimmet, P. Z., Cleeman, J. I., Donato, K. A., et al. (2009). Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 120, 1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644
Aliev, G., Priyadarshini, M., Reddy, V. P., Grieg, N. H., Kaminsky, Y., Cacabelos, R., et al. (2014). Oxidative stress mediated mitochondrial and vascular lesions as markers in the pathogenesis of Alzheimer disease. Curr. Med. Chem. 21, 2208–2217. doi: 10.2174/0929867321666131227161303
Allen, H. B. (2016). Alzheimer's disease: assessing the role of spirochetes, biofilms, the immune system, and amyloid-β with regard to potential treatment and prevention. J. Alzheimers Dis. 53, 1271–1276. doi: 10.3233/JAD-160388
Allen, H., Morales, D., Jones, K., and Joshi, S. (2016). Alzheimer’s disease: a novel hypothesis for the development and the subsequent role of beta amyloid. J. Neuroinfect. Dis. 7. doi: 10.4172/2314-7326.1000211
Almanzar, G., Schwaiger, S., Jenewein, B., Keller, M., Herndler-Brandstetter, D., Würzner, R., et al. (2005). Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J. Virol. 79, 3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005
Ambrosini, Y. M., Borcherding, D., Kanthasamy, A., Kim, H. J., Willette, A. A., Jergens, A., et al. (2019). The gut-brain Axis in neurodegenerative diseases and relevance of the canine model: a review. Front. Aging Neurosci. 11:130. doi: 10.3389/fnagi.2019.00130
Bagasra, O, Pandey, P, McCean, E, and Albrecht, H. (n.d.). Infectivity of Human Olfactory Neurons to SARS-CoV-2: A Link to Anosmia. vol. 19.
Bahar, B., and Singhrao, S. K. (2021). An evaluation of the molecular mode of action of trans-resveratrol in the Porphyromonas gingivalis lipopolysaccharide challenged neuronal cell model. Mol. Biol. Rep. 48, 147–156. doi: 10.1007/s11033-020-06024-y
Ball, M., Mathews, R., Steiner, I., Hill, J., Wisner, T., Murdoch, G., et al. (2001). Latent HSV 1 virus in trigeminal ganglia: the optimal site for linking prevention of Alzheimer’s disease to vaccination. Neurobiol. Aging 22, 705–709. doi: 10.1016/S0197-4580(01)00253-6
Ball, M., Nuttall, K., and Warren, K. (1982). Neuronal and lymphocytic populations in human trigeminal ganglia: implications for ageing and for latent virus. Neuropathol. Appl. Neurobiol. 8, 177–187. doi: 10.1111/j.1365-2990.1982.tb00273.x
Baranova, A., Cao, H., and Zhang, F. (2023). Causal effect of COVID-19 on Alzheimer's disease: a Mendelian randomization study. J. Med. Virol. 95:e28107. doi: 10.1002/jmv.28107
Barnes, L. L., Capuano, A. W., Aiello, A. E., Turner, A. D., Yolken, R. H., Torrey, E. F., et al. (2015). Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J. Infect. Dis. 211, 230–237. doi: 10.1093/infdis/jiu437
Barth, S., Pfuhl, T., Mamiani, A., Ehses, C., Roemer, K., Kremmer, E., et al. (2008). Epstein-Barr virus-encoded microRNA miR-BART2 down-regulates the viral DNA polymerase BALF5. Nucleic Acids Res. 36, 666–675. doi: 10.1093/nar/gkm1080
Bello-Morales, R., Andreu, S., and López-Guerrero, J. A. (2020). The role of herpes simplex virus type 1 infection in demyelination of the central nervous system. Int. J. Mol. Sci. 21:5026. doi: 10.3390/ijms21145026
Berger, J. R., and Houff, S. (2008). Neurological complications of herpes simplex virus type 2 infection. Arch. Neurol. 65, 596–600. doi: 10.1001/archneur.65.5.596
Blader, I. J., and Saeij, J. P. (2009). Communication between toxoplasma gondii and its host: impact on parasite growth, development, immune evasion, and virulence. APMIS 117, 458–476. doi: 10.1111/j.1600-0463.2009.02453.x
Bostancıklıoğlu, M. (2020). SARS-CoV2 entry and spread in the lymphatic drainage system of the brain. Brain Behav. Immun. 87, 122–123. doi: 10.1016/j.bbi.2020.04.080
Bourgade, K., Dupuis, G., Frost, E. H., and Fülöp, T. (2016). Anti-viral properties of amyloid-β peptides. J. Alzheimers Dis. 54, 859–878. doi: 10.3233/JAD-160517
Bourgade, K., Frost, E. H., Dupuis, G., Witkowski, J. M., Laurent, B., Calmettes, C., et al. (2022). Interaction mechanism between the HSV-1 glycoprotein B and the antimicrobial peptide amyloid-β. J. Alzheimers Dis. Rep. 6, 599–606. doi: 10.3233/ADR-220061
Bourgade, K., Garneau, H., Giroux, G., Le Page, A. Y., Bocti, C., Dupuis, G., et al. (2015). β-Amyloid peptides display protective activity against the human Alzheimer's disease-associated herpes simplex virus-1. Biogerontology 16, 85–98. doi: 10.1007/s10522-014-9538-8
Bourgade, K., Le Page, A., Bocti, C., Witkowski, J. M., Dupuis, G., Frost, E. H., et al. (2016). Protective effect of amyloid-β peptides against herpes simplex Virus-1 infection in a neuronal cell culture model. JAD 50, 1227–1241. doi: 10.3233/JAD-150652
Breijyeh, Z., and Karaman, R. (2020). Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules 25:5789. doi: 10.3390/molecules25245789
Bu, X. L., Yao, X. Q., Jiao, S. S., Zeng, F., Liu, Y. H., Xiang, Y., et al. (2015). A study on the association between infectious burden and a lzheimer's disease. Eur. J. Neurol. 22, 1519–1525. doi: 10.1111/ene.12477
Bukhbinder, A. S., Ling, Y., Hasan, O., Jiang, X., Kim, Y., Phelps, K. N., et al. (2022). Risk of Alzheimer's disease following influenza vaccination: a claims-based cohort study using propensity score matching. J. Alzheimers Dis. 88, 1061–1074. doi: 10.3233/JAD-220361
Bulka, C. M., Enggasser, A. E., and Fry, R. C. (2022). Epigenetics at the intersection of COVID-19 risk and environmental chemical exposures. Curr. Environ. Health Reports 9, 477–489. doi: 10.1007/s40572-022-00353-9
Cabral, C. M., McGovern, K. E., MacDonald, W. R., Franco, J., and Koshy, A. A. (2017). Dissecting amyloid Beta deposition using distinct strains of the neurotropic parasite toxoplasma gondii as a novel tool. ASN Neuro 9:1759091417724915. doi: 10.1177/1759091417724915
Cahill-Smith, S., and Li, J. M. (2014). Oxidative stress, redox signalling and endothelial dysfunction in ageing-related neurodegenerative diseases: a role of NADPH oxidase 2. Br. J. Clin. Pharmacol. 78, 441–453. doi: 10.1111/bcp.12357
Camandola, S., and Mattson, M. P. (2007). NF-kappa B as a therapeutic target in neurodegenerative diseases. Expert Opin. Ther. Targets 11, 123–132. doi: 10.1517/14728222.11.2.123
Carbone, I., Lazzarotto, T., Ianni, M., Porcellini, E., Forti, P., Masliah, E., et al. (2014). Herpes virus in Alzheimer's disease: relation to progression of the disease. Neurobiol. Aging 35, 122–129. doi: 10.1016/j.neurobiolaging.2013.06.024
Carocci, A., Catalano, A., Sinicropi, M. S., and Genchi, G. (2018). Oxidative stress and neurodegeneration: the involvement of iron. Biometals 31, 715–735. doi: 10.1007/s10534-018-0126-2
Chacko, A., Delbaz, A., Walkden, H., Basu, S., Armitage, C. W., Eindorf, T., et al. (2022). Chlamydia pneumoniae can infect the central nervous system via the olfactory and trigeminal nerves and contributes to Alzheimer’s disease risk. Sci. Rep. 12:2759. doi: 10.1038/s41598-022-06749-9
Chaves Filho, A. J. M., Gonçalves, F., Mottin, M., Andrade, C. H., Fonseca, S. N. S., and Macedo, D. S. (2021). Repurposing of Tetracyclines for COVID-19 neurological and neuropsychiatric manifestations: a valid option to control SARS-CoV-2-associated Neuroinflammation? J. Neuroimmune Pharmacol. 16, 213–218. doi: 10.1007/s11481-021-09986-3
Chen, L., Huang, Q., Luo, Y., Zhou, Y., Tong, T., Chen, Y., et al. (2023). MiR-184 targeting FOXO1 regulates host-cell oxidative stress induced by Chlamydia psittaci via the Wnt/β-catenin signaling pathway. Infect. Immun. 91:e0033723. doi: 10.1128/iai.00337-23
Choopani, S., Kiani, B., Aliakbari, S., Babaie, J., Golkar, M., Pourbadie, H. G., et al. (2023). Latent toxoplasmosis impairs learning and memory yet strengthens short-term and long-term hippocampal synaptic plasticity at perforant pathway-dentate gyrus, and Schaffer collatterals-CA1 synapses. Sci. Rep. 13:8959. doi: 10.1038/s41598-023-35971-2
Ciaccio, M., Lo Sasso, B., Scazzone, C., Gambino, C. M., Ciaccio, A. M., Bivona, G., et al. (2021). COVID-19 and Alzheimer’s disease. Brain Sci. 11, 1–10. doi: 10.3390/brainsci11030305
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., et al. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923. doi: 10.1126/science.8346443
Costa, M. J. F., de Araújo, I. D. T., da Rocha, A. L., da Silva, R. L., Dos Santos, C. P., Borges, B. C. D., et al. (2021). Relationship of Porphyromonas gingivalis and Alzheimer's disease: a systematic review of pre-clinical studies. Clin. Oral Investig. 25, 797–806. doi: 10.1007/s00784-020-03764-w
Cuomo, P., Papaianni, M., Sansone, C., Iannelli, A., Iannelli, D., Medaglia, C., et al. (2020). An in vitro model to investigate the role of Helicobacter pylori in type 2 diabetes, obesity, Alzheimer's disease and Cardiometabolic disease. Int. J. Mol. Sci. 21:8369. doi: 10.3390/ijms21218369
De Plano, L. M., Saitta, A., Oddo, S., and Caccamo, A. (2024). Epigenetic changes in Alzheimer’s disease: DNA methylation and histone modification. Cells 13:719. doi: 10.3390/cells13080719
Delzenne, N. M., Neyrinck, A. M., and Cani, P. D. (2011). Modulation of the gut microbiota by nutrients with prebiotic properties: consequences for host health in the context of obesity and metabolic syndrome. Microb. Cell Factories 10:S10. doi: 10.1186/1475-2859-10-S1-S10
Desforges, M., Le Coupanec, A., Dubeau, P., Bourgouin, A., Lajoie, L., Dubé, M., et al. (2020). Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 12:14. doi: 10.3390/v12010014
Dezfulian, M. (2018). A new Alzheimer's disease cell model using B cells to induce beta amyloid plaque formation and increase TNF alpha expression. Int. Immunopharmacol. 59, 106–112. doi: 10.1016/j.intimp.2018.04.012
Dezfulian, M., Shokrgozar, M. A., Sardari, S., Parivar, K., and Javadi, G. (2008). Can phages cause Alzheimer's disease? Med. Hypotheses 71, 651–656. doi: 10.1016/j.mehy.2008.07.005
Dhar, D., and Mohanty, A. (2020). Gut microbiota and Covid-19- possible link and implications. Virus Res. 285:198018. doi: 10.1016/j.virusres.2020.198018
Dhungana, S., and Jankovic, J. (2013). Yips and other movement disorders in golfers. Mov. Disord. 28, 576–581. doi: 10.1002/mds.25442
Di Virgilio, F., Dal Ben, D., Sarti, A. C., Giuliani, A. L., and Falzoni, S. (2017). The P2X7 receptor in infection and inflammation. Immunity 47, 15–31. doi: 10.1016/j.immuni.2017.06.020
Dolatshahi, M., Sabahi, M., and Aarabi, M. H. (2021). Pathophysiological clues to how the emergent SARS-CoV-2 can potentially increase the susceptibility to neurodegeneration. Mol. Neurobiol. 58, 2379–2394. doi: 10.1007/s12035-020-02236-2
Domingues, C., da Cruz E Silva, O. A. B., and Henriques, A. G. (2017). Impact of cytokines and chemokines on Alzheimer's disease neuropathological hallmarks. Curr. Alzheimer Res. 14, 870–882. doi: 10.2174/1567205014666170317113606
Dominy, S. S., Lynch, C., Ermini, F., Benedyk, M., Marczyk, A., Konradi, A., et al. (2019). Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5. doi: 10.1126/sciadv.aau3333
Dong, M., Zhang, J., Ma, X., Tan, J., Chen, L., Liu, S., et al. (2020). ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed. Pharmacother. 131:110678. doi: 10.1016/j.biopha.2020.110678
dos Santos Picanco, L., Ozela, P. F., de Fatima de Brito Brito, M., Pinheiro, A. A., Padilha, E. C., Braga, F. S., et al. (2018). Alzheimer's disease: a review from the pathophysiology to diagnosis, new perspectives for pharmacological treatment. Curr. Med. Chem. 25, 3141–3159. doi: 10.2174/0929867323666161213101126
Douros, A., Ante, Z., Fallone, C. A., Azoulay, L., Renoux, C., Suissa, S., et al. (2024). Clinically apparent Helicobacter pylori infection and the risk of incident Alzheimer's disease: a population-based nested case-control study. Alzheimers Dement. 20, 1716–1724. doi: 10.1002/alz.13561
Dulipati, V., Meri, S., and Panelius, J. (2020). Complement evasion strategies of Borrelia burgdorferi sensu lato. FEBS Lett. 594, 2645–2656. doi: 10.1002/1873-3468.13894
Dunn, A. J. (2006). Effects of cytokines and infections on brain neurochemistry. Clin. Neurosci. Res. 6, 52–68. doi: 10.1016/j.cnr.2006.04.002
Esposito, G., Pesce, M., Seguella, L., Sanseverino, W., Lu, J., and Sarnelli, G. (2020). Can the enteric nervous system be an alternative entrance door in SARS-CoV2 neuroinvasion? Brain Behav. Immun. 87, 93–94. doi: 10.1016/j.bbi.2020.04.060
Fan, R., Zhou, Y., Chen, X., Zhong, X., He, F., Peng, W., et al. (2023). Porphyromonas gingivalis outer membrane vesicles promote apoptosis via msRNA-regulated DNA methylation in periodontitis. Microbiol. Spectr. 11:e0328822. doi: 10.1128/spectrum.03288-22
Feng, S., Liu, Y., Zhou, Y., Shu, Z., Cheng, Z., Brenner, C., et al. (2023). Mechanistic insights into the role of herpes simplex virus 1 in Alzheimer’s disease. Front. Aging Neurosci. 15:15. doi: 10.3389/fnagi.2023.1245904
Filon, J. R., Intorcia, A. J., Sue, L. I., Vazquez Arreola, E., Wilson, J., Davis, K. J., et al. (2016). Gender differences in Alzheimer disease: brain atrophy, histopathology burden, and cognition. J. Neuropathol. Exp. Neurol. 75, 748–754. doi: 10.1093/jnen/nlw047
Fu, P., Gao, M., and Yung, K. K. L. (2020). Association of Intestinal Disorders with Parkinson's disease and Alzheimer's disease: a systematic review and Meta-analysis. ACS Chem. Neurosci. 11, 395–405. doi: 10.1021/acschemneuro.9b00607
Gate, D., Saligrama, N., Leventhal, O., Yang, A. C., Unger, M. S., Middeldorp, J., et al. (2020). Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer's disease. Nature 577, 399–404. doi: 10.1038/s41586-019-1895-7
Gaur, S., and Agnihotri, R. (2015). Alzheimer's disease and chronic periodontitis: is there an association? Geriatr Gerontol Int 15, 391–404. doi: 10.1111/ggi.12425
Geng, L., Fan, L. M., Liu, F., Smith, C., and Li, J.-M. (2020). Nox2 dependent redox-regulation of microglial response to amyloid-β stimulation and microgliosis in aging. Sci. Rep. 10, 1–11. doi: 10.1038/s41598-020-58422-8
Gieffers, J., van Zandbergen, G., Rupp, J., Sayk, F., Krüger, S., Ehlers, S., et al. (2004). Phagocytes transmit Chlamydia pneumoniae from the lungs to the vasculature. Eur. Respir. J. 23, 506–510. doi: 10.1183/09031936.04.00093304
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C., and Gage, F. H. (2010). Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934. doi: 10.1016/j.cell.2010.02.016
Griffin, W. S., and Barger, S. W. (2010). Neuroinflammatory cytokines-the common thread in Alzheimer's pathogenesis. US Neurol. 6, 19–27
Griffiths, E. (1991). Iron and bacterial virulence—a brief overview. Biol. Met. 4, 7–13. doi: 10.1007/BF01135551
Guerreiro, R., Wojtas, A., Bras, J., Carrasquillo, M., Rogaeva, E., Majounie, E., et al. (2013). TREM2 variants in Alzheimer's disease. N. Engl. J. Med. 368, 117–127. doi: 10.1056/NEJMoa1211851
Gupta, S. C., Sundaram, C., Reuter, S., and Aggarwal, B. B. (2010). Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 1799, 775–787. doi: 10.1016/j.bbagrm.2010.05.004
Harding, A., Robinson, S., Crean, S., and Singhrao, S. K. (2017). Can better Management of Periodontal Disease Delay the onset and progression of Alzheimer's disease? JAD 58, 337–348. doi: 10.3233/JAD-170046
Haroon, E., Raison, C. L., and Miller, A. H. (2012). Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 37, 137–162. doi: 10.1038/npp.2011.205
Hascup, E. R., and Hascup, K. N. (2020). Does SARS-CoV-2 infection cause chronic neurological complications? Geroscience 42, 1083–1087. doi: 10.1007/s11357-020-00207-y
He, Q., Liu, H., Huang, C., Wang, R., Luo, M., and Lu, W. (2020). Herpes simplex virus 1-induced blood-brain barrier damage involves apoptosis associated with GM130-mediated Golgi stress. Front. Mol. Neurosci. 13:2. doi: 10.3389/fnmol.2020.00002
Heneka, M. T., Golenbock, D., Latz, E., Morgan, D., and Brown, R. (2020). Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res. Ther. 12, 1–3. doi: 10.1186/s13195-020-00640-3
Heneka, M. T., Kummer, M. P., and Latz, E. (2014). Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14, 463–477. doi: 10.1038/nri3705
Heneka, M. T., Kummer, M. P., Stutz, A., Delekate, A., Schwartz, S., Vieira-Saecker, A., et al. (2013). NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678. doi: 10.1038/nature11729
Hjelholt, A. J., Bergh, C., Bhatt, D. L., Fröbert, O., and Kjolby, M. F. (2023). Pleiotropic effects of influenza vaccination. Vaccines 11:1419. doi: 10.3390/vaccines11091419
Hong, Y., Xu, J., Hu, Y., Li, L., Dong, Z., Zhu, T.-k., et al. (2018). Neuroinflammation and Neuroimmunomodulation in Alzheimer’s disease. Curr. Pharmacol. Rep. 4, 408–413. doi: 10.1007/s40495-018-0148-z
Iizasa, H., Wulff, B. E., Alla, N. R., Maragkakis, M., Megraw, M., Hatzigeorgiou, A., et al. (2010). Editing of Epstein-Barr virus-encoded BART6 microRNAs controls their dicer targeting and consequently affects viral latency. J. Biol. Chem. 285, 33358–33370. doi: 10.1074/jbc.M110.138362
Itzhaki, R. F., Wozniak, M. A., Appelt, D. M., and Balin, B. J. (2004). Infiltration of the brain by pathogens causes Alzheimer's disease. Neurobiol. Aging 25, 619–627. doi: 10.1016/j.neurobiolaging.2003.12.021
Jacobs, A. H., and Tavitian, B. (2012). Noninvasive molecular imaging of neuroinflammation. J. Cereb. Blood Flow Metab. 32, 1393–1415. doi: 10.1038/jcbfm.2012.53
Jakhmola, S., Indari, O., Chatterjee, S., and Jha, H. C. (2020). SARS-CoV-2, an underestimated pathogen of the nervous system. SN Comp. Clin. Med. 2, 2137–2146. doi: 10.1007/s42399-020-00522-7
Jang, H., Boltz, D., Sturm-Ramirez, K., Shepherd, K. R., Jiao, Y., Webster, R., et al. (2009). Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc. Natl. Acad. Sci. USA 106, 14063–14068. doi: 10.1073/pnas.0900096106
Jha, H. C., Mehta, D., Lu, J., El-Naccache, D., Shukla, S. K., Kovacsics, C., et al. (2015). Gammaherpesvirus infection of human neuronal cells. MBio 6, e01844–e01815. doi: 10.1128/mBio.01844-15
Ji, Q., Lian, W., Meng, Y., Liu, W., Zhuang, M., Zheng, N., et al. (2023). Cytomegalovirus infection and Alzheimer’s disease: a Meta-analysis. J. Prev Alzheimers Dis. :11. doi: 10.14283/jpad.2023.126
Jiang, M., Zhang, X., Yan, X., Mizutani, S., Kashiwazaki, H., Ni, J., et al. (2021). GSK3β is involved in promoting Alzheimer's disease pathologies following chronic systemic exposure to Porphyromonas gingivalis lipopolysaccharide in amyloid precursor protein(NL-F/NL-F) knock-in mice. Brain Behav. Immun. 98, 1–12. doi: 10.1016/j.bbi.2021.08.213
Ju, Y. E., McLeland, J. S., Toedebusch, C. D., Xiong, C., Fagan, A. M., Duntley, S. P., et al. (2013). Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 70, 587–593. doi: 10.1001/jamaneurol.2013.2334
Jung, Y. J., Choi, H., Kim, H., and Lee, S. K. (2014). MicroRNA miR-BART20-5p stabilizes Epstein-Barr virus latency by directly targeting BZLF1 and BRLF1. J. Virol. 88, 9027–9037. doi: 10.1128/JVI.00721-14
Jung, E. S., and Mook-Jung, I. (2024). “Sex differences in Alzheimer’s disease pathogenesis” in Sex/gender-specific medicine in clinical areas. ed. N. Kim (Singapore: Springer Nature Singapore), 403–421.
Jung, B. K., Pyo, K. H., Shin, K. Y., Hwang, Y. S., Lim, H., Lee, S. J., et al. (2012). Toxoplasma gondii infection in the brain inhibits neuronal degeneration and learning and memory impairments in a murine model of Alzheimer's disease. PLoS One 7:e33312. doi: 10.1371/journal.pone.0033312
Kanakry, J. A., Hegde, A. M., Durand, C. M., Massie, A. B., Greer, A. E., Ambinder, R. F., et al. (2016). The clinical significance of EBV DNA in the plasma and peripheral blood mononuclear cells of patients with or without EBV diseases. Blood 127, 2007–2017. doi: 10.1182/blood-2015-09-672030
Kang, J. S., and Liu, P. P. (2020). Human herpesvirus 4 and adaptive immunity in Alzheimer's disease. Signal Transduct. Target. Ther. 5:1–48. doi: 10.1038/s41392-020-0125-y
Karasneh, G. A., and Shukla, D. (2011). Herpes simplex virus infects most cell types in vitro: clues to its success. Virol. J. 8:481. doi: 10.1186/1743-422X-8-481
Klibaner-Schiff, E., Simonin, E. M., Akdis, C. A., Cheong, A., Johnson, M. M., Karagas, M. R., et al. (2024). Environmental exposures influence multigenerational epigenetic transmission. Clin. Epigenetics 16:145. doi: 10.1186/s13148-024-01762-3
Kloske, C. M., and Wilcock, D. M. (2020). The important interface between apolipoprotein E and neuroinflammation in Alzheimer’s disease. Front. Immunol. 11:754. doi: 10.3389/fimmu.2020.00754
Koch, S., Solana, R., Dela Rosa, O., and Pawelec, G. (2006). Human cytomegalovirus infection and T cell immunosenescence: a mini review. Mech. Ageing Dev. 127, 538–543. doi: 10.1016/j.mad.2006.01.011
Koulintchenko, M., Temperley, R. J., Mason, P. A., Dietrich, A., and Lightowlers, R. N. (2006). Natural competence of mammalian mitochondria allows the molecular investigation of mitochondrial gene expression. Hum. Mol. Genet. 15, 143–154. doi: 10.1093/hmg/ddi435
Kountouras, J. (2009). Helicobacter pylori: An intruder involved in conspiring glaucomatous neuropathy, BMJ Publishing Group Ltd, vol. 93:1413–1415.
Kountouras, J., Boziki, M., Gavalas, E., Zavos, C., Deretzi, G., Chatzigeorgiou, S., et al. (2010). Five-year survival after Helicobacter pylori eradication in Alzheimer disease patients. Cognit. Behav. Neurol. 23, 199–204. doi: 10.1097/WNN.0b013e3181df3034
Kountouras, J., Boziki, M., Zavos, C., Gavalas, E., Giartza-Taxidou, E., Venizelos, I., et al. (2012). A potential impact of chronic Helicobacter pylori infection on Alzheimer's disease pathobiology and course. Neurobiol. Aging 33, e3–e4. doi: 10.1016/j.neurobiolaging.2012.01.003
Kountouras, J., Gavalas, E., Zavos, C., Stergiopoulos, C., Chatzopoulos, D., Kapetanakis, N., et al. (2007). Alzheimer's disease and Helicobacter pylori infection: defective immune regulation and apoptosis as proposed common links. Med. Hypotheses 68, 378–388. doi: 10.1016/j.mehy.2006.06.052
Kountouras, J., Zavos, C., Gavalas, E., Boziki, M., Chatzopoulos, D., and Katsinelos, P. (2007). Normal-tension glaucoma and Alzheimer's disease: Helicobacter pylori as a possible common underlying risk factor. Med. Hypotheses 68, 228–229. doi: 10.1016/j.mehy.2006.07.008
Kristen, H., Santana, S., Sastre, I., Recuero, M., Bullido, M. J., and Aldudo, J. (2015). Herpes simplex virus type 2 infection induces AD-like neurodegeneration markers in human neuroblastoma cells. Neurobiol. Aging 36, 2737–2747. doi: 10.1016/j.neurobiolaging.2015.06.014
Kumar, A., Barrett, J. P., Alvarez-Croda, D.-M., Stoica, B. A., Faden, A. I., and Loane, D. J. (2016). NOX2 drives M1-like microglial/macrophage activation and neurodegeneration following experimental traumatic brain injury. Brain Behav. Immun. 58, 291–309. doi: 10.1016/j.bbi.2016.07.158
Kumar, A., and Singh, A.Ekavali (2015). A review on Alzheimer's disease pathophysiology and its management: an update. Pharmacol. Rep. 67, 195–203. doi: 10.1016/j.pharep.2014.09.004
Kusbeci, O. Y., Miman, O., Yaman, M., Aktepe, O. C., and Yazar, S. (2011). Could toxoplasma gondii have any role in Alzheimer disease? Alzheimer Dis. Assoc. Disord. 25, 1–3. doi: 10.1097/WAD.0b013e3181f73bc2
Lambert, J. C., Grenier-Boley, B., Chouraki, V., Heath, S., Zelenika, D., Fievet, N., et al. (2010). Implication of the immune system in Alzheimer's disease: evidence from genome-wide pathway analysis. JAD 20, 1107–1118. doi: 10.3233/JAD-2010-100018
Lehrer, S., and Rheinstein, P. H. (2022). Vaccination reduces risk of Alzheimer's disease, Parkinson's disease and other neurodegenerative disorders. Discov. Med. 34, 97–101
Letenneur, L., Pérès, K., Fleury, H., Garrigue, I., Barberger-Gateau, P., Helmer, C., et al. (2008). Seropositivity to herpes simplex virus antibodies and risk of Alzheimer's disease: a population-based cohort study. PLoS One 3:e3637. doi: 10.1371/journal.pone.0003637
Levine, K. S., Leonard, H. L., Blauwendraat, C., Iwaki, H., Johnson, N., Bandres-Ciga, S., et al. (2023). Virus exposure and neurodegenerative disease risk across national biobanks. Neuron 111, 1086–93.e2. doi: 10.1016/j.neuron.2022.12.029
Li, Y., Tan, M. S., Jiang, T., and Tan, L. (2014). Microglia in Alzheimer's disease. Biomed. Res. Int. 2014:437483, 1–7. doi: 10.1155/2014/437483
Licastro, F., Carbone, I., Ianni, M., and Porcellini, E. (2011). Gene signature in Alzheimer's disease and environmental factors: the virus chronicle. J. Alzheimers Dis. 27, 809–817. doi: 10.3233/JAD-2011-110755
Lim, S. L., Rodriguez-Ortiz, C. J., and Kitazawa, M. (2015). Infection, systemic inflammation, and Alzheimer's disease. Microbes Infect. 17, 549–556. doi: 10.1016/j.micinf.2015.04.004
Limphaibool, N., Iwanowski, P., Holstad, M. J. V., Kobylarek, D., and Kozubski, W. (2019). Infectious etiologies of parkinsonism: pathomechanisms and clinical implications. Front. Neurol. 10:652. doi: 10.3389/fneur.2019.00652
Lindlau, A., Widmann, C., Putensen, C., Jessen, F., Semmler, A., and Heneka, M. (2015). Predictors of hippocampal atrophy in critically ill patients. Eur. J. Neurol. 22, 410–415. doi: 10.1111/ene.12443
Liu, J. C., Hsu, Y. P., Kao, P. F., Hao, W. R., Liu, S. H., Lin, C. F., et al. (2016). Influenza vaccination reduces dementia risk in chronic kidney disease patients: a population-based cohort study. Medicine 95:e2868. doi: 10.1097/MD.0000000000002868
Liu, X., Jiao, B., and Shen, L. (2018). The epigenetics of Alzheimer’s disease: factors and therapeutic implications. Front. Genet. 9:579. doi: 10.3389/fgene.2018.00579
Liu, H., Qiu, K., He, Q., Lei, Q., and Lu, W. (2019). Mechanisms of blood-brain barrier disruption in herpes simplex encephalitis. J. Neuroimmune Pharmacol. 14, 157–172. doi: 10.1007/s11481-018-9821-6
Liu, J., Wang, F., Liu, S., Du, J., Hu, X., Xiong, J., et al. (2017). Sodium butyrate exerts protective effect against Parkinson's disease in mice via stimulation of glucagon like peptide-1. J. Neurol. Sci. 381, 176–181. doi: 10.1016/j.jns.2017.08.3235
Liu, Y., Wu, Z., Nakanishi, Y., Ni, J., Hayashi, Y., Takayama, F., et al. (2017). Infection of microglia with Porphyromonas gingivalis promotes cell migration and an inflammatory response through the gingipain-mediated activation of protease-activated receptor-2 in mice. Sci. Rep. 7:11759. doi: 10.1038/s41598-017-12173-1
Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., et al. (2017). Dementia prevention, intervention, and care. Lancet 390, 2673–2734. doi: 10.1016/S0140-6736(17)31363-6
Lövheim, H., Olsson, J., Weidung, B., Johansson, A., Eriksson, S., Hallmans, G., et al. (2018). Interaction between cytomegalovirus and herpes simplex virus type 1 associated with the risk of Alzheimer's disease development. JAD 61, 939–945. doi: 10.3233/JAD-161305
Lu, Y., Li, X., Geng, D., Mei, N., Wu, P. Y., Huang, C. C., et al. (2020). Cerebral Micro-structural changes in COVID-19 patients - an MRI-based 3-month follow-up study. EClinicalMedicine 25:100484. doi: 10.1016/j.eclinm.2020.100484
Lung, R. W., Tong, J. H., Sung, Y. M., Leung, P. S., Ng, D. C., Chau, S. L., et al. (2009). Modulation of LMP2A expression by a newly identified Epstein-Barr virus-encoded microRNA miR-BART22. Neoplasia 11, 1174–IN17. doi: 10.1593/neo.09888
Luo, M. (2012). Influenza Virus Entry. Viral Mol. Mach., 726:201–221. doi: 10.1007/978-1-4614-0980-9_9
Luo, C. S., Chi, C. C., Fang, Y. A., Liu, J. C., and Lee, K. Y. (2020). Influenza vaccination reduces dementia in patients with chronic obstructive pulmonary disease: a nationwide cohort study. J. Investig. Med. 68, 838–845. doi: 10.1136/jim-2019-001155
Luong, K. V., and Nguyễn, L. T. (2013). The beneficial role of thiamine in Parkinson disease. CNS Neurosci. Ther. 19, 461–468. doi: 10.1111/cns.12078
Lurain, N. S., Hanson, B. A., Martinson, J., Leurgans, S. E., Landay, A. L., Bennett, D. A., et al. (2013). Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J. Infect. Dis. 208, 564–572. doi: 10.1093/infdis/jit210
Lynch, C. J., and Adams, S. H. (2014). Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 10, 723–736. doi: 10.1038/nrendo.2014.171
Mahami-Oskouei, M., Hamidi, F., Talebi, M., Farhoudi, M., Taheraghdam, A. A., Kazemi, T., et al. (2016). Toxoplasmosis and Alzheimer: can toxoplasma gondii really be introduced as a risk factor in etiology of Alzheimer? Parasitol. Res. 115, 3169–3174. doi: 10.1007/s00436-016-5075-5
Mao, L., Jin, H., Wang, M., Hu, Y., Chen, S., He, Q., et al. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 77, 683–690. doi: 10.1001/jamaneurol.2020.1127
Marques, M., Ramos, B., Albuquerque, H., Pereira, M., Ribeiro, D. R., Nunes, A., et al. (2024). Influenza a virus propagation requires the activation of the unfolded protein response and the accumulation of insoluble protein aggregates. iScience 27:109100. doi: 10.1016/j.isci.2024.109100
Marques, M., Ramos, B., Albuquerque, H., Pereira, M., Ribeiro, D. R., Nunes, A., et al. (2023). Influenza a virus activates the unfolded protein response and induces the accumulation of insoluble protein aggregates that are essential for efficient viral propagation. bioRxiv. doi: 10.1101/2023.09.11.557148
Matschke, J., Lütgehetmann, M., Hagel, C., Sperhake, J. P., Schröder, A. S., Edler, C., et al. (2020). Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 19, 919–929. doi: 10.1016/S1474-4422(20)30308-2
Mawanda, F., and Wallace, R. (2013). Can infections cause Alzheimer's disease? Epidemiol. Rev. 35, 161–180. doi: 10.1093/epirev/mxs007
McCusker, R. H., and Kelley, K. W. (2013). Immune-neural connections: how the immune system's response to infectious agents influences behavior. J. Exp. Biol. 216, 84–98. doi: 10.1242/jeb.073411
Mehrbod, P., Ande, S. R., Alizadeh, J., Rahimizadeh, S., Shariati, A., Malek, H., et al. (2019). The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence 10, 376–413. doi: 10.1080/21505594.2019.1605803
Mielke, M. M. (2018). Sex and Gender Differences in Alzheimer's Disease Dementia. Psychiatr. Times 35, 14–17
Migliore, L., and Coppedè, F. (2022). Gene–environment interactions in Alzheimer disease: the emerging role of epigenetics. Nat. Rev. Neurol. 18, 643–660. doi: 10.1038/s41582-022-00714-w
Miklossy, J. (2008). Chronic inflammation and Amyloidogenesis in Alzheimer's disease -- role of Spirochetes1. JAD 13, 381–391. doi: 10.3233/JAD-2008-13404
Miksys, S., and Tyndale, R. (2006). Nicotine induces brain CYP enzymes: relevance to Parkinson’s disease. Parkinson’s Disease Related Disord., 70:177–180. doi: 10.1007/978-3-211-45295-0_28
Molestina, R. E., and Sinai, A. P. (2005). Detection of a novel parasite kinase activity at the toxoplasma gondii parasitophorous vacuole membrane capable of phosphorylating host IkappaBalpha. Cell. Microbiol. 7, 351–362. doi: 10.1111/j.1462-5822.2004.00463.x
Montagne, A., Zhao, Z., and Zlokovic, B. V. (2017). Alzheimer’s disease: a matter of blood–brain barrier dysfunction? J. Exp. Med. 214, 3151–3169. doi: 10.1084/jem.20171406
Morales, I., Guzmán-Martínez, L., Cerda-Troncoso, C., Farías, G. A., and Maccioni, R. B. (2014). Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 8:8. doi: 10.3389/fncel.2014.00112
Mueed, Z., Tandon, P., Maurya, S. K., Deval, R., Kamal, M. A., and Poddar, N. K. (2018). Tau and mTOR: the hotspots for multifarious diseases in Alzheimer's development. Front. Neurosci. 12:1017. doi: 10.3389/fnins.2018.01017
Muegge, B. D., Kuczynski, J., Knights, D., Clemente, J. C., González, A., Fontana, L., et al. (2011). Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 332, 970–974. doi: 10.1126/science.1198719
Nativel, B., Couret, D., Giraud, P., Meilhac, O., d'Hellencourt, C. L., Viranaïcken, W., et al. (2017). Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human. Sci. Rep. 7:15789. doi: 10.1038/s41598-017-16190-y
Nayeri Chegeni, T., Sarvi, S., Moosazadeh, M., Sharif, M., Aghayan, S. A., Amouei, A., et al. (2019). Is toxoplasma gondii a potential risk factor for Alzheimer's disease? A systematic review and meta-analysis. Microb. Pathog. 137:103751. doi: 10.1016/j.micpath.2019.103751
Nayeri, T., Sarvi, S., Sharif, M., and Daryani, A. (2021). Toxoplasma gondii: a possible etiologic agent for Alzheimer's disease. Heliyon 7:e07151. doi: 10.1016/j.heliyon.2021.e07151
Ni, W., Yang, X., Yang, D., Bao, J., Li, R., Xiao, Y., et al. (2020). Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 24, 1–10. doi: 10.1186/s13054-020-03120-0
Nicola, M., Alsafi, Z., Sohrabi, C., Kerwan, A., Al-Jabir, A., Iosifidis, C., et al. (2020). Las implicaciones socioeconómicas de la pandemia de coronavirus (COVID-19): una revisión. Revista internacional de cirugía 78, 185–193. doi: 10.1016/j.ijsu.2020.04.018
Oh, S. J., Lee, J. K., and Shin, O. S. (2019). Aging and the immune system: the impact of Immunosenescence on viral infection, immunity and vaccine immunogenicity. Immune Netw. 19:e37. doi: 10.4110/in.2019.19.e37
Ou, Y. N., Zhu, J. X., Hou, X. H., Shen, X. N., Xu, W., Dong, Q., et al. (2020). Associations of infectious agents with Alzheimer's disease: a systematic review and Meta-analysis. J. Alzheimers Dis. 75, 299–309. doi: 10.3233/JAD-191337
Pawlica, P., Yario, T. A., White, S., Wang, J., Moss, W. N., Hui, P., et al. (2021). SARS-CoV-2 expresses a microRNA-like small RNA able to selectively repress host genes. Proc. Natl. Acad. Sci. 118:e2116668118. doi: 10.1073/pnas.2116668118
Piacentini, R., Civitelli, L., Ripoli, C., Marcocci, M. E., De Chiara, G., Garaci, E., et al. (2011). HSV-1 promotes Ca2+ −mediated APP phosphorylation and Aβ accumulation in rat cortical neurons. Neurobiol. Aging 32, 2323.e13–2323.e26. doi: 10.1016/j.neurobiolaging.2010.06.009
Piekut, T., Hurła, M., Banaszek, N., Szejn, P., Dorszewska, J., Kozubski, W., et al. (2022). Infectious agents and Alzheimer's disease. J. Integr. Neurosci. 21:73. doi: 10.31083/j.jin2102073
Premkumar, T., and Sajitha, L. S. (2023). Molecular crosstalk between COVID-19 and Alzheimer’s disease using microarray and RNA-seq datasets: a system biology approach. Front. Med. :10. doi: 10.3389/fmed.2023.1151046
Prosswimmer, T., Heng, A., and Daggett, V. (2024). Mechanistic insights into the role of amyloid-β in innate immunity. Sci. Rep. 14:5376. doi: 10.1038/s41598-024-55423-9
Qiu, J., and Thorley-Lawson, D. A. (2014). EBV microRNA BART 18-5p targets MAP3K2 to facilitate persistence in vivo by inhibiting viral replication in B cells. Proc. Natl. Acad. Sci. USA 111, 11157–11162. doi: 10.1073/pnas.1406136111
Qiu, Q., Zhang, F., Wu, J., Xu, N., and Liang, M. (2018). Gingipains disrupt F-actin and cause osteoblast apoptosis via integrin β1. J. Periodontal Res. 53, 762–776. doi: 10.1111/jre.12563
Ransohoff, R. M. (2016). How neuroinflammation contributes to neurodegeneration. Science 353, 777–783. doi: 10.1126/science.aag2590
Ransohoff, R. M., Schafer, D., Vincent, A., Blachère, N. E., and Bar-Or, A. (2015). Neuroinflammation: ways in which the immune system affects the brain. Neurotherapeutics 12, 896–909. doi: 10.1007/s13311-015-0385-3
Ribeiro, D. E., Oliveira-Giacomelli, Á., Glaser, T., Arnaud-Sampaio, V. F., Andrejew, R., Dieckmann, L., et al. (2021). Hyperactivation of P2X7 receptors as a culprit of COVID-19 neuropathology. Mol. Psychiatry 26, 1044–1059. doi: 10.1038/s41380-020-00965-3
Roberts, E. T., Haan, M. N., Dowd, J. B., and Aiello, A. E. (2010). Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am. J. Epidemiol. 172, 363–371. doi: 10.1093/aje/kwq177
Romagnoli, M., Porcellini, E., Carbone, I., Veerhuis, R., and Licastro, F. (2020). Impaired innate immunity mechanisms in the brain of Alzheimer's disease. Int. J. Mol. Sci. 21:1126. doi: 10.3390/ijms21031126
Romeo, M. A., Santarelli, R., Gilardini Montani, M. S., Gonnella, R., Benedetti, R., Faggioni, A., et al. (2020). Viral infection and autophagy dysregulation: the case of HHV-6, EBV and KSHV. Cells 9:2624. doi: 10.3390/cells9122624
Rosenfeld, C. S. (2015). Microbiome disturbances and autism Spectrum disorders. Drug Metab. Dispos. 43, 1557–1571. doi: 10.1124/dmd.115.063826
Rozenfeld, C., Martinez, R., Seabra, S., Sant'Anna, C., Gonçalves, J. G. R., Bozza, M., et al. (2005). Toxoplasma gondii prevents neuron degeneration by interferon-γ-activated microglia in a mechanism involving inhibition of inducible nitric oxide synthase and transforming growth factor-β1 production by infected microglia. Am. J. Pathol. 167, 1021–1031. doi: 10.1016/S0002-9440(10)61191-1
Rutherford, S. T., and Bassler, B. L. (2012). Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2:a012427. doi: 10.1101/cshperspect.a012427
Sadasivan, S., Zanin, M., O’Brien, K., Schultz-Cherry, S., and Smeyne, R. J. (2015). Induction of microglia activation after infection with the non-neurotropic a/CA/04/2009 H1N1 influenza virus. PLoS One 10:e0124047. doi: 10.1371/journal.pone.0124047
Sadrameli, M., Bathini, P., and Alberi, L. (2020). Linking mechanisms of periodontitis to Alzheimer's disease. Curr. Opin. Neurol. 33, 230–238. doi: 10.1097/WCO.0000000000000797
Sait, A., Angeli, C., Doig, A. J., and Day, P. J. R. (2021). Viral involvement in Alzheimer's disease. ACS Chem. Neurosci. 12, 1049–1060. doi: 10.1021/acschemneuro.0c00719
Santana, S., Bullido, M. J., Recuero, M., Valdivieso, F., and Aldudo, J. (2012). Herpes simplex virus type i induces an incomplete autophagic response in human neuroblastoma cells. J. Alzheimers Dis. 30, 815–831. doi: 10.3233/JAD-2012-112000
Santana, S., Recuero, M., Bullido, M. J., Valdivieso, F., and Aldudo, J. (2012). Herpes simplex virus type I induces the accumulation of intracellular β-amyloid in autophagic compartments and the inhibition of the non-amyloidogenic pathway in human neuroblastoma cells. Neurobiol. Aging 33, 430.e19–430.e33. doi: 10.1016/j.neurobiolaging.2010.12.010
Santana, S., Sastre, I., Recuero, M., Bullido, M. J., and Aldudo, J. (2013). Oxidative stress enhances neurodegeneration markers induced by herpes simplex virus type 1 infection in human neuroblastoma cells. PLoS One 8:e75842. doi: 10.1371/journal.pone.0075842
Sasannejad, C., Ely, E. W., and Lahiri, S. (2019). Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit. Care 23:352. doi: 10.1186/s13054-019-2626-z
Scheper, W., and Hoozemans, J. J. (2015). The unfolded protein response in neurodegenerative diseases: a neuropathological perspective. Acta Neuropathol. 130, 315–331. doi: 10.1007/s00401-015-1462-8
Scheperjans, F., Aho, V., Pereira, P. A., Koskinen, K., Paulin, L., Pekkonen, E., et al. (2015). Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov. Disord. 30, 350–358. doi: 10.1002/mds.26069
Schmaltz, H. N., Fried, L. P., Xue, Q. L., Walston, J., Leng, S. X., and Semba, R. D. (2005). Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J. Am. Geriatr. Soc. 53, 747–754. doi: 10.1111/j.1532-5415.2005.53250.x
Schmitz-Esser, S., Linka, N., Collingro, A., Beier, C. L., Neuhaus, H. E., Wagner, M., et al. (2004). ATP/ADP translocases: a common feature of obligate intracellular amoebal symbionts related to Chlamydiae and Rickettsiae. J. Bacteriol. 186, 683–691. doi: 10.1128/JB.186.3.683-691.2004
Schromm, A. B., and Brandenburg, K. (2021). “TLR4 ligands: single molecules and aggregates” in The role of toll-like receptor 4 in infectious and non infectious inflammation. eds. C. Rossetti and F. Peri (Cham: Springer International Publishing), 39–56.
Sekino, N., Selim, M., and Shehadah, A. (2022). Sepsis-associated brain injury: underlying mechanisms and potential therapeutic strategies for acute and long-term cognitive impairments. J. Neuroinflammation 19:101. doi: 10.1186/s12974-022-02464-4
Sequeira, R. C., and Godad, A. (2024). An update on microRNA as a potential blood-based biomarker for Alzheimer’s disease. Nucleus 67, 263–275. doi: 10.1007/s13237-023-00427-5
Shafi, O. (2016). Inverse relationship between Alzheimer’s disease and cancer, and other factors contributing to Alzheimer’s disease: a systematic review. BMC Neurol. 16, 1–17. doi: 10.1186/s12883-016-0765-2
Sharshar, T., Hopkinson, N. S., Orlikowski, D., and Annane, D. (2004). Science review: the brain in sepsis–culprit and victim. Crit. Care 9, 37–38. doi: 10.1186/cc2951
Shiers, S., Ray, P. R., Wangzhou, A., Tatsui, C. E., Rhines, L., Li, Y., et al. (2020). ACE2 expression in human dorsal root ganglion sensory neurons: implications for SARS-CoV-2 virus-induced neurological effects. bioRxiv. doi: 10.1101/2020.05.28.122374
Shim, S. M., Cheon, H. S., Jo, C., Koh, Y. H., Song, J., and Jeon, J. P. (2017). Elevated Epstein-Barr virus antibody level is associated with cognitive decline in the Korean elderly. JAD 55, 293–301. doi: 10.3233/JAD-160563
Shima, K., Kuhlenbäumer, G., and Rupp, J. (2010). Chlamydia pneumoniae infection and Alzheimer's disease: a connection to remember? Med. Microbiol. Immunol. 199, 283–289. doi: 10.1007/s00430-010-0162-1
Shin, J. H., Hwang, Y. S., Jung, B. K., Seo, S. H., Ham, D. W., and Shin, E. H. (2021). Reduction of amyloid burden by proliferated homeostatic microglia in toxoplasma gondii-infected Alzheimer's disease model mice. Int. J. Mol. Sci. 22:2764. doi: 10.3390/ijms22052764
Singh, N., and Ecker, G. F. (2018). Insights into the structure, function, and ligand discovery of the large neutral amino acid transporter 1, LAT1. Int. J. Mol. Sci. 19:1278. doi: 10.3390/ijms19051278
Siu, K. L., Yuen, K. S., Castano-Rodriguez, C., Ye, Z. W., Yeung, M. L., Fung, S. Y., et al. (2019). Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 33, 8865–8877. doi: 10.1096/fj.201802418R
Slats, D., Claassen, J. A., Verbeek, M. M., and Overeem, S. (2013). Reciprocal interactions between sleep, circadian rhythms and Alzheimer's disease: focus on the role of hypocretin and melatonin. Ageing Res. Rev. 12, 188–200. doi: 10.1016/j.arr.2012.04.003
Soscia, S., Kirby, J., Washicosky, K., Tucker, S., and Ingelsson, M. (2010). The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One, 5, e9505. doi: 10.1371/journal.pone.0009505
Spitzer, P., Condic, M., Herrmann, M., Oberstein, T. J., Scharin-Mehlmann, M., Gilbert, D. F., et al. (2016). Amyloidogenic amyloid-β-peptide variants induce microbial agglutination and exert antimicrobial activity. Sci. Rep. 6:32228. doi: 10.1038/srep32228
Srinivasan, M., and Lahiri, D. K. (2015). Significance of NF-κB as a pivotal therapeutic target in the neurodegenerative pathologies of Alzheimer's disease and multiple sclerosis. Expert Opin. Ther. Targets 19, 471–487. doi: 10.1517/14728222.2014.989834
Stein, S. R., Ramelli, S. C., Grazioli, A., Chung, J. Y., Singh, M., Yinda, C. K., et al. (2022). SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 612, 758–763. doi: 10.1038/s41586-022-05542-y
Stowe, R. P., Kozlova, E. V., Yetman, D. L., Walling, D. M., Goodwin, J. S., and Glaser, R. (2007). Chronic herpesvirus reactivation occurs in aging. Exp. Gerontol. 42, 563–570. doi: 10.1016/j.exger.2007.01.005
Subedi, L., Gaire, B. P., Koronyo, Y., Koronyo-Hamaoui, M., and Crother, T. R. (2024). Chlamydia pneumoniae in Alzheimer's disease pathology. Front. Neurosci. 18:1393293. doi: 10.3389/fnins.2024.1393293
Takayama, F., Hayashi, Y., Wu, Z., Liu, Y., and Nakanishi, H. (2016). Diurnal dynamic behavior of microglia in response to infected bacteria through the UDP-P2Y6 receptor system. Sci. Rep. 6:30006. doi: 10.1038/srep30006
Tallóczy, Z., Virgin, I., and Herbert, L. B. (2006). PKR-dependent xenophagic degradation of herpes simplex virus type 1. Autophagy 2, 24–29. doi: 10.4161/auto.2176
Talwar, P., Gupta, R., Kushwaha, S., Agarwal, R., Saso, L., Kukreti, S., et al. (2019). Viral induced oxidative and inflammatory response in Alzheimer's disease pathogenesis with identification of potential drug candidates: a systematic review using systems biology approach. Curr. Neuropharmacol. 17, 352–365. doi: 10.2174/1570159X16666180419124508
Tan, A. H., Mahadeva, S., Thalha, A. M., Gibson, P. R., Kiew, C. K., Yeat, C. M., et al. (2014). Small intestinal bacterial overgrowth in Parkinson's disease. Parkinsonism Relat. Disord. 20, 535–540. doi: 10.1016/j.parkreldis.2014.02.019
Thomas, R., Morris, A. W., and Tai, L. M. (2017). Epidermal growth factor prevents APOE4-induced cognitive and cerebrovascular deficits in female mice. Heliyon 3:e00319. doi: 10.1016/j.heliyon.2017.e00319
Thomas, R., Zuchowska, P., Morris, A. W., Marottoli, F. M., Sunny, S., Deaton, R., et al. (2016). Epidermal growth factor prevents APOE4 and amyloid-beta-induced cognitive and cerebrovascular deficits in female mice. Acta Neuropathol. Commun. 4, 1–14. doi: 10.1186/s40478-016-0387-3
Tiwari, D., Singh, V. K., Baral, B., Pathak, D. K., Jayabalan, J., Kumar, R., et al. (2021). Indication of neurodegenerative Cascade initiation by amyloid-like aggregate-forming EBV proteins and peptide in Alzheimer's disease. ACS Chem. Neurosci. 12, 3957–3967. doi: 10.1021/acschemneuro.1c00584
Torrens-Mas, M., Pons, D. G., Sastre-Serra, J., Oliver, J., and Roca, P. (2020). Sexual hormones regulate the redox status and mitochondrial function in the brain pathological implications. Redox Biol. 31:101505. doi: 10.1016/j.redox.2020.101505
Torres, L., Robinson, S. A., Kim, D. G., Yan, A., Cleland, T. A., and Bynoe, M. S. (2018). Toxoplasma gondii alters NMDAR signaling and induces signs of Alzheimer's disease in wild-type, C57BL/6 mice. J. Neuroinflammation 15:57. doi: 10.1186/s12974-018-1086-8
Tynkkynen, J., Chouraki, V., van der Lee, S. J., Hernesniemi, J., Yang, Q., Li, S., et al. (2018). Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer's disease: a prospective study in eight cohorts. Alzheimers Dement. 14, 723–733. doi: 10.1016/j.jalz.2018.01.003
Uppoor, A. S., Lohi, H. S., and Nayak, D. (2013). Periodontitis and Alzheimer's disease: oral systemic link still on the rise? Gerodontology 30, 239–242. doi: 10.1111/j.1741-2358.2012.00660.x
Vakili, K., Fathi, M., Hajiesmaeili, M., Salari, M., Saluja, D., Tafakhori, A., et al. (2021). Neurological symptoms, comorbidities, and complications of COVID-19: a literature review and Meta-analysis of observational studies. Eur. Neurol. 84, 307–324. doi: 10.1159/000516258
van den Boogaard, M., Kox, M., Quinn, K. L., van Achterberg, T., van der Hoeven, J. G., Schoonhoven, L., et al. (2011). Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit. Care 15:R297. doi: 10.1186/cc10598
van der Lelie, D., and Taghavi, S. (2020). COVID-19 and the gut microbiome: more than a gut feeling. mSystems 5. doi: 10.1128/mSystems.00453-20
Varga, Z., Flammer, A., Steiger, P., Haberecker, M., Andermatt, R., and Zinkernagel, A. (2020). Infecção de células endoteliais e endotelite em COVID-19. Lancet 395, 1417–1418. doi: 10.1016/S0140-6736(20)30937-5
Veronese, N., Demurtas, J., Smith, L., Michel, J. P., Barbagallo, M., Bolzetta, F., et al. (2022). Influenza vaccination reduces dementia risk: a systematic review and meta-analysis. Ageing Res. Rev. 73:101534. doi: 10.1016/j.arr.2021.101534
Villemagne, V. L., Burnham, S., Bourgeat, P., Brown, B., Ellis, K. A., Salvado, O., et al. (2013). Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 12, 357–367. doi: 10.1016/S1474-4422(13)70044-9
Violi, F., Oliva, A., Cangemi, R., Ceccarelli, G., Pignatelli, P., Carnevale, R., et al. (2020). Nox2 activation in COVID-19. Redox Biol. 36:101655. doi: 10.1016/j.redox.2020.101655
Vlad, S. C., Miller, D. R., Kowall, N. W., and Felson, D. T. (2008). Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 70, 1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63
Wagoner, V. A., Cheon, M., Chang, I., and Hall, C. K. (2014). Impact of sequence on the molecular assembly of short amyloid peptides. Proteins 82, 1469–1483. doi: 10.1002/prot.24515
Wang, L., Davis, P. B., Volkow, N. D., Berger, N. A., Kaelber, D. C., and Xu, R. (2022). Association of COVID-19 with new-onset Alzheimer's disease. J. Alzheimers Dis. 89, 411–414. doi: 10.3233/JAD-220717
Wang, Z., Liu, J., Han, J., Zhang, T., Li, S., Hou, Y., et al. (2024). Herpes simplex virus 1 accelerates the progression of Alzheimer’s disease by modulating microglial phagocytosis and activating NLRP3 pathway. J. Neuroinflammation 21:176. doi: 10.1186/s12974-024-03166-9
Wang, Y., Liu, N., and Lu, B. (2019). Mechanisms and roles of mitophagy in neurodegenerative diseases. CNS Neurosci. Ther. 25, 859–875. doi: 10.1111/cns.13140
Weinberg, E. D. (1992). Iron depletion: a defense against intracellular infection and neoplasia. Life Sci. 50, 1289–1297. doi: 10.1016/0024-3205(92)90279-X
White, M. R., Kandel, R., Hsieh, I. N., De Luna, X., and Hartshorn, K. L. (2018). Critical role of C-terminal residues of the Alzheimer's associated β-amyloid protein in mediating antiviral activity and modulating viral and bacterial interactions with neutrophils. PLoS One 13:e0194001. doi: 10.1371/journal.pone.0194001
White, M. R., Kandel, R., Tripathi, S., Condon, D., Qi, L., Taubenberger, J., et al. (2014). Alzheimer's associated β-amyloid protein inhibits influenza a virus and modulates viral interactions with phagocytes. PLoS One 9:e101364. doi: 10.1371/journal.pone.0101364
Woods, J. J., Skelding, K. A., Martin, K. L., Aryal, R., Sontag, E., Johnstone, D. M., et al. (2020). Assessment of evidence for or against contributions of Chlamydia pneumoniae infections to Alzheimer's disease etiology. Brain Behav. Immun. 83, 22–32. doi: 10.1016/j.bbi.2019.10.014
Wozniak, M. A., Frost, A. L., Preston, C. M., and Itzhaki, R. F. (2011). Antivirals reduce the formation of key Alzheimer's disease molecules in cell cultures acutely infected with herpes simplex virus type 1. PLoS One 6:e25152. doi: 10.1371/journal.pone.0025152
Wu, Z., Ni, J., Liu, Y., Teeling, J. L., Takayama, F., Collcutt, A., et al. (2017). Cathepsin B plays a critical role in inducing Alzheimer's disease-like phenotypes following chronic systemic exposure to lipopolysaccharide from Porphyromonas gingivalis in mice. Brain Behav. Immun. 65, 350–361. doi: 10.1016/j.bbi.2017.06.002
Xiao, X., Liu, X., and Jiao, B. (2020). Epigenetics: recent advances and its role in the treatment of Alzheimer's disease. Front. Neurol. 11:11. doi: 10.3389/fneur.2020.538301
Xie, L., Kang, H., Xu, Q., Chen, M. J., Liao, Y., Thiyagarajan, M., et al. (2013). Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. doi: 10.1126/science.1241224
Yang, Y., He, Z., Xing, Z., Zuo, Z., Yuan, L., Wu, Y., et al. (2020). Influenza vaccination in early Alzheimer's disease rescues amyloidosis and ameliorates cognitive deficits in APP/PS1 mice by inhibiting regulatory T cells. J. Neuroinflammation 17:65. doi: 10.1186/s12974-020-01741-4
Yarlagadda, A., Alfson, E., and Clayton, A. H. (2009). The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont) 6, 18–22
Yirün, A., Çakır, D. A., Sanajou, S., Erdemli Köse, S. B., Özyurt, A. B., Zeybek, D., et al. (2023). Evaluation of the effects of herpes simplex glycoprotein B on complement system and cytokines in in vitro models of Alzheimer's disease. J. Appl. Toxicol. 43, 1368–1378. doi: 10.1002/jat.4471
Zavos, C., Kountouras, J., Deretzi, G., Tsona, A., Polyzos, S. A., Mantzoukis, K., et al. (2012). Hpn protein as a mediator between Helicobacter pylori infection and Alzheimer’s disease in sub-populations worldwide. Med. Hypotheses 78, 349–350. doi: 10.1016/j.mehy.2011.10.033
Zeng, Q., Fang, Q., Zhou, X., Yang, H., Dou, Y., Zhang, W., et al. (2021). Cofilin 2 acts as an inflammatory linker between chronic periodontitis and Alzheimer's disease in amyloid precursor protein/Presenilin 1 mice. Front. Mol. Neurosci. 14:728184. doi: 10.3389/fnmol.2021.728184
Zhang, Y., Qu, J., Luo, L., Xu, Z., and Zou, X. (2021). Multigenomics reveals the causal effect of herpes simplex virus in Alzheimer's disease: a two-sample Mendelian randomization study. Front. Genet. 12:773725. doi: 10.3389/fgene.2021.773725
Zhang, S., Yang, F., Wang, Z., Qian, X., Ji, Y., Gong, L., et al. (2020). Poor oral health conditions and cognitive decline: studies in humans and rats. PLoS One 15:e0234659. doi: 10.1371/journal.pone.0234659
Zhang, N., Zuo, Y., Jiang, L., Peng, Y., Huang, X., and Zuo, L. (2022). Epstein-Barr virus and neurological diseases. Front. Mol. Biosci. 8:8. doi: 10.3389/fmolb.2021.816098
Zhao, M., Wang, Y., Shen, Y., Wei, C., Zhang, G., and Sun, L. (2024). A review of the roles of pathogens in Alzheimer's disease. Front. Neurosci. 18:1439055. doi: 10.3389/fnins.2024.1439055
Keywords: Alzheimer’s disease, pathogen, viral infection, bacterial infection, parasite
Citation: Hosseininasab SSM, Ebrahimi R, Yaghoobpoor S, Kazemi K, Khakpour Y, Hajibeygi R, Mohamadkhani A, Fathi M, Vakili K, Tavasol A, Tutunchian Z, Fazel T, Fathi M and Hajiesmaeili M (2025) Alzheimer’s disease and infectious agents: a comprehensive review of pathogenic mechanisms and microRNA roles. Front. Neurosci. 18:1513095. doi: 10.3389/fnins.2024.1513095
Received: 17 October 2024; Accepted: 02 December 2024;
Published: 07 January 2025.
Edited by:
Elena Zenaro, University of Verona, ItalyReviewed by:
Harry Wilhelm Steinbusch, Maastricht University, NetherlandsCopyright © 2025 Hosseininasab, Ebrahimi, Yaghoobpoor, Kazemi, Khakpour, Hajibeygi, Mohamadkhani, Fathi, Vakili, Tavasol, Tutunchian, Fazel, Fathi and Hajiesmaeili. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammadreza Hajiesmaeili, bXJoYWppZXNtYWVpbGlAc2JtdS5hYy5pcg==: Mohammad Fathi, bS5mYXRoaUBzYm11LmFjLmly
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.