
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurosci., 30 October 2024
Sec. Neurodegeneration
Volume 18 - 2024 | https://doi.org/10.3389/fnins.2024.1502779
This article is part of the Research TopicOlfactory dysfunction in neurodegenerative diseasesView all 6 articles
Olfactory dysfunction has emerged as a hallmark feature shared among several neurological conditions, including both neurodevelopmental and neurodegenerative disorders. While diseases of both categories have been extensively studied for decades, their association with olfaction has only recently gained attention. Olfactory deficits often manifest already during prodromal stages of these diseases, yet it remains unclear whether common pathophysiological changes along olfactory pathways cause such impairments. Here we probe into the intricate relationship between olfactory dysfunction and neurodegenerative and neurodevelopmental disorders, shedding light on their commonalities and underlying mechanisms. We begin by providing a brief overview of the olfactory circuit and its connections to higher-associated brain areas. Additionally, we discuss olfactory deficits in these disorders, focusing on potential common mechanisms that may contribute to olfactory dysfunction across both types of disorders. We further debate whether olfactory deficits contribute to the disease propagation or are simply an epiphenomenon. We conclude by emphasizing the significance of olfactory function as a potential pre-clinical diagnostic tool to identify individuals with neurological disorders that offers the opportunity for preventive intervention before other symptoms manifest.
In humans, the sense of smell is often overlooked due to the dominance of vision and hearing in our daily lives. However, olfactory perception plays an important role in modulating cognition and emotions in healthy individuals (Richardson and Zucco, 1989; Sohrabi et al., 2012; Stevenson, 2013; Yahiaoui-Doktor et al., 2019). Olfactory performance decreases with age and correlates with cognitive abilities in the elderly (Attems et al., 2015; Murman, 2015; Uchida et al., 2020). Yet, olfactory impairments are also common symptoms of various neurodevelopmental and neurodegenerative disorders. Key olfactory functions—such as odor identification, odor discrimination, odor detection threshold, and odor memory processing—are frequently affected. Deficits in those olfactory functions can be readily assessed in humans using tests like Sniffin’ Sticks or the University of Pennsylvania Smell Identification Test (Doty et al., 1984; Hummel et al., 1997). Similarly, tests such as the buried pellet test, olfactory habituation/dishabituation tests, and olfactory preference/avoidance assays can be employed to evaluate olfactory impairments in mouse models of neurodevelopmental and neurodegenerative diseases, providing a valuable link between animal studies and human conditions (Yang and Crawley, 2009; Meyer and Alberts, 2016). The prevalence of olfactory deficits as a symptom of numerous neurodevelopmental and neurodegenerative diseases is striking. For instance, approximately 90% of patients with Alzheimer’s Disease (AD) and Parkinson’s disease (PD) exhibit olfactory impairments (Doty et al., 1988; Doty, 2017). Crucially, deficits in odor detection and discrimination alongside pathological changes in olfactory brain areas, often precede cognitive and/or motor symptoms by years (Ross et al., 2008; Devanand et al., 2010). Similarly, a significant proportion of individuals with schizophrenia (SCZ) or autism spectrum disorder (ASD) experience problems with their sense of smell, often without being aware of it (Moberg et al., 1999; Corcoran et al., 2005; Bennetto et al., 2007; Koehler et al., 2018). Given the early onset of olfactory deficits in a broad spectrum of distinct neurological disorders, early damage to the olfactory system could play a significant role in the progression of these diseases. Thus, a deeper understanding of the mechanisms underlying olfactory dysfunction could provide valuable insights into disease progression. Additionally, screening for olfactory deficits may offer a means of pre-clinical diagnostics and intervention before more severe cognitive symptoms emerge.
Olfactory sensing begins when odor molecules bind to diverse olfactory receptors on olfactory sensory neurons (OSNs) located within the olfactory epithelium (OE) in the nasal cavity (Zhang and Firestein, 2002). Sensory afferents from OSNs transmit excitatory signals to the olfactory bulb (OB), the main olfactory processing center. Mitral and tufted cells (M/TCs) in the OB relay this preprocessed information to various cortical and subcortical regions, such as the anterior olfactory nucleus (AON), piriform cortex (PIR), amygdala, and lateral entorhinal cortex (LEC; Sosulski et al., 2011; Igarashi et al., 2012; Imai, 2014). Olfactory cortical areas such as PIR and LEC subsequently project to higher-order brain areas, including the prefrontal cortex (PFC), orbitofrontal cortex (OFC), and hippocampus (HP), which are critical for cognitive functions (Witter et al., 2017; Figure 1). Unlike other sensory modalities, olfactory information bypasses the thalamus and directly connects to these higher-order brain regions. These direct connections are crucial for the processing of odor information. For instance, direct projections from LEC to HP are important for odor discrimination and memory (Leitner et al., 2016; Li et al., 2017), while connections from PIR to OFC are important for learning odor values (Wang et al., 2020). Moreover, slow respiration-driven oscillations in the OB modulate local field potentials in PIR, LEC, HP, and PFC (Zelano et al., 2016; Biskamp et al., 2017; Tort et al., 2018; Heck et al., 2022), and beta oscillations synchronize across olfactory and cognitive brain areas during working memory and decision-making, influencing task performance (Gourévitch et al., 2010; Mori et al., 2013; Igarashi et al., 2014; Rangel et al., 2016; Symanski et al., 2022). Studies have also shown that odor-induced fast oscillations in OB and PIR correlate with odor perception and discrimination (Beshel et al., 2007; Lepousez and Lledo, 2013; Yang et al., 2022).
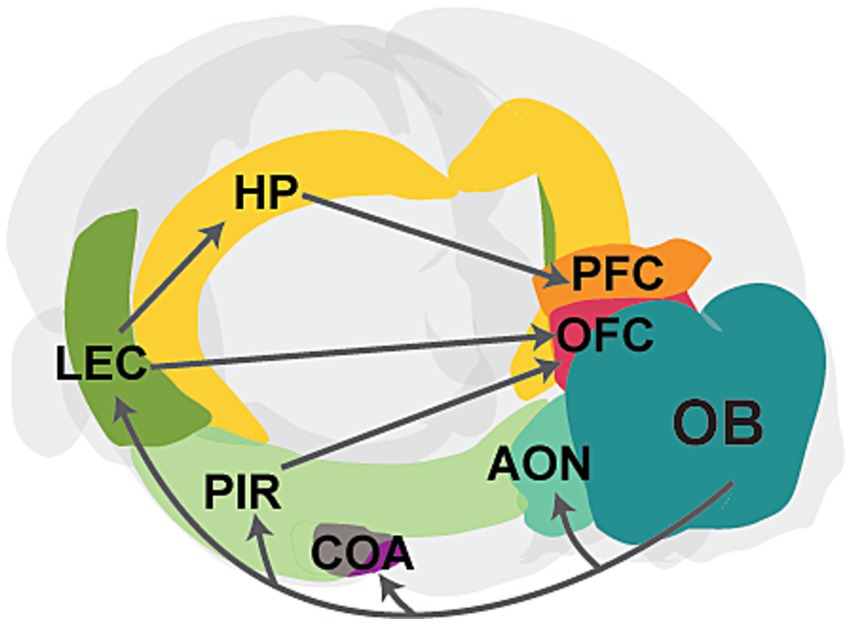
Figure 1. Schematic showing the main connectivity between the olfactory bulb and higher-order brain regions. The OB projects to primary cortical regions, including the anterior olfactory nucleus (AON), cortical amygdaloid nucleus (COA), piriform cortex (PIR), and lateral entorhinal cortex (LEC). Further, PIR and LEC send projections to higher-order cognitive regions, such as the hippocampus (HP), prefrontal cortex (PFC), and orbitofrontal cortex (OFC). The PFC also receives input from the HP. Gray arrows represent axonal projections, and individual areas are highlighted in different colors.
OB networks are also strongly influenced by neuromodulatory inputs such as noradrenergic, serotonergic, and cholinergic inputs, which are involved in odor discrimination and odor learning (Linster and Fontanini, 2014; Brunert and Rothermel, 2021). Sparse dopaminergic (DA) input from the substantia nigra also terminates in the OB (Höglinger et al., 2015). A subpopulation of OB interneurons is both DA and GABAergic (Borisovska et al., 2013; Pignatelli and Belluzzi, 2017; Liu et al., 2019) and undergoes adult neurogenesis (Altman, 1969; Lazarini et al., 2014). These neurons modulate neurotransmitter release from OSNs and lateral inhibition within glomeruli (Hsia et al., 1999; Liu et al., 2013a; McGann, 2013) and are important for odor discrimination (Tillerson et al., 2006). Moreover, granule (GC) and periglomerular (PGC) interneurons in the OB, along with OSNs in the OE, are continuously generated throughout life (Murrell et al., 1996; Hahn et al., 2005; Batista-Brito et al., 2008; Lledo and Valley, 2016).
The olfactory system is anatomically and functionally interconnected with brain regions essential for cognitive processing. Importantly, pathological changes associated with neurodegenerative and neurodevelopmental disorders have been observed throughout the olfactory circuitry - from the OE and OB to primary olfactory cortices and downstream targets like LEC, HP, and PFC.
Neurodegenerative disorders, like AD and PD, are characterized by progressive decline of cognitive and motor functions (Goedert and Spillantini, 2006; Wilson et al., 2023). Emerging evidence indicates that olfactory deficits—such as impaired odor detection and discrimination—manifest early in these diseases, preceding cognitive and motor symptoms by several years (Ross et al., 2008; Doty, 2017).
In AD, olfactory dysfunction correlates closely with the progression of cognitive decline (Roberts et al., 2016; Dintica et al., 2019; Papadatos and Phillips, 2023). Pathological features of AD include amyloid plaques (deposition of amyloid beta (Aβ) protein) and neurofibrillary tangles (aggregates of hyperphosphorylated tau proteins; Goedert and Spillantini, 2006; Ballard et al., 2011; Braak and Del Tredici, 2015). These pathological aggregations affect the OE and brain areas involved in odor processing, such as OB, AON, PIR, and LEC, often before clinical symptoms occur (Attems and Jellinger, 2006; Arnold et al., 2010; Murphy, 2019). Animal studies suggest that overexpression of the Aβ precursor protein causes olfactory deficits by progressive Aβ deposition, starting from the OE and expanding to the OB, PIR, entorhinal cortex (EC), and HP (Wesson et al., 2010; Wu et al., 2013). Similarly, in humans, areas like EC are among the first to be affected by AD pathology (Braak and Braak, 1991). Further, higher levels of phosphorylated tau (P-tau) in the OBs of AD patients correlate with MC loss, impaired dendro-dendritic inhibition, and diminished olfactory detection abilities before cognitive impairments emerged (Li et al., 2019a). Mouse models of Aβ pathology also show early olfactory deficits, alongside a loss of OSNs and decreased odor-evoked potentials in the OE, altered dendro-dendritic inhibition, and increased gamma oscillations in the OB, PIR, and LEC (Wesson et al., 2011; Xu et al., 2015; Li et al., 2019b; Chen et al., 2021b). These symptoms occur before Aβ plaque formation, suggesting that soluble Aβ might be responsible. In line with this, overexpression of a mutated human Aβ precursor protein in OSNs disrupts the glomerular axon targeting of those neurons and causes olfactory deficits before Aβ deposition forms in the OB (Cao et al., 2012). Similarly, injecting soluble Aβ oligomers into the OB damages the olfactory detection abilities of rodents (Alvarado-Martínez et al., 2013).
Similarly, olfactory dysfunction is an early and prominent non-motor symptom of PD (Ross et al., 2008; Doty, 2012, 2017; Haehner et al., 2019). PD patients score lower on the Sniffin’ Sticks Test compared to healthy controls (Haehner et al., 2007, 2009; Trentin et al., 2022), and brain areas such as the OB, AON, PIR and EC show early volume reductions (Wattendorf et al., 2009; Wang et al., 2011; Chen et al., 2014; Lee et al., 2014; Tanik et al., 2016). Characterized by α-synuclein aggregation forming Lewy bodies (Mezey et al., 1998), PD shows early pathological changes in the OB and AON (Braak et al., 2003). A transgenic mouse model of α-synuclein pathology confirms the prevalence of α-synuclein aggregation in the OB, AON, and PIR and shows reduced odor detection and diminished adult neurogenesis in the OB (Martin-Lopez et al., 2023). This aggregation is associated with increased odor-evoked gamma oscillations and altered neuronal firing in the OB (Chen et al., 2021a).
Thus, neurodegenerative disorders like AD and PD exhibit early olfactory deficits that coincide with the initial accumulation of pathological proteins in olfactory-related brain regions, before spreading to other parts of the brain.
Neurodevelopmental disorders, including SCZ and ASD, are characterized by atypical brain development and impaired cognitive, social, or motivation-related behaviors (Owen et al., 2016; Thye et al., 2018; Chini and Hanganu-Opatz, 2021). A prominent feature is impaired sensory processing (Schechter et al., 2003; Chang et al., 2014; Siper et al., 2021). In particular, reduced odor detection early in life, accompanied by anatomical and functional alterations in olfactory and higher-order cortical networks, is typical (Crow et al., 2020).
For instance, SCZ patients exhibit olfactory deficits and reduced OB, PIR EC, HP, and amygdala volumes, which precede the onset of cognitive deficits (Turetsky et al., 2000; Corcoran et al., 2005; Rupp et al., 2005; Nguyen et al., 2010, 2011; Kamath et al., 2018; Yang et al., 2021). Additionally, reduced olfactory-evoked potentials are associated with impaired odor identification in SCZ patients (Turetsky et al., 2003). Both genetic and environmental factors play a significant role in shaping the development of the olfactory system and are implicated in neurodevelopmental disorders. One prominent susceptibility factor for SCZ is the mutation of the Disrupted-in-Schizophrenia 1 (DISC1) gene, which is involved in various neuropsychiatric disorders (Blackwood et al., 2001; Chubb et al., 2008; Brandon et al., 2009) and is highly expressed in M/TCs (Schurov et al., 2004). DISC1 knockdown, combined with a prenatal environmental stressor - maternal immune activation (MIA) - leads to impaired oscillatory activity in the OB and reduced functional connectivity within olfactory-limbic networks of neonatal mice (Parbst et al., 2024; Xu et al., 2021).
In ASD, children often exhibit early olfactory deficits, including impaired odor identification along with reduced odor-evoked activity (Bennetto et al., 2007; Koehler et al., 2018). Like SCZ, both genetic and environmental factors contribute to the etiology of ASD. Genetic mutations, such as those affecting Shank proteins, involved in postsynaptic scaffolding, are prevalent in patients with ASD and are associated with olfactory deficits. Shank3 deficiency impairs odor detection, reduces odor-evoked potentials, and alters synaptic transmission in the OB and PIR (Drapeau et al., 2018; Ryndych et al., 2023; Mihalj et al., 2024). Moreover, mutation of the autism-related gene Tbr leads to smaller OBs, reduced numbers of OB interneurons, and abnormal dendritic morphology of MCs (Huang et al., 2019). Environmental factors like MIA, which largely increases the risks for both ASD and SCZ (Hartung et al., 2016; Schepanski et al., 2022; Dutra et al., 2023; Godavarthi et al., 2024), can impair adult neurogenesis in the OB and contribute to decreased olfactory discrimination abilities (Liu et al., 2013b).
Thus, olfactory dysfunctions accompanied by pathophysiological changes in brain areas associated with olfaction, are frequently observed in neurodevelopmental disorders.
Olfactory deficits in neurodevelopmental and neurodegenerative disorders often coincide with structural alterations across brain regions involved in olfactory processing. For example, reduced OB volume has been documented in AD (Thomann et al., 2009b; Thomann et al., 2009a), PD (Wattendorf et al., 2009; Wang et al., 2011), and SCZ (Turetsky et al., 2000; Nguyen et al., 2011; Yang et al., 2021). This reduction in OB volume might be caused by multiple mechanisms, including altered neuronal morphology and neuronal loss. For example, postmortem OB tissue of PD patients reveals substantial loss of ventral glomerular areas in the OB, correlated with phosphorylated α-synuclein load (Zapiec et al., 2017). This α-synuclein accumulation specifically induces apoptosis of DA neurons (Xu et al., 2002), likely contributing to the reduced size or number of glomeruli in the OB. In AD, the accumulation of P-tau and Aβ drives neuronal atrophy throughout the brain, including M/TCs in the OB (Struble and Clark, 1992; Yao et al., 2017; Li et al., 2019b). This aligns with studies showing that the MC layer is predominantly affected by tau pathology in an AD mouse model (Yang et al., 2016). While reduced OB volume is also common in SCZ, direct evidence for altered neuronal morphology in OB is limited for neurodevelopmental disorders. However, it was recently shown that a mouse model of OE inflammation which closely mimics inflammatory processes in the OE of first-episode psychosis patients shows reduced glomerular size and OB volume, alongside decreased numbers of OSNs and M/TCs (Yang et al., 2024). Further, animal models of SCZ, such as immune-challenged DISC1 knockdown mice and 22q11-deletion mice, show reduced soma size and dendritic arborization of pyramidal neurons in brain regions such as LEC, HP, and PFC (Chini et al., 2020; Kringel et al., 2023; Stark et al., 2008; Fénelon et al., 2013). However, so far it is unknown whether the same holds for M/TCs, which strongly express DISC (Schurov et al., 2004). Impaired neurogenesis might also contribute to OB volume reduction in both neurodegenerative and neurodevelopmental disorders. Neuroblasts generated in the subventricular zone (SVZ) continuously migrate to the OB, where they differentiate into GCs and PGCs (Belluzzi et al., 2003; Livneh et al., 2014). Impairments of adult SVZ neurogenesis are evident early in animal models of AD and PD (Winner et al., 2008, 2011; Rodríguez et al., 2009; Scopa et al., 2020; Esteve et al., 2022; Martin-Lopez et al., 2023). Similarly, disruptions in SVZ neurogenesis are seen in neurodevelopmental disorders. DISC1 knockdown leads to reduced progenitor cell proliferation in the SVZ during embryonic stages (Mao et al., 2009). Additionally, MIA leads to altered proliferation in the SVZ of neonatal mice and further contributes to reduced adult neurogenesis in the OB (Liu et al., 2013b; Loayza et al., 2023).
Aside from structural changes, alterations in the neuronal activity within olfactory circuits are common in these disorders. OSNs, the first neurons to receive odor information, are reduced in numbers and show smaller odor-evoked responses in AD, resulting in diminished excitatory input to the OB (Chen et al., 2021b). This, combined with reduced dendritic spine density in GCs and impaired dendro-dendritic inhibition onto MCs, leads to increased gamma-band OB network activity (Wesson et al., 2011; Chen et al., 2021b; Li et al., 2019a, 2019b). Similarly, overexpression of α-synuclein in the OB, one of the key features of PD, leads to reduced GC activity and impaired dendro-dendritic inhibition onto MCs, along with elevated odor-evoked gamma oscillations (Chen et al., 2021a). In neuropsychiatric disorders, a broadband reduction of oscillatory power in the OBs of a SCZ mouse model was accompanied by reduced firing of M/TCs (Parbst et al., 2024). Furthermore, compromised functional connectivity between brain regions accounting for olfactory and cognitive processing is evident in both neurodegenerative and neurodevelopmental disorders. For instance, patients with SCZ show reduced functional connectivity between PIR, PFC, and nucleus accumbens (Kiparizoska and Ikuta, 2017) as well as between the HP and PFC (Adams et al., 2020). In AD, disruption of functional connectivity between olfactory networks (including PIR and OFC) and HP is linked to cognitive decline (Lu et al., 2019a, 2019b). Already during neonatal development, desynchronization between LEC, HP, and PFC manifests in an animal model of neuropsychiatric disorders such as SCZ (Hartung et al., 2016; Xu et al., 2021). A recent study showed that functional connectivity between OB and HP, as well as, OB and PFC was significantly reduced in the same animal model (Parbst et al., 2024). Similarly, in animal models of ASD, reduced OB activity and altered connectivity between HP and PFC have been reported (Cheaha et al., 2015; Richter et al., 2019). During early development, olfactory inputs are critical in synchronizing brain regions involved in olfactory and cognitive processing (Gretenkord et al., 2019; Kostka and Hanganu-Opatz, 2023). Notably, silencing M/TC activity in the OB during early development impairs the maturation of olfactory-hippocampal networks and cognitive abilities later in life (Chen et al., 2023). These findings suggest that altered olfactory activity can disrupt the development of functional coupling within neuronal networks, potentially contributing to cognitive impairments, seen in many neurodegenerative and neurodevelopmental disorders (Bennetto et al., 1996; Jahn, 2013; Davis and Racette, 2016; Guo et al., 2019).
Alterations in neurotransmitter systems, particularly the DA system, also significantly contribute to olfactory deficits in neurodegenerative and neurodevelopmental disorders. DA neurons in the OB inhibit olfactory transmission in the olfactory glomeruli (Wilson and Sullivan, 1995; Hsia et al., 1999) and are important for the encoding of innate odor values (Kato et al., 2023). In PD and AD, loss of DA neurons in the substantia nigra and ventral tegmental area leads to impaired DA outflow to several brain areas, including the OB (German et al., 1989; Nobili et al., 2017). Interestingly, increased numbers of DA neurons have been observed in the OBs of PD and AD patients (Huisman et al., 2004; Mundiñano et al., 2011). Neurodevelopmental disorders, such as ASD, also exhibit altered DA signaling and DA receptor abnormalities (Pavăl, 2017; Kosillo and Bateup, 2021; Pavăl and Micluția, 2021). Beyond DA, cholinergic transmission plays an important role in olfactory processing and is frequently altered in these disorders (Doty, 2017). For example, acetylcholine dysfunction exacerbates Aβ pathology in AD and cholinergic receptor abnormalities are present in ASD (Gil-Bea et al., 2012; Ovsepian et al., 2019; Vallés and Barrantes, 2021).
Overall, several intertwined mechanisms, such as neuronal loss, reduced neurogenesis, impaired synaptic transmission, and altered neurotransmitter signaling can lead to structural and functional alterations in olfactory circuits, contributing to the olfactory deficits characteristic of both neurodevelopmental and neurodegenerative diseases.
Olfactory deficits emerge early, often preceding the clinical diagnosis of neurodegenerative and neurodevelopmental disorders. Whether this relationship is causal or merely an epiphenomenon remains an open question. However, the presence of olfactory dysfunction and alterations in olfactory-related brain areas well before cognitive and motor symptoms suggest a potential causal relationship. Notably, individuals at high risk for psychiatric disorders, such as relatives of SCZ patients, often exhibit olfactory impairments, indicating that these deficits are unlikely due to secondary effects of treatment (Turetsky et al., 2018).
The OE as well as primary olfactory areas such as OB and AON often show pathological changes in prodromal disease stages before the involvement of other brain areas. In line with the α-synuclein transmission hypothesis (McCann et al., 2016) injection of human α-synuclein fibrils into the OBs of young mice leads to a spread of α-synuclein aggregates across several brain regions, correlating with increasing olfactory deficits (Rey et al., 2016). Similarly, the injection of soluble Aβ in an AD mouse model shows similar spreading patterns (He et al., 2018). This suggests that in neurodegenerative diseases, pathological aggregation of proteins originates in olfactory areas and spreads in a prion-like manner to higher-order cortical regions, contributing to disease progression. Moreover, in both neurodegenerative and neurodevelopmental disorders, olfactory dysfunction is linked to reduced functional connectivity with downstream brain regions, potentially accelerating cognitive decline. For example, layer 2 neurons in LEC, which receive direct OB input and project to HP, are especially vulnerable, showing functional and morphological alterations in AD (Stranahan and Mattson, 2010). Thus disrupted inputs from the OB may, cause structural and functional changes along olfactory pathways. Supporting this, studies have shown that recently acquired sensory loss can alter both morphology and functional connectivity between the PIR and higher-order cortical brain regions (Bitter et al., 2010; Iravani et al., 2021). Thus, olfactory circuits may play a dual role: they could serve as a route for the spread of pathogenic proteins to downstream brain areas, and disruptions in olfactory processing in the OE and OB could have lasting consequences on higher-order cortical regions, potentially contributing to cognitive deficits.
On the other hand, olfactory impairments might coincide with disease progression or result from secondary effects. For example, disruptions in forebrain development and altered neurotransmitter signaling can lead to olfactory dysfunctions (Doty, 2017). Furthermore, the propagation of tau, from the temporal lobe to olfactory circuits was shown to drive the degradation of odor perception as individuals get older (Diez et al., 2024). Moreover, while many patients with neurodevelopmental and neurodegenerative disorders experience impaired olfaction, this is not universal, suggesting that olfactory system involvement is not a necessary feature of disease progression in all cases.
Regardless of whether olfactory dysfunction is a cause or consequence of these diseases, it consistently occurs early, often before a clinical diagnosis is made. Testing olfactory abilities for example with simple Sniffn’ Sticks tests or the University of Pennsylvania Smell Identification Test is an effective and inexpensive way to identify individuals with olfactory deficits. Since reliable biomarkers for early diagnostics are lacking, monitoring olfactory deficits in individuals at risk or incorporating olfactory testing into routine health checks has great potential (Dan et al., 2021). In addition, olfactory testing may serve as a tool for monitoring disease progression or evaluating therapeutic effects (Berendse et al., 2011). Further research is necessary to understand the mechanisms underlying olfactory dysfunctions, as this could offer valuable insights into the etiology and progression of these diseases.
Y-NC: Conceptualization, Validation, Writing – original draft, Writing – review & editing. JK: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by grants from the German Research Foundation (526195732 and FOR5159 TP1: 437610067) and grants from the European Union (Horizon 2020 DEEPER: 101016787 and MSCA-ITN: 860563) to Ileana L. Hanganu-Opatz.
We thank Ileana L. Hanganu-Opatz for comments on the manuscript and financial support. A large language model (ChatGPT 4; open AI) was used to improve grammatical accuracy, and to correct syntactical errors. It was not used to create or curate the scientific content in any way.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adams, R. A., Bush, D., Zheng, F., Meyer, S. S., Kaplan, R., Orfanos, S., et al. (2020). Impaired theta phase coupling underlies frontotemporal dysconnectivity in schizophrenia. Brain 143, 1261–1277. doi: 10.1093/brain/awaa035
Altman, J. (1969). Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 137, 433–457. doi: 10.1002/cne.901370404
Alvarado-Martínez, R., Salgado-Puga, K., and Peña-Ortega, F. (2013). Amyloid Beta inhibits olfactory bulb activity and the ability to smell. PLoS One 8:e75745. doi: 10.1371/journal.pone.0075745
Arnold, S. E., Lee, E. B., Moberg, P. J., Stutzbach, L., Kazi, H., Han, L., et al. (2010). Olfactory epithelium amyloid-β and paired helical filament-tau pathology in Alzheimer disease. Ann. Neurol. 67, 462–469. doi: 10.1002/ana.21910
Attems, J., and Jellinger, K. A. (2006). Olfactory tau pathology in Alzheimer disease and mild cognitive impairment. Clin. Neuropathol. 25, 265–271
Attems, J., Walker, L., and Jellinger, K. A. (2015). Olfaction and aging: a Mini-review. Gerontology 61, 485–490. doi: 10.1159/000381619
Ballard, C., Gauthier, S., Corbett, A., Brayne, C., Aarsland, D., and Jones, E. (2011). Alzheimer’s disease. Lancet 377, 1019–1031. doi: 10.1016/S0140-6736(10)61349-9
Batista-Brito, R., Close, J., Machold, R., and Fishell, G. (2008). The distinct temporal origins of olfactory bulb interneuron subtypes. J. Neurosci. 28, 3966–3975. doi: 10.1523/JNEUROSCI.5625-07.2008
Belluzzi, O., Benedusi, M., Ackman, J., and LoTurco, J. J. (2003). Electrophysiological differentiation of new neurons in the olfactory bulb. J. Neurosci. 23, 10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003
Bennetto, L., Kuschner, E. S., and Hyman, S. L. (2007). Olfaction and taste processing in autism. Biol. Psychiatry 62, 1015–1021. doi: 10.1016/j.biopsych.2007.04.019
Bennetto, L., Pennington, B. F., and Rogers, S. J. (1996). Intact and impaired memory functions in autism. Child Dev. 67, 1816–1835. doi: 10.2307/1131734
Berendse, H. W., Roos, D. S., Raijmakers, P., and Doty, R. L. (2011). Motor and non-motor correlates of olfactory dysfunction in Parkinson’s disease. J. Neurol. Sci. 310, 21–24. doi: 10.1016/j.jns.2011.06.020
Beshel, J., Kopell, N., and Kay, L. M. (2007). Olfactory bulb gamma oscillations are enhanced with task demands. J. Neurosci. 27, 8358–8365. doi: 10.1523/JNEUROSCI.1199-07.2007
Biskamp, J., Bartos, M., and Sauer, J.-F. (2017). Organization of prefrontal network activity by respiration-related oscillations. Sci. Rep. 7:45508. doi: 10.1038/srep45508
Bitter, T., Brüderle, J., Gudziol, H., Burmeister, H. P., Gaser, C., and Guntinas-Lichius, O. (2010). Gray and white matter reduction in hyposmic subjects — a voxel-based morphometry study. Brain Res. 1347, 42–47. doi: 10.1016/j.brainres.2010.06.003
Blackwood, D. H. R., Fordyce, A., Walker, M. T., Porteous, D. J., and Muir, W. J. (2001). Schizophrenia and affective disorders—Cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am. J. Hum. Genet. 69, 428–433. doi: 10.1086/321969
Borisovska, M., Bensen, A. L., Chong, G., and Westbrook, G. L. (2013). Distinct modes of dopamine and GABA release in a dual transmitter neuron. J. Neurosci. 33, 1790–1796. doi: 10.1523/JNEUROSCI.4342-12.2013
Braak, H., and Braak, E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl.) 82, 239–259. doi: 10.1007/BF00308809
Braak, H., and Del Tredici, K. (2015). The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 138, 2814–2833. doi: 10.1093/brain/awv236
Braak, H., Del Tredici, K., Rüb, U., de Vos, R. A. I., Jansen Steur, E. N. H., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211. doi: 10.1016/s0197-4580(02)00065-9
Brandon, N. J., Millar, J. K., Korth, C., Sive, H., Singh, K. K., and Sawa, A. (2009). Understanding the role of DISC1 in psychiatric disease and during Normal development. J. Neurosci. 29, 12768–12775. doi: 10.1523/JNEUROSCI.3355-09.2009
Brunert, D., and Rothermel, M. (2021). Extrinsic neuromodulation in the rodent olfactory bulb. Cell Tissue Res. 383, 507–524. doi: 10.1007/s00441-020-03365-9
Cao, L., Schrank, B. R., Rodriguez, S., Benz, E. G., Moulia, T. W., Rickenbacher, G. T., et al. (2012). Aβ alters the connectivity of olfactory neurons in the absence of amyloid plaques in vivo. Nat. Commun. 3:1009. doi: 10.1038/ncomms2013
Chang, Y.-S., Owen, J. P., Desai, S. S., Hill, S. S., Arnett, A. B., Harris, J., et al. (2014). Autism and sensory processing disorders: shared White matter disruption in sensory pathways but divergent connectivity in social-emotional pathways. PLoS One 9:e103038. doi: 10.1371/journal.pone.0103038
Cheaha, D., Bumrungsri, S., Chatpun, S., and Kumarnsit, E. (2015). Characterization of in utero valproic acid mouse model of autism by local field potential in the hippocampus and the olfactory bulb. Neurosci. Res. 98, 28–34. doi: 10.1016/j.neures.2015.04.006
Chen, M., Chen, Y., Huo, Q., Wang, L., Tan, S., Misrani, A., et al. (2021b). Enhancing GABAergic signaling ameliorates aberrant gamma oscillations of olfactory bulb in AD mouse models. Mol. Neurodegener. 16:14. doi: 10.1186/s13024-021-00434-7
Chen, Y.-N., Kostka, J. K., Bitzenhofer, S. H., and Hanganu-Opatz, I. L. (2023). Olfactory bulb activity shapes the development of entorhinal-hippocampal coupling and associated cognitive abilities. Curr. Biol. 33, 4353–4366.e5. doi: 10.1016/j.cub.2023.08.072
Chen, F., Liu, W., Liu, P., Wang, Z., Zhou, Y., Liu, X., et al. (2021a). α-Synuclein aggregation in the olfactory bulb induces olfactory deficits by perturbing granule cells and granular–mitral synaptic transmission. Npj Park. Dis. 7, 114–115. doi: 10.1038/s41531-021-00259-7
Chen, S., Tan, H., Wu, Z., Sun, C., He, J., Li, X., et al. (2014). Imaging of olfactory bulb and gray matter volumes in brain areas associated with olfactory function in patients with Parkinson’s disease and multiple system atrophy. Eur. J. Radiol. 83, 564–570. doi: 10.1016/j.ejrad.2013.11.024
Chini, M., and Hanganu-Opatz, I. L. (2021). Prefrontal cortex development in health and disease: lessons from rodents and humans. Trends Neurosci. 44, 227–240. doi: 10.1016/j.tins.2020.10.017
Chini, M., Pöpplau, J. A., Lindemann, C., Carol-Perdiguer, L., Hnida, M., Oberländer, V., et al. (2020). Resolving and rescuing developmental Miswiring in a mouse model of cognitive impairment. Neuron 105, 60–74.e7. doi: 10.1016/j.neuron.2019.09.042
Chubb, J. E., Bradshaw, N. J., Soares, D. C., Porteous, D. J., and Millar, J. K. (2008). The DISC locus in psychiatric illness. Mol. Psychiatry 13, 36–64. doi: 10.1038/sj.mp.4002106
Corcoran, C., Whitaker, A., Coleman, E., Fried, J., Feldman, J., Goudsmit, N., et al. (2005). Olfactory deficits, cognition and negative symptoms in early onset psychosis. Schizophr. Res. 80, 283–293. doi: 10.1016/j.schres.2005.07.028
Crow, A. J. D., Janssen, J. M., Vickers, K. L., Parish-Morris, J., Moberg, P. J., and Roalf, D. R. (2020). Olfactory dysfunction in neurodevelopmental disorders: a Meta-analytic review of autism Spectrum disorders, attention deficit/hyperactivity disorder and obsessive-compulsive disorder. J. Autism Dev. Disord. 50, 2685–2697. doi: 10.1007/s10803-020-04376-9
Dan, X., Wechter, N., Gray, S., Mohanty, J. G., Croteau, D. L., and Bohr, V. A. (2021). Olfactory dysfunction in aging and neurodegenerative diseases. Ageing Res. Rev. 70:101416. doi: 10.1016/j.arr.2021.101416
Davis, A. A., and Racette, B. (2016). Parkinson disease and cognitive impairment. Neurol. Clin. Pract. 6, 452–458. doi: 10.1212/CPJ.0000000000000285
Devanand, D. P., Tabert, M. H., Cuasay, K., Manly, J. J., Schupf, N., Brickman, A. M., et al. (2010). Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol. Aging 31, 1593–1600. doi: 10.1016/j.neurobiolaging.2008.09.008
Diez, I., Ortiz-Terán, L., Ng, T. S. C., Albers, M. W., Marshall, G., Orwig, W., et al. (2024). Tau propagation in the brain olfactory circuits is associated with smell perception changes in aging. Nat. Commun. 15:4809. doi: 10.1038/s41467-024-48462-3
Dintica, C. S., Marseglia, A., Rizzuto, D., Wang, R., Seubert, J., Arfanakis, K., et al. (2019). Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology 92, e700–e709. doi: 10.1212/WNL.0000000000006919
Doty, R. L. (2012). Olfaction in Parkinson’s disease and related disorders. Neurobiol. Dis. 46, 527–552. doi: 10.1016/j.nbd.2011.10.026
Doty, R. L. (2017). Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. 16, 478–488. doi: 10.1016/S1474-4422(17)30123-0
Doty, R. L., Deems, D. A., and Stellar, S. (1988). Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 38, 1237–1244. doi: 10.1212/wnl.38.8.1237
Doty, R. L., Shaman, P., Kimmelman, C. P., and Dann, M. S. (1984). University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 94, 176–178. doi: 10.1288/00005537-198402000-00004
Drapeau, E., Riad, M., Kajiwara, Y., and Buxbaum, J. D. (2018). Behavioral phenotyping of an improved mouse model of Phelan–McDermid syndrome with a complete deletion of the Shank3 gene. eneuro 5:ENEURO.0046-18.2018. doi: 10.1523/ENEURO.0046-18.2018
Dutra, M. L., Dias, P., Freiberger, V., Ventura, L., Comim, C. M., Martins, D. F., et al. (2023). Maternal immune activation induces autism-like behavior and reduces brain-derived neurotrophic factor levels in the hippocampus and offspring cortex of C57BL/6 mice. Neurosci. Lett. 793:136974. doi: 10.1016/j.neulet.2022.136974
Esteve, D., Molina-Navarro, M. M., Giraldo, E., Martínez-Varea, N., Blanco-Gandia, M.-C., Rodríguez-Arias, M., et al. (2022). Adult neural stem cell migration is impaired in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 59, 1168–1182. doi: 10.1007/s12035-021-02620-6
Fénelon, K., Xu, B., Lai, C. S., Mukai, J., Markx, S., Stark, K. L., et al. (2013). The pattern of cortical dysfunction in a mouse model of a schizophrenia-related microdeletion. J. Neurosci. 33, 14825–14839. doi: 10.1523/JNEUROSCI.1611-13.2013
German, D. C., Manaye, K., Smith, W. K., Woodward, D. J., and Saper, C. B. (1989). Midbrain dopaminergic cell loss in parkinson’s disease: computer visualization. Ann. Neurol. 26, 507–514. doi: 10.1002/ana.410260403
Gil-Bea, F. J., Gerenu, G., Aisa, B., Kirazov, L. P., Schliebs, R., and Ramírez, M. J. (2012). Cholinergic denervation exacerbates amyloid pathology and induces hippocampal atrophy in Tg2576 mice. Neurobiol. Dis. 48, 439–446. doi: 10.1016/j.nbd.2012.06.020
Godavarthi, S. K., Li, H., Pratelli, M., and Spitzer, N. C. (2024). Embryonic exposure to environmental factors drives transmitter switching in the neonatal mouse cortex causing autistic-like adult behavior. Proc. Natl. Acad. Sci. 121:e2406928121. doi: 10.1073/pnas.2406928121
Goedert, M., and Spillantini, M. G. (2006). A century of Alzheimer’s disease. Science 314, 777–781. doi: 10.1126/science.1132814
Gourévitch, B., Kay, L. M., and Martin, C. (2010). Directional coupling from the olfactory bulb to the Hippocampus during a go/no-go odor discrimination task. J. Neurophysiol. 103, 2633–2641. doi: 10.1152/jn.01075.2009
Gretenkord, S., Kostka, J. K., Hartung, H., Watznauer, K., Fleck, D., Minier-Toribio, A., et al. (2019). Coordinated electrical activity in the olfactory bulb gates the oscillatory entrainment of entorhinal networks in neonatal mice. PLoS Biol. 17:e2006994. doi: 10.1371/journal.pbio.2006994
Guo, J. Y., Ragland, J. D., and Carter, C. S. (2019). Memory and cognition in schizophrenia. Mol. Psychiatry 24, 633–642. doi: 10.1038/s41380-018-0231-1
Haehner, A., Boesveldt, S., Berendse, H. W., Mackay-Sim, A., Fleischmann, J., Silburn, P. A., et al. (2009). Prevalence of smell loss in Parkinson’s disease – a multicenter study. Parkinsonism Relat. Disord. 15, 490–494. doi: 10.1016/j.parkreldis.2008.12.005
Haehner, A., Hummel, T., Hummel, C., Sommer, U., Junghanns, S., and Reichmann, H. (2007). Olfactory loss may be a first sign of idiopathic Parkinson’s disease. Mov. Disord. 22, 839–842. doi: 10.1002/mds.21413
Haehner, A., Masala, C., Walter, S., Reichmann, H., and Hummel, T. (2019). Incidence of Parkinson’s disease in a large patient cohort with idiopathic smell and taste loss. J. Neurol. 266, 339–345. doi: 10.1007/s00415-018-9135-x
Hahn, C.-G., Han, L.-Y., Rawson, N. E., Mirza, N., Borgmann-Winter, K., Lenox, R. H., et al. (2005). In vivo and in vitro neurogenesis in human olfactory epithelium. J. Comp. Neurol. 483, 154–163. doi: 10.1002/cne.20424
Hartung, H., Cichon, N., De Feo, V., Riemann, S., Schildt, S., Lindemann, C., et al. (2016). From shortage to surge: a developmental switch in hippocampal-prefrontal coupling in a gene-environment model of neuropsychiatric disorders. Cereb. Cortex N. Y. N 26, 4265–4281. doi: 10.1093/cercor/bhw274
He, Z., Guo, J. L., McBride, J. D., Narasimhan, S., Kim, H., Changolkar, L., et al. (2018). Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med. 24, 29–38. doi: 10.1038/nm.4443
Heck, D. H., Correia, B. L., Fox, M. B., Liu, Y., Allen, M., and Varga, S. (2022). Recent insights into respiratory modulation of brain activity offer new perspectives on cognition and emotion. Biol. Psychol. 170:108316. doi: 10.1016/j.biopsycho.2022.108316
Höglinger, G. U., Alvarez-Fischer, D., Arias-Carrión, O., Djufri, M., Windolph, A., Keber, U., et al. (2015). A new dopaminergic nigro-olfactory projection. Acta Neuropathol. (Berl.) 130, 333–348. doi: 10.1007/s00401-015-1451-y
Hsia, A. Y., Vincent, J. D., and Lledo, P. M. (1999). Dopamine depresses synaptic inputs into the olfactory bulb. J. Neurophysiol. 82, 1082–1085. doi: 10.1152/jn.1999.82.2.1082
Huang, T.-N., Yen, T.-L., Qiu, L. R., Chuang, H.-C., Lerch, J. P., and Hsueh, Y.-P. (2019). Haploinsufficiency of autism causative gene Tbr1 impairs olfactory discrimination and neuronal activation of the olfactory system in mice. Mol. Autism. 10:5. doi: 10.1186/s13229-019-0257-5
Huisman, E., Uylings, H. B. M., and Hoogland, P. V. (2004). A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 19, 687–692. doi: 10.1002/mds.10713
Hummel, T., Sekinger, B., Wolf, S. R., Pauli, E., and Kobal, G. (1997). “Sniffin” sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 22, 39–52. doi: 10.1093/chemse/22.1.39
Igarashi, K. M., Ieki, N., An, M., Yamaguchi, Y., Nagayama, S., Kobayakawa, K., et al. (2012). Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J. Neurosci. 32, 7970–7985. doi: 10.1523/JNEUROSCI.0154-12.2012
Igarashi, K. M., Lu, L., Colgin, L. L., Moser, M.-B., and Moser, E. I. (2014). Coordination of entorhinal–hippocampal ensemble activity during associative learning. Nature 510, 143–147. doi: 10.1038/nature13162
Imai, T. (2014). Construction of functional neuronal circuitry in the olfactory bulb. Semin. Cell Dev. Biol. 35, 180–188. doi: 10.1016/j.semcdb.2014.07.012
Iravani, B., Peter, M. G., Arshamian, A., Olsson, M. J., Hummel, T., Kitzler, H. H., et al. (2021). Acquired olfactory loss alters functional connectivity and morphology. Sci. Rep. 11:16422. doi: 10.1038/s41598-021-95968-7
Jahn, H. (2013). Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 15, 445–454. doi: 10.31887/DCNS.2013.15.4/hjahn
Kamath, V., Lasutschinkow, P., Ishizuka, K., and Sawa, A. (2018). Olfactory functioning in first-episode psychosis. Schizophr. Bull. 44, 672–680. doi: 10.1093/schbul/sbx107
Kato, A., Ohta, K., Okanoya, K., and Kazama, H. (2023). Dopaminergic neurons dynamically update sensory values during olfactory maneuver. Cell Rep. 42:113122. doi: 10.1016/j.celrep.2023.113122
Kiparizoska, S., and Ikuta, T. (2017). Disrupted olfactory integration in schizophrenia: functional connectivity study. Int. J. Neuropsychopharmacol. 20, 740–746. doi: 10.1093/ijnp/pyx045
Koehler, L., Fournel, A., Albertowski, K., Roessner, V., Gerber, J., Hummel, C., et al. (2018). Impaired odor perception in autism Spectrum disorder is associated with decreased activity in olfactory cortex. Chem. Senses 43, 627–634. doi: 10.1093/chemse/bjy051
Kosillo, P., and Bateup, H. S. (2021). Dopaminergic dysregulation in syndromic autism Spectrum disorders: insights from genetic mouse models. Front. Neural Circuits 15:700968. doi: 10.3389/fncir.2021.700968
Kostka, J. K., and Hanganu-Opatz, I. L. (2023). Olfactory-driven beta band entrainment of limbic circuitry during neonatal development. J. Physiol. 601, 3605–3630. doi: 10.1113/JP284401
Kringel, R., Song, L., Xu, X., Bitzenhofer, S. H., and Hanganu-Opatz, I. L. (2023). Layer-specific impairment in the developing lateral entorhinal cortex of immune-challenged Disc1+/− mice. J. Physiol. 601, 847–857. doi: 10.1113/JP283896
Lazarini, F., Gabellec, M.-M., Moigneu, C., De Chaumont, F., Olivo-Marin, J.-C., and Lledo, P.-M. (2014). Adult neurogenesis restores dopaminergic neuronal loss in the olfactory bulb. J. Neurosci. 34, 14430–14442. doi: 10.1523/JNEUROSCI.5366-13.2014
Lee, E.-Y., Eslinger, P. J., Du, G., Kong, L., Lewis, M. M., and Huang, X. (2014). Olfactory-related cortical atrophy is associated with olfactory dysfunction in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 29, 1205–1208. doi: 10.1002/mds.25829
Leitner, F. C., Melzer, S., Lütcke, H., Pinna, R., Seeburg, P. H., Helmchen, F., et al. (2016). Spatially segregated feedforward and feedback neurons support differential odor processing in the lateral entorhinal cortex. Nat. Neurosci. 19, 935–944. doi: 10.1038/nn.4303
Lepousez, G., and Lledo, P.-M. (2013). Odor discrimination requires proper olfactory fast oscillations in awake mice. Neuron 80, 1010–1024. doi: 10.1016/j.neuron.2013.07.025
Li, W., Li, S., Shen, L., Wang, J., Wu, X., Li, J., et al. (2019b). Impairment of Dendrodendritic inhibition in the olfactory bulb of APP/PS1 mice. Front. Aging Neurosci. 11:2. doi: 10.3389/fnagi.2019.00002
Li, S., Li, W., Wu, X., Li, J., Yang, J., Tu, C., et al. (2019a). Olfactory deficit is associated with mitral cell dysfunction in the olfactory bulb of P301S tau transgenic mice. Brain Res. Bull. 148, 34–45. doi: 10.1016/j.brainresbull.2019.03.006
Li, Y., Xu, J., Liu, Y., Zhu, J., Liu, N., Zeng, W., et al. (2017). A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nat. Neurosci. 20, 559–570. doi: 10.1038/nn.4517
Linster, C., and Fontanini, A. (2014). Functional neuromodulation of chemosensation in vertebrates. Curr. Opin. Neurobiol. 29, 82–87. doi: 10.1016/j.conb.2014.05.010
Liu, G., Froudarakis, E., Patel, J. M., Kochukov, M. Y., Pekarek, B., Hunt, P. J., et al. (2019). Target specific functions of EPL interneurons in olfactory circuits. Nat. Commun. 10:3369. doi: 10.1038/s41467-019-11354-y
Liu, Y.-H., Lai, W.-S., Tsay, H.-J., Wang, T.-W., and Yu, J.-Y. (2013b). Effects of maternal immune activation on adult neurogenesis in the subventricular zone-olfactory bulb pathway and olfactory discrimination. Schizophr. Res. 151, 1–11. doi: 10.1016/j.schres.2013.09.007
Liu, S., Plachez, C., Shao, Z., Puche, A., and Shipley, M. T. (2013a). Olfactory bulb short axon cell release of GABA and dopamine produces a temporally biphasic inhibition-excitation response in external tufted cells. J. Neurosci. 33, 2916–2926. doi: 10.1523/JNEUROSCI.3607-12.2013
Livneh, Y., Adam, Y., and Mizrahi, A. (2014). Odor processing by adult-born neurons. Neuron 81, 1097–1110. doi: 10.1016/j.neuron.2014.01.007
Lledo, P.-M., and Valley, M. (2016). Adult olfactory bulb neurogenesis. Cold Spring Harb. Perspect. Biol. 8:a018945. doi: 10.1101/cshperspect.a018945
Loayza, M., Lin, S., Carter, K., Ojeda, N., Fan, L.-W., Ramarao, S., et al. (2023). Maternal immune activation alters fetal and neonatal microglia phenotype and disrupts neurogenesis in mice. Pediatr. Res. 93, 1216–1225. doi: 10.1038/s41390-022-02239-w
Lu, J., Testa, N., Jordan, R., Elyan, R., Kanekar, S., Wang, J., et al. (2019a). Functional connectivity between the resting-state olfactory network and the Hippocampus in Alzheimer’s disease. Brain Sci. 9:338. doi: 10.3390/brainsci9120338
Lu, J., Yang, Q. X., Zhang, H., Eslinger, P. J., Zhang, X., Wu, S., et al. (2019b). Disruptions of the olfactory and default mode networks in Alzheimer’s disease. Brain Behav. 9:e01296. doi: 10.1002/brb3.1296
Mao, Y., Ge, X., Frank, C. L., Madison, J. M., Koehler, A. N., Doud, M. K., et al. (2009). Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3β/β-catenin signaling. Cell 136, 1017–1031. doi: 10.1016/j.cell.2008.12.044
Martin-Lopez, E., Vidyadhara, D. J., Liberia, T., Meller, S. J., Harmon, L. E., Hsu, R. M., et al. (2023). α-Synuclein pathology and reduced neurogenesis in the olfactory system affect olfaction in a mouse model of Parkinson’s disease. J. Neurosci. 43, 1051–1071. doi: 10.1523/JNEUROSCI.1526-22.2022
McCann, H., Cartwright, H., and Halliday, G. M. (2016). Neuropathology of α-synuclein propagation and braak hypothesis. Mov. Disord. 31, 152–160. doi: 10.1002/mds.26421
McGann, J. P. (2013). Presynaptic inhibition of olfactory sensory neurons: new mechanisms and potential functions. Chem. Senses 38, 459–474. doi: 10.1093/chemse/bjt018
Meyer, P. M., and Alberts, J. R. (2016). Non-nutritive, thermotactile cues induce odor preference in infant mice (Mus musculus). J. Comp. Psychol. Wash. DC 130, 369–379. doi: 10.1037/com0000044
Mezey, E., Dehejia, A. M., Harta, G., Suchy, S. F., Nussbaum, R. L., Brownstein, M. J., et al. (1998). Alpha synuclein is present in Lewy bodies in sporadic Parkinson’s disease. Mol. Psychiatry 3, 493–499. doi: 10.1038/sj.mp.4000446
Mihalj, D., Borbelyova, V., Pirnik, Z., Bacova, Z., Ostatnikova, D., and Bakos, J. (2024). Shank3 deficiency results in a reduction in GABAergic postsynaptic puncta in the olfactory brain areas. Neurochem. Res. 49, 1008–1016. doi: 10.1007/s11064-023-04097-2
Moberg, P. J., Agrin, R., Gur, R. E., Gur, R. C., Turetsky, B. I., and Doty, R. L. (1999). Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Am. College Neuropsychopharmacol. 21, 325–340. doi: 10.1016/S0893-133X(99)00019-6
Mori, K., Manabe, H., Narikiyo, K., and Onisawa, N. (2013). Olfactory consciousness and gamma oscillation couplings across the olfactory bulb, olfactory cortex, and orbitofrontal cortex. Front. Psychol. 4:743. doi: 10.3389/fpsyg.2013.00743
Mundiñano, I.-C., Caballero, M.-C., Ordóñez, C., Hernandez, M., DiCaudo, C., Marcilla, I., et al. (2011). Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol. (Berl.) 122, 61–74. doi: 10.1007/s00401-011-0830-2
Murman, D. L. (2015). The impact of age on cognition. Semin. Hear. 36, 111–121. doi: 10.1055/s-0035-1555115
Murphy, C. (2019). Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol. 15, 11–24. doi: 10.1038/s41582-018-0097-5
Murrell, W., Bushell, G. R., Livesey, J., McGrath, J., MacDonald, K. P. A., Bates, P. R., et al. (1996). Neurogenesis in adult human. Neuroreport 7, 1189–1194. doi: 10.1097/00001756-199604260-00019
Nguyen, A. D., Pelavin, P. E., Shenton, M. E., Chilakamarri, P., McCarley, R. W., Nestor, P. G., et al. (2011). Olfactory sulcal depth and olfactory bulb volume in patients with schizophrenia: an MRI study. Brain Imaging Behav. 5, 252–261. doi: 10.1007/s11682-011-9129-0
Nguyen, A. D., Shenton, M. E., and Levitt, J. J. (2010). Olfactory dysfunction in schizophrenia: a review of neuroanatomy and psychophysiological measurements. Harv. Rev. Psychiatry 18, 279–292. doi: 10.3109/10673229.2010.511060
Nobili, A., Latagliata, E. C., Viscomi, M. T., Cavallucci, V., Cutuli, D., Giacovazzo, G., et al. (2017). Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 8:14727. doi: 10.1038/ncomms14727
Ovsepian, S. V., O’Leary, V. B., Zaborszky, L., Ntziachristos, V., and Dolly, J. O. (2019). Amyloid plaques of Alzheimer’s disease as hotspots of glutamatergic activity. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 25, 288–297. doi: 10.1177/1073858418791128
Owen, M. J., Sawa, A., and Mortensen, P. B. (2016). Schizophrenia. Lancet 388, 86–97. doi: 10.1016/S0140-6736(15)01121-6
Papadatos, Z., and Phillips, N. A. (2023). Olfactory function reflects episodic memory performance and atrophy in the medial temporal lobe in individuals at risk for Alzheimer’s disease. Neurobiol. Aging 128, 33–42. doi: 10.1016/j.neurobiolaging.2023.04.001
Parbst, F., Kostka, J. K., Günther, A., Chen, Y.-N., Hanganu-Opatz, I. L., and Bitzenhofer, S. H. (2024). Developmental olfactory dysfunction and abnormal odor memory in immune-challenged Disc1+/− mice. bioRxiv 2024-05:663. doi: 10.1101/2024.05.17.594663
Pavăl, D. (2017). A dopamine hypothesis of autism Spectrum disorder. Dev. Neurosci. 39, 355–360. doi: 10.1159/000478725
Pavăl, D., and Micluția, I. V. (2021). The dopamine hypothesis of autism Spectrum disorder revisited: current status and future prospects. Dev. Neurosci. 43, 73–83. doi: 10.1159/000515751
Pignatelli, A., and Belluzzi, O. (2017). Dopaminergic Neurones in the Main olfactory bulb: An overview from an electrophysiological perspective. Front. Neuroanat. 11:7. doi: 10.3389/fnana.2017.00007
Rangel, L. M., Rueckemann, J. W., Riviere, P. D., Keefe, K. R., Porter, B. S., Heimbuch, I. S., et al. (2016). Rhythmic coordination of hippocampal neurons during associative memory processing. eLife 5:e09849. doi: 10.7554/eLife.09849
Rey, N. L., Steiner, J. A., Maroof, N., Luk, K. C., Madaj, Z., Trojanowski, J. Q., et al. (2016). Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J. Exp. Med. 213, 1759–1778. doi: 10.1084/jem.20160368
Richardson, J. T. E., and Zucco, G. M. (1989). Cognition and olfaction: a review. Psychol. Bull. 105, 352–360. doi: 10.1037/0033-2909.105.3.352
Richter, M., Murtaza, N., Scharrenberg, R., White, S. H., Johanns, O., Walker, S., et al. (2019). Altered TAOK2 activity causes autism-related neurodevelopmental and cognitive abnormalities through RhoA signaling. Mol. Psychiatry 24, 1329–1350. doi: 10.1038/s41380-018-0025-5
Roberts, R. O., Christianson, T. J. H., Kremers, W. K., Mielke, M. M., Machulda, M. M., Vassilaki, M., et al. (2016). Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. 73, 93–101. doi: 10.1001/jamaneurol.2015.2952
Rodríguez, J. J., Jones, V. C., and Verkhratsky, A. (2009). Impaired cell proliferation in the subventricular zone in an Alzheimer’s disease model. Neuroreport 20, 907–912. doi: 10.1097/WNR.0b013e32832be77d
Ross, G. W., Petrovitch, H., Abbott, R. D., Tanner, C. M., Popper, J., Masaki, K., et al. (2008). Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann. Neurol. 63, 167–173. doi: 10.1002/ana.21291
Rupp, C. I., Fleischhacker, W. W., Kemmler, G., Kremser, C., Bilder, R. M., Mechtcheriakov, S., et al. (2005). Olfactory functions and volumetric measures of orbitofrontal and limbic regions in schizophrenia. Schizophr. Res. 74, 149–161. doi: 10.1016/j.schres.2004.07.010
Ryndych, D., Sebold, A., Strassburg, A., Li, Y., Ramos, R. L., and Otazu, G. H. (2023). Haploinsufficiency of Shank3 in mice selectively impairs target odor recognition in novel background odors. J. Neurosci. 43, 7799–7811. doi: 10.1523/JNEUROSCI.0255-23.2023
Schechter, I., Butler, P. D., Silipo, G., Zemon, V., and Javitt, D. C. (2003). Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophr. Res. 64, 91–101. doi: 10.1016/S0920-9964(03)00008-2
Schepanski, S., Chini, M., Sternemann, V., Urbschat, C., Thiele, K., Sun, T., et al. (2022). Pregnancy-induced maternal microchimerism shapes neurodevelopment and behavior in mice. Nat. Commun. 13:4571. doi: 10.1038/s41467-022-32230-2
Schurov, I. L., Handford, E. J., Brandon, N. J., and Whiting, P. J. (2004). Expression of disrupted in schizophrenia 1 (DISC1) protein in the adult and developing mouse brain indicates its role in neurodevelopment. Mol. Psychiatry 9, 1100–1110. doi: 10.1038/sj.mp.4001574
Scopa, C., Marrocco, F., Latina, V., Ruggeri, F., Corvaglia, V., La Regina, F., et al. (2020). Impaired adult neurogenesis is an early event in Alzheimer’s disease neurodegeneration, mediated by intracellular Aβ oligomers. Cell Death Differ. 27, 934–948. doi: 10.1038/s41418-019-0409-3
Siper, P. M., Layton, C., Levy, T., Lurie, S., Benrey, N., Zweifach, J., et al. (2021). Sensory reactivity symptoms are a Core feature of ADNP syndrome irrespective of autism diagnosis. Gen. Dent. 12:351. doi: 10.3390/genes12030351
Sohrabi, H. R., Bates, K. A., Weinborn, M. G., Johnston, A. N. B., Bahramian, A., Taddei, K., et al. (2012). Olfactory discrimination predicts cognitive decline among community-dwelling older adults. Transl. Psychiatry 2:e118. doi: 10.1038/tp.2012.43
Sosulski, D. L., Bloom, M. L., Cutforth, T., Axel, R., and Datta, S. R. (2011). Distinct representations of olfactory information in different cortical centres. Nature 472, 213–216. doi: 10.1038/nature09868
Stark, K. L., Xu, B., Bagchi, A., Lai, W.-S., Liu, H., Hsu, R., et al. (2008). Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat. Genet. 40, 751–760. doi: 10.1038/ng.138
Stevenson, R. J. (2013). Olfactory perception, cognition, and dysfunction in humans. WIREs Cogn. Sci. 4, 273–284. doi: 10.1002/wcs.1224
Stranahan, A. M., and Mattson, M. P. (2010). Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer’s disease. Neural Plast. 2010, 1–8. doi: 10.1155/2010/108190
Struble, R. G., and Clark, H. B. (1992). Olfactory bulb lesions in Alzheimer’s disease. Neurobiol. Aging 13, 469–473. doi: 10.1016/0197-4580(92)90074-8
Symanski, C. A., Bladon, J. H., Kullberg, E. T., Miller, P., and Jadhav, S. P. (2022). Rhythmic coordination of hippocampal-prefrontal ensembles for odor-place associative memory and decision making. bioRxiv 2020-06:140939. doi: 10.1101/2020.06.08.140939
Tanik, N., Serin, H. I., Celikbilek, A., Inan, L. E., and Gundogdu, F. (2016). Associations of olfactory bulb and depth of olfactory sulcus with basal ganglia and hippocampus in patients with Parkinson’s disease. Neurosci. Lett. 620, 111–114. doi: 10.1016/j.neulet.2016.03.050
Thomann, P. A., Dos Santos, V., Seidl, U., Toro, P., Essig, M., and Schröder, J. (2009a). MRI-derived atrophy of the olfactory bulb and tract in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 17, 213–221. doi: 10.3233/JAD-2009-1036
Thomann, P. A., Dos Santos, V., Toro, P., Schönknecht, P., Essig, M., and Schröder, J. (2009b). Reduced olfactory bulb and tract volume in early Alzheimer’s disease—a MRI study. Neurobiol. Aging 30, 838–841. doi: 10.1016/j.neurobiolaging.2007.08.001
Thye, M. D., Bednarz, H. M., Herringshaw, A. J., Sartin, E. B., and Kana, R. K. (2018). The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev. Cogn. Neurosci. 29, 151–167. doi: 10.1016/j.dcn.2017.04.010
Tillerson, J. L., Caudle, W. M., Parent, J. M., Gong, C., Schallert, T., and Miller, G. W. (2006). Olfactory discrimination deficits in mice lacking the dopamine transporter or the D2 dopamine receptor. Behav. Brain Res. 172, 97–105. doi: 10.1016/j.bbr.2006.04.025
Tort, A. B. L., Brankačk, J., and Draguhn, A. (2018). Respiration-entrained brain rhythms are global but often overlooked. Trends Neurosci. 41, 186–197. doi: 10.1016/j.tins.2018.01.007
Trentin, S., Fraiman de Oliveira, B. S., Ferreira Felloni Borges, Y., and de Mello Rieder, C. R. (2022). Systematic review and meta-analysis of Sniffin sticks test performance in Parkinson’s disease patients in different countries. Eur. Arch. Otorrinolaringol. 279, 1123–1145. doi: 10.1007/s00405-021-06970-8
Turetsky, B. I., Moberg, P. J., Owzar, K., Johnson, S. C., Doty, R. L., and Gur, R. E. (2003). Physiologic impairment of olfactory stimulus processing in schizophrenia. Biol. Psychiatry 53, 403–411. doi: 10.1016/s0006-3223(02)01865-6
Turetsky, B. I., Moberg, P. J., Quarmley, M., Dress, E., Calkins, M. E., Ruparel, K., et al. (2018). Structural anomalies of the peripheral olfactory system in psychosis high-risk subjects. Schizophr. Res. 195, 197–205. doi: 10.1016/j.schres.2017.09.015
Turetsky, B. I., Moberg, P. J., Yousem, D. M., Doty, R. L., Arnold, S. E., and Gur, R. E. (2000). Reduced olfactory bulb volume in patients with schizophrenia. Am. J. Psychiatry 157, 828–830. doi: 10.1176/appi.ajp.157.5.828
Uchida, S., Shimada, C., Sakuma, N., Kagitani, F., Kan, A., and Awata, S. (2020). The relationship between olfaction and cognitive function in the elderly. J. Physiol. Sci. 70:48. doi: 10.1186/s12576-020-00777-8
Vallés, A. S., and Barrantes, F. J. (2021). Dysregulation of neuronal nicotinic acetylcholine receptor-cholesterol crosstalk in autism Spectrum disorder. Front. Mol. Neurosci. 14:744597. doi: 10.3389/fnmol.2021.744597
Wang, P. Y., Boboila, C., Chin, M., Higashi-Howard, A., Shamash, P., Wu, Z., et al. (2020). Transient and persistent representations of odor value in prefrontal cortex. Neuron 108, 209–224.e6. doi: 10.1016/j.neuron.2020.07.033
Wang, J., You, H., Liu, J.-F., Ni, D.-F., Zhang, Z.-X., and Guan, J. (2011). Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. AJNR Am. J. Neuroradiol. 32, 677–681. doi: 10.3174/ajnr.A2350
Wattendorf, E., Welge-Lüssen, A., Fiedler, K., Bilecen, D., Wolfensberger, M., Fuhr, P., et al. (2009). Olfactory impairment predicts brain atrophy in Parkinson’s disease. J. Neurosci. 29, 15410–15413. doi: 10.1523/JNEUROSCI.1909-09.2009
Wesson, D. W., Borkowski, A. H., Landreth, G. E., Nixon, R. A., Levy, E., and Wilson, D. A. (2011). Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer’s β-amyloidosis mouse model. J. Neurosci. 31, 15962–15971. doi: 10.1523/JNEUROSCI.2085-11.2011
Wesson, D. W., Levy, E., Nixon, R. A., and Wilson, D. A. (2010). Olfactory dysfunction correlates with amyloid-β burden in an Alzheimer’s disease mouse model. J. Neurosci. 30, 505–514. doi: 10.1523/JNEUROSCI.4622-09.2010
Wilson, D. M., Cookson, M. R., Van Den Bosch, L., Zetterberg, H., Holtzman, D. M., and Dewachter, I. (2023). Hallmarks of neurodegenerative diseases. Cell 186, 693–714. doi: 10.1016/j.cell.2022.12.032
Wilson, D. A., and Sullivan, R. M. (1995). The D2 antagonist spiperone mimics the effects of olfactory deprivation on mitral/tufted cell odor response patterns. J. Neurosci. 15, 5574–5581. doi: 10.1523/JNEUROSCI.15-08-05574.1995
Winner, B., Melrose, H. L., Zhao, C., Hinkle, K. M., Yue, M., Kent, C., et al. (2011). Adult neurogenesis and neurite outgrowth are impaired in LRRK2 G2019S mice. Neurobiol. Dis. 41, 706–716. doi: 10.1016/j.nbd.2010.12.008
Winner, B., Rockenstein, E., Lie, D. C., Aigner, R., Mante, M., Bogdahn, U., et al. (2008). Mutant α-synuclein exacerbates age-related decrease of neurogenesis. Neurobiol. Aging 29, 913–925. doi: 10.1016/j.neurobiolaging.2006.12.016
Witter, M. P., Doan, T. P., Jacobsen, B., Nilssen, E. S., and Ohara, S. (2017). Architecture of the entorhinal cortex a review of entorhinal anatomy in rodents with some comparative notes. Front. Syst. Neurosci. 11:46. doi: 10.3389/fnsys.2017.00046
Wu, N., Rao, X., Gao, Y., Wang, J., and Xu, F. (2013). Amyloid-β deposition and olfactory dysfunction in an Alzheimer’s disease model. J. Alzheimers Dis. 37, 699–712. doi: 10.3233/JAD-122443
Xu, W., Fitzgerald, S., Nixon, R. A., Levy, E., and Wilson, D. A. (2015). Early hyperactivity in lateral entorhinal cortex is associated with elevated levels of AβPP metabolites in the Tg2576 mouse model of Alzheimer’s disease. Exp. Neurol. 264, 82–91. doi: 10.1016/j.expneurol.2014.12.008
Xu, J., Kao, S.-Y., Lee, F. J. S., Song, W., Jin, L.-W., and Yankner, B. A. (2002). Dopamine-dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat. Med. 8, 600–606. doi: 10.1038/nm0602-600
Xu, X., Song, L., Kringel, R., and Hanganu-Opatz, I. L. (2021). Developmental decrease of entorhinal-hippocampal communication in immune-challenged DISC1 knockdown mice. Nat. Commun. 12:6810. doi: 10.1038/s41467-021-27114-w
Yahiaoui-Doktor, M., Luck, T., Riedel-Heller, S. G., Loeffler, M., Wirkner, K., and Engel, C. (2019). Olfactory function is associated with cognitive performance: results from the population-based LIFE-adult-study. Alzheimers Res. Ther. 11:43. doi: 10.1186/s13195-019-0494-z
Yang, M., and Crawley, J. N. (2009). Simple behavioral assessment of mouse olfaction. Curr. Protoc. Neurosci. 8:48. doi: 10.1002/0471142301.ns0824s48
Yang, K., Hasegawa, Y., Bhattarai, J. P., Hua, J., Dower, M., Etyemez, S., et al. (2024). Inflammation-related pathology in the olfactory epithelium: its impact on the olfactory system in psychotic disorders. Mol. Psychiatry 29, 1453–1464. doi: 10.1038/s41380-024-02425-8
Yang, K., Hua, J., Etyemez, S., Paez, A., Prasad, N., Ishizuka, K., et al. (2021). Volumetric alteration of olfactory bulb and immune-related molecular changes in olfactory epithelium in first episode psychosis patients. Schizophr. Res. 235, 9–11. doi: 10.1016/j.schres.2021.07.016
Yang, S., Kuan, W.-L., and Spillantini, M. G. (2016). Progressive tauopathy in P301S tau transgenic mice is associated with a functional deficit of the olfactory system. Eur. J. Neurosci. 44, 2396–2403. doi: 10.1111/ejn.13333
Yang, Q., Zhou, G., Noto, T., Templer, J. W., Schuele, S. U., Rosenow, J. M., et al. (2022). Smell-induced gamma oscillations in human olfactory cortex are required for accurate perception of odor identity. PLoS Biol. 20:e3001509. doi: 10.1371/journal.pbio.3001509
Yao, Z.-G., Hua, F., Zhang, H.-Z., Li, Y.-Y., and Qin, Y.-J. (2017). Olfactory dysfunction in the APP/PS1 transgenic mouse model of Alzheimer’s disease: morphological evaluations from the nose to the brain. Neuropathology 37, 485–494. doi: 10.1111/neup.12391
Zapiec, B., Dieriks, B. V., Tan, S., Faull, R. L. M., Mombaerts, P., and Curtis, M. A. (2017). A ventral glomerular deficit in Parkinson’s disease revealed by whole olfactory bulb reconstruction. Brain J. Neurol. 140, 2722–2736. doi: 10.1093/brain/awx208
Zelano, C., Jiang, H., Zhou, G., Arora, N., Schuele, S., Rosenow, J., et al. (2016). Nasal respiration entrains human limbic oscillations and modulates cognitive function. J. Neurosci. 36, 12448–12467. doi: 10.1523/JNEUROSCI.2586-16.2016
Keywords: olfaction, olfactory dysfunction, neurodegenerative disorders, neurodevelopmental disorders, Alzheimer’s disease, Parkinson’s disease, schizophrenia, autism spectrum disorder
Citation: Chen Y-N and Kostka JK (2024) Beyond anosmia: olfactory dysfunction as a common denominator in neurodegenerative and neurodevelopmental disorders. Front. Neurosci. 18:1502779. doi: 10.3389/fnins.2024.1502779
Received: 27 September 2024; Accepted: 21 October 2024;
Published: 30 October 2024.
Edited by:
Thomas Heinbockel, Howard University, United StatesReviewed by:
Samir Ranjan Panda, National Institute of Pharmaceutical Education and Research, IndiaCopyright © 2024 Chen and Kostka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johanna Katharina Kostka, am9oYW5uYS5rb3N0a2FAem1uaC51bmktaGFtYnVyZy5kZQ==; Yu-Nan Chen, eXVuYW4uY2hlbkB6bW5oLnVuaS1oYW1idXJnLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.