- 1Department of Rehabilitation Medicine, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
- 2Department of Rehabilitation Medicine, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Gusu School, Nanjing Medical University, Suzhou, China
- 3Department of Radiology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
- 4Department of Neurology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
- 5Department of Nursing, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
- 6National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, China
Background: Multiple system atrophy-cerebellar subtype (MSA-C) is a predominance of cerebellar ataxia and autonomic failure. MSA-C has a rapid progression, with average 9 years from symptom onset to death. Despite its prevalence, there is still a lack of effective treatments. In recent years, it has been established that taVNS has significant therapeutic effects on epilepsy, depression, migraine, insomnia, and other diseases. Hence, we performed taVNS treatment for one MSA-C patient to explore whether taVNS could alleviate patient’s motor and non-motor symptoms.
Case presentation: A 65-year-old woman diagnosed with MSA-C received taVNS treatment for the following duration and course: once a day, 40 min a time, 20 times a month continually for 12 months. Meanwhile, she received assessments of motor and non-motor symptoms at baseline, 4-weeks and 12-months after taVNS treatment. Motor symptoms assessments was made by Scale for the Assessment and Rating of Ataxia (SARA) and Unified Multiple System Atrophy Rating Scale (UMSARS), non-motor symptoms assessment by Pittsburgh Sleep Quality Index (PSQI), Hamilton Anxiety Scale (HAMA), and Hamilton Depression Scale (HAMD). After 4-weeks and 12-months of taVNS treatment, compared to baseline assessments, SARA scores decreased from 13 to 11 and then to 10.5, UMSARS scores from 28 to 24 and then to 23, PSQI scores from 19 to 13 and then to 6, HAMA scores from 13 to 3 and then remained unchanged, and HAMD scores from 7 to 4 and then remained unchanged.
Conclusion: In the case, we found that short-term taVNS treatment can alleviate ataxia, sleep problem, anxiety and depression of the MSA-C patient. The effects can be maintained and some symptoms may be further improved after receiving long-term treatment. Our case report supports the feasibility and effectiveness of taVNS treatment in MSA-C patients.
Introduction
Multiple system atrophy (MSA) is a disseminated and unexplained neurodegenerative disease that affects the extrapyramidal system, pyramidal system, cerebellum, and autonomic nervous system, with Parkinsonian symptoms, ataxia, and autonomic dysfunction as the main clinical manifestations (Foubert-Samier et al., 2020). According to prodromal motor symptoms, MSA is categorized into MSA parkinsonism variant (MSA-P) and MSA cerebellar variant (MSA-C) (Gilman et al., 2008). MSA-C with atrophy of medullo-ponto-cerebellar (MPC) white matter (WM) (Lu et al., 2014) is mainly characterized by gait ataxia, limb ataxia, cerebellar dysarthria, and cerebellar oculomotor disorders, which seriously affects the patient’s quality of life (Zhang et al., 2018). MSA-C has a rapid progression, with average 9 years from symptom onset to death, and there is still a lack of effective treatments (Terao et al., 2017). Therefore, how to improve patients’ motor and balance dysfunction through non-pharmacological treatment is the focus of current clinical research (Lamotte and Kaufmann, 2022).
It has been well established that transcutaneous auricular vagus nerve stimulation (taVNS) plays a vital role in neuromodulation, central projection, and anti-inflammatory pathway effects (Jiakai et al., 2023; Wang et al., 2021; De Smet et al., 2023), mainly by stimulating the branches of auricular vagus nerve (Peuker and Filler, 2002). The auricular branches of the vagus nerve project stimulation to the nucleus tractus solitarius (NTS). Then, the stimulation is projected extensively, directly or indirectly, to the reticular formation, forebrain, mesencephalon, limbic system, cerebellum, and other parts of the brain through other brainstem structures, such as the locus coeruleus (LC), parabrachial nucleus (PBN), and raphe nuclei (RN), in order to regulate the neural functional activities of these areas (Jiakai et al., 2023; Wang et al., 2021; De Smet et al., 2023; Butt et al., 2020). In recent years, taVNS has achieved significant therapeutic effects in epilepsy, depression, migraine, insomnia, and other diseases (Jiakai et al., 2023; Wang et al., 2021). Besides, treatment by means of taVNS is safe, non-invasive, easy to be applied clinically, and high in adherence (Kim et al., 2022; Tan et al., 2023).
Hence, we hypothesized that taVNS may provide beneficial assistance to MSA-C patients. However, there are no previous studies applying taVNS to patients with MSA-C. Therefore, we performed the taVNS treatment for MSA-C patient to explore whether taVNS could alleviate patient’s ataxia as well as other non-motor symptoms.
Case presentation
A 65-year-old woman with MSA-C visited the Outpatient Rehabilitation Department of our hospital with walking instability for 1 year and worsening for 5 months. One year ago, the patient presented with unsteady walking without obvious triggers, accompanied by weakness of both lower limbs, which manifested as shuffling walking, difficulty in lifting the legs, and falling easily. Five months ago, the symptoms of unsteady walking and bilateral lower limb weakness became progressively worse. The patient also had slurred speech and frequent urination at night. Magnetic Resonance Imaging (MRI) of the cranium showed hot cross bun sign (Figure 1A) and cerebellar atrophy (Figure 1B). Nervous system examination of the patient showed that his consciousness is clear but the speech is slightly vague, and the remaining cranial nerve examination proved negative. The muscle strength of both upper limbs was grade 5, while that of both lower limbs was grade 4+. The muscle tone of the extremities was normal. The tendon reflex was symmetrical and active. Besides, no pathological signs were elicited. The left side of the body was unstable and inaccurate in the finger-nose test, while the right side being normal. The result of heel–knee-tibia test was also normal, with positive Romberg Sign and poor straight-line walking. In addition, proprioceptive sensation and superficial sensation were normal and Kernig’s sign is negative.
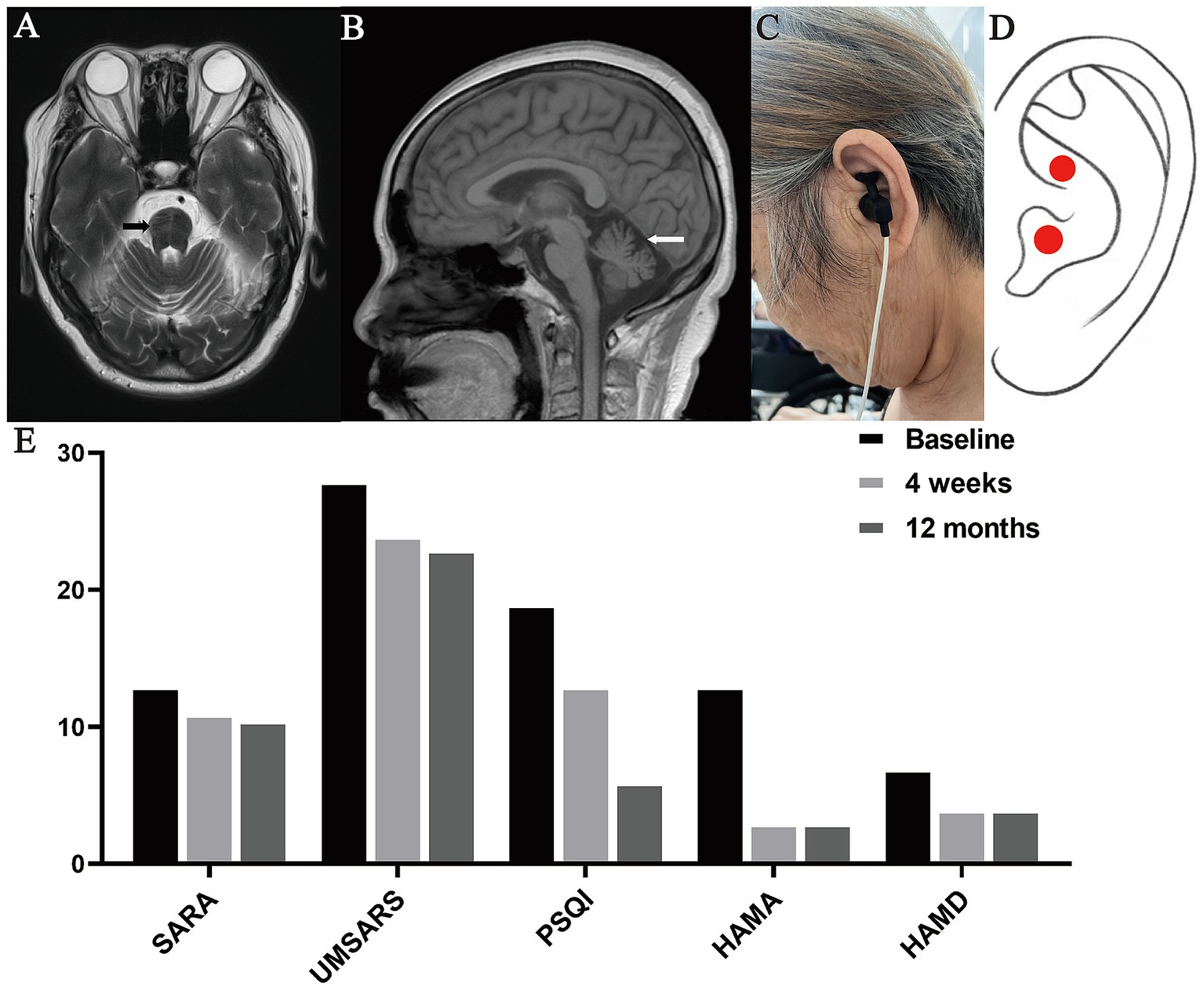
Figure 1. Representative images of the case. (A) T2W1 showed hot cross bun sign, (B) T2 showed cerebellar atrophy, (C) The patient with MSA-C was undergoing the taVNS treatment, (D) Position of the taVNS stimulation (cymba conchae), (E) Scores of SARA, UMSARS, PSQI, HAMA and HAMD at baseline, 4 weeks and 12 months after taVNS treatment.
In addition, it was found that the patient had been suffering from hypertension for one year, and was taking Amlodipine on a regular basis, and her blood pressure was controlled at 110/80 mmHg. She had also been suffering from aortic sclerosis and cerebral ischemia for 5 months, and was prescribed oral Atorvastatin Calcium. At the same time, she took Buspirone Hydrochloride Tablets for improving ataxia.
In the case, the clinical and pharmacologic treatments the patient had previously received did not improve her symptoms and she experienced exacerbations. In order to alleviate the above symptoms, we tried to give the patient taVNS treatment, during which other former medication and lifestyle of the patient remained unchanged and the patient did not accept other therapies through the course. Informed consent has been signed with the patient. All research procedures were approved by Branch for Medical Research and Clinical Technology Application, Ethics Committee of the First Affiliated Hospital of Fujian Medical University (MRCTA, ECFAH of FMU [2022]400).
In this study, we used the taVNS device (Hwato, TENS-200A, Suzhou, China) to stimulate the left cymba conchae in patients with MSA-C (Figures 1C,D), which is in line with established practice (Farmer et al., 2020). The taVNS device used the following parameters: (i) Output pulse waveform: frequency 20 Hz (7 s) and 4 Hz (3 s), alternating between the two; (ii) Output pulse width: 0.2 ms; (iii) Output current limit ≤50 mA. The appropriate intensity was based on the patient experiencing a mild painful stimulation. During treatment, the patient was in supine or sitting position. The patient received taVNS in the Outpatient Rehabilitation Department for the following time and course of treatment: once a day, 40 min a time, 20 times a month for 12 months. Besides, she received assessments of motor and non-motor symptoms at baseline, 4-weeks and 12-months after taVNS treatment. Motor symptoms assessments included Scale for the Assessment and Rating of Ataxia (SARA) and Unified Multiple System Atrophy Rating Scale (UMSARS). Non-motor symptoms assessments included Pittsburgh Sleep Quality Index (PSQI), Hamilton Anxiety Scale (HAMA), and Hamilton Depression Scale (HAMD). Compared to baseline assessments, SARA scores decreased from 13 to 11 and then to 10.5, UMSARS scores from 28 to 24 and then to 23, PSQI scores from 19 to 13 and then to 6, HAMA scores from 13 to 3 and then remained unchanged, and HAMD scores from 7 to 4 and then remained unchanged (Figure 1E).
Discussion
This case report firstly confirmed the efficacy of taVNS treatment in the patient with MSA-C. In the study, the patient suffered mainly from ataxia, walking instability for 1 year and worsening for 5 months. The pharmacologic treatments the patient had previously received did not improve her symptoms and some symptoms continued to progress. After receiving 12 months of taVNS treatment, she showed improvements in ataxia symptoms, sleep, anxiety and depression.
MSA-C is a neurodegenerative disorder with extrapyramidal system, pyramidal system, cerebellum, and autonomic nervous system (Lu et al., 2014; Terao et al., 2017), presenting with motor and non-motor symptoms such as ataxia, anxiety, depression and sleep disorders (Makawita et al., 2024). Cerebellar atrophy and decreased cerebellar blood flow are typical problems in MSA-C (Kimura et al., 2009). The cerebellum involves diverse functions from motor coordination to higher cognitive functions, impairment of which can cause ataxia and cerebellar cognitive affective syndrome (Lu et al., 2014). Previous studies have shown that the auricular branch of the vagus nerve, when stimulated by the taVNS, projects to the cerebellum through NTS (Jiakai et al., 2023; Wang et al., 2021; De Smet et al., 2023; Butt et al., 2020), and in turn causes significant changes of cerebral blood flow in posterior superior cerebellum (Chen et al., 2021). Also, taVNS can modulate the cerebello-thalamo-cortical pathway and the cerebellum may serve as an entry for modulating effects of taVNS (van Midden et al., 2023). Therefore, we infer that taVNS may improve ataxia symptoms of the MSA-C patient by modulating cerebellar function.
Sleep disorders are also one of the common symptoms in MSA-C patients, characterized by insomnia, rapid eye movement sleep behavior disorder (RBD), and daytime sleepiness (Lin et al., 2020). In the case, the patient had reduced total sleep time, decreased sleep efficiency, and prolonged time before sleep prior to the treatment, all of which improved after taVNS treatment. TaVNS may improve RBD of the patient by stimulating the nucleus of the solitary tract producing a long-lasting increase in the theta and beta band power (Martinez-Vargas et al., 2017). In addition, taVNS is a very promising resource for treating emotional disorders (Aranberri, 2024). It has also been applied to improve post-stroke depression and the depressive symptoms of COVID-19 with favorable results (Liu et al., 2024; Guo et al., 2021). Our study found that taVNS is effective in improving anxiety and depression in MSA-C patients, possibly by directly and indirectly modulating the activity and connectivity of key brain regions involved in anxiety and depression, inhibiting central and peripheral inflammation or modulating brain circuits via the hypothalamic–pituitary–adrenal axis (Aranberri, 2024; Guo et al., 2021).
In the above assessments, UMSARS is the most commonly used semiquantitative rating scale to assess symptoms and measure disease progression in MSA (Krismer et al., 2022). The higher the scale score, the more severe the condition (Krismer et al., 2022). MSA patients had a mean increase of approximately 4 points per year in UMSARS score with disease progression (Kaufmann et al., 2017). In the case, the UMSARS of MSA-C patient that received one-year taVNS treatment did not increase but instead decreased from 28 to 24 and then to 23. Also, no adverse effects were observed in the patients during treatment, and the safety of taVNS treatment was further validated. It can be seen that long-term adherence to taVNS treatment helped slow down disease progression and improve prognosis.
The above findings are based solely on this case. The assessment of the case lacked of objective parameters (such as gait analysis or the timed-up-and-go test) and objective markers (e.g., neuroimaging, electroencephalogram, biomarkers like inflammatory or oxidative stress markers), to provide more objective corroboration for our conclusions. Besides, the exact frequency, intensity, and duration of taVNS required for optimal therapeutic outcomes in MSA-C are not well-established. While the case report using a specific protocol (40 min a time, 20 times a month for 12 months) with reference to previous studies, it is unclear whether this protocol is the most effective. Further study is needed to standardize these parameters.
Conclusion
In the case, we found that short-term taVNS treatment can improve ataxia, sleep, anxiety and depression of the MSA-C patient. The effects can be maintained and some symptoms may be further improved after long-term treatment. The findings in this case are of great interest for MSA-C patients with rapid disease progression and poor prognosis. However, taVNS parameters are diverse and it is not clear which one works best, so further validation is required. Our study is merely a case report, hence definitive conclusions cannot be drawn until further studies involving more evidence are done. In the future, there are still needs for myriad high-quality studies to demonstrate the group therapeutic efficacy and explicit treatment mechanisms of taVNS in patients with MSA-C to provide an effective, safe and promising treatment for them.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Z-DW: Writing – original draft, Writing – review & editing. X-PC: Writing – original draft. Z-YL: Writing – original draft. DW: Writing – review & editing. JN: Writing – review & editing. C-JC: Writing – original draft, Writing – review & editing. X-YC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China to Xin-Yuan Chen (grant number: 82402952).
Acknowledgments
The authors sincerely thank the participants for their help and willingness to participate in this study. We also thank the reviewers for their helpful comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aranberri, R. A. (2024). Transcutaneous auricular vagus nerve stimulation to improve emotional state. Biomedicines 12:407. doi: 10.3390/biomedicines12020407
Butt, M. F., Albusoda, A., Farmer, A. D., and Aziz, Q. (2020). The anatomical basis for transcutaneous auricular vagus nerve stimulation. J. Anat. 236, 588–611. doi: 10.1111/joa.13122
Chen, C., Mao, Y., Falahpour, M., Mac Niven, K. H., Heit, G., Sharma, V., et al. (2021). Effects of sub-threshold transcutaneous auricular vagus nerve stimulation on cerebral blood flow. Sci. Rep. 11:24018. doi: 10.1038/s41598-021-03401-w
De Smet, S., Ottaviani, C., Verkuil, B., Kappen, M., Baeken, C., and Vanderhasselt, M. A. (2023). Effects of non-invasive vagus nerve stimulation on cognitive and autonomic correlates of perseverative cognition. Psychophysiology 60:e14250. doi: 10.1111/psyp.14250
Farmer, A. D., Strzelczyk, A., Finisguerra, A., Gourine, A. V., Gharabaghi, A., Hasan, A., et al. (2020). International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (version 2020). Front. Hum. Neurosci. 14:568051. doi: 10.3389/fnhum.2020.568051
Foubert-Samier, A., Pavy-Le, T. A., Guillet, F., Le-Goff, M., Helmer, C., Tison, F., et al. (2020). Disease progression and prognostic factors in multiple system atrophy: a prospective cohort study. Neurobiol. Dis. 139:104813. doi: 10.1016/j.nbd.2020.104813
Gilman, S., Wenning, G. K., Low, P. A., Brooks, D. J., Mathias, C. J., Trojanowski, J. Q., et al. (2008). Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71, 670–676. doi: 10.1212/01.wnl.0000324625.00404.15
Guo, Z. P., Soros, P., Zhang, Z. Q., Yang, M. H., Liao, D., and Liu, C. H. (2021). Use of transcutaneous auricular vagus nerve stimulation as an adjuvant therapy for the depressive symptoms of covid-19: a literature review. Front. Psych. 12:765106. doi: 10.3389/fpsyt.2021.765106
Jiakai, H. E., Jinling, Z., Yu, W., Shaoyuan, L. I., Jiliang, F., Shuai, Z., et al. (2023). Transcutaneous auricular vagus nerve stimulation would be an alternative to implantable cervical vagus nerve stimulation in some situation. J. Tradit. Chin. Med. 43, 627–630. doi: 10.19852/j.cnki.jtcm.20230308.002
Kaufmann, H., Norcliffe-Kaufmann, L., Palma, J. A., Biaggioni, I., Low, P. A., Singer, W., et al. (2017). Natural history of pure autonomic failure: a United States prospective cohort. Ann. Neurol. 81, 287–297. doi: 10.1002/ana.24877
Kim, A. Y., Marduy, A., de Melo, P. S., Gianlorenco, A. C., Kim, C. K., Choi, H., et al. (2022). Safety of transcutaneous auricular vagus nerve stimulation (tavns): a systematic review and meta-analysis. Sci. Rep. 12:22055. doi: 10.1038/s41598-022-25864-1
Kimura, N., Kumamoto, T., Masuda, T., Nomura, Y., Hanaoka, T., Hazama, Y., et al. (2009). Evaluation of regional cerebral blood flow in cerebellar variant of multiple system atrophy using finesrt. Clin. Neurol. Neurosurg. 111, 829–834. doi: 10.1016/j.clineuro.2009.08.014
Krismer, F., Seppi, K., Jonsson, L., Astrom, D. O., Berger, A. K., Simonsen, J., et al. (2022). Sensitivity to change and patient-centricity of the unified multiple system atrophy rating scale items: a data-driven analysis. Mov. Disord. 37, 1425–1431. doi: 10.1002/mds.28993
Lamotte, G., and Kaufmann, H. (2022). Movement disorder society criteria for the diagnosis of multiple system atrophy-what's new? Clin. Auton. Res. 32, 163–165. doi: 10.1007/s10286-022-00869-y
Lin, J. Y., Zhang, L. Y., Cao, B., Wei, Q. Q., Ou, R. W., Hou, Y. B., et al. (2020). Sleep-related symptoms in multiple system atrophy: determinants and impact on disease severity. Chin. Med. J. 134, 690–698. doi: 10.1097/CM9.0000000000001211
Liu, C., Tang, H., Liu, C., Ma, J., Liu, G., Niu, L., et al. (2024). Transcutaneous auricular vagus nerve stimulation for post-stroke depression: a double-blind, randomized, placebo-controlled trial. J. Affect. Disord. 354, 82–88. doi: 10.1016/j.jad.2024.03.005
Lu, C. F., Wang, P. S., Lao, Y. L., Wu, H. M., Soong, B. W., and Wu, Y. T. (2014). Medullo-ponto-cerebellar white matter degeneration altered brain network organization and cortical morphology in multiple system atrophy. Brain Struct. Funct. 219, 947–958. doi: 10.1007/s00429-013-0545-3
Makawita, C., Ananthavarathan, P., de Silva, R., and Malek, N. (2024). A systematic review of the spectrum and prevalence of non-motor symptoms in multiple system atrophy. Cerebellum 23, 1642–1650. doi: 10.1007/s12311-023-01642-1
Martinez-Vargas, D., Valdes-Cruz, A., Magdaleno-Madrigal, V. M., Fernandez-Mas, R., and Almazan-Alvarado, S. (2017). Effect of electrical stimulation of the nucleus of the solitary tract on electroencephalographic spectral power and the sleep-wake cycle in freely moving cats. Brain Stimul. 10, 116–125. doi: 10.1016/j.brs.2016.08.012
Peuker, E. T., and Filler, T. J. (2002). The nerve supply of the human auricle. Clin. Anat. 15, 35–37. doi: 10.1002/ca.1089
Tan, C., Qiao, M., Ma, Y., Luo, Y., Fang, J., and Yang, Y. (2023). The efficacy and safety of transcutaneous auricular vagus nerve stimulation in the treatment of depressive disorder: a systematic review and meta-analysis of randomized controlled trials. J. Affect. Disord. 337, 37–49. doi: 10.1016/j.jad.2023.05.048
Terao, Y., Fukuda, H., Tokushige, S. I., Inomata-Terada, S., Yugeta, A., Hamada, M., et al. (2017). Distinguishing spinocerebellar ataxia with pure cerebellar manifestation from multiple system atrophy (msa-c) through saccade profiles. Clin. Neurophysiol. 128, 31–43. doi: 10.1016/j.clinph.2016.10.012
van Midden, V. M., Pirtosek, Z., and Kojovic, M. (2023). The effect of tavns on the cerebello-thalamo-cortical pathway: a tms study. Cerebellum 23, 1013–1019. doi: 10.1007/s12311-023-01595-5
Wang, Y., Li, S. Y., Wang, D., Wu, M. Z., He, J. K., Zhang, J. L., et al. (2021). Transcutaneous auricular vagus nerve stimulation: from concept to application. Neurosci. Bull. 37, 853–862. doi: 10.1007/s12264-020-00619-y
Keywords: multiple system atrophy-cerebellar subtype, transcutaneous auricular vagus nerve stimulation, case report, ataxia, sleep
Citation: Wang Z-D, Cheng X-P, Liu Z-Y, Wu D, Ni J, Chen C-J and Chen X-Y (2025) Effect of long-term transcutaneous auricular vagus nerve stimulation in multiple system atrophy-cerebellar subtype: a case report. Front. Neurosci. 18:1499793. doi: 10.3389/fnins.2024.1499793
Edited by:
Muthuraju Sangu, Universiti Sains Malaysia Health Campus, MalaysiaReviewed by:
Francesco Motolese, Campus Bio-Medico University, ItalyHongyu Xu, Virginia Commonwealth University, United States
Copyright © 2025 Wang, Cheng, Liu, Wu, Ni, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan-Juan Chen, Zmpjd2otMDU5OEAxNjMuY29t; Xin-Yuan Chen, ZnljaGVueGlueXVhbkBmam11LmVkdS5jbg==
†These authors share first authorship
‡ORCID: Xin-Yuan Chen, https://orcid.org/0000-0002-3241-6719
 Zhao-Di Wang
Zhao-Di Wang Xiao-Ping Cheng
Xiao-Ping Cheng Zhen-Yi Liu
Zhen-Yi Liu Di Wu
Di Wu Jun Ni
Jun Ni Chuan-Juan Chen
Chuan-Juan Chen Xin-Yuan Chen
Xin-Yuan Chen