- 1Department of Neurology, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 2Anhui Province Key Laboratory of Cognition and Neuropsychiatric Disorders, Hefei, China
- 3Collaborative Innovation Center of Neuropsychiatric Disorders and Mental Health, Hefei, China
- 4Wuhan WuDong Hospital, Wuhan, China
- 5The School of Mental Health and Psychological Sciences, Anhui Medical University, Hefei, China
- 6Department of Psychology and Sleep Medicine, The Second Affiliated Hospital of Anhui Medical University, Hefei, China
- 7Institute of Artificial Intelligence, Hefei Comprehensive National Science Center, Hefei, China
Background: The Montreal Cognitive Assessment (MoCA) is a valuable tool for detecting cognitive impairment, widely used in many countries. However, there is still a lack of large sample normative data and whose cut-off values for detecting cognitive impairment is considerable controversy.
Methods: The assessment conducted in this study utilizes the MoCA scale, specifically employing the Mandarin-8.1 version. This study recruited a total of 3,097 healthy adults aged over 20 years. We performed multiple linear regression analysis, incorporating age, gender, and education level as predictor variables, to examine their associations with the MoCA total score and subdomain scores. Subsequently, we established normative values stratified by age and education level. Finally, we included 242 patients with vascular cognitive impairment (VCI) and 137 controls with normal cognition, and determined the optimal cut-off value of VCI through ROC curves.
Results: The participants in this study exhibit a balanced gender distribution, with an average age of 54.46 years (SD = 14.38) and an average education period of 9.49 years (SD = 4.61). The study population demonstrates an average MoCA score of 23.25 points (SD = 4.82). The multiple linear regression analysis indicates that MoCA total score is influenced by age and education level, collectively accounting for 46.8% of the total variance. Higher age and lower education level are correlated with lower MoCA total scores. A score of 22 is the optimal cut-off value for diagnosing vascular cognitive impairment (VCI).
Conclusion: This study offered normative MoCA values specific to the Chinese adults. Furthermore, this study indicated that a score of 26 may not represent the most optimal cut-off value for VCI. And for detecting VCI, a score of 22 may be a better cut-off value.
Background
Age-related cognitive impairment represents a significant public health and social challenge in the contemporary era. In 2018 alone, approximately 50 million individuals worldwide were affected by this condition, with projections estimating that the number will triple by 2050, leading to a staggering economic loss of nearly $4 trillion (Rundek et al., 2022). Cerebrovascular pathology is the most important factor in cognitive impairment and has an additional or synergistic effect with neurodegenerative pathology (Rundek et al., 2022). Vascular cognitive impairment (VCI) includes a series of varying degrees of cognitive impairment, ranging from mild cognitive impairment to vascular dementia caused by cerebral ischemic (or hemorrhagic) etiology or vascular factors alone or in combination with neurodegenerative changes (Zhang et al., 2019). VCI accounts for approximately 20–40% of all dementia patients (Wolf, 2012). For cognitive disorders, early identification is of paramount importance to maximize the effectiveness of interventions, which may include counseling, psychoeducation, cognitive training, and medication (De Roeck et al., 2016). Owing to the absence of effective treatments for advanced dementia, the significance of early diagnosis and intervention during the mild cognitive impairment (MCI) stage has gained widespread recognition as a pivotal approach in disease management. These strategies hold the potential to impact long-term outcomes (Zhuang et al., 2021). The early detection and identification of cognitive decline necessitates a straightforward, easily comprehensible, and highly diagnostic tool. Historically, the Mini-Mental Status Examination (MMSE) served as the widely adopted assessment tool (Folstein et al., 1975). Nevertheless, owing to the absence of executive function assessment (Fu et al., 2017), the MMSE demonstrates limited sensitivity in identifying mild cognitive impairment (Roalf et al., 2013; Ciesielska et al., 2016). Subsequently, the Montreal Cognitive Assessment (MoCA) emerged as a viable alternative (Nasreddine et al., 2005), a widely utilized international neuropsychological screening tool, proficient in evaluating a subject’s global cognitive function. In comparison to the MMSE, MoCA incorporates additional tasks to assess visuospatial abilities, as well as specific evaluations of executive function, attention and delayed recall (Smith et al., 2007; Dong et al., 2010). Consequently, it exhibits heightened sensitivity and specificity in detecting cognitive impairment, particularly mild cognitive impairment (Damian et al., 2011; Oudman et al., 2014), and it has been widely recognized and widely used in clinical and scientific research.
The MoCA scale employs cognitive tasks that are rapid, sensitive, and easily manageable, and it has undergone multiple revisions throughout its usage. Over the past decade, the scale has undergone translation into multiple languages and validation in diverse populations encompassing various ages and educational backgrounds (Kenny et al., 2013; Narazaki et al., 2013; Conti et al., 2015; Malek-Ahmadi et al., 2015; Santangelo et al., 2015; Konstantopoulos et al., 2016; Larouche et al., 2016; Borland et al., 2017; Kopecek et al., 2017; Rossetti et al., 2017; Apolinario et al., 2018; Thomann et al., 2018; Cesar et al., 2019; Hayek et al., 2020; Serrano et al., 2020; Davis et al., 2021; Muayqil et al., 2021; Classon et al., 2022; Gagnon et al., 2022; Gaete et al., 2023). However, owing to variations in cultural and linguistic practices across different countries and regions, the performance of MoCA may exhibit variability. As a result, it is particularly important to make reasonable localization revisions to the MoCA scale and develop appropriate local normative values, this approach has been implemented in numerous countries (Rossetti et al., 2011; Narazaki et al., 2013; Conti et al., 2015; Larouche et al., 2016; Borland et al., 2017; Kopecek et al., 2017; Rossetti et al., 2017; Apolinario et al., 2018; Thomann et al., 2018; Cesar et al., 2019; Hayek et al., 2020; Muayqil et al., 2021; Classon et al., 2022; Gaete et al., 2023). At present, MoCA has multiple Chinese versions, including Beijing, Changsha, Cantonese, Hong Kong, Taiwan, and Mandarin version.1 However, it must be mentioned that the MoCA has been updated to version 8.1, but there has been a lack of matching Chinese Mandarin version scales, as well as a lack of matching large sample based normative data for the Chinese population. To address this gap, this study used Mandarin-8.1 version (Chinese Mandarin version), which was localized and revised based on the language and cultural characteristics of the Chinese population, some tests, such as alternating connection (“A,” “B,” “C,” “D,” and “E” are adjusted to the “one,” “two,” “three,” “four,” and “five” of Chinese characters) and memory (“Velvet” and “Church” adjusted to “Silk” and “School”), were adjusted to common Chinese characters, while the sentence repetition part was not only adjusted to common words, but also modified the expression of sentences (see footnote 1) Therefore, the main objective of this study is to recruit a large, geographically diverse, and population-based sample based on a higher quality Mandarin-8.1 version of the MoCA scale, in order to develop normative MoCA data stratified by age and education level that is suitable for the Chinese population.
Over an extended period of MoCA implementation, scores below 26 out of 30 were deemed indicative of cognitive impairment; however, empirical investigations have established that despite its high sensitivity, this threshold exhibits limited specificity (Carson et al., 2018; Davis et al., 2021), so it may not be applicable to people with VCI (Pendlebury et al., 2012). At the same time, studies have shown that a slightly lower cut-off value seems to improve the overall testing properties of VCI compared to a score of 26 (Pendlebury et al., 2013; Lees et al., 2014). Overall, it seems meaningful to further clarify more appropriate cut-off values. Therefore, the secondary objective is to further validate the effectiveness of the Mandarin-8.1 version of the MoCA scale in the Chinese population with VCI and explore optimal cut-off value for detecting VCI in the Chinese population.
Materials and methods
Study design and population
This study comprised a total of 3,097 cognitively healthy adults from various provinces, autonomous regions, and municipalities directly under the Central Government of Mainland China. Simultaneously include 242 patients with VCI and 137 controls without cognitive impairment who are matched with general conditions.
Participants who meet the following eligibility criteria can undergo MoCA assessment. The participants of cognitively healthy individuals met the following inclusion criteria: (1) age of 20 years or older, (2) proficiency in speaking and understanding Mandarin Chinese, (3) provision of informed consent, (4) capability to effectively complete the MoCA scale, (5) ability to take care of themselves in daily life, and (6) no complaints of cognitive impairment. Exclusion criteria encompassed: (1) presence of dementia, mild cognitive impairment, or any known cognitive impairment attributed to neurological or non-neurological disorders or treatments (e.g., chemotherapy), (2) history of stroke within the past 5 years, (3) manifestation of moderate or severe mental disorders, and (4) presence of other conditions precluding successful completion of the MoCA test.
The participants of patients with or without VCI met the following inclusion criteria (1) age of 40 years or older, (2) proficiency in speaking and understanding Mandarin Chinese, (3) provision of informed consent, (4) capability to effectively complete a battery of neuropsychological tests, (5) the Hachinski scores above 7, and (6) two neuropsychologists assessed that it meets the definition of The Vascular Impairment of Cognition Classification Consensus Study (VICCCS) (Rundek et al., 2022). Exclusion criteria encompassed: (1) There are other diseases and factors that affect cognitive function, such as brain tumors, normal intracranial pressure hydrocephalus, depression, mental illness, and Alzheimer’s disease, Parkinson’s disease, brain trauma caused by drug abuse; (2) Severe visual, auditory, language, and physical impairments, and inability to complete corresponding test scales; (3) Illiteracy, family members refusing to evaluate with a complete set of neuropsychological scales, or lack of cooperation in the evaluation process; (4) Contraindications to MRI or factors that affect imaging quality, such as pacemakers, cochlear implants, metal dentures, etc.; (5) History of head and neck stent implantation, balloon dilation, carotid endarterectomy, aneurysm embolization, arteriovenous malformation embolization, or open head and neck surgery.
Diagnosis of VCI is as follows: (1) Cognitive impairment is defined as: a set of neuropsychological tests in which one or more cognitive dimensions score below the mean by 1.5 standard deviation or above (Chen et al., 2016; Huang et al., 2018); (2) MRI examination clearly shows cerebrovascular pathological changes (white matter hyperintensities (WMH) or ischemic stroke patients); (3) Cognitive impairment is causally related to cerebrovascular pathological changes; (4) The Hachinski scores above 7 (Johnson et al., 2014).
All participants provided written informed consent was obtained in accordance with the Declaration of Helsinki, and this study was approved by the Anhui Medical University Ethics Committee.
Neuropsychological assessment
Translation and cultural modification of the MoCA
Compared with the original English version of MoCA 8.1, the Mandarin-8.1 version has been localized and revised based on the language characteristics and cultural habits of the Chinese population, mainly manifested in the vocabulary used in alternating connection tests, memory tests, attention tests, sentence repetition tests, and word fluency tests (see footnote 1). The total score comprises 30 points, and the assessment can be completed in approximately 10 min. The scoring is divided into various cognitive domains, including visual space/executive function (0–5 points), naming function (0–3 points), attention function (0–6 points), language function (0–3 points), abstraction function (0–2 points), delayed recall function (0–5 points), and orientation function (0–6 points).
Besides the cognitive screening tests of MoCA (Mandarin-8.1 version), all the participants in detecting VCI cut-off values underwent a battery of standardized neuropsychological tests of memory, language, attention, executive function, and visuospatial ability. The tests included: memory (Auditory Verbal Learning Test [AVLT]-immediate recall, delayed recall, and recognition) (Maj et al., 1994), language (Verbal Fluency Test-categories of animals, fruits, and vegetables) (Mok et al., 2004), attention (Digit Span Test [DST] forward and Color Trails Test [CTT]-1) (Dai et al., 1990), executive function (Stroop Color Word Test [SCWT]-dot, word, colored word, Color Trails Test [CTT]-2 and DST backward) (Dai et al., 1990; Lee and Chan, 2000; Cong et al., 2023), and visuospatial ability (Clock Drawing Test) (Sunderland et al., 1989).
All recruiters and evaluators have received training from neurologists well-versed in Neuropsychology testing methodologies.
Statistical analysis
This study used SPSS 20.0 for statistical analysis, and multiple linear regression methods were used to analyze the relationship between age, gender, education level, MoCA total score, and various sub cognitive domain scores. To obtain reliable normative values, age was categorized into six groups (<40 years old, 40–49 years old, 50–59 years old, 60–69 years old, 70–79 years old, and ≥ 80 years old), while education level was classified into four groups (illiteracy, primary school, middle school, and high school and above). We also used receiver operating characteristic (ROC) curve analysis for sensitivity and specificity analysis. The comparison of ROC curves was performed using Hanley and McNeil’s (1983) area under the curve (AUC) method (0.5 ≤ AUC <0.7, no apparent accuracy, 0.7 ≤ f < 0.8, modal accuracy, 0.8 ≤ f < 1, good accuracy) (Hanley and McNeil, 1983; Huang et al., 2023). Based on research data, the corresponding score that produces the highest Youden’s Index was selected as the optimal cut-off value. p < 0.05 indicates a significant statistical difference.
Results
Demographic information of normative data
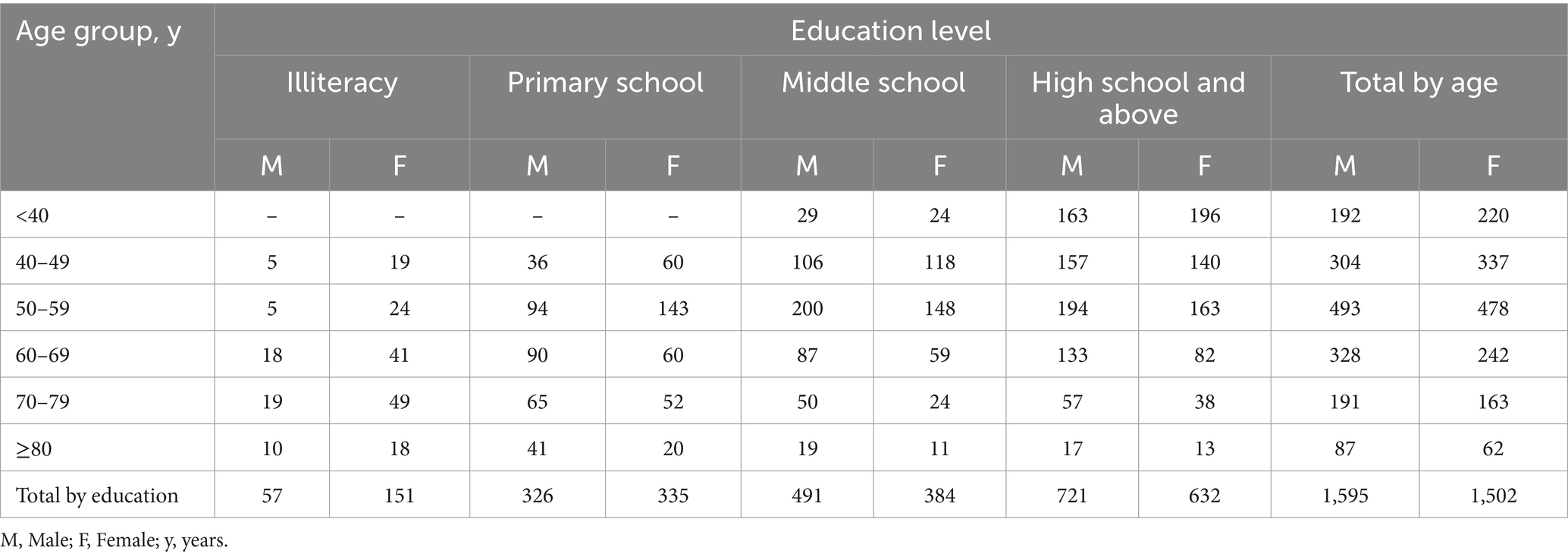
Among the 3,097 participants in this study, 1,595 were male (approximately 51.50%). The average age of the sample is 54.46 years (SD = 14.38), and the average educational is 9.49 years (SD = 4.61). The specific distribution can be seen in Table 1.
Demographic influences on the MoCA score
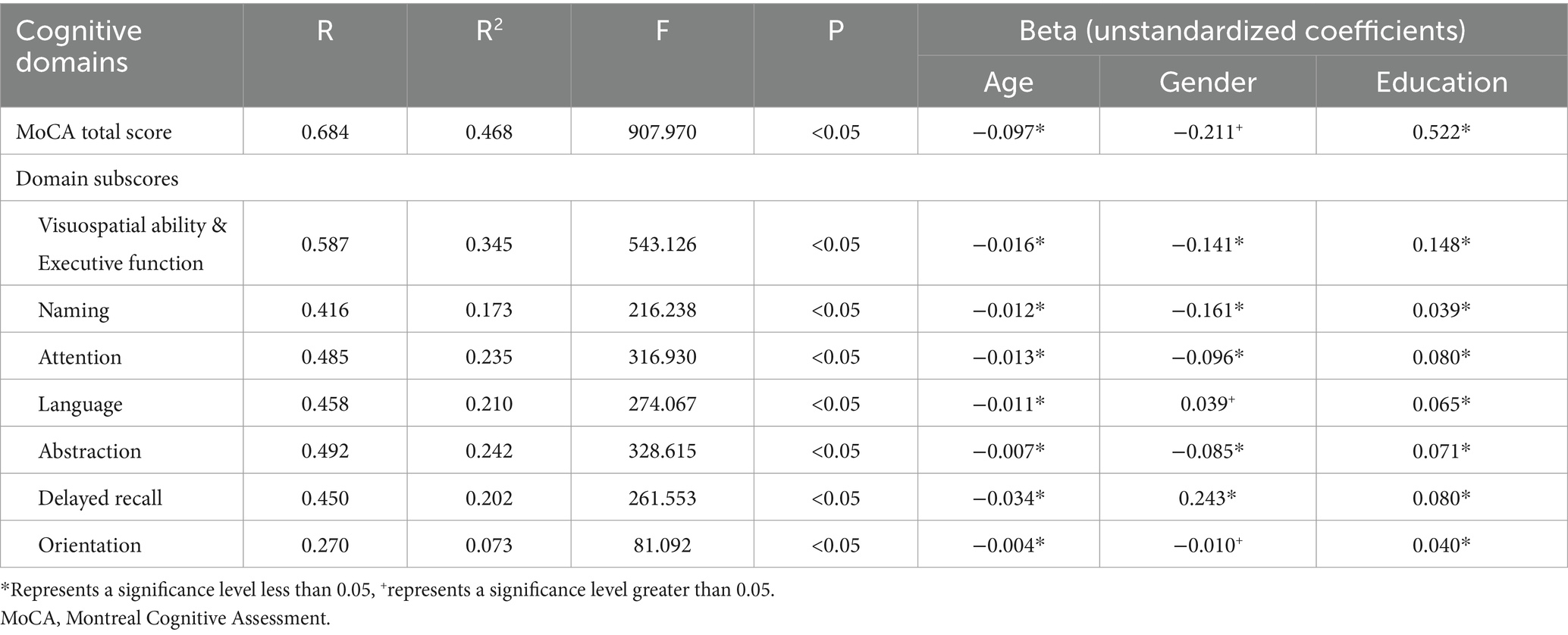
Multivariate linear regression analysis was used to assess the impact of demographic information on the MoCA total score and subdomain scores. The regression model incorporating age and education demonstrated the highest predictive capability for the MoCA total score (MoCA = 23.900–0.097*Age+0.522*Education, adjusted R2 = 0.468, F = 907.970, p<0.05), explaining 46.8% of the variance. In the regression analysis, increasing age (p < 0.05), less education (p < 0.05) were associated with a lower MoCA total score, as shown in Table 2 and Figure 1. It was also found that age, education, and gender predicted the scores of each sub cognitive domain to varying degrees. The detailed results of multiple linear regression analysis can be seen in Table 2. In addition, Supplementary Figure S1 shows the standard regression coefficients for predicting the MoCA total score and subdomain scores based on demographic factors.
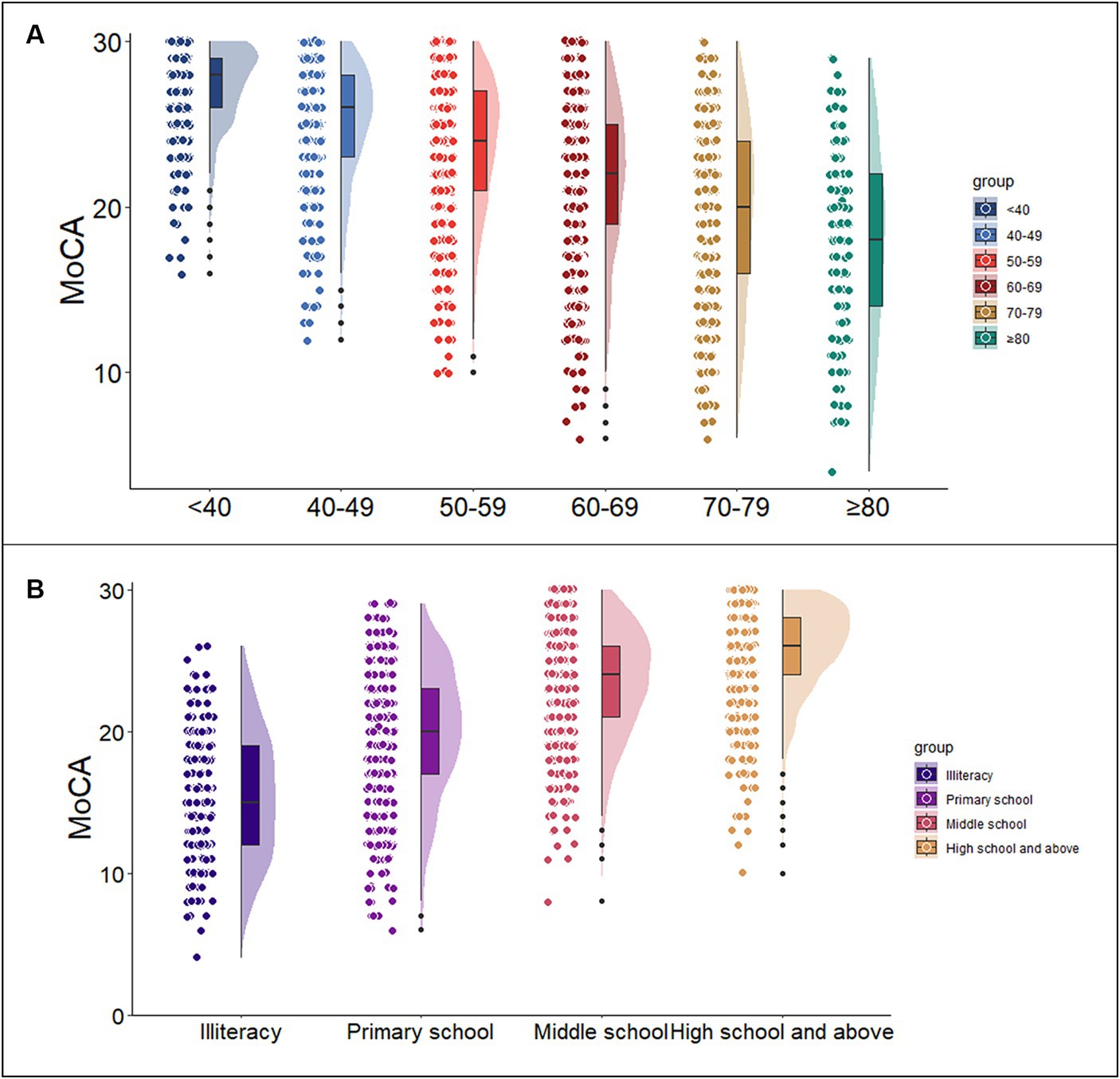
Figure 1. Association of the MoCA total score with age and education. Figures (A,B) show the trends of MoCA scores with age group and education level, respectively. (MoCA = 23.900–0.097 * Age+0.522 * Education). MoCA, Montreal Cognitive Assessment.
MoCA total score
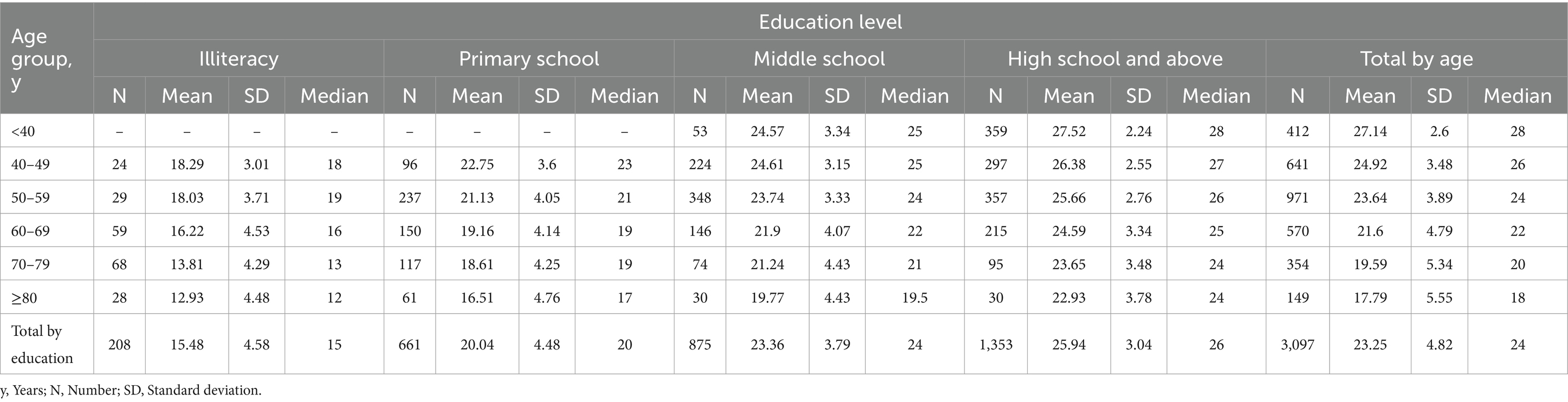
Normative data stratified by age and education were derived. The mean MoCA score of the participants was 23.25 (SD = 4.82) and was higher among the youngest participants and those with the highest education. Due to the popularization of education, no participants under the age of 40 with an education level of illiterate or elementary school were recruited. Normative data stratified by age and education can be seen in Table 3. As education level decreases, MoCA total score decrease faster with age, as shown in Figure 2.
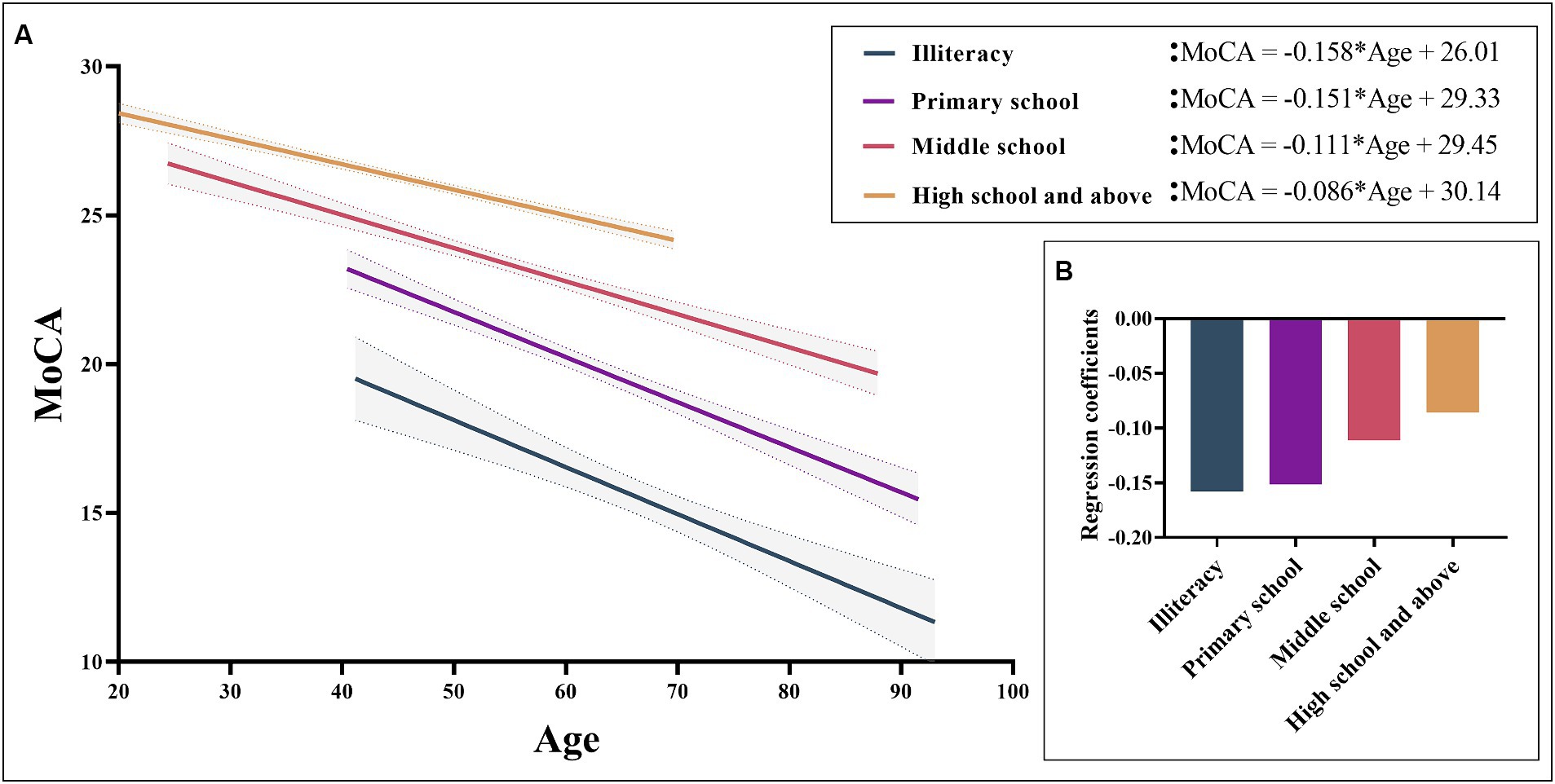
Figure 2. MoCA total scores based on age for different levels of education. Figure (A) shows a negative correlation between MoCA total scores and age can be observed in different educational levels. Figure (B) shows the regression coefficients of different groups, showing that the lower the education level, the faster the cognitive decline with age. MoCA, Montreal Cognitive Assessment.
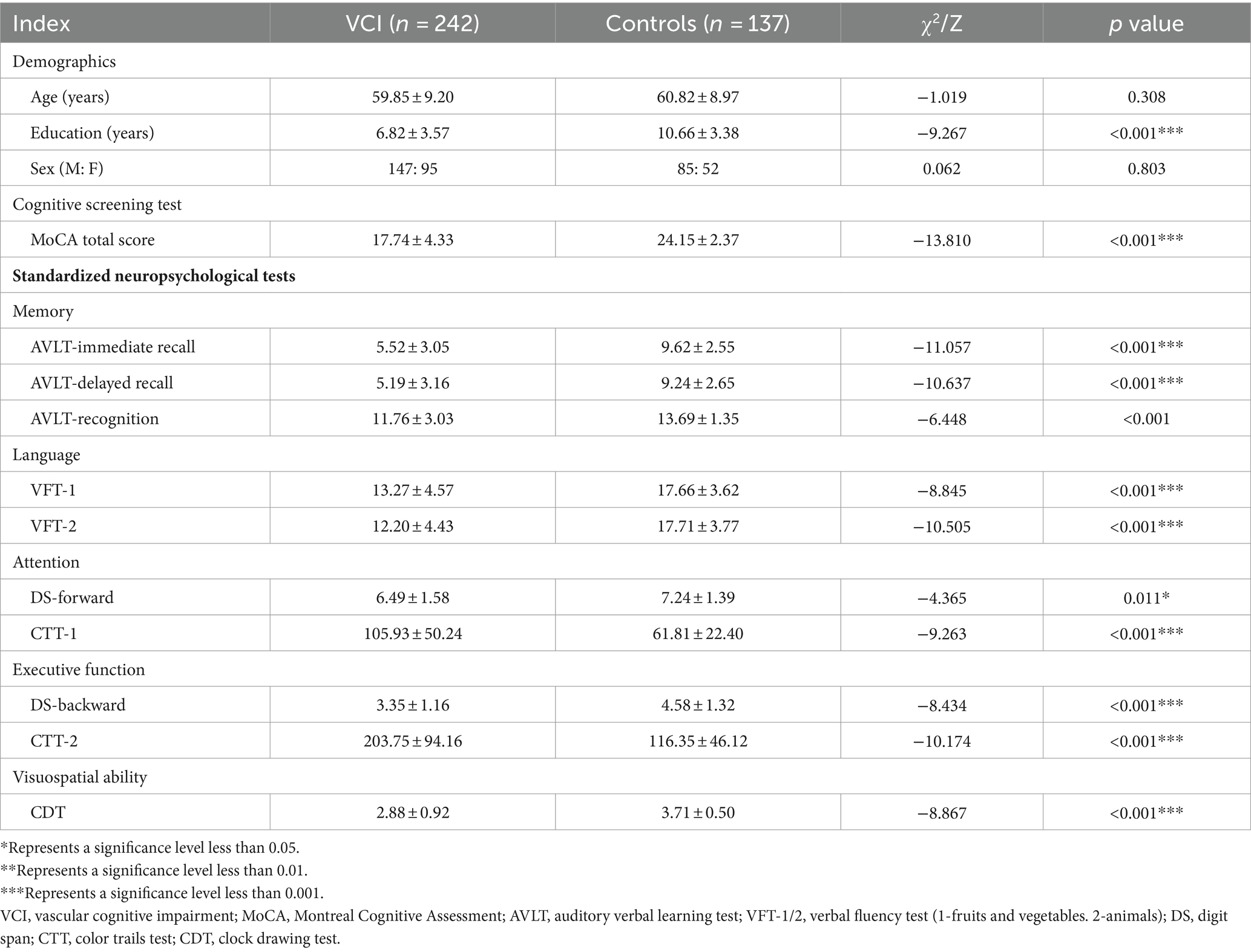
Demographics and neuropsychological tests for controls and VCI
A total of 242 VCI patients and 137 age, education, and gender matched control groups were included. Detailed demographic information, MoCA total scores, and multidimensional standardized neuropsychological tests can be found in Table 4.
Cut-off value for detecting VCI
ROC analysis was used to evaluate the screening accuracy of MoCA total score and subdomain scores in detecting VCI and controls. The MoCA total score has excellent screening ability for detecting VCI, with an AUC of 0.9257 (95% confidence interval = 0.9007–0.9507). The optimal cut-off value for detecting VCI using MoCA total score and subdomain scores was selected and summarized in Table 5. The detailed ROC curve can be seen in Figure 3. In addition, we also summarized the screening ability of MoCA total score and the combinations of subdomains for detecting VCI in Supplementary Table S1 and Supplementary Figure S2.
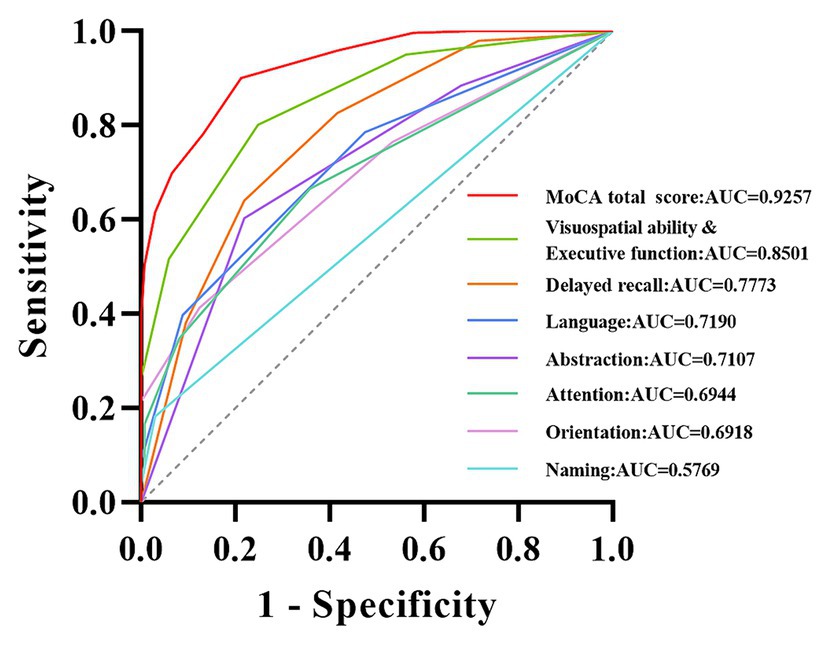
Figure 3. ROC curve of MoCA total score and subdomain scores for detecting VCI. The curves of different colors, respectively, demonstrate the ability of MoCA total score and subdomain scores for detecting VCI. MoCA, Montreal Cognitive Assessment; VCI, vascular cognitive impairment; AUC, area under the curve.
Discussion
This study presents normative MoCA (Mandarin-8.1 version) data stratified by age and education for individuals in Mainland China. The data were collected from a large, population-based sample, including healthy adults from 22 provinces, autonomous regions, and municipalities, thus providing a good representation of the Chinese population to a certain extent. The average age of the participants in this study was 54.46 years old (SD = 14.38), with an average education period of 9.49 years (SD = 4.61). The average MoCA score of the study population was 23.25 points (SD = 4.82). Table 3 comprehensively presents the MoCA norm data based on age and education level. Meanwhile, this study further indicates that MoCA total score is mainly influenced by age and education level, and the relationship with gender is not significant. In addition, this study found that a score of 22 was the optimal threshold for detecting VCI, with high sensitivity, specificity, and AUC value.
It is widely recognized that demographic factors such as age and educational attainment exert a substantial influence on cognitive function. In this study, multiple linear regression analysis demonstrated that MoCA scores are predominantly influenced by years of education and age, while no statistically significant relationship was observed with gender. These results are consistent with previous studies on MoCA, which have sparked considerable debate over the potential impact of gender on MoCA total score (Freitas et al., 2011; Lu et al., 2011; Kenny et al., 2013; Narazaki et al., 2013; Conti et al., 2015; Malek-Ahmadi et al., 2015; Kopecek et al., 2017; Rossetti et al., 2017). Age and education accounted for 46.8% of the variance in MoCA raw scores, a proportion that is notably higher than the normative data reported in recent years in some other countries (Borland et al., 2017; Thomann et al., 2018; Classon et al., 2022; Gagnon et al., 2022). This could be attributed to our substantial sample size and comprehensive representation of various age groups and education levels (Thomann et al., 2018). This study aligns with the findings of previous research, as it indicates that younger age and higher education levels are associated with higher MoCA scores (Rossetti et al., 2011; Kenny et al., 2013; Narazaki et al., 2013; Santangelo et al., 2015; Tan et al., 2015; Konstantopoulos et al., 2016; Larouche et al., 2016; Mellor et al., 2016; Borland et al., 2017; Kopecek et al., 2017). Hence, when assessing cognitive impairment using MoCA, it is imperative to take into comprehensive account the subjects’ age and educational background. Across the four distinct educational strata examined in this study, a discernible pattern emerged wherein MoCA scores exhibited a reduction in tandem with advancing age. Moreover, our investigation revealed a noteworthy observation: individuals possessing higher educational qualifications demonstrated comparatively attenuated cognitive decline in relation to age. This overarching trend is graphically depicted in Figure 2. This aligns coherently with prior research outcomes that have similarly underscored the positive correlation between higher education levels and superior cognitive function. Essentially, education serves as a protective factor for cognitive function (Zhu et al., 2017; Kim, 2020; Liu et al., 2021; Rahe and Quaiser-Pohl, 2023).
In earlier work Nasreddine et al. (2005), previously set a score of 26 as the cut-off value for cognitive impairment, a benchmark that has garnered widespread recognition among researchers for an extended period of time. However, it is worth noting that there is still considerable doubt as to whether 26 points are suitable as a cut-off value for cognitive impairment in different countries or ethnic groups. This did not surpass our anticipated psychological expectations, as this has been mentioned in previous studies focused on normative MoCA data across diverse countries. And these investigations have revealed that relatively few participants attain a score of 26, and a substantial proportion of participants exhibit younger ages or possess higher education levels (Freitas et al., 2011; Borland et al., 2017; Kopecek et al., 2017; Rossetti et al., 2017; Davis et al., 2021). Our study further supports this viewpoint from the perspective of the Chinese population and revealed that the previously established cut-off value of 26 points may not be suitable for the Chinese population. For among the 3,097 participants in this study, the overall mean was 23.25, merely 1,204 (38.88%) achieved a score equal to or higher than the conventional cut-off points of 26, as proposed by Nasreddine et al. (2005). Meanwhile, a recent study demonstrated that although the critical value of 26 points exhibits high sensitivity in detecting dementia, its specificity is comparatively suboptimal (Davis et al., 2021). Therefore, it is even more important for the Chinese population to develop more realistic MoCA standardized values. It is important to highlight that the study conducted by Nasreddine et al. (2005) differs significantly from our research in terms of research design and objectives. Their study primarily aimed to ascertain the most effective score for distinguishing between healthy volunteers, individuals with mild cognitive impairment, and dementia patients, with a relatively higher educational level among the subjects (Engedal et al., 2022). In contrast, our research focused on generating normative MoCA data applicable to diverse age groups and education levels among the Chinese population. As illustrated in Table 1, this study encompasses potential reasons for the substantial variance observed in the 26-point cut-off when compared to our research findings. The MoCA scale is composed of multiple subdomain tests, representing various dimensions of cognition. In this study, we explored the efficacy of individual subdomain scores and combined subdomain scores in detecting VCI. As shown in Table 5 and Figure 3, the screening ability of single subdomain scores for detecting VCI is relatively insufficient. Notably, Supplementary materials indicate that certain combinations of subdomains exhibit promising AUC, sensitivity, and specificity, particularly the combination of visuospatial ability & executive function with delayed recall. Additionally, the screening efficiency improves with the inclusion of more subdomains. These findings align well with the characteristics of vascular cognitive impairment (Badji et al., 2023). However, it is clear that the MoCA total score remains the optimal choice for screening.
There are currently many types of threshold values for the detecting VCI, which has caused great trouble (Koski, 2013; Salvadori et al., 2013; Tu et al., 2013; Chiti and Pantoni, 2014). It should be noted that due to the complex etiology of VCI, it may even belong to different stages. Although high threshold values can achieve high sensitivity, their specificity is relatively low (Nasreddine et al., 2005), resulting in poor clinical practicality. This study determined the detecting VCI by integrating the results of multidimensional neuropsychological tests. Subtracting 1.5SD below the mean is defined as a more stable result and a false positive diagnosis of farmland. Our research results show that the cut-off value (≤ 22 points) of the Mandarin 8.1 version of the MoCA scale for detecting VCI has a high AUC. This is consistent with the results of previous related studies, which have also found that a cutoff value of 22/23 is more reasonable, and their sensitivity and specificity are similar to those of this study (Lees et al., 2014; Potocnik et al., 2020).
It must be mentioned that this study also has some limitations. Firstly, despite the wide geographical distribution of participants, there exist substantial variations in the number of enrolled individuals across different regions. Secondly, even if there are no obvious complaints of cognitive impairment, we did not screen for too many potential factors that may affect cognitive function (excluding stroke) and did not use strict cognitive assessments to evaluate participants’ cognitive function. Therefore, it remains possible that latent cognitive impairments among the subjects cannot be entirely ruled out. Thirdly, we did not employ MMSE to further evaluate the consistency of the revised MoCA, a choice that also partially mitigated the potential bias stemming from the learning effects associated with administering analogous tests.
Conclusion
This study provides MoCA with normative values based on age and education years for the Chinese population. Our research findings further confirm the contribution of age and years of education to MoCA total score, and further indicate that the cut-off value of 26 points is not suitable for the Chinese population. Moreover, for patients with vascular cognitive impairment, a score of ≤22 may be a better cut-off value.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: The anonymous raw data of the normative data in this study has been publicly available on Science Data Bank (https://doi.org/10.57760/sciencedb.10751) and can be obtained under the Creative Commons Attribution-Non-Commercial 4.0 International License (CC BY-NC 4.0).
Ethics statement
The studies involving humans were approved by Anhui Medical University Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
QW: Data curation, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. BD: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. YL: Formal analysis, Investigation, Methodology, Writing – original draft. SC: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. SY: Formal analysis, Investigation, Methodology, Writing – original draft. YZ: Formal analysis, Investigation, Methodology, Writing – original draft. RY: Data curation, Methodology, Formal analysis, Writing – review & editing. TB: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. XW: Conceptualization, Data curation, Methodology, Writing – original draft, Investigation. YT: Conceptualization, Data curation, Methodology, Writing – original draft. PH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Writing – review & editing. KW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Clinical Medical Research and Transformation Project of Anhui (Grant Nos.202204295107020028 and 202204295107020006), the Anhui Provincial Key Research and Development Project (Population Health Special Project, 202104j07020033), the National Natural Science Foundation of China (Grant Nos. 82171917) and the Science and Technology Innovation 2030-"Brain Science and Brain-like Research" Major Project (Grant Nos.2021ZD0201801).
Acknowledgments
The authors appreciate the contributions from all the investigators, participants for their cooperation during this study. The normative data of this study can be found on the preprint website (https://www.medrxiv.org/content/10.1101/2023.12.18.23300135v1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2024.1455129/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | The contribution of demographic factors to the total score and sub domain scores of MoCA. The standard regression coefficients of age, education, and gender on the total score and subdomains of MoCA. *Represents a significant level less than 0.05, +represents a significant level greater than 0.05, MoCA, Montreal Cognitive Assessment.
SUPPLEMENTARY FIGURE S2 | ROC curve of MoCA total score and combination score of subdomains for detecting VCI. Combine subdomains based on AUC and gradually eliminate the subdomain with the lowest AUC. The curves of different colors respectively demonstrate the ability of total MoCA score and the combination of subdomains for detecting VCI. MoCA, Montreal Cognitive Assessment, VCI, vascular cognitive impairment, AUC, area under the curve, Combination 1: Visuospatial ability& Executive function & Delayed recall & Language & Abstraction & Attention& Orientation, Combination 2: Visuospatial ability& Executive function& Delayed recall & Language & Abstraction & Attention, Combination 3: Visuospatial ability& Executive function& Delayed recall & Language & Abstraction, Combination 4: Visuospatial ability& Executive function& Delayed recall & Language, Combination 5: Visuospatial ability& Executive function& Delayed recall.
Footnotes
References
Apolinario, D., Dos Santos, M. F., Sassaki, E., Pegoraro, F., Pedrini, A. V. A., Cestari, B., et al. (2018). Normative data for the Montreal cognitive assessment (MoCA) and the memory index score (MoCA-MIS) in Brazil: adjusting the nonlinear effects of education with fractional polynomials. Int. J. Geriatr. Psychiatry 33, 893–899. doi: 10.1002/gps.4866
Badji, A., Youwakim, J., Cooper, A., Westman, E., and Marseglia, A. (2023). Vascular cognitive impairment - past, present, and future challenges. Ageing Res. Rev. 90:102042. doi: 10.1016/j.arr.2023.102042
Borland, E., Nägga, K., Nilsson, P. M., Minthon, L., Nilsson, E. D., and Palmqvist, S. (2017). The Montreal cognitive assessment: normative data from a large Swedish population-based cohort. J. Alzheimers Dis. 59, 893–901. doi: 10.3233/jad-170203
Carson, N., Leach, L., and Murphy, K. J. (2018). A re-examination of Montreal cognitive assessment (MoCA) cutoff scores. Int. J. Geriatr. Psychiatry 33, 379–388. doi: 10.1002/gps.4756
Cesar, K. G., Yassuda, M. S., Porto, F. H. G., Brucki, S. M. D., and Nitrini, R. (2019). MoCA test: normative and diagnostic accuracy data for seniors with heterogeneous educational levels in Brazil. Arq. Neuropsiquiatr. 77, 775–781. doi: 10.1590/0004-282x20190130
Chen, K. L., Xu, Y., Chu, A. Q., Ding, D., Liang, X. N., Nasreddine, Z. S., et al. (2016). Validation of the Chinese version of Montreal cognitive assessment basic for screening mild cognitive impairment. J. Am. Geriatr. Soc. 64, e285–e290. doi: 10.1111/jgs.14530
Chiti, G., and Pantoni, L. (2014). Use of Montreal cognitive assessment in patients with stroke. Stroke 45, 3135–3140. doi: 10.1161/strokeaha.114.004590
Ciesielska, N., Sokołowski, R., Mazur, E., Podhorecka, M., Polak-Szabela, A., and Kędziora-Kornatowska, K. (2016). Is the Montreal cognitive assessment (MoCA) test better suited than the Mini-mental state examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatria Polska 50, 1039–1052. doi: 10.12740/pp/45368
Classon, E., van den Hurk, W., Lyth, J., and Johansson, M. M. (2022). Montreal cognitive assessment: normative data for cognitively healthy Swedish 80- to 94-year-olds. J. Alzheimers Dis. 87, 1335–1344. doi: 10.3233/jad-215629
Cong, L., Ren, Y., Wang, Y., Hou, T., Dong, Y., Han, X., et al. (2023). Mild cognitive impairment among rural-dwelling older adults in China: a community-based study. Alzheimers Dement. 19, 56–66. doi: 10.1002/alz.12629
Conti, S., Bonazzi, S., Laiacona, M., Masina, M., and Coralli, M. V. (2015). Montreal cognitive assessment (MoCA)-Italian version: regression based norms and equivalent scores. Neurol. Sci. 36, 209–214. doi: 10.1007/s10072-014-1921-3
Dai, X. Y., Ryan, J. J., Paolo, A. M., and Harrington, R. G. (1990). Factor analysis of the mainland Chinese version of the Wechsler adult intelligence scale (WAIS-RC) in a brain-damaged sample. Int. J. Neurosci. 55, 107–111. doi: 10.3109/00207459008985956
Damian, A. M., Jacobson, S. A., Hentz, J. G., Belden, C. M., Shill, H. A., Sabbagh, M. N., et al. (2011). The Montreal cognitive assessment and the mini-mental state examination as screening instruments for cognitive impairment: item analyses and threshold scores. Dement. Geriatr. Cogn. Disord. 31, 126–131. doi: 10.1159/000323867
Davis, D. H., Creavin, S. T., Yip, J. L., Noel-Storr, A. H., Brayne, C., and Cullum, S. (2021). Montreal cognitive assessment for the detection of dementia. Cochrane Database Syst. Rev. 2021:CD010775. doi: 10.1002/14651858.CD010775.pub3
De Roeck, E. E., Engelborghs, S., and Dierckx, E. (2016). Next generation brain health depends on early Alzheimer disease diagnosis: from a timely diagnosis to future population screening. J. Am. Med. Dir. Assoc. 17, 452–453. doi: 10.1016/j.jamda.2016.02.015
Dong, Y., Sharma, V. K., Chan, B. P., Venketasubramanian, N., Teoh, H. L., Seet, R. C., et al. (2010). The Montreal cognitive assessment (MoCA) is superior to the Mini-mental state examination (MMSE) for the detection of vascular cognitive impairment after acute stroke. J. Neurol. Sci. 299, 15–18. doi: 10.1016/j.jns.2010.08.051
Engedal, K., Gjora, L., Benth, J. S., Wagle, J., Ronqvist, T. K., and Selbaek, G. (2022). The Montreal cognitive assessment: normative data from a large, population-based sample of cognitive healthy older adults in Norway-the HUNT study. J. Alzheimers Dis. 86, 589–599. doi: 10.3233/JAD-215442
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Freitas, S., Simões, M. R., Alves, L., and Santana, I. (2011). Montreal cognitive assessment (MoCA): normative study for the Portuguese population. J. Clin. Exp. Neuropsychol. 33, 989–996. doi: 10.1080/13803395.2011.589374
Fu, C., Jin, X., Chen, B., Xue, F., Niu, H., Guo, R., et al. (2017). Comparison of the Mini-mental state examination and Montreal cognitive assessment executive subtests in detecting post-stroke cognitive impairment. Geriatr Gerontol Int 17, 2329–2335. doi: 10.1111/ggi.13069
Gaete, M., Jorquera, S., Bello-Lepe, S., Mendoza, Y. M., Véliz, M., Alonso-Sanchez, M. F., et al. (2023). Standardised results of the Montreal cognitive assessment (MoCA) for neurocognitive screening in a Chilean population. Neurologia 38, 246–255. doi: 10.1016/j.nrleng.2020.08.021
Gagnon, C., Olmand, M., Dupuy, E. G., Besnier, F., Vincent, T., Grégoire, C. A., et al. (2022). Videoconference version of the Montreal cognitive assessment: normative data for Quebec-French people aged 50 years and older. Aging Clin. Exp. Res. 34, 1627–1633. doi: 10.1007/s40520-022-02092-1
Hanley, J. A., and McNeil, B. J. (1983). A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 148, 839–843. doi: 10.1148/radiology.148
Hayek, M., Tarabey, L., Abou-Mrad, T., Fadel, P., and Abou-Mrad, F. (2020). Normative data for the Montreal cognitive assessment in a Lebanese older adult population. J. Clin. Neurosci. 74, 81–86. doi: 10.1016/j.jocn.2020.01.050
Huang, L., Chen, K. L., Lin, B. Y., Tang, L., Zhao, Q. H., Lv, Y. R., et al. (2018). Chinese version of Montreal cognitive assessment basic for discrimination among different severities of Alzheimer's disease. Neuropsychiatr. Dis. Treat. 14, 2133–2140. doi: 10.2147/NDT.S174293
Huang, L., Mei, Z., Ye, J., and Guo, Q. (2023). AMES: an automated self-administered scale to detect incipient cognitive decline in primary care settings. Assessment 30, 2247–2257. doi: 10.1177/10731911221144774
Johnson, L. A., Cushing, B., Rohlfing, G., Edwards, M., Davenloo, H., D'Agostino, D., et al. (2014). The Hachinski ischemic scale and cognition: the influence of ethnicity. Age Ageing 43, 364–369. doi: 10.1093/ageing/aft189
Kenny, R. A., Coen, R. F., Frewen, J., Donoghue, O. A., Cronin, H., and Savva, G. M. (2013). Normative values of cognitive and physical function in older adults: findings from the Irish longitudinal study on ageing. J. Am. Geriatr. Soc. 61, S279–S290. doi: 10.1111/jgs.12195
Kim, Y. (2020). Risk and protective factors for the onset of cognitive impairment in Korea: a 10-year longitudinal panel study. Psychiatry Investig. 17, 769–776. doi: 10.30773/pi.2020.0070
Konstantopoulos, K., Vogazianos, P., and Doskas, T. (2016). Normative data of the Montreal cognitive assessment in the Greek population and parkinsonian dementia. Arch. Clin. Neuropsychol. 31, 246–253. doi: 10.1093/arclin/acw002
Kopecek, M., Stepankova, H., Lukavsky, J., Ripova, D., Nikolai, T., and Bezdicek, O. (2017). Montreal cognitive assessment (MoCA): normative data for old and very old Czech adults. Appl. Neuropsychol. Adult 24, 23–29. doi: 10.1080/23279095.2015.1065261
Koski, L. (2013). Validity and applications of the Montreal cognitive assessment for the assessment of vascular cognitive impairment. Cerebrovasc. Dis. 36, 6–18. doi: 10.1159/000352051
Larouche, E., Tremblay, M. P., Potvin, O., Laforest, S., Bergeron, D., Laforce, R., et al. (2016). Normative data for the Montreal cognitive assessment in middle-aged and elderly Quebec-French people. Arch. Clin. Neuropsychol. 31, 819–826. doi: 10.1093/arclin/acw076
Lee, T. M., and Chan, C. C. (2000). Comparison of the trail making and color trails tests in a Chinese context: a preliminary report. Percept. Mot. Skills 90, 187–190. doi: 10.2466/pms.2000.90.1.187
Lees, R., Selvarajah, J., Fenton, C., Pendlebury, S. T., Langhorne, P., Stott, D. J., et al. (2014). Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke 45, 3008–3018. doi: 10.1161/STROKEAHA.114.005842
Liu, L. Y., Lu, Y., Shen, L., Li, C. B., Yu, J. T., Yuan, C. R., et al. (2021). Prevalence, risk and protective factors for mild cognitive impairment in a population-based study of Singaporean elderly. J. Psychiatr. Res. 145, 111–117. doi: 10.1016/j.jpsychires.2021.11.041
Lu, J., Li, D., Li, F., Zhou, A., Wang, F., Zuo, X., et al. (2011). Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J. Geriatr. Psychiatry Neurol. 24, 184–190. doi: 10.1177/0891988711422528
Maj, M., Satz, P., Janssen, R., Zaudig, M., Starace, F., D'Elia, L., et al. (1994). WHO neuropsychiatric AIDS study, cross-sectional phase II. Neuropsychological and neurological findings. Arch. Gen. Psychiatry 51, 51–61. doi: 10.1001/archpsyc.1994.03950010051007
Malek-Ahmadi, M., Powell, J. J., Belden, C. M., O'Connor, K., Evans, L., Coon, D. W., et al. (2015). Age- and education-adjusted normative data for the Montreal cognitive assessment (MoCA) in older adults age 70-99. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 22, 755–761. doi: 10.1080/13825585.2015.1041449
Mellor, D., Lewis, M., McCabe, M., Byrne, L., Wang, T., Wang, J., et al. (2016). Determining appropriate screening tools and cut-points for cognitive impairment in an elderly Chinese sample. Psychol. Assess. 28, 1345–1353. doi: 10.1037/pas0000271
Mok, E. H., Lam, L. C., and Chiu, H. F. (2004). Category verbal fluency test performance in chinese elderly with Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 18, 120–124. doi: 10.1159/000079190
Muayqil, T. A., Alamri, N. K., Alqahtani, A. M., Julaidan, S. S., Alsuhaibani, R., Nafisah, I., et al. (2021). Normative and equated data of the original and basic versions of the Montreal cognitive assessment among community dwelling Saudi Arabians. Behav. Neurol. 2021:5395627. doi: 10.1155/2021/5395627
Narazaki, K., Nofuji, Y., Honda, T., Matsuo, E., Yonemoto, K., and Kumagai, S. (2013). Normative data for the Montreal cognitive assessment in a Japanese community-dwelling older population. Neuroepidemiology 40, 23–29. doi: 10.1159/000339753
Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Oudman, E., Postma, A., Van der Stigchel, S., Appelhof, B., Wijnia, J. W., and Nijboer, T. C. (2014). The Montreal cognitive assessment (MoCA) is superior to the Mini mental state examination (MMSE) in detection of Korsakoff's syndrome. Clin. Neuropsychol. 28, 1123–1132. doi: 10.1080/13854046.2014.960005
Pendlebury, S. T., Mariz, J., Bull, L., Mehta, Z., and Rothwell, P. M. (2012). MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian stroke network vascular cognitive impairment harmonization standards neuropsychological battery after TIA and stroke. Stroke 43, 464–469. doi: 10.1161/STROKEAHA.111.633586
Pendlebury, S. T., Mariz, J., Bull, L., Mehta, Z., and Rothwell, P. M. (2013). Impact of different operational definitions on mild cognitive impairment rate and MMSE and MoCA performance in transient ischaemic attack and stroke. Cerebrovasc. Dis. 36, 355–362. doi: 10.1159/000355496
Potocnik, J., Ovcar Stante, K., and Rakusa, M. (2020). The validity of the Montreal cognitive assessment (MoCA) for the screening of vascular cognitive impairment after ischemic stroke. Acta Neurol. Belg. 120, 681–685. doi: 10.1007/s13760-020-01330-5
Rahe, M., and Quaiser-Pohl, C. (2023). Protective effects of education on the cognitive decline in a mental rotation task using real models: a pilot study with middle and older aged adults. Psychol. Res. 87, 1284–1292. doi: 10.1007/s00426-022-01719-2
Roalf, D. R., Moberg, P. J., Xie, S. X., Wolk, D. A., Moelter, S. T., and Arnold, S. E. (2013). Comparative accuracies of two common screening instruments for classification of Alzheimer's disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 9, 529–537. doi: 10.1016/j.jalz.2012.10.001
Rossetti, H. C., Lacritz, L. H., Cullum, C. M., and Weiner, M. F. (2011). Normative data for the Montreal cognitive assessment (MoCA) in a population-based sample. Neurology 77, 1272–1275. doi: 10.1212/WNL.0b013e318230208a
Rossetti, H. C., Lacritz, L. H., Hynan, L. S., Cullum, C. M., Van Wright, A., and Weiner, M. F. (2017). Montreal cognitive assessment performance among community-dwelling African Americans. Arch. Clin. Neuropsychol. 32, 238–244. doi: 10.1093/arclin/acw095
Rundek, T., Tolea, M., Ariko, T., Fagerli, E. A., and Camargo, C. J. (2022). Vascular Cognitive Impairment (VCI). Neurotherapeutics 19, 68–88. doi: 10.1007/s13311-021-01170-y
Salvadori, E., Pasi, M., Poggesi, A., Chiti, G., Inzitari, D., and Pantoni, L. (2013). Predictive value of MoCA in the acute phase of stroke on the diagnosis of mid-term cognitive impairment. J. Neurol. 260, 2220–2227. doi: 10.1007/s00415-013-6962-7
Santangelo, G., Siciliano, M., Pedone, R., Vitale, C., Falco, F., Bisogno, R., et al. (2015). Normative data for the Montreal cognitive assessment in an Italian population sample. Neurol. Sci. 36, 585–591. doi: 10.1007/s10072-014-1995-y
Serrano, C. M., Sorbara, M., Minond, A., Finlay, J. B., Arizaga, R. L., Iturry, M., et al. (2020). Validation of the argentine version of the Montreal cognitive assessment test (MOCA): a screening tool for mild cognitive impairment and mild dementia in elderly. Dement. Neuropsychol. 14, 145–152. doi: 10.1590/1980-57642020dn14-020007
Smith, T., Gildeh, N., and Holmes, C. (2007). The Montreal cognitive assessment: validity and utility in a memory clinic setting. Can. J. Psychiatry 52, 329–332. doi: 10.1177/070674370705200508
Sunderland, T., Hill, J. L., Mellow, A. M., Lawlor, B. A., Gundersheimer, J., Newhouse, P. A., et al. (1989). Clock drawing in Alzheimer's disease. A novel measure of dementia severity. J. Am. Geriatr. Soc. 37, 725–729. doi: 10.1111/j.1532-5415.1989.tb02233.x
Tan, J. P., Li, N., Gao, J., Wang, L. N., Zhao, Y. M., Yu, B. C., et al. (2015). Optimal cutoff scores for dementia and mild cognitive impairment of the Montreal cognitive assessment among elderly and oldest-old Chinese population. J. Alzheimers Dis. 43, 1403–1412. doi: 10.3233/jad-141278
Thomann, A. E., Goettel, N., Monsch, R. J., Berres, M., Jahn, T., Steiner, L. A., et al. (2018). The Montreal cognitive assessment: normative data from a German-speaking cohort and comparison with international normative samples. J. Alzheimers Dis. 64, 643–655. doi: 10.3233/jad-180080
Tu, Q. Y., Jin, H., Ding, B. R., Yang, X., Lei, Z. H., Bai, S., et al. (2013). Reliability, validity, and optimal cutoff score of the Montreal cognitive assessment (Changsha version) in ischemic cerebrovascular disease patients of Hunan province, China. Dement. Geriatr. Cogn. Disord. Extra 3, 25–36. doi: 10.1159/000346845
Wolf, P. A. (2012). Contributions of the Framingham heart study to stroke and dementia epidemiologic research at 60 years. Arch. Neurol. 69, 567–571. doi: 10.1001/archneurol.2011.977
Zhang, X., Su, J., Gao, C., Ni, W., Gao, X., Li, Y., et al. (2019). Progression in vascular cognitive impairment: pathogenesis, neuroimaging evaluation, and treatment. Cell Transplant. 28, 18–25. doi: 10.1177/0963689718815820
Zhu, X., Qiu, C., Zeng, Y., and Li, J. (2017). Leisure activities, education, and cognitive impairment in Chinese older adults: a population-based longitudinal study. Int. Psychogeriatr. 29, 727–739. doi: 10.1017/S1041610216001769
Keywords: Montreal cognitive assessment, healthy adults, normative data, vascular cognitive impairment, cut-off value
Citation: Wei Q, Du B, Liu Y, Cao S, Yin S, Zhang Y, Ye R, Bai T, Wu X, Tian Y, Hu P and Wang K (2024) The Montreal cognitive assessment: normative data from a large, population-based sample of Chinese healthy adults and validation for detecting vascular cognitive impairment. Front. Neurosci. 18:1455129. doi: 10.3389/fnins.2024.1455129
Edited by:
Peng Lei, Sichuan University, ChinaReviewed by:
Sijia Zhao, University of Oxford, United KingdomJiu Chen, Nanjing University, China
Liang Gong, Chengdu Second People’s Hospital, China
Copyright © 2024 Wei, Du, Liu, Cao, Yin, Zhang, Ye, Bai, Wu, Tian, Hu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Wang, d2FuZ2thaTE5NjRAMTI2LmNvbQ==; Panpan Hu, aHBwcGFuZGFAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Qiang Wei
Qiang Wei Baogen Du
Baogen Du Yuanyuan Liu4†
Yuanyuan Liu4† Shanshan Cao
Shanshan Cao Tongjian Bai
Tongjian Bai Xingqi Wu
Xingqi Wu Yanghua Tian
Yanghua Tian Panpan Hu
Panpan Hu Kai Wang
Kai Wang