
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurosci., 31 July 2024
Sec. Sleep and Circadian Rhythms
Volume 18 - 2024 | https://doi.org/10.3389/fnins.2024.1451219
This article is part of the Research TopicLong-term effects of adolescent stress, sleep deprivation, or circadian disruption on mood and anxietyView all 7 articles
Light-at-night is known to produce a wide variety of behavioral outcomes including promoting anxiety, depression, hyperactivity, abnormal sociability, and learning and memory deficits. Unfortunately, we all live in a 24-h society where people are exposed to light-at-night or light pollution through night-shift work - the need for all-hours emergency services – as well as building and street-lights, making light-at-night exposure practically unavoidable. Additionally, the increase in screentime (tvs and smart devices) during the night also contributes to poorer sleep and behavioral impairments. Compounding these factors is the fact that adolescents tend to be “night owls” and prefer an evening chronotype compared to younger children and adults, so these teenagers will have a higher likelihood of being exposed to light-at-night. Making matters worse is the prevalence of high-school start times of 8 am or earlier – a combination of too early school start times, light exposure during the night, and preference for evening chronotypes is a recipe for reduced and poorer sleep, which can contribute to increased susceptibility for behavioral issues for this population. As such, this mini-review will show, using both human and rodent model studies, how light-at-night affects behavioral outcomes and stress responses, connecting photic signaling and the circadian timing system to the hypothalamic–pituitary adrenal axis. Additionally, this review will also demonstrate that adolescents are more likely to exhibit abnormal behavior in response to light-at-night due to changes in development and hormone regulation during this time period, as well as discuss potential interventions that can help mitigate these negative effects.
One unfortunate here-to-stay part of modern life is exposure to light-at-night either through shift- work or exposure to artificial building lights and streetlights – light pollution – which can cause disruptions to the endogenous biological clock. The increased use of light-emitting devices during the night, something prevalent among adolescents, also contributes to light-at-night exposure. Current research in humans of both biological sexes has consistently shown that exposure to light-at-night leads to abnormal behavior, including anxiety (Paksarian et al., 2020), depression (Helbich et al., 2020), social issues (Deibel et al., 2020), and exacerbates the symptoms of existing psychiatric disorders (Esaki et al., 2022). Studies using nocturnal rodents have corroborated the anti-social, anxiogenic, and depression-inducing effects of light-at-night, similar to what was found in diurnal humans (Zhou et al., 2018; Michaud et al., 2022; Medeiros Contini and Seggio, 2023). Furthermore, the altered timing of light exposure disrupts melatonin secretion (a hormone that regulates sleep and its levels depressed by light-at-night) leading to increased cortisol during the night (Rahman et al., 2019). These changes in mood due to light-at-night can be attributed to poorer sleep and increased stress responses. Therefore, minimizing light-at-night exposure is essential for preserving mental health and reducing the risk of the development of psychiatric disorders, particularly among adolescents, which exhibit increased evening preference and sensitivity to light-at-night.
Light exposure allows for the entrainment of circadian rhythm to the 24-h day. Morning light shifts the circadian timekeeping system earlier (phase-advance) and evening light shifts it later (phase-delay). The time at which the circadian system promotes sleep or wake can be influenced by internal factors (endogenous circadian period and circadian photosensitivity) as well as external factors (light exposure). The circadian timing system relies on the photopigment melanopsin (Opn4), rather than the visual photoreceptor rhodopsin, to relay light signals to the suprachiasmatic nucleus (SCN) through intrinsically photosensitive retinal ganglion cells (ipRGCs) in the retinohypothalamic tract. Within the SCN core, vasoactive intestinal peptide (VIP)-containing neurons receive direct retinal inputs, whereas the vasopressin (AVP)-containing neurons within the SCN shell receive GABAergic and VIP input from the core. Coupling the oscillatory network between core and shell generates self-sustaining pacemaker rhythms, with SCN outputs of VIP and GABA being sent to other hypothalamic nuclei, while GABA and AVP are sent as outputs to other parts of the brain (Figure 1A; Barko et al., 2019). Lastly, photic signals to the SCN core can directly affect circadian clock genes expression (e.g., period), leading to the determination of the phase of the mammal (Figure 1B).
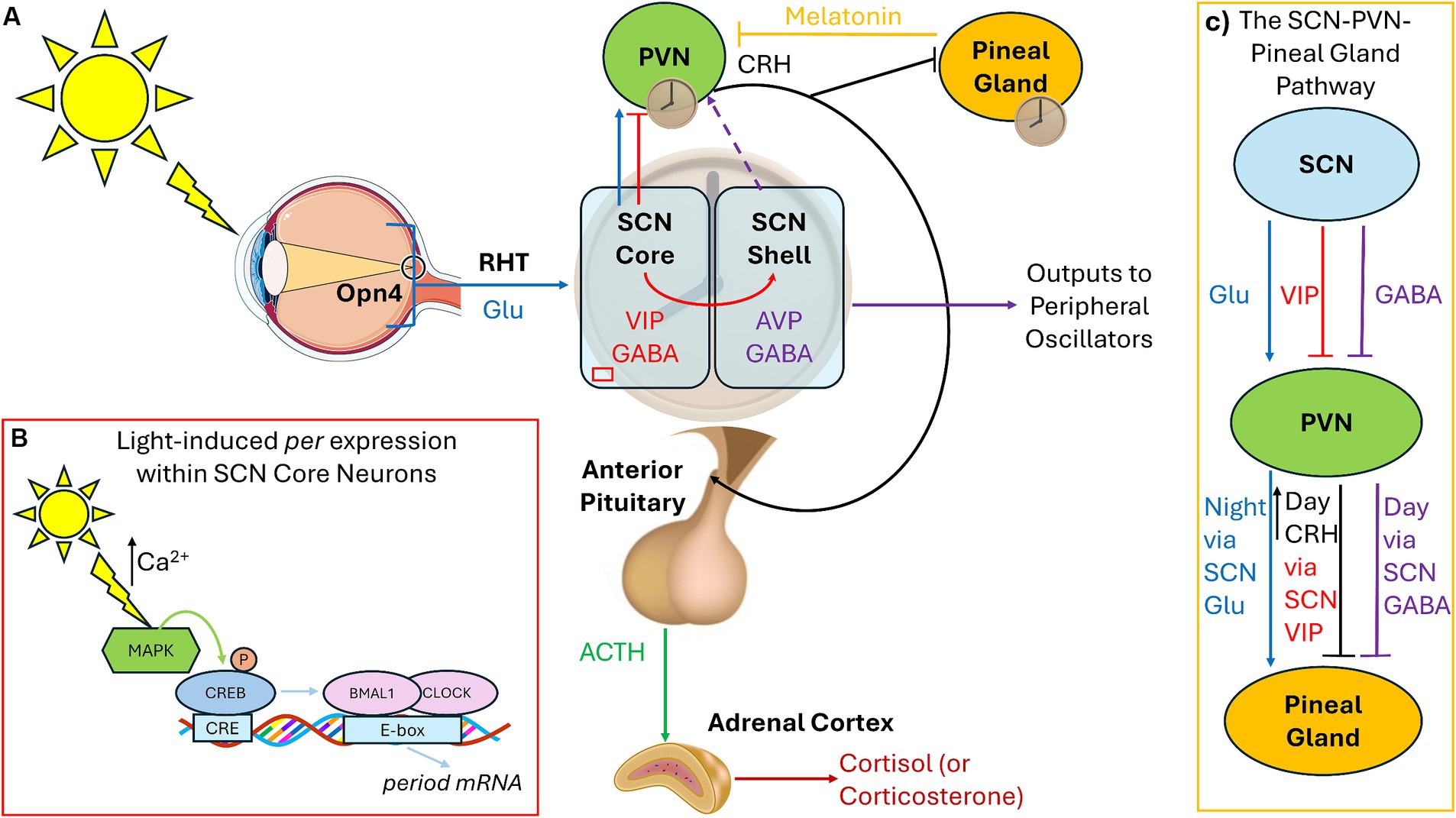
Figure 1. Connections among the circadian pacemaker, pineal gland, and HPA axis. (A) Under standard light:dark cycles, the SCN receives photic signals through the RHT via specialized Opn4-containing retinal ganglion cells, which conveys glutamatergic signals to the SCN core (blue). The SCN core contains VIP-containing neurons, which express circadian clock genes in response to light input. The SCN shell contains AVP-containing cells, receives no retinal photic input, but receives GABA and VIP signaling from the core (red). GABAergic signals from the SCN shell inhibit the PVN, and AVP is used as outputs to synchronize peripheral oscillators (purple). Direct glutamate and VIP projections from the SCN core to the hypothalamic PVN also exist. Hypophysiotropic neurons within the PVN release CRH (black) activating the anterior pituitary to release ACTH (green) to modulate the adrenal gland to release cortisol/corticosterone. AVP outputs exert control over the HPA axis by regulating CRH, depending upon whether the animal is nocturnal (inhibition) or diurnal (stimulation), leading to release of cort at the beginning of the active phase (dashed purple). (B) Light via glutamatergic signaling leads to calcium entry and activation of CREB leading to the transcription of the period genes within the SCN core during the night. (C) The SCN sends excitatory glutamatergic signals to the PVN during the night which activates melatonin synthesis and release within the pineal gland. During the day, the SCN sends inhibitory GABA inhibit melatonin release. CRH from the PVN also inhibits melatonin via modulation of VIP. RHT, Retinohypothalamic tract; Glu, Glutamate; Opn4, Melanopsin; SCN, Suprachiasmatic Nucleus; VIP, Vasoactive Intestinal Peptide; AVP, Vasopressin; PVN, Paraventricular Nucleus of the Hypothalamus; CRH, Corticotropin Releasing Hormone; ACTH, Adrenocorticotrophic Hormone; CREB, cAMP response element binding protein.
Connections exist among the hypothalamic–pituitary–adrenal (HPA) axis and the circadian rhythm, both of which can be modulated by light exposure. For normal responses to stressors, corticotropin-releasing hormone (CRH) from paraventricular nucleus of the hypothalamus (PVN) stimulates the pituitary gland to secrete adrenocorticotropic hormone (ACTH), which in turn prompts the adrenal glands to release cortisol (or the rodent equivalent corticosterone/cort; Figure 1A). Cort then binds to glucocorticoid receptors (GRs), which are expressed throughout the body, to regulate glucose metabolism and brain function during times of stress. Chronic stress, regardless of the source, is associated with increased CRH (Chappell et al., 1986). In both diurnal and nocturnal animals under normal lighting conditions, cort levels surge at the start of the active period to promote waking, then drop during the remainder of the day, starting to rise again immediately before the start of the active cycle. Recent studies illustrate that VIP-containing SCN-core neurons directly innervate the PVN and that VIP from the SCN is necessary to convey light cues to the PVN to regulate the HPA axis. Ablation of molecular clock in the PVN leads to desynchronization of CRH release (Jones et al., 2021). Silencing VIP during the early day increases corticosterone release later in the subjective day (Paul et al., 2020). A second pathway exists involving Neuromendin S (NMS) from the SCN to dopaminergic neurons in the PVN which can also regulate CRH levels. SCN NMS is upregulated under short-day photoperiods (<5 h), which activates dopaminergic PVN neurons and inhibits CRH production (Porcu et al., 2022). As such, the role of the SCN is to inhibit HPA axis activation after the cort surge at the start of the active period through the synchronization of PVN “core clock gene” rhythms, inhibiting CRH-producing neurons.
Under standard light:dark cycles, inhibitory and stimulatory signals from the SCN regulate the pineal gland so that melatonin is produced during the night. Melatonin is produced via SCN innervation of the PVN, initiating norepinephrine release, which then activates the pineal gland to synthesize melatonin. GABAergic signals from the SCN inhibit melatonin release from the pineal gland during the day via the PVN (Kalsbeek et al., 2000). At night, glutamatergic signaling from the SCN leads to melatonin synthesis and release (Perreau-Lenz et al., 2004). Therefore, the SCN uses GABAergic daytime inhibitory signals and nighttime glutamatergic stimulatory signals on the PVN-pineal pathway – GABA responsible for the (daytime) inhibitory signal and glutamate for the (nighttime) stimulation and release of melatonin (Figure 1C). Melatonin also has a role in inhibiting the CRH-induced release of ACTH and cortisol (Tsukamoto et al., 2013), so when melatonin levels are low during morning light, the HPA axis can exert its effects. In turn, CRH from the PVN inhibits melatonin release from the pineal gland (Kellner et al., 1997) and stimulation of the PVN and CRH by GABAergic neurons in the SCN core promotes wakefulness (Ono et al., 2021).
Regardless of age, light-at-night increases both CRH and cort levels and the number of cells that produce them (Milosević et al., 2005; Dulcis et al., 2013). Even short-term exposure to light-at-night (0.5–3 h) can lead to increased anxiety due to increased GRs and cort (Loh et al., 2008; Wang et al., 2023). Recent research also illustrates that the mood-altering effects of light-at-night are mediated through Opn4 inputs to the amygdala, the perihabenula nucleus of the thalamus, and prefrontal cortex (PFC; Fernandez et al., 2018; Li et al., 2024a; Lazzerini Ospri et al., 2024). Additionally, individuals with mood disorders exhibit reduced nighttime melatonin secretion (Li et al., 2024b). In summary, research suggests a mechanism whereby: (1) the circadian timing system regulates the release of CRH from the PVN (and subsequently ACTH and cort) through photic signaling pathways, (2) evening light suppresses melatonin, which can stimulate nighttime HPA activation to promote wakefulness during the inactive phase, and (3) that exposure to light-at-night can lead to abnormal emotionality due to changes in the HPA axis, melatonin secretion, and the function of brain areas associated with mood regulation.
Adolescents are particularly prone to mood disruptions and increased stress due to light-at-night for several reasons related to their developmental stage and behavioral patterns. Evening light (both standard room-level light and dim-light through screentime) has greater delaying and suppression effects on melatonin in adolescents, indicating greater sensitivity to light-at-night compared to adults (Higuchi et al., 2014; Crowley et al., 2015; Figueiro and Overington, 2016). Melatonin suppression is correlated with increased prevalence and exacerbated symptoms of psychiatric disorders, which tend to present during adolescence, including bipolar disorder and depression (Chauhan et al., 2023). In animal models, exposure to light-at-night prior to adolescence can result in anxiety-like and depressive-like behaviors during adulthood, even when the evening light was removed (Borniger et al., 2014; Cissé et al., 2016; Chen et al., 2021). Adolescent exposure to light-at-night also leads to poorer memory, increased anxiety, and activation of the amygdala (Bonilla et al., 2024). In addition to altered melatonin secretion and sensitivity to light-at-night, teenagers also exhibit altered phase and levels of cortisol, particularly during the morning surge to promote wakefulness (Carpenter et al., 2015). Adolescent girls with conduct disorder exhibit reduced morning cortisol levels compared to girls without conduct disorder, indicating a reduced or phase-delayed HPA axis (Helleman et al., 2023). Stressors also produce reduced morning cortisol secretion and dampened HPA axis function in response to aberrant light exposure in both teenagers (Chiang et al., 2016) and juvenile mice (Miller et al., 2022). These results underscore the heightened vulnerability of adolescents to the adverse impacts of light-at-night on the regulation of the HPA axis, making them particularly susceptible to its effects in altering emotionality.
There is a propensity towards evening chronotypes in adolescence partially due to hormonally-driven changes in circadian rhythmicity, which is aggravated by a further delay in wakefulness due to their increased sensitivity to exposure to light-at-night (Hagenauer and Lee, 2013). In particular, sex hormone increases during puberty is associated with increased evening preference in teenagers of both sexes, which is correlated with impaired mood (Dolsen et al., 2019). This change is driven by the timing of melatonin release (even if good light-hygiene is practiced), which occurs later in the evening in teenagers compared to pre-adolescence and adulthood, leading to delays in the timing of sleep–wake cycles, making adolescents feel more alert and less sleepy at night (Carskadon et al., 2004). Teenagers with rhythms delayed toward eveningness exhibit shorter melatonin secretion periods and increased risk of developing mood disorders (Jääkallio et al., 2024). These studies all illustrate that adolescents exhibit an endogenous shifting towards eveningness compared to both younger children and adults, making them more likely to be exposed to light-at-night as well as being more sensitive to its negative effects.
Compounding the tendency for evening chronotypes, teenagers tend to stay awake later and sleep-in on the weekends, when free from mandatory school schedules, leading to further circadian desynchronization in a way circadian scientists call “social jet-lag.” One study reported that teenagers had a school night bedtime approximately 22:30, but during the weekends it was delayed to after midnight with longer sleep; this alteration to the time and duration of sleep was associated with increased anxiety and depression (Zhang et al., 2017). The use of electronic devices during the night influences adolescent sleep timing and quality by inducing circadian phase-delays and exacerbating social jet-lag (Lemola et al., 2015; Hena and Garmy, 2020). Teenagers with mood disorders are especially prone to the adverse effects of light-at-night, as a recent study reported increased likelihood of shorter sleep duration and social jet-lag in clinically-depressed adolescents compared to individuals without depression (Tonon et al., 2022). The cumulative effect of these disruptions – altered melatonin levels, increased cortisol, and impaired emotional regulation – leads to chronic stress, further exacerbating sleep issues and leading to poor mental health.
Sleep and circadian disruption through exposure to light-at-night are associated with alterations in brain chemistry and structure, which can lead to abnormal behaviors. Variability in sleeping patterns, including social jet-lag, is associated with reduced white matter integrity within the superior longitudinal fasciculus and posterior thalamatic radiation, areas that modulate emotionality (Telzer et al., 2015; Uy et al., 2023). White matter integrity changes have been observed in people with mood disorders, including depression (Uy et al., 2023). One possible reason for the reduced white matter integrity found in individuals with social jet-lag, mood disorders, and sleep issues through alterations in Brain-derived Neurotrophic Factor (BDNF). BDNF is known to have neuroprotective effects to white matter integrity (Husson et al., 2005; Weinstock-Guttman et al., 2007) and evidence illustrates that anti-psychotic drugs and antidepressants can ameliorate white matter lesions and increase myelination through the activation of BDNF pathways (Xiao et al., 2010; Sun et al., 2022). Meanwhile, light-at-night has been shown to reduce BDNF levels within the PFC (Capri et al., 2019), while increasing BDNF within the amygdala (Li et al., 2024a). As adolescence is a crucial period for brain development, particularly in areas involved in emotional regulation, light-at-night can impair the development and functioning of these regions, making adolescents more vulnerable to developing mood disorders.
Stressors can create increased inflammatory responses within the amygdala and PFC, which is associated with white matter lesions and mood disorders in adolescents (Thomas et al., 2021; Doney et al., 2022; Poletti et al., 2022). In rodents, light-at-night is known to produce increases to inflammatory cytokines in the amygdala and PFC, which is positively correlated with the intensity of behavioral issues (Walker et al., 2020; Kumari et al., 2021; Jerigova et al., 2023). Increased BDNF is associated with reduced inflammation in individuals with depression (Zhang et al., 2016), while reduced BDNF and increased inflammation are associated with exacerbated symptoms of mood disorders in adolescents (Karthikeyan et al., 2022). Therefore, reducing the amount of inflammation may be a method to alleviate some of the negative behavioral outcomes associated with mood disorders.
While numerous studies have linked the negative loop of the molecular clock (i.e., the BMAL/CLOCK regulation of period – Figure 1B) to mood disorders, recent work is linking the effects of light-at-night on the positive loop (regulation of BMAL1 via rev-erbα and rorα – Figure 2A) on mood and stress responses. Light-at-night decreases and alters rhythmicity of rev-erbα expression not only within the SCN, but also within areas that control emotionality, including other hypothalamic nuclei, the PFC, and the amygdala (Cissé et al., 2016; Otsuka et al., 2020). Part of the reason for this impaired emotionality seen in individuals exposed to light-at-night is due to the desynchronization of expression patterns of core clock mRNA and protein levels between the SCN and other brain areas, including the amygdala (Ikeno and Yan, 2016; Bonilla et al., 2024). Additionally, both rev-erbα knockout mice and knock-down of rev-erbα are associated with increased anxiety-like, and depression-like behaviors, through modulation of dopaminergic and serotonergic signaling, neurotransmitter pathways associated with emotional health (Chung et al., 2014; Zhao and Gammie, 2018; Otsuka et al., 2022; Chen et al., 2023). REV-ERB agonists also have anxiolytic effects (Banerjee et al., 2014). Connections exist between rev-erbα expression and the HPA axis, wherein GR agonists and stressors suppress rev-erbα expression (Murayama et al., 2019). Lastly, rev-erbα and inflammation are also connected as reductions in rev-erbα lead to increased inflammatory responses, while rev-erbα itself can reduce inflammation (Sato et al., 2014; Figure 2B). Although these aforementioned studies provide good evidence for the potential therapeutic effects of targeting rev-erbα, very few studies have investigated the role of the positive loop of the molecular clock on the development of mood disorders and stress responses in adolescents, emphasizing the urgency of addressing this gap of knowledge specifically within this age group.
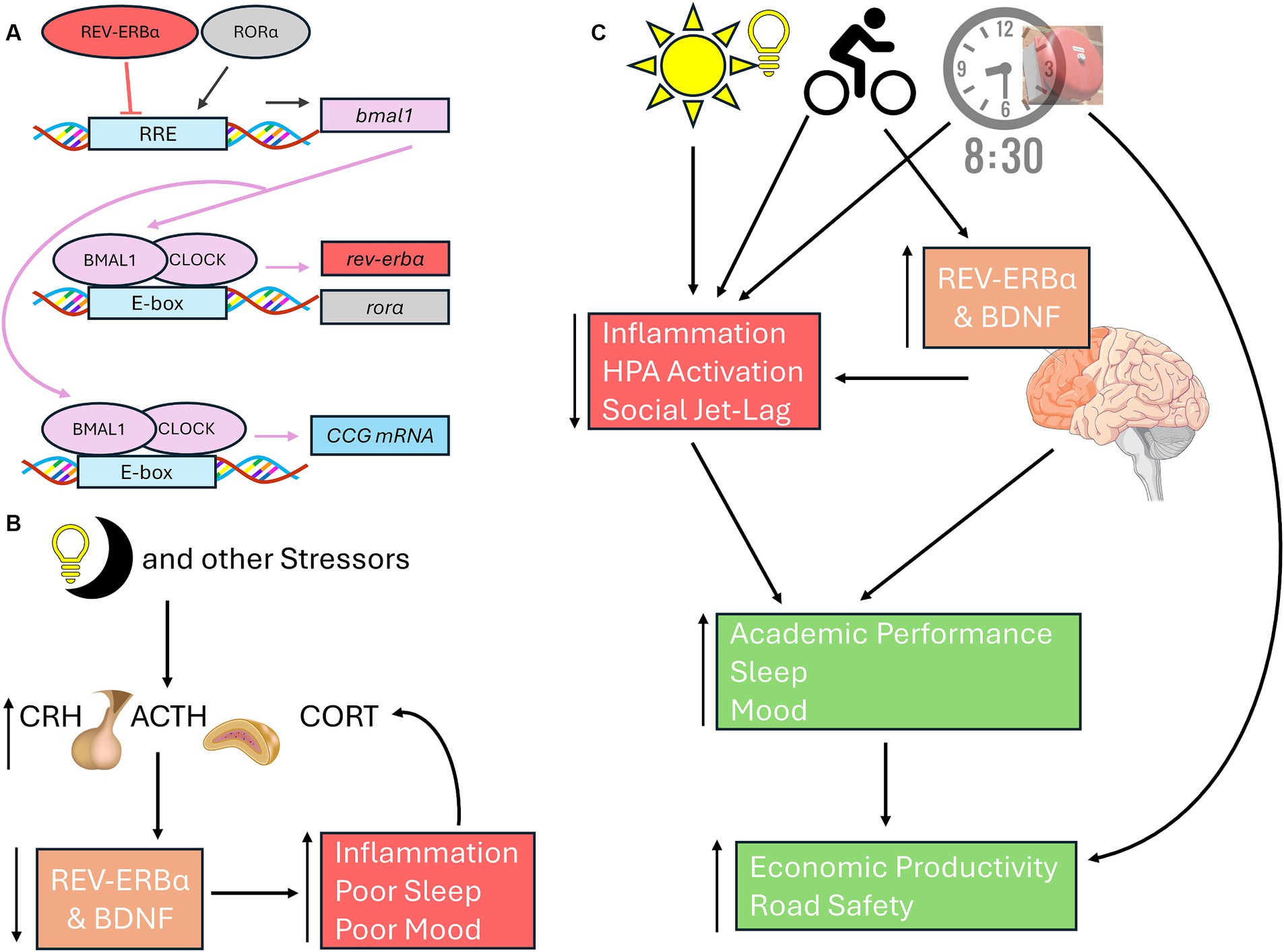
Figure 2. Connections among neuronal health, rev-erbα, and light-at-night. (A) In the positive loop of the molecular clock under standard light:dark cycles, BMAL1 and CLOCK heterodimers bind to the E-boxes of the rev-erbα and rorα to promote their transcription. RORα induces bmal1 transcription, REV-ERBα represses bmal1 expression through activating or inhibiting RREs, respectively. Once translated, BMAL1 can form heterodimers with CLOCK to promote the transcription of CCGs, including period. (B) Light-at-night, HPA axis activation, and other stressors inhibit BDNF and rev-erbα, leading to poorer sleep, inflammation, and mood issues, each of which in turn can further exacerbate all three issues. (C) Exposure to morning light, high school start times of 8:30 am or later, and morning exercise leads to improved emotionality in adolescents through better sleep and reduced social jet-lag, reduced stress, and better academic performance. Both morning light and exercise increases and stabilizes BDNF and rev-erbα, leading to improved neuronal health in areas associated with mood regulation, including PFC. Exercise, BDNF and rev-erbα rhythmicity, are all positively correlated cognitive performance. Later school start times improves academic achievement, road safety, and economic outcomes for both students and their communities. Basic Helix-Loop-Helix ARNT Like 1 (BMAL1 also known as ARNTL); CLOCK, circadian locomotor output cycles kaput; REV-ERBα (also known as nuclear receptor subfamily 1 group D member 1 or NR1D1); RAR-related orphan receptor alpha (RORα); CCG, Core Clock Genes; BDNF, brain derived neurotrophic factor; PFC, Prefrontal cortex.
The first step to mitigating these issues is to reduce light exposure during the night. One study indicated that evening chronotype in adolescents is less severe in rural locations where there is less exposure to artificial light-at-night (Vollmer et al., 2012). Reducing light-at-night is not as simple as it sounds, as adolescents face various psychosocial challenges, including academic pressures, social interactions, and athletic competitions which promote a trend toward eveningness. The best way to improve sleep and cognitive performance in teenagers during the day is to stop using screens after 9 pm (Perrault et al., 2019). Additionally, schools can add the biological and health consequences of light-at-night exposure to the curriculum, as preliminary evidence supports that students would be interested in pursuing methods that reduce light pollution (Yüzbaşıoğlu et al., 2020).
Early school start times force adolescents to wake up earlier than their biological clocks dictate, leading to sleep deprivation, social jet-lag, and stress. School start times of 8:30 am or later lead to improved academic performance, less sleepiness, and improved mood in high school students (Yip et al., 2022; Yeo et al., 2023). Longer school commute time is associated with increased HPA axis activation, particularly in adolescents with evening chronotypes (Karan et al., 2021). Additionally, later school start times can contribute to improved road safety, especially for adolescents (Bin-Hasan et al., 2020). Advocating for policy changes by involving key stakeholders, including parents, teachers, school administrators, and students, in the decision-making process and sharing success stories from other school districts that have implemented later high school start times and experienced positive outcomes could lead to this change (Watson et al., 2017). Delaying school start times also have positive economic impacts for both communities and students’ future salaries (Hafner et al., 2017). Though it may pose logistical challenges including interfering with extracurricular activities, transportation, and misaligning with parental schedules, solutions could be discussed to help convince stakeholders of the importance of making this change for adolescent well-being and success.
If school times were to start later, adolescents will have to resist the urge to stay-up later, thinking that they can compensate the later school day with a later bedtime, necessitating a discussion about the importance of good sleep hygiene with teenagers. Poor sleep hygiene is associated with mood disorders in adolescents (Short et al., 2020). One method in promoting sleep hygiene among adolescents is for caregivers to set an example by practicing healthy sleep habits themselves (Brand et al., 2009). Exposure to daytime light can help promote better sleep at night and lead to a phase-advance that can prevent social jet-lag, and impairments in mood (Misiunaite et al., 2020). Low-intensity exercise during the morning can also lead to phase-advances and improvements in mood, while sedentary lifestyles can exacerbate evening chronotypes in adolescents (Lang et al., 2022). Exercise is associated with increases in BDNF and rev-erbα, enhanced sleep, and reduced inflammation, leading to improved mood (Brand et al., 2010; Dopp et al., 2012; Wunram et al., 2021; da Rocha et al., 2022; Figure 2C).
In conclusion, the relationship among adolescent mood, stress, and light-at-night, highlights the critical importance of ensuring proper sleep, health, and well-being for this age group. While the precise anatomic links among the HPA axis, the biological clock, and photic pathways are known, these studies have been conducted predominately using adult animal models, highlighting the need for additional preclinical studies which investigate the effects of aberrant light exposure on adolescent development. Reducing light pollution, particularly exposure to artificial light-at-night, plays a vital role in promoting healthy sleep patterns as adolescents are more sensitive to it. By implementing these strategies, adolescents can minimize their exposure to light-at-night and promote healthier sleep patterns and reductions in stress.
GG: Conceptualization, Writing – original draft, Writing – review & editing. CM: Conceptualization, Writing – original draft. MM: Conceptualization, Writing – review & editing. JS: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Banerjee, S., Wang, Y., Solt, L. A., Griffett, K., Kazantzis, M., Amador, A., et al. (2014). Pharmacological targeting of the mammalian clock regulates sleep architecture and emotional behaviour. Nat. Commun. 5:5759. doi: 10.1038/ncomms6759
Barko, K., Shelton, M. A., Seggio, J. A., and Logan, R. W. (2019). “Circadian rhythms and addiction” in Neural mechanisms of addiction. ed. M. M. Torregrossa (San Diego, CA, USA: Academic Press), 189–212.
Bin-Hasan, S., Kapur, K., Rakesh, K., and Owens, J. (2020). School start time change and motor vehicle crashes in adolescent drivers. J. Clin. Sleep Med. 16, 371–376. doi: 10.5664/jcsm.8208
Bonilla, P., Shanks, A., Nerella, Y., and Porcu, A. (2024). Effects of chronic light cycle disruption during adolescence on circadian clock, neuronal activity rhythms, and behavior in mice. Front. Neurosci. 18:1418694. doi: 10.3389/fnins.2024.1418694
Borniger, J. C., McHenry, Z. D., Abi Salloum, B. A., and Nelson, R. J. (2014). Exposure to dim light at night during early development increases adult anxiety-like responses. Physiol. Behav. 133, 99–106. doi: 10.1016/j.physbeh.2014.05.012
Brand, S., Gerber, M., Beck, J., Hatzinger, M., Pühse, U., and Holsboer-Trachsler, E. (2010). High exercise levels are related to favorable sleep patterns and psychological functioning in adolescents: a comparison of athletes and controls. J. Adolesc. Health 46, 133–141. doi: 10.1016/j.jadohealth.2009.06.018
Brand, S., Gerber, M., Hatzinger, M., Beck, J., and Holsboer-Trachsler, E. (2009). Evidence for similarities between adolescents and parents in sleep patterns. Sleep Med. 10, 1124–1131. doi: 10.1016/j.sleep.2008.12.013
Capri, K. M., Maroni, M. J., Deane, H. V., Concepcion, H. A., DeCourcey, H., Logan, R. W., et al. (2019). Male C57BL6/N and C57BL6/J mice respond differently to constant light and running-wheel access. Front. Behav. Neurosci. 13:268. doi: 10.3389/fnbeh.2019.00268
Carpenter, J. S., Robillard, R., and Hickie, I. B. (2015). Variations in the sleep–wake cycle from childhood to adulthood: chronobiological perspectives. Chrono Physiol. Therapy 5, 37–49. doi: 10.2147/CPT.S41765
Carskadon, M. A., Acebo, C., and Jenni, O. G. (2004). Regulation of adolescent sleep: implications for behavior. Ann. N. Y. Acad. Sci. 1021, 276–291. doi: 10.1196/annals.1308.032
Chappell, P. B., Smith, M. A., Kilts, C. D., Bissette, G., Ritchie, J., Anderson, C., et al. (1986). Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J. Neurosci. 6, 2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986
Chauhan, S., Barbanta, A., Ettinger, U., and Kumari, V. (2023). Pineal abnormalities in psychosis and mood disorders: A systematic review. Brain Sci. 13:827. doi: 10.3390/brainsci13050827
Chen, R., Routh, B. N., Straetker, J. E., Gibson, C. R., Weitzner, A. S., Bell, K. S., et al. (2023). Microglia depletion ameliorates neuroinflammation, anxiety-like behavior, and cognitive deficits in a sex-specific manner in rev-erbα knockout mice. Brain Behav. Immun. 114, 287–298. doi: 10.1016/j.bbi.2023.08.029
Chen, R., Weitzner, A. S., McKennon, L. A., and Fonken, L. K. (2021). Light at night during development in mice has modest effects on adulthood behavior and neuroimmune activation. Behav. Brain Res. 405:113171. doi: 10.1016/j.bbr.2021.113171
Chiang, J. J., Tsai, K. M., Park, H., Bower, J. E., Almeida, D. M., Dahl, R. E., et al. (2016). Daily family stress and HPA axis functioning during adolescence: the moderating role of sleep. Psychoneuroendocrinology 71, 43–53. doi: 10.1016/j.psyneuen.2016.05.009
Chung, S., Lee, E. J., Yun, S., Choe, H. K., Park, S. B., Son, H. J., et al. (2014). Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell 157, 858–868. doi: 10.1016/j.cell.2014.03.039
Cissé, Y. M., Peng, J., and Nelson, R. J. (2016). Dim light at night prior to adolescence increases adult anxiety-like behaviors. Chronobiol. Int. 33, 1473–1480. doi: 10.1080/07420528.2016.1221418
Crowley, S. J., Cain, S. W., Burns, A. C., Acebo, C., and Carskadon, M. A. (2015). Increased sensitivity of the circadian system to light in early/mid-puberty. J. Clin. Endocrinol. Metab. 100, 4067–4073. doi: 10.1210/jc.2015-2775
da Rocha, A. L., Pinto, A. P., Bedo, B. L. S., Morais, G. P., Oliveira, L. C., Carolino, R. O. G., et al. (2022). Exercise alters the circadian rhythm of REV-ERB-α and downregulates autophagy-related genes in peripheral and central tissues. Sci. Rep. 12:20006. doi: 10.1038/s41598-022-24277-4
Deibel, S. H., McDonald, R. J., and Kolla, N. J. (2020). Are owls and larks different when it comes to aggression? Genetics, neurobiology, and behavior. Front. Behav. Neurosci. 14:39. doi: 10.3389/fnbeh.2020.00039
Dolsen, E. A., Deardorff, J., and Harvey, A. G. (2019). Salivary pubertal hormones, sleep disturbance, and an evening circadian preference in adolescents: risk across health domains. J. Adolesc. Health 64, 523–529. doi: 10.1016/j.jadohealth.2018.10.003
Doney, E., Cadoret, A., Dion-Albert, L., Lebel, M., and Menard, C. (2022). Inflammation-driven brain and gut barrier dysfunction in stress and mood disorders. Eur. J. Neurosci. 55, 2851–2894. doi: 10.1111/ejn.15239
Dopp, R. R., Mooney, A. J., Armitage, R., and King, C. (2012). Exercise for adolescents with depressive disorders: a feasibility study. Depress. Res. Treat. 2012:257472. doi: 10.1155/2012/257472
Dulcis, D., Jamshidi, P., Leutgeb, S., and Spitzer, N. C. (2013). Neurotransmitter switching in the adult brain regulates behavior. Science 340, 449–453. doi: 10.1126/science.1234152
Esaki, Y., Obayashi, K., Saeki, K., Fujita, K., Iwata, N., and Kitajima, T. (2022). Effect of nighttime bedroom light exposure on mood episode relapses in bipolar disorder. Acta Psychiatr. Scand. 146, 64–73. doi: 10.1111/acps.13422
Fernandez, D. C., Fogerson, P. M., Lazzerini Ospri, L., Thomsen, M. B., Layne, R. M., Severin, D., et al. (2018). Light affects mood and learning through distinct retina-brain pathways. Cell 175, 71–84.e18. doi: 10.1016/j.cell.2018.08.004
Figueiro, M., and Overington, D. (2016). Self-luminous devices and melatonin suppression in adolescents. Light. Res. Technol. 48, 966–975. doi: 10.1177/1477153515584979
Hafner, M., Stepanek, M., and Troxel, W. M. (2017). The economic implications of later school start times in the United States. Sleep Health 3, 451–457. doi: 10.1016/j.sleh.2017.08.007
Hagenauer, M. H., and Lee, T. M. (2013). Adolescent sleep patterns in humans and laboratory animals. Horm. Behav. 64, 270–279. doi: 10.1016/j.yhbeh.2013.01.013
Helbich, M., Browning, M. H. E. M., and Huss, A. (2020). Outdoor light at night, air pollution and depressive symptoms: A cross-sectional study in the Netherlands. Sci. Total Environ. 744:140914. doi: 10.1016/j.scitotenv.2020.140914
Helleman, A., Rubin, R. T., Gardner, W., Lourie, A., Taylor, A. N., Cochran, J., et al. (2023). Circadian cortisol secretion in adolescent girls with conduct disorder. Psychoneuroendocrinology 148:105972. doi: 10.1016/j.psyneuen.2022.105972
Hena, M., and Garmy, P. (2020). Social jetlag and its association with screen time and nighttime texting among adolescents in Sweden: A cross-sectional study. Front. Neurosci. 14:122. doi: 10.3389/fnins.2020.00122
Higuchi, S., Nagafuchi, Y., Lee, S. I., and Harada, T. (2014). Influence of light at night on melatonin suppression in children. J. Clin. Endocrinol. Metab. 99, 3298–3303. doi: 10.1210/jc.2014-1629
Husson, I., Rangon, C. M., Lelièvre, V., Bemelmans, A. P., Sachs, P., Mallet, J., et al. (2005). BDNF-induced white matter neuroprotection and stage-dependent neuronal survival following a neonatal excitotoxic challenge. Cereb. Cortex 15, 250–261. doi: 10.1093/cercor/bhh127
Ikeno, T., and Yan, L. (2016). Chronic light exposure in the middle of the night disturbs the circadian system and emotional regulation. J. Biol. Rhythm. 31, 352–364. doi: 10.1177/0748730416642065
Jääkallio, P., Kuula, L., and Pesonen, A. K. (2024). Temporal pathways between circadian rhythm, depression and anxiety in the transition from adolescence to early adulthood. J. Affect. Disord. 350, 656–664. doi: 10.1016/j.jad.2024.01.141
Jerigova, V., Zeman, M., and Okuliarova, M. (2023). Chronodisruption of the acute inflammatory response by night lighting in rats. Sci. Rep. 13:14109. doi: 10.1038/s41598-023-41266-3
Jones, J. R., Chaturvedi, S., Granados-Fuentes, D., and Herzog, E. D. (2021). Circadian neurons in the paraventricular nucleus entrain and sustain daily rhythms in glucocorticoids. Nat. Commun. 12:5763. doi: 10.1038/s41467-021-25959-9
Kalsbeek, A., Garidou, M. L., Palm, I. F., Van Der Vliet, J., Simonneaux, V., Pévet, P., et al. (2000). Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur. J. Neurosci. 12, 3146–3154. doi: 10.1046/j.1460-9568.2000.00202.x
Karan, M., Rahal, D., Almeida, D. M., Bower, J. E., Irwin, M. R., McCreath, H., et al. (2021). School commute time, chronotype, and altered HPA axis functioning during adolescence. Psychoneuroendocrinology 133:105371. doi: 10.1016/j.psyneuen.2021.105371
Karthikeyan, S., Dimick, M. K., Fiksenbaum, L., Jeong, H., Birmaher, B., Kennedy, J. L., et al. (2022). Inflammatory markers, brain-derived neurotrophic factor, and the symptomatic course of adolescent bipolar disorder: A prospective repeated-measures study. Brain Behav. Immun. 100, 278–286. doi: 10.1016/j.bbi.2021.11.020
Kellner, M., Yassouridis, A., Manz, B., Steiger, A., Holsboer, F., and Wiedemann, K. (1997). Corticotropin-releasing hormone inhibits melatonin secretion in healthy volunteers--a potential link to low-melatonin syndrome in depression? Neuroendocrinology 65, 284–290. doi: 10.1159/000127186
Kumari, R., Verma, V., Kronfeld-Schor, N., and Singaravel, M. (2021). Differential response of diurnal and nocturnal mammals to prolonged altered light-dark cycle: a possible role of mood associated endocrine, inflammatory and antioxidant system. Chronobiol. Int. 38, 1618–1630. doi: 10.1080/07420528.2021.1937200
Lang, C., Richardson, C., Short, M. A., and Gradisar, M. (2022). Low-intensity scheduled morning exercise for adolescents with a late chronotype: a novel treatment to advance circadian phase? Sleep Adv. 3:zpac 021. doi: 10.1093/sleepadvances/zpac021
Lazzerini Ospri, L., Zhan, J. J., Thomsen, M. B., Wang, H., Komal, R., Tang, Q., et al. (2024). Light affects the prefrontal cortex via intrinsically photosensitive retinal ganglion cells. Sci. Adv. 10:eadh9251. doi: 10.1126/sciadv.adh9251
Lemola, S., Perkinson-Gloor, N., Brand, S., Dewald-Kaufmann, J. F., and Grob, A. (2015). Adolescents' electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J. Youth Adolesc. 44, 405–418. doi: 10.1007/s10964-014-0176-x
Li, Z., Lee, C. S., Chen, S., He, B., Chen, X., Peng, H. Y., et al. (2024a). Blue light at night produces stress-evoked heightened aggression by enhancing brain-derived neurotrophic factor in the basolateral amygdala. Neurobiol. Stress 28:100600. doi: 10.1016/j.ynstr.2023.100600
Li, Z., Lee, C. S., Peng, H. Y., Lin, T. B., Hsieh, M. C., Lai, C. Y., et al. (2024b). Lights at night mediate depression-like behavioral and molecular phenotypes in a glucocorticoid-dependent manner in male rats. Neuropharmacology 248:109888. doi: 10.1016/j.neuropharm.2024.109888
Loh, D. H., Abad, C., Colwell, C. S., and Waschek, J. A. (2008). Vasoactive intestinal peptide is critical for circadian regulation of glucocorticoids. Neuroendocrinology 88, 246–255. doi: 10.1159/000140676
Medeiros Contini, F., and Seggio, J. A. (2023). Constant light and single housing alter novelty-induced locomotor activity and sociability in female Swiss Webster mice. Neuro Endocrinol. Lett. 44, 117–122
Michaud, J. M., Price, J. C., Deane, H. V., Concepcion, H. A., Coronella, J. A., DeCourcey, H., et al. (2022). The effects of Ovariectomy on the behavioral and physiological responses to constant light in C57BL6/J mice. Biol. Rhythm. Res. 53, 921–938. doi: 10.1080/09291016.2020.1842970
Miller, L., Bodemeier Loayza Careaga, M., Handa, R. J., and Wu, T. J. (2022). The effects of chronic variable stress and photoperiod alteration on the hypothalamic-pituitary-adrenal Axis response and behavior of mice. Neuroscience 496, 105–118. doi: 10.1016/j.neuroscience.2022.06.011
Milosević, V., Trifunović, S., Sekulić, M., Sosić-Jurjević, B., Filipović, B., Negić, N., et al. (2005). Chronic exposure to constant light affects morphology and secretion of adrenal zona fasciculata cells in female rats. Gen. Physiol. Biophys. 24, 299–309
Misiunaite, I., Eastman, C. I., and Crowley, S. J. (2020). Circadian phase advances in response to weekend morning light in adolescents with Short sleep and late bedtimes on school nights. Front. Neurosci. 14:99. doi: 10.3389/fnins.2020.00099
Murayama, Y., Yahagi, N., Takeuchi, Y., Aita, Y., Mehrazad Saber, Z., Wada, N., et al. (2019). Glucocorticoid receptor suppresses gene expression of rev-erbα (Nr1d1) through interaction with the CLOCK complex. FEBS Lett. 593, 423–432. doi: 10.1002/1873-3468.13328
Ono, D., Honma, K. I., and Honma, S. (2021). GABAergic mechanisms in the suprachiasmatic nucleus that influence circadian rhythm. J. Neurochem. 157, 31–41. doi: 10.1111/jnc.15012
Otsuka, T., Le, H. T., Thein, Z. L., Ihara, H., Sato, F., Nakao, T., et al. (2022). Deficiency of the circadian clock gene rev-erbα induces mood disorder-like behaviours and dysregulation of the serotonergic system in mice. Physiol. Behav. 256:113960. doi: 10.1016/j.physbeh.2022.113960
Otsuka, T., Thi Le, H., Kohsaka, A., Sato, F., Ihara, H., Nakao, T., et al. (2020). Adverse effects of circadian disorganization on mood and molecular rhythms in the prefrontal cortex of mice. Neuroscience 432, 44–54. doi: 10.1016/j.neuroscience.2020.02.013
Paksarian, D., Rudolph, K. E., Stapp, E. K., Dunster, G. P., He, J., Mennitt, D., et al. (2020). Association of Outdoor Artificial Light at night with mental disorders and sleep patterns among US adolescents. JAMA Psychiatry 77, 1266–1275. doi: 10.1001/jamapsychiatry.2020.1935
Paul, S., Hanna, L., Harding, C., Hayter, E. A., Walmsley, L., Bechtold, D. A., et al. (2020). Output from VIP cells of the mammalian central clock regulates daily physiological rhythms. Nat. Commun. 11:1453. doi: 10.1038/s41467-020-15277-x
Perrault, A. A., Bayer, L., Peuvrier, M., Afyouni, A., Ghisletta, P., Brockmann, C., et al. (2019). Reducing the use of screen electronic devices in the evening is associated with improved sleep and daytime vigilance in adolescents. Sleep 42, 1–10. doi: 10.1093/sleep/zsz125
Perreau-Lenz, S., Kalsbeek, A., Pévet, P., and Buijs, R. M. (2004). Glutamatergic clock output stimulates melatonin synthesis at night. Eur. J. Neurosci. 19, 318–324. doi: 10.1111/j.0953-816X.2003.03132.x
Poletti, S., Paolini, M., Ernst, J., Bollettini, I., Melloni, E., Vai, B., et al. (2022). Long-term effect of childhood trauma: role of inflammation and white matter in mood disorders. Brain Behav. Immun. Health 26:100529. doi: 10.1016/j.bbih.2022.100529
Porcu, A., Nilsson, A., Booreddy, S., Barnes, S. A., Welsh, D. K., and Dulcis, D. (2022). Seasonal changes in day length induce multisynaptic neurotransmitter switching to regulate hypothalamic network activity and behavior. Sci. Adv. 8:eabn9867. doi: 10.1126/sciadv.abn9867
Rahman, S. A., Wright, K. P., Lockley, S. W., Czeisler, C. A., and Gronfier, C. (2019). Characterizing the temporal dynamics of melatonin and cortisol changes in response to nocturnal light exposure. Sci. Rep. 9:19720. doi: 10.1038/s41598-019-54806-7
Sato, S., Sakurai, T., Ogasawara, J., Shirato, K., Ishibashi, Y., Oh-ishi, S., et al. (2014). Direct and indirect suppression of interleukin-6 gene expression in murine macrophages by nuclear orphan receptor REV-ERBα. ScientificWorldJournal 2014:685854. doi: 10.1155/2014/685854
Short, M. A., Booth, S. A., Omar, O., Ostlundh, L., and Arora, T. (2020). The relationship between sleep duration and mood in adolescents: A systematic review and meta-analysis. Sleep Med. Rev. 52:101311. doi: 10.1016/j.smrv.2020.101311
Sun, Z., Jia, L., Shi, D., He, Y., Ren, Y., Yang, J., et al. (2022). Deep brain stimulation improved depressive-like behaviors and hippocampal synapse deficits by activating the BDNF/mTOR signaling pathway. Behav. Brain Res. 419:113709. doi: 10.1016/j.bbr.2021.113709
Telzer, E. H., Goldenberg, D., Fuligni, A. J., Lieberman, M. D., and Gálvan, A. (2015). Sleep variability in adolescence is associated with altered brain development. Dev. Cogn. Neurosci. 14, 16–22. doi: 10.1016/j.dcn.2015.05.007
Thomas, M., Savitz, J., Zhang, Y., Burrows, K., Smith, R., and Figueroa-Hall, L. (2021). Elevated systemic inflammation is associated with reduced corticolimbic white matter integrity in depression. Life (Basel) 12. doi: 10.3390/life12010043
Tonon, A. C., Constantino, D. B., Amando, G. R., Abreu, A. C., Francisco, A. P., de Oliveira, M. A. B., et al. (2022). Sleep disturbances, circadian activity, and nocturnal light exposure characterize high risk for and current depression in adolescence. Sleep 45, 1–10. doi: 10.1093/sleep/zsac104
Tsukamoto, N., Otsuka, F., Ogura-Ochi, K., Inagaki, K., Nakamura, E., Toma, K., et al. (2013). Melatonin receptor activation suppresses adrenocorticotropin production via BMP-4 action by pituitary AtT20 cells. Mol. Cell. Endocrinol. 375, 1–9. doi: 10.1016/j.mce.2013.05.010
Uy, J. P., Ho, T. C., Buthmann, J. L., Coury, S. M., and Gotlib, I. H. (2023). Early life stress, sleep disturbances, and depressive symptoms during adolescence: the role of the cingulum bundle. Dev. Cogn. Neurosci. 63:101303. doi: 10.1016/j.dcn.2023.101303
Vollmer, C., Michel, U., and Randler, C. (2012). Outdoor light at night (LAN) is correlated with eveningness in adolescents. Chronobiol. Int. 29, 502–508. doi: 10.3109/07420528.2011.635232
Walker, W. H., Borniger, J. C., Gaudier-Diaz, M. M., Hecmarie Meléndez-Fernández, O., Pascoe, J. L., Courtney DeVries, A., et al. (2020). Acute exposure to low-level light at night is sufficient to induce neurological changes and depressive-like behavior. Mol. Psychiatry 25, 1080–1093. doi: 10.1038/s41380-019-0430-4
Wang, G., Liu, Y. F., Yang, Z., Yu, C. X., Tong, Q., Tang, Y. L., et al. (2023). Short-term acute bright light exposure induces a prolonged anxiogenic effect in mice via a retinal ipRGC-CeA circuit. Sci. Adv. 9:eadf4651. doi: 10.1126/sciadv.adf4651
Watson, N. F., Martin, J. L., Wise, M. S., Carden, K. A., Kirsch, D. B., Kristo, D. A., et al. (2017). Delaying middle school and high school start times promotes student health and performance: an American Academy of sleep medicine position statement. J. Clin. Sleep Med. 13, 623–625. doi: 10.5664/jcsm.6558
Weinstock-Guttman, B., Zivadinov, R., Tamaño-Blanco, M., Abdelrahman, N., Badgett, D., Durfee, J., et al. (2007). Immune cell BDNF secretion is associated with white matter volume in multiple sclerosis. J. Neuroimmunol. 188, 167–174. doi: 10.1016/j.jneuroim.2007.06.003
Wunram, H. L., Oberste, M., Ziemendorff, A., Hamacher, S., Kapanci, T., Heller, R., et al. (2021). Differential effects of ergometer-cycling and whole-body-vibration training on serological BDNF and IGF-1 in the treatment of adolescent depression - is there an impact of BDNFp.Val 66Met variants? Physiol. Behav. 241:113596. doi: 10.1016/j.physbeh.2021.113596
Xiao, J., Wong, A. W., Willingham, M. M., van den Buuse, M., Kilpatrick, T. J., and Murray, S. S. (2010). Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals 18, 186–202. doi: 10.1159/000323170
Yeo, S. C., Yabuki, H., Charoenthammanon, R. S., and Gooley, J. J. (2023). University students' diurnal learning-directed behavior is strongly influenced by school start times with implications for grades. Sleep 46, 1–11. doi: 10.1093/sleep/zsad141
Yip, T., Wang, Y., Xie, M., Ip, P. S., Fowle, J., and Buckhalt, J. (2022). School start times, sleep, and youth outcomes: a meta-analysis. Pediatrics 149, 62–91. doi: 10.1542/peds.2021-054068
Yüzbaşıoğlu, M. K., Doğanay, K., and Avan, C. (2020). Science, engineering and entrepreneurship applications: designing a lighting tool for reducing the light pollution and students’ views. Online Sci. Ed. J. 5, 60–72.
Zhang, J., Paksarian, D., Lamers, F., Hickie, I. B., He, J., and Merikangas, K. R. (2017). Sleep patterns and mental health correlates in US adolescents. J. Pediatr. 182, 137–143. doi: 10.1016/j.jpeds.2016.11.007
Zhang, J. C., Yao, W., and Hashimoto, K. (2016). Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr. Neuropharmacol. 14, 721–731. doi: 10.2174/1570159X14666160119094646
Zhao, C., and Gammie, S. C. (2018). The circadian gene Nr1d1 in the mouse nucleus accumbens modulates sociability and anxiety-related behavior. Eur. J. Neurosci, 48, 1924–1943. doi: 10.1111/ejn.14066
Keywords: light, circadian, HPA axis, teenagers, clock genes, sleep
Citation: Guindon GE, Murphy CA, Milano ME and Seggio JA (2024) Turn off that night light! Light-at-night as a stressor for adolescents. Front. Neurosci. 18:1451219. doi: 10.3389/fnins.2024.1451219
Received: 18 June 2024; Accepted: 19 July 2024;
Published: 31 July 2024.
Edited by:
Marianne Seney, University of Pittsburgh, United StatesReviewed by:
Alessandra Porcu, University of South Carolina, United StatesCopyright © 2024 Guindon, Murphy, Milano and Seggio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph A. Seggio, anNlZ2dpb0BicmlkZ2V3LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.