- 1Department of Cardiovascular Medicine, People's Hospital of Xiangxi Tujia and Miao Autonomous Prefecture, The First Affiliated Hospital of Jishou University, Jishou, China
- 2Department of Neurology, Zixi Hospital of Jiangxi Provincial People’s Hospital, Zixi County People’s Hospital, Fuzhou, China
- 3Department of Neurology, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
Background and purpose: To evaluate the association between sleep-related factors, including sleep duration, self-reported sleep disturbances, and diagnosed sleep disorders, and the risk of cardiovascular disease (CVD) in US participants.
Methods: The data of this study from the National Health and Nutrition Examination Survey (NHANES) conducted between 2007 and 2014. Sleep factors were assessed using a standardized questionnaire, and overall sleep scores were calculated on a scale of 0 to 3. The participants were classified into three sleep pattern groups: poor sleep pattern (overall sleep score ≤ 1), intermediate sleep pattern (overall sleep score = 2), and healthy sleep pattern (overall sleep score = 3). CVD was defined based on self-reported questionnaire responses. Logistic regression models were used to investigate the association between sleep factors and CVD.
Results: Among 21,115 participants, 2,245 (10.6%) were diagnosed with CVD. Participants with poor sleep patterns had a significantly higher risk of CVD (OR = 1.82, 95% CI: 1.52–2.16, p < 0.001). Self-reported trouble sleeping (OR = 1.53, 95% CI: 1.32–1.78, p < 0.001), and sleep disorder (OR = 2.09, 95% CI: 1.75–2.50, p < 0.001) were related to an increased risk of CVD. However, no such association was observed for either short (OR = 1.12, 95% CI: 0.95–1.33, p = 0.174) or long sleep durations (OR = 1.14, 95% CI: 0.90–1.45, p = 0.266). Our study also suggested an interaction between sleep patterns and age (P for interaction = 0.002).
Conclusion: This study highlights the significant association between poor sleep patterns and an increased risk of CVD in US participants.
1 Introduction
Cardiovascular disease (CVD), which includes congestive heart failure (CHF), coronary heart disease (CHD), angina, myocardial infarction (MI), and stroke, continues to be the primary reason for mortality and disease burden in numerous countries around the world (Joseph et al., 2017). Over the last decade, the global death toll from CVD has risen by 12.5%, contributing to almost one-third of all worldwide deaths (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, 2016). The exact causes of CVD are complex and involve an interplay of genetic and environmental factors. The predominant share of CVD cases can be mitigated by addressing modifiable behavioral risk factors, including poor sleep patterns, tobacco use, unhealthy dietary habits, obesity, and physical inactivity. Therefore, timely recognition of CVD is crucial to initiate prevention and control measures, commencing with counseling and addressing risk factors (WHO, 2021).
Sleep behaviors represent a vital metric, serving as a pivotal indicator of physiological health. Individual sleep behaviors typically exhibit interrelated patterns in a compensatory manner (Li et al., 2021; Fan et al., 2020). Human sleep patterns are crucial for evaluating general health and functionality. Sleep plays a critical role in modulating cardiovascular function, affecting the development of CVD in both the physiological and pathological states. Prior research (Saz-Lara et al., 2022; Miller and Howarth, 2023) have demonstrated that sleep can have a profound impact on the human autonomic nervous system, vascular tone, cardiac function, endothelial function, and coagulation. These effects correlate with alterations in sympathetic nerve activity (Panza et al., 1991), plasma catecholamine levels, and blood flow shear stress.
In most previous studies, sleep behaviors were examined in isolation, neglecting the intricacies and interrelationships among sleep parameters. There is a paucity of studies that have comprehensively examined sleep behaviors collectively. Investigations indicate that the combination of insufficient sleep duration and other adverse sleep behaviors, such as difficulty falling asleep and sleep disorders, increases the likelihood of developing CVD.
Therefore, using the NHANES database, we separately analyzed the relationships between sleep duration, sleep difficulties, sleep disorders, and CVD risk. We also defined the combination of these three factors as “sleep patterns,” which were also evaluated in relation to CVD risk.
2 Materials and methods
2.1 Data sources and study population
This cross-sectional study utilized data from NHANES, which was collected during the 2007–2008, 2009–2010, 2011–2012, and 2013–2014 cycles by the Centers for Disease Control and Prevention. NHANES was designed in a stratified, multistage probability sample survey to assess health and nutrition statistics. This survey is known for its nationally representative nature. The ethical clearance for NHANES was obtained from the NCHS Ethics Review Committee, and all participants provided written informed consent before their involvement.
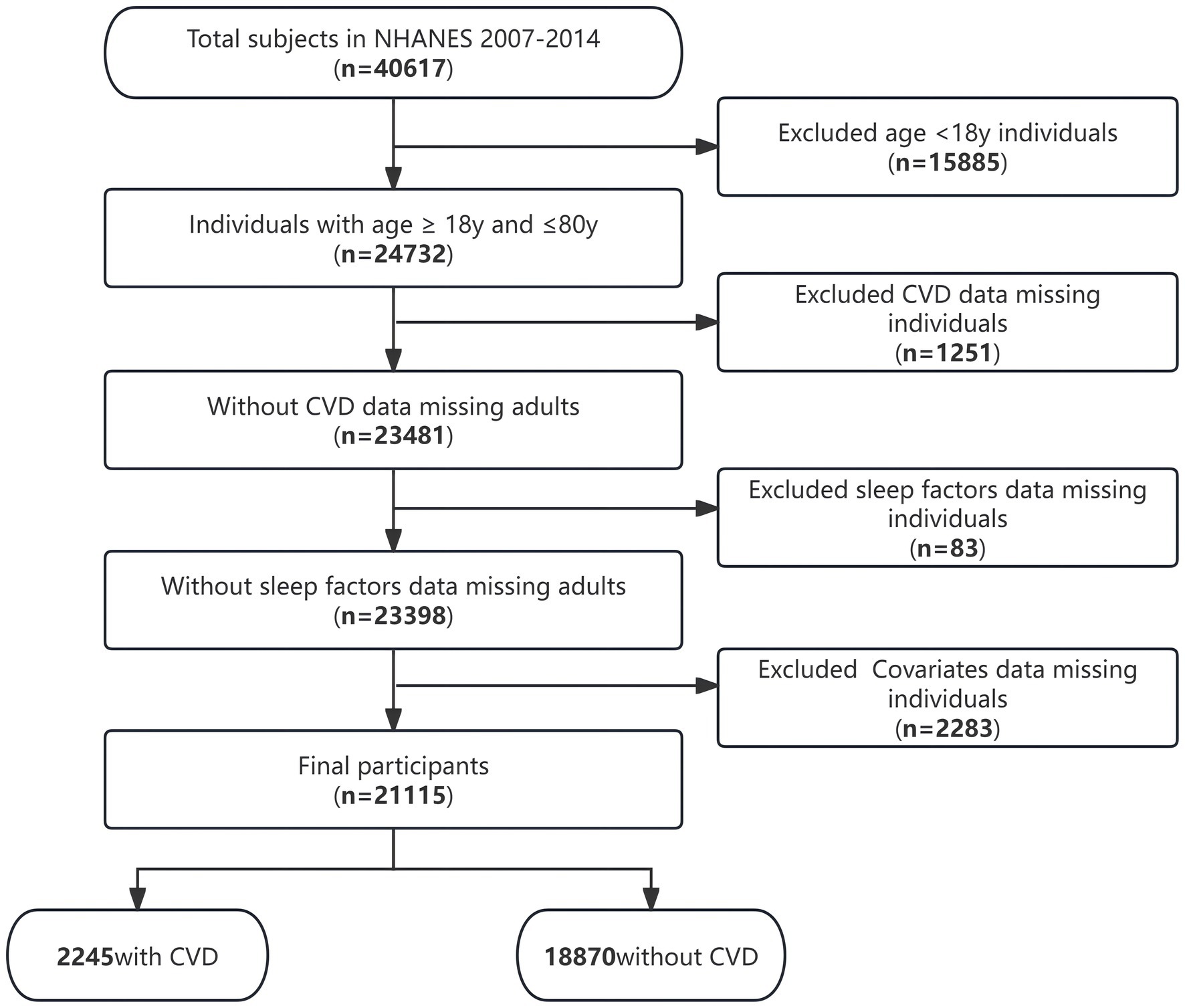
Our study exclusively included individuals aged ≥18 years who had undergone an interview. Exclusion criteria comprised individuals with missing data related to cardiovascular disease, sleep factors, or covariates. This research followed the guidelines established by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).
2.2 Definition of CVD
Our outcome is whether diagnosed with CVD. CVD is defined as at least one diagnosis of CHF, CHD, angina, MI, or stroke; the hard criteria include MI and stroke (Cai et al., 2020). We used a participant’s responses to the medical conditions questionnaire to determine whether they had CVD: “Has a doctor or other health professional ever told you had angina/heart attack/stroke/ CHD?” (Zhang et al., 2023).
2.3 Sleep factors and sleep pattern
Data regarding sleep duration were collected by asking the question, “How much sleep do you typically get on weekdays or workdays?” The duration was then classified into three categories: short (less than 7 h per night), normal (7–9 h per night), or long (more than 9 h per night), with 7–9 h per night serving as the reference group. To assess trouble sleeping and sleep disorders, responses to questions such as “Have you ever told a doctor or other health professional that you have trouble sleeping?” and “Have you ever been told by a doctor or other health professional that you have a sleep disorder?” were analyzed, respectively. To determine a comprehensive sleep score, several aspects of sleep were taken into account, including sleep duration, self-reported sleep difficulty, and diagnosed sleep disorders. A low-risk sleep score was assigned to individuals who slept between 7 and 9 h each night without experiencing any trouble sleeping or being diagnosed with a sleep disorder. A binary categorization was assigned to each sleep factor, with a low risk being indicated by 1 and a high risk being indicated by 0. The overall sleep patterns were determined to be either healthy, with an overall sleep score of 3, intermediate, with an overall sleep score of 2, or poor, with an overall sleep score ranging from 0 to 1. This resulted in a cumulative score that could range from 0 to 3 (Li and Shang, 2021).
2.4 Other covariates
This data was acquired through a computer-assisted automated approach, employing the U.S. Department of Agriculture (USDA) automated multiple-pass method to accommodate day-to-day variations. According to prior research (Fan et al., 2020; Saz-Lara et al., 2022; Kizilkilic et al., 2023), we evaluated various potential confounding factors, which included age, sex, race, education level, marital status, insurance, BMI (body mass index), hypertension, DM (diabetes mellitus), smoking status, physical activity, HbA1c, TC (total cholesterol), and HDL-C (high-density lipoprotein). Our analysis focused on these factors, as they have been identified as important in previous studies. The classification of race or ethnicity encompasses Mexican-American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other races. Based on the U.S. education system and previous literature, the education level was divided into three categories: less than 9 years, 9–11 years, and more than 12 years (Hamad et al., 2019; Gurgel et al., 2021; Fleming et al., 2021; Liu et al., 2022). Married, never married, and others make up the different categories of marital status. Covered by health insurance (any insurance, no). BMI is calculated by dividing an individual’s weight in kilograms by their height squared (kg/m2). Hypertension is diagnosed when a person’s systolic blood pressure is 140 mmHg or higher and/or their diastolic blood pressure is 90 mmHg or higher, or if they have a prior diagnosis of high blood pressure (Xiao and Xiao, 2023). DM was determined on the basis of a questionnaire question about whether the patient had a history of diabetes. The smoking status of individuals was categorized into three categories, namely never, former, and current smokers, based on their responses to two questions from a questionnaire. The questions were: “Have you smoked at least 100 cigarettes in your entire life?” and “Do you currently smoke cigarettes?” Drinking habits were defined as having at least 12 alcoholic drinks per year (Yang et al., 2024). Physical activity was classified into three levels: sedentary, moderate, or vigorous (Liu et al., 2022).
2.5 Statistical analysis
In accordance with the guidelines set by NHANES for analysis, this study took into account the complexities of sampling designs and the associated weights (Johnson et al., 2013). The sample weight for the four NHANES cycles was determined by taking the initial two-year sample weight, dividing it by four, and subsequently assigning this adjusted weight to each individual participant. Furthermore, the variable denoting masked variance pseudo-PSU (SDMVPSU) is used to identify the primary sampling units (PSU), while the variable indicating masked variance pseudo-stratum (SDMVSTRA) is responsible for identifying the strata from which the PSUs are chosen.
Participants were categorized by sleep pattern for baseline characteristics. The normal distribution of continuous variables was evaluated using the Shapiro–Wilk statistical test, while differences in both continuous and categorical variables were investigated through independent t-tests and chi-squared tests. Logistic regression was employed to determine the relationship between each sleep factor and CVD by computing odds ratios (ORs) and 95% confidence intervals (95% CIs). In separate analyses, the connection between sleep patterns and CVD was explored.
Prior to conducting statistical power estimates, no a priori determination was made regarding the sample size, as it was solely based on the available data. The statistical software packages used for all analyses were R 4.2.2 (http://www.R-project.org, The R Foundation, Shanghai, China) (accessed on November 24, 2023) and Free Statistics software version 1.9. All participants underwent a descriptive study. The two-tailed test yielded a p-value of less than 0.05, indicating a significant result.
3 Results
3.1 Baseline characteristics of participants
The present research encompassed 21,115 individuals who were subjected to an extensive screening procedure guided by predetermined inclusive and exclusive criteria (Figure 1). Among these individuals, there was an overall 10.6% prevalence of CVD.
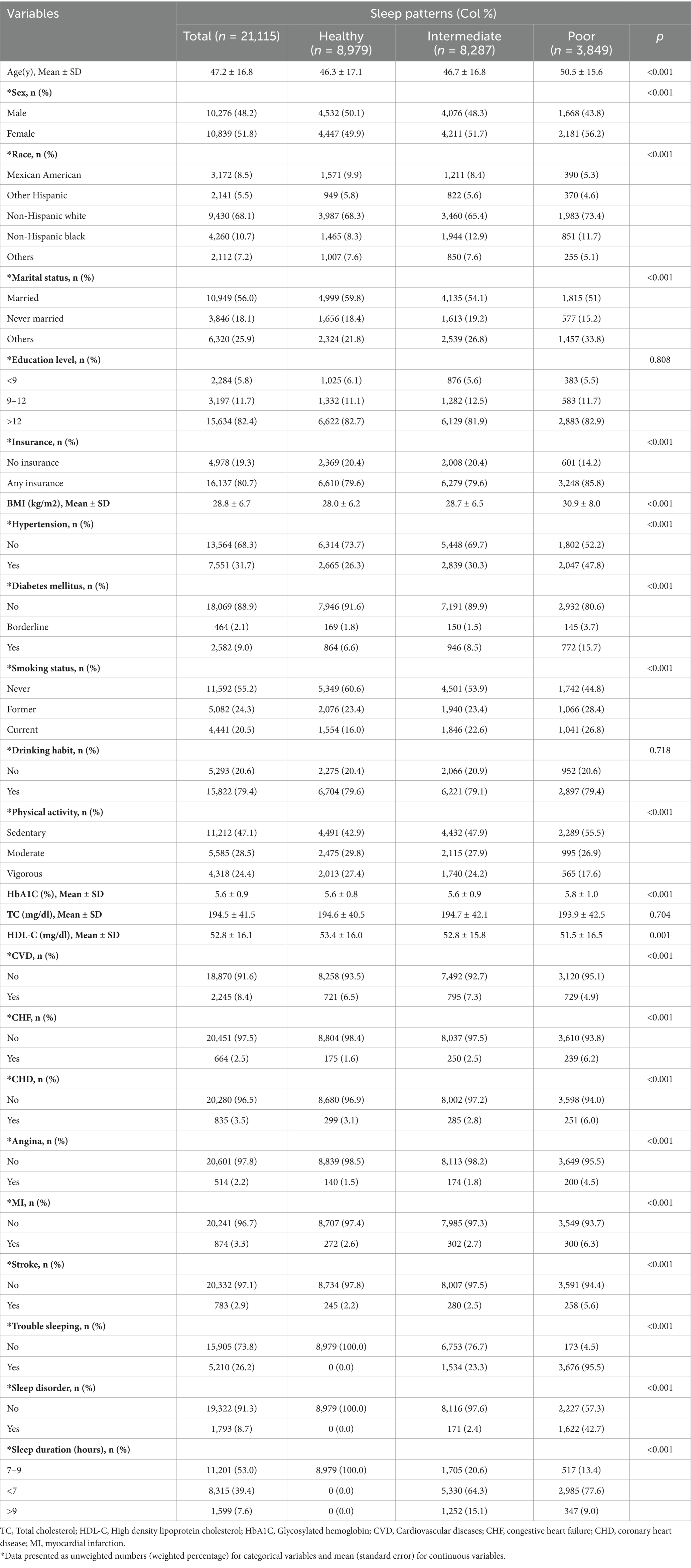
Table 1 displays the baseline characteristics of the participants in the study, which were categorized according to their sleep patterns. According to research, individuals with disrupted sleep patterns seem to have a greater likelihood of developing CVD. Among the 21,115 participants, 48.2% were male, with an average age (SD) of 47.2 (16.8) years. According to the data, 42.5% of individuals exhibited healthy sleep patterns, while 39.3 and 18.2% had intermediate and poor sleep patterns, respectively. It was observed that individuals with healthy sleep patterns tended to be younger, have a lower BMI, and were more likely to be male, married, more physically active (vigorous), and non-smokers. There were no statistically significant differences in education level, drinking habit, and TC subgroups.
3.2 Relationship between sleep and CVD
Univariate analysis showed that all covariates, except race, were associated with CVD (Supplementary Table S1).
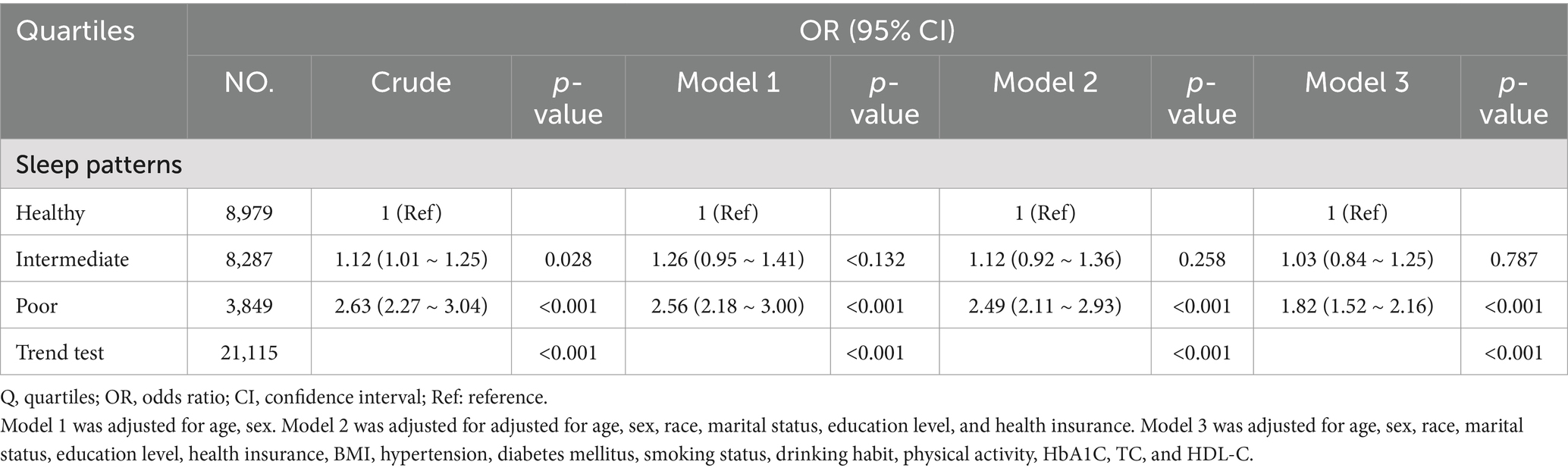
Upon categorizing sleep patterns into three levels, healthy, intermediate, and poor, we observed a noteworthy positive correlation between sleep patterns and CVD, even after accounting for potential confounding factors. In comparison to those with a healthy sleep pattern, the adjusted OR for CVD risk in individuals with intermediate and poor sleep patterns were 1.03 (95% CI: 0.84 ~ 1.25, p = 0.787) and 1.82 (95% CI: 1.52 ~ 2.16, p < 0.001), respectively (Table 2).
To assess potential modifications in the connection between sleep patterns and CVD, stratified analyses were conducted in multiple subgroups, including the variables: age (20–60y vs. >60y), sex (male vs. female), insurance (no vs. yes), smoking status (never vs. former or current), and physical activity (sedentary vs. moderate or vigorous). There is a significant interaction between sleep patterns and age (P for interaction = 0.002) shown in Figure 2.
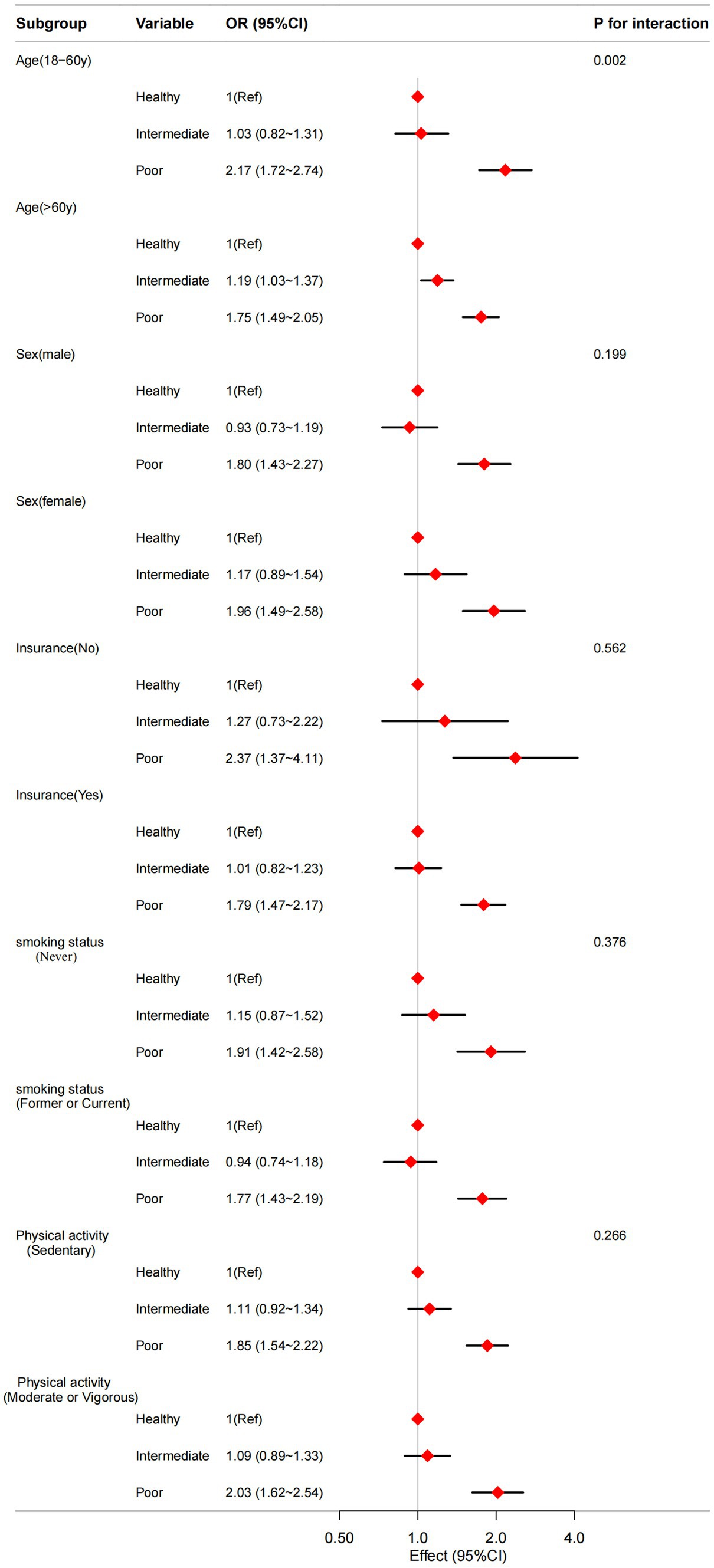
Figure 2. The relationship between sleep patterns and CVD according to basic features. Except for the stratification component itself, each stratification factor was adjusted for all other variables (age, sex, race, marital status, education level, health insurance, BMI, hypertension, diabetes mellitus, smoking status, drinking habit, physical activity, HbA1C, TC, and HDL-C).
We conducted multivariable analysis to examine the associations between each of the three elements (sleep duration, self-reported trouble sleeping, and diagnosed sleep disorder) within sleep patterns and CVD. In sleep duration, compared with individuals with normal sleep duration, the adjusted OR values in short and long sleep duration were 1.12 (95%CI: 0.95–1.33, p = 0.174), and 1.14 (95%CI:0.90–1.45, p = 0.266) (Supplementary Table S2). In self-reported trouble sleeping, compared with individuals with no trouble sleeping, the adjusted OR values in trouble sleeping were 1.53 (95%CI: 1.32–1.78, p < 0.001) (Supplementary Table S3). In diagnosed sleep disorder, compared with individuals with no sleep disorder, the adjusted OR values in sleep disorder were 2.09 (95%CI: 1.75–2.50, p < 0.001) (Supplementary Table S4). Additionally, a significant U-shaped curve (nonlinear, p < 0.001) was observed in the correlation between sleep duration and CVD in the restricted cubic spline (RCS) analysis (Supplementary Figure S1).
As shown in Supplementary Table S5, following the adjustment for all covariates within our full model (Model 3), it was found that poor sleep patterns exhibited a positive correlation with all subtypes of CVD risk in comparison to healthy sleep patterns (all p < 0.05).
4 Discussion
In this nationally representative study, a clear correlation was found between sleep factors and increased risk of cardiovascular disease. Moreover, a positive dose–response relationship was observed, indicating that the risk of CVD increased with poorer sleep patterns. Intermediate and poor sleep patterns were associated with a 3 and 82% higher incidence of CVD, respectively, compared to participants with healthy sleep patterns.
There’s some debate about the relationship between sleep duration and the risk of cardiovascular diseases. In three cohort studies (Wang et al., 2016; Yang et al., 2016; Song et al., 2017) involving Chinese populations, both cardiovascular diseases (CHD and atrial fibrillation) had been reported to have a relationship with variations in sleep duration. Similarly, sleep durations that are both short and long have been connected with increased risks of stroke morbidity and mortality (Brunetti et al., 2022). However, it is worth noting that individuals who sleep for extended periods are more likely to experience negative consequences than those who sleep for shorter durations (Wang et al., 2022). In a cross-sectional (Chen et al., 2022) study utilizing the NHANES database, it was discovered that sleep duration exhibited a significant relationship with an increased incidence of chest pain. Both short and long sleep durations were found to heighten the risk of chest pain, while the optimal quantity of sleep to mitigate this risk was approximately 6.5 h. However, a few studies have reported inconsistent results. In a study of the British population by Wannamethee et al. (2016), only patients with short sleep durations were found to be positively associated with the risk of hypertension, coronary artery calcification, and heart failure. A meta-analysis (Cappuccio et al., 2011) found that only long sleep duration was positively associated with the risk of CVD. In a study conducted by Song et al. (2016) there was no reported association between myocardial infarction and abnormal sleep duration. In our study, neither short nor long sleep durations were positively correlated with CVD risk. These discrepancies may be attributed to the inconsistent definitions of normal sleep duration, as well as the varying methods used to assess sleep duration and the diversity of study populations. This underscores the need for further research to establish the optimal sleep duration and to standardize the measurement methods for sleep duration.
Wang et al.’s study (Wang et al., 2020) did not identify an association between sleep duration and CVD, however, a comprehensive examination of the relationship between short sleep duration, insomnia/poor sleep, and cardiovascular disease did reveal such an association. In several previous studies (Bertisch et al., 2018; Chandola et al., 2010; Chien et al., 2010) on combinations of sleep factors, it was found that participants with short or long sleep durations, frequent symptoms of insomnia, as well as snoring and daytime sleepiness, had the highest risk of cardiovascular disease and were associated with all-cause mortality. This finding suggests that, in addition to sleep duration, altered sleep quality (insomnia/poor sleep) may also influence CVD. Hence, a composite of sleep factors may offer a more precise depiction of this relationship. In light of this, we introduced a novel metric, the healthy sleep score, which considers the combined impacts of three sleep behaviors (Li and Shang, 2021) (sleep duration, sleep disorders, and trouble sleeping) on the risk of CVD. This score captures a more holistic view of the sleep patterns. The healthy sleep pattern, as defined in our study (7–9 h of sleep per day, absence of sleep disorders and no reported trouble sleeping), establishes a favorable benchmark for sleep, serving as a useful reference to identify populations at risk and promote health management. From a public health standpoint, a straightforward scoring algorithm enhances the interpretability of epidemiological findings, facilitating their translation into practical insights for the general population. In an observational study by Fan et al. (2020), which also used sleep scores to assess their effect on CVD, it was concluded that healthy sleep patterns reduced the risk of CVD, CHD, and stroke in participants; however, this study was only conducted on a European population, and our study complements the U.S. population.
The results of our subgroup analyses revealed an interaction between sleep patterns and CVD risk across different age groups, indicating that the impact of sleep patterns on cardiovascular health varies by age. Among individuals under 60 years of age, poorer sleep patterns were linked to a significantly increased risk of CVD. This finding aligns with existing literature (Cappuccio et al., 2011), suggesting that inadequate sleep habits can adversely affect cardiovascular health even in younger adults. The relationship between sleep patterns and CVD risk appears to become more complex with advancing age. In individuals aged 60 and older, poorer sleep patterns and intermediate sleep patterns were associated with an increased risk of CVD, potentially reflecting the cumulative effects of chronic sleep disturbances on the cardiovascular system (Laksono et al., 2022).
The various mechanisms underlying the relationship between sleep and the onset of CVD are remain unclear at present. These Include dysregulation of the hypothalamus-pituitary–adrenal (HPA) axis (Grandner et al., 2016), abnormalities in autonomic nervous system regulation (Johnson et al., 2021), increased sympathetic nervous system (SNS) activity (Parthasarathy et al., 2015; Seravalle et al., 2018), insulin resistance (Kline et al., 2018), changes in inflammatory and hormonal markers (due to increased secretion of pro-inflammatory cytokines or an inverse correlation with the ratio of vascular collagen elastin proteins) (D'Antono and Bouchard, 2019), and impaired endothelial function (De Nys et al., 2022). Hong et al. (2023) found that fibrinogen may be a mechanism for the development of CHD in participants with long sleep duration, although the exact mechanism is unknown, and may be related to depressive symptoms, low socioeconomic status, unemployment, and low physical activity. A meta-analysis (Irwin et al., 2016) conducted on cohort studies discovered that sleep disruption and extended sleep durations (as opposed to insufficient sleep) were linked to increased levels of systemic inflammatory biomarkers such as CRP and IL-6, which may contribute to the higher incidence of CVD. Several biological mechanisms have been suggested for sleep disorders that can affect the regulation of the central nervous system (Tamisier et al., 2018), hemodynamics (Yang et al., 2019), ventilatory function (Chowdhuri and Badr, 2017), and biorhythms (Makarem et al., 2019), leading to altered cardiac physiology and pathological changes in blood pressure.
In addition to sleep patterns, dietary habits represent another critical factor influencing CVD risk. Specifically, a diet abundant in fruits, vegetables, whole grains, nuts, and fish, while limiting the intake of processed foods, as well as foods high in salt and sugar, has been demonstrated to lower CVD risk (Ghaedi et al., 2019). Moreover, the Mediterranean dietary pattern, characterized by high olive oil consumption and moderate alcohol intake, is linked to reduced morbidity and mortality associated with cardiovascular diseases (Estruch et al., 2018). Therefore, future research should aim to incorporate dietary factors into their analyses to facilitate a more comprehensive assessment of cardiovascular disease risk.
The study boasts several strengths, notably the utilization of a substantial and nationally representative sample from the NHANES 2007–2014 database, offering an extensive array of potential confounders for analysis. In addition, by consolidating sleep quality and quantity (including sleep duration, self-reported sleep difficulties, and sleep disorders) into a unified sleep metric, we comprehensively address the intricate nature of sleep. Nevertheless, certain limitations are acknowledged in this study. Firstly, given its cross-sectional design, causal relationships between sleep and CVD cannot be established. Secondly, reliance on self-reported sleep data introduces potential memory bias, and sleep duration information is confined to weekdays, omitting details on shift work and weekend sleep duration. Ambiguities in the categorization of self-reported sleep disorders preclude a precise examination of their association with CVD. Thirdly, despite employing regression models and stratification analyses, we cannot exclude the possibility that the observed associations are due to unmeasured confounders. Fourthly, the current findings stem from surveys conducted among U.S. adults, and further research is needed to determine generalizability to other populations. It is imperative to recognize the observational nature of this study, warranting cautious interpretations of the results. Future clinical trials on sleep interventions are imperative to discern the causal nature of the observed associations.
5 Conclusion
Participants who exhibit poor sleep patterns have been found to be at a significantly higher risk for developing CVD. Sleep, as a modifiable behavioral factor, plays a crucial role in the prevention of primary cardiovascular disease. To establish a causal relationship between sleep and cardiovascular disease, further prospective studies are necessary.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YW: Data curation, Methodology, Resources, Software, Writing – original draft, Formal analysis. ZL: Conceptualization, Writing – review & editing, Writing – original draft. PZ: Data curation, Investigation, Writing – review & editing. JX: Formal analysis, Writing – review & editing. MY: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We gratefully thank Dr. Jie Liu of Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital for his contribution to the statistical support, study deign consultations and comments regarding the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2024.1447543/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Non-linear relationship of sleep duration and CVD. Solid and dashed lines represent the predicted value and 95% confidence intervals. They were adjusted for age, sex, race, marital status, education level, health insurance, BMI, hypertension, diabetes mellitus, smoking status, drinking habit, physical activity, HbA1C, TC, and HDL-C. All of the data is shown.
References
Bertisch, S. M., Pollock, B. D., Mittleman, M. A., Buysse, D. J., Bazzano, L. A., Gottlieb, D. J., et al. (2018). Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: sleep heart health study. Sleep 41:zsy047. doi: 10.1093/sleep/zsy047
Brunetti, V., Rollo, E., Broccolini, A., Frisullo, G., Scala, I., and Della, M. G. (2022). Sleep and stroke: opening our eyes to current knowledge of a key relationship. Curr. Neurol. Neurosci. Rep. 22, 767–779. doi: 10.1007/s11910-022-01234-2
Cai, X., Zhang, Y., Li, M., Wu, J. H., Mai, L., Li, J., et al. (2020). Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ 370:m2297. doi: 10.1136/bmj.m2297
Cappuccio, F. P., Cooper, D., D'Elia, L., Strazzullo, P., and Miller, M. A. (2011). Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur. Heart J. 32, 1484–1492. doi: 10.1093/eurheartj/ehr007
Chandola, T., Ferrie, J. E., Perski, A., Akbaraly, T., and Marmot, M. G. (2010). The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: a prospective study from the Whitehall II cohort. Sleep 33, 739–744. doi: 10.1093/sleep/33.6.739
Chen, W., Wang, J. P., Wang, Z. M., Hu, P. C., and Chen, Y. (2022). Association between sleep duration and chest pain in US adults: a cross-sectional study. Front. Public Health 10:952075. doi: 10.3389/fpubh.2022.952075
Chien, K. L., Chen, P. C., Hsu, H. C., Su, T. C., Sung, F. C., Chen, M. F., et al. (2010). Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep 33, 177–184. doi: 10.1093/sleep/33.2.177
Chowdhuri, S., and Badr, M. S. (2017). Control of ventilation in health and disease. Chest 151, 917–929. doi: 10.1016/j.chest.2016.12.002
D'Antono, B., and Bouchard, V. (2019). Impaired sleep quality is associated with concurrent elevations in inflammatory markers: are post-menopausal women at greater risk. Biol. Sex Differ. 10:34. doi: 10.1186/s13293-019-0250-x
De Nys, L., Anderson, K., Ofosu, E. F., Ryde, G. C., Connelly, J., and Whittaker, A. C. (2022). The effects of physical activity on cortisol and sleep: a systematic review and meta-analysis. Psychoneuroendocrinology 143:105843. doi: 10.1016/j.psyneuen.2022.105843
Estruch, R., Ros, E., Salas-Salvadó, J., Covas, M. I., Corella, D., Arós, F., et al. (2018). Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 378:e34. doi: 10.1056/NEJMoa1800389
Fan, M., Sun, D., Zhou, T., Heianza, Y., Lv, J., Li, L., et al. (2020). Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur. Heart J. 41, 1182–1189. doi: 10.1093/eurheartj/ehz849
Fleming, M., McLay, J. S., Clark, D., King, A., Mackay, D., Minnis, H., et al. (2021). Educational and health outcomes of schoolchildren in local authority care in Scotland: a retrospective record linkage study. PLoS Med. 18:e1003832. doi: 10.1371/journal.pmed.1003832
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet 388, 1545–1602. doi: 10.1016/S0140-6736(16)31678-6
Ghaedi, E., Mohammadi, M., Mohammadi, H., Ramezani-Jolfaie, N., Malekzadeh, J., Hosseinzadeh, M., et al. (2019). Effects of a Paleolithic diet on cardiovascular disease risk factors: a systematic review and Meta-analysis of randomized controlled trials. Adv. Nutr. 10, 634–646. doi: 10.1093/advances/nmz007
Grandner, M. A., Alfonso-Miller, P., Fernandez-Mendoza, J., Shetty, S., Shenoy, S., and Combs, D. (2016). Sleep: important considerations for the prevention of cardiovascular disease. Curr. Opin. Cardiol. 31, 551–565. doi: 10.1097/HCO.0000000000000324
Gurgel, M., Reijneveld, S., Geboers, B., Navis, G., and Winter, A. (2021). Low health literacy is associated with the onset of CKD during the life course. J. Am. Soc. Nephrol. 32, 1436–1443. doi: 10.1681/ASN.2020081155
Hamad, R., Nguyen, T. T., Bhattacharya, J., Glymour, M. M., and Rehkopf, D. H. (2019). Educational attainment and cardiovascular disease in the United States: a quasi-experimental instrumental variables analysis. PLoS Med. 16:e1002834. doi: 10.1371/journal.pmed.1002834
Hong, C., Zhu, H., Zhou, X., Zhai, X., Li, S., Ma, W., et al. (2023). Association of Blood Urea Nitrogen with cardiovascular diseases and all-cause mortality in USA adults: results from NHANES 1999-2006. Nutrients 15:461. doi: 10.3390/nu15020461
Irwin, M. R., Olmstead, R., and Carroll, J. E. (2016). Sleep disturbance, sleep duration, and inflammation: a systematic review and Meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry 80, 40–52. doi: 10.1016/j.biopsych.2015.05.014
Johnson, K. A., Gordon, C. J., Chapman, J. L., Hoyos, C. M., Marshall, N. S., Miller, C. B., et al. (2021). The association of insomnia disorder characterised by objective short sleep duration with hypertension, diabetes and body mass index: a systematic review and meta-analysis. Sleep Med. Rev. 59:101456. doi: 10.1016/j.smrv.2021.101456
Johnson, C. L., Paulose-Ram, R., Ogden, C. L., Carroll, M. D., Kruszon-Moran, D., Dohrmann, S. M., et al. (2013). National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat., 1–24.
Joseph, P., Leong, D., McKee, M., Anand, S. S., Schwalm, J. D., Teo, K., et al. (2017). Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ. Res. 121, 677–694. doi: 10.1161/CIRCRESAHA.117.308903
Kizilkilic, S. E., Falter, M., and Dendale, P. (2023). The power of movement: how physical activity can mitigate the risks of inadequate sleep. Eur. J. Prev. Cardiol. 30, 830–831. doi: 10.1093/eurjpc/zwad121
Kline, C. E., Hall, M. H., Buysse, D. J., Earnest, C. P., and Church, T. S. (2018). Poor sleep quality is associated with insulin resistance in postmenopausal women with and without metabolic syndrome. Metab. Syndr. Relat. Disord. 16, 183–189. doi: 10.1089/met.2018.0013
Laksono, S., Yanni, M., Iqbal, M., and Prawara, A. S. (2022). Abnormal sleep duration as predictor for cardiovascular diseases: a systematic review of prospective studies. Sleep Disord. 2022, 1–10. doi: 10.1155/2022/9969107
Li, C., and Shang, S. (2021). Relationship between sleep and hypertension: findings from the NHANES (2007-2014). Int. J. Environ. Res. Public Health 18:7867. doi: 10.3390/ijerph18157867
Li, X., Xue, Q., Wang, M., Zhou, T., Ma, H., Heianza, Y., et al. (2021). Adherence to a healthy sleep pattern and incident heart failure: a prospective study of 408 802 UK biobank participants. Circulation 143, 97–99. doi: 10.1161/CIRCULATIONAHA.120.050792
Liu, H., Wang, L., Chen, C., Dong, Z., and Yu, S. (2022). Association between dietary niacin intake and migraine among American adults: National Health and nutrition examination survey. Nutrients 14:3052. doi: 10.3390/nu14153052
Makarem, N., Shechter, A., Carnethon, M. R., Mullington, J. M., Hall, M. H., and Abdalla, M. (2019). Sleep duration and blood pressure: recent advances and future directions. Curr. Hypertens. Rep. 21:33. doi: 10.1007/s11906-019-0938-7
Miller, M. A., and Howarth, N. E. (2023). Sleep and cardiovascular disease. Emerg. Top. Life Sci. 7, 457–466. doi: 10.1042/ETLS20230111
Panza, J. A., Epstein, S. E., and Quyyumi, A. A. (1991). Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N. Engl. J. Med. 325, 986–990. doi: 10.1056/NEJM199110033251402
Parthasarathy, S., Vasquez, M. M., Halonen, M., Bootzin, R., Quan, S. F., Martinez, F. D., et al. (2015). Persistent insomnia is associated with mortality risk. Am. J. Med. 128, 268–75.e2. doi: 10.1016/j.amjmed.2014.10.015
Saz-Lara, A., Lucerón-Lucas-Torres, M., Mesas, A. E., Notario-Pacheco, B., López-Gil, J. F., and Cavero-Redondo, I. (2022). Association between sleep duration and sleep quality with arterial stiffness: a systematic review and meta-analysis. Sleep Health 8, 663–670. doi: 10.1016/j.sleh.2022.07.001
Seravalle, G., Mancia, G., and Grassi, G. (2018). Sympathetic nervous system, sleep, and hypertension. Curr. Hypertens. Rep. 20:74. doi: 10.1007/s11906-018-0874-y
Song, Q., Liu, X., Hu, W., Zhou, W., Liu, A., Wang, X., et al. (2017). Long sleep duration is an independent risk factor for incident atrial fibrillation in a Chinese population: a prospective cohort study. Sci. Rep. 7:3679. doi: 10.1038/s41598-017-04034-8
Song, Q., Liu, X., Wang, X., and Wu, S. (2016). Age-and gender-specific associations between sleep duration and incident hypertension in a Chinese population: the Kailuan study. J. Hum. Hypertens. 30, 503–507. doi: 10.1038/jhh.2015.118
Tamisier, R., Weiss, J. W., and Pépin, J. L. (2018). Sleep biology updates: hemodynamic and autonomic control in sleep disorders. Metabolism 84, 3–10. doi: 10.1016/j.metabol.2018.03.012
Wang, X., Liu, X., Song, Q., and Wu, S. (2016). Sleep duration and risk of myocardial infarction and all-cause death in a Chinese population: the Kailuan study. Sleep Med. 19, 13–16. doi: 10.1016/j.sleep.2015.09.027
Wang, H., Sun, J., Sun, M., Liu, N., and Wang, M. (2022). Relationship of sleep duration with the risk of stroke incidence and stroke mortality: an updated systematic review and dose-response meta-analysis of prospective cohort studies. Sleep Med. 90, 267–278. doi: 10.1016/j.sleep.2021.11.001
Wang, Y. H., Wang, J., Chen, S. H., Li, J. Q., Lu, Q. D., Vitiello, M. V., et al. (2020). Association of Longitudinal Patterns of habitual sleep duration with risk of cardiovascular events and all-cause mortality. JAMA Netw. Open 3:e205246. doi: 10.1001/jamanetworkopen.2020.5246
Wannamethee, S. G., Papacosta, O., Lennon, L., and Whincup, P. H. (2016). Self-reported sleep duration, napping, and incident heart failure: prospective associations in the British regional heart study. J. Am. Geriatr. Soc. 64, 1845–1850. doi: 10.1111/jgs.14255
WHO. (2021). Cardiovascular diseases (CVDs). Available at: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
Xiao, Y., and Xiao, Z. (2023). Association between serum klotho and kidney stones in US middle-aged and older individuals with diabetes mellitus: results from 2007 to 2016 National Health and nutrition survey. Am. J. Nephrol. 54, 224–233. doi: 10.1159/000531045
Yang, H., Liao, Z., Zhou, Y., Gao, Z., and Mao, Y. (2024). Non-linear relationship of serum albumin-to-globulin ratio and cognitive function in American older people: a cross-sectional national health and nutrition examination survey 2011-2014 (NHANES) study. Front. Public Health 12:1375379. doi: 10.3389/fpubh.2024.1375379
Yang, D., Rundek, T., Patel, S. R., Cabral, D., Redline, S., Testai, F. D., et al. (2019). Cerebral hemodynamics in sleep apnea and Actigraphy-determined sleep duration in a sample of the Hispanic community health study/ study of Latinos. J. Clin. Sleep Med. 15, 15–21. doi: 10.5664/jcsm.7560
Yang, L., Yang, H., He, M., Pan, A., Li, X., Min, X., et al. (2016). Longer sleep duration and midday napping are associated with a higher risk of CHD incidence in middle-aged and older Chinese: the Dongfeng-Tongji cohort study. Sleep 39, 645–652. doi: 10.5665/sleep.5544
Keywords: sleep disorders, trouble sleeping, cardiovascular disease, association, clinical epidemiology
Citation: Wu Y, Li Z, Zhao P, Xu J and Yuan M (2025) Sleep patterns and cardiovascular disease risk in US participants: a comprehensive analysis. Front. Neurosci. 18:1447543. doi: 10.3389/fnins.2024.1447543
Edited by:
Eleonora Rollo, Catholic University of the Sacred Heart, Rome, ItalyReviewed by:
Dorela Doris Shuboni-Mulligan, Eastern Virginia Medical School, United StatesEmyr Reisha Isaura, Airlangga University, Indonesia
Yan Zhuang, The Second Affiliated Hospital of Xi’an Jiaotong University, China
Copyright © 2025 Wu, Li, Zhao, Xu and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Yuan, eXVhbm1pbjIwMTMxNEBzaW5hLmNvbQ==; eXVhbm1pbkBuY21jLmVkdS5jbg==
 Yue Wu1
Yue Wu1 Min Yuan
Min Yuan