
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 23 July 2024
Sec. Neuropharmacology
Volume 18 - 2024 | https://doi.org/10.3389/fnins.2024.1430447
 Kyle Richard Gossman1
Kyle Richard Gossman1 Camryn Serra Lowe1
Camryn Serra Lowe1 Adrianna Kirckof1,2
Adrianna Kirckof1,2 Sydney Vanmeerhaeghe1
Sydney Vanmeerhaeghe1 Adam Steven Smith1,2*
Adam Steven Smith1,2*Introduction: The mesolimbic reward system is associated with the promotion and rewarding benefits of social relationships. In the socially monogamous prairie vole (Microtus ochrogaster), the establishment of a pair bond can be displayed by a robust preference for a breeding partner and aggressive rejection of unfamiliar conspecifics. Mesolimbic dopamine signaling influences bond-related behaviors within the vole through dopamine transmission and receptor activity in the nucleus accumbens. However, only one experiment has examined how the ventral tegmental area (VTA), a region that produces much of the fore- and mid-brain dopamine, regulates these social behaviors. Specifically, inhibition of either glutamate or GABA neurons in the VTA during a brief courtship promoted a partner preference formation in male prairie voles. The VTA is a heterogeneous structure that contains dopamine, GABA, and glutamate neurons as well as receives a variety of projections including corticotropin-releasing factor (CRF) suggested to modulate dopamine release.
Methods: We used pharmacological manipulation to examine how GABA and CRF signaling in the VTA modulate partner preference formation in male and female prairie voles. Specifically, we used a 3 h partner preference test, a social choice test, to assess the formation of a partner preference following an infused bicuculline and CRF during a 1 h cohabitation and muscimol and CP154526, a CRFR1 antagonist, during a 24 h cohabitation with an opposite-sex conspecific.
Results: Our study demonstrated that bicuculline, a GABAA receptor antagonist, and CRF in the VTA promoted a partner preference, whereas low-dose muscimol, a GABAA receptor agonist, and CP154526, a CRFR1 antagonist, inhibited a partner preference in both male and female prairie voles.
Conclusion: This study demonstrated that GABA and CRF inputs into the VTA is necessary for the formation of a partner preference in male and female prairie voles.
The functional significance of social attachment in humans, including pair bonding between partners and parental care toward offspring, has been documented cross-culturally (Jankowiak and Fischer, 1992; Young et al., 2011). Paired individuals, particularly those in stable marital relationships, have been shown to live longer compared to their unpaired counterparts, a finding noted across demographic groups (House et al., 1988; Lillard and Waite, 1995). High levels of intimacy between pairs have also been correlated inversely with negative psychological states, such as depressed moods, and positively with immune function and cardiovascular health (Waltz et al., 1988; Kiecolt-Glaser and Newton, 2001). Thus, intimate relationships represent significant aspects of our social world (Yamaguchi et al., 2015). It is critical that social partners display “commitment” through context-appropriate behaviors, such as selective affiliation to a current partner and rejection (or aggression in some species) of potential partners. Classically, the mesolimbic reward system has been associated with motivation and assigning salience to situational cues. In addition, this network has been shown to regulate the rewarding aspects of social attachment (Aragona and Wang, 2009). Recent functional connectivity research has also demonstrated that the regions of the mesolimbic reward system cluster together or show similar neural activation with one another when interacting with a partner, which is lost when interacting with a stranger conspecific (Ortiz et al., 2018; Gossman et al., 2021). However, research studying social attachment mainly focuses on the activity of the anterior cingulate cortex and nucleus accumbens, which use the neurotransmitter dopamine to control the formation of attachment, reward, and processing of emotions (Nestler and Carlezon, 2006; Baik, 2013; Hostetler et al., 2017; Yin et al., 2018; Horie et al., 2020). Surprisingly, few studies have examined the role of the ventral tegmental area (VTA), a dopamine-rich source hub for the mesolimbic reward system, in regulating social attachment. However, models that form these types of social attachments or pair bonds, as well as display distinct social behaviors associated with these bonds are limited in animal research.
The prairie vole (Microtus ochrogaster) is a socially monogamous rodent that forms long-term social attachments or pair bonds between breeding pairs, thus providing a unique model to characterize the role the VTA has in the regulation of pair bond formation. The vole model has been utilized for more than three decades to study the neurobiology of pair bonding, which has led to well-defined behavioral characteristics of pair bonding and much of the current knowledge of neuromechanisms of these behaviors (Smith et al., 2013; Tickerhoof and Smith, 2017). Specifically, dopamine signaling in the nucleus accumbens has been shown to regulate pair bond-associated behaviors, such that activation of dopamine receptor 2 regulates pair bond formation (i.e., partner preference) and dopamine receptor 1 regulates pair bond maintenance (i.e., aggression) (Gingrich et al., 2000; Aragona et al., 2006). Although dopamine has been well established in the regulation of pair bond formation, there is currently only one study that has focused on the VTA, a dopamine-rich nucleus of the mesolimbic system, during pair bond formation in prairie voles (Curtis and Wang, 2005). This study showed that inhibition of either glutamate or GABA neurons promotes a partner preference formation in male prairie voles after a 6 h cohabitation, a length of time insufficient for male voles to form a partner preference (Curtis and Wang, 2005). This study suggests a local circuit within the VTA—glutamate neurons project to and stimulate GABA neurons which project to and inhibit dopamine neurons. Thus, inhibition of glutamate or GABA could disinhibit dopamine neurons allowing for greater VTA dopamine release to downstream target sites. While the VTA is a heterogeneous structure that contains glutamate and GABA neurons that modulate dopamine neurons, the VTA also receives a variety of inputs such as corticotrophin-releasing factor (CRF) that are suggested to stimulate dopamine release (Morales and Margolis, 2017; Cai and Tong, 2022). Specifically, it has been estimated that there is CRHR1 on approximately 98% of dopamine-expressing neurons in the VTA (Refojo et al., 2011; Zalachoras et al., 2022). Thus far, one study demonstrated that the CRF into the nucleus accumbens accelerated a partner preference in male prairie voles (Lim et al., 2007); however, it has yet to be shown if CRF into the VTA has an influence on pair bond formation and maintenance in prairie voles. Thus, this study looks to further our understanding of how the local circuit and neurochemical inputs into the VTA influence partner preference formation in prairie voles. Here, we use pharmacological manipulation in the VTA to assess how GABA and CRF modulate partner preference in male and female prairie voles.
Male and female subjects were captive-bred descendants from populations captured in southern Illinois. Voles were weaned on postnatal day 21 and housed with a non-sibling, same-sex conspecific in cages (29.2 L × 19.1 W × 12.7 H cm) which contained corn cob bedding and crinkle paper nesting material. Voles had access to food (LabDiet Rabbit Diet 5321) and water ad libitum. Colony rooms were kept on a 14L:10D photoperiod (Lights on at 0600 h) and at a temperature range of 21 ± 1°C. The subjects were sexually naïve, 60–90 days old at the beginning of the experiment. All procedures were conducted in accordance with the National Institutes of Health Guide and Use of Laboratory Animals and the Institutional Animal Care and Use Committee and the University of Kansas.
One week prior to pairing with an opposite-sex conspecific, subjects were anesthetized with an intraperitoneal injection of ketamine/dexmedetomidine cocktail (75/1 mg/kg) in sterile saline. Subjects were then head-fixed into a stereotaxic instrument (Stoelting) and a small incision was made to expose the skull. The skull was leveled using bregma and lambda as reference points. Once the head was aligned, holes were drilled, bilaterally, to target the VTA (ML: ± 0.79, DV: −4.91, AP: −3.20). Stereotaxic coordinates were validated with the use of the Allen Mouse Brain Atlas. Guide cannulae (26-gauge, Cat #: 62129, RWD Life Science) were fixed to the skull with a surgical screw, ethyl-2-cyanoacrylate-based adhesive, and dental cement. Once, the dental cement was set, a dummy cannula (RWD Life Scienc)e was placed in the guide cannula, and a cap was placed over the top of the dummy and guide cannulae (RWD Life Science).
The drugs used in this experiment included bicuculline (GABAA receptor antagonist, Cat #: ALX-550-515-M050, Lot #: 03072218, Enzo), muscimol (GABAA receptor agonist, Cat #: 2763-96-4, Lot #: 3938537, EMD Millipore), CRF (Cat #: C3042, Lot #: 166977, Sigma), and CP154526 (CRFR1 antagonist, Cat #: 2779, Lot #: 3B1265193, Tocris). All drugs were aliquoted in either ddH20 (bicuculline), artificial cerebrospinal fluid (aCSF; 150mM Na+, 3.0 mM K+, 1.4mM Ca2+, 0.8 mM Mg2+, 1.0mM P3–, 155mM Cl−) (CRF), or DMSO (0.025% or 0.0025% muscimol and 0.05% or 0.1% CP154526) at a 1 mg/ml concentration. On the day of testing, drug stocks were diluted to reach the appropriate working concentration in aCSF.
Thirty minutes prior to cohabitation, 200 nL aCSF containing no drug (aCSF), 5 ng bicuculline, 0.1 pg CRF, 5 ng (low dose) or 50 ng (high dose) muscimol, or 10 pg (low dose) or 100 pg (high dose) CP154526 was bilaterally injected into the VTA with a 1mm projection using a 33-gauge internal cannula (RWD Life Science) through the guide cannula. The rate of infusion was 200 nl/min, which was controlled with an infusion pump (KD Scientific). Following the infusion, the internal cannula was left for an additional minute before retracting it from the brain. After infusions, the subjects were returned to their home cage. The drug doses were selected based on previous literature using these drugs in prairie voles and other rodent models (Laviolette et al., 2004; Curtis and Wang, 2005; Lim et al., 2007; Iyilikci et al., 2016). Brains were harvested after behavioral testing for histological validation of cannula placement. Partial or complete histological misses were removed from the data analysis.
Thirty minutes after infusions, subjects were paired with a reproductively-sterilized, novel opposite-sex conspecific for either a 1 h or 24 h cohabitation (DeVries et al., 1995; Bales and Carter, 2003; Aragona and Wang, 2004). Female partners were estrogen primed with daily subcutaneous injection of estradiol benzoate at 1 μg/100 μL sesame oil for three consecutive days (Carter et al., 1988; Amadei et al., 2017; Gossman et al., 2021). Subjects that received either bicuculline or CRF were paired for 1 h, and subjects that received either muscimol or CP154526 were paired for 24 h. A partner preference test (PPT) was used, which is a well-documented behavioral assessment of pair bonding in prairie voles (Williams et al., 1992; Beery, 2021). In short, PPT utilizes a three-chambered arena (75 L x 20 W x 25 H cm) with a layer of corn cob bedding. The two stimulus animals, which include the partner of the subject and a novel opposite-sex conspecific, were tethered to the outer chambers. The female stimulus animals were estradiol benzoate primed as described above. The subject was restricted to the center chamber until the behavioral assessment began. Once the test began, the subject was allowed access to all chambers for a total of 3 h. Behavioral testing was completed between 1200 and 1600 h. Duration of stationary contact (i.e., side-by-side contact plus huddling) between the subject and either stimulus voles and the number of chamber crosses by the subject were recorded. All behavioral tests were manually scored by an experimenter blinded to experimental conditions using Solomon Coder (andraspeter.com).
Three-way mixed-model ANOVAs were used to assess the effects of drug condition, biological sex of subject, and social stimulus on stationary contact duration (side-by-side contact plus huddling) and chamber crosses during the PPT. Bonferroni post-hoc comparisons were used to determine significant differences between conditions when ANOVAs included significant main effects or interactions. Effects were considered significant at p < 0.05.
There were main effects for social choice (partner vs stranger, F(1,55) = 52.698, p < 0.001) and drug conditions (F(2,55) = 5.900, p < 0.005). Moreover, there was a significant interaction between social choice and drug conditions (F(2,55) = 8.651, p < 0.001). Specifically, subjects injected with bicuculline or CRF in the VTA before a 1 h cohabitation with a new partner spent an increased amount of time in stationary contact with their partner compared to a stranger vole (Figure 1A). However, control-treated voles did not differ in contact time with either stimulus vole. Also, overall stationary contact was higher in the bicuculline and CRF-treated voles compared to the voles in the control (aCSF) group (p < 0.001). Validation of guide cannula was confirmed for each subject in the pharmacological groups (Figures 1B, C). Lastly, the aCSF group had more total cage crosses compared to the drug-treated groups (F(2,58) = 6.164, p = 0.004; Figure 1D). This is likely due to the decrease in overall stationary contact time in this group compared to the drug conditions. There were no main effects or interactions with biological sex of the subject observed for any of the statistical analyses. Altogether, these data suggest that inhibition of GABAA receptors and activation of CRF receptors in the VTA was sufficient to promote a partner preference, as well as promote overall social interaction in both male and female prairie voles.
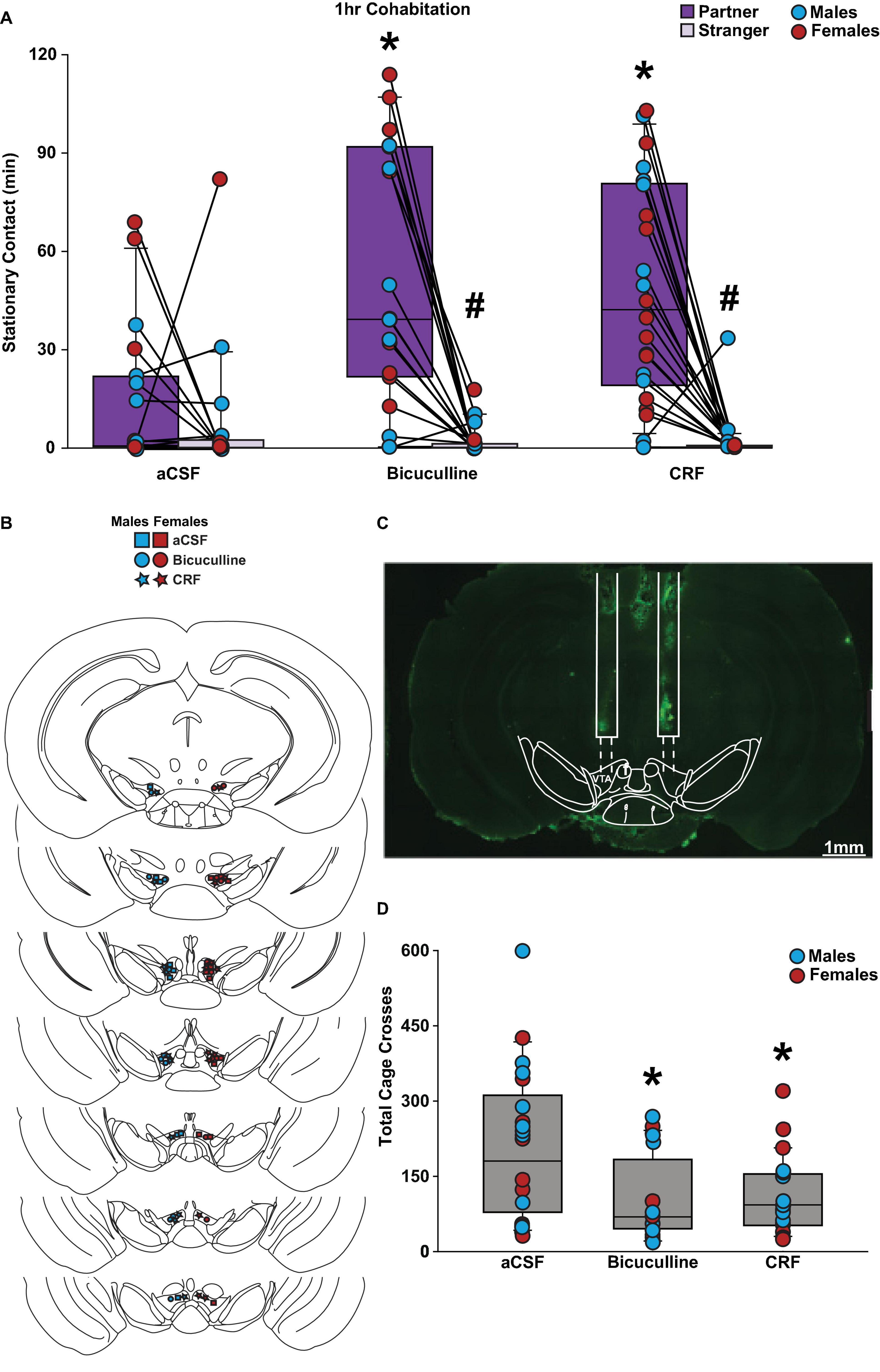
Figure 1. Bicuculline and CRF treatments promoted a partner preference in male and female prairie voles after a 1 h cohabitation. Stationary contact refers to the total of side-by-side and huddling. (A) Male and female voles infused with bicuculline (n = 9–10 per sex) and CRF (n = 10–12 per sex) demonstrated a significant preference toward their partner compared to a novel opposite-sex conspecific. Bicuculline and CRF groups exhibited significantly higher stationary contact time with their partner compared to the artificial cerebrospinal fluid (aCSF) (n = 9–11 per sex) group. (B) Placement of successful stereotactic hits that were included in the data analysis. (C) Representative image of cannula placement. (D) The aCSF group demonstrated an increase in total cage crosses comparted to both bicuculline and CRF groups. Three-way mixed-model ANOVA with Bonferroni post-hoc were used to analyze behavioral data. #p < 0.05 vs. social stimulus. *p < 0.05 vs. aCSF. No sex differences were observed.
To demonstrate that GABA and CRF in the VTA are sufficient and necessary for partner preference formation in prairie voles, we infused muscimol, a GABAA receptor agonist, or CP154526, a CRFR1 antagonist, into the VTA before a 24 h cohabitation with a new opposite-sex partner and a partner preference test was conducted after cohabitation. We observed main effects for social choice (partner vs. stranger, F(1,80) = 62.893, p < 0.001) and drug conditions (F(4,80) = 11.671, p < 0.001). There was a significant interaction between social choice and drug conditions (F(4,80) = 12.160, p < 0.001). Specifically, control voles formed a preference for the new partner followed 24 h cohabitation; however, low dose muscimol inhibited a partner preference in both male and female prairie voles, but high dose muscimol did not. In addition, both low and high doses of CP154526 inhibited partner preference formation in male and female prairie voles (Figure 2A). Placement of the guide cannula was confirmed for each male and female subject (Figure 2B). There was no main effect for biological sex of the subject. There were interactions between sex and social choice (F(1,80) = 7.091, p < 0.01) as well as sex and drug condition (F(4,80) = 4.041, p = 0.005). However, post-hoc analyses revealed no group differences for either interaction. Therefore, no sex differences were observed. There were no differences between total cage crosses (F(4,85) = 2.112, p = 0.086, Figure 2C).
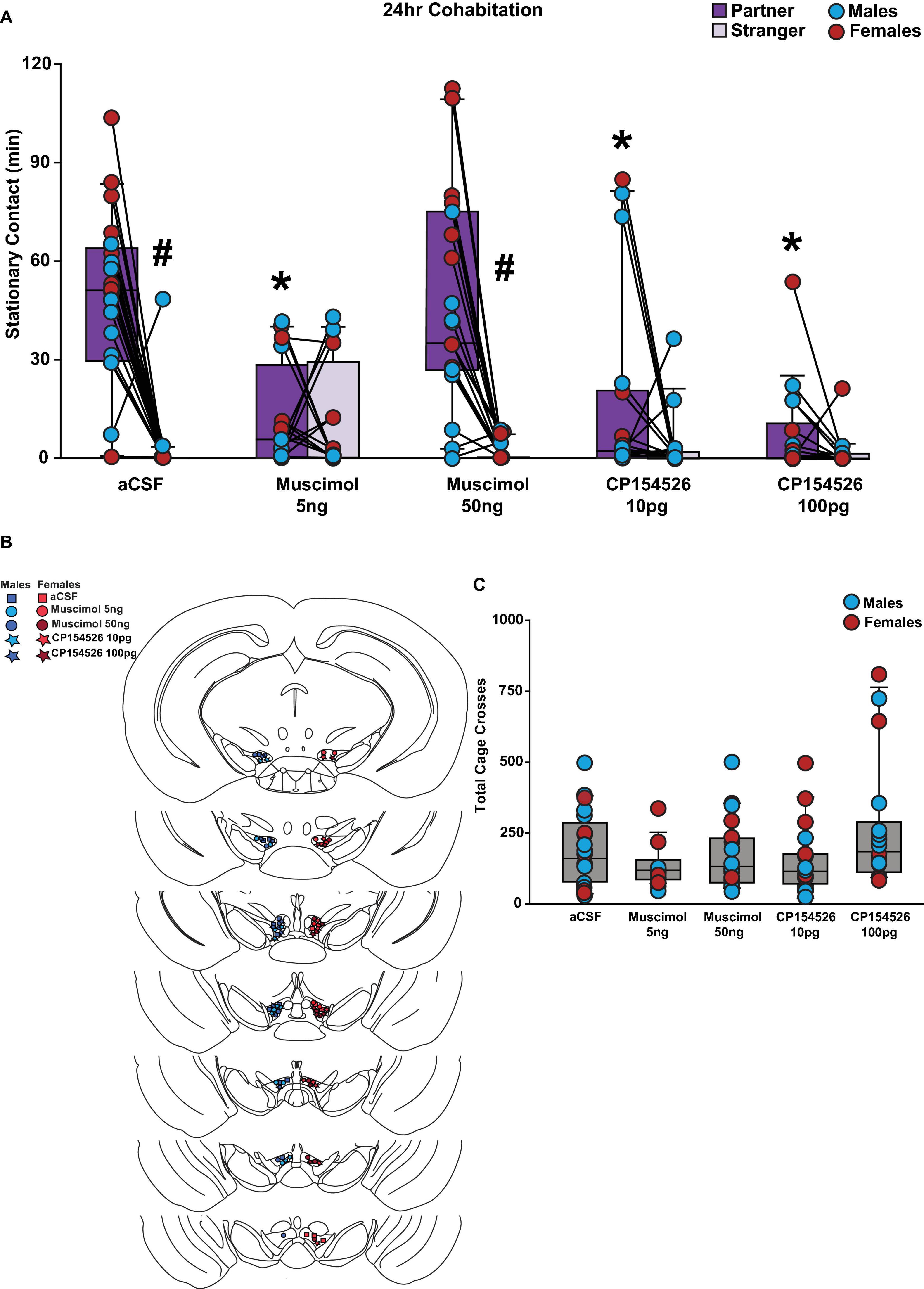
Figure 2. Low-dose muscimol and both low and high dose CP154526 inhibited a partner preference in male and female prairie voles after a 24 h cohabitation. Stationary contact refers to the total of side-by-side and huddling. (A) Male and female voles infused with low (5 ng) dose muscimol (n = 8 per sex) and low (10pg) or high (100pg) dose CP154526 (n = 8–10 per sex) did not exhibit a significant partner preference. However, subjects infused with high (50ng) dose muscimol (n = 9–10 per sex) or artificial cerebrospinal fluid (aCSF) (n = 9–11 per sex) demonstrated a significant preference toward their partner compared to a novel opposite-sex conspecific. Low dose muscimol and low or high dose CP154526 treated voles exhibited significantly lower stationary contact time with their partner compared to the aCSF and high dose muscimol groups. (B) Placement of successful stereotactic hits for the low or high dose muscimol, CP154526, and aCSF groups that were included in the data analysis. (C) Drug groups did not demonstrate a difference in total cage crosses when compared to the aCSF groups. Three-way mixed-model ANOVA with Bonferroni post-hoc were used to analyze behavioral data. #p < 0.05 vs. social stimulus. *p < 0.05 vs. aCSF. No sex differences were observed.
Social attachments, particularly those in long-term marriages and other romantic partnerships, present well-documented positive health benefits (House et al., 1988; Kiecolt-Glaser and Newton, 2001). One particular neural network thought to regulate various aspects of social attachment is the mesolimbic reward system (Aragona and Wang, 2009). However, there has been limited research to assess the role of the VTA, a dopamine-rich hub of the mesolimbic reward system, regulation of social attachment and commitment behaviors. Here we report that CRF promotes and GABA inhibits pair bond formation in both male and female prairie voles. Specifically, CRF and bicuculline administration in the VTA at the start of a short-term (1 h) cohabitation with a new partner facilitated the formation of a partner preference in males and females. Conversely, muscimol and CP154526 injections in the VTA at the start of a long-term (24 h) cohabitation with a new partner blocked the formation of a partner preference. These pharmacological effects were observed in both male and female prairie voles suggesting that, in a stress-naïve state, CRF signaling into the VTA may be appetitive and facilitates a pair bond while GABA signaling is aversive and hinders such social attachment.
Consistent with research from Curtis and Wang (2005), our study demonstrated that VTA GABA inhibited partner preference in male prairie voles. We also included females in our study and documented for the first time that VTA GABA inhibits partner preference formation in female prairie voles. Surprisingly, bicuculline was able to promote a partner preference in male and female prairie voles after a 1 h cohabitation compared to a 6 h cohabitation used in the previous study, as it has been well-documented that female prairie voles require 6–12 h of cohabitation and male prairie voles need a 24 h cohabitation to form this social preference (Aragona and Wang, 2004; Beery, 2021). There are limited studies that examine how VTA GABA neurons regulate the rewarding aspects and motivation for social attachment behaviors. One study proposed a circuit and demonstrated an increase in the activity of the GABAergic pathway from the medial preoptic area to the VTA during maternal behaviors including hovering, crouching, and body licking (Caba et al., 2019). Another study demonstrated that activation of GABA, by the use of muscimol, in the VTA decreased pup retrieval, increased the latency to retrieve all the pups, and decreased both nursing duration and crouch duration (Numan et al., 2009). Similar to our study, these studies suggest that the activation of VTA GABA, during a short-time period (i.e., 15 to 60 mins), in social attachments such as pair bonding and mother-infant bonds, regulate the reward aspects and motivation for social interaction and the display of attachment behaviors. Apart from social attachments, VTA GABA neurons have been shown to regulate a variety of behaviors in response to reward and stress (Bouarab et al., 2019). Stimulation of VTA GABA has been shown to regulate reward consumption and increase aversive behaviors in mice (van Zessen et al., 2012). Also, VTA GABA interneurons have been suggested to regulate and encode the expectation of reward and reward outcomes (Pan et al., 2013; Eshel et al., 2015). Beyond reward, there is an increase in the activity of VTA GABA neurons during stressful experiences, including foot shock or a looming stimulus (Tan et al., 2012; Zhou et al., 2019). VTA GABA neurons also project throughout the mesolimbic reward system, particularly the nucleus accumbens (Bouarab et al., 2019). GABA projections from the VTA to the nucleus accumbens regulates associative learning by acting on cholinergic interneurons and inhibition of these neurons rescues reward-seeking and reward reinforcement (Creed et al., 2014; Al-Hasani et al., 2021; Lowes et al., 2021). Together, this work identifies a role for VTA GABA in reward, stress, and motivation, however our study is one of the first to expands the role of VTA GABA function in regulating social attachment. Further studies should be conducted to examine how VTA GABA regulates the coding of a new partner as a rewarding and motivating stimulus during social attachment formation and maintenance behaviors.
We also demonstrated that CRF administration in the VTA facilitated partner preference formation in male and female prairie voles. Thus far, CRF system is well-known and is particularly studied in stress-related behaviors (Hostetler and Ryabinin, 2013). In the VTA, CRF is suggested to regulate behaviors associated with stress and addiction. CRF in the VTA specifically regulates social stress, mediates cocaine seeking, and modulates food reward in rats as well as controls aversive effects and nicotine intake in mice (Blacktop et al., 2011; Wanat et al., 2013; Grieder et al., 2014; Holly et al., 2016). CRF inputs into the VTA also act on VTA GABA neurons. Specifically, two studies have shown CRF modulates GABA neurons to mediate stress-induced ethanol and cocaine seeking (Blacktop et al., 2016; Harlan et al., 2018). Recently, however, studies have shown that CRF can promote appetitive motivation and positive valence in a stress naïve state. Specifically in the limbic system, CRF microinjections into the nucleus accumbens can promote partner preference formation, cue-triggered motivation for sucrose, conditioned place preference, and DA release in stress naïve males (Pecina et al., 2006; Lim et al., 2007; Chen et al., 2012; Lemos et al., 2012). A few studies in the prairie vole have shown similar effects of CRF through other routes of administration, including intracerebroventricular and site-specific nucleus accumbens injections (DeVries et al., 2002; Lim et al., 2007; Bosch et al., 2009). In addition, CRF receptor antagonist into the nucleus accumbens has been shown to decrease overall sociability (DeVries et al., 2002). Beyond pair bonding, CRF in the extended amygdala and hypothalamus modulates maternal behaviors including maternal neglect and stress in rats (Klampfl et al., 2014, 2016, 2018). However, our study is one of the first to document that CRF signaling in the VTA promotes social attachments, specifically pair bonding, in prairie voles in a stress-naïve state. Given that VTA CRF signaling can produce appetitive or aversive effects, more research will be required to determine the mechanism of these effects, which will likely be stress-dependent.
Social interactions and attachments have many rewarding aspects that are suggested to be processed through the mesolimbic reward system, and in particular, regulated by the neurotransmitter dopamine. As the VTA is a dopamine-rich region, this would suggest that our pharmacological manipulation of GABA and CRF have either local projections to or directly modulate dopamine neurons that would project downstream to other regions of the mesolimbic reward system. VTA GABA projections to dopamine neurons have been a well-established local circuit in certain motivational and learned behaviors (Creed et al., 2014; Yang et al., 2018; Ohta et al., 2022; Song et al., 2024). However, it also has been shown that different GABA and dopamine populations in the VTA regulate specific memory activities, as well as VTA dopamine projects to other regions such as the basolateral amygdala during positive experiences, suggesting that different circuits can be activated during varying behaviors and these circuits should be examined during social attachment behaviors (Sun et al., 2021; Glykos and Fujisawa, 2023). Like GABA, CRF can directly modulate VTA dopamine release but also has indirect effects on dopamine through glutamate and GABA (Wanat et al., 2008; Wise and Morales, 2010; Refojo et al., 2011; Boyson et al., 2014; Zalachoras et al., 2022). Although dopamine has been shown to regulate motivation and rewarding behaviors and prairie vole pair bond formation specifically (Aragona et al., 2003, 2006; Liu and Wang, 2003; Lee and Beery, 2021), it remains uncertain how and if GABA and CRF directly modulate VTA dopamine neurons to promote a partner preference. Further investigation should be completed to investigate this potential circuit.
In conclusion, GABA and CRF in the VTA exhibit a novel and critical role in the formation of a partner preference in male and female prairie voles. This elucidates a particular local circuit in the VTA that may increase the positive valence coding of a new social partner, promoting a choice toward a partner over another novel social stimulus after a short cohabitation. With the rewarding benefits of social attachments, this would suggest that GABA and CRF act on VTA dopamine neurons to promote dopamine downstream; however, these circuits need further attention. Taken together, these findings further demonstrate how a region within the mesolimbic reward system modulates social attachment behaviors as well as provide future directions to examine how neurochemical systems interact with one another to modulate such behaviors.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The animal study was approved by the National Institutes of Health Guide and Use of Laboratory Animals and the Institutional Animal Care and Use Committee and the University of Kansas. The study was conducted in accordance with the local legislation and institutional requirements.
KG: Conceptualization, Data curation, Formal analysis, Project administration, Validation, Writing−original draft, Writing−review and editing. CL: Data curation, Writing−review and editing. AK: Data curation, Writing−review and editing. SV: Data curation, Validation, Writing−review and editing. AS: Supervision, Conceptualization, Formal analysis, Funding acquisition, Resources, Writing−original draft, Writing−review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of the article. We would like to thank the funding that supported this work, the BRAIN Initiative through the National Institute of Health (NIH) (R01-NS113104), the National Institute of Mental Health (R01-MH133123), and KU PREP Program through the National Institute of General Medical Sciences (NIGMS) (R25-GM078441).
We would like to thank the KU ACU staff for veterinary and husbandry care of the vole colony at KU.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al-Hasani, R., Gowrishankar, R., Schmitz, G. P., Pedersen, C. E., Marcus, D. J., Shirley, S. E., et al. (2021). Ventral tegmental area GABAergic inhibition of cholinergic interneurons in the ventral nucleus accumbens shell promotes reward reinforcement. Nat. Neurosci. 24, 1414–1428.
Amadei, E. A., Johnson, Z. V., Jun Kwon, Y., Shpiner, A. C., Saravanan, V., Mays, W. D., et al. (2017). Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature 546, 297–301. doi: 10.1038/nature22381
Aragona, B. J., and Wang, Z. (2004). The prairie vole (Microtus ochrogaster): An animal model for behavioral neuroendocrine research on pair bonding. ILAR J. 45, 35–45. doi: 10.1093/ilar.45.1.35
Aragona, B. J., and Wang, Z. (2009). Dopamine regulation of social choice in a monogamous rodent species. Front. Behav Neurosci. 3, 15. doi: 10.3389/neuro.08.015.2009
Aragona, B. J., Liu, Y., Curtis, J. T., Stephan, F. K., and Wang, Z. (2003). A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J. Neurosci. 23, 3483–3490.
Aragona, B. J., Liu, Y., Yu, Y. J., Curtis, J. T., Detwiler, J. M., Insel, T. R., et al. (2006). Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci. 9, 133–139.
Baik, J. H. (2013). Dopamine signaling in reward-related behaviors. Front. Neural Circuits 7:152. doi: 10.3389/fncir.2013.00152
Bales, K. L., and Carter, C. S. (2003). Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster). Behav. Neurosci. 117, 854–859. doi: 10.1037/0735-7044.117.4.854
Beery, A. K. (2021). Familiarity and mate preference assessment with the partner preference test. Curr. Protoc. 1:e173.
Blacktop, J. M., Seubert, C., Baker, D. A., Ferda, N., Lee, G., Graf, E. N., et al. (2011). Augmented cocaine seeking in response to stress or Crf delivered into the ventral tegmental area following long-access self-administration is mediated by Crf receptor type 1 but not Crf receptor type 2. J. Neurosci. 31, 11396–11403.
Blacktop, J. M., Vranjkovic, O., Mayer, M., Van Hoof, M., Baker, D. A., and Mantsch, J. R. (2016). Antagonism of Gaba-B but not Gaba-A receptors in the Vta prevents stress- and intra-VTA CRF-induced reinstatement of extinguished cocaine seeking in rats. Neuropharmacology 102, 197–206.
Bosch, O. J., Nair, H. P., Ahern, T. H., Neumann, I. D., and Young, L. J. (2009). The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology 34, 1406–1415. doi: 10.1038/npp.2008.154
Bouarab, C., Thompson, B., and Polter, A. M. (2019). VTA GABA neurons at the interface of stress and reward. Front. Neural Circuits 13:78. doi: 10.3389/fncir.2019.00078
Boyson, C. O., Holly, E. N., Shimamoto, A., Albrechet-Souza, L., Weiner, L. A., Debold, J. F., et al. (2014). Social stress and Crf-dopamine interactions in the VTA: Role in long-term escalation of cocaine self-administration. J. Neurosci. 34, 6659–6667. doi: 10.1523/JNEUROSCI.3942-13.2014
Caba, M., Melo, A. I., Fleming, A., and Meza, E. (2019). Maternal care activates the ventral tegmental area but not dopaminergic cells in the rat. J. Neuroendocrinol. 31:12713.
Cai, J., and Tong, Q. (2022). Anatomy and function of ventral tegmental area glutamate neurons. Front. Neural Circuits 16:867053. doi: 10.3389/fncir.2022.867053
Carter, C. S., Witt, D. M., Thompson, E. G., and Carlstead, K. (1988). Effects of hormonal, sexual, and social history on mating and pair bonding in prairie voles. Physiol. Behav. 44, 691–697. doi: 10.1016/0031-9384(88)90049-2
Chen, Y. W., Rada, P. V., Butzler, B. P., Leibowitz, S. F., and Hoebel, B. G. (2012). Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience 206, 155–166. doi: 10.1016/j.neuroscience.2011.12.009
Creed, M. C., Ntamati, N. R., and Tan, K. R. (2014). VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front. Behav. Neurosci. 8:8. doi: 10.3389/fnbeh.2014.00008
Curtis, J. T., and Wang, Z. (2005). Ventral tegmental area involvement in pair bonding in male prairie voles. Physiol. Behav. 86, 338–346.
DeVries, A. C., Devries, M. B., Taymans, S., and Carter, C. S. (1995). Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proc. Natl. Acad. Sci. U.S.A. 92, 7744–7748. doi: 10.1073/pnas.92.17.7744
DeVries, A. C., Guptaa, T., Cardillo, S., Cho, M., and Carter, C. S. (2002). Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology 27, 705–714.
Eshel, N., Bukwich, M., Rao, V., Hemmelder, V., Tian, J., and Uchida, N. (2015). Arithmetic and local circuitry underlying dopamine prediction errors. Nature 525, 243–246.
Gingrich, B., Liu, Y., Cascio, C., Wang, Z., and Insel, T. R. (2000). Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav. Neurosci. 114, 173–183. doi: 10.1037//0735-7044.114.1.173
Glykos, V., and Fujisawa, S. (2023). Memory-specific encoding activities of the ventral tegmental area dopamine and GABA neurons. Cold Spring Harb. Lab. 12:R89743. doi: 10.7554/eLife.89743
Gossman, K. R., Dykstra, B., Garcia, B. H., Swopes, A. P., Kimbrough, A., and Smith, A. S. (2021). Pair bond-induced affiliation and aggression in male prairie voles elicit distinct functional connectivity in the social decision-making network. Front. Neurosci. 15:748431. doi: 10.3389/fnins.2021.748431
Grieder, T. E., Herman, M. A., Contet, C., Tan, L. A., Vargas-Perez, H., Cohen, A., et al. (2014). VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat. Neurosci. 17, 1751–1758. doi: 10.1038/nn.3872
Harlan, B. A., Becker, H. C., Woodward, J. J., and Riegel, A. C. (2018). Opposing actions of CRF-R1 and CB1 receptors on VTA-Gabaergic plasticity following chronic exposure to ethanol. Neuropsychopharmacology 43, 2064–2074. doi: 10.1038/s41386-018-0106-9
Holly, E. N., Boyson, C. O., Montagud-Romero, S., Stein, D. J., Gobrogge, K. L., Debold, J. F., et al. (2016). Episodic social stress-escalated cocaine self-administration: Role of phasic and tonic corticotropin releasing factor in the anterior and posterior ventral tegmental area. J. Neurosci. 36, 4093–4105. doi: 10.1523/JNEUROSCI.2232-15.2016
Horie, K., Inoue, K., Nishimori, K., and Young, L. J. (2020). Investigation of oxtr-expressing neurons projecting to nucleus accumbens using oxtr-ires-Cre Knock-in prairie voles (Microtus ochrogaster). Neuroscience 448, 312–324. doi: 10.1016/j.neuroscience.2020.08.023
Hostetler, C. M., and Ryabinin, A. E. (2013). The CRF system and social behavior: A review. Front. Neurosci. 7:92. doi: 10.3389/fnins.2013.00092
Hostetler, C. M., Hinde, K., Maninger, N., Mendoza, S. P., Mason, W. A., Rowland, D. J., et al. (2017). Effects of pair bonding on dopamine D1 receptors in monogamous male titi monkeys (Callicebus cupreus). Am. J. Primatol. 79, 1–9. doi: 10.1002/ajp.22612
House, J. S., Landis, K. R., and Umberson, D. (1988). Social relationships and health. Science 241, 540–545. doi: 10.1136/sextrans-2018-053935
Iyilikci, O., Balthazart, J., and Ball, G. F. (2016). Medial preoptic regulation of the ventral tegmental area related to the control of sociosexual behaviors. eNeuro 3, 1–12. doi: 10.1523/ENEURO.0283-16.2016
Jankowiak, W. R., and Fischer, E. F. (1992). A cross-cultural perspective on romantic love. Ethnology 31, 149–155.
Kiecolt-Glaser, J. K., and Newton, T. L. (2001). Marriage and health: His and hers. Psychol. Bull. 127, 472–503.
Klampfl, S. M., Brunton, P. J., Bayerl, D. S., and Bosch, O. J. (2014). Hypoactivation of CRF receptors, predominantly type 2, in the medial-posterior Bnst is vital for adequate maternal behavior in lactating rats. J. Neurosci. 34, 9665–9676. doi: 10.1523/JNEUROSCI.4220-13.2014
Klampfl, S. M., Brunton, P. J., Bayerl, D. S., and Bosch, O. J. (2016). CRF-R1 activation in the anterior-dorsal BNST induces maternal neglect in lactating rats via an Hpa axis-independent central mechanism. Psychoneuroendocrinology 64, 89–98. doi: 10.1016/j.psyneuen.2015.11.015
Klampfl, S. M., Schramm, M. M., Gassner, B. M., Hubner, K., Seasholtz, A. F., Brunton, P. J., et al. (2018). Maternal stress and the Mpoa: Activation of Crf receptor 1 impairs maternal behavior and triggers local oxytocin release in lactating rats. Neuropharmacology 133, 440–450. doi: 10.1016/j.neuropharm.2018.02.019
Laviolette, S. R., Gallegos, R. A., Henriksen, S. J., and Van Der Kooy, D. (2004). Opiate state controls bi-directional reward signaling via Gabaa receptors in the ventral tegmental area. Nat. Neurosci. 7, 160–169. doi: 10.1038/nn1182
Lee, N. S., and Beery, A. K. (2021). The role of dopamine signaling in prairie vole peer relationships. Horm. Behav. 127:104876.
Lemos, J. C., Wanat, M. J., Smith, J. S., Reyes, B. A., Hollon, N. G., Van Bockstaele, E. J., et al. (2012). Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 490, 402–406. doi: 10.1038/nature11436
Lillard, L. A., and Waite, L. J. (1995). Til death do us part: Marital disruption and mortality. Am. J. Sociol. 100, 1131–1156.
Lim, M. M., Liu, Y., Ryabinin, A. E., Bai, Y., Wang, Z., and Young, L. J. (2007). CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Horm. Behav. 51, 508–515. doi: 10.1016/j.yhbeh.2007.01.006
Liu, Y., and Wang, Z. X. (2003). Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121, 537–544. doi: 10.1016/s0306-4522(03)00555-4
Lowes, D. C., Chamberlin, L. A., Kretsge, L. N., Holt, E. S., Abbas, A. I., Park, A. J., et al. (2021). Ventral tegmental area GABA neurons mediate stress-induced blunted reward-seeking in mice. Nat. Commun. 12:3539. doi: 10.1038/s41467-021-23906-2
Morales, M., and Margolis, E. B. (2017). Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 18, 73–85.
Nestler, E. J., and Carlezon, W. A. Jr. (2006). The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 59, 1151–1159.
Numan, M., Stolzenberg, D. S., Dellevigne, A. A., Correnti, C. M., and Numan, M. J. (2009). Temporary inactivation of ventral tegmental area neurons with either muscimol or baclofen reversibly disrupts maternal behavior in rats through different underlying mechanisms. Behav. Neurosci. 123, 740–751.
Ohta, Y., Murakami, T. E., Kawahara, M., Haruta, M., Takehara, H., Tashiro, H., et al. (2022). Investigating the influence of GABA neurons on dopamine neurons in the ventral tegmental area using optogenetic techniques. Int. J. Mol. Sci. 23:1114. doi: 10.3390/ijms23031114
Ortiz, J. J., Portillo, W., Paredes, R. G., Young, L. J., and Alcauter, S. (2018). Resting state brain networks in the prairie vole. Sci. Rep. 8:1231.
Pan, W. X., Brown, J., and Dudman, J. T. (2013). Neural signals of extinction in the inhibitory microcircuit of the ventral midbrain. Nat. Neurosci. 16, 71–78. doi: 10.1038/nn.3283
Pecina, S., Schulkin, J., and Berridge, K. C. (2006). Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: Paradoxical positive incentive effects in stress? BMC Biol. 4:8. doi: 10.1186/1741-7007-4-8
Refojo, D., Schweizer, M., Kuehne, C., Ehrenberg, S., Thoeringer, C., Vogl, A. M., et al. (2011). Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science 333, 1903–1907. doi: 10.1126/science.1202107
Smith, A. S., Lei, K., and Wang, Z. (2013). The neurobiology of social attachment. Oxford: Oxford University Press, 1112–1126.
Song, Q., Wei, A., Xu, H., Gu, Y., Jiang, Y., Dong, N., et al. (2024). An Acc-Vta-Acc positive-feedback loop mediates the persistence of neuropathic pain and emotional consequences. Nat. Neurosci. 27, 272–285. doi: 10.1038/s41593-023-01519-w
Sun, L., You, J., Sun, F., Cui, M., Wang, J., Wang, W., et al. (2021). Reactivating a positive feedback loop VTA-BLA-NAc circuit associated with positive experience ameliorates the attenuated reward sensitivity induced by chronic stress. Neurobiol. Stress 15:100370. doi: 10.1016/j.ynstr.2021.100370
Tan, K. R., Yvon, C., Turiault, M., Mirzabekov, J. J., Doehner, J., Labouebe, G., et al. (2012). GABA neurons of the Vta drive conditioned place aversion. Neuron 73, 1173–1183.
Tickerhoof, M. C., and Smith, A. S. (2017). Vasopressinergic neurocircuitry regulating social attachment in a monogamous species. Front. Endocrinol. 8:265. doi: 10.3389/fendo.2017.00265
van Zessen, R., Phillips, J. L., Budygin, E. A., and Stuber, G. D. (2012). Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194.
Waltz, M., Badura, B., Pfaff, H., and Schott, T. (1988). Marriage and the psychological consequences of a heart attack: A longitudinal study of adaptation to chronic illness after 3 years. Soc. Sci. Med. 27, 149–158. doi: 10.1016/0277-9536(88)90323-1
Wanat, M. J., Bonci, A., and Phillips, P. E. (2013). CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat. Neurosci. 16, 383–385. doi: 10.1038/nn.3335
Wanat, M. J., Hopf, F. W., Stuber, G. D., Phillips, P. E., and Bonci, A. (2008). Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of IH. J. Physiol. 586, 2157–2170. doi: 10.1113/jphysiol.2007.150078
Williams, J. R., Catania, K. C., and Carter, C. S. (1992). Development of partner preferences in female prairie voles (Microtus ochrogaster): The role of social and sexual experience. Horm. Behav. 26, 339–349. doi: 10.1016/0018-506x(92)90004-f
Wise, R. A., and Morales, M. (2010). A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Res. 1314, 38–43. doi: 10.1016/j.brainres.2009.09.101
Yamaguchi, M., Smith, A., and Ohtsubo, Y. (2015). Commitment signals in friendship and romantic relationships. Evol. Hum. Behav. 36, 467–474. doi: 10.1037//0022-3514.81.2.247
Yang, H., De Jong, J. W., Tak, Y., Peck, J., Bateup, H. S., and Lammel, S. (2018). Nucleus accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron 97:434–449.e4. doi: 10.1016/j.neuron.2017.12.022
Yin, J., Zou, Z., Song, H., Zhang, Z., Yang, B., and Huang, X. (2018). Cognition, emotion and reward networks associated with sex differences for romantic appraisals. Sci. Rep. 8:2835. doi: 10.1038/s41598-018-21079-5
Young, K. A., Gobrogge, K. L., Liu, Y., and Wang, Z. (2011). The neurobiology of pair bonding: Insights from a socially monogamous rodent. Front. Neuroendocrinol. 32:53–69. doi: 10.1016/j.yfrne.2010.07.006
Zalachoras, I., Astori, S., Meijer, M., Grosse, J., Zanoletti, O., De Suduiraut, I. G., et al. (2022). Opposite effects of stress on effortful motivation in high and low anxiety are mediated by Crhr1 in the Vta. Sci. Adv. 8:eabj9019. doi: 10.1126/sciadv.abj9019
Keywords: prairie voles, ventral tegmental area, CRF, GABA, pair bond, partner preference
Citation: Gossman KR, Lowe CS, Kirckof A, Vanmeerhaeghe S and Smith AS (2024) Corticotropin-releasing factor and GABA in the ventral tegmental area modulate partner preference formation in male and female prairie voles (Microtus ochrogaster). Front. Neurosci. 18:1430447. doi: 10.3389/fnins.2024.1430447
Received: 09 May 2024; Accepted: 09 July 2024;
Published: 23 July 2024.
Edited by:
Gabriela Rodriguez-Manzo, Center for Research and Advanced Studies, National Polytechnic Institute of Mexico (CINVESTAV), MexicoReviewed by:
Genaro Alfonso Coria-Avila, Universidad Veracruzana, MexicoCopyright © 2024 Gossman, Lowe, Kirckof, Vanmeerhaeghe and Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam Steven Smith, YWRhbXNtaXRoQGt1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.