
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurosci. , 14 June 2024
Sec. Brain Imaging Methods
Volume 18 - 2024 | https://doi.org/10.3389/fnins.2024.1415411
 Lulu Cheng1,2†
Lulu Cheng1,2† Jianxin Zhang1†
Jianxin Zhang1† Hongyu Xi3
Hongyu Xi3 Mengting Li4,5
Mengting Li4,5 Su Hu4,5
Su Hu4,5 Wenting Yuan3,6
Wenting Yuan3,6 Peng Wang7,8
Peng Wang7,8 Lanfen Chen9*
Lanfen Chen9* Linlin Zhan3*
Linlin Zhan3* Xize Jia4,5*
Xize Jia4,5*Background: Previous neuroimaging studies have revealed structural and functional brain abnormalities in patients with cervical spondylosis (CS). However, the results are divergent and inconsistent. Therefore, the present study conducted a multi-modal meta-analysis to investigate the consistent structural and functional brain alterations in CS patients.
Methods: A comprehensive literature search was conducted in five databases to retrieve relevant resting-state functional magnetic resonance imaging (rs-fMRI), structural MRI and diffusion tensor imaging (DTI) studies that measured brain functional and structural differences between CS patients and healthy controls (HCs). Separate and multimodal meta-analyses were implemented, respectively, by employing Anisotropic Effect-size Signed Differential Mapping software.
Results: 13 rs-fMRI studies that used regional homogeneity, amplitude of low-frequency fluctuations (ALFF) and fractional ALFF, seven voxel-based morphometry (VBM) studies and one DTI study were finally included in the present research. However, no studies on surface-based morphometry (SBM) analysis were included in this research. Due to the insufficient number of SBM and DTI studies, only rs-fMRI and VBM meta-analyses were conducted. The results of rs-fMRI meta-analysis showed that compared to HCs, CS patients demonstrated decreased regional spontaneous brain activities in the right lingual gyrus, right middle temporal gyrus (MTG), left inferior parietal gyrus and right postcentral gyrus (PoCG), while increased activities in the right medial superior frontal gyrus, bilateral middle frontal gyrus and right precuneus. VBM meta-analysis detected increased GMV in the right superior temporal gyrus (STG) and right paracentral lobule (PCL), while decreased GMV in the left supplementary motor area and left MTG in CS patients. The multi-modal meta-analysis revealed increased GMV together with decreased regional spontaneous brain activity in the left PoCG, right STG and PCL among CS patients.
Conclusion: This meta-analysis revealed that compared to HCs, CS patients had significant alterations in GMV and regional spontaneous brain activity. The altered brain regions mainly included the primary visual cortex, the default mode network and the sensorimotor area, which may be associated with CS patients' symptoms of sensory deficits, blurred vision, cognitive impairment and motor dysfunction. The findings may contribute to understanding the underlying pathophysiology of brain dysfunction and provide references for early diagnosis and treatment of CS.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, CRD42022370967.
Cervical spondylosis (CS) is a common degenerative condition of the cervical spine that predominantly affects middle-aged and elderly populations (Reddy et al., 2019). The disorder may develop into different conditions depending on the location of nerve compression and the stage of the disease development (Takagi et al., 2011). For example, pure spondylosis was usually accompanied by axial neck pain or stiffness (Takagi et al., 2011), cervical radiculopathy was caused by compression of spinal nerve and showed symptoms of shooting or burning pain in the neck, paresthesia or motor weakness related to the disordered nerve root (Shedid and Benzel, 2007; Theodore, 2020), while cervical myelopathy was generated by compression of spinal cord due to the deterioration of cervical spine, which may lead to neck pain or stiffness, weakness or numbness in the upper and/or lower extremity (Takagi et al., 2011; Kalsi-Ryan et al., 2013; Theodore, 2020). Studies have found that people may suffer from one or several types of CS such as both cervical radiculopathy and myelopathy (Yu et al., 1986), all of which have significant impacts on our daily life (Badhiwala and Wilson, 2018). The symptoms of CS are mostly considered to be caused by cervical spine or spinal cord function injury, so previous studies on CS mainly focused on local lesions of cervical spine and spinal cord (Berberat et al., 2023). However, scholars have detected that the dysfunction of cervical spine or spinal cord alone could not explain the connections between CS-related symptoms, and cervical spinal decompression sometimes did not relieve the symptoms of some patients and even worsened the condition (Sun L. et al., 2016; Wu and Wang, 2023; Fard et al., 2024). On this basis, scholars began to explore related changes of the brain after the degeneration or injury of cervical spine and found that the brain function and structure of CS patients would undergo remodeling changes, which may then affect the clinical manifestations and prognosis of patients (Wu and Wang, 2023). However, the neurobiological mechanisms of CS remain unclear, indicating the need for further research to fully understand the disease.
Resting-state functional magnetic resonance imaging (rs-fMRI) is an effective tool for exploring the neural mechanisms of various diseases (Khan et al., 2024). It examines the spontaneous fluctuations in the blood oxygen level-dependent (BOLD) signal (Biswal et al., 1997). Among the analytical methods of rs-fMRI, functional connectivity (FC) (including region of interest and seed-based FC) examines the synchronicity or similarity of functional activities between remote brain regions through the calculation of the correlation of time series (Friston et al., 1993; Biswal et al., 1995). However, it could not identify the specific abnormal areas of the brain (Zang et al., 2007). To complementing this, the amplitude of low-frequency fluctuation (ALFF), fractional ALFF (fALFF) and regional homogeneity (ReHo) are well-established and widely-utilized for examining regional spontaneous brain activity (Yang et al., 2019; Wang et al., 2022; Chang et al., 2023). Specifically, ALFF and fALFF gauge the intensity of spontaneous brain activity within a single voxel during rest (Zou et al., 2008), whereas ReHo evaluates the synchronization of the BOLD signal across a focal voxel and its 26 surroundings (Zang et al., 2004). The integrative application of ALFF/fALFF and ReHo has been evidenced to provide more comprehensive complementary insights into regional spontaneous brain activity (Salvia et al., 2019; Yao et al., 2021). Besides, voxel-based morphometry (VBM) and surface-based morphometry (SBM) are effective approaches to measure the indexes of cortical morphology by using T1-weighted MRI scans (Goto et al., 2022). Specifically, VBM offers a standardized approach to assessing gray matter volume (GMV) (Whitwell, 2009), while SBM calculate such morphological characteristics as cortical thickness, surface area, sulcus depth, gyrification index and fractal dimension (Riccelli et al., 2017). Scholars also have found that VBM and SBM can be used as complementary methods to detect the morphological alterations of the gray matter, which can improve the accuracy of the detection results (Goto et al., 2022). In addition to the study of the function and structure of gray matter, research on white matter has also been paid more attention. Recently, diffusion tensor imaging (DTI) has become an effective means to investigate the microstructure of white matter beyond the structural dimensions evaluated by T1 and T2 weighted MRI (Qiu et al., 2015). Combining the structural and functional studies on gray matter, we can get a more comprehensive picture of gray matter (Dang et al., 2022), while the combination of studies on gray matter and white matter could advance our understanding of the cerebral microstructure.
Recently, a growing body of research has employed rs-fMRI, structural MRI and DTI to explore functional and structural brain anomalies in CS patients (Bernabéu-Sanz et al., 2020; Chang et al., 2023), which have advanced our knowledge of the pathophysiology of CS. However, the findings of previous neuroimaging research varied, leading to diverse and inconsistent evidence, and the persistent neurological alterations related with CS remain largely unknown. For example, previous studies on regional spontaneous brain activity in the middle frontal gyrus (MFG) in CS patients have produced mixed outcomes, with some finding hyperactivity, some finding hypoactivity while the other finding no abnormal change in this area (Xu et al., 2018; Yue and Du, 2020; Bai et al., 2022). Complex results were also found in structural neuroimaging studies on CS (Woodworth et al., 2019; Wang et al., 2023). The inconsistency among different studies could be due to small sample sizes, different data processing methods, publication bias toward positive results and flexible analytical methods (Tahmasian et al., 2019; Sun et al., 2020; Liu et al., 2021). To account for this, meta-analysis has emerged as an objective, effective and efficient method to integrate the findings of prior studies and identify more definitive brain regions that are persistently involved in the pathophysiology of a specific disorder, namely, to create the “collective mind” (Fox et al., 2014), thus enhancing sample size, statistical power, the reliability and replicability of findings (Radua and Mataix-Cols, 2012; Tahmasian et al., 2019). Recently, it has become increasingly popular in addressing discrepancies in clinical research and has been employed to investigate the persistent brain alterations in a variety of disease such as anxiety disorder, major depression and autism spectrum disorder (Serra-Blasco et al., 2021; Wang et al., 2022), but there is no systematic meta-analysis of neuroimaging studies related to cervical spondylosis. Although three systematic reviews on CS, which have been published recently, have provided an overview of CS-related neuroimaging studies from a macroscopic perspective, they did not examine the most consistent and core brain alterations in CS patients (Wu and Wang, 2023; Fard et al., 2024; Khan et al., 2024). However, the identification of the consistent and core brain alterations may help us understand its underlying neuropathological basis, further explain the symptoms of CS patients, and facilitate the diagnosis and treatment of the disease (Tahmasian et al., 2019). In this sense, it is of vital clinical and research significance to conduct a meta-analysis to reach a consistent conclusion.
Therefore, in the present study, we conducted a multi-modal voxel-based meta-analysis of rs-fMRI, structural MRI and DTI studies to investigate the most consistent brain alterations for each modality in CS patients, aiming to advance the understanding of CS pathogenesis. Given that different conditions of CS may occur simultaneously and share some key clinical manifestations such as neck pain, numbness or stiffness (Wang et al., 2016), we included studies on different stages of the disease, encompassing pure spondylosis, cervical radiculopathy and cervical myelopathy.
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021) (Supplementary Tables S1, S2) and registered in the PROSPERO International Prospective Register of Systematic Reviews (register number: CRD42022370967) (https://www.crd.york.ac.uk/PROSPERO/). To collate relevant studies on brain structural and functional differences between CS and healthy controls (HCs), a comprehensive and systematic literature search was executed up until April 30, 2024 across five Chinese and English databases including Embase, PubMed, Web of Science, Chinese National Knowledge Infrastructure (CNKI) and Wanfang Data. The following keywords were employed to identify related rs-fMRI studies: (“cervical spondylosis” OR “CS” OR “CSD” OR “cervical spondylotic” OR “cervical radiculopathy” OR “CSR” OR “cervical myelopathy” OR “CSM” OR “DCM”) AND (“ReHo” OR “regional homogeneity” OR “amplitude of low-frequency fluctuations” OR “ALFF” OR “fractional amplitude of low-frequency fluctuations” OR “fALFF”), while the following ones were for VBM studies: (“cervical spondylosis” OR “cervical spondylotic” OR “cervical radiculopathy” OR “cervical myelopathy” OR “CS” OR “CSD” OR “CSM” OR “DCM”) AND (“voxel-based morphometry” OR “VBM” OR “voxel-wise” OR “voxel-based” OR “volumetric” OR “morphometry” OR “gray matter”) AND (“magnetic resonance imaging” OR “MRI” OR “neuroimaging”). As for relevant SBM studies, we used such keywords as (“cervical spondylosis” OR “cervical spondylotic” OR “cervical myelopathy” OR “cervical radiculopathy”) AND (“SBM” OR “surface-based morphometry” OR “cortical thickness” OR “surface area” OR “sulcus depth” OR “gyrification index” OR “fractal dimension”). Meanwhile, relevant DTI studies were retrieved by the following keywords: (“cervical spondylosis” OR “cervical spondylotic” OR “cervical myelopathy” OR “cervical radiculopathy”) AND (“DTI” OR “diffusion tensor imaging” OR “diffusion tensor magnetic resonance imaging” OR “diffusion tensor MRI” OR “diffusion tensor MRIs” OR “Diffusion Tractography”) AND (“brain” OR “cerebral” OR “cortex” OR “subcortex” OR “cortical” OR “subcortical” OR “cerebrum”). Detailed search strategies for each database were shown in Supplementary Table S3. To ensure an exhaustive coverage, the references of selected studies and review articles were also scrutinized.
Inclusion criteria were as follows: (1) Diagnosis of CS in patients, including pure spondylosis, cervical myelopathy, and cervical radiculopathy; (2) rs-fMRI studies using ReHo, ALFF, or fALFF analytical methods, structural MRI studies including VBM or SBM research, and relevant DTI studies; (3) Conducting brain imaging comparisons between CS and HCs; (4) Reporting whole-brain results in Montreal Neurological Institute (MNI) or Talairach coordinates (Radua and Mataix-Cols, 2009; Müller et al., 2018). Exclusion criteria included: (1) Non-empirical or nonhuman research such as review, conference abstract and animal research; (2) Studies only reporting region of interest results, which would bias the meta-analytic findings (Radua and Mataix-Cols, 2009; Müller et al., 2018); (3) Studies failing to report MNI or Talairach coordinates. Longitudinal or intervention studies were included only for their baseline data. Among studies with overlapping samples, preference was given to the study with the largest sample size and most comprehensive information (Zhao et al., 2023).
The quality of each included study was evaluated using a 20-point checklist in previous studies since it can reflect key variables that are significant for evaluating neuroimaging studies (Iwabuchi et al., 2015; Pan et al., 2017). Specifically, the checklist, as shown in Supplementary Table S4, contains two categories with 13 questions assessing the sample characteristics (e.g., the diagnostic criteria, demographic and clinical information on the study samples of the included study), methodology (e.g., neuroimaging acquisition parameters) and the quality of reporting results (e.g., statistical correction methods). Meanwhile, the characteristics of the included studies, including the diagnostic criteria, types of CS, the number, gender ratio and mean age of participants in both CS and HCs groups, analytical methods, statistical thresholds, research design, the peak coordinates of differential brain regions and their corresponding effect sizes were extracted from each included study. The process of literature search, selection, quality assessment and data extraction were independently executed by two authors. Discrepancies were resolved through consultation with a third author to reach consensus.
Due to the insufficient number of SBM and DTI studies that could be included in this meta-analysis, we mainly detailed the meta-analysis of rs-fMRI and VBM studies in the methodological part. Individual meta-analysis was conducted to discern brain structural or functional differences using Anisotropic Effect-size Signed Differential Mapping (AES-SDM) software (version 5.15, https://www.sdmproject.com/software/). Specifically, for each meta-analysis, peak coordinates of statistically significant clusters between CS and HCs together with their corresponding effect sizes (T-values) were extracted from each included study and compiled into separate text files (Radua et al., 2014). Here, we included whole-brain analyses both with and without multiple comparison correction since according to Radua and Mataix-Cols (2009), the inclusion of analysis without multiple comparison correction would not bias the possibility to identify significant findings. An effect size map for each study was then generated using an anisotropic Gaussian kernel (Radua et al., 2014). Subsequently, a mean map was computed employing a random-effects model, accounting for sample size, intra-study variability, and inter-study heterogeneity (Radua and Mataix-Cols, 2012; Pan et al., 2017; Su et al., 2022). The threshold of p < 0.005, peak height Z > 1, and cluster extent > 10 voxels was selected to balance sensitivity and specificity against false positives (Radua and Mataix-Cols, 2009; Radua et al., 2012b; Pan et al., 2017; Wang et al., 2022). The results of abnormal brain areas among CS patients in each neuroimaging modality were finally presented in MNI coordinates.
Based on the two probability maps (PF and PV) generated by rs-fMRI and VBM meta-analyses, respectively, a multi-modal meta-analysis was conducted to explore the overlapping or conjunction of functional and structural brain alterations in CS patients (Long et al., 2023). The usual multimodal approach was to overlap the regions of statistical significance in the two modes (Nichols et al., 2005), in other words, to obtain the intersection of the two maps. However, this approach assumes that p-values were calculated without error, which may not be the case in neuroimaging data where different statistical means, such as permutations and randomizations, evaluating the same hypothesis generate significantly different p-values (Radua et al., 2013). Therefore, we conducted the multimodal meta-analysis according to the refined overlap approach (Radua et al., 2012a, 2013). Specifically, the two probability maps (PF and PV) generated by unimodal meta-analysis were combined so that the p-values could be amalgamated to determine a union of changes in the two modes (U), with U estimated as U = PV + PF – PV × PF (Radua et al., 2012a, 2013). However, this statistic of U in its original form would be obviously conservative, so it was optimized by P = U + (1 – U) × ln (1 – U), thus, reducing the disequilibrium of false positive and negative rates (Radua et al., 2012a, 2013). Meanwhile, the threshold of p < 0.0025 was employed for multimodal meta-analysis since in the unimodal meta-analysis, we used the threshold of p < 0.005, as suggested in previous studies (Radua et al., 2012a, 2013).
To ascertain the reliability and stability of the findings, jackknife sensitivity analysis was performed for each meta-analysis. This entailed iterative statistical re-evaluation, excluding one different dataset each time (Radua and Mataix-Cols, 2009; Pan et al., 2017). A finding was deemed robust if brain regions remained significant across most combinations of studies (Radua and Mataix-Cols, 2009). Heterogeneity analyses were separately executed for each meta-analysis, using a random-effects model with Q-statistics to determine the presence of unexplained inter-study variance (Iwabuchi et al., 2015; Wang et al., 2022). A voxel threshold of p < 0.005 with a peak height Z > 1 and a cluster extent > 10 voxels was set for identifying significant heterogeneity (Radua et al., 2012b; Su et al., 2022). Additionally, Egger test was performed to assess publication bias, utilizing peak coordinates from clusters where significant differences were observed between CS and HCs (Ioannidis et al., 2014; Wang et al., 2022). A p-value < 0.05 in the Egger test was indicative of significant publication bias (Pan et al., 2017; Wang et al., 2018, 2022).
Two subgroup analyses were conducted to examine the possible sources of heterogeneity that occurred in rs-fMRI meta-analysis and to assess the potential impact of different analytical methods in rs-fMRI data processing and different conditions of the disease. Specifically, the subgroup analyses of ALFF and ReHo were performed to analyze the effects of methods on abnormal brain areas. In addition, given that cervical myelopathy was caused by compression of spinal cord, a part of the central nervous system, which is different from the generation of pure spondylosis and cervical radiculopathy (Takagi et al., 2011; Wang et al., 2016; Theodore, 2020), a subgroup analysis of different types of the disease was performed to explore specific functional brain changes for different conditions, mainly including cervical myelopathy and CS without myelopathy. However, the subgroup analysis was not conducted in the VBM meta-analysis due to the small sample number of each subgroup.
Based on AES-SDM, general linear meta-regression of random effects was conducted to analyze the potential effects of related clinical and demographic characteristics on alterations in brain structure and function among CS patients. The independent variables of meta-regression include the Japanese Orthopedic Association (JOA) scores, the mean age and the percentage of female, while the dependent variable was the SDM values for meta-analysis of each mode. To reduce false positives, we set the significance threshold to p < 0.0005, Z > 1, and cluster size > 10 according to previous studies (Radua et al., 2012a; Yao et al., 2021; Cheng et al., 2023). Only those clusters demonstrating significant changes in one of the extremes of the regressor and slope were reported (Radua et al., 2012a). Meanwhile, the results that fall outside the main meta-analysis were discarded (Radua and Mataix-Cols, 2009; Radua et al., 2012a).
A total of 21 studies were included in this meta-analysis (Table 1; Figure 1; Supplementary Figure S1): 13 rs-fMRI studies encompassing 486 CS patients (mean age ± SD: 46.21 ± 13.86 years) and 439 HCs (44.86 ± 13.68 years), seven VBM studies (eight datasets) comprising 262 CS patients (50.32 ± 11.54 years) and 221 HCs (49.13 ± 12.30 years), as well as 1 DTI study with 42 CS patients (42.8 ± 9.3 years) and 42 HCs (42.4 ± 9.4 years). However, no SBM studies were included in this research. In the rs-fMRI cohort, eight studies focused on cervical spondylotic myelopathy (CSM) (Tan et al., 2015; Chen Z. et al., 2018; Kuang and Zha, 2019; Ge et al., 2021; Fan et al., 2022; Zhao et al., 2022; Su et al., 2023; Wu et al., 2024), three on cervical spondylotic radiculopathy (CSR) (Yu et al., 2017; Xu et al., 2018; Yue and Du, 2020), and the remaining two on pure spondylosis (Chen J. et al., 2018; Bai et al., 2022), while the VBM cohort included four studies on CSM (Chen et al., 2022; Tian et al., 2023; Kuang and Zha, 2024; Wang et al., 2024), one study on CSR (Yu et al., 2017), and the remaining two focusing on pure spondylosis (Bernabéu-Sanz et al., 2020; Yang et al., 2020). Besides, among the 13 rs-fMRI studies, one research used fALFF method (Wu et al., 2024), four studies utilized ReHo method (Tan et al., 2015; Yu et al., 2017; Chen J. et al., 2018; Xu et al., 2018), six studies employed ALFF method (Yue and Du, 2020; Ge et al., 2021; Bai et al., 2022; Fan et al., 2022; Zhao et al., 2022; Su et al., 2023), while the remaining two researches utilized both ReHo and ALFF methods (Chen Z. et al., 2018; Kuang and Zha, 2019). In cases where both ReHo and ALFF were used, only results from the more statistically significant method were included in our meta-analysis. The quality assessment of the included studies indicated acceptable levels, with each study scoring at least 17 points (Wang et al., 2018). Details of the included studies were presented in Table 1.
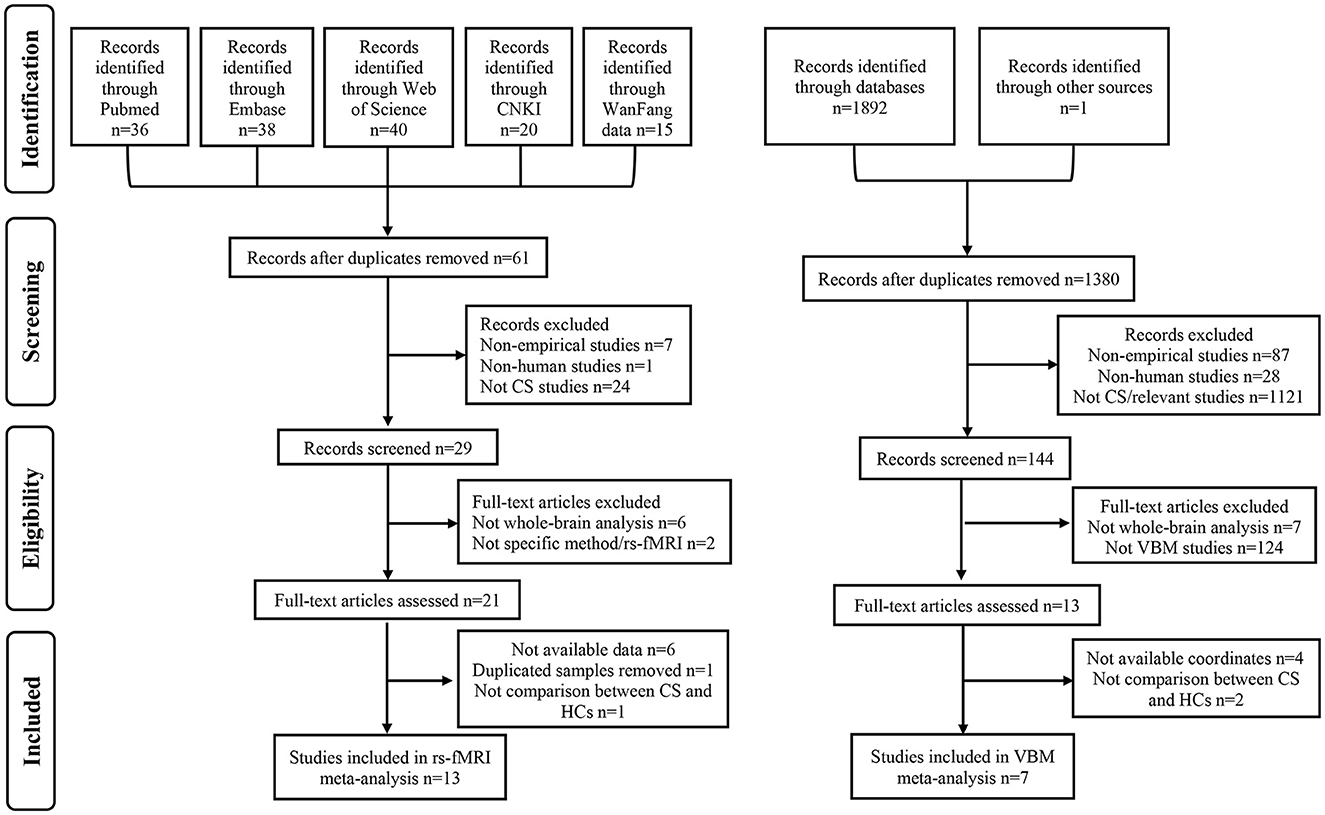
Figure 1. Flow diagram of literature search and study selection. CNKI, Chinese National Knowledge Infrastructure; CS, cervical spondylosis; rs-fMRI, resting-state functional magnetic resonance imaging; HCs, healthy controls; VBM, voxel-based morphometry.
Due to the small sample size of DTI study (N = 1), the meta-analysis was not conducted for this modality. Specifically, meta-analysis was conducted only for rs-fMRI and VBM studies. In rs-fMRI meta-analysis, the results showed that decreased activities were found in the right lingual gyrus (LING), right middle temporal gyrus (MTG), left inferior parietal gyri (IPL) and right postcentral gyrus (PoCG) among CS patients when compared to HCs, while increased activities were detected in the right medial superior frontal gyrus (SFGmed), bilateral MFG and right precuneus (PCUN) among CS patients (Table 2; Figure 2). Complementing these findings, the VBM meta-analysis revealed that compared to HCs, CS patients demonstrated increased GMV in the right superior temporal gyrus (STG) and right paracentral lobule (PCL) while decreased GMV in the left supplementary motor area (SMA) and left MTG (Table 3; Figure 3). These alterations highlight significant functional and structural differences in CS patients.
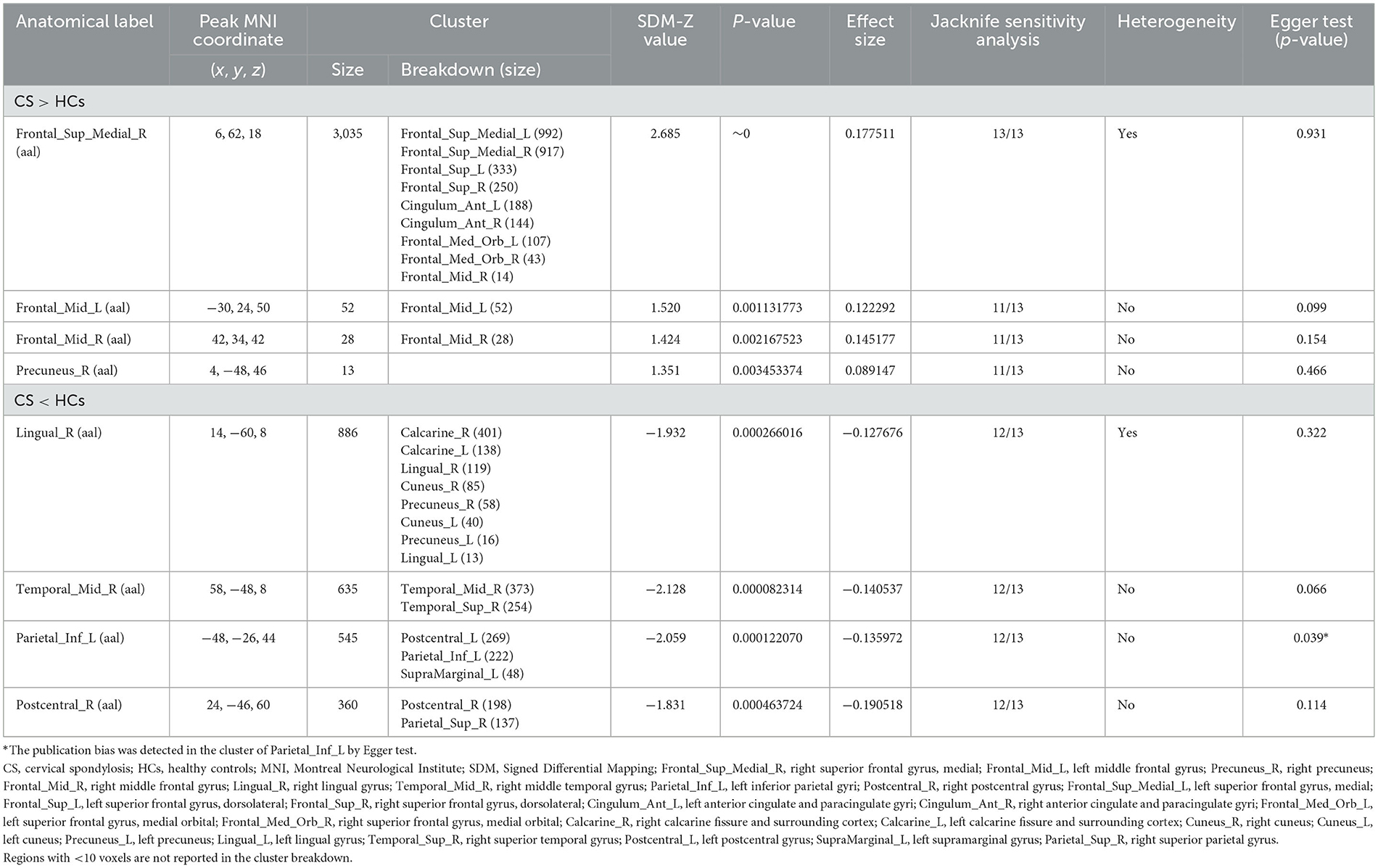
Table 2. Regions with abnormal regional spontaneous brain activities in CS patients relative to HCs.

Figure 2. Brain regions showing increased and decreased regional spontaneous brain activities in patients with cervical spondylosis compared to healthy controls. Frontal_Sup_Medial_R, right superior frontal gyrus, medial; Frontal_Mid_L, left middle frontal gyrus; Precuneus_R, right precuneus; Frontal_Mid_R, right middle frontal gyrus; Lingual_R, right lingual gyrus; Temporal_Mid_R, right middle temporal gyrus; Parietal_Inf_L, left inferior parietal gyri; Postcentral_R, right postcentral gyrus.
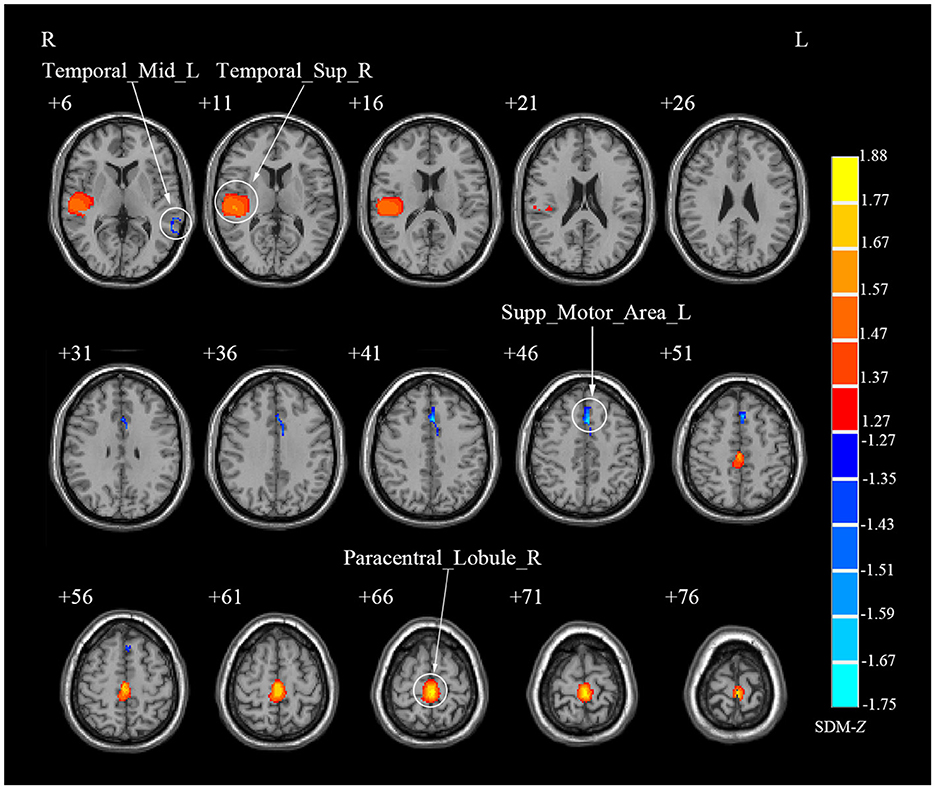
Figure 3. Brain regions showing abnormal gray matter volume in patients with cervical spondylosis compared to healthy controls. Temporal_Sup_R, right superior temporal gyrus; Paracentral_Lobule_R, right paracentral lobule; Supp_Motor_Area_L, left supplementary motor area; Temporal_Mid_L, left middle temporal gyrus.
Our multi-modal meta-analysis revealed a unique pattern in CS patients when compared to HCs. Specifically, compared to HCs, CS patients showed a conjoint increase of GMV and decreased regional spontaneous brain activity in the left PoCG, right STG and right PCL (Table 4; Figure 4; Supplementary Table S5; Supplementary Figures S2, S3).
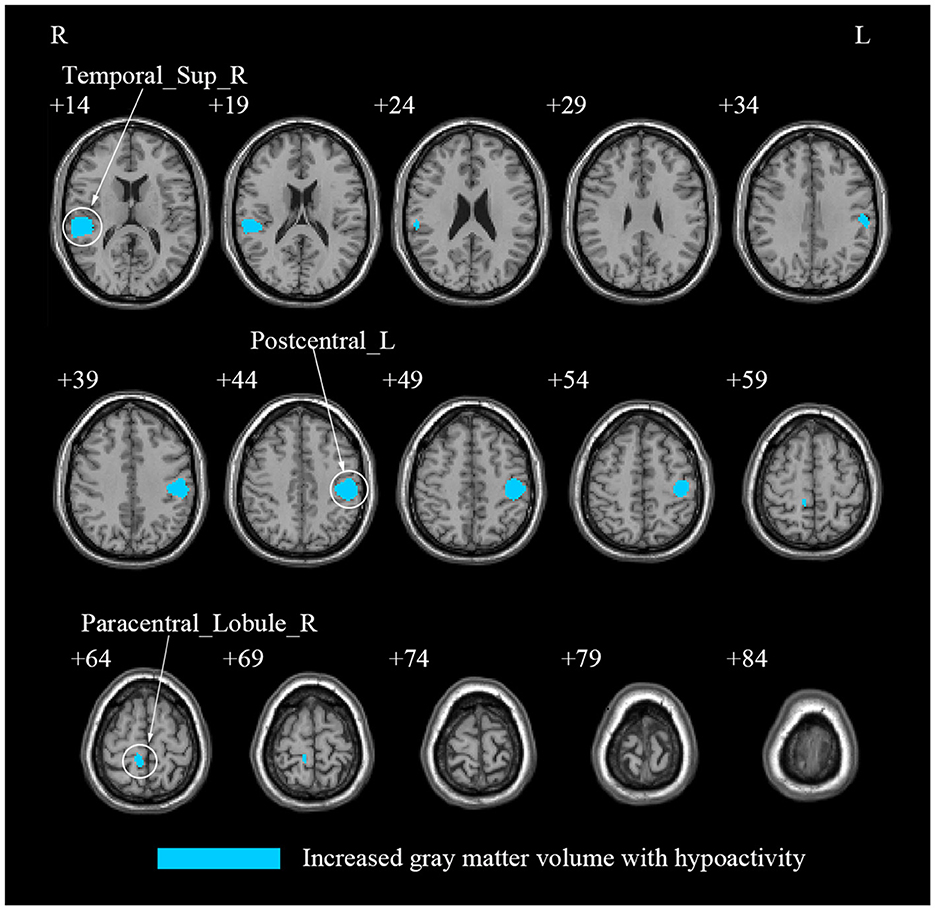
Figure 4. A multi-modal meta-analysis of combined structural and functional alterations in patients with cervical spondylosis. Postcentral_L, left postcentral gyrus; Temporal_Sup_R, right superior temporal gyrus; Paracentral_Lobule_R, right paracentral lobule; Parietal_Inf_L, left inferior parietal, but supramarginal and angular gyri; Precentral_L, left precentral gyrus; SupraMarginal_L, left supramarginal gyrus; Rolandic_Oper_R, right rolandic operculum; SupraMarginal_R, right supramarginal gyrus; Temporal_Mid_R: right middle temporal gyrus; Heschl_R, right heschl gyrus; Postcentral_R, right postcentral gyrus; Precentral_R, right precentral gyrus; Precuneus_R, right precuneus.
The jackknife sensitivity analysis affirmed the reliability of our findings. In rs-fMRI meta-analysis, the right SFGmed emerged as the most consistently altered region, showing replicable changes across all 13 dataset combinations (Table 2). The right LING, right MTG, left IPL and right PoCG demonstrated significant alterations in 12/13 combinations. Besides, the bilateral MFG and right PCUN also showed significant changes in 11/13 combinations. In VBM meta-analysis, the right PCL and left SMA displayed altered GMV consistently across all study combinations while the right STG and left MTG exhibited altered GMV in 7/8 dataset combinations (Table 3).
In rs-fMRI meta-analysis, we identified significant heterogeneity in the right SFGmed and right LING (Table 2). Similarly, in the VBM meta-analysis, the right PCL displayed significant heterogeneity (Table 3). Furthermore, our Egger test only revealed publication bias in the left IPL through rs-fMRI meta-analysis (p = 0.039) (Table 2).
The subgroup analysis of ALFF (N = 7) revealed that compared to HCs, CS patients showed increased activities in the right SFGmed and PCUN, but decreased activity in the right LING (Table 5; Figure 5a). The subgroup analysis of ReHo (N = 5) indicated increased activities in the left cerebellum lobule VIIB, bilateral MFG and right MTG, while decreased activities in the left PoCG and right MTG in CS (Table 5; Figure 5b). Subgroup analysis on CSM studies (N = 8) showed that compared to HCs, CSM patients demonstrated decreased brain activity in the right LING while increased brain activity in the right SFGmed (Table 6; Figure 6a). Additionally, the subgroup analysis of studies measuring differences between HCs and CS patients without myelopathy (N = 5) detected decreased brain activities in the right MTG and bilateral PoCG, while increased neural activities in the bilateral MFG among CS patients when compared to HCs (Table 6; Figure 6b). The findings revealed different impacts of the analytical methods and conditions of the disease.
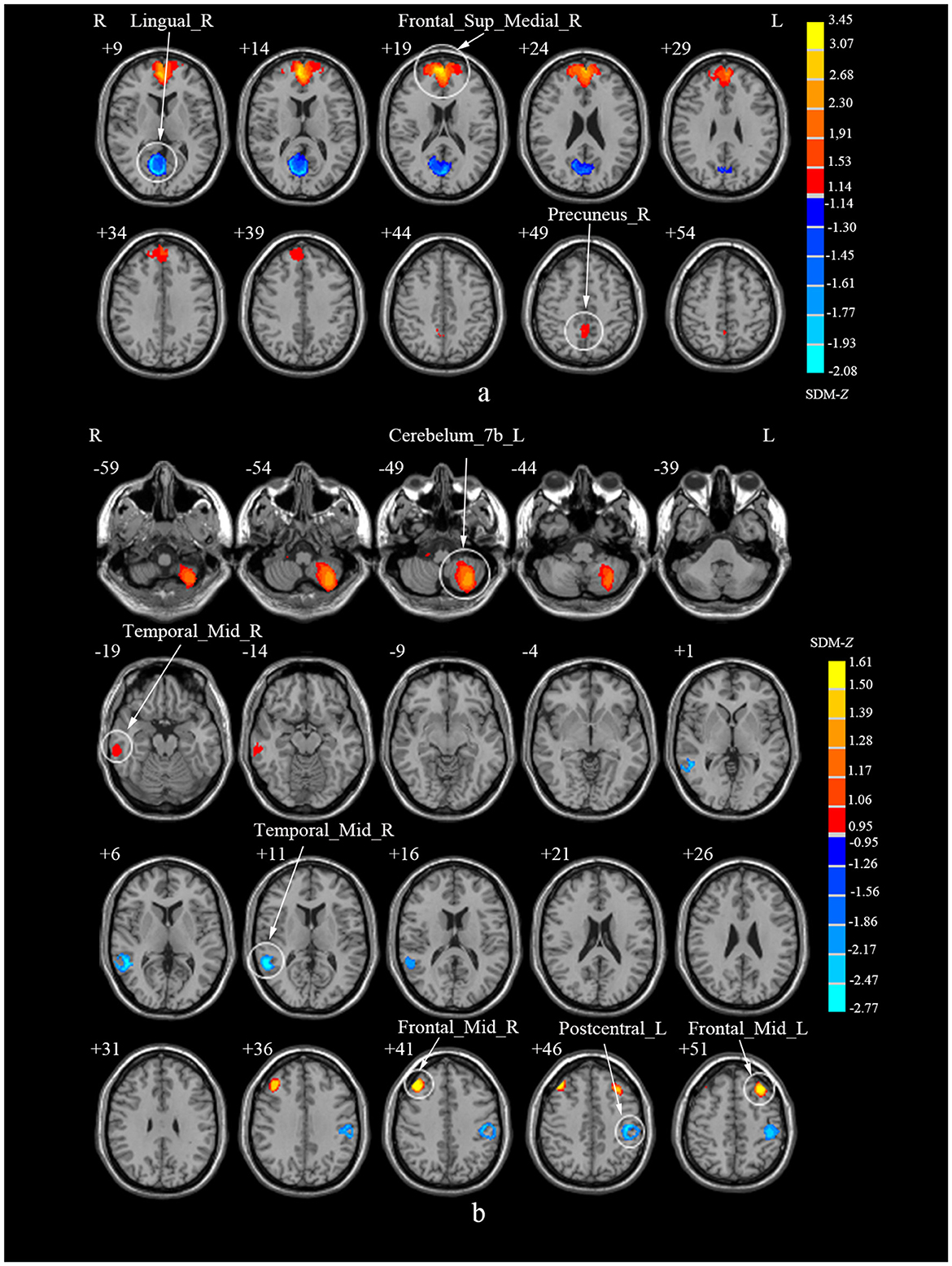
Figure 5. Subgroup analyses of ALFF and ReHo studies in CS patients compared to HCs. (a) Subgroup analysis of ALFF studies. (b) Subgroup analysis of ReHo studies. ALFF, amplitude of low-frequency fluctuations; ReHo, regional homogeneity; CS, cervical spondylosis; HCs, healthy controls; Frontal_Sup_Medial_R, right superior frontal gyrus, medial; Precuneus_R, right precuneus; Lingual_R, right lingual gyrus; ReHo, regional homogeneity; Cerebellum_7b_L, left cerebellum, hemispheric lobule VIIB; Frontal_Mid_R, right middle frontal gyrus; Frontal_Mid_L, left middle frontal gyrus; Temporal_Mid_R, right middle temporal gyrus; Postcentral_L, left postcentral gyrus; Temporal_Mid_R, right middle temporal gyrus.
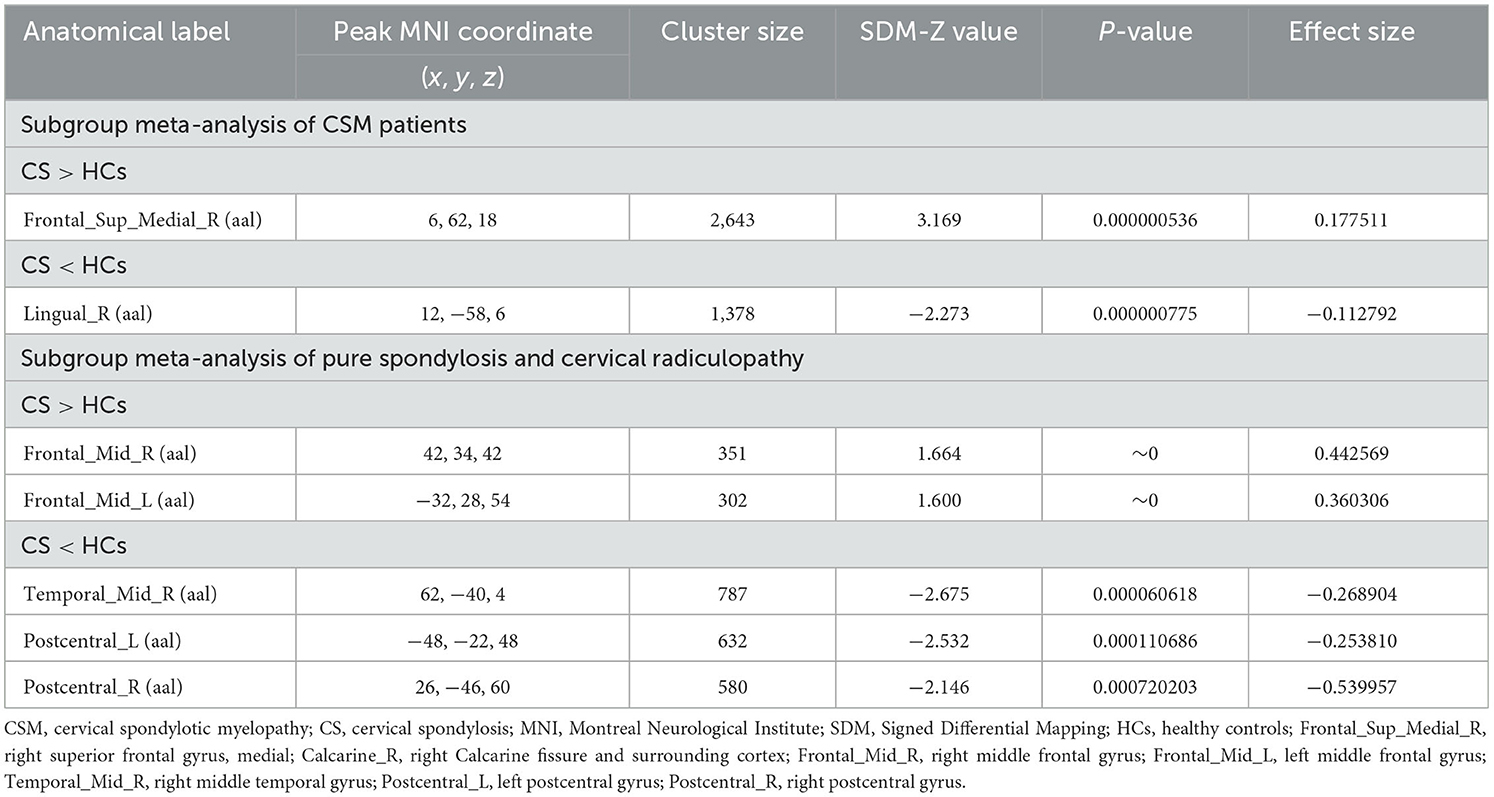
Table 6. Subgroup meta-analysis of CSM patients and CS patients with/without radiculopathy, respectively.
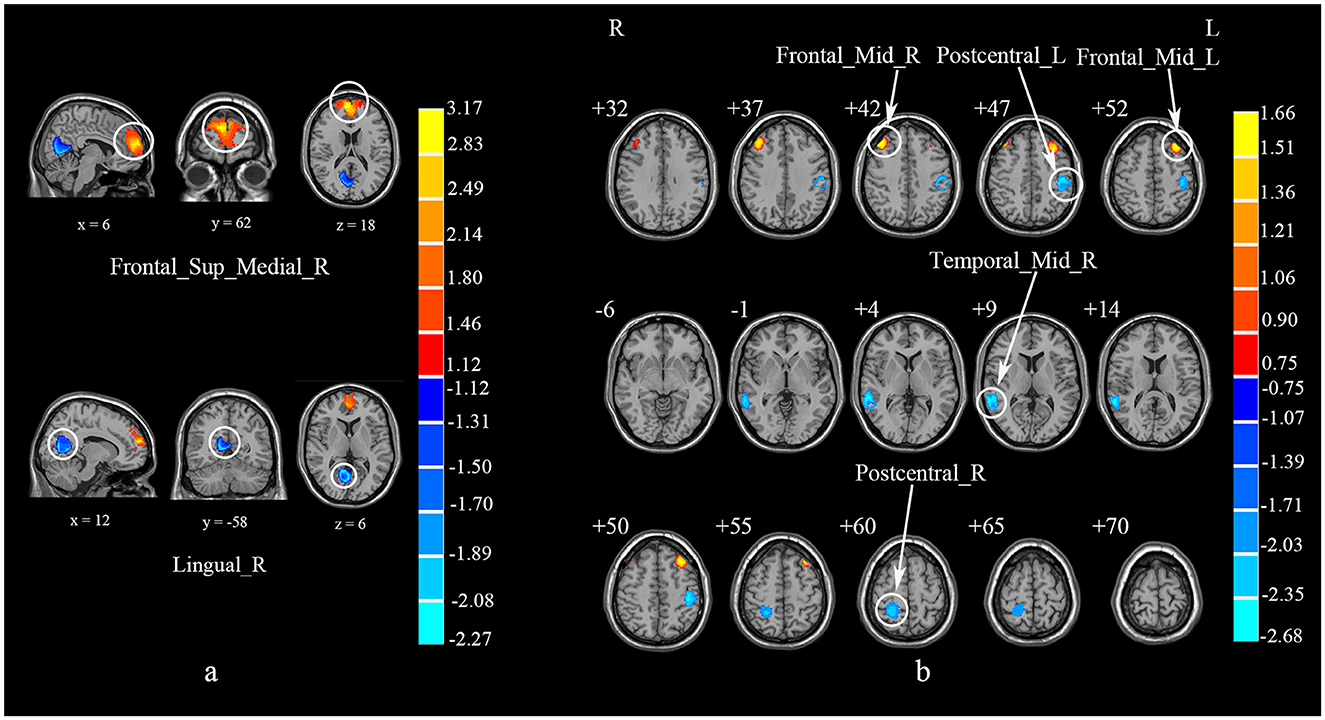
Figure 6. Subgroup analyses of CSM studies and studies on CS patients without myelopathy. (a) Subgroup meta-analysis of CSM studies. (b) Subgroup meta-analysis of studies on CS patients without myelopathy. CSM, cervical spondylotic myelopathy; CS, cervical spondylosis; Frontal_Sup_Medial_R, right superior frontal gyrus, medial; Calcarine_R, right Calcarine fissure and surrounding cortex; Frontal_Mid_R, right middle frontal gyrus; Frontal_Mid_L, left middle frontal gyrus; Temporal_Mid_R, right middle temporal gyrus; Postcentral_L, left postcentral gyrus; Postcentral_R, right postcentral gyrus.
The meta-regression analyses revealed that increased mean age of patients in rs-fMRI studies (available in all rs-fMRI studies) was associated with decreased regional spontaneous brain activity in the left IPL (MNI coordinates: −52, −28, 42; Number of voxels: 55; Peak intensity: 3.658; p = 0.000053644), but the percentage of female (available in all rs-fMRI studies) and the JOA score of patients (available in seven rs-fMRI studies) were not associated with CS-related regional spontaneous brain activity changes. Besides, the percentage of female patients (available in all VBM studies), the mean age (available in all VBM studies) and the JOA scores (available in five VBM studies) of patients were not associated with CS-related GMV alterations.
This study represented the first comprehensive meta-analysis that investigated the consistent alterations in brain structure and function among CS patients by combining voxel-based rs-fMRI and VBM studies. Although the meta-analyses of SBM and DTI studies were not conducted due to the insufficient sample sizes, previous studies still indicated that these measures also provided alternative neuroimaging approach for assessing the pathophysiological features of CS. The main discoveries of the present study are as follows: (1) The rs-fMRI meta-analysis indicated significantly lower regional spontaneous brain activities in the right LING, right MTG, left IPL and right PoCG, while higher brain activities in the right SFGmed, bilateral MFG and right PCUN among CS patients when compared to HCs. (2) The VBM meta-analysis revealed decreased GMV in the left SMA and MTG while increased GMV within the right STG and PCL in CS patients. (3) Our multi-modal meta-analysis uncovered conjoint increased GMV but decreased regional brain activity in the left PoCG, right STG and right PCL.
In CS patients, decreased regional spontaneous brain activity was noted in the right LING, right MTG, left IPL and right PoCG, while increased regional spontaneous brain activity was observed in the right PCUN, right SFGmed, and bilateral MFG. The right LING, belonging to the primary visual cortex (V1), is responsible for receiving and transmitting visual stimulation (Chang et al., 2023). The hypoactivity of the right LING may suggest the visual processing impairment in CS patients since studies have found the symptom of blurred vision in CS patients, especially in those with CSM (Sun et al., 2013; Sun Y. et al., 2016; Chen Z. et al., 2018), which could also explain our finding of hypoactivity in the right LING by the subgroup analysis of studies on CSM patients. In other words, we speculate that the activation of this brain region in different groups may be related to the severity of the disease. Considering that CSM is not a primary visual disorder, the right LING may be a potential neurobiological marker for the diagnosis and recovery of CSM (Takenaka et al., 2019). In addition, our subgroup meta-analysis of methods revealed that this brain area also depended on the analytical method of ALFF. This may indicate strong regional spontaneous activity in this brain area at the single-voxel level, rather than synchronized activity with neighboring voxels, since ALFF gauges the intensity of spontaneous brain activity within a single voxel (Zang et al., 2007; Jia et al., 2020), while ReHo measures the similarity of time courses within clusters consisting of 27 adjacent voxels (Song et al., 2011).
The MTG, IPL, and PCUN are key constituents of the default mode network which involves cognitive and emotional processing (Usui et al., 2020; Yue and Du, 2020; Wu et al., 2023). Specifically, the MTG takes part in diverse functions including cognitive, visual and sensory processing due to its anterior association with the visual network and default mode network (Wu et al., 2020). Studies have indicated that CS patients would suffer from memory loss, poor attention, depression and the absence of visual cues (Theodore, 2020; Zhao et al., 2020). The findings of decreased regional spontaneous brain activity and GMV in the right MTG may be associated with the above symptoms or disorders of CS patients, explaining their underlying neurophysiological bases, since previous studies indicated that these symptoms could not be explained by the degeneration of cervical spine alone (Sun et al., 2018; Fard et al., 2024). The IPL, a key part of the parietal-integrated region and somatosensory association cortex, is involved in translating various sensory modes into actions, namely, sensorimotor transformation (Zhou et al., 2015; Chang et al., 2023). For example, Patri et al. (2020) noted that IPL disruption can impair its role in actualizing motor intentions. Scholars also reported loss of manual dexterity as well as impaired gait and balance in CS patients (Theodore, 2020), so the hypoactivity of the left IPL may indicate patient's impaired function in sensorimotor transformation. Furthermore, our meta-regression analysis revealed that older patients were significantly more likely to report decreased regional spontaneous brain activity in this area, which may provide evidence for the diagnosis of CS in elderly populations. This was consistent with previous studies which also detected the association between age and the prognosis of CS (Lv Y. et al., 2018). However, the publication bias was detected in this area, which may be caused by incomplete research data (Cheng et al., 2023) or underlying differences between smaller and larger studies (Egger et al., 1997; Lau et al., 2006), so the results should be interpreted with scrutiny (Wang et al., 2022). Further studies are needed in the future to verify the functional brain alterations in the left IPL. The PCUN is linked to advanced cognitive functions like episodic memory and visuospatial imagination (Cavanna and Trimble, 2006). Woodworth et al. (2019) also found decreased cortical thickness in the bilateral PCUN among patients with cervical myelopathy. In view of the cognitive impairments reported in CS patients, like hypomnesia and affective disturbance, the hyperactivity of the right PCUN may be a functional compensation for its structural impairment in dealing with cognitive information.
The SFGmed and MFG, integral to the prefrontal lobe, are responsible for diverse functions encompassing sensation, emotion and cognition (Garcia-Larrea and Peyron, 2013). Prior research indicated that the prefrontal cortex integrates sensory and emotional pain information through modulating cortex-subcortical and intercortical nociceptive pathways (Yang et al., 2013). It was also found that the region was involved in processing cognition and negative emotions (Tyborowska et al., 2018; Magon et al., 2019). For example, Gong et al. (2020) found that the heightened ALFF in this area was related to major depression and bipolar disorder. While neck pain is the predominant symptom in CS patients (Takagi et al., 2011), this discomfort, along with other CS-associated ailments, could elicit negative emotions, typically represented by anxiety and depression (Zhao et al., 2020; Chu et al., 2022; Pei et al., 2022). Furthermore, scholars also found cortical thinning in the sensorimotor and pain-related areas (e.g., the superior frontal cortex) of patients (Wang et al., 2024). Therefore, the increased brain activity in the SFGmed and MFG may be an indication of their compensatory role in regulating neck pain, negative emotions and cognitive disorders in CS patients. The results also supported the findings of previous study that the regional brain activity in the superior frontal cortex had impact on the prognosis of patients (Fan et al., 2022). It is worth noting that the right SFGmed also demonstrated significant heterogeneity in the rs-fMRI meta-analysis. Our exploratory subgroup analyses revealed that this region was also dependent on the method of ALFF and the stage of cervical myelopathy, similar to the heterogeneity sources of right LING. As stated in previous studies, patients with pure spondylosis usually have symptoms of neck pain while patients with cervical radiculopathy often reported paresthesia such as tingling, burning or shooting pain (Takagi et al., 2011; Theodore, 2020). However, the sensory impairment in patients with cervical myelopathy is more serious than the first two conditions since CSM patients usually suffer from the symptom of numbness or loss of position sensation (Shedid and Benzel, 2007), namely, they could not feel light touch, pain, temperature, or vibrations, or even not know where their body parts are, which would then weaken their abilities in balance and coordination. In this sense, there may be significant differences in functional brain activity between patients with or without myelopathy in the right SFGmed. Besides, the heterogeneity of this brain region is also derived from the analytical methods of ALFF and ReHo, which may be due to their focus on different characteristics of brain activity (An et al., 2013; Lv H. et al., 2018).
Decreased GMV alone was also found in the left SMA in CS patients. The SMA plays a crucial role in bridging cognitive processes with motor actions (Yu et al., 2017) and managing self-initiated movements (Martín-Signes et al., 2019) by projecting its neuron to the spinal cord (Roy et al., 2009). Specifically, it is primarily involved in self-generated and controlled movement, such as in preparing and executing the practical action sequence (Bhagavatula et al., 2016). Meanwhile, this region is also strongly influenced by other aspects of the movements including attention and performance (Bhagavatula et al., 2016). In view of such symptoms as loss of hand movement flexibility and visual impairment in CS patients (Takagi et al., 2011), decreased GMV in the left SMA among CS patients may represent the underlying neurobiological basis of their motor dysfunction. The PCL, located between the marginal branch of the cingulate sulcus and the paracentral sulcus, controls motor and sensory nerve innervation and is mainly associated with motor of lower extremity, especially with the movement and sensation of the opposite leg and foot (Zhou et al., 2018; Choi et al., 2024). The hypoactivity in this brain area among CS patients, CSM patients in particular, may indicate their decreased sensory and motor abilities, especially in the lower extremity. In addition, scholars also revealed cortical atrophy in the right PCL (Wang et al., 2021), so the increase of GMV in this region may be a compensation for its functional impairment and cortical thinning. Significantly, the additional analysis also revealed heterogeneity in the right PCL. To explore the sources of the heterogeneity, we conducted meta-regression analyses rather than subgroup analyses due to the relatively small sample sizes of the subgroups in VBM studies. No correlation was detected between the three regressor variables, namely, the mean age, the percentage of female and the JOA score and the changes of this region, so the findings of heterogeneity in this area warrant cautious interpretation (Ronaldson et al., 2020).
In addition, we also found an increase in GMV paired with decreased regional spontaneous brain activity in the left PoCG and right STG through the multimodal meta-analysis. The PoCG, representative of the primary somatosensory cortex (S1) (Oni-Orisan et al., 2016), processes and interprets a variety of sensory information from other regions, such as proprioception (Cai et al., 2022) and pain perception (Tseng et al., 2013). The decreased regional spontaneous brain activity in the bilateral PoCG may be a neural signal of sensory disturbance in CS patients, since typical symptoms such as numbness or sensory loss in the feet or hands have been reported in CS patients (Theodore, 2020). Furthermore, previous studies also found significant cortical atrophy and decreased sulcus depth in the PoCG among CS patients (Woodworth et al., 2019; Wang et al., 2021, 2023; Chang et al., 2023). Based on this, the increased GMV of the right PoCG may play a compensatory role in sensory processing. In addition, increased ALFF values in the right PoCG was detected to be related with decreased fractional anisotropy (FA) values at the C2 level of spinal cord, demonstrating the pathophysiological interaction between cerebral cortex and spinal cord (Zhou et al., 2014). These findings could further explain the remodeling of cerebral cortex in response to spinal cord injury and facilitate the clinical treatment decisions of physicians. The STG was also found active in pain-inducing studies, indicating its role in sensory processing (Rottmann et al., 2010). Meanwhile, the cortical thickness of STG among CS patients was also detected to be thinner when compared with HCs (Wang et al., 2023). We speculated that the decreased regional spontaneous brain activity of the right STG in CS patients may contribute to their difficulties in accurately recognizing, integrating and processing sensory information, potentially leading to numbness, paresthesia or sensory loss commonly reported (Theodore, 2020), whereas the increased GMV in STG may represent compensations for its decreased regional spontaneous brain activity and cortical atrophy.
The findings of the present meta-analytic study suggest consistent and core alterations in brain structure and function among CS patients as well as a complex interplay between different neuroimaging modalities, regional spontaneous brain activity and GMV in particular, which could provide evidence and reference for clinical practice. Firstly, neuroimaging technique has been increasingly used to explore brain abnormalities in structure and function among CS patients, which generated diverse and inconsistent results. The consistent brain changes in CS patients identified by this meta-analysis could consolidate new insights that are clinically useful, thus contributing to the understanding of the complex pathophysiology of CS (Tahmasian et al., 2019), especially the underlying neuropathological mechanisms of cervical spondylosis. Secondly, the integration of previous studies may provide conclusive evidence for the diagnosis of CS and improve the diagnostic accuracy, thus reducing the waste of medical resources (Fan et al., 2022). For example, according to Fan et al. (2022), the accuracy of support vector machine analysis and linear analysis which were based on DTI and fMRI data for identifying CS patients from HCs has exceeded 97%. Thirdly, the persistent neuroimaging findings of the present study may provide prognostic insights to physicians and facilitate their treatment decision, thus reducing unnecessary sufferings of patients with CS (Fan et al., 2022; Fard et al., 2024). However, some limitations must be acknowledged. First, due to the cross-sectional nature of the included studies, a causal link between CS and brain abnormalities couldn't be established (Gong et al., 2020). Second, this meta-analysis relies on peak coordinates and effect sizes extracted from published studies rather than on raw statistical brain maps, which could affect the precision of the spatial localization of the reported effects (Radua et al., 2012b; Jiang et al., 2017). Third, our meta-analysis may include some studies without correction for multiple comparisons. However, according to Radua and Mataix-Cols (2009), the inclusion of analyses that were not corrected for multiple comparisons did not bias the likelihood of finding significant results. Future research could include more homogeneous studies and provide a more granular understanding of the mechanisms underlying CS.
The present study conducted a multi-modal meta-analysis to identify the consistent structural and functional brain alterations in CS patients. The results showed that compared to HCs, CS patients demonstrated a significant change in GMV and regional spontaneous brain activity mainly in the visual cortex, the default mode network and the sensorimotor area, with a complex interplay between the two modalities. These findings could provide fresh insights into the pathophysiology of CS, potentially directing future research on its diagnosis and therapeutic approaches.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
LCheng: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. JZ: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. HX: Data curation, Writing – review & editing. ML: Writing – review & editing. SH: Writing – review & editing. WY: Data curation, Supervision, Validation, Resources, Writing – review & editing. PW: Writing – review & editing. LChen: Writing – review & editing. LZ: Writing – review & editing. XJ: Conceptualization, Funding acquisition, Software, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by Taishan Scholars Project Special Fund (No. tsqn202306134), Shandong Province Social Science Planning Humanities and Social Science Basic Theory Research Project “A study on family language planning for children with delayed speaking in Shandong Province” (grant number 23CRWJ20), and the National Natural Science Foundation of China (No. 82001898).
We would like to thank all the authors of the research included in this meta-analysis for the use of their data and coordinates.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2024.1415411/full#supplementary-material
An, L., Cao, Q. J., Sui, M. Q., Sun, L., Zou, Q. H., Zang, Y. F., et al. (2013). Local synchronization and amplitude of the fluctuation of spontaneous brain activity in attention-deficit/hyperactivity disorder: a resting-state fMRI study. Neurosci. Bull. 29, 603–613. doi: 10.1007/s12264-013-1353-8
Badhiwala, J. H., and Wilson, J. R. (2018). The natural history of degenerative cervical myelopathy. Neurosurg. Clin. N. Am. 29, 21–32. doi: 10.1016/j.nec.2017.09.002
Bai, L., Zhang, L., Chen, Y., Li, Y., Ma, D., Li, W., et al. (2022). Middle cingulate cortex function contributes to response to non-steroidal anti-inflammatory drug in cervical spondylosis patients: a preliminary resting-state fMRI study. Neuroradiology 64, 1401–1410. doi: 10.1007/s00234-022-02964-3
Berberat, J., Andereggen, L., Gruber, P., Hausmann, O., Reza Fathi, A., and Remonda, L. (2023). A diagnostic biomarker for cervical myelopathy based on dynamic magnetic resonance imaging. Spine 48, 1041–1046. doi: 10.1097/BRS.0000000000004667
Bernabéu-Sanz, Á., Mollá-Torró, J. V., López-Celada, S., Moreno López, P., and Fernández-Jover, E. (2020). MRI evidence of brain atrophy, white matter damage, and functional adaptive changes in patients with cervical spondylosis and prolonged spinal cord compression. Eur. Radiol. 30, 357–369. doi: 10.1007/s00330-019-06352-z
Bhagavatula, I. D., Shukla, D., Sadashiva, N., Saligoudar, P., Prasad, C., and Bhat, D. I. (2016). Functional cortical reorganization in cases of cervical spondylotic myelopathy and changes associated with surgery. Neurosurg. Focus 40:E2. doi: 10.3171/2016.3.FOCUS1635
Biswal, B., Yetkin, F. Z., Haughton, V. M., and Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541. doi: 10.1002/mrm.1910340409
Biswal, B. B., Van Kylen, J., and Hyde, J. S. (1997). Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 10, 165–170. doi: 10.1002/(SICI)1099-1492(199706/08)10:4/5<165::AID-NBM454>3.0.CO;2-7
Cai, M., Wang, R., Liu, M., Du, X., Xue, K., Ji, Y., et al. (2022). Disrupted local functional connectivity in schizophrenia: an updated and extended meta-analysis. Schizophrenia 8:93. doi: 10.1038/s41537-022-00311-2
Cavanna, A. E., and Trimble, M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129(Pt 3), 564–583. doi: 10.1093/brain/awl004
Chang, J., Zhu, K., Zhang, S., Wang, Y., Li, Y., Zuo, J., et al. (2023). Dysregulated neural activity between the thalamus and cerebral cortex mediates cortical reorganization in cervical spondylotic myelopathy. Brain Res. Bull. 205:110837. doi: 10.1016/j.brainresbull.2023.110837
Chen, J., Wang, Z., Tu, Y., Liu, X., Jorgenson, K., Ye, G., et al. (2018). Regional homogeneity and multivariate pattern analysis of cervical spondylosis neck pain and the modulation effect of treatment. Front. Neurosci. 12:900. doi: 10.3389/fnins.2018.00900
Chen, S., Wang, Y., Wu, X., Chang, J., Jin, W., Li, W., et al. (2022). Degeneration of the sensorimotor tract in degenerative cervical myelopathy and compensatory structural changes in the brain. Front. Aging Neurosci. 14:784263. doi: 10.3389/fnagi.2022.784263
Chen, Z., Wang, Q., Liang, M., Zhao, R., Zhu, J., Xiong, W., et al. (2018). Visual cortex neural activity alteration in cervical spondylotic myelopathy patients: a resting-state fMRI study. Neuroradiology 60, 921–932. doi: 10.1007/s00234-018-2061-x
Cheng, L., Xi, H., Gu, H., Gao, Y., Hu, S., Li, M., et al. (2023). Abnormalities of regional spontaneous brain activity in poststroke aphasia: a meta-analysis. Cereb. Cortex 33, 7771–7782. doi: 10.1093/cercor/bhad078
Choi, M., Kim, H. C., Youn, I., Lee, S. J., and Lee, J. H. (2024). Use of functional magnetic resonance imaging to identify cortical loci for lower limb movements and their efficacy for individuals after stroke. J. Neuroeng. Rehabil. 21:58. doi: 10.1186/s12984-024-01319-8
Chu, Y., Zhang, Y., Wang, S., and Dai, H. (2022). Resilience mediates the influence of hope, optimism, social support, and stress on anxiety severity among Chinese patients with cervical spondylosis. Front. Psychiatry 13:997541. doi: 10.3389/fpsyt.2022.997541
Dang, J., Tao, Q., Niu, X., Zhang, M., Gao, X., Yang, Z., et al. (2022). Meta-analysis of structural and functional brain abnormalities in cocaine addiction. Front. Psychiatry 13:927075. doi: 10.3389/fpsyt.2022.927075
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Fan, N., Zhao, B., Liu, L., Yang, W., Chen, X., and Lu, Z. (2022). Dynamic and static amplitude of low-frequency fluctuation is a potential biomarker for predicting prognosis of degenerative cervical myelopathy patients: a preliminary resting-state fMRI study. Front. Neurol. 13:829714. doi: 10.3389/fneur.2022.829714
Fard, A. R., Mowforth, O. D., Yuan, M., Myrtle, S., Lee, K. S., Banerjee, A., et al. (2024). Brain MRI changes in degenerative cervical myelopathy: a systematic review. Ebiomedicine 99:104915. doi: 10.1016/j.ebiom.2023.104915
Fox, P. T., Lancaster, J. L., Laird, A. R., and Eickhoff, S. B. (2014). Meta-analysis in human neuroimaging: computational modeling of large-scale databases. Annu. Rev. Neurosci. 37, 409–434. doi: 10.1146/annurev-neuro-062012-170320
Friston, K. J., Frith, C. D., Liddle, P. F., and Frackowiak, R. S. (1993). Functional connectivity: the principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 13, 5–14. doi: 10.1038/jcbfm.1993.4
Garcia-Larrea, L., and Peyron, R. (2013). Pain matrices and neuropathic pain matrices: a review. Pain 154(Suppl. 1), S29–S43. doi: 10.1016/j.pain.2013.09.001
Ge, Z. C., Wu, J. J., Sun, H., and Zhao, Q. (2021). Changes of intrinsic brain activity in patients with cervical spondylotic myelopathy based on fMRI classification. J. Tongji Univ. 42, 785–790. doi: 10.12289/j.issn.1008-0392.21082
Gong, J., Wang, J., Qiu, S., Chen, P., Luo, Z., Wang, J., et al. (2020). Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: voxel-based meta-analysis. Transl. Psychiatry 10:353. doi: 10.1038/s41398-020-01036-5
Goto, M., Abe, O., Hagiwara, A., Fujita, S., Kamagata, K., Hori, M., et al. (2022). Advantages of using both voxel- and surface-based morphometry in cortical morphology analysis: a review of various applications. Magn. Reson. Med. Sci. 21, 41–57. doi: 10.2463/mrms.rev.2021-0096
Ioannidis, J. P., Munafò, M. R., Fusar-Poli, P., Nosek, B. A., and David, S. P. (2014). Publication and other reporting biases in cognitive sciences: detection, prevalence, and prevention. Trends Cogn. Sci. 18, 235–241. doi: 10.1016/j.tics.2014.02.010
Iwabuchi, S. J., Krishnadas, R., Li, C., Auer, D. P., Radua, J., and Palaniyappan, L. (2015). Localized connectivity in depression: a meta-analysis of resting state functional imaging studies. Neurosci. Biobehav. Rev. 51, 77–86. doi: 10.1016/j.neubiorev.2015.01.006
Jia, X. Z., Sun, J. W., Ji, G. J., Liao, W., Lv, Y. T., Wang, J., et al. (2020). Percent amplitude of fluctuation: a simple measure for resting-state fMRI signal at single voxel level. PLoS ONE 15:e0227021. doi: 10.1371/journal.pone.0227021
Jiang, J., Zhao, Y. J., Hu, X. Y., Du, M. Y., Chen, Z. Q., Wu, M., et al. (2017). Microstructural brain abnormalities in medication-free patients with major depressive disorder: a systematic review and meta-analysis of diffusion tensor imaging. J. Psychiatry Neurosci. 42, 150–163. doi: 10.1503/jpn.150341
Kalsi-Ryan, S., Karadimas, S. K., and Fehlings, M. G. (2013). Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist 19, 409–421. doi: 10.1177/1073858412467377
Khan, A. F., Muhammad, F., Mohammadi, E., O'Neal, C., Haynes, G., Hameed, S., et al. (2024). Beyond the aging spine - a systematic review of functional changes in the human brain in cervical spondylotic myelopathy. Geroscience 46, 1421–1450. doi: 10.1007/s11357-023-00954-8
Kuang, C., and Zha, Y. (2019). Abnormal intrinsic functional activity in patients with cervical spondylotic myelopathy: a resting-state fMRI study. Neuropsychiatr. Dis. Treat. 15, 2371–2383. doi: 10.2147/NDT.S209952
Kuang, C. L., and Zha, Y. F. (2024). Neurodegeneration within the rostral spinal cord is associated with brain gray matter volume atrophy in the early stage of cervical spondylotic myelopathy. Spinal Cord 29, 1–7. doi: 10.1038/s41393-024-00971-0
Lau, J., Ioannidis, J. P., Terrin, N., Schmid, C. H., and Olkin, I. (2006). The case of the misleading funnel plot. BMJ 333, 597–600. doi: 10.1136/bmj.333.7568.597
Li, D., Xu, H., Yang, Q., Zhang, M., and Wang, Y. (2022). Cerebral white matter alterations revealed by multiple diffusion metrics in cervical spondylotic patients with pain: a TBSS study. Pain Med. 23, 895–901. doi: 10.1093/pm/pnab227
Liu, X., Lai, H., Li, J., Becker, B., Zhao, Y., Cheng, B., et al. (2021). Gray matter structures associated with neuroticism: a meta-analysis of whole-brain voxel-based morphometry studies. Hum. Brain Mapp. 42, 2706–2721. doi: 10.1002/hbm.25395
Long, Y., Pan, N., Yu, Y., Zhang, S., Qin, K., Chen, Y., et al. (2023). Shared and distinct neurobiological bases of bipolar disorder and attention-deficit/hyperactivity disorder in children and adolescents: a comparative meta-analysis of structural abnormalities. J. Am. Acad. Child Adolesc. Psychiatry 37, 586–604. doi: 10.1016/j.jaac.2023.09.551
Lv, H., Wang, Z., Tong, E., Williams, L. M., Zaharchuk, G., Zeineh, M., et al. (2018). Resting-state functional MRI: everything that nonexperts have always wanted to know. Am. J. Neuroradiol. 39, 1390–1399. doi: 10.3174/ajnr.A5527
Lv, Y., Tian, W., Chen, D., Liu, Y., Wang, L., and Duan, F. (2018). The prevalence and associated factors of symptomatic cervical Spondylosis in Chinese adults: a community-based cross-sectional study. BMC Musculoskelet. Disord. 19:325. doi: 10.1186/s12891-018-2234-0
Magon, S., May, A., Stankewitz, A., Goadsby, P. J., Schankin, C., Ashina, M., et al. (2019). Cortical abnormalities in episodic migraine: a multi-center 3T MRI study. Cephalalgia 39, 665–673. doi: 10.1177/0333102418795163
Martín-Signes, M., Pérez-Serrano, C., and Chica, A. B. (2019). Causal Contributions of the SMA to Alertness and Consciousness Interactions. Cereb. Cortex 29, 648–656. doi: 10.1093/cercor/bhx346
Müller, V. I., Cieslik, E. C., Laird, A. R., Fox, P. T., Radua, J., Mataix-Cols, D., et al. (2018). Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 84, 151–161. doi: 10.1016/j.neubiorev.2017.11.012
Nichols, T., Brett, M., Andersson, J., Wager, T., and Poline, J. B. (2005). Valid conjunction inference with the minimum statistic. Neuroimage 25, 653–660. doi: 10.1016/j.neuroimage.2004.12.005
Oni-Orisan, A., Kaushal, M., Li, W., Leschke, J., Ward, B. D., Vedantam, A., et al. (2016). Alterations in cortical sensorimotor connectivity following complete cervical spinal cord injury: a prospective resting-state fMRI study. PLoS ONE 11:e0150351. doi: 10.1371/journal.pone.0150351
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. doi: 10.1136/bmj.n71
Pan, P., Zhu, L., Yu, T., Shi, H., Zhang, B., Qin, R., et al. (2017). Aberrant spontaneous low-frequency brain activity in amnestic mild cognitive impairment: a meta-analysis of resting-state fMRI studies. Ageing Res. Rev. 35, 12–21. doi: 10.1016/j.arr.2016.12.001
Patri, J. F., Cavallo, A., Pullar, K., Soriano, M., Valente, M., Koul, A., et al. (2020). Transient disruption of the inferior parietal lobule impairs the ability to attribute intention to action. Curr. Biol. 30, 4594–4605.e4597. doi: 10.1016/j.cub.2020.08.104
Pei, F., Hu, W. J., Mao, Y. N., and Zhao, Y. L. (2022). The efficacy of acupuncture combined with Bailemian capsule in the treatment of cervical spondylosis accompanied by headache, anxiety, and depression. Explore 18, 533–538. doi: 10.1016/j.explore.2021.10.012
Qiu, A., Mori, S., and Miller, M. I. (2015). Diffusion tensor imaging for understanding brain development in early life. Annu. Rev. Psychol. 66, 853–876. doi: 10.1146/annurev-psych-010814-015340
Radua, J., Borgwardt, S., Crescini, A., Mataix-Cols, D., Meyer-Lindenberg, A., McGuire, P. K., et al. (2012a). Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci. Biobehav. Rev. 36, 2325–2333. doi: 10.1016/j.neubiorev.2012.07.012
Radua, J., and Mataix-Cols, D. (2009). Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatry 195, 393–402. doi: 10.1192/bjp.bp.108.055046
Radua, J., and Mataix-Cols, D. (2012). Meta-analytic methods for neuroimaging data explained. Biol. Mood Anxiety Disord. 2:6. doi: 10.1186/2045-5380-2-6
Radua, J., Mataix-Cols, D., Phillips, M. L., El-Hage, W., Kronhaus, D. M., Cardoner, N., et al. (2012b). A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry 27, 605–611. doi: 10.1016/j.eurpsy.2011.04.001
Radua, J., Romeo, M., Mataix-Cols, D., and Fusar-Poli, P. (2013). A general approach for combining voxel-based meta-analyses conducted in different neuroimaging modalities. Curr. Med. Chem. 20, 462–466. doi: 10.2174/0929867311320030017
Radua, J., Rubia, K., Canales-Rodríguez, E. J., Pomarol-Clotet, E., Fusar-Poli, P., and Mataix-Cols, D. (2014). Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front. Psychiatry 5:13. doi: 10.3389/fpsyt.2014.00013
Reddy, R. S., Tedla, J. S., Dixit, S., and Abohashrh, M. (2019). Cervical proprioception and its relationship with neck pain intensity in subjects with cervical spondylosis. BMC Musculoskelet. Disord. 20:447. doi: 10.1186/s12891-019-2846-z
Riccelli, R., Toschi, N., Nigro, S., Terracciano, A., and Passamonti, L. (2017). Surface-based morphometry reveals the neuroanatomical basis of the five-factor model of personality. Soc. Cogn. Affect. Neurosci. 12, 671–684. doi: 10.1093/scan/nsw175
Ronaldson, A., Elton, L., Jayakumar, S., Jieman, A., Halvorsrud, K., and Bhui, K. (2020). Severe mental illness and health service utilisation for nonpsychiatric medical disorders: a systematic review and meta-analysis. PLoS Med. 17:e1003284. doi: 10.1371/journal.pmed.1003284
Rottmann, S., Jung, K., Vohn, R., and Ellrich, J. (2010). Long-term depression of pain-related cerebral activation in healthy man: an fMRI study. Eur. J. Pain 14, 615–624. doi: 10.1016/j.ejpain.2009.10.006
Roy, M., Piché, M., Chen, J. I., Peretz, I., and Rainville, P. (2009). Cerebral and spinal modulation of pain by emotions. Proc. Natl. Acad. Sci. U. S. A. 106, 20900–20905. doi: 10.1073/pnas.0904706106
Salvia, E., Tissier, C., Charron, S., Herent, P., Vidal, J., Lion, S., et al. (2019). The local properties of bold signal fluctuations at rest monitor inhibitory control training in adolescents. Dev. Cogn. Neurosci. 38:100664. doi: 10.1016/j.dcn.2019.100664
Serra-Blasco, M., Radua, J., Soriano-Mas, C., Gómez-Benlloch, A., Porta-Casteràs, D., Carulla-Roig, M., et al. (2021). Structural brain correlates in major depression, anxiety disorders and post-traumatic stress disorder: a voxel-based morphometry meta-analysis. Neurosci. Biobehav. Rev. 129, 269–281. doi: 10.1016/j.neubiorev.2021.07.002
Shedid, D., and Benzel, E. C. (2007). Cervical spondylosis anatomy: pathophysiology and biomechanics. Neurosurgery 60(1 Supp1, 1), S7–S13. doi: 10.1227/01.NEU.0000215430.86569.C4
Song, X. W., Dong, Z. Y., Long, X. Y., Li, S. F., Zuo, X. N., Zhu, C. Z., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE 6:e25031. doi: 10.1371/journal.pone.0025031
Su, C., Liu, W., Wang, Q., Qiu, S., Li, M., Lv, Y., et al. (2022). Abnormal resting-state local spontaneous functional activity in irritable bowel syndrome patients: a meta-analysis. J. Affect. Disord. 302, 177–184. doi: 10.1016/j.jad.2022.01.075
Su, Q., Li, J., Chu, X., and Zhao, R. (2023). Preoperative pain hypersensitivity is associated with axial pain after posterior cervical spinal surgeries in degenerative cervical myelopathy patients: a preliminary resting-state fMRI study. Insights Imaging 14:16. doi: 10.1186/s13244-022-01332-2
Sun, L., Li, M., and Li, Y. M. (2016). Predictors for surgical outcome of laminoplasty for cervical spondylotic myelopathy. World Neurosurg. 94, 89–96. doi: 10.1016/j.wneu.2016.06.092
Sun, R., Zhou, J., Qu, Y., Zhou, J., Xu, G., and Cheng, S. (2020). Resting-state functional brain alterations in functional dyspepsia: protocol for a systematic review and voxel-based meta-analysis. Medicine 99:e23292. doi: 10.1097/MD.0000000000023292
Sun, Y., Muheremu, A., and Tian, W. (2018). Atypical symptoms in patients with cervical spondylosis: comparison of the treatment effect of different surgical approaches. Medicine 97:e10731. doi: 10.1097/MD.0000000000010731
Sun, Y., Muheremu, A., Yan, K., Yu, J., Zheng, S., and Tian, W. (2016). Effect of double-door laminoplasty on atypical symptoms associated with cervical spondylotic myelopathy/radiculopathy. BMC Surg. 16:31. doi: 10.1186/s12893-016-0146-1
Sun, Y. Q., Zheng, S., Yu, J., Yan, K., and Tian, W. (2013). Effect of total disc replacement on atypical symptoms associated with cervical spondylosis. Eur. Spine J. 22, 1553–1557. doi: 10.1007/s00586-013-2785-6
Tahmasian, M., Sepehry, A. A., Samea, F., Khodadadifar, T., Soltaninejad, Z., Javaheripour, N., et al. (2019). Practical recommendations to conduct a neuroimaging meta-analysis for neuropsychiatric disorders. Hum. Brain Mapp. 40, 5142–5154. doi: 10.1002/hbm.24746
Takagi, I., Eliyas, J. K., and Stadlan, N. (2011). Cervical spondylosis: an update on pathophysiology, clinical manifestation, and management strategies. Dis. Mon. 57, 583–591. doi: 10.1016/j.disamonth.2011.08.024
Takenaka, S., Kan, S., Seymour, B., Makino, T., Sakai, Y., Kushioka, J., et al. (2019). Towards prognostic functional brain biomarkers for cervical myelopathy: a resting-state fMRI study. Sci. Rep. 9:10456. doi: 10.1038/s41598-019-46859-5
Tan, Y. M., Zhou, F. Q., He, L. C., Wu, L., Zeng, X. J., and Gong, H. H. (2015). Alteration of cerebral regional homogeneity in cervical spondylotic myelopathy: a resting state functional magnetic resonance imaging study. J. Clin. Radiol. 34, 1544–1548. doi: 10.1155/2015/647958
Theodore, N. (2020). Degenerative cervical spondylosis. N. Engl. J. Med. 383, 159–168. doi: 10.1056/NEJMra2003558
Tian, A. X., Gao, H. Z., Wang, Z., Li, N., Ma, J. X., Guo, L., et al. (2023). Brain structural correlates of postoperative axial pain in degenerative cervical myelopathy patients following posterior cervical decompression surgery: a voxel-based morphometry study. BMC Med. Imaging 23:136. doi: 10.1186/s12880-023-01057-8
Tseng, M. T., Chiang, M. C., Yazhuo, K., Chao, C. C., Tseng, W. I., and Hsieh, S. T. (2013). Effect of aging on the cerebral processing of thermal pain in the human brain. Pain 154, 2120–2129. doi: 10.1016/j.pain.2013.06.041
Tyborowska, A., Volman, I., Niermann, H. C. M., Pouwels, J. L., Smeekens, S., Cillessen, A. H. N., et al. (2018). Early-life and pubertal stress differentially modulate grey matter development in human adolescents. Sci. Rep. 8:9201. doi: 10.1038/s41598-018-27439-5
Usui, C., Kirino, E., Tanaka, S., Inami, R., Nishioka, K., Hatta, K., et al. (2020). Music intervention reduces persistent fibromyalgia pain and alters functional connectivity between the insula and default mode network. Pain Med. 21, 1546–1552. doi: 10.1093/pm/pnaa071
Wang, C., Ellingson, B. M., Islam, S., Laiwalla, A., Salamon, N., and Holly, L. T. (2021). Supraspinal functional and structural plasticity in patients undergoing surgery for degenerative cervical myelopathy. J. Neurosurg. Spine 35, 185–193. doi: 10.3171/2020.11.SPINE201688
Wang, C., Pan, Y., Liu, Y., Xu, K., Hao, L., Huang, F., et al. (2018). Aberrant default mode network in amnestic mild cognitive impairment: a meta-analysis of independent component analysis studies. Neurol. Sci. 39, 919–931. doi: 10.1007/s10072-018-3306-5
Wang, C., Sanvito, F., Oughourlian, T. C., Islam, S., Salamon, N., Holly, L. T., et al. (2023). Structural relationship between cerebral gray and white matter alterations in degenerative cervical myelopathy. Tomography 9, 315–327. doi: 10.3390/tomography9010025
Wang, C., Tian, F., Zhou, Y., He, W., and Cai, Z. (2016). The incidence of cervical spondylosis decreases with aging in the elderly, and increases with aging in the young and adult population: a hospital-based clinical analysis. Clin. Interv. Aging 11, 47–53. doi: 10.2147/CIA.S93118
Wang, Q., Wang, C., Deng, Q., Zhan, L., Tang, Y., Li, H., et al. (2022). Alterations of regional spontaneous brain activities in anxiety disorders: a meta-analysis. J. Affect. Disord. 296, 233–240. doi: 10.1016/j.jad.2021.09.062
Wang, Y., Zhao, R., Zhu, D., Fu, X., Sun, F., Cai, Y., et al. (2024). Voxel- and tensor-based morphometry with machine learning techniques identifying characteristic brain impairment in patients with cervical spondylotic myelopathy. Front. Neurol. 15:1267349. doi: 10.3389/fneur.2024.1267349
Whitwell, J. L. (2009). Voxel-based morphometry: an automated technique for assessing structural changes in the brain. J. Neurosci. 29, 9661–9664. doi: 10.1523/JNEUROSCI.2160-09.2009
Woodworth, D. C., Holly, L. T., Mayer, E. A., Salamon, N., and Ellingson, B. M. (2019). Alterations in cortical thickness and subcortical volume are associated with neurological symptoms and neck pain in patients with cervical spondylosis. Neurosurgery 84, 588–598. doi: 10.1093/neuros/nyy066
Wu, K., Liu, M., He, L., and Tan, Y. (2020). Magnetic resonance imaging study on spinal cord structural damage and brain functional organization in patients with cervical spondylotic myelopathy. J. Clin. Radiol. 39, 2364–2369. doi: 10.13437/j.cnki.jcr.2020.12.003
Wu, K., and Wang, X. (2023). Research progress of MRI on brain in patients with cervical spondylotic myelopathy. Chin. J. Magn. Reson. Imaging 14, 187–191. doi: 10.12015/issn.1674-8034.2023.06.034
Wu, K. F., Li, H., Xie, Y. L., Zhang, S. T., and Wang, X. (2024). Fractional amplitude of low-frequency fluctuation alterations in patients with cervical spondylotic myelopathy: a resting-state fMRI study. Neuroradiology 66, 847–854. doi: 10.1007/s00234-024-03337-8
Wu, X., Wang, Y., Chang, J., Zhu, K., Zhang, S., Li, Y., et al. (2023). Remodeling of the brain correlates with gait instability in cervical spondylotic myelopathy. Front. Neurosci. 17:1087945. doi: 10.3389/fnins.2023.1087945
Xu, Y. C., Pan, J. L., Li, B., and Yu, X. C. (2018). Regional homogeneity of brain in patients suffering from chronic neck and shoulder pain caused by cervical spondylotic radiculopathy: a resting-state fMRI study. Radiol. Pract. 33, 549–554. doi: 10.13609/j.cnki.1000-0313.2018.06.001
Yang, F. C., Chou, K. H., Fuh, J. L., Huang, C. C., Lirng, J. F., Lin, Y. Y., et al. (2013). Altered gray matter volume in the frontal pain modulation network in patients with cluster headache. Pain 154, 801–807. doi: 10.1016/j.pain.2013.02.005
Yang, J., Li, B., Yu, Q. Y., Ye, L., Zhu, P. W., Shi, W. Q., et al. (2019). Altered intrinsic brain activity in patients with toothaches using the amplitude of low-frequency fluctuations: a resting-state fMRI study. Neuropsychiatr. Dis. Treat. 15, 283–291. doi: 10.2147/NDT.S189962
Yang, Q., Xu, H., Zhang, M., Wang, Y., and Li, D. (2020). Volumetric and functional connectivity alterations in patients with chronic cervical spondylotic pain. Neuroradiology 62, 995–1001. doi: 10.1007/s00234-020-02413-z
Yao, L., Yang, C., Zhang, W., Li, S., Li, Q., Chen, L., et al. (2021). A multimodal meta-analysis of regional structural and functional brain alterations in type 2 diabetes. Front. Neuroendocrinol. 62:100915. doi: 10.1016/j.yfrne.2021.100915
Yu, C. X., Ji, T. T., Song, H., Li, B., Han, Q., Li, L., et al. (2017). Abnormality of spontaneous brain activities in patients with chronic neck and shoulder pain: a resting-state fMRI study. J. Int. Med. Res. 45, 182–192. doi: 10.1177/0300060516679345
Yu, Y. L., du Boulay, G. H., Stevens, J. M., and Kendall, B. E. (1986). Computed tomography in cervical spondylotic myelopathy and radiculopathy: visualisation of structures, myelographic comparison, cord measurements and clinical utility. Neuroradiology 28, 221–236. doi: 10.1007/BF00548196
Yue, X., and Du, Y. (2020). Altered intrinsic brain activity and regional cerebral blood flow in patients with chronic neck and shoulder pain. Pol. J. Radiol. 85, e155–e162. doi: 10.5114/pjr.2020.94063
Zang, Y., Jiang, T., Lu, Y., He, Y., and Tian, L. (2004). Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394–400. doi: 10.1016/j.neuroimage.2003.12.030
Zang, Y. F., He, Y., Zhu, C. Z., Cao, Q. J., Sui, M. Q., Liang, M., et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83–91. doi: 10.1016/j.braindev.2006.07.002
Zhao, M., Hao, Z., Li, M., Xi, H., Hu, S., Wen, J., et al. (2023). Functional changes of default mode network and structural alterations of gray matter in patients with irritable bowel syndrome: a meta-analysis of whole-brain studies. Front. Neurosci. 17:1236069. doi: 10.3389/fnins.2023.1236069
Zhao, R., Guo, X., Wang, Y., Song, Y., Su, Q., Sun, H., et al. (2022). Functional MRI evidence for primary motor cortex plasticity contributes to the disease's severity and prognosis of cervical spondylotic myelopathy patients. Eur. Radiol. 32, 3693–3704. doi: 10.1007/s00330-021-08488-3
Zhao, R., Su, Q., Chen, Z., Sun, H., Liang, M., and Xue, Y. (2020). Neural correlates of cognitive dysfunctions in cervical spondylotic myelopathy patients: a resting-state fMRI study. Front. Neurol. 11:596795. doi: 10.3389/fneur.2020.596795
Zhou, F., Gong, H., Liu, X., Wu, L., Luk, K. D., and Hu, Y. (2014). Increased low-frequency oscillation amplitude of sensorimotor cortex associated with the severity of structural impairment in cervical myelopathy. PLoS ONE 9:e104442. doi: 10.1371/journal.pone.0104442
Zhou, F., Huang, M., Wu, L., Tan, Y., Guo, J., Zhang, Y., et al. (2018). Altered perfusion of the sensorimotor cortex in patients with cervical spondylotic myelopathy: an arterial spin labeling study. J. Pain Res. 11, 181–190. doi: 10.2147/JPR.S148076
Zhou, F. Q., Tan, Y. M., Wu, L., Zhuang, Y., He, L. C., and Gong, H. H. (2015). Intrinsic functional plasticity of the sensory-motor network in patients with cervical spondylotic myelopathy. Sci. Rep. 5:9975. doi: 10.1038/srep09975
Keywords: amplitude of low-frequency fluctuations, cervical spondylosis, diffusion tensor imaging, meta-analysis, regional homogeneity, surface-based morphometry, voxel-based morphometry
Citation: Cheng L, Zhang J, Xi H, Li M, Hu S, Yuan W, Wang P, Chen L, Zhan L and Jia X (2024) Abnormalities of brain structure and function in cervical spondylosis: a multi-modal voxel-based meta-analysis. Front. Neurosci. 18:1415411. doi: 10.3389/fnins.2024.1415411
Received: 10 April 2024; Accepted: 27 May 2024;
Published: 14 June 2024.
Edited by:
Mingrui Xia, Beijing Normal University, ChinaReviewed by:
Nanfang Pan, Sichuan University, ChinaCopyright © 2024 Cheng, Zhang, Xi, Li, Hu, Yuan, Wang, Chen, Zhan and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xize Jia, amlheGl6ZUBmb3htYWlsLmNvbQ==; Linlin Zhan, emhhbmxpbmxpbjA5MjBAcy5obGp1LmVkdS5jbg==; Lanfen Chen, Y2hlbmxhbmZlbkB3Zm1jLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.