- 1Faculty of Pharmaceutical Sciences (FOP), University of Central Punjab, Lahore, Pakistan
- 2Qatar University Young Scientists Center (QUYSC), Qatar University, Doha, Qatar
Objective: The purpose of this study was to compare donepezil at 5 mg and 10 mg/day against a placebo to systematically evaluate its effectiveness in improving cognitive function among patients suffering from dementia at any stage.
Method: For this systematic review and meta-analysis, we looked up Medline, Scopus, Embase, Web of Science, and The Cochrane Library for articles on the efficacy of donepezil in dementia published in the past 20 years and summarized the placebo and intervention data. Initially, a total of 2,272 articles were extracted using our search query and after the inclusion and exclusion criteria set for extraction of data, 18 studies were included in this review using PRISMA flowchart. The ADAS-cog and MMSE assessment scales were used for measuring the outcomes using IBM SPSS 29.0 for the meta-analysis.
Result: The meta-analysis comprised a total of 18 RCTs (randomized controlled trials) that were randomized to receive either donepezil 5 mg/day (n = 1,556), 10 mg/day (n = 2050) or placebo (n = 2,342). Meta-analysis concerning efficacy showed that donepezil at 10 mg/day significantly improved the MMSE score (g: 2.27, 95%CI: 1.25–3.29) but could not substantially reduce the ADAS-cog. At 5 mg/day donepezil, an overall slight improvement in MMSE score (Hedges’ g: 2.09, 95%CI: 0.88–3.30) was observed.
Conclusion: Both donepezil 5 mg/day and 10 mg/day doses demonstrated improved cognitive functions for patients with dementia, however results indicated that the 10 mg/day dose was more efficacious.
1 Introduction
Dementia is a neurological disorder that usually progresses over time. It is marked by cognitive impairment (language and memory) and non-cognitive alterations (psychosis and behavioral abnormalities; Jin and Liu, 2019; Ohno et al., 2019; Moreno-Morales et al., 2020). According to the World Health Organization (WHO) (WHO, 2023), currently over 55 million individuals are affected by dementia worldwide (WHO, 2023). The most prevalent form of dementia is Alzheimer’s disease (AD; Weller and Budson, 2018; Breijyeh and Karaman, 2020) responsible for about 70% of dementia cases (Aarsland, 2020; WHO, 2023), followed by vascular dementia (VaD; Hu et al., 2022; Morris et al., 2022) constituting about 20% (Aarsland, 2020) of the total cases. Whereas, mild cognitive impairment (MCI) may be considered an early-stage disease in AD as well as VaD (Kasper et al., 2020) with 39.2% of cases progressing to dementia (Bai et al., 2022).
AD is an irreversible and gradual impairment in memory and cognitive functions. It is believed that a decrease in acetylcholine (ACh) production and accumulation of β-amyloid (Aβ) have a major role in its pathogenesis (Cholinesterase inhibitors as Alzheimer’s therapeutics (Review), 2019; Argueta et al., 2022). Unfortunately, AD currently lacks a disease-modifying therapy (DMT; Joe and Ringman, 2019; Noufi et al., 2019; Takramah and Asem, 2022) due to its complex and unclear pathophysiology i.e., genetic or environmental factors (Zhang H, et al., 2022). The success rate of drug development against complete AD progression has been very low and is almost 0% against DMT (Cummings et al., 2019). However, its progression can be slowed and its symptoms can be improved through pharmacotherapy but their therapeutic effects are limited too (Joe and Ringman, 2019; Noufi et al., 2019; Takramah and Asem, 2022). Some studies show that physical exercise can also help improve cognitive symptoms and decrease their rate (Cheng, 2016; Pisani et al., 2021).
Currently, three acetylcholinesterase inhibitors (AChEI; i.e., donepezil, galantamine, and rivastigmine) at any stage of AD and memantine (Argueta et al., 2022) for mild to moderate AD have been authorized by FDA (US Food and Drug Administration) as symptomatic pharmacotherapies (Howard et al., 2012; Grossberg et al., 2013; Tisher and Salardini, 2019). Acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) are candidate enzymes that are responsible for catabolism of ACh in the brain and therefore, acetylcholinesterase inhibitors (AChEI) are used to increase brain Ach levels by inhibiting these enzymes (Birks and Harvey, 2018; Noufi et al., 2019) as well as inhibit Aβ aggregation (Mezeiova et al., 2019). However, out of the three AChEI, donepezil has shown the most hopeful results and therefore has been the most studied drug (Colwell et al., 2022) approved by the FDA in the late 20th century for mild to moderate AD (Marucci et al., 2021) at doses of 5 mg/day and 10 mg/day. Donepezil is a reversible AChE inhibitor having 1,250 times greater affinity toward AChE than BuChE and an inhibitory effect toward Aβ formation at the same time. It is known to be well-tolerated with a high ability to improve cognitive deficits and global function in AD patients showing minimal side effects (Barfejani et al., 2020; Marucci et al., 2021).
In addition, new monoclonal antibodies against amyloid-β i.e., Bapineuzumab and Solanezumab were introduced for the treatment of AD but their development was terminated by Pfizer and Johnson & Johnson in 2012 (Perng et al., 2018). These drugs failed to execute any better results as compared to placebo in trials conducted at late-stage in patients with mild to moderate AD. One study conducted (Abushouk et al., 2017) for Bapineuzumab and a phase 3 trial study conducted by Eli Lily (Sperling et al., 2023) for Solanezumab confirmed these results. However, aducanumab (BiogenInc), another monoclonal antibody recently got approval from the FDA in 2021 for AD treatment in 20 years (Dunn et al., 2021; Argueta et al., 2022) but is still under investigation concerning its risk–benefit evaluation. Besides this, recent studies have also suggested combination therapies effectively treating cognitive impairment. A very famous combination of donepezil and memantine has shown its effect in improving behavioral and psychological symptoms in dementia (Guo et al., 2020; Rong et al., 2021; Yaghmaei et al., 2024) as well as in moderate to severe AD (Knorz and Quante, 2022a) by activation of glutamatergic neurons. Furthermore, according to the latest studies by (Knorz and Quante, 2022), a neuroprotective agent EGb 761 has been shown to reduce concentrations of amyloid-β and AChE significantly (Rong et al., 2021; Knorz and Quante, 2022). In addition to this, various vitamins (Knorz and Quante, 2022) i.e., 25 OH vitamin D in combination with donepezil (Ware et al., 2016; Rong et al., 2021) have demonstrated a greater response to cognition in the case of AD.
In contrast to AD, currently, there are no approved drugs for the treatment of VaD (Patel and Holland, 2022) and MCI. The progress in the development of effective pharmacotherapy for VaD has been a challenge due to its not so well understood pathogenesis i.e., the relation between cerebrovascular pathology and cognitive impairment (Lagunin et al., 2020). Donepezil 5 and 10 mg/day is currently the most widely used drug for the treatment of cognitive symptoms of VaD (Tisher and Salardini, 2019) and MCI (Chen et al., 2021). However, further study is required to determine its efficacy in the treatment of MCI (Devanand et al., 2018). According to certain studies, there is currently insufficient evidence to warrant using cholinesterase inhibitors to treat MCI (Pisani et al., 2021). Therefore, this meta-analysis lays the groundwork for further investigation on the efficacy of donepezil for the treatment of dementia (mainly AD, VaD, and MCI) in improving cognitive symptoms, comparing the dosages of 5 mg/day and 10 mg/day. To achieve this, the present study addresses the following research questions:
1. What are the most effective assessments to measure cognitive symptoms in dementia?
2. Among 5 mg and 10 mg donepezil doses, which one is more effective in managing cognitive symptoms in patients with dementia, as measured through systematic assessments?
2 Methods
For this meta-analysis, a systematic literature review approach was used following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). These guidelines were used because of their ability to review and summarize previous studies while also highlighting areas in which more study is required.
2.1 Literature search strategy
To comprehensively cover all the available research, we searched Medline, Scopus, Embase, Web of Science, and The Cochrane Library. These databases were chosen because of their global reputation, offering an ideal blend of resources. We included studies from the past 20 years to make this study timely. Several trial searches were conducted and then in August 2023, the final search was executed. The search terminology followed was: [(“effic*” OR “saf*” OR “outcom*” OR “resul*” OR “effec*” OR “respons*”) AND (“donepezil” OR “aricept”) AND (“alzheime*” OR “dement*” OR “cognitive”) AND (“random* contro*” OR “placebo contro*”)]. The purpose of this search query was to elicit the study’s title, abstract, or keywords. Additionally, these search terms were chosen because of their frequent usage in medical research related to our topic. The search string was simplified which led to the more extensive search, producing the most publications possible. Afterward, these were narrowed down by using article-specific standards.
2.2 Inclusion and exclusion criteria
This study included all randomized, double-blind, or placebo-controlled trials randomized either as groups or individuals with both intervention and observation groups. Patients diagnosed with any type of dementia showing cognitive symptoms with no restriction to age, origin, gender, etiology, and cognitive impairment severity were considered research participants. Any intervention that compared donepezil with a placebo in patients with dementia was included in this review. In the treatment group where there were doses other than 5 mg/day or 10 mg/day were also inquired (i.e., 1 mg/day or 3 mg/day) in some studies, only doses of donepezil at 5 mg/day or 10 mg/day and the control group given placebo were considered in present study. Additionally, assessment scales for measurement of cognitive symptoms included in this meta-analysis were the Mini-Mental State Examination Scale (MMSE), and the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-cog) at both doses. For the exclusion criteria, the following were considered: (1) Studies that were originally a review, a retrospective study, a case report, or a meta-analysis were excluded; (2) Articles with incomplete, ongoing, or unavailable information were also excluded; and (3) Interventions in which either group was given cholinesterase other than donepezil or any non-pharmacological interventions (i.e., physical or cognitive therapy, music therapy, aromatherapy or physical exercise) were also excluded.
2.3 Cognition
Cognitive assessment scales are frequently used in clinical research of geriatric settings to assess the likelihood of dementia (Mahendran et al., 2015). ADAS-Cog and MMSE assessment scales were used in the present study to evaluate the severity and improvement of cognitive impairment in patients.
2.3.1 ADAS-Cog
The purpose of ADAS-Cog is to quantify and track the progression of cognitive symptoms and has been a cornerstone of substantial clinical research and trials since its introduction in the 1980s (Cogo-Moreira et al., 2021, 2023). ADAS is considered more reliable and accurate than MMSE (Kaufman et al., 2023). It mainly evaluates cognitive areas of memory, language, and orientation consisting of 11 tasks scoring of which is done from 0 to 70 as a single scale. Scoring is based on patient-performed tasks and observer-based evaluation and takes about 30–45 min for its completion (Kueper et al., 2018).
2.3.2 MMSE
Mini-Mental State Examination (MMSE) is the most often used screening instrument commonly used for assessing the extent of cognitive impairment in patients and takes about 7–10 min. It has a total of 30 tasks with subgroups covering memory skills, comprehension, reading, writing, and illustrating skills as well as visual construction attention (Saczynski et al., 2015; Chiu et al., 2021; Jia et al., 2021; Su et al., 2021).
2.4 Data extraction
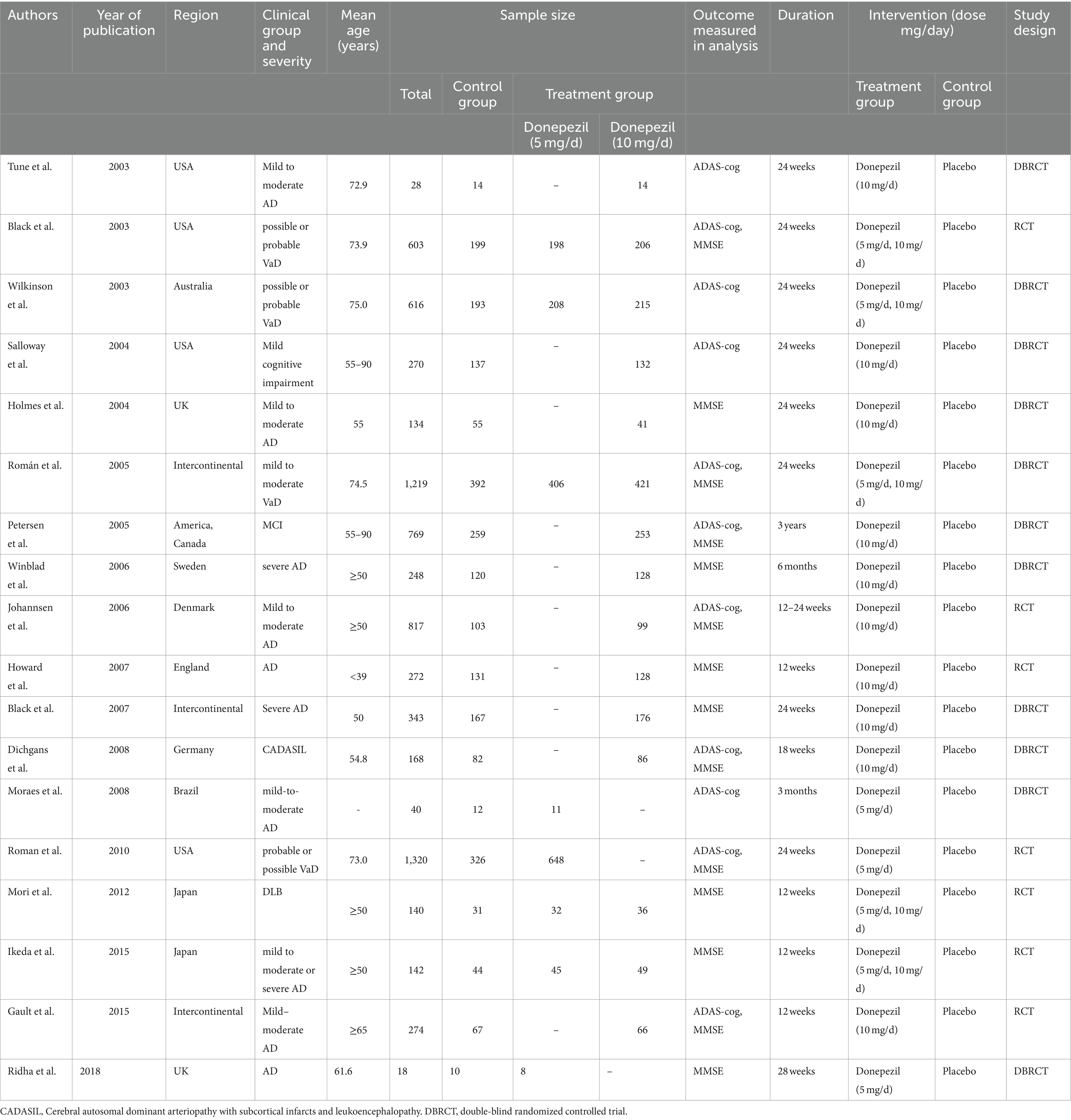
Relevant items were retrieved and cross-checked after one independent researcher examined the entirety of the included literature. The gathered data was then examined by a senior researcher. The following data from included trials was added: initial author, eligibility criteria, publishing year, region of research, research design, sample size, clinical group with severity, age, follow-up period, intervention (dose, duration), and methods of outcome assessments. If accessible, we documented the ITT findings.
2.5 Statistical methods
The goal of a meta-analysis is to compile the statistical data acquired from several research investigations. We used IBM SPSS 29.0 for statistical analysis of the data. When performing a meta-analysis, there is a trade-off between increasing the number of studies to boost strength as well as narrowing the selection to lower heterogeneity. For this meta-analysis, the raw data retrieved from studies was first inserted into a Microsoft Excel template created according to the design of the present study and was then entered in the form of means and standard deviations in SPSS software. This allowed SPSS to identify heterogeneity between studies using the Q test, p-value, and I2, which shows the proportion of overall variance caused by heterogeneity across trials (Higgins et al., 2003; Ioannidis, 2008; DerSimonian and Laird, 2015). Hedges’s g was preferred in this study over Cohen’s d because it is usually used for sample sizes with significant differences and eliminates bias by a correction factor (Hedge’s g Statistic, 2017; Lin and Aloe, 2021). I2 ≥ 50%, p < 0.1 was regarded as substantial heterogeneity between studies, therefore heterogeneity source between them was further analyzed. Moreover, Forest Plots were made using a Forest Plot viewer to see ES distributions and identify outliers. Additionally, 95% confidence intervals were computed for the mean impact size estimate of each study to provide an accuracy metric. Egger’s regression test was utilized by visual examination of funnel plots using the trim-and-fill method to ascertain publication bias (Egger et al., 1997; Duval and Tweedie, 2000). The resultant figure was used to calculate the number of research articles that were overlooked in the meta-analysis and their possible influence on the outcome, as well as to look into possible publication bias.
3 Results
3.1 Characteristics of included studies
The search query yielded 652 hits from the Web of Science repository, 785 hits from the Scopus database, and 366 hits from the Medline database. We also searched Embase returning with 85 hits and The Cochrane Library yielding 384 hits. Using the literature search approach, a total of 2,272 articles were found. After the first search, titles and abstracts were manually reviewed, followed by an inclusion/exclusion criterion. Out of these, 18 articles met the requirements for inclusion. A total of 3,770 patients were assigned to a control group (placebo), while 5,989 patients were assigned to a treatment group (donepezil). Figure 1 illustrates the literature screening procedure whereas Table 1 lists the basic characteristics of included articles.
3.2 Efficacy outcomes
We conducted the MMSE and ADAS-cog tests to evaluate donepezil’s efficacy in patients with cognitive impairment We examined the impact of two commonly administered doses, i.e., 5 mg and 10 mg, on changes in the cognitive function of the patients in comparison to placebo. To more precisely assess the effectiveness of donepezil, we further divided the studies into additional subgroups.
3.2.1 MMSE
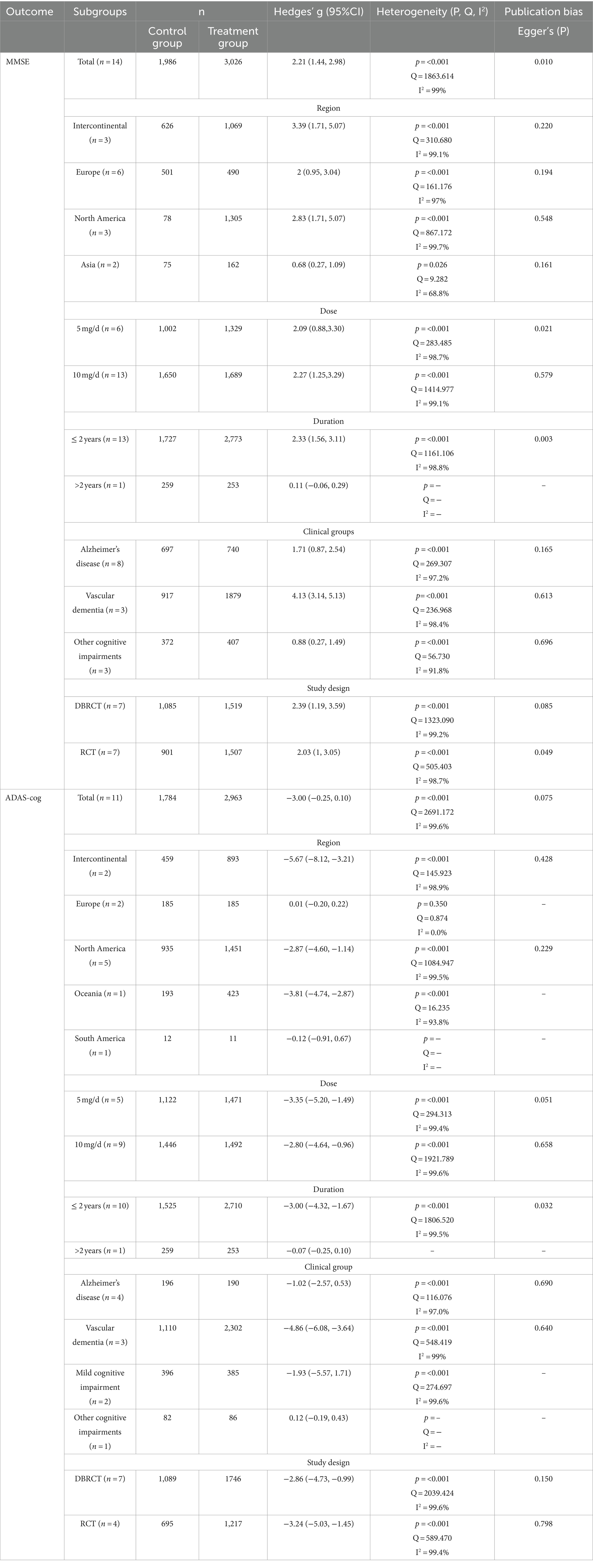
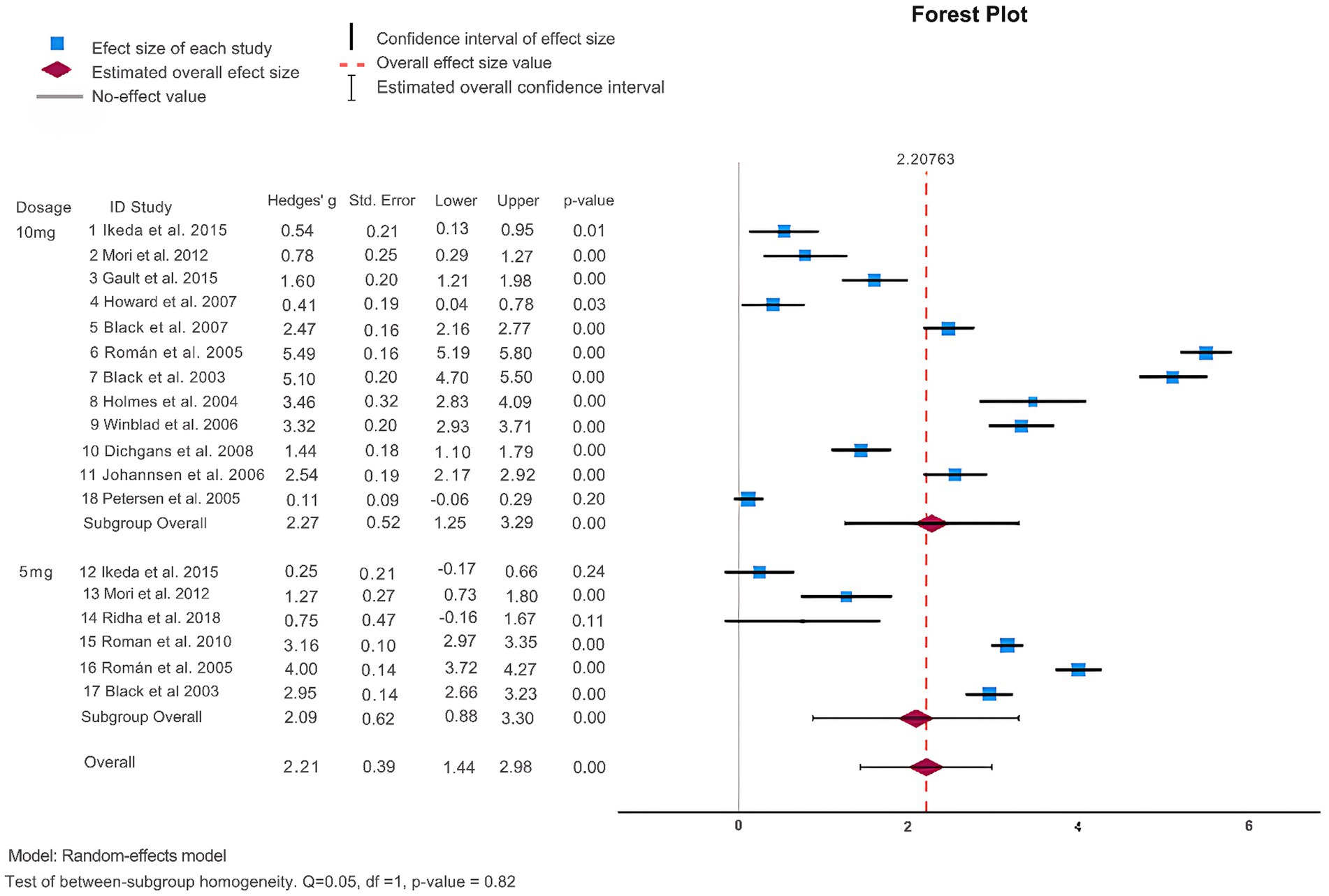
As shown in Table 2 and Figure 2, 14 articles (Black et al., 2003; Holmes et al., 2004; Petersen et al., 2005; Román et al., 2005; Johannsen et al., 2006; Winblad et al., 2006; Black et al., 2007; Howard et al., 2007; Dichgans et al., 2008; Román et al., 2010; Mori et al., 2012; Gault et al., 2015; Ikeda et al., 2015; Ridha et al., 2018) reported the MMSE score change from baseline to endpoint to assess donepezil’s effectiveness in comparison to a placebo. The results of the heterogeneity test indicated a high level of heterogeneity among these studies (I2 = 99%) and the Q-test demonstrated statistical significance (Q = 1863.61, p < 0.001), indicating that a sizable amount of the variability seems to represent true variation. Consequently, for this meta-analysis, a random effects model was used because the observed heterogeneity did not diminish when we observed different study designs i.e., DBRCT and RCT. The analysis indicated that patients undergoing donepezil treatment significantly improved their MMSE score (Hedges’ g: 2.21, 95%CI: [1.44, 2.98]) favoring intervention over the control group (see Supplementary Figure S4). Furthermore, the results of Egger’s test (p = 0.010) showed the absence of publication bias in the included studies.
Subsequently, we performed a subgroup analysis to assess the efficacy of donepezil when administered in doses of 5 mg and 10 mg. We analyzed those patients receiving donepezil 10 mg/day had a significant increase in their MMSE score (Hedges’ g: 2.27, 95%CI: [1.25,3.29]) while 5 mg/day donepezil only slightly managed to increase their MMSE score (Hedges’ g: 2.09, 95%CI: [0.88, 3.30]) as shown in Figure 2. Next, we divided studies into further subgroups for more detailed analysis. When categorized into regions (see Supplementary Figure S1), it was observed that donepezil significantly led to an increase in the MMSE score (Hedges’ g: 3.39, 95%CI: [1.71, 5.07]) in studies conducted intercontinental. In terms of study duration, studies of more than 2 years showed an increase in MMSE score, while no significant difference was observed in studies of less than 2 years (Hedges’ g: 2.33, 95% CI: [1.56, 3.11], I2 = 98.8%) indicating that study duration might be a source of heterogeneity (see Supplementary Figure S2). Furthermore, when analysis was performed in subgroups based on clinical groups, patients with VaD demonstrated an increase in MMSE score (Hedges’ g: 4.13, 95%CI: [3.14, 5.13], I2 = 98.4%) whereas other clinical groups did not exhibit significant difference (see Supplementary Figure S3).
3.2.2 ADAS-cog
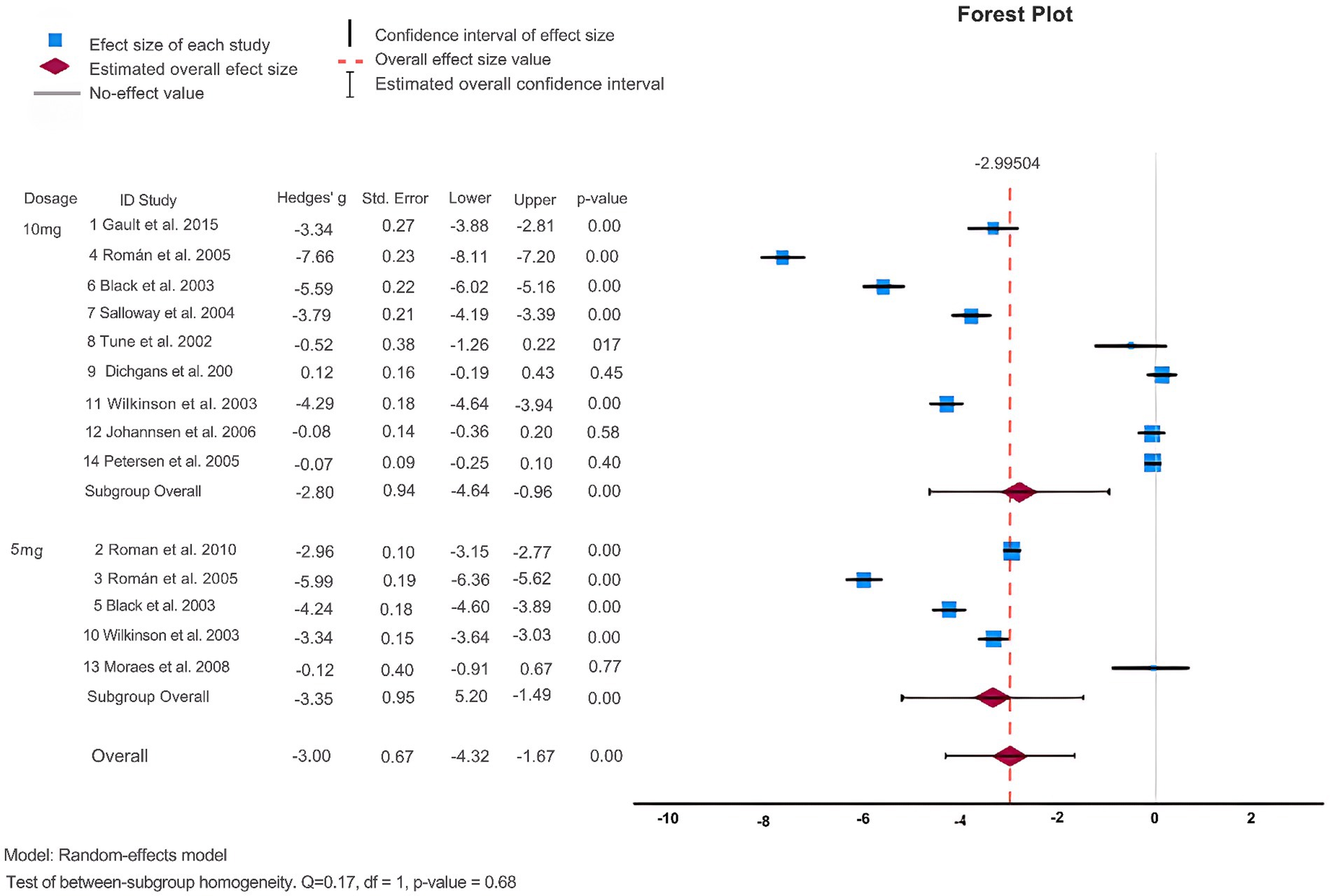
Additionally, ADAS-cog scores were also examined. As shown in Figure 3 and Table 2, 11 articles (Black et al., 2003; Holmes et al., 2004; Salloway et al., 2004; Petersen et al., 2005; Román et al., 2005; Johannsen et al., 2006; Dichgans et al., 2008; Román et al., 2010; Gault et al., 2015Tune et al., 2003; Wilkinson et al., 2003) employed ADAS-cog scores to assess the effectiveness of donepezil in treating dementia. The heterogeneity test revealed a high degree of heterogeneity among the studies (p = <0.001, Q = 2691.172, I2 = 99.6%). When the random effects model was used for the meta-analysis, Egger’s test (p = 0.075) revealed that publication bias was absent. The findings demonstrated that donepezil tended to lower ADAS-cog scores and enhance cognitive function in patients as compared to the control group. The studies were divided into subgroups, and ADAS-cog scores were analyzed. When categorized into regions, donepezil led to an increase in ADAS-cog scores (Hedges’ g: –5.67, 95%CI: [−8.12, −3.21]) only in studies with subjects from intercontinental locations (see Supplementary Figure S5). The heterogeneity test indicated a high degree of heterogeneity (p = <0.001, Q = 145.923, I2 = 98.9%) in these subgroups. When distinguished by the dose of donepezil (5 mg vs. 10 mg), statistically there was no substantial difference in ADAS-cog scores (Hedges’ g: -2.80, 95%CI: [−4.64, −0.96]) as shown in Figure 3. When different clinical groups were considered, there was a substantial increase in heterogeneity (p = <0.001, Q = 548.419, I2 = 99%) among VaD patients compared to other clinical groups (see Supplementary Figure S7). Similarly, the division into RCT and DBRCT groups did not reveal a significant difference in the heterogeneity test (see Supplementary Figure S8).
3.3 Safety outcomes
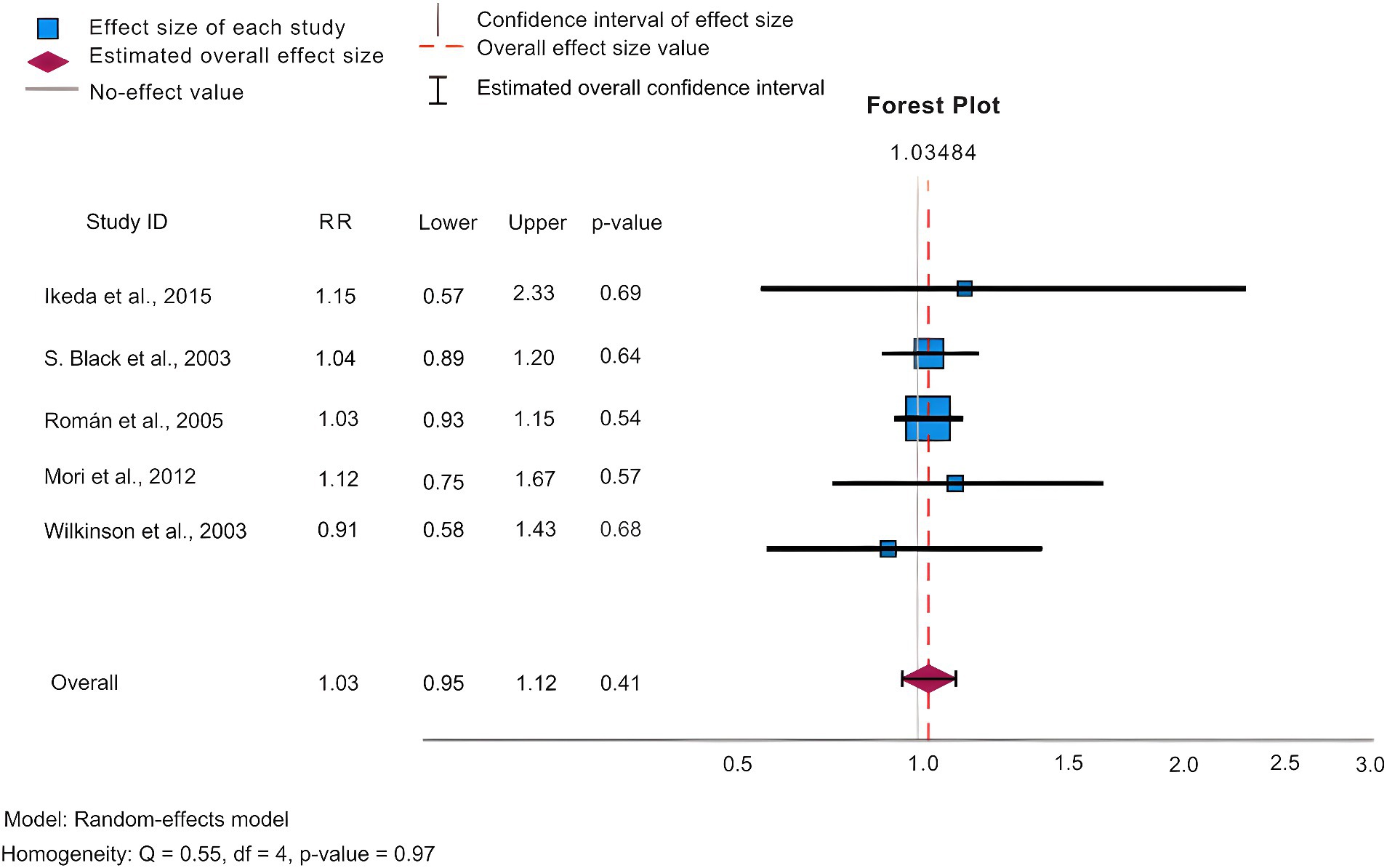
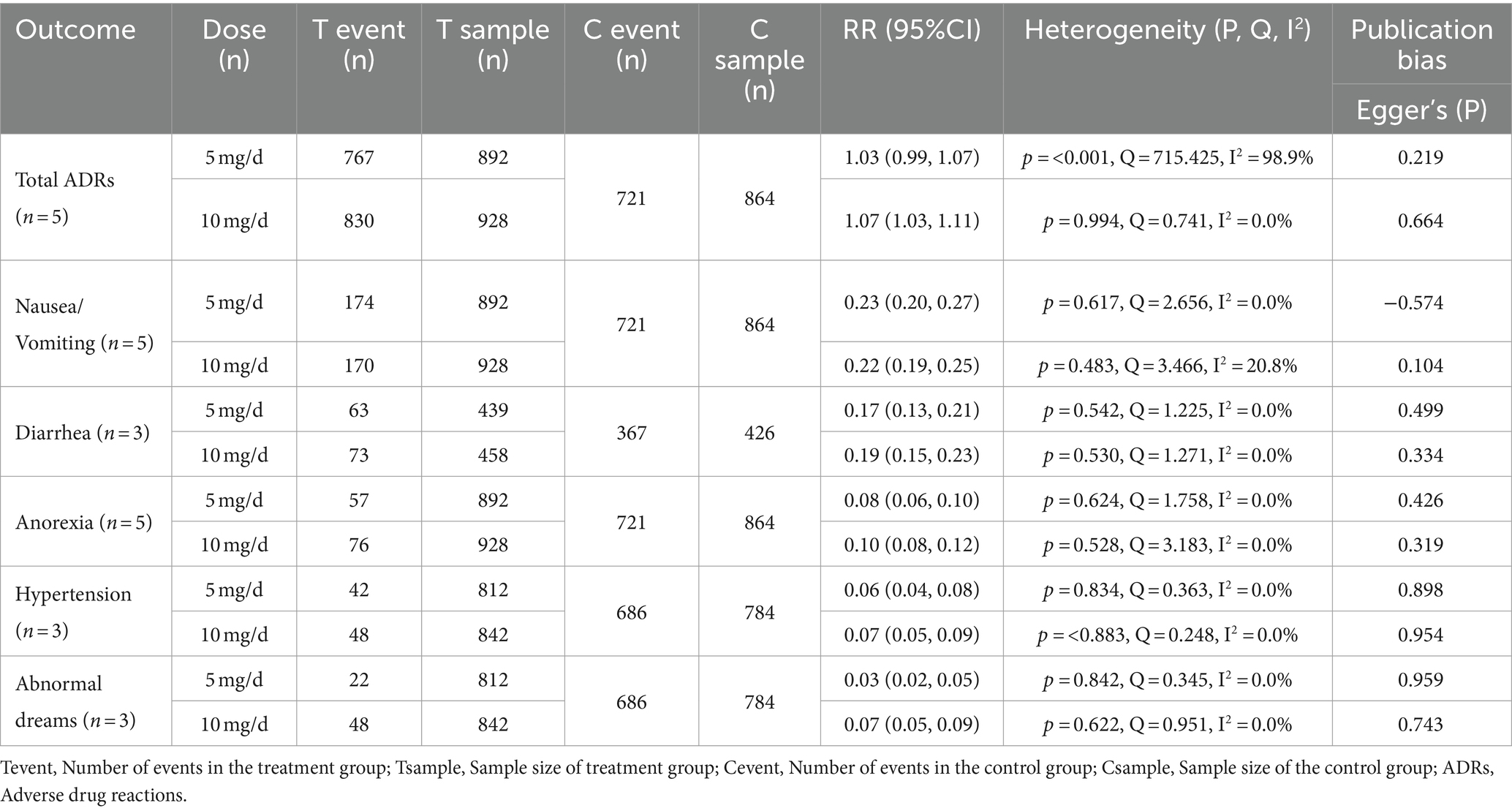
In our analysis, we conducted statistics of common adverse effects observed during the trial, with 5 articles (Black et al., 2003; Wilkinson et al., 2003; Román et al., 2005; Mori et al., 2012; Ikeda et al., 2015) contributing to the analysis. As depicted in Figure 4 for 5 mg/day, Figure 5 for 10 mg/day, and Table 3. The heterogeneity test for the 10 mg/day dosage indicated significant variation among the studies (p = <0.001, Q = 715.425, I2 = 98.9%), using the random effects model for the meta-analysis. Our findings revealed that the likelihood of experiencing adverse reactions with 10 mg/day of donepezil (RR = 1.07, 95%CI = 1.03, 1.11) was higher compared to 5 mg/day (RR = 1.03, 95%CI = 0.99, 1.07). The Egger’s test (p = 0.219) in the case of 5 mg/day, and (p = 0.664) in the case of 10 mg/day suggested no publication bias. Furthermore, we conducted thorough observations of adverse reactions during the trials, selecting adverse effects based on their prevalence and notable differences between the 5 mg/day and 10 mg/day doses. As presented in Table 3, the findings revealed that the 10 mg/day treatment group had a higher risk for adverse events such as nausea/vomiting, diarrhea, anorexia, hypertension, and abnormal dreams. The differences were statistically significant across most adverse events, except for nausea where the 5 mg/day group showed slightly higher results (RR = 0.23, CI95% = 0.20, 0.27).
4 Discussion
This study was intended to ascertain the effectiveness of donepezil in cognitive impairments in doses. This meta-analysis of placebo-controlled trials revealed substantial differences between the donepezil and placebo groups based on MMSE (14 studies, 5,012 participants) and for the ADAS-Cog analysis (11 studies, 4,747 participants). A combination of comparative trials evaluating donepezil’s dose–response at 5 mg/d and 10 mg/d suggested that a higher dosage i.e., 10 mg/day of donepezil is favorable for maintaining or slowing down the progress of cognitive impairment in patients with any stage of dementia. All the patients with AD, Vad, and other cognitive impairments showed significant improvement in MMSE scores and a moderate improvement in ADAS-cog scores and thus these enhancements in cognition appear to have a positive effect on the functioning of the patients with dementia.
Our findings are similar to previous done meta-analyses studies including control vs. treatment group results; however, we did not just limit our study to only one factor. We divided our study into subgroups of duration, dose, region, clinical group, and study design to get more precise results of donepezil efficacy in patients. Previous meta-analysis has been done with either only a specific stage of dementia or its specific type, however, we selected all available studies with dementia i.e., AD, VaD, MCI, CADASIL, and DLB to expand our research, making our conclusions more reliable.
Our main focus throughout the studies was the dose effectiveness of donepezil measured by MMSE and ADAS-Cog scores. Through our results, we found out that 10 mg/day of donepezil was more effective than 5 mg/day and this is concurrent with the results of studies done by Whitehead et al. (2004), Birks and Harvey (2018), and Govind (2020) in case of AD. However, if compared to other treatment strategies both 5 mg and 10 mg donepezil are the most effective strategies for treatment according to results in studies (Kobayashi et al., 2016; Blanco-Silvente et al., 2017; Zhang et al., 2020) in the case of AD. In the case of severe AD, MMSE scores were improved at 10 mg/day of donepezil and this result is also shown by Adlimoghaddam et al. (2018).
Our study also revealed that in the case of MCI, giving 10 mg/day of donepezil showed significant improvement in MMSE scores but no substantial progress in ADAS-cog score and this result was in line with a study done by Zhang X. et al. (2022). Whereas in the case of VaD, our results showed improved ADAS-cog scores, which are in line with the results of Chen et al. (2016), however, our results showed moderate improvement in MMSE scores too which was not concurrent in the latter but was in line with the studies done by Shi et al. (2022) at dose of 10 mg. We could not conclude decisively in the case of DLB and CADSIL due to limited available research as well as very small sample sizes. However, our limited results showed no statistically significant improvement on cognitive scales. ADAS-cog scores were moderately improved, though MMSE scores did not show much improvement.
When subgroups were distinguished by geography, patients from Europe showed significantly enhanced MMSE scores. The subjects’ Asian origin rendered this distinction insignificant. However, studies performed across multiple continents and in North America alone showed moderate improvement as well. Furthermore, subgroup analyses based on clinical groups, dose, region, duration, and type of intervention could not mitigate the high level of heterogeneity among studies. This indicated that individual studies were highly likely to be the source of heterogeneity. Furthermore, one of the possible reasons for heterogeneity among 5 mg and 10 mg studies can be due to the different stages of severity in each study.
Moreover, as the results of this study and previous trials included in this review, are evidence of the fact that donepezil 10 mg/day is more efficacious than donepezil 5 mg/day, the adverse drug reactions (ADRs) of donepezil 10 mg/day, however, are more than donepezil 5 mg/day. One study suggests that ADRs associated with donepezil 10 mg/day are thought to be connected to a quick escalation up to 10 mg/day within 1 week of commencing medication, which was the usual procedure in the donepezil pivotal trials (Doody et al., 2008). However, if we compare 10 mg with higher doses of donepezil, many recent studies show that higher doses, i.e., 15 mg/day up to 23 mg/day of donepezil have more serious side effects and poor safety profiles besides the fast improvement of cognitive symptoms (Study Results, 2007; Hong et al., 2019; jia et al., 2020; Study Results, 2021; Mori et al., 2024a,b). When discussing ADRs, similar studies and a study by Birks and Harvey (2018) suggest that 10 mg/day of donepezil shows mild to moderate ADRs commonly nausea, vomiting, diarrhea, and dizziness as compared to higher doses responsible for ADRs like bradycardia, and urinary incontinence. Given all of these findings, donepezil 10 mg/day appears to be an optimal choice for reducing cognitive problems compared to donepezil 5 mg/day and better tolerability and safety profile compared to higher doses. Although to overcome ADRs of 10 mg/day of donepezil, studies suggest that 10 mg/day of donepezil in combination therapy with agents like memantine shows similar improvement in cognitive symptoms with a better safety profile and tolerable ADRs in contrast to monotherapy of donepezil at 10 mg and higher doses (Rong et al., 2021; Knorz and Quante, 2022).
4.1 Study strengths
This meta-analysis attempted to collate all the published RCT studies on dementia conducted according to inclusion criteria. Previous meta-analyses have opted to limit their analysis to a certain severity range, such as mild to moderate or severe. This was done with the idea that the severity of the disease may have an impact on the medication’s effectiveness. Regardless of the length of the trials or the severity of dementia in the patients, our research found very little evidence that the treatment effects varied between studies. This would corroborate our choice to incorporate all research, regardless of the degree of severity.
4.2 Study limitations
The current study has some limitations, which should be taken into account when interpreting its results. This study was intended to determine the efficacy of donepezil at standard doses; therefore, this meta-analysis excluded the studies that were conducted at higher doses of donepezil, i.e., more than 10 mg. The precision of results for dementia types other than AD and VaD was compromised. This might be due to a limited number of studies that fulfilled the inclusion criteria as well as the small size of these studies. Additionally, the present meta-analysis did not encompass unpublished research or data, which could not be made available even after the requests to the authors.
5 Conclusion
This meta-analysis suggests that as compared to placebo, commonly prescribed cholinesterase inhibitors, donepezil (5 and 10 mg/day) is effective for symptomatic treatment for patients with dementia. All studies included in this meta-analysis showed a positive impact of donepezil on stabilizing and delaying the development of cognitive impairment, with some studies showing statistical significance over others. Donepezil at both doses is efficacious, however, 10 mg/day at 24 weeks is more likely to execute the utmost gain.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MS: Writing – original draft, Formal analysis, Methodology, Software. MA: Writing – review & editing, Validation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
This work was made possible through the Summer Research Internship Program 2023 organized by Qatar University Young Scientists Center (QUYSC). The authors would like to acknowledge Zubair Ahmad for his invaluable guidance, expertise, and support throughout this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2024.1398952/full#supplementary-material
References
Aarsland, D. (2020). Epidemiology and pathophysiology of dementia-related psychosis. J. Clin. Psychiatry 81:27625. doi: 10.4088/JCP.AD19038BR1C
Abushouk, A. I., Elmaraezy, A., Aglan, A., Salama, R., Fouda, S., Fouda, R., et al. (2017). Bapineuzumab for mild to moderate Alzheimer’s disease: a meta-analysis of randomized controlled trials. BMC Neurol. 17, 1–13. doi: 10.1186/S12883-017-0850-1/FIGURES/6
Adlimoghaddam, A., Neuendorff, M., Roy, B., and Albensi, B. C. (2018). A review of clinical treatment considerations of donepezil in severe Alzheimer’s disease. CNS Neurosci. Ther. 24, 876–888. doi: 10.1111/CNS.13035
Argueta, N., Notari, E., and Szigeti, K. (2022). Role of pharmacogenomics in individualizing treatment for Alzheimer’s disease. CNS Drugs 36, 365–376. doi: 10.1007/s40263-022-00915-3
Bai, W., Chen, P., Cai, H., Zhang, Q., Su, Z., Cheung, T., et al. (2022). Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: a meta-analysis and systematic review of epidemiology studies. Age Ageing 51, 1–14. doi: 10.1093/AGEING/AFAC173
Barfejani, A. H., Jafarvand, M., Seyedsaadat, S. M., and Rasekhi, R. T. (2020). Donepezil in the treatment of ischemic stroke: review and future perspective. Life Sci. 263:118575. doi: 10.1016/J.LFS.2020.118575
Birks, J. S., and Harvey, R. J. (2018). Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018:CD001190. doi: 10.1002/14651858.CD001190.pub3
Black, S. E., Doody, R., Li, H., McRae, T., Jambor, K. M., Xu, Y., et al. (2007). Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology 69, 459–469. doi: 10.1212/01.WNL.0000266627.96040.5A
Black, S., Román, G. C., Geldmacher, D. S., Salloway, S., Hecker, J., Burns, A., et al. (2003). Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke 34, 2323–2330. doi: 10.1161/01.STR.0000091396.95360.E1
Blanco-Silvente, L., Castells, X., Saez, M., Barceló, M. A., Garre-Olmo, J., Vilalta-Franch, J., et al. (2017). Discontinuation, efficacy, and safety of cholinesterase inhibitors for Alzheimer’s disease: a Meta-analysis and Meta-regression of 43 randomized clinical trials enrolling 16 106 patients. Int. J. Neuropsychopharmacol. 20, 519–528. doi: 10.1093/IJNP/PYX012
Breijyeh, Z., and Karaman, R. (2020). Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules (Basel, Switzerland) 25:5789. doi: 10.3390/MOLECULES25245789
Chen, Y. X., Liang, N., Li, X. L., Yang, S. H., Wang, Y. P., and Shi, N. N. (2021). Diagnosis and treatment for mild cognitive impairment: a systematic review of clinical practice guidelines and consensus statements. Front. Neurol. 12:719849. doi: 10.3389/fneur.2021.719849
Chen, Y. D., Zhang, J., Wang, Y., Yuan, J. L., and Hu, W. L. (2016). Efficacy of cholinesterase inhibitors in vascular dementia: an updated Meta-analysis. Eur. Neurol. 75, 132–141. doi: 10.1159/000444253
Cheng, S. T. (2016). Cognitive reserve and the prevention of dementia: the role of physical and cognitive activities. Curr. Psychiatry Rep. 18, 1–12. doi: 10.1007/S11920-016-0721-2/METRICS
Chiu, E. C., Chien, T. H., and Lee, Y. C. (2021). Measurement equivalence between the original and estimated Mini-mental state examination in people with dementia. Int. J. Environ. Res. Public Health 18:7616. doi: 10.3390/IJERPH18147616
Cholinesterase inhibitors as Alzheimer’s therapeutics (Review) . (2019). Retrieved 23 October 2023, from https://www.spandidos-publications.com/10.3892/mmr.2019.10374#b5-mmr-20-02-1479
Cogo-Moreira, H., Krance, S. H., Black, S. E., Herrmann, N., Lanctôt, K. L., MacIntosh, B. J., et al. (2021). Questioning the meaning of a change on the Alzheimer’s disease assessment scale–cognitive subscale (ADAS-cog): noncomparable scores and item-specific effects over time. Assessment 28, 1708–1722. doi: 10.1177/1073191120915273
Cogo-Moreira, H., Krance, S. H., Wu, C. Y., Lanctôt, K. L., Herrmann, N., Black, S. E., et al. (2023). State, trait, and accumulated features of the Alzheimer’s disease assessment scale cognitive subscale (ADAS-cog) in mild Alzheimer’s disease. Alzheimer’s and Dementia: Translational Res. Clin. Interven. 9:e12376. doi: 10.1002/TRC2.12376
Colwell, M. J., Tagomori, H., Chapman, S., Gillespie, A. L., Cowen, P. J., Harmer, C. J., et al. (2022). Pharmacological targeting of cognitive impairment in depression: recent developments and challenges in human clinical research. Transl. Psychiatry 12, 484–416. doi: 10.1038/s41398-022-02249-6
Cummings, J., Feldman, H. H., and Scheltens, P. (2019). The “rights” of precision drug development for Alzheimer’s disease. Alzheimers Res. Ther. 11, 76–14. doi: 10.1186/S13195-019-0529-5
DerSimonian, R., and Laird, N. (2015). Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 45, 139–145. doi: 10.1016/J.CCT.2015.09.002
Devanand, D. P., Pelton, G. H., D’Antonio, K., Ciarleglio, A., Scodes, J., Andrews, H., et al. (2018). Donepezil treatment in patients with depression and cognitive impairment on stable antidepressant treatment: a randomized controlled trial. American J.f Geriatric Psychiatry: Official J. American Association for Geriatric Psychiatry 26, 1050–1060. doi: 10.1016/J.JAGP.2018.05.008
Dichgans, M., Markus, H. S., Salloway, S., Verkkoniemi, A., Moline, M., Wang, Q., et al. (2008). Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet. Neurol. 7, 310–318. doi: 10.1016/S1474-4422(08)70046-2
Doody, R. S., Corey-Bloom, J., Zhang, R., Li, H., Ieni, J., and Schindler, R. (2008). Safety and tolerability of donepezil at doses up to 20 mg/day: results from a pilot study in patients with Alzheimer’s disease. Drugs Aging 25, 163–174. doi: 10.2165/00002512-200825020-00008
Dunn, B., Stein, P., and Cavazzoni, P. (2021). Approval of Aducanumab for Alzheimer disease—the FDA’s perspective. JAMA Intern. Med. 181, 1276–1278. doi: 10.1001/JAMAINTERNMED.2021.4607
Duval, S., and Tweedie, R. (2000). Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication Bias in Meta-analysis. Biometrics 56, 455–463. doi: 10.1111/J.0006-341X.2000.00455.X
Egger, M., Smith, G. D., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/BMJ.315.7109.629
Gault, L. M., Ritchie, C. W., Robieson, W. Z., Pritchett, Y., Othman, A. A., and Lenz, R. A. (2015). A phase 2 randomized, controlled trial of the α7 agonist ABT-126 in mild-to-moderate Alzheimer’s dementia. Alzheimer’s & Dementia (New York) 1, 81–90. doi: 10.1016/J.TRCI.2015.06.001
Govind, N. (2020). Donepezil for dementia due to Alzheimer's disease. Br J Community Nurs 25, 148–149. doi: 10.12968/bjcn.2020.25.3.148
Grossberg, G. T., Manes, F., Allegri, R. F., Gutiérrez-Robledo, L. M., Gloger, S., Xie, L., et al. (2013). The safety, tolerability, and efficacy of once-daily memantine (28 mg): a multinational, randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer’s disease taking cholinesterase inhibitors. CNS Drugs 27, 469–478. doi: 10.1007/S40263-013-0077-7
Guo, J., Wang, Z., Liu, R., Huang, Y., Zhang, N., and Zhang, R. (2020). Memantine, donepezil, or combination therapy—what is the best therapy for Alzheimer’s disease? A Network Meta-Analysis. Brain Behav. 10:e01831. doi: 10.1002/BRB3.1831
Hedge’s g Statistic . (2017). Retrieved 29 October 2023, from https://www.itl.nist.gov/div898/software/dataplot/refman2/auxillar/hedgeg.htm
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ: British Medical J. 327, 557–560. doi: 10.1136/BMJ.327.7414.557
Holmes, C., Wilkinson, D., Dean, C., Vethanayagam, S., Olivieri, S., Langley, A., et al. (2004). The efficacy of donepezil in the treatment of neuropsychiatric symptoms in Alzheimer disease. Neurology 63, 214–219. doi: 10.1212/01.WNL.0000129990.32253.7B
Hong, Y. J., Han, H. J., Youn, Y. C., Park, K. W., Yang, D. W., Kim, S., et al. (2019). Safety and tolerability of donepezil 23 mg with or without intermediate dose titration in patients with Alzheimer’s disease taking donepezil 10 mg: a multicenter, randomized, open-label, parallel-design, three-arm, prospective trial. Alzheimers Res. Ther. 11:37. doi: 10.1186/S13195-019-0492-1
Howard, R. J., Juszczak, E., Ballard, C. G., Bentham, P., Brown, R. G., Bullock, R., et al. (2007). Donepezil for the treatment of agitation in Alzheimer’s disease. N. Engl. J. Med. 357, 1382–1392. doi: 10.1056/NEJMOA066583
Howard, R., McShane, R., Lindesay, J., Ritchie, C., Baldwin, A., Barber, R., et al. (2012). Donepezil and Memantine for moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 366, 893–903. doi: 10.1056/NEJMoa1106668
Hu, Y., Zhang, L., Guan, X., Hou, H., Bi, S., Liu, C., et al. (2022). Gunao-Yizhi decoction combined with donepezil for vascular dementia: a systematic review and meta-analysis. Medicine 101:E30971. doi: 10.1097/MD.0000000000030971
Ikeda, M., Mori, E., Matsuo, K., Nakagawa, M., and Kosaka, K. (2015). Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled, confirmatory phase III trial. Alzheimers Res. Ther. 7:4. doi: 10.1186/S13195-014-0083-0
Ioannidis, J. P. A. (2008). Interpretation of tests of heterogeneity and bias in meta-analysis. J. Eval. Clin. Pract. 14, 951–957. doi: 10.1111/J.1365-2753.2008.00986.X
Jia, X., Wang, Z., Huang, F., Su, C., Du, W., Jiang, H., et al. (2021). A comparison of the Mini-mental state examination (MMSE) with the Montreal cognitive assessment (MoCA) for mild cognitive impairment screening in Chinese middle-aged and older population: a cross-sectional study. BMC Psychiatry 21:485. doi: 10.1186/S12888-021-03495-6
Jia, J., Wei, C., Chen, W., Jia, L., Zhou, A., Wang, F., et al. (2020). Safety and efficacy of donepezil 10 mg/day in patients with mild to moderate Alzheimer’s disease. J. Alzheimer’s Dis.: JAD 74, 199–211. doi: 10.3233/JAD-190940
Jin, B., and Liu, H. (2019). Comparative efficacy and safety of therapy for the behavioral and psychological symptoms of dementia: a systemic review and Bayesian network meta-analysis. J. Neurol. 266, 2363–2375. doi: 10.1007/s00415-019-09200-8
Joe, E., and Ringman, J. M. (2019). Cognitive symptoms of Alzheimer’s disease: clinical management and prevention. BMJ 367:l6217. doi: 10.1136/BMJ.L6217
Johannsen, P., Salmon, E., Hampel, H., Xu, Y., Richardson, S., Qvitzau, S., et al. (2006). Assessing therapeutic efficacy in a progressive disease: a study of donepezil in Alzheimer’s disease. CNS Drugs 20, 311–325. doi: 10.2165/00023210-200620040-00005
Kasper, S., Bancher, C., Eckert, A., Förstl, H., Frölich, L., Hort, J., et al. (2020). Management of mild cognitive impairment (MCI): the need for national and international guidelines. World J. Biol. Psychiatry 21, 579–594. doi: 10.1080/15622975.2019.1696473
Kaufman, D. M., Geyer, H. L., Milstein, M. J., and Rosengard, J. L. (2023). Dementia. Kaufman’s Clinical Neurol. Psychiatrists 111–158. doi: 10.1016/B978-0-323-79680-4.00007-3
Knorz, A. L., and Quante, A. (2022). Alzheimer’s disease: efficacy of mono- and combination therapy. A systematic review. J. Geriatr. Psychiatry Neurol. 35, 475–486. doi: 10.1177/08919887211044746
Kobayashi, H., Ohnishi, T., Nakagawa, R., and Yoshizawa, K. (2016). The comparative efficacy and safety of cholinesterase inhibitors in patients with mild-to-moderate Alzheimer’s disease: a Bayesian network meta-analysis. Int. J. Geriatr. Psychiatry 31, 892–904. doi: 10.1002/GPS.4405
Kueper, J. K., Speechley, M., and Montero-Odasso, M. (2018). The Alzheimer's disease assessment scale-cognitive subscale (ADAS-cog): modifications and responsiveness in pre-dementia populations. A narrative review. J. Alzheimer’s Dis. 63, 423–444. doi: 10.3233/JAD-170991
Lagunin, A. A., Ivanov, S. M., Gloriozova, T. A., Pogodin, P. V., Filimonov, D. A., Kumar, S., et al. (2020). Combined network pharmacology and virtual reverse pharmacology approaches for identification of potential targets to treat vascular dementia. Scientific Reports 10, 257–216. doi: 10.1038/s41598-019-57199-9
Lin, L., and Aloe, A. M. (2021). Evaluation of various estimators for standardized mean difference in meta-analysis. Stat. Med. 40, 403–426. doi: 10.1002/SIM.8781
Mahendran, R., Chua, J., Feng, L., Kua, E. H., and Preedy, V. R. (2015). The Mini-mental state examination and other neuropsychological assessment tools for detecting cognitive decline. Diet and Nutrit. Dementia and Cogn. Decline 1159–1174. doi: 10.1016/B978-0-12-407824-6.00109-9
Marucci, G., Buccioni, M., Ben, D. D., Lambertucci, C., Volpini, R., and Amenta, F. (2021). Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 190:108352. doi: 10.1016/J.NEUROPHARM.2020.108352
Mezeiova, E., Chalupova, K., Nepovimova, E., Gorecki, L., Prchal, L., Malinak, D., et al. (2019). Donepezil derivatives targeting amyloid-β Cascade in Alzheimer’s disease. Curr. Alzheimer Res. 16, 772–800. doi: 10.2174/1567205016666190228122956
Moreno-Morales, C., Calero, R., Moreno-Morales, P., and Pintado, C. (2020). Music therapy in the treatment of dementia: a systematic review and meta-analysis. Front. Med. 7, 1–11. doi: 10.3389/FMED.2020.00160/BIBTEX
Mori, E., Ikeda, M., Iseki, E., Katayama, S., Nagahama, Y., Ohdake, M., et al. (2024a). Efficacy and safety of donepezil in patients with dementia with Lewy bodies: results from a 12-week multicentre, randomised, double-blind, and placebo-controlled phase IV study. Psychogeriatrics: Official J. Japanese Psychogeriatric Society 24, 542–554. doi: 10.1111/PSYG.13091
Mori, E., Ikeda, M., and Kosaka, K. (2012). Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled trial. Ann. Neurol. 72, 41–52. doi: 10.1002/ANA.23557
Mori, E., Ikeda, M., and Ohdake, M. (2024b). Donepezil for dementia with Lewy bodies: meta-analysis of multicentre, randomised, double-blind, placebo-controlled phase II, III, and, IV studies. Psychogeriatrics: Official J. Japanese Psychogeriatric Society 24, 589–596. doi: 10.1111/PSYG.13101
Morris, J. C., Chang Wong, E., and Chang Chui, H. (2022). Vascular cognitive impairment and dementia. Continuum (Minneapolis, Minn.) 28, 750–780. doi: 10.1212/CON.0000000000001124
Noufi, P., Khoury, R., Jeyakumar, S., and Grossberg, G. T. (2019). Use of cholinesterase inhibitors in non-Alzheimer’s dementias. Drugs Aging 36, 719–731. doi: 10.1007/S40266-019-00685-6
Ohno, Y., Kunisawa, N., and Shimizu, S. (2019). Antipsychotic treatment of behavioral and psychological symptoms of dementia (BPSD): management of extrapyramidal side effects. Front. Pharmacol. 10:478465. doi: 10.3389/FPHAR.2019.01045/BIBTEX
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. doi: 10.1136/BMJ.N71
Patel, B. B., and Holland, N. W. (2022). Vascular dementia. The 5-minute clinical consult standard 2016. 24th Edn. Wolters Kluwer Health.
Perng, C. H., Chang, Y. C., and Tzang, R. F. (2018). The treatment of cognitive dysfunction in dementia: a multiple treatments meta-analysis. Psychopharmacology 235, 1571–1580. doi: 10.1007/s00213-018-4867-y
Petersen, R. C., Thomas, R. G., Grundman, M., Bennett, D., Doody, R., Ferris, S., et al. (2005). Study questions effectiveness of Alzheimer’s drug. Nat. Rev. Drug Discov. 4:361. doi: 10.1056/NEJMOA050151/SUPPL_FILE/NEJMOA050151SA1.PDF
Pisani, S., Mueller, C., Huntley, J., Aarsland, D., and Kempton, M. J. (2021). A meta-analysis of randomised controlled trials of physical activity in people with Alzheimer’s disease and mild cognitive impairment with a comparison to donepezil. Int. J. Geriatr. Psychiatry 36, 1471–1487. doi: 10.1002/GPS.5581
Ridha, B. H., Crutch, S., Cutler, D., Frost, C., Knight, W., Barker, S., et al. (2018). A double-blind placebo-controlled cross-over clinical trial of DONepezil in posterior cortical atrophy due to underlying Alzheimer’s disease: DONIPAD study. Alzheimer’s Res. Therapy 10, 1–9. doi: 10.1186/S13195-018-0363-1/FIGURES/3
Román, G. C., Salloway, S., Black, S. E., Royall, D. R., Decarli, C., Weiner, M. W., et al. (2010). Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke 41, 1213–1221. doi: 10.1161/STROKEAHA.109.570077
Román, G. C., Wilkinson, D. G., Doody, R. S., Black, S. E., Salloway, S. P., and Schindler, R. J. (2005). Donepezil in vascular dementia: combined analysis of two large-scale clinical trials. Dement. Geriatr. Cogn. Disord. 20, 338–344. doi: 10.1159/000088494
Rong, X., Jiang, L., Qu, M., and Hassan, S. S. (2021). Enhancing therapeutic efficacy of donepezil by combined therapy: a comprehensive review. Curr. Pharm. Des. 27, 332–344. doi: 10.2174/1381612826666201023144836
Saczynski, J. S., Inouye, S. K., Guess, J., Jones, R. N., Fong, T. G., Nemeth, E., et al. (2015). The Montreal cognitive assessment (MoCA): creating a crosswalk with the Mini-mental state examination. J. Am. Geriatr. Soc. 63, 2370–2374. doi: 10.1111/JGS.13710
Salloway, S., Ferris, S., Kluger, A., Goldman, R., Griesing, T., Kumar, D., et al. (2004). Efficacy of donepezil in mild cognitive impairment. Neurology 63, 651–657. doi: 10.1212/01.WNL.0000134664.80320.92
Shi, X., Ren, G., Cui, Y., and Xu, Z. (2022). Comparative efficacy and acceptability of cholinesterase inhibitors and Memantine based on dosage in patients with vascular cognitive impairment: a network Meta-analysis. Curr. Alzheimer Res. 19, 133–145. doi: 10.2174/1567205019666220120112301
Sperling, R. A., Donohue, M. C., Raman, R., Rafii, M. S., Johnson, K., Masters, C. L., et al. (2023). Trial of Solanezumab in preclinical Alzheimer’s disease. N. Engl. J. Med. 389, 1096–1107. doi: 10.1056/NEJMoa2305032
Study Results . Comparison of 23 mg donepezil sustained release (SR) to 10 mg donepezil immediate release (IR) in patients with moderate to severe Alzheimer’s disease | ClinicalTrials.gov. (2007). Retrieved 2 June 2024, from https://clinicaltrials.gov/study/NCT00478205?cond=Dementia&term=Alzheimer%27s%20Disease&intr=Donepezil&page=3&rank=30&tab=results
Study Results . Open-label extension study of 23 mg donepezil SR in participants with moderate to severe Alzheimer’s disease | ClinicalTrials.gov. (2021). Retrieved 2 June 2024, from https://clinicaltrials.gov/study/NCT00566501?cond=Dementia&term=Alzheimer%27s%20Disease&intr=Donepezil&page=3&rank=25&tab=results#results-overview
Su, Y., Dong, J., Sun, J., Zhang, Y., Ma, S., Li, M., et al. (2021). Cognitive function assessed by Mini-mental state examination and risk of all-cause mortality: a community-based prospective cohort study. BMC Geriatr. 21, 1–10. doi: 10.1186/S12877-021-02471-9/TABLES/3
Takramah, W. K., and Asem, L. (2022). The efficacy of pharmacological interventions to improve cognitive and behavior symptoms in people with dementia: a systematic review and meta-analysis. Health Sci. Reports 5:e913. doi: 10.1002/HSR2.913
Tisher, A., and Salardini, A. (2019). A comprehensive update on treatment of dementia. Semin. Neurol. 39, 167–178. doi: 10.1055/S-0039-1683408/ID/JR180075-20/BIB
Tune, L., Tiseo, P. J., Ieni, J., Perdomo, C., Pratt, R. D., Votaw, J. R., et al. (2003). Donepezil HCl (E2020) maintains functional brain activity in patients with Alzheimer disease: results of a 24-week, double-blind, placebo-controlled study. Am. J. Geriatr. Psychiatry 11, 169–177. doi: 10.1097/00019442-200303000-00007
Ware, C., Assoun, P.-L., Cristancho, V., Djabelkhir, L., Wu, Y.-H., and Rigaud, A.-S. (2016). P4-384: donepezil and vitamin d in Alzheimer’S disease: the Donde study. Alzheimers Dement. 12, P1184–P1185. doi: 10.1016/J.JALZ.2016.07.130
Weller, J., and Budson, A. (2018). Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 7:1161. doi: 10.12688/f1000research.14506.1
Whitehead, A., Perdomo, C., Pratt, R. D., Birks, J., Wilcock, G. K., and Evans, J. G. (2004). Donepezil for the symptomatic treatment of patients with mild to moderate Alzheimer’s disease: a meta-analysis of individual patient data from randomised controlled trials. Int. J. Geriatr. Psychiatry 19, 624–633. doi: 10.1002/GPS.1133
WHO . (2023). Dementia. Retrieved 11 October 2023, from https://www.who.int/news-room/fact-sheets/detail/dementia/?gclid=CjwKCAjwyY6pBhA9EiwAMzmfwTj8cCAWwNAL88FEQGo5lIGW0vZyKkq7O424hQCUoUa40ldL1EQ4uBoCBQcQAvD_BwE
Wilkinson, D., Doody, R., Helme, R., Taubman, K., Mintzer, J., Kertesz, A., et al. (2003). Donepezil in vascular dementia. Neurology 61, 479–486. doi: 10.1212/01.WNL.0000078943.50032.FC
Winblad, B., Kilander, L., Eriksson, S., Minthon, L., Båtsman, S., Wetterholm, A. L., et al. (2006). Donepezil in patients with severe Alzheimer’s disease: double-blind, parallel-group, placebo-controlled study. Lancet (London, England) 367, 1057–1065. doi: 10.1016/S0140-6736(06)68350-5
Yaghmaei, E., Lu, H., Ehwerhemuepha, L., Zheng, J., Danioko, S., Rezaie, A., et al. (2024). Combined use of donepezil and Memantine increases the probability of five-year survival of Alzheimer’s disease patients. Communications Med. 4, 99–98. doi: 10.1038/s43856-024-00527-6
Zhang, X., Lian, S., Zhang, Y., and Zhao, Q. (2022). Efficacy and safety of donepezil for mild cognitive impairment: a systematic review and meta-analysis. Clin. Neurol. Neurosurg. 213:107134. doi: 10.1016/J.CLINEURO.2022.107134
Zhang, T., Liu, N., Cao, H., Wei, W., Ma, L., and Li, H. (2020). Different doses of pharmacological treatments for mild to moderate Alzheimer’s disease: a bayesian network meta-analysis. Front. Pharmacol. 11:529266. doi: 10.3389/FPHAR.2020.00778/BIBTEX
Keywords: systematic review, meta-analysis, cognition, donepezil, dementia, Alzheimer’s disease, vascular dementia, cognitive impairment
Citation: Sheikh M and Ammar M (2024) Efficacy of 5 and 10 mg donepezil in improving cognitive function in patients with dementia: a systematic review and meta-analysis. Front. Neurosci. 18:1398952. doi: 10.3389/fnins.2024.1398952
Edited by:
Sadayuki Hashioka, Asahikawa Medical University, JapanReviewed by:
Ronald Glenn Thomas, University of California, San Diego, United StatesSyed Shams ul Hassan, Shanghai Jiao Tong University, China
Copyright © 2024 Sheikh and Ammar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehak Sheikh, bWVoLnNoMjExQGdtYWlsLmNvbQ==; Mohammad Ammar, bS5hbW1hckBxdS5lZHUucWE=
 Mehak Sheikh
Mehak Sheikh Mohammad Ammar
Mohammad Ammar