- 1Adaptive Neural Systems Group, The Institute for Integrative and Innovative Research, University of Arkansas, Fayetteville, AR, United States
- 2Biomedical Engineering Department, University of Arkansas, Fayetteville, AR, United States
Novel bioelectronic medical devices that target neural control of visceral organs (e.g., liver, gut, spleen) or inflammatory reflex pathways are innovative class III medical devices like implantable cardiac pacemakers that are lifesaving and life-sustaining medical devices. Bringing innovative neurotechnologies early into the market and the hands of treatment providers would benefit a large population of patients inflicted with autonomic and chronic immune disorders. Medical device manufacturers and software developers widely use the Waterfall methodology to implement design controls through verification and validation. In the Waterfall methodology, after identifying user needs, a functional unit is fabricated following the verification loop (design, build, and verify) and then validated against user needs. Considerable time can lapse in building, verifying, and validating the product because this methodology has limitations for adjusting to unanticipated changes. The time lost in device development can cause significant delays in final production, increase costs, and may even result in the abandonment of the device development. Software developers have successfully implemented an Agile methodology that overcomes these limitations in developing medical software. However, Agile methodology is not routinely used to develop medical devices with implantable hardware because of the increased regulatory burden of the need to conduct animal and human studies. Here, we provide the pros and cons of the Waterfall methodology and make a case for adopting the Agile methodology in developing medical devices with physical components. We utilize a peripheral nerve interface as an example device to illustrate the use of the Agile approach to develop neurotechnologies.
1 Introduction
Healthcare professionals routinely use various medical devices to save, support, and sustain patients’ lives. The United States of America Food and Drug Administration (FDA) and other international regulatory institutions provide guidance documents to medical device industry professionals for developing safe and effective medical devices and approve them for use by persons in need. Traditional medical devices have physical (electronics and/or mechanical) and sometimes also software components [referred to as “software in medical devices (SiMD)” (FDA, 2020)]. Recent advances in digital health have created a new device category, “software as a medical device (SaMD)” (FDA, 2018b), which works independently without the need for physical components (e.g., software that extracts diagnostic information from X-rays).
The Waterfall methodology has been considered a de facto method for developing medical devices (hardware devices, SiMD, and SaMD) among established medical manufacturers. It follows sequential processes with strictly defined milestones (Figure 1A) with clear decision points for moving to the next steps. It is popular not only because the FDA suggested it in their guidance document but also because it provides a concise pathway for documenting the quality of the device design to ensure the safety and efficacy of the product. However, one of the primary criticisms of the Waterfall methodology is that the entire development process is too strict and has little room for adapting to unanticipated changes, which causes significant delays (Lee et al., 2006; Antonini et al., 2021; Slattery et al., 2022). To address such delays, the developers of SiMDs and SaMDs have adopted Agile, V-model, and other lean methodologies (Lee et al., 2006; Rottier and Rodrigues, 2008; Antonini et al., 2021; ScaledAgile, 2022; Slattery et al., 2022). In the Agile methodology (Figure 1B), the product is developed in increments, called Sprints, to produce testable and deployable products by allowing the requirements to evolve during the development to reduce delays.
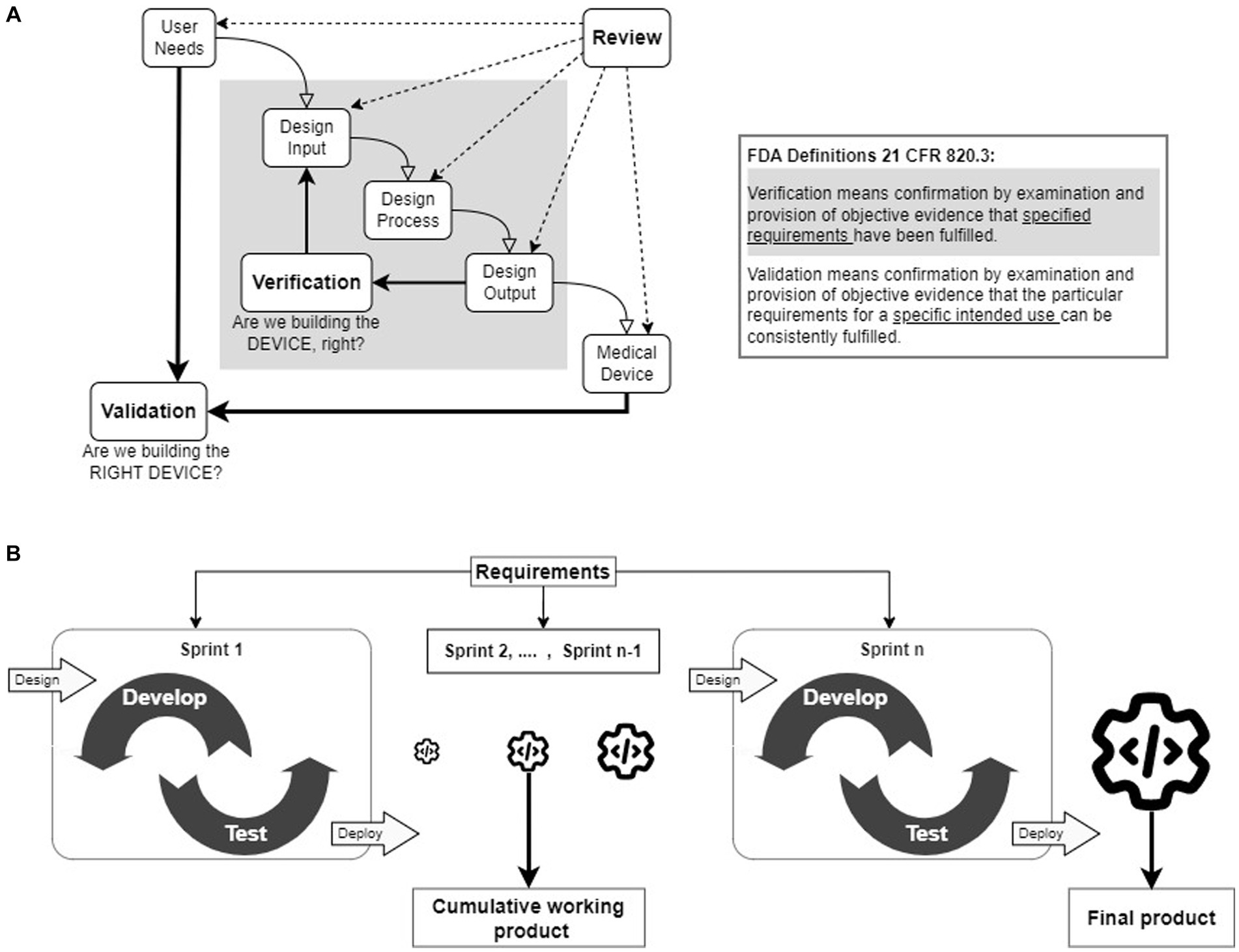
Figure 1. (A) Waterfall design control process. The waterfall methodology follows a step-by-step linear progression of logical sequences of design control processes. The Design Verification process, highlighted in gray, is an iterative process to confirm that the Design Inputs match the Design Outputs. Design Validation process is also an iterative process to prove that the device’s intended purpose is met by evaluating User Needs. The inset provides FDA-specified definitions of verification and validation processes. (B) The Agile framework. The Agile frameworks were developed from the Agile manifesto, which gives importance to flexibility to adapt to changes and quick delivery of the functional product for deployment. The primary concept of an Agile framework is to divide the project into small functional units (referred to as sprints) for development and testing. Each successful sprint enables the deployment of working software products. The final product is the summation of all the working software products.
The Agile inspired “Crisis-Responsive Framework” was developed during the COVID-19 pandemic to tackle the global scarcity crisis of personal protective equipment (PPE) by accelerating the production using unconventional sources for supply, engaging stakeholders throughout the development process, and modifying verification and validation processes (Antonini et al., 2021). Although this framework was successful in developing PPE, a Class I device, adopting Agile in developing high-risk (Class II/III) medical devices is challenging. One of the limiting factors is the need for preclinical and clinical validation studies to obtain regulatory approval (Famm et al., 2013; McHugh et al., 2013; Jung et al., 2017; Horn et al., 2019; Pavlov and Tracey, 2019; Cho et al., 2020; Larson and Meng, 2020; Magisetty and Park, 2022). These studies are time-consuming; hence, delivering a functional product quickly to test is challenging.
This review provides salient aspects and shortcomings of the Waterfall and Agile methodologies. It may spur a dialog among academic researchers and business leaders in neurotechnology development on the applicability and usefulness of Agile Frameworks. We illustrate the use of the Agile methodology as an alternative to the established Waterfall methodology using the development of electrodes for peripheral nerve interfaces (PNI) as an example.
2 Waterfall methodology
The FDA provides detailed regulations (21 CFR 800–898) and guidelines for developing, prescribing, and using medical devices (FDA, 2018a). The Design Controls (21CFR 820.30) are crucial for ensuring the quality of medical devices and user safety under quality management system regulations. The FDA released the “Design Control Guidance for Medical Device Manufacturers” guidance document, which introduced the use of design controls through the Waterfall methodology (FDA, 1998; Figure 1A). The Waterfall methodology follows a logical sequence (User Needs, Design Input, Design Process, and Design Output) followed by Design Verification and Design Validation iterative processes (FDA, 1998; Fry et al., 2016). Throughout the device development cycle, reviews are regularly conducted to compile the comprehensive documentation necessary for FDA approval.
Compiling a comprehensive list of User Needs at the beginning of the development process is critical for successful medical device development and deployment. To achieve this, multiple interviews and reviews are conducted to gather input from all the stakeholders (patients, doctors, device manufacturers, and others). The User Needs include intended uses of the device (primary purpose), indications for use (medical conditions aimed to diagnose, treat, or cure), and other end-user needs, such as operator and environmental needs.
After the development team approves the User Needs list, the Design Inputs are defined from the User Needs and are translated to Design Specifications. The Design Specifications are reviewed and approved to ensure that the specifications can be tested, measured, or observed.
In the Design Process, the approved Design Specifications are used to develop detailed documentation of product drawings, tools used, and instructions to produce tangible products (physical devices or computer models/programs). The design reviews are conducted and approved to ensure the proposed tools can produce the designed product. Design using the design documents developed in the Design Process.
The iterative Design Verification process ensures that the Design Outputs meet the Design Inputs, aka Design Specifications (see: gray shaded inner loop in Figure 1A). To achieve this, testing protocols are written for each Design Specification, and tests are run to compare with the corresponding Design Output. The design refinement continues iteratively until the Design Outputs meet the Design Specifications. At the end of each iteration, Design Reviews are conducted to monitor the quality of the designs and the ability of the designs to produce products to the specifications. A fully verified device is produced for Design Validation.
The iterative Design Validation process is the most critical development process (see: outer loop in Figure 1A). It ensures that the verified medical device meets all the defined User Needs, and is safe and effective for use. This process also continues until the finished product satisfies all the User Needs and may require conducting preclinical or clinical studies.
3 Need for an alternative device design control methodology
The primary reason for significant delays in utilizing the Waterfall method (FDA, 1998) is the need to make unanticipated changes during validation studies (Rottier and Rodrigues, 2008; Stare, 2014; Papadakis and Tsironis, 2020; Salisbury, 2021; Slattery et al., 2022). The unanticipated changes in User Needs and Design Specifications can cause significant delays in the development of the device, can become expensive, and could entirely stop the development process. The compiled comprehensive list of User Needs at the beginning of the development process may be incomplete or have incorrect entries due to misinterpreting stakeholders’ responses or other constraints in engaging various stakeholder groups. This leads to identifying new User Needs at later stages of device development, causing significant delays. Inadequate engagement with various stakeholders, insufficient literature review, or limited data from pilot studies may lead to errors in translating User Needs into Design Specifications, resulting in flaws in defining each design specification’s accuracy, precision, and tolerance metrics. This may, in turn, lead to failure in design verification or require an increased number of verification loops, causing delays and or depletion of resources.
Changes during medical device development are inevitable. Hence, there is a need for an alternative design control methodology that can adapt to the unanticipated changes during the product development process.
4 Agile methodology as an alternative
A group of software developers who found the Waterfall methodology highly ineffective authored the “Agile Manifesto” (Beck et al., 2001) with four values and twelve principles. Based on these values and principles, software developers have created multiple Agile frameworks such as Scrum, Extreme Programming, and Kanban (Stare, 2014; Theobald et al., 2019; Reiff and Schlegel, 2022). These Agile frameworks promote flexibility in following predefined processes, delivering products quickly, collaborating with end-users for validation tests, and in adjusting plans in response to changing requirements (Antonini et al., 2021). In Agile (Figure 1B), the project is divided into short, repeatable phases (Rottier and Rodrigues, 2008; Theobald et al., 2019; Birk, 2021; Holden et al., 2021; Reiff and Schlegel, 2022; ScrumAlliance, 2023), often referred to as Sprints. Each successful Sprint incorporates one or more requirements to build and evaluate a functional unit. The outcomes of the first Sprint are integrated into subsequent Sprints to develop an incrementally improved functional unit until the completion of the project. Thus, the effect of unanticipated changes in User Needs and Design Specifications can be mitigated by adopting the Agile methodology.
5 Barriers to adopting Agile for traditional medical devices
The Agile process, as used for general software development, does not meet the needs for the development of medical device software, which includes extensive planning for use, assuring traceability of requirements, rigorous documentation of the development process, and ability to meet regulatory compliance. However, some developers of SiMD and SaMD have overcome the above-mentioned operational barriers and are either already using or actively pursuing the Agile methodology (Birk, 2021) for developing medical device software to reduce time-to-market (Antonini et al., 2021; Salisbury, 2021). The authors of the Agile-inspired tailored “Crisis Crisis-Responsive Framework” described two significant Barriers to developing Class I traditional medical devices with physical components: increased verification and validation tasks, including biocompatibility testing, because at least one physical component (structural, mechanical, electronic, or a combination thereof) is in contact with living tissue, and extensive safety and efficacy documentation for regulatory approval (Antonini et al., 2021).
6 Adoption of the Agile methodology for developing peripheral nerve interfaces
Recent innovations in computer-aided programs for developing designs, finite element analysis for testing designs in realistic simulated conditions, and 3D printing technology offer opportunities for accelerating the development of devices with physical components utilizing the Agile process (FDA, 2017; Milovanović et al., 2019; Ghilan et al., 2020; Al-Dulimi et al., 2021; Calvo-Haro et al., 2021; Kumar et al., 2021; Algorri et al., 2022). Here, we use a peripheral nerve interface (PNI) design as an exemplar of the Agile methodology for medical hardware device development. An Agile methodology may accelerate the development of novel PNIs for use in emerging bioelectronic medicine therapies. To successfully adopt the methodology, the functional product delivery (design and fabrication) and testing (verification and validation) from each Sprint should be accelerated. This will permit the development team to learn about the product’s performance in a realistic end-user environment and allow consideration of the end-user’s experience in the use of the product. The identified barriers of the need for increased testing and the lack of comprehensive documentation for FDA review must also be overcome.
6.1 Accelerating sprints
6.1.1 Design process
Reviewing existing PNIs provides insights into design considerations and could accelerate the design of new PNIs. The authors of several review articles (Ghafoor et al., 2017; Giagka and Serdijn, 2018; Russell et al., 2019; Wu and Peng, 2019; Cho et al., 2020; Larson and Meng, 2020; Wu and Guo, 2020; Yildiz et al., 2020; del Valle et al., 2021; Eiber et al., 2021; Farina et al., 2021; Paggi et al., 2021; Selim et al., 2021) provide different perspectives but a convergent view on primary design requirements for PNI. The three primary requirements are identifying the target nerve for interfacing, choosing the electrode type for interfacing, and determining the electronic hardware to stimulate or record neural activity. The other components of PNIs include electronics packaging and its connections with electrodes. Here, we focus our discussion on the development of neural electrodes.
Considerations for identifying the target nerve for interfacing with the electrode depend on the intended use of the proposed medical device. The selection of the electrode type (extraneural vs. intraneural) depends on the trade-off between the electrode performance properties (selectivity and specificity) versus invasiveness. The selectivity of the electrode (Ottestad and Orlovich, 2020) is defined as the ability to interface with a distinct group of nerve fibers. The specificity (Overstreet et al., 2019; Verma et al., 2023) is defined as the ability to activate a specific targeted group of nerve fibers to elicit a desired neural function. All the reviews cited above suggest that intrafascicular (intraneural) electrodes can achieve higher selectivity and specificity but at the cost of invasiveness.
The material considerations for fabricating electrodes depend on the stimulation and/or recording function of the PNI (Cogan, 2008; Grill et al., 2009). For stimulating, the electrode material should have sufficient charge-carrying capacity to stimulate and/or block neural activity without causing electrode material dissolution or neural tissue damage. For recording, the distance between the electrode and neural signal source and the impedance of the electrode should be chosen such that the signal-to-noise ratio is high.
6.1.2 Device fabrication
3D printing, emerging bioprinting technologies, and off-the-shelf electronic or mechanical components can be used to fabricate functional devices to accelerate the verification and validation processes (Pena et al., 2017; Shepherd et al., 2018; Larson and Meng, 2020). This approach enables the development team to modify the designs quickly to conduct verification/validation studies. For example, biocompatible material was utilized to 3D print implantable components to verify and validate a quick to implant intrafascicular electrode (Q-PINE) array (Strauss et al., 2020), and a direct laser writing technique was used to print a nanoclip electrode capable of interfacing with ~50 μm diameter nerves (Lissandrello et al., 2017; Otchy et al., 2020).
6.1.3 Verification process
Depending on the proposed medical device’s intended use for a specific PNI, one or more in-vitro and bench-top testing procedures in Table 1 could accelerate the verification process. In many instances, various jigs/fixtures may be needed for conducting verification tests. Instead of using conventional machining tools to build the jigs/fixtures, a 3D printer could offer a quick fabrication turnaround. 3D-printed fixtures are easier to scale if the device needs to be verified for varied sizes. In some cases, digital twins, i.e., computational models of the device (Morrison et al., 2018; Ahmed et al., 2023), can be used for verification. These testing procedures could also be used to verify the mechanical (e.g., electronics package hermicity) and electrical performances (e.g., communication protocols, power delivery) of the stimulating/recording electronics and electrode leads.
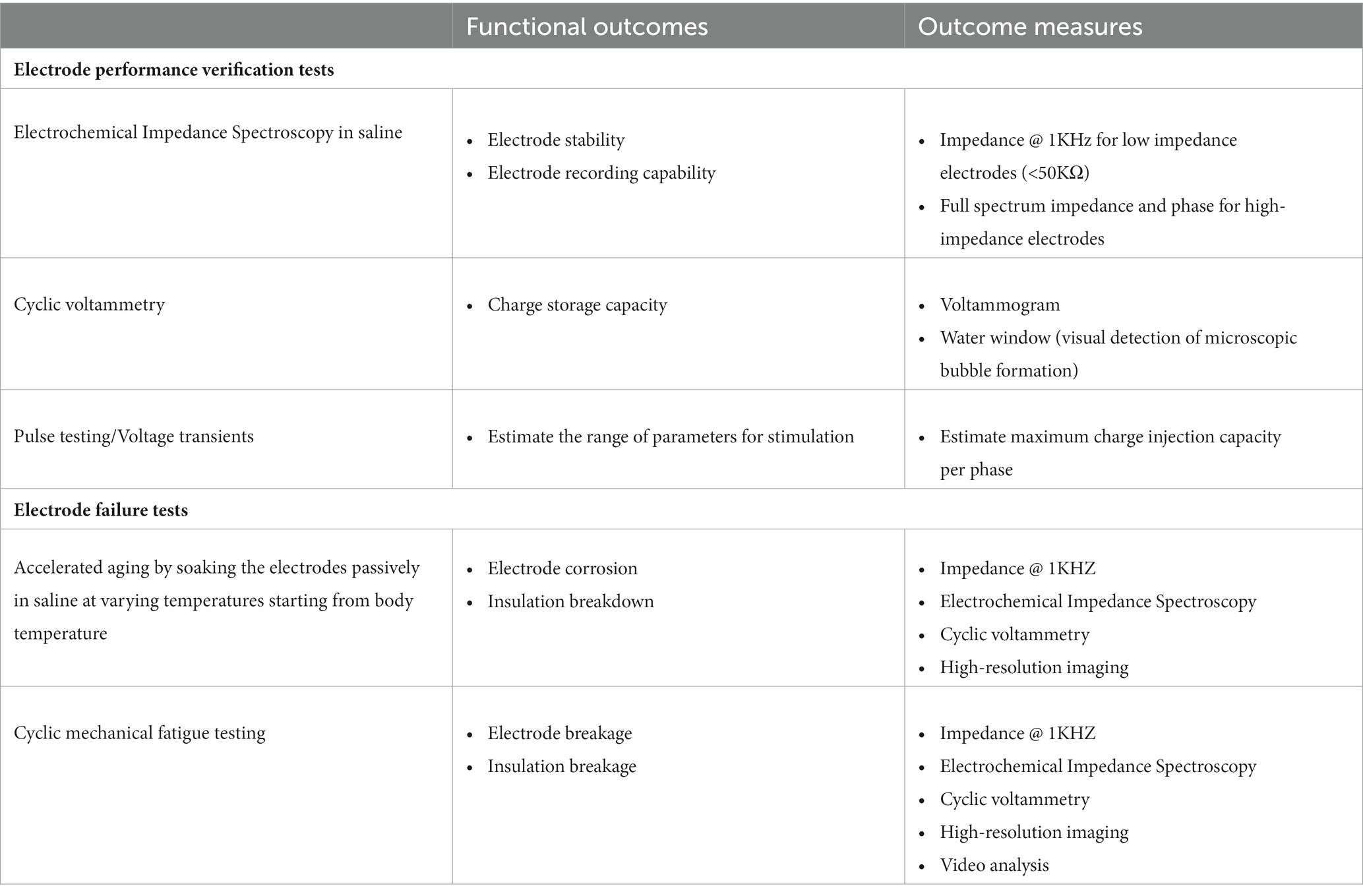
Table 1. The most common in-vitro tests utilized during the development of neural electrodes are listed (Cogan, 2008; Shepherd et al., 2018; Larson and Meng, 2020).
6.1.4 Validation process
Ex-vivo studies in animal tissue followed by human cadaver tissue provide a fast turnaround for evaluating working prototypes in a real-world surgical environment, accelerating the validation process (Shepherd et al., 2018; Gupta et al., 2020). Multiple types of electrodes have been tested in porcine models because of the comparable neuroanatomy to humans (Shepherd et al., 2018; Trevathan et al., 2019; Settell et al., 2020; Strauss et al., 2020), allowing testing of scaled-up prototypes for human use. Ex-vivo animal studies can provide preliminary but critical validation data on several performance metrics that include but are not limited to passive electrical (impedance) performances, tethering forces on the leads connected to the stimulation/recording electronics package (Pena et al., 2017), and surgical planning of the implantation process. After ex-vivo studies, in-vivo animal studies can further reduce inadvertent and unknown risks.
6.2 Overcoming barriers
6.2.1 Regulatory and ethical compliance during the validation process
Regulatory and ethical compliance in accelerating the validation process can be achieved by choosing an appropriate living tissue or simulated environment and following the research institute’s/company’s regulatory/ethical compliance procedures. The living tissue environment can be simulated using ex-vivo animal cadaver tissue sourced from other terminal in-vivo studies or an abattoir. These simulated but realistic environments help in the verification and validation process with a limited regulatory burden and provide valuable feedback to the development team. Following ex-vivo cadaver studies in appropriately sized tissue samples, it may be prudent to do studies in human cadaver tissue to validate the product further.
Following ex-vivo studies, in-vivo animal studies can be performed to evaluate the biofunctionality and biocompatibility of the device. The principles of the three R’s (Replace, Reduce, and Refine) can guide the developers in choosing the animal models with suitable anatomy and physiology needed for testing the device’s intended uses in in-vivo studies.
6.2.2 Comprehensive documentation for regulatory approval
Regular and systematic reviews are conducted in the Waterfall methodology to generate comprehensive documentation. The same process can be implemented at the end of each Sprint for compiling documentation required by the FDA (Antonini et al., 2021; Slattery et al., 2022). In addition, documentation generated during the verification/validation process using digital twins or physical models can be submitted to the FDA through the Pre-Submisson/Q-Sub program to ensure appropriate validation is conducted with the final product, thereby accelerating the overall product development timeline.
7 Discussion
Navigating the complex medical device development (MDD) pathway (Guan et al., 2017; FDA, 2021; Lottes et al., 2022; Ashfaq, 2023), which involves translating innovative ideas into medical products, can be a challenging and resource-intensive task. Using the traditional Waterfall methodology can be slow and costly because the development team plans extensively and spends considerable upfront resources to make a fully functional product before releasing it to the users for testing. Further, if any unanticipated changes occur during testing (validation studies), then the development of the device has to revert to the beginning stage or product development has to be abandoned. Utilizing the Agile methodology can help accelerate the MDD pathway by delivering a verified product quicker for the users to test and for the product development team to revise iteratively, leading to cost reduction and faster delivery, ultimately benefiting patients in need. The enabling factors for adopting the Agile methodology for MDD include accelerating the verification and validation processes utilizing 3D printing and food-grade tissue for ex-vivo validation studies.
A review of hybrid design control methodologies (Schlauderer et al., 2015; Papadakis and Tsironis, 2020; Reiff and Schlegel, 2022; Slattery et al., 2022) such as hybrid V-model, hybrid Waterfall-Agile methodology, and a tailored Agile framework (Antonini et al., 2021), and white papers (Ashfaq, 2023) can provide insights into best practices for integrating the methodologies into existing new product introduction processes, and to overcome the barriers of managing regulatory compliance and approval. In addition, implementing the Agile methodology would foster the development and use of novel alternate methods (NAMs: in chemico, in vitro, and in silico) for the verification/validation processes, which could potentially reduce the use of animal models in biomedical research. Ultimately, the approach best suited for developing medical device hardware involving animal and human testing will depend on the verification and validation requirements as well as ethical and regulatory requirements. Further, emerging technologies, such as DevSecOps, which incorporates security and increased collaboration between the development team and operations, can be implemented for continued medical device development beyond the product’s initial release.
In conclusion, the Agile methodology with iterative verification and validation cycles across the design features can significantly improve de-risking a medical device’s clinical translation path by refining the design specifications to achieve the device’s intended use.
Author contributions
AT: Conceptualization, Writing – original draft, Writing – review & editing. RJ: Funding acquisition, Supervision, Writing – review & editing, Conceptualization, Resources.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by US National Institute of Biomedical Imaging and Bioengineering (Grant Nos. R01-EB027584 and R01-EB023261).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, K. B. R., Pathmanathan, P., Kabadi, S. V., Drgon, T., and Morrison, T. M. (2023). Editorial on the Fda report on “successes and opportunities in Modeling & Simulation for Fda”. Ann. Biomed. Eng. 51, 6–9. doi: 10.1007/s10439-022-03112-x
Al-Dulimi, Z., Wallis, M., Tan, D. K., Maniruzzaman, M., and Nokhodchi, A. (2021). 3D printing technology as innovative solutions for biomedical applications. Drug Discovery Today 26, 360–383. doi: 10.1016/j.drudis.2020.11.013
Algorri, M., Abernathy, M. J., Cauchon, N. S., Christian, T. R., Lamm, C. F., and Moore, C. M. V. (2022). Re-envisioning pharmaceutical manufacturing: increasing agility for global patient access. J. Pharm. Sci. 111, 593–607. doi: 10.1016/j.xphs.2021.08.032
Antonini, M.-J., Plana, D., Srinivasan, S., Atta, L., Achanta, A., Yang, H., et al. (2021). A crisis-responsive framework for medical device development applied to the Covid-19 pandemic. Front. Digital Health 3:617106. doi: 10.3389/fdgth.2021.617106
Ashfaq, A. (2023). Understanding the 5 phases of medical device development [online]. greenlight guru. Available at: https://www.greenlight.guru/blog/5-phases-of-medical-device-development (Accessed Ferbruary 19, 2023)
Beck, K., Beedle, M., Van Bennekum, A., Cockburn, A., Cunningham, W., Fowler, M., et al. (2001). The Agile Manifesto. Agile Alliance. Available at: http://agilemanifesto.org/
Calvo-Haro, J. A., Pascau, J., Asencio-Pascual, J. M., Calvo-Manuel, F., Cancho-Gil, M. J., Del Cañizo López, J. F., et al. (2021). Point-of-care manufacturing: a single university hospital’s initial experience. 3D Print. Med. 7:11. doi: 10.1186/s41205-021-00101-z
Cho, Y., Park, J., Lee, C., and Lee, S. (2020). Recent progress on peripheral neural interface technology towards bioelectronic medicine. Bioelectron. Med. 6:23. doi: 10.1186/s42234-020-00059-z
Cogan, S. F. (2008). Neural stimulation and recording electrodes. Ann. Rev. Biomed. Eng. 10, 275–309. doi: 10.1146/annurev.bioeng.10.061807.160518
Del Valle, J., Rodríguez-Meana, B., and Navarro, X. (2021). “Chapter 16 – neural electrodes for long-term tissue interfaces” in Somatosensory feedback for Neuroprosthetics. ed. B. Güçlü (Cambridge, MA: Academic Press)
Eiber, C. D., Delbeke, J., Cardoso, J., Neeling, M. D., John, S. E., Lee, C. W., et al. (2021). Preliminary minimum reporting requirements for in-vivo neural Interface research: I. Implantable neural interfaces. IEEE Open J. Eng. Med. Biol. 2, 74–83. doi: 10.1109/OJEMB.2021.3060919
Famm, K., Litt, B., Tracey, K. J., Boyden, E. S., and Slaoui, M. (2013). Drug discovery: a jump-start for electroceuticals. Nature 496, 159–161. doi: 10.1038/496159a
Farina, D., Vujaklija, I., Brånemark, R., Bull, A. M. J., Dietl, H., Graimann, B., et al. (2021). Toward higher-performance bionic limbs for wider clinical use. Nat. Biomed. Eng. 7, 473–485. doi: 10.1038/s41551-021-00732-x
FDA. (1998). Design control guidance for medical device manufacturers. Center for Drug Evaluation and Research. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/design-control-guidance-medical-device-manufacturers (Accessed May 1, 2023)
FDA. (2017). Process of 3D printing medical devices. Center for Drug Evaluation and Research. Available at: https://www.fda.gov/medical-devices/3d-printing-medical-devices/process-3d-printing-medical-devices (Accessed October 21, 2021)
FDA. (2018a). Code of Federal Regulations. Center for Drug Evaluation and Research. Available at: https://www.fda.gov/medical-devices/medical-device-databases/code-federal-regulations-title-21-food-and-drugs (Accessed Febrauary 17, 2023)
FDA. (2018b). Software as a medical device (Samd) [online]. Center for Drug Evaluation and Research. Available at: https://www.fda.gov/medical-devices/digital-health-center-excellence/software-medical-device-samd (Accessed February 17, 2023)
FDA. (2020). What is digital health?. Center for Drug Evaluation and Research. Available at: https://www.fda.gov/medical-devices/digital-health-center-excellence/what-digital-health (Accessed April 10, 2023)
FDA. (2021). Cdrh innovation initiative: Medical device innovation initiative white paper [online]. Center for Drug Evaluation and Research. Available at: https://www.fda.gov/about-fda/cdrh-innovation/medical-device-innovation-initiative-white-paper (Accessed May1, 2023)
Fry, A. R., Krentz, A. J., and Hompesch, M. (2016). Considerations in biosimilar insulin device development. Biosimilars 6, 9–15. doi: 10.2147/BS.S77034
Ghafoor, U., Kim, S., and Hong, K.-S. (2017). Selectivity and longevity of peripheral-nerve and machine interfaces: a review. Front. Neurorobot 11:59. doi: 10.3389/fnbot.2017.00059
Ghilan, A., Chiriac, A. P., Nita, L. E., Rusu, A. G., Neamtu, I., and Chiriac, V. M. (2020). Trends in 3D printing processes for biomedical field: opportunities and challenges. J. Polymers Environ. 28, 1345–1367. doi: 10.1007/s10924-020-01722-x
Giagka, V., and Serdijn, W. A. (2018). Realizing flexible bioelectronic medicines for accessing the peripheral nerves – technology considerations. Bioelectron. Med. 4:8. doi: 10.1186/s42234-018-0010-y
Grill, W. M., Norman, S. E., and Bellamkonda, R. V. (2009). Implanted neural interfaces: biochallenges and engineered solutions. Ann. Rev. Biomed. Eng. 11, 1–24. doi: 10.1146/annurev-bioeng-061008-124927
Guan, A., Hamilton, P., Wang, Y., Gorbet, M., Li, Z., and Phillips, K. S. (2017). Medical devices on chips. Nat. Biomed. Eng. 1:0045. doi: 10.1038/s41551-017-0045
Gupta, I., Cassará, A. M., Tarotin, I., Donega, M., Miranda, J. A., Sokal, D. M., et al. (2020). Quantification of clinically applicable stimulation parameters for precision near-organ neuromodulation of human splenic nerves. Commun. Biol. 3:577. doi: 10.1038/s42003-020-01299-0
Holden, R. J., Boustani, M. A., and Azar, J. (2021). Agile innovation to transform healthcare: innovating in complex adaptive systems is an everyday process, not a light bulb event. BMJ Innov. 7, 499–505. doi: 10.1136/bmjinnov-2020-000574
Horn, C. C., Ardell, J. L., and Fisher, L. E. (2019). Electroceutical targeting of the autonomic nervous system. Physiology 34, 150–162. doi: 10.1152/physiol.00030.2018
Jung, R., Abbas, J. J., Kuntaegowdanahalli, S., and Thota, A. K. (2017). Bionic intrafascicular interfaces for recording and stimulating peripheral nerve fibers. Bioelectron. Med. 1, 55–69. doi: 10.2217/bem-2017-0009
Kumar, P., Rajak, D. K., Abubakar, M., Ali, S. G. M., and Hussain, M. (2021). 3D printing Technology for Biomedical Practice: a review. J. Materials Eng. Perform. 30, 5342–5355. doi: 10.1007/s11665-021-05792-3
Larson, C. E., and Meng, E. (2020). A review for the peripheral nerve interface designer. J. Neurosci. Methods 332:108523. doi: 10.1016/j.jneumeth.2019.108523
Lee, I., Pappas, G. J., Cleaveland, R., Hatcliff, J., Krogh, B. H., Lee, P., et al. (2006). High-confidence medical device software and systems. Computer 39, 33–38. doi: 10.1109/MC.2006.180
Lissandrello, C. A., Gillis, W. F., Shen, J., Pearre, B. W., Vitale, F., Pasquali, M., et al. (2017). A micro-scale printable nanoclip for electrical stimulation and recording in small nerves. J. Neural Eng. 14:036006.
Lottes, A. E., Cavanaugh, K. J., Chan, Y. Y.-F., Devlin, V. J., Goergen, C. J., Jean, R., et al. (2022). Navigating the regulatory pathway for medical devices—a conversation with the Fda, clinicians, researchers, and industry experts. J. Cardiovasc. Translat. Res. 15, 927–943. doi: 10.1007/s12265-022-10232-1
Magisetty, R., and Park, S.-M. (2022). New era of electroceuticals: clinically driven smart implantable electronic devices moving towards precision therapy. Micromachines 13:161. doi: 10.3390/mi13020161
Mchugh, M., Cawley, O., Mccaffcry, F., Richardson, I., and Wang, X. An Agile V-model for medical device software development to overcome the challenges with plan-driven software development lifecycles. 2013 5th International Workshop on Software Engineering in Health Care (Sehc), 20–21 May 2013. (2013). 12–19.
Milovanović, A., Milošević, M., Mladenović, G., Likozar, B., Čolić, K., and Mitrović, N. Experimental dimensional accuracy analysis of reformer prototype model produced by Fdm and Sla 3D printing technology. (2019). Cham: Springer International Publishing, 84–95.
Morrison, T. M., Pathmanathan, P., Adwan, M., and Margerrison, E. (2018). Advancing regulatory science with computational modeling for medical devices at the FDA's Office of Science and engineering laboratories. Front. Med. 5:241. doi: 10.3389/fmed.2018.00241
Otchy, T. M., Michas, C., Lee, B., Gopalan, K., Nerurkar, V., Gleick, J., et al. (2020). Printable microscale interfaces for long-term peripheral nerve mapping and precision control. Nat. Commun. 11:4191.
Ottestad, E., and Orlovich, D. S. (2020). History of peripheral nerve stimulation—update for the 21st century. Pain Med. 21, S3–S5. doi: 10.1093/pm/pnaa165
Overstreet, C. K., Cheng, J., and Keefer, E. W. (2019). Fascicle specific targeting for selective peripheral nerve stimulation. J. Neural Eng. 16:066040. doi: 10.1088/1741-2552/ab4370
Paggi, V., Akouissi, O., Micera, S., and Lacour, S. P. (2021). Compliant peripheral nerve interfaces. J. Neural Eng. 18:031001. doi: 10.1088/1741-2552/abcdbe
Papadakis, E., and Tsironis, L. (2020). Towards a hybrid project management framework: a systematic literature review on traditional, Agile and hybrid techniques. J. Modern Project Manag. 8, 125–139.
Pavlov, V. A., and Tracey, K. J. (2019). Bioelectronic medicine: updates, challenges and paths forward. Bioelectron. Med. 5:1. doi: 10.1186/s42234-019-0018-y
Pena, A. E., Kuntaegowdanahalli, S. S., Abbas, J. J., Patrick, J., Horch, K. W., and Jung, R. (2017). Mechanical fatigue resistance of an implantable branched lead system for a distributed set of longitudinal intrafascicular electrodes. J. Neural Eng. 14:066014. doi: 10.1088/1741-2552/aa814d
Reiff, J., and Schlegel, D. (2022). Hybrid project management–a systematic literature review. Int. J. Inform. Syst. Project Manag. 10, 45–63. doi: 10.12821/ijispm100203
Rottier, P. A., and Rodrigues, V. Agile development in a medical device company. Agile 2008 Conference, 4–8 August 2008. (2008). 218–223.
Russell, C., Roche, A. D., and Chakrabarty, S. (2019). Peripheral nerve bionic interface: a review of electrodes. Int. J. Intell. Robot. Appl. 3, 11–18. doi: 10.1007/s41315-019-00086-3
Salisbury, J. P. (2021). Using medical device standards for design and risk Management of Immersive Virtual Reality for at-home therapy and remote patient monitoring. JMIR Biomed. Eng. 6:e26942. doi: 10.2196/26942
ScaledAgile. (2022). Safe® for healthcare. Available at: https://scaledagile.com/business-solutions/industries/healthcare/ (Accessed February 17, 2023)
Schlauderer, S., Overhage, S., and Fehrenbach, B. (2015). Widely used but also highly valued? Acceptance factors and their perceptions in water-scrum-fall projects. Thirty Sixth International Conference on Information Systems, Fort Worth.
ScrumAlliance. (2023). Scrum – an Agile framework. Scrum Alliance Inc. Available at: https://www.scrumalliance.org/about-scrum#!section3 (Accessed February 17, 2023)
Selim, O. A., Lakhani, S., Midha, S., Mosahebi, A., and Kalaskar, D. M. (2021). Three-dimensional engineered peripheral nerve: toward a new era of patient-specific nerve repair solutions. Tissue Eng. B 28, 295–335. doi: 10.1089/ten.teb.2020.0355
Settell, M. L., Pelot, N. A., Knudsen, B. E., Dingle, A. M., Mcconico, A. L., Nicolai, E. N., et al. (2020). Functional vagotopy in the cervical vagus nerve of the domestic pig: implications for the study of vagus nerve stimulation. J. Neural Eng. 17:026022. doi: 10.1088/1741-2552/ab7ad4
Shepherd, R. K., Villalobos, J., Burns, O., and Nayagam, D. A. X. (2018). The development of neural stimulators: a review of preclinical safety and efficacy studies. J. Neural Eng. 15:041004. doi: 10.1088/1741-2552/aac43c
Slattery, O., Trubetskaya, A., Moore, S., and Mcdermott, O. (2022). A review of lean methodology application and its integration in medical device new product introduction processes. Processes 10:2005. doi: 10.3390/pr10102005
Stare, A. (2014). Agile Project Management in product development projects. Procedia Soc. Behav. Sci. 119, 295–304. doi: 10.1016/j.sbspro.2014.03.034
Strauss, I., Niederhoffer, T., Giannotti, A., Panarese, A. M., Bernini, F., Gabisonia, K., et al. (2020). Q-Pine: a quick to implant peripheral intraneural electrode. J. Neural Eng. 17:066008. doi: 10.1088/1741-2552/abc52a
Theobald, S., Schmitt, A., and Diebold, P. Comparing scaling Agile frameworks based on underlying practices. (2019). Cham. Springer International Publishing, 88–96.
Trevathan, J. K., Baumgart, I. W., Nicolai, E. N., Gosink, B. A., Asp, A. J., Settell, M. L., et al. (2019). An injectable neural stimulation electrode made from an in-body curing polymer/metal composite. Adv. Healthcare Mat. 8:e1900892. doi: 10.1002/adhm.201900892
Verma, N., Knudsen, B. E., Gholston, A., Skubal, A. C., Blanz, S. L., Settell, M. L., et al. (2023). Microneurography as a minimally invasive method to assess target engagement during neuromodulation. J. Neural Eng. 20:026036. doi: 10.1088/1741-2552/acc35c
Wu, Y., and Guo, L. (2020). “Peripheral nerve electrodes” in Neural Interface engineering: Linking the physical world and the nervous system. ed. L. Guo (Cham: Springer International Publishing)
Wu, X., and Peng, H. (2019). Polymer-based flexible bioelectronics. Sci. Bulletin 64, 634–640. doi: 10.1016/j.scib.2019.04.011
Keywords: medical device development, design controls, verification and validation, waterfall, Agile, neurotechnology, peripheral neural interfaces
Citation: Thota AK and Jung R (2024) Accelerating neurotechnology development using an Agile methodology. Front. Neurosci. 18:1328540. doi: 10.3389/fnins.2024.1328540
Edited by:
Aaron M. Dingle, University of Wisconsin-Madison, United StatesReviewed by:
James Morizio, Duke University, United StatesCopyright © 2024 Thota and Jung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ranu Jung, cmp1bmdAdWFyay5lZHU=
 Anil Kumar Thota
Anil Kumar Thota Ranu Jung
Ranu Jung