- 1National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
- 2First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
Neurodegenerative diseases refer to a battery of medical conditions that affect the survival and function of neurons in the brain, which are mainly presented with progressive loss of cognitive and/or motor function. Acupuncture showed benign effects in improving neurological deficits, especially on movement and cognitive function impairment. Here, we reviewed the therapeutic mechanisms of acupuncture at the neural circuit level in movement and cognition disorders, summarizing the influence of acupuncture in the dopaminergic system, glutamatergic system, γ-amino butyric acid-ergic (GABAergic) system, serotonergic system, cholinergic system, and glial cells at the circuit and synaptic levels. These findings can provide targets for clinical treatment and perspectives for further studies.
1 Introduction
Neurodegenerative diseases are characterized by progressive brain dysfunction and overlapping clinical syndromes induced by damage to neurons and synapses (Gan et al., 2018). Movement disorder and cognitive deficits are common complaints in neurodegenerative disorders, such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) (Shi et al., 2020). Given the considerable prevalence of neurodegenerative diseases, more than 52 million people were affected by AD and dementia globally in 2019, including 3.9 million individuals with PD, with a 1.61- and 1.56-fold increase from 1990, respectively (Cieza et al., 2021). With the booming population and prolonged life expectancy, it is estimated that there will be 42.5 million people with AD in China by 2050, and the number will be 152 million worldwide (Nichols et al., 2022). The impairment of motor and cognitive function frustrates normal daily life and lowers the quality of life (Tahami Monfared et al., 2022; Leite Silva et al., 2023). The societal expenditure on dementia has increased by 161% from 2010 to 2015 in the USA (Dauphinot et al., 2022), and economic studies forecast 332 billion RMB costs of AD in 2050 in China (Clay et al., 2019). For PD, the individual healthcare economic burden on physiotherapy ranges from €2056 to €2,586 annually (Ypinga et al., 2018), and the projected PD expense will be at least $79 billion in the USA by 2037 (Yang et al., 2020). Regarding the growing demographic trends, the burden of people with neurodegenerative diseases will continue to grow.
As the aggregation and spreading of abnormal proteins are shared pathogenetic mechanisms in the neural degeneration process, similar metabolic connectivity changes have been reported at the brain network level (Boccalini et al., 2022). Well-organized neural circuits via precise complex connectivity between neurons have been recognized as the foundation of specific neural functions. Classical neural circuits are neural networks comprising axons, dendritic terminals, and glial cells, which perform functions using specific excitation or inhibition signal flow pathways (Luo, 2021). For motor control, the basal ganglia neural circuit accounts for subtle movement modulation, whose dysfunction following dopamine (DA) loss plays a critical role in PD (Mcgregor and Nelson, 2019). In cognitive function, the cholinergic circuit from the medial septal and vertical limb of the diagonal band (VDB) to the hippocampus with cholinergic neuron damage contributes to learning deficits and memory impairment in AD model rats (Liu et al., 2022). Synaptic plasticity provides a rehabilitation foundation for motor learning and cognitive function improvement at the synapse level (Chu, 2020). Sufficient evidence has proven adaptive alterations in synapses in neurodegenerative disorders, such as synaptic changes in the striatum and the subthalamic nucleus in PD (Shen et al., 2022) and long-term potentiation (LTP) alterations in the hippocampal neural circuit in cognition modulation (Cuestas Torres and Cardenas, 2020).
Acupuncture provides an adjunctive approach to the rehabilitation of neurological disorders (Shi, 2021; Lu et al., 2022; Zhang Z. et al., 2023). Clinical studies have validated the neurological functional improvement effect of acupuncture in cognitive impairment (Yang et al., 2019) and movement disorders. For the patients with vascular cognitive impairment, acupuncture showed indeed effects in improving cognitive symptom and preventing the process of dementia (Lei et al., 2016; Jang et al., 2020a). As for PD patients with gait disturbance, acupuncture benefits the hypometric gait and functional brain activity, providing a practical approach for PD. Acupuncture plays a role in improving anatomical and functional changes in the brain network via multiple signaling pathways, on both the neural circuit and synapse level (Lin et al., 2022; Liu and Zhu, 2023). Recently, the therapeutic mechanisms of acupuncture at the neural circuit level have been frequently reported in neurological deficits, especially in the dopaminergic system, glutamatergic system, γ-amino butyric acid-ergic (GABAergic) system (Fan et al., 2022), serotonergic system, and cholinergic system (Li L. et al., 2021). In addition, glial cells such as astrocytes, microglia, and oligodendrocytes (OLs) contribute to neural plasticity by facilitating neural circuit rehabilitation (Allen and Lyons, 2018), of which the mechanism of acupuncture interference has been demonstrated (Chang et al., 2022). At the synapse level, mechanisms of acupuncture on synaptic plasticity have been revealed in modulating ion channels, functional proteins, and neurotransmitters (Xiao et al., 2018).
Although sufficient evidence has proven the mechanistic pathways of acupuncture in neurodegenerative diseases, limited reviews focus on motor disorders and cognitive impairment at the circuit and synaptic levels. In this review, we summarized the neural protective effects and therapeutic mechanisms of acupuncture on movement and cognitive disorders in neurodegenerative diseases.
2 Influence of different neuronal and glial systems on movement and cognitive disorders
2.1 Neuronal systems
2.1.1 Dopaminergic system
Dopaminergic neurons are mainly distributed in the dense region of the substantia nigra (SN) of the midbrain, generating neurofibers that project to the telencephalon, diencephalon, brainstem, and spinal cord, and form dopaminergic neuronal pathways, including the nigra-striatum network, mesocortical pathway, mesolimbic pathway, and tuberoinfundibular system (Morita and Kawaguchi, 2019).
The basal ganglia modulates the control and initiation of movement via two neural circuits projecting from the striatum (Rui et al., 2013): one is the direct circuit comprised of dopaminergic neurons with the D1 receptor, and the other is the indirect pathway comprised of dopaminergic neurons with the D2 receptor (Yan, 2021). The dynamic balance of the basal ganglia-thalamocortical circuits is crucial for normal activity.
In neurodegenerative diseases, the integrity of dopaminergic neural circuits and the activation of the dopamine receptor account for normal movement performance (Klein et al., 2019). One hypothesis is the release of striatal dopamine regulates movement control, which accounts for movement disorders such as Parkinson’s (Geibl et al., 2019); another is the distribution of dopamine receptors plays a role in the formation of LTP or long-term depression (LTD), which contributes to the cognitive impairment (Jay, 2003).
2.1.2 Glutamatergic system
The glutamatergic system contains glutamatergic neurons, glutamate transporters, glutamate receptors, and glutamate neurotransmitters. Glutamatergic (Hussan et al., 2022) neurons form a complex neural network primarily comprising the cortex-to-striatum pathway, the hippocampus-to-septum pathway, the visual and auditory systems, the olfactory system, and the cerebellum (Broman et al., 2004; Hussan et al., 2022).
In neurodegenerative disorders, two vital processes related to the glutamatergic system have been reported, one is the excessive accumulation of glutamic acid (Glu) causing neuronal swelling and membrane integrity destruction, hence leading to movement disorders (Hirschberg et al., 2022) and cognitive impairment (Conway, 2020); another is the imbalance between synaptic and extrasynaptic glutamatergic receptors (Babaei, 2021), including the N-methyl-D-aspartate (NMDA) receptors that induce excitatory postsynaptic potential (EPSP), and the aminomethylphosphonic acid (AMPA) receptors that facilitate the formation of late long-term potentiation (L-LTP) (Bonansco et al., 2023; Figure 1).
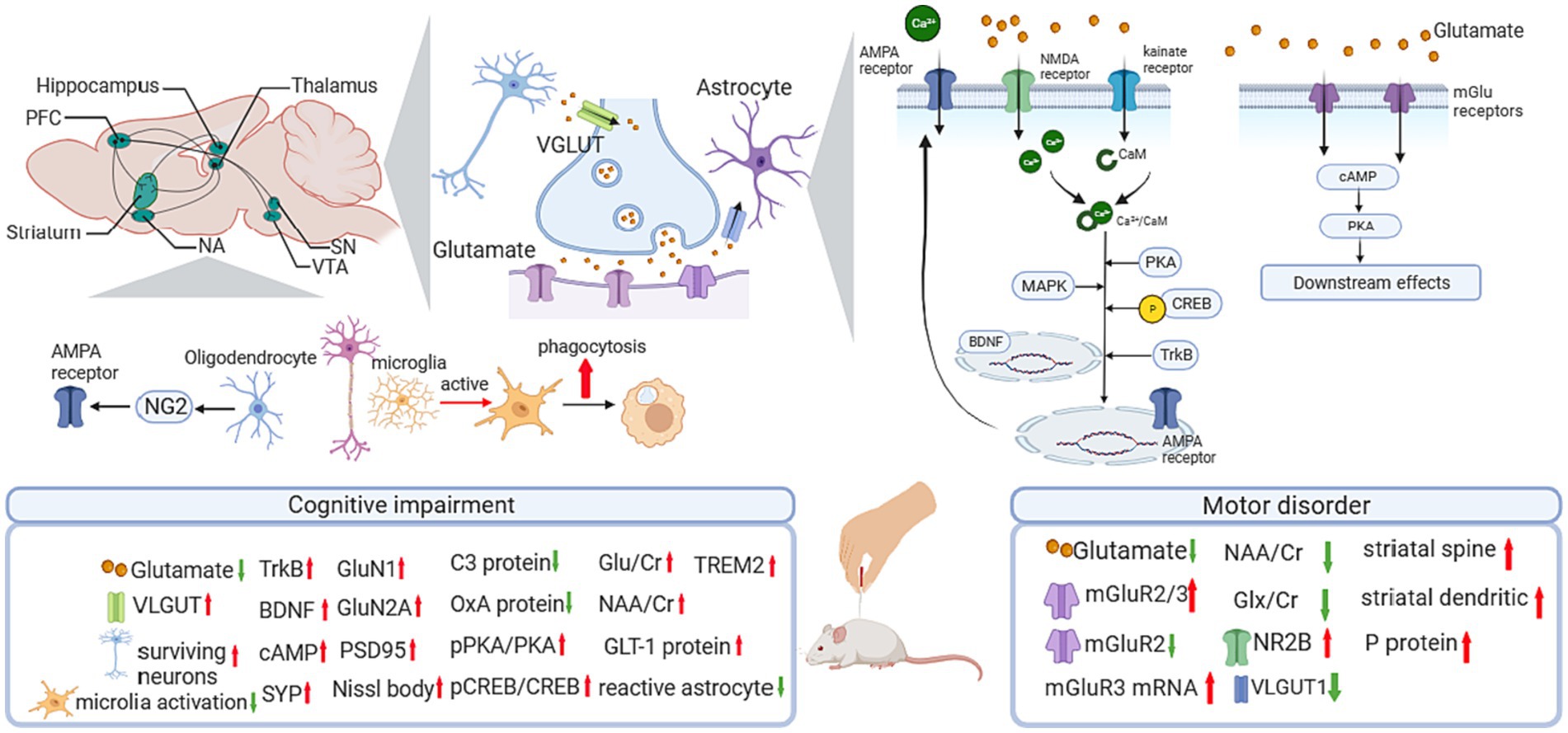
Figure 1. Influence of acupuncture on the glutamatergic system. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; BDNF, brain-derived neurotrophic factor; C3, compliment component 3; CaM, calmodulin; cAMP, cyclic adenosine monophosphate; Cr, creatine; CREB, cAMP response element-binding protein; GLT-1, glutamate transporter 1; Glu, glutamine; Glx, glutamate + glutamine; mGluR, metabotropic glutamate receptor; NA, nucleus accumbens; NAA, N-acetylaspartate; NG2, nerve-glia antigen 2; NMDA, N-methyl-D-aspartate; NR2B, receptor subunit 2B; OxA, Orexin A; PFC, prefrontal cortex; PKA, cAMP response element-binding protein; pPKA, cAMP response element-binding protein; pPKA, phosphorylated PKA; PSD95, postsynaptic density protein 95; SN, substantia nigra; TrkB, Tyrosine Kinase receptor B; VTA, ventral tegmental area; VGLUT, vesicular Glu transporter.
2.1.3 GABAergic system
The GABAergic system comprises γ-amino butyric acid (GABA), GABA transporters, and GABA receptors. As the cardinal inhibitory system in the central neural system (CNS), the GABAergic system contributes to the formation of synaptic plasticity, participating in the modulation of learning and memory (Zhang et al., 2021). Ligand-gated ion channel GABA type A receptors are the major mediator of fast inhibition in the CNS, regulating the balance of excitatory and inhibitory (E/I) synapses alone with Glu receptors (Chapman et al., 2022).
Growing evidence has proven the relationship between dysfunction of the GABAergic system and neural disorders, especially in the over-activity of hippocampal neurons in cognitive impairment, and the enhanced excitability in the primary motor cortex in movement disorder.
2.1.4 Serotonergic system
Serotonin is also known as 5-hydroxytryptamine (5-HT), which is a neuromodulatory neurotransmitter secreted by serotonergic neurons. Serotonergic neurons are mainly distributed in brainstem raphe nuclei, from which projections are sent to wide areas of the brain (Paquelet et al., 2022; Figure 2). A battery of canonical signaling pathways is involved in the 5-HT-mediated system, including the phosphatidylinositol 3-kinase/protein kinase B/ mammalian target of rapamycin (PI3K-Akt/mTOR) signaling pathway, extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) signaling pathway (Albert and Vahid-Ansari, 2019), and cAMP response element-binding protein/cAMP response element-binding protein (PKA/CREB) pathway (Yue et al., 2022; Lin et al., 2023).
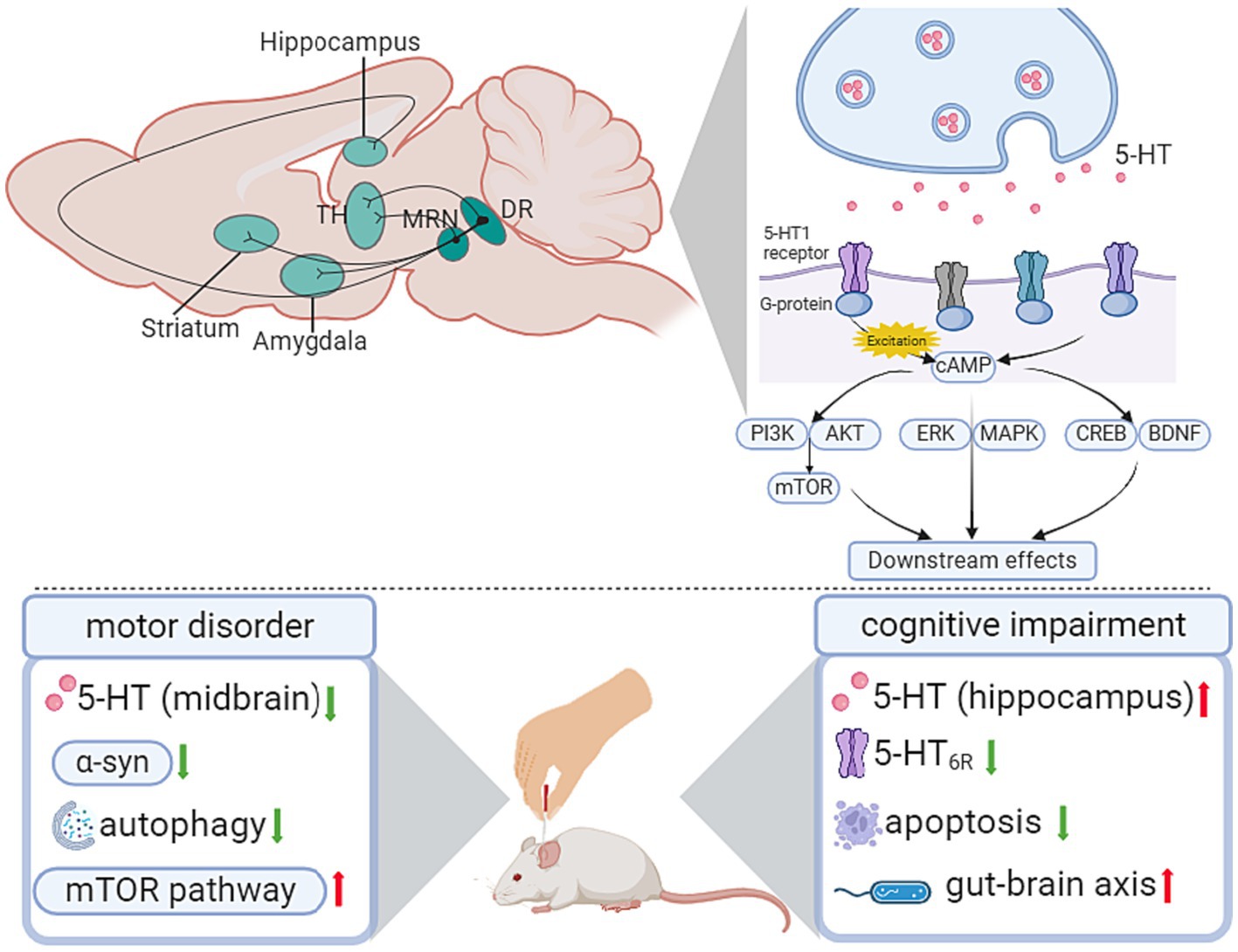
Figure 2. Influence of acupuncture on the serotonergic system. AKT, protein kinase B; cAMP, cyclic adenosine monophosphate; BDNF, brain-derived neurotrophic factor; CREB, cAMP response element-binding protein; DR, dorsal raphe; ERK, extracellular signal-regulated kinase; LDT, laterodorsal tegmental nucleus; MAPK, mitogen-activated protein kinase; MRN, median raphe nucleus; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase; TH, thalamus.
The dysfunction of 5-HT and its receptors has been validated to be associated with different phases of neurodegenerative disease. In movement disorder-related research, the inactivation of 5-HT neurons of the dorsal raphe nucleus (DRN) accounts for the impaired behavioral flexibility in the DRN-cerebral cortex projection (Hale and Lowry, 2011). As for cognitive impairment, the expression level of 5-HT receptors in the hippocampus contributes to spatial memory deficits (Crispino et al., 2020).
2.1.5 Cholinergic system
The cholinergic system is known to be associated with memory and learning ability. In the CNS, cholinergic neurons are mainly distributed in the basal forebrain, brainstem pedunculopontine and lateral dorsal tegmental nuclei, striatum, and cortex. The basal forebrain cholinergic system comprises 4 distinct nuclei projecting to specific forebrain targets, including the medial septal (Ch1), VDB (Ch2), horizontal limb of the diagonal band (Ch3), and basalis of Meynert (Ch4)(Mesulam, 2013). Among them, Ch4 has been reported to be correlated with cognitive impairment progression in patients with PD (Zhang P. et al., 2023). The projection from the basal forebrain cholinergic system to the hippocampus, amygdala, and cortex plays a crucial role in cognitive function and motor modulation.
Previous studies have illustrated the relationship between cholinergic neuron degeneration and cognitive dysfunction in dementia patients (Berry and Harrison, 2023), thus proposing the classical “cholinergic hypothesis”(Hampel et al., 2018). Recently, studies reported enlightening findings on the cholinergic system in AD treatment. Pharmaceutical treatments, such as acetylcholinesterase inhibitors, have modest effects in different phases of dementia (Joe and Ringman, 2019).
In the nucleus basalis-cortical projection, the tauopathy in the cholinergic system leads to the degeneration of the cholinergic axons, accounting for cognitive impairment in AD (Mesulam, 2013). As for PD, the activation of striatal cholinergic system accelerates the suppression of the direct pathway and the overexcitation of the indirect pathway, which aggravates the symptom of PD (Liu, 2020).
2.2 Glial cells
In the CNS, neuroglial cells consist of astrocytes, microglia, and oligodendrocytes and facilitate neural functions at the neural circuit, cellular, and molecular levels (Oliveira and Araque, 2022). Neuroglia performs function through alternating neuroprotective and neurotoxic effects, which are essential to the homeostasis of the CNS (Sancho et al., 2021).
2.2.1 Astrocytes
Astrocytes are the major type of glial cells in the CNS and facilitate brain activity, and mediate neuronal activity by controlling the extracellular microenvironment of ions and neurotransmitters at the synaptic level. The activity of Ca2+ in the astrocyte network has been proven to be influenced by neurotransmitters in different neural circuits, including the prefrontal cortex with the GABAergic neural pathway, the cortex and hippocampus with the cholinergic circuit, and the cortex and inferior colliculus with the glutamatergic pathway (Oliveira and Araque, 2022).
Research has demonstrated that the enhanced intracellular Ca2+ in hippocampal astrocytes alters the memory formation process. In the medial prefrontal cortex, activated astrocyte Ca2+ increased cortical activity and improved cognition (Mederos et al., 2021). In addition, astrocytes interfere with neuronal oscillations by modulating ion content and ion buffering and regulating neuronal firing patterns and neuronal synchronization in several neural networks (Brockett et al., 2018). In prefrontal cortex astrocytes, the deletion of GABAB receptors altered low-gamma oscillations and firing properties of cortical neurons, which resulted in poor working memory (Mederos et al., 2021).
2.2.2 Microglia
Microglia are commonly recognized as the brain’s resident immune cells, which are crucial for inflammatory/repair responses in the CNS. Studies have investigated the signaling pathways of microglia-synapse interactions and uncovered the synaptic plasticity regulatory mechanisms of microglia (Yu et al., 2023). The microglia-dependent synapse elimination is a vital phase in the development of neural circuits (Hansen et al., 2018). On the one hand, microglia prompt the formation of synapses by secreting cytokines (Andoh and Koyama, 2021); on the other hand, microglia facilitate synapse elimination via phagocytosis (Hansen et al., 2018).
In PD model rats, activated microglia appeared in the substantia nigra pars reticulata (SNr), and microglia phagocytosed glutamatergic synapses of the STN neurons (Aono et al., 2017). Through phagocytosis, microglia might alleviate PD symptoms by eliminating hyperactive synapses (Choudhury et al., 2021). In an AD model, pathological changes in microglia were observed before tau aggregation pathology (Leng and Edison, 2021).
2.2.3 Oligodendrocytes
Oligodendrocytes are derived from oligodendrocyte progenitor cells (OPCs) (Shengjiao et al., 2022), and act as receptors of GABA and Glu neurotransmitters and facilitate neural plasticity by promoting the function of synaptic receptors. Recent studies have reported that the formation of learning-related neuron circuits is associated with the OPC subgroups. In brain-injured mice that received motor learning stimulation, the formation of Oligodendrocyte Transcription Factor 2 (Olig2)-negative OPCs is related to the establishment of neural circuits in the dentate gyrus (DG) and CA1 regions (Fang et al., 2023).
3 Neural circuit mechanism of acupuncture in movement disorders via different neuronal and glial systems
3.1 The impact of acupuncture on the dopaminergic system
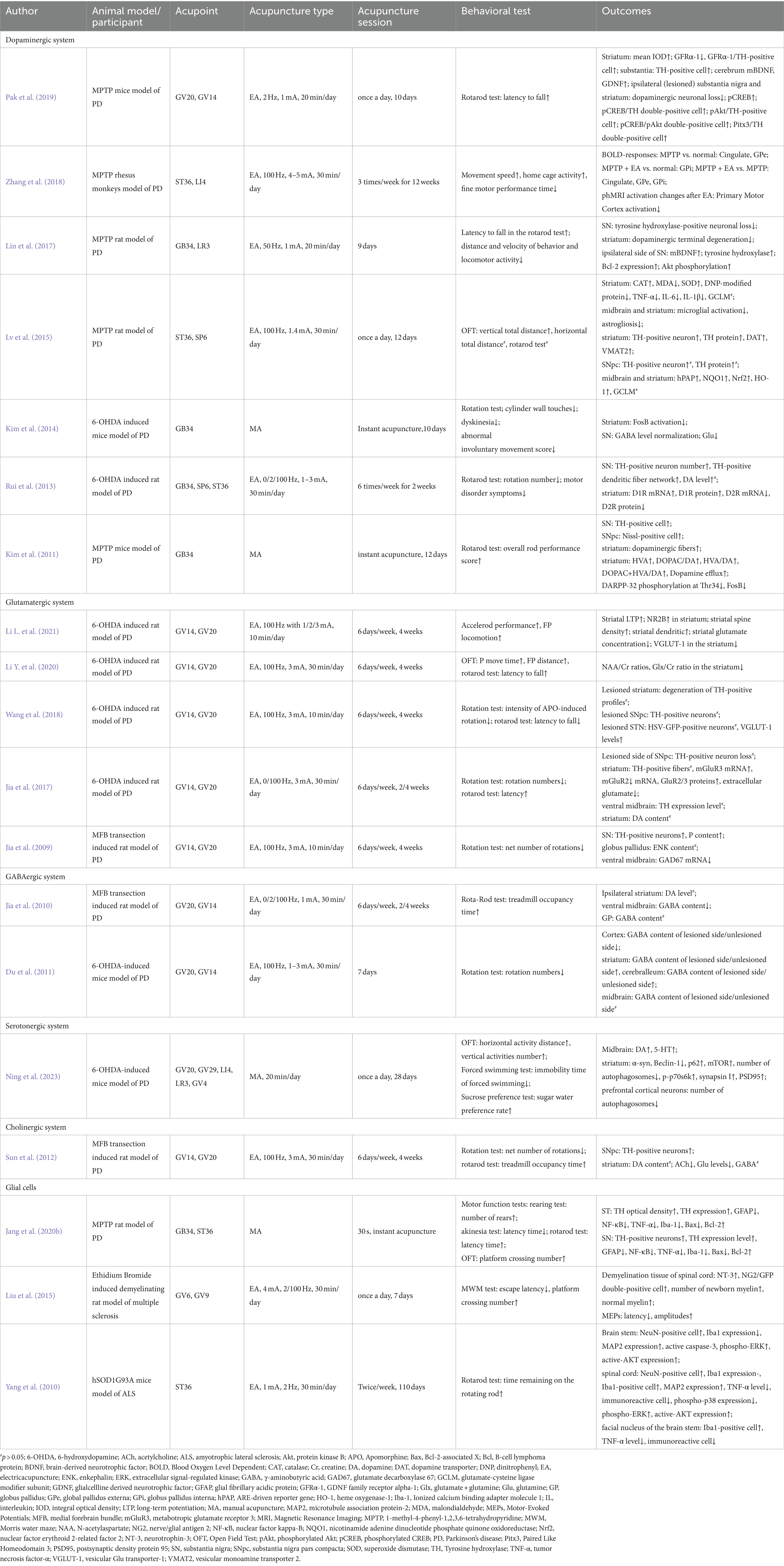
Acupuncture indicated benign effects in improving motor performance and behavioral disorders, and showed protective effects dopaminergic signaling pathways, including regulating the expression of Tyrosine hydroxylase (TH), DA, and DA receptors targeting the signaling pathways relating to oxidative stress activity, and modulating the expression level of neurotrophic factors (Table 1; Figure 3).
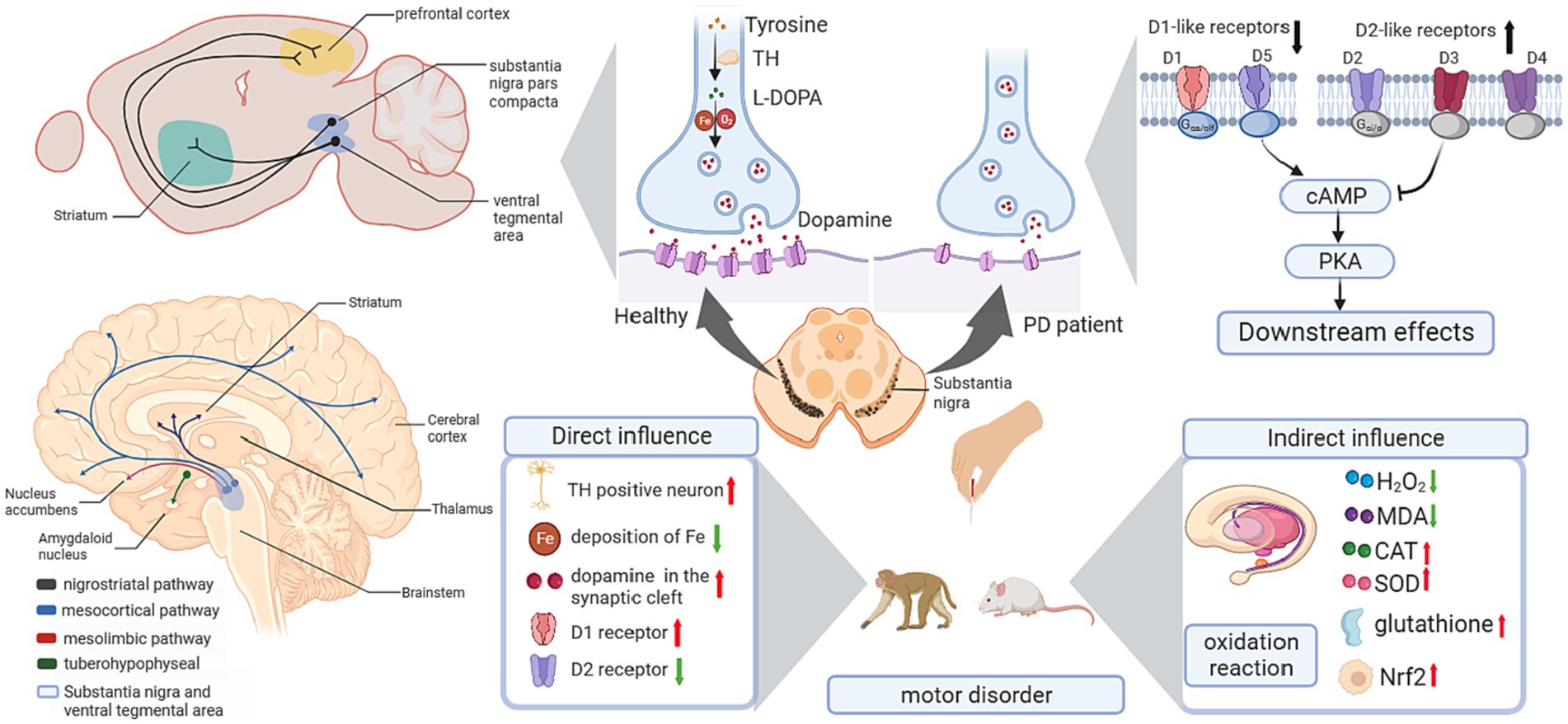
Figure 3. Influence of acupuncture on the dopaminergic system. cAMP, cyclic adenosine monophosphate; CAT, catalase; L-DOPA, levodopa; MDA, malondialdehyde; Nrf2, nuclear factor erythroid 2-related factor 2; PD, Parkinson’s disease; PKA, cAMP response element-binding protein; SOD, superoxide dismutase; TH, Tyrosine hydroxylase.
Animal research has reported that acupuncture alleviates behavioral disorders in PD model rats (Haiyang et al., 2023), enhances the number of TH-positive neurons in the SN, and decreases the deposition of Fe in the SN (Li et al., 2019). Regarding Tourette syndrome (TS), the “DA hypothesis” was proposed for the pathophysiology of TS (Nespoli et al., 2018), in which the hypersensitivity of DA receptors and the hyperactivity of dopaminergic neurons in the nigrostriatum were the basic mechanisms (Dwyer, 2018). In TS model mice, acupuncture reduced the overexpression of TH in the substantia nigra pars compacta (SNpc) and reduced behavioral stereotypies (Lin et al., 2019). Interestingly, there is a benign modulatory effect of DA after acupuncture treatment. In a PD mouse model, acupuncture enhanced dopamine release in the synaptic cleft in the basal ganglia neural circuit, which benefited postsynaptic dopamine neurotransmission (Kim et al., 2011). Moreover, the combination therapy of acupuncture and levodopa (L-DOPA) significantly improved movement speed and fine motor performance in Parkinsonian rhesus monkeys (Zhang et al., 2018). In addition, studies using the combination of acupuncture with a 50% reduced dose of L-DOPA (7.5 mg/kg) showed an equivalent effect with the standard dose of L-DOPA alone in motor function improvement, and the combination of acupuncture with L-DOPA was superior in behavioral benefits compared to L-DOPA alone (Kim et al., 2014). For basal ganglia-thalamocortical circuits, 6-hydroxydopamine (6-OHDA)-lesioned rats showed decreased D1R expression and upregulated D2R expression in the striatum. High-frequency (100 Hz) electroacupuncture (EA) inhibited the upregulation of D2R and significantly restored striatal D1R mRNA and protein levels (Rui et al., 2013). Considering the important role of D1R in the direct pathway and the dominant role of D2R in basal ganglia circuits, EA showed a benign effect in balancing dopaminergic neural circuits.
In the dopaminergic system, dopaminergic neuron loss in the SNpc is the major cause of PD. Sufficient studies have supported the crucial role of oxidative stress in the development of PD. Hence, reinforcing the antioxidative capacity of dopaminergic neurons is considered a practicable strategy for the prevention and treatment of PD. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD model mice, lipid and protein oxidation was upregulated in the striatum, while antioxidants were downregulated. After applying high-frequency EA (100 Hz), there was a significant decrease in hydrogen peroxide and malondialdehyde (MDA) levels and an increase in glutathione, catalase (CAT) and superoxide dismutase (SOD) levels in the striatum. Meanwhile, EA enhanced the nuclear factor erythroid 2-related factor 2 (Nrf2)-regulated downstream antioxidative response and upregulated the expression of nicotinamide adenine dinucleotide phosphate quinone oxidoreductase (NQO1) and heme oxygenase-1 (HO-1) in the midbrain and/or striatum (Lv et al., 2015). These findings support the antioxidant role of acupuncture in the dopaminergic system.
Neurotrophic factors are important endogenous biological mediators due to their crucial roles in neural development and functional regulation. Targeting neurotrophic factors in neural circuits has been reported to facilitate recovery in neurodegenerative diseases. For instance, brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) have been proven to benefit the survival and development of dopaminergic neurons and thus may bolster PD treatments through the nigrostriatal pathway. In PD model mice, a 3-week EA in GV20 and GV14 ameliorated motor impairments and dopaminergic neuron loss and increased the levels of BDNF and GDNF in the substantia nigra and striatum (Pak et al., 2019). Similarly, another study conducted EA in GB34 and LR3 in PD model mice showed consistent results in behavioral improvement and BDNF enhancement (Lin et al., 2017).
3.2 The impact of acupuncture on the glutamatergic system
In PD-related studies (Table 1; Figure 1), a 24-session EA treatment at GV14 and GV20 showed significant improvement in motor dysfunction. Meanwhile, EA upregulated the expression of NMDA receptor subunit 2B (NR2B) and reversed the decrease in dendritic arborization, spine density, and LTP in the striatum. In addition, striatal glutamate and vesicular Glu transporter-1 (VGLUT-1) were downregulated after EA intervention in corticostriatal glutamatergic projections (Li et al., 2022). Regarding the classical pathway of the basal ganglia circuit, studies have demonstrated the effect of acupuncture on PD by modulating the Glu system in the motor cortex-subthalamic nucleus (M1-STN) pathway. In 6-hydroxydopamine (6-OHDA)-lesioned rats, there is a significant increase in glutamate content in the lesion-side nigrostriatal system, and a decreased metabotropic glutamate receptor 2/3 (mGluR2/3) protein level and mGluR3 mRNA expression were observed. Four weeks of EA treatment on GV14 and GV20 alleviated motor deficits, downregulated glutamate levels in the cortex and striatum, and rescued mGluR3 mRNA expression and mGluR2/3 protein expression (Jia et al., 2017). Another study reported the glutamate-dependent mechanism focused on VGLUT-1. In a PD rat model, 100-Hz EA treatment significantly restored the loss of VGLUT-1 in the STN and alleviated motor symptoms induced by 6-OHDA (Wang et al., 2018). Apart from the direct influence of the Glu system on PD, acupuncture showed a protective effect on the dopaminergic system by modulating the Glu system (Li et al., 2022). Jia’s team proved EA at 100 HZ reduced the mRNA expression of glutamate decarboxylase 67 (GAD67) and enhanced the TH-positive neurons in SN in the rat model of PD (Jia et al., 2009).
These findings proved the direct regulation effect of acupuncture on the glutamate receptors, and the indirect modulation effect on the dopaminergic system via the glutamatergic system.
3.3 The impact of acupuncture on the GABAergic system
Given that GABAergic neurons account for the impressive modulation of synapses, in the basal ganglia neural circuit, they are crucial transmitters of inhibitory projections in both the direct pathway and indirect pathway. The dysfunction of GABA has been revealed to be a vital process in PD pathogenesis. Recent studies demonstrated that acupuncture facilitated the normalization of the GABA content in PD (Table 1; Figure 4).
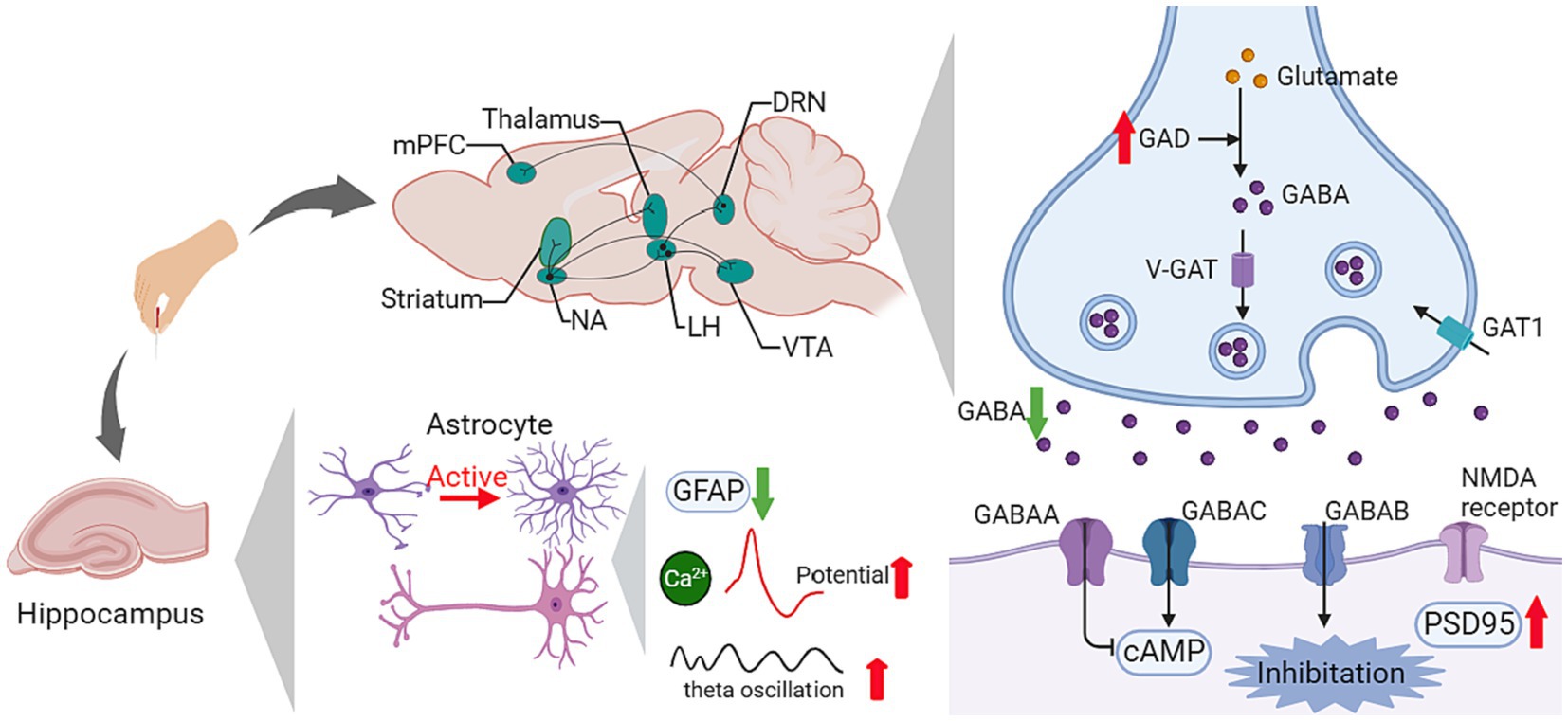
Figure 4. Influence of acupuncture on the GABAergic system. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; cAMP, cyclic adenosine monophosphate; DRN, dorsal raphe nucleus; GABA, γ-aminobutyric acid; GAD, glutamate decarboxylase; GAT1, GABA transporter 1; GFAP, glial fibrillary acidic protein; LH, lateral hypothalamus; MAPK, mitogen-activated protein kinase; mPFC, medial prefrontal cortex; NA, nucleus accumbens; NMDA, N-methyl-D-aspartate; PSD95, postsynaptic density protein 95; V-GAT, Vesicular GABA transporter; VTA, ventral tegmental area.
In 6-OHDA-induced PD model rats, elevated GABA content was found in the lesion-side cortex and striatum. After EA for 24 sessions, the GABA ratio of the normal side to the lesion side in the cortex was reduced significantly (Du et al., 2011). Another study investigated the therapeutic mechanism of EA in a rat hemiparkinsonian model and reported similar results. High-frequency (100 Hz) EA attenuated the medial forebrain bundle (MFB) lesion-induced increase in midbrain GABA content, increased GABAergic inhibition in the basal ganglia, and improved motor coordination (Jia et al., 2010). Regarding the mutual influence between the GABAergic system and the dopaminergic system in the PD process, PD treatment normalized the GABA content in the SNr. EA had a beneficial effect on these two systems. In Kim’s study, EA showed an equivalent effect on motor function improvement to L-DOPA, and the combination treatment of L-DOPA and EA ameliorated the abnormally increased GABA in the SNr (Kim et al., 2014).
3.4 The impact of acupuncture on the serotonergic system
In PD, the degeneration of suture nucleus neurons and the decrease in serotonergic innervation are mutual intervention processes, of which the dysfunction of 5-HTergic neurons affects the conversion of L-DOPA to DA, thus leading to the aggravation of PD (Nemade et al., 2021). In the rat PD model, Sun’s study reported that EA reduced the abnormally elevated acetylcholine (ACh) and Glu in the lesioned side of the striatum, and the Glu level was correlated with the survival ratios of dopaminergic neurons in the SNpc and behavioral improvement (Sun et al., 2012). In Ning’s study (Ning et al., 2023), EA alleviated motor and depressive symptoms. At the same time, EA reversed the decreased 5-HT and DA content in the midbrain of PD rats, attenuated the increased α-syn, activated the mTOR pathway, and inhibited autophagy in the striatum (Table 1; Figure 2).
3.5 The impact of acupuncture on the cholinergic system
In PD, cholinergic interneurons within the striatum play an important role in balancing dopamine signaling and regulating movement. In the striatum, cholinergic interneurons form axon-axonal connections with afferent glutamatergic and dopaminergic terminals, exerting influences on striatal function together with GABAergic interneurons and DA neurons (Liu, 2020). As the neurotransmitter, ACh has been recognized as a critical mediator in the transmission of striatal glutamate, which influences the release of DA in the striatum. Currently, sufficient findings have proven the contribution of the cholinergic system to the pathophysiology of PD. However, there is limited evidence in acupuncture-related studies, which requires further investigation (Table 1). In Sun’s research, high-frequency EA (100 Hz) stimulation at GV14 and GV20 ameliorated rotational behavior in Parkinsonian rats, attenuated the abnormally increased content of Glu and ACh in the lesioned side of the striatum, and enhanced the survival of dopaminergic neurons in the SNpc (Sun et al., 2012).
3.6 The impact of acupuncture via the microglia-related pathways
In PD-related studies (Table 1), Jang’s team proved that acupuncture at GB34 and ST36 improved motor function in PD model mice, restored dopaminergic fiber and neuron damage induced by MPTP, attenuated the overexpression of microglia and astrocytes, and ameliorated the inflammatory responses and apoptosis in the striatum and the substantia nigra (Jang et al., 2020b). In Lv’s study, EA at ST36 and SP6 demonstrated consistent results, relieving abnormal movement in MPTP mice and reducing microglial activation and astrogliosis in the striatum and midbrain (Lv et al., 2015). In addition, acupuncture demonstrated an adjunctive role in the transplantation therapy of multiple sclerosis using bone marrow mesenchymal stem cells. EA at GV9 and GV6 increased neurotrophin-3 (NT-3) levels, facilitated oligodendrocyte-like cell differentiation from grafted NT-3 and retinoic acid (RA) preinduced mesenchymal stem cells, and promoted remyelination in the demyelinated spinal cord. Meanwhile, EA speeds up the conduction of cortical motor-evoked potentials, improving nerve conduction function in multiple sclerosis (Liu et al., 2015). Regarding amyotrophic lateral sclerosis (ALS), EA at ST36 reduced microglial cell activation and restored motor neuronal cell survival in the brain stem and spinal cord of mutant ALS model mice (Yang et al., 2010).
The above studies provide evidence for acupuncture’s therapeutic mechanism of protecting dopaminergic neurons, interfering with inflammatory responses and apoptosis in PD, prompting remyelination and nerve conduction function in multiple sclerosis, and facilitating neuronal survival in ALS.
4 Neural circuit mechanism of acupuncture on cognitive impairment via different neuronal and glial systems
4.1 The impact of acupuncture on the glutamatergic system
In animal studies, acupuncture showed indeed effect on improving short-term memory, long-term memory, and spatial memory ability in mice models of cognition impairment. Beneath acupuncture’s effect, the potential mechanism might related to the regulation of Glu expression level (Table 2; Figure 1).
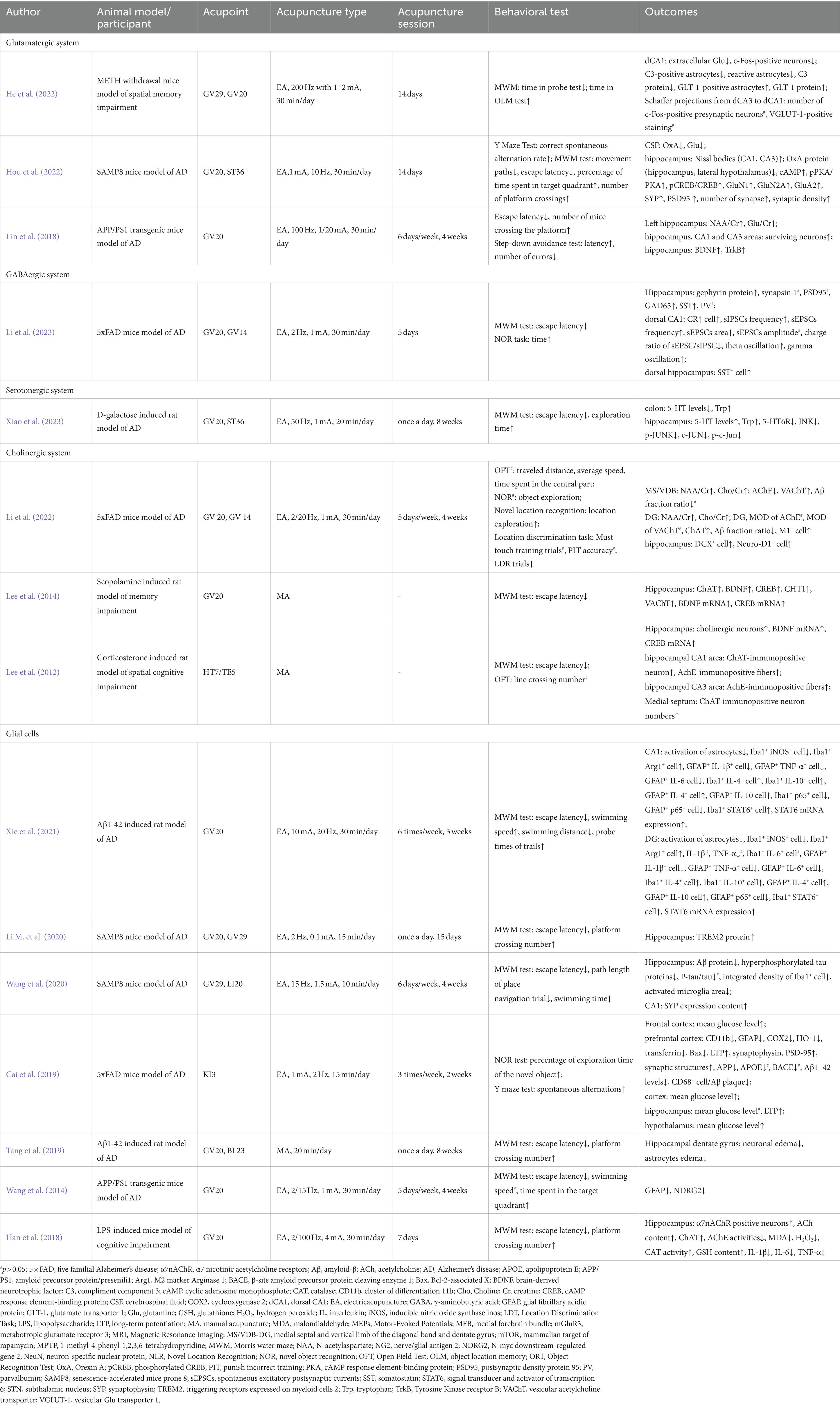
Table 2. The effect and mechanisms of acupuncture on cognitive disorders in neurodegenerative diseases.
In the senescence-accelerated mouse prone 8 (SAMP8) model, a 14-session 10 Hz EA treatment restored the Glu level in the hippocampus, improved the synaptic structure, and enhanced synaptic transmission, thus ameliorating the memory deficit (Hou et al., 2022). In amyloid precursor protein/presenili1 (APP/PS1) mice, EA at GV20 demonstrated similar results. EA upregulated Glu metabolism and the survival rate of neurons in the hippocampus and alleviated cognitive dysfunction (Lin et al., 2018). In Methamphetamine (METH)-withdrawal mice, EA at GV20 and GV29 alleviated impaired spatial memory. Meanwhile, EA attenuated the increased Glu level in the dorsal CA1 (dCA1) of the hippocampus by enhancing astrocyte-mediated Glu clearance and upregulating the expression of glutamate transporter 1 and glutamine synthase, which are responsible for astrocytic glutamatergic transportation (He et al., 2022).
4.2 The impact of acupuncture on the GABAergic system
In AD, the impairment of GABA interneurons contributes to theE/I imbalance and related excitotoxicity, which accelerates amyloid plaque deposition and hyperphosphorylated tau sequestration, thus resulting in cognitive impairment. Meanwhile, based on electroencephalogram (EEG), the oscillatory activities in targeted brain regions are related to specific behaviors. In cognitive-related studies, the θ rhythm is regulated by working memory processes, and the γ rhythm is associated with cognitive processes (Hamm et al., 2015). Abnormal γ and θ rhythms in brain oscillatory activities have been demonstrated among patients with AD, while interfering with GABAergic interneurons could restore γ oscillation (Verret et al., 2012). In five familial Alzheimer’s disease (5 × FAD) mutation mice, EA at GV20 and GV14 ameliorates recognition learning and memory function deficits, restores glutamate decarboxylase 65 (GAD65) and somatostatin (SST) in the hippocampus, attenuates the loss of somatostatin-positive interneurons in the dorsal hippocampus, and reverses the decreased θ and γ brain oscillations (Li et al., 2023; Table 2; Figure 4).
4.3 The impact of acupuncture on the serotonergic system
In D-galactose-induced AD-like rats, an eight-week EA pretreatment of GV20 and ST36 showed a therapeutic effect on improving cognitive dysfunction. Meanwhile, there was a significant increase in hippocampal 5-HT levels and a decrease in 5-HT6R expression after EA pretreatment. The upregulation of 5-HT6R has been associated with cognitive dysfunction and participates in the c-jun N-terminal kinase (JNK) pathway to modulate downstream cell apoptosis. In Xiao’s study (Xiao et al., 2023), EA downregulated the abnormally increased expression of JNK-related molecules, including JNK, p-JNK, c-JUN, and p-c-Jun, in the hippocampus of AD rats (Table 2; Figure 2).
4.4 The impact of acupuncture on the cholinergic system
Acupuncture showed improvement in spatial recognition memory in cognition impaired model mice. As for the therapeutic mechanism, four main contributions have been reported on the impact of acupuncture on the cholinergic system: the neuronal protective effect on the basal forebrain-hippocampus circuit, benign regulation effect on the neural plasticity-related neurotransmitters and proteins, modulation of the inflammatory cholinergic-dependent pathway, and improvement of the oxidative stress activity.
Focusing on the basal forebrain-hippocampus circuit, Li’s study (Li L. et al., 2021) highlighted the mechanism of EA treatment on the medial septal and vertical limbs of the diagonal band and dentate gyrus (medial septal/VDB-DG) cholinergic neural circuit. After applying EA treatment in 5 × FAD mice for 20 sessions, there was a significant improvement in spatial recognition memory and the medial septal/VDB-DG cholinergic neural circuit regulation, which promoted hippocampal neurogenesis in DG. Regarding mechanisms via synaptic plasticity pathways, one of Lee’s studies reported the cholinergic system-related mechanism of EA therapy in scopolamine-induced cognitive impairment rats. After conducting 14 sessions of EA on GV20, neuronal impairment and memory dysfunction improved significantly. Meanwhile, EA increased choline acetyltransferase (ChAT), BDNF and CREB protein levels and restored the decreased mRNA expression of choline transporter 1(CHT1), vesicular acetylcholine transporter (VAChT), BDNF and CREB in the hippocampus (Lee et al., 2014). Another study conducted by their team proved that EA at HT7 attenuated the loss of cholinergic neurons, decreased cholinergic immunoreactivity, and increased the mRNA expression of BDNF and CREB in a corticosterone-induced memory dysfunctional rat model (Lee et al., 2012). In addition, Liu’s study demonstrated the anti-inflammatory cholinergic-dependent pathway in the neuroprotection mechanism of acupuncture, thus improving cognitive function. In addition, accumulating research has focused on oxidative stress and neuroinflammation signaling pathways in EA-mediated prevention of cognitive impairment. Cao’s team observed neuronal damage, inflammation, and downregulation of α7nAChR in the hippocampus of chronic cerebral hypoperfusion (CCH) rats. EA restored neuronal survival and regulated the expression of α7nAChR, together with its downstream Janus Kinase 2 (JAK2)/Signal Transducer and Activator of Transcription 3 (STAT3) pathways. Meanwhile, EA suppressed microglia activation and inflammatory cytokines (Cao et al., 2021). In the lipopolysaccharide (LPS)-induced cognitive impairment mouse model, EA pretreatment on GV 20 ameliorated the decrease in α7nAChR protein, ACh content and ChAT activity and enhanced the activity of AChE in the cholinergic pathway of the hippocampus. Regarding oxidative stress, EA inhibited the expression of MDA and hydrogen peroxide (H2O2) and downregulated the levels of catalase (CAT) and glutathione (GSH) in the hippocampus (Table 2; Figure 5).
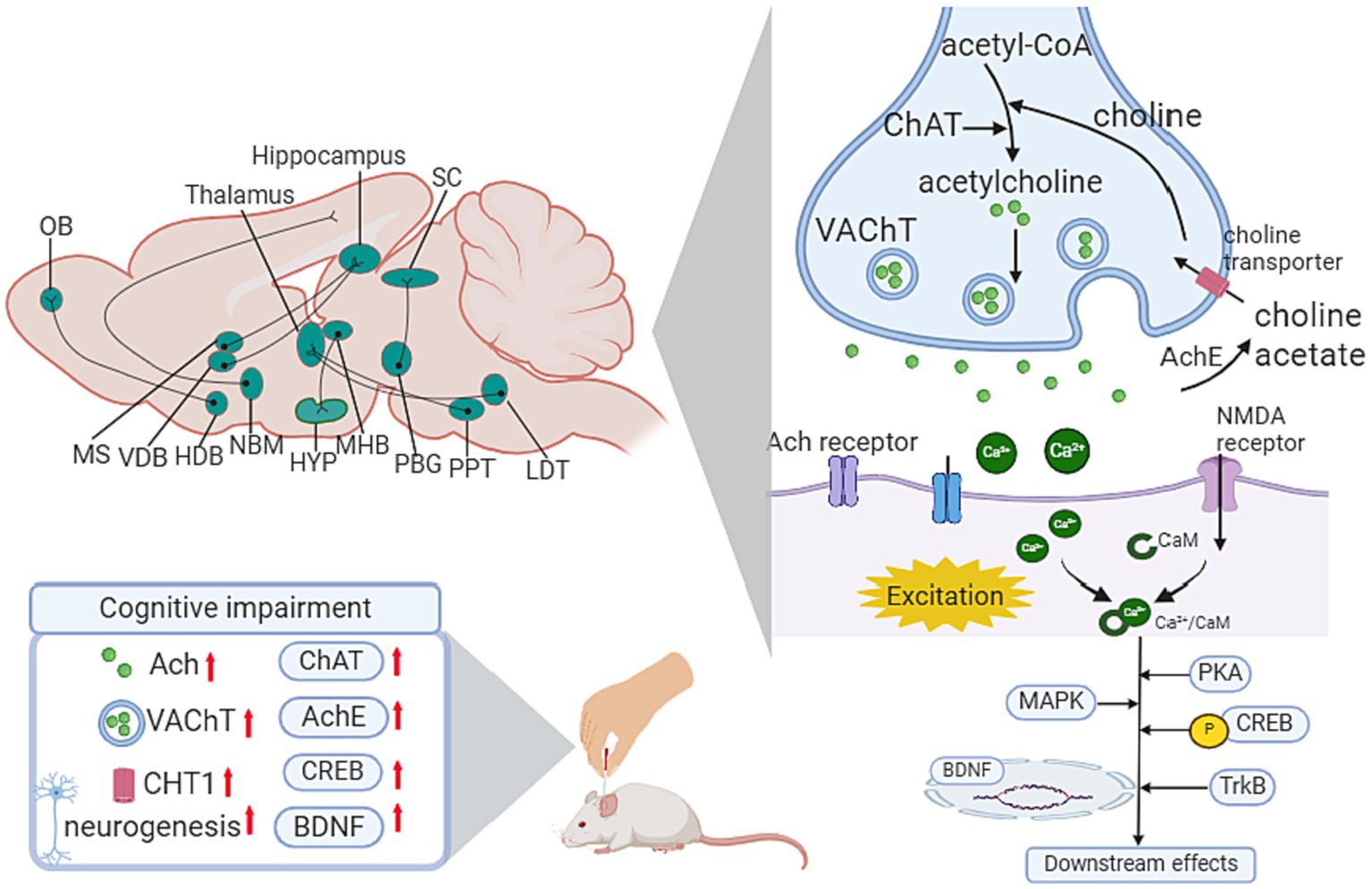
Figure 5. Influence of acupuncture on the cholinergic system. Ach, Acetylcholine; ChAT, choline acetyltransferase; AChE, acetylcholinesterase; BDNF, brain-derived neurotrophic factor; CaM, calmodulin; CHT1, choline transporter 1; CREB, cAMP response element-binding protein; HDB, horizontal diagonal band; HYP, hypothalamus; LDT, laterodorsal tegmental; MAPK, mitogen-activated protein kinase; MHB, medial habenula; MS, medial septal; NBM, Nucleus basalis of Meynert; OB, olfactory bulb; PBG, parabigeminal nucleus; PPT, peduculo-pontine tegmental nucleus; SC, superior colliculus; VDB, vertical limb of the diagonal band; VTA, Ventral tegmental area.
4.5 The impact of acupuncture via the astrocyte-related pathways
Acupuncture demonstrated improvements in cognitive impairment including delaying memory decline and enhancing memory capacity. Current studies have investigated the role of astrocytes in acupuncture’s effect mechanisms in AD, such as protecting astrocytes and neurons, and modulating the expression level of AD-related proteins (Table 2; Figure 4).
Tang’s team showed that acupuncture at GV20 and BL23 altered morphological damage in astrocytes and neurons in the hippocampal dentate gyrus and improved cognitive dysfunction in Aβ1-42-induced AD model rats (Tang et al., 2019). In Wang’s study, EA at GV20 for 4 weeks suppressed astrocytosis in APP mice, ameliorated AD-induced cognitive impairment and memory decline, and attenuated the upregulation of glial fibrillary acidic protein (GFAP) in reactive astrocytes, implying the therapeutic pathway of EA via astrocytic alteration (Wang et al., 2014). Similar results have been reported in Li’s study, which applied EA at ST36 in vascular dementia rats and found a decreasing tendency of astrocytes and an increased pyramidal neuron number in the hippocampal CA1 area (Li et al., 2015).
4.6 The impact of acupuncture via the microglia-related pathways
In cognitive impairment-related studies focusing on microglia, acupuncture elevated learning ability, memory capacity, and working memory. The potential mechanism related to the anti-inflammatory pathway, the modulation of the microglia activation, and the regulation of the AD-related protein expression (Table 2; Figure 1).
In an AD animal model, EA at GV20 and GV29 upregulated the expression of triggering receptors expressed on myeloid cells 2 (TREM2) in the hippocampus, ameliorating learning and memory dysfunction and protecting neurons (Li Y. et al., 2020). TREM2 contributes to neuroinflammation in AD (Kober and Brett, 2017) and is expressed by microglia in the brain (Wolfe et al., 2019). The TREM2-mediated anti-inflammatory pathway might be the potential mechanism by which EA exerts its neuroprotective effect. In AD-related studies, Xie’s team (Xie et al., 2021) summarized the effect of EA in inhibiting the activation of glia and the M2 phenotype polarization of microglia. Meanwhile, there was a reduction in pro-inflammatory cytokines and an elevation in anti-inflammatory cytokines after EA intervention in the hippocampus of AD rats. In addition, Cai’s study reported a co-occurrence reduction in microglia-mediated Aβ deposition and amyloid precursor protein in the prefrontal cortex of 5 × FAD rats after EA treatment (Cai et al., 2019). Similarly, Wang’s team (Wang et al., 2020) proved that the “olfactory three-needle” reduced the deposition of Aβ, upregulated the expression of synaptophysin (SYP), and inhibited the excessive activation of microglia in the hippocampus of SAMP8 mice. Regarding neuroinflammation, EA reduced the content of IL-1β, interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in the hippocampus (Han et al., 2018).
4.7 The impact of acupuncture via the OL-related pathways
Acupuncture showed a positive effect on the learning ability and memory function in cognitive impaired model animals, and the mechanism might be the enhancement of cerebral perfusion, the improvement of oligodendrocyte regeneration, and regulation of neurotrophin-4/5- tyrosine receptor kinase B (NT4/5-TrkB) signaling pathway.
In CCH rats, Zhang’s team observed learning and memory abilities and the number of Oli2-marked OL-positive cells after acupuncture of GV20 and EX-HN1 for 4 weeks. The study results indicated that acupuncture induced a significant increase in OL-positive cells in the corpus callosum, an improvement in the structure of cerebral white matter fibers, and decreased learning and memory dysfunction (Li Z. et al., 2021). Ahn’s study demonstrated similar results after applying EA at GV20 and GV14. The 7-session EA stimulation attenuated memory impairment, strengthened OL differentiation from OPCs, improved white matter structure recovery in the corpus callosum, and activated the NT4/5-TrkB signaling pathway (Ahn et al., 2016). Additionally, EA at GV20, GV16, and BL23 showed a benign effect on promoting the proliferation and differentiation of endogenous neural stem cells to neurons and oligodendrocytes in the hippocampal dentate gyrus of AD model rats (Table 2).
5 Acupuncture details
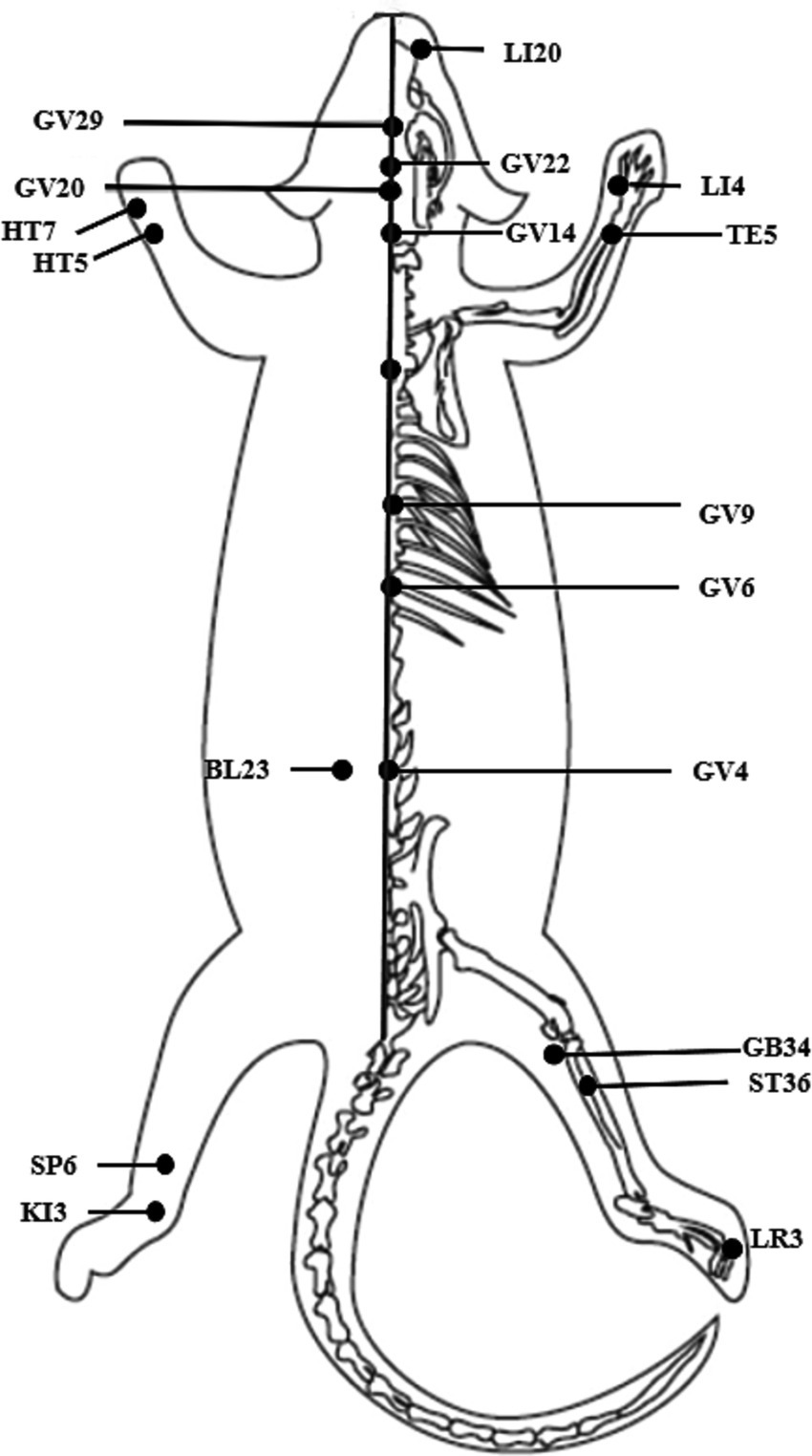
Among the included 34 studies, 7 studies used manual acupuncture, and the other 27 studies conducted electroacupuncture. The duration of acupuncture ranged from 7 days to 110 days, except for one study applying instant acupuncture. Twelve acupoints were mentioned in the movement disorders, of which the GV20 and GV14 were the most frequently used; and 10 acupoints were mentioned in the cognitive impairment, of which the GV20 and GV29 were the most frequently used. Figure 6 illustrates the position of the above acupoints.
6 Discussion
6.1 The characteristics and innovation of this study
This review summarized current evidence related to acupuncture’s therapeutic mechanism in neurodegenerative disease at the neural circuit level. We focused on movement disorder and cognitive impairment, which are the most frequently occurring symptoms in patients with neurodegenerative disorders. Regarding targeted disease, among the 38 included studies, 20 focused on motor disorders involving PD, ALS, and multiple sclerosis; 18 studies reported cognitive impairment, including AD, memory deficits, and spatial cognition impairment.
Previously, a literature review reported the therapeutic mechanism of acupuncture on PD in the dopaminergic neural pathway and summarized the efficacy and dopaminergic neuron protection of acupuncture (Zhao et al., 2021). In this review, we found that apart from the dopaminergic neural circuit, neural pathways such as the glutamatergic system, GABAergic system, and glial cells play roles in acupuncture improving motor disorders in PD.
In PD-related motor deficit studies, current findings support the hypothesis based on the basal ganglia circuit, and the targeted pathways projecting from the midbrain SNpc, striatum, cortex, and thalamus, of which the direct pathway comprises the basal ganglia circuit and the indirect pathway of basal ganglia-thalamus-cortex (Mcgregor and Nelson, 2019). At the circuit level, electroacupuncture treatment enhanced brain region activation in the striatum, primary motor cortex (M1), cingulate gyrus, and global pallidus externa (GPe) (Zhang et al., 2018). The results supported were consistent with the classical circuit model in the pathogenesis of PD. In molecular studies, dopaminergic neurons and DA receptors provide a structural basis at the circuit level. Acupuncture indicated a beneficial impact on restoring the survival of dopaminergic neurons and the metabolism of DA receptors, especially in the protein level and mRNA expression of D1R and D2R, which are the functional receptors in the direct and indirect pathways. Regarding the input pathway of prefrontal cortical-striatum projections, acupuncture demonstrated a similar protective effect on dopaminergic neurons and facilitated the elimination of α-syn in the striatum.
Apart from the direct influence on the dopaminergic system, neurons with other neurotransmitters displayed indirect effects on acupuncture’s mechanistic pathways. In the PD process, current studies proposed the hypotheses that the overexcitation of glutamatergic neurons in cortex-striatum projections deteriorates the degeneration of dopaminergic neurons, and the impairment of Glu receptors inhibits the information transmission efficacy in synaptic communication. Acupuncture showed a benign modulation of LTP and synaptic plasticity in the cortex-striatum circuits. GABAergic neurons account for inhibitory information transmission as interneurons in the indirect pathway. The imbalance of E/I information conduction resulted in dysfunction of the motor control system. Acupuncture demonstrated benign regulation of GABAergic neurons in the output pathway of the basal ganglia, and the cerebellum-basal ganglia-cortical circuit.
For cognitive impairment, which is a frequently reported symptom caused by a variety of diseases with different phases, our review concentrated on neurodegenerative diseases, which share common pathogenesis and rehabilitation mechanisms to some degree. Previous review reported the mechanistic pathways of different interventions on acupoints, and summarized the molecular mechanisms of neuroprotective effects on cognitive deficits (Zhang Z. et al., 2023). This study focused on acupuncture and electroacupuncture details, and highlighted the frequently studied acupoints and their targeted mechanistic pathways.
For cognitive impairment, some hypotheses stimulating acupoints in periphery could change the neurochemistry/neurocircuitry in the brain of rodents. The hippocampal Schaffer projection and hippocampal DG were the most frequently reported circuits. At the circuit level, acupuncture attenuated memory deficits by modulating the E/I balance of hippocampal GABAergic interneurons and facilitated the modulation of brain oscillations. For the basal forebrain-hippocampus cholinergic neural circuit, acupuncture displayed benign regulation of the molecular metabolism of the medial septal/VDB-DG pathway, which contributed to pattern separation impairment in cognitive impairment (Li et al., 2022). There are bidirectional connections in the basal forebrain-hippocampus projection, of which the hippocampal interneurons receive input from GABAergic neurons and cholinergic neurons and project to the medial septal/VDB in the basal forebrain from the CA1 region (Zheng et al., 2023). In our review, we found that the medial septal/VDB-DG pathway was a modulation target of acupuncture via the cholinergic neural circuit. Interestingly, according to brain-network research, abnormal functional connectivity within the medial septal/VDB circuit has been reported in PD patients with mild cognitive impairment (MCI) (Zhang P. et al., 2023). In addition, the volumes of cholinergic cells in the basal forebrain showed a relationship with disorders of brain rhythm (α-rhythm activity, θ oscillations) in MCI (Rea et al., 2021), and AD (Liu et al., 2022).
At the molecular level, the imbalance of Glu metabolism prevented neural information transmission efficacy, and acupuncture showed a bidirectional benign regulatory effect on synaptic plasticity. On the one hand, in the METH withdrawal model of spatial memory impairment mice, acupuncture accelerated the elimination of abnormally enhanced extracellular Glu in the dCA1 and suppressed irregular spontaneous excitatory postsynaptic currents (EPSCs) in Schaffer projections. On the other hand, in AD model mice, acupuncture restored the level of Glu receptors and promoted synaptic density in the hippocampus. In addition, acupuncture regulated the brain-gut axis via the serotonergic system, improving the learning and memory ability of AD model rats (Xiao et al., 2023).
Regarding glial cells, there is limited evidence of their direct influences on neural circuits in specific neural circuits. The indirect effect on acupuncture-related mechanisms involved neuron protective effects and energy metabolic modulation through astrocytes and microglia. Recent studies have proposed a new communication pathway in the neuron–glia–neuron system, the energy signaling pathway. Apart from classical signaling molecules, energy support plays a vital role in astrocyte connections with neurons (Illes et al., 2019). In general, adenosine triphosphate (ATP) derived from astrocytes inhibits neuronal excitability and signal transmission by modulating synapse formation and energy demands (Lezmy, 2023). At the circuit level, astrocyte-derived ATP indicated an increase in LTP and LTD in Schaffer collaterals (Obot et al., 2023). In the VD model, acupuncture has been proven to restore the ATP level and attenuate mitochondrial dysfunction from the accumulation of Aβ (Su et al., 2018). However, few studies have reported the astrocyte-induced energy metabolic mechanism of acupuncture intervention. Microglia are the crucial target of acupuncture intervention in the neuroimmune system, mainly involving inflammation and oxidative stress. Current evidence supports the neuroprotective effect of acupuncture by mediating the activation and polarization of microglia. Nevertheless, there have been limited findings focusing on specific neural circuits, except for the Schaffer collaterals and DG in cognitive impairment-related studies and the nigra-striatum network in movement disorder studies. Given the contribution of glial cells to the development and repair of neural function, relevant explorations in this field are needed in further studies.
7 Limitation
There are some limitations in this review. Firstly, this literature review describes the neural circuit mechanism in different chemical transmitters under acupuncture therapy. Due to the study type, we reported the included studies in a qualitative way rather than a quantitative evaluation. Secondly, though motor disorder and cognitive impairment were the common symptoms in neurodegenerative disorders, acupuncture-related studies mainly focused on PD and AD, except for three studies on TS, ALS, and multiple sclerosis separately. Given the increasing number of patients with neurodegenerative diseases, further studies should pay more attention to these diseases. Thirdly, since the neural circuit-related studies were mainly conducted on animal models, our findings mainly focused on rodents-related research, evidence based on humans are needed in the future.
8 Conclusion
In this review study, we summarized the acupuncture-related neural circuits in different chemical transmitters and glial cells on motor disorders and cognitive impairment in neurodegenerative diseases. In movement dysfunction, dopaminergic circuits, such as the cortex-basal ganglia-midbrain circuit, were the direct target of acupuncture’s mechanistic pathway, which was interfered with by other chemical transmitters and glial systems, and the glutamatergic pathway played crucial roles at the synaptic level. For cognitive impairment, acupuncture showed a neuroprotective role via the glutamatergic and cholinergic systems at the circuit level, including the forebrain-hippocampus and hippocampal circuits, and facilitated glial cells. Given the high occurrence of motor disorder and cognitive impairment in neurodegenerative disorders, there are shared pathogenesis in these two symptoms, and the behavioral tests indicated coincident improvement after acupuncture intervention. Currently, there is limited evidence in neural circuit research on both motor disorders and cognitive impairment, and acupuncture’s mechanism at the circuit level is in the preliminary stage. Considering that the high coincidence of acupoint selection implied a common recovery process in neurodegenerative diseases, the common mechanistic pathways under acupuncture intervention should be explored.
Author contributions
BL: Conceptualization, Writing – original draft, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software. SD: Data curation, Validation, Writing – review & editing. HJ: Validation, Writing – review & editing. WZ: Validation, Writing – review & editing. BZ: Visualization, Writing – review & editing. YD: Project administration, Writing – review & editing. ZM: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This review was supported by grants from the Ministry of Science and Technology of the People’s Republic of China, National Key Research and Development Program (2018YFC1706001, 2010CB530506); Tianjin Municipal Education Commission (2022BKYZ046); The Science & Technology Development Fund of Tianjin Education Commission for Higher Education (2022KJ170); Scientific research and innovation projects of Tianjin University of Traditional Chinese Medicine (YJSKC-20222002); Open Project of National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion (NCRCOP2023003). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
Thanks to BioRender for helping with figures production.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahn, S. M., Kim, Y. R., Kim, H. N., Shin, Y., Shin, H. K., and Choi, B. T. (2016). Electroacupuncture ameliorates memory impairments by enhancing oligodendrocyte regeneration in a mouse model of prolonged cerebral hypoperfusion. Sci. Rep. 6:28646. doi: 10.1038/srep28646
Albert, P. R., and Vahid-Ansari, F. (2019). The 5-HT1A receptor: signaling to behavior. Biochimie 161, 34–45. doi: 10.1016/j.biochi.2018.10.015
Allen, N. J., and Lyons, D. A. (2018). Glia as architects of central nervous system formation and function. Science 362, 181–185. doi: 10.1126/science.aat0473
Andoh, M., and Koyama, R. (2021). Microglia regulate synaptic development and plasticity. Dev. Neurobiol. 81, 568–590. doi: 10.1002/dneu.22814
Aono, H., Choudhury, M. E., Higaki, H., Miyanishi, K., Kigami, Y., Fujita, K., et al. (2017). Microglia may compensate for dopaminergic neuron loss in experimental parkinsonism through selective elimination of glutamatergic synapses from the subthalamic nucleus. Glia 65, 1833–1847. doi: 10.1002/glia.23199
Babaei, P. (2021). NMDA and AMPA receptors dysregulation in Alzheimer's disease. Eur. J. Pharmacol. 908:174310. doi: 10.1016/j.ejphar.2021.174310
Berry, A. S., and Harrison, T. M. (2023). New perspectives on the basal forebrain cholinergic system in Alzheimer’s disease. Neurosci. Biobehav. Rev. 150:105192. doi: 10.1016/j.neubiorev.2023.105192
Boccalini, C., Bortolin, E., Carli, G., Pilotto, A., Galbiati, A., Padovani, A., et al. (2022). Metabolic connectivity of resting-state networks in alpha synucleinopathies, from prodromal to dementia phase. Front. Neurosci. 16:930735. doi: 10.3389/fnins.2022.930735
Bonansco, C., Cerpa, W., and Inestrosa, N. C. (2023). How are synapses born? A functional and molecular view of the role of the Wnt signaling pathway. Int. J. Mol. Sci. 24:708. doi: 10.3390/ijms24010708
Brockett, A. T., Kane, G. A., Monari, P. K., Briones, B. A., Vigneron, P., Barber, G. A., et al. (2018). Evidence supporting a role for astrocytes in the regulation of cognitive flexibility and neuronal oscillations through the Ca2+ binding protein S100β. PLoS One 13:e195726:e0195726. doi: 10.1371/journal.pone.0195726
Broman, J., Rinvik, E., Sassoe-Pognetto, M., Shandiz, H. K., and Ottersen, O. P. (2004). “Chapter 36 – Glutamate” in The rat nervous system (third edition). ed. G. Paxinos (Burlington: Academic Press), 1269–1292.
Cai, M., Lee, J., and Yang, E. J. (2019). Electroacupuncture attenuates cognition impairment via anti-neuroinflammation in an Alzheimer’s disease animal model. J. Neuroinflamm. 16:264. doi: 10.1186/s12974-019-1665-3
Cao, Y., Wang, L., Lin, L., Wang, X., Ma, S., Yang, N., et al. (2021). Acupuncture attenuates cognitive deficits through α7nAChR mediated anti-inflammatory pathway in chronic cerebral hypoperfusion rats. Life Sci. 266:118732. doi: 10.1016/j.lfs.2020.118732
Chang, X., Chen, K., Cheng, T., Lai, P., Zhang, L., So, K., et al. (2022). In vivo neuronal and astrocytic activation in somatosensory cortex by acupuncture stimuli. Neural Regen. Res. 17, 2526–2529. doi: 10.4103/1673-5374.339003
Chapman, C. A., Nuwer, J. L., and Jacob, T. C. (2022). The Yin and Yang of GABAergic and glutamatergic synaptic plasticity: opposites in balance by Crosstalking mechanisms. Front. Synaptic Neurosci. 14:911020. doi: 10.3389/fnsyn.2022.911020
Choudhury, M. E., Kigami, Y., and Tanaka, J. (2021). Dual roles of microglia in the basal ganglia in Parkinson’s disease. Int. J. Mol. Sci. 22:3907. doi: 10.3390/ijms22083907
Chu, H. (2020). Synaptic and cellular plasticity in Parkinson's disease. Acta Pharmacol. Sin. 41, 447–452. doi: 10.1038/s41401-020-0371-0
Cieza, A., Causey, K., Kamenov, K., Hanson, S. W., Chatterji, S., and Vos, T. (2021). Global estimates of the need for rehabilitation based on the global burden of disease study 2019: a systematic analysis for the global burden of disease study 2019. Lancet 396, 2006–2017. doi: 10.1016/S0140-6736(20)32340-0
Clay, E., Zhou, J., Yi, Z., Zhai, S., and Toumi, M. (2019). Economic burden for Alzheimer’s disease in China from 2010 to 2050: a modelling study. J. Market Access Health Policy 7:1667195. doi: 10.1080/20016689.2019.1667195
Conway, M. E. (2020). Alzheimer’s disease: targeting the glutamatergic system. Biogerontology 21, 257–274. doi: 10.1007/s10522-020-09860-4
Crispino, M., Volpicelli, F., and Perrone-Capano, C. (2020). Role of the serotonin receptor 7 in brain plasticity: from development to disease. Int. J. Mol. Sci. 21:505. doi: 10.3390/ijms21020505
Cuestas Torres, D. M., and Cardenas, F. P. (2020). Synaptic plasticity in Alzheimer's disease and healthy aging. Rev. Neurosci. 31, 245–268. doi: 10.1515/revneuro-2019-0058
Dauphinot, V., Potashman, M., Levitchi-Benea, M., Su, R., Rubino, I., and Krolak-Salmon, P. (2022). Economic and caregiver impact of Alzheimer’s disease across the disease spectrum: a cohort study. Alzheimers Res. Ther. 14:34. doi: 10.1186/s13195-022-00969-x
Du, J., Sun, Z., Jia, J., Wang, X., and Wang, X. (2011). High-frequency electro-acupuncture stimulation modulates intracerebral γ-aminobutyric acid content in rat model of Parkinson's disease. Acta Physiologica Sinica 63, 305–310. doi: 10.13294/j.aps.2011.04.003
Dwyer, J. B. (2018). A developmental perspective of dopaminergic dysfunction in Tourette syndrome. Biol. Psychiatry 84, e33–e35. doi: 10.1016/j.biopsych.2018.07.008
Fan, J., Lu, W., Tan, W., Feng, W., and Zhuang, L. (2022). Acupuncture for Parkinson’s disease: from theory to practice. Biomed. Pharmacother. 149:112907. doi: 10.1016/j.biopha.2022.112907
Fang, L. P., Liu, Q., Meyer, E., Welle, A., Huang, W., Scheller, A., et al. (2023). A subset ofOPCs do not express Olig2 during development which can be increased in the adult by brain injuries and complex motor learning. Glia 71, 415–430. doi: 10.1002/glia.24284
Gan, L., Cookson, M. R., Petrucelli, L., and La Spada, A. R. (2018). Converging pathways in neurodegeneration, from genetics to mechanisms. Nat. Neurosci. 21, 1300–1309. doi: 10.1038/s41593-018-0237-7
Geibl, F. F., Henrich, M. T., and Oertel, W. H. (2019). Mesencephalic and extramesencephalic dopaminergic systems in Parkinson's disease. J. Neural Transm. 126, 377–396. doi: 10.1007/s00702-019-01970-9
Haiyang, W. U., Ying, W., Wei, H., Huihui, L. I., Haisheng, J. I., and Xiuxiu, L. (2023). Protective effect of Tongdu Tiaoshen acupuncture combined with Xiaoxuming decoction on dopaminergic neurons in Parkinson's disease model. J. Tradit. Chin. Med. 43, 484–493. doi: 10.19852/j.cnki.jtcm.20230214.005
Hale, M. W., and Lowry, C. A. (2011). Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology 213, 243–264. doi: 10.1007/s00213-010-2089-z
Hamm, V., Héraud, C., Cassel, J., Mathis, C., and Goutagny, R. (2015). Precocious alterations of brain oscillatory activity in Alzheimer’s disease: a window of opportunity for early diagnosis and treatment. Front. Cell. Neurosci. 9:491. doi: 10.3389/fncel.2015.00491
Hampel, H., Mesulam, M. M., Cuello, A. C., Khachaturian, A. S., Vergallo, A., Farlow, M. R., et al. (2018). Revisiting the cholinergic hypothesis in ALZHEIMER’S disease: emerging evidence from translational and clinical research. J. Prev Alzheimers Dis. 6, 1–14. doi: 10.14283/jpad.2018.43
Han, Y., Qin, X., Zhang, T., Lei, M., Sun, F., Sun, J., et al. (2018). Electroacupuncture prevents cognitive impairment induced by lipopolysaccharide via inhibition of oxidative stress and neuroinflammation. Neurosci. Lett. 683, 190–195. doi: 10.1016/j.neulet.2018.06.003
Hansen, D. V., Hanson, J. E., and Sheng, M. (2018). Microglia in Alzheimer's disease. J. Cell Biol. 217, 459–472. doi: 10.1083/jcb.201709069
He, T., Li, N., Shi, P., Xu, X., Nie, J., Lu, X., et al. (2022). Electroacupuncture alleviates spatial memory deficits in METH withdrawal mice by enhancing astrocyte-mediated glutamate clearance in the dCA1. Addict. Biol. 27:e13068. doi: 10.1111/adb.13068
Hirschberg, S., Dvorzhak, A., Rasooli-Nejad, S. M. A., Angelov, S., Kirchner, M., Mertins, P., et al. (2022). Uncoupling the excitatory amino acid transporter 2 from its C-terminal Interactome restores synaptic glutamate clearance at Corticostriatal synapses and alleviates mutant huntingtin-induced Hypokinesia. Front. Cell. Neurosci. 15:792652. doi: 10.3389/fncel.2021.792652
Hou, Z., Yang, X., Li, Y., Chen, J., and Shang, H. (2022). Electroacupuncture enhances neuroplasticity by regulating the orexin A-mediated cAMP/PKA/CREB signaling pathway in senescence-accelerated mouse prone 8 (SAMP8) mice. Oxidative Med. Cell. Longev. 2022, 1–15. doi: 10.1155/2022/8694462
Hussan, M. T., Sakai, A., and Matsui, H. (2022). Glutamatergic pathways in the brains of turtles: a comparative perspective among reptiles, birds, and mammals. Front. Neuroanat. 16:937504. doi: 10.3389/fnana.2022.937504
Illes, P., Burnstock, G., and Tang, Y. (2019). Astroglia-derived ATP modulates CNS neuronal circuits. Trends Neurosci. 42, 885–898. doi: 10.1016/j.tins.2019.09.006
Jang, J., Park, S., An, J., Choi, J., Seol, I. C., Park, G., et al. (2020a). Gait disturbance improvement and cerebral cortex rearrangement by acupuncture in Parkinson’s disease: a pilot Assessor-blinded, randomized, controlled, Parallel-Group Trial. Neurorehabil. Neural Repair 34, 1111–1123. doi: 10.1177/1545968320969942
Jang, J., Yeom, M., Ahn, S., Oh, J., Ji, S., Kim, T., et al. (2020b). Acupuncture inhibits neuroinflammation and gut microbial dysbiosis in a mouse model of Parkinson’s disease. Brain Behav. Immun. 89, 641–655. doi: 10.1016/j.bbi.2020.08.015
Jay, T. M. (2003). Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog. Neurobiol. 69, 375–390. doi: 10.1016/S0301-0082(03)00085-6
Jia, Y. J., Deng, J. H., Zhang, W. Z., Sun, Z. L., Yang, J., Yu, Y., et al. (2017). The role of group II metabotropic glutamate receptors in the striatum in Electroacupuncture treatment of parkinsonian rats. CNS Neurosci. Ther. 23, 23–32. doi: 10.1111/cns.12587
Jia, J., Li, B., Sun, Z., Yu, F., Wang, X., and Wang, X. (2010). Electro-acupuncture stimulation acts on the basal ganglia output pathway to ameliorate motor impairment in parkinsonian model rats. Behav. Neurosci. 124, 305–310. doi: 10.1037/a0018931
Jia, J., Sun, Z., Li, B., Pan, Y., Wang, H., Wang, X., et al. (2009). Electro-acupuncture stimulation improves motor disorders in parkinsonian rats. Behav. Brain Res. 205, 214–218. doi: 10.1016/j.bbr.2009.06.024
Joe, E., and Ringman, J. M. (2019). Cognitive symptoms of Alzheimer’s disease: clinical management and prevention. BMJ :l6217. doi: 10.1136/bmj.l6217
Kim, S. N., Doo, A. R., Park, J. Y., Bae, H., Chae, Y., Shim, I., et al. (2011). Acupuncture enhances the synaptic dopamine availability to improve motor function in a mouse model of Parkinson's disease. PLoS One 6:e27566. doi: 10.1371/journal.pone.0027566
Kim, S. N., Doo, A. R., Park, J. Y., Choo, H. J., Shim, I., Park, J. J., et al. (2014). Combined treatment with acupuncture reduces effective dose and alleviates adverse effect of L-dopa by normalizing Parkinson's disease-induced neurochemical imbalance. Brain Res. 1544, 33–44. doi: 10.1016/j.brainres.2013.11.028
Klein, M. O., Battagello, D. S., Cardoso, A. R., Hauser, D. N., Bittencourt, J. C., and Correa, R. G. (2019). Dopamine: functions, signaling, and association with neurological diseases. Cell. Mol. Neurobiol. 39, 31–59. doi: 10.1007/s10571-018-0632-3
Kober, D. L., and Brett, T. J. (2017). TREM2-ligand interactions in health and disease. J. Mol. Biol. 429, 1607–1629. doi: 10.1016/j.jmb.2017.04.004
Lee, B., Sur, B., Kwon, S., Jung, E., Shim, I., Lee, H., et al. (2012). Acupuncture stimulation alleviates corticosterone-induced impairments of spatial memory and cholinergic neurons in rats. Evid.-based complement. Altern. Med. 2012, 1–14. doi: 10.1155/2012/670536
Lee, B., Sur, B., Shim, J., Hahm, D. H., and Lee, H. (2014). Acupuncture stimulation improves scopolamine-induced cognitive impairment via activation of cholinergic system and regulation of BDNF and CREB expressions in rats. BMC Complement. Altern. Med. 14:338. doi: 10.1186/1472-6882-14-338
Lei, H., Toosizadeh, N., Schwenk, M., Sherman, S., Karp, S., Sternberg, E., et al. (2016). A pilot clinical trial to objectively assess the efficacy of Electroacupuncture on gait in patients with Parkinson's disease using body worn sensors. PLoS One 11:e155613:e0155613. doi: 10.1371/journal.pone.0155613
Leite Silva, A. B. R., Gonçalves De Oliveira, R. W., Diógenes, G. P., de Castro Aguiar, M. F., Sallem, C. C., Lima, M. P. P., et al. (2023). Premotor, nonmotor and motor symptoms of Parkinson's disease: a new clinical state of the art. Ageing Res. Rev. 84:101834. doi: 10.1016/j.arr.2022.101834
Leng, F., and Edison, P. (2021). Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nature reviews. Neurology 17, 157–172. doi: 10.1038/s41582-020-00435-y
Lezmy, J. (2023). How astrocytic ATP shapes neuronal activity and brain circuits. Curr. Opin. Neurobiol. 79:102685. doi: 10.1016/j.conb.2023.102685
Li, Y., Jiang, J., Tang, Q., Tian, H., Wang, S., Wang, Z., et al. (2020). Microglia TREM2: a potential role in the mechanism of action of Electroacupuncture in an Alzheimer’s disease animal model. Neural Plast. 2020, 1–8. doi: 10.1155/2020/8867547
Li, H., Lai, L., Li, X., Wang, R., Fang, X., Xu, N., et al. (2023). Electroacupuncture ameliorates cognitive impairment by regulating γ-amino butyric Acidergic interneurons in the Hippocampus of 5 familial Alzheimer’s disease mice. Neuromodulation Technol. Neural Interface. 1–12. doi: 10.1016/j.neurom.2022.11.014
Li, L., Li, J., Dai, Y., Yang, M., Liang, S., Wang, Z., et al. (2021). Electro-acupuncture improve the early pattern separation in Alzheimer's disease mice via basal forebrain-Hippocampus cholinergic neural circuit. Front. Aging Neurosci. 13:770948. doi: 10.3389/fnagi.2021.770948
Li, L., Lu, J., Sun, Y., and Jin, X. (2019). Acupuncture protects from 6-OHDA-induced neuronal damage by balancing the ratio of DMT1/Fpn1. Saudi J. Biol. Sci. 26, 1948–1955. doi: 10.1016/j.sjbs.2019.07.003
Li, M., Wang, K., Su, W., Jia, J., and Wang, X. (2020). Effects of Electroacupuncture on metabolic changes in motor cortex and striatum of 6-Hydroxydopamine-induced parkinsonian rats. Chin. J. Integr. Med. 26, 701–708. doi: 10.1007/s11655-017-2975-x
Li, M., Wang, K., Su, W., Jia, J., and Wang, X. (2022). The modulatory effect of 100 Hz electroacupuncture on striatal synaptic plasticity in unilateral lesioned 6-OHDA rats. Brain Res. Bull. 186, 123–135. doi: 10.1016/j.brainresbull.2022.06.004
Li, Z., Xiaojing, G., Yemeng, G., Ji, L. I., Lu, W., and Guile, X. (2021). Effects of acupuncture-rehabilitation therapy on learning and memory ability and OLGs in callus of rats with CCH. J. Clin. Acupunc. Moxibustion 37, 63–68. doi: 10.19917/j.cnki.1005-0779.021120
Li, F., Yan, C., Lin, L., Li, H., Zeng, X., Liu, Y., et al. (2015). Acupuncture attenuates cognitive deficits and increases pyramidal neuron number in hippocampal CA1 area of vascular dementia rats. BMC Complement. Altern. Med. 15:133. doi: 10.1186/s12906-015-0656-x
Lin, J. G., Chen, C. J., Yang, H. B., Chen, Y. H., and Hung, S. Y. (2017). Electroacupuncture promotes recovery of motor function and reduces dopaminergic neuron degeneration in rodent models of Parkinson's disease. Int. J. Mol. Sci. 18:1846. doi: 10.3390/ijms18091846
Lin, R., Li, L., Zhang, Y., Huang, S., Chen, S., Shi, J., et al. (2018). Electroacupuncture ameliorate learning and memory by improving N-acetylaspartate and glutamate metabolism in APP/PS1 mice. Biol. Res. 51:21. doi: 10.1186/s40659-018-0166-7
Lin, J., Liu, W., Guan, J., Cui, J., Shi, R., Wang, L., et al. (2023). Latest updates on the serotonergic system in depression and anxiety. Front. Synaptic Neurosci. 15:1124112. doi: 10.3389/fnsyn.2023.1124112
Lin, L., Yu, L., Xiang, H., Hu, X., Yuan, X., Zhu, H., et al. (2019). Effects of acupuncture on behavioral stereotypies and brain dopamine system in mice as a model of Tourette syndrome. Front. Behav. Neurosci. 13:239. doi: 10.3389/fnbeh.2019.00239
Lin, B. B., Zhang, L. L., Yin, X. L., Chen, X. C., Ruan, C. D., Wu, T. C., et al. (2022). Modulation of entorhinal cortex-hippocampus connectivity and recognition memory following electroacupuncture on 3xTg-AD model: evidence from multimodal MRI and electrophysiological recordings. Front. Neurosci. 16:968767. doi: 10.3389/fnins.2022.968767
Liu, C. (2020). Targeting the cholinergic system in Parkinson's disease. Acta Pharmacol. Sin. 41, 453–463. doi: 10.1038/s41401-020-0380-z
Liu, Z., He, B., Zhang, R., Zhang, K., Ding, Y., Ruan, J., et al. (2015). Electroacupuncture promotes the differentiation of transplanted bone marrow mesenchymal stem cells Preinduced with Neurotrophin-3 and retinoic acid into oligodendrocyte-like cells in demyelinated spinal cord of rats. Cell Transplant. 24, 1265–1281. doi: 10.3727/096368914X682099
Liu, W., Li, J., Yang, M., Ke, X., Dai, Y., Lin, H., et al. (2022). Chemical genetic activation of the cholinergic basal forebrain hippocampal circuit rescues memory loss in Alzheimer’s disease. Alzheimers Res. Ther. 14:53. doi: 10.1186/s13195-022-00994-w
Liu, K., and Zhu, B. (2023). Significance of pleasant touch and state-of-the-art neuroscience technologies in acupuncture research. Acupunc. Herbal Med. 3, 55–58. doi: 10.1097/HM9.0000000000000058
Lu, L., Zhang, Y., Tang, X., Ge, S., Wen, H., Zeng, J., et al. (2022). Evidence on acupuncture therapies is underused in clinical practice and health policy. BMJ 376:e067475. doi: 10.1136/bmj-2021-067475
Luo, L. (2021). Architectures of neuronal circuits. Science 373:eabg7285. doi: 10.1126/science.abg7285
Lv, E., Deng, J., Yu, Y., Wang, Y., Gong, X., Jia, J., et al. (2015). Nrf2-ARE signals mediated the anti-oxidative action of electroacupuncture in an MPTP mouse model of Parkinson's disease. Free Radic. Res. 49, 1296–1307. doi: 10.3109/10715762.2015.1067696
McGregor, M. M., and Nelson, A. B. (2019). Circuit mechanisms of Parkinson’s disease. Neuron (Cambridge, Mass.) 101, 1042–1056. doi: 10.1016/j.neuron.2019.03.004
Mederos, S., Sánchez-Puelles, C., Esparza, J., Valero, M., Ponomarenko, A., and Perea, G. (2021). GABAergic signaling to astrocytes in the prefrontal cortex sustains goal-directed behaviors. Nat. Neurosci. 24, 82–92. doi: 10.1038/s41593-020-00752-x
Mesulam, M. M. (2013). Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer's disease. J. Comp. Neurol. 521, 4124–4144. doi: 10.1002/cne.23415
Morita, K., and Kawaguchi, Y. (2019). A dual role hypothesis of the Cortico-basal-ganglia pathways: Opponency and temporal difference through dopamine and adenosine. Front. Neural Circuits 12:111. doi: 10.3389/fncir.2018.00111
Nemade, D., Subramanian, T., and Shivkumar, V. (2021). An update on medical and surgical treatments of Parkinson’s disease. Aging Dis. 12, 1021–1035. doi: 10.14336/AD.2020.1225
Nespoli, E., Rizzo, F., Boeckers, T., Schulze, U., and Hengerer, B. (2018). Altered dopaminergic regulation of the dorsal striatum is able to induce tic-like movements in juvenile rats. PLoS One 13:e196515:e0196515. doi: 10.1371/journal.pone.0196515
Nichols, E., Steinmetz, J. D., Vollset, S. E., Fukutaki, K., Chalek, J., Abd-Allah, F., et al. (2022). Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health 7, e105–e125. doi: 10.1016/S2468-2667(21)00249-8
Ning, B., Wang, Z., Wu, Q., Deng, Q., Yang, Q., Gao, J., et al. (2023). Acupuncture inhibits autophagy and repairs synapses by activating the mTOR pathway in Parkinson’s disease depression model rats. Brain Res. 1808:148320. doi: 10.1016/j.brainres.2023.148320
Obot, P., Subah, G., Schonwald, A., Pan, J., Velíšek, L., Velíšková, J., et al. (2023). Astrocyte and neuronal Panx1 support long-term reference memory in mice. ASN Neuro 15:1759. doi: 10.1177/17590914231184712
Oliveira, J. F., and Araque, A. (2022). Astrocyte regulation of neural circuit activity and network states. Glia 70, 1455–1466. doi: 10.1002/glia.24178
Pak, M. E., Ahn, S. M., Jung, D. H., Lee, H. J., Ha, K. T., Shin, H. K., et al. (2019). Electroacupuncture therapy ameliorates motor dysfunction via brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor in a mouse model of Parkinson's disease. J. Gerontol. Series A 75, 712–721. doi: 10.1093/gerona/glz256
Paquelet, G. E., Carrion, K., Lacefield, C. O., Zhou, P., Hen, R., and Miller, B. R. (2022). Single-cell activity and network properties of dorsal raphe nucleus serotonin neurons during emotionally salient behaviors. Neuron 110, 2664–2679.e8. doi: 10.1016/j.neuron.2022.05.015
Rea, R. C., Berlot, R., Martin, S. L., Craig, C. E., Holmes, P. S., Wright, D. J., et al. (2021). Quantitative EEG and cholinergic basal forebrain atrophy in Parkinson's disease and mild cognitive impairment. Neurobiol. Aging 106, 37–44. doi: 10.1016/j.neurobiolaging.2021.05.023
Rui, G., Zhang, G. J., Yong, W., Jie, F., Cui, Y. C., Xi, J., et al. (2013). High frequency electro-acupuncture enhances striatum DAT and D1 receptor expression, but decreases D2 receptor level in 6-OHDA lesioned rats. Behav. Brain Res. 237, 263–269. doi: 10.1016/j.bbr.2012.09.047
Sancho, L., Contreras, M., and Allen, N. J. (2021). Glia as sculptors of synaptic plasticity. Neurosci. Res. 167, 17–29. doi: 10.1016/j.neures.2020.11.005
Shen, W., Zhai, S., and Surmeier, D. J. (2022). Striatal synaptic adaptations in Parkinson's disease. Neurobiol. Dis. 167:105686. doi: 10.1016/j.nbd.2022.105686
Shengjiao, S., Juan, L., Wencheng, W., Yun, X., Baoying, L., and Xing, L. (2022). Function of oligodendrocytes and demyelinating disease. Chin. J. Tissue Eng. Res. 26, 3108–3116. doi: 10.12307/2022.394
Shi, X. (2021). Study of the relationship between acupuncture dose and effect. Acupunc. Herbal Med. 1, 3–9. doi: 10.1097/HM9.0000000000000009
Shi, J., Sabbagh, M. N., and Vellas, B. (2020). Alzheimer’s disease beyond amyloid: strategies for future therapeutic interventions. BMJ 371:m3684. doi: 10.1136/bmj.m3684
Su, X., Wu, Z., Mai, F., Fan, Z., Du, S., Qian, H., et al. (2018). ‘Governor vessel-unblocking and mind-regulating’ acupuncture therapy ameliorates cognitive dysfunction in a rat model of middle cerebral artery occlusion. Int. J. Mol. Med. 43, 221–232. doi: 10.3892/ijmm.2018.3981
Sun, Z., Jia, J., Gong, X., Jia, Y., Deng, J., Wang, X., et al. (2012). Inhibition of glutamate and acetylcholine release in behavioral improvement induced by electroacupuncture in parkinsonian rats. Neurosci. Lett. 520, 32–37. doi: 10.1016/j.neulet.2012.05.021
Tahami Monfared, A. A., Byrnes, M. J., White, L. A., and Zhang, Q. (2022). The humanistic and economic burden of Alzheimer's disease. Neurol. Ther. 11, 525–551. doi: 10.1007/s40120-022-00335-x
Tang, S. H., Du, Y. J., Tao, Y. M., Tian, Q., and Kong, L. H. (2019). Effect of acupuncture on the ultrastructure of neurons and astrocytes in the hippocampal dentate gyrus in rats with Alzheimer's disease induced by Aβ(1-42). Zhongguo Zhen Jiu 39, 281–286. doi: 10.13703/j.0255-2930.2019.03.015
Verret, L., Mann, E. O., Hang, G. B., Barth, A. M. I., Cobos, I., Ho, K., et al. (2012). Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721. doi: 10.1016/j.cell.2012.02.046
Wang, Y., Wang, Y., Liu, J., and Wang, X. (2018). Electroacupuncture alleviates motor symptoms and up-regulates vesicular glutamatergic transporter 1 expression in the subthalamic nucleus in a unilateral 6-Hydroxydopamine-lesioned hemi-parkinsonian rat model. Neurosci. Bull. 34, 476–484. doi: 10.1007/s12264-018-0213-y
Wang, Y., Wang, Q., Ren, B., Guo, T., Qiang, J., Cao, H., et al. (2020). “Olfactory three-needle” enhances spatial learning and memory ability in SAMP8 mice. Behav. Neurol. 2020, 1–11. doi: 10.1155/2020/2893289
Wang, F., Zhong, H., Li, X., Peng, Y., Kinden, R., Liang, W., et al. (2014). Electroacupuncture attenuates reference memory impairment associated with astrocytic NDRG2 suppression in APP/PS1 transgenic mice. Mol. Neurobiol. 50, 305–313. doi: 10.1007/s12035-013-8609-1
Wolfe, C., Fitz, N., Nam, K., Lefterov, I., and Koldamova, R. (2019). The role of APOE and TREM2 in Alzheimer′s disease—current understanding and perspectives. Int. J. Mol. Sci. 20:81. doi: 10.3390/ijms20010081
Xiao, M., Wang, X. S., He, C., Huang, Z. S., Chen, H. R., and Kong, L. H. (2023). The gut-brain axis: effect of electroacupuncture pretreatment on learning, memory, and JNK signaling in D-galactose-induced AD-like rats. Iran. J. Basic Med. Sci. 26, 532–539. doi: 10.22038/IJBMS.2023.66954.14683
Xiao, L., Wang, X., Ye, Y., Yang, J., Cao, Y., Ma, S., et al. (2018). Applications of acupuncture therapy in modulating plasticity of central nervous system. Neuromodulation Technol. Neural Interface 21, 762–776. doi: 10.1111/ner.12724
Xie, L., Liu, Y., Zhang, N., Li, C., Sandhu, A. F., Williams, G., et al. (2021). Electroacupuncture improves M2 microglia polarization and glia anti-inflammation of Hippocampus in Alzheimer’s disease. Front. Neurosci. 15:689629. doi: 10.3389/fnins.2021.689629
Yan, C. (2021) Dynamic analysis of the cortical-basal ganglia-thalamus loop. Hohhot: Inner Mongolia University.
Yang, W., Hamilton, J. L., Kopil, C., Beck, J. C., Tanner, C. M., Albin, R. L., et al. (2020). Current and projected future economic burden of Parkinson's disease in the U.S. NPJ Parkinsons Dis. 6:15. doi: 10.1038/s41531-020-0117-1
Yang, E. J., Jiang, J. H., Lee, S. M., Hwang, H. S., Lee, M. S., and Choi, S. M. (2010). Electroacupuncture reduces neuroinflammatory responses in symptomatic amyotrophic lateral sclerosis model. J. Neuroimmunol. 223, 84–91. doi: 10.1016/j.jneuroim.2010.04.005
Yang, J., Shi, G., Zhang, S., Tu, J., Wang, L., Yan, C., et al. (2019). Effectiveness of acupuncture for vascular cognitive impairment no dementia: a randomized controlled trial. Clin. Rehabil. 33, 642–652. doi: 10.1177/0269215518819050
Ypinga, J., de Vries, N. M., Boonen, L., Koolman, X., Munneke, M., Zwinderman, A. H., et al. (2018). Effectiveness and costs of specialised physiotherapy given via ParkinsonNet: a retrospective analysis of medical claims data. Lancet Neurol. 17, 153–161. doi: 10.1016/S1474-4422(17)30406-4
Yu, C., Wang, M., Li, R., Wei, T., Yang, H., Yin, Y., et al. (2023). TREM2 and microglia contribute to the synaptic plasticity: from physiology to pathology. Mol. Neurobiol. 60, 512–523. doi: 10.1007/s12035-022-03100-1
Yue, Y., Zhong, M., An, X., Feng, Q., Lai, Y., Yu, M., et al. (2022). Serotonin (5-HT) 2A receptor involvement in melanin synthesis and transfer via activating the PKA/CREB signaling pathway. Int. J. Mol. Sci. 23:6111. doi: 10.3390/ijms23116111
Zhang, R., Andersen, A. H., Hardy, P. A., Forman, E., Evans, A., Ai, Y., et al. (2018). Objectively measuring effects of electro-acupuncture in parkinsonian rhesus monkeys. Brain Res. 1678, 12–19. doi: 10.1016/j.brainres.2017.10.006
Zhang, Z., Chen, L., Guo, Y., Li, D., Zhang, J., Liu, L., et al. (2023). The neuroprotective and neural circuit mechanisms of acupoint stimulation for cognitive impairment. Chin. Med. 18:8. doi: 10.1186/s13020-023-00707-x
Zhang, P., Rong, S., He, C., Li, Y., Li, X., Chen, Z., et al. (2023). Cortical connectivity of cholinergic basal forebrain in Parkinson’s disease with mild cognitive impairment. Quant. Imaging Med. Surg. 13, 2167–2182. doi: 10.21037/qims-22-582
Zhang, W., Xiong, B., Zhang, L., Huang, X., Yuan, X., Tian, Y., et al. (2021). The role of the GABAergic system in diseases of the central nervous system. Neuroscience 470, 88–99. doi: 10.1016/j.neuroscience.2021.06.037
Zhao, Y., Zhang, Z., Qin, S., Fan, W., Li, W., Liu, J., et al. (2021). Acupuncture for Parkinson's disease: efficacy evaluation and mechanisms in the dopaminergic neural circuit. Neural Plast. 2021:9926445. doi: 10.1155/2021/9926445
Zheng, J., Zhu, H., Zhao, Z., Du, M., Wang, Z., Lan, L., et al. (2023). Vesicular acetylcholine transporter in the basal forebrain improves cognitive impairment in chronic cerebral hypoperfusion rats by modulating theta oscillations in the hippocampus. Neurosci. Lett. 807:137281. doi: 10.1016/j.neulet.2023.137281
Glossary
Keywords: acupuncture, neural circuit, movement disorder, cognitive impairment, neurodegenerative disease
Citation: Li B, Deng S, Jiang H, Zhu W, Zhuo B, Du Y and Meng Z (2024) The mechanistic effects of acupuncture in rodent neurodegenerative disease models: a literature review. Front. Neurosci. 18:1323555. doi: 10.3389/fnins.2024.1323555
Edited by:
Nemanja Jovicic, University of Kragujevac, SerbiaReviewed by:
Christophe Melon, UMR7289 Institut de Neurosciences de la Timone (INT), FranceYan Li, Apellis Pharmaceuticals, United States
Copyright © 2024 Li, Deng, Jiang, Zhu, Zhuo, Du and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuzheng Du, ZHJkdXl1emhlbmdAMTI2LmNvbQ==; Zhihong Meng, cHJvZm1lbmd6aGlob25nQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Boxuan Li
Boxuan Li Shizhe Deng
Shizhe Deng Hailun Jiang
Hailun Jiang Weiming Zhu
Weiming Zhu Bifang Zhuo
Bifang Zhuo Yuzheng Du1,2*
Yuzheng Du1,2* Zhihong Meng
Zhihong Meng