
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Neurosci., 28 November 2023
Sec. Neurodevelopment
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1338853
This article is a correction to:
PlexinD1 signaling controls domain-specific dendritic development in newborn neurons in the postnatal olfactory bulb
 Masato Sawada1,2†
Masato Sawada1,2† Ayato Hamaguchi1
Ayato Hamaguchi1 Naomichi Mano1
Naomichi Mano1 Yutaka Yoshida3,4,5
Yutaka Yoshida3,4,5 Akiyoshi Uemura6
Akiyoshi Uemura6 Kazunobu Sawamoto1,2*†
Kazunobu Sawamoto1,2*†A corrigendum on
PlexinD1 signaling controls domain-specific dendritic development in newborn neurons in the postnatal olfactory bulb
by Sawada, M., Hamaguchi, A., Mano, N., Yoshida, Y., Uemura, A., and Sawamoto, K. (2023). Front. Neurosci. 17:1143130. doi: 10.3389/fnins.2023.1143130
In the published article, there was an error in Figures 2 and 3 as published. In Figures 2E and 3F, “Wilt-type” is a typographical error of “Wild-type”. The corrected Figures 2 and 3 appear below.
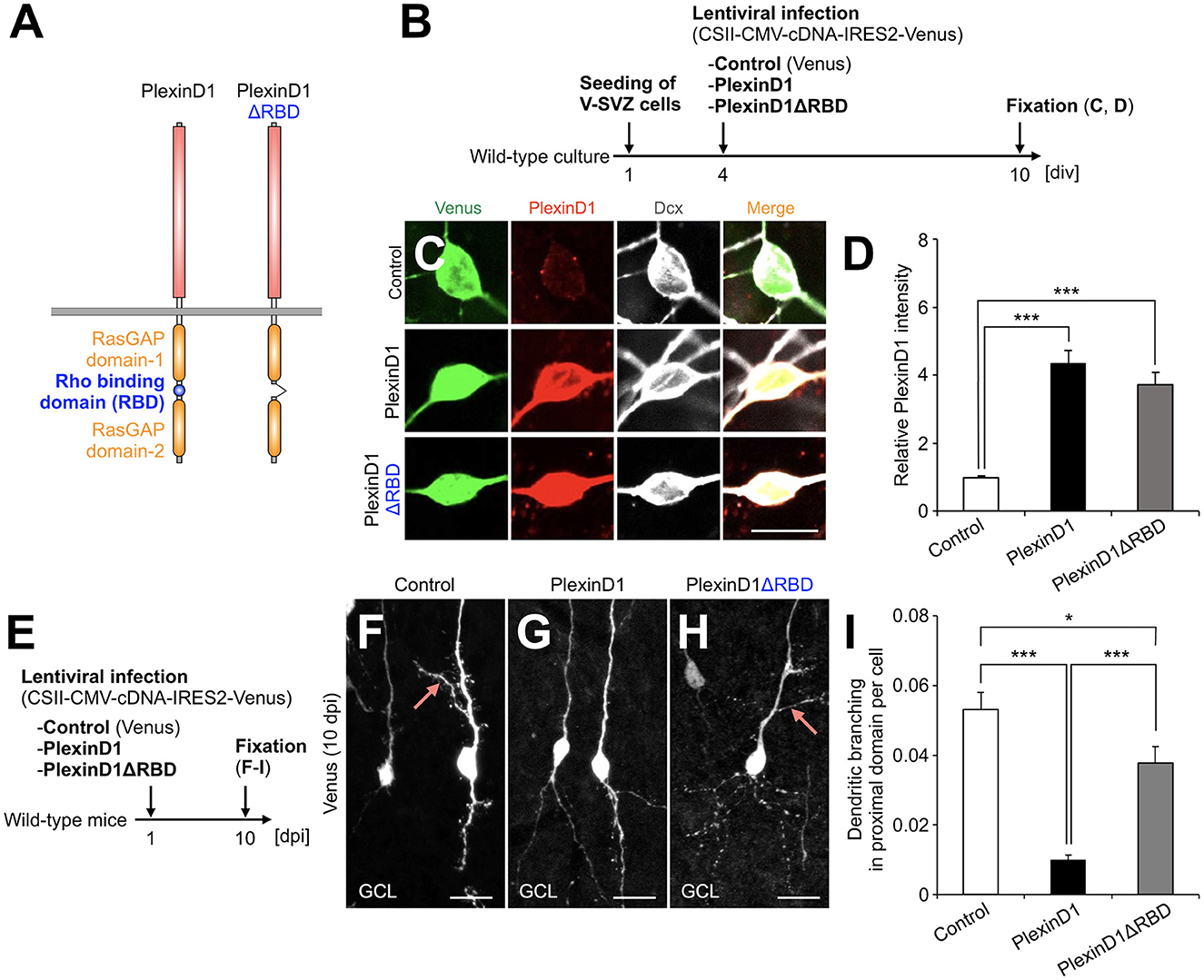
Figure 2. PlexinD1's RBD is involved in the PlexinD1-mediated suppression of lateral dendrite formation in granule cells in the postnatal OB. (A) Molecular structure of PlexinD1. (B) Experimental scheme of PlexinD1- and PlexinD1ΔRBD-overexpressing neuronal culture. (C) Representative images of Venus+ (green) Dcx+ (white) cultured control, PlexinD1-overexpressing, and PlexinD1ΔRBD-overexpressing neurons. Red indicates PlexinD1. (D) Relative PlexinD1 intensity in the infected neurons (control, n = 49 cells; PlexinD1, n = 38 cells; PlexinD1ΔRBD, n = 30 cells; three independent experiments). (E) Experimental scheme for PlexinD1 overexpression in vivo. (F–H) Representative projection images of Venus+ control (F), PlexinD1-overexpressing (G), and PlexinD1ΔRBD-overexpressing (H) granule cells at 10 dpi. (I) Proportions of lateral dendrite-bearing granule cells at 10 dpi (control, n = 2,217 cells from 5 mice; PlexinD1, n = 4,204 cells from 5 mice; PlexinD1ΔRBD, n = 1,641 cells from 5 mice). Pink arrows indicate dendritic branches in the proximal domain of the apical dendrite. GCL, granule cell layer; RBD, Rho binding domain. *p < 0.05, ***p < 0.005. Scale bars: (C), 10 μm; (F–H), 20 μm. Bars indicate mean ± SEM.

Figure 3. RhoJ is expressed in migrating and differentiating granule cells in the postnatal OB and involved in the suppression of their dendritic branching in the proximal domain of the apical dendrite. (A) Representative images of the coronal V-SVZ sections in RhoJ+/GFP mice stained for GFP (green), Dcx (red), and CD31 (cyan). Nuclei were stained with Hoechst 33342 (Blue). (B–D) Representative images of the coronal OB sections in RhoJ+/GFP mice stained for GFP (green), Dcx (red), and NeuN (cyan). Boxed area in (B) was enlarged in (C) and (D). White arrows, yellow arrowheads, and cyan arrowheads (C) and (D) indicate GFP + Dcx + NeuN-, GFP + Dcx + NeuN+, and GFP + Dcx-NeuN+ granule cells, respectively. (E) Proportions of RhoJ+/GFP-positive cells in the OB (n = 3 mice; 144 cells analyzed). (F) Experimental scheme for RhoJ overexpression experiment. (G) Representative dendritic tracings of control (n = 32 cells from 4 mice) and RhoJ-overexpressing (n = 36 cells from 8 mice) granule cells at 10 day-post injection (dpi). (H–J) Dendritic branch numbers of distal [(H); control, n = 32 cells from 4 mice; RhoJ, n = 36 cells from 8 mice], proximal [(I); control, n = 231 cells from 4 mice; RhoJ, n = 67 cells from 8 mice], and basal [(J); control, n = 32 cells from 4 mice; RhoJ, n = 36 cells from 8 mice] domains in control and RhoJ-overexpressing granule cells at 10 dpi. (K) Experimental scheme for RhoJ loss-of-function experiment. (L) Representative dendritic tracings of control (n = 43 cells from 3 mice), RhoJ-KO (n = 47 cells from 3 mice), and PlexinD1-overexpressing RhoJ-KO (n = 24 cells from 4 mice) granule cells at 14 dpi. (M–O) Dendritic branch numbers of distal [(M); control, n = 43 cells from 3 mice; RhoJ-KO, n = 47 cells from 3 mice; RhoJ-KO + PlexinD1, n = 24 cells from 4 mice], proximal [(N); control, n = 137 cells from 3 mice; RhoJ-KO, n = 140 cells from 3 mice; RhoJ-KO + PlexinD1, n = 128 cells from 4 mice], and basal [(O); control, n = 43 cells from 3 mice; RhoJ-KO, n = 47 cells from 3 mice; RhoJ-KO + PlexinD1, n = 24 cells from 4 mice] domains in control, RhoJ-KO, and PlexinD1-overexpressing RhoJ-KO granule cells at 14 dpi. (P) Mechanism of dendritic branching in the proximal domain of the apical dendrite in granule cells in the postnatal OB. Pink arrows indicate dendritic branches in the proximal domain of the apical dendrite. V-SVZ, ventricular-subventricular zone; LV, lateral ventricle; RMS, rostral migratory stream; GCL, granule cell layer. *p < 0.05, ***p < 0.005. Scale bars: (A), (B), (G), (L), 20 μm; (C), (D), 10 μm. Bars indicate mean ± SEM.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: postnatal neurogenesis, ventricular-subventricular zone, olfactory bulb, newborn neurons, dendrites, PlexinD1, RhoJ
Citation: Sawada M, Hamaguchi A, Mano N, Yoshida Y, Uemura A and Sawamoto K (2023) Corrigendum: PlexinD1 signaling controls domain-specific dendritic development in newborn neurons in the postnatal olfactory bulb. Front. Neurosci. 17:1338853. doi: 10.3389/fnins.2023.1338853
Received: 15 November 2023; Accepted: 16 November 2023;
Published: 28 November 2023.
Approved by:
Frontiers Editorial Office, Frontiers Media SA, SwitzerlandCopyright © 2023 Sawada, Hamaguchi, Mano, Yoshida, Uemura and Sawamoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazunobu Sawamoto, c2F3YW1vdG9AbWVkLm5hZ295YS1jdS5hYy5qcA==
†ORCID: Masato Sawada orcid.org/0000-0002-8694-8526
Kazunobu Sawamoto orcid.org/0000-0003-1984-5129
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.