- Manipal Institute of Regenerative Medicine (MIRM), Bengaluru, Manipal Academy of Higher Education (MAHE), Manipal, Karnataka, India
Lysosomes primarily recognized as center for cellular ‘garbage-disposing-unit’, which has recently emerged as a crucial regulator of cellular metabolism. This organelle is a well-known vital player in the pathology including neurodegenerative disorders. In pathological context, removal of intracellular damaged misfolded proteins, organelles and aggregates are ensured by ‘Autophagy’ pathway, which initially recognizes, engulfs and seals the toxic cargo at the cytosolic environment. Thereafter the cell completes the task of encapsulated cargo elimination upon delivery of them to the terminal compartment - lysosome, which contains acid hydrolases, that are capable of degrading the abnormal protein-lipid-repertoire. The merge between inseparable ‘Autophagy’ and ‘Lysosomal’ pathways evolved into ‘Autophagy-Lysosome Pathway (ALP)’, through which cell ultimately degrades and recycles bio-materials for metabolic needs. Dysregulation of any of the steps of the multi-step ALP can contribute to the development and progression of disorders including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD). Therefore, targeting differential steps of ALP or directly lysosomes using nano-bioengineering approaches holds great promise for therapeutic interventions. This review aims to explore the role of distal autophagy pathway and proximal lysosomal function, as cellular degradative and metabolic hubs, in healing neurological disorders and highlights the contributions of nano-bioengineering in this field. Despite multiple challenges, this review underscores the immense potential of integrating autophagy-lysosomal biology with nano-bioengineering to revolutionize the field and provide novel therapeutic avenues for tackling neurological-neurodegenerative-disorders.
Introduction
Historical perspective and summary
Cellular health relies on maintenance of proper cellular-metabolic function, which is heavily dependent on clearance of damaged cellular materials. Lysosomes are the key organelle for cellular protein degradation and clearance. Malfunction of lysosome is one of the major causes for multiple degenerative-disorders, including, neurodegeneration, which manifests the importance of this organelle in pathophysiological contexts (Wong and Cuervo, 2010). The cluster of neurological disorders pose significant challenge in healthcare, requiring special clinical attention in combatting these complex multi-faceted diseases. In this regard lysosomes can play pivotal role in designing innovative therapeutic-strategies against neurodegenerative disorders, as lysosome is recently conferred with multi-modal role in cellular-metabolism. It is important to highlight that, apart from its conventional role in ‘garbage-recycling’, the newly-emerging concept is further establishing lysosome as the major signaling-hub for cellular metabolite storage and homeostasis, which is vital for sensing and maintaining the intracellular-extracellular metabolite balance. However, relevance of all these aspects in the context of neurodegeneration and neurotrauma is a mysterious black-box. The primary feature of this review is to explore the avenues to therapeutically target the multi-dimensional role of autophagy-pathways and lysosomes in catabolism and in conventional and/or unconventional metabolism, using newly-developed ‘Nanotechniques and Bioengineering’ approaches, to better tackle the various neurodegenerative disorders.
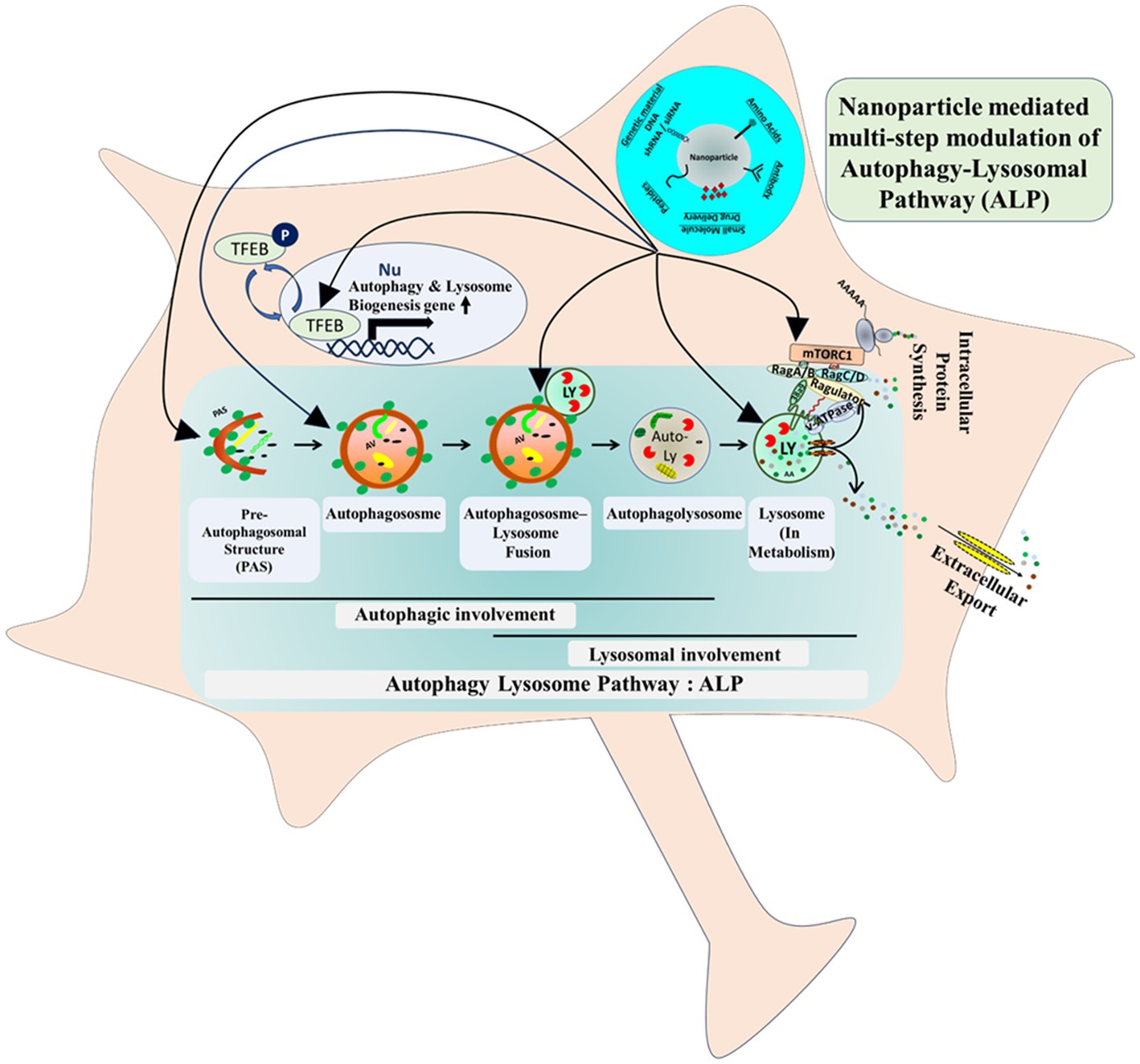
The hallmark of neurodegeneration is cellular and lysosomal aggregate formation. There are contradictory concepts prevailing in the neurobiology field, supporting the advantages and disadvantages of aggregate formation in neurodegeneration. No matter whatever the cause and effect of the aggregation process is, subsequent shielding and removal of the aggregated-proteins have been shown to be beneficial even at the clinical level (Karran and De Strooper, 2022). So far, the aggregate removal process is tightly inter-linked with the autophagy pathway, more precisely bulk-degradative macroautophagy pathway, where aggregates and damaged proteins are being sequestered by pre-autophagosomal structures (PAS/phagophore), which upon sealing (autophagosome formation) delivers the luminal cargo to lysosomes for degradation in a fusion dependent manner (He and Klionsky, 2009; Yamamoto et al., 2023) (Figure 1). In brief, the ALP is a complex multi-step pathway. At the distal-end or upstream of it, initial PAS formation occurs followed by engulfment of cytosolic protein-aggregate/abnormal-protein/organelle (cargo). This is then followed by sealing of the double-membraned autophagosomal structures that completes the autophagosome/autophagic-vacuole formation. At the proximal-end or downstream of it, this cargo-carrying autophagosomes then delivers their cargo to lysosomes upon fusion, that ultimately generates autophagolysosomes/autolysosome. Finally, within the autolysosome the entrapped materials get degraded by variety of lysosome-residing acid-hydrolases. With the advent of the field of nano-biotechnology, alteration of this multi-step, highly complex, fine-tuned clearance pathway using bioengineering-nanotechnology could potentially be an effective strategy to better tackle progressive neurodegenerative disorder (Figure 1).
On the other hand, ours and other groups’ research have shown lysosomal-metabolite-storage in response to altered nutrient composition in cellular-niche (Abu-Remaileh et al., 2017; Bandyopadhyay et al., 2022). This lysosomal metabolite-storage-reservoir has been shown to be utilized for cellular protein synthesis that decides the cell fate - growth, proliferation and survival. In contrast to the proliferating cells, lysosomal metabolite storage capacity is compromised in senescent cells. These suggest possibility of varying cellular metabolite homeostasis, requirements and utilization-pattern for cellular growth, proliferation and survival in different cell sub-types within a cell-populations. This could be far more relevant in neurodegenerative disorders, where mixed neuronal cell-population with varying abnormalities exists in a particular brain-niche. Further the significance of the niche-nutrient composition in nurturing the metabolically diverse cell-population is highlighted from the recent clinical trials in neurodegeneration. Recently, in case of the most devastating neurodegenerative disorder, AD (tauopathy), alteration of amino acid (AA) levels in patient diet has turned out to be a clinically effective strategy (Sato et al., 2021). However, variation of metabolic state at the individual cell level within a mixed neuronal cell-population, could render the wholistic niche-metabolite alteration less effective and could even be detrimental. Therefore, between the affected versus unaffected neuronal population in neurodegeneration, local concentration of AAs/metabolites are needed to be altered, leaving the surrounding unperturbed. In that case, ultimately adjustment of local, targeted nutrient-level will be therapeutically far more effective. Moreover, the global change in nutrients in the microenvironment can eventually alter mTOR-dependent as well as mTOR-independent AA-homeostasis-pathways in cell (Abu-Remaileh et al., 2017; Bandyopadhyay et al., 2022). This will ultimately lead to unwanted alteration of lysosome-autophagy-pathways at the molecular level, which could have disastrous effect on healthy versus affected neuronal populations. These poorly effective universal flooding-strategy should be essentially replaced with the restricted targeted delivery approach to particular cell-populations, using bioengineered-nanoparticles carrying selective set of biomolecules, e.g., bioengineered AAs/metabolites/drugs/genetic-modifiers (siRNA/shRNA gene silencing tools).
Specifically, targeting the newly-emerging signaling hub – lysosome and related ALP to curb neurodegeneration with the latest bioengineering-nanotechnologies, could potentially be an efficient strategy from therapeutic standpoint and is being discussed in this review.
Current state of the art
Tackling neurodegeneration with bioengineering-nanotechnologies
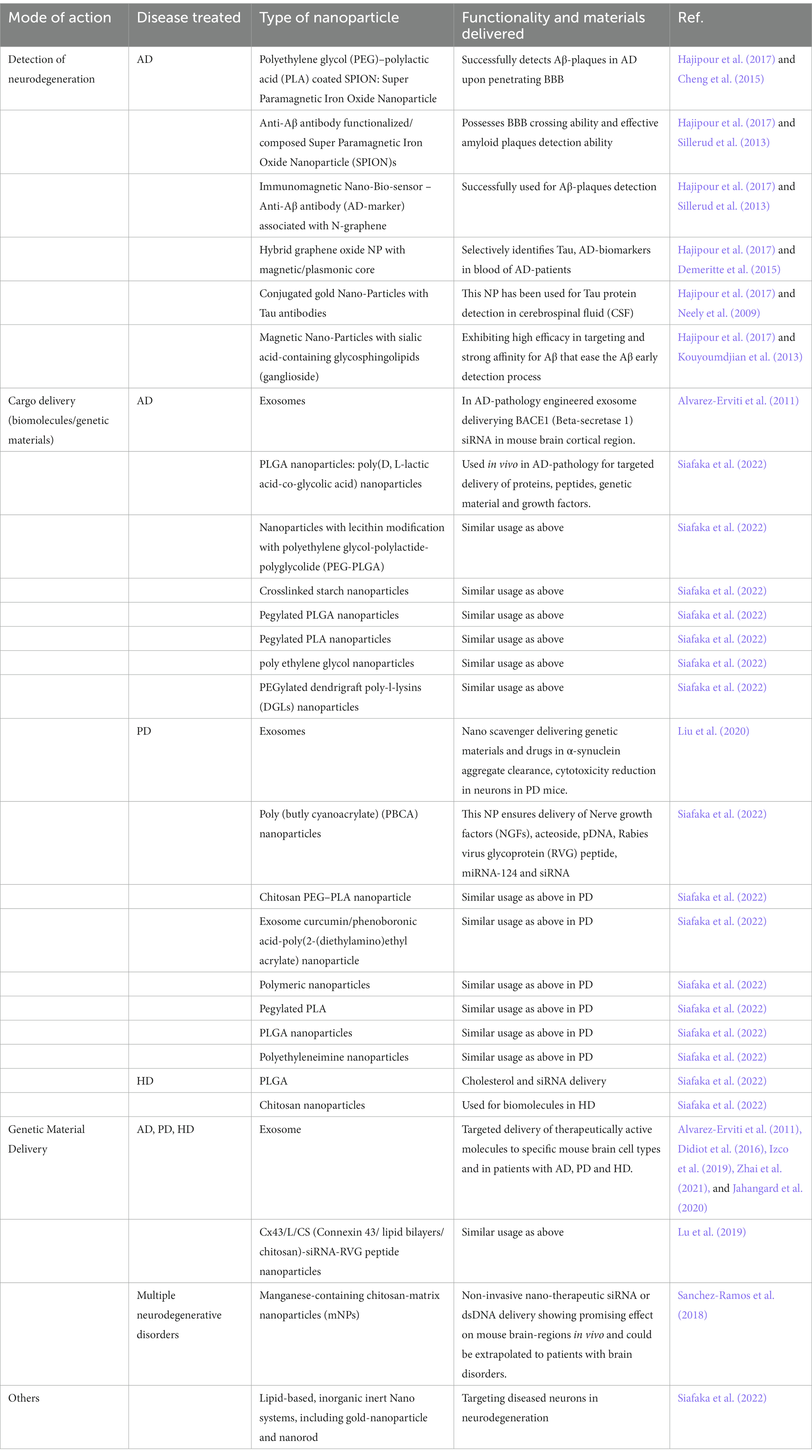
The trademark of the neurodegenerative disorders is the formation of small/large aggregates at the cellular level, in the cytosol as well as within the lysosomes of the diseased-neurons, that are residing within the heterogeneous cluster of healthy and abnormal neurons. Therefore, therapeutically early-detection of abnormality in a targeted manner within particular brain-region or in particular set of neurons, will facilitate the treatment process manifold. Recently, the advent of theranostics dual-strategic approach, unraveled new hope for simultaneous targeting of affected cell detection as well as the localized treatment options for the diseased neuronal-population. This strategy has already been successfully applied for detecting amyloid-beta (Aβ) plaques at the initial stage in AD-patient brain-neurons, just by delivering magnetic resonance imaging (MRI)-competent anti-Aβ antibody/peptide/probe-functionalized nanoparticles (NPs), coupled with simultaneous treatment-effective drug-targeting to the diseased-neurons, using bioengineered-nanoparticles having blood brain barrier (BBB)-crossing ability (Moorthy and Govindaraju, 2021). From this kind of study, it can be easily appreciated that for successful, targeted localized-drug-delivery, accuracy on the defect detection front is vital. Moreover, as early detection can lead to better cure probability, many researchers have been focusing at the detection end of the broad spectrum of neurological disorders treatment. Conceptually, all these precise aggregate detection processes, including antibody-conjugated-nanoparticles, peptide/probe-functionalized nanoparticles, nanobodies, nanocrystals, liposomes, polymer micelle, gold nanoparticles, dendrimer, iron-oxide nanoparticle, carbon nanotubes, will not only detect the aggregates but also simultaneously isolate the undigested-toxic materials in cytosol. This can prepare them for their bulk-engulfment and further degradation by ALP in cell. In brief, mechanistically the antibody-coated aggregates can be recognized as intracellular substrate and ultimately being encapsulated in cytosol by autophagosome, which will subsequently be delivered to lysosome for further degradation and removal.
In that scenario, in order to ensure a successful cellular aggregate removal, one can imagine alteration of multiple steps of autophagy-lysosomal-machinery - either by targeting autophagy machinery upstream or lysosomal-activity downstream. Therefore, the latest bioengineering-nanotechnological approaches, will turn out to be an effective strategy from clinical angle (Figure 1).
Lysosome-autophagy pathway modulation: targeting autophagy pathway
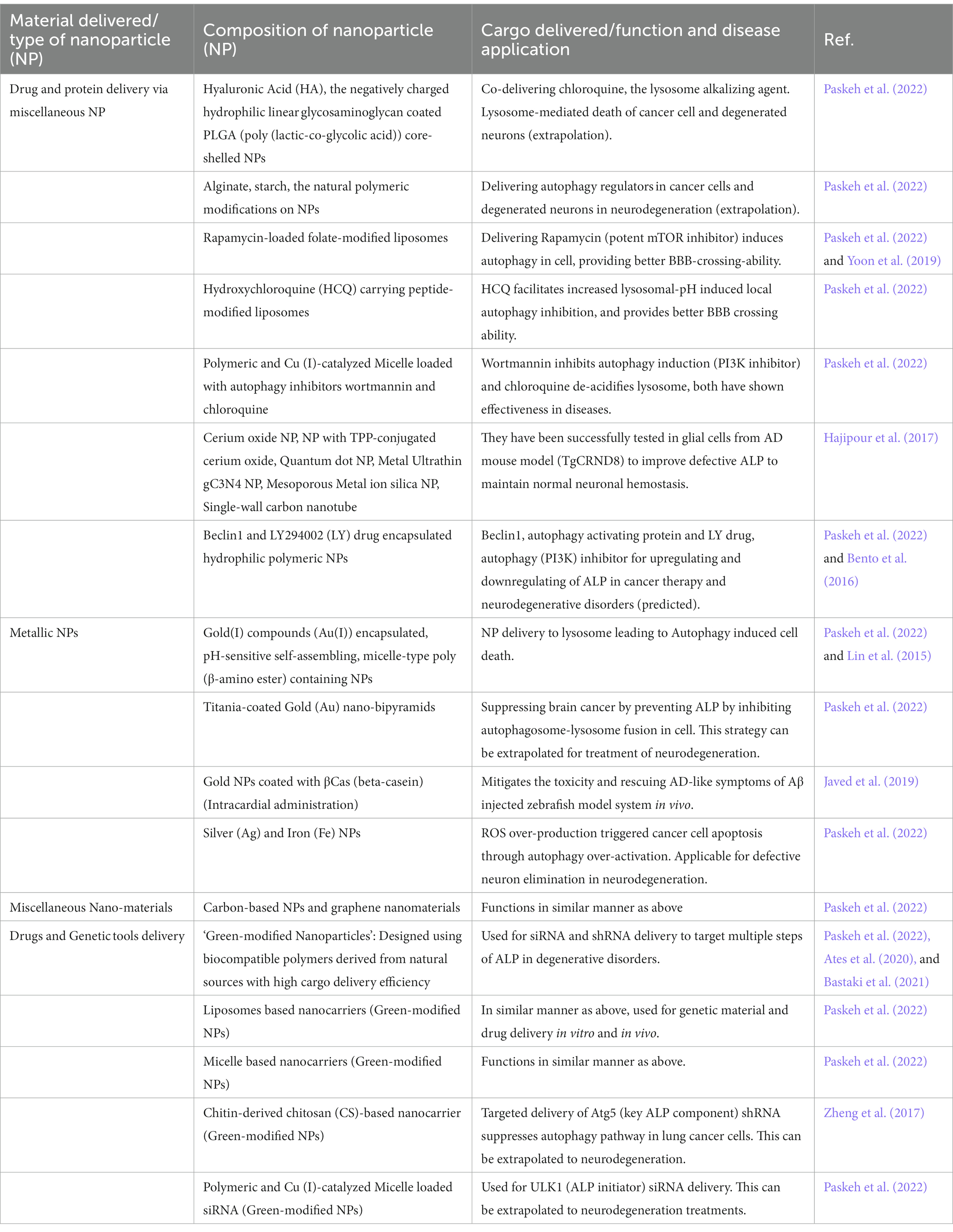
As discussed earlier, the distal-end of the lysosome-autophagy pathway – the initiation of autophagosome, PAS/phagophore formation, cargo-entrapment and sealing-processes, can be modulated and already proven to be beneficial in case of multiple neurodegenerative disorders. With the advent of the field of nano-biotechnology, alteration of this multi-step, highly complex, fine-tuned clearance pathway using nanotechnology could potentially be an effective strategy to better tackle progressive neurodegenerative disorders (Figure 1). In Table 1 multiple precedence of targeting autophagy pathway at different steps, via newly-emerging bioengineered-nanotechnological tools, in neurological-disorders and in brain-cancer (e.g., neuroglioma and glioblastoma), has been illustrated.
Additionally, neuroprotective nano-biological treatment avenues with rapamycin-loaded liposome induced inhibition of master AA homeostatic regulator - mTOR signaling pathway could potentially be effective (Table 1). The reciprocal relationship between mTOR and autophagy in this scenario could indirectly lead to ALP activation in neuronal system, which in turn can facilitate aggregate removal from diseased neuronal-population. This vital nanotechnology-based modulatory way of mTOR-autophagy-lysosome pathway, could potentially turn out to be effective potential therapeutic intervention and may open up new avenues for neurodegenerative disorder treatment by exploring the cellular metabolism and degradative juncture in future (Maiese, 2016).
Lysosome-autophagy pathway modulation: targeting directly the organelle - lysosome
So far, the autophagy pathway modulation with nanotechnological advances has been highlighted. However, it is needless to emphasize that the downstream part or the end-point of ALP, majorly the lysosomal compartment, is of prime importance. Therefore, change in lysosome directly through nanotechnological devices, could be advantageous for arresting degenerative disorders. Recent research in AD field, uncovered effectiveness of immunotherapy-driven aggregate removal conceivably by lysosome (Karran and De Strooper, 2022). This emphasizes the importance of boosting the compromised lysosomal degradative-efficiency with the help of bioengineered-nanotechnological tools. Ideally, this can be achieved by generating bioengineered super-functional lysosomes by nanoparticle-mediated direct delivery of activated lysosome-residing enzymes, including lysosomal acid-hydrolases (proteases-cathepsins/phosphatases/nucleases/glycosidases/peptidases/sulphatases/lipases), lysosomal-enzyme activators, metabolites, AAs, peptides, proteins, directly to the lysosomal compartments, which might have higher ability to dissolve cellular-lysosomal aggregates. As a treatment measure of Covid-19, inhibition of lysosomal luminal proteases by endocytic entry and subsequent lysosomal delivery of negatively surface-charged mefloquine loaded poly-(glycerol monostearate-co-ε-caprolactone) nanoparticles (MFQ-NPs) to lungs-cells, have been shown to mitigate SARS-CoV2 infection (Petcherski et al., 2023). Identical nanoparticle-mediated intervention can be applied to alter lysosomal-activity in the context of neurodegeneration.
Although, impaired lysosomal activity/degradation due to lysosomal acidification-loss in multiple familial neurodegenerative disorders is detrimental (Colacurcio and Nixon, 2016), this can be hypothetically utilized for therapeutic advantage upon combining with the alkalinizing MFQ-NP-delivery strategy. Here, the lysosomal de-acidification can be further aggravated in neurodegeneration. This in one hand can be harmful for neurons, whereas counterintuitively can trigger pathogenic aggregate excretion and clearance from the defective neurons by lysosomal exocytosis, due to their complete loss of protein-degradation-ability of modified lysosomes in extreme pH-escalation. In fact, lysosomal exocytosis-mediated aggregate removal strategy has already been proven to be effective in synucleinopathy model system (Xie et al., 2022). Essentially the deleterious effect of lysosomal pH-hike is counterbalanced by its over-induction, which in turn lead to better neuronal health.
Additionally, it is worth highlighting couple more ALP-components, that have been showing significant promise in the field of nanoparticle-mediated therapeutics.
i. Upstream of ALP, TFEB (transcription factor EB), the master transcriptional controller of lysosomal-biogenesis and autophagy-induction, plays a vital role in multiple neurodegenerative disorders. At the molecular level, phosphorylation-dephosphorylation-cycle dependent cytosol-nuclear shuttling of TFEB regulates its activity, therefore ALP-activity, mTORC1-kinase-activity and hence overall cellular metabolism (Figure 1). Given the pivotal roles of TFEB, researchers have considered TFEB-level manipulations, using Ceria-nanoparticles. Cerium oxide nanoparticles, which has been shown to be activating TFEB and ultimately promotes the clearance of toxic aggregates in diseased cells (Song et al., 2014). Moreover, the anti-oxidant property of this nanoparticle can potentially mitigate the ROS-related-toxicity in defective-neurons, which is the common feature of affected neurons in neurodegenerative disorders.
ii. Currently histone deacetylase (HDAC), as a TFEB-deacetylating component became a lucrative target for ongoing clinical trial for HD, ALS and SMA (spinal-muscular-atrophy), where nanoparticle-mediated delivery of HDAC-inhibitors/siRNAs was successfully able to alter ALP in-vivo (Li et al., 2022).
Overall, this kind of multi-modal approaches would provide great promise in treating brain-disorders, where proper restoration of dysregulated autophagy, lysosomal activity and biogenesis will potentially be fruitful.
Targeting lysosomes in a ‘Macrophagic Way’ using nano-bioengineering
Recently nano-bioengineered macrophage in therapeutically tackling bacterial-infections in a transfer-based approach shows great clinical impact, which can be extrapolated to the neurodegeneration field. Directed delivery of drugs/proteinaceous/genetic-materials to the endolysosomal-systems or to the affected cell population niche can be programmed by exploiting the over-active engulfment-endocytotic-exocytotic property of macrophages, which are triggered by immuno-inflammation during infection, injury and neurodegeneration (Huang et al., 2022; Wang et al., 2023). In support, recently intravenous administration of macrophages carrying particular gene-modifying nanoparticles within their endolysosomal compartments has been shown to be efficiently targeted to the PD-affected inflamed mouse brain-regions to ensure local delivery (Haney et al., 2020). Further the elevated engulfment-export strategy of macrophages can be utilized therapeutically for futuristic AA/metabolite-filled UV irradiated apoptotic corpse delivery to the affected neuronal-niche in neurodegeneration (Weavers et al., 2016). Perhaps this could preferentially alter niche-AA and cellular-metabolism in neurodegenerative disorders. Alternatively, macrophages carrying nanoparticles filled with AA/metabolite-cocktail can be targeted directly to the site of disorder, which can ensure direct transfer of nanoparticle-conjugated-AA/metabolite from carrier macrophage (donor) to the recipient neighboring diseased neurons (acceptor). Targeted delivery of peptides/hormones/drugs/genetic-materials (siRNA/shRNA) can be executed in similar manner to regulate metabolism in neurodegeneration.
Regulation of cellular-lysosomal metabolism by targeted AA/metabolite delivery using nanotechnology-bioengineering in disease
As the undeniable importance of lysosomal function and metabolism in neurodegeneration is beginning to emerge, further discussion is needed to find out the role of nano-bioengineering in cellular-lysosomal metabolism in neurodegeneration. It can be speculated that damaged protein aggregation can alter neuronal environment and ultimately manifest metabolic abnormality in neurodegeneration. In support, niche-guided compromised lysosomal metabolite-storage and related aberrant protein synthesis in senescent cells, mimicking post-mitotic neurons, have been noticed (unpublished data). Moreover, the impact of extracellular-AAs/metabolites in controlling intra-cellular metabolism is supported by multiple clinical trials in neurodegeneration. Recently, researchers have, (i) identified symptom-relieving effect of dietary administration of selective-essential-AAs in aging and tauopathy mouse-models (Sato et al., 2021); (ii) uncovered AD-symptom manifestation in patients is tightly coupled to lower blood levels of essential-AAs/Brunched-Chain-AAs (BCAAs) in a cohort study; (iii) identified effective reversal of ASD (Autism-spectrum-Disorder) behavior-symptoms upon dietary interventions with AA-cocktails in patients and mice. At the molecular level this could be an effect of mTOR-signaling suppression by autophagic upregulation (Wu et al., 2017; van Sadelhoff et al., 2019). Based on these studies, the promising strategy of external-AA/metabolite level modulation could be applied for treatment of neurodegenerative disorders. However, the wholistic AA/metabolite concentration change as well as global delivery of proteins and genetic materials (including, plasmids/DNA/shRNA/siRNA/miRNA; Siafaka et al., 2022; Duan et al., 2023), within a mixed neuronal-population could have limited efficacy, as region-specificity has been evidenced in patient-brains in neurodegeneration. For example, HD affects the basal ganglia region, whereas hippocampal, entorhinal and cerebral cortical zone is affected in AD, substantia nigra is affected in PD and set of brain-spinal cord motor neurons are the vulnerable population in ALS. This emphasizes the importance of targeted-delivery-mode in order to better control this cluster of disease.
As discussed earlier, uniform spreading-strategy of AAs/metabolites could even be detrimental where local alteration is preferred. At the molecular level, the global, non-targeted, uncontrolled change in the nutrients in the microenvironment can eventually alter mTOR-dependent as well as mTOR-independent AA-homeostasis that can lead to aberrant ALP alteration causing neuronal health deterioration within a population. Therefore, it is essential to replace the existing global AA/metabolite-treatment-strategy with futuristic restricted, targeted delivery-approach to the particular cell populations in a region-specific manner. In this scenario, contribution from the Bioengineering-Nanotherapeutic-technological advances will be extremely beneficial for targeted delivery of selective set of AAs/proteinoids/lysosomal-activity-modifying-agents, through nanoparticle-based carriers. The bi-modal effectiveness of nanotechnology-based carriers is undeniable, as not only they are ensuring targeted delivery to specific neuronal populations in particular affected brain regions, but also capable of protecting the molecular integrity of the modifiable and degradation-prone proteinaceous and genetic-materials. To this end, examples of nanoparticles are highlighted in Table 2, which can either carry protein/peptide/genetic-materials/drugs in an encapsulated or surface conjugated manner (Kang et al., 2018; Siafaka et al., 2022; Duan et al., 2023), to facilitate BBB-crossing to initiate localized treatment for neurodegeneration.
Altogether, all the above-mentioned nano-bioengineering strategies could be applied to alter autophagy-lysosome regulated cellular metabolism in disease context. Either genetic alteration of candidates of canonical mTORC1-pathway (Abu-Remaileh et al., 2017) and/or noncanonical lysosomal-AA-storage-homeostasis-pathway (Bandyopadhyay et al., 2022); or direct local AA/metabolite delivery (lysosomal/cytosolic/extracellular-niche), or combination of both, could potentially be helpful to change the course and progression of neurodegenerative-disorders.
Discussion
Highlight of future directions
The emerging field of bioengineering-nanotechnology offers enormous potential in treating complex multi-dimensional age-dependent neurodegenerative disorders. The role of cellular-metabolic and quality-control regulatory, lysosome and autophagy-lysosomal-pathway has been unequivocally established in life-threatening neurological disorders. This imposes substantial importance on therapeutically targeting this wing (Bonam et al., 2019). In order to have a successful approach, it is extremely important to know all the intricate details of nanotechnology-biology juncture to produce functional nanoparticles that can be safely delivered in a targeted manner avoiding toxicity. The toxicity-factor is a major obstacle in nanoparticle delivery process, which can be bypassed by thoughtful choice of the nano-materials for manufacturing nanoparticles.
Apart from the non-toxicity feature, consideration of safe delivery mode will be crucial for successful nanoparticle-mediated treatment strategy. Moreover, in the context of autophagy-lysosome-biology, the stability versus biodegradability balance could be a major paradigm that needed to be considered. This can potentially determine the state of lysosomal function and ultimately fate of cellular health. As for example, long-term stability of nanoparticles within the lysosomal lumen can eventually hamper lysosomal function, possibly by altering lysosomal low-pH environment. In fact, similar lysosomal-pH-spoiling effect has been found upon long-term lysosomal gold-nanoparticle accumulation in AD-pathology (Javed et al., 2019). Therefore, careful selection of nanoparticles is crucial to balance their therapeutic benefits versus potential adverse effects. This kind of nanoparticle-mediated lysosomal-poisoning can affect substrate-clearance and consequently perturb lysosomal metabolite storage-homeostasis and ultimately the cellular-metabolic balance. This can be detrimental in case of neurodegeneration, where diseased-neurons are already under severe metabolic stress. In that scenario, designing potentially biodegradable, short-life-spanned, non-toxic nanoparticles would be far more effective in the context of treatment of neurodegenerative disorders.
Another important aspect that requires further discussion is that whether the role of Autophagy manipulation be detrimental or beneficial in the context of neurodegeneration? Being the double-edged sword, any generalized mode of ALP alteration could be tricky and the modulation of the catabolic-pathway would rather have to be case specific. In general, the notion in the field of neurodegeneration is that the upregulation of ALP could be potentially advantageous. However, uncontrolled hyper-activation of this degradative-machinery could have undesired over-catabolic side-effects, which can potentially result in the collapse of the healthy cellular-metabolic environment. Already, in ALS, effectiveness of the hyperactivation of autophagy-lysosomal-wing in improving motor neuronal health is vastly questionable (Bandyopadhyay et al., 2014). Varying choice of the directionality of ALP-regulation in case of particular neurodegenerative disorder would be critical. Although the upregulation of lysosomal-activity upon nanoparticle-mediated lysosomal cathepsin delivery is undeniable, recent studies have identified CathepsinB as a negative regulator of lysosomal calcium-channel, TRPML1. This channel is the upstream regulator/activator of TFEB-activity, that controls autophagy-lysosomal-biogenesis genes, needed for ALP function (Qi et al., 2016). These kinds of dual players have to be considered with care while designing the nano-bioengineered therapeutics.
Thus, the educated selection of targeted intervention avenues, the treatment-delivery mode through bioengineering-nanotechnological advances would be crucial and should be the future focus for successful treatment purposes to better tackle the neurodegenerative and neurological diseases.
Key concepts
Tackling neurodegeneration with bioengineering-nanotechnologies: novel prospective study
The hallmark of neurodegeneration is aggregate formation at the cellular-cytosolic-lysosomal level in specific neuronal-population within a pool of healthy-abnormal neuronal-cluster. Applying Nano-Bioengineering driven theranostics - targeted aggregate detection, followed by ‘Autophagy-Lysosomal Pathway’-mediated engulfment-removal, could be an effective treatment-strategy for neurodegeneration.
Lysosome-autophagy pathway (ALP) modulation: targeting autophagy pathway
The ‘ALP’ consists of upstream cargo-capture and downstream lysosomal-degradation of the captured substrates. Combinatorial genetic and drug targeted modulation of this multi-step clearance pathway consist of autophagosome formation and cargo delivery processes, by using nano-bioengineering devices, could potentially be an effective strategy to better tackle progressive neurodegenerative disorders.
Lysosome-autophagy pathway (ALP) modulation: targeting directly the organelle - lysosome
For the treatment of neurological-disorder, the contribution of the lysosome, the terminal point of ALP, is vital. Lysosomal alteration through nanotechnology, especially by generating bioengineered super-functional lysosomes with higher aggregate removal ability, by nanoparticle-mediated delivery of activated lysosome-residing enzymes, could be beneficial to arrest neurodegenerative disorders.
Targeting lysosomes in a ‘Macrophagic Way’ using nano-bioengineering
Smooth targeted delivery of drugs, AA/metabolites, peptides, hormones, and genetic materials (siRNA/shRNA) to the cellular/endo-lysosomal systems of the affected neuronal-population in case of neurodegeneration, can be ensured by bio-engineered macrophages with hyperactivated engulfment-endocytotic-exocytotic property, caused by immuno-inflammatory response triggered in this degenerative disorder.
Regulation of cellular-lysosomal metabolism regulation by targeted AA/metabolite delivery using nanotechnology-bioengineering in disease
Alteration of niche AAs in treating neurodegeneration are gaining popularity. However, therapeutically, targeted local AA perturbation is far more effective than global change. Therefore, targeted delivery of AA/lysosome-modifiers to alter canonical/noncanonical metabolic pathways, through bioengineered nanoparticle carriers, in affected neurons in neurodegeneration will be highly beneficial.
Author contributions
AR: Writing – original draft. UB: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by DBT Ramalingaswami Re-entry Fellowship (BT/RLF/Re-entry/57/2021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu-Remaileh, M., Wyant, G. A., Kim, C., Laqtom, N. N., Abbasi, M., Chan, S. H., et al. (2017). Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science 358, 807–813. doi: 10.1126/science.aan6298
Alvarez-Erviti, L., Seow, Y., Yin, H. F., Betts, C., Lakhal, S., and Wood, M. J. A. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345. doi: 10.1038/nbt.1807
Ates, B., Koytepe, S., Ulu, A., Gurses, C., and Thakur, V. K. (2020). Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 120, 9304–9362. doi: 10.1021/acs.chemrev.9b00553
Bandyopadhyay, U., Nagy, M., Fenton, W. A., and Horwich, A. L. (2014). Absence of lipofuscin in motor neurons of SOD1-linked ALS mice. Proc. Natl. Acad. Sci. U. S. A. 111, 11055–11060. doi: 10.1073/pnas.1409314111
Bandyopadhyay, U., Todorova, P., Pavlova, N. N., Tada, Y., Thompson, C. B., Finley, L. W. S., et al. (2022). Leucine retention in lysosomes is regulated by starvation. Proc. Natl. Acad. Sci. U. S. A. 119:e2114912119. doi: 10.1073/pnas.2114912119
Bastaki, S., Aravindhan, S., Ahmadpour Saheb, N., Afsari Kashani, M., Evgenievich Dorofeev, A., Karoon Kiani, F., et al. (2021). Codelivery of STAT3 and PD-L1 siRNA by hyaluronate-TAT trimethyl/thiolated chitosan nanoparticles suppresses cancer progression in tumor-bearing mice. Life Sci. 266:118847. doi: 10.1016/j.lfs.2020.118847
Bento, C. F., Renna, M., Ghislat, G., Puri, C., Ashkenazi, A., Vicinanza, M., et al. (2016). Mammalian autophagy: how does it work? Annu. Rev. Biochem. 85, 685–713. doi: 10.1146/annurev-biochem-060815-014556
Bonam, S. R., Wang, F., and Muller, S. (2019). Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 18, 923–948. doi: 10.1038/s41573-019-0036-1
Cheng, K. K., Chan, P. S., Fan, S., Kwan, S. M., Yeung, K. L., Wáng, Y. X. J., et al. (2015). Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer's disease mice using magnetic resonance imaging (MRI). Biomaterials 44, 155–172. doi: 10.1016/j.biomaterials.2014.12.005
Colacurcio, D. J., and Nixon, R. A. (2016). Disorders of lysosomal acidification-the emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res. Rev. 32, 75–88. doi: 10.1016/j.arr.2016.05.004
Demeritte, T., Viraka Nellore, B. P., Kanchanapally, R., Sinha, S. S., Pramanik, A., Chavva, S. R., et al. (2015). Hybrid graphene oxide based Plasmonic-magnetic multifunctional Nanoplatform for selective separation and label-free identification of Alzheimer's disease biomarkers. ACS Appl. Mater. Interfaces 7, 13693–13700. doi: 10.1021/acsami.5b03619
Didiot, M. C., Hall, L. M., Coles, A. H., Haraszti, R. A., Godinho, B. M. D. C., Chase, K., et al. (2016). Exosome-mediated delivery of Hydrophobically modified siRNA for huntingtin mRNA silencing. Mol. Ther. 24, 1836–1847. doi: 10.1038/mt.2016.126
Duan, L., Li, X., Ji, R., Hao, Z., Kong, M., Wen, X., et al. (2023). Nanoparticle-based drug delivery systems: an inspiring therapeutic strategy for neurodegenerative diseases. Polymers (Basel) 15:2196. doi: 10.3390/polym15092196
Hajipour, M. J., Santoso, M. R., Rezaee, F., Aghaverdi, H., Mahmoudi, M., and Perry, G. (2017). Advances in Alzheimer's diagnosis and therapy: the implications of nanotechnology. Trends Biotechnol. 35, 937–953. doi: 10.1016/j.tibtech.2017.06.002
Haney, M. J., Zhao, Y., Fay, J., Duhyeong, H., Wang, M., Wang, H., et al. (2020). Genetically modified macrophages accomplish targeted gene delivery to the inflamed brain in transgenic Parkin Q311X(a) mice: importance of administration routes. Sci. Rep. 10:11818. doi: 10.1038/s41598-020-68874-7
He, C., and Klionsky, D. J. (2009). Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93. doi: 10.1146/annurev-genet-102808-114910
Huang, X., Liu, C., Kong, N., Xiao, Y., Yurdagul, A. Jr., Tabas, I., et al. (2022). Synthesis of siRNA nanoparticles to silence plaque-destabilizing gene in atherosclerotic lesional macrophages. Nat. Protoc. 17, 748–780. doi: 10.1038/s41596-021-00665-4
Izco, M., Blesa, J., Schleef, M., Schmeer, M., Porcari, R., al-Shawi, R., et al. (2019). Systemic Exosomal delivery of shRNA Minicircles prevents parkinsonian pathology. Mol. Ther. 27, 2111–2122. doi: 10.1016/j.ymthe.2019.08.010
Jahangard, Y., Monfared, H., Moradi, A., Zare, M., Mirnajafi-Zadeh, J., and Mowla, S. J. (2020). Therapeutic effects of transplanted exosomes containing miR-29b to a rat model of Alzheimer's disease. Front. Neurosci. 14:564. doi: 10.3389/fnins.2020.00564
Javed, I., Peng, G., Xing, Y., Yu, T., Zhao, M., Kakinen, A., et al. (2019). Inhibition of amyloid beta toxicity in zebrafish with a chaperone-gold nanoparticle dual strategy. Nat. Commun. 10:3780. doi: 10.1038/s41467-019-11762-0
Kang, Y. J., Cutler, E. G., and Cho, H. (2018). Therapeutic nanoplatforms and delivery strategies for neurological disorders. Nano Converg 5:35. doi: 10.1186/s40580-018-0168-8
Karran, E., and De Strooper, B. (2022). The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat. Rev. Drug Discov. 21, 306–318. doi: 10.1038/s41573-022-00391-w
Kouyoumdjian, H., Zhu, D. C., el-Dakdouki, M. H., Lorenz, K., Chen, J., Li, W., et al. (2013). Glyconanoparticle aided detection of beta-amyloid by magnetic resonance imaging and attenuation of beta-amyloid induced cytotoxicity. ACS Chem. Neurosci. 4, 575–584. doi: 10.1021/cn3002015
Li, T., Yin, L., Kang, X., Xue, W., Wang, N., Zhang, J., et al. (2022). TFEB acetylation promotes lysosome biogenesis and ameliorates Alzheimer's disease-relevant phenotypes in mice. J. Biol. Chem. 298:102649. doi: 10.1016/j.jbc.2022.102649
Lin, Y. X., Gao, Y. J., Wang, Y., Qiao, Z. Y., Fan, G., Qiao, S. L., et al. (2015). pH-sensitive polymeric nanoparticles with gold(I) compound payloads synergistically induce Cancer cell death through modulation of autophagy. Mol. Pharm. 12, 2869–2878. doi: 10.1021/acs.molpharmaceut.5b00060
Liu, L., Li, Y., Peng, H., Liu, R., Ji, W., Shi, Z., et al. (2020). Targeted exosome coating gene-chem nanocomplex as "nanoscavenger" for clearing alpha-synuclein and immune activation of Parkinson's disease. Sci. Adv. 6:eaba3967. doi: 10.1126/sciadv.aba3967
Lu, M., Zhao, X., Xing, H., Liu, H., Lang, L., Yang, T., et al. (2019). Cell-free synthesis of connexin 43-integrated exosome-mimetic nanoparticles for siRNA delivery. Acta Biomater. 96, 517–536. doi: 10.1016/j.actbio.2019.07.006
Maiese, K. (2016). Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br. J. Clin. Pharmacol. 82, 1245–1266. doi: 10.1111/bcp.12804
Moorthy, H., and Govindaraju, T. (2021). Dendrimer architectonics to treat Cancer and neurodegenerative diseases with implications in Theranostics and personalized medicine. ACS Appl Bio Mater 4, 1115–1139. doi: 10.1021/acsabm.0c01319
Neely, A., Perry, C., Varisli, B., Singh, A. K., Arbneshi, T., Senapati, D., et al. (2009). Ultrasensitive and highly selective detection of Alzheimer's disease biomarker using two-photon Rayleigh scattering properties of gold nanoparticle. ACS Nano 3, 2834–2840. doi: 10.1021/nn900813b
Paskeh, M. D. A., Entezari, M., Clark, C., Zabolian, A., Ranjbar, E., Farahani, M. V., et al. (2022). Targeted regulation of autophagy using nanoparticles: new insight into cancer therapy. Biochim. Biophys. Acta Mol. basis Dis. 1868:166326. doi: 10.1016/j.bbadis.2021.166326
Petcherski, A., Tingley, B. M., Martin, A., Adams, S., Brownstein, A. J., Steinberg, R. A., et al. (2023). Endo-lysosome-targeted nanoparticle delivery of antiviral therapy for coronavirus infections. bioRxiv. doi: 10.1101/2023.05.08.539898
Qi, X., Man, S. M., Malireddi, R. K. S., Karki, R., Lupfer, C., Gurung, P., et al. (2016). Cathepsin B modulates lysosomal biogenesis and host defense against Francisella novicida infection. J. Exp. Med. 213, 2081–2097. doi: 10.1084/jem.20151938
Sanchez-Ramos, J., Song, S., Kong, X., Foroutan, P., Martinez, G., Dominguez-Viqueria, W., et al. (2018). Chitosan-Mangafodipir nanoparticles designed for intranasal delivery of siRNA and DNA to brain. J Drug Deliv Sci Technol 43, 453–460. doi: 10.1016/j.jddst.2017.11.013
Sato, H., Takado, Y., Toyoda, S., Tsukamoto-Yasui, M., Minatohara, K., Takuwa, H., et al. (2021). Neurodegenerative processes accelerated by protein malnutrition and decelerated by essential amino acids in a tauopathy mouse model. Sci. Adv. 7:eabd5046. doi: 10.1126/sciadv.abd5046
Siafaka, P. I., Okur, M. E., Erim, P. D., Çağlar, E. Ş., Özgenç, E., Gündoğdu, E., et al. (2022). Protein and gene delivery Systems for Neurodegenerative Disorders: where do we stand today? Pharmaceutics 14:2425. doi: 10.3390/pharmaceutics14112425
Sillerud, L. O., Solberg, N. O., Chamberlain, R., Orlando, R. A., Heidrich, J. E., Brown, D. C., et al. (2013). SPION-enhanced magnetic resonance imaging of Alzheimer's disease plaques in AbetaPP/PS-1 transgenic mouse brain. J. Alzheimers Dis. 34, 349–365. doi: 10.3233/JAD-121171
Song, W., Soo Lee, S., Savini, M., Popp, L., Colvin, V. L., and Segatori, L. (2014). Ceria nanoparticles stabilized by organic surface coatings activate the lysosome-autophagy system and enhance autophagic clearance. ACS Nano 8, 10328–10342. doi: 10.1021/nn505073u
van Sadelhoff, J. H. J., Perez Pardo, P., Wu, J., Garssen, J., van Bergenhenegouwen, J., Hogenkamp, A., et al. (2019). The gut-immune-brain Axis in autism Spectrum disorders; a focus on amino acids. Front Endocrinol (Lausanne) 10:247. doi: 10.3389/fendo.2019.00247
Wang, P., Wu, B., Li, M., Song, Y., Chen, C., Feng, G., et al. (2023). Lysosome-targeting aggregation-induced emission nanoparticle enables adoptive macrophage transfer-based precise therapy of bacterial infections. ACS Nano 17, 10365–10375. doi: 10.1021/acsnano.3c00796
Weavers, H., Evans, I. R., Martin, P., and Wood, W. (2016). Corpse engulfment generates a molecular memory that primes the macrophage inflammatory response. Cells 165, 1658–1671. doi: 10.1016/j.cell.2016.04.049
Wong, E., and Cuervo, A. M. (2010). Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 13, 805–811. doi: 10.1038/nn.2575
Wu, J., de Theije, C. G. M., da Silva, S. L., Abbring, S., van der Horst, H., Broersen, L. M., et al. (2017). Dietary interventions that reduce mTOR activity rescue autistic-like behavioral deficits in mice. Brain Behav. Immun. 59, 273–287. doi: 10.1016/j.bbi.2016.09.016
Xie, Y. X., Naseri, N. N., Fels, J., Kharel, P., Na, Y., Lane, D., et al. (2022). Lysosomal exocytosis releases pathogenic alpha-synuclein species from neurons in synucleinopathy models. Nat. Commun. 13:4918. doi: 10.1038/s41467-022-32625-1
Yamamoto, H., Zhang, S., and Mizushima, N. (2023). Autophagy genes in biology and disease. Nat. Rev. Genet. 24, 382–400. doi: 10.1038/s41576-022-00562-w
Yoon, H. Y., Chang, I. H., Goo, Y. T., Kim, C. H., Kang, T. H., Kim, S. Y., et al. (2019). Intravesical delivery of rapamycin via folate-modified liposomes dispersed in thermo-reversible hydrogel. Int. J. Nanomedicine 14, 6249–6268. doi: 10.2147/IJN.S216432
Zhai, L., Shen, H., Sheng, Y., and Guan, Q. (2021). ADMSC Exo-MicroRNA-22 improve neurological function and neuroinflammation in mice with Alzheimer's disease. J. Cell. Mol. Med. 25, 7513–7523. doi: 10.1111/jcmm.16787
Keywords: lysosome, autophagy, amino acid, nanotechnology, bioengineering, neurodegenerative disorder
Citation: Raj A and Bandyopadhyay U (2024) Role of lysosome in healing neurological disorders by nano-bioengineering. Front. Neurosci. 17:1331211. doi: 10.3389/fnins.2023.1331211
Edited by:
Kumar Vaibhav, Augusta University, United StatesReviewed by:
Srinivasa Reddy Bonam, University of Texas Medical Branch at Galveston, United StatesCopyright © 2024 Raj and Bandyopadhyay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Urmi Bandyopadhyay, dWJhbmR5b3BAZ21haWwuY29t; dXJtaS5iYW5keW9wYWRoeWF5QG1hbmlwYWwuZWR1
 Aiswarya Raj
Aiswarya Raj Urmi Bandyopadhyay
Urmi Bandyopadhyay