- 1USC Stevens Neuroimaging and Informatics Institute, Keck School of Medicine of USC, University of Southern California, Los Angeles, CA, United States
- 2Department of Neurology, Inje University College of Medicine, Ilsan Paik Hospital, Goyang, Republic of Korea
- 3Department of Otorhinolaryngology—Head and Neck Surgery, Korea University College of Medicine, Korea University Ansan Hospital, Ansan, Republic of Korea
- 4Department of Otorhinolaryngology—Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 5Department of Neurology, Neuroscience Center, Samsung Medical Center, Samsung Biomedical Research Institute, School of Medicine, Sungkyunkwan University, Seoul, Republic of Korea
Study objectives: Obstructive sleep apnea (OSA) is a prevalent clinical problem significantly affecting cognitive functions. Surgical treatment is recommended for those unable to use continuous positive airway pressure. We aimed to investigate the therapeutic effect of upper airway surgery on the white matter (WM) microstructure and brain connectivity in patients with OSA.
Methods: Twenty-one male patients with moderate-to-severe OSA were recruited for multi-level upper airway surgery. Overnight polysomnography (PSG), neuropsychiatric tests, and brain MRI scans were acquired before and 6.1 ± 0.8 months after surgery. Nineteen male patients with untreated OSA were also included as a reference group. We calculated the longitudinal changes of diffusion tensor imaging (DTI) parameters, including fractional anisotropy (ΔFA) and mean/axial/radial diffusivity (ΔMD/AD/RD). We also assessed changes in network properties based on graph theory.
Results: Surgically treated patients showed improvement in PSG parameters and verbal memory after surgery. Globally, ΔFA was significantly higher and ΔRD was lower in the surgery group than in the untreated group. Especially ΔFA of the tracts involved in the limbic system was higher after surgery. In network analysis, higher Δbetweenness and lower Δclustering coefficients were observed in the surgical group than in the untreated group. Finally, the improvement of verbal memory after surgery positively correlated with ΔFA in superior thalamic radiation (p = 0.021), fronto aslant tracts (p = 0.027), and forceps minor tracts (p = 0.032).
Conclusion: Surgical treatment of OSA can alleviate alterations in WM integrity and disruptions in local networks, particularly for the tracts involved in the limbic system. These findings may further explain the cognitive improvement observed after the treatment of OSA.
Introduction
Obstructive sleep apnea (OSA) is one of the most prevalent sleep disorders, characterized by repetitive interruptions of breathing during sleep due to complete or partial airway obstruction. Cyclic apneas and hypopneas cause intermittent hypoxemia, hypercapnia, microarousals, and fragmented sleep, resulting in oxidative stress on the brain and subsequent brain structural and functional alterations (Park et al., 2011; Jordan et al., 2014). OSA is associated with cognitive impairments in various domains, including impaired memory, executive function, and attention (Lal et al., 2012; Sforza, 2012). Several neuroimaging studies with different modalities have observed that OSA is associated with loss of gray matter (GM) volume (Macey et al., 2002; Joo et al., 2010), disruption of white matter (WM) integrity (Macey et al., 2008; Koo et al., 2020), limited cerebral blood flow (Joo et al., 2007; Yan et al., 2021), and alterations in functional connectivity (Peng et al., 2014; Park et al., 2022).
Continuous positive airway pressure (CPAP) is the most widely used treatment for patients with OSA. CPAP use has been linked with positive outcomes such as a reduced risk of cardiovascular disease (Grimm et al., 2000; Pepperell et al., 2002; Kaneko et al., 2003; Vennelle et al., 2010) and improved daytime functioning and cognitive performance (Kushida et al., 2012; Olaithe and Bucks, 2013). Neuroimaging studies showed recovery of brain volume, regional hypoperfusion, or change in functional connectivity after treatment (Prilipko et al., 2014; Shiota et al., 2014; Kim et al., 2017; Maresky et al., 2019). However, patients' low adherence to CPAP is one of the most challenging issues in OSA treatment (Haniffa et al., 2004; Lin et al., 2007). Due to high levels of non-adherence, patients and providers often seek alternative treatment options, including upper airway surgery. Several studies found that surgical modifications of the upper airway alleviate the severity of OSA in adults (Sundaram et al., 2005; Caples et al., 2010) as well as improve cardiovascular outcomes (Halle et al., 2017; Kang et al., 2022).
The therapeutic effect of upper airway surgery on the central nervous system (CNS) has been rarely documented. One recent study demonstrated improved attention and driver performance in patients with OSA following upper airway surgery (Alkan et al., 2021). Among studies using magnetic resonance imaging (MRI), only one voxel-based morphometry (VBM) study investigated post-operative changes in OSA patients (Lin et al., 2016). It found improved cognitive performance after treatment and decreased GM volumes in the regions that might suffer from oxidative stress-led vasogenic edema, including the precuneus, cingulate gyrus, and cerebellum (Lin et al., 2016). No research has investigated the WM structure and integrity changes after surgical treatment of OSA.
Diffusion tensor imaging (DTI) is a powerful imaging technique that measures the movement and diffusion of water molecules and in brain tissues (Basser et al., 1994a,b) and can reveal alterations in the integrity of WM tissues in terms of axonal and myelin microstructures and brain region-to-region connectivity. Several scalar indices such as fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) (Chenevert et al., 1990; Le Bihan et al., 2001) have been used to characterize diffusion anisotropy (Le Bihan et al., 2001). The most commonly used invariant metric is FA, which measures the fraction of the magnitude of diffusion coefficients (Le Bihan et al., 2001). Diffusion in WM is less restricted along the axon and tends to be anisotropic (directionally dependent). Therefore, FA is commonly used in studying various neurological diseases and is related to alterations in structure pointing to specific myelination levels and axonal injury (Basser and Pierpaoli, 1996; White et al., 2008; Budde et al., 2009; Jones et al., 2013).
Here, our study aimed to investigate the effects of surgical treatment on WM tissue integrity and connectivity in patients with OSA by quantitatively analyzing longitudinal DTI scans acquired before and after surgical treatment. To statistically assess whether there is recovery resulting from treatment, we compared the trajectory of changes in FA (ΔFA) between surgically treated patients and untreated patients. We also assessed network/connectivity property changes based on graph theory (Bullmore and Sporns, 2009).
Methods
Participants and surgical procedure
Twenty-five male patients diagnosed with moderate-to-severe OSA were recruited for multi-level upper airway surgery at the sleep clinic of Samsung Medical Center, Seoul, South Korea. The severity of OSA was defined by the apnea–hypopnea index (AHI), and patients with AHI ≥ 15 were considered moderate to severe. These patients displayed failure or refusal to use CPAP and were willing to undergo upper airway surgery, clinical interview, sleep questionnaire, overnight polysomnography (PSG), neuropsychiatric tests, and brain MRI scans. The neuropsychological tests consisted of digit span tests from the Wechsler Memory Scale-Revised (Elwood, 1991), the Corsi block tapping tests (forward and backward) (Kessels et al., 2000), the trail-making tests A and B (Bowie and Harvey, 2006), the Stroop test for attention and executive function (Scarpina and Tagini, 2017), the Controlled Oral Word Association Test for verbal fluency function (Ross et al., 2007), the Korean California Verbal Test (Kim and Kang, 1999), and the Rey Complex Figure Test for verbal and visual memory function (Meyers et al., 1996). Composite scores were computed for each domain by transforming the neuropsychological test scores into standardized z-scores and averaging this data (Table 2).
All patients received a complete presurgical otolaryngologic evaluation, including tonsil grade and modified Mallampati score and a drug-induced sleep endoscopy (DISE) as previously described (Hong et al., 2013). In tailoring retropalatal narrowing during the surgical procedure, expansion sphincter pharyngoplasty was conducted with tonsillectomy. A midline glossectomy or radiofrequency tongue base reduction procedure was performed in the retrolingual region. At the same time, septoplasty and/or turbinoplasty were added for patients with significant nasal septal deviation or hypertrophy of inferior turbinates if necessary. These surgical procedures were selected based on DISE results and presurgical assessment by a surgeon. The follow-up PSG, neuropsychiatric tests, and MRI scans were performed 6 months after the surgery.
As a reference group, we recruited 26 male patients who initiated CPAP treatment but were shortly found to be not compliant and thus discontinued the treatment. We included these “untreated” OSA subjects only when they stopped the treatment within the first 2 weeks. If these patients returned to the sleep clinic after a certain period had elapsed to restart proper management for OSA, we registered them in the study and conducted a follow-up MRI before resuming the treatment.
Both the surgical group and the reference group (from here on, namely, the untreated group) share the same inclusion criteria: (1) adult males aged 20 years or older and (2) OSA with an AHI ≥ 15 in the initial PSG. They were excluded if they exhibited any of the following: (1) known sleep disorders other than OSA, (2) heart or respiratory disease, (3) history of cerebrovascular disease, (4) other neurological or psychiatric diseases, (5) history of OSA treatment (CPAP, oral device) for more than 2 weeks, or (6) a structural lesion on brain MRI. Four patients with surgical treatment and seven untreated patients were excluded according to the exclusion criteria mentioned above. Finally, 21 patients with surgery and 19 untreated patients were analyzed in the study, as shown in Table 1. The Institutional Review Board of Samsung Medical Center approved the study protocol (IRB No. 2017-02-126), and informed consent was obtained from all subjects. Study methods were carried out in accordance with approved guidelines.
Brain MRI acquisition
Within 1 week of the lab visit, sets of axial diffusion-weighted images (DWI) were acquired from all patients on a Philips Achieva 3 Tesla scanner with the following parameters: 128 × 128 acquisition matrix, 1.72 × 1.72 × 2 mm3, 70 axial slices, 220 × 220 mm2 field-of-view, TE = 60 ms, TR = 7,383 ms, flip angle = 90°, slice gap 0 mm, and b-value of 600 s mm−2 with 32 directions. All axial sections were acquired parallel to the anterior commissure—posterior commissure line (AC-PC line). Follow-up scans were performed half a year after the baseline scans for patients in the surgical group and a year or longer after baseline for the untreated group. Even though the two groups of patients were age- and sex-matched, the mean time interval between baseline and follow-up scans in the untreated group was significantly longer than in the surgical group. This difference arises due to the retrospective nature of the untreated group; follow-up MRI scans were performed when patients revisited the sleep clinic after a certain period of follow-up loss. Consequently, the time intervals are generally longer, often exceeding 1 year and vary across individuals.
Image processing and tractography
We visually inspected each DWI volume to identify outliers due to severe motion or signal dropouts. Then, four-dimensional images were corrected for eddy-current-introduced geometric distortions and motion artifacts using the FMRI Software Library (FSL) (Andersson and Sotiropoulos, 2016). Diffusion tensor modeling was performed using the Quantitative Imaging Toolkit (QIT; http://cabeen.io/qitwiki) diffusion workflow (Cabeen et al., 2018), which included multi-fiber diffusion modeling with FSL bedpost (Behrens et al., 2007). Finally, the FA and MD maps were estimated using weighted linear least squares at each curve vertex.
In this study, we performed deterministic multi-fiber streamline tractography from multi-fiber model data in native DWI space (Cabeen et al., 2016; Cabeen and Toga, 2020). This process generated geometric models of WM pathways from fiber orientation data, which were subsequently subdivided by their target brain area as the region of interest (ROI) and then characterized based on DTI parameters (FA and MD maps). More specifically, the ROIs were defined using the John Hopkins University white matter tractography atlas (Mori et al., 2008, 2009), which included GM, superficial WM, and subcortical nuclei with a separate label per ROI. When FA maps were non-linearly registered to the template (Desikan et al., 2006) using Advanced Normalization Tools (ANTs) (Avants et al., 2011), the resultant deformation maps were generated. To analyze each ROI, we warped ROIs into each subject's native DWI space by inverting the deformation map. Finally, the tracts were produced for each ROI in native space, and the FA, MD, AD, and RD values were averaged within each entire bundle and along-tract segmentation for statistical analysis.
Network construction
To construct structural brain networks, we adopted the parcellation scheme based on the Desikan–Killiany atlas (Desikan et al., 2006), resulting in 82 unilateral cortical and deep GM ROIs. Each ROI represented a node of the brain network. Two nodes were connected if the two endpoints of a given fiber tract were identified at these nodes. The weight of each connection (edge) between the two connected ROIs was calculated as the average of FA values on all voxels in the corresponding tract.
To evaluate whether surgery resulted in a better brain network organization, we computed global scale network metrics, including clustering coefficient (used as a measure of segregation reflecting the prevalence of clustered connectivity), degree centrality (used as a measure of integration and defined as an average of the degree centrality across all nodes), small-worldness [characterizing an ensemble of networks where a high small-worldness value represents a simultaneously highly segregated and integrated network (Humphries and Gurney, 2008)], and modularity (as a measurement of segregation). We quantified the degree to which the network may be subdivided into distinct groups and compared these global network properties between the untreated and the surgical groups (Rubinov and Sporns, 2010). In addition, we computed the brain regional connectivity metrics, such as betweenness (as a measurement of integration; a high betweenness value represents hub nodes with a high number of shortest paths) (Rubinov and Sporns, 2010), node degree centrality, and regional clustering coefficient and compared these regional properties between the surgical and non-compliant groups. The mathematical details and related equations regarding the aforementioned brain connectivity metrics are found in Supplementary Methods S1.
Statistical analysis
We first analyzed global white matter metrics and then tract-wise measurements. To examine group differences in clinical and polysomnographic characteristics, we performed two-sample t-tests for continuous variables and chi-square tests for categorical variables. In the DTI connectivity analysis, each ΔFA/ΔMD/ΔAD/ΔRD was calculated as “post-operative metric–pre-operative metric” in the surgical group and “follow-up metric–baseline metric” in the untreated group. Because of various scan intervals across patients and a significantly longer average scan interval in the untreated group than in the surgical group, all the ΔFA/ΔMD/ΔAD/ΔRD metrics were divided by the scan intervals in the unit of months.
The ΔFA/ΔMD/ΔAD/ΔRD metrics and the brain global/regional connectivity metrics were statistically analyzed using linear regression models to assess differences between surgical and untreated groups. Linear regression models were used to correct for age and body mass index (BMI) in the two groups.
To perform a more in-depth analysis, we split the surgical group into a responder group (AHI reduction > 50% and postop AHI < 20; n = 9) and a non-responder group (AHI reduction < 50% or postop AHI ≥ 20; n = 12). The cutoff value of AHI reduction < 50% or postop AHI ≥ 20 was selected based on the literature (Won et al., 2008; Zaghi et al., 2016). We then compared each ΔFA/ΔMD/ΔAD/ΔRD in each tract between the two subgroups. The p-values were adjusted for multiple comparisons using Tukey's honestly significant difference (HSD) test (Tukey, 1949).
Finally, we correlated each tract's ΔFA/ΔMD/ΔAD/ΔRD metrics with cognitive function changes after surgery (Δcognitive function score) using Spearman's ρ test.
Results
Demographics and sleep parameters
Detailed clinical characteristics and polysomnographic findings for the surgical and untreated groups are summarized in Table 1. Demographic characteristics and PSG parameters were not significantly different between the two groups. The time interval between baseline and the follow-up MRI scans was longer in the non-compliant group than in the surgically treated group (43.7 ± 16.3 vs. 6.1 ± 0.8 months, p < 0.001).
Changes in sleep parameters and cognitive performances after surgery
Patients who underwent surgery (n = 21) showed improvement in daytime sleepiness (Epworth sleepiness score 10.7 ± 5.1 vs. 7.9 ± 3.2, p = 0.013) and objective sleep quality; AHI, arousal index, and proportion of shallow sleep (N1%) significantly decreased after surgery, while total sleep time increased after surgery. Regarding the neuropsychiatric tests, the composite verbal memory score improved significantly after surgery (p = 0.002). The details of PSG parameters and the neuropsychological tests before and after surgery are summarized in Table 2. Because the untreated patients (n = 19) did not perform follow-up PSG and neuropsychiatric tests, we did not assess changes in sleep parameters and cognitive performance for this group.
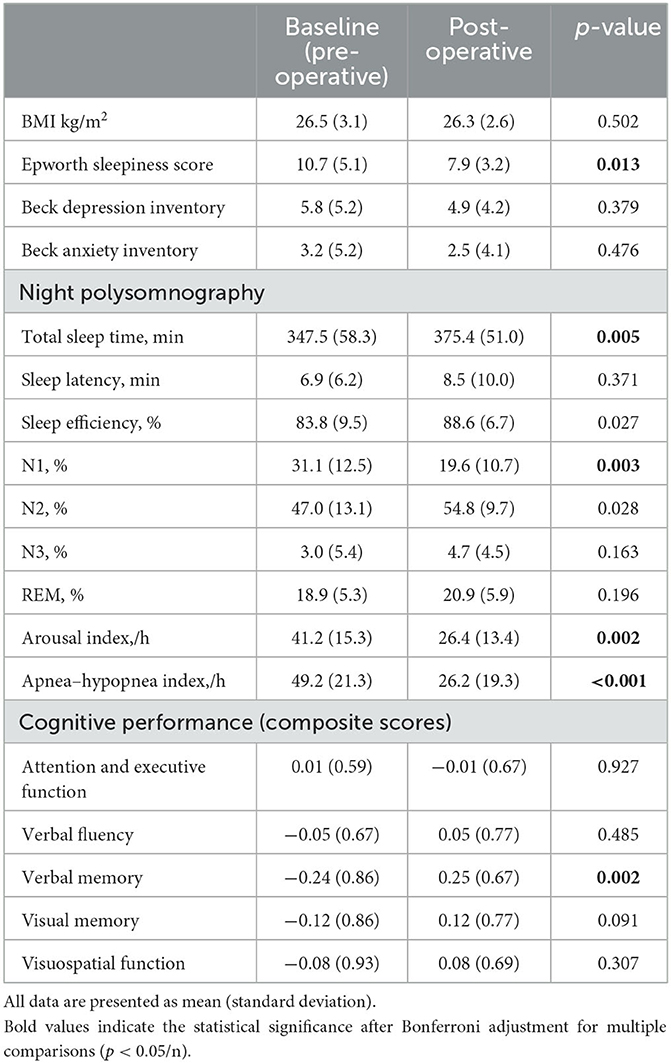
Table 2. Comparison of sleep parameters and cognitive performance before and after surgery in the treatment group (n = 21).
White matter microstructural changes
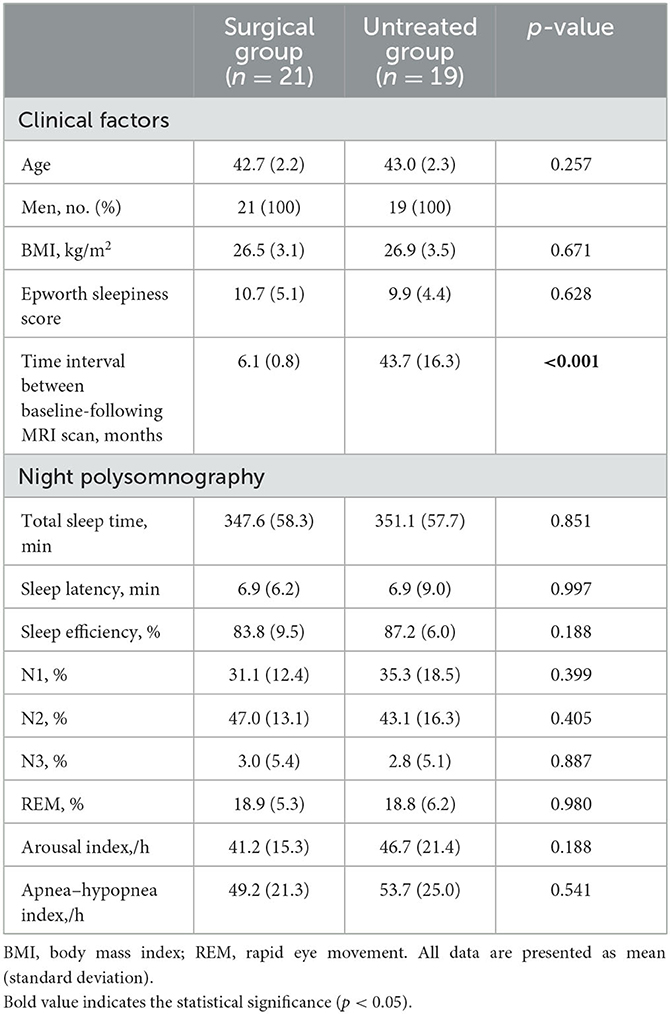
For the analysis of the global white matter metrics, we found significantly higher ΔFA and lower ΔRD in the surgical group than in the untreated group and then performed regional analyses.
ΔFA was significantly higher (Figure 1) in the surgical group than in the untreated group in the uncinate fasciculus (t-ratio = −2.42, p = 0.046), middle longitudinal fasciculus (t-ratio = −2.10, p = 0.043), fornix (t-ratio = −2.42, p = 0.031), forceps minor (t-ratio = −7.28, p = 0.024), the cingulum bundle (t-ratio = −1.98, p = 0.046), and superior longitudinal fasciculus (t-ratio = −3.86, p = 0.033). ΔFA of these tracts in the untreated group was significantly negative (i.e., decrease in FA over time), whereas ΔFA remained near-zero in the surgical group. In contrast, ΔFA of the forceps major (t-ratio = 2.17, p = 0.044), inferior longitudinal fasciculus (ILF) (t-ratio = 3.79, p = 0.030), superior cerebellar peduncle (t-ratio = 6.37, p = 0.009), and corona radiata (t-ratio = 3.14, p = 0.039) were lower in the surgical group than in the untreated group (Figure 2). On the contrary, the untreated OSA group showed positive ΔFA (i.e., increase in FA over time) in these tracts, except forceps major, while the surgical group showed near-zero ΔFA.
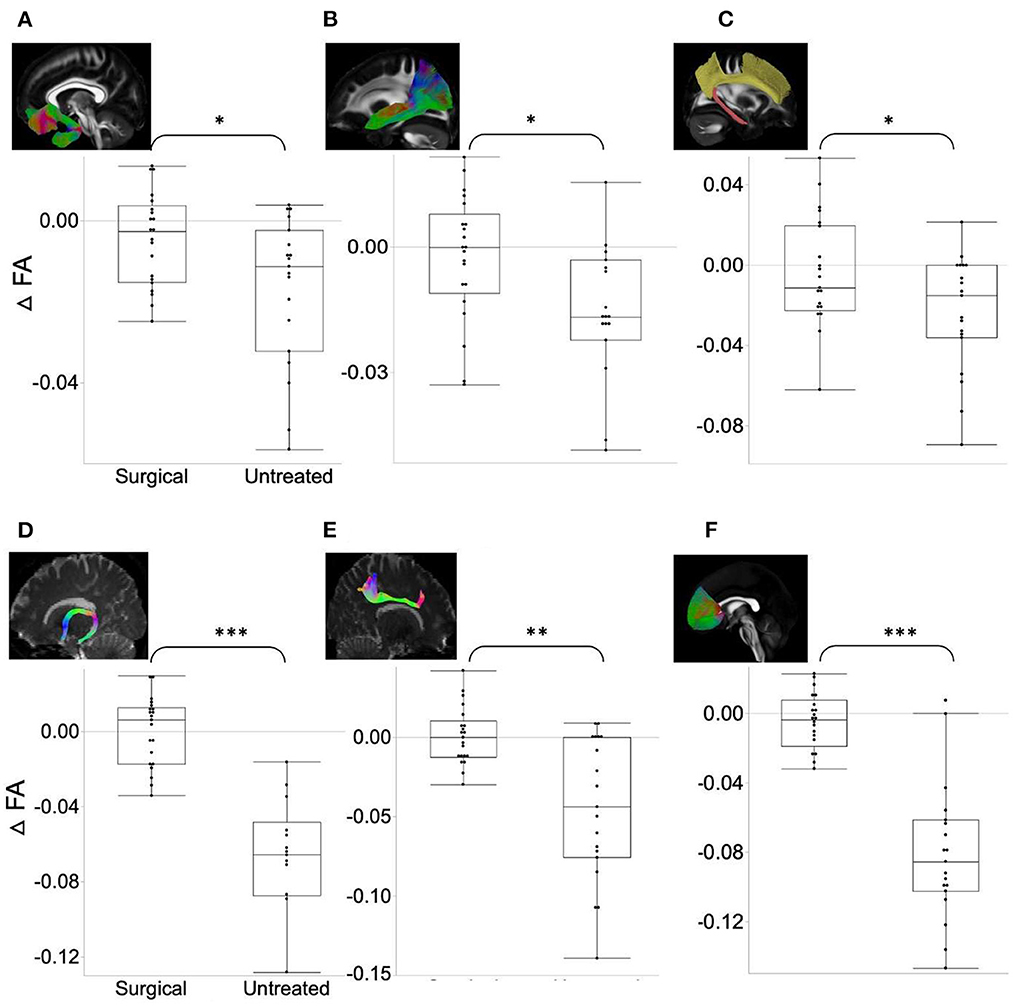
Figure 1. ΔFA in the surgical group (FA changes between post- and pre-operative measures) is significantly higher (relative increase in connectivity or myelination after surgery) than in the untreated group (difference between follow-up and baseline). (A) Uncinate fasciculus. (B) Middle longitudinal fasciculus. (C) Cingulum bundle. (D) Fornix. (E) Superior longitudinal fasciculus. (F) Forceps minor. Asterisk indicates the significance level (*p < 0.05, **p < 0.001, ***p < 0.0001).
Figure 2. ΔFA in the surgical group is significantly lower (relative decrease in connectivity after surgery) than in the untreated group. (A) Forceps major. (B) Inferior longitudinal fasciculus. (C) Superior cerebellar peduncle. (D) Corona radiata. Asterisk indicates the significance level (*p < 0.05).
Among those ROIs in which we found higher ΔFA in the surgical group than in the untreated group, forceps minor displayed significantly higher ΔFA in the responder group after surgery (AHI reduction > 50% and postop AHI < 20; n = 9) than in the non-responders (p = 0.033, Supplementary Figure S1). In other tracts, ΔFA was not different between the responder and non-responder groups (p > 0.2). Additionally, the fronto aslant tract showed significantly higher ΔFA in the responders than in the non-responders (p = 0.045). The results of ΔRD are shown in Supplementary Tables S1, S2.
Structural brain connectivity changes
Globally, there was no significant difference between the two groups for all the measures, including strength, clustering coefficient, degree, small-worldness, and modularity. However, in the analysis of regional network property metrics, we found relatively increased hubness and decreased segregation after surgical treatment compared to the follow-up measures in untreated patients (Figure 3); ΔBetweenness of the cuneus (t-ratio = −2.42) and frontal pole (t-ratio = −3.83) was significantly higher, and Δclustering coefficient of bilateral postcentral area (t-ratio = 2.26) and cuneus (t-ratio = −2.14) was lower in the surgical group than in the untreated group.
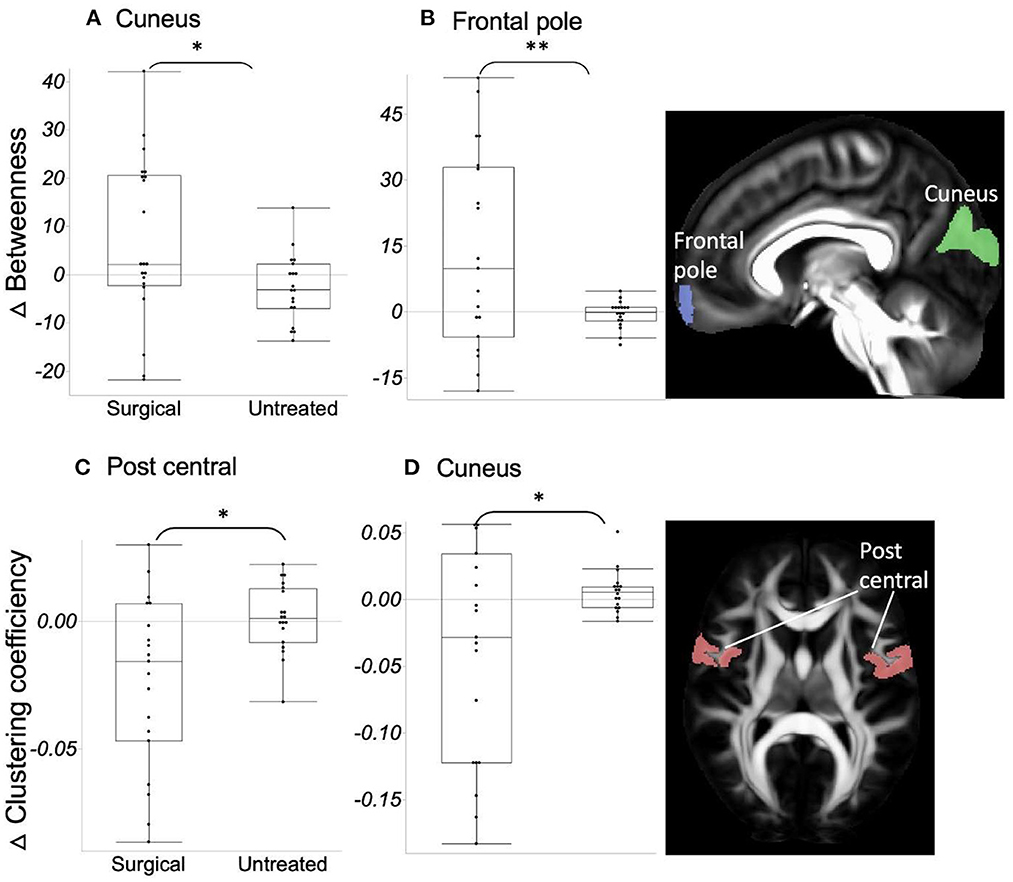
Figure 3. (A, B) ΔBetweenness in the surgical group is significantly higher (=increased network integration) than in the untreated group. (C, D) ΔClustering coefficient in the surgical group is significantly lower (=decreased network segregation) than in the untreated group. Asterisk indicates the significance level (*p < 0.05, **p < 0.001).
Correlation between WM microstructural changes and PSG parameters and cognitive performance
We correlated ΔFA with ΔPSG and Δcognitive performance (i.e., change of composite score of each cognitive domain) in the surgical group (Figure 4). The Δverbal memory of them positively correlated with ΔFA in superior thalamic radiation (p = 0.021), fronto aslant tract (p = 0.027), and forceps minor (p = 0.032). We found no correlations between Δbrain connectivity changes and ΔPSG and Δcognitive performance.
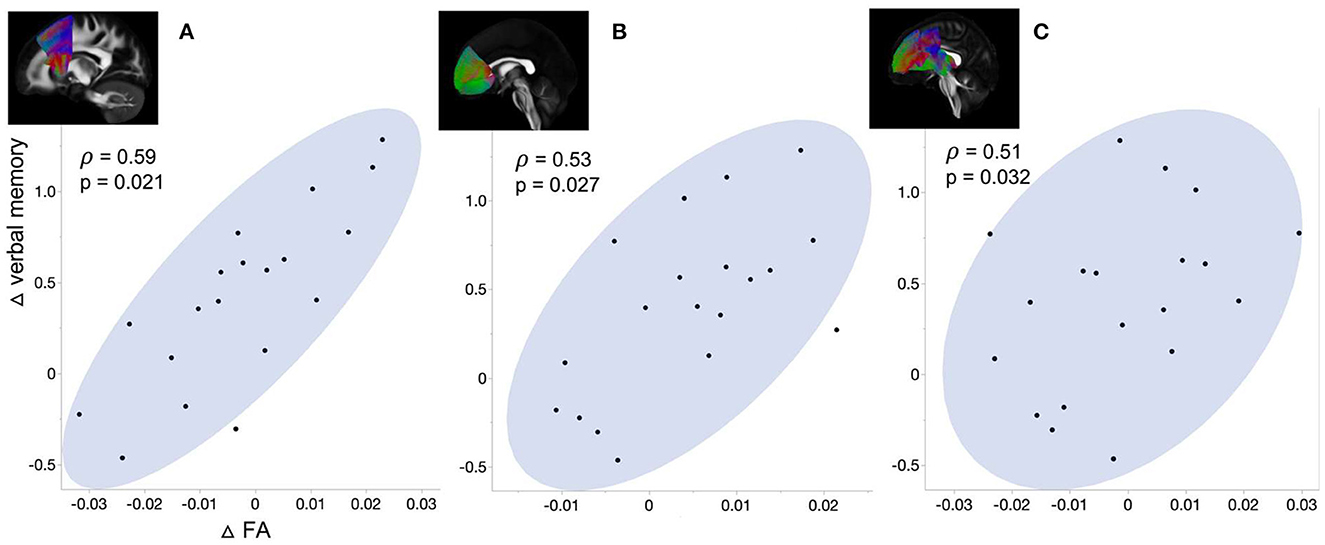
Figure 4. Correlation between the change of verbal memory and ΔFA in (A) Forceps minor, (B) Frontoaslant tract, and (C) Superior thalamic radiation.
Discussion
Summary
The current study is the first study to compare the trajectory of white matter microstructure and connectivity changes between untreated (total CPAP use: <2 weeks) and surgically treated OSA patients. We found significantly higher ΔFA and lower ΔRD in the surgical group than in the untreated group for numerous tracts. Furthermore, we found significantly decreased segregation and increased integration in the surgical group compared to the untreated group. Finally, the improvement of verbal memory after surgery positively correlated with ΔFA in some WM tracts.
Recovery of OSA-led disruptions in WM microstructural integrity following treatment
We found higher ΔFA values globally in the surgical group. In regional analysis, higher ΔFA to the “near-zero” level was observed in the right uncinate fasciculus, middle longitudinal fasciculus, fornix, forceps minor, the left cingulum bundle, and superior longitudinal fasciculus in the surgical group. Our findings of higher ΔFA (=the relative increase) in these WM tracts, which are mainly connected to the limbic system, suggest that surgical treatment of OSA could alleviate the WM integrity disruptions and prevent further impairment in these tracts. The improvement of the verbal memory score observed in the surgery patient group of our study (p = 0.02) may thus result from the alleviated alterations in the WM tracts, which link to the limbic system, which is involved in verbal and language memory processing. Our findings in the correlation between improved verbal memory and ΔFA values go in line with the previous studies (Castronovo et al., 2014; Salsone et al., 2021). Specifically, these studies noted FA differences and Rey word lists associations between healthy controls and post-treatment in arcuate fasciculus, superior longitudinal fasciculus, and uncinate. However, these studies did not observe the association with ΔFA between follow-up and baseline. Nevertheless, while distinct brain regions have been identified across various investigations, our findings mark a notable advancement in unraveling the significance of WM modifications resulting from OSA treatment within the context of cognitive impairment's pathogenesis.
In addition to significantly higher ΔFA, we also observed lower ΔRD (=relative decrease) after surgical treatment in a few tracts. Our finding of reduced RD following surgical treatment likely indicates that the myelin damage led by OSA can be alleviated by treatment.
Our study demonstrated a significant reduction of AHI after surgery in patients with OSA, thus suggesting that the prevention of apneic events stops imposing hypoxic burdens on the brain and improves cerebral perfusion, which consequently alleviates myelin damage and encourages recovery of WM microstructural integrity. Previous studies showed that CPAP, the first-line treatment for OSA, can lead to recovery of WM microstructural integrity and brain volume in OSA (Castronovo et al., 2014; Kim et al., 2016; Maresky et al., 2019). It is thus reasonable to speculate that a similar mechanism is involved in the WM integrity recovery after surgical treatment.
Brain network reorganization due to pathophysiology of OSA and compensatory mechanisms and normalization after surgical treatment
In contrast to the several structures mentioned above and global ΔFA, forceps major, ILF, superior cerebellar peduncle, and corona radiata displayed significantly increased FA values over time (higher ΔFA) in untreated patients, whereas ΔFA was maintained at “near-zero” after surgical treatment. One possible explanation is that the brain network at the system level continues to become less integrated and more segregated in the organization in relation to the pathophysiology of OSA, by which some tracts undergo a connectivity increase in a compensatory manner, as opposed to the larger number of tracts showing decreased connectivity (lower ΔFA). However, this abnormal connectivity enhancement was normalized to have no more pathologic WM alterations (i.e., near-zero ΔFA) after eliminating the source of pathophysiology in OSA by surgery. Furthermore, we observed increased integration (betweenness) and decreased segregation (clustering coefficient) after surgical treatment (Figure 3).
Corona radiata conveys sensorimotor information from the pre/postcentral gyrus to the thalamus and brain stem. Decreased segregation of the bilateral postcentral area after surgical treatment was observed in the present study. Increased descending information in these areas due to network segregation may change microstructures of the connected WM tracts, such as corona radiata in the untreated group. Similarly, decreased FA in forceps major and ILF, which connect the ipsilateral occipital lobe to the contralateral occipital lobe and ipsilateral anterior temporal lobe, may explain the network reorganization of cuneus (i.e., decreased segregation and increased hubness) after surgical treatment.
Association between WM microstructural changes and cognitive performance after surgery
In addition to recovery of WM disruption connected to the limbic structure following surgery, we found improvement of verbal memory that further correlated with the increase in FA in superior thalamic radiation, frontoaslant tract, and forceps minor. These tracts were associated with consciousness, language function, and cognitive behavior, respectively (Zhou et al., 2011; Mamiya et al., 2018; La Corte et al., 2021). In particular, forceps minor, which showed higher ΔFA in the surgical group of the present study, was implicated in cognitive dysfunctions in various conditions such as neurodegenerative disease and attention deficit hyperactivity disorder (Haller et al., 2010; Qiu et al., 2011; Lillo et al., 2012). These findings support the potential recovery of the initial WM disruption as one of the underlying mechanisms of cognitive improvement following treatment in patients with OSA.
These findings highlight the significance of surgical treatment in terms of cognition for OSA patients. Particularly, they can provide a rationale for more active surgical intervention in pediatric OSA patients, who often experience delays in surgery for various reasons (Richards and Ferdman, 2000). However, it should be noted that our study solely focused on adult OSA patients. Therefore, future research targeting pediatric populations is necessary to validate these recommendations for pediatric patients.
Limitations and potential future work
Despite the overall improvements in WM integrity after surgery, we note that ΔFA in the surgical group was quite variable (i.e., large standard deviation) among patients (Figure 1). This may indicate that some patients did not benefit from surgery, which may be explained by the following two factors: First, the degree of eliminating obstruction after surgery is variable depending on the level of obstruction, tissue collapsibility, and weight gain (Carvalho et al., 2012). Our in-depth analysis indeed suggests that non-responders (e.g., insufficiently decreased AHI) might recover less in regional WM integrity. However, a future study is required to clarify further due to a small sample size (n = 12 vs. 9). Second, the reversibility of WM damages may contribute to the inter-individual difference. Oligodendrocytes have minimal regenerative capacity; thus, in the event that they are severely damaged by recurrent hypoxia-ischemia, at least some myelin damage would be hard to recover after alleviating the airway obstruction. These two possibilities require further clarification in future work.
Our longitudinal study of WM microstructural integrity using both surgical and untreated OSA groups is novel. However, there were limitations in this study that the Number of participants was relatively small, and a short-term follow-up (6 months) was performed after surgery. We also note that the scanning time intervals in the untreated group (mean = 7 years) are significantly longer than in the treated group (mean = 0.5 years). To address this issue, we divided all the WM diffusion metrics by the scan intervals in months and analyzed this normalized metric using linear models. In addition, we conducted a paired t-test for WM characteristics and connectivity between pre- and post-surgical scans within the surgical group. These tests did not yield significant differences, which align with the results presented in Figures 1, 2, highlighting that ΔFA in the surgical group were distributed around zero. However, this may not fully address the deviations between the two groups. Including healthy subjects in future studies can further clarify the effects of treatment compared to the normal aging trajectory.
While the application of thresholds to connectivity matrices has been a common practice in network studies to reduce false positive connections (de Reus and van den Heuvel, 2013; Roberts et al., 2017; Buchanan et al., 2020), note that we employed a deterministic tensor-based tracking approach. This method may benefit less from network density thresholding compared to probabilistic tractography (Baum et al., 2017). However, the interplay between tracking algorithms, reconstruction methods, and thresholding strategies requires further investigation to fully comprehend their influence on connectivity outcomes.
Conclusion
Our study demonstrated that surgical treatment alleviated destructive changes following persistent OSA, and WM microstructural integrity may be recovered and/or reorganized after surgery, at least for some patients. The positive effects of surgery were particularly prominent for the tracts involved in the limbic system, which may further explain cognitive improvement after the surgical treatment of OSA. This longitudinal study provides novel insights into the effects of surgical treatment on WM microstructure, brain connectivity, and cognitive performance.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2017-02-126). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HYK, HK, and EYJ contributed to conception and design of the study. HYK, HRP, MS, and EYJ organized the database. YC contributed to image processing and figure making. HRP, YC, and HK wrote the first draft of the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
Funding
This study was supported by the Samsung Medical Center Grant (OTC1190671) and the National Institutes of Health Grant (P41EB015922). HK was funded by the BrightFocus Foundation Award (A2019052S).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1221290/full#supplementary-material
References
Alkan, U., Nachalon, Y., Weiss, P., Ritter, A., Feinmesser, R., Gilat, H., et al. (2021). Effects of surgery for obstructive sleep apnea on cognitive function and driving performance. Sleep Breath. 25, 1593–1600. doi: 10.1007/s11325-020-02285-w
Andersson, J. L. R., and Sotiropoulos, S. N. (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063–1078. doi: 10.1016/j.neuroimage.2015.10.019
Avants, B. B., Tustison, N. J., Song, G., Cook, P. A., Klein, A., Gee, J. C., et al. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044. doi: 10.1016/j.neuroimage.2010.09.025
Basser, P. J., Mattiello, J., and Le Bihan, D. (1994a). Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B 103, 247–254. doi: 10.1006/jmrb.1994.1037
Basser, P. J., Mattiello, J., and Le Bihan, D. (1994b). MR diffusion tensor spectroscopy and imaging. Biophys. J. 66, 259–267. doi: 10.1016/S0006-3495(94)80775-1
Basser, P. J., and Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B 111, 209–219. doi: 10.1006/jmrb.1996.0086
Baum, G. L., Ciric, R., Roalf, D. R., Betzel, R. F., Moore, T. M., Shinohara, R. T., et al. (2017). Modular segregation of structural brain networks supports the development of executive function in youth. Curr. Biol. 27, 1561–1572.e8. doi: 10.1016/j.cub.2017.04.051
Behrens, T. E., Berg, H. J., Jbabdi, S., Rushworth, M. F., and Woolrich, M. W. (2007). Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34, 144–155. doi: 10.1016/j.neuroimage.2006.09.018
Bowie, C. R., and Harvey, P. D. (2006). Administration and interpretation of the Trail Making Test. Nat. Protoc. 1, 2277–2281. doi: 10.1038/nprot.2006.390
Buchanan, C. R., Bastin, M. E., Ritchie, S. J., Liewald, D. C., Madole, J. W., Tucker-Drob, E. M., et al. (2020). The effect of network thresholding and weighting on structural brain networks in the UK Biobank. Neuroimage.211, 116443. doi: 10.1016/j.neuroimage.2019.116443
Budde, M. D., Xie, M., Cross, A. H., and Song, S. K. (2009). Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J. Neurosci. 29, 2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009
Bullmore, E., and Sporns, O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198. doi: 10.1038/nrn2575
Cabeen, R. P., Bastin, M. E., and Laidlaw, D. H. (2016). Kernel regression estimation of fiber orientation mixtures in diffusion MRI. Neuroimage 127, 158–172. doi: 10.1016/j.neuroimage.2015.11.061
Cabeen, R. P., Laidlaw, D. H., and Toga, A. W. (2018). “Quantitative imaging toolkit: software for interactive 3D visualization, processing, and analysis of neuroimaging datasets,” in Proceedings of the International Society for Magnetic Resonance in Medicine.
Cabeen, R. P., and Toga, A. W. (2020). “Reinforcement tractography: a hybrid approach for robust segmentation of complex fiber bundles,” in 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI) (IEEE), 999–1003.
Caples, S. M., Rowley, J. A., Prinsell, J. R., Pallanch, J. F., Elamin, M. B., Katz, S. G., et al. (2010). Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 33, 1396–1407. doi: 10.1093/sleep/33.10.1396
Carvalho, B., Hsia, J., and Capasso, R. (2012). Surgical therapy of obstructive sleep apnea: a review. Neurotherapeutics 9, 710–716. doi: 10.1007/s13311-012-0141-x
Castronovo, V., Scifo, P., Castellano, A., Aloia, M. S., Iadanza, A., Marelli, S., et al. (2014). White matter integrity in obstructive sleep apnea before and after treatment. Sleep 37, 1465–1475. doi: 10.5665/sleep.3994
Chenevert, T. L., Brunberg, J. A., and Pipe, J. G. (1990). Anisotropic diffusion in human white matter: demonstration with MR techniques in vivo. Radiology 177, 401–405. doi: 10.1148/radiology.177.2.2217776
de Reus, M. A., and van den Heuvel, M. P. (2013). Estimating false positives and negatives in brain networks. Neuroimage 70, 402–409. doi: 10.1016/j.neuroimage.2012.12.066
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Elwood, R. W. (1991). The Wechsler Memory Scale-Revised: psychometric characteristics and clinical application. Neuropsychol. Rev. 2, 179–201. doi: 10.1007/BF01109053
Grimm, W., Koehler, U., Fus, E., Hoffmann, J., Menz, V., Funck, R., et al. (2000). Outcome of patients with sleep apnea-associated severe bradyarrhythmias after continuous positive airway pressure therapy. Am. J. Cardiol. 86, 688–692. doi: 10.1016/S0002-9149(00)01055-9
Halle, T. R., Oh, M. S., Collop, N. A., Quyyumi, A. A., Bliwise, D. L., Dedhia, R. C., et al. (2017). Surgical treatment of OSA on cardiovascular outcomes: a systematic review. Chest 152, 1214–1229. doi: 10.1016/j.chest.2017.09.004
Haller, S., Nguyen, D., Rodriguez, C., Emch, J., Gold, G., Bartsch, A., et al. (2010). Individual prediction of cognitive decline in mild cognitive impairment using support vector machine-based analysis of diffusion tensor imaging data. J. Alzheimers Dis. 22, 315–327. doi: 10.3233/JAD-2010-100840
Haniffa, M., Lasserson, T. J., and Smith, I. (2004). Interventions to improve compliance with continuous positive airway pressure for obstructive sleep apnoea. Cochr. Database Syst. Rev. CD003531. doi: 10.1002/14651858.CD003531.pub2
Hong, S. D., Dhong, H. J., Kim, H. Y., Sohn, J. H., Jung, Y. G., Chung, S. K., et al. (2013). Change of obstruction level during drug-induced sleep endoscopy according to sedation depth in obstructive sleep apnea. Laryngoscope 123, 2896–2899. doi: 10.1002/lary.24045
Humphries, M. D., and Gurney, K. (2008). Network “small-world-ness” a quantitative method for determining canonical network equivalence. PLoS ONE 3, e0002051. doi: 10.1371/journal.pone.0002051
Jones, D. K., Knosche, T. R., and Turner, R. (2013). White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage.73, 239–254. doi: 10.1016/j.neuroimage.2012.06.081
Joo, E. Y., Tae, W. S., Han, S. J., Cho, J.-.W., and Hong, S. B. (2007). Reduced cerebral blood flow during wakefulness in obstructive sleep apnea-hypopnea syndrome. Sleep 30, 1515–1520. doi: 10.1093/sleep/30.11.1515
Joo, E. Y., Tae, W. S., Lee, M. J., Kang, J. W., Park, H. S., Lee, J. Y., et al. (2010). Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep 33, 235–241. doi: 10.1093/sleep/33.2.235
Jordan, A. S., McSharry, D. G., and Malhotra, A. (2014). Adult obstructive sleep apnoea. Lancet 383, 736–747. doi: 10.1016/S0140-6736(13)60734-5
Kaneko, Y., Floras, J. S., Usui, K., Plante, J., Tkacova, R., Kubo, T., et al. (2003). Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N. Engl. J. Med. 348, 1233–1241. doi: 10.1056/NEJMoa022479
Kang, K. T., Yeh, T. H., Ko, J. Y., Lee, C. H., Lin, M. T., Hsu, W. C., et al. (2022). Effect of sleep surgery on blood pressure in adults with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med. Rev. 62, 101590. doi: 10.1016/j.smrv.2022.101590
Kessels, R. P., Van Zandvoort, M. J., Postma, A., Kappelle, L. J., and De Haan, E. H. (2000). The Corsi block-tapping task: standardization and normative data. Appl. Neuropsychol. 7, 252–258. doi: 10.1207/S15324826AN0704_8
Kim, H., Joo, E., Suh, S., Kim, J. H., Kim, S. T., Hong, S. B., et al. (2016). Effects of long-term treatment on brain volume in patients with obstructive sleep apnea syndrome. Hum. Brain Mapp. 37, 395–409. doi: 10.1002/hbm.23038
Kim, J. K., and Kang, Y. (1999). Normative study of the Korean-California Verbal Learning Test (K-CVLT). Clin. Neuropsychol. 13, 365–369. doi: 10.1076/clin.13.3.365.1740
Kim, J. S., Seo, J. H., Kang, M. R., Seong, M. J., Lee, W. G., Joo, E. Y., et al. (2017). Effect of continuous positive airway pressure on regional cerebral blood flow in patients with severe obstructive sleep apnea syndrome. Sleep Med. 32, 122–128. doi: 10.1016/j.sleep.2016.03.010
Koo, D. L., Kim, H. R., Kim, H., Seong, J. K., and Joo, E. Y. (2020). White matter tract-specific alterations in male patients with untreated obstructive sleep apnea are associated with worse cognitive function. Sleep. 43. doi: 10.1093/sleep/zsz247
Kushida, C. A., Nichols, D. A., Holmes, T. H., Quan, S. F., Walsh, J. K., Gottlieb, D. J., et al. (2012). Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 35, 1593–1602. doi: 10.5665/sleep.2226
La Corte, E., Eldahaby, D., Greco, E., Aquino, D., Bertolini, G., Levi, V., et al. (2021). The frontal aslant tract: a systematic review for neurosurgical applications. Front. Neurol. 12, 641586. doi: 10.3389/fneur.2021.641586
Lal, C., Strange, C., and Bachman, D. (2012). Neurocognitive impairment in obstructive sleep apnea. Chest 141, 1601–1610. doi: 10.1378/chest.11-2214
Le Bihan, D., Mangin, J. F., Poupon, C., Clark, C. A., Pappata, S., Molko, N., et al. (2001). Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging 13, 534–546. doi: 10.1002/jmri.1076
Lillo, P., Mioshi, E., Burrell, J. R., Kiernan, M. C., Hodges, J. R., Hornberger, M., et al. (2012). Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS ONE 7, e43993. doi: 10.1371/journal.pone.0043993
Lin, H. S., Zuliani, G., Amjad, E. H., Prasad, A. S., Badr, M. S., Pan, C. J., et al. (2007). Treatment compliance in patients lost to follow-up after polysomnography. Otolaryngol Head Neck Surg. 136, 236–240. doi: 10.1016/j.otohns.2006.08.007
Lin, W. C., Huang, C. C., Chen, H. L., Chou, K. H., Chen, P. C., Tsai, N. W., et al. (2016). Longitudinal brain structural alterations and systemic inflammation in obstructive sleep apnea before and after surgical treatment. J. Transl. Med. 14, 139. doi: 10.1186/s12967-016-0887-8
Macey, P. M., Henderson, L. A., Macey, K. E., Alger, J. R., Frysinger, R. C., Woo, M. A., et al. (2002). Brain morphology associated with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 166, 1382–1387. doi: 10.1164/rccm.200201-050OC
Macey, P. M., Kumar, R., Woo, M. A., Valladares, E. M., Yan-Go, F. L., Harper, R. M., et al. (2008). Brain structural changes in obstructive sleep apnea. Sleep 31, 967–977. doi: 10.5665/sleep/31.7.967
Mamiya, P. C., Richards, T. L., and Kuhl, P. K. (2018). Right forceps minor and anterior thalamic radiation predict executive function skills in young bilingual adults. Front. Psychol. 9, 118. doi: 10.3389/fpsyg.2018.00118
Maresky, H. S., Shpirer, I., Klar, M. M., Levitt, M., Sasson, E., Tal, S., et al. (2019). Continuous positive airway pressure alters brain microstructure and perfusion patterns in patients with obstructive sleep apnea. Sleep Med. 57, 61–69. doi: 10.1016/j.sleep.2018.12.027
Meyers, J. E., Bayless, J. D., and Meyers, K. R. (1996). Rey complex figure: memory error patterns and functional abilities. Appl. Neuropsychol. 3, 89–92. doi: 10.1207/s15324826an0302_8
Mori, S., Oishi, K., and Faria, A. V. (2009). White matter atlases based on diffusion tensor imaging. Curr. Opin. Neurol. 22, 362–369. doi: 10.1097/WCO.0b013e32832d954b
Mori, S., Oishi, K., Jiang, H., Jiang, L., Li, X., Akhter, K., et al. (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40, 570–582. doi: 10.1016/j.neuroimage.2007.12.035
Olaithe, M., and Bucks, R. S. (2013). Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep 36, 1297–1305. doi: 10.5665/sleep.2950
Park, H. R., Cha, J., Joo, E. Y., and Kim, H. (2022). Altered cerebrocerebellar functional connectivity in patients with obstructive sleep apnea and its association with cognitive function. Sleep. 45. doi: 10.1093/sleep/zsab209
Park, J. G., Ramar, K., and Olson, E. J. (2011). Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin. Proc. 86, 549–54. quiz 54–5. doi: 10.4065/mcp.2010.0810
Peng, D. C., Dai, X. J., Gong, H. H., Li, H. J., Nie, X., Zhang, W., et al. (2014). Altered intrinsic regional brain activity in male patients with severe obstructive sleep apnea: a resting-state functional magnetic resonance imaging study. Neuropsychiatr. Dis. Treat. 10, 1819–1826. doi: 10.2147/NDT.S67805
Pepperell, J. C., Ramdassingh-Dow, S., Crosthwaite, N., Mullins, R., Jenkinson, C., Stradling, J. R., et al. (2002). Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 359, 204–210. doi: 10.1016/S0140-6736(02)07445-7
Prilipko, O., Huynh, N., Thomason, M. E., Kushida, C. A., and Guilleminault, C. (2014). An fMRI study of cerebrovascular reactivity and perfusion in obstructive sleep apnea patients before and after CPAP treatment. Sleep Med. 15, 892–898. doi: 10.1016/j.sleep.2014.04.004
Qiu, M. G., Ye, Z., Li, Q. Y., Liu, G. J., Xie, B., Wang, J., et al. (2011). Changes of brain structure and function in ADHD children. Brain Topogr. 24, 243–252. doi: 10.1007/s10548-010-0168-4
Richards, W., and Ferdman, R. M. (2000). Prolonged morbidity due to delays in the diagnosis and treatment of obstructive sleep apnea in children. Clin. Pediatr. 39, 103–108. doi: 10.1177/000992280003900205
Roberts, J. A., Perry, A., Roberts, G., Mitchell, P. B., and Breakspear, M. (2017). Consistency-based thresholding of the human connectome. Neuroimage 145, 118–129. doi: 10.1016/j.neuroimage.2016.09.053
Ross, T. P., Calhoun, E., Cox, T., Wenner, C., Kono, W., Pleasant, M., et al. (2007). The reliability and validity of qualitative scores for the Controlled Oral Word Association Test. Arch. Clin. Neuropsychol. 22, 475–488. doi: 10.1016/j.acn.2007.01.026
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Salsone, M., Caligiuri, M. E., Castronovo, V., Canessa, N., Marelli, S., Quattrone, A., et al. (2021). Microstructural changes in normal-appearing white matter in male sleep apnea patients are reversible after treatment: a pilot study. J. Neurosci. Res. 99, 2646–2656. doi: 10.1002/jnr.24858
Scarpina, F., and Tagini, S. (2017). The stroop color and word test. Front. Psychol. 8, 557. doi: 10.3389/fpsyg.2017.00557
Sforza, E. (2012). Sleep apnea syndrome and cognition. Front. Neurol. 3, 87. doi: 10.3389/fneur.2012.00087
Shiota, S., Inoue, Y., Takekawa, H., Kotajima, M., Nakajyo, M., Usui, C., et al. (2014). Effect of continuous positive airway pressure on regional cerebral blood flow during wakefulness in obstructive sleep apnea. Sleep Breath. 18, 289–295. doi: 10.1007/s11325-013-0881-9
Sundaram, S., Bridgman, S. A., Lim, J., and Lasserson, T. J. (2005). Surgery for obstructive sleep apnoea. Cochr. Database Syst. Rev. CD001004. doi: 10.1002/14651858.CD001004.pub2
Tukey, J. W. (1949). Comparing individual means in the analysis of variance. Biometrics 5, 99–114. doi: 10.2307/3001913
Vennelle, M., White, S., Riha, R. L., Mackay, T. W., Engleman, H. M., Douglas, N. J., et al. (2010). Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep 33, 267–271. doi: 10.1093/sleep/33.2.267
White, T., Nelson, M., and Lim, K. O. (2008). Diffusion tensor imaging in psychiatric disorders. Top. Magn. Reson. Imaging 19, 97–109. doi: 10.1097/RMR.0b013e3181809f1e
Won, C. H., Li, K. K., and Guilleminault, C. (2008). Surgical treatment of obstructive sleep apnea: upper airway and maxillomandibular surgery. Proc. Am. Thorac. Soc. 5, 193–199. doi: 10.1513/pats.200708-121MG
Yan, L., Park, H. R., Kezirian, E. J., Yook, S., Kim, J. H., Joo, E. Y., et al. (2021). Altered regional cerebral blood flow in obstructive sleep apnea is associated with sleep fragmentation and oxygen desaturation. J. Cereb. Blood Flow Metab. 41, 2712–2724. doi: 10.1177/0271678X211012109
Zaghi, S., Holty, J.-E. C., Certal, V., Abdullatif, J., Guilleminault, C., Powell, N. B., et al. (2016). Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis. JAMA Otolaryngol. Head Neck Surg. 142, 58–66. doi: 10.1001/jamaoto.2015.2678
Keywords: obstructive sleep apnea (OSA), upper airway surgery in OSA, cognitive function, white matter (WM), network, diffusion tensor imaging (DTI)
Citation: Chai Y, Park HR, Jo H, Seo MY, Kim HY, Joo EY and Kim H (2023) White matter microstructure and connectivity changes after surgery in male adults with obstructive sleep apnea: recovery or reorganization? Front. Neurosci. 17:1221290. doi: 10.3389/fnins.2023.1221290
Received: 23 May 2023; Accepted: 06 September 2023;
Published: 28 September 2023.
Edited by:
Mark Wagshul, Albert Einstein College of Medicine, United StatesReviewed by:
Min-Hee Lee, Wayne State University, United StatesShani Stern, University of Haifa, Israel
Copyright © 2023 Chai, Park, Jo, Seo, Kim, Joo and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eun Yeon Joo, ZWpvb0Bza2t1LmVkdQ==; Hyo Yeol Kim, c2lhbWtoeUBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Yaqiong Chai
Yaqiong Chai