- 1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China
- 3Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4Clinical & Technical Solutions, Philips Healthcare, Beijing, China
Background and purpose: Executive function impairment, a slight but noticeable cognitive deficit in mild cognitive impairment (MCI) patients, is influenced by gamma-aminobutyric acid (GABA) levels. Reduced cognitive function is accompanied by thinning of the cerebral cortex, which has higher GABA levels than white matter. However, the relationships among GABA levels, cortical thickness, and executive function in MCI patients have not yet been elucidated. We investigated the relationships among GABA levels, cortical thickness, and executive function in MCI patients.
Methods: In this study, a total of 36 MCI patients and 36 sex-, age-, and education-matched healthy controls (HC) were recruited. But 33 MCI patients and 35 HC were included because of head motion or poor data quality for three MCI patients and one HC. The levels of gamma-aminobutyric acid plus relative to creatine (GABA+/Cr) and glutamate-glutamine relative to creatine (Glx/Cr) in the anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC) were measured using the Meshcher-Garwood point resolved spectroscopy (MEGA-PRESS) sequence. Metabolite ratios, cortical thickness, and executive function and their interrelationships were determined in the MCI and HC groups.
Results: Patients with MCI showed lower GABA+/Cr levels in the ACC and PCC. Combined levels of GABA+ and Glx in the ACC and GABA+ in the PCC showed good diagnostic efficacy for MCI (AUC: 0.82). But no differences in cortical thickness were found between the two groups. In the MCI group, lower GABA+/Cr level was correlated to worse performance on the digit span test backward, and the shape trail test-B. The cortical thickness was not associated with GABA+ levels and executive function in patients.
Conclusion: These results implied that decreased GABA levels in the ACC and PCC had a critical role in the early diagnosis of impaired executive function of MCI. Therefore, GABA in the ACC and PCC could be a potential diagnostic marker of the executive function decline of MCI.
1. Introduction
Mild cognitive impairment (MCI) is an intermediate state between normal aging and dementia, characterized by the manifestation of mild cognitive symptoms along with alterations in the neurobiological function that may eventually result in neurodegeneration. The worldwide prevalence of MCI was over 15% among community dwellers aged 50 years and older (Albert et al., 2011; Bai et al., 2022). Epidemiological studies from China showed that the overall prevalence of dementia was 6.0%, and the MCI was 15.5% in adults over 60 years (Jia et al., 2020). In a previous study, MCI progressed to dementia in one-third of the people observed for 3–5 years (Manly et al., 2008). In order to offer appropriate support and prevent the quality of life from declining for MCI patients, diagnosis and intervention at the early stage are necessary.
MCI patients are commonly evaluated using neuropsychological scales, cerebrospinal fluid (CSF) biomarkers, and imaging methods, such as positron emission tomography (PET) and magnetic resonance imaging (MRI) (Ottoy et al., 2019; Lauretani et al., 2020; Santangelo et al., 2020). The evaluation of MCI patients using neuropsychological scales is crucial, as it involves assessing overall cognitive function and specific cognitive domains. Among these, executive function impairment leads to a slight but noticeable cognitive deficit in MCI besides memory impairment (Kirova et al., 2015; Junquera et al., 2020). The CSF and positron emission tomography (PET) biomarkers are considered critical not only for the diagnosis of MCI but also for the prediction of possible dementia progression (Albert et al., 2011). Research demonstrated that MCI patients exhibited decreased levels of Aβ1–42 in CSF, while PET showed reduced cerebral metabolism on [18F] fludeoxyglucose (FDG) PET and increased amyloid deposition on amyloid PET (Giau et al., 2019; Spallazzi et al., 2019). A study demonstrated that the CSF p-tau/Aβ (42) ratio and brain FDG-PET may reliably detect MCI “imminent” converters to AD (Santangelo et al., 2020). In addition, several MRI imaging studies found volumetric atrophy in MCI, such as the hippocampus, medial temporal lobe, etc. (Lehmann et al., 2013; Mak et al., 2017). However, the frequent clinical use of CSF and PET is limited due to the invasiveness of the procedures, cost, and radiation exposure. The MRI volume atrophy measurement may be insensitive to some MCI patients (Delli Pizzi et al., 2019). Therefore, it is necessary to explore the other markers for MCI patients.
The levels of neurotransmitters and the cortical thickness can reflect cognitive function. Thinning of the cortex in specific brain regions is associated with cognitive dysfunction dominated by that brain region (Ren et al., 2022). Gamma (γ)-aminobutyric acid (GABA) and glutamate (Glu) are important inhibitory and excitatory neurotransmitters in the central nervous system (CNS) and their abnormal levels can contribute to cognitive impairment (Bi et al., 2020). The known pathophysiology of MCI/AD includes amyloid β plaques (Aβ) deposition on several brain regions, including the anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC) (Small et al., 2006; Wu et al., 2021) leading to cognitive deficits and neuropsychiatric symptoms (Hirao et al., 2015). However, inflammation, another pathogenesis in MCI/AD, may contribute to the disruption of GABA and Glu levels (Bradburn et al., 2019; Bi et al., 2020; Sood et al., 2021). Previous studies demonstrated that impaired GABAergic inhibitory networks (Long and Holtzman, 2019) and glutamate toxicity (Zott and Konnerth, 2023) played a role in the pathogenesis of AD. Thus, besides cortical thickness, the levels of these two neurotransmitters might identify MCI and subsequent cognitive progression. Furthermore, the stability of executive function is influenced by GABAergic processes (Hatoum et al., 2023). Brain regions relevant to executive function included the prefrontal cortex [mainly dorsolateral prefrontal cortex (DLPFC)] and the ACC, where the changes in GABA levels determined using magnetic resonance spectroscopy (MRS) (Li et al., 2022a) and cortical thinning determined using structural MRI (Makris et al., 2007) were reported. And the PCC is a crucial region of the default mode network, which was closely related to executive function (Brown et al., 2019). However, most of the existing studies have only focused on the correlation between GABA levels or cortical thickness and memory function in AD or MCI. The relationships among GABA levels, cortical thickness, and executive function in MCI have not yet been elucidated.
A molecular quantification technique, proton-MRS (1H-MRS) is available to measure the levels of endogenous brain neurochemicals (Mitolo et al., 2019). The GABA level in the brain is extremely low and the GABA level peaks by the traditional 1H-MRS overlap with other metabolites, such as N-acetyl aspartic acid (NAA), Glu, and glutamine (Gln) (Weis et al., 2021). Therefore, the Meshcher-Garwood point resolved spectroscopy (MEGA-PRESS) sequence (Mescher et al., 1998), using J-difference spectrum editing technology, was introduced to provide relatively rapid and reliable quantification of the CNS GABA concentration. But the detected signal was referred to as GABA plus (GABA+) instead of GABA, as it included contributions from both macromolecules (MM) as well as smaller homocysteine molecules. Additionally, this sequence could effectively include the glutamate-glutamine (Glx) signal due to the significant structural and chemical similarities between Glx and GABA (Harris et al., 2017). Further, 3D high-resolution T1-weighted sequence is mainly used to observe anatomical structures (Alhazmi et al., 2020), which in combination with advanced post-processing technique, such as FreeSurfer, a well-documented semi-automatic software for the cortical surface reconstruction and cortical thickness calculation (Fischl and Dale, 2000), allows for accurate determination of cortical thickness.
Combining molecular and structural imaging is a potential step for distinguishing MCI patients and evaluating their executive function impairment. The present study aimed to (i) compare the differences in GABA levels and cortical thickness in the ACC and PCC and executive function between the MCI patients and healthy older adults; (ii) assess the sensitivity and specificity for the diagnosis of MCI by utilizing combined neurometabolic content; (iii) explore the relationships among GABA levels, cortical thickness, and executive function in both groups.
2. Materials and methods
2.1. Participants
Thirty-six patients with MCI and 36 healthy controls (HC) were recruited from the neurology clinics of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, and underwent neuropsychological assessment and MRI scanning. But one participant with MCI was excluded due to motion artifacts during the scanning process, and two MCI patients and one HC were excluded based on post-processing spectral fitting criteria. Therefore, the study included 33 MCI patients and 35 HC. All participants enrolled in this study were (a) between 50 and 80 (included) years of age; (b) were right-handed; (c) had no major psychiatric disease (e.g., severe major depression, severe anxiety, bipolar disorder, and substance abuse); (d) had no active cancer; and (e) had no traumatic injuries, serious cardiovascular diseases, or hydrocephalus according to medical history and MRI images of all participants. Besides, the inclusion criteria for patients with MCI were as follows; (a) MCI reported by patients or insiders, or diagnosed by an experienced clinician; (b) Patients with cognitive dysfunction confirmed by objective tests [Montreal cognitive assessment, MoCA score of ≤24; ≤19 for ≥7; 1–6 years of education (Nasreddine et al., 2005; Lu et al., 2011), clinical dementia rating (CDR) of 0.5]; (c) Patients with preservation of independence in functional abilities; and (d) Patients who were not demented. The inclusion criteria for the HC group were as follows. Age-, sex-, and education-matched subjects to MCI group with normal cognitive function (MoCA score of ≥25; ≥20 for ≥7; 1–6 years of education; CDR of 0). Participants for both groups were excluded if they had (a) cognitive impairment due to other etiologies (Parkinson’s disease, Huntington’s disease, stroke, severe thyroid diseases, and metabolic diseases) and (b) contraindications to MRI scanning. Before the onset of the study, approval from the Institutional Ethics Committee and informed consent from each subject were obtained.
2.2. Neuropsychological assessment
All participants underwent neuropsychological tests which were conducted by an experienced neuropsychologist. The MoCA was used for global cognitive screening (Yu et al., 2012; Pinto et al., 2019), including for orientation, attention, memory, visuospatial/executive function, and naming function. Individual episodic memory performance, including immediate recall and delayed recall, was measured by the auditory verbal learning test (AVLT) (Zhao et al., 2012; Xu et al., 2020). The digit span test (Chincotta and Chincotta, 1996) consisted of a digit forward (FDS) assessment of attention and a backward (BDS) assessment of executive function. The shape trail test (STT) is a new version of the traditional trail-making test, in which attention is tested by the STT-A while the executive function is tested by the STT-B (Zhao et al., 2013). The Hamilton depression rating scale (HAMD) and the Hamilton anxiety rating scale (HAMA) were implemented to assess the emotional state. Participants were excluded if they had HAMD of ≥24 (Zimmerman et al., 2013) or HAMA of ≥29 that were considered severe depression and anxiety.
2.3. Neuroimaging
All subjects were scanned on a 3.0 T scanner (Philips Achieva TX; Philips, Best, Netherlands) using a 32-channel phased-array head coil. 3D high-resolution T1-weighted images were acquired using a magnetization-prepared turbo field echo sequence using the following parameters, repetition time/echo time (TR/TE) of 5.8/2.7 ms, flip angle of 8°, the field of view of 220× 220 × 180 mm3, slice thickness of 1 mm, and in-plane resolution of 1 × 1 mm2.
Concentrations of GABA+, Glx to creatine (Cr) were obtained using the MEGA-PRESS sequence. A frequency-selective, refocusing Gauss pulse resonating at 1.89 ppm (the ON spectrum) and 7.46 ppm (the OFF spectrum) during odd and even-numbered acquisitions were applied, respectively. The brain volume of interest (VOI) was in the ACC (volume size = 30 × 30 × 20 mm3) and the PCC (volume size = 30 × 30 × 20 mm3) (Figures 1A,B) based on 3D high-resolution T1-weighted images, avoiding the lateral ventricle and skull. The median sagittal plane was selected as the reference plane for voxel localization. The voxels of the ACC and PCC were, respectively, positioned superior to the genu of the corpus callosum and posterior and superior to the splenium of the corpus callosum, both of which were aligned with the shape of the corpus callosum and positioned medial to the axial plane. The parameters of this sequence were as follows, TR/TE of 2000/68 ms; averages of 160 (consisting of 80 ON and 80 OFF resonance pulses); spectral bandwidth of 2000 Hz; acquisition time of 5:40 min per voxel (total scanning time of 11:20 min). Water suppression was conducted using the variable power and optimized relaxations delays (VAPOR) method. The checklist for MRS protocol and data quality control in Supplementary Table S1.
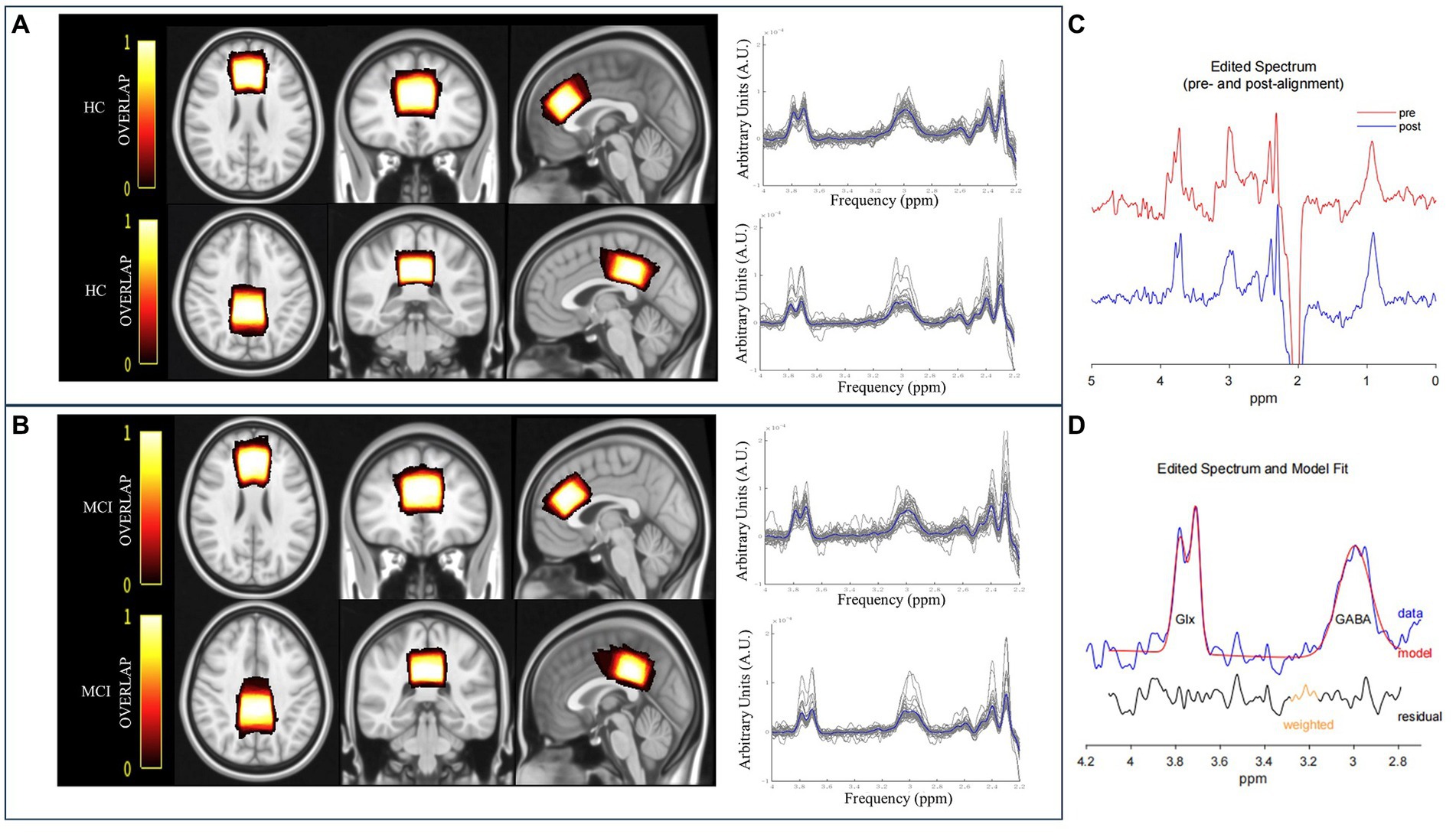
Figure 1. The overlay of the MRS voxel positions and raw spectra [individual (grey) and group mean (blue)] for HC (A) and MCI patients (B). The color bar indicates the overlap of the individual voxels, with bright indicating a high overlap and dark color a low overlap. (C) GannetLoad output of the edited spectra. The red line indicates preprocessed spectrum before frequency and phase correction, and the blue line denotes the post-processed spectrum after frequency and phase correction. (D) GannetFit output of fitted GABA+ and Glx signals. The blue line represents the experimental data, the red line denotes the fit model, and the black line indicates the residual between the experimental data and the curve-fitted data.
2.4. Data postprocessing
2.4.1. MRS processing
The quantification of metabolite signals was assessed using a MATLAB-based GABA analysis toolkit, Gannet3.1.1 Subsequently, GABA+ and Glx signals were obtained at 3.02 ppm and 3.74 ppm, from the subtracted spectrum image of (ON–OFF) because the majority of the signals in the spectra were undisturbed by the editing pulse (Mullins et al., 2014) (Figures 1C,D). Meanwhile, individual and group mean of raw spectra were shown in Figures 1A,B. Metabolite ratios that were investigated were primarily referenced to Cr, obtained at 3.0 ppm from the OFF spectrum because it was more stable and robust than water referencing (Valenzuela and Sachdev, 2001; Bogner et al., 2010; Graff-Radford and Kantarci, 2013). The signal detected by the MEGA-PRESS at 3.02 ppm was a combination of contributions from GABA, macromolecules, and homocarnosine. Therefore, it was referred to as “GABA+” instead of GABA.
Head motion was checked when artifacts were present in the spectra, such as line splitting, or if there was signal from outside the organ of interest (e.g., subcutaneous lipid signals for brain MRS) (Andronesi et al., 2021). And we used (a) head immobilization; (b) prospective correction; and (c) retrospective correction to correct head motion during MRS acquisition for data analysis. The specific methods were included in Supplementary Table S1.
Data preprocessing included the automated frequency and phase correction, artifact rejection based on frequency deviation (>3 SD from the mean), and 3 Hz exponential line broadening. Metabolite concentrations were estimated by fitting Gaussian curves to the 3.02 ppm (GABA+) and double Gaussian curves to the 3.74 ppm (Glx) peaks and scaling them relative to creatine. Concentrations for GABA+ and Glx were reported in institutional units (i.u.). We kept the full width at half maximum (FWHM) value smaller than 20 Hz in order to control the quality of the MRS data. Meanwhile, individual metabolite ratios with a relative FitError above 15% were excluded (Liu et al., 2020).
2.4.2. Volumes of interest position and segmentation
The overlapping individual spectra and an average voxel localization for both voxels, after transformation to Montreal Neurological Institute (MNI) space FSL software (FMRIB SOFTWARE LIBRARY, Oxford UK, FSL version 6.0) were shown in Figures 1A,B. Bright represented greater overlap between participants, and dark represented less overlap.
The group differences in metabolite levels were modulated by the proportions of white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF) in volumes of interest (VOIs). Therefore, preprocessing of 3D high-resolution T1-weighted images format conversion was first performed (from DICOM to NIfTI using MRIcroGL, https://www.nitrc.org/projects/mricrogl/), after which the SPM12 was applied to measure the GM, WM, and CSF volumes within MRS voxels (Figure 2).2 The GM, WM, and CSF fractions were obtained, and the ratio of GM/ (GM + WM) of each voxel was calculated.
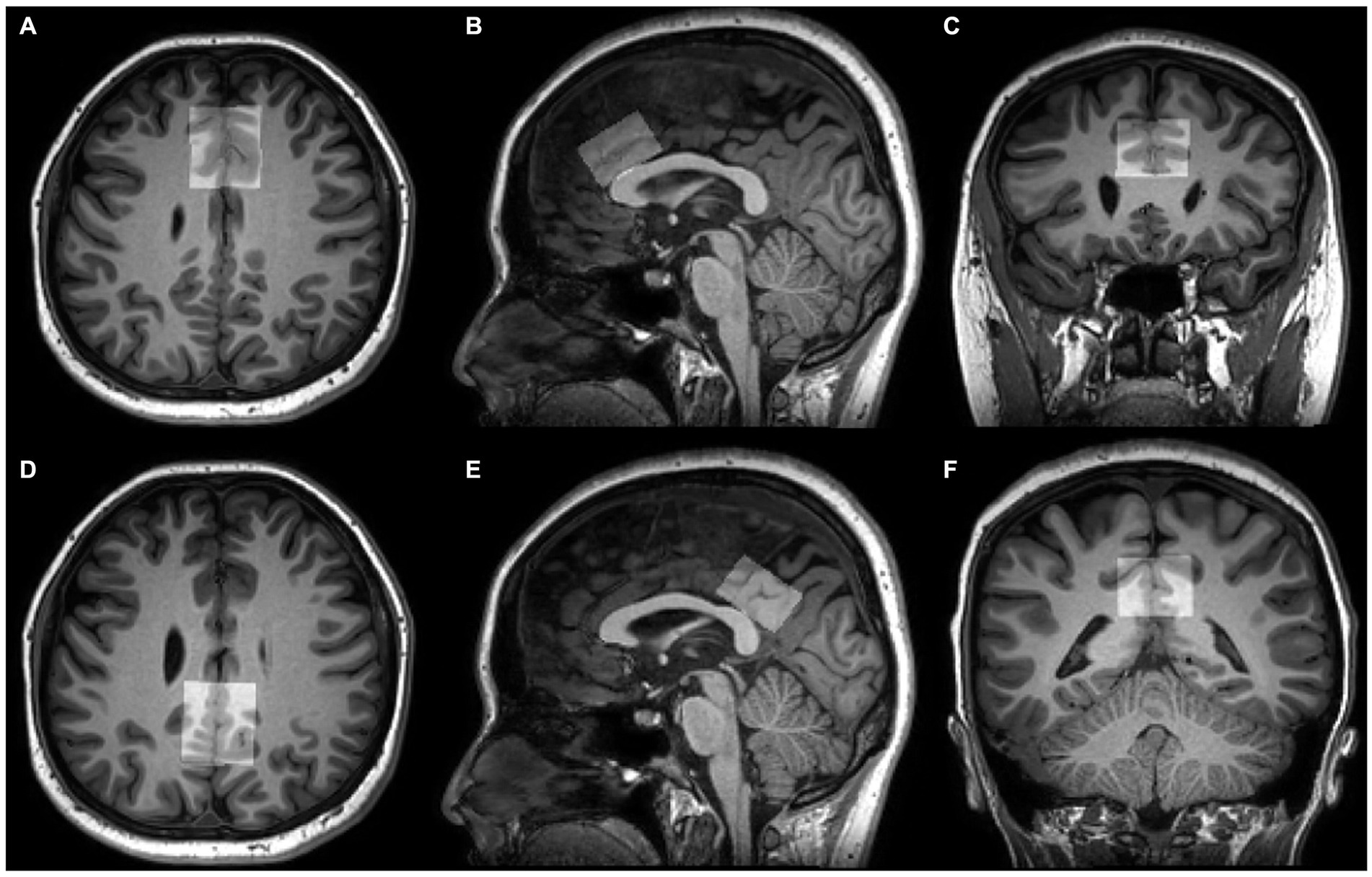
Figure 2. Voxel positions of tissue segmentation using SPM12. 3D T1-weighted images show the position and size of VOI in the ACC (A–C) and PCC (D–F) on axial, sagittal, and coronal planes.
2.4.3. Cortical thickness measurement
All 3D high-resolution T1-weighted images were automatically segmented using FreeSurfer version 7.2.0.3 All subjects were processed using the command recon-all default parameters. The measurement process involved several steps, including transformation to Talairach and MNI coordinate systems, removal of non-brain tissue, and segmentation of subcortical white and deep grey matter (Fischl et al., 2002; Ségonne et al., 2004). After intense normalization, the boundary between white and gray matter was separated and the topological defects were automatically corrected (Fischl et al., 2001). The grey/white matter pial boundary was identified as the greatest intense shift (Fischl et al., 2001), and any segmentation inaccuracies were manually edited. The resulting surface models were registered in the Bainnetome Atlas,4 and the cortical thickness was determined by averaging the shortest distance between a vertex on the pial surface and the grey/white matter boundary (Clarkson et al., 2011). In the present study, the cortex thickness of the A32p_l and A23d_l was extracted as the left ACC and left PCC thickness, similarly, A32p_r and A23d_r as the right ACC and right PCC thickness in all participants (Figure 3).
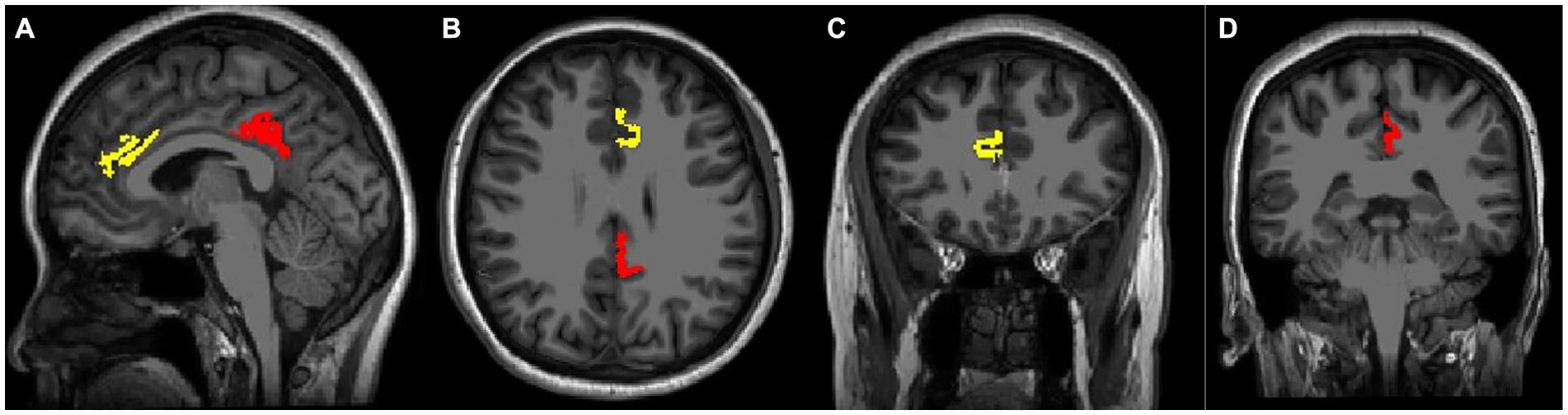
Figure 3. Voxel positions using Bainnetome Atlas. 3D T1-weighted images show the position of left cortical thickness in sagittal (A), axial (B), and coronal images (C,D). The yellow mark represents the ACC thickness, the red color indicates the PCC thickness.
2.5. Statistical analysis
All statistical analyses were performed using SPSS software (version 25.0; SPSS Inc., Chicago, IL, United States). The normal distributions of demographic and neurocognitive scores, spectral data, segmentation results, and cortical thickness were verified by the Shapiro–Wilk test. Differences between the groups were assessed by the Mann–Whitney U test and independent two-sample t-test. A χ2 test was used to analyze the count data. A general linear model was applied to determine the group differences in metabolite ratios, cortical thicknesses, and neurocognitive scores, with age and gender, and education in years as covariates. The paired test was used to compare the distribution of metabolite levels of the ACC and PCC, as well as cortical thickness of different hemispheres within groups. Partial correlation analyses were performed to detect correlations of metabolites, cortical thickness in regions of interest, and executive function, adjusting for age, gender, and education in years. The receiver operating characteristic (ROC) analysis was used to evaluate the diagnostic efficacy of MCI using combined neurometabolic content, with significant difference of metabolite between groups as independent variables, age, gender, and education as covariates. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Demographic and neuropsychological characteristics of participants
Demographic data and neurocognitive results of subjects are summarized in Table 1. No significant differences in age (p = 0.153), gender (p = 0.959), education (p = 0.139), HAMA (p = 0.179), and HAMD (p = 0.407) were found between groups. MoCA score in the MCI group was significantly lower than in the HC group (p < 0.001). MCI patients performed worse on AVLT (including AVLT-immediate recall and AVLT-delayed recall; all p-values were <0.001), FDS (p = 0.007), BDS (p = 0.038), STT-A (p = 0.005), and STT-B (p<0.001), relative to the HC.
3.2. Comparisons of metabolite concentrations
Metabolite data for all participants are shown in Table 2 and Supplementary Figure S1. After adjusting for age, gender, and education, significant group differences were found in the levels of GABA+/Cr in the VOIs. Compared to the HC group, patients with MCI had a significant decrease in GABA+/Cr ratios in the ACC (p = 0.001) and PCC (p = 0.009), and Glx/Cr ratios in the ACC (p = 0.046). However, no significant group difference was found in Glx/Cr levels in the PCC (p = 0.542). The GABA+/Cr levels in the ACC were significantly lower compared to the PCC in each group (MCI, t = −7.69, p<0.001; HC, t = −7.92, p<0.001), but no significant difference in Glx/Cr levels.
3.3. The VOIs segmental results and MRS quality
The average GM tissue fraction GM/(GM + WM) was 55.67% and 59.73% in the ACC and PCC, respectively, in MCI patients, while in HC, it was 55.57% and 59.51%, respectively (Table 2). No significant differences in GM/(GM + WM) in the VOIs within groups (ACC, p = 0.936; PCC, p = 0.811). The fitting errors of metabolites and the FWHM in both VOIs did not differ between patients and HC (all p-values were > 0.05) (Table 2).
3.4. Accuracy of metabolite concentrations in diagnosing MCI
The largest area under the ROC curve for the combined content of GABA+ and Glx in the ACC and GABA+ in the PCC was 0.82 (95% confidence interval, 0.72–0.92, and with 78.8% sensitivity and 74.3% specificity), with age, gender, and education as covariates (Figure 4).
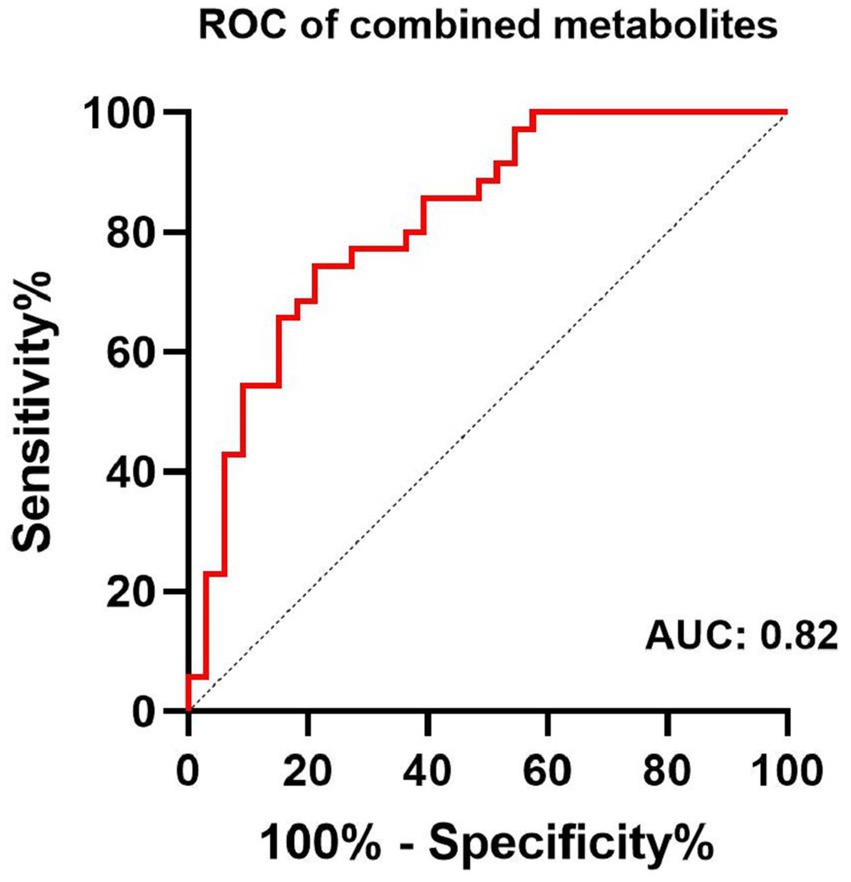
Figure 4. Receiver-operator-characteristic (ROC) analysis of combined content of GABA+ and Glx in the ACC, and GABA+ in the PCC for the diagnosis of MCI.
3.5. Comparison of cortical thickness
No significant differences in bilateral ACC and PCC thickness were found between groups (all p-value were >0.05) (Supplementary Table S2, Supplementary Figure S2). The right ACC thickness was thicker than left ACC thickness within groups (MCI, t = −2.90, p<0.01; HC, t = −5.40, p<0.001), while no significant difference was observed in the PCC.
3.6. Correlations among neuro metabolites, cortical thickness, and executive function
3.6.1. Correlation between neuro metabolites and executive function
Correlations between GABA+ in both VOIs and executive function are summarized in Figure 5. In the MCI group, significant positive correlations between GABA+/Cr and BDS (ACC, r = 0.439, p = 0.015; PCC, r = 0.483, p = 0.007), and negative correlations between GABA+/Cr and STT-B (ACC, r = −0.527, p = 0.003; PCC, r = −0.462, p = 0.010), adjusting for age, gender, and education, were found. The relationship between GABA+/Cr ratios in the two regions and executive function did not reach statistical significance (all p values were > 0.05) in the HC group. Meanwhile, we found no relationships between Glx/Cr ratios in the VOIs and executive function within both groups (all p values were > 0.05).
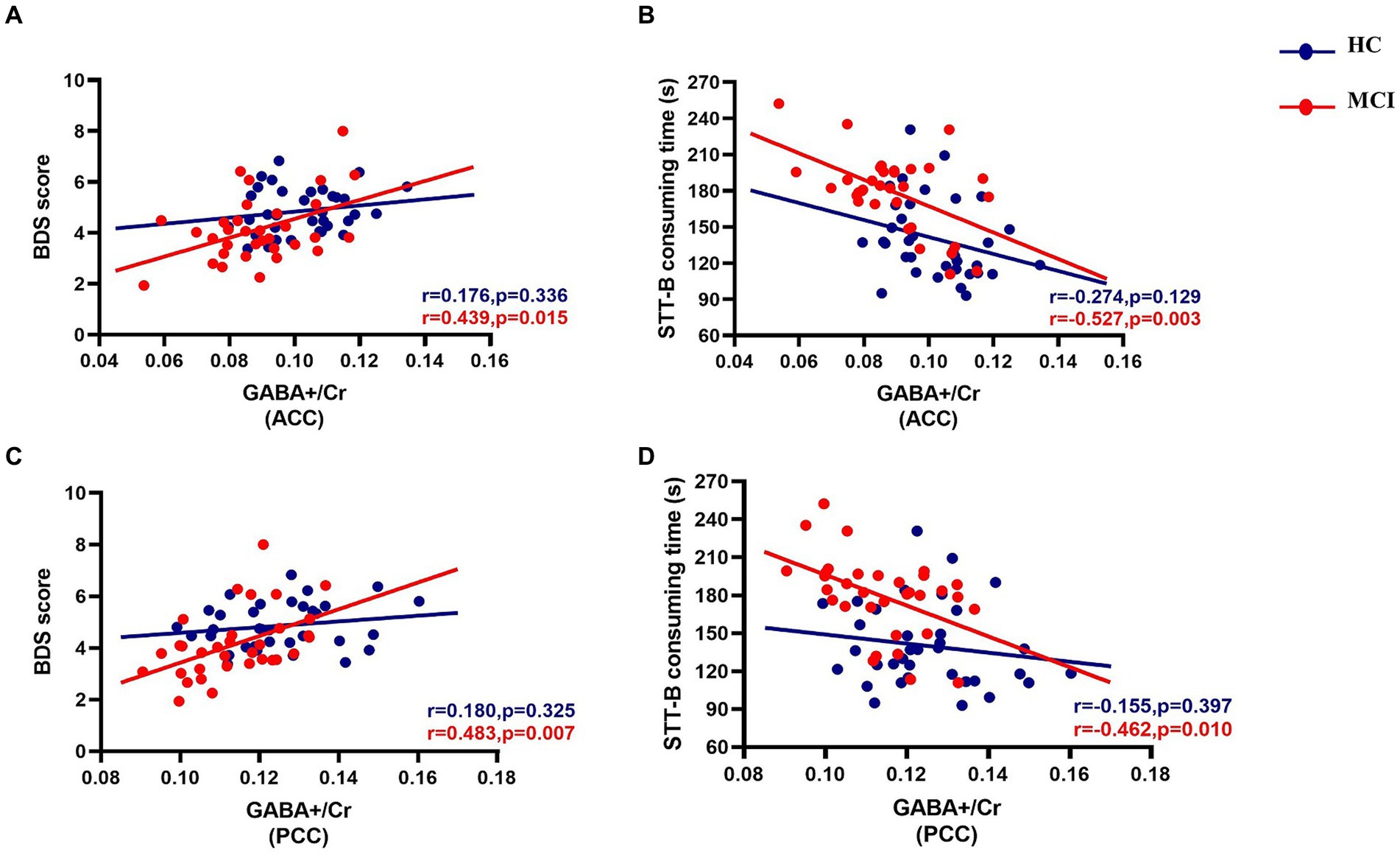
Figure 5. Partial correlations between GABA+/Cr levels of the ACC and PCC and executive function in both groups, adjusting for age, gender, and education (in years). The MCI group show statistically significant positive correlations between GABA+/Cr and BDS [ACC, r = 0.439, p = 0.015 (A); PCC, r = 0.483, p = 0.007 (C)] and negative correlations between GABA+/Cr and STT-B [ACC, r = −0.527, p = 0.003 (B); PCC, r = −0.462, p = 0.010 (D)] consuming time.
3.6.2. Correlation between cortical thickness and executive function
In the MCI group, no correlations were found between cortical thickness in VOIs and executive function, with age, gender, and education as covariates (all p values were > 0.05) (Supplementary Table S3). However, in the HC group, no correlation was found between cortical thickness and executive function, except for a positive correlation between the right PCC thickness and STT-B (r = 0.369, p = 0.038) (Supplementary Table S4).
3.6.3. Correlation between neuro metabolites and cortical thickness
Within the ACC and PCC, the GABA+ and Glx levels were not significantly related to cortical thickness in the MCI patients, adjusting for age, gender, and education (all p-values were > 0.05) (Supplementary Tables S5, S6). In the HC group, only the Glx content of the ACC was positively correlated with the thickness of the right ACC (r = 0.371, p = 0.036) (Supplementary Tables S7, S8).
4. Discussion
This study found decreased GABA+ levels in the ACC and PCC and decreased Glx levels in the ACC in the MCI group. Combined levels of GABA+ and Glx in the ACC and GABA+ in the PCC showed good diagnostic efficacy for MCI (AUC: 0.82). Further, executive function was positively related to the GABA+ concentrations in the ACC and PCC within MCI patients. In contrast, cortical thickness could not distinguish MCI and healthy adults, and it was not related to the GABA level as well as executive function. Therefore, decreased GABA levels in the ACC and PCC had an important role in the early recognition of impaired executive function in the MCI patients, but not the cortical thickness.
GABA+ and Glx levels in the ACC were decreased in patients with MCI. MCI is the transition state from normal cognition to dementia, characterized by single or multiple cognitive dysfunctions but basic activities of daily living being preserved (Lissek and Suchan, 2021). About one-third of MCI people progress into irreversible dementia within 3–5 years (Manly et al., 2008), therefore, early and timely intervention at this stage provides significant benefits. An imbalance of neurotransmitters (such as GABA and Glu or Gln) in the brain of MCI or dementia patients exists and this may be due to inflammatory responses involving astrocytes. For example, neuroimaging studies found decreased GABA, Glx or Glu levels in the MCI (Riese et al., 2015; Oeltzschner et al., 2019) and AD patients (Lanctôt et al., 2004; Bai et al., 2015), although the affected brain regions were different. This study found that decreased GABA+ levels in the ACC and PCC and Glx levels in the ACC in the MCI group compared to the HC group were consistent with the abovementioned studies. Contrastingly, another study (Huang et al., 2017) found no significant difference in the GABA+/Cr and Glx/Cr ratios of ACC and right hippocampus between the MCI group and the elderly normal, which was different from our results. The discrepancy may have been due to population heterogeneity, differences in location, and the size of selected brain regions.
The decreased GABA levels may also have been due to GABAergic neuronal loss or/and dysfunction because the single photon emission computed tomography (SPECT) demonstrated reduced GABAA/benzodiazepine (BZD) receptor density in the parietal lobe of patients with MCI (Pappata et al., 2010). Additionally, the excitatory and inhibitory interneurons use Gln, the precursor of Glu synthesized in astrocytes, to synthesize Glu and GABA, and the released neurotransmitters are cleared by astrocytes (Czapski and Strosznajder, 2021; Sood et al., 2021; Kilb and Kirischuk, 2022). Therefore, the decline in GABA+ and Glx found in this study could be due to loss of interneurons, decreased GABA/Glu synthesis, or altered astroglial cycling of GABA, Glu, and Gln. Interestingly, we found that the GABA levels in the ACC were lower compared to the PCC within groups. But this observation required further validation through large-scale studies. Meanwhile, the diagnostic efficacy of combining GABA+ and Glx levels in the ACC and GABA+ levels in the PCC was excellent, suggesting the potential role of neuro metabolites in diagnosing MCI.
Further, executive function was correlated to the GABA+ levels in the ACC and PCC in MCI patients. The cingulate cortex is an essential component of the default mode network, and the PCC performs a complex exchange of information with the ACC to control cognitive function and emotion (Bubb et al., 2018; Aponik-Gremillion et al., 2022). The executive function represents a series of advanced cognitive control abilities necessary for achieving goals (Li et al., 2022a). Its impairment is a small but noticeable cognitive deficit in MCI besides impaired memory. Most of the existing imaging studies on executive impairment focused on the prefrontal/cerebellar cortex or white matter (Friedman and Robbins, 2022; Li et al., 2022b; Gustavson et al., 2023), and there are a few studies on the correlation between neuro metabolites and executive function in the ACC and PCC. The relationship between the execution and GABA, which has been discussed previously, was controversial (Marenco et al., 2018; Oeltzschner et al., 2019). A genome-wide association study found that executive function was influenced by GABAergic processes (Hatoum et al., 2023). In another research (Marenco et al., 2018) with middle-aged healthy volunteers, higher GABA levels in dorsal ACC were associated with better executive function measured by the Wisconsin card sorting test. Our results were consistent with the abovementioned studies. A positive correlation between execution and GABA was not only found in the ACC but also in the PCC. However, a previous study (Gozdas et al., 2022) examined the association between proton MRS metabolites in the DLPFC and cognitive function in amnestic mild cognitive impairment patients (aMCI). They identified that Glx/total Cr (tCr) levels, rather than GABA/tCr, were significantly associated with executive function in patients with aMCI. In previous research (Oeltzschner et al., 2019), the GABA levels of the ACC and PCC in the aMCI group were not related to the executive function measured by the symbol digit modalities test (SDMT). Different methods of assessing executive function, brain regions, and sample size might have contributed to the heterogeneity of outcomes.
In this study, brain atrophy was explored using the MRI modality. A study (Zhu et al., 2021) showed the left ACC and left PCC in MCI patients with moderate or severe WMH became thinning. They also found the cortical thickness of the middle frontal gyrus mediated the association between WMH presence and worse processing speed and executive function. We found no significant thinning of the ACC and PCC between groups and no correlation between executive function and cortical thickness, although the right ACC thickness was thicker than the left ACC thickness within groups. These observations could be attributed to the absence of WMH in MCI patients of our study. These differences also indicated that the brain atrophy pattern probably differed between MCI with and without WMH. There was an initial observation of atrophy occurring in the medial temporal lobe, including the entorhinal cortex and hippocampus in MCI and AD patients (Chandra et al., 2019). Since this study only measured the ACC and PCC, other brain areas may be needed to be explored in the future. For the first time, we also did not observe any correlation between cortical thickness and GABA+ or Glx levels in the ACC and PCC. Consequently, measuring neurometabolites might offer additive value in reflecting the executive impairment of MCI patients, rather than the cortical thickness of the ACC and PCC.
In light of the notable advancements in the outcomes, this study had several limitations. First, the non-significant findings in this study might have resulted from the small sample size. Second, whether GABA level changed with the MCI progression was unclear. These two limitations could be addressed by conducting longitudinal studies with larger sample sizes. Additionally, the measured metabolites did not represent the pure GABA and Glu content because GABA+ was the mixture of GABA, macromolecular, and homocarnosine, and Glx was the complex of Glu and Gln, which might have introduced bias into the results. The isolated changes in homocarnosine are unlikely to be the main driver of the observed changes, as the concentration of homocarnosine in vivo is considerably lower than GABA (Govindaraju et al., 2000). Therefore, new methods for macromolecule suppression of GABA-edited MRS (Chan et al., 2021), detecting “pure GABA,” should be used in future studies of MCI. Finally, the inclusion of additional regions is important in future studies, because the single-voxel MRS was not able to capture the relationships among GABA levels, cortical thickness, and executive function in other brain regions in this study.
5. Conclusion
The associations among GABA levels, cortical thickness, and executive function were investigated in MCI patients. Lower levels of GABA in the ACC and PCC were significantly associated with worse executive function in MCI patients. The study also demonstrated that GABA levels were more sensitive than structural changes in the ACC and PCC as indicators of executive function decline in MCI patients. These results suggested that GABA levels in the ACC and PCC could serve as potential diagnostic markers of executive function impairment in MCI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XF: data curation, data analysis and interpretation, methodology, and writing original draft. MQ: conceptualization, writing original draft, writing-review, and editing. XL, LC, LZ, and XZ: data analysis and interpretation. YL and QZ: data curation. PS and LL: software, methodology, and statistical analysis. YS: supervision, study design, project administration, and funding acquisition. JW: supervision, study design, project administration, writing-review and editing, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Ministry of Science and Technology of the People’s Republic of China (STI2030-Major Projects 2021ZD0201900) and the National Science Foundation of Hubei Province (2021CFB447).
Acknowledgments
The authors thank all participants who volunteered for this study as well as the researchers who participated in the data collection for this project. Further, we express our gratitude to Jiazheng Wang (Philips Healthcare), Xingjie Hao, and Jing Wu for their helpful suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1220122/full#supplementary-material
Footnotes
1. ^https://github.com/richardedden/Gannet3.1
2. ^http://www.fil.ion.ucl.ac.uk/spm/software/spm12/
References
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 270–279. doi: 10.1016/j.jalz.2011.03.008
Alhazmi, F. H., Abdulaal, O. M., Qurashi, A. A., Aloufi, K. M., and Sluming, V. (2020). The effect of the MR pulse sequence on the regional corpus callosum morphometry. Insights Imaging 11:17. doi: 10.1186/s13244-019-0821-8
Andronesi, O. C., Bhattacharyya, P. K., Bogner, W., Choi, I. Y., Hess, A. T., Lee, P., et al. (2021). Motion correction methods for MRS: experts' consensus recommendations. NMR Biomed. 34:e4364. doi: 10.1002/nbm.4364
Aponik-Gremillion, L., Chen, Y. Y., Bartoli, E., Koslov, S. R., Rey, H. G., Weiner, K. S., et al. (2022). Distinct population and single-neuron selectivity for executive and episodic processing in human dorsal posterior cingulate. elife 11:e80722. doi: 10.7554/eLife.80722
Bai, W., Chen, P., Cai, H., Zhang, Q., Su, Z., Cheung, T., et al. (2022). Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: a meta-analysis and systematic review of epidemiology studies. Age Ageing 51:afac173. doi: 10.1093/ageing/afac173
Bai, X., Edden, R. A., Gao, F., Wang, G., Wu, L., Zhao, B., et al. (2015). Decreased gamma-aminobutyric acid levels in the parietal region of patients with Alzheimer's disease. J. Magn. Reson. Imaging 41, 1326–1331. doi: 10.1002/jmri.24665
Bi, D., Wen, L., Wu, Z., and Shen, Y. (2020). GABAergic dysfunction in excitatory and inhibitory (E/I) imbalance drives the pathogenesis of Alzheimer's disease. Alzheimers Dement. 16, 1312–1329. doi: 10.1002/alz.12088
Bogner, W., Gruber, S., Doelken, M., Stadlbauer, A., Ganslandt, O., Boettcher, U., et al. (2010). In vivo quantification of intracerebral GABA by single-voxel (1)H-MRS-how reproducible are the results? Eur. J. Radiol. 73, 526–531. doi: 10.1016/j.ejrad.2009.01.014
Bradburn, S., Murgatroyd, C., and Ray, N. (2019). Neuroinflammation in mild cognitive impairment and Alzheimer's disease: a meta-analysis. Ageing Res. Rev. 50, 1–8. doi: 10.1016/j.arr.2019.01.002
Brown, C. A., Schmitt, F. A., Smith, C. D., and Gold, B. T. (2019). Distinct patterns of default mode and executive control network circuitry contribute to present and future executive function in older adults. NeuroImage 195, 320–332. doi: 10.1016/j.neuroimage.2019.03.073
Bubb, E. J., Metzler-Baddeley, C., and Aggleton, J. P. (2018). The cingulum bundle: anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 92, 104–127. doi: 10.1016/j.neubiorev.2018.05.008
Chan, K. L., Hock, A., Edden, R. A. E., MacMillan, E. L., and Henning, A. (2021). Improved prospective frequency correction for macromolecule-suppressed GABA editing with metabolite cycling at 3T. Magn. Reson. Med. 86, 2945–2956. doi: 10.1002/mrm.28950
Chandra, A., Dervenoulas, G., and Politis, M. (2019). Magnetic resonance imaging in Alzheimer's disease and mild cognitive impairment. J. Neurol. 266, 1293–1302. doi: 10.1007/s00415-018-9016-3
Chincotta, M., and Chincotta, D. (1996). Digit span, articulatory suppression, and the deaf: a study of the Hong Kong Chinese. Am. Ann. Deaf 141, 252–257. doi: 10.1353/aad.2012.0289
Clarkson, M. J., Cardoso, M. J., Ridgway, G. R., Modat, M., Leung, K. K., Rohrer, J. D., et al. (2011). A comparison of voxel and surface based cortical thickness estimation methods. NeuroImage 57, 856–865. doi: 10.1016/j.neuroimage.2011.05.053
Czapski, G. A., and Strosznajder, J. B. (2021). Glutamate and GABA in microglia-neuron cross-talk in Alzheimer's disease. Int. J. Mol. Sci. 22:11677. doi: 10.3390/ijms222111677
Delli Pizzi, S., Punzi, M., and Sensi, S. L. (2019). Functional signature of conversion of patients with mild cognitive impairment. Neurobiol. Aging 74, 21–37. doi: 10.1016/j.neurobiolaging.2018.10.004
Fischl, B., and Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 97, 11050–11055. doi: 10.1073/pnas.200033797
Fischl, B., Liu, A., and Dale, A. M. (2001). Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging 20, 70–80. doi: 10.1109/42.906426
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. doi: 10.1016/s0896-6273(02)00569-x
Friedman, N. P., and Robbins, T. W. (2022). The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 47, 72–89. doi: 10.1038/s41386-021-01132-0
Giau, V. V., Bagyinszky, E., and An, S. S. A. (2019). Potential fluid biomarkers for the diagnosis of mild cognitive impairment. Int. J. Mol. Sci. 20:4149. doi: 10.3390/ijms20174149
Govindaraju, V., Young, K., and Maudsley, A. A. (2000). Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 13, 129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v
Gozdas, E., Hinkley, L., Fingerhut, H., Dacorro, L., Gu, M., Sacchet, M., et al. (2022). (1)H-MRS neurometabolites and associations with neurite microstructures and cognitive functions in amnestic mild cognitive impairment. Neuroimage Clin. 36:103159. doi: 10.1016/j.nicl.2022.103159
Graff-Radford, J., and Kantarci, K. (2013). Magnetic resonance spectroscopy in Alzheimer's disease. Neuropsychiatr. Dis. Treat. 9, 687–696. doi: 10.2147/NDT.S35440
Gustavson, D. E., Archer, D. B., Elman, J. A., Puckett, O. K., Fennema-Notestine, C., Panizzon, M. S., et al. (2023). Associations among executive function abilities, free water, and white matter microstructure in early old age. Neuroimage Clin. 37:103279. doi: 10.1016/j.nicl.2022.103279
Harris, A. D., Saleh, M. G., and Edden, R. A. (2017). Edited (1) H magnetic resonance spectroscopy in vivo: methods and metabolites. Magn. Reson. Med. 77, 1377–1389. doi: 10.1002/mrm.26619
Hatoum, A. S., Morrison, C. L., Mitchell, E. C., Lam, M., Benca-Bachman, C. E., Reineberg, A. E., et al. (2023). Genome-wide association study shows that executive functioning is influenced by GABAergic processes and is a neurocognitive genetic correlate of psychiatric disorders. Biol. Psychiatry 93, 59–70. doi: 10.1016/j.biopsych.2022.06.034
Hirao, K., Pontone, G. M., and Smith, G. S. (2015). Molecular imaging of neuropsychiatric symptoms in Alzheimer's and Parkinson's disease. Neurosci. Biobehav. Rev. 49, 157–170. doi: 10.1016/j.neubiorev.2014.11.010
Huang, D., Liu, D., Yin, J., Qian, T., Shrestha, S., and Ni, H. (2017). Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur. Radiol. 27, 2698–2705. doi: 10.1007/s00330-016-4669-8
Jia, L., Du, Y., Chu, L., Zhang, Z., Li, F., Lyu, D., et al. (2020). Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health 5, e661–e671. doi: 10.1016/s2468-2667(20)30185-7
Junquera, A., García-Zamora, E., Olazarán, J., Parra, M. A., and Fernández-Guinea, S. (2020). Role of executive functions in the conversion from mild cognitive impairment to dementia. J. Alzheimers Dis. 77, 641–653. doi: 10.3233/jad-200586
Kilb, W., and Kirischuk, S. (2022). GABA release from astrocytes in health and disease. Int. J. Mol. Sci. 23:15859. doi: 10.3390/ijms232415859
Kirova, A. M., Bays, R. B., and Lagalwar, S. (2015). Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer's disease. Biomed. Res. Int. 2015:748212. doi: 10.1155/2015/748212
Lanctôt, K. L., Herrmann, N., Mazzotta, P., Khan, L. R., and Ingber, N. (2004). GABAergic function in Alzheimer's disease: evidence for dysfunction and potential as a therapeutic target for the treatment of behavioural and psychological symptoms of dementia. Can. J. Psychiatr. 49, 439–453. doi: 10.1177/070674370404900705
Lauretani, F., Ruffini, L., Scarlattei, M., and Maggio, M. (2020). Relationship between comprehensive geriatric assessment and amyloid PET in older persons with MCI. BMC Geriatr. 20:337. doi: 10.1186/s12877-020-01746-x
Lehmann, M., Koedam, E. L., Barnes, J., Bartlett, J. W., Barkhof, F., Wattjes, M. P., et al. (2013). Visual ratings of atrophy in MCI: prediction of conversion and relationship with CSF biomarkers. Neurobiol. Aging 34, 73–82. doi: 10.1016/j.neurobiolaging.2012.03.010
Li, H., Heise, K. F., Chalavi, S., Puts, N. A. J., Edden, R. A. E., and Swinnen, S. P. (2022a). The role of MRS-assessed GABA in human behavioral performance. Prog. Neurobiol. 212:102247. doi: 10.1016/j.pneurobio.2022.102247
Li, W., Li, Y., Chen, Y., Yue, L., and Xiao, S. (2022b). Association between physical exercise, executive function, and cerebellar cortex: a cross-sectional study among the elderly in Chinese communities. Front. Aging Neurosci. 14:975329. doi: 10.3389/fnagi.2022.975329
Lissek, V., and Suchan, B. (2021). Preventing dementia? Interventional approaches in mild cognitive impairment. Neurosci. Biobehav. Rev. 122, 143–164. doi: 10.1016/j.neubiorev.2020.12.022
Liu, B., Wang, Z., Lin, L., Yang, H., Gao, F., Gong, T., et al. (2020). Brain GABA+ changes in primary hypothyroidism patients before and after levothyroxine treatment: a longitudinal magnetic resonance spectroscopy study. Neuroimage Clin. 28:102473. doi: 10.1016/j.nicl.2020.102473
Long, J. M., and Holtzman, D. M. (2019). Alzheimer disease: An update on pathobiology and treatment strategies. Cells 179, 312–339. doi: 10.1016/j.cell.2019.09.001
Lu, J., Li, D., Li, F., Zhou, A., Wang, F., Zuo, X., et al. (2011). Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J. Geriatr. Psychiatry Neurol. 24, 184–190. doi: 10.1177/0891988711422528
Mak, E., Gabel, S., Su, L., Williams, G. B., Arnold, R., Passamonti, L., et al. (2017). Multi-modal MRI investigation of volumetric and microstructural changes in the hippocampus and its subfields in mild cognitive impairment, Alzheimer's disease, and dementia with Lewy bodies. Int. Psychogeriatr. 29, 545–555. doi: 10.1017/S1041610216002143
Makris, N., Biederman, J., Valera, E. M., Bush, G., Kaiser, J., Kennedy, D. N., et al. (2007). Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb. Cortex 17, 1364–1375. doi: 10.1093/cercor/bhl047
Manly, J. J., Tang, M. X., Schupf, N., Stern, Y., Vonsattel, J. P., and Mayeux, R. (2008). Frequency and course of mild cognitive impairment in a multiethnic community. Ann. Neurol. 63, 494–506. doi: 10.1002/ana.21326
Marenco, S., Meyer, C., van der Veen, J. W., Zhang, Y., Kelly, R., Shen, J., et al. (2018). Role of gamma-amino-butyric acid in the dorsal anterior cingulate in age-associated changes in cognition. Neuropsychopharmacology 43, 2285–2291. doi: 10.1038/s41386-018-0134-5
Mescher, M., Merkle, H., Kirsch, J., Garwood, M., and Gruetter, R. (1998). Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 11, 266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j
Mitolo, M., Stanzani-Maserati, M., Capellari, S., Testa, C., Rucci, P., Poda, R., et al. (2019). Predicting conversion from mild cognitive impairment to Alzheimer's disease using brain (1)H-MRS and volumetric changes: a two- year retrospective follow-up study. Neuroimage Clin. 23:101843. doi: 10.1016/j.nicl.2019.101843
Mullins, P. G., McGonigle, D. J., O'Gorman, R. L., Puts, N. A., Vidyasagar, R., Evans, C. J., et al. (2014). Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage 86, 43–52. doi: 10.1016/j.neuroimage.2012.12.004
Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal cognitive assessment, MoCA a brief screening. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Oeltzschner, G., Wijtenburg, S. A., Mikkelsen, M., Edden, R. A. E., Barker, P. B., Joo, J. H., et al. (2019). Neurometabolites and associations with cognitive deficits in mild cognitive impairment: a magnetic resonance spectroscopy study at 7 tesla. Neurobiol. Aging 73, 211–218. doi: 10.1016/j.neurobiolaging.2018.09.027
Ottoy, J., Niemantsverdriet, E., Verhaeghe, J., De Roeck, E., Struyfs, H., Somers, C., et al. (2019). Association of short-term cognitive decline and MCI-to-AD dementia conversion with CSF, MRI, amyloid- and (18)F-FDG-PET imaging. Neuroimage Clin. 22:101771. doi: 10.1016/j.nicl.2019.101771
Pappata, S., Varrone, A., Vicidomini, C., Milan, G., De Falco, C., Sansone, V., et al. (2010). SPECT imaging of GABA(a)/benzodiazepine receptors and cerebral perfusion in mild cognitive impairment. Eur. J. Nucl. Med. Mol. Imaging 37, 1156–1163. doi: 10.1007/s00259-010-1409-1
Pinto, T. C. C., Machado, L., Bulgacov, T. M., Rodrigues-Junior, A. L., Costa, M. L. G., Ximenes, R. C. C., et al. (2019). Is the Montreal cognitive assessment (MoCA) screening superior to the Mini-mental state examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer's disease (AD) in the elderly? Int. Psychogeriatr. 31, 491–504. doi: 10.1017/S1041610218001370
Ren, H., Wang, Q., Li, C., Li, Z., Li, J., Dai, L., et al. (2022). Differences in cortical thickness in schizophrenia patients with and without auditory verbal hallucinations. Front. Mol. Neurosci. 15:845970. doi: 10.3389/fnmol.2022.845970
Riese, F., Gietl, A., Zolch, N., Henning, A., O'Gorman, R., Kalin, A. M., et al. (2015). Posterior cingulate gamma-aminobutyric acid and glutamate/glutamine are reduced in amnestic mild cognitive impairment and are unrelated to amyloid deposition and apolipoprotein E genotype. Neurobiol. Aging 36, 53–59. doi: 10.1016/j.neurobiolaging.2014.07.030
Santangelo, R., Masserini, F., Agosta, F., Sala, A., Caminiti, S. P., Cecchetti, G., et al. (2020). CSF p-tau/Aβ(42) ratio and brain FDG-PET may reliably detect MCI "imminent" converters to AD. Eur. J. Nucl. Med. Mol. Imaging 47, 3152–3164. doi: 10.1007/s00259-020-04853-4
Ségonne, F., Dale, A. M., Busa, E., Glessner, M., Salat, D., Hahn, H. K., et al. (2004). A hybrid approach to the skull stripping problem in MRI. NeuroImage 22, 1060–1075. doi: 10.1016/j.neuroimage.2004.03.032
Small, G. W., Kepe, V., Ercoli, L. M., Siddarth, P., Bookheimer, S. Y., Miller, K. J., et al. (2006). PET of brain amyloid and tau in mild cognitive impairment. N. Engl. J. Med. 355, 2652–2663. doi: 10.1056/NEJMoa054625
Sood, A., Preeti, K., Fernandes, V., Khatri, D. K., and Singh, S. B. (2021). Glia: a major player in glutamate-GABA dysregulation-mediated neurodegeneration. J. Neurosci. Res. 99, 3148–3189. doi: 10.1002/jnr.24977
Spallazzi, M., Barocco, F., Michelini, G., Immovilli, P., Taga, A., Morelli, N., et al. (2019). CSF biomarkers and amyloid PET: concordance and diagnostic accuracy in a MCI cohort. Acta Neurol. Belg. 119, 445–452. doi: 10.1007/s13760-019-01112-8
Valenzuela, M. J., and Sachdev, P. (2001). Magnetic resonance spectroscopy in AD. Neurology 56, 592–598. doi: 10.1212/wnl.56.5.592
Weis, J., Persson, J., Frick, A., Ahs, F., Versluis, M., and Alamidi, D. (2021). GABA quantification in human anterior cingulate cortex. PLoS One 16:e0240641. doi: 10.1371/journal.pone.0240641
Wu, J., Dong, Q., Gui, J., Zhang, J., Su, Y., Chen, K., et al. (2021). Predicting brain amyloid using multivariate morphometry statistics, sparse coding, and Correntropy: validation in 1,101 individuals from the ADNI and OASIS databases. Front. Neurosci. 15:669595. doi: 10.3389/fnins.2021.669595
Xu, Y., Chen, K., Zhao, Q., Li, F., and Guo, Q. (2020). Short-term delayed recall of auditory verbal learning test provides equivalent value to long-term delayed recall in predicting MCI clinical outcomes: a longitudinal follow-up study. Appl. Neuropsychol. Adult 27, 73–81. doi: 10.1080/23279095.2018.1481067
Yu, J., Li, J., and Huang, X. (2012). The Beijing version of the Montreal cognitive assessment as a brief screening tool for mild cognitive impairment a community-based study. BMC Psychiatry 12:156. doi: 10.1186/1471-244X-12-156
Zhao, Q., Guo, Q., Li, F., Zhou, Y., Wang, B., and Hong, Z. (2013). The Shape Trail test: application of a new variant of the trail making test. PLoS One 8:e57333. doi: 10.1371/journal.pone.0057333
Zhao, Q., Lv, Y., Zhou, Y., Hong, Z., and Guo, Q. (2012). Short-term delayed recall of auditory verbal learning test is equivalent to long-term delayed recall for identifying amnestic mild cognitive impairment. PLoS One 7:e51157. doi: 10.1371/journal.pone.0051157
Zhu, W., Huang, H., Yang, S., Luo, X., Zhu, W., Xu, S., et al. (2021). Cortical and subcortical Grey matter abnormalities in white matter Hyperintensities and subsequent cognitive impairment. Neurosci. Bull. 37, 789–803. doi: 10.1007/s12264-021-00657-0
Zimmerman, M., Martinez, J. H., Young, D., Chelminski, I., and Dalrymple, K. (2013). Severity classification on the Hamilton depression rating scale. J. Affect. Disord. 150, 384–388. doi: 10.1016/j.jad.2013.04.028
Keywords: mild cognitive impairment, anterior cingulate cortex, posterior cingulate cortex, GABA, executive function, MEGA-PRESS
Citation: Fu X, Qin M, Liu X, Cheng L, Zhang L, Zhang X, Lei Y, Zhou Q, Sun P, Lin L, Su Y and Wang J (2023) Decreased GABA levels of the anterior and posterior cingulate cortex are associated with executive dysfunction in mild cognitive impairment. Front. Neurosci. 17:1220122. doi: 10.3389/fnins.2023.1220122
Edited by:
Federico Giove, Centro Fermi – Museo storico della fisica e Centro studi e ricerche Enrico Fermi, ItalyReviewed by:
Koen Cuypers, University of Hasselt, BelgiumDeepika Shukla, National Brain Research Centre (NBRC), India
Copyright © 2023 Fu, Qin, Liu, Cheng, Zhang, Zhang, Lei, Zhou, Sun, Lin, Su and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Su, c3V5aW5nMDExMEAxMjYuY29t; Jing Wang, eGh3YW5namluZ0BodXN0LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Xiaona Fu
Xiaona Fu Mengting Qin3†
Mengting Qin3† Xiaoming Liu
Xiaoming Liu Lan Zhang
Lan Zhang Yu Lei
Yu Lei Peng Sun
Peng Sun Liangjie Lin
Liangjie Lin Ying Su
Ying Su Jing Wang
Jing Wang