
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurosci., 19 June 2023
Sec. Neural Technology
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1199299
This article is part of the Research TopicTherapeutic Potential of Smart Hydrogel and Nanomaterial Carriers in Neurogenic DiseaseView all 5 articles
Repairing injuries to the nervous system has always been a prominent topic in clinical research. Direct suturing and nerve displacement surgery are the primary treatment options, but they may not be suitable for long nerve injuries and may require sacrificing the functionality of other autologous nerves. With the emergence of tissue engineering, hydrogel materials have been identified as a promising technology with clinical translation potential for repairing nervous system injuries due to their excellent biocompatibility and ability to release or deliver functional ions. By controlling their composition and structure, hydrogels can be Functionalized and almost fully matched with nerve tissue and even simulate nerve conduction function and mechanical properties. Thus, they are suitable for repairing injuries to both the central and peripheral nervous systems. This article provides a review of recent research progress in functionalized hydrogels for nerve injury repair, highlighting the design differences among various materials and future research directions. We strongly believe that the development of functionalized hydrogels has great potential for improving the clinical treatment of nerve injuries.
The nervous system is a crucial component of living organisms, composed of the brain, spinal cord, and peripheral nervous tissue, and consists of abundant neurons and supportive glial cells that form neural circuits, regulating life activities and transmitting physiological information (Brodal, 2004; Sanes et al., 2011). In general, nerve damage or injury may result in the death of endogenous nerve cells at the lesion site, making spontaneous regeneration challenging and leading to abnormal or deteriorating organ functions, and even patient death. For instance, spinal cord fractures or dislocations due to accidents such as trauma, traffic accidents, falls, or sports injuries can cause nerve damage, leading to gradual deterioration of organ functions and consciousness below the injury site, and even paralysis in severe cases. Moreover, the high morbidity and mortality rates of nerve damage make it a significant public health issue worldwide, causing enormous psychological pain and economic burden to patients and their families (Karsy et al., 2019). Therefore, nerve injury repair is a topic of great interest, and the induction of nerve cell regeneration in the damaged area to restore the neural circuit and recover patients’ motor function is currently a crucial issue in the field of basic medical research and clinical transformation practice related to nerve damage treatment.
In the meantime, recent advances in tissue engineering and regenerative medicine have highlighted three crucial factors that play a key role in reshaping neural tissue structure and function. These include seed cells, biomaterial scaffolds, and bioactive factors (Schmidt and Leach, 2003; Gu et al., 2014). Usually, neural stem cells are used as seed cells for neural repair, and biomaterial scaffolds act as carriers for both seed cells and bioactive factors during stem cell transplantation. Other than that, an ideal biomaterial scaffold should provide anchoring sites for stem cells to adhere and grow, as well as induce their proliferation and differentiation within the microenvironment, ultimately leading to the formation of functional neural tissue with mechanical stability.
As a scaffold material widely used in neural tissue engineering, hydrogel has the following properties. Hydrogels are highly porous network materials that result from crosslinking hydrophilic polymers through both physical and chemical processes (Ullah et al., 2015). They possess excellent biocompatibility, and as such, are widely used in the diagnosis and treatment of various diseases (Hoffman, 2012; Caló and Khutoryanskiy, 2015). The physicochemical and structural microenvironment of hydrogels resembles that of the extracellular matrix of neural tissue, and their viscoelastic property is highly compatible with biological tissue. Moreover, hydrogels provide attachment sites and a three-dimensional space for the growth, migration, proliferation, and differentiation of transplanted neural cells. Additionally, hydrogels exhibit tissue affinity on their soft and wet surfaces, making them ideal for in vivo cell scaffolding (Nisbet et al., 2008). To clarify, this mini review is structured around various strategies for distinct hydrogel material designs; however, it is important to note that due to differences in cellular microenvironments, cell types, and post-injury repair capabilities between the central and peripheral nervous systems (CNS and PNS, respectively), the responses to biomaterials vary. Consequently, the requirements for material design diverge as well. For instance, when designing biomaterials, the degradability of the material is a crucial factor. Biomaterials designed for CNS repair must degrade within an appropriate time frame to prevent long-term disruption to surrounding tissues, while in PNS repair, the degradation rate of biomaterials should correspond with the rate of nerve regeneration to ensure adequate support for nerve growth. Another example is the variation in biomaterial types for CNS and PNS injuries. CNS injury repair materials may encompass scaffolds, gels, nanoparticles, and other components with the primary aim of providing physical support, promoting cell growth and differentiation, and releasing growth factors. In contrast, PNS injury repair materials typically involve nerve conduits, biofilms, and the like, with the main functions of guiding nerve growth and supplying extracellular matrix support. Furthermore, drug delivery to the CNS can be challenging due to the blood–brain barrier. As a result, when designing biomaterials for CNS repair, it is worth considering the development of materials with drug carrier capabilities to enhance the efficiency of drug delivery to the damaged site. In PNS repair, however, the limitation imposed by the blood–brain barrier is less significant. In summary, the design of biomaterials for CNS and PNS injury repair necessitates a comprehensive understanding of their distinct biological, biomechanical, and biocompatibility aspects. Thus, this article reviews the fundamental research in neural injury repair that utilizes various functionalized hydrogels, explores their potential for clinical practice, and discusses the synthesis strategies and application conditions of different types of hydrogels (Figure 1).
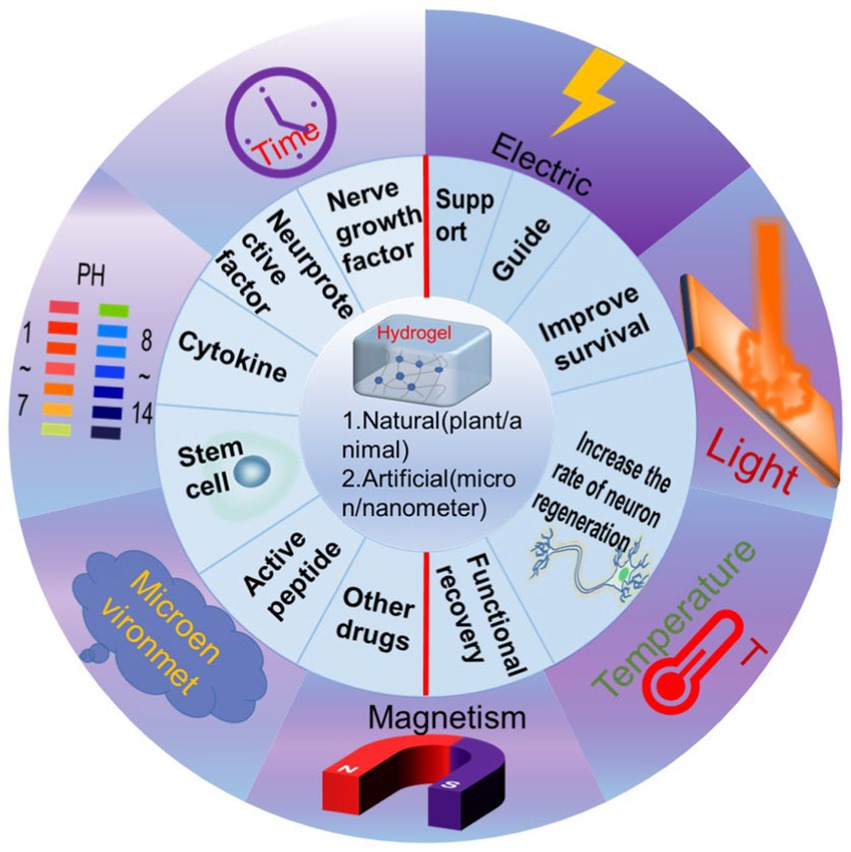
Figure 1. Schematic illustration showing the classification of functionalized hydrogels and remote control to realize the various functions.
Hydrogels play a crucial role in nerve injury repair by providing physical support, enhancing mechanical and guiding capabilities, and promoting nerve regeneration and repair. The polymer chains within hydrogels can be crosslinked to form a three-dimensional network that enhances their mechanical properties, such as toughness, strength, and elasticity, allowing them to closely mimic physiological tissue structures (Lesný et al., 2002; Ma et al., 2022). Furthermore, the strength and elasticity of hydrogels can be adjusted by controlling their crosslinking density and structure, and subsequent adsorption of water molecules can increase their toughness and durability. The three-dimensional network structure formed by hydrogels at the microscale provides physical support to the surrounding tissues and cells, promoting oriented cell growth, facilitating the generation of new neural tissue and blood vessels, and enhancing the supply of oxygen and nutrients to support neural cell growth and regeneration (Deligkaris et al., 2010; Xia and Chen, 2022). Moreover, hydrogels can effectively fill damaged areas of neural tissue, reducing tissue gaps, forming supportive structures, and aiding in neural tissue repair (Štulík and Syková, 2008). Hydrogels can also be used as a carrier for bioactive molecules, making them a promising biomaterial for promoting neural tissue regeneration and repair (Ma et al., 2022).
Overall, the supportive role of hydrogels in neural injury repair is based on their formation of a three-dimensional network with uniform micro-pores, excellent biocompatibility, and the ability to promote neural regeneration and repair. The combination of these advantageous properties makes hydrogels a crucial and effective material for nerve repair, resulting in the survival and functional recovery of neurons, and providing hope for the millions of people affected by nerve injuries.
Dopamine, a neurotransmitter, participates in numerous physiological and pathological processes of the CNS, including emotion, behavior, memory, attention, motivation, and reward (Wise, 2004; Iversen and Iversen, 2007). Furthermore, dopamine plays a crucial role in regulating the morphology, growth, and migration of neurons to the site of injury, which impacts the formation and connection of neurons and contributes to the growth, development, and repair of neurons (He et al., 2022; Lindholm and Saarma, 2022; Silva et al., 2022). Recent studies have revealed that dopamine also facilitates the growth and development of neurons, promotes synaptic formation and connection, and encourages the differentiation of neurons, allowing them to assume different roles in the nervous system (Kim et al., 2002; Chinta and Andersen, 2005). Therefore, incorporating dopamine into hydrogels can potentially enhance the ability of hydrogels to promote neural regeneration and repair.
The neural regulatory function of dopamine makes it a promising candidate for combining with hydrogels for nerve injury repair. Studies have shown that the failure of axonal regeneration in the CNS is closely related to the formation of glial scar after injury (Chen and Zhu 2016). However, dopamine-functionalized hydrogels have been found to be effective in promoting the differentiation of neural stem cells (NSCs) and the growth of synapses, inhibiting the formation of glial scars following spinal cord injury, and facilitating axon regeneration (Zhou, 2018).
Furthermore, regarding the optimization of dopamine binding to hydrogels, several studies have shown that it can be achieved through chemical reactions (Carballo-Molina et al., 2016; Adil et al., 2017), or by physisorption (Yang et al., 2012; Pei et al., 2020). The most common approach for dopamine-functionalized hydrogels is the oxidative polymerization reaction of dopamine under alkaline conditions, which generates a polymer compound called polydopamine (Tang et al., 2019; Chen et al., 2020). Polydopamine can then covalently bond with numerous hydrogel materials, such as dopamine-functionalized poly (lactic-co-glycolic acid) (PLGA) hydrogel. Another approach to combine dopamine with hydrogels is physical adsorption, which depends on the interaction between the aromatic ring structure of dopamine molecules and the aromatic functional groups on the hydrogel surface (Kim et al., 2014; Soylu et al., 2021). For instance, dopamine can be physically adsorbed onto gelatin hydrogels to impart them with dopamine-functionalized characteristics. A thorough comprehension of the chemical and physical interactions between dopamine and hydrogels is crucial in developing dopamine-functionalized hydrogels for neural repair applications.
Dopamine-functionalized hydrogels have shown promising potential in promoting neural stem cell adhesion and proliferation, with the ability to modulate the proliferation of neural stem cells through dopamine content adjustments. Moreover, these hydrogels have demonstrated an increase in the rate of neuronal differentiation, synapse formation promotion, and neural network development facilitation. Additionally, dopamine-functionalized hydrogels have been found to improve the survival rate of neural stem cells and enhance the regenerative capacity of neural tissue by modulating the extracellular environment of neural stem cells. As shown in Table 1, more and more studies have shown that the combination of dopamine and hydrogel is effective in repairing nerve injury.
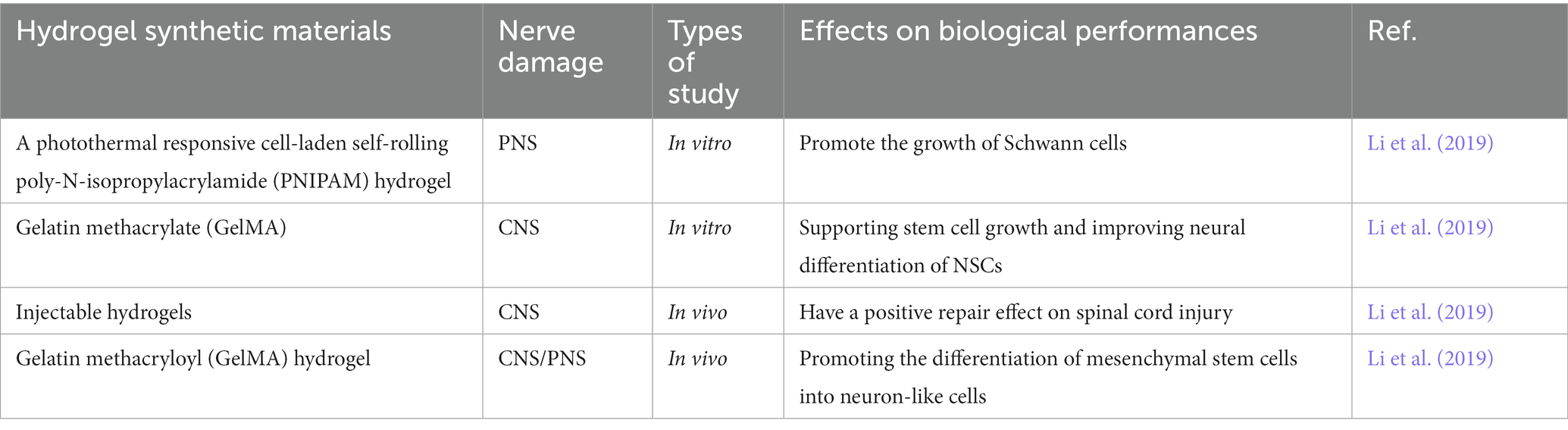
Table 1. Representative studies of dopamine-functionalized different hydrogels and their biological properties in nerve injury repair.
The nervous system primarily communicates through electrical signals, which are critical in the development, maturation, and regeneration of nervous tissue. Inter-cellular signal transmission predominantly occurs via the extracellular matrix, and the integration of conductive substrates into the cellular microenvironment promotes inter-cellular electrical signal transmission. Therefore, maintaining the function of neurons heavily relies on the stimulation and transmission of electrical signals. Peripheral nerve injury can result in neurologic disorders, chronic pain, paralysis, and even disability by disrupting the electrical signal transmission between the brain and the body. Autologous nerve transplantation is commonly used to repair peripheral nerves; however, this method is affected by various factors such as a shortage of donors, long-term excessive tension, synaptic regeneration disorder, and severe nerve interruption that is difficult to suture. Conductive hydrogels have become a preferred alternative to autologous nerve transplantation due to their biocompatibility and the advantages of conductive polymers that can transmit electrical signals within nerve tissue. Additionally, conductive hydrogels have been found to effectively use electrical signals to promote neural tissue regeneration (Dong et al., 2020).
Extensive research has been conducted on biocompatible and conductive biomaterials that promote neural tissue regeneration (Guarino et al., 2013; Uz and Mallapragada, 2019; Park et al., 2020). Conductive hydrogels are a specific type of hydrogel material with electrical conductivity, containing conductive substances such as conductive polymers (polypyrrole, polyaniline, and polystyrene), metallic elements (silver, copper, gold, and iron), carbon materials (graphene, carbon nanotubes), and metal oxides (indium tin oxide, and aluminum oxide), as compared to traditional hydrogel materials (Wang et al., 2018; Zhou et al., 2022). These conductive substances can be combined with hydrogels through direct mixing, chemical reduction, or electrochemical deposition.
Conductive hydrogels find application in electronic devices, biosensors, and smart medical fields (Xu et al., 2020). However, it is worth noting that the conductive properties of these materials can vary greatly. For example, while metals display excellent conductivity, their biocompatibility is relatively poor. On the other hand, carbon-based materials can enhance the mechanical strength of hydrogels, but achieving homogeneous dispersion is challenging, which subsequently affects the conductivity performance. Conductive hydrogels have the potential to establish a neural-electronic interface that connects electrons and nerve cells, enabling electronic and ionic transport to simulate electrical signal transmission between nerve cells. Research has demonstrated that the conductive properties of conductive hydrogels can create an electrical stimulation environment that promotes the growth and regeneration of neural cells (Liu et al., 2017; Park et al., 2020; Cai et al., 2022). For example, conductive hydrogel conduits with a gradient of growth factors can promote the regeneration of peripheral nerves and muscle fibers in mice, holding significant potential for repairing peripheral nerve injuries (PNIs) and muscle atrophy in diabetic patients (Liu et al., 2017; Park et al., 2020; Cai et al., 2022). Furthermore, using conductive polymer hydrogels to repair spinal cord injuries in mice has resulted in the regeneration of spinal cord neurons and the restoration of limb motor function (Zhou et al., 2018; Guo et al., 2019).
Conductive hydrogels, specifically poly (acrylic acid)/polypyrrole (PAA/PPy) hydrogels, have been successfully utilized as scaffold materials for facilitating the differentiation of neural stem cells into neurons under electrical stimulation, as reported by (Milani et al., 2022). Additionally, the combination of these hydrogels and electrodes can be employed for neural electrical stimulation, modulation, and recording of neural electrical activity signals in the cerebral cortex, as mentioned in (Khan et al., 2022). Conductive hydrogels not only possess suitable physicochemical properties for cell growth but also exhibit electrical conductivity, which enables them to provide additional electrical stimulation to nerve cells. This capability can support the restoration of interrupted conduction pathways and maintain the endogenous electrical microenvironment for nerve regeneration. Therefore, the interaction between conductive hydrogels and nerve cells is a critical area of research in the field of neural repair and is considered one of the popular technologies for achieving nerve regeneration and repair. As shown in Table 2, more and more studies have shown the effectiveness of conductive hydrogels for repairing nerve injury.
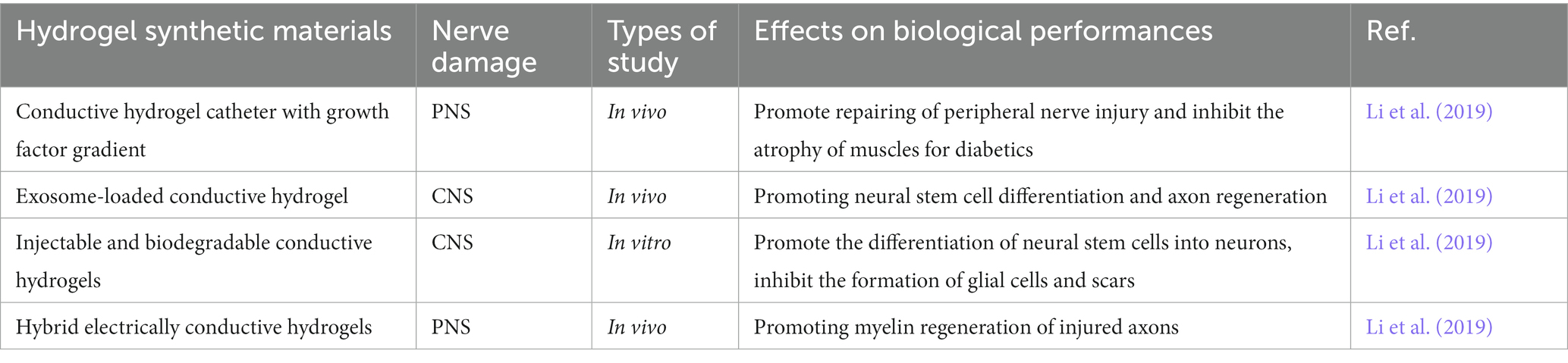
Table 2. Representative study of conductive hydrogels and their biological properties in nerve injury repair.
However, conductive materials present various challenges in their application within the field of biomedicine. Firstly, they may be difficult to process and degrade, with poor uniform dispersion of carbon-based materials. Surface modification and controlling polymerization conditions have been suggested as potential solutions to these issues. Secondly, further study is needed to fully understand the synergistic mechanism between electrical stimulation and conductive materials, to better meet the challenges of long-distance nerve injury repair. Finally, the long-term cytotoxicity, biocompatibility, and metabolism of conductive materials within the human body remain areas of active research focus (Khan et al., 2022).
The nervous system communicates mainly through electrical signals, and in the CNS, neurons are responsible for signal transduction. The severe inflammatory reaction after nerve injury can lead to the death of neurons and the formation of glial scar, which affects the repair of CNS injury. Therefore, ideal CNS repair materials need to have properties that minimize the occurrence of inflammatory reactions (Raposo and Stahl, 2019). Studies have shown that extracellular vesicles (EVs) contain a diverse array of biological molecules, such as cell factors, proteins, and nucleic acids, and have multiple biological functions, including promoting cell proliferation and reducing inflammation (Raposo and Stahl, 2019). Previous studies have indicated that EVs derived from cells possess robust regenerative and reparative capabilities in various systems, including the musculoskeletal (Alcaraz et al., 2019), cardiovascular (Adamiak et al., 2018), liver injury (Psaraki et al., 2022), kidney injury (Aghajani Nargesi et al., 2017), traumatic brain injury (Mondello et al., 2018) and others. These vesicles have significant potential as a “cell-free” therapeutic approach for regenerative medicine and may serve as a replacement for stem cells. Notably, many cells in the peripheral nervous system, such as small glial cells and astrocytes, are capable of secreting different types of EVs, particularly following nerve injury.
And EVs can participate in protein synthesis in neurons, promote axonal regeneration, and inhibit axonal degeneration through various mechanisms. One such mechanism involves the binding of vesicular membrane proteins with target cell membrane proteins through ligand-receptor interactions (Liu et al., 2021). Another mechanism is that vesicular proteins can activate signaling pathway proteins on the surface of target cells after vesicle degradation, leading to a series of biological responses (Guo et al., 2021; Lee et al., 2022). Loading functionalized hydrogels with EVs is a novel therapeutic approach that can promote neural repair through various pathways such as enhancing the proliferation and migration of neural cells, improving neuronal survival, and restoring function. Studies have shown that EVs derived from skin-derived precursor Schwann cells (SKP-SC-EVs) accelerate the recovery of motor, sensory and electrophysiological functions in rats, promote the growth of regenerative axons and the formation of myelin sheaths, reduce the atrophy of target muscles caused by denervation, and promote the neurite growth of motor neurons (MNs) and sensory neurons, which is helpful for PNI repair (Yu et al., 2021; Jiang et al., 2022; Zhang et al., 2022). This promising approach may hold significant potential for the development of regenerative medicine therapies for neural injury and degenerative disorders.
Various studies have demonstrated the potential of stem cell-derived extracellular vesicles (EVs) and exosomes to reduce inflammation, inhibit cell apoptosis, and mitigate the impact of neural damage on surrounding tissues. For instance, researchers have loaded human mesenchymal stem cell-derived EVs onto chitosan-based functional hydrogels to repair peripheral nerve injury in rats. The results of the study indicated that the EV-loaded functional hydrogels promoted neuronal cells proliferation and migration, while reducing inflammation and cell apoptosis, leading to a significant improvement in neural damage repair (Ju et al., 2023). Another study utilized exosomes derived from mouse neural stem cells loaded onto poly (ε-caprolactone) based functional hydrogels and reported a significant increase in the survival and regeneration of spinal cord neurons, accompanied by a reduction in inflammation and glial scar formation (Xie et al., 2022). Moreover, there have been studies loading exosomes containing miRNA into collagen hydrogels for neural injury repair, demonstrating that they not only promote neuronal growth and regeneration but also improve the structure and function of damaged neural tissue (Lei et al., 2019; Xu et al., 2021; Zhang et al., 2021). In this study, the 3D fiber-hydrogel scaffold delivered axon microRNA (miR) to the injured site and repaired it in a non-viral manner. In the presence of methylprednisolone, the role of axon miRs in promoting mature axon regeneration is not affected, which can reduce the inflammatory response. More importantly, axon miRs in the presence of methylprednisolone reduced cyst formation and provided a tendency to improve functional recovery (Zhang et al., 2021).
Despite the promising therapeutic effects of EVs in regenerative medicine research, such as tissue repair and regeneration, their naturally low quantity and difficulty in controlling them present challenges in terms of extraction efficiency and purification yield, which has limited their research and application (Ingato et al., 2016; Usman et al., 2018). Therefore, it is crucial to improve the quality and yield of EVs, which will be a key focus for future research. Furthermore, before they can be effectively applied in clinical settings, more studies are needed to validate their safety and efficacy. But with the progress of technology, we believe that these issues will be addressed soon, and look forward to further development in this field.
In recent years, research on nano-functionalized hydrogels for neural injury repair has gained significant attention due to the expanding and enhancing of hydrogel functionality by nanomaterials (Rafieian et al., 2019; Kailasa et al., 2022). Nanomaterials can provide more growth factors and cell adhesion molecules, promoting the regeneration and growth of neural cells, while hydrogels provide a supportive and protective environment that facilitates the directional growth of neural cells. Additionally, nanomaterials can serve as drug carriers, enhancing drug loading and release efficiency for neural repair drugs, and even enabling intelligent and precise controlled release. As a result, nanomaterial-based hydrogels offer promising potential for the development of effective neural injury repair strategies. Hydrogels can limit drug diffusion and degradation, thus increasing drug concentration and duration at the treatment site. Furthermore, nanomaterials can enhance the mechanical properties and stability of hydrogels, resulting in improved durability and lifespan of the material (Li and Mooney, 2016). With their large and specific surface area, nanoparticles can increase drug delivery efficiency, and in combination with hydrogels, the use of nanomaterials can minimize immune reactions. Composites of nanoparticle-metal-hydrogel have been found to promote neuronal growth and have the potential to facilitate nerve regeneration and repair.
Nanoparticle biosensors have been combined with hydrogels to promote nerve regeneration and repair by enhancing neuronal proliferation and differentiation. Nanocarbon tube hydrogels have shown promise in nerve regeneration by promoting neuronal proliferation, reducing inflammation, and increasing the rate of neuronal regeneration (Ye et al., 2021). Similarly, nanoparticle proton pump hydrogels have been developed to reduce inflammation during nerve regeneration and promote neuronal regeneration, improving the effectiveness of nerve injury repair (Mendiratta et al., 2019). Graphene oxide nanosheets have also been incorporated into gelatin hydrogels to create a composite material for nerve regeneration, which has been shown to promote the proliferation and differentiation of neural stem cells and achieve nerve regeneration in vivo. The incorporation of micropatterns and bioactive substances into the inner wall of nerve-guide conduits (NGCs) can effectively regulate the behavior of Schwann cells, axon elongation, and macrophage phenotype, ultimately promoting the regeneration of injured nerves. In a recent study, 3/3, 5/5/, 10/10, and 30/30 μm linear micro-ribbons were prepared on poly (D, l-lactic acid-co-caprolactone) (PLCL) films. Surface ammonolysis and electrostatic adsorption of graphene oxide (GO) nanosheets were performed. This material has demonstrated great potential for promoting nerve regeneration (Zhang et al., 2020). Additionally, magnetic nanoparticle (MNPs) and gelatin hydrogel composites have been developed, which exhibit a significant magnetic guiding effect, facilitating the directional differentiation of neural stem cells and promoting nerve regeneration (Pavón et al., 2019). Curcumin-loaded mesoporous silica nanoparticles (MSN-CCM) dispersed in hydrogels have shown potential for assisting in the treatment of neurodegenerative diseases such as Alzheimer’s disease (Ribeiro et al., 2022). Additionally, a dual responsive hydrogel based on poly N-isopropylacrylamide (PNIPAM) and polyacrylic acid (PAA) functionalized mesoporous silica nanoparticles (MSNs) has been shown to be effective for killing tumor cells while also promoting tissue regeneration (Chen et al., 2017). Further, studies have demonstrated that the incorporation of lipid nanoparticles into hyaluronic acid-functionalized hydrogels can help create an anti-inflammatory microenvironment and reduce immune response (Pavón et al., 2019). Currently, there is relatively limited research on the combination of hydrogels with nanomagnetic hyperthermia for neural injury repair. However, due to the potential benefits of hyperthermia, there may be significant applications in this area. We have found previous research demonstrating that a novel thermosensitive heparin-poloxamer (HP) hydrogel, delivering basic fibroblast growth factor (bFGF) and nerve growth factor (NGF), can be used for sciatic nerve compression injury in diabetic rats, promoting axonal and myelin sheath regeneration, and improving motor function recovery (Li et al., 2018). We believe that the combination of hydrogels with nanomagnetic hyperthermia has tremendous potential in neural injury repair and look forward to further progress in future studies (Pavón et al., 2019). Nanomaterials have shown promise in inhibiting the activity of inflammatory cells, which can help reduce inflammation, minimize immune reactions, and decrease immune rejection.
Table 3 provides a comprehensive overview of the growing body of research showcasing the effectiveness of using nanomaterials in combination with hydrogels for repairing neural injuries. These applications have been preliminarily validated in laboratory experiments, and some studies have progressed to animal and human trials. The combination of nanomaterials and hydrogels offers multiple advantages and has broad application prospects in neural tissue engineering. With further technological development, these materials are expected to play an even more significant role in the field of neural injury repair. It is excited to witness the potential of these materials and look forward to further advancements in this field.
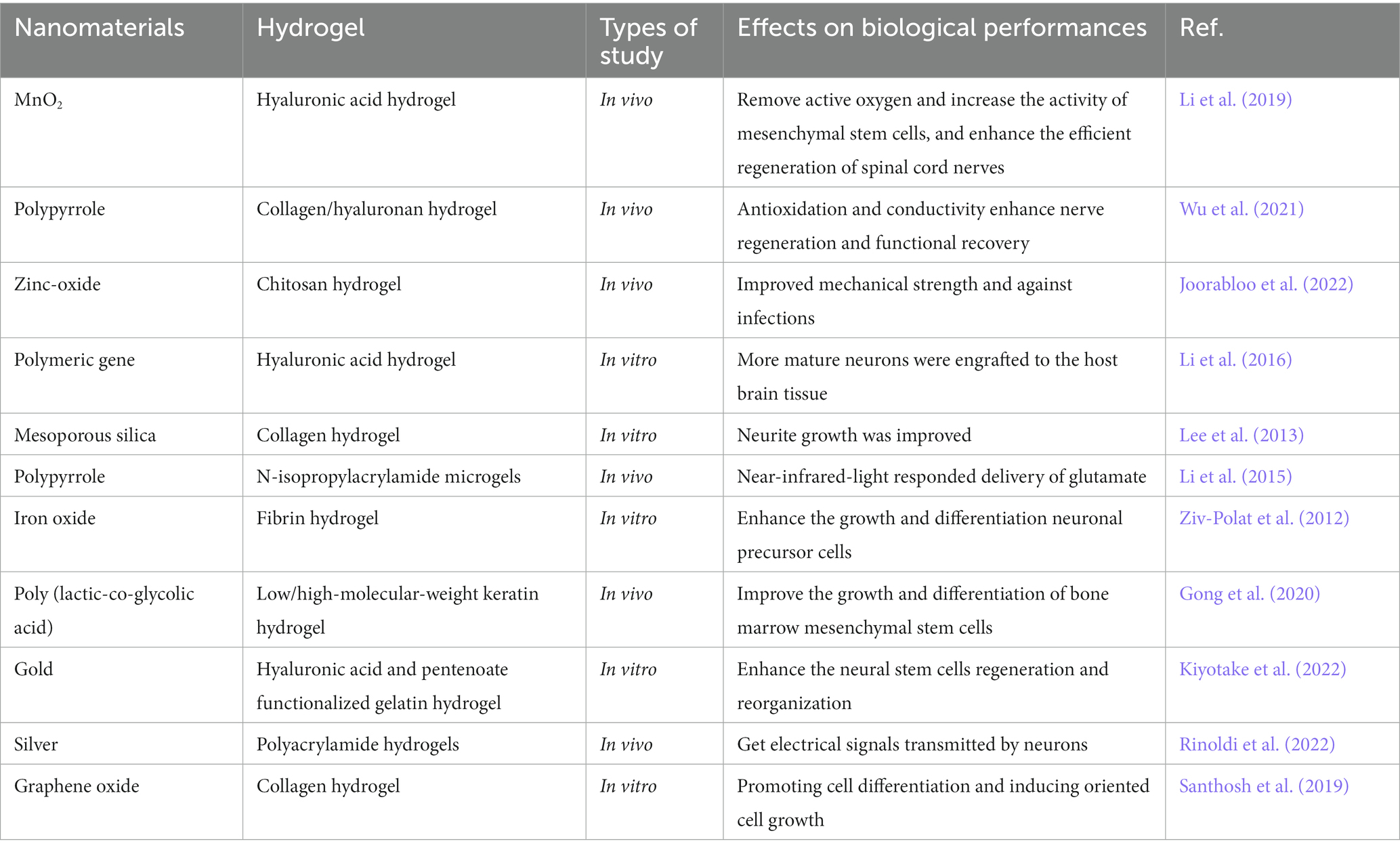
Table 3. Representative research of varying hydrogels functionalized by nanoparticles and their biological performances in neural injury repairing.
Although nanomaterials hold great potential for the repair and regeneration of damaged tissues, their use in synthesizing new materials must consider potential immune responses and toxicity to tissues and organs, as well as issues of instability. Therefore, ensuring the biosafety and stability of nanomaterials is a crucial direction for future research in the field (Zheng et al., 2021).
The above paragraph describes different types of functionalized hydrogels, and the following materials have been specifically applied in cases of CNS or peripheral nervous system injuries. For instance, dopamine-modified chitosan hydrogels have been shown to improve cell survival, modulate immunity, and promote axonal regeneration (Liu et al., 2022). Additionally, the construction of injectable silk fibroin/dopamine hydrogels has been found to be helpful in promoting the repair of spinal cord injuries (Ye et al., 2021).
A novel conductive hydrogel made from gelatin methacrylate (GelMA), hyaluronic acid methacrylate (HAMA), and poly (3,4-ethylenedioxythiophene): sulfonated lignin (PEDOT: LS) has shown promise in repairing spinal cord injuries and promoting neuronal differentiation of neural stem cells (Gao et al., 2023). Additionally, bone marrow stem cell-derived exosomes (BMSC-exosomes) have been found to bind to conductive hydrogels, enhancing axonal growth and promoting tissue repair (Fan et al., 2022). The combination of a conductive hydrogel with chitosan to form a multifunctional double-layer hydrogel catheter has been shown to be effective for peripheral nerve injury repair (Deng et al., 2022). Finally, a rubber-like conductive hydrogel composed of gelatin, conductive polypyrrole, and tannic acid (referred to as GPT) has demonstrated the ability to promote peripheral nerve regeneration (Ye et al., 2021).
Injectable thermosensitive hydrogels containing immunoregulatory extracellular vesicles have been found to help alleviate inflammation and promote nerve regeneration in cases of spinal cord injury (Zhang et al., 2022). Additionally, the combination of exosomes derived from mouse neural stem cells with functional hydrogels based on poly (ε-caprolactone) has shown promise in promoting the survival and regeneration of spinal cord neurons, as well as reducing inflammation and glial scar formation (Xie et al., 2022). Another study found that the use of human mesenchymal stem cell-derived extracellular vesicles combined with chitosan-based functional hydrogels is effective in repairing peripheral nerve injuries in rats (Ju et al., 2023).
A composite hydrogel consisting of polyvinyl alcohol (PVA) and molybdenum sulfide/graphene oxide (MoS2/GO) nanomaterials has been shown to promote the differentiation of neural stem cells into neurons for use in spinal cord injury repair (Chen et al., 2022). Additionally, multifunctional biomimetic hydrogels based on graphene nanoparticles and sodium alginate have demonstrated the ability to simulate the microenvironment of nerve growth, reduce inflammatory factors, and contribute to the repair of peripheral nerve injuries (Ju et al., 2023).
Biomedical materials for neural tissue engineering must possess not only excellent biocompatibility but also specific neural conduction functions that promote cell-to-cell signaling. Hydrogels, which are gel-like polymers containing water, represent the most water-like scaffold materials that closely resemble human soft tissue and are crosslinked by either physical or covalent bonds. However, conventional hydrogels exhibit low strength, weak biological activity, and a single function, limiting their ability to meet the complex demands of neural injury repair. Recent studies have demonstrated that functionalized hydrogels, created through combination with other functional materials, can improve biocompatibility and responsiveness to various stimuli by regulating their composition and structure. These functionalized hydrogels effectively mimic the in vivo neural extracellular matrix microenvironment, facilitating efficient repair, replication, or differentiation of neural cells and achieving high matching of the elastic modulus between the biomaterials and neural tissue. Despite their potential for aiding in the repair of nerve tissue after injury, functionalized hydrogels are still plagued by various deficiencies, such as concerns related to biosafety and stability. Additionally, their practical implementation in clinical applications remains an area of ongoing exploration. However, with continued advancements in technology, I am optimistic that these issues will be successfully resolved, and look forward to the further development and refinement of this exciting field.
Conceptualization was made by JL and FZ. WZ and HT contributed equally to manuscript writing. HL and JW drafted the picture and summary the table. JC organized the material. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (no. 31800836) and Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (Project number: 2019PY087).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adamiak, M., Cheng, G., Bobis-Wozowicz, S., Zhao, L., Kedracka-Krok, S., Samanta, A., et al. (2018). Induced pluripotent stem cell (iPSC)–derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ. Res. 122, 296–309. doi: 10.1161/CIRCRESAHA.117.311769
Adil, M. M., Vazin, T., Ananthanarayanan, B., Rodrigues, G. M., Rao, A. T., Kulkarni, R. U., et al. (2017). Engineered hydrogels increase the post-transplantation survival of encapsulated hESC-derived midbrain dopaminergic neurons. Biomaterials 136, 1–11. doi: 10.1016/j.biomaterials.2017.05.008
Aghajani Nargesi, A., O Lerman, L., and Eirin, A. (2017). Mesenchymal stem cell-derived extracellular vesicles for renal repair. Curr. Gene Ther. 17, 29–42. doi: 10.2174/1566523217666170412110724
Alcaraz, M. J., Compañ, A., and Guillén, M. I. (2019). Extracellular vesicles from mesenchymal stem cells as novel treatments for musculoskeletal diseases. Cells 9:98. doi: 10.3390/cells9010098
Cai, Y., Huang, Q., Wang, P., Ye, K., Zhao, Z., Chen, H., et al. (2022). Conductive hydrogel conduits with growth factor gradients for peripheral nerve repair in diabetics with non-suture tape. Adv. Healthc. Mater. 11:2200755. doi: 10.1002/adhm.202200755
Caló, E., and Khutoryanskiy, V. V. (2015). Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 65, 252–267. doi: 10.1016/j.eurpolymj.2014.11.024
Carballo-Molina, O. A., Sánchez-Navarro, A., López-Ornelas, A., Lara-Rodarte, R., Salazar, P., Campos-Romo, A., et al. (2016). Semaphorin 3C released from a biocompatible hydrogel guides and promotes axonal growth of rodent and human dopaminergic neurons. Tissue Engineering Part A 22, 850–861.
Chen, S., Liu, S., Zhang, L., Han, Q., Liu, H., Shen, J., et al. (2020). Construction of injectable silk fibroin/polydopamine hydrogel for treatment of spinal cord injury. Chem. Eng. J. 399:125795. doi: 10.1016/j.cej.2020.125795
Chen, L., Wang, W., Lin, Z., Lu, Y., Chen, H., Li, B., et al. (2022). Conducting molybdenum sulfide/graphene oxide/polyvinyl alcohol nanocomposite hydrogel for repairing spinal cord injury. J. Nanobiotechnol. 20:210. doi: 10.1186/s12951-022-01396-8
Chen, X., Yuan, P., Liu, Z., Bai, Y., and Zhou, Y. (2017). Dual responsive hydrogels based on functionalized mesoporous silica nanoparticles as an injectable platform for tumor therapy and tissue regeneration. J. Mater. Chem. B 5, 5968–5973. doi: 10.1039/C7TB01225J
Chen, X., and Zhu, W. (2016). A Mathematical Model of Regenerative Axon Growing along Glial Scar after Spinal Cord Injury. Comput. Math. Methods Med. 2016:3030454. doi: 10.1155/2016/3030454
Chinta, S. J., and Andersen, J. K. (2005). Dopaminergic neurons. Int. J. Biochem. Cell Biol. 37, 942–946. doi: 10.1016/j.biocel.2004.09.009
Deligkaris, K., Tadele, T. S., Olthuis, W., and Van Den Berg, A. (2010). Hydrogel-based devices for biomedical applications. Sensors Actuators B Chem. 147, 765–774. doi: 10.1016/j.snb.2010.03.083
Deng, P., Chen, F., Zhang, H., Chen, Y., and Zhou, J. (2022). Multifunctional Double-Layer Composite Hydrogel Conduit Based on Chitosan for Peripheral Nerve Repairing. Advanced Healthcare Materials 11:2200115.
Dong, M., Shi, B., Liu, D., Liu, J. H., Zhao, D., Yu, Z. H., et al. (2020). Conductive hydrogel for a photothermal-responsive stretchable artificial nerve and coalescing with a damaged peripheral nerve. ACS nano 14, 16565–16575. doi: 10.1021/acsnano.0c05197
Fan, L., Liu, C., Chen, X., Zheng, L., Zou, Y., Wen, H., et al. (2022). Exosomes-Loaded Electroconductive Hydrogel Synergistically Promotes Tissue Repair after Spinal Cord Injury via Immunoregulation and Enhancement of Myelinated Axon Growth. Advanced Science 9:2105586.
Gao, C., Li, Y., Liu, X., Huang, J., and Zhang, Z. (2023). 3D bioprinted conductive spinal cord biomimetic scaffolds for promoting neuronal differentiation of neural stem cells and repairing of spinal cord injury. Chem. Eng. J. 451:138788. doi: 10.1016/j.cej.2022.138788
Gong, Y., Wang, Y., Qu, Q., Hou, Z., Guo, T., Xu, Y., et al. (2020). Nanoparticle encapsulated core-shell hydrogel for on-site BMSCs delivery protects from iron overload and enhances functional recovery. J. Control. Release 320, 381–391. doi: 10.1016/j.jconrel.2020.01.029
Gu, X., Ding, F., and Williams, D. F. (2014). Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 35, 6143–6156. doi: 10.1016/j.biomaterials.2014.04.064
Guarino, V., Alvarez-Perez, M. A., Borriello, A., Napolitano, T., and Ambrosio, L. (2013). Conductive PANi/PEGDA macroporous hydrogels for nerve regeneration. Adv. Healthc. Mater. 2, 218–227. doi: 10.1002/adhm.201200152
Guo, B., Ma, Z., Pan, L., and Shi, Y. (2019). Properties of conductive polymer hydrogels and their application in sensors. J. Polym. Sci. B Polym. Phys. 57, 1606–1621. doi: 10.1002/polb.24899
Guo, S., Redenski, I., and Levenberg, S. (2021). Spinal cord repair: from cells and tissue engineering to extracellular vesicles. Cells 10:1872. doi: 10.3390/cells10081872
He, X., Zhu, Y., Ma, B., Xu, X., Huang, R., Cheng, L., et al. (2022). Bioactive 2D nanomaterials for neural repair and regeneration. Adv. Drug Deliv. Rev. 187:114379. doi: 10.1016/j.addr.2022.114379
Hoffman, A. S. (2012). Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 64, 18–23. doi: 10.1016/j.addr.2012.09.010
Ingato, D., Lee, J. U., Sim, S. J., and Kwon, Y. J. (2016). Good things come in small packages: Overcoming challenges to harness extracellular vesicles for therapeutic delivery. J. Control Release. 241, 174–185. doi: 10.1016/j.jconrel.2016.09.016
Iversen, S. D., and Iversen, L. L. (2007). Dopamine: 50 years in perspective. Trends Neurosci. 30, 188–193. doi: 10.1016/j.tins.2007.03.002
Jiang, Y., Wang, R., Wang, C., Guo, Y., Xu, T., Zhang, Z., et al. (2022). Brain microenvironment responsive and pro-Angiogenic extracellular vesicle-hydrogel for promoting neurobehavioral recovery in type 2 diabetic mice after stroke. Adv. Healthc. Mater. 11:2201150. doi: 10.1002/adhm.202201150
Joorabloo, A., Khorasani, M. T., Adeli, H., Milan, P. B., and Amoupour, M. (2022). Using artificial neural network for design and development of PVA/chitosan/starch/heparinized nZnO hydrogels for enhanced wound healing. J. Ind. Eng. Chem. 108, 88–100. doi: 10.1016/j.jiec.2021.12.027
Ju, Y., Hu, Y., Yang, P., Xie, X., and Fang, B. (2023). Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater Today Bio 18:100522. doi: 10.1016/j.mtbio.2022.100522
Kailasa, S. K., Joshi, D. J., Kateshiya, M. R., Koduru, J. R., and Malek, N. I. (2022). Review on the biomedical and sensing applications of nanomaterial-incorporated hydrogels. Mater Today Chem 23:100746. doi: 10.1016/j.mtchem.2021.100746
Karsy, M., Watkins, R., Jensen, M. R., Guan, J., Brock, A. A., and Mahan, M. A. (2019). Trends and cost analysis of upper extremity nerve injury using the national (nationwide) inpatient sample. World Neurosurg. 123, e488–e500. doi: 10.1016/j.wneu.2018.11.192
Khan, Z. M., Wilts, E., Vlaisavljevich, E., Long, T. E., and Verbridge, S. S. (2022). Electroresponsive hydrogels for therapeutic applications in the brain. Macromol. Biosci. 22:2100355. doi: 10.1002/mabi.202100355
Kim, J.-H., Auerbach, J. M., Rodríguez-Gómez, J. A., Velasco, I., Gavin, D., Lumelsky, N., et al. (2002). Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature 418, 50–56. doi: 10.1038/nature00900
Kim, H. H., Park, J. B., Kang, M. J., and Park, Y. H. (2014). Surface-modified silk hydrogel containing hydroxyapatite nanoparticle with hyaluronic acid–dopamine conjugate. Int. J. Biol. Macromol. 70, 516–522. doi: 10.1016/j.ijbiomac.2014.06.052
Kiyotake, E. A., Thomas, E. E., Homburg, H. B., Milton, C. K., Smitherman, A. D., Donahue, N. D., et al. (2022). Conductive and injectable hyaluronic acid/gelatin/gold nanorod hydrogels for enhanced surgical translation and bioprinting. J. Biomed. Mater. Res. A 110, 365–382. doi: 10.1002/jbm.a.37294
Lee, E. J., Choi, Y., Lee, H. J., Hwang, D. W., and Lee, D. S. (2022). Human neural stem cell-derived extracellular vesicles protect against Parkinson’s disease pathologies. J. Nanobiotechnol. 20, 1–20. doi: 10.1186/s12951-022-01356-2
Lee, J. H., Park, J.-H., Eltohamy, M., Perez, R., Lee, E.-J., and Kim, H.-W. (2013). Collagen gel combined with mesoporous nanoparticles loading nerve growth factor as a feasible therapeutic three-dimensional depot for neural tissue engineering. RSC Adv. 3, 24202–24214. doi: 10.1039/c3ra43534b
Lei, L., Liu, Z., Yuan, P., Jin, R., Wang, X., Jiang, T., et al. (2019). Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of micro RNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J. Mater. Chem. B 7, 2722–2735. doi: 10.1039/C9TB00025A
Lesný, P., De Croos, J., Přádný, M., Vacık, J., Michalek, J., Woerly, S., et al. (2002). Polymer hydrogels usable for nervous tissue repair. J. Chem. Neuroanat. 23, 243–247. doi: 10.1016/S0891-0618(02)00011-X
Li, R., Li, Y., Wu, Y., Zhao, Y., Chen, H., Yuan, Y., et al. (2018). Heparin-poloxamer thermosensitive hydrogel loaded with bFGF and NGF enhances peripheral nerve regeneration in diabetic rats. Biomaterials 168, 24–37. doi: 10.1016/j.biomaterials.2018.03.044
Li, W., Luo, R., Lin, X., Jadhav, A. D., Zhang, Z., Yan, L., et al. (2015). Remote modulation of neural activities via near-infrared triggered release of biomolecules. Biomaterials 65, 76–85. doi: 10.1016/j.biomaterials.2015.06.041
Li, J., and Mooney, D. J. (2016). Designing hydrogels for controlled drug delivery. Nature Reviews Materials 1:16071. doi: 10.1038/natrevmats.2016.71
Li, X., Tzeng, S. Y., Liu, X., Tammia, M., Cheng, Y.-H., Rolfe, A., et al. (2016). Nanoparticle-mediated transcriptional modification enhances neuronal differentiation of human neural stem cells following transplantation in rat brain. Biomaterials 84, 157–166. doi: 10.1016/j.biomaterials.2016.01.037
Li, L., Xiao, B., Mu, J., Zhang, Y., Zhang, C., Cao, H., et al. (2019). A MnO2 nanoparticle-dotted hydrogel promotes spinal cord repair via regulating reactive oxygen species microenvironment and synergizing with mesenchymal stem cells. ACS Nano 13, 14283–14293. doi: 10.1021/acsnano.9b07598
Lindholm, P., and Saarma, M. (2022). Cerebral dopamine neurotrophic factor protects and repairs dopamine neurons by novel mechanism. Mol. Psychiatry 27, 1310–1321. doi: 10.1038/s41380-021-01394-6
Liu, H., Feng, Y., Che, S., Guan, L., Yang, X., Zhao, Y., et al. (2022). An Electroconductive Hydrogel Scaffold with Injectability and Biodegradability to Manipulate Neural Stem Cells for Enhancing Spinal Cord Injury Repair. Biomacromolecules 24, 86–97. doi: 10.1021/acs.biomac.2c00920
Liu, L., Guo, S., Shi, W., Liu, Q., Huo, F., Wu, Y., et al. (2021). Bone marrow mesenchymal stem cell-derived small extracellular vesicles promote periodontal regeneration. Tissue Eng. A 27, 962–976. doi: 10.1089/ten.tea.2020.0141
Liu, X., Miller, A. L., Park, S., Waletzki, B. E., Zhou, Z., Terzic, A., et al. (2017). Functionalized carbon nanotube and graphene oxide embedded electrically conductive hydrogel synergistically stimulates nerve cell differentiation. ACS Appl. Mater. Interfaces 9, 14677–14690. doi: 10.1021/acsami.7b02072
Ma, X., Wang, M., Ran, Y., Wu, Y., Wang, J., Gao, F., et al. (2022). Design and fabrication of polymeric hydrogel carrier for nerve repair. Polymers 14:1549. doi: 10.3390/polym14081549
Mendiratta, S., Hussein, M., Nasser, H. A., and Ali, A. A. A. (2019). Multidisciplinary role of mesoporous silica nanoparticles in brain regeneration and cancers: from crossing the blood–brain barrier to treatment. Part. Part. Syst. Charact. 36:1900195. doi: 10.1002/ppsc.201900195
Milani, G. M., Coutinho, I. T., Ambrosio, F. N., Monteiro Do Nascimento, M. H., Lombello, C. B., Venancio, E. C., et al. (2022). Poly (acrylic acid)/polypyrrole interpenetrated network as electro-responsive hydrogel for biomedical applications. J. Appl. Polym. Sci. 139:52091. doi: 10.1002/app.52091
Mondello, S., Thelin, E. P., Shaw, G., Salzet, M., Visalli, C., Cizkova, D., et al. (2018). Extracellular vesicles: pathogenetic, diagnostic and therapeutic value in traumatic brain injury. Expert Rev. Proteomics 15, 451–461. doi: 10.1080/14789450.2018.1464914
Nisbet, D. R., Crompton, K. E., Horne, M. K., Finkelstein, D. I., and Forsythe, J. S. (2008). Neural tissue engineering of the CNS using hydrogels. J Biomed Mater Res Part B Appl Biomater 87B, 251–263. doi: 10.1002/jbm.b.31000
Park, J., Jeon, J., Kim, B., Lee, M. S., Park, S., Lim, J., et al. (2020). Electrically conductive hydrogel nerve guidance conduits for peripheral nerve regeneration. Adv. Funct. Mater. 30:2003759. doi: 10.1002/adfm.202003759
Pavón, J. J., Allain, J. P., Verma, D., Echeverry-Rendón, M., Cooper, C. L., Reece, L. M., et al. (2019). In situ study unravels bio-Nanomechanical behavior in a magnetic bacterial Nano-cellulose (MBNC) hydrogel for neuro-endovascular reconstruction. Macromol. Biosci. 19:1800225. doi: 10.1002/mabi.201800225
Pei, X., Zhang, H., Zhou, Y., Zhou, L., and Fu, J. (2020). Stretchable, self-healing and tissue-adhesive zwitterionic hydrogels as strain sensors for wireless monitoring of organ motions. Mater Horiz 7, 1872–1882. doi: 10.1039/D0MH00361A
Psaraki, A., Ntari, L., Karakostas, C., Korrou-Karava, D., and Roubelakis, M. G. (2022). Extracellular vesicles derived from mesenchymal stem/stromal cells: the regenerative impact in liver diseases. Hepatology 75, 1590–1603. doi: 10.1002/hep.32129
Rafieian, S., Mirzadeh, H., Mahdavi, H., and Masoumi, M. E. (2019). A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 26, 154–174. doi: 10.1515/secm-2017-0161
Raposo, G., and Stahl, P. D. (2019). Extracellular vesicles: a new communication paradigm? Nat. Rev. Mol. Cell Biol. 20, 509–510. doi: 10.1038/s41580-019-0158-7
Ribeiro, T. D. C., Sábio, R. M., Luiz, M. T., de Souza, L. C., Fonseca-Santos, B., Cides da Silva, L. C., et al. (2022). Curcumin-Loaded Mesoporous Silica Nanoparticles Dispersed in Thermo-Responsive Hydrogel as Potential Alzheimer Disease Therapy. Pharmaceutics 14:1976. doi: 10.3390/pharmaceutics14091976
Rinoldi, C., Ziai, Y., Zargarian, S. S., Nakielski, P., Zembrzycki, K., Haghighat Bayan, M. A., et al. (2022). In vivo chronic brain cortex signal recording based on a soft conductive hydrogel biointerface. ACS Appl. Mater. Interfaces 15, 6283–6296. doi: 10.1021/acsami.2c17025
Sanes, D. H., Reh, T. A., and Harris, W. A. (2011). Development of the Nervous System. Academic Press.
Santhosh, M., Choi, J.-H., and Choi, J.-W. (2019). Magnetic-assisted cell alignment within a magnetic nanoparticle-decorated reduced graphene oxide/collagen 3D nanocomposite hydrogel. Nano 9:1293. doi: 10.3390/nano9091293
Schmidt, C. E., and Leach, J. B. (2003). Neural tissue engineering: strategies for repair and regeneration. Annu. Rev. Biomed. Eng. 5, 293–347. doi: 10.1146/annurev.bioeng.5.011303.120731
Silva, R. C., Domingues, H. S., Salgado, A. J., and Teixeira, F. G. (2022). From regenerative strategies to pharmacological approaches: can we fine-tune treatment for Parkinson's disease? Neural Regen. Res. 17:933. doi: 10.4103/1673-5374.324827
Soylu, H. M., Chevallier, P., Copes, F., Ponti, F., Candiani, G., Yurt, F., et al. (2021). A novel strategy to coat dopamine-functionalized titanium surfaces with agarose-based hydrogels for the controlled release of gentamicin. Front. Cell. Infect. Microbiol. 11:678081. doi: 10.3389/fcimb.2021.678081
Štulík, J., and Syková, E. (2008). Biocompatible hydrogels in spinal cord injury repair. Physiol. Res. 57, S121–S132. doi: 10.33549/physiolres.931606
Tang, Z., Jiang, F., Zhang, Y., Zhang, Y., Huang, X., Wang, Y., et al. (2019). Mussel-inspired injectable hydrogel and its counterpart for actuating proliferation and neuronal differentiation of retinal progenitor cells. Biomaterials 194, 57–72. doi: 10.1016/j.biomaterials.2018.12.015
Ullah, F., Othman, M. B. H., Javed, F., Ahmad, Z., and Akil, H. M. (2015). Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 57, 414–433. doi: 10.1016/j.msec.2015.07.053
Usman, W. M., Pham, T. C., Kwok, Y. Y., Vu, L. T., Ma, V., Peng, B., et al. (2018). Efficient RNA drug delivery using red blood cell extracellular vesicles. Nature Comm. 9:2359. doi: 10.1038/s41467-018-04791-8
Uz, M., and Mallapragada, S. K. (2019). Conductive polymers and hydrogels for neural tissue engineering. J Indian Inst Sci 99, 489–510. doi: 10.1007/s41745-019-00126-8
Wang, Y., Huang, F., Chen, X., Wang, X.-W., Zhang, W.-B., Peng, J., et al. (2018). Stretchable, conductive, and self-healing hydrogel with super metal adhesion. Chem. Mater. 30, 4289–4297. doi: 10.1021/acs.chemmater.8b01260
Wise, R. A. (2004). Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483–494. doi: 10.1038/nrn1406
Wu, C., Chen, S., Zhou, T., Wu, K., Qiao, Z., Zhang, Y., et al. (2021). Antioxidative and conductive nanoparticles-embedded cell niche for neural differentiation and spinal cord injury repair. ACS Appl. Mater. Interfaces 13, 52346–52361. doi: 10.1021/acsami.1c14679
Xia, B., and Chen, G. (2022). Research progress of natural tissue-derived hydrogels for tissue repair and reconstruction. Int. J. Biol. Macromol. 214, 480–491. doi: 10.1016/j.ijbiomac.2022.06.137
Xie, J., Li, J., Ma, J., Li, M., Wang, X., Fu, X., et al. (2022). Magnesium oxide/poly (l-lactide-co-ε-caprolactone) scaffolds loaded with neural morphogens promote spinal cord repair through targeting the calcium influx and neuronal differentiation of neural stem cells. Adv. Healthc. Mater. 11:e2200386. doi: 10.1002/adhm.202200386
Xu, J., Tsai, Y.-L., and Hsu, S.-H. (2020). Design strategies of conductive hydrogel for biomedical applications. Molecules 25:5296. doi: 10.3390/molecules25225296
Xu, X.-H., Yuan, T.-J., Dad, H. A., Shi, M.-Y., Huang, Y.-Y., Jiang, Z.-H., et al. (2021). Plant exosomes as novel nanoplatforms for microRNA transfer stimulate neural differentiation of stem cells in vitro and in vivo. Nano Lett. 21, 8151–8159. doi: 10.1021/acs.nanolett.1c02530
Yang, K., Lee, J. S., Kim, J., Lee, Y. B., Shin, H., Um, S. H., et al. (2012). Polydopamine-mediated surface modification of scaffold materials for human neural stem cell engineering. Biomaterials 33, 6952–6964. doi: 10.1016/j.biomaterials.2012.06.067
Ye, L., Ji, H., Liu, J., Tu, C.-H., Kappl, M., Koynov, K., et al. (2021). Carbon nanotube–hydrogel composites facilitate neuronal differentiation while maintaining homeostasis of network activity. Adv. Mater. 33:2102981. doi: 10.1002/adma.202102981
Yu, M., Gu, G., Cong, M., Du, M., Wang, W., Shen, M., et al. (2021). Repair of peripheral nerve defects by nerve grafts incorporated with extracellular vesicles from skin-derived precursor Schwann cells. Acta Biomater. 134, 190–203. doi: 10.1016/j.actbio.2021.07.026
Zhang, N., Lin, J., Lin, V. P. H., Milbreta, U., Chin, J. S., Chew, E. G. Y., et al. (2021). A 3D Fiber-hydrogel based non-viral gene delivery platform reveals that microRNAs promote axon regeneration and enhance functional recovery following spinal cord injury. Adv Sci 8:2100805. doi: 10.1002/advs.202100805
Zhang, D., Yao, Y., Duan, Y., Yu, X., Shi, H., Nakkala, J. R., et al. (2020). Surface-anchored graphene oxide nanosheets on cell-scale micropatterned poly (d, l-lactide-co-caprolactone) conduits promote peripheral nerve regeneration. ACS Appl. Mater. Interfaces 12, 7915–7930. doi: 10.1021/acsami.9b20321
Zhang, Z., Zhang, X., Wang, C., Teng, W., Xing, H., Wang, F., et al. (2022). Enhancement of motor functional recovery using immunomodulatory extracellular vesicles-loaded injectable thermosensitive hydrogel post spinal cord injury. Chem. Eng. J. 433:134465. doi: 10.1016/j.cej.2021.134465
Zheng, X., Zhang, P., Fu, Z., Meng, S., Dai, L., and Yang, H. (2021). Applications of nanomaterials in tissue engineering. RSC Advances 11, 19041–19058.
Zhou, L. (2018). Construction of neurotransmission function hydrogel and its application in spinal cord injury repair. [Doctoral dissertation]. South China University of Technology.
Zhou, L., Fan, L., Yi, X., Zhou, Z., Liu, C., Fu, R., et al. (2018). Soft conducting polymer hydrogels cross-linked and doped by tannic acid for spinal cord injury repair. ACS Nano 12, 10957–10967. doi: 10.1021/acsnano.8b04609
Zhou, C., Wu, T., Xie, X., Song, G., Ma, X., Mu, Q., et al. (2022). Advances and challenges in conductive hydrogels: from properties to applications. Eur. Polym. J. 177:111454. doi: 10.1016/j.eurpolymj.2022.111454
Keywords: functionalized hydrogel, nerve injury, tissue engineering, nerve regeneration, nanomaterial
Citation: Zhao W, Tu H, Chen J, Wang J, Liu H, Zhang F and Li J (2023) Functionalized hydrogels in neural injury repairing. Front. Neurosci. 17:1199299. doi: 10.3389/fnins.2023.1199299
Received: 03 April 2023; Accepted: 27 April 2023;
Published: 19 June 2023.
Edited by:
Yi Cao, Xiangtan University, ChinaReviewed by:
Yunhuan Yuan, Harbin Institute of Technology (Shenzhen), ChinaCopyright © 2023 Zhao, Tu, Wang, Liu, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengshou Zhang, ZmVuZ3Nob3V6aGFuZ0AxNjMuY29t; Jing Li, OTk0MzIxNEBoYXVzdC5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.