
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 18 May 2023
Sec. Neural Technology
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1167612
This article is part of the Research TopicAdvanced Deep Learning Approaches for Medical Neuroimaging Data with LimitationView all 5 articles
 Dongnan Liu1,2*
Dongnan Liu1,2* Mariano Cabezas2
Mariano Cabezas2 Dongang Wang2,3
Dongang Wang2,3 Zihao Tang1,2
Zihao Tang1,2 Lei Bai2,4
Lei Bai2,4 Geng Zhan2,3
Geng Zhan2,3 Yuling Luo2,3
Yuling Luo2,3 Kain Kyle2,3
Kain Kyle2,3 Linda Ly2,3
Linda Ly2,3 James Yu2,3
James Yu2,3 Chun-Chien Shieh2,3
Chun-Chien Shieh2,3 Aria Nguyen2,3
Aria Nguyen2,3 Ettikan Kandasamy Karuppiah5
Ettikan Kandasamy Karuppiah5 Ryan Sullivan6
Ryan Sullivan6 Fernando Calamante2,6,7
Fernando Calamante2,6,7 Michael Barnett2,3
Michael Barnett2,3 Wanli Ouyang4
Wanli Ouyang4 Weidong Cai1
Weidong Cai1 Chenyu Wang2,3
Chenyu Wang2,3Background and introduction: Federated learning (FL) has been widely employed for medical image analysis to facilitate multi-client collaborative learning without sharing raw data. Despite great success, FL's applications remain suboptimal in neuroimage analysis tasks such as lesion segmentation in multiple sclerosis (MS), due to variance in lesion characteristics imparted by different scanners and acquisition parameters.
Methods: In this work, we propose the first FL MS lesion segmentation framework via two effective re-weighting mechanisms. Specifically, a learnable weight is assigned to each local node during the aggregation process, based on its segmentation performance. In addition, the segmentation loss function in each client is also re-weighted according to the lesion volume for the data during training.
Results: The proposed method has been validated on two FL MS segmentation scenarios using public and clinical datasets. Specifically, the case-wise and voxel-wise Dice score of the proposed method under the first public dataset is 65.20 and 74.30, respectively. On the second in-house dataset, the case-wise and voxel-wise Dice score is 53.66, and 62.31, respectively.
Discussions and conclusions: The Comparison experiments on two FL MS segmentation scenarios using public and clinical datasets have demonstrated the effectiveness of the proposed method by significantly outperforming other FL methods. Furthermore, the segmentation performance of FL incorporating our proposed aggregation mechanism can achieve comparable performance to that from centralized training with all the raw data.
Multiple sclerosis (MS) is a chronic inflammatory and degenerative disease of the central nervous system, characterized by the appearance of focal lesions in the white and gray matter that topographically correlate with an individual patient's neurological symptoms and disability. Globally there are an estimated 2.3 million people with MS and, besides trauma, the disease constitutes the most common cause of neurological disability in young adults (Prinster et al., 2006; Coles et al., 2008; Plantone et al., 2015; Mills et al., 2018). Lesion characteristics, such as number and volume, are principal imaging metrics for both MS clinical trials and monitoring of the disease in clinical practice (Carass et al., 2017; Filippi et al., 2019; Schwenkenbecher et al., 2019; Pontillo et al., 2021). To this end, automatic, robust, and accurate MS lesion segmentation with Magnetic Resonance (MR) imaging is crucial to both MS research and patient management (Zijdenbos et al., 2002; Lladó et al., 2012; Brosch et al., 2016; Aslani et al., 2019; Cerri et al., 2021).
In classical MS lesion segmentation methods, the brain tissues types, such as white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF), are firstly segmented based on the raw MR images via statistical methods, e.g., the Expectation-Maximization (EM) algorithm (Catanese et al., 2015; Beaumont et al., 2016) or Gaussian Mixture Modeling (Doyle et al., 2016; Knight and Khademi, 2016). Then, lesions are detected as outliers based on the tissue masks (Catanese et al., 2015; Beaumont et al., 2016; Doyle et al., 2016; Knight and Khademi, 2016). With the advent of deep learning-based medical data computing (Plis et al., 2014; Livne et al., 2019; Sun et al., 2019), deep learning models that learn representative features via convolutional modules have been widely employed for automatic MS lesion segmentation, achieving competitive performance (Brosch et al., 2016; Ghafoorian et al., 2017; Valverde et al., 2017; Wang et al., 2018; Zhang et al., 2018; Aslani et al., 2019; McKinley et al., 2020; Nair et al., 2020; Isensee et al., 2021; Ma et al., 2022).
Despite this, there remain significant challenges in the current methods (Danelakis et al., 2018; Ma et al., 2022). In clinical practice, the data quality of brain MRI varies across MRI scanners due to variance in image geometry, resolution, tissue intensity, and contrast conferred by differences in hardware (scanner and coil) and acquisition protocols (Kamnitsas et al., 2017; Dewey et al., 2019; Valverde et al., 2019; Ackaouy et al., 2020). These domain differences limit the performance of supervised learning methods when applied to images from new scanners (Kamnitsas et al., 2017; Ackaouy et al., 2020; Ma et al., 2022). Such phenomenon is referred to as the domain shift issue, which exists in various medical image analyses applications for multiple datasets from different resources (e.g, modalities, sites) (Valverde et al., 2019; Chen et al., 2020; Liu et al., 2020). Recently, cross-domain MS lesion segmentation methods have been further explored to enhance the models' generalization ability. In particular, the domain differences are alleviated by inducing the model to generate scanner-invariant features (Kamnitsas et al., 2017; Ackaouy et al., 2020), learning from synthetic images that follow the distribution of the target scanners (Palladino et al., 2020), and cross-scanner data harmonization (Dewey et al., 2019). A crucial prerequisite of these methods is that all the data from multiple scanners should be fed into the framework simultaneously. However, sharing clinical data across sites invokes privacy issues, which limit the practical applications of these methods in large collaborative studies (Li et al., 2020b; Guo et al., 2021).
Federated learning (FL) techniques where training is decentralized were proposed for multi-center computer vision while preserving data privacy and security (McMahan et al., 2017; Li et al., 2020a, 2021). Briefly, at the beginning of the FL process, each participating client is firstly assigned an initialized model. Note that throughout the paper, we use the notion “client” to represent the data in each distinct scanner or clinical center. Next, these models are trained using the local data in each client. After several training iterations, each client is required to share their private model weights with a central server, which aggregates these local weights and distributes them back to each client. Initialized by the updated weights from the server, the model in each client continues their local training for another round of FL process. By enriching the knowledge learned in each local model without sharing the raw data, the server side can eventually obtain a model for each client which can achieve a good performance simultaneously. FL methods have also been widely employed for multi-client medical image analysis (Li et al., 2020b; Guo et al., 2021; Liu et al., 2021a; Shen et al., 2021). In Li et al. (2020b) and Guo et al. (2021), each local model is incorporated with an adversarial domain discriminator to alleviate the inter-client distribution bias. However, the intermediate features in each local client are required to be shared across clients. Despite these privacy-preserving strategies, distributing features still incur the risk of data leakage. To solve this problem, FedBN (Li et al., 2021) has been proposed for domain adaptive FL by only processing the parameters outside the batch normalization layers of each local model.
Although FL methods are effective to address these concerns in many medical imaging scenarios, their applicability is limited to MS lesion segmentation. Particularly, they have not considered the weighting strategies for the global aggregation and local training, which is crucial for FL MS segmentation. First, during aggregation, the central server averages the model parameters from all the local clients, assuming each local model has the same importance and performance. For MS lesion segmentation, the datasets from multiple clients, their data distribution and the lesion morphology and signal characteristics can vary greatly (Kamnitsas et al., 2017; Ackaouy et al., 2020), which can lead to divergence of the private local models, thereby conferring distinct segmentation characteristics when they are aggregated in the central server. By fusing a model with inferior segmentation performance to others with superior ability, the segmentation performance for the entire updated model may be compromised (Shen et al., 2021). Second, differences in the clinical distribution of patients can impact lesion burden, size, and morphology at a client level, generating significant inter-site variance in multi-client studies, as shown in Figure 1. As explored in Nichyporuk et al. (2021); Shirokikh et al. (2020), a model trained on a dataset with smaller lesions will usually present a lower performance due to the lack of lesion samples for training. However, the task loss functions in each client are optimized with the same importance in previous FL methods (McMahan et al., 2017; Li et al., 2020b, 2021), which would induce the inferior performance of the central model on the clients with smaller lesion sizes, and further influence the overall FL segmentation accuracy.
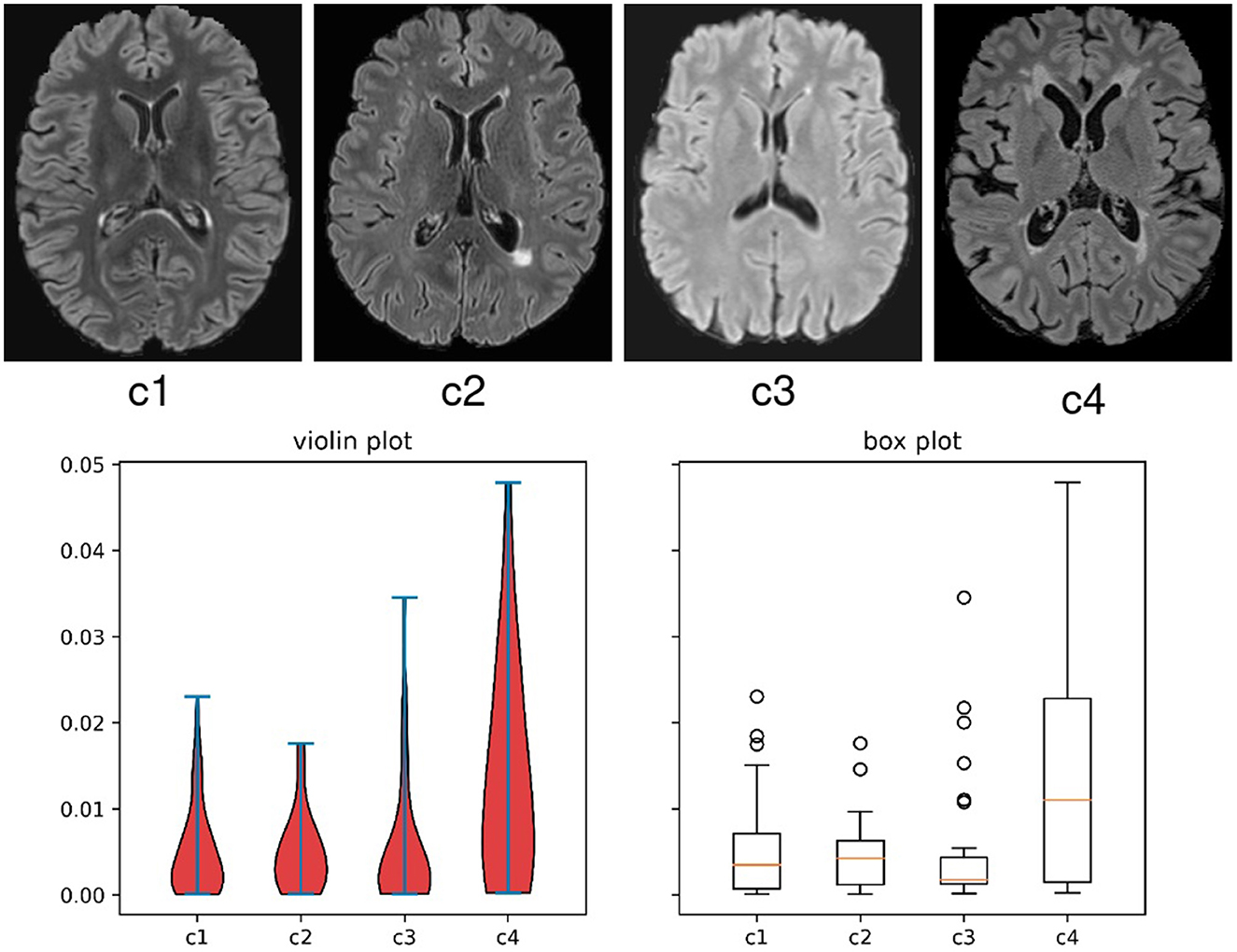
Figure 1. Evidence of the variance on appearance and lesion volume in multi-client studies in scenario 2 of this work, where cases are from clinical trials. The top images are examples of 2D slices from each client in the study. The bottom graphs are the violin and box plots for the lesion volume to brain volume ratio distributions per client for all the subjects in this FL study.
To solve the aforementioned issues, we propose a Federated MS lesion segmentation framework based on two dynamic Re-Weighting mechanisms (FedMSRW). Our FedMSRW method can alleviate the cross-client data distinctions caused by both image distributions and label variance. Specifically, we first alleviate the negative influence from the domain shift on the MRI data from different clients, by employing aggregation mechanisms from FedBN (Li et al., 2021). Second, during the model aggregation process, the model parameters from each client are assigned a weight based on their segmentation abilities during local training, including the segmentation performance and confidence. Models with higher abilities are assigned a higher weight and vice versa. To solve the lesion volume imbalance across different clients, we further propose to re-weight the task loss function in each client based on the average case-wise lesion volume ratio, i.e., the ratio of lesion volume to the brain volume, of the training data for that client. Motivated by Shirokikh et al. (2020), where more attention should be paid to smaller lesion objects during model training, the weights for the overall loss functions in clients with a smaller lesion volume are enlarged, and vice versa.
The major contributions of this work are summarized as follows:
• To the best of our knowledge, this work is the first application of privacy-preserving FL methods to the task of MS lesion segmentation and, in particular, to multi-client MS datasets featured with different data characteristics.
• We propose uncertainty-aware re-weighting mechanisms during the central model aggregation process to prevent the negative influence of the inferior local models.
• We further propose to re-weight the segmentation loss functions in each local client/center based on its local lesion volume ratio, addressing the impact of client-specific lesion variance in the multi-client MS datasets.
• We have conducted extensive experiments in two FL MS lesion segmentation scenarios using both public and real-world clinical MS datasets. Our FedMSRW method outperforms typical FL methods significantly.
In this work, we have conducted experiments on two FL MS lesion segmentation scenarios. We first conduct experiments on a public MS lesion segmentation dataset from multiple clients, in favor of reproducibility. Second, we conduct experiments using our own multi-site MS lesion segmentation from different hospitals labeled following clinical trial standard, to further demonstrate the effectiveness of our proposed method in clinical practice. The study is approved by the University of Sydney Human Research and Ethics Committee.
First, we conducted experiments on the MSSEG-2016 MS lesion segmentation challenge from MICCAI (Commowick et al., 2018, 2021), containing a totally of 53 cases from 4 different sites, as illustrated in Table 1. In each case, different MR imaging modalities are available, including a FLAIR sequence, a T1 weighted sequence pre and post-Gadolinium injection, a T2 sequence, and a PD sequence. All sequences are co-registered to FLAIR sequences at a similar resolution via rigid registration. In addition, the pre-processing steps are conducted including denoising with the NL-means algorithm, brain extraction via the volBrain platform, and the N4 bias correction. In our experiments, we only use the FLAIR sequence. All experiments were performed in two-fold cross-validation. At each iteration, 3D patches of size 64 × 64 × 64 were randomly cropped from the original FLAIR images, with random flipping and rotation augmentations.
To further indicate the effectiveness of our proposed framework on the FL MS lesion segmentation tasks in a practical clinical scenario, we conducted experiments using in-house and public multi-scanner MS datasets from 4 different scanners.
Among them, the data from C1, C2, and C3 are obtained from three different hospitals using different scanners. as indicated in Table 1. All the cases are acquired from patients with relapsing and remitting MS, which is diagnosed based on the McDonald 2010 criteria (Polman et al., 2011). Additionally, the disease duration is less than 10 years, with an expanded disability status scale (EDSS) score of less than 4. Each case contains 3D MRI sequences in two modalities, including a T1 sequence without gadolinium administration and a FLAIR sequence. For all the cases, they are acquired under several different geometrics and timing protocols. For the lesion labeling process, the T1 and FLAIR sequences of each case are resampled to a 3 mm slice thickness for accelerated labeling and to provide a common labeling space). First, the automatic Jim 5.0 (http://www.xinapse.com/home.php) is employed to detect and delineate the lesions on the FLAIR images in a semi-automatic manner. For each case, at least two trained neuroimaging analysts at the Sydney Neuroimaging Analysis Centre (Sydney, Australia) confirmed all the segmentations based on the T1 and FLAIR images, to generate final, gold standard reference masks.
To further increase the diversity of the multi-client MS data, we included a public dataset from a new site acquired with a new type of scanner (Lesjak et al., 2018), in addition to the private data from different scanners. This dataset consists of 30 cases imaged from MS patients under 3 different modalities, consisting of a 2D T1-weighted sequence, 2D T2-weighted sequence, and a 3D FLAIR sequence.
For the data usage, we follow the same settings in Scenario 1, where only the FLAIR sequence for each case is employed. To further simulate the practical multi-client scenario, we use the data in their original resolutions, without any registration process. Given the larger scale of the dataset compared with those in Scenario 1, all experiments under these settings were conducted in a three-fold cross-validation manner. During training, the 32 × 32 × 32 patches were randomly cropped from the original MRI data, with the augmentations of flipping and rotations.
The framework of our proposed FedMSRW method is shown in Figure 2. We denote Di = {Xi, Yi}i = 1, 2, ..., N as the set of MS lesion segmentation datasets from N different clients, where X and Y represent the MR images and the corresponding lesion annotations. In the ith client, the local model Mi with the parameters θi is optimized via:
where Ldice is the soft Dice loss function for probabilistic binary segmentations (Milletari et al., 2016):
Due to the data distribution differences in multi-client MR images, we establish our proposed FedMSRW on FedBN (Li et al., 2021), which tackles the domain bias issues in FL processes that only require sharing of the model parameters. Based on the assumption that the parameters of the normalization layers in deep learning models represent the domain-specific information (Huang et al., 2018; Chang et al., 2019), FedBN prevents the central model from domain shift by aggregating the parameters in the convolutional layers, while ignoring those in the batch normalization layers. Specifically, each θi can be represented as: , where are the parameters for all the batch normalization layers, and are those for the rest layers. After collecting the local weights, the central server aggregates model through:
Then the central server distributes the updated weights to each local client. At the beginning of the next round of local segmentation training, each Mi is then initialized as .
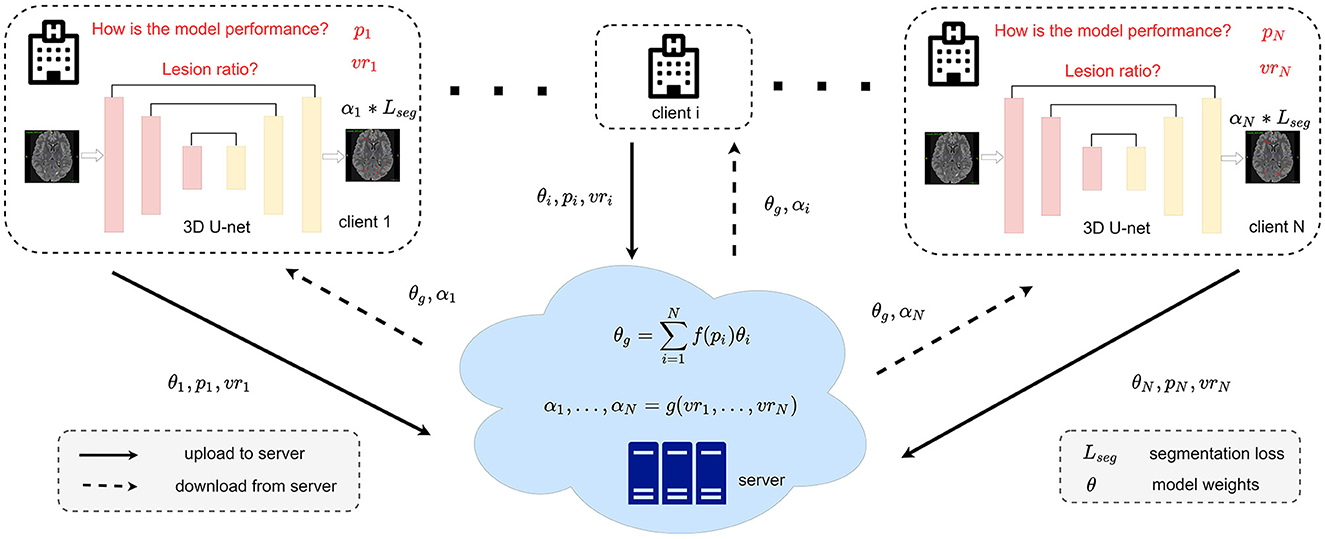
Figure 2. Detailed framework of our FedMSRW method. The f(.) for calculating the weighting factors during model aggregation can be referred to Equation (4). The details of g(.) for the segmentation task re-weighting are in Equation (6).
Due to distinct, client-specific characteristics of both the MRI data and the MS lesions, the difficulty of lesion segmentation tasks differs across clients. To this end, the segmentation ability for the various Mi is different after each round of local training. According to Equation (3), both the low-performance and high-performance models are assigned equal importance during the aggregation process at the central server. This is suboptimal since the local models with inferior segmentation ability influence the updated model from the server and further limit collaborative knowledge learning in FL. A trivial solution to this problem is to adjust the number of training samples for each client, as indicated in McMahan et al. (2017). However, there is no simple, non-biased sample selection mechanism to alleviate the negative effects of the models with inferior performance. Additionally, selecting auxiliary hyperparameters manually in FL would limit the model's robustness.
To this end, we propose an aggregation re-weighting mechanism based on the segmentation performance of each Mi during the training process in the local clients. For each training iteration in client i, we define the input data and corresponding labels as x and y, respectively. The segmentation ability for probabilistic lesion segmentation Mi is measured as:
As indicated in Equation (4), the first item represents the models' confidence in the predicted lesion segmentation. Since the MS lesion region of interest occupies only a tiny fraction (around 1% on average) of the whole brain volume, the confidence value within the true positive lesion regions better reflects the models' lesion prediction certainty relative to traditional methods that measure the models' confidence based on the entropy of the whole prediction map. For the second part (1 − Ldice(Mi(x), y)) in Equation (4), the model's segmentation performance is further considered for re-weighting. If the model has a better segmentation accuracy, its attribute during aggregation is upgraded, and vice versa. Finally, the average Pi for all the local training iterations is able to indicate the segmentation ability for the Mi. Considering Pi, the central aggregation process in Equation (3) is re-formulated as:
Another challenge in FL MS lesion segmentation tasks is the heterogeneity of lesion size across different clients. As indicated in Nichyporuk et al. (2021); Shirokikh et al. (2020), lesions with smaller sizes should be assigned a larger weight during model training. To this end, we further propose to re-weight the segmentation loss functions in each client defined in Equation (1) based on the lesion volume.
For the kth round of local training in client i, we first calculate the average lesion volume ratio of all the data samples for training. Specifically, the lesion ratio in each training patch is the ratio of the lesion volume to the brain volume. Compared with only counting the voxel number of lesions, the lesion volume ratio can avoid inaccurate estimations when the proportions of the brain volume in some specific training patches are small. Next, the is accumulated with the average lesion volume ratio from the previous k − 1 round, denoted as vri. With the increase of k, the accumulated vri can represent the true lesion volume ratio for the data used during the model training process in each client. In the K + 1 th round of local training, the segmentation loss in Equation (1) is then reformulated as:
The overall training algorithm of our proposed FedMSRW method is indicated in Algorithm 1. In each local client, the lesion segmentation task is trained with a 3D U-Net (Çiçek et al., 2016). During training, we employ the SGD optimizer with a momentum of 0.9, a weight decay of 0.0005, and a learning rate of 0.0002. After every 800 training iteration, the local models are sent to the central server for aggregation. During inference, the model in each client is constructed by the central aggregated convolutional weights and the client-private batch normalization weights.
Regarding the data splits, N-fold cross-validation has been conducted on all the experiments to ensure all the cases are evaluated. First, all the images in each client are randomly split into N-folds. For the experiments on each fold, the (N-1) folds are used for training and validation, while the rest fold of the data is employed only for testing. Such a process has been repeated N times and the average segmentation performance of all cases is reported as the final results for each method. During testing, each case is first cropped into patches of the same size as the training inputs. The segmentation results of the patches of each case are then constructed together to form the final segmentation prediction of this case. Our experiment is implemented with PyTorch (Paszke et al., 2017) on 4 RTX 6000 GPU devices with 24 GB memory. The CPU device is an AMD EPYC 7302 16-Core Processor, and the total memory for the RAM is 256 GB.
To evaluate the segmentation performance of our proposed method, we first employed the case-level and voxel-level Dice coefficient, defined as:
where TP, FP, and FN indicate the number of true positive, false positive, and false negative voxel predictions, respectively. The case-wise Dice score (C-Dice) was obtained by the average Dice score for all cases. For the voxel-level Dice score (V-Dice), we first calculate the total voxel numbers of the TP, FN, and FP predictions for all the testing cases. Next, the V-Dice score is obtained using these accumulated metrics. Additionally, we also evaluated the performance based on the true positive rate (TPR) and false positive rate (FPR) at the voxel level via the accumulated TP, FN, and FP, defined as:
In this section, we present the detailed MS lesion segmentation performance under two FL scenarios. Following typical FL methods (Li et al., 2021; Liu et al., 2021a), we also present two common multi-center learning settings as references, including single-client training, and the centralized training. Specifically, the single-client training indicates each client train and test their models locally, without any cross-client communications (Single), and the centralized training indicates the model is optimized directly on all the data from all clients (Central). For a fair comparison, the Single and Central methods are implemented via the N-fold cross-validation settings as our proposed FedMSRW under the same data split. The experimental results are shown in Tables 2, 3. Compared with the single client training, our proposed FedMSRW method can achieve stable performance gain under the majority of metrics in both scenarios. Specifically, our FedMSRW method outperformed the single-client training under the case-wise and voxel-wise dice scores, and the voxel-wise true positive rate. In addition, we notice our proposed FedMSRW method can even outperform the centralized training method in the second scenario, without sharing the data across clients.
To demonstrate the superiority of our proposed FedMSRW method over other FL methods on FL MS lesion segmentation tasks, we present the experimental results in comparison with typical FL methods, including (1) FedAvg (McMahan et al., 2017), a fundamental FL method by central aggregation via averaging of model weights; (2) FedProx (Li et al., 2020a), a FL framework introducing an auxiliary regularization mechanism in each client to stabilize learning, (3) FedBN (Li et al., 2021), an FL framework which can alleviate the cross-site data distribution bias by ignoring parameters in the normalization layers during aggregation, and (4) DWA (Shen et al., 2021), a dynamic re-weighting mechanism for the central model aggregation process based on the changes of the loss functions in each client. For a fair comparison, we re-implement the DWA on the same FL baseline as our proposed FedMSRW method, i.e., FedBN. We also report the results by training within each local client (Single), and joint training with the raw data from all clients (Central). We maintained the same data split on the N-fold cross-validation for all methods. The experimental results under two FL MS segmentation scenarios are shown in Table 4 and Figure 3.
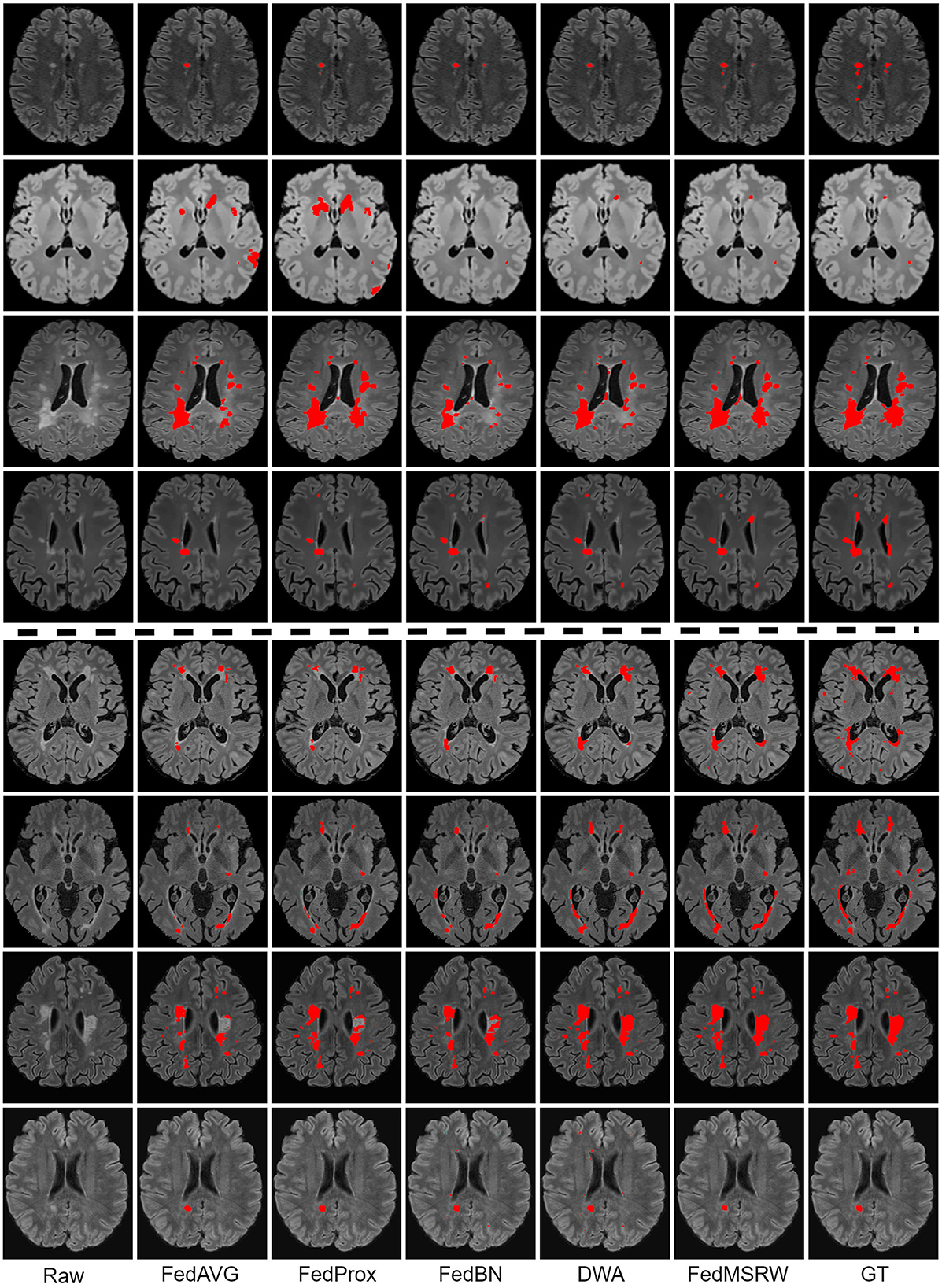
Figure 3. Qualitative results on the comparison FL methods. Lesion masks are overlaid on the original images. The top four rows are the visualization for the Scenario 1, and the bottom four rows are for the Scenario 2. The examples in all rows are from different patients.
To indicate the effectiveness of our proposed weighting mechanism for the central aggregation (CA) process and local training (LT) process, we present ablation experiments and the results are shown in Table 5. For both two scenarios, we notice that solely employing the CA or LT mechanism can sometimes incur performance drop. However, by jointly incorporating the two re-weighting mechanisms, we can consistently improves the baseline (FedBN) method by a large margin, indicating the effectiveness and robustness of our method on the FL MS segmentation tasks.
For deep learning-based medical image analysis models, there can be multiple design selections even under the similar motivation. In this section, we investigate different design choices of our FedMSRW method on the two scenarios. These experiments were conducted on both scenarios and the results are shown in Table 6.
First, we replace the model's segmentation confidence in Equation (4) with the entropy map of the whole segmentation predictions (“Ours-ent” in Table 6), following typical uncertainty learning methods in medical image segmentation (Yu et al., 2019; Liu et al., 2021b). Equation (4) is then re-formulated as:
Finally, each local model in the central aggregation process in Equation (5) is assigned a weight of . In addition, we conducted experiments in which lesion volume was employed for local-level re-weighting on the task learning, referred to as the “Ours-vol” method in Table 6. Specifically, the volume ratio vri in Equation (6) is replaced by the total number of lesion voxels vi. The results in Table 6 indicate the “Ours-ent” and “Ours-vol” are less robust than the FedMSRW method, since their performance drops on Scenario 2, while our FedMSRW can improve the baseline on both two scenarios.
For typical deep learning MS lesion segmentation methods (Brosch et al., 2016; Ghafoorian et al., 2017; Valverde et al., 2017; Zhang et al., 2018; Aslani et al., 2019; McKinley et al., 2020; Nair et al., 2020; Isensee et al., 2021; Ma et al., 2022), MR sequences under different modalities are jointly employed to achieve an outstanding segmentation performance. In this section, we have explored whether such implementations are still effective under the FL scenarios. Specifically, we have evaluated the performance of our methods using different MRI modalities. Our experiments are conducted on the MSSEG-2016 challenge, where each subject has five imaging modalities (T1, FLAIR, T2, DP, and GADO). The results are shown in Table 7, where we have presented the results using T1 and FLAIR, and all five modalities. This tables shows that the FL method trained on FLAIR MRI cases can achieve a better performance than on more modalities.
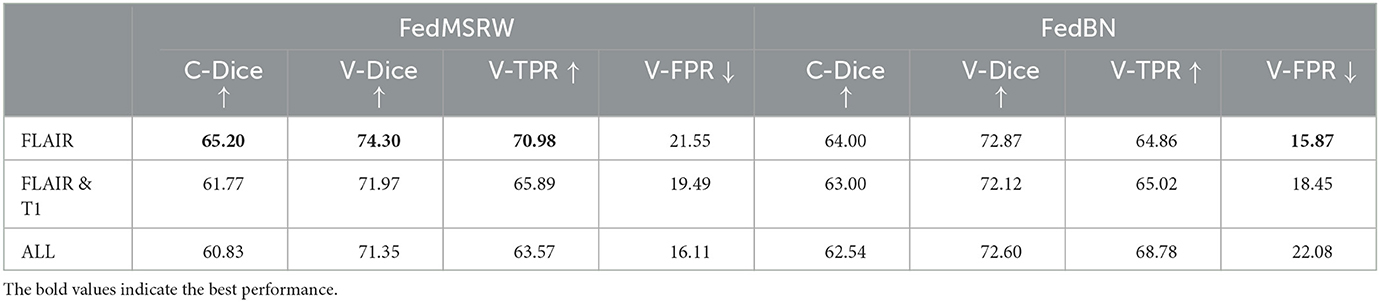
Table 7. Details of the experimental results on using different imaging modalities on the MSSEG-2016 dataset.
We have presented here an FL MS lesion segmentation framework, FedMSRW, which includes two innovative re-weighting mechanisms for improved performance of the FL aggregated model. Specifically, a learnable weight is assigned to each local node during the aggregation process, based on its segmentation performance. In addition, the segmentation loss function in each client is also re-weighted according to the lesion volume for the data during training.
In contrast to typical FL benchmark tasks, which assume the disease burden/lesion loads for each client are in the same distribution space (Li et al., 2021), the MS lesion segmentation task is confounded by substantial inter-client lesion heterogeneity / distinctions. For the multi-client MS lesion segmentation dataset, the data distributions for each client are distinct, reflecting variance in hardware and image acquisition protocols. This results in domain bias issues when optimizing the aggregated model on each local client. For MS lesion segmentation task, the foreground objects (i.e., lesions) are almost always small and numerous, with a heterogenous spatial distribution. For specific clients whose MR images generally contain smaller lesions with more noise, it is more challenging for a 3D U-Net to segment lesions accurately. In the first scenario of our work, the MS lesion segmentation experiments were conducted on images the MSSEG-2016 dataset. As shown in Table 4, the performance of the typical FedAvg and FedProx methods is worse than the models solely trained with the data in each specific client, which did not demonstrate the benefit of inclusion of additional dataset through federated learning. Subsequently, the domain shifts incur inaccurate segmentation performance for the FedAvg and FedProx methods. By preserving the domain-specific batch normalization in each client, FedBN can alleviate the issue and improve the locally trained models. With the two proposed re-weighting mechanisms at the global and local levels, our FedMSRW method can further outperforms FedBN.
In the second scenario, FL methods were conducted on the in-house and data and a public dataset, where the data differences across the clients are more distinct, and therefore an overall reduced performance is expected. The experimental results are presented in Table 4. We observed a similar phenomenon as the first scenario, namely that cross-client distribution bias in multi-client MS datasets degrades the collaborative performance of the FedAvg and FedProx, while FedBN achieves much better performance by alleviating the domain bias. However, incorporating the DWA with the FedBN baseline has incurred a severe performance drop. The relatively larger dataset used from each client in the second scenario, which exaggerates client-specific differences in data distribution, may explain this observation. Compared to the limited performance of other comparison FL methods in scenario 2, our FedMSRW can improve FedBN by a large margin, which further indicates the robustness of our proposed method. In addition, Shen et al. (2021) recently proposed an FL method with re-weighting schemes for each local model's training based on the loss value changes. However, its dynamic weighting strategy is sensitive to hyperparameter selections, which lacks robustness. Rather, our proposed re-weighting mechanisms at the global and local levels are effective and simple, without auxiliary hyperparameters. On the other hand, the superiority of our proposed FedMSRW method also indicates its effectiveness.
According to Table 4, FedBN can improve the segmentation performance since it alleviates the distinctions for the cross-client MR images. However, its performance is still limited by ignoring the bias of labeling space on MS lesion segmentation tasks. To solve this problem, we propose a re-evaluation of the weighting mechanism for the central aggregation (CA) process and local training (LT) process. As shown in Table 5, solely employing the CA or LT mechanism incurs an unstable performance gain. In Scenario 1, the LT module marginally degrades the Dice score, and incurs an even larger performance drop in the second scenario. A similar phenomenon has been observed in Shen et al. (2021), namely that re-weighting the training loss functions in each client generates unstable FL performance. For the CA module, this introduces a slight performance gain under all the segmentation metrics. Conversely, in the proposed FedMSRW framework, jointly incorporating the two re-weighting mechanisms consistently improves the baseline (FedBN) method by a large margin, indicating the effectiveness and robustness of our method on the FL MS segmentation tasks. Moreover, our proposed even outperformed centralized training on the voxel- and case-wise dice scores in Scenario 2. This is an important finding to emphasize the superiority and data privacy preserving capability of our proposed FedMSRW method on the MS lesion segmentation data from clinical trials and with large cross-client data distinctions. Figure 3 illustrates a visual comparison of FedMSRW with other methods, which indicates the outstanding segmentation performance of our method from the qualitative perspective.
We further conduct experiments to investigate whether different model design strategy can introduce performance variance, as indicated in Table 5. Due to the severe imbalance of MS lesions in the brain MRI from the clinical practice, utilizing entropy maps incurs inaccurate representations of the model's segmentation confidence, and further degrades the FL segmentation performance in both two scenarios. Therefore, we select the global-level re-weighting mechanism based on the mask probability as defined in Equation (5), due to the consistent performance gain. For the local level re-weighting based on true lesion volume, the “Ours-vol” method degrades the segmentation accuracies under all metrics in the two FL scenarios. It is potentially because the inaccurate estimation of the true MS lesion distributions in brain MRI patches for model training. For both the “Ours-ent” and “Ours-vol” selections, we notice although they can improve the FedBN baseline in the Scenario 1, a severe performance drop has been incurred in the Scenario 2. The potential reasons for this phenomenon are two-folds: (1) each client of the Scenario 2 has more data than those in Scenario 1; (2) the multi-client MS dataset in Scenario 2 is constructed by various datasets from in-house scanners and the public resources, which brings more distinctions for the cross-client data distributions.
Furthermore, as illustrated in Table 7, we have evaluated the performance of our framework using different MRI modalities. We notice that although introducing auxiliary modalities can bring more imaging contrast information for segmentation learning, the models actually suffer from performance drop under the FL settings. A potential reason is that, during the FL process, the data distributions across different clients are heavily distinct, which limits the models' segmentation performance on these clients. In addition, including auxiliary data in multiple modalities also introduce more noise and variences from data processing and registration processes. To this end, the FLAIR only approach in our experiments remains the most effective input imaging modality for FL MS lesion segmentation.
We have conducted a computational complexity study on the aggregation process of the proposed FL methods. Specifically, each aggregation process of our proposed FedMSRW method costs 73 ms, while the baseline FedBN costs 72 ms. Since our proposed method has not included auxiliary trainable modules, no extra parameters are introduced. Given the superior performance of our method indicated in Table 4, we think the auxiliary computational cost of our FedMSRW method is negligible, and our proposed aggregation mechanisms at the global and local levels are effective and efficient.
One limitation of this work is the potential bias for the MS lesion masks. In our experiments, the labeling was done with trained Neuroimaging analysts and tested in simulated FL settings. In real-world FL application scenarios, labeling from different sites can have more variance. In addition, the segmentation models for each client in practical FL might also be different, which limits the usage of the model aggregation mechanisms in our work, as well as the typical FL methods. One of the future directions of this work is to implement our FL method on broad computer vision studies and beyond MS applications, to further explore the utility and generalization ability of the two adaptive aggregating mechanisms. The second future direction is to implement the algorithm on the practical computational platform with multiple servers, since existing FL research studies (e.g., our method, FedAVG, and FedBN) are implemented on a single server for the simulated FL setting. This might overlook some issues due to the distinctions among local servers in real-world scenarios. For example, the performance of each local hardware device varies in practical applications. This brings auxiliary communication costs, although does not affect the segmentation accuracies, since the central server has to wait for every client to finish their local training before aggregation. To address this problem, our third future potential is to facilitate the computational efficiency of the FL framework in practical applications, such as introducing lightweight deep learning models for each local client.
In this work, we proposed a novel FedMSRW method for MS lesions segmentation under the federated learning settings. Our FedMSRW is featured with global and local reweighting mechanisms to adjust the variance of the MR data and annotations across clients. Extensive experiments in two FL MS lesion segmentation scenarios indicated the superiority of our proposed re-weighting mechanism compared with typical FL methods. The demand for privacy-preserving FL in clinical scenarios heightens the imperative to refine existing approaches. FedMSRW is an important methodological advance for analyzing heterogenous multi-client imaging datasets with FL.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the University of Sydney Human Research and Ethics Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
DL designed the research method, conducted the code implementation, and wrote the draft. MC, DW, ZT, LB, and GZ have been involved in the research design and discussions, and the manuscript revision. YL, KK, LL, and JY have been involved in in-house data processing and annotations. C-CS and AN have been involved in the project management. EK, RS, FC, MB, WO, WC, and CW have been involved in the project supervision, project support, research design, and manuscript revision. All authors contributed to the article and approved the submitted version.
This research was supported by Australia Medical Research Future Fund under Grant (MRFFAI000085).
We thank the organizers of the public datasets used in this paper for providing the data and annotations. We also thank the contributions of the staff at Sydney Neuroimaging Analysis Centre for the in-house data processing and annotation.
EK is employed by NVIDIA Corporation, Singapore. DW, GZ, YL, KK, LL, JY, C-CS, AN, and CW are employees at Sydney Neuroimaging Analysis Centre.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1167612/full#supplementary-material
Ackaouy, A., Courty, N., Vallee, E., Commowick, O., Barillot, C., and Galassi, F. (2020). Unsupervised domain adaptation with optimal transport in multi-site segmentation of multiple sclerosis lesions from MRI data. Front. Comput. Neurosci. 14, 19. doi: 10.3389/fncom.2020.00019
Aslani, S., Dayan, M., Storelli, L., Filippi, M., Murino, V., Rocca, M. A., et al. (2019). Multi-branch convolutional neural network for multiple sclerosis lesion segmentation. Neuroimage 196, 1–15. doi: 10.1016/j.neuroimage.2019.03.068
Beaumont, J., Commowick, O., and Barillot, C. (2016). “Automatic multiple sclerosis lesion segmentation from intensity-normalized multi-channel MRI,” in Proceedings of the 1st MICCAI Challenge on Multiple Sclerosis Lesions Segmentation Challenge Using a Data Management and Processing Infrastructure - MICCAI-MSSEG (Beaumont: Springer).
Brosch, T., Tang, L. Y., Yoo, Y., Li, D. K., Traboulsee, A., and Tam, R. (2016). Deep 3d convolutional encoder networks with shortcuts for multiscale feature integration applied to multiple sclerosis lesion segmentation. IEEE Trans. Med. Imaging 35, 1229–1239. doi: 10.1109/TMI.2016.2528821
Carass, A., Roy, S., Jog, A., Cuzzocreo, J. L., Magrath, E., Gherman, A., et al. (2017). Longitudinal multiple sclerosis lesion segmentation: resource and challenge. Neuroimage 148, 77–102. doi: 10.1016/j.neuroimage.2016.12.064
Catanese, L., Commowick, O., and Barillot, C. (2015). “Automatic graph cut segmentation of multiple sclerosis lesions,” in ISBI Longitudinal Multiple Sclerosis Lesion Segmentation Challenge (IEEE).
Cerri, S., Puonti, O., Meier, D. S., Wuerfel, J., Mühlau, M., Siebner, H. R., et al. (2021). A contrast-adaptive method for simultaneous whole-brain and lesion segmentation in multiple sclerosis. Neuroimage 225, 117471. doi: 10.1016/j.neuroimage.2020.117471
Chang, W.-G., You, T., Seo, S., Kwak, S., and Han, B. (2019). “Domain-specific batch normalization for unsupervised domain adaptation,” in Proceedings of the IEEE/CVF conference on Computer Vision and Pattern Recognition, 7354–7362.
Chen, C., Dou, Q., Chen, H., Qin, J., and Heng, P. A. (2020). Unsupervised bidirectional cross-modality adaptation via deeply synergistic image and feature alignment for medical image segmentation. IEEE Trans. Med. Imaging 39, 2494–2505. doi: 10.1109/TMI.2020.2972701
Çiçek, Ö., Abdulkadir, A., Lienkamp, S. S., Brox, T., and Ronneberger, O. (2016). “3D U-net: learning dense volumetric segmentation from sparse annotation,” in International Conference on Medical Image Computing and Computer-Assisted Intervention (Springer), 424–432.
Coles, A. J., Compston, D., Selmaj, K. W., Lake, S. L., Moran, S., Margolin, D. H., et al. (2008). Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N. Engl. J. Med. 359, 1786–1801. doi: 10.1056/NEJMoa0802670
Commowick, O., Istace, A., Kain, M., Laurent, B., Leray, F., Simon, M., et al. (2018). Objective evaluation of multiple sclerosis lesion segmentation using a data management and processing infrastructure. Sci. Rep. 8, 1–17. doi: 10.1038/s41598-018-31911-7
Commowick, O., Kain, M., Casey, R., Ameli, R., Ferré, J.-C., Kerbrat, A., et al. (2021). Multiple sclerosis lesions segmentation from multiple experts: the miccai 2016 challenge dataset. Neuroimage 244, 118589. doi: 10.1016/j.neuroimage.2021.118589
Danelakis, A., Theoharis, T., and Verganelakis, D. A. (2018). Survey of automated multiple sclerosis lesion segmentation techniques on magnetic resonance imaging. Comput. Med. Imaging Graph. 70, 83–100. doi: 10.1016/j.compmedimag.2018.10.002
Dewey, B. E., Zhao, C., Reinhold, J. C., Carass, A., Fitzgerald, K. C., Sotirchos, E. S., et al. (2019). Deepharmony: a deep learning approach to contrast harmonization across scanner changes. Magn. Reson. Imaging 64, 160–170. doi: 10.1016/j.mri.2019.05.041
Doyle, S., Forbes, F., and Dojat, M. (2016). “Automatic multiple sclerosis lesion segmentation with p-locus,” in Proceedings of the 1st MICCAI Challenge on Multiple Sclerosis Lesions Segmentation Challenge Using a Data Management and Processing Infrastructure - MICCAI-MSSEG (Springer), 17–21.
Filippi, M., Preziosa, P., and Rocca, M. A. (2019). Brain mapping in multiple sclerosis: lessons learned about the human brain. Neuroimage 190, 32–45. doi: 10.1016/j.neuroimage.2017.09.021
Ghafoorian, M., Karssemeijer, N., Heskes, T., Bergkamp, M., Wissink, J., Obels, J., et al. (2017). Deep multi-scale location-aware 3D convolutional neural networks for automated detection of lacunes of presumed vascular origin. Neuroimage Clin. 14, 391–399. doi: 10.1016/j.nicl.2017.01.033
Guo, P., Wang, P., Zhou, J., Jiang, S., and Patel, V. M. (2021). “Multi-institutional collaborations for improving deep learning-based magnetic resonance image reconstruction using federated learning,” in Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, 2423–2432.
Huang, X., Liu, M.-Y., Belongie, S., and Kautz, J. (2018). “Multimodal unsupervised image-to-image translation,” in Proceedings of the European Conference on Computer Vision (ECCV), 172–189.
Isensee, F., Jaeger, P. F., Kohl, S. A. A., and Maier-Hein, K. H. (2021). nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat. Methods 18, 203–211. doi: 10.1038/s41592-020-01008-z
Kamnitsas, K., Baumgartner, C., Ledig, C., Newcombe, V., Simpson, J., Kane, A., et al. (2017). “Unsupervised domain adaptation in brain lesion segmentation with adversarial networks,” in International Conference on Information Processing in Medical Imaging (Springer), 597–609.
Knight, J., and Khademi, A. (2016). “MS lesion segmentation using FLAIR MRI only,” in Proceedings of the 1st MICCAI Challenge on Multiple Sclerosis Lesions Segmentation Challenge Using a Data Management and Processing Infrastructure-MICCAI-MSSEG, 21–28.
Lesjak, Ž., Galimzianova, A., Koren, A., Lukin, M., Pernuš, F., Likar, B., et al. (2018). A novel public MR image dataset of multiple sclerosis patients with lesion segmentations based on multi-rater consensus. Neuroinformatics 16, 51–63. doi: 10.1007/s12021-017-9348-7
Li, T., Sahu, A. K., Zaheer, M., Sanjabi, M., Talwalkar, A., and Smith, V. (2020a). Federated optimization in heterogeneous networks. Proc. Mach. Learn. Syst. 2, 429–450.
Li, X., Gu, Y., Dvornek, N., Staib, L. H., Ventola, P., and Duncan, J. S. (2020b). Multi-site fMRI analysis using privacy-preserving federated learning and domain adaptation: abide results. Med. Image Anal. 65, 101765. doi: 10.1016/j.media.2020.101765
Li, X., Jiang, M., Zhang, X., Kamp, M., and Dou, Q. (2021). “FedBN: federated learning on non-IID features via local batch normalization,” in International Conference on Learning Representations. Available online at: https://openreview.net/forum?id=6YEQUn0QICG
Liu, D., Zhang, D., Song, Y., Zhang, F., O'Donnell, L., Huang, H., et al. (2020). PDAM: a panoptic-level feature alignment framework for unsupervised domain adaptive instance segmentation in microscopy images. IEEE Trans. Med. Imaging 40, 154–165. doi: 10.1109/TMI.2020.3023466
Liu, Q., Chen, C., Qin, J., Dou, Q., and Heng, P.-A. (2021a). “FedDG: federated domain generalization on medical image segmentation via episodic learning in continuous frequency space,” in Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, 1013–1023.
Liu, X., Xing, F., Yang, C., El Fakhri, G., and Woo, J. (2021b). “Adapting off-the-shelf source segmenter for target medical image segmentation,” in International Conference on Medical Image Computing and Computer-Assisted Intervention (Springer), 549–559.
Livne, M., Rieger, J., Aydin, O. U., Taha, A. A., Akay, E. M., Kossen, T., et al. (2019). A U-Net deep learning framework for high performance vessel segmentation in patients with cerebrovascular disease. Front. Neurosci. 13, 97. doi: 10.3389/fnins.2019.00097
Lladó, X., Oliver, A., Cabezas, M., Freixenet, J., Vilanova, J. C., Quiles, A., et al. (2012). Segmentation of multiple sclerosis lesions in brain MRI: a review of automated approaches. Inform. Sci. 186, 164–185. doi: 10.1016/j.ins.2011.10.011
Ma, Y., Zhang, C., Cabezas, M., Song, Y., Tang, Z., Liu, D., et al. (2022). Multiple sclerosis lesion analysis in brain magnetic resonance images: techniques and clinical applications. IEEE J. Biomed. Health Inform. 26. doi: 10.1109/JBHI.2022.3151741
McKinley, R., Wepfer, R., Grunder, L., Aschwanden, F., Fischer, T., Friedli, C., et al. (2020). Automatic detection of lesion load change in multiple sclerosis using convolutional neural networks with segmentation confidence. Neuroimage Clin. 25, 102104. doi: 10.1016/j.nicl.2019.102104
McMahan, B., Moore, E., Ramage, D., Hampson, S., and y Arcas, B. A. (2017). “Communication-efficient learning of deep networks from decentralized data,” in Artificial Intelligence and Statistics (PMLR), 1273–1282.
Milletari, F., Navab, N., and Ahmadi, S.-A. (2016). “V-Net: fully convolutional neural networks for volumetric medical image segmentation,” in 2016 Fourth International Conference on 3D Vision (3DV) (IEEE), 565–571.
Mills, E. A., Ogrodnik, M. A., Plave, A., and Mao-Draayer, Y. (2018). Emerging understanding of the mechanism of action for dimethyl fumarate in the treatment of multiple sclerosis. Front. Neurol. 9, 5. doi: 10.3389/fneur.2018.00005
Nair, T., Precup, D., Arnold, D. L., and Arbel, T. (2020). Exploring uncertainty measures in deep networks for multiple sclerosis lesion detection and segmentation. Med. Image Anal. 59, 101557. doi: 10.1016/j.media.2019.101557
Nichyporuk, B., Szeto, J., Arnold, D., and Arbel, T. (2021). “Optimizing operating points for high performance lesion detection and segmentation using lesion size reweighting,” in Medical Imaging with Deep Learning.
Palladino, J. A., Slezak, D. F., and Ferrante, E. (2020). “Unsupervised domain adaptation via cyclegan for white matter hyperintensity segmentation in multicenter MR images,” in 16th International Symposium on Medical Information Processing and Analysis (International Society for Optics and Photonics), 1158302.
Paszke, A., Gross, S., Chintala, S., Chanan, G., Yang, E., DeVito, Z., et al. (2017). “Automatic differentiation in pytorch,” in NeurIPS 2017 Autodiff Workshop.
Plantone, D., Renna, R., Sbardella, E., and Koudriavtseva, T. (2015). Concurrence of multiple sclerosis and brain tumors. Front. Neurol. 6, 40. doi: 10.3389/fneur.2015.00040
Plis, S. M., Hjelm, D. R., Salakhutdinov, R., Allen, E. A., Bockholt, H. J., Long, J. D., et al. (2014). Deep learning for neuroimaging: a validation study. Front. Neurosci. 8, 229. doi: 10.3389/fnins.2014.00229
Polman, C. H., Reingold, S. C., Banwell, B., Clanet, M., Cohen, J. A., Filippi, M., et al. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the mcdonald criteria. Ann. Neurol. 69, 292–302. doi: 10.1002/ana.22366
Pontillo, G., Tommasin, S., Cuocolo, R., Petracca, M., Petsas, N., Ugga, L., et al. (2021). A combined radiomics and machine learning approach to overcome the clinicoradiologic paradox in multiple sclerosis. Am. J. Neuroradiol. 42. doi: 10.3174/ajnr.A7274
Prinster, A., Quarantelli, M., Orefice, G., Lanzillo, R., Brunetti, A., Mollica, C., et al. (2006). Grey matter loss in relapsing–remitting multiple sclerosis: a voxel-based morphometry study. Neuroimage 29, 859–867. doi: 10.1016/j.neuroimage.2005.08.034
Schwenkenbecher, P., Wurster, U., Konen, F. F., Gingele, S., Sühs, K.-W., Wattjes, M. P., et al. (2019). Impact of the McDonald criteria 2017 on early diagnosis of relapsing-remitting multiple sclerosis. Front. Neurol. 10, 188. doi: 10.3389/fneur.2019.00188
Shen, C., Wang, P., Roth, H. R., Yang, D., Xu, D., Oda, M., et al. (2021). “Multi-task federated learning for heterogeneous pancreas segmentation,” in Clinical Image-Based Procedures, Distributed and Collaborative Learning, Artificial Intelligence for Combating COVID-19 and Secure and Privacy-Preserving Machine Learning (Springer), 101–110.
Shirokikh, B., Shevtsov, A., Kurmukov, A., Dalechina, A., Krivov, E., Kostjuchenko, V., et al. (2020). “Universal loss reweighting to balance lesion size inequality in 3d medical image segmentation,” in International Conference on Medical Image Computing and Computer-Assisted Intervention (Springer), 523–532.
Sun, L., Zhang, S., Chen, H., and Luo, L. (2019). Brain tumor segmentation and survival prediction using multimodal MRI scans with deep learning. Front. Neurosci. 13, 810. doi: 10.3389/fnins.2019.00810
Valverde, S., Cabezas, M., Roura, E., González-Villà, S., Pareto, D., Vilanova, J. C., et al. (2017). Improving automated multiple sclerosis lesion segmentation with a cascaded 3d convolutional neural network approach. Neuroimage 155, 159–168. doi: 10.1016/j.neuroimage.2017.04.034
Valverde, S., Salem, M., Cabezas, M., Pareto, D., Vilanova, J. C., Ramió-Torrentà, L., et al. (2019). One-shot domain adaptation in multiple sclerosis lesion segmentation using convolutional neural networks. Neuroimage Clin. 21, 101638. doi: 10.1016/j.nicl.2018.101638
Wang, S.-H., Tang, C., Sun, J., Yang, J., Huang, C., Phillips, P., et al. (2018). Multiple sclerosis identification by 14-layer convolutional neural network with batch normalization, dropout, and stochastic pooling. Front. Neurosci. 12, 818. doi: 10.3389/fnins.2018.00818
Yu, L., Wang, S., Li, X., Fu, C.-W., and Heng, P.-A. (2019). “Uncertainty-aware self-ensembling model for semi-supervised 3d left atrium segmentation,” in International Conference on Medical Image Computing and Computer-Assisted Intervention (Springer), 605–613.
Zhang, C., Song, Y., Liu, S., Lill, S., Wang, C., Tang, Z., et al. (2018). “MS-GAN: GAN-based semantic segmentation of multiple sclerosis lesions in brain magnetic resonance imaging,” in 2018 Digital Image Computing: Techniques and Applications (DICTA) (IEEE), 39–46.
Keywords: deep learning, federated learning, multiple sclerosis, segmentation, MRI
Citation: Liu D, Cabezas M, Wang D, Tang Z, Bai L, Zhan G, Luo Y, Kyle K, Ly L, Yu J, Shieh C-C, Nguyen A, Kandasamy Karuppiah E, Sullivan R, Calamante F, Barnett M, Ouyang W, Cai W and Wang C (2023) Multiple sclerosis lesion segmentation: revisiting weighting mechanisms for federated learning. Front. Neurosci. 17:1167612. doi: 10.3389/fnins.2023.1167612
Received: 16 February 2023; Accepted: 24 April 2023;
Published: 18 May 2023.
Edited by:
Ming Li, Hong Kong Polytechnic University, Hong Kong SAR, ChinaReviewed by:
Wenhan Liu, Wuhan University, ChinaCopyright © 2023 Liu, Cabezas, Wang, Tang, Bai, Zhan, Luo, Kyle, Ly, Yu, Shieh, Nguyen, Kandasamy Karuppiah, Sullivan, Calamante, Barnett, Ouyang, Cai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongnan Liu, ZG9uZ25hbi5saXVAc3lkbmV5LmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.