
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurosci., 09 May 2023
Sec. Perception Science
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1164145
This article is part of the Research TopicThe Brain in Pain: A Multidimensional ApproachView all 11 articles
Objective: Although neuroimaging investigations have revealed significant changes in brain structure in fibromyalgia (FM) patients, these findings are inconsistent. The current study conducted a systematic review and meta-analysis of voxel-based morphometric studies in order to comprehend those alterations in brain structure in FM patients.
Methods: Voxel-based morphometric (VBM) studies published up to January 17, 2023 were searched in the Web of Science, PubMed, EMBASE, Cochrane Library (CENTRAL), China National Knowledge Infrastructure (CNKI), Chongqing VIP, Wanfang Database. Two independent researchers carried out study screening, quality assessment, clinical data and neuroimaging data extraction. The whole-brain voxel-based gray matter (GM) data of FM patients were collected from eligible studies, and meta-analyzed using anisotropic effect size-signed differential mapping (AES-SDM).
Results: Twelve researches were included in this study, including 289 FM patients (mean age: 47.36 years) and 272 HS (mean age: 47.34 years). According to the meta-analysis, FM patients had increased GM in the right postcentral gyrus and left angular gyrus, and decreased GM in the right cingulate gyrus, right paracingulate gyrus, left cerebellum, and left gyrus rectus.
Conclusion: Our study suggests that fibromyalgia patients have altered gray matter in several brain regions that are involved in affective, cognitive functions, and in motor adaptations to pain processing.
Fibromyalgia (FM) is a chronic condition characterized by widespread musculoskeletal pain, along with fatigue, cognitive problems and sleep disturbances (Clauw, 2014; Winslow et al., 2023). FM affects 2 to 4% of the general population on average (Jones et al., 2015), with more female than male being diagnosed (Branco et al., 2010; Winslow et al., 2023). Fibromyalgia patients have high level of health care utilization and high costs associated with medical visits and diagnostic test, which bring heavy economic burden to society and family (Boonen et al., 2005; Pinto et al., 2023). Fibromyalgia is underdiagnosed due to the uncertainty surrounding its etiology (Bair and Krebs, 2020; Gatta et al., 2021).
According to previous studies, FM is a disorder of pain regulation and central sensitization (O'Brien et al., 2018; Siracusa et al., 2021). FM patients showed alterations in gray matter, along with aberrant activity and functional connections in brain regions involving pain processing (Pomares et al., 2017; Aster et al., 2022). Voxel-based morphometric analysis showed that FM patients had increased gray matter in the angular gyrus, cuneus, postcentral gyrus, insula, and putamen (Ceko et al., 2013; Pomares et al., 2017), and decreased gray matter in the bilateral hippocampus, anterior insula, posterior cingulate cortex (PCC), medial prefrontal cortex (MPFC), anterior cingulate cortex (ACC), precentral gyrus, and precuneus (Ceko et al., 2013; Pomares et al., 2017; Boehme et al., 2020). Because of the heterogeneous of the anomalies in gray matters, it is challenging to reconcile the findings of different researches. Although three meta-analyses have been published in 2016, the synthesized results were also heterogeneous (Dehghan et al., 2016; Lin et al., 2016; Shi et al., 2016).
Since 2016, a growing number of neuroimaging researches have helped us better understand the brain underpinnings of FM. Hence, the aim of the present study was to identify the most prominent and replicable GM regions that involved in FM patients from all the whole-brain VBM research published to date using the anisotropic effect size signed differential mapping (AES-SDM), which employs anisotropic kernel during the reconstruction of effect size maps to account for the anisotropy in the spatial covariance of the neuroimaging investigations (Radua et al., 2012a, 2014).
Systematic searches were conducted from origin to January 17, 2023 in seven electronic databases, including Web of Science, PubMed, EMBASE, Cochrane Library (CENTRAL), China National Knowledge Infrastructure (CNKI), Chongqing VIP, and Wanfang Database. The search terms in PubMed were “fibromyalgia” AND (“voxel-based morphometry” OR “VBM” OR “gray matter” OR “gray matter” OR “voxel wise” OR “voxel-wise”). This search strategy was modified to be suitable for the other six electronic databases. In addition, the review articles and references in the included publications were examined to identify any potential researches that might have been missed in the systematic searches.
The article was included if: (1) the abnormalities of gray matter volume or density in adult fibromyalgia patients were investigated using VBM analysis; and (2) the control group were healthy subjects; and (3) the neuroimaging outcomes were reported in three-dimensional coordinates (x, y, z) in Montreal Neurological Institute (MNI) or Talairach space; and (4) magnet strength of the magnetic resonance imaging (MRI) scanner was at least 1.5 Tesla. The article was excluded if: (1) publications were not original article; or (2) the analysis was confined to regions of interests in brain; or (3) the number of participants in any group was fewer than 10.
If the data was ambiguous or confusing, the corresponding author of the research was contacted through email. If two or more researches used the same data source, only the article with the largest sample size and most thorough information was included. Only baseline data were included in longitudinal or intervention studies. The current study adhered to the PRISMA (preferred reporting items for systematic review and meta-analysis) guidelines (Figure 1).
To evaluate the quality of the included researches, a specialized checklist based on those in prior neuroimaging meta-analyses was used in present study (Supplementary Table S1). The 12-point checklist covered diagnostic procedures, clinical and demographic characteristics, sample size, scanning parameters, analysis methods, and the caliber of the given outcomes. Each research was evaluated separately by two authors (MX, YQ). If there were any rating disputes, the papers were considered by the authors' group to get a decision on a final score.
The two authors (MX and YQ) independently extracted data from each study, using a predetermined data extraction form. Any discrepancies were discussed in the authors' group in order to be rectified. The authors' name, year of the publication, sample size, age and gender of the study population, disease duration, and the technical information about neuroimaging (MRI scanner, analysis software, full width at half maximum, thresholds, and significant gray matter alterations) were all extracted (Table 1). The peak coordinates in each research were collected following the standards of AES-SDM. In cases of significant results from both corrected and uncorrected thresholds in the VBM statistical analysis of one trial, only the corrected results were collected.
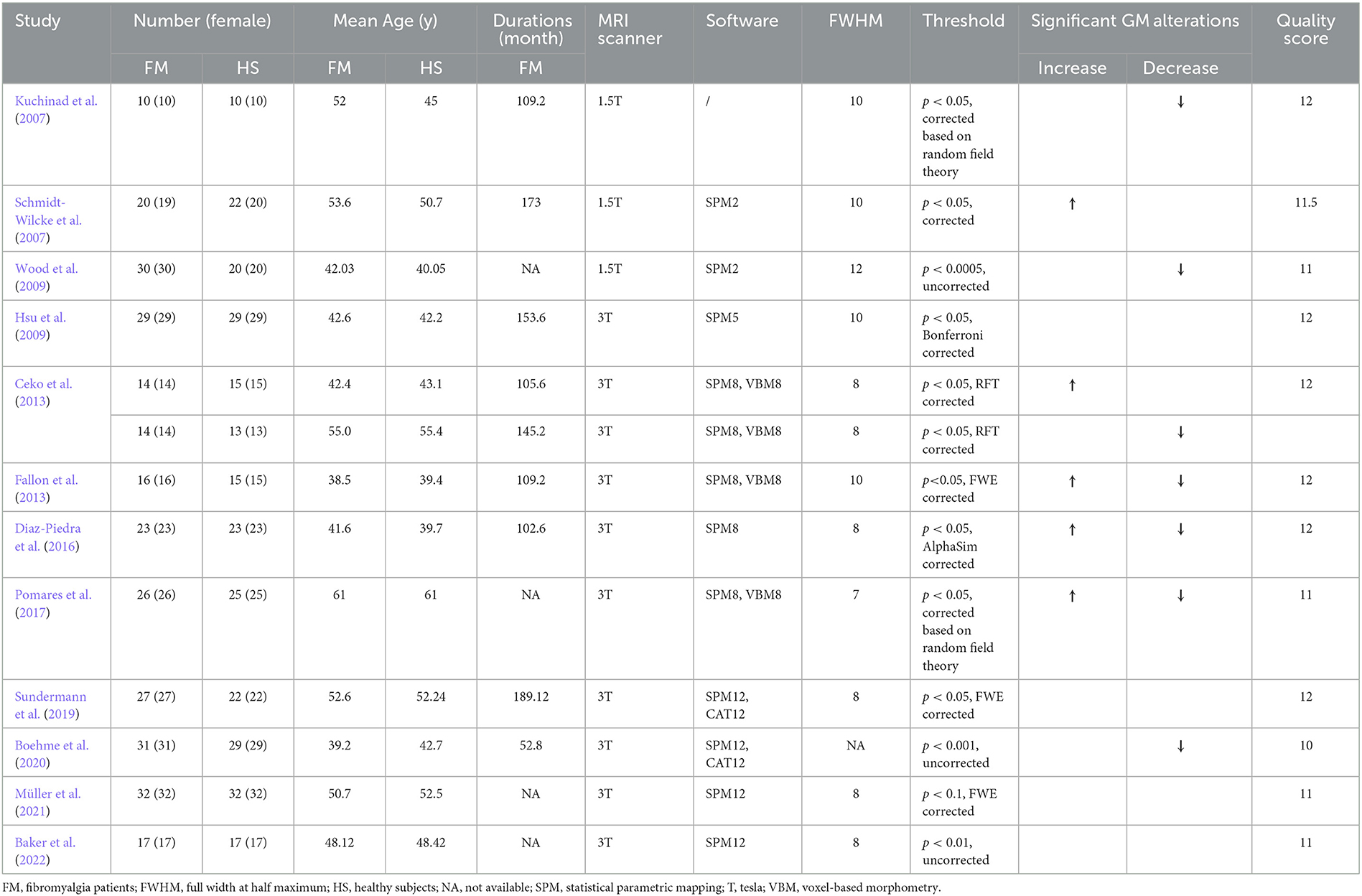
Table 1. Demographic and clinical characteristics of subjects in VBM studies included in the meta-analysis.
Alterations in brain structure were subjected to the whole-brain voxel-wise meta-analysis by AES-SDM (www.sdmproject.com/software) (Radua et al., 2012a,b). First, a Gaussian kernel was used to integrate the retrieved peak information to rebuild the effect-size and variance maps, which gave voxels closer to the peaks larger effect sizes. To prevent false-positive results, the assignment's full width at half maximum (FWHM) was fixed at 20 mm (Radua et al., 2012b). Study maps were computed voxel-wise to determining the random-effects mean while taking the sample size, intra-study variability, and between-study heterogeneity into consideration. After determining the meta-analysis means, thresholds were applied using the default parameters (voxel threshold p < 0.005, peak height threshold z > 1.00, and cluster size threshold > 10 voxels) (Radua et al., 2012b). The meta-analysis effect-size map was then statistically assessed by comparison to a null distribution created using a permutation algorithm. The reproducibility of VBM research results was examined using a leave-one-out Jackknife sensitivity analysis, which did the mean analysis again after methodically removing each research. We furtherly performed a subgroup analysis to rule out any potential heterogeneity originating from different MRI scanning techniques (1.5T or 3.0T scanner). To see if the results could have been influenced by a few or tiny researches, funnel plots of the peaks of the main findings were conducted. Additionally, the Egger test was also conducted to look for any potential publication bias (Radua and Mataix-Cols, 2009).
An evaluation of relationships between changes in the brain and subject characteristics (age and duration of FM patients) was carried out using a meta-regression analysis, weighted by sample size and intra- and between-study variances, in order to look for any potential impacts (Radua et al., 2012b). The probability threshold was lowered to 0.005 to reduce the detection of false associations. Results for the slope and one of the regressor's extremes were considered, while results for regions that were not detected in the main analysis were discarded. Fits that were obviously driven by an insufficient number of studies were also discarded by examining the regression plot (Radua et al., 2012b).
The search strategy resulted in 1,324 articles, and 12 articles were included in this meta-analysis (Figure 1) (Kuchinad et al., 2007; Schmidt-Wilcke et al., 2007; Hsu et al., 2009; Wood et al., 2009; Ceko et al., 2013; Fallon et al., 2013; Diaz-Piedra et al., 2016; Pomares et al., 2017; Sundermann et al., 2019; Boehme et al., 2020; Müller et al., 2021; Baker et al., 2022). One study included two subgroups of fibromyalgia and did separate comparison analyses, so the study was considered separately into two studies for the meta-analysis (Ceko et al., 2013). As a result, the number of studies in meta-analysis was elevated to 13. Among these studies, 9 studies reported gray matter decrease or increase or both in FM patients (Kuchinad et al., 2007; Schmidt-Wilcke et al., 2007; Wood et al., 2009; Ceko et al., 2013; Fallon et al., 2013; Diaz-Piedra et al., 2016; Pomares et al., 2017; Boehme et al., 2020), while 4 studies reported no abnormalities between FM patients and HS (Hsu et al., 2009; Sundermann et al., 2019; Müller et al., 2021; Baker et al., 2022). A total of 561 subjects were considered in this study, including 289 FM patients (mean age: 47.36 years) and 272 HS (mean age: 47.34 years). There was no significant difference in age or gender between the FM patients and HS (p > 0.05). The studies had a mean quality score of 11.5 out of a total possible score of 12, indicating that they were of high quality. Details of the literature search and criteria for article inclusion are shown in Figure 1. The clinical variables and technical details of the included studies were presented in Table 1.
The AES-SDM results showed that FM patients exhibited increased GM in the right postcentral gyrus (p = 0.001, z = 1.040), and left angular gyrus (p = 0.001, z = 1.017), and decreased GM in the right cingulate gyrus and right paracingulate gyrus (p = 0.000, z = −2.105), left cerebellum (p = 0.001, z = −1.486), and left gyrus rectus (p = 0.004, z = −1.300) compared with HS (Table 2; Figure 2).
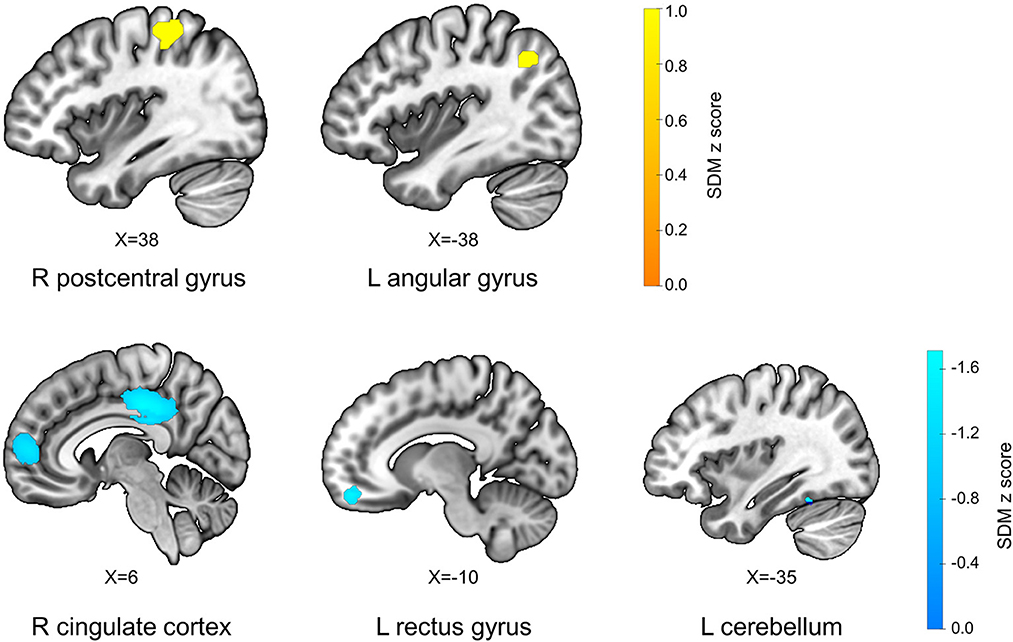
Figure 2. Differences in gray matter between fibromyalgia patients and healthy subjects. L, left; R, right; X, x-axis MNI coordinates of this section of the brain region.
The subgroup analysis of VBM studies using 1.5 T scanners revealed structural abnormality in the right cingulate gyrus, right paracingulate gyrus (p = 0.001, z = −1.333), left cerebellum, hemispheric lobule IV / V (p = 0.001, z = −1.222) of FM patients (Table 3). The subgroup analysis of VBM studies using 3.0 T scanners revealed structural abnormality in the right postcentral gyrus (p = 0.000, z = 1.162), left angular gyrus (p = 0.001, z = 1.016), right cingulate gyrus and right paracingulate gyrus (p = 0.000, z = −1.844), and left gyrus rectus (p = 0.002, z = −1.452) of FM patients (Table 3).
A meta-regression analysis was conducted to examine potential confounding variables (mean age, disease duration, and pain intensity). The mean age of FM patients was associated with changed GM in the left angular gyrus (p = 0.001, z = 2.112), and the right cingulate gyrus, right paracingulate gyrus (p = 0.001, z = −2.696) in VBM studies (Table 4). The disease duration was associated with altered GM in the left cerebellum (p = 0.001, z = 2.401) (Table 4). The mean pain intensity of FM patients was associated with abnormal GM in the right cingulate gyrus and right paracingulate gyrus (p = 0.000, z = 2.054) (Table 4).
There was no significant heterogeneity among the VBM studies with GM alterations according to the heterogeneity analysis (p > 0.005, Table 2). The leave-one-out Jackknife sensitivity analysis indicated that the left angular gyrus and right cingulate gyrus, right paracingulate gyrus were preserved in 12 combinations (Supplementary Table S2). Publication bias were checked using the funnel plots and the Egger test. The funnel plots demonstrated that the main findings were driven by at least 10 VBM studies (Supplementary Figure S1). Analysis of publication bias revealed that the Egger tests were insignificant in the peaks of the altered brain regions in the VBM meta-analysis (p = 0.591).
In order to evaluate the changes of gray matter in FM patients compared with HS, we performed an update meta-analysis using AES-SDM to pool VBM data. FM patients had increased GM in right postcentral gyrus and left angular gyrus, and decreased GM in right cingulate gyrus, right paracingulate gyrus, left cerebellum, and left gyrus rectus. These results remained consistent when each study was eliminated in the Jackknife sensitivity analysis.
Lin et al. included 6 voxel-wise VBM studies (156 FM patients vs. 147 HS), used activation likelihood estimation (ALE) to synthesize the altered gray matter of FM patients, and regional GM loss in left medial prefrontal cortex and right dorsal posterior cingulate cortex in FM patients was discovered (Lin et al., 2016). Shi et al. synthesized the abnormalities of 7 VBM studies (180 FM patients vs. 126 HS), and found GM decreases in the bilateral ACC, MPFC, PCC, paracingulate cortex, and parahippocampal gyrus (Shi et al., 2016). Dehghan et al. synthesized the structural changes in 6 MRI studies, including 4 VBM studies (92 FM patients vs. 92 HS), 1 DTI study and 1 cortical thickness study, and showed variations in the left midcingulate gyrus (Dehghan et al., 2016). In the present study, 12 researches (289 FM patients vs. 272 HS) were analyzed using AES-SDM. Compared with Lin's study, five papers published recently have been added in present study, and the meta-analysis methods were different. The screening criteria used in Shi et al. SDM meta-analysis were inconsistent with our study. Dehghan's study included three kinds of structural MRI researches, and synthesized using ALE. There might be several reasons for the inconsistency of the results in these four meta-analyses. First, only whole-brain gray-matter VBM studies have been included in our study in order to reduce the heterogeneity, and the heterogeneity analysis demonstrated that the main results were robust. Second, the added five studies increased the proportion of 3.0T MRI scanner in present meta-analysis, which influence the results according to our subgroup analysis in studies using 1.5 T scanner and 3.0 T scanner. Third, the SDM and ALE, based on different algorithms, may have a non-negligible impact on the results.
The postcentral gyrus plays a critical role in the perception of pain, its gray matter was increased in people suffering from fibromyalgia (Lutz et al., 2008) and other chronic pain disorders (Ogino et al., 2005). The angular gyrus is involved in visual and sensorimotor information convergence (Prado et al., 2005). The increased gray matter in angular gyrus and postcentral gyrus, implicated in attention to the body and visuo-motor coordination (Macaluso and Maravita, 2010), might relate to increased attentional resources allocated in FM patients to nociceptive and other unpleasant sensory inputs (Schweinhardt et al., 2008). However, none of the three meta-analysis that were published in 2016 showed any increased modification in the gray matter of the postcentral gyrus or the angular gyrus (Dehghan et al., 2016; Lin et al., 2016; Shi et al., 2016), indicating that these alterations may result from the literatures published in recent years (Pomares et al., 2017). The subgroup analysis revealed that the structural MRI scanner (1.5T / 3.0T) had an impact on the increased GM in postcentral gyrus and angular gyrus in FM patients. Three researches using 1.5 T MRI scanners were published in 2007 (Kuchinad et al., 2007; Schmidt-Wilcke et al., 2007) and 2009 (Wood et al., 2009), which had a high proportion of all included researches in the three meta-analysis of VBM studies in 2016 (Dehghan et al., 2016; Lin et al., 2016; Shi et al., 2016). It may also be the reason why there was no such outcome in the three VBM meta-analysis. Additionally, the meat-regression analysis revealed a relationship between age and the increase in gray matter of the angular gyrus in FM patients.
The statistically most robust gray matter declines were observed in the cingulate gyrus and paracingulate gyrus, which were consistent with Dehghan's (Dehghan et al., 2016) and Shi's meta-analysis (Shi et al., 2016). The altered gray matter in the cingulate gyrus was correlated with pain intensity. Cingulate cortex is involved in pain perception, pain modulation, selective attention, error awareness, working memory, and recognition (Kuchinad et al., 2007; Turriziani et al., 2008). Paracingulate gyrus was a significant anatomical marker in the medial prefrontal cortex, and when it is reduced, the ACC around them is increased in gray matter volume (Fornito et al., 2008).
Furthermore, it was noteworthy that gray matter loss in the gyrus rectus was similar to that seen in the cingulate cortex. The anterior cingulate was thought to extend into the frontal lobe through the gyrus rectus (Ballmaier et al., 2004), which may assist to explain why the gray matter abnormalities in both areas are identical. Approximately 30–60% of fibromyalgia patients have psychological comorbidities, which are often characterized by depression and anxiety (Hudson et al., 1985; Boissevain and McCain, 1991; Schmidt-Wilcke and Clauw, 2011). The cingulate cortex and gyrus rectus have also been previously identified as crucial regions implicated in pain catastrophizing and related psychiatric illnesses according to the structural neuroimaging researches conducted to date (Ballmaier et al., 2004; Diaz-Piedra et al., 2016).
In this context, it is also interesting to discuss our findings in the cerebellum. The cerebellum, which is now generally regarded as a cardinal area for pain processing, showed decreased gray matter in FM patients. Researches on both animals and humans has demonstrated that the cerebellum had a role in pain perception and regulation, in addition to motor adaptability, cognitive, and affective activities (Diano et al., 2016; Aster et al., 2022). The activation of cerebellum in FM patients was associated with catastrophizing scores (Gracely et al., 2004), indicating that the cerebellum was involved in pain expectancy and assessment (Schmidt-Wilcke and Clauw, 2011). Several studies have shown increased cerebellar gray matter (Kuchinad et al., 2007; Schmidt-Wilcke et al., 2007), in agreement with the findings from Shi et al. (2016), while Boehme et al. found reduced gray matter density in the cerebellum (Boehme et al., 2020). Studies with larger sample sizes contributed more since the square root of each study's sample size was used to weight the mean map in the SDM analysis. Based on this, the reduction of gray matter in the cerebellum in current meta-analysis was partially influenced by the conclusion of Boehme et al.'s study (Boehme et al., 2020).
There are several limitations that need to be considered when interpreting our results. FM is a chronic pain disorder and shows a wide range of symptoms and severity. The included articles in present study used several pain-related scales that couldn't be converted amongst one another, so the results of meta-regression analysis might not be robust. Besides, only 5 of 13 researches reported the medication used in the FM patients (Ceko et al., 2013; Diaz-Piedra et al., 2016; Boehme et al., 2020; Müller et al., 2021; Baker et al., 2022) and fewer researches reported the emotion state of FM patients, so we can't perform meta-regression analysis to observe the effect of medication taken and emotion state on gray matter changes of FM patients. Furthermore, it is evident that the prevalence of FM in females is obviously higher than that in males (Jones et al., 2015). We are unable to conduct a gender subgroup analysis to compare the differences in gray matter changes between females and males because the majority of the participants in the current study are females.
In summary, our study suggests that FM patients have altered gray matter in several brain regions that are involved in affective, cognitive functions, and motor adaptations to pain processing. These results might reflect the alterations of chronic pain disorders.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
SC and MX contributed to the study conception and design, and conceived the data analysis strategy. MX and YQ acquired the data, collated and analyzed the data, and drafted the manuscript. SC, XP, and DZ discussed, read, and revised the manuscript. All authors approved the publication of this manuscript.
This study is financially supported by the National Natural Science Foundation of China (No. 82205288), China Postdoctoral Science Foundation (No. 2021MD703796), Sichuan Science and Technology Program (No. 2022NSFSC0856), and Medical Technology Project of Health Commission of Sichuan Province (No. 21PJ110). The funders did not play any role in the study design, data collection and analysis, preparation of the manuscript, or decision to publish.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1164145/full#supplementary-material
Aster, H. C., Evdokimov, D., Braun, A., Üçeyler, N., Kampf, T., Pham, M., et al. (2022). CNS imaging characteristics in fibromyalgia patients with and without peripheral nerve involvement. Sci. Rep. 12, 6707. doi: 10.1038/s41598-022-10489-1
Bair, M. J., and Krebs, E. E. (2020). Fibromyalgia. Ann. Intern. Med. 172, Itc33–48. doi: 10.7326/AITC202003030
Baker, A. K., Nanda, M., Park, S. H., and Martucci, K. T. (2022). Attempt to replicate voxel-based morphometry analysis in fibromyalgia: detection of below threshold differences framed by contributions of variable clinical presentation to low reproducibility. medRxiv. doi: 10.1101/2022.03.04.22271900
Ballmaier, M., Toga, A. W., Blanton, R. E., Sowell, E. R., Lavretsky, H., Peterson, J., et al. (2004). Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am. J. Psychiatry. 161, 99–108. doi: 10.1176/appi.ajp.161.1.99
Boehme, R., Ettinger-Veenstra, H. V., Olausson, H., Gerdle, B., and Nagi, S. S. (2020). Anhedonia to gentle touch in fibromyalgia: Normal sensory processing but abnormal evaluation. Brain Sci. 10, 306. doi: 10.3390/brainsci10050306
Boissevain, M. D., and McCain, G. A. (1991). Toward an integrated understanding of fibromyalgia syndrome. II. Psychological and phenomenological aspects. Pain. 45, 239–248. doi: 10.1016/0304-3959(91)90048-3
Boonen, A., van den Heuvel, R., van Tubergen, A., Goossens, M., Severens, J. L., van der Heijde, D., et al. (2005). Large differences in cost of illness and wellbeing between patients with fibromyalgia, chronic low back pain, or ankylosing spondylitis. Ann. Rheum. Dis. 64, 396–402. doi: 10.1136/ard.2003.019711
Branco, J. C., Bannwarth, B., Failde, I., Abello Carbonell, J., Blotman, F., Spaeth, M., et al. (2010). Prevalence of fibromyalgia: a survey in five European countries. Semin. Arthritis Rheum. 39, 448–453. doi: 10.1016/j.semarthrit.2008.12.003
Ceko, M., Bushnell, M. C., Fitzcharles, M. A., and Schweinhardt, P. (2013). Fibromyalgia interacts with age to change the brain. NeuroImage Clin. 3, 249–260. doi: 10.1016/j.nicl.2013.08.015
Clauw, D. J. (2014). Fibromyalgia: a clinical review. JAMA. 311, 1547–1555. doi: 10.1001/jama.2014.3266
Dehghan, M., Schmidt-Wilcke, T., Pfleiderer, B., Eickhoff, S. B., Petzke, F., Harris, R. E., et al. (2016). Coordinate-based. (ALE). meta-analysis of brain activation in patients with fibromyalgia. Hum. Brain Mapp. 37, 1749–1758. doi: 10.1002/hbm.23132
Diano, M., D'Agata, F., Cauda, F., Costa, T., Geda, E., Sacco, K., et al. (2016). Cerebellar clustering and functional connectivity during pain processing. Cerebellum. 15, 343–356. doi: 10.1007/s12311-015-0706-4
Diaz-Piedra, C., Guzman, M. A., Buela-Casal, G., and Catena, A. (2016). The impact of fibromyalgia symptoms on brain morphometry. Brain Imaging Behav. 10, 1184–1197. doi: 10.1007/s11682-015-9485-2
Fallon, N., Alghamdi, J., Chiu, Y., Sluming, V., Nurmikko, T., and Stancak, A. (2013). Structural alterations in brainstem of fibromyalgia syndrome patients correlate with sensitivity to mechanical pressure. NeuroImage Clin. 3, 163–170. doi: 10.1016/j.nicl.2013.07.011
Fornito, A., Wood, S. J., Whittle, S., Fuller, J., Adamson, C., Saling, M. M., et al. (2008). Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume, and sulcal depth. Hum. Brain Mapp. 29, 222–236. doi: 10.1002/hbm.20381
Gatta, G., La Forgia, D., Fanizzi, A., Massafra, R., Somma, F., Belfiore, M. P., et al. (2021). Prevalence of patients affected by fibromyalgia in a cohort of women underwent mammography screening. Healthcare. 9, 1340. doi: 10.3390/healthcare9101340
Gracely, R. H., Geisser, M. E., Giesecke, T., Grant, M. A., Petzke, F., Williams, D. A., et al. (2004). Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 127, 835–843. doi: 10.1093/brain/awh098
Hsu, M. C., Harris, R. E., Sundgren, P. C., Welsh, R. C., Fernandes, C. R., Clauw, D. J., et al. (2009). No consistent difference in gray matter volume between individuals with fibromyalgia and age-matched healthy subjects when controlling for affective disorder. Pain. 143, 262–267. doi: 10.1016/j.pain.2009.03.017
Hudson, J. I., Hudson, M. S., Pliner, L. F., Goldenberg, D. L., and Pope, H. G. Jr. (1985). Fibromyalgia and major affective disorder: a controlled phenomenology and family history study. Am. J. Psychiatry. 142, 441–446. doi: 10.1176/ajp.142.4.441
Jones, G. T., Atzeni, F., Beasley, M., Flüß, E., Sarzi-Puttini, P., and Macfarlane, G. J. (2015). The prevalence of fibromyalgia in the general population: a comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol. 67, 568–575. doi: 10.1002/art.38905
Kuchinad, A., Schweinhardt, P., Seminowicz, D. A., Wood, P. B., Chizh, B. A., and Bushnell, M. C. (2007). Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J. Neurosci. 27, 4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007
Lin, C., Lee, S. H., and Weng, H. H. (2016). Gray matter atrophy within the default mode network of fibromyalgia: a meta-analysis of voxel-based morphometry studies. Biomed Res. Int. 2016, 7296125. doi: 10.1155/2016/7296125
Lutz, J., Jäger, L., de Quervain, D., Krauseneck, T., Padberg, F., Wichnalek, M., et al. (2008). White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheum. 58, 3960–3969. doi: 10.1002/art.24070
Macaluso, E., and Maravita, A. (2010). The representation of space near the body through touch and vision. Neuropsychologia. 48, 782–795. doi: 10.1016/j.neuropsychologia.2009.10.010
Müller, M., Wüthrich, F., Federspiel, A., Wiest, R., Egloff, N., Reichenbach, S., et al. (2021). Altered central pain processing in fibromyalgia-A multimodal neuroimaging case-control study using arterial spin labelling. PloS ONE. 16, e0235879. doi: 10.1371/journal.pone.0235879
O'Brien, A. T., Deitos, A., Triñanes Pego, Y., Fregni, F., and Carrillo-de-la-Peña, M. T. (2018). Defective endogenous pain modulation in fibromyalgia: a meta-analysis of temporal summation and conditioned pain modulation paradigms. J. Pain. 19, 819–836. doi: 10.1016/j.jpain.2018.01.010
Ogino, Y., Nemoto, H., and Goto, F. (2005). Somatotopy in human primary somatosensory cortex in pain system. Anesthesiology. 103, 821–827. doi: 10.1097/00000542-200510000-00021
Pinto, A. M., Geenen, R., Wager, T. D., Lumley, M. A., Häuser, W., Kosek, E., et al. (2023). Emotion regulation and the salience network: a hypothetical integrative model of fibromyalgia. Nat. Rev. Rheumatol. 19, 44–60. doi: 10.1038/s41584-022-00873-6
Pomares, F. B., Funck, T., Feier, N. A., Roy, S., Daigle-Martel, A., Ceko, M., et al. (2017). Histological underpinnings of grey matter changes in fibromyalgia investigated using multimodal brain imaging. J. Neurosci. 37, 1090–1101. doi: 10.1523/JNEUROSCI.2619-16.2016
Prado, J., Clavagnier, S., Otzenberger, H., Scheiber, C., Kennedy, H., and Perenin, M. T. (2005). Two cortical systems for reaching in central and peripheral vision. Neuron. 48, 849–858. doi: 10.1016/j.neuron.2005.10.010
Radua, J., Borgwardt, S., Crescini, A., Mataix-Cols, D., Meyer-Lindenberg, A., McGuire, P. K., et al. (2012a). Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci. Biobehav. Rev. 36, 2325–2333. doi: 10.1016/j.neubiorev.2012.07.012
Radua, J., and Mataix-Cols, D. (2009). Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatry. 195, 393–402. doi: 10.1192/bjp.bp.108.055046
Radua, J., Mataix-Cols, D., Phillips, M. L., El-Hage, W., Kronhaus, D. M., Cardoner, N., et al. (2012b). A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry. 27, 605–611. doi: 10.1016/j.eurpsy.2011.04.001
Radua, J., Rubia, K., Canales-Rodríguez, E. J., Pomarol-Clotet, E., Fusar-Poli, P., and Mataix-Cols, D. (2014). Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front. Psychiatry. 5, 13. doi: 10.3389/fpsyt.2014.00013
Schmidt-Wilcke, T., and Clauw, D. J. (2011). Fibromyalgia: from pathophysiology to therapy. Nat. Rev. Rheumatol. 7, 518–527. doi: 10.1038/nrrheum.2011.98
Schmidt-Wilcke, T., Luerding, R., Weigand, T., Juergens, T., Schuierer, G., Leinisch, E., et al. (2007). Striatal grey matter increase in patients suffering from fibromyalgia - A voxel-based morphometry study. Pain. 132, S109–S116. doi: 10.1016/j.pain.2007.05.010
Schweinhardt, P., Sauro, K. M., and Bushnell, M. C. (2008). Fibromyalgia: a disorder of the brain? Neuroscientist. 14, 415–421. doi: 10.1177/1073858407312521
Shi, H., Yuan, C., Dai, Z., Ma, H., and Sheng, L. (2016). Gray matter abnormalities associated with fibromyalgia: a meta-analysis of voxel-based morphometric studies. Semin. Arthritis Rheum. 46, 330–337. doi: 10.1016/j.semarthrit.2016.06.002
Siracusa, R., Paola, R. D., Cuzzocrea, S., and Impellizzeri, D. (2021). Fibromyalgia: pathogenesis, mechanisms, diagnosis and treatment options update. Int. J. Mol. Sci. 22, 3891. doi: 10.3390/ijms22083891
Sundermann, B., Nayyeri, M. D., Pfleiderer, B., Stahlberg, K., Juenke, L., Baie, L., et al. (2019). Subtle changes of gray matter volume in fibromyalgia reflect chronic musculoskeletal pain rather than disease-specific effects. Eur. J. Neurosci. 50, 3958–3967. doi: 10.1111/ejn.14558
Turriziani, P., Oliveri, M., Salerno, S., Costanzo, F., Koch, G., Caltagirone, C., et al. (2008). Recognition memory and prefrontal cortex: dissociating recollection and familiarity processes using rTMS. Behav. Neurol. 19, 23–27. doi: 10.1155/2008/568057
Winslow, B. T., Vandal, C., and Dang, L. (2023). Fibromyalgia: diagnosis and management. Am. Fam. Physician. 107, 137–144.
Keywords: fibromyalgia, neuroimaging, meta-analysis, signed differential mapping, voxel-based morphometry
Citation: Xin M, Qu Y, Peng X, Zhu D and Cheng S (2023) A systematic review and meta-analysis of voxel-based morphometric studies of fibromyalgia. Front. Neurosci. 17:1164145. doi: 10.3389/fnins.2023.1164145
Received: 12 February 2023; Accepted: 17 April 2023;
Published: 09 May 2023.
Edited by:
Fausta Lui, University of Modena and Reggio Emilia, ItalyReviewed by:
Gilda Sandri, University of Modena and Reggio Emilia, ItalyCopyright © 2023 Xin, Qu, Peng, Zhu and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shirui Cheng, Y2hlbmdzaGlydWlAY2R1dGNtLmVkdS5jbg==; Deliang Zhu, MzY5MDg2MDY5QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.