
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 02 June 2023
Sec. Neuroendocrine Science
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1148971
This article is part of the Research Topic Neuroendocrine Research in Health and Disease Volume II View all 7 articles
Introduction: Obsessive–compulsive disorder (OCD), characterized by the presence of obsessions and/or compulsions, is often difficult to diagnose and treat in routine clinical practice. The candidate circulating biomarkers and primary metabolic pathway alteration of plasma in OCD remain poorly understood.
Methods: We recruited 32 drug-naïve patients with severe OCD and 32 compared healthy controls and applied the untargeted metabolomics approach by ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS) to assess their circulating metabolic profiles. Both univariate and multivariate analyses were then utilized to filtrate differential metabolites between patients and healthy controls, and weighted Correlation Network Analysis (WGCNA) was utilized to screen out hub metabolites.
Results: A total of 929 metabolites were identified, including 34 differential metabolites and 51 hub metabolites, with an overlap of 13 metabolites. Notably, the following enrichment analyses underlined the importance of unsaturated fatty acids and tryptophan metabolism alterations in OCD. Metabolites of these pathways in plasma appeared to be promising biomarkers, such as Docosapentaenoic acid and 5-Hydroxytryptophan, which may be biomarkers for OCD identification and prediction of sertraline treatment outcome, respectively.
Conclusion: Our findings revealed alterations in the circulating metabolome and the potential utility of plasma metabolites as promising biomarkers in OCD.
Obsessive–compulsive disorder (OCD) is a common, chronic and severe neuropsychiatric disorder characterized by the presence of obsessions and/or compulsions that lead to various degrees of the subjective experience of anxiety (Veale and Roberts, 2014). About 2–3% of the population suffer from OCD (Fontenelle et al., 2006), and their social relations, quality of life, and occupational functioning were disturbed, costing heavy burdens for their families and society (Yang et al., 2021). Patients with OCD have distinctly heterogeneous obsessive–compulsive symptom dimensions, including contamination, symmetry, checking and unacceptability (Mataix-Cols et al., 2005), which are often obscured by symptoms of anxiety or depression, contributing to the difficulty of OCD diagnosis in routine clinical practice. Whereas up to 40–60% of patients are unable to have a satisfactory outcome through selective serotonin reuptake inhibitors (SSRIs) monotherapy, the proven first-line treatment of OCD (Pallanti and Quercioli, 2006), but may benefit from the combination therapy, cognitive-behavioral therapy or other treatment options. So, it would be helpful if patients who would respond to SSRIs could be identified. Given that, the demand for reliable biomarkers of OCD identification and prediction of sertraline treatment outcome has always been pressing.
Integrating existing neurobiology, genetics and neurochemistry findings, the perturbation of serotonergic (Goddard et al., 2008), dopaminergic (Koo et al., 2010) and glutamatergic (Pittenger et al., 2011) neurotransmitter systems, oxidative imbalance (Maia et al., 2019; Mohammadi et al., 2021) as well as neuroinflammation (Attwells et al., 2017; Gerentes et al., 2019) have been regarded as the underlying neural and pathophysiological underpinnings of OCD (Pauls et al., 2014). Magnetic Resonance Spectroscopy (MRS) studies has revealed glutamatergic dysfunction in cortico-striatal-thalamo-cortical (CSTC) circuit in OCD (Brennan et al., 2013) and SSRIs might act by modulating cortico-striatal glutamatergic activity(Lázaro et al., 2012). But to date, the details of the metabolic pathways and peripheral biomarkers in OCD remain poorly understood. A preceding study applied mendelian randomization design to data of genome-wide association studies (GWAS) to screen blood metabolome among psychiatric disorders but failed to identify any significant biomarker in OCD (Jia et al., 2022). Whilst, a couple of transmitters and their precursors and primary metabolites were probed in blood and Cerebrospinal Fluid (CSF) samples of OCD patients(Benkelfat et al., 1991; Hollander et al., 1992; Swedo et al., 1992; Leckman et al., 1995; Zohar et al., 2000; Chakrabarty et al., 2005; Delorme et al., 2005; Bhattacharyya et al., 2009). Among them, CSF glutamate level (Chakrabarty et al., 2005; Bhattacharyya et al., 2009), plasma homovanillic acid level (the major terminal metabolite of dopamine) (Benkelfat et al., 1991; Hollander et al., 1992) and whole-blood serotonin level (Delorme et al., 2005) were detected to be different in drug-naïve patients compared to healthy controls in some cases. However, these findings were either equivocal or conflicting. To discover biomarkers of OCD, more precise studies will be needed.
High-throughput sequencing technologies have revolutionized medical research. Varieties of omics technologies have been utilized for biomarkers detection and potential pathogenesis interpretation (Hasin et al., 2017). Metabolomics, the newest generation of omics technology, presents the profiles of small molecular compounds in biological samples and reflects directly the underlying biochemical activity and state, as a result of high-throughput characterization of metabolites (Rinschen et al., 2019; Wishart, 2019). The most commonly used metabolomics method is Ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS), developed from Liquid Chromatography-Mass Spectrometry (LC–MS), which provides accurate mass information and high mass resolution; helps the analysis of trace compounds in complex multicomponent mixtures like plasma (Alseekh et al., 2021).
Nowadays, metabolomics technologies have been widely applied to discover biomarkers and key pathways in many mental diseases, such as schizophrenia, bipolar disease and depression. However, there has been no study directly and systematically detecting the plasma metabolome in OCD patients yet. In the present study, we aimed to identify the disturbing profile of metabolome in OCD. Consequently, we applied the untargeted metabolomics approach based on UPLC-Q-TOF/MS technology to disclose the primary metabolic pathway alteration in OCD compared with healthy control, and then discovered the candidate biomarker for OCD diagnosis and outcome prediction of sertraline monotherapy.
Figure 1 depicted the integrated workflow of our research. All analyses were conducted by IBM Statistical Product and Service Solutions (SPSS) for Windows, version 26.0 (IBM Corp., Armonk, N.Y., United States), R software, version 4.2.2 and MetaboAnalyst 5.0 Web Server.1 The networks were visualized by Cytoscape version 3.9.1.
Patients with OCD were recruited at psychiatric clinics of Shanghai Mental Health Center (Shanghai, China) from January 2021 to October 2022 (based on “A Pragmatic Trial of Pharmacotherapy Options Following Unsatisfactory Initial Treatment in OCD” (NCT04539951)). All participants were assessed by experienced psychiatrists who received regular training to ensure consistency. The study was approved by the Institutional Review Board.
Individuals were included in the current study if they: (1) met the Diagnostic and Statistical Manual of Mental Disorder, fifth Edition (DSM-5) criteria for OCD as the primary diagnosis(American Psychological Association, 2013); (2) were OCD patients with a Yale-Brown Obsessive–Compulsive Scale (Y-BOCS) (Goodman et al., 1989) total severity score of ≥24; (3) were 18–65 years old; (4) neither had been exposed to any psychiatric medication nor had received any form of psychotherapy; and (5) had provided written informed consent.
Individuals were excluded if they: (1) met the DSM-5 diagnostic criteria for Schizophrenia Spectrum and Other Psychotic Disorders, or Bipolar and Related Disorders; (2) ever had moderate to severe suicidal ideation or suicide attempts; (3) had psychoactive substance use; (4) had comorbid other medical disorders that may impact on metabolites; (5) were pregnant or lactating females.
Healthy controls, without any DSM-5 diagnosis, were recruited by advertisements. Other inclusion criteria included: (1) between the age of 18 and 65 years; (2) neither had been exposed to any psychiatric medication nor had received any form of psychotherapy; and (3) had provided written informed consent. Individuals were excluded if they: (1) had psychoactive substance abuse; (2) had medical disorders that may impact metabolites; (3) had a family history of mental disorders; or (4) were experiencing pregnancy or lactation.
From January 2021 to October 2022, 58 patients were recruited in the Pragmatic Trial of Obsessive–compulsive Disorder at Shanghai Mental Health Center. Among them, 19 were excluded because their Y-BOCS scores were under 24; 5 were excluded because they received psychiatric medicine before; 2 were excluded because their plasma samples appeared to show signs of hemolysis. As a result, 32 patients with OCD and 32 matched healthy controls (HCs) were included in the present study.
Besides, according to the protocol of “A Pragmatic Trial of Pharmacotherapy Options Following Unsatisfactory Initial Treatment in OCD” (Wang et al., 2022), all included patients with OCD received sertraline (a commonly used SSRIs drug) monotherapy in 12 weeks, initially at 50 mg/d, with a weekly 50 mg/d further increase, to the maximum recommended dosage (200 mg/d) or the maximum tolerated dosage (less than 200 mg/d). Patients would be on their maximum dose by week 4, so allowing an assessment of response at 12 weeks. Till October 2022, there were 28 out of 32 included patients with OCD received 12-week sertraline treatment and finished follow-up visits. Three patients withdrew because of side effects; one patient dropped out because she had an unexpected pregnancy.
As for assessments, the OCD symptom severity of the patient was assessed using the Y-BOCS(Goodman et al., 1989); the Mini International Neuropsychiatric Interview (MINI) was used to screen history of comorbid DSM-5 psychiatric disorders; moreover, the Obsessive–Compulsive Inventory-Revised (OCI-R) (Foa et al., 2002) assesses distress associated with common OCD symptoms, including washing, checking, ordering, obsessing, hoarding, and neutralizing. The Beck Depression Inventory-II (BDI-II) (Beck et al., 1961) and the Beck Anxiety Inventory (BAI) (Beck et al., 1996) were employed to assess the severity of depression and anxiety symptoms, respectively. The details of their clinical characteristic and assessments at baseline were shown in Table 1. The assessments of patients with OCD at the 12-week follow-up visit were shown in Table 2. Responders were defined as patients with the reduction rate of Y-BOCS ≥35%.
All participants were asked to fast for up to 8 hours before blood sampling. About 2 mL venous blood samples were collected from all participants in Ethylenediaminetetraacetic acid (EDTA) anticoagulant polypropylene tubes from an antecubital vein between 9 am and 4 pm. Blood samples were centrifuged at 3,000 rpm at 4°C for 15 min for the separation of blood cells and plasma. The separated plasma then was pipetted into respective vials and stored at −80°C until thawed for analysis.
To extract metabolites, 64 plasma samples (100 μL each) were individually mixed with prechilled methanol (400 μL) by well vortexing. The samples were incubated on ice for 5 min and then were centrifuged at 15,000 rpm, 4°C for 5 min. Then the supernatant was diluted to a final concentration containing 53% methanol by LC–MS grade water. The samples were subsequently transferred to a fresh Eppendorf tube and then were centrifuged at 15000 g, 4°C for 10 min. Finally, the supernatant was injected into the UPLC-Q-TOF/MS system.
UPLC-Q-TOF/MS analyses were performed using a Vanquish UHPLC system (Thermo Fisher, Germany) coupled with an Orbitrap Q Exactive™ HF mass spectrometer (Thermo Fisher, Germany) in Biozeron Co., Ltd. (Shanghai, China). Samples were injected onto a Hypesil Gold column (100 × 2.1 mm, 1.9 μm) using a 17-min linear gradient at a flow rate of 0.2 mL/min. The eluents for the positive polarity mode were eluent A (0.1% FA in Water) and eluent B (Methanol). The eluents for the negative polarity mode were eluent A (5 mM ammonium acetate, pH 9.0) and eluent B (Methanol). The solvent gradient was set as follows: 2% B, 1.5 min; 2–100% B, 12.0 min; 100% B, 14.0 min; 100–2% B, 14.1 min; 2% B, 17 min. Q Exactive™ HF mass spectrometer was operated in positive/negative polarity mode with a spray voltage of 3.2 kV, capillary temperature of 320°C, sheath gas flow rate of 40 arb and aux gas flow rate of 10 arb.
To ensure data quality, pooled quality control (QC) samples were first prepared by mixing all of the plasma samples and were analysed throughout the run every 10 injections. Plasma samples were run alongside QC samples to identify impurities of either the solvents or extraction procedure and for checking carryover contamination from intense analytes.
The raw data files generated by UPLC-Q-TOF/MS were processed using Compound Discoverer 3.1 (CD3.1, Thermo Fisher) to perform peak alignment, peak picking, and quantitation for each metabolite. Core parameters were set as follows: retention time tolerance, 0.2 min; actual mass tolerance, 5 ppm; signal intensity tolerance, 30%; signal/noise ratio, 3; and minimum intensity, 100,000. After that, peak intensities were normalized to the total spectral intensity, and metabolites with the Coefficient of Variance (CV) less than 30% in QC samples were retained. The normalized data were used to predict the formula based on additive ions, molecular ion peaks and fragment ions. And then peaks were matched with the mzCloud,2 mzVault and MassList databases to obtain accurate and relative quantitative results. These metabolites were annotated by the Human Metabolome Database (HMDB, https://hmdb.ca/metabolites).
Afterward, the dataset was normalized by Probabilistic Quotient Normalization (HC group), log transformation and auto-scaling to reach optimal normal distribution (Chen et al., 2017). The principal component analysis (PCA) and heatmaps were first used to observe possible outliers in both data of positive and negative polarity modes; as well as the QC presentation on PCA plots was used for analytical validation.
Metabolic profiling and differential metabolites between the OCD group and HC group were performed by MetaboAnalyst. After outlier exclusion and normalization, the metabolic profiling was presented by PCA and orthogonal partial least-squares-discriminant analysis (OPLS-DA). Meanwhile, OPLS-DA enables to model separately multivariate data (X-predictors) correlated and uncorrelated to the group labels (Y-responses) and thus the first component of OPLS-DA containing group information is suitable for biomarkers detection. To address the overfitting issue, a 2000-time permutation test of the OPLS-DA model was conducted.
As for differential metabolites, we performed both univariate (t-test and fold change, FC) and multivariate level statistical analysis (OPLS-DA) to screen out. The selection was according to the following criteria: (1) False Discovery Rate (FDR) adjusted p-value<0.05; (2) FC (OCD/HCs) > 2.0 or < 0.5; and (3) variable importance in the projection value from the OPLS-DA model (VIP) >1.5.
Weighted Correlation Network Analysis (WGCNA) (Langfelder and Horvath, 2008), a systems biology method, has been applied in many high-dimensional data sets, including metabolomics. Briefly, WGCNA constructs a weighted metabolites correlation network and identifies several modules of highly interconnected metabolites. We can relate these modules to sample traits, to filter biologically interested modules and hub metabolites of each interested module. Hub metabolites were defined as the metabolites with almost the highest connectivity in the metabolite interaction network, which are more likely to be biologically relevant markers according to prior studies.
In the current study, we conducted a weighted metabolite correlation network and related it with dichotomous grouping variables, Y-BOCS scores and other clinical information accessed at baseline. To be specific, soft thresholding power β was estimated based on the criterion of approximate scale-free topology (R2 fit >0.9), aiming to calculate the adjacency matrix. To minimize the effects of noise and spurious associations, adjacency values were then transformed into a signed topological overlap matrix (TOM). We used the dissimilarity matrix (1-TOM) to produce the hierarchical clustering trees (dendrogram) of metabolites, and module identification was based on the identification of individual brunches by the Dynamic Tree Cut. Each module’s expression profile was summarized into a module eigengene (ME) using the matched module’s first principal component. After constructing the metabolite correlation network and identifying modules, we selected biologically interested modules from module-trait relationship plots. Hub metabolites were then filtrated according to Module Membership (MM) which measured how correlated each metabolite was to the ME of the related module, and Gene Significances (GS) which was defined as the correlation of a metabolite profile with grouping variables in this case. Metabolites in interesting modules with MM > 0.8 or < −0.8 and GS >0.2 or < −0.2 were considered as hub metabolites.
After filtering differential and hub metabolites, we performed enrichment analysis on the basis of the KEGG (Kyoto Encyclopedia of Genes and Genomes, https://www.genome.jp/kegg/) database (Kanehisa and Goto, 2000).
Potential biomarkers for diagnosis were selected from metabolites that were both differential and hub metabolites. To avoid multicollinearity, we used stepwise logistic regression to filtrate and remain solid significant predictor variables. A nomogram was then established by combining screened-out plasma metabolites based on the logistic regression model. The multivariate receiver operating characteristic (ROC) curve and the area under the curve (AUC) was also calculated to evaluate the discriminative ability of the model. Additionally, the Hosmer–Lemeshow test was performed to further evaluate the consistency between the predicted efficacy and the actual response rate (p > 0.05 was considered a good consistency).
To filtrate potential biomarkers of sertraline monotherapy, the dataset of 28 patients with OCD who had finished 12-week follow-up visits with all detected metabolites was enrolled for the least absolute shrinkage and selection operator (LASSO) regression. LASSO is a penalization method that shrinks all regression coefficients and sets the coefficients of many irrelevant features that have no discriminatory power between the classes exactly to zero. It is suitable for high-dimensional data with large features but small samples and also avoids overfitting. To provide a robust generalized performance of a model that best fitted our data, 10-fold cross-validation with a minimum criterion was applied, with these folds being randomly picked. The minimum lambda value was then defined as a cut-off point to minimize the mean cross-validated error and was utilized to filtrate metabolites.
Subsequently, the selected metabolites were used to perform an outcome prediction nomogram based on the logistic regression model. Similar to the diagnostic nomogram mentioned before, we conducted a ROC curve to evaluate the predictive discriminative performance of the model and the Hosmer–Lemeshow test to describe its consistency.
Through the untargeted UPLC-Q-TOF/MS metabolomics analysis, 430 metabolite features in positive polarity mode and 499 in negative polarity mode were extracted. Integrating each sample’s performance in the PCA scores plots and heatmaps (Supplementary Figures S1A–D), 3 samples (X1177, X1202 in the HC group and X89 in the OCD group) were excluded from the following analysis. Besides, the PCA scores plots revealed a close clustering of all QC samples, indicating that the analytical system performance was of good quality.
In total, among 929 detected metabolites, there were 475 metabolites showed higher levels in the OCD group and 454 counterparts in the HC group. Metabolic profiling comparing the OCD group with the HC group was first presented in the PCA scores plot (Figure 2A). We can observe an overlap between these two groups, and the HC group seemed to have a more concentrated cluster than the OCD group. A clearer discrimination of metabolic profiling between the OCD and HC groups was presented in Figure 2B, suggesting a better separation with OPLS-DA. The result of the permutation test showed that the classification of the OCD group and HC group was significantly better than any other random classification in two arbitrary groups (Figure 2C; Q2 = 0.796, p < 5e-04; R2Y = 0.985, p < 5e-04).
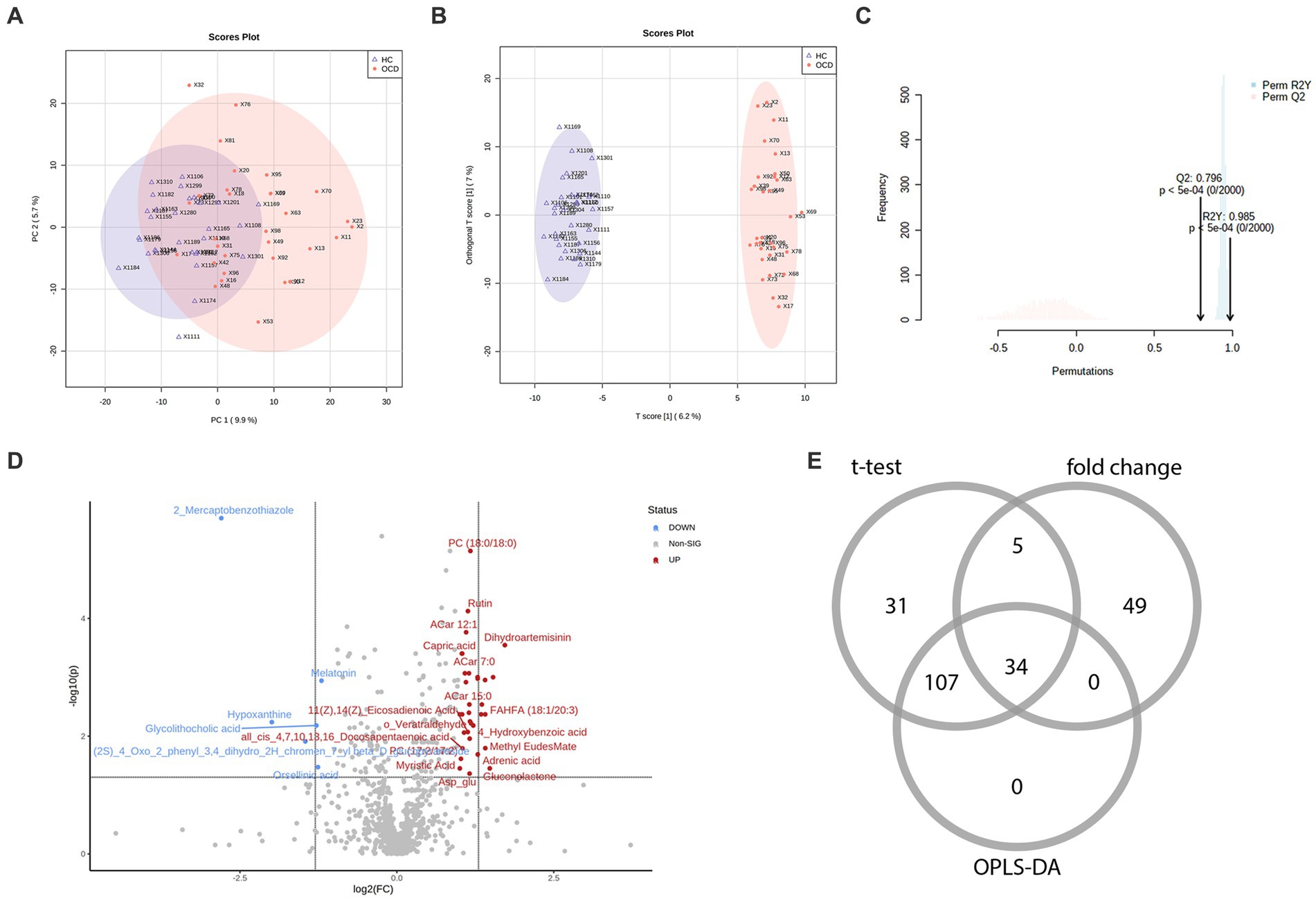
Figure 2. The metabolic profiling between the OCD and HC group and the selection of differential metabolites. (A) The scores plot of the principal component analysis between the OCD group and the HC group. (B) The scores plot of the orthogonal partial least-squares-discriminant analysis between the OCD and HC group. (C) The result of the 2000-time permutation test in the orthogonal partial least-squares-discriminant analysis. (D) The volcano plot. (E) The Venn diagram of the selection of differential metabolites.
According to the univariate and multivariate analysis, 177 metabolites were significantly altered after FDR correction; 88 metabolites met the criterion of FC >2/<0.5. The volcano plot was shown in Figure 2D. There were 141 metabolites detected from the OPLS-DA model. As illustrated in the Venn diagram (Figure 2E), we identified 34 differential metabolites listed in Table 3.
To construct the weighted metabolite correction network, power 3, which was the lowest power for which the scale-free topology fit index reaches 0.90, was chosen (Figures 3A,B). Subsequently, there were 11 distinct modules clustered and labeled with colors (Figure 3C) in the weighted metabolite correction network. The grey module was reserved for unassigned metabolites.
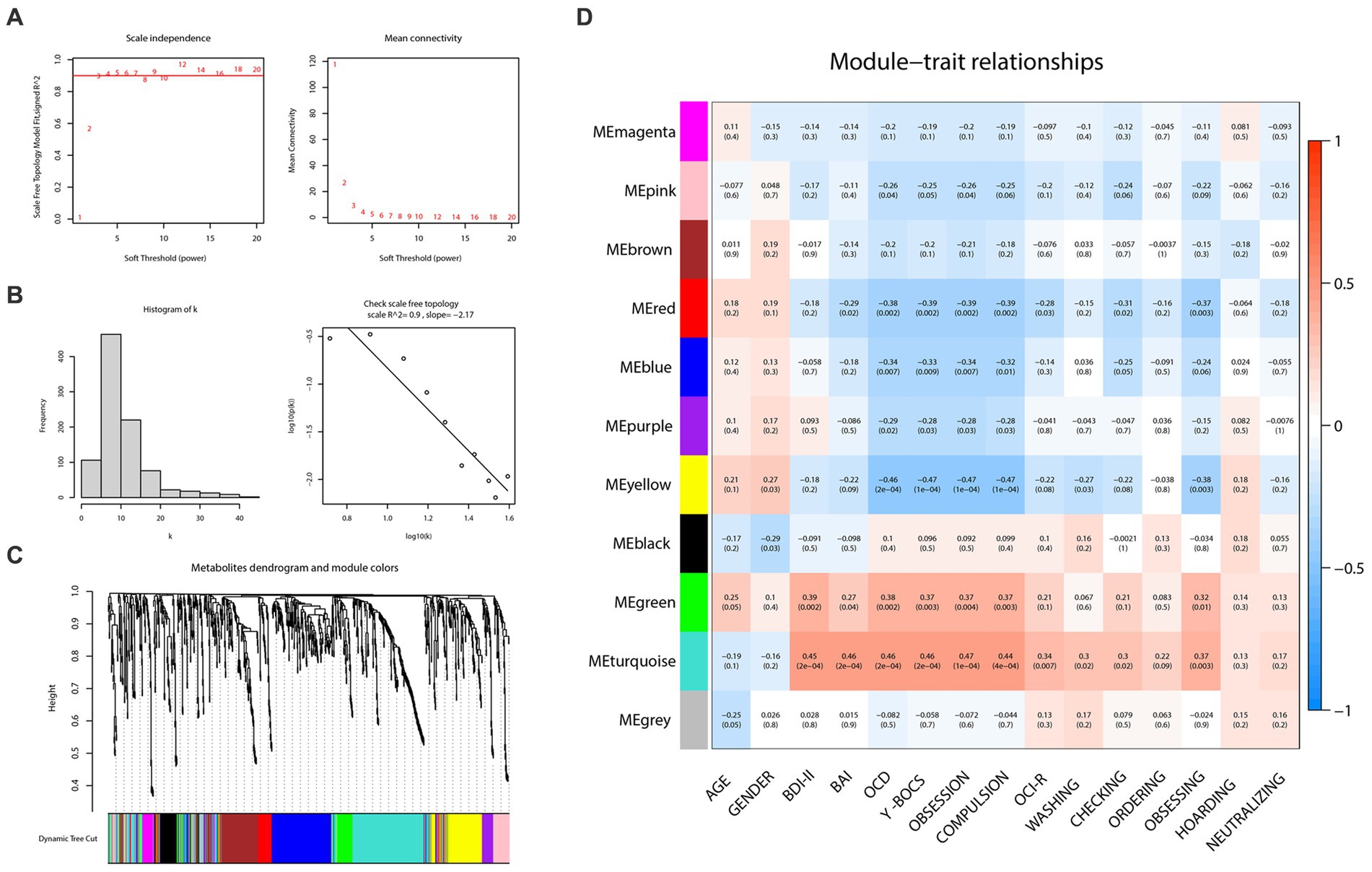
Figure 3. Weighted correlation network analysis and the selection of hub metabolites. (A) Scale-free fitting index analysis and mean connectivity of soft threshold power from 1 to 20. (B) Checking the scale free topology (power = 3). (C) Clustering dendrograms. (D) Correlation heatmap between module eigengenes and clinical traits.
As the plot of the module-trait relationship (Figure 3D) showed, grouping variables (namely “OCD” in the plot) and Y-BOCS scores had the highest correction with turquoise (cor = 0.46, p = 2e-04; cor = 0.46, p = 2e-04, respectively), yellow (cor = −0.46, p = 2e-04; cor = −0.47, p = 1e-04, respectively), red (cor = −0.38, p = 0.002; cor = −0.39, p = 0.002, respectively), green (cor = 0.38, p = 0.002; cor = 0.37, p = 0.003, respectively) and blue modules (cor = −0.34, p = 0.007; cor = −0.33, p = 0.009, respectively). With regard to OCI-R, it turned out to be similar but slightly different. The total scores of OCI-R saw significant associations with turquoise (cor = 0.34, p = 0.007) and red modules (cor = −0.28, p = 0.02) rather than the other three mentioned modules. The results in each subscale of OCI-R also differed. Subscales of ordering, hoarding and neutralizing were uncorrected with any module, while the subscale of obsessing was relevant with red (cor = −0.37, p = 0.003), yellow (cor = −0.38, p = 0.003) and turquoise (cor = 0.37, p = 0.003) modules. In addition, the turquoise module was closely related to the BDI-II (cor = 0.45, p = 2e-04) and BAI (cor = 0.46, p = 2e-04) scores; the green module also represented a prominent positive correlation with BDI-II scores (cor = 0.39, p = 0.002).
In the light of this, red, blue, yellow, green and turquoise modules were considered as interested modules. Figures 4A–E presented the correlation between MM and GS of all metabolites in each selected module. 51 hub metabolites were filtrated, on the basis of the standards mentioned above in the method, listed in Table 4. None was screened out in the blue module. The correlation network of these hub metabolites was in Figure 4F.
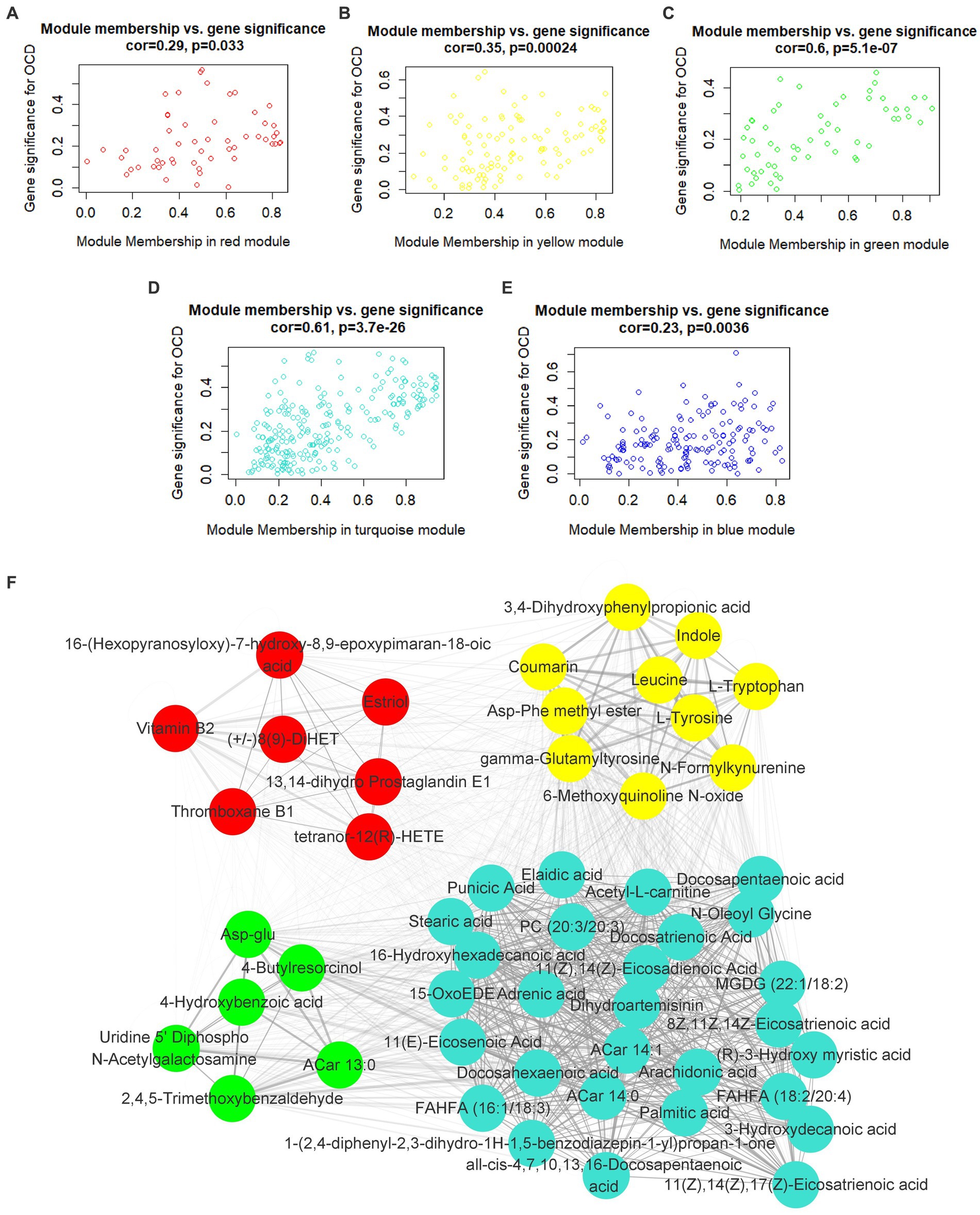
Figure 4. Hub metabolites. Correlation between module membership and gene significance of all metabolites in (A) red, (B) yellow, (C) green, (D) turquoise and (E) blue module. (F) correlation network of 51 selected hub metabolites.
Interestingly, as Figure 4D illustrated, most of the hub metabolites in the turquoise module were fatty acids, which included polyunsaturated fatty acids (PUFAs) such as Arachidonic acid, Docosahexaenoic acid (DHA), Docosapentaenoic acid (DPA), all-cis-4,7,10,13,16-Docosapentaenoic acid, 8Z,11Z,14Z-Eicosatrienoic acid, 11(Z),14(Z)-Eicosadienoic Acid, 11(Z),14(Z),17(Z)-Eicosatrienoic acid, Punicic Acid, Adrenic acid and Docosatrienoic Acid; saturated fatty acids such as Palmitic acid, Stearic acid and Capric acid. In red module (Figure 4A), several eicosanoids, such as tetranor-12(R)-HETE, Thromboxane B1, 13,14-dihydro Prostaglandin E1 and (+/−)8(9)-DiHET were screened as hub metabolites. In the yellow module (Figure 4B), there were several amino acids, such as L-Tryptophan (Trp), L-Tyrosine (Tyr) and Leucine, and related precursors or primary metabolites.
72 metabolites in total were selected as interested metabolites. The heatmap was shown in Figure 5A and the Venn diagrams were illustrated in Figure 5B. While only 19 differential metabolites and 32 hub metabolites were annotated by HMDB (Tables 3, 4) and were included in the following enrichment analysis (Figures 5C,D). As Table 5 listed, in both pathway analysis of differential metabolites and hub metabolites, biosynthesis of unsaturated fatty acids and tryptophan metabolism were detected.
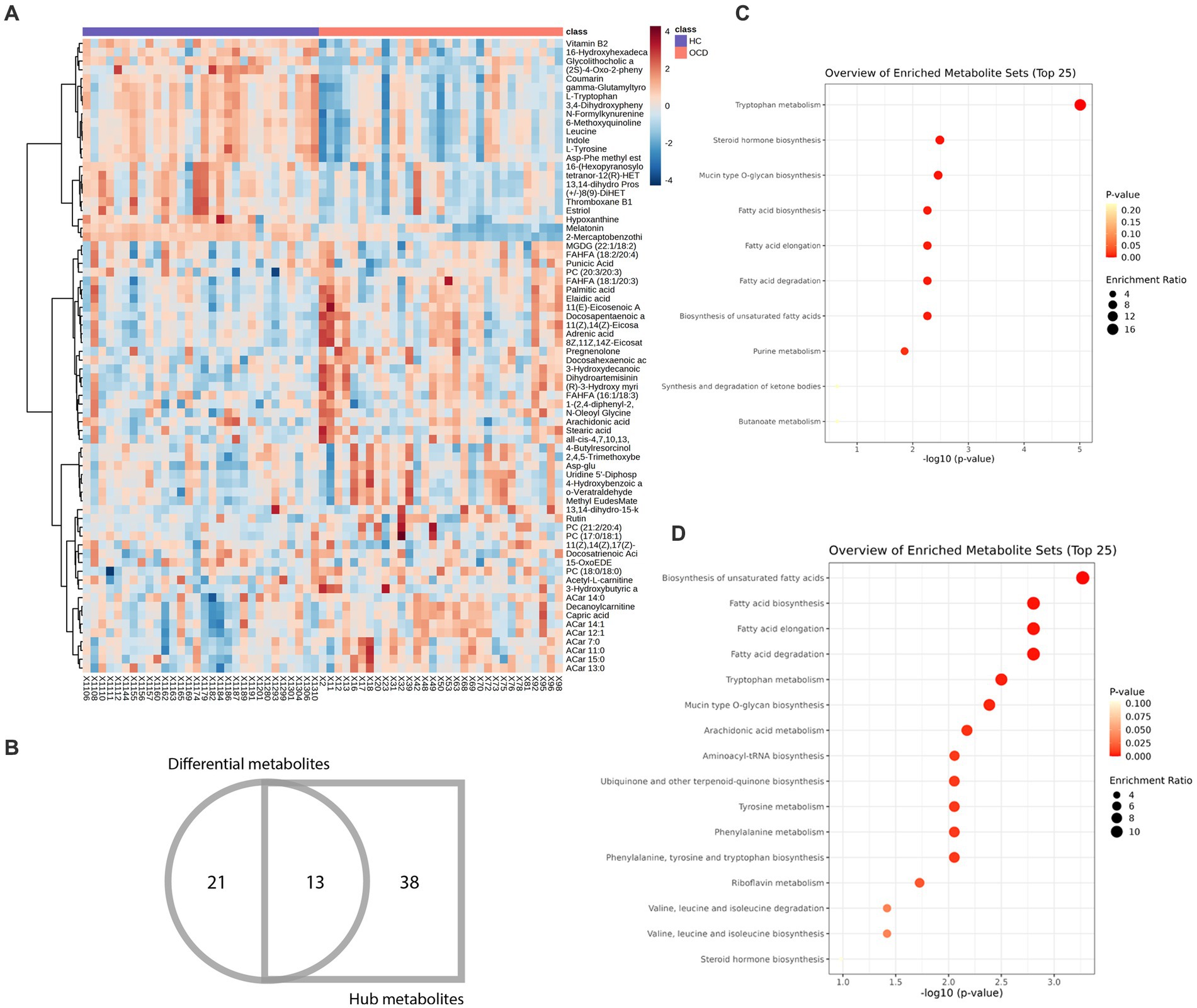
Figure 5. Heatmap and enrichment analyses of differential and hub metabolites. (A) Heatmap of differential and hub metabolites. (B) The Venn diagram of differential and hub metabolites. (C) Enrichment analyses of differential metabolites. (D) Enrichment analyses of hub metabolites.
As Figure 5B and Tables 3, 4 illustrated, there were 13 potential biomarkers which were both differential metabolites and hub metabolites. After stepwise regression, 8 of them were selected as predictor variables which were Uridine 5’-Diphospho-N-Acetylgalactosamine, Palmitic acid, all-cis-4,7,10,13,16-Docosapentaenoic acid, DPA, 8Z,11Z,14Z-Eicosatrienoic acid, 11(Z),14(Z)-Eicosadienoic Acid, Dihydroartemisinin, and 11(E)-Eicosenoic Acid. The nomogram was illustrated in the Figure 6A. The diagnostic performance was evaluated by the ROC curve (Figure 6B), with the AUC value of 96.7% and the 95% confidence interval (CI) ranging from 83.9 to 100%. Besides, the Hosmer–Lemeshow test (p-value = 0.676, Figure 6C) showed the goodness of fit for this model.
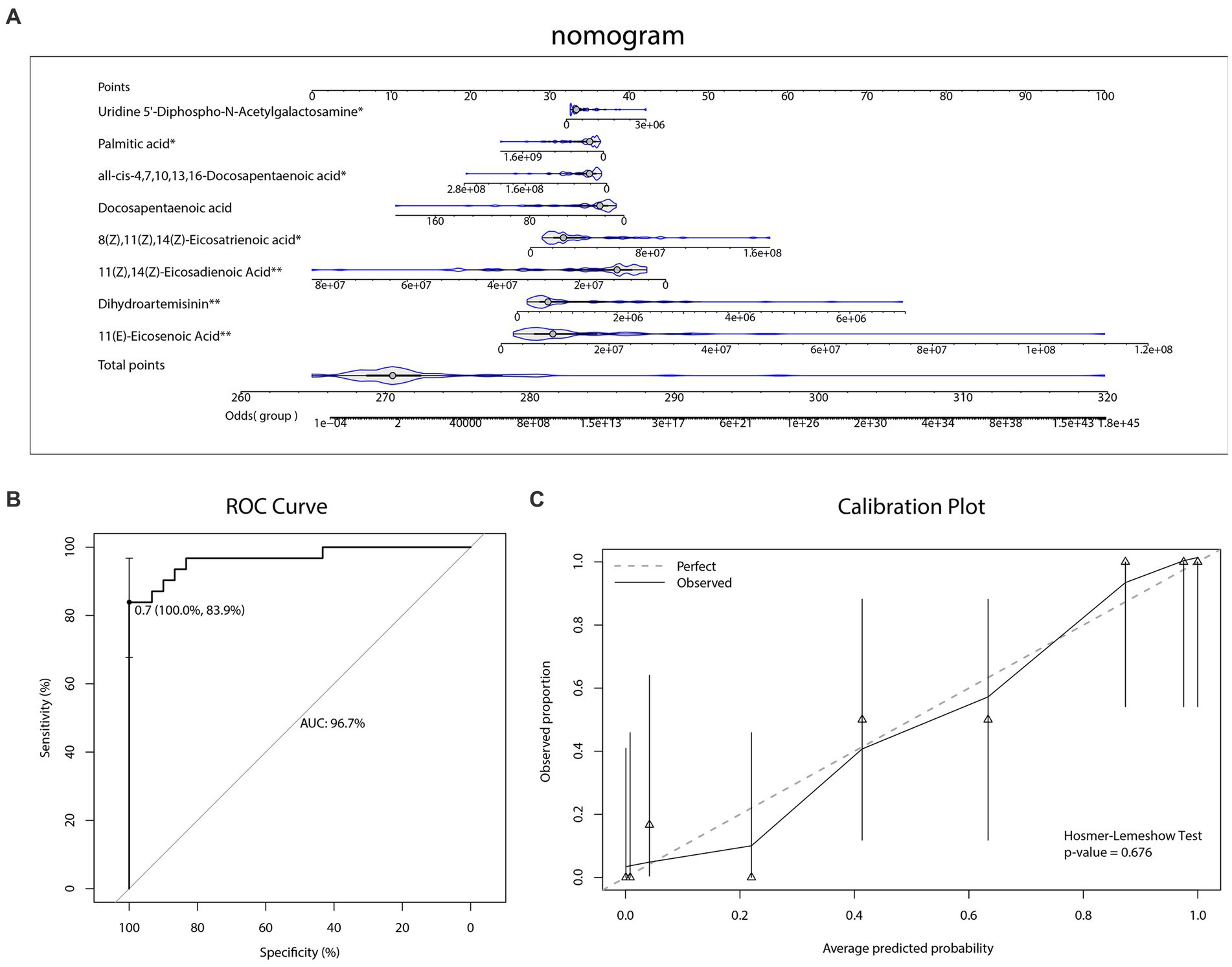
Figure 6. Diagnostic biomarker analysis. (A) Diagnostic nomogram. (B) The receiver operating characteristic (ROC) curve and the area under the curve (AUC) of diagnostic model. (C) The Hosmer–Lemeshow test and calibration plot of diagnostic model.
As for the outcome prediction of the sertraline therapeutic effect, a dataset of 28 patients with OCD who received 12-week sertraline monotherapy and finished follow-up visits was utilized for the LASSO analysis (Figures 7A,B). Among a total of 929 recognizable metabolites, three kinds of plasma metabolites were selected, namely 5-Hydroxytryptophan, 11-dehydro Thromboxane B2, 2,3-Bisphospho-D-glyceric acid. The heatmap (Figure 7C) illustrated the level of the selected metabolites in patients with OCD and their reduction rates of Y-BOCS scores.
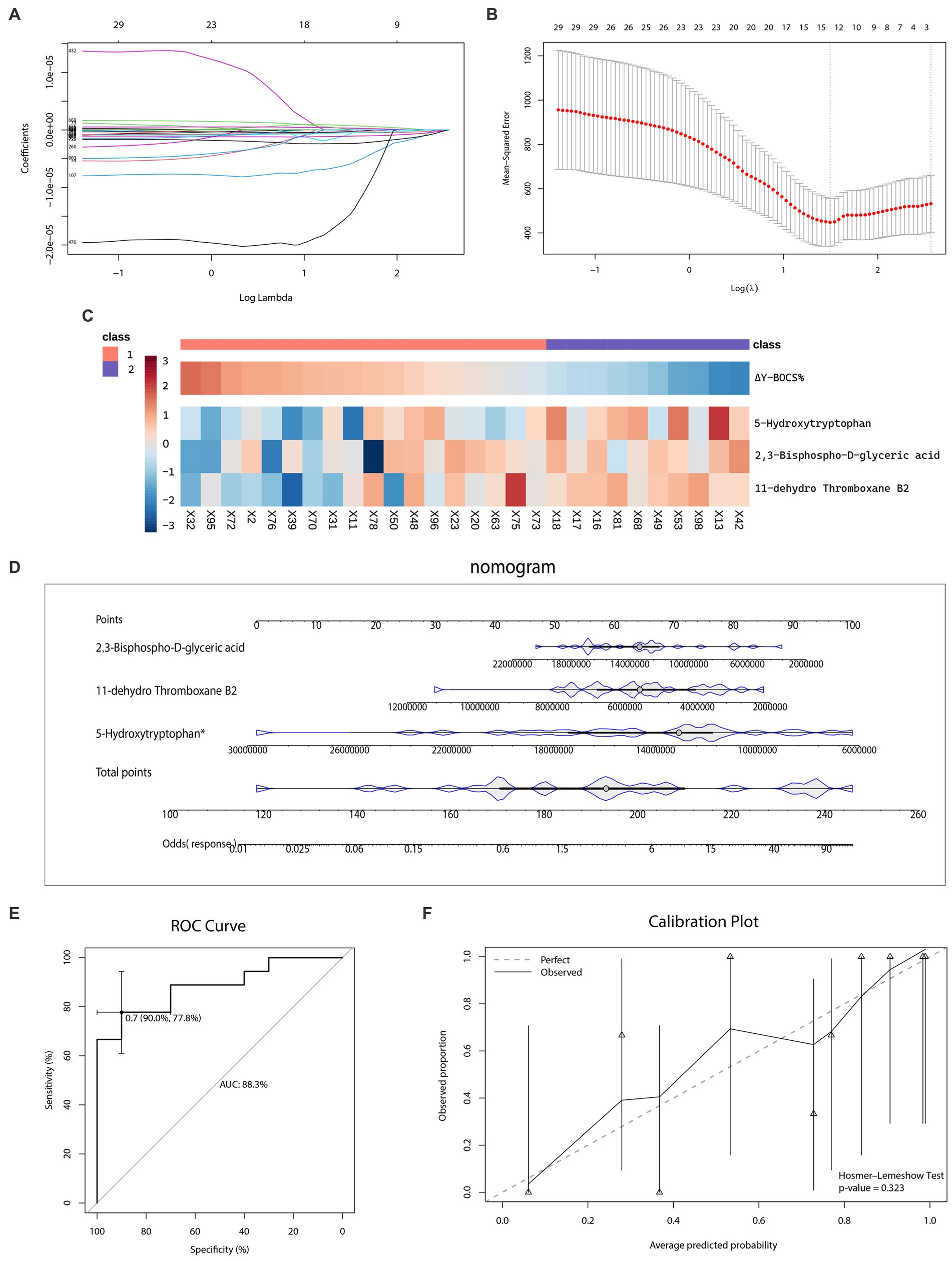
Figure 7. Selection of metabolic predictors and nomogram for predicting the therapeutic effect of sertraline. (A–B) Feature selection by the least absolute shrinkage and selection operator (LASSO) regression model. (C) The heatmap of metabolic predictors. (D) The outcome prediction nomogram. (E) The receiver operating characteristic (ROC) curve and the area under the curve (AUC) of outcome prediction model. (F) The Hosmer–Lemeshow test and calibration plot of outcome prediction model.
The nomogram (Figure 7D) visualized the effect of each metabolic predictor. By adding up points of every metabolic predictor, the total points were then calculated and positioned in the bottom line to evaluate the probability of response to sertraline monotherapy in patients with severe OCD. The predictability and consistency of this nomogram were also verified. The accumulated points summarized by the nomogram presented the possibility of the sertraline treatment response. The predictive ability of this model was, evaluated by the AUC value, 88.3% (CI: 77.8–90.0%) (Figure 7E). Meanwhile, we also conducted the Hosmer–Lemeshow test (p-value = 0.323), which showed the goodness of fit for this model. The calibration curve was shown in Figure 7F.
To our knowledge, this article was the first application of the untargeted metabolomics approach based on UPLC-Q-TOF/MS technology to analyze the plasma metabolic signature of OCD patients. Our analyses indicated that some amino acids and fatty acids, particularly in the biosynthesis of unsaturated fatty acids and tryptophan metabolism, were significantly different in OCD patients compared with HCs, and may play important roles in the pathogenesis of OCD. Besides, to explore the utility of circulating metabolites as biomarkers, we conducted nomograms of selected metabolites for diagnosis and sertraline treatment prediction based on the logistic regression model and achieved great discriminative abilities.
PUFAs, comprising 25–30% of the fatty acids in the human brain, have been shown to fulfill vital roles in regulating brain inflammation (Janssen and Kiliaan, 2014). Among them, omega-6 PUFAs (n-6) and omega-3 PUFAs (n-3) are two predominant kinds of PUFAs in the brain but play different roles. Omega-6 PUFAs, such as Arachidonic Acid, Docosatrienoic Acid and Eicosatrienoic Acid, produce n-6 eicosanoids (including prostaglandins, leukotrienes, thromboxanes, etc.) which mediate broadly pro-inflammatory effects. Omega-3 PUFAs, such as DHA, Eicosapentaenoic Acid (EPA) and DPA, produce n-3 eicosanoids (including resins and resolvins) which are generally anti-inflammatory (Haag, 2003). Hence, lower levels of omega-6 PUFAs and higher levels of omega-3 PUFAs might have particular importance in maintaining brain inflammation and functioning. Besides, PUFAs cannot be synthesized de novo and are mainly supplied by the blood; therefore, levels of circulating PUFAs might reflect on levels of counterparts in the brain and serve as potential biomarkers of several psychiatric diseases (Spector, 2001). Deficiency of circulating omega-3 PUFAs has been found in patients with Major Depression Disorder, Bipolar Disease, Schizophrenia and Alzheimer’s disease and has been seen to be closely associated with mood and cognitive function (Gao et al., 2022).
In the current study, we observed an alteration of unsaturated fatty acids and eicosanoids network (turquoise and red module) among patients with OCD. As shown in the turquoise module, both omega-6 PUFAs (Arachidonic acid, 11(Z),14(Z)-Eicosadienoic Acid, all-cis-4,7,10,13,16-Docosapentaenoic acid, Adrenic acid, 8Z,11Z,14Z-Eicosatrienoic acid, Docosatrienoic Acid) and omega-3 PUFAs (DHA, Docosapentaenoic acid, 11(Z),14(Z),17(Z)-Eicosatrienoic acid) were selected as hub metabolites and tended to be elevated compared to HCs. Whilst, levels of several omega-6 PUFAs (11(Z),14(Z)-Eicosadienoic Acid, all-cis-4,7,10,13,16-Docosapentaenoic acid, Adrenic acid, 8(Z),11(Z),14(Z)-Eicosatrienoic acid) significant increased comparing with HCs. These results suggested that patients with OCD have higher levels of circulating PUFAs but a relatively lower ratio of omega-3 to omega-6, consistent with the inflammatory hypothesis of OCD.
The unbalanced omega-3/omega-6 and pro- and anti- inflammatory processes might be new treatment targets in OCD. According to a randomized double-blind clinical trial, when taking celecoxib as an adjuvant to fluoxetine to inhibit cyclooxygenase-2 (COX-2), an enzyme converts arachidonic acid to eicosanoids, it presents rapid-onset anti–obsession and –compulsion effects in patients with OCD (Sayyah et al., 2011). Whereas, a preliminary placebo-controlled crossover trial adjunctive Omega-3 PUFAs (EPA in this case) failed to bring improvement in obsessive–compulsive, anxiety and depressive symptoms in patients with OCD, unlike its therapeutic benefit in other psychiatry diseases (Janssen and Kiliaan, 2014). What needs to be emphasized is that there were insufficient participants in the two trials mentioned above and more associated studies are called for in the future.
Tryptophan metabolism might be a key driver of the neurobiological mechanism of OCD. As we mentioned before, in the yellow module, Trp, N-Formylkynurenine were screened as hub metabolites and decreased compared with HCs. Trp, the essential amino acid, is a key precursor of serotonin (5-hydroxytryptamine or 5-HT), which is transported into the brain via the transporter located in capillaries of the blood–brain barrier and thus different plasma levels of Trp can affect cerebral 5-HT levels (Carneiro et al., 2018). In parallel to existing studies, we found lower but inapparent plasma Trp levels in OCD than HCs (Lucca et al., 1992). Furthermore, acute Trp depletion, which might lead to a short-term increase in presynaptic 5-HT availability, fails to improve the core impulsive-compulsive symptom in a short time (Berney et al., 2006; Külz et al., 2007), but cause a significant decrease perceived control and increase in interfering thoughts at the time of provocation (Hood et al., 2017).
Whereas, only a minor fraction of Trp is utilized for 5-HT synthesis; nearly 95% of the Trp pool enters the kynurenic pathway (Platten et al., 2019). The first stage of the Trp-kynurenic pathway is catalyzed by the hepatic enzyme tryptophan 2,3-dioxygenase (TDO) and the extrahepatic enzyme indoleamine 2,3-dioxygenase (IDO), enzymes that are induced by glucocorticoids and pro-inflammatory cytokines, respectively. Thus, chronic stress and infections can shunt available Trp toward the kynurenic pathway and thereby lower 5-HT synthesis (Sorgdrager et al., 2019), which in combination with the neuroinflammatory hypothesis may contribute to the hyposerotonin status in people with OCD.
Melatonin, which can be biosynthesized by Trp or serotonin and is involved in the control of the sleep–wake cycle, saw significantly lower plasma levels in patients with OCD. Previous studies have also demonstrated a decreased 24-h production of melatonin compared patients with OCD to HCs (Catapano et al., 1992; Millet et al., 1998), contributing to disruptions in circadian rhythms of the sleep–wake cycle. In particular, Delayed bedtimes are associated with more severe OCD symptoms (Coles et al., 2020; Nota et al., 2020).
Notwithstanding, these mentioned metabolites might not be suitable as biomarkers for diagnosis. Melatonin was one of the differential metabolites, but was not a stable predictor variable in our diagnostic nomogram model. Therefore, we proposed that there were wide, subtle and mostly poorly detectable (in separate comparisons) alterations in tryptophan metabolism in the plasma of patients with OCD, but drive an important role through the perturbation of serotonergic neurotransmitters and neuroinflammation in OCD.
We performed the logistic regression model using a combination of several selected metabolites to evaluate the discriminative ability of metabolites as promising biomarkers for diagnosis and outcome prediction of sertraline treatment efficacy. The results seemed promising. Both diagnostic and predictive nomogram model had impressive discrimination (AUC value) and calibration (p-value from the Hosmer–Lemeshow test) performances.
We discovered, for the first time, the capacity of fatty acids as potential biomarkers for OCD diagnosis. Four out of eight predictor variables of the diagnostic nomogram model are PUFAs. Thereinto, decreased circulating DPA, omega-3 PUFAs, was associated with a lower risk of OCD. Similarly, the protective effects of omega-3 PUFAs have been also found in other psychiatric diseases, such as attention deficit hyperactivity disorder, bipolar disorder, and schizophrenia (Bozzatello et al., 2016).
5-Hydroxytryptophan (5-HTP), meanwhile, was a significant predictor variable of the predictive nomogram model for sertraline treatment. A lower plasma level of 5-HTP was related to higher odds of response after receiving sertraline according to the predictive nomogram model. Also, recent studies revealed the potential benefits of 5-HTP as an augmentative medication of SSRIs (Yousefzadeh et al., 2020). 5-HTP is the intermediate metabolite of the Trp-serotonin pathway and can rapidly be converted to 5-HT. Since 5-HTP passively crosses the blood–brain barrier while 5-HT does not, the low plasma level of 5-HTP might present the hyposerotonin state in the brain which could be reversed by SSRIs.
There were some limitations in our research. Firstly, we only included patients with severe obsessive–compulsive symptoms, because we assumed that the metabolomic profile alterations would be more prominent and typical in patients with severe OCD. But the metabolomic profile of patients with mild to moderate OCD is still unknown; comparisons between patients with severe OCD and mild OCD need to be explored, which might offer fresh and novel insights into the mechanisms of OCD. Secondly, OCD is a heterogeneous disorder with distinct symptom dimensions and the pathological profiles of OCD may differ across symptom dimensions. Dimension-based analysis would be meaningful in future studies with larger sample sizes. As for biomarker analyses, we only validated our diagnostic and predictive model within the given dataset. We lack large-scale, multi-center data and external validation to access models’ reproducibility and generalizability, and therefore, our models should be interpreted with caution. The last but not the least, we did not control the body’s dietary intake, Body Mass Index or other Nutritional Status Indicators during studies.
Based on untargeted UPLC-Q-TOF/MS analysis, we discovered unsaturated fatty acids and tryptophan metabolism as key metabolic pathways alteration in OCD, and circulating metabolites of these pathways, such as DPA and 5-HTP, appeared to be promising biomarkers for OCD identification and sertraline treatment prediction.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Institutional Review Boards of Shanghai Mental Health Center. The patients/participants provided their written informed consent to participate in this study.
ZL and ZW were involved in the study design and writing of the manuscript. JG, LL, ZZ, and SY contributed to the acquisition of data for the work. ZL, DS, WW, and ZW were involved in the analysis and interpretation of the results. All authors contributed to the article and approved the submitted version.
This study was funded by the Shanghai Municipal Education Commission-Gao Feng Clinical Medicine Grant (20161321), the grants from Shanghai Municipal Health Commission (2019ZB0201), and the Shanghai Key Laboratory of Psychotic Disorders (No.13dz2260500).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1148971/full#supplementary-material
American Psychological Association. Diagnostic and statistical manual of mental disorders. 5th Edn Am Psychiatric Assoc. Washington, DC (2013).
Alseekh, S., Aharoni, A., Brotman, Y., Contrepois, K., D’Auria, J., Ewald, J., et al. (2021). Mass spectrometry-based metabolomics: a guide for annotation, quantification and best reporting practices. Nat. Methods 18, 747–756. doi: 10.1038/s41592-021-01197-1
Attwells, S., Setiawan, E., Wilson, A. A., Rusjan, P. M., Mizrahi, R., Miler, L., et al. (2017). Inflammation in the Neurocircuitry of obsessive-compulsive disorder. JAMA Psychiat. 74, 833–840. doi: 10.1001/jamapsychiatry.2017.1567
Beck, A. T., Steer, R. A., Ball, R., and Ranieri, W. (1996). Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 67, 588–597. doi: 10.1207/s15327752jpa6703_13
Beck, A. T., Ward, C., Mendelson, M., Mock, J., and Erbaugh, J. (1961). Beck depression inventory (BDI). Arch. Gen. Psychiatry 4, 561–571. doi: 10.1001/archpsyc.1961.01710120031004
Benkelfat, C., Mefford, I. N., Masters, C. F., Nordahl, T. E., King, A. C., Cohen, R. M., et al. (1991). Plasma catecholamines and their metabolites in obsessive-compulsive disorder. Psychiatry Res. 37, 321–331. doi: 10.1016/0165-1781(91)90067-Y
Berney, A., Sookman, D., Leyton, M., Young, S. N., and Benkelfat, C. (2006). Lack of effects on core obsessive-compulsive symptoms of tryptophan depletion during symptom provocation in remitted obsessive-compulsive disorder patients. Biol. Psychiatry 59, 853–857. doi: 10.1016/j.biopsych.2005.08.023
Bhattacharyya, S., Khanna, S., Chakrabarty, K., Mahadevan, A., Christopher, R., and Shankar, S. K. (2009). Anti-brain autoantibodies and altered excitatory neurotransmitters in obsessive-compulsive disorder. Neuropsychopharmacology 34, 2489–2496. doi: 10.1038/npp.2009.77
Bozzatello, P., Brignolo, E., De Grandi, E., and Bellino, S. (2016). Supplementation with Omega-3 fatty acids in psychiatric disorders: a review of literature data. J. Clin. Med. 5:67. doi: 10.3390/jcm5080067
Brennan, B. P., Rauch, S. L., Jensen, J. E., and Pope, H. G. (2013). A critical review of magnetic resonance spectroscopy studies of obsessive-compulsive disorder. Biol. Psychiatry 73, 24–31. doi: 10.1016/j.biopsych.2012.06.023
Carneiro, I. B. C., Toscano, A. E., Lacerda, D. C., da Cunha, M., De, S. B., De Castro, R. M., et al. (2018). L-tryptophan administration and increase in cerebral serotonin levels: systematic review. Eur. J. Pharmacol. 836, 129–135. doi: 10.1016/j.ejphar.2018.08.009
Catapano, F., Monteleone, P., Fuschino, A., Maj, M., and Kemali, D. (1992). Melatonin and cortisol secretion in patients with primary obsessive-compulsive disorder. Psychiatry Res. 44, 217–225. doi: 10.1016/0165-1781(92)90025-X
Chakrabarty, K., Bhattacharyya, S., Christopher, R., and Khanna, S. (2005). Glutamatergic dysfunction in OCD. Neuropsychopharmacology 30, 1735–1740. doi: 10.1038/sj.npp.1300733
Chen, J., Zhang, P., Lv, M., Guo, H., Huang, Y., Zhang, Z., et al. (2017). Influences of normalization method on biomarker discovery in gas chromatography–mass spectrometry-based untargeted metabolomics: what should be considered? Anal. Chem. 89, 5342–5348. doi: 10.1021/acs.analchem.6b05152
Coles, M. E., Schubert, J., Stewart, E., Sharkey, K. M., and Deak, M. (2020). Sleep duration and timing in obsessive-compulsive disorder (OCD): evidence for circadian phase delay. Sleep Med. 72, 111–117. doi: 10.1016/j.sleep.2020.03.021
Delorme, R., Betancur, C., Callebert, J., Chabane, N., Laplanche, J. L., Mouren-Simeoni, M. C., et al. (2005). Platelet serotonergic markers as endophenotypes for obsessive-compulsive disorder. Neuropsychopharmacology 30, 1539–1547. doi: 10.1038/sj.npp.1300752
Foa, E. B., Huppert, J. D., Leiberg, S., Langner, R., Kichic, R., Hajcak, G., et al. (2002). The obsessive-compulsive inventory: development and validation of a short version. Psychol. Assess. 14, 485–496. doi: 10.1037/1040-3590.14.4.485
Fontenelle, L. F., Mendlowicz, M. V., and Versiani, M. (2006). The descriptive epidemiology of obsessive-compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 30, 327–337. doi: 10.1016/j.pnpbp.2005.11.001
Gao, X., Su, X., Han, X., Wen, H., Cheng, C., Zhang, S., et al. (2022). Unsaturated fatty acids in mental disorders: an umbrella review of meta-analyses. Adv. Nutr. 13, 2217–2236. doi: 10.1093/advances/nmac084
Gerentes, M., Pelissolo, A., Rajagopal, K., Tamouza, R., and Hamdani, N. (2019). Obsessive-compulsive disorder: autoimmunity and Neuroinflammation. Curr. Psychiatry Rep. 21:78. doi: 10.1007/s11920-019-1062-8
Goddard, A. W., Shekhar, A., Whiteman, A. F., and McDougle, C. J. (2008). Serotoninergic mechanisms in the treatment of obsessive-compulsive disorder. Drug Discov. Today 13, 325–332. doi: 10.1016/j.drudis.2007.12.009
Goodman, W. K., Price, L. H., Rasmussen, S. A., Mazure, C., Fleischmann, R. L., Hill, C. L., et al. (1989). The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Arch. Gen. Psychiatry 46, 1006–1011. doi: 10.1001/archpsyc.1989.01810110048007
Haag, M. (2003). Essential fatty acids and the brain. Can. J. Psychiatr. 48, 195–203. doi: 10.1177/070674370304800308
Hasin, Y., Seldin, M., and Lusis, A. (2017). Multi-omics approaches to disease. Genome Biol. 18:83. doi: 10.1186/s13059-017-1215-1
Hollander, E., Stein, D. J., Saoud, J. B., DeCaria, C. M., Cooper, T. B., Trungold, S., et al. (1992). Effects of fenfluramine on plasma HVA in OCD. Psychiatry Res. 42, 185–188. doi: 10.1016/0165-1781(92)90081-D
Hood, S. D., Broyd, A., Robinson, H., Lee, J., Hudaib, A. R., and Hince, D. A. (2017). Effects of tryptophan depletion on selective serotonin reuptake inhibitor-remitted patients with obsessive compulsive disorder. J. Psychopharmacol. 31, 1615–1623. doi: 10.1177/0269881117736916
Janssen, C. I., and Kiliaan, A. J. (2014). Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 53, 1–17. doi: 10.1016/j.plipres.2013.10.002
Jia, Y., Hui, L., Sun, L., Guo, D., Shi, M., Zhang, K., et al. (2022). Association between human blood metabolome and the risk of psychiatric disorders. Schizophr. Bull. 49:sbac130. doi: 10.1093/schbul/sbac130
Kanehisa, M., and Goto, S. (2000). KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27 –30. doi: 10.1093/nar/28.1.27
Koo, M. S., Kim, E. J., Roh, D., and Kim, C. H. (2010). Role of dopamine in the pathophysiology and treatment of obsessive-compulsive disorder. Expert. Rev. Neurother. 10, 275–290. doi: 10.1586/ern.09.148
Külz, A. K., Meinzer, S., Kopasz, M., and Voderholzer, U. (2007). Effects of tryptophan depletion on cognitive functioning, obsessive-compulsive symptoms and mood in obsessive-compulsive disorder: preliminary results. Neuropsychobiology 56, 127–131. doi: 10.1159/000115778
Langfelder, P., and Horvath, S. (2008). WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9:559. doi: 10.1186/1471-2105-9-559
Lázaro, L., Bargalló, N., Andrés, S., Falcón, C., Morer, A., Junqué, C., et al. (2012). Proton magnetic resonance spectroscopy in pediatric obsessive-compulsive disorder: longitudinal study before and after treatment. Psychiatry Res. 201, 17–24. doi: 10.1016/j.pscychresns.2011.01.017
Leckman, J. F., Goodman, W. K., Anderson, G. M., Riddle, M. A., Chappell, P. B., McSwiggan-Hardin, M. T., et al. (1995). Cerebrospinal fluid biogenic amines in obsessive compulsive disorder, Tourette’s syndrome, and healthy controls. Neuropsychopharmacology 12, 73–86. doi: 10.1038/sj.npp.1380241
Lucca, A., Lucini, V., Piatti, E., Ronchi, P., and Smeraldi, E. (1992). Plasma tryptophan levels and plasma tryptophan/neutral amino acids ratio in patients with mood disorder, patients with obsessive-compulsive disorder, and normal subjects. Psychiatry Res. 44, 85–91. doi: 10.1016/0165-1781(92)90043-3
Maia, A., Oliveira, J., Lajnef, M., Mallet, L., Tamouza, R., Leboyer, M., et al. (2019). Oxidative and nitrosative stress markers in obsessive-compulsive disorder: a systematic review and meta-analysis. Acta Psychiatr. Scand. 139, 420–433. doi: 10.1111/acps.13026
Mataix-Cols, D., Rosario-Campos, M. C., and Leckman, J. F. (2005). A multidimensional model of obsessive-compulsive disorder. Am. J. Psychiatry 162, 228–238. doi: 10.1176/appi.ajp.162.2.228
Millet, B., Touitou, Y., Poirier, M. F., Bourdel, M. C., Hantouche, E., Bogdan, A., et al. (1998). Plasma melatonin and cortisol in patients with obsessive-compulsive disorder: relationship with axillary temperature, physical activity, and clinical symptoms. Biol. Psychiatry 44, 874–881. doi: 10.1016/S0006-3223(97)00512-X
Mohammadi, A. H., Balandeh, E., and Milajerdi, A. (2021). Malondialdehyde concentrations in obsessive-compulsive disorder: a systematic review and meta-analysis. Ann. General Psychiatry 20:34. doi: 10.1186/s12991-021-00354-2
Nota, J. A., Potluri, S., Kelley, K. N., Elias, J. A., and Krompinger, J. W. (2020). Delayed bedtimes are associated with more severe obsessive-compulsive symptoms in intensive residential treatment. Behav. Ther. 51, 559–571. doi: 10.1016/j.beth.2019.12.006
Pallanti, S., and Quercioli, L. (2006). Treatment-refractory obsessive-compulsive disorder: methodological issues, operational definitions and therapeutic lines. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 30, 400–412. doi: 10.1016/j.pnpbp.2005.11.028
Pauls, D. L., Abramovitch, A., Rauch, S. L., and Geller, D. A. (2014). Obsessive–compulsive disorder: an integrative genetic and neurobiological perspective. Nat. Rev. Neurosci. 15, 410–424. doi: 10.1038/nrn3746
Pittenger, C., Bloch, M. H., and Williams, K. (2011). Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacol. Ther. 132, 314–332. doi: 10.1016/j.pharmthera.2011.09.006
Platten, M., Nollen, E. A. A., Röhrig, U. F., Fallarino, F., and Opitz, C. A. (2019). Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 18, 379–401. doi: 10.1038/s41573-019-0016-5
Rinschen, M. M., Ivanisevic, J., Giera, M., and Siuzdak, G. (2019). Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 20, 353–367. doi: 10.1038/s41580-019-0108-4
Sayyah, M., Boostani, H., Pakseresht, S., and Malayeri, A. (2011). A preliminary randomized double-blind clinical trial on the efficacy of celecoxib as an adjunct in the treatment of obsessive-compulsive disorder. Psychiatry Res. 189, 403–406. doi: 10.1016/j.psychres.2011.01.019
Sorgdrager, F. J. H., Naudé, P. J. W., Kema, I. P., Nollen, E. A., and Deyn, P. P. D. (2019). Tryptophan metabolism in Inflammaging: from biomarker to therapeutic target. Front. Immunol. 10:2565. doi: 10.3389/fimmu.2019.02565
Spector, A. A. (2001). Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain. J. Mol. Neurosci. 16, 159–165; discussion 215-21. doi: 10.1385/JMN:16:2-3:159
Swedo, S. E., Leonard, H. L., Kruesi, M. J., Rettew, D. C., Listwak, S. J., Berrettini, W., et al. (1992). Cerebrospinal fluid neurochemistry in children and adolescents with obsessive-compulsive disorder. Arch. Gen. Psychiatry 49, 29–36. doi: 10.1001/archpsyc.1992.01820010029004
Veale, D., and Roberts, A. (2014). Obsessive-compulsive disorder. BMJ 348:g2183. doi: 10.1136/bmj.g2183
Wang, P., Gu, W., Gao, J., Wang, C., Fang, J., Hu, M., et al. (2022). Protocol for a pragmatic trial of pharmacotherapy options following unsatisfactory initial treatment in OCD (PROCEED). Front. Psych. 13:822976. doi: 10.3389/fpsyt.2022.1046821
Wishart, D. S. (2019). Metabolomics for investigating physiological and pathophysiological processes. Physiol. Rev. 99, 1819–1875. doi: 10.1152/physrev.00035.2018
Yang, W., Tang, Z., Wang, X., Ma, X., Cheng, Y., Wang, B., et al. (2021). The cost of obsessive-compulsive disorder (OCD) in China: a multi-center cross-sectional survey based on hospitals. Gen Psychiatr. 34:e100632. doi: 10.1136/gpsych-2021-100632
Yousefzadeh, F., Sahebolzamani, E., Sadri, A., Mortezaei, A., Aqamolaei, A., Mortazavi, S. H., et al. (2020). 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int. Clin. Psychopharmacol. 35, 254–262. doi: 10.1097/YIC.0000000000000321
Keywords: obsessive–compulsive disorder (OCD), metabolomics, untargeted metabolomics analysis, biomarker, WGCNA (weighted gene co- expression network analyses), Unsaturared fatty acid, amino acid, neuroinflammation
Citation: Li Z, Gao J, Lin L, Zheng Z, Yan S, Wang W, Shi D and Wang Z (2023) Untargeted metabolomics analysis in drug-naïve patients with severe obsessive–compulsive disorder. Front. Neurosci. 17:1148971. doi: 10.3389/fnins.2023.1148971
Received: 20 January 2023; Accepted: 15 May 2023;
Published: 02 June 2023.
Edited by:
Lei Sha, China Medical University, ChinaReviewed by:
Shaohua Hu, Zhejiang University, ChinaCopyright © 2023 Li, Gao, Lin, Zheng, Yan, Wang, Shi and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Wang, d2FuZ3poZW5Ac21oYy5vcmcuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.