
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci. , 18 April 2023
Sec. Neuropharmacology
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1126004
This article is part of the Research Topic Respiratory Control and Dysfunction in Neurological Patients, Volume II View all 5 articles
Recent changes in cannabis accessibility have provided adjunct therapies for patients across numerous disease states and highlights the urgency in understanding how cannabinoids and the endocannabinoid (EC) system interact with other physiological structures. The EC system plays a critical and modulatory role in respiratory homeostasis and pulmonary functionality. Respiratory control begins in the brainstem without peripheral input, and coordinates the preBötzinger complex, a component of the ventral respiratory group that interacts with the dorsal respiratory group to synchronize burstlet activity and drive inspiration. An additional rhythm generator: the retrotrapezoid nucleus/parafacial respiratory group drives active expiration during conditions of exercise or high CO2. Combined with the feedback information from the periphery: through chemo- and baroreceptors including the carotid bodies, the cranial nerves, stretch of the diaphragm and intercostal muscles, lung tissue, and immune cells, and the cranial nerves, our respiratory system can fine tune motor outputs that ensure we have the oxygen necessary to survive and can expel the CO2 waste we produce, and every aspect of this process can be influenced by the EC system. The expansion in cannabis access and potential therapeutic benefits, it is essential that investigations continue to uncover the underpinnings and mechanistic workings of the EC system. It is imperative to understand the impact cannabis, and exogenous cannabinoids have on these physiological systems, and how some of these compounds can mitigate respiratory depression when combined with opioids or other medicinal therapies. This review highlights the respiratory system from the perspective of central versus peripheral respiratory functionality and how these behaviors can be influenced by the EC system. This review will summarize the literature available on organic and synthetic cannabinoids in breathing and how that has shaped our understanding of the role of the EC system in respiratory homeostasis. Finally, we look at some potential future therapeutic applications the EC system has to offer for the treatment of respiratory diseases and a possible role in expanding the safety profile of opioid therapies while preventing future opioid overdose fatalities that result from respiratory arrest or persistent apnea.
A functioning respiratory system is critical to survival (Prabhakar and Semenza, 2015; Agrawal and Mabalirajan, 2016) and preserved across many species. The role of the endocannabinoid (EC) system in respiratory homeostasis remains to be fully elucidated. The infancy of our understanding of the interactions of the EC system and respiratory physiology should not equal an assumed lack of influence over each other. Recent studies have shown that administration of a cannabinoid2 receptor (CB2R) inverse agonist (Wiese et al., 2020) or a brain penetrant cannabinoid1 receptor agonist (CB1R; Wiese et al., 2021) induced respiratory depression (Wiese et al., 2021) – suggesting a tonic role of the CB2R and CB1R in modulation of respiratory functionality. The EC system has repeatedly been shown to play a holistic regulatory role in many other activities, from experiencing pleasure, to cognitive abilities, and even in the perception of pain (Gardner and Vorel, 1998), making it no surprise that the EC system is also involved in respiratory behavior. It is well established that cannabinoids from the cannabis plant act on our EC system, lending to the discovery of the EC system itself (Gaoni and Mechoulam, 1964). To date all but three states in the US participate in some form of legal cannabis access (Control CfD, 2017), and a recent Gallup poll showed that ~12% of US adults report consistently smoking cannabis (Hrynowski, 2019). Researchers have long been working to understand the impact of cannabis on the body as well as in combination with other medication therapies, including opioids (Epstein and Preston, 2015; Hurd et al., 2015; Jicha et al., 2015; Manini et al., 2015; Mayet et al., 2015; Hrynowski, 2019). While some observational studies have found conflicting results (Ghasemiesfe et al., 2018; Darke et al., 2019), studies since have been able to delineate some of the changes cannabis smoke can have on the body, such as upper lobe emphysematous changes (Gates et al., 2014), hyperinflation (Hancox et al., 2022), bronchiolitis (Gates et al., 2014), alveolar cell hyperplasia with atypia and fibrosis (Darke et al., 2019), sputum production and increased cough (Ghasemiesfe et al., 2018). It is worth stating that this was seen with traditional combustion delivery methods, as opposed to other routes of cannabis administration, and no evidence to date suggests cannabis smoke leads to chronic obstructive pulmonary disease like tobacco smoke does (Owen et al., 2014). But these changes alone do not paint the full picture of the role the EC system has in respiratory functionality. With the increasing number of people utilizing cannabis and cannabinoids on their own or as an adjunct to other treatments (Mauro et al., 2019; Goodwin et al., 2021), especially in combination with analgesics, it is imperative to understand how the EC system and cannabinoids influence our respiratory system.
This review will explore cannabinoids and the EC system in the context of respiratory regulation, highlighting CB1R and CB2R influence in the context of central versus peripheral activation, followed by the effects of organic and synthetic cannabinoids on breathing. A summary of cannabinoids effects on breathing is laid out in Figure 1. While additional research is available on cannabinoid tolerance (Deng et al., 2015; Bradford and Bradford, 2016; Bradford et al., 2018; Wiese and Wilson-Poe, 2018; Soliman et al., 2021) or sex differences (De Fonseca et al., 1994; Craft et al., 2013; Channappanavar et al., 2017; Cooper and Craft, 2018; Gargaglioni et al., 2019; Silver and Hur, 2020; Albrechet-Souza et al., 2021; Boullon et al., 2021; Levine et al., 2021; Silveyra et al., 2021; Llorente-Berzal et al., 2022; Simone et al., 2022) could affect breathing, they are outside the scope of this review. We will discuss future possible therapeutic applications for treatment of respiratory diseases as well as a possible role in preventing future opioid overdose fatalities that result from respiratory arrest or persistent apnea.
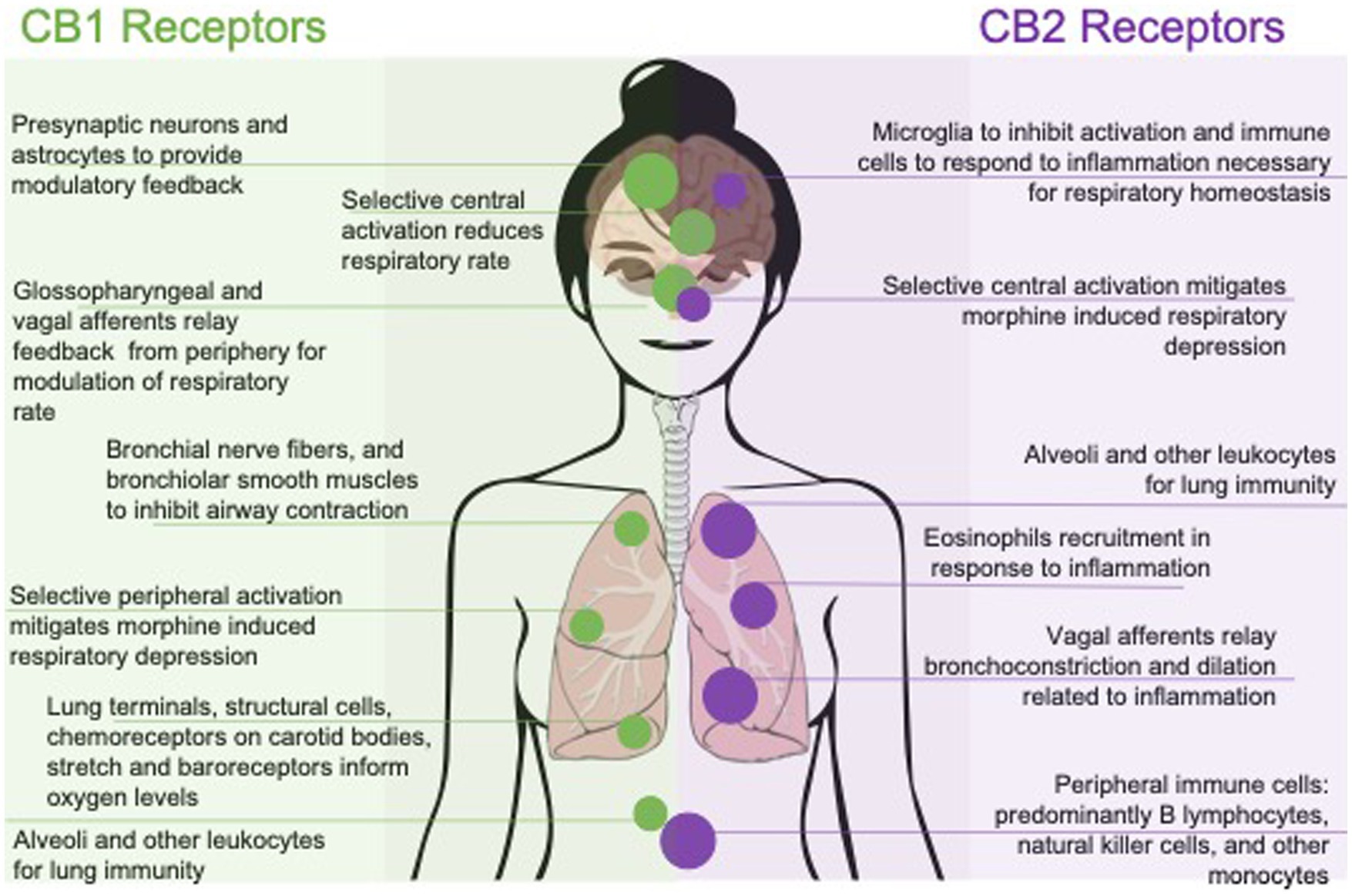
Figure 1. Effects of pharmacologically targeting central or peripheral CB1 and CB2 receptors on respiratory function. Respiratory outcomes are represented by their mechanism of action; with CB1 selective affinity to the left and CB2 selective affinity to the right. Outcomes are also represented with peripherally mediated outcomes along the bottom and centrally, or systemic outcomes, along the top.
The respiratory system is made up of two main components, a reflexive and cognitive component. The reflexive component is always at work; meticulously monitoring carbon dioxide (CO2) levels, pH changes, and expelling waste 24/7 without any conscious thought or input (Guyenet, 2011; Ogoh, 2019). The other component is the cognitive side. The reflexive component can be overridden and altered by a conscious choice to intervene such as when engaging in breathing exercises, holding ones’ breath, or smoking of a substance (Evans et al., 1999; Mckay et al., 2003; Butler, 2007; Raux et al., 2007). It is these intentional altered inhales that aid the gas exchange of inhaled compounds from the lungs into the bloodstream, such as cannabis or other inhalants The effects felt through inhalation are almost immediate thanks to the efficiency of this fine-tuned respiratory system (Hickey, 2020). The most common route of cannabis and synthetic cannabinoid (SC) consumption is through inhalation, making it vital to public safety that we understand the effects these compounds have on lung tissue and function, as well as how our endogenous cannabinoids influence our respiratory behavior for future therapeutic discovery.
The EC system is highly integrated in multiple organ systems of the brain and body, and involved in multiple ways in all homeostatic regulation (Evans et al., 1999; Mckay et al., 2003; Butler, 2007; Raux et al., 2007; Guyenet, 2011; Soliman et al., 2021). Both cannabinoid receptors, CB1R and CB2R, have varying distributions in the body, purporting different roles between the two. Within the central nervous system (CNS) CB1R are primarily localized within the CNS on presynaptic cells for inhibitory feedback to the cell (Ghasemiesfe et al., 2018), as well as some non-neural tissue. CB2Rs are involved in inflammation and immunology in the periphery, as well as the CNS, where they are highly expressed in immune cells (Galiègue et al., 1995) and microglia (Schmöle et al., 2015) on the CNS, regulating immune functions (Soliman et al., 2021). With the ubiquitous distribution of cannabinoid receptors within the CNS, especially the CB1R, it is no surprise that the EC system plays a direct role in fine tuning the process of breathing and can be manipulated by exogenous or endogenous cannabinoids. Locations of known EC system influence are shown in Figure 2. While drug administration through inhalation is fast and effective, cannabinoids have been shown to influence respiratory rate through other routes of administration (Ryberg et al., 2007; Webster and Schmidt, 2019), as well as offering a more personalized treatment plan for patients who do not tolerate inhalation, or an alternative to traditional combustion based methods of drug delivery. While reports of direct CNS administration of the dual CB1/CB2R agonist, WIN 55212-2, produced respiratory depression (Pfitzer et al., 2004), imagining studies for cannabinoid receptors have been inconsistent in confirming receptor presence and exact concentration levels in brainstem respiratory nuclei (Ryberg et al., 2007; Huxtable et al., 2010; Lorea-Hernandez et al., 2016).
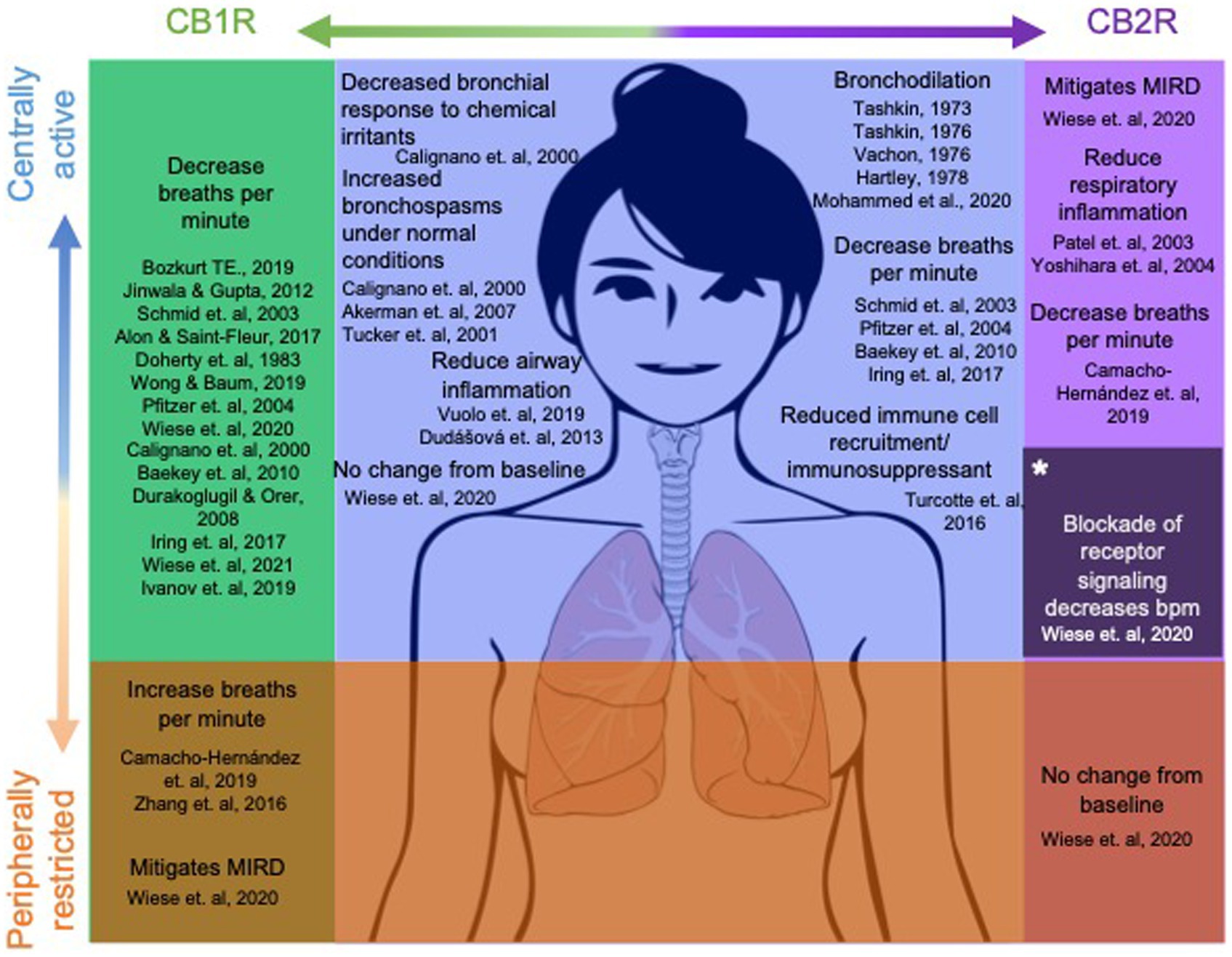
Figure 2. CB1/CB2 receptor distribution and current understanding of their role in respiratory function. Dots in the brain represent centrally mediated effects, dots in the lungs and abdomen represent peripherally mediated effects. Dot size corresponds to concentration levels of the receptor within the region.
The most studied and well understood EC lipids are anandamide (AEA), a partial agonist, and 2-arachidonoylglycerol (2-AG), a full agonist (Soliman et al., 2021), at both CB1R and CB2R. Both ligands are produced on demand in the postsynaptic cell for retrograde regulation of presynaptic activity and glial cell function. Endogenous cannabinoid ligands, AEA and 2-AG, are synthesized intracellularly (Matias et al., 2002), further supporting a critical role of the EC system in the homeostatic process. Cannabinoids and EC lipids are not the only active compounds to consider about this system, as some of their metabolites, like 2-arachidonoyglycerol ether (Levine et al., 2021), have also been shown to activate and modulate CB1R and CB2R (Turcotte et al., 2016). Other endogenous molecules have been shown to activate CB1R and CB2R, such as oleamide, N-oleoyl dopamine, N-arachidonoyl-dopamine, O-arachidonoyl-ethanolamine, noladin, and virodhamine, just to name a few (Porter et al., 2002; Ralevic, 2003; Bradshaw and Walker, 2005); for review of EC metabolites (see Pertwee, 1997; Bradshaw and Walker, 2005; Turcotte et al., 2016). All of these lipids predominantly target the CB1R and CB2R, but have been shown to bind to other targets such as orphan receptors; GPCR GPR-55 (Ryberg et al., 2007; Turcotte et al., 2016), GPR18 (McHugh et al., 2012), GPR110 (Lee et al., 2016), GPR119 (Brown, 2007) transient receptor potential channels (TRP; De Petrocellis et al., 2012), and peroxisome proliferator-activated receptors (PPARs; Brown, 2007; Bozkurt, 2019). Orphan GPCRs which respond to cannabinoid ligands have emerged as putative cannabinoid receptors. While cannabinoids have been shown to bind to these GPCRs and ion channels, their effect on breathing have yet to be fully elucidated.
Both CB1R and CB2R are GPCRs that negatively couple to adenylyl cyclase, activate potassium (K+) channels, and inhibit calcium influx to hyperpolarize the cell and attenuate vesicular release (Schmid et al., 2003; Yoshihara et al., 2004; De Petrocellis et al., 2012; Jinwala and Gupta, 2012; Gamage et al., 2018; Bozkurt, 2019). CB1R and CB2R also stimulate the mitogen-activated protein kinase (MAPK) pathway (Carroll et al., 2012; Jinwala and Gupta, 2012), potentially explaining how this system communicates to recruit necessary support cells to regulate neuronal behavior. Specifically, agonism of CB1Rs activates the MAPK pathway, impacting cell transcription, translation, motility, shape, proliferation, and differentiation from the resulting phosphorylation of nuclear transcription factors, and that can cause CB1R desensitization and internalization if prolonged (Carroll et al., 2012; Jinwala and Gupta, 2012). For a list of receptors and location see table 1 by Bozkurt (2019) review (Bozkurt, 2019). Given the wide-spread distribution of CB1R and CB2Rs, understanding their contributions to respiratory control can be evaluated by peripheral versus central contributions.
CB1R activation in the periphery is involved in the functional reactivity of the airways through stimulation that inhibits the contraction of airway smooth muscle via acetylcholine inhibition from cholinergic nerves (Bozkurt, 2019). The CB1Rs are found to couple to Gαi that can lead to GIRK coupling similar to mu opioid receptors (MOR), hyperpolarizing the neurons (Pertwee et al., 2010; Merighi et al., 2012) to reduce respiratory rate (Doherty et al., 1983; Schmid et al., 2003; Alon and Saint-Fleur, 2017) yet, unlike that of opioids, lack the ability to cause a persistent apnea. This is thought to be due to MORs found both pre- and post synaptically (Alon and Saint-Fleur, 2017; Cohen and Weinstein, 2018) producing additional inhibitory feedback while CB1Rs are predominantly found presynaptically (Vanderah, 2007; Tree et al., 2010). Additionally, the ability to track and observe our oxygen (O2) saturation and respiratory rate appear to be a CB1R driven effect, yet whether the CB1R effect is peripherally or centrally mediated remains to be uncovered.
Literature has also shown CB2Rs enhance the release of anti-inflammatory factors, as well as modulate respiratory drive (Komorowska-Müller and Schmöle, 2020). Complimentary qRT-PCR assay confirmed heavy populations of CB2Rs in the preBötzinger complex (pBc; Schmöle et al., 2015; Wiese et al., 2020); unpublished immunohistochemistry assays revealed an abundance of CB2Rs co-localized with Iba1, not GFAP, suggesting their location to be on microglia and not astrocytes (unpublished data, Largent-Milnes lab) aligning with prior reports. Activated microglia decrease the amplitude of nearby neuron action potentials, including those of the pBc (Camacho-Hernández et al., 2019) suggesting a possible protective mechanism for CB2R agonists to inhibit activated microglia. CB2R have also been found on multiple types of immune cells, including white blood cells, B lymphocytes, natural killer cells (Fernández-Ruiz et al., 2007), polymorphonuclear leukocytes such as eosinophils (Oka et al., 2004; Chouinard et al., 2011, 2013) and other monocytes (Galiègue et al., 1995). They are implicated in inflammatory responses of the periphery and CNS, acting through sensory nerves (Ogoh, 2019). The predominant cell type expressing CB2R are B lymphocytes (Fernández-Ruiz et al., 2007), with the level of expression contingent on the type and strength of the stimuli (Muller et al., 2018). Robust localization of CB2R in immune cells may purport a role for immunosuppression. Studies across several disease states have shown the role of cannabinoids in immunosuppression through induction of apoptosis, inhibition of cell proliferation, inhibition of the production of cyto- and chemokines, reduce cytokine activation and T cell proliferation, as well as induction of regulatory T lymphocytes. Antagonists of the CB2R have shown to prevent THC-induced apoptosis (Fernández-Ruiz et al., 2007), while antagonism of the CB1R failed to show similar results (Malfait et al., 2000; Croxford and Miller, 2003) further highlighting the immunoprotective effects of the CB2R (Fernández-Ruiz et al., 2007). Chronic inflammatory respiratory conditions, such as allergic asthma, recruit eosinophils to the airways in response (Bozkurt, 2019). These densely packed white blood cells with CB2Rs that respond to such inflammatory conditions further suggest a homeostatic role of the EC system in respiratory function and warrants continued investigations of ways to manipulate this mechanism therapeutically for people with inflammatory respiratory conditions.
Inspiratory drive comes from the central pattern generator (CPG) in the brainstem via the preBötzinger Complex (pBc), a nucleus located in the ventral lateral medulla and part of the ventral respiratory group. The pBc interacts with the dorsal respiratory group and the central termini of the hypoglossal and vagal nerves to generate respiratory rhythm (Del Negro et al., 2018; Varga et al., 2020) In the dorsal region of the pons, also known as the pneumotaxic center, the parabrachial nucleus, containing Kölliker-Fuse nucleus, provides tonic excitatory inputs to the pBc to provide smooth transitions from inspiration to expiration by inhibiting the rhythmic burstlet conversion to motor output bursts arising from the pBc (Varga et al., 2019). The dorsal respiratory group receives inputs from the apneustic center in the lower pons, as well as feedback from the periphery to inhibit expiration and allow for inspiration to, again, occur. The stretch mechanoreceptors from the lungs, diaphragm, and intercostal muscles (Del Negro et al., 2018; Webster and Schmidt, 2019; Varga et al., 2020), as well as inputs from chemoreceptors and baroreceptors of the carotid bodies and aortic arch, all relay this feedback to the nucleus tractus solitarius, heavily populated with CB1Rs (Glass et al., 1997), and the dorsal respiratory group, for modulation of respiratory rate. Additional feedback is provided by the vagal and glossopharyngeal nerves to the nucleus tractus solitarius about O2, CO2, and pH levels from lung mechanoreceptors and peripheral chemoreceptors to further refine the necessary burstlets to maintain O2 levels for cell survival.
The pneumotaxic center inhibits the pBc and apneustic center, while the apneustic center promotes activity of the pBc. The pBc then sends signals to inhibit the pneumotaxic center, moving the tongue out of the way via the hypoglossal nerve during inspiration (Ghali, 2019). The nucleus ambiguous controls the pharynx, larynx, and soft palate during inspiration, while the nucleus retroambiguus sends signals to the diaphragm and intercostal muscles in response to inspiration and expiration. While cannabinoid receptors were not traditionally thought to exist in respiratory nuclei, recent studies have confirmed their presence in the pBc (Wiese et al., 2020), as well as neighboring regions controlling motor output has been well established (Glass et al., 1997).
The other CPG, is known as the retrotrapezoid nucleus (Eljaschewitsch et al., 2006)/parafacial (Pfitzer et al., 2004) respiratory group, which controls active expiration, during conditions of exercise or high CO2 concentrations (Janczewski and Feldman, 2006). The retrotrapezoid nucleus is believed to promote breathing immediately following birth (Shi et al., 2021) and is opioid insensitive since the endogenously released opioids to comfort the mother and baby during the birthing process (Chernick and Craig, 1982) would be detrimental on the opioid sensitive pBc (Gray et al., 1999), making this potentially opioid insensitive region an area of promise for future research into prevention of the deadly effects of over ingestion of opioids. Genetic knockout mice for selective genes that play a role in active expiration as early as birth (Chatonnet et al., 2007), are unable to survive 24 h post-delivery without administration of naloxone to maintain rhythmic properties of the opioid sensitive pBc (Jacquin et al., 1996).
The pBc is responsible for the synchronization of the neuronal burstlets that control automatic inspiration, but not expiration. The pBc neurons are characterized neurokinin 1 receptor (NK1) containing cells that are the targets of neurotransmitters such as substance P, GABA and glutamate. These input neurons as well as the pBc neurons themselves are provided support by astrocytes and microglia (Feldman and Del Negro, 2006; Montandon et al., 2011; Anderson et al., 2016; Sun et al., 2019). Substance P activation of NK1 neurons in somatostatin-containing neurons of the pBc is reported to drive bursts, while Mu opioid receptors (MOR) on these same neurons, when activated, inhibit these same events. In addition, the pBc burslet activity for synchronized inspiration has been shown to be modulated by multiple additional receptors including CB1Rs and CB2Rs (Feldman and Del Negro, 2006; Montandon et al., 2011; Anderson et al., 2016), purinergic receptors, TRP subtype channel 3, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Jacquin et al., 1996), N-methyl-D-aspartate (NMDA) receptors, developing brain homeobox protein 1 (DBX1) receptors, gastrin releasing peptide receptors, adenosine receptors, nicotinic acetylcholine receptors, and muscarinic acetylcholine receptors (Smith et al., 1991; Feldman and Del Negro, 2006; Montandon et al., 2011; Anderson et al., 2016; Camacho-Hernández et al., 2019).
The pBc and other respiratory nuclei, including the Bötzinger complex just anterior to the pBc, and a subset of neurons in the ventrolateral medulla are heavily populated with MORs (Del Negro et al., 2018), and NK1Rs, which are colocalized on somatostatin neurons within the pBc (Del Negro et al., 2018). A fatal opioid overdose occurs following activation of MORs within the pBc causing desynchronization of burstlets, and consequently respiratory arrest or persistent apnea. This increase in time it takes to synchronize burstlets slows inspiration but has no impact on the frequency of expiration (Feldman and Del Negro, 2006; Sun et al., 2019), eventually resulting in death; this outcome from hyperpolarization following MOR activation can be reversed with the opioid antagonist, naloxone (Montandon et al., 2011). The fact that levels of CB1Rs and CB2Rs in the central respiratory nuclei are less than MORs is partly due to their restricted expression to only presynaptic inhibition instead of pre- and postsynaptic inhibition like MORs, possibly explaining why no deaths have been reported by cannabis despite the many similarities in actions opioid and cannabinoid receptors share (Glass et al., 1997).
Glia, support cells found in the extracellular environment that are involved in neuronal homeostasis, include microglia and astrocytes (Jäkel and Dimou, 2017). Astrocytes have been shown to support this environment via Kir4.1 channels that regulate baseline potassium (K+) levels in the pBc (Neusch et al., 2006). Kir4.1 is an inwardly rectifying K+ channel exclusively expressed in glial cells within the CNS that modulates extracellular K+ homeostasis, maintains astrocyte resting membrane potential, and facilitates glutamate uptake (Funk et al., 2015). Additionally, astrocytes have been well defined in supportive roles maintaining water and ion concentrations, blood–brain barrier integrity, and membership in the tripartite synapse (Jäkel and Dimou, 2017). Astrocytes within the pBc are morphologically different compared to astrocytes within the brainstem by their different K+ channel expression patterns (Glass et al., 1997; Funk et al., 2015), likely reflective of their role in respiratory modulation (SheikhBahaei et al., 2018). While not fully understood, studies to date suggest astrocytes assist in the exchange of K+ and Cl− on neighboring GABAergic neurons and regulate extrasynaptic glutamate concentrations via an exchange with cystine. It has also been shown that declines in this glutamate/cystine exchange can promote trafficking of mGLU5 receptors to the extrasynaptic membrane and are believed to be directly involved in the onset of long-term depression. This integrated involvement of astrocytes and the behavior of glutamatergic and GABAergic neurons support evidence that they may be actively involved in respiratory rhythm generation within the pBc. A certain subset of astrocytes within the pBc have demonstrated increased rises of calcium immediately preceding inspiratory neuronal firing (Okada et al., 2012). Inhibition of astrocytes has been shown to depress breathing in vivo (Young et al., 2005). Astrocytes in the pBc release ATP which under hypoxic conditions increases respiratory activity (Rajani et al., 2018). Though the exact role of astrocytes in the pBc is poorly understood, it is thought that they modulate the respiratory network (Funk et al., 2015).
Prior studies have shown that either the inhibition (or depletion) of microglia, or their activation, reduces the respiratory rates (Lorea-Hernandez et al., 2016). Beyond extracellular homeostatic maintenance, phenotypically classified as M0, microglia are also critical mediators of neuroinflammation (Hülsmann et al., 2000; Erlichman and Leiter, 2010; Huxtable et al., 2010) and respond quickly to small extracellular K+ changes to become activated (Varga et al., 2020). Once activated, phenotypically classified as M1, microglia mediated neuroinflammation by phagocytizing pathogens, recruiting inflammatory cells, and the production of chemokines and cytokines (Hickman et al., 2018), as well as upregulating adaptive immune responses (Orihuela et al., 2016). Once the threat has dampened, the healing process begins via polarization into an M2 microglia phenotype, or alternative activation state. Here microglia begin to express growth factors and anti-inflammatory mediators to aid in recovery (Franco et al., 1988). While three distinct phenotype classification states have been established, it is understood that these states exist on a spectrum and are not an all or none classification. Microglia, when activated, decrease the amplitude of the action potentials in nearby neurons (Camacho-Hernández et al., 2019) so microglia activation in the pBc could have a negative impact on burstlets that reach burst threshold.
Further research has uncovered CB2R knockout (KO) mice to be unable to fully polarize to an M2 microglia phenotype (Komorowska-Müller and Schmöle, 2020), further supporting the necessity of CB2Rs to facilitate polarization to an M2 phenotype. The M2 phenotype is further stratified into M2a and M2c activation states. Stimuli typical of these activation states have been shown to increase synthesis of endogenous EC ligands such as 2-AG and AEA further suggesting a role for the EC signaling system (Mecha et al., 2015) in the M2 phenotype and anti-inflammatory effects. Studies comparing administration of AEA as well as administration of a CB1/2 receptor agonist, such as WIN 55212-2, has also shown to suppress proinflammatory cytokines such as IL-5 and inducible nitric oxide synthetase. Conversely, inhibition of CB2R in the setting of inflammation is known to exacerbate neuronal damage more so than CB1R inhibition allowing microglia to pursue a pro-inflammatory response (Eljaschewitsch et al., 2006). Despite initial reports that CB2R expression was present only in the periphery, studies have shown CB2R expression in the brain in both pathological and nonpathological conditions (Schmöle et al., 2015). Furthermore, the implication of ECs producing an anti-inflammatory state postulates a role for ECs in respiratory homeostasis within the CNS (Cabral et al., 2008; Montandon et al., 2016; Varga et al., 2020). For a review of microglia CB2Rs (see Komorowska-Müller and Schmöle, 2020).
In the periphery, receptors and neurotransmitters work together to regulate sympathetic activation instead of the maintenance mechanisms seen in the CNS. Among the cannabinoid receptors, CB1R plays a role in the functional reactivity of the airways through stimulation that inhibits the contraction of airway smooth muscle via inhibition of acetylcholine from cholinergic nerves (Bozkurt, 2019). It is believed that AEA activation of peripheral CB1Rs is one means to control bronchial contractility. This control is dependent on the current state of the bronchial muscle. During the capsaicin-evoked bronchospasm, when the muscle is contracted, AEA can ease this contraction, likely by inhibiting prejunctional release of excitatory neurotransmitters and neuropeptides (Bozkurt, 2019). Alternatively, bronchoconstriction can be seen because of CB1R activation when the smooth muscle is relaxed following the removal of a constricting influence on the vagus nerve (Calignano et al., 2000). CB2Rs are likely to play a role in the mechanisms for neurogenic inflammation, acting through sensory nerves (Bozkurt, 2019). Chemoreceptors located on carotid arteries respond to changes in blood O2 levels, baroreceptors sense blood pressure changes, and activated pulmonary stretch receptors release surfactant, reducing surface tension for the transition to expiration (Calignano et al., 2000). These stretch receptors interact with chemoreceptors and baroreceptors to continuously inform the central respiratory centers, via the vagal and glossopharyngeal nerves, to maintain respiratory homeostasis (Rice et al., 1997; Calignano et al., 2000; Niederhoffer et al., 2003; Schmid et al., 2003). ECs are produced and their receptors are expressed in each of these areas.
As a first line of defense, the respiratory system contains numerous immune cells, the abundance of those cells being alveolar macrophages (Adams et al., 2017) to protect us against aerosolized bacteria, viruses and toxins are bronchial epithelial cells, alveolar macrophages and dendritic cells of the lungs (Turcotte et al., 2016). While most abundant in alveoli, other leukocytes express CB1Rs and CB2Rs and play a role in lung immunity (Turcotte et al., 2016). Recent literature has begun to uncover the role the EC system plays in this line of defense. CB1Rs and CB2Rs have been found via mRNA and proteins (Galiègue et al., 1995) detection in vagal afferents (Niederhoffer et al., 2003), nerve fibers that innervate bronchioles (Calignano et al., 2000), and bronchiolar smooth muscle cells (Pertwee, 1997; Ständer et al., 2005; Izzo and Sharkey, 2010; Szymaszkiewicz et al., 2018), as well as the peripheral termini of lung tissue (Rice et al., 1997; Calignano et al., 2000; Niederhoffer et al., 2003; Schmid et al., 2003) and are believed to play a homeostatic role in bronchial contractility (Calignano et al., 2000). This is important given that current literature has not reported CB1Rs and CB2Rs expression in the epithelial cells of the primary airway despite the presence of their mRNA being found in the human bronchial epithelial cell line, 16HBE14o (Turcotte et al., 2016). Recently the lung tissue of patients with adenocarcinoma was utilized to isolate macrophages and revealed CB1Rs and CB2R mRNA and proteins in macrophages associated with the tumor and non-tumor collected samples (Staiano et al., 2016). Levels of CB2R were higher than CB1R in alveolar and monocyte macrophages, but they were found to be functionally opposite in extracellular signal-regulated kinases ½ (ERK1/2) phosphorylation assays. Airway epithelial cells are part of that first line of defense that can identify pathogens and activate leukocytes in conditions of inflammation (Weitnauer et al., 2016) making their role and response to cannabinoids and ECs of great interest for future directions and potential future therapies. Thus, highlighting the multitude of outcomes the EC system can produce through different mechanisms within the respiratory defense system of the periphery.
The ability of the mammals to track and observe our O2 saturation and respiratory rate appear to also be a CB1R driven effect, but the mechanisms underlying these effects remain unknown. In one study the cannabinoid reuptake inhibitor, AM404, was administered to investigate the effects of the endogenous cannabinoids on breathing. This increase in cannabinoid availability reduced respiratory rate and arterial O2 saturation. This effect was completely abolished in CB1R KO mice (Iring et al., 2017). While other studies have reported respiratory benefits from peripherally restricted CB1R agonism when coadministered alongside morphine (Wiese et al., 2021). These data lend support for further investigation of peripheral versus brain penetrant CB1R agonism to play different roles in respiratory functionality. Below we detail some of these actions in the periphery.
Cannabinoids may act on peripheral sites such as on chemoreceptors and baroreceptors. Through in-situ hybridization, CB1Rs have been found to have some expression in the nodose-petrosal-jugular ganglia, superior cervical ganglia, and some sparse localization within the carotid body (Roy et al., 2012). Chemoreceptors are what sense gas and pH levels. They respond when O2 levels rise and fall, as well as CO2 levels. They also provide feedback on pH levels in the blood so alterations can be made if necessary. Central chemoreceptors are located below the ventrolateral surface of the medulla. As arterial partial pressure of carbon dioxide (PCO2) rises, it diffuses across the blood brain barrier to raise the CO2 content of cerebrospinal fluid where it eventually hydrates to carbonic acid and ionizes to reduce the pH of cerebrospinal fluid. These pH sensitive receptors within the medulla detect this change and release L-glutamate (Mifflin, 1992), along with ATP, relay to the pBc to increase the respiratory rate in order to decrease arterial PCO2 (Machado and Bonagamba, 2005; Braga et al., 2007). It has also been suggested that respiratory depression resulting from CB1R activation may involve peripheral arterial chemoreceptors; other studies have reported a protective benefit from peripheral CB1R activation (Wiese et al., 2021) leaving future studies to fully parse out the role of the peripheral CB1R. Expression of CB1R within the carotid body implicates a role for blood flow regulation thereby affecting respiratory control. Central chemoreceptors do not directly respond to partial pressure of oxygen (PO2), only PCO2. Peripheral chemoreceptors, on the other hand, which are located on carotid and aortic bodies, are stimulated by increased PCO2, decreased blood pH, and decreased PO2 (Machado and Bonagamba, 2005) to alert the central respiratory centers of necessary alterations needed to maintain homeostasis. In addition, CB1Rs can act through the carotid body by forming heterodimers with other GPCRs present such as delta opioid receptors, MOR, adenosine 2A receptors, and dopamine 2 receptors (Porzionato et al., 2018).
Baroreceptors are rapid acting mechanoreceptors located in the carotid sinus and aortic arch sensing changes in arterial blood pressure. Previous studies imply that increases in blood pressure may abruptly prolong expiration in response to baroreceptor activation (Baekey et al., 2010). Cannabinoids may also act via a modulating role in the baroreceptor reflex. Activation of cannabinoid receptors in the nucleus of tractus solitarius (Distribution Po, n.d.; Cohen and Weinstein, 2018), through administration of WIN 55212-2 and CP 55940, elicits a baroreflex-like response through a decrease in arterial pressure and sympathetic inhibition, which is antagonized with pretreatment of the CB1R antagonist, AM281 (Baekey et al., 2010). An intact baroreceptor reflex was required to demonstrate the baroreflex-like response as sino-aortic-denervated rats demonstrated attenuated responses to WIN 55212-2, implicating a more modulatory role of cannabinoids (Durakoglugil and Orer, 2008).
Few studies have focused solely on synthetic cannabinoids (SCs) effects on peripheral receptors within the respiratory system, such as chemoreceptors and baroreceptors. Prior literature has shown activation of chemo- and baroreceptors can increase bronchial airway resistance, reducing overall respiratory functions. This has been explored as a possible mechanism of central CB1R stimulation to explain the respiratory depression seen from SCs (Alon and Saint-Fleur, 2017).
Cannabinoids, both exogenous and endogenous, have shown to have potentially therapeutic benefits due to their inhibitory effects on immune functions and cell recruitment in lung inflammation. Conversely, cannabinoids have also shown to slow respiratory pathogen clearance and be deleterious on lung function (Turcotte et al., 2016). Other conflicting findings have shown an absence of cannabinoid effects altogether, but many of these studies were conducted in naïve animals, while studies in pathological models have demonstrated beneficial effects from cannabinoids (De Petrocellis et al., 2017).
Lung tissue terminals, structural cells, and leukocytes (Turcotte et al., 2016) have all been shown to contain CB1Rs (Rice et al., 1997; Calignano et al., 2000) and control bronchial contractility and function in a homeostatic role (Calignano et al., 2000). In one study, capsaicin-evoked bronchospasm was relaxed following a local administration of a CB1R agonist via inhibition of vagal input, suggesting CB1Rs role as a homeostatic respiratory regulator (Calignano et al., 2000). This promotion of homeostatic respiration by peripheral CB1R activation may explain how a peripherally restricted CB1R agonist can have a different effect than a brain penetrant CB1R agonist (Wiese et al., 2020, 2021). Alveolar macrophages extracted from people who consume cannabis via combustion regularly have decreased capability to ingest/remove staphylococcus aureus (Baldwin et al., 1997), produce less nitric oxide (Shay et al., 2003), and caused a weakened host defense through decreased cytokine priming (Roth et al., 2004). Following capture of these antigens, dendritic cells migrate to lymph nodes to pass the antigen to naïve T cells (Turcotte et al., 2016). CB2Rs have been shown to facilitate this migration to the lymph nodes (Lu et al., 2006). This migration process becomes impaired following tetrahydrocannabinol (THC) exposure and could leave the individual open to impaired immune responses from pulmonary pathogens (Lu et al., 2006). As with the rest of the EC system, there are still future studies necessary to fully uncover the mechanisms by which cannabis consumption impacts the respiratory immune system as other data have found benefits from THC on the severity of acute respiratory distress syndrome through alterations of lungs microbiota (Mohammed et al., 2020).
The sensory nerves of the respiratory system include the vagal, glossopharyngeal, phrenic, and intercostal nerves (Del Negro et al., 2018). The vagal and glossopharyngeal nerves relay all the necessary information in response to peripheral respiratory actions to the central respiratory nuclei to make needed modifications and signal when to transition to the next phase in the respiratory cycle. Expression of CB1Rs within nuclei of the glossopharyngeal and vagal nerve have suggested a peripheral role in sensory and autonomic function for ECs (Burdyga et al., 2004; Yoshihara et al., 2004; Ye et al., 2019). The phrenic and intercostal nerves send information from the diaphragm while the internal intercostal nerves relay additional stretch information from the intercostal muscles. These nerves bring in information from mechanoreceptors that sense pressure and stretch changes in the lungs, as well as O2 saturation, CO2 saturation, and pH levels via chemoreceptors all to fine tune the respiration sequence (Del Negro et al., 2018).
The most common, and well known organic cannabinoid is Δ9-tetrahydrocannabinol (THC), a mixed CB1R/CB2R partial agonist (Makriyannis, 2014; Nikas et al., 2015), that does not cause respiratory depression (Tree et al., 2010), and has been shown to be beneficial in the treatment of chronic pain, migraines, anorexia, nausea, just to name a few (Wills and Parker, 2016; Yuill et al., 2017; Wiese and Wilson-Poe, 2018; Mohammed et al., 2020). The experienced psychoactive effects seen with cannabis use are largely attributed to the result of THC activation on the multiple receptor targets it may occupy, including CB1R, CB2R, as well as GPR55 (Ryberg et al., 2007), GPR18 (McHugh et al., 2012), serotonin 3A (Barann et al., 2002), PPARγ (Vara et al., 2013), and TRP channels 2, 3, and 4 (De Petrocellis et al., 2011, 2012), explaining just how THC can have such a wide spectrum of therapeutic benefits for such a broad list of ailments, as well as impacts on cognitive functioning, motor movements, and possible immunosuppression (Vachon et al., 1976; Calignano et al., 2000; Owen et al., 2014). Medicinal benefits of THC also appear to be easily modulated by other cannabinoids (LaVigne et al., 2021), for review (see Morales et al., 2017), making fine tuning individual therapies with THC a very promising and future public health benefit. Multiple studies have shown in healthy volunteers and volunteers with chronic bronchial asthma, of minimal or moderate severity, that the use of THC results in bronchodilation (Lee et al., 2001; Croxford and Miller, 2003; Vanderah, 2007; Rieder et al., 2010), and the concentrations of THC that demonstrate this protective finding are concentrations that do not result in central or cardiovascular effects (Tashkin et al., 1973, 1976; Vachon et al., 1976; Hartley et al., 1978) suggesting a possible peripherally driven mechanism. Conversely, disruption of the alveolar epithelium and vascular endothelium of any kind is known as an acute lung injury (Lu et al., 2006). Under these conditions the use of cannabinoids as a treatment option proved beneficial in all (Ribeiro et al., 2015; Fujii et al., 2019) but one study showed CBD to be pro-inflammatory under these conditions (Karmaus et al., 2013).
Another common organic cannabinoid is cannabidiol (CBD). CBD has also shown promising effects, likely also due to the promiscuous affinity CBD has to multiple receptors, for review (see Morales et al., 2017). Studies of systemic administration have shown that CBD reduces the inflammation response and structural changes that take place during the remodeling process of asthma (Vuolo et al., 2019), as well as stunt inflammatory parameters following acute lung injury (Ribeiro et al., 2015). Reductions in airway responsiveness have also been observed following CBD treatment (Vuolo et al., 2019). CBD also influences airway smooth muscle tone and reduces contractions caused by endogenous cannabinoids suggesting beneficial effects for the treatment of obstructive airway disorders (Dudášová et al., 2013). Furthermore, in respiratory studies, CBD was shown to prevent morphine-induced respiratory depression in room air but lost those protective effects under a CO2 challenge (Wiese et al., 2021).
Synthetic cannabinoids (SCs) are a class of cannabinoids that were developed by chemists to investigate and further understand the EC system (Pertwee, 1997; Carroll et al., 2012; Yuill et al., 2017; Szymaszkiewicz et al., 2018). They were not designed for human consumption (Jinwala and Gupta, 2012), as many of these compounds are selective to the CB1R with an ability to cross the blood–brain barrier and can be dangerous (Gunderson et al., 2014), while some experiences have been unpleasant to the person, examples of these compounds being utilized by people outside the laboratory have been reported as case studies (AAPCC, n.d.; Gunderson et al., 2014; Tait et al., 2016; Alon and Saint-Fleur, 2017; Cohen and Weinstein, 2018; Darke et al., 2019; Mathews et al., 2019) and have been equally important in understanding the mechanisms by which the EC system functions (Schmid et al., 2003; Robson, 2005; Jinwala and Gupta, 2012; Trecki et al., 2015; Adams et al., 2017; Tournebize et al., 2017; Ivanov et al., 2019). An overview of these different outcomes on breathing can be seen in Figure 1. Preclinical and clinical studies have shown CB1R brain penetrant SCs to result in respiratory depression (Schmid et al., 2003; Jinwala and Gupta, 2012; Alon and Saint-Fleur, 2017; Wong and Baum, 2019). Inhalation of SCs can damage bronchiolar epithelium and the protective surfactant layer within alveoli causing hypoxia and acidosis from the resulting interference in effective gas exchange (Pertwee et al., 2010). These results have been shown to influence respiratory function by increasing airway resistance (Alon and Saint-Fleur, 2017) and reductions in blood pressure and circulating noradrenaline resulting in sympathetic inhibition and increased vagal tone (Niederhoffer et al., 2003; Schmid et al., 2003). SCs have also been shown to suppress cough and bronchospasms through inhibition of the excitatory effects of noradrenaline in the airways, which may provide an explanation for respiratory depression through vagal transmission (Calignano et al., 2000). Additionally, other adverse effects have been seen with the use of SCs such as tachycardia, paranoia, acute kidney injury, seizures, nausea and vomiting, calls to poison control, and trips to the emergency room (Tait et al., 2016; Cohen and Weinstein, 2018; Darke et al., 2019; Mathews et al., 2019). If peripheral CB1Rs also assist in the suppression of respirations, this may be the mechanism at which they are able to do so (Calignano et al., 2000). Yet, other studies have found protective benefits from selective CB1R activation in combination with morphine (Wiese et al., 2021). Since many phytocannabinoids, as well as mixed cannabinoid agonists, also show an affinity for the CB1R but do not induce respiratory depression (Wiese et al., 2020), understanding how CB1R activation drives respiratory depression is vital to ensuring safe consumption of these opioid adjuncts.
While SCs have shown respiratory depression through CB1R activation in prior studies, there has not been a clear delineation as to whether these effects are directly a cause of central or peripheral CB1R activation (Pfitzer et al., 2004). The CB1R mechanism of action is similar to the MOR to reduce the neuron’s ability to depolarize (Pertwee et al., 2010; Merighi et al., 2012) and lends itself that selective, central CB1R activation could induce respiratory depression (Doherty et al., 1983; Schmid et al., 2003; Alon and Saint-Fleur, 2017). Furthermore, with only presynaptic CB1Rs, compared to MORs that are found on pre and postsynaptic terminals (Lopez-Moreno et al., 2010; Scavone et al., 2010), may explain why fatal respiratory depression has not been seen from central CB1R activation compared to MOR activation in this region. As with other potent cannabinoid agonists at the CB1R (Gunderson et al., 2014; Ivanov et al., 2019), SCs activate the MAPK pathway, impacting cell transcription, translation, motility, shape, proliferation, and differentiation from the resulting phosphorylation of nuclear transcription factors, that if prolonged, can cause CB1R desensitization and internalization (Carroll et al., 2012; Jinwala and Gupta, 2012) Administration of the cannabinoid, WIN 55212-2, a mixed CB1R/CB2R agonist, in preclinical models has been shown to produce a depressed effect on respirations (Schmid et al., 2003; Pfitzer et al., 2004). Following the inhibition of respiratory depression with the administration of SR-141716, a CB1R inverse agonist, it was concluded that the depressive effect was a CB1R mediated mechanism (Pfitzer et al., 2004).
Prior literature has shown that CB1R activation reduces airway contraction and cholinergic induced contractions, while still providing an improvement of static lung elastance and reduced collagen fiber content helping to keep the alveoli from collapsing (Wang et al., 2016). However, other studies have postulated other mechanisms of the pulmonary pathways (Tucker et al., 2001; Akerman et al., 2007). In a condition of capsaicin induced cough, the endogenous cannabinoid, AEA, inhibited the cough response as well as the associated bronchoconstriction, but when administered on its own induced bronchoconstriction (Calignano et al., 2000). These effects were only reversed following the administration of the CB1R inverse agonist, SR-141716, suggesting a CB1R mediated effect. It is worth noting that AEA has been shown to activate TRPV receptors, giving pause for speculation that these results were completely CB1R driven (Tucker et al., 2001; Akerman et al., 2007).
In one study using intraesophageal HCl instillation to assess cannabinoid receptor inhibitory effects on the sensory nerve pathways involved in bronchoconstriction and airway microvascular leakage found administration of WIN 55212-2 (CB1/CB2 agonist) or JWH 133 (CB2R agonist) abolished all associated neurogenic inflammation (Cui et al., 2007). These data support the prior literature that has found administration of the CB2R agonist, JWH 133, inhibits citric acid induced coughing (Patel et al., 2003) and main bronchi contraction induced by capsaicin in preclinical models (Yoshihara et al., 2004). These findings all suggest a role for the CB2R as a potential therapeutic for inflammatory respiratory conditions.
The SC, FUB-AMB, is reportedly over 80 times as potent at the CB1R as THC (Ivanov et al., 2019) in addition to a 9-13-fold greater affinity for the CB2R compared to CB1R (Gamage et al., 2018). FUB-AMB was reportedly involved in multiple mass casualties and “zombie outbreaks” from New York to New Zealand (Adams et al., 2017; Gamage et al., 2018; Ivanov et al., 2019). It is also possible that SCs have additional unknown receptor selectivity and binding affinity themselves, or by their metabolites (Trecki et al., 2015; Gamage et al., 2018) with non-cannabinoid receptors (Jinwala and Gupta, 2012) setting the stage for an unpredictable experience. In addition to the unpredictability of the synthetic compound, the vehicle or carrier oil it is in can also have a variety of additional compounds as well. Everything from THC, cannabidiol (CBD), nicotine, caffeine, and tocopherol – a class of compounds containing vitamin E that was associated with multiple hospitalizations from vaping (Dresen et al., 2010) – have been found in mixtures said to contain SCs. These adulterations with additional ingredients lead to misidentification of the substance being used by the consumer and increase the chances for unknown toxicities (Tofighi and Lee, 2012).
Promise with cannabinoids as a therapeutic intervention for respiratory ailments has also been seen as recently as with the COVID-19 pandemic, although with some conflicting outcomes (Pascual Pastor et al., 2020; Malinowska et al., 2021; Paland et al., 2021; Beasley, 2022; Pereira et al., 2022). Recent publications have reported therapeutic cannabis has shown protective effects at preventing contracting COVID-19 (Pascual Pastor et al., 2020; Malinowska et al., 2021; Pereira et al., 2022), the ease of COVID-19 symptom severity (Pascual Pastor et al., 2020), as well as increased susceptibility to COVID-19 infection and exacerbation of COVID-19 symptoms (Paland et al., 2021; Beasley, 2022; Pereira et al., 2022). These contradicting results further highlight the importance and need of further research aimed at understanding all the ways in which cannabis and the EC system can be utilized therapeutically, and where possible cultivation manipulations stand to increase the safety of cannabis consumption through targeted manipulations in the cannabinoid makeup and profiles of cultivated cannabis.
Another potential mechanism for the treatment of a vast array of respiratory ailments comes from the role the central CB1R plays in O2 saturation and its impact on respiratory rate (Iring et al., 2017). This means pharmacological manipulations to the respiratory system through altered endogenous cannabinoid availability may be plausible treatments for respiratory conditions that involve low levels of O2 saturation or irregular breathing patterns. Cannabinoid reuptake inhibitors are becoming another area of promise to increase endogenous cannabinoid concentrations by increasing the available upstream synthesizing enzymes available to produce the endogenous ligands (Hülsmann et al., 2000). While previous clinical trials found adverse effects from brain penetrant CB1R antagonists (Nguyen et al., 2019), future drug development may hold the key to well tolerated central CB1R antagonists for use by humans (Lazary et al., 2011). Additionally, administration of synthetic cannabinoid agonists and antagonists offer a similar potential outcome for means of treatment of respiratory ailments.
Further research has begun to dive into the pharmacodynamics of cannabis terpenes and their analogs (LaVigne et al., 2021; Liktor-Busa et al., 2021). With over 500 independent compounds identified to exist within cannabis (Rock and Parker, 2021) and more than 700 cultivated varieties (Hazekamp and Fischedick, 2012) that all offer a unique combination of cannabinoid compounds and concentrations. With some of the most common compounds, CBD, THC, and beta-caryophyllene to name a few, showing effects in conditions of pain or anxiety (Gertsch et al., 2008; LaVigne et al., 2021; Soliman et al., 2021), the full scope of outcomes and future therapeutics from specific poly-cannabinoid compositions are only beginning to be investigated. It will be important to continue investigations with these cannabinoids individually and in conjunction with other compounds, as some synergistic actions are found between some cannabinoids and other drugs, such as opioids, that allow for reductions in necessary dosing to achieve pain relief (Fattore et al., 2005; Abrams et al., 2011; Scavone et al., 2013; Bachhuber et al., 2014; Kral et al., 2015; Kazantzis et al., 2016; Lucas and Walsh, 2017; Maher et al., 2017; Hughes et al., 2018; Liang et al., 2018; Wiese and Wilson-Poe, 2018; Lake et al., 2019; Liktor-Busa et al., 2021). Our current understanding of these compounds is promising at possible future potential therapeutic targets able to influence the EC system, and other systems through physiological agonism/antagonism modulation, such as is seen with cannabinoid terpenes that can directly modulate cannabinoid receptor activity through actions on the receptors themselves or through off target influence, as seen with such activity at the TRP channels or on the adenosine system (LaVigne et al., 2021).
Another potential area of promise is the interaction between the EC system and the opioid system. While the receptors of both systems are of the GPCR family and result in inhibition of neuronal activity (Bushlin et al., 2010; Quirion et al., 2020; Zhang et al., 2020), there are some key differences that highlight a potential point of intervention to reduce the negative side effects of opioids, that include the escalating number of fatal overdoses seen with the current overdose epidemic. The most notable difference is the location of receptors, specifically within the pBc, where the location of receptors on the pre- and postsynaptic neuron can completely abolish the burstlet activity of the pBc CPG that reflexively controls breathing (Sun et al., 2019), while CB1Rs are only found presynaptically, preventing complete inhibition of this vital respiratory nuclei (Alon and Saint-Fleur, 2017; Iring et al., 2017; Wiese et al., 2021). Furthermore, microglial CB2Rs appear to have an ability to override some of this inhibition offering another point of intervention to prevent the fatal effects seen from over ingestion of opioids currently (Wiese et al., 2020).
The hyperpolarization of pBc neurons following MOR activation increases the extracellular K+ (Montandon et al., 2016; Varga et al., 2020) and may sufficiently activate nearby microglia, switching from M0 to M1 phenotype. CB2R activation can facilitate the anti-inflammatory effects of microglia through downstream cascade events. The anti-inflammatory effects of CB2R activation are regulated through microglial polarization (switch from M1 to M2 microglia phenotype), demonstrating a switch from a pro-inflammatory to an anti-inflammatory state (Mecha et al., 2015; Komorowska-Müller and Schmöle, 2020). Use of THC in multiple sclerosis has been shown to increase TNF-α, congruent with an anti-inflammatory state (Bradford and Bradford, 2016). In addition to the established modulatory role that microglia play in the pBc (Hülsmann et al., 2000; Erlichman and Leiter, 2010; Huxtable et al., 2010), it is likely the activation of microglial CB2Rs is necessary for respiratory modulation and the physiological antagonism of MOR agonism in the pBc that would otherwise inhibit inspiration. Microglia are activated by opioid administration via toll-like4 and toll-like9 receptor agonism (de Oliveira et al., 2022; Reusch et al., 2022). Activation of microglia initiates proinflammatory responses as a result (Merighi et al., 2012). Co-administration of CB2R agonists with opioids has shown to reduce opioid induced proinflammatory responses (Tumati et al., 2012) and to be synergistic as pain therapeutics across acute, neuropathic, and complex pain states (Grenald et al., 2017; Yuill et al., 2017). Thus, selective CB2R agonism mitigation of opioid-induced respiratory depression by inhibiting microglial activation (Camacho-Hernández et al., 2019) to resynchronize pBc neurons is plausible. Growing evidence suggests that glia-derived proinflammatory mediators enhance tolerance to the anti-nociceptive properties of MOR activation (Watkins et al., 2009). Antagonizing these pro-inflammatory mediators, such as IL-1β, IL-6 and TNF-α, attenuate the development of MOR induced tolerance as well as attenuation of opioid withdrawal induced hyperalgesia (Raghavendra et al., 2002, 2004; Cui et al., 2006) and may be related to the explanation of downstream effects that allow for CB2R mitigation of opioid induced respiratory depression. Moreover, endogenous CB2R ligands could create a physiological antagonism to opioid induced desynchronization of pBc neurons (Zhang et al., 2017).
Recent publications have shown an opposing role of central versus peripheral CB1R activation, with coadministration of the peripherally restricted CB1R agonist, PrNMI, and morphine, morphine-induced respiratory depression was completely prevented, while administration of the brain penetrant CB1R agonist, AM356, alongside morphine enhanced the already seen respiratory depression (Wiese et al., 2021). Conversely, administration of the brain penetrant CB2R agonist, AM2301, in combination with morphine was also able to prevent morphine induced respiratory depression, while the peripherally restricted CB2R agonist, AM1710, was not (Wiese et al., 2020), supporting the CB2Rs ability to prevent respiratory depression to be completely mediated through central CB2Rs. In another study the administration of AM404, an EC reuptake inhibitor, in wild-type and CB1R KO mice uncovered the CB1R dependent manner of respiratory depression and arterial hypoxia, further supporting limitations of brain penetrant CB1R agonists and reuptake and hydrolysis inhibitors (Iring et al., 2017).
In this review we explored the respiratory system in the context of central versus peripheral control and how the EC system is currently known to influence that control. Next, we reviewed the literature available on organic and synthetic cannabinoids effects on breathing and how that has shaped our understanding of the role the EC system has in respiratory homeostasis. Finally, we looked at some potential future therapeutic applications the EC system has to offer for treatment of respiratory diseases and a possible role in preventing future opioid overdose fatalities that result from respiratory arrest or persistent apnea.
It will be important to fully characterize the cell type and location within the central respiratory nuclei, as well as in the periphery if viable therapeutics are going to be developed. It will also be vital to understand dose response curves and off target binding affinity for other cannabinoid receptors, or even more selective agonists. Specifically in the case of the CB1R, as it appears to have opposed roles in the periphery and central nervous system. This means understanding the dosing of peripherally restricted CB1R agonists to ensure they do not cross the blood brain barrier will also be of high importance for public safety since central CB1R activation enhances respiratory depression instead of mitigating it when administered alongside opioids (Wiese et al., 2021). Additionally, with the similarities in the cannabinoid receptors, many ligands will spill over to bind the other cannabinoid receptor once the intended targets are all full or activate the other cannabinoid receptor in conditions of genetic deletions of the intended cannabinoid receptor (Wiese et al., 2020). This is what was seen with escalating doses of CB2R agonists administered alongside morphine. The CB2R agonist began to leak over and activate central CB1Rs at the same time, causing enhanced respiratory depression and abolishing the protective feature of central CB2R activation alongside morphine. The ability to mitigate morphine induced respiratory depression through CB2R activation appears to be mediated centrally, as these effects have not been shown through activation of peripherally restricted CB2R. Activation of CB2R plays an additional role in modulating the immune system through the release of anti-inflammatory factors. There may also be a role for immunosuppression as studies across several disease states have shown downstream effects of cannabinoid receptor activation to include induction of apoptosis, inhibition of cell proliferation, inhibition of cyto- and chemokine production, reduced cytokine activation, T cell proliferation, and induction of regulatory T lymphocytes. With the expansion of cannabis access, it is essential that investigations continue to uncover the underpinnings and mechanistic workings of the EC system, the impact cannabis, and exogenous cannabinoids have on these systems, and how some of these compounds can mitigate respiratory depression when combined with opioids.
Respiratory control is complex and begins in the brainstem without peripheral input (Del Negro et al., 2018). The key regions are coordinated through a CPG, the pBc, a component of the ventral respiratory group that interacts with the dorsal respiratory group to synchronize burstlet activity and produce inspirations (Ghali, 2019). An additional rhythm generator: the retrotrapezoid nucleus (Eljaschewitsch et al., 2006)/parafacial respiratory group drives active expiration during conditions of exercise or high CO2 (Janczewski and Feldman, 2006). Combined with the feedback information from the periphery: through carotid bodies, stretch of the diaphragm or intercostal muscles, chemo- and baroreceptors, lung tissue, immune cells, and the cranial nerves, our respiratory system can fine tune motor outputs that ensure we have the O2 necessary to survive and can expel the CO2 waste we produce. It is important that we understand all the ways we can treat and protect our respiratory system to ensure its ability to function for the duration of our lifetime. It is vital to public safety that we understand the effects these compounds have on lung tissue and function, as well as how our endogenous cannabinoids influence our respiratory behavior for future therapeutic discovery.
BW and AAR contributed equally to the writing and revisions and comments were provided by TV and TL-M funding. All authors contributed to the article and approved the submitted version.
This work was funded by R01DA056608 to TL-M and TV, Comprehensive Pain and Addiction Center, and Department of Pharmacology NIH/NIDA 1P30DA051355.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AAPCC. (n.d.). Synthetic cannabinoids National Poison Data System, American Association of Poison Control Centers: American Association of Poison Control Centers; 2020 (updated February 29, 2020; cited 2020 12, 2020). Available at: https://aapcc.org/track/synthetic-cannabinoids
Abrams, D. I., Couey, P., Shade, S. B., Kelly, M. E., and Benowitz, N. L. (2011). Cannabinoid-opioid interaction in chronic pain. Clin. Pharmacol. Ther. 90, 844–851. doi: 10.1038/clpt.2011.188
Adams, A. J., Banister, S. D., Irizarry, L., Trecki, J., Schwartz, M., and Gerona, R. (2017). "zombie" outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N. Engl. J. Med. 376, 235–242. doi: 10.1056/NEJMoa1610300
Agrawal, A., and Mabalirajan, U. (2016). Rejuvenating cellular respiration for optimizing respiratory function: targeting mitochondria. Am. J. Phys. Lung Cell. Mol. Phys. 310, L103–L113. doi: 10.1152/ajplung.00320.2015
Akerman, S., Holland, P. R., and Goadsby, P. J. (2007). Cannabinoid (CB1) receptor activation inhibits trigeminovascular neurons. J. Pharmacol. Exp. Ther. 320, 64–71. doi: 10.1124/jpet.106.106971
Albrechet-Souza, L., Nastase, A. S., Hill, M. N., and Gilpin, N. W. (2021). Amygdalar endocannabinoids are affected by predator odor stress in a sex-specific manner and modulate acoustic startle reactivity in female rats. Neurobiol Stress. 15:100387. doi: 10.1016/j.ynstr.2021.100387
Alon, M. H., and Saint-Fleur, M. O. (2017). Synthetic cannabinoid induced acute respiratory depression: case series and literature review. Respir. Med. Case. Rep. 22, 137–141. doi: 10.1016/j.rmcr.2017.07.011
Anderson, T. M., Garcia, A. J., Baertsch, N. A., Pollak, J., Bloom, J. C., Wei, A. D., et al. (2016). A novel excitatory network for the control of breathing. Nature 536, 76–80. doi: 10.1038/nature18944
Bachhuber, M. A., Saloner, B., Cunningham, C. O., and Barry, C. L. (2014). Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999-2010. JAMA Intern. Med. 174, 1668–1673. doi: 10.1001/jamainternmed.2014.4005
Baekey, D. M., Molkov, Y. I., Paton, J. F., Rybak, I. A., and Dick, T. E. (2010). Effect of baroreceptor stimulation on the respiratory pattern: insights into respiratory-sympathetic interactions. Respir. Physiol. Neurobiol. 174, 135–145. doi: 10.1016/j.resp.2010.09.006
Baldwin, G. C., Tashkin, D. P., Buckley, D. M., Park, A. N., Dubinett, S. M., and Roth, M. D. (1997). Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am. J. Respir. Crit. Care Med. 156, 1606–1613. doi: 10.1164/ajrccm.156.5.9704146
Barann, M., Molderings, G., Brüss, M., Bönisch, H., Urban, B. W., and Göthert, M. (2002). Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br. J. Pharmacol. 137, 589–596. doi: 10.1038/sj.bjp.0704829
Beasley, M. B. (2022). Acute lung injury-from cannabis to COVID. Mod. Pathol. 35, 1–7. doi: 10.1038/s41379-021-00915-6
Boullon, L., Finn, D. P., and Llorente-Berzal, Á. (2021). Sex differences in a rat model of peripheral neuropathic pain and associated levels of endogenous cannabinoid ligands. Front. Pain Res. (Lausanne). 2:673638. doi: 10.3389/fpain.2021.673638
Bozkurt, T. E. (2019). Endocannabinoid system in the airways. Molecules 24:24. doi: 10.3390/molecules24244626
Bradford, A. C., and Bradford, W. D. (2016). Medical marijuana laws reduce prescription medication use in Medicare part D. Health Aff. 35, 1230–1236. doi: 10.1377/hlthaff.2015.1661
Bradford, A. C., Bradford, W. D., Abraham, A., and Adams, G. B. (2018). Association between US state medical cannabis laws and opioid prescribing in the Medicare part D population. JAMA Intern. Med. 178, 667–672. doi: 10.1001/jamainternmed.2018.0266
Bradshaw, H. B., and Walker, J. M. (2005). The expanding field of cannabimimetic and related lipid mediators. Br. J. Pharmacol. 144, 459–465. doi: 10.1038/sj.bjp.0706093
Braga, V. A., Soriano, R. N., Braccialli, A. L., de Paula, P. M., Bonagamba, L. G., Paton, J. F., et al. (2007). Involvement of L-glutamate and ATP in the neurotransmission of the sympathoexcitatory component of the chemoreflex in the commissural nucleus tractus solitarii of awake rats and in the working heart-brainstem preparation. J. Physiol. 581, 1129–1145. doi: 10.1113/jphysiol.2007.129031
Brown, A. J. (2007). Novel cannabinoid receptors. Br. J. Pharmacol. 152, 567–575. doi: 10.1038/sj.bjp.0707481
Burdyga, G., Lal, S., Varro, A., Dimaline, R., Thompson, D. G., and Dockray, G. J. (2004). Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J. Neurosci. 24, 2708–2715. doi: 10.1523/jneurosci.5404-03.2004
Bushlin, I., Rozenfeld, R., and Devi, L. A. (2010). Cannabinoid–opioid interactions during neuropathic pain and analgesia. Curr. Opin. Pharmacol. 10, 80–86. doi: 10.1016/j.coph.2009.09.009
Butler, J. E. (2007). Drive to the human respiratory muscles. Respir. Physiol. Neurobiol. 159, 115–126. doi: 10.1016/j.resp.2007.06.006
Cabral, G., Raborn, E. S., Griffin, L., Dennis, J., and Marciano-Cabral, F. (2008). CB2 receptors in the brain: role in central immune function. Br. J. Pharmacol. 153, 240–251. doi: 10.1038/sj.bjp.0707584
Calignano, A., Kátona, I., Désarnaud, F., Giuffrida, A., La Rana, G., Mackie, K., et al. (2000). Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature 408, 96–101. doi: 10.1038/35040576
Camacho-Hernández, N. P., Lorea-Hernández, J. J., and Peña-Ortega, F. (2019). Microglial modulators reduce respiratory rhythm long-term facilitation in vitro. Respir. Physiol. Neurobiol. 265, 9–18. doi: 10.1016/j.resp.2018.07.012
Carroll, F. I., Lewin, A. H., Mascarella, S. W., Seltzman, H. H., and Reddy, P. A. (2012). Designer drugs: a medicinal chemistry perspective. Ann. N. Y. Acad. Sci. 1248, 18–38. doi: 10.1111/j.1749-6632.2011.06199.x
Channappanavar, R., Fett, C., Mack, M., Ten Eyck, P. P., Meyerholz, D. K., and Perlman, S. (2017). Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. 198, 4046–4053. doi: 10.4049/jimmunol.1601896
Chatonnet, F., Wrobel, L. J., Mézières, V., Pasqualetti, M., Ducret, S., Taillebourg, E., et al. (2007). Distinct roles of Hoxa2 and Krox20 in the development of rhythmic neural networks controlling inspiratory depth, respiratory frequency, and jaw opening. Neural Dev. 2:19. doi: 10.1186/1749-8104-2-19
Chernick, V., and Craig, R. J. (1982). Naloxone reverses neonatal depression caused by fetal asphyxia. Science 216, 1252–1253. doi: 10.1126/science.7200636
Chouinard, F., Lefebvre, J. S., Navarro, P., Bouchard, L., Ferland, C., Lalancette-Hébert, M., et al. (2011). The endocannabinoid 2-arachidonoyl-glycerol activates human neutrophils: critical role of its hydrolysis and de novo leukotriene B4 biosynthesis. J. Immunol. 186, 3188–3196. doi: 10.4049/jimmunol.1002853
Chouinard, F., Turcotte, C., Guan, X., Larose, M. C., Poirier, S., Bouchard, L., et al. (2013). 2-Arachidonoyl-glycerol- and arachidonic acid-stimulated neutrophils release antimicrobial effectors against E. coli, S. aureus, HSV-1, and RSV. J. Leukoc. Biol. 93, 267–276. doi: 10.1189/jlb.0412200
Cohen, K., and Weinstein, A. M. (2018). Synthetic and non-synthetic cannabinoid drugs and their adverse effects-a review from public health prospective. Front. Public Health 6:162. doi: 10.3389/fpubh.2018.00162
Control CfD (2017). Prevention. Understanding the epidemic. 11. Available at: https://www.cdcgov/drugoverdose/epidemic/indexhtml
Cooper, Z. D., and Craft, R. M. (2018). Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology 43, 34–51. doi: 10.1038/npp.2017.140
Craft, R. M., Marusich, J. A., and Wiley, J. L. (2013). Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci. 92, 476–481. doi: 10.1016/j.lfs.2012.06.009
Croxford, J. L., and Miller, S. D. (2003). Immunoregulation of a viral model of multiple sclerosis using the synthetic cannabinoid R+WIN55,212. J. Clin. Invest. 111, 1231–1240. doi: 10.1172/jci17652
Cui, Y., Chen, Y., Zhi, J.-L., Guo, R.-X., Feng, J.-Q., and Chen, P.-X. (2006). Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 1069, 235–243. doi: 10.1016/j.brainres.2005.11.066
Cui, Y. Y., D'Agostino, B., Risse, P. A., Marrocco, G., Naline, E., Zhang, Y., et al. (2007). Cannabinoid CB(2) receptor activation prevents bronchoconstriction and airway oedema in a model of gastro-oesophageal reflux. Eur. J. Pharmacol. 573, 206–213. doi: 10.1016/j.ejphar.2007.06.040
Darke, S., Duflou, J., Farrell, M., Peacock, A., and Lappin, J. (2019). Characteristics and circumstances of synthetic cannabinoid-related death. Clin. Toxicol. (Phila.) 58, 368–374. doi: 10.1080/15563650.2019.1647344
De Fonseca, F. R., Cebeira, M., Ramos, J., Martin, M., and Fernandez-Ruiz, J. (1994). Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 54, 159–170. doi: 10.1016/0024-3205(94)00585-0
de Oliveira, A. C. P., Moreira, F. A., and Fiebich, B. L. (2022). CB(2) and toll-like receptors crosstalk in microglia. Trends Neurosci. 45, 1–2. doi: 10.1016/j.tins.2021.10.012
De Petrocellis, L., Ligresti, A., Moriello, A. S., Allarà, M., Bisogno, T., Petrosino, S., et al. (2011). Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163, 1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x
De Petrocellis, L., Nabissi, M., Santoni, G., and Ligresti, A. (2017). Actions and regulation of ionotropic cannabinoid receptors. Adv. Pharmacol. 80, 249–289. doi: 10.1016/bs.apha.2017.04.001
De Petrocellis, L., Orlando, P., Moriello, A. S., Aviello, G., Stott, C., Izzo, A. A., et al. (2012). Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol (Oxf.) 204, 255–266. doi: 10.1111/j.1748-1716.2011.02338.x
Del Negro, C. A., Funk, G. D., and Feldman, J. L. (2018). Breathing matters. Nat. Rev. Neurosci. 19, 351–367. doi: 10.1038/s41583-018-0003-6
Deng, L., Guindon, J., Cornett, B. L., Makriyannis, A., Mackie, K., and Hohmann, A. G. (2015). Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol. Psychiatry 77, 475–487. doi: 10.1016/j.biopsych.2014.04.009
Distribution Po (n.d.). Syringes [sales catalog]. https://pointsofdistribution.org/marketplace2022. Available at: https://pointsofdistribution.org/marketplace (Accessed August 19, 2022).
Doherty, P. A., McCarthy, L. E., and Borison, H. L. (1983). Respiratory and cardiovascular depressant effects of nabilone, N-methyllevonantradol and delta 9-tetrahydrocannabinol in anesthetized cats. J. Pharmacol. Exp. Ther. 227, 508–516.
Dresen, S., Ferreirós, N., Pütz, M., Westphal, F., Zimmermann, R., and Auwärter, V. (2010). Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J. Mass Spectrom. 45, 1186–1194. doi: 10.1002/jms.1811
Dudášová, A., Keir, S. D., Parsons, M. E., Molleman, A., and Page, C. P. (2013). The effects of cannabidiol on the antigen-induced contraction of airways smooth muscle in the Guinea-pig. Pulm. Pharmacol. Ther. 26, 373–379. doi: 10.1016/j.pupt.2013.02.002
Durakoglugil, M. S., and Orer, H. S. (2008). Cannabinoid receptor activation in the nucleus tractus solitaries produces baroreflex-like responses in the rat. Int. J. Biomedical Sci. 4, 229–237.
Eljaschewitsch, E., Witting, A., Mawrin, C., Lee, T., Schmidt, P. M., Wolf, S., et al. (2006). The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron 49, 67–79. doi: 10.1016/j.neuron.2005.11.027
Epstein, D. H., and Preston, K. L. (2015). No evidence for reduction of opioid-withdrawal symptoms by cannabis smoking during a methadone dose taper. Am. J. Addict. 24, 323–328. doi: 10.1111/ajad.12183
Erlichman, J. S., and Leiter, J. (2010). Glia modulation of the extracellular milieu as a factor in central CO2 chemosensitivity and respiratory control. J. Appl. Physiol. 108, 1803–1811. doi: 10.1152/japplphysiol.01321.2009
Evans, K. C., Shea, S. A., and Saykin, A. J. (1999). Functional MRI localisation of central nervous system regions associated with volitional inspiration in humans. J. Physiol. 520, 383–392. doi: 10.1111/j.1469-7793.1999.00383.x
Fattore, L., Deiana, S., Spano, S. M., Cossu, G., Fadda, P., Scherma, M., et al. (2005). Endocannabinoid system and opioid addiction: behavioural aspects. Pharmacol. Biochem. Behav. 81, 343–359. doi: 10.1016/j.pbb.2005.01.031
Feldman, J. L., and Del Negro, C. A. (2006). Looking for inspiration: new perspectives on respiratory rhythm. Nat. Rev. Neurosci. 7, 232–241. doi: 10.1038/nrn1871
Fernández-Ruiz, J., Romero, J., Velasco, G., Tolón, R. M., Ramos, J. A., and Guzmán, M. (2007). Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol. Sci. 28, 39–45. doi: 10.1016/j.tips.2006.11.001
Franco, R., Centelles, J., and Canela, E. (1988). Determination of the characteristics, properties and homogeneity of rat brain microsomes. Binding of lactate dehydrogenase, malate dehydrogenase and 5'nucleotidase to microsomal membranes. Biochem. Int. 16, 689–699.
Fujii, K., Koshidaka, Y., Adachi, M., and Takao, K. (2019). Effects of chronic fentanyl administration on behavioral characteristics of mice. Neuropsychopharmacol Rep. 39, 17–35. doi: 10.1002/npr2.12040
Funk, G. D., Rajani, V., Alvares, T. S., Revill, A. L., Zhang, Y., Chu, N. Y., et al. (2015). Neuroglia and their roles in central respiratory control; an overview. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 186, 83–95. doi: 10.1016/j.cbpa.2015.01.010
Galiègue, S., Mary, S., Marchand, J., Dussossoy, D., Carrière, D., Carayon, P., et al. (1995). Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 232, 54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x
Gamage, T. F., Farquhar, C. E., Lefever, T. W., Marusich, J. A., Kevin, R. C., McGregor, I. S., et al. (2018). Molecular and behavioral pharmacological characterization of abused synthetic cannabinoids MMB- and MDMB-FUBINACA, MN-18, NNEI, CUMYL-PICA, and 5-fluoro-CUMYL-PICA. J. Pharmacol. Exp. Ther. 365, 437–446. doi: 10.1124/jpet.117.246983
Gaoni, Y., and Mechoulam, R. (1964). Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 86, 1646–1647. doi: 10.1021/ja01062a046
Gardner, E. L., and Vorel, S. R. (1998). Cannabinoid transmission and reward-related events. Neurobiol. Dis. 5, 502–533. doi: 10.1006/nbdi.1998.0219
Gargaglioni, L. H., Marques, D. A., and Patrone, L. G. A. (2019). Sex differences in breathing. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 238:110543. doi: 10.1016/j.cbpa.2019.110543
Gates, P., Jaffe, A., and Copeland, J. (2014). Cannabis smoking and respiratory health: consideration of the literature. Respirology 19, 655–662. doi: 10.1111/resp.12298
Gertsch, J., Leonti, M., Raduner, S., Racz, I., Chen, J. Z., Xie, X. Q., et al. (2008). Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. U. S. A. 105, 9099–9104. doi: 10.1073/pnas.0803601105
Ghali, M. G. Z. (2019). Respiratory rhythm generation and pattern formation: oscillators and network mechanisms. J. Integr. Neurosci. 18, 481–517. doi: 10.31083/j.jin.2019.04.188
Ghasemiesfe, M., Ravi, D., Vali, M., Korenstein, D., Arjomandi, M., Frank, J., et al. (2018). Marijuana use, respiratory symptoms, and pulmonary function: a systematic review and meta-analysis. Ann. Intern. Med. 169, 106–115. doi: 10.7326/m18-0522
Glass, M., Dragunow, M., and Faull, R. L. (1997). Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77, 299–318. doi: 10.1016/s0306-4522(96)00428-9
Goodwin, R. D., Kim, J. H., Cheslack-Postava, K., Weinberger, A. H., Wu, M., Wyka, K., et al. (2021). Trends in cannabis use among adults with children in the home in the United States, 2004–2017: impact of state-level legalization for recreational and medical use. Addiction 116, 2770–2778. doi: 10.1111/add.15472
Gray, P. A., Rekling, J. C., Bocchiaro, C. M., and Feldman, J. L. (1999). Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science 286, 1566–1568. doi: 10.1126/science.286.5444.1566
Grenald, S. A., Young, M. A., Wang, Y., Ossipov, M. H., Ibrahim, M. M., Largent-Milnes, T. M., et al. (2017). Synergistic attenuation of chronic pain using mu opioid and cannabinoid receptor 2 agonists. Neuropharmacology 116, 59–70. doi: 10.1016/j.neuropharm.2016.12.008
Gunderson, E. W., Haughey, H. M., Ait-Daoud, N., Joshi, A. S., and Hart, C. L. (2014). A survey of synthetic cannabinoid consumption by current cannabis users. Subst. Abus. 35, 184–189. doi: 10.1080/08897077.2013.846288
Guyenet, P. G. (2011). Regulation of breathing and autonomic outflows by chemoreceptors. Compr. Physiol. 4, 1511–1562. doi: 10.1002/cphy.c140004
Hancox, R. J., Gray, A. R., Zhang, X., Poulton, R., Moffitt, T. E., Caspi, A., et al. (2022). Differential effects of cannabis and tobacco on lung function in mid-adult life. Am. J. Respir. Crit. Care Med. 205, 1179–1185. doi: 10.1164/rccm.202109-2058OC
Hartley, J. P., Nogrady, S. G., and Seaton, A. (1978). Bronchodilator effect of delta1-tetrahydrocannabinol. Br. J. Clin. Pharmacol. 5, 523–525. doi: 10.1111/j.1365-2125.1978.tb01667.x
Hazekamp, A., and Fischedick, J. T. (2012). Cannabis – from cultivar to chemovar. Drug Test. Anal. 4, 660–667. doi: 10.1002/dta.407
Hickey, A. J. (2020). Emerging trends in inhaled drug delivery. Adv. Drug Deliv. Rev. 157, 63–70. doi: 10.1016/j.addr.2020.07.006
Hickman, S., Izzy, S., Sen, P., Morsett, L., and El Khoury, J. (2018). Microglia in neurodegeneration. Nat. Neurosci. 21, 1359–1369. doi: 10.1038/s41593-018-0242-x
Hrynowski, Z. (2019). What percentage of Americans smoke marijuana? news.gallup.com: Gallup.
Hughes, B., Wiessing, L., Des Jarlais, D., and Griffiths, P. (2018). Could cannabis liberalisation lead to wider changes in drug policies and outcomes? Int. J. Drug Policy 51, 156–159. doi: 10.1016/j.drugpo.2017.10.004
Hülsmann, S., Oku, Y., Zhang, W., and Richter, D. W. (2000). Metabolic coupling between glia and neurons is necessary for maintaining respiratory activity in transverse medullary slices of neonatal mouse. Eur. J. Neurosci. 12, 856–862. doi: 10.1046/j.1460-9568.2000.00973.x
Hurd, Y. L., Yoon, M., Manini, A. F., Hernandez, S., Olmedo, R., Ostman, M., et al. (2015). Early phase in the development of Cannabidiol as a treatment for addiction: opioid relapse takes initial Center stage. Neurotherapeutics 12, 807–815. doi: 10.1007/s13311-015-0373-7
Huxtable, A. G., Zwicker, J. D., Alvares, T. S., Ruangkittisakul, A., Fang, X., Hahn, L. B., et al. (2010). Glia contribute to the purinergic modulation of inspiratory rhythm-generating networks. J. Neurosci. 30, 3947–3958. doi: 10.1523/JNEUROSCI.6027-09.2010
Iring, A., Hricisak, L., and Benyo, Z. (2017). CB1 receptor-mediated respiratory depression by endocannabinoids. Respir. Physiol. Neurobiol. 240, 48–52. doi: 10.1016/j.resp.2017.02.011
Ivanov, I. D., Stoykova, S., Ivanova, E., Vlahova, A., Burdzhiev, N., Pantcheva, I., et al. (2019). A case of 5F-ADB/FUB-AMB abuse: drug-induced or drug-related death? Forensic Sci. Int. 297, 372–377. doi: 10.1016/j.forsciint.2019.02.005
Izzo, A. A., and Sharkey, K. A. (2010). Cannabinoids and the gut: new developments and emerging concepts. Pharmacol. Ther. 126, 21–38. doi: 10.1016/j.pharmthera.2009.12.005
Jacquin, T. D., Borday, V., Schneider-Maunoury, S., Topilko, P., Ghilini, G., Kato, F., et al. (1996). Reorganization of pontine rhythmogenic neuronal networks in Krox-20 knockout mice. Neuron 17, 747–758. doi: 10.1016/S0896-6273(00)80206-8
Jäkel, S., and Dimou, L. (2017). Glial cells and their function in the adult brain: a journey through the history of their ablation. Front. Cell. Neurosci. 11:11. doi: 10.3389/fncel.2017.00024
Janczewski, W. A., and Feldman, J. L. (2006). Distinct rhythm generators for inspiration and expiration in the juvenile rat. J. Physiol. 570, 407–420. doi: 10.1113/jphysiol.2005.098848
Jicha, C. J., Lofwall, M. R., Nuzzo, P. A., Babalonis, S., Elayi, S. C., and Walsh, S. L. (2015). Safety of Oral Dronabinol during opioid withdrawal in humans. Drug Alcohol Depend. 157, 179–183. doi: 10.1016/j.drugalcdep.2015.09.031
Jinwala, F. N., and Gupta, M. (2012). Synthetic cannabis and respiratory depression. J. Child Adolesc. Psychopharmacol. 22, 459–462. doi: 10.1089/cap.2011.0122
Karmaus, P. W., Wagner, J. G., Harkema, J. R., Kaminski, N. E., and Kaplan, B. L. (2013). Cannabidiol (CBD) enhances lipopolysaccharide (LPS)-induced pulmonary inflammation in C57BL/6 mice. J. Immunotoxicol. 10, 321–328. doi: 10.3109/1547691x.2012.741628
Kazantzis, N. P., Casey, S. L., Seow, P. W., Mitchell, V. A., and Vaughan, C. W. (2016). Opioid and cannabinoid synergy in a mouse neuropathic pain model. Br. J. Pharmacol. 173, 2521–2531. doi: 10.1111/bph.13534
Komorowska-Müller, J. A., and Schmöle, A.-C. (2020). CB2 receptor in microglia: the Guardian of self-Control. Int. J. Mol. Sci. 22:19. doi: 10.3390/ijms22010019
Kral, A. H., Wenger, L., Novak, S. P., Chu, D., Corsi, K. F., Coffa, D., et al. (2015). Is cannabis use associated with less opioid use among people who inject drugs? Drug Alcohol Depend. 153, 236–241. doi: 10.1016/j.drugalcdep.2015.05.014
Lake, S., Walsh, Z., Kerr, T., Cooper, Z. D., Buxton, J., Wood, E., et al. (2019). Frequency of cannabis and illicit opioid use among people who use drugs and report chronic pain: a longitudinal analysis. PLoS Med. 16:e1002967. doi: 10.1371/journal.pmed.1002967
LaVigne, J. E., Hecksel, R., Keresztes, A., and Streicher, J. M. (2021). Cannabis sativa terpenes are cannabimimetic and selectively enhance cannabinoid activity. Sci. Rep. 11:8232. doi: 10.1038/s41598-021-87740-8
Lazary, J., Juhasz, G., Hunyady, L., and Bagdy, G. (2011). Personalized medicine can pave the way for the safe use of CB1 receptor antagonists. Trends Pharmacol. Sci. 32, 270–280. doi: 10.1016/j.tips.2011.02.013
Lee, J. W., Huang, B. X., Kwon, H., Rashid, M. A., Kharebava, G., Desai, A., et al. (2016). Orphan GPR110 (ADGRF1) targeted by N-docosahexaenoylethanolamine in development of neurons and cognitive function. Nat. Commun. 7:13123. doi: 10.1038/ncomms13123
Lee, S. F., Newton, C., Widen, R., Friedman, H., and Klein, T. W. (2001). Differential expression of cannabinoid CB(2) receptor mRNA in mouse immune cell subpopulations and following B cell stimulation. Eur. J. Pharmacol. 423, 235–241. doi: 10.1016/s0014-2999(01)01122-0
Levine, A., Liktor-Busa, E., Lipinski, A. A., Couture, S., Balasubramanian, S., Aicher, S. A., et al. (2021). Sex differences in the expression of the endocannabinoid system within V1M cortex and PAG of Sprague Dawley rats. Biol. Sex Differ. 12:60. doi: 10.1186/s13293-021-00402-2
Liang, D., Bao, Y., Wallace, M., Grant, I., and Shi, Y. (2018). Medical cannabis legalization and opioid prescriptions: evidence on US Medicaid Enrollees during 1993-2014. Addiction 113, 2060–2070. doi: 10.1111/add.14382
Liktor-Busa, E., Keresztes, A., LaVigne, J., Streicher, J. M., and Largent-Milnes, T. M. (2021). Analgesic potential of terpenes derived from Cannabis sativa. Pharmacol. Rev. 73, 98–126. doi: 10.1124/pharmrev.120.000046
Llorente-Berzal, A., McGowan, F., Gaspar, J. C., Rea, K., Roche, M., and Finn, D. P. (2022). Sexually dimorphic expression of fear-conditioned analgesia in rats and associated alterations in the endocannabinoid system in the periaqueductal Grey. Neuroscience 480, 117–130. doi: 10.1016/j.neuroscience.2021.11.005
Lopez-Moreno, J., Lopez-Jimenez, A., Gorriti, M., and de Fonseca, F. R. (2010). Functional interactions between endogenous cannabinoid and opioid systems: focus on alcohol, genetics and drug-addicted behaviors. Curr. Drug Targets 11, 406–428. doi: 10.2174/138945010790980312
Lorea-Hernandez, J. J., Morales, T., Rivera-Angulo, A. J., Alcantara-Gonzalez, D., and Pena-Ortega, F. (2016). Microglia modulate respiratory rhythm generation and autoresuscitation. Glia 64, 603–619. doi: 10.1002/glia.22951
Lu, T., Newton, C., Perkins, I., Friedman, H., and Klein, T. W. (2006). Cannabinoid treatment suppresses the T-helper cell-polarizing function of mouse dendritic cells stimulated with legionella pneumophila infection. J. Pharmacol. Exp. Ther. 319, 269–276. doi: 10.1124/jpet.106.108381
Lucas, P., and Walsh, Z. (2017). Medical cannabis access, use, and substitution for prescription opioids and other substances: a survey of authorized medical cannabis patients. Int. J. Drug Policy 42, 30–35. doi: 10.1016/j.drugpo.2017.01.011
Machado, B. H., and Bonagamba, L. G. (2005). Antagonism of glutamate receptors in the intermediate and caudal NTS of awake rats produced no changes in the hypertensive response to chemoreflex activation. Auton. Neurosci. 117, 25–32. doi: 10.1016/j.autneu.2004.10.004
Maher, D. P., Carr, D. B., Hill, K., McGeeney, B., Weed, V., Jackson, W. C., et al. (2017). Cannabis for the treatment of chronic pain in the era of an opioid epidemic: a symposium-based review of Sociomedical science. Pain Med. 20, 2311–2323. doi: 10.1093/pm/pnx143
Makriyannis, A. (2014). 2012 division of medicinal chemistry award address. Trekking the cannabinoid road: a personal perspective. J. Med. Chem. 57, 3891–3911. doi: 10.1021/jm500220s
Malfait, A. M., Gallily, R., Sumariwalla, P. F., Malik, A. S., Andreakos, E., Mechoulam, R., et al. (2000). The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. U. S. A. 97, 9561–9566. doi: 10.1073/pnas.160105897
Malinowska, B., Baranowska-Kuczko, M., Kicman, A., and Schlicker, E. (2021). Opportunities, Challenges and pitfalls of using Cannabidiol as an adjuvant drug in COVID-19. Int. J. Mol. Sci. 22:1986. doi: 10.3390/ijms22041986
Manini, A. F., Yiannoulos, G., Bergamaschi, M. M., Hernandez, S., Olmedo, R., Barnes, A. J., et al. (2015). Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J. Addict. Med. 9, 204–210. doi: 10.1097/ADM.0000000000000118
Mathews, E. M., Jeffries, E., Hsieh, C., Jones, G., and Buckner, J. D. (2019). Synthetic cannabinoid use among college students. Addict. Behav. 93, 219–224. doi: 10.1016/j.addbeh.2019.02.009
Matias, I., Pochard, P., Orlando, P., Salzet, M., Pestel, J., and Di Marzo, V. (2002). Presence and regulation of the endocannabinoid system in human dendritic cells. Eur. J. Biochem. 269, 3771–3778. doi: 10.1046/j.1432-1033.2002.03078.x
Mauro, C. M., Newswanger, P., Santaella-Tenorio, J., Mauro, P. M., Carliner, H., and Martins, S. S. (2019). Impact of medical marijuana laws on state-level marijuana use by age and gender, 2004–2013. Prev. Sci. 20, 205–214. doi: 10.1007/s11121-017-0848-3
Mayet, A., Lions, C., Roux, P., Mora, M., Maradan, G., Morel, A., et al. (2015). Variations in cannabis use level and correlates in opiate-users on methadone maintenance treatment: a French prospective study. J. Subst. Abus. Treat. 58, 100–105. doi: 10.1016/j.jsat.2015.06.015
McHugh, D., Page, J., Dunn, E., and Bradshaw, H. B. (2012). Δ(9) -tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 165, 2414–2424. doi: 10.1111/j.1476-5381.2011.01497.x
Mckay, L. C., Evans, K. C., Frackowiak, R. S., and Corfield, D. R. (2003). Neural correlates of voluntary breathing in humans. J. Appl. Physiol. 95, 1170–1178. doi: 10.1152/japplphysiol.00641.2002
Mecha, M., Feliú, A., Carrillo-Salinas, F., Rueda-Zubiaurre, A., Ortega-Gutiérrez, S., de Sola, R. G., et al. (2015). Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav. Immun. 49, 233–245. doi: 10.1016/j.bbi.2015.06.002
Merighi, S., Gessi, S., Varani, K., Fazzi, D., Mirandola, P., and Borea, P. A. (2012). Cannabinoid CB(2) receptor attenuates morphine-induced inflammatory responses in activated microglial cells. Br. J. Pharmacol. 166, 2371–2385. doi: 10.1111/j.1476-5381.2012.01948.x
Mifflin, S. W. (1992). Arterial chemoreceptor input to nucleus tractus solitarius. Am. J. Phys. 263, R368–R375. doi: 10.1152/ajpregu.1992.263.2.R368
Mohammed, A., Alghetaa, H. K., Zhou, J., Chatterjee, S., Nagarkatti, P., and Nagarkatti, M. (2020). Protective effects of Δ9-tetrahydrocannabinol against enterotoxin-induced acute respiratory distress syndrome are mediated by modulation of microbiota. Br. J. Pharmacol. 177, 5078–5095. doi: 10.1111/bph.15226
Montandon, G., Qin, W., Liu, H., Ren, J., Greer, J. J., and Horner, R. L. (2011). PreBötzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J. Neurosci. 31, 1292–1301. doi: 10.1523/JNEUROSCI.4611-10.2011
Montandon, G., Ren, J., Victoria, N. C., Liu, H., Wickman, K., Greer, J. J., et al. (2016). G-protein–gated inwardly rectifying potassium channels modulate respiratory depression by opioids. Anesthesiology 124, 641–650. doi: 10.1097/ALN.0000000000000984
Morales, P., Hurst, D. P., and Reggio, P. H. (2017). Molecular targets of the Phytocannabinoids: a complex picture. Prog. Chem. Org. Nat. Prod. 103, 103–131. doi: 10.1007/978-3-319-45541-9_4
Muller, C., Morales, P., and Reggio, P. H. (2018). Cannabinoid ligands targeting TRP channels. Front. Mol. Neurosci. 11:487. doi: 10.3389/fnmol.2018.00487
Neusch, C., Papadopoulos, N., Müller, M., Maletzki, I., Winter, S. M., Hirrlinger, J., et al. (2006). Lack of the Kir4. 1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: impact on extracellular K+ regulation. J. Neurophysiol. 95, 1843–1852. doi: 10.1152/jn.00996.2005
Nguyen, T., Thomas, B. F., and Zhang, Y. (2019). Overcoming the psychiatric side effects of the cannabinoid CB1 receptor antagonists: current approaches for therapeutics development. Curr. Top. Med. Chem. 19, 1418–1435. doi: 10.2174/1568026619666190708164841
Niederhoffer, N., Schmid, K., and Szabo, B. (2003). The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn Schmiedeberg's Arch. Pharmacol. 367, 434–443. doi: 10.1007/s00210-003-0755-y
Nikas, S. P., Sharma, R., Paronis, C. A., Kulkarni, S., Thakur, G. A., Hurst, D., et al. (2015). Probing the carboxyester side chain in controlled deactivation (−)-delta(8)-tetrahydrocannabinols. J. Med. Chem. 58, 665–681. doi: 10.1021/jm501165d
Ogoh, S. (2019). Interaction between the respiratory system and cerebral blood flow regulation. J. Appl. Physiol. 127, 1197–1205. doi: 10.1152/japplphysiol.00057.2019
Oka, S., Ikeda, S., Kishimoto, S., Gokoh, M., Yanagimoto, S., Waku, K., et al. (2004). 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces the migration of EoL-1 human eosinophilic leukemia cells and human peripheral blood eosinophils. J. Leukoc. Biol. 76, 1002–1009. doi: 10.1189/jlb.0404252
Okada, Y., Sasaki, T., Oku, Y., Takahashi, N., Seki, M., Ujita, S., et al. (2012). Preinspiratory calcium rise in putative pre-Botzinger complex astrocytes. J. Physiol. 590, 4933–4944. doi: 10.1113/jphysiol.2012.231464
Orihuela, R., McPherson, C. A., and Harry, G. J. (2016). Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 173, 649–665. doi: 10.1111/bph.13139
Owen, K. P., Sutter, M. E., and Albertson, T. E. (2014). Marijuana: respiratory tract effects. Clin. Rev. Allergy Immunol. 46, 65–81. doi: 10.1007/s12016-013-8374-y
Paland, N., Pechkovsky, A., Aswad, M., Hamza, H., Popov, T., Shahar, E., et al. (2021). The immunopathology of COVID-19 and the cannabis paradigm. Front. Immunol. 12:631233. doi: 10.3389/fimmu.2021.631233
Pascual Pastor, F., Isorna Folgar, M., Carvalho, N., Carvalho, F., and Arias, H. F. (2020). Therapeutic cannabis and COVID-19: between opportunism and infoxication. Adicciones 32, 167–172. doi: 10.20882/adicciones.1603
Patel, H. J., Birrell, M. A., Crispino, N., Hele, D. J., Venkatesan, P., Barnes, P. J., et al. (2003). Inhibition of Guinea-pig and human sensory nerve activity and the cough reflex in Guinea-pigs by cannabinoid (CB2) receptor activation. Br. J. Pharmacol. 140, 261–268. doi: 10.1038/sj.bjp.0705435
Pereira, C. F., Vargas, D., Toneloto, F. L., Ito, V. D., and Volpato, R. J. (2022). Implications of cannabis and cannabinoid use in COVID-19: scoping review. Rev. Bras Enferm. 75:e20201374. doi: 10.1590/0034-7167-2020-1374
Pertwee, R. G. (1997). Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 74, 129–180. doi: 10.1016/S0163-7258(97)82001-3
Pertwee, R. G., Howlett, A. C., Abood, M. E., Alexander, S. P. H., Di Marzo, V., Elphick, M. R., et al. (2010). International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol. Rev. 62, 588–631. doi: 10.1124/pr.110.003004
Pfitzer, T., Niederhoffer, N., and Szabo, B. (2004). Central effects of the cannabinoid receptor agonist WIN55212-2 on respiratory and cardiovascular regulation in anaesthetised rats. Br. J. Pharmacol. 142, 943–952. doi: 10.1038/sj.bjp.0705874
Porter, A. C., Sauer, J. M., Knierman, M. D., Becker, G. W., Berna, M. J., Bao, J., et al. (2002). Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 301, 1020–1024. doi: 10.1124/jpet.301.3.1020
Porzionato, A., Stocco, E., Guidolin, D., Agnati, L., Macchi, V., and De Caro, R. (2018). Receptor–receptor interactions of G protein-coupled receptors in the carotid body: a working hypothesis. Front. Physiol. 9:697. doi: 10.3389/fphys.2018.00697
Prabhakar, N. R., and Semenza, G. L. (2015). Oxygen sensing and homeostasis. Physiology 30, 340–348. doi: 10.1152/physiol.00022.2015
Quirion, B., Bergeron, F., Blais, V., and Gendron, L. (2020). The Delta-opioid receptor; a target for the treatment of pain. Front. Mol. Neurosci. 13:13. doi: 10.3389/fnmol.2020.00052
Raghavendra, V., Rutkowski, M. D., and DeLeo, J. A. (2002). The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J. Neurosci. 22, 9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002
Raghavendra, V., Tanga, F. Y., and DeLeo, J. A. (2004). Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology 29, 327–334. doi: 10.1038/sj.npp.1300315
Rajani, V., Zhang, Y., Jalubula, V., Rancic, V., SheikhBahaei, S., Zwicker, J. D., et al. (2018). Release of ATP by pre-Bötzinger complex astrocytes contributes to the hypoxic ventilatory response via a ca(2+) -dependent P2Y(1) receptor mechanism. J. Physiol. 596, 3245–3269. doi: 10.1113/JP274727
Ralevic, V. (2003). Cannabinoid modulation of peripheral autonomic and sensory neurotransmission. Eur. J. Pharmacol. 472, 1–21. doi: 10.1016/s0014-2999(03)01813-2
Raux, M., Straus, C., Redolfi, S., Morelot-Panzini, C., Couturier, A., Hug, F., et al. (2007). Electroencephalographic evidence for pre-motor cortex activation during inspiratory loading in humans. J. Physiol. 578, 569–578. doi: 10.1113/jphysiol.2006.120246
Reusch, N., Ravichandran, K. A., Olabiyi, B. F., Komorowska-Müller, J. A., Hansen, J. N., Ulas, T., et al. (2022). Cannabinoid receptor 2 is necessary to induce toll-like receptor-mediated microglial activation. Glia 70, 71–88. doi: 10.1002/glia.24089
Ribeiro, A., Almeida, V. I., Costola-de-Souza, C., Ferraz-de-Paula, V., Pinheiro, M. L., Vitoretti, L. B., et al. (2015). Cannabidiol improves lung function and inflammation in mice submitted to LPS-induced acute lung injury. Immunopharmacol. Immunotoxicol. 37, 35–41. doi: 10.3109/08923973.2014.976794
Rice, W., Shannon, J. M., Burton, F., and Fiedeldey, D. (1997). Expression of a brain-type cannabinoid receptor (CB1) in alveolar type II cells in the lung: regulation by hydrocortisone. Eur. J. Pharmacol. 327, 227–232. doi: 10.1016/S0014-2999(97)89665-3
Rieder, S. A., Chauhan, A., Singh, U., Nagarkatti, M., and Nagarkatti, P. (2010). Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 215, 598–605. doi: 10.1016/j.imbio.2009.04.001
Robson, P. Human studies of cannabinoids and medicinal cannabis. Cannabinoids: Springer; (2005). 719–756.
Rock, E. M., and Parker, L. A. (2021). Constituents of cannabis Sativa. Adv. Exp. Med. Biol. 1264, 1–13. doi: 10.1007/978-3-030-57369-0_1
Roth, M. D., Whittaker, K., Salehi, K., Tashkin, D. P., and Baldwin, G. C. (2004). Mechanisms for impaired effector function in alveolar macrophages from marijuana and cocaine smokers. J. Neuroimmunol. 147, 82–86. doi: 10.1016/j.jneuroim.2003.10.017
Roy, A., Mandadi, S., Fiamma, M.-N., Rodikova, E., Ferguson, E. V., Whelan, P. J., et al. (2012). Anandamide modulates carotid sinus nerve afferent activity via TRPV1 receptors increasing responses to heat. J. Appl. Physiol. 112, 212–224. doi: 10.1152/japplphysiol.01303.2010
Ryberg, E., Larsson, N., Sjögren, S., Hjorth, S., Hermansson, N. O., Leonova, J., et al. (2007). The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 152, 1092–1101. doi: 10.1038/sj.bjp.0707460
Scavone, J. L., Mackie, K., and Van Bockstaele, E. J. (2010). Characterization of cannabinoid-1 receptors in the locus coeruleus: relationship with mu-opioid receptors. Brain Res. 1312, 18–31. doi: 10.1016/j.brainres.2009.11.023
Scavone, J., Sterling, R., and Van Bockstaele, E. (2013). Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neuroscience 248, 637–654. doi: 10.1016/j.neuroscience.2013.04.034
Schmid, K., Niederhoffer, N., and Szabo, B. (2003). Analysis of the respiratory effects of cannabinoids in rats. Naunyn Schmiedeberg's Arch. Pharmacol. 368, 301–308. doi: 10.1007/s00210-003-0787-3
Schmöle, A.-C., Lundt, R., Gennequin, B., Schrage, H., Beins, E., Krämer, A., et al. (2015). Correction: expression analysis of CB2-GFP BAC transgenic mice. PLoS One 10:e0145472. doi: 10.1371/journal.pone.0145472
Shay, A. H., Choi, R., Whittaker, K., Salehi, K., Kitchen, C. M., Tashkin, D. P., et al. (2003). Impairment of antimicrobial activity and nitric oxide production in alveolar macrophages from smokers of marijuana and cocaine. J. Infect. Dis. 187, 700–704. doi: 10.1086/368370
SheikhBahaei, S., Morris, B., Collina, J., Anjum, S., Znati, S., Gamarra, J., et al. (2018). Morphometric analysis of astrocytes in brainstem respiratory regions. J. Comp. Neurol. 526, 2032–2047. doi: 10.1002/cne.24472
Shi, Y., Stornetta, D. S., Reklow, R. J., Sahu, A., Wabara, Y., Nguyen, A., et al. (2021). A brainstem peptide system activated at birth protects postnatal breathing. Nature 589, 426–430. doi: 10.1038/s41586-020-2991-4
Silver, E. R., and Hur, C. (2020). Gender differences in prescription opioid use and misuse: implications for men's health and the opioid epidemic. Prev. Med. 131:105946. doi: 10.1016/j.ypmed.2019.105946
Silveyra, P., Fuentes, N., and Rodriguez Bauza, D. E. (2021). Sex and gender differences in lung disease. Adv. Exp. Med. Biol. 1304, 227–258. doi: 10.1007/978-3-030-68748-9_14
Simone, J. J., Green, M. R., and McCormick, C. M. (2022). Endocannabinoid system contributions to sex-specific adolescent neurodevelopment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 113:110438. doi: 10.1016/j.pnpbp.2021.110438
Smith, J. C., Ellenberger, H. H., Ballanyi, K., Richter, D. W., and Feldman, J. L. (1991). Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729. doi: 10.1126/science.1683005
Soliman, N., Haroutounian, S., Hohmann, A. G., Krane, E., Liao, J., Macleod, M., et al. (2021). Systematic review and meta-analysis of cannabinoids, cannabis-based medicines, and endocannabinoid system modulators tested for antinociceptive effects in animal models of injury-related or pathological persistent pain. Pain 162, S26–s44. doi: 10.1097/j.pain.0000000000002269
Staiano, R. I., Loffredo, S., Borriello, F., Iannotti, F. A., Piscitelli, F., Orlando, P., et al. (2016). Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J. Leukoc. Biol. 99, 531–540. doi: 10.1189/jlb.3HI1214-584R
Ständer, S., Schmelz, M., Metze, D., Luger, T., and Rukwied, R. (2005). Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J. Dermatol. Sci. 38, 177–188. doi: 10.1016/j.jdermsci.2005.01.007
Sun, X., Thörn Pérez, C., Halemani, D. N., Shao, X. M., Greenwood, M., Heath, S., et al. (2019). Opioids modulate an emergent rhythmogenic process to depress breathing. eLife 8:e50613. doi: 10.7554/eLife.50613
Szymaszkiewicz, A., Zielinska, M., Li, K., Ramanathan, M., Alam, S., Hou, D. R., et al. (2018). Novel derivatives of 1,2,3-triazole, cannabinoid-1 receptor ligands modulate gastrointestinal motility in mice. Naunyn Schmiedeberg's Arch. Pharmacol. 391, 435–444. doi: 10.1007/s00210-018-1465-9
Tait, R. J., Caldicott, D., Mountain, D., Hill, S. L., and Lenton, S. (2016). A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin. Toxicol. (Phila.) 54, 1–13. doi: 10.3109/15563650.2015.1110590
Tashkin, D. P., Shapiro, B. J., and Frank, I. M. (1973). Acute pulmonary physiologic effects of smoked marijuana and oral (Delta)9 -tetrahydrocannabinol in healthy young men. N. Engl. J. Med. 289, 336–341. doi: 10.1056/nejm197308162890702
Tashkin, D. P., Shapiro, B. J., Reiss, S., Olsen, J. L., and Lodge, J. W. (1976). “Bronchial effects of Aerosolized Delta-9-tetrahydrocannabinol” in The therapeutic potential of marihuana. eds. S. Cohen and R. C. Stillman (Boston, MA: Springer US), 97–109.
Tofighi, B., and Lee, J. D. (2012). Internet highs--seizures after consumption of synthetic cannabinoids purchased online. J. Addict. Med. 6, 240–241. doi: 10.1097/ADM.0b013e3182619004
Tournebize, J., Gibaja, V., and Kahn, J.-P. (2017). Acute effects of synthetic cannabinoids: update 2015. Subst. Abus. 38, 344–366. doi: 10.1080/08897077.2016.1219438
Trecki, J., Gerona, R. R., and Schwartz, M. D. (2015). Synthetic cannabinoid–related illnesses and deaths. N. Engl. J. Med. 373, 103–107. doi: 10.1056/NEJMp1505328
Tree, K., Caravagna, C., Hilaire, G., Peyronnet, J., and Cayetanot, F. (2010). Anandamide centrally depresses the respiratory rhythm generator of neonatal mice. Neuroscience 170, 1098–1109. doi: 10.1016/j.neuroscience.2010.08.045
Tucker, R., Kagaya, M., Page, C., and Spina, D. (2001). The endogenous cannabinoid agonist, anandamide stimulates sensory nerves in Guinea-pig airways. Br. J. Pharmacol. 132, 1127–1135. doi: 10.1038/sj.bjp.0703906
Tumati, S., Largent-Milnes, T. M., Keresztes, A. I., Yamamoto, T., Vanderah, T. W., Roeske, W. R., et al. (2012). Tachykinin NK₁ receptor antagonist co-administration attenuates opioid withdrawal-mediated spinal microglia and astrocyte activation. Eur. J. Pharmacol. 684, 64–70. doi: 10.1016/j.ejphar.2012.03.025
Turcotte, C., Blanchet, M. R., Laviolette, M., and Flamand, N. (2016). Impact of cannabis, cannabinoids, and endocannabinoids in the lungs. Front. Pharmacol. 7:317. doi: 10.3389/fphar.2016.00317
Vachon, L., Robins, A., and Gaensler, E. A. (1976). “Airways response to Aerosolized Delta-9-tetrahydrocannabinol: preliminary report” in The therapeutic potential of marihuana. eds. S. Cohen and R. C. Stillman (Boston, MA: Springer US), 111–121.
Vanderah, T. W. (2007). Pathophysiology of pain. Med. Clin. N. Am. 91, 1–12. doi: 10.1016/j.mcna.2006.10.006
Vara, D., Morell, C., Rodríguez-Henche, N., and Diaz-Laviada, I. (2013). Involvement of PPARγ in the antitumoral action of cannabinoids on hepatocellular carcinoma. Cell Death Dis. 4:e618. doi: 10.1038/cddis.2013.141
Varga, D. P., Menyhart, A., Posfai, B., Csaszar, E., Lenart, N., Cserep, C., et al. (2020). Microglia alter the threshold of spreading depolarization and related potassium uptake in the mouse brain. J. Cereb. Blood Flow Metab. 40, S67–S80. doi: 10.1177/0271678x19900097
Varga, A. G., Reid, B. T., Kieffer, B. L., and Levitt, E. S. (2019). Differential impact of two critical respiratory centers in opioid-induced respiratory depression in awake mice. J. Physiol. 598, 189–205. doi: 10.1113/jp278612
Vuolo, F., Abreu, S. C., Michels, M., Xisto, D. G., Blanco, N. G., Hallak, J. E., et al. (2019). Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur. J. Pharmacol. 843, 251–259. doi: 10.1016/j.ejphar.2018.11.029
Wang, L. L., Zhao, R., Li, J. Y., Li, S. S., Liu, M., Wang, M., et al. (2016). Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re-epithelialization during skin wound healing. Eur. J. Pharmacol. 786, 128–136. doi: 10.1016/j.ejphar.2016.06.006
Watkins, L. R., Hutchinson, M. R., Rice, K. C., and Maier, S. F. (2009). The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol. Sci. 30, 581–591. doi: 10.1016/j.tips.2009.08.002
Webster, L., and Schmidt, W. K. (2019). Dilemma of addiction and respiratory depression in the treatment of pain: a prototypical Endomorphin as a new approach. Pain Med. 21, 992–1004. doi: 10.1093/pm/pnz122
Weitnauer, M., Mijošek, V., and Dalpke, A. H. (2016). Control of local immunity by airway epithelial cells. Mucosal Immunol. 9, 287–298. doi: 10.1038/mi.2015.126
Wiese, B. M., Liktor-Busa, E., Couture, S. A., Nikas, S. P., Ji, L., Liu, Y., et al. (2021). Brain penetrant, but not peripherally restricted, synthetic cannabinoid 1 receptor agonists promote morphine-mediated respiratory depression. Cannabis Cannabinoid Res. 7, 621–627. doi: 10.1089/can.2021.0090
Wiese, B. M., Liktor-Busa, E., Levine, A., Couture, S. A., Nikas, S. P., Ji, L., et al. (2020). Cannabinoid-2 Agonism with AM2301 mitigates morphine-induced respiratory depression. Cannabis Cannabinoid Res. 6, 401–412. doi: 10.1089/can.2020.0076
Wiese, B. M., and Wilson-Poe, A. R. (2018). Emerging evidence for cannabis' role in opioid use disorder. Cannabis Cannabinoid Res. 3, 179–189. doi: 10.1089/can.2018.0022
Wills, K. L., and Parker, L. A. (2016). Effect of pharmacological modulation of the endocannabinoid system on opiate withdrawal: a review of the preclinical animal literature. Front. Pharmacol. 7:187. doi: 10.3389/fphar.2016.00187
Wong, K. U., and Baum, C. R. (2019). Acute cannabis toxicity. Pediatr. Emerg. Care 35, 799–804. doi: 10.1097/pec.0000000000001970
Ye, L., Cao, Z., Wang, W., and Zhou, N. (2019). New insights in cannabinoid receptor structure and Signaling. Curr. Mol. Pharmacol. 12, 239–248. doi: 10.2174/1874467212666190215112036
Yoshihara, S., Morimoto, H., Yamada, Y., Abe, T., and Arisaka, O. (2004). Cannabinoid receptor agonists inhibit sensory nerve activation in Guinea pig airways. Am. J. Respir. Crit. Care Med. 170, 941–946. doi: 10.1164/rccm.200306-775OC
Young, J. K., Dreshaj, I. A., Wilson, C. G., Martin, R. J., Zaidi, S. I., and Haxhiu, M. A. (2005). An astrocyte toxin influences the pattern of breathing and the ventilatory response to hypercapnia in neonatal rats. Respir. Physiol. Neurobiol. 147, 19–30. doi: 10.1016/j.resp.2005.01.009
Yuill, M. B., Hale, D. E., Guindon, J., and Morgan, D. J. (2017). Anti-nociceptive interactions between opioids and a cannabinoid receptor 2 agonist in inflammatory pain. Mol. Pain 13:1744806917728227. doi: 10.1177/1744806917728227
Zhang, H. Y., Gao, M., Shen, H., Bi, G. H., Yang, H. J., Liu, Q. R., et al. (2017). Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addict. Biol. 22, 752–765. doi: 10.1111/adb.12367
Zhang, L., Zhang, J.-T., Hang, L., and Liu, T. (2020). Mu opioid receptor heterodimers emerge as novel therapeutic targets: recent Progress and future perspective. Front. Pharmacol. 11:11. doi: 10.3389/fphar.2020.01078
Keywords: endocannabinoid system, respiratory system, opioids, cannabinoids, cannabinoid receptors
Citation: Wiese BM, Alvarez Reyes A, Vanderah TW and Largent-Milnes TM (2023) The endocannabinoid system and breathing. Front. Neurosci. 17:1126004. doi: 10.3389/fnins.2023.1126004
Received: 16 December 2022; Accepted: 16 March 2023;
Published: 18 April 2023.
Edited by:
Vanessa Costhek Abilio, Federal University of São Paulo, BrazilReviewed by:
David Cabañero, Miguel Hernández University of Elche, SpainCopyright © 2023 Wiese, Alvarez Reyes, Vanderah and Largent-Milnes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tally M. Largent-Milnes, dGxhcmdlbnRAZW1haWwuYXJpem9uYS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.