
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 06 February 2023
Sec. Neurodegeneration
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1098441
This article is part of the Research TopicAdvances in understanding the pathogenetic mechanisms of neurodevelopmental disorders and neurodegenerative disease - The environment as a putative risk factorView all 12 articles
 Azzurra Invernizzi1*†
Azzurra Invernizzi1*† Elza Rechtman1†
Elza Rechtman1† Kristie Oluyemi1,2,3,4
Kristie Oluyemi1,2,3,4 Stefano Renzetti5
Stefano Renzetti5 Paul Curtin1
Paul Curtin1 Elena Colicino1
Elena Colicino1 Claudia Ambrosi6
Claudia Ambrosi6 Lorella Mascaro7
Lorella Mascaro7 Alessandra Patrono5
Alessandra Patrono5 Daniele Corbo5
Daniele Corbo5 Giuseppa Cagna5
Giuseppa Cagna5 Roberto Gasparotti5
Roberto Gasparotti5 Abraham Reichenberg8
Abraham Reichenberg8 Cheuk Y. Tang9
Cheuk Y. Tang9 Donald R. Smith10
Donald R. Smith10 Donatella Placidi5
Donatella Placidi5 Roberto G. Lucchini5,11
Roberto G. Lucchini5,11 Robert O. Wright1
Robert O. Wright1 Megan K. Horton1
Megan K. Horton1Introduction: Adolescent exposure to neurotoxic metals adversely impacts cognitive, motor, and behavioral development. Few studies have addressed the underlying brain mechanisms of these metal–associated developmental outcomes. Furthermore, metal exposure occurs as a mixture, yet previous studies most often consider impacts of each metal individually. In this cross–sectional study, we investigated the relationship between exposure to neurotoxic metals and topological brain metrics in adolescents.
Methods: In 193 participants (53% females, ages: 15–25 years) enrolled in the Public Health Impact of Metals Exposure (PHIME) study, we measured concentrations of four metals (manganese, lead, copper, and chromium) in multiple biological media (blood, urine, hair, and saliva) and acquired resting–state functional magnetic resonance imaging scans. Using graph theory metrics, we computed global and local efficiency (global:GE; local:LE) in 111 brain areas (Harvard Oxford Atlas). We used weighted quantile sum (WQS) regression models to examine association between metal mixtures and each graph metric (GE or LE), adjusted for sex and age.
Results: We observed significant negative associations between the metal mixture and GE and LE [βGE = −0.076, 95% CI (−0.122, −0.031); βLE= −0.051, 95% CI (−0.095, −0.006)]. Lead and chromium measured in blood contributed most to this association for GE, while chromium measured in hair contributed the most for LE.
Discussion: Our results suggest that exposure to this metal mixture during adolescence reduces the efficiency of integrating information in brain networks at both local and global levels, informing potential neural mechanisms underlying the developmental toxicity of metals. Results further suggest these associations are due to combined joint effects to different metals, rather than to a single metal.
Exposure to neurotoxic metals and their impact on the brain is a growing worldwide health concern (Carmona et al., 2021). Metals such as lead and manganese have been shown to readily pass the blood-brain barrier and accumulate within various brain areas, where they exert neurotoxic effects (Balali-Mood et al., 2021; Carmona et al., 2021) and are associated with altered neurotransmission, disrupted synaptic structure (Sadiq et al., 2012; Karri et al., 2016; Carmona et al., 2021; Goel and Aschner, 2021) and accelerated neurodegeneration (Caito and Aschner, 2015; Karri et al., 2016; Lim et al., 2019; Twohig and Nielsen, 2019). These among others key features of the above mentioned metals contributed to define the brain as the target organ for exposure (Chandra et al., 1983; Caito and Aschner, 2015; Gilani et al., 2015; Chen et al., 2022). Growing research has identified adolescence as a critical window (Schalbetter et al., 2022) that is vulnerable to environmental exposure including metals (Rechtman et al., 2020). Few studies investigated the neural mechanisms of metal neurotoxicity throughout this extended window of vulnerability. Findings from these studies have linked metal exposure with alterations in regional brain volume (Claus et al., 2012, 2014; Horton et al., 2014, 2018; Levin-Schwartz et al., 2021; Heng et al., 2022; Migneron-Foisy et al., 2022), and brain metabolite concentrations (Trope et al., 2001; Meng et al., 2005; Thomason et al., 2019, 2021; Cecil, 2022) during this period. This heightened vulnerability may be due to rapid growth and differentiation of the brain throughout childhood. Neurotoxic exposures during this critical period can also disrupt behavioral, cognitive, and motor development (Claus et al., 2012, 2014; Horton et al., 2018; Rechtman et al., 2020; Levin-Schwartz et al., 2021; Heng et al., 2022). Despite the breadth of research on the developmental effects of childhood metal exposure, the underlying brain mechanisms behind these observed metal-associated outcomes are still not clear.
Over the past decade, increasing use of functional magnetic resonance imaging (fMRI) provides insight into the mechanisms linking metal exposure and alterations in brain functions (Horton et al., 2014). In particular, resting-state functional MRI - task-independent assessment of spontaneous fluctuations in blood oxygen level dependent (BOLD) signal from the brain at rest–has emerged as a novel tool in pediatric populations to investigate the intrinsic functional connectivity of the brain. Different from task-based fMRI which requires participants to engage or respond to stimuli (Canario et al., 2021), in rs-fMRI participants are instructed to simply lay still in the scanner with their eyes closed, while allowing their mind to roam freely (i.e., not focusing their thought on anything in particular). This facilitates research in younger populations, who may have difficulty completing complex tasks in the scanner (Canario et al., 2021). Results from rs-fMRI studies have shown a topological organization of the brain in a highly efficient manner with a high level of local clustering, together with long-distance connections (van den Heuvel et al., 2009). Graph theory analysis of rs-fMRI data characterizes the topological organization of the brain at rest (Wang et al., 2010) using metrics such as global and local efficiency, which quantify how efficient the brain is at integrating information at global and local levels, respectively (Wang et al., 2010). Global efficiency (GE) provides an indication of how efficiently the information is integrated and exchanged between the different regions of the brain (Stanley et al., 2015; Rakesh et al., 2020). In contrast, local efficiency (LE) measures the ability of the brain to perform functionally specialized and segregated processing within a network, requiring densely interconnected regions within modules (Stanley et al., 2015; Rakesh et al., 2020). Previous results have demonstrated the utility to characterize the topological network organization of the brain by using graph metrics based on rs-fMRI and link them with human behavior (Xu et al., 2015; Liu et al., 2022), cognition (van den Heuvel et al., 2009; Uehara et al., 2013), and diseases (Liu et al., 2008; Supekar et al., 2008). Recent studies have used rs-fMRI to demonstrate intrinsic functional connectivity patterns in a priori selected brain regions associated with early life exposure to individual metals (i.e., lead, manganese) (de Water et al., 2018, 2019; Thomason et al., 2019). Our data-driven graph theory approach builds on this foundational research by informing potential neural mechanisms underlying the developmental toxicity of metal mixture exposure during adolescence.
To investigate the impact of metal exposure on the brain, it is critical to consider not only single metal exposures but the mixture of co-occurring neurotoxic metals (Bauer et al., 2020). Historically, studies measure individual chemical concentrations in individual biological media (i.e., blood, urine, etc.). These exposure biomarkers are used as surrogates of total exposure from the environment. However, metals distribute unevenly among biological media that represent different aspects of each chemical’s toxicokinetics. Therefore, each medium provides complementary information on different biological processes. Recent studies have started to combine information from multiple biomarkers using statistical methods, like multi-media biomarker approaches, that resulted in an improved measure of the total body burden and thus improved exposure characterization (Levin-Schwartz et al., 2020, 2021; Bauer et al., 2021). Exposure, defined as metal mixtures, has been observed to more negatively impact neurodevelopment than exposure to a single metal component (Claus et al., 2012, 2014; Freire et al., 2018; Horton et al., 2018). Therefore, examining the effects of metal mixtures on brain function is crucial to better understand the real-world impact of metal exposure on cognition and behavior. In this study, we will use an integrated measure of metal mixtures across multiple media, called multi-media biomarker (MMB) (Levin-Schwartz et al., 2020), to analyze the impact on the brain of each metal across multiple media.
In this study, we investigate how metal exposure impacts brain network properties in adolescents. We use graph theory metrics to quantify how the brain integrates globally (GE) and locally segregates (LE) information and assess associations between these metrics with metal mixture exposure. To define our metal mixtures, we measured concentrations of four metals [lead (Pb), manganese (Mn), chromium (Cr), and copper (Cu)] in four biological media (blood, urine, hair, and saliva) from 193 adolescent participants living nearby ferro-manganese industry/alloy plant activity in northern Italy enrolled in the Public Health Impact of Metals Exposure (PHIME) study. Then, using weighted quantile sum (WQS) regression, a statistical method commonly used to assess the impact of chemical mixtures on various health outcomes (Tanner et al., 2019), we examined associations between the metal mixture and each graph metric (GE and LE), adjusting for sex and age. This paper contributes to further understanding the impacts of environmental exposures to a mixture of neurotoxic metals in developmental windows like adolescence.
The Public Health Impact of Metal Exposure (PHIME) cohort investigates associations between metal exposure from anthropogenic emissions and developmental health outcomes in adolescents and young adults living nearby the ferro-manganese industry in northern Italy. Details of the study have been described elsewhere (Lucchini et al., 2012a; Lucas et al., 2015). Inclusion criteria were: birth in the areas of interest; family residence in Brescia for at least two generations; residence in the study areas since birth. The exclusion criteria were: having a neurological, hepatic, metabolic, endocrine, or psychiatric disorder; using medications (in particular with neuro-psychological side effects); having clinically diagnosed motor deficits or cognitive impairment and having visual deficits that are not adequately corrected. Detailed description of this recruitment process and study design can be found in previous publications (Lucchini et al., 2012a,b). A convenience based sample of 202 participants (53% female, ages 15–25 years) were selected to participate in a multi-modal magnetic resonance imaging (MRI) study, PHIME-MRI. They completed multimodal MRI scans, neuropsychological tests, including measures of IQ [Kaufman Brief Intelligence Test, Second Edition (KBIT-2)] (Kaufman and Kaufman, 2014; Reynolds et al., 2014), memory and motor functions. All participants satisfied eligibility criteria for MRI scanning [i.e., metal implants or shrapnel, claustrophobia, no prior history of traumatic brain injury, body mass index (BMI) ≤40]. Mn, Pb, Cr, and Cu were measured in saliva, hair, blood and urine, for each PHIME-MRI participant. Complete exposure data (i.e., all metals in all media for a total of 16 components), MRI and covariates data were available for 193 participants included in this analysis. A total of 193 participants were included in this analysis, 9 were missing at least one biological marker (Supplementary Figure 1).
Written informed consent was obtained from parents, while participants provided written assent. Study procedures were approved by the Institutional Review Board of the University of California, Santa Cruz and the ethical committees of the University of Brescia, and the Icahn School of Medicine at Mount Sinai.
Biological samples including venous whole blood, spot urine, saliva and hair were collected from each subject upon enrollment, as described in detail in previous studies (Smith et al., 2007; Eastman et al., 2013; Lucas et al., 2015; Butler et al., 2019). Complete overview of biomarkers can be found in Supplementary Figure 1 and Table 1. Biological samples were processed and analyzed for metal concentrations using magnetic sector inductively coupled plasma mass spectroscopy (Thermo Element XR ICP-MS), as described elsewhere (Smith et al., 2007; Eastman et al., 2013; Lucas et al., 2015; Butler et al., 2019).
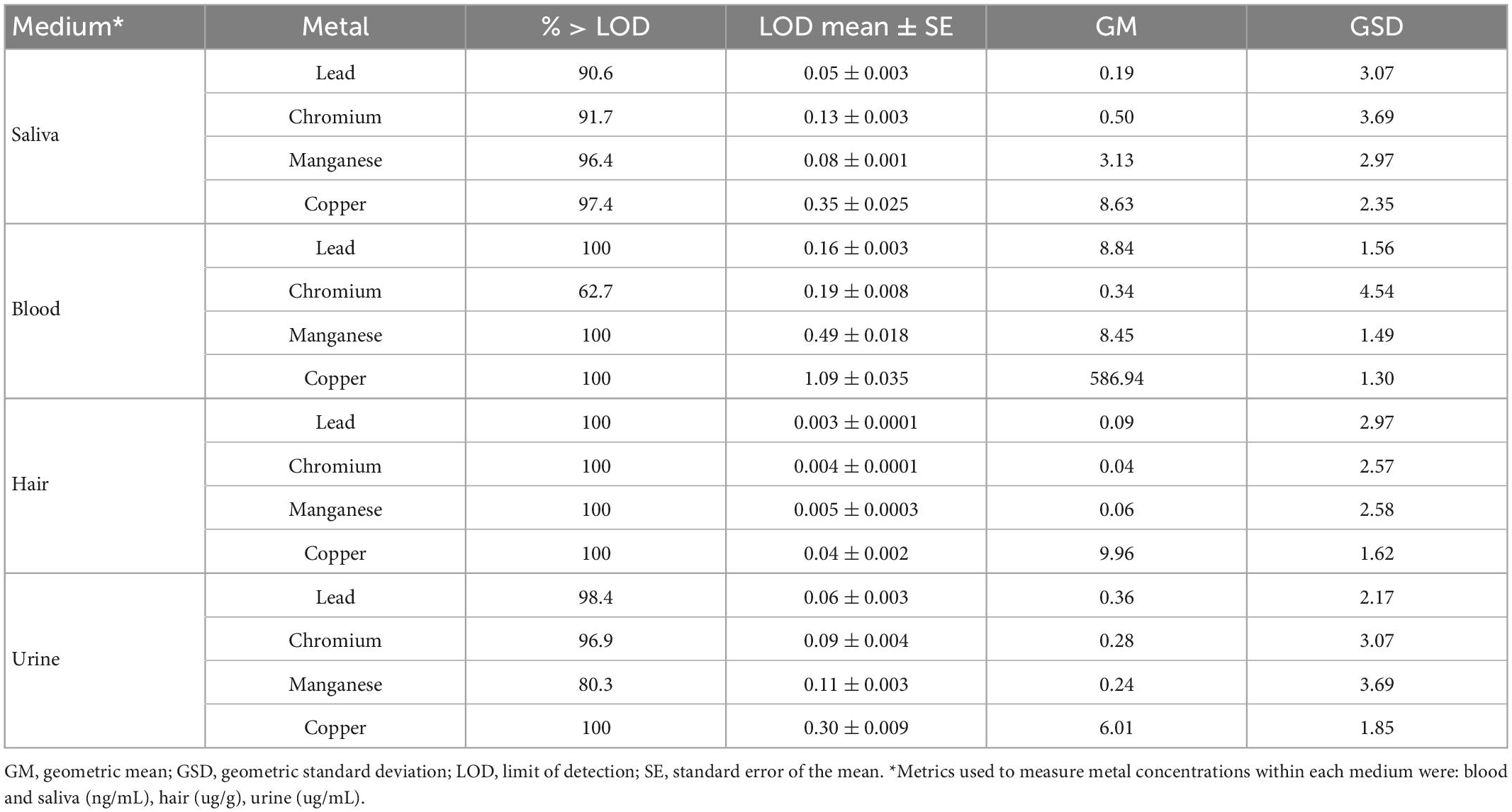
Table 1. Metal concentrations (Mn, Pb, Cr, and Cu) measured in blood, urine, hair, and saliva collected from 193 adolescents participants PHIME-MRI included in the current study.
Magnetic resonance imaging (MRI) and functional MRI (fMRI) data acquisition was performed on a high-resolution 3-Tesla SIEMENS Skyra scanner using a 64-channel phased array head and neck coil, at the Neuroimaging Division of ASST Spetali Civili Hospital of Brescia. For each participant, a high-resolution 3D T1-weighted structural scan was acquired using a MPRAGE sequence (TR = 2.4 ms, TE = 2.06 ms, TI = 230 ms, acquisition matrix = 256 × 256 and 224 sagittal slices with final voxel size = 0.9 mm3). Fifty contiguous oblique-axial sections were used to cover the whole brain where the first four images were discarded to allow the magnetization to reach equilibrium. For each subject, a single 10-min continuous functional sequence using a T2*weighted echo-planar imaging (EPI) sequence (TR = 1.0 ms, TE = 27 ms, 70 axial slices, 2.1 mm thickness, matrix size 108 × 108, covering the brain from vertex to cerebellum) was acquired. During resting-state scans, lights of the MRI room were off and participants were instructed to stay awake, relax and daydream (not think about anything) with their eyes open. They were presented with an image of a night skyline figure projected on a MRI compatible monitor. Padding was used for comfort and reduction of head motion. Earplugs were used to reduce noise. Data were read by a board-certified radiologist to determine quality and possible incidental findings–no findings were reported.
Image pre-processing, global and local efficiency calculations, and statistical analyses were performed using SPM12 (Wellcome Department of Imaging Neuroscience, London, UK), Brain Connectivity toolbox (Rubinov et al., 2009; Rubinov and Sporns, 2010) and customized scripts, implemented in MatLab 2016b (The Mathworks Inc., Natick, MA, USA) and R (v3.4).
For each subject, the structural magnetic resonance image was co-registered and normalized against the Montreal Neurological Institute (MNI) template and segmented to obtain white matter (WM), gray matter (GM) and cerebrospinal fluid (CSF) probability maps in the MNI space. FMRI data were spatially realigned, co-registered to the MNI-152 EPI template and subsequently normalized utilizing the segmentation option for EPI images in SPM12. All normalized data were denoised using ICA-AROMA (Pruim et al., 2015). Additionally, spatial smoothing was applied (8 millimeters) to the fMRI data. As further quality check of fMRI data, large head motion in any direction or rotation (>3 mm or 3°) was used as exclusion criteria in our study–no participants were excluded in this study. No global signal regression was applied.
Based on the Harvard-Oxford (Desikan et al., 2006) atlas, 111 regions of interest (ROI; 48 left and 48 right cortical areas; 7 left and 7 right subcortical regions and 1 brainstem) were defined. In this atlas, the brain areas were defined using T1-weighted images of 21 healthy male and 16 healthy female subjects (ages 18–50). The T1-weighted images were segmented and affine-registered to MNI152 space using FLIRT (FSL), and the transforms then applied to the individual brain areas’ label. Finally, these were combined across subjects to form population probability maps for each ROI (Desikan et al., 2006). For each ROI, a time-series was extracted by averaging across voxels per time point. To facilitate statistical inference, data were “pre-whitened” by removing the estimated autocorrelation structure in a two-step generalized linear model (GLM) procedure (Monti, 2011; Bright and Murphy, 2015). In the first step, the raw data were filtered against the 6 motion parameters (three translations and three rotations). Using the resulting residuals, the autocorrelation structures present in the data were estimated using an Auto-Regressive model of order 1 [AR (1)] and then removed from the raw data. Next, the realignment parameters, white matter (WM) and cerebrospinal fluid (CSF) signals were removed as confounders on the whitened data.
Global and Local Efficiency (GE and LE) were computed using the Brain Connectivity toolbox (Rubinov et al., 2009; Rubinov and Sporns, 2010) on the defined ROI time course data per subject. GE and LE build on the concept of efficient integration of communication in a network at local (LE) and whole (GE) level. Based on the average inverse shortest path length in the brain or network, GE is defined as the inverse of the average characteristic path length between all nodes in the networks (Latora and Marchiori, 2001; Bullmore and Sporns, 2012). For each individual node defined as ROI, the shortest number of steps required to go from one node to another was computed. Then, the average number of shortest steps to all defined nodes was computed separately for each node. To correct for the total number of connections between nodes, the inverse of the average number of shortest steps for each node was summed across all network nodes and normalized. LE is computed on the neighborhood of each single ROI/node (Rubinov et al., 2009; Rubinov and Sporns, 2010) and defined as the inverse of the shortest average path length of all neighbors of nodes among themselves (Latora and Marchiori, 2001). First we identified a set of nodes which are directly connected with a given node, then we removed that node from the identified subgraph and calculated the averaged shortest path between all remaining nodes. GE and LE are scaled measures ranging from 0 to 1, with a value of 1 indicating maximum GE/LE in the brain.
Visual inspection and descriptive statistics (geometric mean, geometric standard deviation and Pearson’s correlation) were used to characterize the metal concentrations in different media. All descriptive statistical analyses were performed using R version 4.2.1.
To examine associations between our multi-media metal mixture (four metals, four media) and graph theory outcomes (GE and LE), we used a WQS-based multi-media biomarker (MMB) approach (Lee et al., 2019; Levin-Schwartz et al., 2021). Figure 1 shows the complete flowchart of the analysis performed. Briefly, WQS is a data driven, mixture-based ensemble modeling strategy that tests for associations between the combined effect of multiple, correlated variables and an outcome of interest. The WQS MMB approach builds on WQS, by incorporating exposure information across different biological media, providing an integrated estimate of total bodily exposure to a given chemical as well as identifying the chemical-matrix specific combination that contributes most to the overall association with the graph theory based outcomes (GE and LE) (Levin-Schwartz et al., 2021). The MMB WQS is hierarchical with two levels. The first level estimates a weighted index across all biological media for a single metal and the outcome (i.e., Pb MMB = blood Pb, urine Pb, saliva Pb, hair Pb). Our model estimated across 50 bootstrap samples, and 100 repeated holdouts (Tanner et al., 2019) for each individual MMB. By using the repeated holdouts WQS (Tanner et al., 2019), the data are randomly partitioned 100 times to produce a distribution of validated results where the mean is taken as the final estimate. The directionality of the association of the WQS index was constrained to be negative. Note that the WQS assumptions of linearity and directional homogeneity were validated through visual inspection of residuals (Levin-Schwartz et al., 2021). The second level estimates a weighted index across the different metals (i.e., Pb MMB, Mn MMB, Cr MMB, Cu MMB; (Levin-Schwartz et al., 2019, 2021)). First level MMBs are included in the WQS regression model predicting the association between the metal biomarker “mixture” and GE or LE. A significance test for the WQS index provided an estimate of the association with the metal mixture, while the weights associated with each metal MMB provided an indicator of each individual metal contribution to the overall effect. All weights are constrained to sum to one, enabling sorting by relative importance. Metals that impact the outcome have larger weights; factors with little or no impact on the outcome have near-zero weights. These models were adjusted for age and sex, and prior to model estimation, all exposures were grouped into deciles.
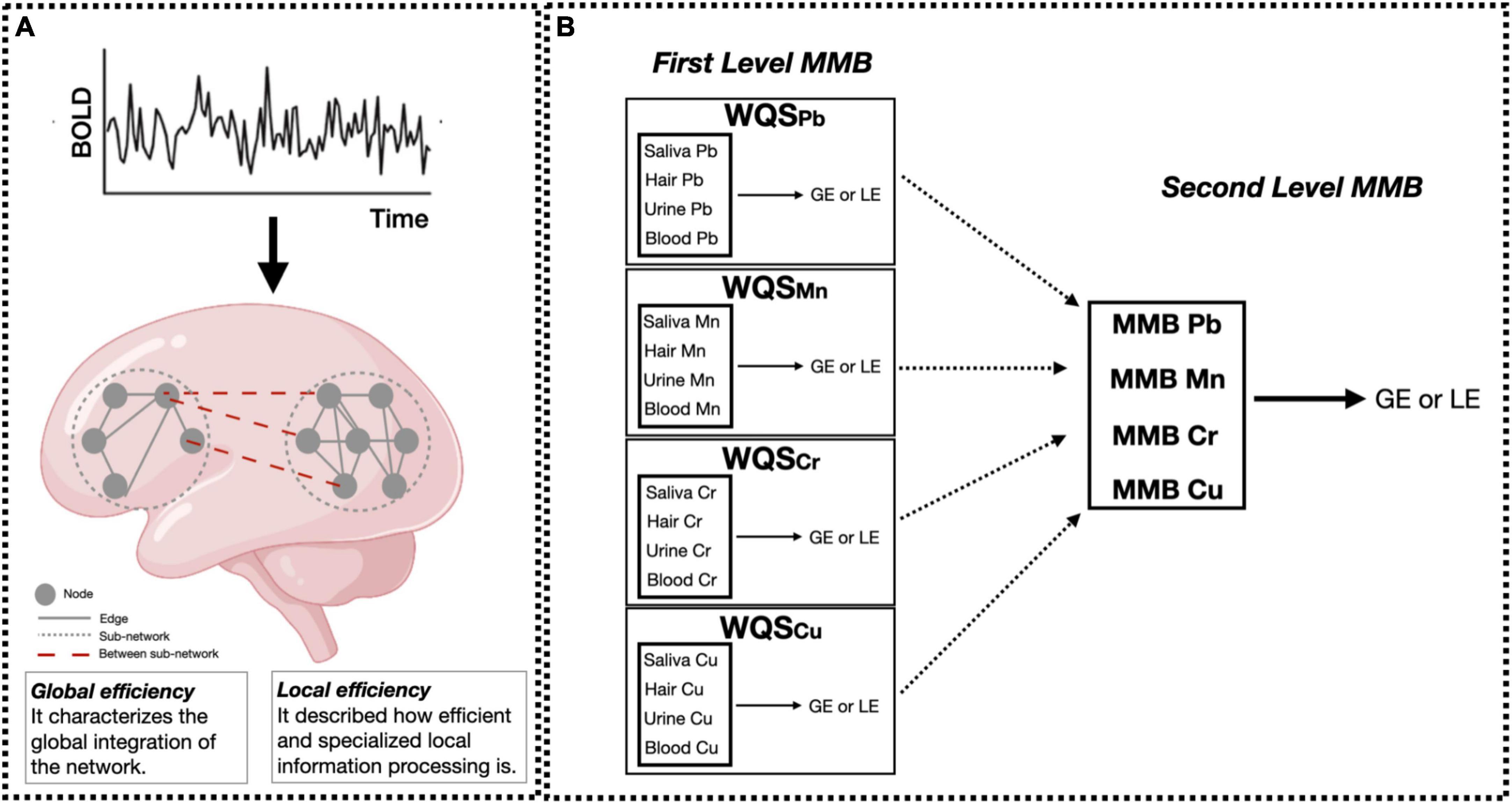
Figure 1. Data analysis flowchart. (A) Resting-state fMRI data were preprocessed and the averaged time-series were extracted using the Harvard-Oxford atlas. Then, global and local efficiency (GE and LE, respectively) metrics were computed for each participant using graph theory. Small solid gray circles represent nodes of the graphs (brain regions), while gray connecting lines are the edges of the graph (functional connections). Larger dotted circles represent segregated sub-graphs/networks (functional network characterized by highly connected brain areas), while dashed red lines are the within-network connections at the whole brain level. Panel (B) shows the two hierarchical levels of analysis performed using the MMB WQS approach to measure the effect of Pb, Mn, Cr, Cu on brain metrics (GE and LE). At the first level, WQS was performed to measure and derive the MMB metric for each metal individually (Pb, Mn, Cr, Cu) on brain metrics (GE and LE). Then, the joint effect of Pb, Mn, Cr, Cu on brain metrics was assessed by applying WQS to Pb, Mn, Cr, and Cu MMBs. All models were adjusted for sex and age. Figure adapted from Levin-Schwartz et al. (2019) and Rakesh et al. (2020).
This study included 193 participants (53% female) living in Northern Italy, with an average age of 19.2 years (range = 15–25). Metal concentrations in the different media are reported in Table 1 while Pearson’s correlations between them is reported in Supplementary Figure 3.
We first examined the association between each individual metal in all media with GE and LE (Figure 2). For all metals, urinary metal concentrations contributed most to the association between the first level MMB (i.e., individual metal in each matrix) and GE. Urinary Pb contributed 46% of the association between Pb exposure and GE. Urinary Mn, Cr and Cu contributed 51, 34, and 68%, respectively to the association with GE. For LE, the most heavily weighted metal-matrix combination differed by metal; blood Pb concentration contributed most to the association with LE (34%). Hair Mn and hair Cr contributed 31 and 43% to the association with LE. Urine Cu contributed the most to the Cu-LE association. Beta coefficients and 95% confidence intervals obtained for each individual MMB WQS model are reported in Figure 2 and Supplementary Figure 2.
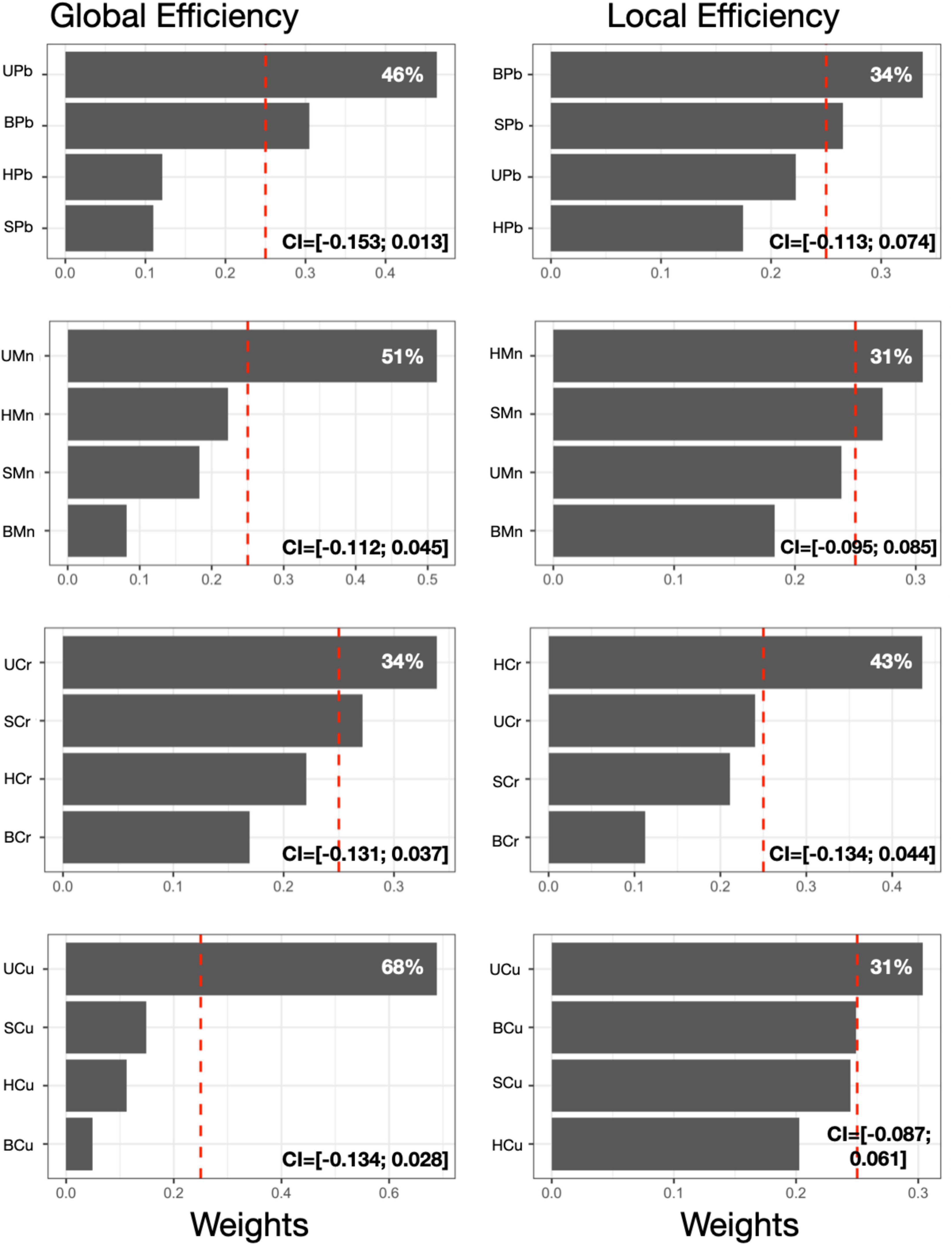
Figure 2. First level MMB models. Results obtained from the MMB WQS association between each metal’s respective exposure biomarker (e.g., blood Pb) and GE or LE was estimated among 193 adolescents included in the current study. Bar plots show estimated weights for each component of the mixture in the WQS regressions. Red dotted lines represent the significant thresholds for each WQS model. 95% confidence intervals obtained for each individual MMB WQS model are reported. All models were adjusted for sex and age. Components abbreviations: the first letter represents the medium (S, saliva; B, blood; U, urine; H, hair) and the second and third letters represent the metals (Mn, manganese; Pb, lead; Cr, chromium; Cu, copper).
Results from second level repeated holdout WQS analyses revealed associations between the overall metal mixture and GE and LE (Figure 3A), and the contribution of each metal MMB to these associations (Figure 3). We observed significant negative associations between the combined metal mixture MMB and both GE [βGE = −0.076, 95% CI (−0.122, −0.031); Figure 3A], and LE [βLE = −0.048, 95% CI (−0.095, −0.006); Figure 3A]. We observed that Cr and Pb contributed most to the association between the combined metal mixture and GE (29%; Figure 3B), whereas Cr contributed most to the association with LE (38%; Figure 3C).

Figure 3. Second level MMBs approach. Beta coefficients (A) and weights (B,C) obtained from the WQS association between each MMB metal and GE or LE was estimated among 193 adolescents included in the current study. Panel a reports the beta coefficients for GE (orange) and LE (blue), respectively. In panels (B,C), data points indicate weights for each of the 100 holdouts; box plots show the 25, 50, and 75th percentiles while the whiskers show the 10 and 90th percentiles of weights for the 100 holdouts. Diamonds show the mean weights for the 100 holdouts. Dotted lines indicate the thresholds. Mn, manganese; Pb, lead; Cr, chromium; Cu, copper.
This is the first study to use rs-fMRI to investigate global and local connectivity in adolescents exposed to a neurotoxic metal mixture. Using graph-theory based network metrics and a multimedia biomarker (MMB) approach, we observed a significant negative association between exposure to a mixture of five neurotoxic metals (lead, manganese, copper, chromium, and zinc) and global efficiency (GE), with lead and chromium contributing most to this association. Significant negative associations between the metal mixture and both GE and LE were found [βGE = −0.076, 95% CI (−0.122, −0.031); βLE = −0.048, 95% CI (−0.095, −0.006)]. We also observed that urinary lead and chromium contributed most to the association with GE (29 and 24%, respectively); while hair chromium contributed most to the association with LE (38%). Overall, our results substantiate previous findings of associations between metal exposure and altered brain connectivity, and further suggest that environmental exposure to a mixture of neurotoxic metals during adolescence reduces the brain ability to efficiently integrate and segregate information, highlighting the need to further study the impacts of environmental exposures in developmental windows like adolescence (Golub, 2000; Spear, 2007; Rechtman et al., 2020). Furthermore, our results suggest these associations are due to the combined joint effects of multiple metals, rather than to a single metal, emphasizing the importance of analyzing metal mixtures to better understand the real-world impact of metal exposure on brain health.
Our findings show that urinary lead and chromium were the top contributing metals in the association between the metal mixture and GE, and hair chromium contributed most to the association with LE, suggesting that urine and hair may be critical biomarkers for estimating the effects of metal mixtures on brain connectivity and further, these metals may exert a greater influence on global and/or local functional connectivity across/within topological brain networks. Lead exposure is known for causing the disruption of neuronal activity, in particular to alter the release and storage of neurotransmitters from the presynaptic nerve endings, that may change the developmental processes of synapse formation in children and young adults and results in altered brain functions (Bressler and Goldstein, 1991). Previous human neuroimaging studies have observed associations between lead exposure and altered structural connectivity and functional activation patterns in both children and adults (Thomason et al., 2019, 2021; Cecil, 2022). In particular, Thomason et al. (2019) found prenatal lead (Pb) exposure was associated with altered age-related intrinsic functional connectivity patterns in developing fetuses. Furthermore, previous studies in animal models have found lead exposure to disrupt multiple neurotransmitter systems (Goel and Aschner, 2021) (e.g., glutamatergic, dopaminergic, cholinergic), as well as neurotransmitter and synaptic function in various areas of the brain, including the hippocampus (Sadiq et al., 2012; Carmona et al., 2021), and prefrontal cortex (Mansouri et al., 2013). Therefore, our finding of lead being a top contributor to the negative association between the metal mixture and global efficiency, could in part be explained by its impact on structural connectivity (e.g., white matter integrity) and synaptic function and neurotransmission within/across the brain.
The underlying mechanism for neurotoxicity of chromium is still not fully understood (Xu et al., 2021). Increased oxidative stress, chromosome disruptions and DNA-adduct formation are some of the many cellular damages found to be caused by high level exposure to Cr in the brain (Wise et al., 2022). While there are no neuroimaging studies investigating the impact of chromium exposure to date, previous studies in humans have observed evidence linking chromium exposure to neurological and psychiatric conditions, including olfactory dysfunction, autism spectrum disorder, and acute schizophrenia (Watanabe and Fukuchi, 1981; Kitamura et al., 2003; Saghazadeh et al., 2020; Wise et al., 2022). These findings suggest an impact of chromium exposure on underlying neurobiological function. Furthermore, previous studies across various animal models have observed brain-wide neurodegeneration following chromium exposure, again suggesting an impact of chromium exposure on neurobiological function via its neurodegenerative effects (Soudani et al., 2012; Hao et al., 2017; Wise et al., 2022). Therefore, our finding of chromium being a top contributor to the negative association between the metal mixture and both local and global efficiency is consistent with these prior studies suggesting its widespread neurodegenerative effects, which could potentially contribute to changes in functional connectivity across brain networks.
Several studies have also detailed the potential synergistic neurotoxic effects of certain metals upon co-exposure, based on their unique chemical properties and similar neurobiological mechanisms of action (de Andrade et al., 2021). Metals within our mixture that have been shown to produce such synergistic neurotoxic effects include lead and manganese (Tao et al., 1999; Chen et al., 2016; Lu et al., 2018), whose co-exposure has been observed to increase disruptions to neurodevelopment in both animal (Chandra et al., 1981, 1983; Shukla and Chandra, 1987; Levin-Schwartz et al., 2021) and human studies (Kim et al., 2009; Claus et al., 2012; Lin et al., 2013; Levin-Schwartz et al., 2021). de Water et al. (2018) found that early postnatal manganese (Mn) concentrations were associated with altered intrinsic functional connectivity within cognitive control and motor brain areas of adolescents. Additionally, in another study, de Water et al. (2019) found prenatal Mn concentrations were associated with reduced intrinsic functional connectivity of brain areas involved in emotion processing and regulation in children. Furthermore, co-exposures of certain metals have been reported to potentially increase accumulation, retention and distribution of individual metal components in animal models (Chen et al., 2016). In particular, manganese has been shown to increase accumulation of various metals in the brain, notably lead (Chandra et al., 1983; Chen et al., 2016), and copper (Mercadante et al., 2016). Therefore, while lead and chromium were found to contribute most to the association between the metal mixture and GE, and chromium contributed most to the association with LE, the higher influence of these metals may be due to synergistic interactions with other metals in the mixture (e.g., manganese). This possibility highlights the importance of analyzing metal mixtures rather than single metals in environmental epidemiological studies, as the influence of a single metal exposure may be affected by other metals an individual is exposed to. Further, our findings show that urine contributed most to the association between both lead, chromium and GE, and hair contributed most to the association between chromium and LE, suggesting that urine and hair may be critical for estimating the effects of metal mixtures on brain connectivity. Previous studies that analyzed one metal at the time, have indicated blood as the most reliable biomarker to assess lead exposure (Levin-Schwartz et al., 2021), and blood has also been used previously as an exposure biomarker for other metals such as chromium (Alexopoulos et al., 2008; Wise et al., 2022) and manganese (Levin-Schwartz et al., 2021). By using novel techniques like MMB WQS, we can increase the accuracy in measuring mixture effects compared to individual biomarkers and provide a data-driven biomarker selection (Levin-Schwartz et al., 2019, 2020). Finally, as previous neuroimaging studies have mainly examined associations between brain function and a single metal exposure, future studies should aim to utilize metal mixtures to better account for these potential synergistic effects due to metal co-exposure, which would ultimately help better understand the real-world impact of metal exposure on the brain.
In this study, while we found robust associations between metals and GE and LE metrics, our small sample size resulted in relatively small effect sizes (Figure 3). While it would be beneficial to repeat our analysis in a larger dataset, to our knowledge no such dataset with multi-media biomarkers and fMRI data exists. Further, we assumed that all metals have a linear association with both global and local efficiency metrics. Our MMB WQS approach does not assume linear associations between outcomes but only considers additive effects. Future studies should investigate non-linear associations between outcomes and possible multiplicative effects. Finally, MMB WQS might suffer from overfitting issues, since two WQS models are performed on the same set of data. To compensate for this, we split our data into training and testing datasets in both MMB WQS analysis levels.
Using a multimedia biomarker (MMB) approach, we were able to estimate the associations between a complex metal mixture and brain metrics. This method allows us to leverage the complementary information provided by each medium on different biological processes and therefore, to improve the exposure characterization. Our findings that urine contributed most to the associations between both lead and chromium and GE, and hair contributed most to the associations between chromium and LE, suggests that urine and hair may be critical overlooked biomarkers for estimating the effects of metal mixtures on brain connectivity. Given our results, we suggest that future neuroimaging studies on metal mixture exposure aim to collect multiple media, including urine and hair specimens, to explore the effects of metal mixtures on the brain. Altogether, our research supports the notion of adolescence being a timepoint of vulnerability to environmental exposures. More specifically, our results suggest that the adolescent brain connectivity is vulnerable to metal mixture exposures during this period. Given that adolescence is a period of rapid brain development, our results suggest that metal exposure may have the potential to alter neurodevelopment via changes to global and local connectivity. These connectivity changes may potentially lead to alterations in cognition and neurobehavior in adolescence. Therefore, future environmental neuroimaging studies should focus on adolescents to further characterize how metal mixture exposure during this period can lead to potential alterations in brain development (e.g., brain volume, functional connectivity), and ultimately neurobehavior and cognition.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the University of California, Santa Cruz and the Ethical Committees of the University of Brescia, and the Icahn School of Medicine at Mount Sinai. Written informed consent to participate in this study was provided by the participants or their legal guardian/next of kin.
All authors listed in this work have made a substantial contribution to the concept or design of the article, the acquisition, analysis, or interpretation of data for the article, drafted the article or revised it critically for important intellectual content, approved the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The authors would like to acknowledge support from the National Institutes of Environmental Health Sciences (NIEHS) grant numbers R01 ES019222, R01 ES013744, and P30ES023515, and the European Union through its Sixth Framework Programme for RTD (contract number FOOD-CT-2006-016253).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1098441/full#supplementary-material
Alexopoulos, E., Cominos, X., Trougakos, I., Lourda, M., Gonos, E., and Makropoulos, V. (2008). Biological monitoring of hexavalent chromium and serum levels of the senescence biomarker apolipoprotein j/clusterin in welders. Bioinorg. Chem. Appl. 2008:420578. doi: 10.1155/2008/420578
Balali-Mood, M., Naseri, K., Tahergorabi, Z., Khazdair, M., and Sadeghi, M. (2021). Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 12:643972. doi: 10.3389/fphar.2021.643972
Bauer, J., Devick, K., Bobb, J., Coull, B., Bellinger, D., Benedetti, C., et al. (2020). Associations of a metal mixture measured in multiple biomarkers with IQ: Evidence from italian adolescents living near ferroalloy industry. Environ. Health Perspect. 128:097002. doi: 10.1289/EHP6803
Bauer, J., White, R., Coull, B., Austin, C., Oppini, M., Zoni, S., et al. (2021). Critical windows of susceptibility in the association between manganese and neurocognition in Italian adolescents living near ferro-manganese industry. Neurotoxicology 87, 51–61. doi: 10.1016/j.neuro.2021.08.014
Bressler, J., and Goldstein, G. (1991). Mechanisms of lead neurotoxicity. Biochem. Pharmacol. 41, 479–484. doi: 10.1016/0006-2952(91)90617-e
Bright, M., and Murphy, K. (2015). Is fMRI “noise” really noise? Resting state nuisance regressors remove variance with network structure. Neuroimage 114, 158–169. doi: 10.1016/j.neuroimage.2015.03.070
Bullmore, E., and Sporns, O. (2012). The economy of brain network organization. Nat. Rev. Neurosci. 13, 336–349.
Butler, L., Gennings, C., Peli, M., Borgese, L., Placidi, D., Zimmerman, N., et al. (2019). Assessing the contributions of metals in environmental media to exposure biomarkers in a region of ferroalloy industry. J. Exp. Sci. Environ. Epidemiol. 29, 674–687. doi: 10.1038/s41370-018-0081-6
Caito, S., and Aschner, M. (2015). Neurotoxicity of metals. Handb. Clin. Neurol. 131, 169–189. doi: 10.1016/B978-0-444-62627-1.00011-1
Canario, E., Chen, D., and Biswal, B. A. (2021). review of resting-state fMRI and its use to examine psychiatric disorders. Psychoradiology 1, 42–53.
Carmona, A., Roudeau, S., and Ortega, R. (2021). Molecular mechanisms of environmental metal neurotoxicity: A focus on the interactions of metals with synapse structure and function. Toxics 9:198. doi: 10.3390/toxics9090198
Cecil, K. (2022). Pediatric exposures to neurotoxicants: A Review of magnetic resonance imaging and spectroscopy findings. Diagnostics 12:641.
Chandra, A., Ali, M., Saxena, D., and Murthy, R. (1981). Behavioral and neurochemical changes in rats simultaneously exposed to manganese and lead. Arch. Toxicol. 49, 49–56. doi: 10.1007/BF00352071
Chandra, S., Murthy, R., Saxena, D., and Lal, B. (1983). Effects of pre- and postnatal combined exposure to Pb and Mn on brain development in rats. Ind. Health 21, 273–279. doi: 10.2486/indhealth.21.273
Chen, M., Zhang, Y., Adams, T., Ji, D., Jiang, W., Wain, L. V., et al. (2022). Integrative analyses for the identification of idiopathic pulmonary fibrosis-associated genes and shared loci with other diseases. Thorax thoraxjnl–2021–217703. doi: 10.1136/thorax-2021-217703 [Epub ahead of print].
Chen, P., Miah, M., and Aschner, M. (2016). Metals and neurodegeneration. F1000Res. 5:F1000FacultyRev–36. doi: 10.12688/f1000research.7431.1
Claus, H., Coull, B., and Wright, R. (2014). Chemical mixtures and children’s health. Curr. Opin. Pediatr. 26, 223–229. doi: 10.1097/MOP.0000000000000067
Claus, H., Schnaas, L., Ettinger, A., Schwartz, J., Lamadrid-Figueroa, H., Hernández-Avila, M., et al. (2012). Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ. Health Perspect. 120, 126–131. doi: 10.1289/ehp.1003300
de Andrade, V., dos Santos, A., and Aschner, M. (2021). NEUROTOXICITY OF METAL MIXTURES. Adv. Neurotoxicol. 5, 329–364.
de Water, E., Papazaharias, D., Ambrosi, C., Mascaro, L., Iannilli, E., Gasparotti, R., et al. (2019). Early-life dentine manganese concentrations and intrinsic functional brain connectivity in adolescents: A pilot study. PLoS One 14:e0220790. doi: 10.1371/journal.pone.0220790
de Water, E., Proal, E., Wang, V., Medina, S., Schnaas, L., Téllez-Rojo, M., et al. (2018). Prenatal manganese exposure and intrinsic functional connectivity of emotional brain areas in children. Neurotoxicology 64, 85–93. doi: 10.1016/j.neuro.2017.06.006
Desikan, R., Ségonne, F., Fischl, B., Quinn, B., Dickerson, B., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Eastman, R., Jursa, T., Benedetti, C., Lucchini, R., and Smith, D. (2013). Hair as a biomarker of environmental manganese exposure. Environ. Sci. Technol. 47, 1629–1637. doi: 10.1021/es3035297
Freire, C., Amaya, E., Gil, F., Fernández, M., Murcia, M., Llop, S., et al. (2018). Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: The environment and childhood (INMA) project. Sci. Total Environ. 621, 340–351. doi: 10.1016/j.scitotenv.2017.11.273
Gilani, S., Zaidi, S., Batool, M., Bhatti, A., Durrani, A., and Mahmood, Z. (2015). Report: Central nervous system (CNS) toxicity caused by metal poisoning: Brain as a target organ. Pak. J. Pharm Sci. 28, 1417–1423.
Goel, A., and Aschner, M. (2021). The effect of lead exposure on autism development. Int. J. Mol. Sci. 22:1637. doi: 10.3390/ijms22041637
Hao, P., Zhu, Y., Wang, S., Wan, H., Chen, P., Wang, Y., et al. (2017). Selenium administration alleviates toxicity of chromium(VI) in the chicken brain. Biol. Trace Elem. Res. 178, 127–135. doi: 10.1007/s12011-016-0915-9
Heng, Y., Asad, I., Coleman, B., Menard, L., Benki-Nugent, S., Were, F., et al. (2022). Heavy metals and neurodevelopment of children in low and middle-income countries: A systematic review. PLoS One 17:e0265536. doi: 10.1371/journal.pone.0265536
Horton, M., Hsu, L., Claus, H., Margolis, A., Austin, C., Svensson, K., et al. (2018). Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environ. Int. 121(Pt 1), 148–158. doi: 10.1016/j.envint.2018.08.045
Horton, M., Margolis, A., Tang, C., and Wright, R. (2014). Neuroimaging is a novel tool to understand the impact of environmental chemicals on neurodevelopment. Curr. Opin. Pediatr. 26, 230–236. doi: 10.1097/MOP.0000000000000074
Karri, V., Schuhmacher, M., and Kumar, V. (2016). Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 48, 203–213. doi: 10.1016/j.etap.2016.09.016
Kaufman, A., and Kaufman, N. (2014). “Kaufman brief intelligence test, Second Edition,” in Encyclopedia of special education, (Hoboken, NJ: Wiley). doi: 10.1002/9781118660584.ese1325
Kim, Y., Kim, B., Hong, Y., Shin, M., Yoo, H., Kim, J., et al. (2009). Co-exposure to environmental lead and manganese affects the intelligence of school-aged children. Neurotoxicology 30, 564–571. doi: 10.1016/j.neuro.2009.03.012
Kitamura, F., Yokoyama, K., Araki, S., Nishikitani, M., Choi, J., Yum, Y., et al. (2003). Increase of olfactory threshold in plating factory workers exposed to chromium in Korea. Ind. Health 41, 279–285. doi: 10.2486/indhealth.41.279
Latora, V., and Marchiori, M. (2001). Efficient behavior of small-world networks. Phys. Rev. Lett. 87:198701. doi: 10.1103/PhysRevLett.87.198701
Lee, M., Rahbar, M., Samms-Vaughan, M., Bressler, J., Bach, M., Hessabi, M., et al. (2019). A generalized weighted quantile sum approach for analyzing correlated data in the presence of interactions. Biom J. 61, 934–954. doi: 10.1002/bimj.201800259
Levin-Schwartz, Y., Gennings, C., Claus, H., Coull, B., Placidi, D., Lucchini, R., et al. (2020). Multi-media biomarkers: Integrating information to improve lead exposure assessment. Environ. Res. 183:109148. doi: 10.1016/j.envres.2020.109148
Levin-Schwartz, Y., Gennings, C., Schnaas, L., Del Carmen Hernández Chávez, M., Bellinger, D., Téllez-Rojo, M., et al. (2019). Time-varying associations between prenatal metal mixtures and rapid visual processing in children. Environ. Health 18:92. doi: 10.1186/s12940-019-0526-y
Levin-Schwartz, Y., Henn, B., Gennings, C., Coull, B., Placidi, D., Horton, M., et al. (2021). Integrated measures of lead and manganese exposure improve estimation of their joint effects on cognition in Italian school-age children. Environ. Int. 146:106312. doi: 10.1016/j.envint.2020.106312
Lim, E., Aarsland, D., Ffytche, D., Taddei, R., van Wamelen, D., Wan, Y., et al. (2019). Amyloid-β and Parkinson’s disease. J. Neurol. 266, 2605–2619. doi: 10.1007/s00415-018-9100-8
Lin, C., Chen, Y., Su, F., Lin, C., Liao, H., Hwang, Y., et al. (2013). In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ. Res. 123, 52–57. doi: 10.1016/j.envres.2013.03.003
Liu, M., Amey, R., Backer, R., Simon, J., and Forbes, C. (2022). Behavioral Studies using large-scale brain networks – methods and validations. Front. Hum. Neurosci. 16:87520. doi: 10.3389/fnhum.2022.875201
Liu, Y., Liang, M., Zhou, Y., He, Y., Hao, Y., Song, M., et al. (2008). Disrupted small-world networks in schizophrenia. Brain 131(Pt 4), 945–961. doi: 10.1093/brain/awn018
Lu, C., Svoboda, K., Lenz, K., Pattison, C., and Ma, H. (2018). Toxicity interactions between manganese (Mn) and lead (Pb) or cadmium (Cd) in a model organism the nematode C. elegans. Environ. Sci. Pollut. Res. Int. 25, 15378–15389. doi: 10.1007/s11356-018-1752-5
Lucas, E., Bertrand, P., Guazzetti, S., Donna, F., Peli, M., Jursa, T., et al. (2015). Impact of ferromanganese alloy plants on household dust manganese levels: Implications for childhood exposure. Environ. Res. 138, 279–290. doi: 10.1016/j.envres.2015.01.019
Lucchini, R., Guazzetti, S., Zoni, S., Donna, F., Peter, S., Zacco, A., et al. (2012a). Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology 33, 687–696. doi: 10.1016/j.neuro.2012.01.005
Lucchini, R., Zoni, S., Guazzetti, S., Bontempi, E., Micheletti, S., Broberg, K., et al. (2012b). Inverse association of intellectual function with very low blood lead but not with manganese exposure in Italian adolescents. Environ. Res. 118, 65–71. doi: 10.1016/j.envres.2012.08.003
Mansouri, M., Naghizadeh, B., López-Larrubia, P., and Cauli, O. (2013). Behavioral deficits induced by lead exposure are accompanied by serotonergic and cholinergic alterations in the prefrontal cortex. Neurochem. Int. 62, 232–239. doi: 10.1016/j.neuint.2012.12.009
Meng, X., Zhu, D., Ruan, D., She, J., and Luo, L. (2005). Effects of chronic lead exposure on 1H MRS of hippocampus and frontal lobes in children. Neurology 64, 1644–1647. doi: 10.1212/01.WNL.0000160391.58004.D4
Mercadante, C., Herrera, C., Pettiglio, M., Foster, M., Johnson, L., Dorman, D., et al. (2016). The effect of high dose oral manganese exposure on copper, iron and zinc levels in rats. Biometals 29:417. doi: 10.1007/s10534-016-9924-6
Migneron-Foisy, V., Muckle, G., Jacobson, J., Ayotte, P., Jacobson, S., and Saint-Amour, D. (2022). Impact of chronic exposure to legacy environmental contaminants on the corpus callosum microstructure: A diffusion MRI study of Inuit adolescents. Neurotoxicology 92, 200–211. doi: 10.1016/j.neuro.2022.08.010
Monti, M. (2011). Statistical analysis of fMRI time-series: A critical review of the GLM approach. Front. Hum. Neurosci. 5:28. doi: 10.3389/fnhum.2011.00028
Pruim, R., Mennes, M., Buitelaar, J., and Beckmann, C. (2015). Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage 112, 278–287. doi: 10.1016/j.neuroimage.2015.02.063
Rakesh, D., Fernando, K., and Mansour, L. (2020). Functional dedifferentiation of the brain during healthy aging. J. Neurophysiol. 123, 1279–1282. doi: 10.1152/jn.00039.2020
Rechtman, E., Curtin, P., Papazaharias, D., Renzetti, S., Cagna, G., Peli, M., et al. (2020). Sex-specific associations between co-exposure to multiple metals and visuospatial learning in early adolescence. Transl. Psychiatry 10, 1–10. doi: 10.1038/s41398-020-01041-8
Reynolds, C., Vannest, K., and Fletcher-Janzen, E. (2014). Encyclopedia of special education, 4 Volume Set: A reference for the education of children, adolescents, and adults disabilities and other exceptional individuals. New York, NY: Wiley.
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 52, 1059–1069.
Rubinov, M., Kötter, R., Hagmann, P., and Sporns, O. (2009). Brain connectivity toolbox: A collection of complex network measurements and brain connectivity datasets. Neuroimage 47:S169. doi: 10.1016/S1053-8119(09)71822-1
Sadiq, S., Ghazala, Z., Chowdhury, A., and Büsselberg, D. (2012). Metal toxicity at the synapse: Presynaptic, postsynaptic, and long-term effects. J. Toxicol. 2012:132671. doi: 10.1155/2012/132671
Saghazadeh, A., Mahmoudi, M., Shahrokhi, S., Mojarrad, M., Dastmardi, M., Mirbeyk, M., et al. (2020). Trace elements in schizophrenia: A systematic review and meta-analysis of 39 studies (N = 5151 participants). Nutr. Rev. 78, 278–303. doi: 10.1093/nutrit/nuz059
Schalbetter, S., von Arx, A., Cruz-Ochoa, N., Dawson, K., Ivanov, A., Mueller, F., et al. (2022). Adolescence is a sensitive period for prefrontal microglia to act on cognitive development. Sci. Adv. 8:eabi6672. doi: 10.1126/sciadv.abi6672
Shukla, G., and Chandra, S. (1987). Concurrent exposure to lead, manganese, and cadmium and their distribution to various brain regions, liver, kidney, and testis of growing rats. Arch. Environ. Contam. Toxicol. 16, 303–310. doi: 10.1007/BF01054947
Smith, D., Gwiazda, R., Bowler, R., Roels, H., Park, R., Taicher, C., et al. (2007). Biomarkers of Mn exposure in humans. Am. J. Ind. Med. 50, 801–811. doi: 10.1002/ajim.20506
Soudani, N., Troudi, A., Amara, I., Bouaziz, H., Boudawara, T., and Zeghal, N. (2012). Ameliorating effect of selenium on chromium (VI)-induced oxidative damage in the brain of adult rats. J. Physiol. Biochem. 68, 397–409. doi: 10.1007/s13105-012-0152-4
Spear, L. (2007). Assessment of adolescent neurotoxicity: Rationale and methodological considerations: An introduction to the special issue on “risk of neurobehavioral toxicity in adolescence.”. Neurotoxicol. Teratol. 29:1.
Stanley, M., Simpson, S., Dagenbach, D., Lyday, R., Burdette, J., and Laurienti, P. (2015). Changes in brain network efficiency and working memory performance in aging. PLoS One 10:e0123950. doi: 10.1371/journal.pone.0123950
Supekar, K., Menon, V., Rubin, D., Musen, M., and Greicius, M. (2008). Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput. Biol. 4:e1000100. doi: 10.1371/journal.pcbi.1000100
Tanner, E., Bornehag, C., and Gennings, C. (2019). Repeated holdout validation for weighted quantile sum regression. MethodsX 6, 2855–2860. doi: 10.1016/j.mex.2019.11.008
Tao, S., Liang, T., Cao, J., Dawson, R., and Liu, C. (1999). Synergistic effect of copper and lead uptake by fish. Ecotoxicol. Environ. Saf. 44, 190–195. doi: 10.1006/eesa.1999.1822
Thomason, M., Hect, J., Rauh, V., Trentacosta, C., Wheelock, M., Eggebrecht, A., et al. (2019). Prenatal lead exposure impacts cross-hemispheric and long-range connectivity in the human fetal brain. Neuroimage 191, 186–192. doi: 10.1016/j.neuroimage.2019.02.017
Thomason, M., Hect, J., Waller, R., and Curtin, P. (2021). Interactive relations between maternal prenatal stress, fetal brain connectivity, and gestational age at delivery. Neuropsychopharmacology. 46:1839. doi: 10.1038/s41386-021-01066-7
Trope, I., Lopez-Villegas, D., Cecil, K., and Lenkinski, R. (2001). Exposure to lead appears to selectively alter metabolism of cortical gray matter. Pediatrics 107, 1437–1442.
Twohig, D., and Nielsen, H. (2019). α-synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 14, 1–19.
Uehara, T., Yamasaki, T., Okamoto, T., Koike, T., Kan, S., Miyauchi, S., et al. (2013). Efficiency of a “Small-World” brain network depends on consciousness level: A resting-state fMRI study. Cereb. Cortex 24, 1529–1539. doi: 10.1093/cercor/bht004
van den Heuvel, M., Stam, C., Kahn, R., and He, H. (2009). Efficiency of functional brain networks and intellectual performance. J. Neurosci. 29, 7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009
Wang, J., Zuo, X., and He, Y. (2010). Graph-based network analysis of resting-state functional MRI. Front. Syst. Neurosci. 4:16. doi: 10.3389/fnsys.2010.00016
Watanabe, S., and Fukuchi, Y. (1981). [Occupational impairment of the olfactory sense of chromate producing workers (author’s transl)]. Sangyo Igaku 23, 606–611. doi: 10.1539/joh1959.23.606
Wise, J., Young, J., Cai, J., and Cai, L. (2022). Current understanding of hexavalent chromium [Cr(VI)] neurotoxicity and new perspectives. Environ. Int. 158:106877. doi: 10.1016/j.envint.2021.106877
Xu, J., Yin, X., Ge, H., Han, Y., Pang, Z., Tang, Y., et al. (2015). Attentional performance is correlated with the local regional efficiency of intrinsic brain networks. Front. Behav. Neurosci. 9:200. doi: 10.3389/fnbeh.2015.00200
Keywords: resting state–fMRI, graph theory, global and local efficiency, topological network properties, exposure, neurotoxic metals
Citation: Invernizzi A, Rechtman E, Oluyemi K, Renzetti S, Curtin P, Colicino E, Ambrosi C, Mascaro L, Patrono A, Corbo D, Cagna G, Gasparotti R, Reichenberg A, Tang CY, Smith DR, Placidi D, Lucchini RG, Wright RO and Horton MK (2023) Topological network properties of resting-state functional connectivity patterns are associated with metal mixture exposure in adolescents. Front. Neurosci. 17:1098441. doi: 10.3389/fnins.2023.1098441
Received: 14 November 2022; Accepted: 17 January 2023;
Published: 06 February 2023.
Edited by:
Anna Maria Lavezzi, University of Milan, ItalyReviewed by:
Chuncheng Lu, Nanjing Medical University, ChinaCopyright © 2023 Invernizzi, Rechtman, Oluyemi, Renzetti, Curtin, Colicino, Ambrosi, Mascaro, Patrono, Corbo, Cagna, Gasparotti, Reichenberg, Tang, Smith, Placidi, Lucchini, Wright and Horton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Azzurra Invernizzi,  YXp6dXJyYS5pbnZlcm5penppQG1zc20uZWR1
YXp6dXJyYS5pbnZlcm5penppQG1zc20uZWR1
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.