
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 25 January 2023
Sec. Translational Neuroscience
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1077858
This article is part of the Research TopicBridging the Gap in Neuroscience and Neurotherapeutics: from Fundamental Research to Clinical Translational ApplicationsView all 11 articles
 Weiliang Chen1†
Weiliang Chen1† Chunyu Yao1†
Chunyu Yao1† Shengwen Li2†
Shengwen Li2† Hongguang Huang3
Hongguang Huang3 Zujian Zhu1
Zujian Zhu1 Rui Chen1
Rui Chen1 Wen Su1
Wen Su1 Xiao Huang1
Xiao Huang1 Lisheng Xu1
Lisheng Xu1 Kaijie Sun1
Kaijie Sun1 Jiannan Song1
Jiannan Song1 Rongcai Jiang4*
Rongcai Jiang4* Guanjun Wang1*
Guanjun Wang1*Background and purpose: Traumatic brain injury (TBI), especially the severe TBI are often followed by persistent cognitive sequalae, including decision-making difficulties, reduced neural processing speed and memory deficits. Diffuse axonal injury (DAI) is classified as one of the severe types of TBI. Part of DAI patients are marginalized from social life due to cognitive impairment, even if they are rated as favorable outcome. The purpose of this study was to elucidate the specific type and severity of cognitive impairment in DAI patients with favorable outcome.
Methods: The neurocognition of 46 DAI patients with favorable outcome was evaluated by the Chinese version of the Montreal Cognitive Assessment Basic (MoCA-BC), and the differences in the domains of cognitive impairment caused by different grades of DAI were analyzed after data conversion of scores of nine cognitive domains of MoCA-BC by Pearson correlation analysis.
Results: Among the 46 DAI patients with favorable outcome, eight had normal cognitive function (MoCA-BC ≥ 26), and 38 had cognitive impairment (MoCA-BC < 26). The MoCA-BC scores were positively correlated with pupillary light reflex (r = 0.361, p = 0.014), admission Glasgow Coma Scale (GCS) (r = 0.402, p = 0.006), and years of education (r = 0.581, p < 0.001). Return of consciousness (r = −0.753, p < 0.001), Marshall CT (r = −0.328, p = 0.026), age (r = −0.654, p < 0.001), and DAI grade (r = −0.403, p = 0.006) were found to be negatively correlated with the MoCA-BC scores. In patients with DAI grade 1, the actually deducted scores (Ads) of memory (r = 0.838, p < 0.001), abstraction (r = 0.843, p < 0.001), and calculation (r = 0.782, p < 0.001) were most related to the Ads of MoCA-BC. The Ads of nine cognitive domains and MoCA-BC were all proved to be correlated, among patients with DAI grade 2. However, In the DAI grade 3 patients, the highest correlation with the Ads of MoCA-BC were the Ads of memory (r = 0.904, p < 0.001), calculation (r = 0.799, p = 0.006), orientation (r = 0.801, p = 0.005), and executive function (r = 0.869, p = 0.001).
Conclusion: DAI patients with favorable outcome may still be plagued by cognitive impairment, and different grades of DAI cause different domains of cognitive impairment.
DAI is caused by acceleration-deceleration or rotational forces on brain tissues, resulting in axonal shear injuries and delayed axonal disconnection, categorized as a special type of traumatic brain injury (TBI) (Mu et al., 2021; Zhou et al., 2021; Chen et al., 2022; Kim et al., 2022). A total of 20–38% of TBI patients with DAI were followed up with unfavorable outcome (Extended Glasgow Outcome Scale, GOSE 1–4) (Humble et al., 2018; Lohani et al., 2020), but over 85% suffered from persistent cognitive impairment (Macruz et al., 2022; RaukolaLindblom et al., 2022). Significant differences appeared in the incidence of unfavorable outcome and cognitive impairment, reflecting that part of DAI patients classified as favorable outcome were still plagued by cognitive impairment and could not return to normal social life. However, at present, the prevalence and specificity of cognitive impairment in diffuse axonal injury patients with favorable outcome remain unclear.
“Neurocognition” is defined as a collection of interrelated domains such as executive function (EF), language and perceptual motor function, calculation, complex attention, memory, and visuoperception (George et al., 2018; Holguin et al., 2022; RaukolaLindblom et al., 2022). Found that the Finnish KAT test is a valuable tool to detect cognitive-linguistic deficits by comparing the cognitive function of 48 adults with moderate to severe DAI and 27 healthy controls. The Hopkins Language Learning Test (HVLT), Trail Making Test (TMT), and Rey–Osterrieth Complex Figure test were used to assess neuropsychological results in 25 DAI patients at 6 and 12 months post-traumatic, revealed that patients’ episodic verbal memory, attention, and executive function were improved at 6 and 12 months after the trauma (Macruz et al., 2022). All of the above test methods could only evaluate certain domains of Neurocognition, and the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005) was considered to be a more appropriate scale to comprehensively assess cognitive function after traumatic brain injury, subarachnoid hemorrhage, stroke and Alzheimer’s disease (Cornea et al., 2022; Holguin et al., 2022; Wang et al., 2022). MoCA and resting-state perfusion magnetic resonance imaging were performed in 40 patients with acute mild TBI and 40 healthy controls within 14 days following injury to elucidate the relationship between cerebral blood flow connectivity differences and cognitive outcomes in the acute phase after mild TBI (Duan et al., 2022). Further more, a study from Shanghai Huashan Hospital confirmed that the Chinese version of the Montreal Cognitive Assessment Basic (MoCA-BC), as a reliable cognitive screening test across all education levels, has the advantages of high acceptance and good reliability, and is more suitable than MoCA for Chinese elderly adults with low years of education (Chen et al., 2016).
The favorable outcome (GOSE 5–8) can not be used as the final prognostic indicator because a large part of DAI patients is still troubled by cognitive impairment at several months after TBI (George et al., 2018). We hypothesized that DAI patients with favorable outcomes still have cognitive impairment in different domains. To test this hypothesis, we analyzed the functional status of nine cognitive domains in patients with DAI at 6 months after injury.
Patients with DAI diagnosed by magnetic resonance imaging (MRI) within 30 days after injury were screened in this retrospective observational study between January 2019 and December 2021. Neuropsychological of DAI patients were assessed by MoCA-BC at 6 months after TBI. This data collection site was approved by the local Institutional Review Board, and written informed consent was obtained from all participants or their representatives.
The inclusion criteria were the following: TBI patients’ age between 18 and 70 years, diagnosed as DAI by clinical MRI scan within 30 days after injury, 6-month GOSE score ≥5, years of education ≥4. The exclusion criteria were the following: Progressive brain illness (Dementia, Parkinson disease, multiple sclerosis, seizure disorder, and brain tumor) (2/8), history of brain surgery or stroke without full recovery (1/8), Unable to complete or cooperate with the cognitive function test (speech disorder or mental illness) (5/8).
Demographic and clinical characteristics were collected during hospitalization, including age, sex, causes of trauma (road traffic accident, fall, and others), years of education, admission GCS score, pupillary light reflex (none, unilateral or bilateral), Marshall CT classification (evaluated on a scale from 1 to 6) from the first head CT scan, time of the return of consciousness.
The clinical MRI was performed with a 1.5T scanner (Siemens Symphony, ATim) within 30 days after injury. DAI were defined as TBI patients with lesions in gray-white matter junction of the cerebrum, corpus callosum, or brain stem with T2-weighted imaging (T2WI), T2-weighted fluid attenuated inversion recovery (T2 FLAIR), and diffusion-weighted imaging (DWI) in magnetic resonance imaging (MRI) (Subash et al., 2020; Yue et al., 2020). These lesions were defined as having hypointense focus presented on T2WI (hemorrhagic DAI), or hyperintense focus presented on DWI, T2 FLAIR, and T2WI (non-hemorrhagic DAI) (Rutman et al., 2017; Benjamini et al., 2021). The MRI and CT images were independently analyzed by two experienced neuroradiologists, who had access to patient clinical information but were blinded to the cognitive function.
The presence of DAI in the hemispheres or cerebellum was recorded as DAI grade 1, in the corpus callosum with or without lesions of grade 1 as DAI grade 2, and in the brainstem with or without lesions of grade 1 and/or 2 as DAI grade 3. Patients without DAI were assigned to grade 0 (Adams et al., 1989; Figure 1).
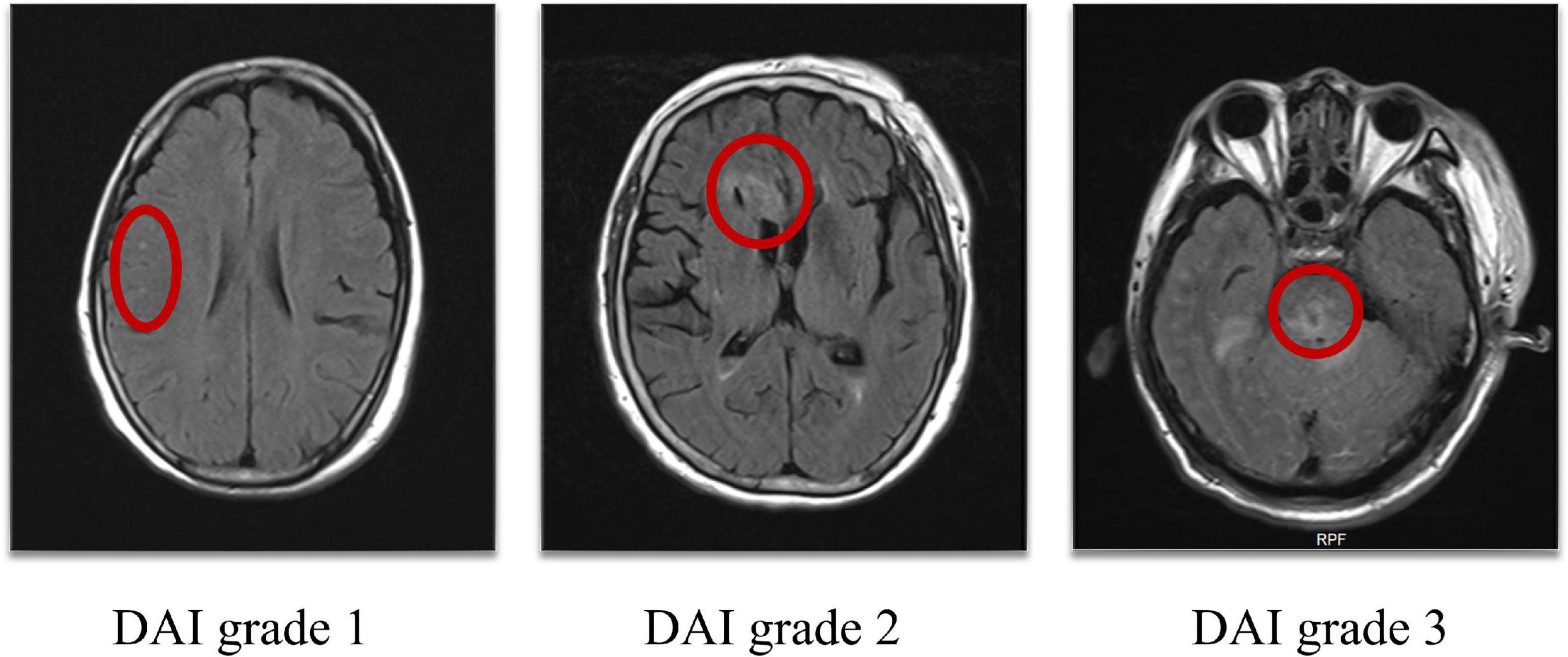
Figure 1. DAI grade 1: Lesions in the hemispheres, DAI grade 2: Lesions in the corpus callosum, DAI grade 3: Lesions in the brainstem (as shown in the red circle). DAI, Diffuse axonal injury.
GOSE was used to quantify 6-month outcome as favorable outcome (GOSE 5–8; no or moderate disability) or unfavorable outcome (GOSE 1–4; severe disability or death). GOSE was divided into eight levels (Wilson et al., 1998; Table 1).
The MoCA-BC was translated from the original English version with subtle linguistic and cultural modifications and regarded as an effective cognitive test for Chinese elderly adults with low years of education (Chen et al., 2016; Huang et al., 2018). The MoCA-BC assesses nine cognitive domains including executive function, language, orientation, calculation, abstraction, memory, visuoperception, naming, and attention. It takes about 10 min to complete the test, with a maximum score of 30 points. More than 26 points was regarded as normal and with a lower score indicating greater cognitive impairment (Zhang et al., 2019). Each patient’s test was performed by trained staff in a quiet environment at 6 months after injury.
The scores of nine cognitive domains of MoCA-BC were all converted into the actually deducted scores (Ads), because the study focused on analyzing the negative impact of cognitive function (Rainer et al., 2006). The specific conversion method was to subtract the total score of each cognitive domain from the actual score of this domain, that is, the negative value of the score deducted from each domain, for example, if the patient’s orientation score was four, then his a Ads of orientation was four minus six (the total score of orientation), which equals to negative two points.
All statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). A value of p < 0.05 with a two-tailed test was considered statistically significant. Categorical data are presented as frequency or percentage and compared by Fisher exact test. Continuous data are presented as the median and interquartile range (IQR) and were compared by the Mann–Whitney U-test. Pearson correlation analysis was employed to show the association of potential risk factors with neurocognition and the Ads of nine cognitive domains with MoCA-BC after data conversion in different grades of DAI patients, and presented in the form of a forestplot.
A total of 79 DAI patients diagnosed by clinical MRI within 30 days after injury were enrolled during the study period. At 6 months after injury, 54 patients met all the criteria for inclusion, eight patients were excluded due to the exclusion criteria, and a total of 46 DAI patients with favorable outcome were analyzed. Among them, eight had normal cognitive function (MoCA-BC ≥ 26) and 38 had cognitive impairment (MoCA-BC < 26). Comparing the two groups of DAI patients with 6-month favorable outcome with or without cognitive impairment, it was found that there were significant statistical differences in patients’ age, years of education, and time to return of consciousness (p = 0.003, p = 0.009, and p < 0.001). But surprisingly, there were no significant statistical differences in pupillary light reflex, admission GCS, Marshall CT score, and DAI grade (Table 2).
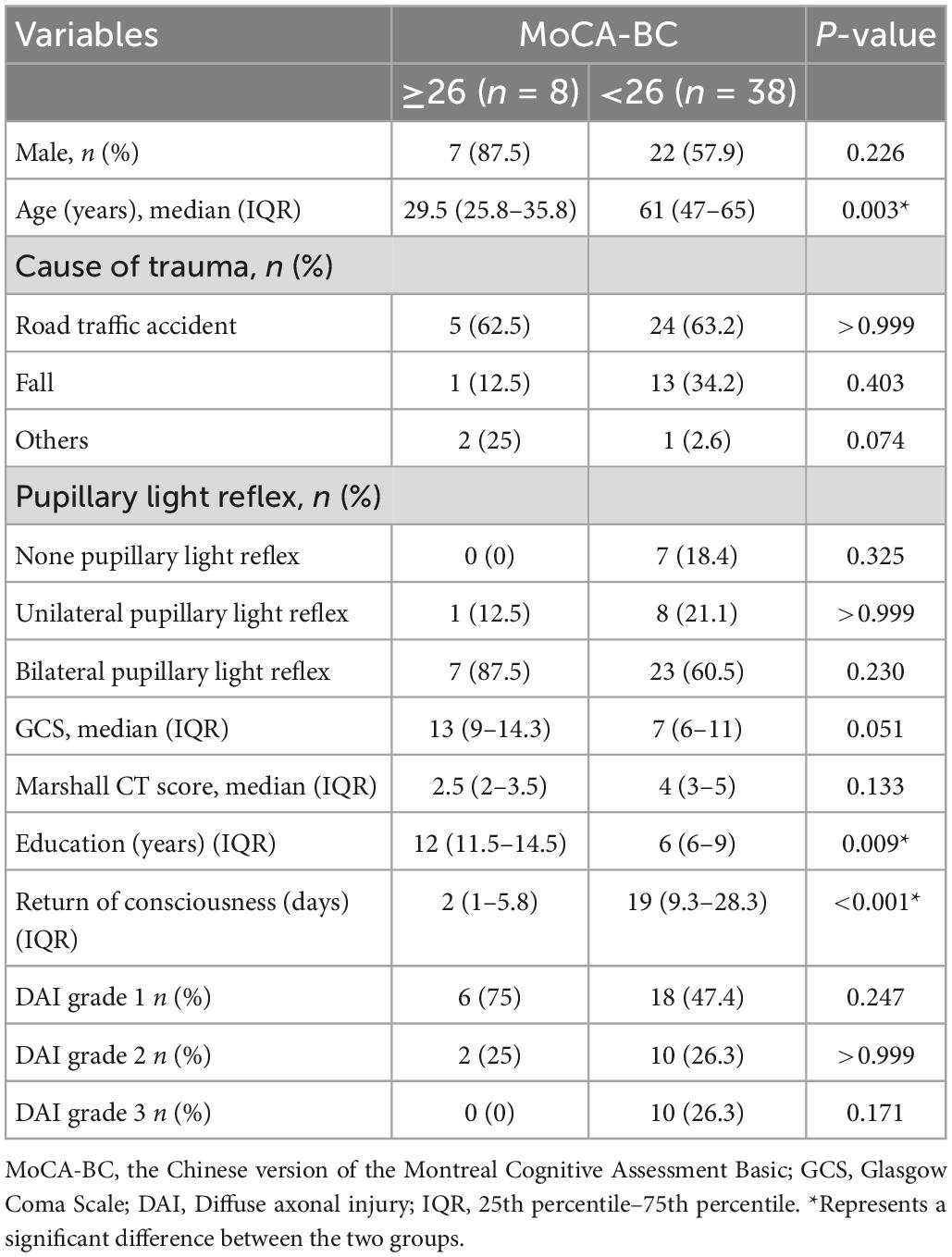
Table 2. Demographic and clinical characteristics of eligible patients with or without cognitive impairment.
Pearson correlation analysis was employed to define the relationship of potential risk factors with the scores of MoCA-BC (Figure 2). The following four factors were found to be negatively correlated with the MoCA-BC scores: Return of consciousness (r = −0.753, p < 0.001), Marshall CT (r = −0.328, p = 0.026), age (r = −0.654, p < 0.001), and DAI grade (r = −0.403, p = 0.006). The MoCA-BC scores were positively correlated with pupillary light reflex (r = 0.361, p = 0.014), admission GCS (r = 0.402, p = 0.006) and years of education (r = 0.581, p < 0.001), Non-significant correlations were observed between sex, causes of trauma and the MoCA-BC scores.
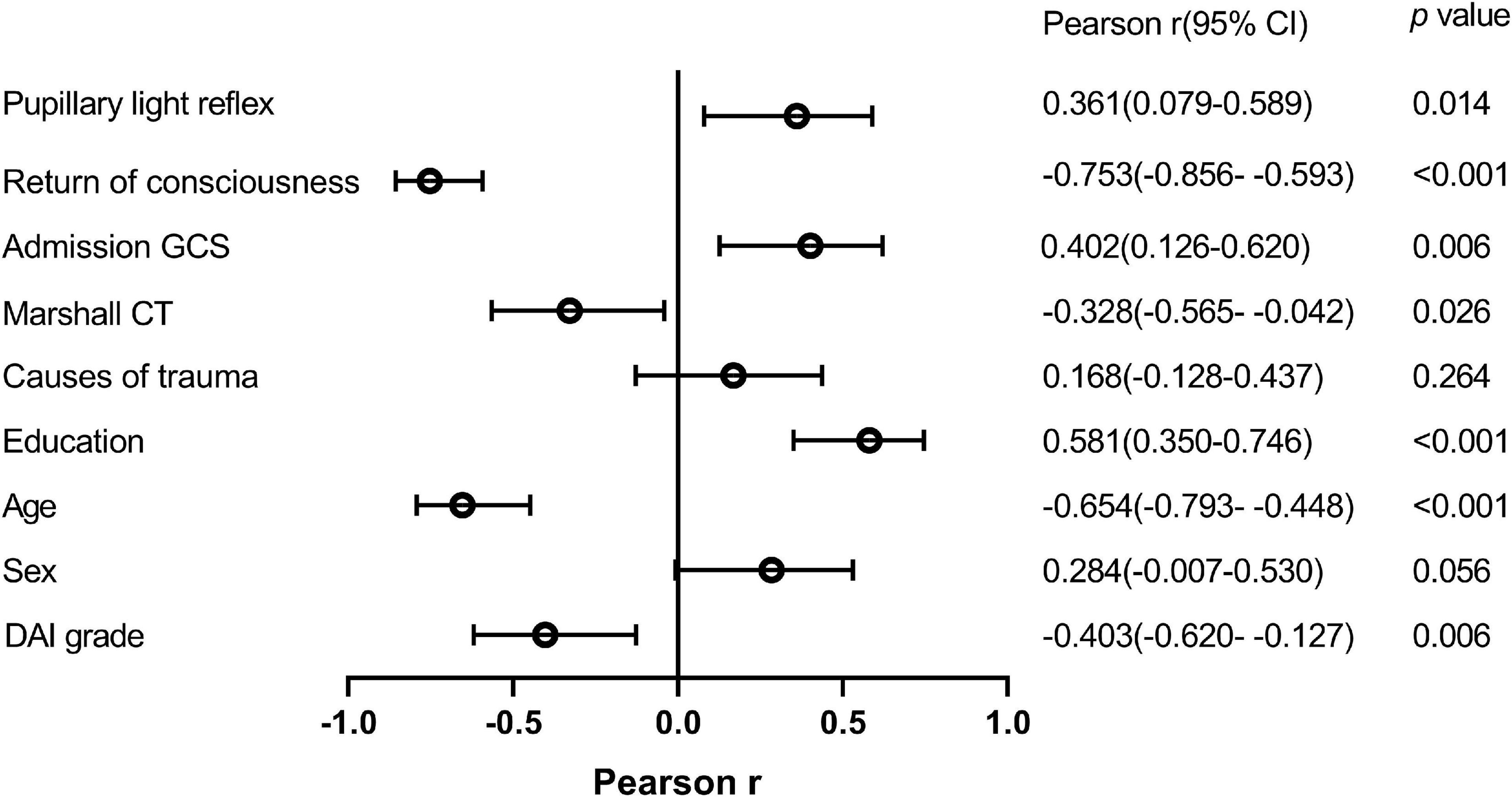
Figure 2. Relationship between potential risk factors and MoCA-BC scores. MoCA-BC, the Chinese version of the Montreal Cognitive Assessment Basic; GCS, Glasgow Coma Scale.
Moreover, to identify the association of cognitive impairment and each cognitive domain, Pearson correlation analysis was performed between the Ads of nine cognitive domains and MoCA-BC after data conversion in different grades of DAI patients (Figure 3). In patients with DAI grade 1, the Ads of memory (r = 0.838, p < 0.001), abstraction (r = 0.843, p < 0.001), and calculation (r = 0.782, p < 0.001) were most related to the Ads of MoCA-BC. The Ads of nine cognitive domains and MoCA-BC were all proved to be correlated, among patients with DAI grade 2. However, in the DAI grade 3 patients, the highest correlation with the Ads of MoCA-BC were the Ads of memory (r = 0.904, p < 0.001), calculation (r = 0.799, p = 0.006), orientation (r = 0.801, p = 0.005), and executive function (r = 0.869, p = 0.001). The whole correlation analysis results can be found in Supplementary Table 1.
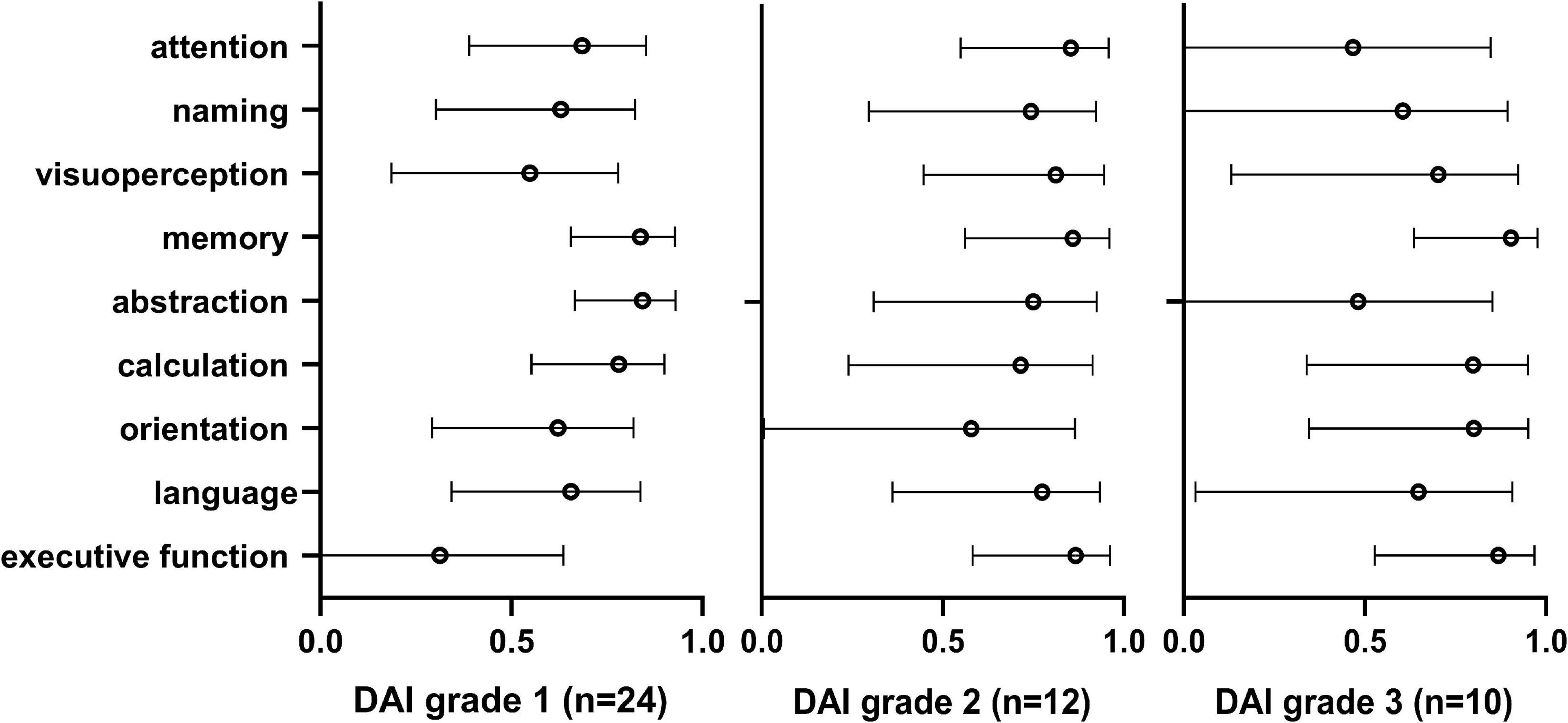
Figure 3. Pearson correlation analysis was performed between the Ads of nine cognitive domains and MoCA-BC after data conversion in different grades of DAI patients. MoCA-BC, the Chinese version of the Montreal Cognitive Assessment Basic; DAI, Diffuse axonal injury.
This retrospective study indicated that 82.6% (38/46) of DAI patients with 6-month GOSE ≥5 were still plagued by cognitive impairment, and the major domains of cognitive impairment caused by different grades of DAI were also distinctive.
More than 50 million people are suffering from TBI each year worldwide, and most TBI patients are diagnosed by clinical MRI with some degree of DAI, especially severe TBI among them (Jiang et al., 2019; Lammy, 2020). DAI manifests in the form of focal axonal shear injuries and axonal breakage, so patients with DAI are more likely to have long-term sequelae or serious neurological deficits, even death (Lang et al., 2021; Palmieri et al., 2021).
GOSE or GOS are usually used by neurosurgeons to evaluate the prognosis of patients with DAI, but these assessment methods are not detailed and comprehensive, because previous studies have found that the rate of cognitive impairment is much higher than that of unfavorable outcome (Humble et al., 2018; Lohani et al., 2020; Macruz et al., 2022; RaukolaLindblom et al., 2022). In our study, MoCA-BC was performed to evaluate the cognitive function of 46 patients with DAI who were classified as favorable outcome, and 38 patients were found to have some degree of cognitive impairment. By comparing the two groups of patients with or without cognitive impairment in the present study, it was concluded that there were significant differences in age, education and return of consciousness, but there were no significant differences in admission GCS, Marshall CT score, pupillary light reflex and DAI grades.
In (Macruz et al., 2022) article, admission GCS and Marshall CT score had significant impact on cognitive function. Outcome was better in patients with DAI grade 1 and DAI grade 2 than in patients with DAI grade 3 (Skandsen et al., 2010; Moe et al., 2020). Have confirmed that age, GCS score, pupillary dilatation, traumatic axonal injury (TAI) grade on clinical MRI were significant predictors for poor outcome. Some of the results in our research were not consistent with the recent literature, which may be due to the deviation caused by the insufficient sample size.
Followed the long-term functional outcome of 134 patients with DAI and found independent prognostic factors were age, return of consciousness ≤7 days, pupillary reaction and DAI grade (van Eijck et al., 2018). Education level has been considered to be the strongest non-cognitive factor influencing performance on the MoCA (Chen et al., 2016). Analysis of 228 patients with TBI showed that education status was correlated with MoCA scores: Those patients with higher level of education had significant association with higher MoCA scores (p = 0.012) (Panwar et al., 2019). Marshall CT score, pupillary light reflex, duration of coma and admission GCS were predictors of clinical outcome in DAI (Palmieri et al., 2021; Xie et al., 2021; Chen et al., 2022). In the present study we also confirmed that return of consciousness, Marshall CT score, age and DAI grade have a negative correlation with cognitive function in DAI patients with 6-month GOSE 5–8. The more sensitive the pupillary light reflex, the higher the admission GCS and the longer the education, the better the long-term cognitive function of DAI patients. The correlation of these risk factors was basically consistent for the cognitive function of TBI patients and DAI patients with favorable outcome.
Severe cognitive impairment and physical disability were caused by lateral hypothalamus and medial hypothalamus damage during DAI (Wang et al., 2021). HVLT, TMT, and Rey–Osterrieth Complex Figure test were performed by Macruz et al. (2022) to reveal the impairment and recovery of episodic verbal memory, attention, and executive function of DAI patients. Further, scholars found that cognitive impairment was associated with brain tissue lesions displayed on MRI (Yue et al., 2020). Twenty-four patients with moderate or severe DAI were evaluated at 2, 6, and 12 months post-injury (Stewan et al., 2018) found out that microhaemorrhage load (MHL) was correlated only with white matter volume (WMV) reduction, executive function and episodic verbal memory were not correlated with MHL, but were, in part, correlated with WMV and total brain volume reduction. Not only severe TBI, but repetitive mild brain injury impair cognitive abilities and increase risk of neurodegenerative disorders in humans (Sai et al., 2020). So far, the persistent cognitive sequelae of patients suffering from DAI have been identified by the current studies, but the specificity of the impairment of nine cognitive domains caused by different grades of DAI have not been noticed by scholars. In our study, according to the diagnostic results of clinical MRI, DAI was divided into three grades for hierarchical interpretation. Finally, it was found that different degrees of DAI lead to significant differences in the domains of cognitive impairment. The decline of cognitive function in patients with DAI grade 1 was mainly caused by dysfunction in the three cognitive domains of memory, abstraction and calculation. But for patients with DAI grade 2, after the damage of the important structure (corpus callosum) connecting the left and right sides of the brain (Shah et al., 2021; Lewis et al., 2022), the cognitive impairment was most widely distributed, and none of the nine cognitive domains was immune. Patients with DAI grade 3 suffered from brainstem (the bridge of morphological and functional connection between telencephalon, diencephalon, cerebellum, and spinal cord) (Henson et al., 2020; Paton, 2020) injury, showing cognitive impairment dominated by memory, calculation and orientation and executive function dysfunction.
There are several limitations in our present study. First, insufficient sample size may lead to deviation and compromise the statistical power. Second, taking 6-month GOSE score ≥5 as the inclusion criteria may exclude some DAI patients who are classified as unfavorable outcome but can cooperate with the cognitive function test, thus affecting the final cognitive domain analysis results. Third, MoCA-BC was translated according to Chinese culture and may not be suitable for people in other countries.
Our current research has revealed that DAI patients with favorable outcome may still be plagued by cognitive impairment. Different grades of DAI cause different domains of cognitive impairment, which requires neurosurgeons to provide patients with targeted cognitive rehabilitation programs.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Haining People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
WC, CY, SL, JS, GW, and RJ: conceptualization and writing – review and editing. HH, ZZ, WS, and RC: data curation. XH and LX: formal analysis. LX, KS, JS, WC, CY, and HH: investigation. WC, SL, RJ, and GW: methodology. RJ and GW: supervision. HH and ZZ: visualization. WC and CY: writing – original draft. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1077858/full#supplementary-material
Adams, J. H., Doyle, D., Ford, I., Gennarelli, T. A., Graham, D. I., and McLellan, D. R. (1989). Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology 15, 49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x
Benjamini, D., Iacono, D., Komlosh, M. E., Perl, D. P., Brody, D. L., and Basser, P. J. (2021). Diffuse axonal injury has a characteristic multidimensional MRI signature in the human brain. Brain 144, 800–816. doi: 10.1093/brain/awaa447
Chen, K., Xu, Y., Chu, A., Ding, D., Liang, X., Nasreddine, Z. S., et al. (2016). Validation of the Chinese version of montreal cognitive assessment basic for screening mild cognitive impairment. J. Am. Geriat. Soc. 64, e285–e290. doi: 10.1111/jgs.14530
Chen, W., Wang, G., Yao, C., Zhu, Z., Chen, R., Su, W., et al. (2022). The ratio of serum neuron-specific enolase level to admission glasgow coma scale score is associated with diffuse axonal injury in patients with moderate to severe traumatic brain injury. Front. Neurol. 13:887818. doi: 10.3389/fneur.2022.887818
Cornea, A., Simu, M., and Rosca, E. C. (2022). Montreal cognitive assessment for evaluating cognitive impairment in subarachnoid hemorrhage: A systematic review. J. Clin. Med. 11:4679. doi: 10.3390/jcm11164679
Duan, M., Liu, Y., Li, F., Lu, L., and Chen, Y. (2022). Cerebral blood flow network differences correlated with cognitive impairment in mild traumatic brain injury. Front. Neurosci. 16:969971. doi: 10.3389/fnins.2022.969971
George, S., David, K. M., Emmanuel, A. S., John, D. P., and Barbara, J. S. (2018). Personalised treatments for traumatic brain injury: Cognitive, emotional and motivational targets. Psychol. Med. 48, 1397–1399. doi: 10.17863/CAM.25958
Henson, J. W., Benkers, T., and McCormick, C. (2020). Brainstem ischemic syndrome in juvenile NF2. Neurol. Genet. 6:e446. doi: 10.1212/NXG.0000000000000446
Holguin, J. A., Margetis, J. L., Narayan, A., Yoneoka, G. M., and Irimia, A. (2022). Vascular cognitive impairment after mild stroke: Connectomic insights, neuroimaging, and knowledge translation. Front. Neurosci. 16:905979. doi: 10.3389/fnins.2022.905979
Huang, L., Chen, K., Lin, B., Tang, L., Zhao, Q., Lv, Y., et al. (2018). Chinese version of montreal cognitive assessment basic for discrimination among different severities of Alzheimer’s disease. Neuropsych. Dis. Treat. 14, 2133–2140. doi: 10.2147/NDT.S174293
Humble, S. S., Wilson, L. D., Wang, L., Long, D. A., Smith, M. A., Siktberg, J. C., et al. (2018). Prognosis of diffuse axonal injury with traumatic brain injury. J. Trauma Acute Care Surg. 85, 155–159. doi: 10.1097/TA.0000000000001852
Jiang, J. Y., Gao, G. Y., Feng, J. F., Mao, Q., Chen, L. G., Yang, X. F., et al. (2019). Traumatic brain injury in China. Lancet Neurol. 18, 286–295. doi: 10.1016/S1474-4422(18)30469-1
Kim, M., Hong, S. K., Jeon, S. R., Roh, S. W., and Lee, S. (2022). Treatment outcome and risk factors associated with diffuse axonal injury in patients with moderate to severe head injury. Turk Neurosurg. 32, 6–15. doi: 10.5137/1019-5149.JTN.28132-19.4
Lammy, S. (2020). Management of traumatic brain injury in China versus Europe. Lancet Neurol. 19:886. doi: 10.1016/S1474-4422(20)30345-8
Lang, S. S., Kilbaugh, T., Friess, S., Sotardi, S., Kim, C. T., Mazandi, V., et al. (2021). Trajectory of long-term outcome in severe pediatric diffuse axonal injury: An exploratory study. Front. Neurol. 12:704576. doi: 10.3389/fneur.2021.704576
Lewis, J. D., Acosta, H., Tuulari, J. J., Fonov, V. S., Collins, D. L., Scheinin, N. M., et al. (2022). Allometry in the corpus callosum in neonates: Sexual dimorphism. Hum. Brain Mapp. 43, 4609–4619. doi: 10.1002/hbm.25977
Lohani, S., Bhandari, S., Ranabhat, K., and Agrawal, P. (2020). Does diffuse axonal injury MRI grade really correlate with functional outcome? World Neurosurg. 135, e424–e426.
Macruz, F. B. D. C., Feltrin, F. S., Zaninotto, A., Guirado, V. M. D. P., Otaduy, M. C. G., Tsunemi, M. H., et al. (2022). Longitudinal assessment of magnetization transfer ratio, brain volume, and cognitive functions in diffuse axonal injury. Brain Behav. 12:e2490. doi: 10.1002/brb3.2490
Moe, H. K., Limandvik Myhr, J., Moen, K. G., Håberg, A. K., Skandsen, T., and Vik, A. (2020). Association of cause of injury and traumatic axonal injury: A clinical MRI study of moderate and severe traumatic brain injury. J. Neurosurg. 133, 1559–1567. doi: 10.3171/2019.6.JNS191040
Mu, J., Wang, T., Li, M., Guan, T., Guo, Y., Zhang, X., et al. (2021). Ketogenic diet protects myelin and axons in diffuse axonal injury. Nutr. Neurosci. 28, 1–14. doi: 10.1080/1028415X.2021.1875300
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Palmieri, M., Frati, A., Santoro, A., Frati, P., Fineschi, V., and Pesce, A. (2021). Diffuse axonal injury: Clinical prognostic factors, molecular experimental models and the impact of the trauma related oxidative stress. An extensive review concerning milestones and advances. Int. J. Mol. Sci. 22:10865. doi: 10.3390/ijms221910865
Panwar, N., Purohit, D., Deo, S. V., and Joshi, M. (2019). Evaluation of extent and pattern of neurocognitive functions in mild and moderate traumatic brain injury patients by using montreal cognitive assessment (MoCA) score as a screening tool: An observational study from India. Asian J. Psychiatr. 41, 60–65. doi: 10.1016/j.ajp.2018.08.007
Paton, J. (2020). Clarity of the rhythmic brainstem. J. Physiol. 598, 2045–2046. doi: 10.1113/JP279732
Rainer, S., Kathrin, W., Thomas, G., Christoph, P., and von Cramon, D. (2006). Cognitive sequelae of diffuse axonal injury. Arch. Neurol. 63, 418–424. doi: 10.1001/archneur.63.3.418
RaukolaLindblom, M., Ljungqvist, L., Kurki, T., Tenovuo, O., and Laasonen, M. (2022). Cognitive-Linguistic outcome in moderate to severe diffuse axonal injury and association with fatigue. Brain Injury 35, 1674–1681. doi: 10.1080/02699052.2021.2012824
Rutman, A. M., Rapp, E. J., Hippe, D. S., Vu, B., and Mossa-Basha, M. (2017). T2*-weighted and diffusion magnetic resonance imaging differentiation of cerebral fat embolism from diffuse axonal injury. J. Comput. Assist. Tomogr. 41, 877–883. doi: 10.1097/RCT.0000000000000635
Sai, A. T., Zsolt, K. B., Nóra, B., Lili, V. N., Krisztina, A., Bálint, F., et al. (2020). Long-term cognitive impairment without diffuse axonal injury following repetitive mild traumatic brain injury in rats. Behav. Brain Res. 378:112268. doi: 10.1016/j.bbr.2019.112268
Shah, A., Jhawar, S., Goel, A., and Goel, A. (2021). Corpus callosum and its connections: A fiber dissection study. World Neurosurg. 151, e1024–e1035. doi: 10.1016/j.wneu.2021.05.047
Skandsen, T., Kvistad, K. A., Solheim, O., Strand, I. H., Folvik, M., and Vik, A. (2010). Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: A cohort study of early magnetic resonance imaging findings and 1-year outcome. J. Neurosurg. 113, 556–563. doi: 10.3171/2009.9.JNS09626
Stewan, F. F., Zaninotto, A. L., Guirado, V. M. P., Macruz, F., Sakuno, D., Dalaqua, M., et al. (2018). Longitudinal changes in brain volumetry and cognitive functions after moderate and severe diffuse axonal injury. Brain Injury 32, 1208–1217. doi: 10.1080/02699052.2018.1494852
Subash, L., Shreeram, B., Kajan, R., and Prity, A. (2020). Does diffuse axonal injury MRI grade really correlate with functional outcome? World Neurosurg. 135, e424–e426.
van Eijck, M., van der Naalt, J., de Jongh, M., Schoonman, G., Oldenbeuving, A., Peluso, J., et al. (2018). Patients with diffuse axonal injury can recover to a favorable long-term functional and quality of life outcome. J. Neurotrauma 35, 2357–2364. doi: 10.1089/neu.2018.5650
Wang, G., Estrella, A., Hakim, O., Milazzo, P., Patel, S., Pintagro, C., et al. (2022). Mini-mental state examination and montreal cognitive assessment as tools for following cognitive changes in Alzheimer’s disease neuroimaging initiative participants. J. Alzheimer’s Dis. JAD 90, 263–270. doi: 10.3233/JAD-220397
Wang, Y., Zhou, F., Li, Y., Li, J., Kuang, H., Chen, Q., et al. (2021). Functional plasticity in lateral hypothalamus and its prediction of cognitive impairment in patients with diffuse axonal injury: Evidence from a resting-state functional connectivity study. Neuro. Rep. 32, 7588–7595. doi: 10.1097/WNR.0000000000001630
Wilson, J. T., Pettigrew, L. E., and Teasdale, G. M. (1998). Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: Guidelines for their use. J. Neurotrauma 15, 573–585. doi: 10.1089/neu.1998.15.573
Xie, Q., Huang, W., Shen, L., Wang, M., Liu, K., and Liu, F. (2021). The combination of neutrophil-to-lymphocyte ratio and admission GCS score is an independent predictor of clinical outcome in diffuse axonal injury. World Neurosurg. 152:248. doi: 10.1016/j.wneu.2021.05.060
Yue, J. K., Yuh, E., Stein, M., Winkler, E., Deng, H., Ore, C. L. D., et al. (2020). Diffuse axonal injury and cerebral contusions on MRI are associated with decreased functional outcome in CT-negative TBI: A TRACK-TBI pilot study. Neurosurgery 67, (Supplement_1). doi: 10.1093/neuros/nyaa447_442
Zhang, Y., Ding, Y., Chen, K., Liu, Y., Wei, C., Zhai, T., et al. (2019). The items in the Chinese version of the montreal cognitive assessment basic discriminate among different severities of Alzheimer’s disease. BMC Neurol. 19:269. doi: 10.21203/rs.2.10497/v5
Keywords: diffuse axonal injury, cognitive impairment, outcome, Montreal cognitive assessment, cognitive domain
Citation: Chen W, Yao C, Li S, Huang H, Zhu Z, Chen R, Su W, Huang X, Xu L, Sun K, Song J, Jiang R and Wang G (2023) Cognitive impairment in diffuse axonal injury patients with favorable outcome. Front. Neurosci. 17:1077858. doi: 10.3389/fnins.2023.1077858
Received: 23 October 2022; Accepted: 09 January 2023;
Published: 25 January 2023.
Edited by:
Na Li, Central South University, ChinaReviewed by:
Zhou Zhou, Royal Institute of Technology, SwedenCopyright © 2023 Chen, Yao, Li, Huang, Zhu, Chen, Su, Huang, Xu, Sun, Song, Jiang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanjun Wang,  aGFpbmluZ3d3d2hrQDE2My5jb20=; Rongcai Jiang,
aGFpbmluZ3d3d2hrQDE2My5jb20=; Rongcai Jiang,  amlhbmcxMTYyMTZAMTYzLmNvbQ==
amlhbmcxMTYyMTZAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.