- 1Department of Neurology, Beijing Luhe Hospital, Capital Medical University, Beijing, China
- 2Department of Neurosurgery, School of Medicine, Wayne State University, Detroit, MI, United States
- 3Department of Neurosurgery, Munson Healthcare, Munson Medical Center, Traverse City, MI, United States
Objective: This study assesses whether stress-induced hyperglycemia is a predictor of poor outcome at 3 months for patients with acute ischemic stroke (AIS) treated by endovascular treatment (EVT) and impacted by their previous blood glucose status.
Methods: This retrospective study collected data from 576 patients with AIS due to large vessel occlusion (LVO) treated by EVT from March 2019 to June 2022. The sample was composed of 230 and 346 patients with and without diabetes mellitus (DM), respectively, based on their premorbid diabetic status. Prognosis was assessed with modified Rankin Scale (mRS) at 3-month after AIS. Poor prognosis was defined as mRS>2. Stress-induced hyperglycemia was assessed by fasting glucose-to-glycated hemoglobin ratio (GAR). Each group was stratified into four groups by quartiles of GAR (Q1–Q4). Binary logistic regression analysis was used to identify relationship between different GAR quartiles and clinical outcome after EVT.
Results: In DM group, a poor prognosis was seen in 122 (53%) patients and GAR level was 1.27 ± 0.44. These variables were higher than non-DM group and the differences were statistically significant (p < 0.05, respectively). Patients with severe stress-induced hyperglycemia demonstrated greater incidence of 3-month poor prognosis (DM: Q1, 39.7%; Q2, 45.6%; Q3, 58.6%; Q4, 68.4%; p = 0.009. Non-DM: Q1, 31%; Q2, 32.6%; Q3, 42.5%; Q4, 64%; p < 0.001). However, the highest quartile of GAR was independently associated with poor prognosis at 3 months (OR 3.39, 95% CI 1.66–6.96, p = 0.001), compared to the lowest quartile in non-DM patients after logistic regression. This association was not observed from DM patients.
Conclusion: The outcome of patients with acute LVO stroke treated with EVT appears to be influenced by premorbid diabetes status. However, the poor prognosis at 3-month in patients with DM is not independently correlated with stress-induced hyperglycemia. This could be due to the long-term damage of persistent hyperglycemia and diabetic patients’ adaptive response to stress following acute ischemic damage to the brain.
Introduction
Approximately one-third of patients with acute ischemic stroke (AIS) develop hyperglycemia regardless of history of diabetes (Li et al., 2013; Yao et al., 2016; Almobarak et al., 2020; Jiang et al., 2021). The acute stress response of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in response to brain injuries are believed to be responsible (Christensen et al., 2004). Hyperglycemia is associated with increased hemorrhagic transformation and can be detrimental to patients with AIS (Robbins and Swanson, 2014; Rosso et al., 2018; Suissa et al., 2020). Larger ischemic areas, worse functional, and cognitive outcomes, and increased mortalities are found to be independently linked to increased blood glucose levels at the time of hospital admission (Tsivgoulis et al., 2019). Stress-induced hyperglycemia increases the risk of stroke recurrence and overall mortality in AIS patients even without history of diabetes (Zhu et al., 2019).
Patients without premorbid diabetes mellitus (DM) tend to develop other morbidities when subjected to stress-induced hyperglycemia than those with history of DM. SITS study notes potential disparities of early mortality and symptomatic intracranial hemorrhage (sICH) in association of hyperglycemia and history of DM; in patients without history of diabetes, admission hyperglycemia was linked to a higher rate of sICH and mortality as well as worse functional independence at 3 months (Ahmed et al., 2010). Similar results have been found in AIS patients who received intravenous thrombolysis (IVT); risk of death, hemorrhagic complications, and functional dependency drastically increase with severe stress-induced hyperglycemia in those without history of DM (Merlino et al., 2022).
High glucose levels have been considered as a predictor of poor functional outcome after IVT and mechanical thrombectomy (MT) (Chen et al., 2019; Tsivgoulis et al., 2019; Suissa et al., 2020; Merlino et al., 2021a; Shen et al., 2021; Cannarsa et al., 2022). Merlino et al. (2022) have found that risk of functional dependence, mortality, and hemorrhagic complications was increased in patients with more severe stress hyperglycemia after IVT only in the absence of prior diabetic history. It is not clear whether the same results would occur to those after MT instead of IVT. In another study, the same research group has concluded that patients with AIS and severe stress hyperglycemia tend to have poor outcome at 3 months after undergoing MT. However, whether the poor outcome was due to premorbid diabetes mellitus (DM) or stress hyperglycemia remains largely unknown (Merlino et al., 2021a). One available study notes that the predictive power of stress-induced hyperglycemia ratio (>0.96) for adverse outcome is not significant in diabetic patients. However, this finding is based on a small sample size (n = 39) (Chen et al., 2019). Overall, there is an insufficient number of studies to determine an association between hyperglycemia and clinical outcomes after MT.
This study was designed to assess whether stress-induced hyperglycemia could function as a predictor of AIS patients’ poor outcomes who had undergone emergent endovascular treatment (EVT). Large samples were obtained to definitively address differences in stress-induced hyperglycemia and prognosis between DM and non-DM patients with AIS.
Materials and methods
Study population
A retrospective observational study was performed by collecting data from patients with AIS due to large vessel occlusion (LVO) from March 2019 to June 2022. The patients underwent EVT (mechanical thrombectomy and/or stent implantation) in Beijing Luhe Hospital, Capital Medical University, within 24 h from the onset of symptom(s). Each patient was assessed by an experienced neurologist. The inclusion criteria for enrollment were: (1) Age ≥ 18 years, (2) diagnosis of AIS based on the definition established by the World Health Organization, and (3) EVT. The exclusion criteria were: (1) no data on fasting plasma glucose or HbA1C and (2) lost to follow up.
Data collection and clinical assessment
Diagnostic studies were obtained, including neurologic examinations, blood tests, echocardiography, electrocardiograph, and appropriate brain imaging studies. Relevant past medical histories were acquired, such as hypertension, DM, coronary heart disease, atrial fibrillation, smoking, hyperlipemia, and past ischemic stroke. Fasting plasma specimens were collected within 24 h from the onset of symptoms. Diabetic patients were defined as those with a history of diabetes mellitus or HbA1c ≥ 6.5%. The stroke subtypes were confirmed by 2 neurologists according to TOAST. Stroke severity was assessed on admission and at discharge by certified examiners, using the National Institutes of Health Stroke Scale (NIHSS). All patients were assessed on 3 months, using modified Rankin Scale (mRS) and poor prognosis was defined as mRS>2. Mortality within 7 and 30 days and presence of sICH or intracranial hemorrhage (ICH) were checked as part of the routine clinical practice.
Assessment of stress hyperglycemia
There is no standardized diagnostic criteria for acute stress hyperglycemia. Random blood glucose or fasting blood glucose levels are generally measured when analyzing stress-induced hyperglycemia after AIS. However, these serum studies are often influenced by eating and/or previous glucose status.
Fasting glucose levels were measured after admission to diagnose “absolute” stress hyperglycemia since most of the blood samples were collected from 12 to 24 h after AIS. Fasting plasma glucose (mmol/l) to HbA1c ratio (GAR) was measured to diagnose “relative” hyperglycemia since GAR minimizes the impact of diet and previous blood glucose status and was associated with critical illness (Roberts et al., 2015, 2021; Su et al., 2017; Li et al., 2020). The diabetic and non-diabetic groups were divided by applying quartiles of GAR. The quartiles were used to assess whether higher GAR was associated with worse prognosis in these two groups. GAR quartiles were applied to divide the patients into four groups (Q1–Q4).
Statistical analysis
Statistic package for social science (SPSS) 19.0 software was used to analyze the data. Categorical variables were presented in frequencies and percentages from chi-square test or Fisher’s exact test. Continuous variables were expressed as means with standard deviations and statistical analyses were performed using one-way ANOVA test and t-test. Binary logistic regression analysis was used to assess the effect of stress hyperglycemia, using the lowest GAR index as a reference and comparing prognosis. The regression model was adjusted for all potential confounders. The probability value for univariate analysis was less than 0.1. The specific variables for the adjustments considered in each analysis were indicated in Table 1. p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 589 patients were treated by EVT. Seven patients without fasting glucose or HbA1c values and six patients who were lost to follow-up were excluded. The remaining patients (n = 576) were divided into DM (n = 230) and non-DM (n = 376) group. The two groups were then divided into 4 quartiles according to GAR (Figure 1).
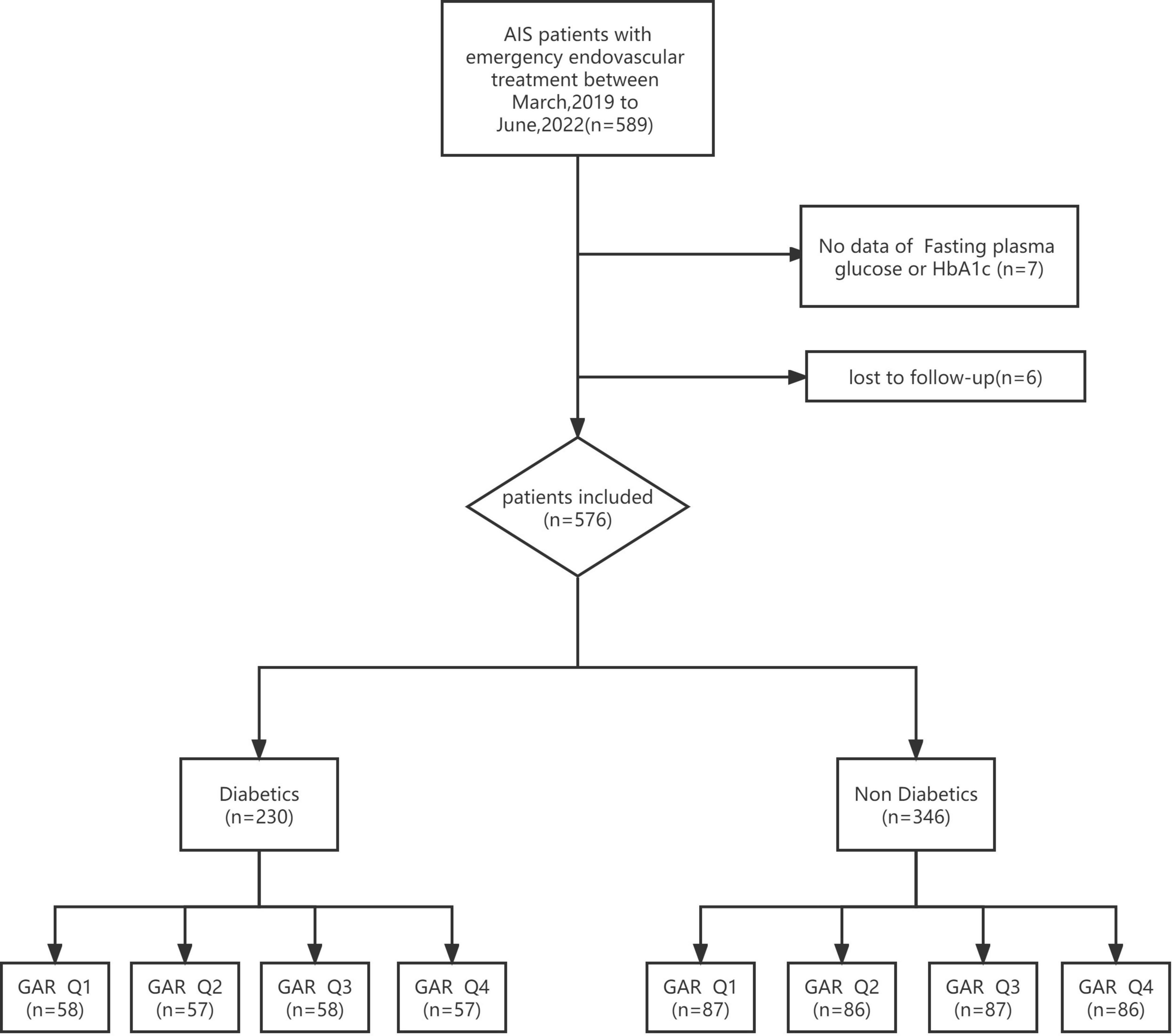
Figure 1. Flowchart of study participants. AFIS, acute ischemic stroke; HbA1c, glycated hemoglobin; GAR Q1, first glucose-to-glycated hemoglobin ratio quartiles; GAR Q2, second glucose-to-glycated hemoglobin ratio quartiles; GAR Q3, third glucose-to-glycated hemoglobin ratio quartiles; GAR Q4, fourth glucose-to-glycated hemoglobin ratio quartiles.
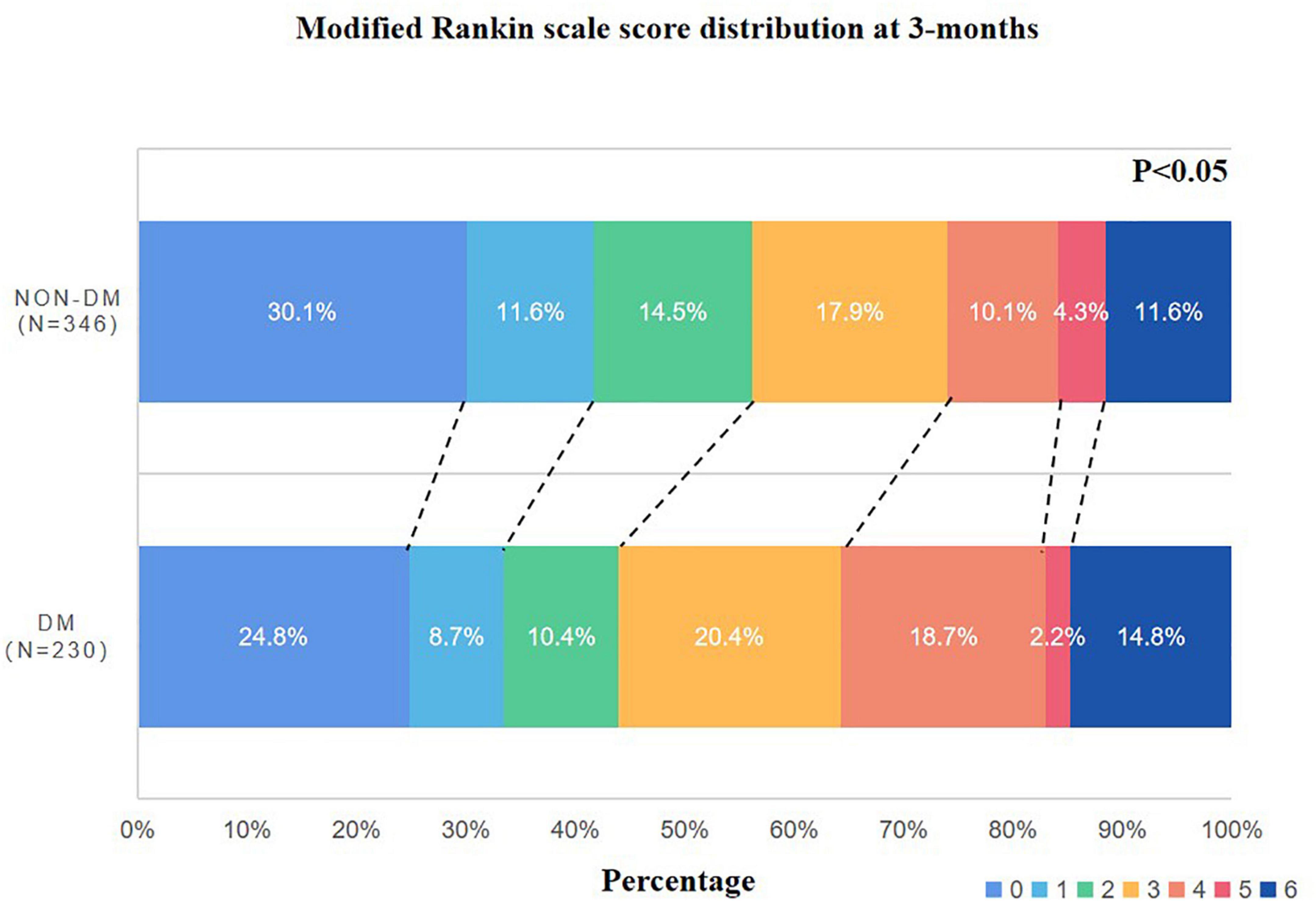
In the DM group, poor outcomes were found in 122 patients (53%) and the GAR level was 1.27 ± 0.44. These two parameters were higher than the non-DM group (p < 0.05, respectively) (Table 2). The mRS distribution was different between the two groups; more patients with DM had higher mRS scores (Figure 2).
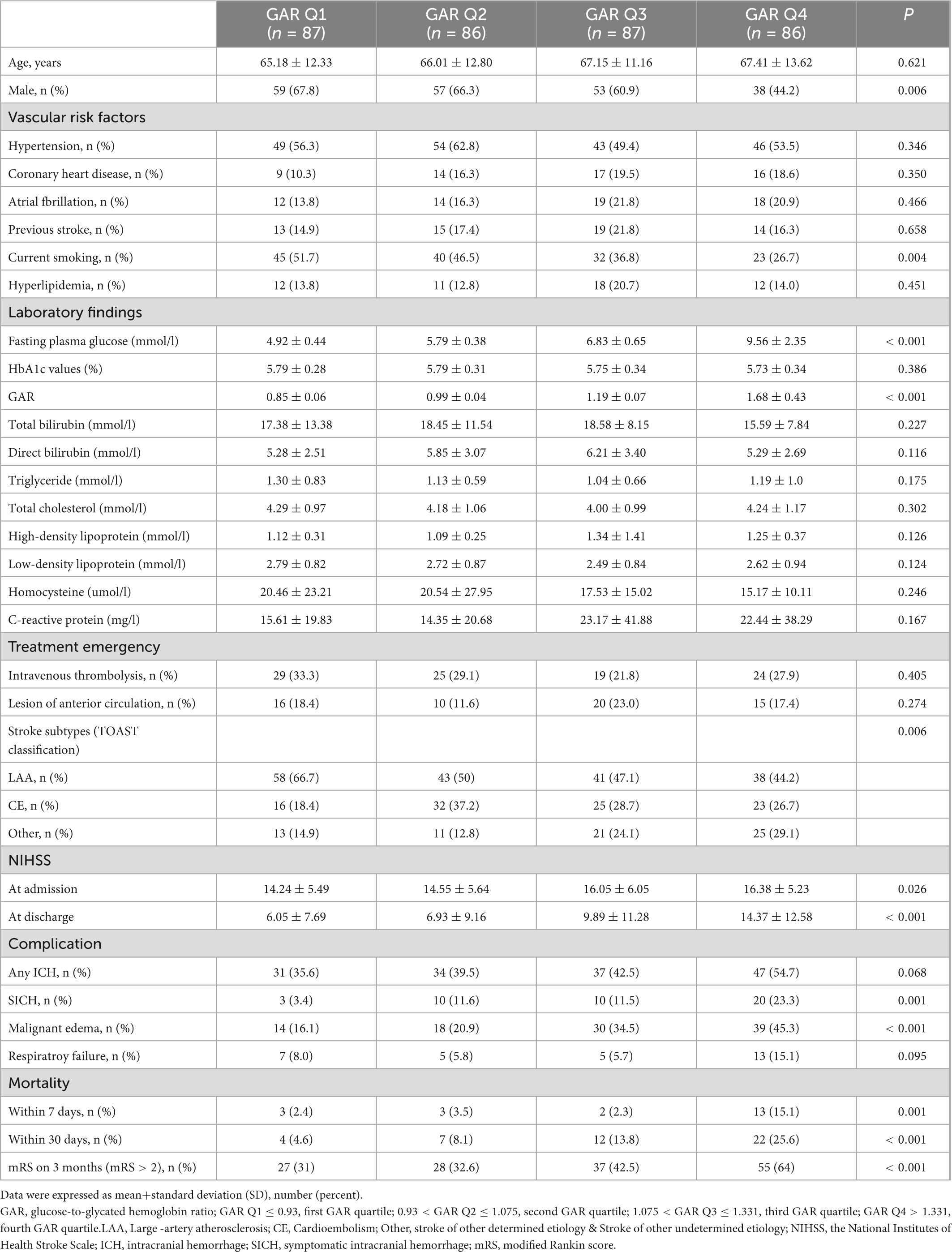
Table 2. Demographics, clinical data, complications and functional outcomes of non-diabetic patients.
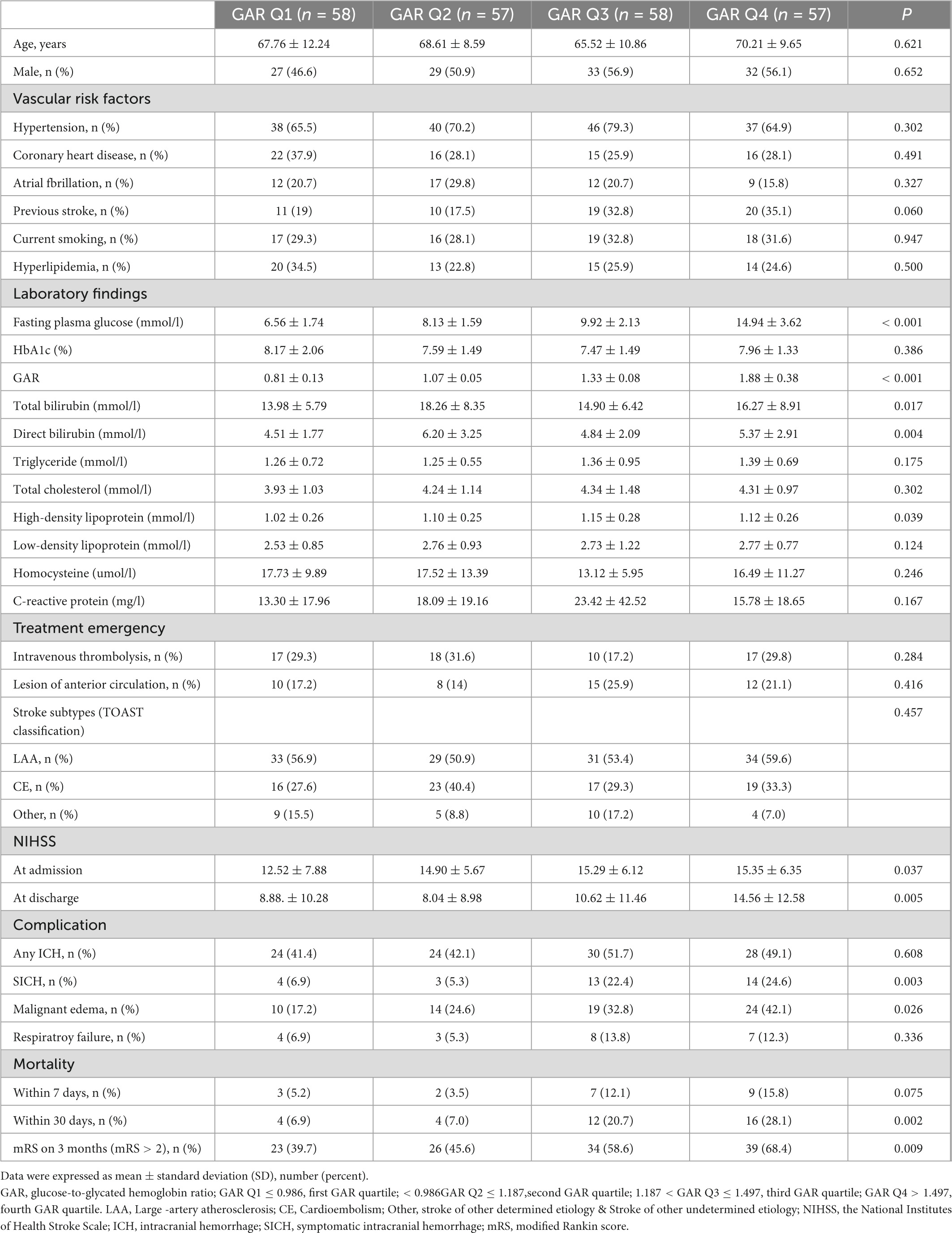
The following numbers of GAR quartiles represented patients without DM: 87 (25.1%) in the first and third quartiles, and 86 patients (24.9%) in the second and fourth quartiles. The general characteristics of each GAR quartile were assessed (Table 3). Compared to the other three quartiles, the fourth quartile had a significantly lower rate of male gender, current smoking history, and large-artery atherosclerosis (LAA) stroke, but higher rate of fasting plasma glucose, NIHSS at admission and at discharge, sICH, malignant edema, mortality within 7 and 30 days, and poor outcome in 3-month (p < 0.05, respectively). Patients with more severe stress hyperglycemia had a higher prevalence of poor outcome at 3 months (GAR Q1, 31%; Q2, 32.6%; Q3, 42.5%; Q4, 64%; p < 0.01) (Figure 3).
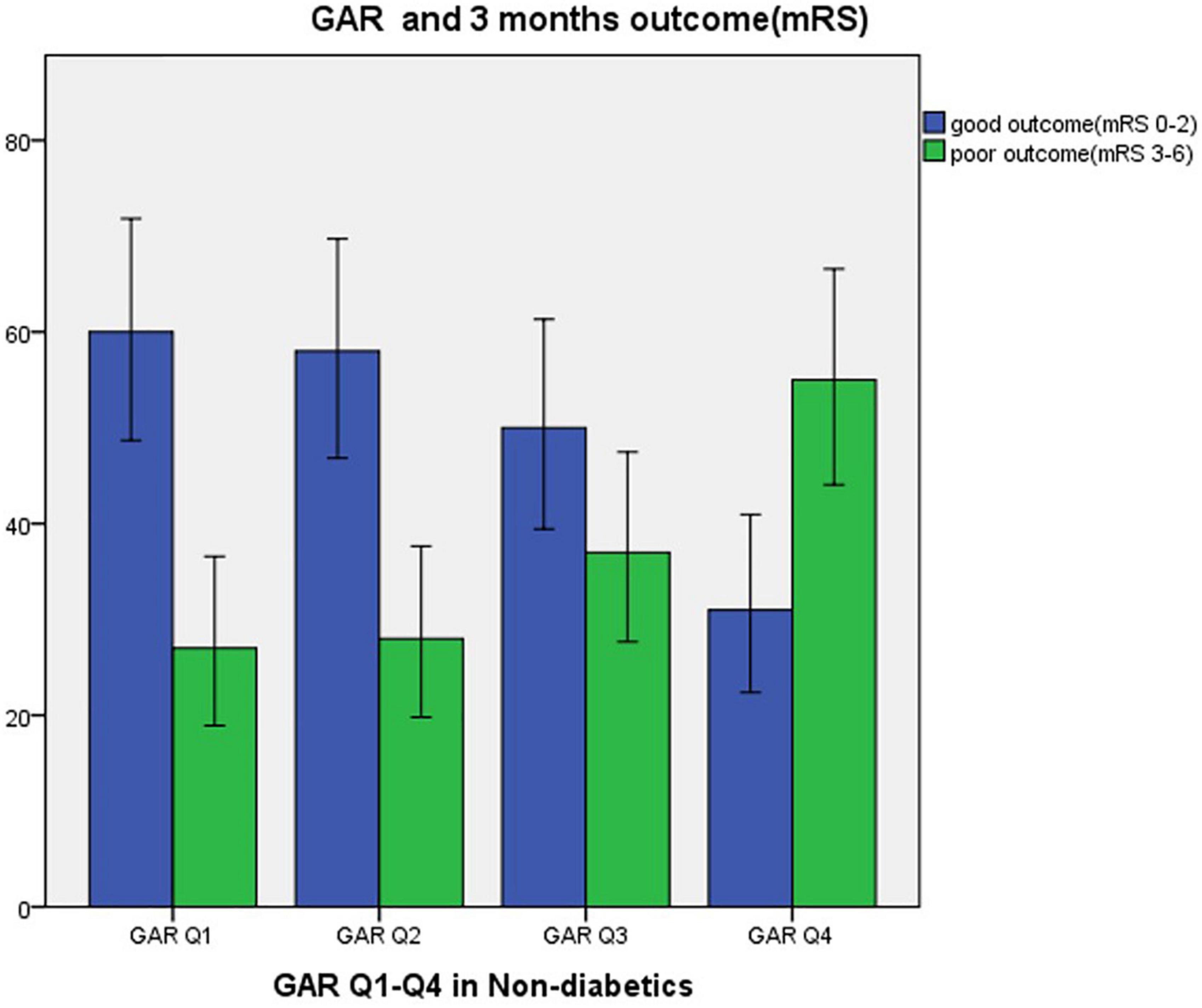
Figure 3. Three months outcome by GAR quartile in non-diabetic patients. GAR Q1, first glucose-to-glycated hemoglobin ratio quartile; GAR Q2, second glucose-to-glycated hemoglobin ratio quartile; GAR Q3, third glucose-to-glycated hemoglobin ratio quartile; GAR Q4, fourth glucose-to-glycated hemoglobin ratio quartile.
The following numbers of GAR quartiles represented patients with DM: 58 (25.2%) in the first and third quartiles, and 57 (24.8%) in the second and fourth quartiles (Table 4). There were no significant differences in age, sex, or vascular risk factors among the four groups. The fourth GAR quartile had higher fasting plasma glucose, total bilirubin, direct bilirubin, and NIHSS at admission and discharge. The fourth quartile had a significantly higher rate of sICH, malignant edema, mortality within 30 days, and poor outcome at 3-month. The etiology of AIS was assessed and there was no significant difference among the four quartiles.
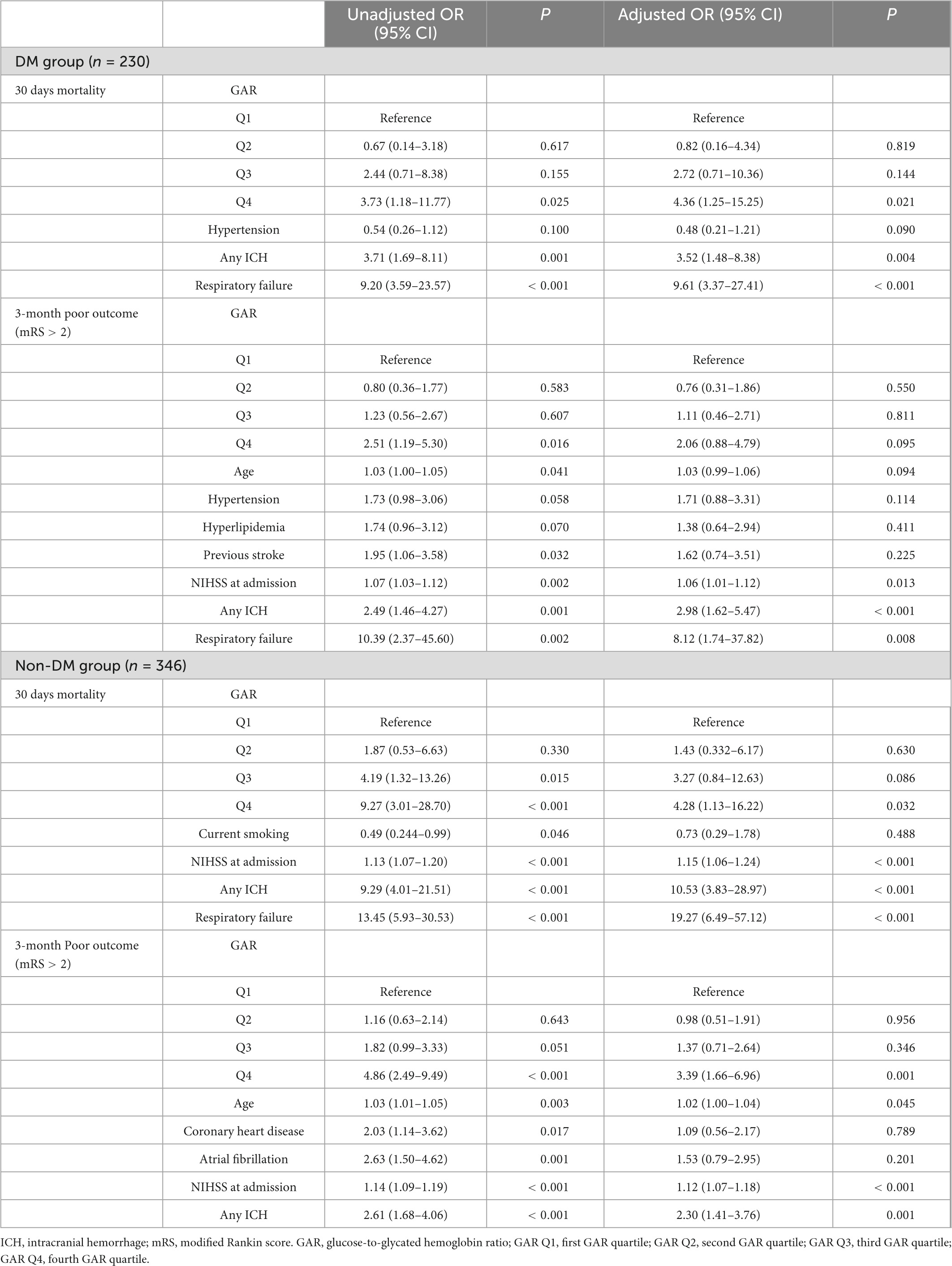
Table 4. Outcome Complications by Logistic Regression Model [ORs (95% CIs) of outcome measures adjusted for GAR quartiles].
Binary logistic regression analysis of stress-induced hyperglycemia and clinical outcomes
The results noted, by comparing the lowest quartile, the fourth quartile of GAR in the non-DM group was independently associated with poor prognosis on 3-month (OR 3.39, 95% CI 1.66–6.96, p = 0.001) after adjusting risk factors. In addition, by comparing to the lowest quartile in both DM and non-DM group, GAR in the fourth quartile were found to be independently linked with 30 days mortality (OR 4.36, 95% CI 1.25–15.25, p = 0.021) and (OR 4.28, 95% CI 1.13–16.22, p = 0.032) after adjusting the confounders. Additionally, intracranial hemorrhage was an independent indicator for 30 days mortality and poor prognosis at 3-month both in DM and non-DM group. NIHSS at admission independently associated with poor prognosis at 3-month in two groups while not associated with 30 days mortality in DM group. Respiratory failure during hospitalization had a relation to 30 days mortality in the two groups and was associated with poor prognosis at 3-month in the DM group (Table 1).
Discussion
The present study has demonstrated that the impact of stress hyperglycemia on 3-month prognosis in AIS patients after EVT was influenced by premorbid diabetic status. Although overall outcomes of DM patients appear to be worse than non-DM patients, those without history of diabetes showed significantly worse outcomes at 3 months after developing stress-induced hyperglycemia.
Recent clinical studies have defined stress hyperglycemia using the fasting blood glucose within 24 h (Merlino et al., 2021a,b; Mi et al., 2022; Ngiam et al., 2022; Tao et al., 2022). In fact, an animal study revealed a leak level of glucose after 24 h of focal cerebral ischemia and this practically normalized on the third day (Harada et al., 2009). In addition, a recent study of rats with global cerebral ischemia demonstrated decreases in hepatic expression of mRNA for pyruvate carboxylase (50%), PEPCK (56%), and G6Pase (80%) at 24 h after ischemia, which resulted in profoundly reduced gluconeogenesis in the liver (Sá-Nakanishi et al., 2020). A clinical study showed that persistent hyperglycemia in 24–48 h after stroke onset was not associated with worse functional prognosis at 3 months, regardless of patients’ diabetic history (Ntaios et al., 2011).
Glycated hemoglobin ratio has been considered a novel marker of poor outcomes in patients with ischemic stroke after IVT and MT (Chen et al., 2019; Tsivgoulis et al., 2019; Suissa et al., 2020; Merlino et al., 2021a; Shen et al., 2021; Cannarsa et al., 2022). This study compared GAR and poor prognosis in patients treated by EVT, based on their history of DM. DM group had more patients with poor prognosis and higher GAR. Patients with DM showed more severe stress-related hyperglycemia and worse prognosis at 3-month than non-DM group. Each group was divided into four quartiles to detect associations between GAR and clinical outcomes and any confounding factors for the above findings.
The results initially noted that elevated GAR was associated with a 3-month poor prognosis and mortality at 30 days in DM and non-DM groups. However, elevated GAR did not directly indicate a 3-month poor prognosis in DM group after adjusting several concomitant variables by logistics regression. This, overall, illustrated the different responses from DM vs. non-DM patients to stress-induced hyperglycemia associated with LVO stroke. Furthermore, types of stroke appeared to be different in non-DM patients (less LAA) with the highest GAR quartile, suggesting an alternative etiology that needs further investigation.
Controlling glucose levels to the range of 140–180 mg/dl (7.7–10 mmol/L) after AIS is advised by the American Heart Association/American Stroke Association and the European Stroke Organization [European Stroke Organisation (Eso) Executive Committee, 2008; Rabinstein et al., 2019]. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018 suggest maintaining blood glucose from 7.8 to 10 mmol/l (Zhong et al., 2018). Many randomized and open observational studies, however, refute these suggestions. The 24-h post-ischemic intense insulin injection for AIS patients has not shown any advantage in the randomized UK Glucose Insulin in Stroke Trial (GIST-UK) (Gray et al., 2007). There has been no discernible positive functional effect from intensive insulin therapy keeping blood glucose within the range of 4–7.5 mmol/L (Bellolio et al., 2014; Ntaios et al., 2014; Yamada et al., 2017; Johnston et al., 2019; Kamal et al., 2020; Hollist et al., 2021).
However, patients without history of DM who develop stress-induced hyperglycemia appear to benefit greatly from the glycemic control treatment. For instance, critical patients without pre-existing DM have shown better clinical outcomes with blood glucose management than those with history DM (Krinsley, 2006). Also, differences between patients with and without DM have been found in time-weighted average glucose levels, the occurrence of hypoglycemia, and variability of blood glucose levels, ultimately impacting their clinical outcomes; patients with DM have a more blunted response to hypo- and hyperglycemia and changes in blood glucose levels (Fong et al., 2022). Similar findings seem to be present with stress-induced hyperglycemia associated with AIS, which emphasizes the importance of previous glycemic status. This may guide future studies on therapeutic options for patients with AIS.
The different clinical outcomes from stress-induced hyperglycemia in patients with and without diabetes can also be attributed to pathologic changes in response to chronic hyperglycemia (Ma et al., 2020; Ferrari et al., 2022). High serum blood glucose can notably influence cerebral vascular structure and body metabolism (Ergul et al., 2012). The hyperglycemic state may promote high levels of plasma plasminogen activator inhibitor-1 (PAI-1), which can be responsible for hypercoagulative status in ischemic stroke (Tjärnlund-Wolf et al., 2012; Chen et al., 2017). Advanced glycation end products (AGEs) and their receptors are found in patients with DM and they increase the production of reactive oxygen species (ROS), resulting in the activation of the nuclear transcription factor (NF-κB) and transcription of pro-inflammatory genes, IL-1, IL-6, and TNF-α (Indyk et al., 2021). AGEs are associated with diabetic complications from atherosclerosis and involved in the aging of blood vessels and numerous sequential damages (Toprak and Yigitaslan, 2019; Ahmad et al., 2020). More studies are necessary to determine if stress hyperglycemia is responsible for worse outcomes with or without EVT or if this is a marker of sympathetic/metabolic derangement. This question may ultimately help target the root cause of the poor outcomes.
The mechanism of stress-induced hyperglycemia after stroke appears to be due to consequences of increased hepatic gluconeogenesis and reduced sensitivity to insulin (Liao et al., 2020). Upregulation of hepatic gluconeogenesis has been noted in hyperglycemic animal models after focal brain ischemia (Harada et al., 2012; Wang et al., 2014). Increased levels of glucagon, corticosterone, and norepinephrine in response to post-stroke hyperglycemia promotes hepatic gluconeogenesis (Chen et al., 2016). Acute brain ischemia has been found to enhance inflammatory pathways, the expression of hepatic TNF-α, and activities of intracellular NF-κB from catecholamine release, which ultimately results in hepatic insulin resistance (Wong et al., 2011; Belayev et al., 2020; Wu et al., 2021). Furthermore, levels of NF-κB and TNF-α in diabetic patients are different from those in non-diabetic patients. Chronic hyperglycemia and the changes of stress hormones after AIS could lead to higher GAR and worse prognosis in diabetic patients in addition to the damages caused by chronic metabolism derangement and vascular damage.
Drugs for glycemic control may have neuroprotective effects. Acarbose can exert neuroprotective effects by preventing mitochondrial and lysosomal dysfunction. It also modifies gene expression related to inflammation, cell survival, and regeneration by suppressing P53 protein which performs as a signaling point for the convergence of necrosis and apoptosis in cerebral ischemia (Tseng, 2020; Cozene et al., 2021; Das et al., 2022). Meformin improves neurological functions of acute stroke patients with DM II and the oxidative stress levels (Zhao et al., 2019). Selective agonists of glucagon-like peptide-1 receptors (GLP-1Ras) have shown neuroprotective effects by exerting anti-apoptotic and anti-edema actions and promoting microcirculation and the brain-blood-barrier integrity (Zhu et al., 2016; Shan et al., 2019; Zhao et al., 2020). Clinical studies have confirmed that the beneficial impact of GLP-1 receptor agonists in treating hyperglycemia of AIS patient (Daly et al., 2013).
There were a few limitations in this study. First, this was a retrospective and single-center study. Unforeseen errors may have occurred because of the nature of the study design and discrepancies in practicing medicine among different geographic regions. Secondly, studies in the past have shown that stress-induced hyperglycemia peaks in 24 h and returns to normal on the third day (Harada et al., 2009); fasting glucose levels were measured within 24 h of the onset of symptom in this study. Finally, glycemic variability (GV), another important component of abnormal glucose levels associated with worse functional outcomes in AIS patients with DM (Kim et al., 2017), was not included in this study.
Conclusion
History of DM seems to influence outcomes of patients with AIS treated by EVT. Contrary to the non-DM group, stress-induced hyperglycemia is not an independent predictor of poor outcome for patients with DM. This may be due to adaptive responses to chronically high serum glucose levels and glucose metabolism. Highest quartile of non-DM group stress hyperglycemic responders appear to behave differently than the other groups from cerebrovascular etiology perspective. These findings may have roles in future study about neuroprotection and improving outcomes of advanced stress hyperglycemic responders.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
Each study participant signed a consent form before undergoing EVT. A consent waiver from the Ethics Committee of Beijing Luhe Hospital was received because of the retrospective study design.
Author contributions
HD: conceptualization, methodology, software, formal analysis, and writing—original draft. HY: writing and editing original draft. GR: writing and review. FC: investigation and software. YW: data curation and methodology. JL: data curation and investigation. LC: resources and supervision. YT: software and validation. ZC: visualization. XG and YD: conceptualization, funding acquisition, resources, supervision, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by the Beijing Tongzhou District Financial Fund (2022), the Laboratory Development Funds of Beijing Luhe Hospital, and the Yunhe talent Program of Beijing Tongzhou District (2022).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, M. N., Farah, A. I., and Al-Qirim, T. M. (2020). The cardiovascular complications of diabetes: A striking link through protein glycation. Rom. J. Intern. Med. 58, 188–198. doi: 10.2478/rjim-2020-0021
Ahmed, N., Dávalos, A., Eriksson, N., Ford, G., Glahn, J., Hennerici, M., et al. (2010). association of admission blood glucose and outcome in patients treated with intravenous thrombolysis: Results from the safe implementation of treatments in stroke international stroke thrombolysis register (SITS-ISTR). Arch. Neurol. 67, 1123–1130. doi: 10.1001/archneurol.2010.210
Almobarak, A., Badi, S., Elmadhoun, W., Tahir, H., and Ahmed, M. (2020). The prevalence and risk factors of stroke among sudanese individuals with diabetes: Cross-sectional survey. Brain Circ. 6, 26–30. doi: 10.4103/bc.bc_15_19
Belayev, L., Obenaus, A., Mukherjee, P., Knott, E., Khoutorova, L., Reid, M., et al. (2020). Blocking pro-inflammatory platelet-activating factor receptors and activating cell survival pathways: A novel therapeutic strategy in experimental ischemic stroke. Brain Circ. 6, 260–268. doi: 10.4103/bc.bc_36_20
Bellolio, M. F., Gilmore, R. M., and Ganti, L. (2014). Insulin for glycaemic control in acute ischaemic stroke. Cochrane Database Syst. Rev. Cd005346. doi: 10.1002/14651858.CD005346.pub4
Cannarsa, G., Wessell, A., Chryssikos, T., Stokum, J., Kim, K., De Paula Carvalho, H., et al. (2022). Initial stress hyperglycemia is associated with malignant cerebral edema, hemorrhage, and poor functional outcome after mechanical thrombectomy. Neurosurgery 90, 66–71. doi: 10.1227/NEU.0000000000001735
Chen, R., Yan, J., Liu, P., Wang, Z., and Wang, C. (2017). Plasminogen activator inhibitor links obesity and thrombotic cerebrovascular diseases: The roles of PAI-1 and obesity on stroke. Metab. Brain Dis. 32, 667–673. doi: 10.1007/s11011-017-0007-3
Chen, W., Mao, F., Liu, C., Kuan, Y., Lai, N., Wu, C., et al. (2016). Chromium supplementation improved post-stroke brain infarction and hyperglycemia. Metab. Brain Dis. 31, 289–297. doi: 10.1007/s11011-015-9749-y
Chen, X., Liu, Z., Miao, J., Zheng, W., Yang, Q., Ye, X., et al. (2019). High stress hyperglycemia ratio predicts poor outcome after mechanical thrombectomy for ischemic stroke. J. Stroke Cerebrovasc. Dis. 28, 1668–1673. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.022
Christensen, H., Boysen, G., and Johannesen, H. H. (2004). Serum-cortisol reflects severity and mortality in acute stroke. J. Neurol. Sci. 217, 175–180. doi: 10.1016/j.jns.2003.09.013
Cozene, B., Russo, E., Anzalone, R., Rocca, G., and Borlongan, C. (2021). Mitochondrial activity of human umbilical cord mesenchymal stem cells. Brain Circ. 7, 33–36. doi: 10.4103/bc.bc_15_21
Daly, S., Chemmanam, T., Loh, P., Gilligan, A. K., Dear, A. E., Simpson, R., et al. (2013). Exenatide in acute ischemic stroke. Int. J. Stroke 8:E44. doi: 10.1111/ijs.12073
Das, J., Mahammad, F. S., and Krishnamurthy, R. G. (2022). An integrated chemo-informatics and in vitro experimental approach repurposes acarbose as a post-ischemic neuro-protectant. 3 Biotech. 12:71. doi: 10.1007/s13205-022-03130-5
Ergul, A., Alhusban, A., and Fagan, S. C. (2012). Angiogenesis: A harmonized target for recovery after stroke. Stroke 43, 2270–2274. doi: 10.1161/STROKEAHA.111.642710
European Stroke Organisation (Eso) Executive Committee, and Eso Writing Committee. (2008). Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc. Dis. 25, 457–507. doi: 10.1159/000131083
Ferrari, F., Moretti, A., and Villa, R. F. (2022). Hyperglycemia in acute ischemic stroke: Physiopathological and therapeutic complexity. Neural Regen. Res. 17, 292–299. doi: 10.4103/1673-5374.317959
Fong, K. M., Au, S. Y., and Ng, G. W. Y. (2022). Glycemic control in critically ill patients with or without diabetes. BMC Anesthesiol. 22:227. doi: 10.1186/s12871-022-01769-4
Gray, C., Hildreth, A., Sandercock, P., O’Connell, J., Johnston, D., Cartlidge, N., et al. (2007). Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: The UK glucose insulin in stroke trial (GIST-UK). Lancet Neurol. 6, 397–406. doi: 10.1016/S1474-4422(07)70080-7
Harada, S., Fujita, W., Shichi, K., and Tokuyama, S. (2009). The development of glucose intolerance after focal cerebral ischemia participates in subsequent neuronal damage. Brain Res. 1279, 174–181. doi: 10.1016/j.brainres.2009.05.014
Harada, S., Fujita-Hamabe, W., and Tokuyama, S. (2012). Ischemic stroke and glucose intolerance: A review of the evidence and exploration of novel therapeutic targets. J. Pharmacol. Sci. 118, 1–13. doi: 10.1254/jphs.11R04CR
Hollist, M., Morgan, L., Cabatbat, R., Au, K., Kirmani, M., and Kirmani, B. (2021). Acute stroke management: Overview and recent updates. Aging Dis. 12, 1000–1009. doi: 10.14336/AD.2021.0311
Indyk, D., Bronowicka-Szydełko, A., Gamian, A., and Kuzan, A. (2021). Advanced glycation end products and their receptors in serum of patients with type 2 diabetes. Sci. Rep. 11:13264. doi: 10.1038/s41598-021-92630-0
Jiang, Y., Liu, N., Han, J., Li, Y., Spencer, P., Vodovoz, S., et al. (2021). Diabetes mellitus/poststroke hyperglycemia: A detrimental factor for tPA thrombolytic stroke therapy. Transl. Stroke Res. 12, 416–427. doi: 10.1007/s12975-020-00872-3
Johnston, K., Bruno, A., Pauls, Q., Hall, C., Barrett, K., Barsan, W., et al. (2019). Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: The shine randomized clinical trial. JAMA 322, 326–335. doi: 10.1001/jama.2019.9346
Kamal, H., Ahmed, M., Zha, A., Lail, N., Shirani, P., Sawyer, R., et al. (2020). Strokes occurring in the hospital: Symptom recognition and eligibility for treatment in the intensive care units versus hospital wards. Brain Circ. 6, 196–199. doi: 10.4103/bc.bc_24_20
Kim, Y., Kim, C., Jung, K., Kwon, H., Heo, S., Kim, B., et al. (2017). Range of glucose as a glycemic variability and 3-month outcome in diabetic patients with acute ischemic stroke. PLoS One 12:e0183894. doi: 10.1371/journal.pone.0183894
Krinsley, J. S. (2006). Glycemic control, diabetic status, and mortality in a heterogeneous population of critically ill patients before and during the era of intensive glycemic management: Six and one-half years experience at a university-affiliated community hospital. Semin. Thorac. Cardiovasc. Surg. 18, 317–325. doi: 10.1053/j.semtcvs.2006.12.003
Li, J., Quan, K., Wang, Y., Zhao, X., Li, Z., Pan, Y., et al. (2020). Effect of stress hyperglycemia on neurological deficit and mortality in the acute ischemic stroke people with and without diabetes. Front. Neurol. 11:576895. doi: 10.3389/fneur.2020.576895
Li, W., Moore-Langston, S., Chakraborty, T., Rafols, J., Conti, A., and Ding, Y. (2013). Hyperglycemia in stroke and possible treatments. Neurol. Res. 35, 479–491. doi: 10.1179/1743132813Y.0000000209
Liao, K., Chen, C., Hsieh, S., Pan, P., and Chen, W. (2020). Interleukin-13 ameliorates postischemic hepatic gluconeogenesis and hyperglycemia in rat model of stroke. Metab. Brain Dis. 35, 1201–1210. doi: 10.1007/s11011-020-00596-1
Ma, W., Tang, J., Lei, Z., Li, C., Zhao, L., Lin, C., et al. (2020). Potential biochemical mechanisms of brain injury in diabetes mellitus. Aging Dis. 11, 978–987. doi: 10.14336/AD.2019.0910
Merlino, G., Pez, S., Gigli, G. L., Sponza, M., Lorenzut, S., Surcinelli, A., et al. (2021a). Stress hyperglycemia in patients with acute ischemic stroke due to large vessel occlusion undergoing mechanical thrombectomy. Front. Neurol. 12:725002. doi: 10.3389/fneur.2021.725002
Merlino, G., Pez, S., Tereshko, Y., Gigli, G., Lorenzut, S., Surcinelli, A., et al. (2022). Stress hyperglycemia does not affect clinical outcome of diabetic patients receiving intravenous thrombolysis for acute ischemic stroke. Front. Neurol. 13:903987. doi: 10.3389/fneur.2022.903987
Merlino, G., Smeralda, C., Gigli, G., Lorenzut, S., Pez, S., Surcinelli, A., et al. (2021b). Stress hyperglycemia is predictive of worse outcome in patients with acute ischemic stroke undergoing intravenous thrombolysis. J. Thromb. Thrombolysis 51, 789–797. doi: 10.1007/s11239-020-02252-y
Mi, D., Li, Z., Gu, H., Jiang, Y., Zhao, X., Wang, Y., et al. (2022). Stress hyperglycemia is associated with in-hospital mortality in patients with diabetes and acute ischemic stroke. CNS Neurosci. Ther. 28, 372–381. doi: 10.1111/cns.13764
Ngiam, J., Cheong, C., Leow, A., Wei, Y., Thet, J., Lee, I., et al. (2022). Stress hyperglycaemia is associated with poor functional outcomes in patients with acute ischaemic stroke after intravenous thrombolysis. QJM 115, 7–11. doi: 10.1093/qjmed/hcaa253
Ntaios, G., Abatzi, C., Alexandrou, M., Lambrou, D., Chatzopoulos, S., Egli, M., et al. (2011). Persistent hyperglycemia at 24-48 h in acute hyperglycemic stroke patients is not associated with a worse functional outcome. Cerebrovasc. Dis. 32, 561–566.
Ntaios, G., Papavasileiou, V., Bargiota, A., Makaritsis, K., and Michel, P. (2014). Intravenous insulin treatment in acute stroke: A systematic review and meta-analysis of randomized controlled trials. Int. J. Stroke 9, 489–493. doi: 10.1111/ijs.12225
Rabinstein, A., Ackerson, T., Adeoye, O., Bambakidis, N., Becker, K., Biller, J., et al. (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 50, e344–e418. doi: 10.1161/STR.0000000000000215
Robbins, N. M., and Swanson, R. A. (2014). Opposing effects of glucose on stroke and reperfusion injury: Acidosis, oxidative stress, and energy metabolism. Stroke 45, 1881–1886. doi: 10.1161/STROKEAHA.114.004889
Roberts, G., Quinn, S., Valentine, N., Alhawassi, T., O’Dea, H., Stranks, S., et al. (2015). Relative hyperglycemia, a marker of critical illness: Introducing the stress hyperglycemia ratio. J. Clin. Endocrinol. Metab. 100, 4490–4497. doi: 10.1210/jc.2015-2660
Roberts, G., Sires, J., Chen, A., Thynne, T., Sullivan, C., Quinn, S., et al. (2021). A comparison of the stress hyperglycemia ratio, glycemic gap, and glucose to assess the impact of stress-induced hyperglycemia on ischemic stroke outcome. J. Diabetes 13, 1034–1042. doi: 10.1111/1753-0407.13223
Rosso, C., Baronnet, F., Diaz, B., Le Bouc, R., Frasca Polara, G., Moulton, E., et al. (2018). The silver effect of admission glucose level on excellent outcome in thrombolysed stroke patients. J. Neurol. 265, 1684–1689. doi: 10.1007/s00415-018-8896-6
Sá-Nakanishi, A., de Oliveira, M., Pateis, V., Silva, L., Pereira-Maróstica, H., Gonçalves, G., et al. (2020). Glycemic homeostasis and hepatic metabolism are modified in rats with global cerebral ischemia. Biochim. Biophys. Acta Mol. Basis Dis. 1866:165934. doi: 10.1016/j.bbadis.2020.165934
Shan, Y., Tan, S., Lin, Y., Liao, S., Zhang, B., Chen, X., et al. (2019). The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke. J. Neuroinflammation 16:242. doi: 10.1186/s12974-019-1638-6
Shen, C., Xia, N., Wang, H., and Zhang, W. (2021). Association of stress hyperglycemia ratio with acute ischemic stroke outcomes post-thrombolysis. Front. Neurol. 12:785428. doi: 10.3389/fneur.2021.785428
Su, Y., Hsu, C., Guo, Y., and Chen, H. (2017). Usefulness of the plasma glucose concentration-to-HbA1c ratio in predicting clinical outcomes during acute illness with extreme hyperglycaemia. Diabetes Metab. 43, 40–47. doi: 10.1016/j.diabet.2016.07.036
Suissa, L., Panicucci, E., Perot, C., Romero, G., Gazzola, S., Laksiri, N., et al. (2020). Effect of hyperglycemia on stroke outcome is not homogeneous to all patients treated with mechanical thrombectomy. Clin. Neurol. Neurosurg. 194:105750. doi: 10.1016/j.clineuro.2020.105750
Tao, J., Hu, Z., Lou, F., Wu, J., Wu, Z., Yang, S., et al. (2022). Higher stress hyperglycemia ratio is associated with a higher risk of stroke-associated pneumonia. Front. Nutr. 9:784114. doi: 10.3389/fnut.2022.784114
Tjärnlund-Wolf, A., Brogren, H., Lo, E., and Wang, X. (2012). Plasminogen activator inhibitor-1 and thrombotic cerebrovascular diseases. Stroke 43, 2833–2839. doi: 10.1161/STROKEAHA.111.622217
Toprak, C., and Yigitaslan, S. (2019). Alagebrium and Complications of Diabetes Mellitus. Eurasian J. Med. 51, 285–292. doi: 10.5152/eurasianjmed.2019.18434
Tseng, C. H. (2020). Dementia risk in type 2 diabetes patients: Acarbose use and its joint effects with metformin and pioglitazone. Aging Dis. 11, 658–667. doi: 10.14336/AD.2019.0621
Tsivgoulis, G., Katsanos, A., Mavridis, D., Lambadiari, V., Roffe, C., Macleod, M., et al. (2019). Association of baseline hyperglycemia with outcomes of patients with and without diabetes with acute ischemic stroke treated with intravenous thrombolysis: A propensity score-matched analysis from the SITS-ISTR registry. Diabetes 68, 1861–1869. doi: 10.2337/db19-0440
Wang, Y., Lin, S., Chuang, Y., Sheu, W., Tung, K., and Chen, C. (2014). Activation of hepatic inflammatory pathways by catecholamines is associated with hepatic insulin resistance in male ischemic stroke rats. Endocrinology 155, 1235–1246. doi: 10.1210/en.2013-1593
Wong, C., Jenne, C., Lee, W., Léger, C., and Kubes, P. (2011). Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 334, 101–105. doi: 10.1126/science.1210301
Wu, J., Wu, Z., He, A., Zhang, T., Zhang, P., Jin, J., et al. (2021). Genome-wide screen and validation of microglia pro-inflammatory mediators in stroke. Aging Dis. 12, 786–800. doi: 10.14336/AD.2020.0926
Yamada, T., Shojima, N., Noma, H., Yamauchi, T., and Kadowaki, T. (2017). Glycemic control, mortality, and hypoglycemia in critically ill patients: A systematic review and network meta-analysis of randomized controlled trials. Intensive Care Med. 43, 1–15. doi: 10.1007/s00134-016-4523-0
Yao, M., Ni, J., Zhou, L., Peng, B., Zhu, Y., Cui, L., et al. (2016). Elevated fasting blood glucose is predictive of poor outcome in non-diabetic stroke patients: A sub-group analysis of smart. PLoS One 11:e0160674. doi: 10.1371/journal.pone.0160674
Zhao, M., Li, X., Chen, Z., Hao, F., Tao, S., Yu, H., et al. (2019). Neuro-protective role of metformin in patients with acute stroke and type 2 diabetes mellitus via AMPK/mammalian target of rapamycin (mTOR) Signaling pathway and oxidative stress. Med. Sci. Monit. 25, 2186–2194. doi: 10.12659/MSM.911250
Zhao, W., Wu, C., Dornbos, D. III, Li, S., Song, H., and Wang, Y. (2020). Multiphase adjuvant neuroprotection: A novel paradigm for improving acute ischemic stroke outcomes. Brain Circ. 6, 11–18. doi: 10.4103/bc.bc_58_19
Zhong, D., Zhang, S., and Wu, B. (2018). Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin. J. Neurol. 51, 666–682.
Zhu, B., Pan, Y., Jing, J., Meng, X., Zhao, X., Liu, L., et al. (2019). Stress hyperglycemia and outcome of non-diabetic patients after acute ischemic stroke. Front. Neurol. 10:1003. doi: 10.3389/fneur.2019.01003
Keywords: stress-induced hyperglycemia, GAR index, premorbid diabetic status, large vessel occlusion stroke, outcome
Citation: Duan H, Yun HJ, Rajah GB, Che F, Wang Y, Liu J, Tong Y, Cheng Z, Cai L, Geng X and Ding Y (2023) Large vessel occlusion stroke outcomes in diabetic vs. non-diabetic patients with acute stress hyperglycemia. Front. Neurosci. 17:1073924. doi: 10.3389/fnins.2023.1073924
Received: 19 October 2022; Accepted: 06 January 2023;
Published: 27 January 2023.
Edited by:
Guo-Yuan Yang, Shanghai Jiao Tong University, ChinaReviewed by:
Guangwen Li, The Affiliated Hospital of Qingdao University, ChinaYang-Min Zheng, Capital Medical University, China
Copyright © 2023 Duan, Yun, Rajah, Che, Wang, Liu, Tong, Cheng, Cai, Geng and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaokun Geng,  eGdlbmdAY2NtdS5lZHUuY24=; Yuchuan Ding,
eGdlbmdAY2NtdS5lZHUuY24=; Yuchuan Ding,  eWRpbmdAbWVkLndheW5lLmVkdQ==
eWRpbmdAbWVkLndheW5lLmVkdQ==
 Honglian Duan
Honglian Duan Ho Jun Yun
Ho Jun Yun Gary Benjamin Rajah
Gary Benjamin Rajah Fengli Che
Fengli Che Yanling Wang1
Yanling Wang1 Yanna Tong
Yanna Tong Lipeng Cai
Lipeng Cai Xiaokun Geng
Xiaokun Geng Yuchuan Ding
Yuchuan Ding