
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci., 19 January 2023
Sec. Gut-Brain Axis
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1059552
This article is part of the Research TopicGut biodiversity and its influence in brain healthView all 6 articles
Necrotizing enterocolitis (NEC) is a common gastrointestinal disease of preterm infants with high morbidity and mortality. In survivors of NEC, one of the leading causes of long-term morbidity is the development of severe neurocognitive injury. The exact pathogenesis of neurodevelopmental delay in NEC remains unknown, but microbiota is considered to have dramatic effects on the development and function of the host brain via the gut-brain axis. In this review, we discuss the characteristics of microbiota of NEC, the impaired neurological outcomes, and the role of the complex interplay between the intestinal microbiota and brain to influence neurodevelopment in NEC. The increasing knowledge of microbial-host interactions has the potential to generate novel therapies for manipulating brain development in the future.
Necrotizing enterocolitis (NEC) is an inflammation intestinal disorder, with variable damage to the intestinal tract including mucosal injury, necrosis, and intestinal perforation (Zani and Pierro, 2015). The typical neonate with NEC is a preterm infant who suddenly presents with feeding intolerance, abdominal distension, bloody stools and signs of sepsis (Neu and Walker, 2011). Despite improvements in neonatal intensive care, the mortality rates for NEC survivors remained 30–50% (Hall et al., 2013). NEC survivors often have life-long consequences, including neurodevelopmental delay, failure to thrive, gastrointestinal strictures and adhesions, cholestasis, short bowel syndrome, or intestinal failure (Bazacliu and Neu, 2019). Although the pathophysiology of NEC is not clear, it is likely to be multifactorial. Immaturity of the gut, intestinal ischemia, formula feeding and microbial dysbiosis contribute to inducing an uncontrolled inflammatory response in the gut and lead to the development of NEC. During the critical period of development, intestinal inflammation may adversely affect the maturation process of neurons and immune system (Strunk et al., 2014; Gussenhoven et al., 2018).
Recent evidence suggests a relation between intestinal microbiota, dysbiosis, and NEC (Niemarkt et al., 2015; Neu and Pammi, 2018). The occurrence of NEC is due to bacterial colonization in the immature host environment. It may be that the baseline response of intestinal mucosa of preterm infants to microbial ligands is enhanced, resulting in mucosal destruction and impaired mesenteric perfusion, increased expression of bacterial receptor Toll like receptor 4 (TLR4), and changes in other high response related factors. The recognition of NEC-specific dysbiosis can guide the prevention of NEC, timely diagnosis and targeted therapy. New technological advances provide more knowledge for the study of human microbiota and metabolic activities. The microbiota in newborns is of interest because it may be related to genetics, reproduction and the development of the immune system and brain.
Neurocognitive development is often impaired in patients with NEC. Epidemiological studies showed that 45% of NEC survivors suffered from a degree of neurodevelopmental delay such as cerebral palsy, hearing, visual, cognitive, and psychomotor impairments at 20 months of age (Rees et al., 2007; van Vliet et al., 2013). Severe neurocognitive impairment in preterm infants with NEC is more severe and persist than that in preterm infants without NEC (Shah et al., 2012; Wadhawan et al., 2014), suggesting a link between intestinal injury and toxicity to the brain of preterm infants. The exact mechanism of communication between the gut microbiota and the brain has not been elucidated yet. Moreover, NEC patients requiring surgery had worse mental and psychomotor developmental index scores around 2 years of age (Hintz et al., 2005)and various cognitive deficits at school age (Roze et al., 2011).
The severity of neuroinflammation is related to severity of NEC. The pro-inflammatory response in the brain leads to cell homeostasis and brain cell density changes in specific areas. These findings suggest that early intervention with NEC may reduce the incidence of acute neuroinflammation and brain injury.
The mechanisms underlying NEC-associated brain changes are largely unknown. The microbiota induced inflammatory cascade in NEC affects not only the intestine, but also the brain development through the so-called bidirectional gut-brain axis (Biouss et al., 2019). In this review, we review the current knowledge on microbiota in NEC, mechanisms of gut-brain axis, the neurocognitive consequences of NEC and explore the mechanisms of NEC related brain development impairments.
The gut-brain axis is a communication system that integrates the central nervous system (CNS), autonomic nervous system, the enteric nervous system, the hypothalamus - pituitary–adrenal axis (HPA), vagus nerve, intestinal microbiota, metabolic system and immunological signaling between the gut and the brain. It provides a potential route for intestinal microbiota and its metabolites to influence brain function. This communication system is bidirectional and enables the brain to influence gastrointestinal functions (such as motility, secretion and mucin production) and mucosal immune system cells which produced cytokines (Tracey, 2009).
Clinical observation found that orally administered antibiotics can reverse encephalopathy in patients with decompensated liver disease, this proves that commensal intestinal microbiota can influence brain function (Schiano, 2010). Studies reveal that the presence of intestinal microbiota in mice affects the development of neuronal circuitry involved in a wide range of activities, including memory, learning, motor control, and anxiety-like behavior (Gareau et al., 2011; Collins et al., 2012). Experimental studies demonstrated that intestinal microbiota in germfree mice (gnotobiotic mice) have the ability to influence brain development. Studies have shown that germ-free mice exhibit exaggerated stress-anxiety behavior with increased corticosteroids levels in response to mild stress and is normalized when germ-free mice were colonized with Bifidobacterium longum subsp. Infantis (Sudo et al., 2004).
Germ-free mice showed different levels of brain-derived neurotrophic factor, synaptophysin and synaptogenesis protein PDS-95, and neurotransmitters, such as noradrenalin, dopamine and serotonin, compared to colonized mice (Collins et al., 2012).
Probiotics have been shown to affect the brain through the vagus effect (Kunze et al., 2009; Bercik et al., 2010, 2011; Bravo et al., 2011). For example, a study showed that chronic administration of Lactobacillus rhamnosus activated exploratory behavior in mice and changes of GABA. This effect was not seen in vagotomized mice, indicating that the probiotic effects on the brain were vagal dependence (Bravo et al., 2011).
Bacterial metabolites have multiple effects on regulating the function of gut-brain axis, and microbiota is a major source of circulating organic acids and tryptophan metabolites (Wikoff et al., 2009). Short-chain fatty acids (SCFAs), which are produced by bacterial fermentation processes in the gut, include acetic, propionic and butyric acids. SCFAs are of importance in maintaining the integrity of the gut barrier and the blood-brain barrier (Diaz Heijtz, 2016). For example, high fecal concentrations of propionic acid correlate with anxiety in patients with irritable bowel disease (Tana et al., 2010) and may lead to abnormal behavior in children with autism spectrum disorder.
Tryptophan is metabolized through three metabolic pathways in the intestinal tract and balances with each other to maintain the homeostasis of intestinal immunity. The inflammatory stress response leads to the imbalance of metabolic pathways and the loss of intestinal immune homeostasis, which may produce neurotoxicity and lead to abnormal mental behavior.
Microbiota can produce multiple neurotransmitter compounds: serotonin, dopamine, norepinephrine, acetylcholine, and gamma-aminobutyric acid (GABA) (Lyte, 2011; Holzer and Farzi, 2014; Lu and Claud, 2019). 5-HT can not only regulate gastrointestinal peristalsis, secretion, vasodilation and nutrient absorption, but also regulate brain emotion, cognition and other functions. GABA is an important inhibitory neurotransmitter in CNS, has recently been shown to be produced by commensal Lactobacilli and Bifidobacteria (Barrett et al., 2012).
Endocrine cells located in the epithelial lining of the gut secrete hormones such as gastrin, cholecystokinin and 5-HT. The proximity of these cells to gut microbes raises the possibility of functional communication between gut microbes and gut endocrine cells. The microbiota can affect the number of intestinal endocrine cells and the release of active peptides (Uribe et al., 1994).
There is a delicate balance between the gut microbiota and the intestinal innate mucosal immune system under normal conditions. Changes in microbial composition caused by environmental factors, including diet, probiotics or antibiotics, disrupt this balance (Honda and Takeda, 2009). In NEC, LPS-containing microbes can induce inflammation in the brain by Toll-like receptor 4 (TLR4) activation-mediated cytokine release. The expression level of TLR4 in the intestinal tract of premature infants is higher than that in the full-term intestinal tract (Sodhi et al., 2010). Activation of TLR4 on the intestinal lining by gram-negative bacteria colonized in the intestinal tract of premature infants results in a number of harmful effects, including increased intestinal cell apoptosis, impaired mucosal healing, and enhanced release of pro-inflammatory cytokines, which overall contributes to the development of NEC (Lu et al., 2014).
In addition, factors involved in Toll-like receptor signaling include nuclear factor κB1, SIGIRR genetic variants (Sampath et al., 2015), myeloid differentiation-2 (MD-2) and GM2 activator protein (GM2A) genetic polymorphisms (Zhou W. et al., 2015), IL-4ra mutant allele which associated with immune phenotype shift from type 1 to type 2 T helper cells (Treszl et al., 2003). These gene expressions could all influence the development of NEC.
Central nervous system disorders also influence the composition of gut microbes. Stress-induced changes in intestinal motility and mucus secretion lead to changes in microbial habitat (Collins et al., 2012). Stress also increases the concentration of norepinephrine in the gut, which may lead to changes in microbial composition during stress.
Necrotizing enterocolitis is associated with inappropriate intestinal colonization in preterm infants. Experimental models showed that NEC occurs in conventionally raised animals but not in germ-free animals (Lawrence et al., 1982; Afrazi et al., 2011).
Following delivery, the gut becomes rapidly colonized with commensal bacteria derived from maternal colonic and vaginal flora (Patel and Denning, 2015), and from the surrounding environment. These commensal bacteria act on the immune system and the metabolism of microorganism, maintain intestinal homeostasis, and protect the gut from injury (Rakoff-Nahoum et al., 2004; Tokarz-Deptula et al., 2016). A study showed that gut colonization of the premature infants took place in an orderly process, from Bacilli to Gammaproteobacteria to Clostridia (La Rosa et al., 2014). Exogenous factors such as antibiotics, delivery mode, feeding and age could influence the pace, but not the “predestined” sequence, of progression. Compared with full-term infants, premature infants have lower microbial diversity, lower presence of intestinal commensal microbes (such as B. longum species and Bacteroidetes) and higher numbers of potential pathogenic bacteria Clostridiaceae and Enterobacteriaceae (Vongbhavit and Underwood, 2016; Wandro et al., 2018). The rate of microbial populations aggregate depends on gestational age. The more premature the infant was, the slower the process of bacterial colonization was. Several factors play a role in the development of the gut microbiota after delivery such as antibiotics, probiotic supplementation, types of infant feeding, gestational age and infant hospitalization (Penders et al., 2006; Madan et al., 2012; Raba et al., 2021). These factors influence the composition of the microbiota measured at 1 month of full term, and remained different at 4 months of age but converged at 1 year of age (Azad et al., 2013).
Overall, this dysbiosis in NEC was characterized by increased abundances of Proteobacteria and low abundances of Firmicutes and Bacteroidetes (Pammi et al., 2017; Olm et al., 2019). Studies provide further evidence that the microbiome shifts prior to the onset of NEC (Sim et al., 2015). A study revealed that there were more Klebsiella, bacteria encoding fimbriae, and bacteria encoding for gene clusters of secondary metabolites associated with quorum sensing and bacteriocin production (Olm et al., 2019). Bacterial replication rates are significantly higher before NEC development especially Enterobacteriaceae.
Some studies also found that the specific microbiota profile differ depending on the age of disease onset. NEC preceded by Firmicutes dysbiosis (increased abundances of Firmicutes and accompanied by decreased Gammaproteobacteria) occurred earlier (onset, days 7 to 21) than NEC preceded by Proteobacteria dysbiosis (increase in Gammaproteobacteria and accompanied by decrease in Firmicutes) (onset, days 19 to 39) (Morrow et al., 2013; Zhou W. et al., 2015).
Antibiotic therapy has been suggested to increased risk of NEC in preterm infants by reducing the diversity of microbiota, which might lead to pathogenic microbes overgrow over the commensal species. The relative abundance of Proteobacteria increased and Firmicutes and Actinobacteria decreased in infants treated with antibiotics (Pammi et al., 2017).
The mode of delivery is the most important factor affecting the composition of microbiota. Bifidobacterium, Escherichia (Escherichia coli), Bacteroides and Parabacteroides were higher after vaginal delivery (Shao et al., 2019). By contrast, the relative abundance of Firmicutes (Enterococcus faecalis, Enterococcus faecium, Staphylococcus epidermis, Streptococcus parasanguinis, and Clostridium perfringens) and Klebsiella oxytoca, Klebsiella pneumoniae, Enterobacter cloacae were higher after Caesarean section (Shao et al., 2019). But it is not clear whether this has anything to do with NEC’s development.
Feeding style affects the composition of intestinal microbiota. Formula-fed infants showed a higher abundance of Firmicutes, and breast-fed infants showed higher relative abundances of Proteobacteria, but its association with NEC development is not for sure (Pammi et al., 2017). Breast milk contains certain macronutrients, polyunsaturated fatty acids, lactoferrin, immune cells, and immunoglobulins and growth factors. An outstanding research reports that IgA in maternal milk shapes the host–microbiota relationship of preterm neonates and that maternal IgA is a critical for the prevention of NEC (Gopalakrishna et al., 2019).
Probiotics may reduce the risk of necrotizing necrosis by regulating the gut microbiome. But its effect is not entirely determined. A Cochrane Database in Sharif et al. (2020) reviewed probiotics in very preterm or very low birth weight infants to prevent necrotizing enterocolitis. Meta-analysis showed that probiotics may reduce the risk of NEC: RR 0.54, 95% CI 0.45 to 0.65 (54 trials, 10,604 infants). The effects of probiotics of different subgenus species on the reduction of NEC and nervous system in preterm infants are shown in Table 1. However, the certainty of the evidence is low because of the limitations of trial design (most trials are small in size) and funnel plot asymmetry consistent with publication bias. Large-scale, high-quality trials are therefore needed. Another meta-analysis (Morgan et al., 2020) exploited that among interventions compared with placebo, the combinations of Lactobacillus spp and Bifidobacterium spp (OR 0.35, 95% CI 0.20 to 0.59), Bifidobacterium animalis subsp, lactis (OR 0.31, 95% CI 0.13 to 0.74), Lactobacillus reuteri (OR 0.55, 95% CI 0.34 to 0.91), or L. rhamnosus (OR 0.44, 95% CI 0.21 to 0.90) significantly reduced severe NEC. Although probiotics may reduce the risk of NEC, their safety or long-term efficacy has not been adequately studied in premature infants.
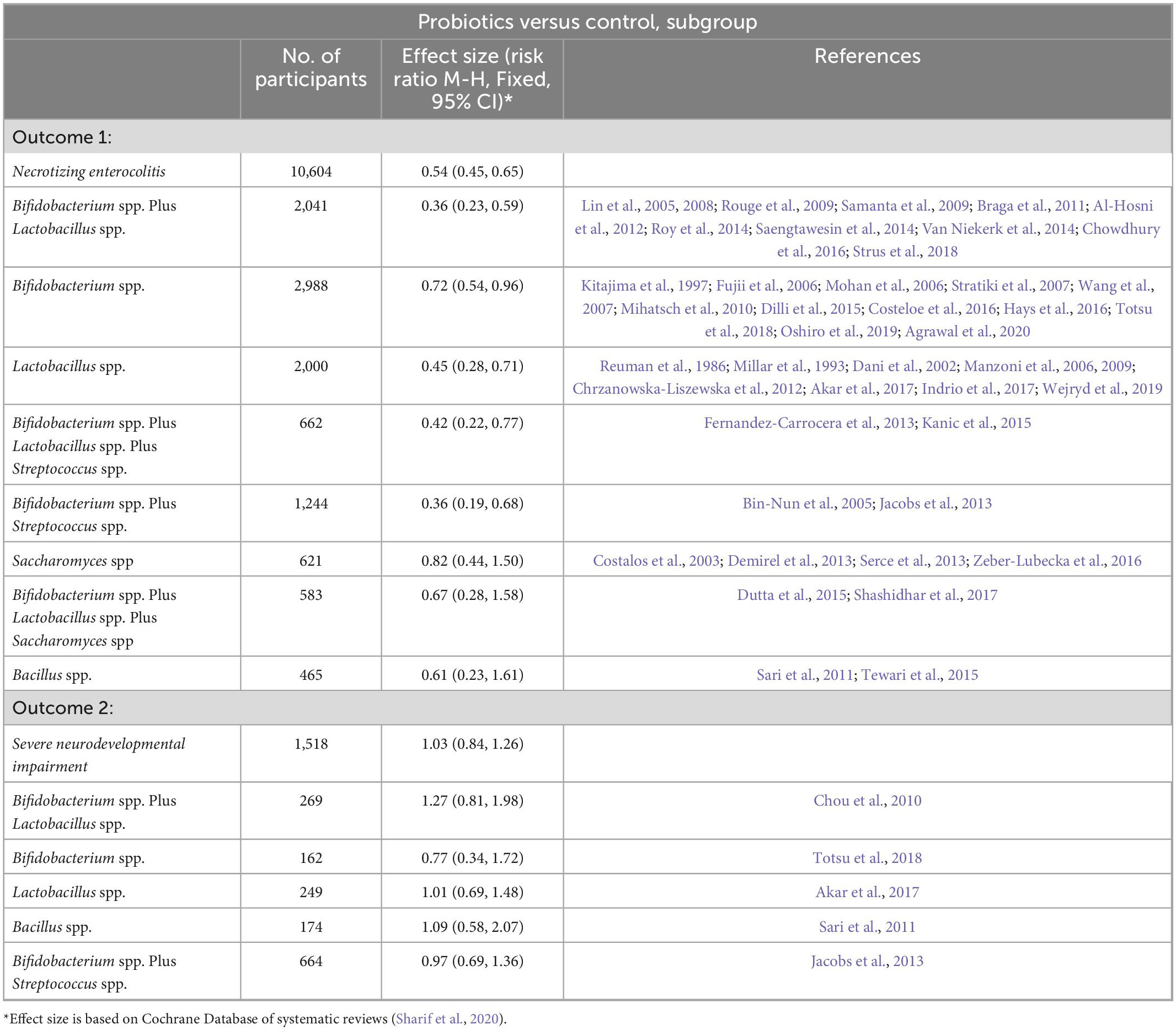
Table 1. Probiotics for very preterm or very low birth weight infants to prevent Necrotizing enterocolitis (NEC) based on Cochrane Database.
Premature infants with NEC have a high incidence of morbidity and mortality. Complications include gastrointestinal problems (including strictures and adhesions, cholestasis, motility disturbances, short bowel syndrome, intestinal failure, feeding difficulties), poor growth and more important worse neurodevelopment outcome [intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL) and white matter injury, predictive of long-term neurodevelopmental impairment]. There is still a possibility of extended hospitalization, repeated surgeries, closer follow-up, and treatment of long-term complications of NEC (Hayakawa et al., 2015; Bazacliu and Neu, 2019).
Necrotizing enterocolitis has a substantial impact on motor, cognitive and behavioral performance outcomes in early childhood later in life (Waugh et al., 1996; Chacko et al., 1999; Sonntag et al., 2000; Castro et al., 2004; Salhab et al., 2004; Hintz et al., 2005; Schulzke et al., 2007; Lodha et al., 2010; Pike et al., 2012; Shah et al., 2012; Zozaya et al., 2021). The association between NEC and neurocognitive impairment has been confirmed by many researchers. Schulzke et al. (2007) analyzed eleven studies and revealed a strong correlation between neurodevelopmental delay and children with stage II or higher NEC, especially if they require surgery for the illness. Another systematic review by Rees et al. (2007) included data from 7,843 preterm infants (821 with NEC) from ten studies found significantly worse neurodevelopmental outcomes in NEC compared with than that of prematurity alone. Characteristics of neurodevelopmental impairment (NDI) related studies on NEC are summarized in Table 2.
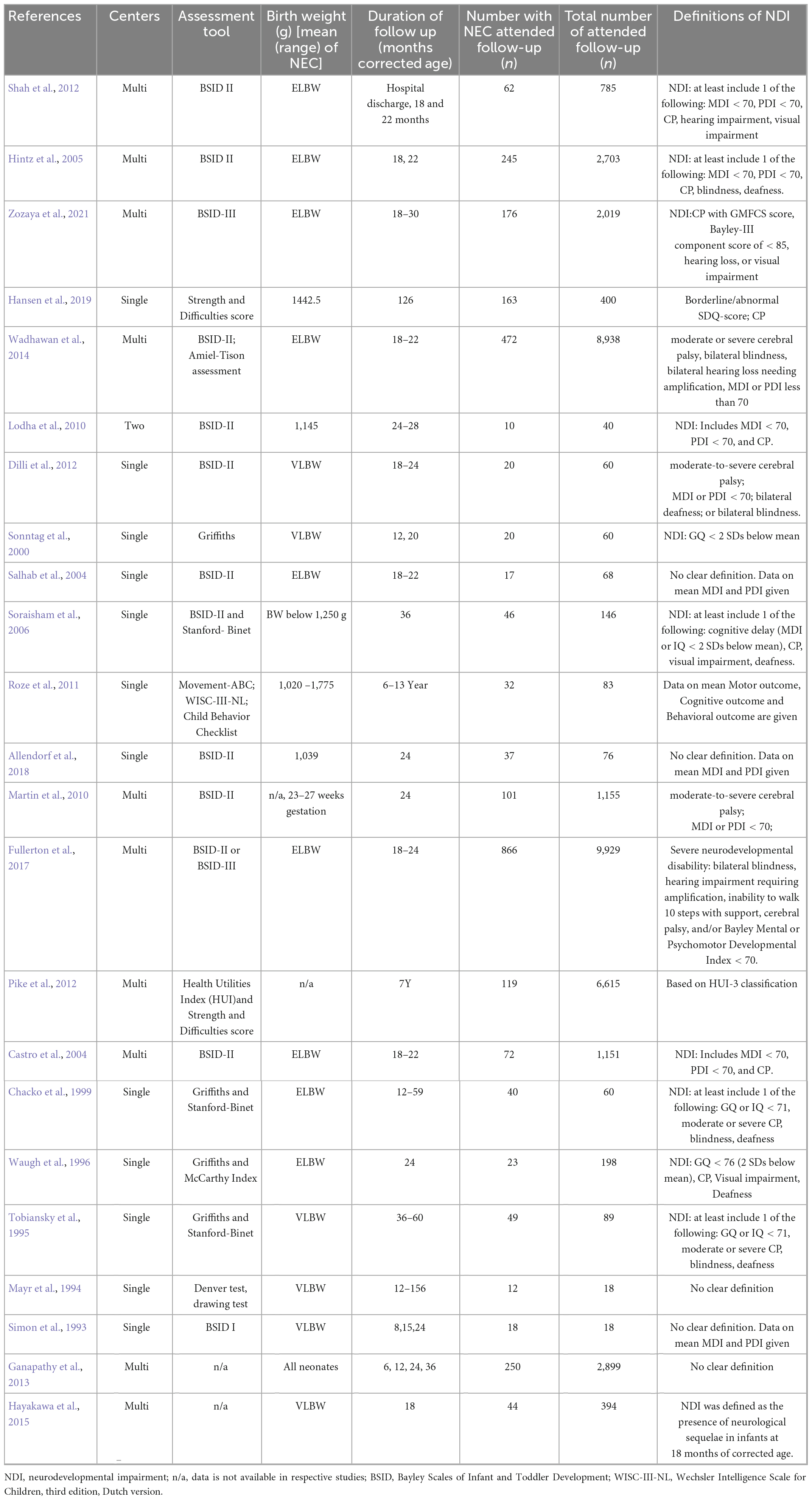
Table 2. Neurodevelopmental impairment (NDI) studies of Necrotizing enterocolitis (NEC) among preterm infants.
Several small matched control studies revealed a significant correlation between NEC and brain injury (Simon et al., 1993; Mayr et al., 1994; Tobiansky et al., 1995; Ganapathy et al., 2013; Allendorf et al., 2018; Hansen et al., 2019). A recent study showed that stage II or III NEC infants had a significantly higher risk of cognitive delay and visual impairment compared to age-matched controls at 36 months (Soraisham et al., 2006). Another study found a significant neurodevelopmental delay at 12 and 20 months in NEC infants compared to age-matched controls (Sonntag et al., 2000). Very low birth-weight babies with NEC had increased risk of severe head growth failure (Regev et al., 2016). Moreover, recent studies demonstrated that NEC was correlated with lower psychomotor developmental index (PDI) scores (Salhab et al., 2004; Dilli et al., 2012) and mental developmental index (MDI) (Dilli et al., 2012). The evidence for their impaired performance in cognitive and developmental assessments is supported by Bayley Scales of Infant Development, the Griffiths Quotient and the Stanford–Binet test. Studies using brain magnetic resonance imaging (MRI) showed that white matter and cortical abnormalities in infants with NEC (Shah et al., 2008; Lee et al., 2014; Merhar et al., 2014; Shin et al., 2016).
The presence of advanced NEC or need for surgery increase the risk of neurological impairment. A large retrospective analysis showed that surgical NEC but not medically managed NEC was a significant independent risk factor for worse mental and psychomotor developmental index scores at 18–22 months corrected age compared with control (Hintz et al., 2005). Several studies indicate that children with surgical NEC have poorer neurodevelopmental outcomes (Rees et al., 2007; Schulzke et al., 2007; Martin et al., 2010; Fullerton et al., 2017) and more brain injury on MRI compared to medical infants (Merhar et al., 2014). But not all the data fully support this conclusion (Dilli et al., 2012). Shah’s team found an increased risk of neurodevelopmental disorders measured by Bayley test in ELBW infants with NEC and spontaneous intestinal perforation (SIP), and there were no significant differences in neurodevelopmental outcomes between NEC medical and surgical groups observed in the study (Shah et al., 2012).
Experimental NEC mice study further explored the morphologic changes in the brain. NEC pups had smaller brain weight and a thinner cerebral cortex compared to the control group (Biouss et al., 2019). In particular, in specific regions of the NEC brain, the number of neurons, oligodendrocytes, and neural progenitors were reduced. There is an impairment in neurogenesis and loss of myelin (Nino et al., 2018; Zhou et al., 2021) which may lead to poor performance in adult life. Levels of apoptosis and ER stress were increased in NEC. In addition, pro-inflammatory cytokines (IL-6 and TNFα) and the density of activated microglia and astrocytes were increased in the brain, which were positively correlated with the increase of intestinal proinflammatory cytokines and the severity of NEC injury, respectively (Biouss et al., 2019).
Necrotizing enterocolitis is often associated with sepsis, which itself is associated with adverse neurocognitive outcomes (Salhab et al., 2004; Soraisham et al., 2006; Martin et al., 2010). Other preterm complications associated with NEC may also conspire to affect brain development.
Early life is critical period of rapid brain development, including neurogenesis, neuronal migration, maturation, apoptosis and synaptogenesis (Hickey et al., 2018). These processes are also influenced by several other factors, such as malnutrition, hypoxia and ischemia, and the inflammatory cytotoxic mediators. The development of NEC at a critical stage of brain development can cause neurodevelopmental disorders and their association is multifactorial (Keunen et al., 2015; Hickey et al., 2018; Niemarkt et al., 2019).
Necrotizing enterocolitis intestinal injury send intestinal microbiological signals associated with systemic inflammation transmitted to the brain and limbic system via enteric nervous system, autonomic nervous system, and hypothalamic-pituitary axis Moschopoulos et al. (2018) suggest that primary laparotomy rather than primary peritoneal drainage should be used in children with advanced NEC, as the continued damaging of residual necrotic intestine is harmful to the neonatal brain.
In experimental NEC brain, there are fewer neurons, oligodendrocytes, and neural progenitors that may affect neurological function in specific regions of the brain (Biouss et al., 2019) (Figure 1). The study found that in NEC’s piglet model, a series of hippocampal differentially expressed genes related to neuroinflammation and hypoxia are increased and expressions of genes related to protection against oxidative stress and oligodendrocytes are downregulated (Sun et al., 2018).
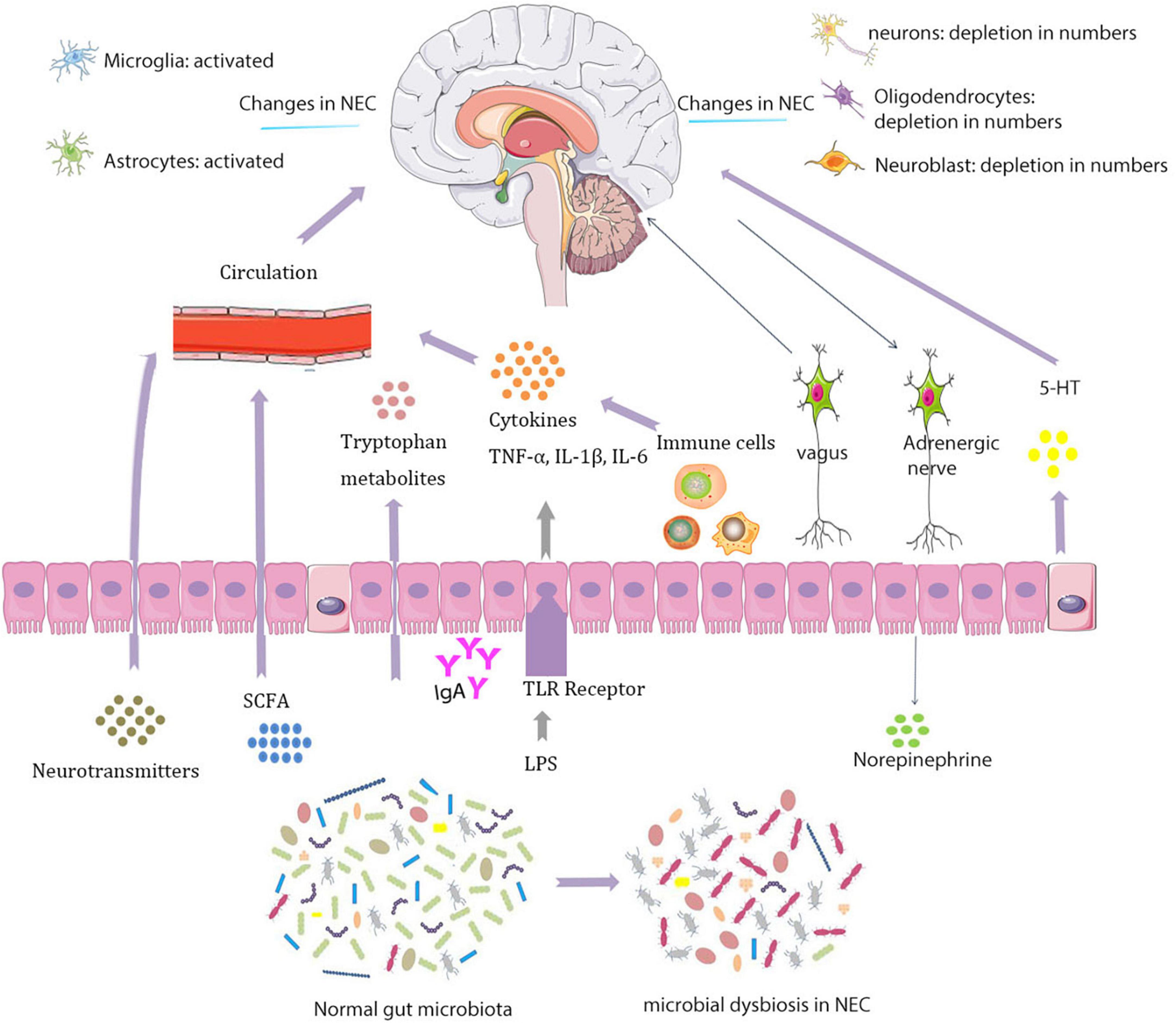
Figure 1. Interactions between the microbiota and nervous systems in necrotizing enterocolitis (NEC).
Studies found that NEC-associated brain injury is characterized by microglial activation, loss of white matter and cognitive dysfunction, the mechanism of which remains unclear (Nino et al., 2018). The underlying mechanisms of white matter injury may include cerebral ischemia, systemic infection or inflammation, and the inherent vulnerability of premyelinated white matter oligodendrocytes. Premyelinating oligodendrocytes are very vulnerable to ischemia and inflammation in the process of encephalopathy (Volpe, 2011). White matter injury is often manifested as periventricular leucomalacia (PVL) (Hintz et al., 2015), which is sign of disrupted brain development and neurological dysfunction in premature babies. Neuronal dysfunction may be the result of excessive cell degeneration or lack of neurogenesis. Neuronal degeneration is in part by exposure to factors predisposed to NEC, such as hypoxic ischemic injury, which increases cell apoptosis and promotes inflammatory response.
Intestinal injury and barrier dysfunction provide bacteria and inflammatory mediators a potential route to transfer to the systemic circulation, leading to a systemic inflammatory response. Cytokines produced during this process disrupt preterm brain development (Lodha et al., 2010; Hickey et al., 2018). Levels of pro-inflammatory cytokines and activated microglia and astrocytes density are increased in NEC-associated brain injury (Zhou et al., 2021). Cytokines have dramatic effects on the development of neurogenesis, neuronal migration, synaptogenesis, and synaptic plasticity (Williamson et al., 2011; Bilbo and Schwarz, 2012; Donzis and Tronson, 2014). Studies serve to illustrate that early life exposure to lipopolysaccharide (LPS) results in dysregulated neurogenesis, increased microglia, reduced hippocampal volume, axonal injury and memory impairment in rats (Bilbo et al., 2005; Wang et al., 2013; Smith et al., 2014; Sun et al., 2018).
The response of TLR4 to LPS of gut bacteria (most often Gammaproteobacteria) is exaggerated in NEC model (Hackam et al., 2019; Niemarkt et al., 2019), resulting in signaling cascades and activated nuclear translocation of nuclear factor kappa-β (NFκB). NFκB signaling pathway is involved in plasticity and survival in neurons, and mediates pro-inflammatory responses in glial cells (Crampton and O’Keeffe, 2013). TLR4 is of critical importance in NEC. NEC is not induced in TLR mutants or knockout animals. Clinical trials have hinted that probiotics activate TLR9 and in turn limit TLR4, thereby relieving NEC (Hackam and Sodhi, 2018; Hackam et al., 2019).
Activated microglia has a central role in white matter injury in newborn mice (Tahraoui et al., 2001). Changes in the early stage of acute inflammation may be related to TLR4-mediated microglia activation (Liddelow et al., 2017; Nino et al., 2018). Activated microglia induce an increase in astrocyte numbers in the late stages of inflammation, a process known as reactive astrocytosis (Liddelow et al., 2017; Sochocka et al., 2017). Neuroinflammatory astrocytes could also release toxic factors that can lead to progressive neuronal loss (Bi et al., 2013; Liddelow et al., 2017; Biouss et al., 2019). TLR4-dependent microglia and astrocytes are activated in the brain of NEC. The excessive astrocyte and microglia activation has been shown to lead to a loss of oligodendrocyte progenitor cells in response to TNFα exposure (Jurewicz et al., 2005; Nino et al., 2018; Biouss et al., 2019) and exacerbate white matter and periventricular injury (Billiards et al., 2006; Selip et al., 2012).
Furthermore, intracerebroventricular injection of NEC enteric-derived CD4 + T lymphocytes into Rag1-/- recipient mice lacking CD4 + T cells induced brain injury, suggesting that enteric-derived T lymphocytes may mediate neuroinflammation of NEC. These findings indicate that enterogenic IFN-γ- releasing CD4 + T cells may induce NEC-related brain injury. This was confirmed in human specimens, where the brains of infants with NEC were examined and compared with those of the control group, exhibiting an increased accumulation of CD4 + T lymphocytes (Zhou et al., 2021).
Microbiota and brain-gut axis are crucial for NEC brain development. The role of the intestinal microbiota in the prevention, pathogenesis and potential neurodevelopmental consequences of NEC are necessary to review here (Hickey et al., 2018). The mechanisms underlying microbiota on brain functioning are several.
First, microbiota cause and may exacerbate systemic inflammation. In addition, dysbiosis may result in reduced intestinal and blood-brain barrier function via SCFA production (Diaz Heijtz et al., 2011; Lu et al., 2018). SCFAs have been shown to influence barrier function of the intestinal epithelial cells. Dysbiosis may disturb the content difference of SCFA in the gut (Neu and Pammi, 2018). Both high and low SCFA levels were associated with NEC (Lin, 2004). SCFA and bile acids affect the production of serotonin, which in turn regulates intestinal motility (Niemarkt et al., 2019). Intestinal microbial metabolites (mainly SCFA) prevent microglial activation and subsequent neuroinflammation through immune modulation (Erny et al., 2015).
Besides, microbiota may influence the brain through the production of neurotransmitters (serotonin, melatonin, GABA, dopamine) or by stimulating intestinal cells to do so. Gut microbes transmit signals to the brain via the vagus nervous system, and vice versa. Although it is known that probiotics may mitigate the effect of dysbiosis and further improve brain development, more research is needed into the relationship between NEC microbiota and brain development in preterm infants.
Necrotizing enterocolitis is often accompanied by malnutrition, which can lead to poor brain development. Children with NEC not only had insufficient nutritional intake, but also sustained mucosal injury resulted in reduced nutritional absorption. Studies have shown that high protein provision in infants with surgical NEC was associated with increased head circumference, an index of brain growth (Lin et al., 2017). Protein status is important due to its role in fat-free mass (FFM) accretion, neurogenesis, neuronal differentiation and involved in cognition related insulin-like growth factor-1 (IGF-1) expression (Hansen-Pupp et al., 2013; Pfister et al., 2013). Micronutrients iron and zinc deficiency also contribute to abnormal neurodevelopment and poor growth (Ramel et al., 2014).
Premature infants with NEC have poor neurodevelopmental outcomes, but the underlying pathogenic mechanism has not been clearly established and it is likely to be multifactorial. Microglial proliferation, astrocyte proliferation, decreased neurogenesis and demyelination are important characteristics of NEC brain changes. Gut microbiota alterations contribute to the pathogenesis of NEC. Dysbiosis causes changes in the brain through TLR4-mediated inflammation, SCFA and neurotransmitters. The recognition of NEC-specific gut-brain axis can guide the prevention of NEC, timely diagnosis and targeted therapy. Studies on how NEC causes neurological impairment and its therapeutics are few. There is ample room for pathophysiological mechanism and clinical application research aimed at improving the prognosis of NEC.
Biomarkers predicted by microbiome and metabolomics are promising tools for accurate and timely prediction of NEC and selection of appropriate treatments. More studies with longer follow-up periods are needed to better understand the brain development of these patients. Further studies are needed to determine the most effective type of probiotics, dosage, administration time, and duration to reduce the incidence of NEC.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This work was supported by the Zhejiang Key Laboratory for Diagnosis and Therapy of Neonatal Diseases, Key Laboratory of Reproductive Genetics (Zhejiang University), Ministry of Education, and by grants from the National Natural Science Foundation of China (No. 82001587).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
NEC, necrotizing enterocolitis; TLR4, Toll like receptor 4; CNS, central nervous system; SCFAs, short-chain fatty acids; NDI, neurodevelopmental impairment; PDI, psychomotor developmental index; MDI, mental developmental index; PVL, periventricular leucomalacia; LPS, lipopolysaccharide; NF κ B, nuclear factor kappa- β.
Afrazi, A., Sodhi, C., Richardson, W., Neal, M., Good, M., Siggers, R., et al. (2011). New insights into the pathogenesis and treatment of necrotizing enterocolitis: Toll-like receptors and beyond. Pediatr. Res. 69, 183–188. doi: 10.1203/PDR.0b013e3182093280
Agrawal, S., Pestell, C., Granich, J., Rao, S., Nathan, E., Wray, J., et al. (2020). Difficulties in developmental follow-up of preterm neonates in a randomised-controlled trial of Bifidobacterium breve M16-V – experience from Western Australia. Early Hum. Dev. 151:105165. doi: 10.1016/j.earlhumdev.2020.105165
Akar, M., Eras, Z., Oncel, M., Arayici, S., Guzoglu, N., Canpolat, F., et al. (2017). Impact of oral probiotics on neurodevelopmental outcomes in preterm infants. J. Matern. Fetal Neonatal Med. 30, 411–415. doi: 10.1080/14767058.2016.1174683
Al-Hosni, M., Duenas, M., Hawk, M., Stewart, L., Borghese, R., Cahoon, M., et al. (2012). Probiotics-supplemented feeding in extremely low-birth-weight infants. J. Perinatol. 32, 253–259. doi: 10.1038/jp.2011.51
Allendorf, A., Dewitz, R., Weber, J., Bakthiar, S., Schloesser, R., and Rolle, U. (2018). Necrotizing enterocolitis as a prognostic factor for the neurodevelopmental outcome of preterm infants – match control study after 2years. J. Pediatr. Surg. 53, 1573–1577. doi: 10.1016/j.jpedsurg.2018.01.006
Azad, M., Konya, T., Maughan, H., Guttman, D., Field, C., Chari, R., et al. (2013). Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. CMAJ 185, 385–394. doi: 10.1503/cmaj.121189
Barrett, E., Ross, R., O’Toole, P., Fitzgerald, G., and Stanton, C. (2012). gamma-aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 113, 411–417. doi: 10.1111/j.1365-2672.2012.05344.x
Bazacliu, C., and Neu, J. (2019). Necrotizing enterocolitis: Long term complications. Curr. Pediatr. Rev. 15, 115–124. doi: 10.2174/1573396315666190312093119
Bercik, P., Park, A., Sinclair, D., Khoshdel, A., Lu, J., Huang, X., et al. (2011). The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 23, 1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x
Bercik, P., Verdu, E., Foster, J., Macri, J., Potter, M., Huang, X., et al. (2010). Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139, 2102–2112.e1. doi: 10.1053/j.gastro.2010.06.063
Bi, F., Huang, C., Tong, J., Qiu, G., Huang, B., Wu, Q., et al. (2013). Reactive astrocytes secrete lcn2 to promote neuron death. Proc. Natl. Acad. Sci. U.S.A. 110, 4069–4074. doi: 10.1073/pnas.1218497110
Bilbo, S., and Schwarz, J. (2012). The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol. 33:267–286. doi: 10.1016/j.yfrne.2012.08.006
Bilbo, S., Biedenkapp, J., Der-Avakian, A., Watkins, L., Rudy, J., and Maier, S. (2005). Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J. Neurosci. 25, 8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005
Billiards, S., Haynes, R., Folkerth, R., Trachtenberg, F., Liu, L., Volpe, J., et al. (2006). Development of microglia in the cerebral white matter of the human fetus and infant. J. Comp. Neurol. 497, 199–208. doi: 10.1002/cne.20991
Bin-Nun, A., Bromiker, R., Wilschanski, M., Kaplan, M., Rudensky, B., Caplan, M., et al. (2005). Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J. Pediatr. 147, 192–196. doi: 10.1016/j.jpeds.2005.03.054
Biouss, G., Antounians, L., Li, B., O’Connell, J., Seo, S., Catania, V., et al. (2019). Experimental necrotizing enterocolitis induces neuroinflammation in the neonatal brain. J. Neuroinflammation 16:97. doi: 10.1186/s12974-019-1481-9
Braga, T., da Silva, G., de Lira, P., and de Carvalho Lima, M. (2011). Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: A double-blind, randomized, controlled trial. Am. J. Clin. Nutr. 93, 81–86. doi: 10.3945/ajcn.2010.29799
Bravo, J., Forsythe, P., Chew, M., Escaravage, E., Savignac, H., Dinan, T., et al. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U.S.A. 108, 16050–16055. doi: 10.1073/pnas.1102999108
Castro, L., Yolton, K., Haberman, B., Roberto, N., Hansen, N., Ambalavanan, N., et al. (2004). Bias in reported neurodevelopmental outcomes among extremely low birth weight survivors. Pediatrics 114, 404–410. doi: 10.1542/peds.114.2.404
Chacko, J., Ford, W., and Haslam, R. (1999). Growth and neurodevelopmental outcome in extremely-low-birth-weight infants after laparotomy. Pediatr. Surg. Int. 15, 496–499. doi: 10.1007/s003830050648
Chou, I., Kuo, H., Chang, J., Wu, S., Chiu, H., Su, B., et al. (2010). Lack of effects of oral probiotics on growth and neurodevelopmental outcomes in preterm very low birth weight infants. J. Pediatr. 156, 393–396. doi: 10.1016/j.jpeds.2009.09.051
Chowdhury, T., Ali, M., Hossain, M., Singh, J., Yousuf, A., Yasmin, F., et al. (2016). Efficacy of probiotics versus placebo in the prevention of necrotizing enterocolitis in preterm very low birth weight infants: A double-blind randomized controlled trial. J. Coll. Physicians Surg. Pak. 26, 770–774.
Chrzanowska-Liszewska, D., Seliga-Siwecka, J., and Kornacka, M. (2012). The effect of Lactobacillus rhamnosus GG supplemented enteral feeding on the microbiotic flora of preterm infants-double blinded randomized control trial. Early Hum. Dev. 88, 57–60. doi: 10.1016/j.earlhumdev.2011.07.002
Collins, S., Surette, M., and Bercik, P. (2012). The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 10, 735–742. doi: 10.1038/nrmicro2876
Costalos, C., Skouteri, V., Gounaris, A., Sevastiadou, S., Triandafilidou, A., Ekonomidou, C., et al. (2003). Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum. Dev. 74, 89–96. doi: 10.1016/s0378-3782(03)00090-2
Costeloe, K., Hardy, P., Juszczak, E., Wilks, M., and Millar, M. (2016). Probiotics in preterm infants study collaborative G. Bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 387, 649–660. doi: 10.1016/S0140-6736(15)01027-2
Crampton, S., and O’Keeffe, G. (2013). NF-kappaB: Emerging roles in hippocampal development and function. Int. J. Biochem. Cell Biol. 45, 1821–1824. doi: 10.1016/j.biocel.2013.05.037
Dani, C., Biadaioli, R., Bertini, G., Martelli, E., and Rubaltelli, F. (2002). Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol. Neonate 82, 103–108. doi: 10.1159/000063096
Demirel, G., Erdeve, O., Celik, I., and Dilmen, U. (2013). Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: A randomized, controlled study. Acta Paediatr. 102, e560–e565. doi: 10.1111/apa.12416
Diaz Heijtz, R. (2016). Fetal, neonatal, and infant microbiome: Perturbations and subsequent effects on brain development and behavior. Semin. Fetal Neonatal Med. 21, 410–417. doi: 10.1016/j.siny.2016.04.012
Diaz Heijtz, R., Wang, S., Anuar, F., Qian, Y., Bjorkholm, B., Samuelsson, A., et al. (2011). Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad Sci. U.S.A. 108, 3047–3052. doi: 10.1073/pnas.1010529108
Dilli, D., Aydin, B., Fettah, N., Ozyazici, E., Beken, S., Zenciroglu, A., et al. (2015). The propre-save study: Effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J. Pediatr. 166, 545–551.e1. doi: 10.1016/j.jpeds.2014.12.004
Dilli, D., Eras, Z., Ozkan Ulu, H., Dilmen, U., and Durgut Sakrucu, E. (2012). Does necrotizing enterocolitis affect growth and neurodevelopmental outcome in very low birth weight infants? Pediatr. Surg. Int. 28, 471–476. doi: 10.1007/s00383-012-3051-4
Donzis, E., and Tronson, N. (2014). Modulation of learning and memory by cytokines: Signaling mechanisms and long term consequences. Neurobiol. Learn. Mem. 115, 68–77. doi: 10.1016/j.nlm.2014.08.008
Dutta, S., Ray, P., and Narang, A. (2015). Comparison of stool colonization in premature infants by three dose regimes of a probiotic combination: A randomized controlled trial. Am. J. Perinatol. 32, 733–740. doi: 10.1055/s-0034-1395473
Erny, D., Hrabe de Angelis, A., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., et al. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977. doi: 10.1038/nn.4030
Fernandez-Carrocera, L., Solis-Herrera, A., Cabanillas-Ayon, M., Gallardo-Sarmiento, R., Garcia-Perez, C., Montano-Rodriguez, R., et al. (2013). Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 98, F5–F9. doi: 10.1136/archdischild-2011-300435
Fujii, T., Ohtsuka, Y., Lee, T., Kudo, T., Shoji, H., Sato, H., et al. (2006). Bifidobacterium breve enhances transforming growth factor beta1 signaling by regulating Smad7 expression in preterm infants. J. Pediatr. Gastroenterol. Nutr. 43, 83–88. doi: 10.1097/01.mpg.0000228100.04702.f8
Fullerton, B., Hong, C., Velazco, C., Mercier, C., Morrow, K., Edwards, E., et al. (2017). Severe neurodevelopmental disability and healthcare needs among survivors of medical and surgical necrotizing enterocolitis: A prospective cohort study. J. Pediatr. Surg. S0022–3468, 30651–30656. doi: 10.1016/j.jpedsurg.2017.10.029
Ganapathy, V., Hay, J., Kim, J., Lee, M., and Rechtman, D. (2013). Long term healthcare costs of infants who survived neonatal necrotizing enterocolitis: A retrospective longitudinal study among infants enrolled in Texas Medicaid. BMC Pediatr. 13:127. doi: 10.1186/1471-2431-13-127
Gareau, M., Wine, E., Rodrigues, D., Cho, J., Whary, M., Philpott, D., et al. (2011). Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317. doi: 10.1136/gut.2009.202515
Gopalakrishna, K., Macadangdang, B., Rogers, M., Tometich, J., Firek, B., Baker, R., et al. (2019). Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 25, 1110–1115. doi: 10.1038/s41591-019-0480-9
Gussenhoven, R., Westerlaken, R., Ophelders, D., Jobe, A., Kemp, M., Kallapur, S., et al. (2018). Chorioamnionitis, neuroinflammation, and injury: Timing is key in the preterm ovine fetus. J. Neuroinflammation 15:113. doi: 10.1186/s12974-018-1149-x
Hackam, D., and Sodhi, C. (2018). Toll-like receptor-mediated intestinal inflammatory imbalance in the pathogenesis of necrotizing enterocolitis. Cell. Mol. Gastroenterol. Hepatol. 6, 229–238.e1. doi: 10.1016/j.jcmgh.2018.04.001
Hackam, D., Sodhi, C., and Good, M. (2019). New insights into necrotizing enterocolitis: From laboratory observation to personalized prevention and treatment. J. Pediatr. Surg. 54, 398–404. doi: 10.1016/j.jpedsurg.2018.06.012
Hall, N., Eaton, S., and Pierro, A. (2013). Royal australasia of surgeons guest lecture. Necrotizing enterocolitis: Prevention, treatment, and outcome. J. Pediatr. Surg. 48, 2359–2367. doi: 10.1016/j.jpedsurg.2013.08.006
Hansen, M., Jensen, I., Gregersen, R., Juhl, S., and Greisen, G. (2019). Behavioural and neurodevelopmental impairment at school age following necrotising enterocolitis in the newborn period. PLoS One 14:e0215220. doi: 10.1371/journal.pone.0215220
Hansen-Pupp, I., Hovel, H., Lofqvist, C., Hellstrom-Westas, L., Fellman, V., Huppi, P., et al. (2013). Circulatory insulin-like growth factor-I and brain volumes in relation to neurodevelopmental outcome in very preterm infants. Pediatr. Res. 74, 564–569. doi: 10.1038/pr.2013.135
Hayakawa, M., Taguchi, T., Urushihara, N., Yokoi, A., Take, H., Shiraishi, J., et al. (2015). Outcome in VLBW infants with surgical intestinal disorder at 18 months of corrected age. Pediatr. Int. 57, 633–638. doi: 10.1111/ped.12594
Hays, S., Jacquot, A., Gauthier, H., Kempf, C., Beissel, A., Pidoux, O., et al. (2016). Probiotics and growth in preterm infants: A randomized controlled trial, PREMAPRO study. Clin. Nutr. 35, 802–811. doi: 10.1016/j.clnu.2015.06.006
Hickey, M., Georgieff, M., and Ramel, S. (2018). Neurodevelopmental outcomes following necrotizing enterocolitis. Semin. Fetal Neonatal Med. 23, 426–432. doi: 10.1016/j.siny.2018.08.005
Hintz, S., Barnes, P., Bulas, D., Slovis, T., Finer, N., Wrage, L., et al. (2015). Health SSGotEKSNIoC, human development neonatal research N. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics 135, e32–e42. doi: 10.1542/peds.2014-0898
Hintz, S., Kendrick, D., Stoll, B., Vohr, B., Fanaroff, A., Donovan, E., et al. (2005). Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 115, 696–703. doi: 10.1542/peds.2004-0569
Holzer, P., and Farzi, A. (2014). Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 817, 195–219. doi: 10.1007/978-1-4939-0897-4_9
Honda, K., and Takeda, K. (2009). Regulatory mechanisms of immune responses to intestinal bacteria. Mucosal Immunol. 2, 187–196. doi: 10.1038/mi.2009.8
Indrio, F., Riezzo, G., Tafuri, S., Ficarella, M., Carlucci, B., Bisceglia, M., et al. (2017). Probiotic supplementation in preterm: Feeding intolerance and hospital cost. Nutrients 9:965. doi: 10.3390/nu9090965
Jacobs, S., Tobin, J., Opie, G., Donath, S., Tabrizi, S., Pirotta, M., et al. (2013). Probiotic effects on late-onset sepsis in very preterm infants: A randomized controlled trial. Pediatrics 132, 1055–1062. doi: 10.1542/peds.2013-1339
Jurewicz, A., Matysiak, M., Tybor, K., Kilianek, L., Raine, C., and Selmaj, K. (2005). Tumour necrosis factor-induced death of adult human oligodendrocytes is mediated by apoptosis inducing factor. Brain 128(Pt 11), 2675–2688. doi: 10.1093/brain/awh627
Kanic, Z., Micetic Turk, D., Burja, S., Kanic, V., and Dinevski, D. (2015). Influence of a combination of probiotics on bacterial infections in very low birthweight newborns. Wien. Klin Wochenschr. 127(Suppl. 5) S210–S215. doi: 10.1007/s00508-015-0845-0
Keunen, K., van Elburg, R., van Bel, F., and Benders, M. (2015). Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr. Res. 77, 148–155. doi: 10.1038/pr.2014.171
Kitajima, H., Sumida, Y., Tanaka, R., Yuki, N., Takayama, H., and Fujimura, M. (1997). Early administration of Bifidobacterium breve to preterm infants: Randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 76, F101–F107. doi: 10.1136/fn.76.2.f101
Kunze, W., Mao, Y., Wang, B., Huizinga, J., Ma, X., Forsythe, P., et al. (2009). Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J. Cell. Mol. Med. 13, 2261–2270. doi: 10.1111/j.1582-4934.2009.00686.x
La Rosa, P., Warner, B., Zhou, Y., Weinstock, G., Sodergren, E., Hall-Moore, C., et al. (2014). Patterned progression of bacterial populations in the premature infant gut. Proc. Natl. Acad. Sci. U.S.A. 111, 12522–12527.
Lawrence, G., Bates, J., and Gaul, A. (1982). Pathogenesis of neonatal necrotising enterocolitis. Lancet 1, 137–139.
Lee, I., Neil, J., Huettner, P., Smyser, C., Rogers, C., Shimony, J., et al. (2014). The impact of prenatal and neonatal infection on neurodevelopmental outcomes in very preterm infants. J. Perinatol. 34, 741–747. doi: 10.1038/jp.2014.79
Liddelow, S., Guttenplan, K., Clarke, L., Bennett, F., Bohlen, C., Schirmer, L., et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. doi: 10.1038/nature21029
Lin, G., Robinson, D., Olsen, S., Reber, K., Moallem, M., DiGeronimo, R., et al. (2017). Nutritional practices and growth in premature infants after surgical necrotizing enterocolitis. J. Pediatr. Gastroenterol. Nutr. 65, 111–116. doi: 10.1097/MPG.0000000000001504
Lin, H., Hsu, C., Chen, H., Chung, M., Hsu, J., Lien, R., et al. (2008). Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: A multicenter, randomized, controlled trial. Pediatrics 122, 693–700. doi: 10.1542/peds.2007-3007
Lin, H., Su, B., Chen, A., Lin, T., Tsai, C., Yeh, T., et al. (2005). Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 115, 1–4.
Lin, J. (2004). Too much short chain fatty acids cause neonatal necrotizing enterocolitis. Med. Hypotheses 62, 291–293. doi: 10.1016/S0306-9877(03)00333-5
Lodha, A., Asztalos, E., and Moore, A. (2010). Cytokine levels in neonatal necrotizing enterocolitis and long-term growth and neurodevelopment. Acta Paediatr. 99, 338–343. doi: 10.1111/j.1651-2227.2009.01600.x
Lu, J., and Claud, E. (2019). Connection between gut microbiome and brain development in preterm infants. Dev. Psychobiol. 61, 739–751. doi: 10.1002/dev.21806
Lu, J., Lu, L., Yu, Y., Cluette-Brown, J., Martin, C., and Claud, E. (2018). Effects of intestinal microbiota on brain development in humanized gnotobiotic mice. Sci. Rep. 8:5443. doi: 10.1038/s41598-018-23692-w
Lu, P., Sodhi, C., and Hackam, D. (2014). Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology 21, 81–93. doi: 10.1016/j.pathophys.2013.11.007
Lyte, M. (2011). Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 33, 574–581. doi: 10.1002/bies.201100024
Madan, J., Farzan, S., Hibberd, P., and Karagas, M. (2012). Normal neonatal microbiome variation in relation to environmental factors, infection and allergy. Curr. Opin. Pediatr. 24, 753–759.
Manzoni, P., Mostert, M., Leonessa, M., Priolo, C., Farina, D., Monetti, C., et al. (2006). Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by candida species in preterm neonates: A randomized study. Clin. Infect. Dis. 42, 1735–1742. doi: 10.1086/504324
Manzoni, P., Rinaldi, M., Cattani, S., Pugni, L., Romeo, M., Messner, H., et al. (2009). Italian task force for the s, prevention of neonatal fungal infections ISoN. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: A randomized trial. JAMA 302, 1421–1428. doi: 10.1001/jama.2009.1403
Martin, C., Dammann, O., Allred, E., Patel, S., O’Shea, T., Kuban, K., et al. (2010). Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J. Pediatr. 157, 751–756.e1. doi: 10.1016/j.jpeds.2010.05.042
Mayr, J., Fasching, G., and Hollwarth, M. (1994). Psychosocial and psychomotoric development of very low birthweight infants with necrotizing enterocolitis. Acta Paediatr. Suppl. 396, 96–100. doi: 10.1111/j.1651-2227.1994.tb13256.x
Merhar, S., Ramos, Y., Meinzen-Derr, J., and Kline-Fath, B. (2014). Brain magnetic resonance imaging in infants with surgical necrotizing enterocolitis or spontaneous intestinal perforation versus medical necrotizing enterocolitis. J Pediatr. 164, 410–412.e1. doi: 10.1016/j.jpeds.2013.09.055
Mihatsch, W., Vossbeck, S., Eikmanns, B., Hoegel, J., and Pohlandt, F. (2010). Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: A randomized controlled trial. Neonatology 98, 156–163. doi: 10.1159/000280291
Millar, M., Bacon, C., Smith, S., Walker, V., and Hall, M. (1993). Enteral feeding of premature infants with Lactobacillus GG. Arch. Dis. Child. 69, 483–487.
Mohan, R., Koebnick, C., Schildt, J., Schmidt, S., Mueller, M., Possner, M., et al. (2006). Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: A double-blind, placebo-controlled, randomized study. J. Clin. Microbiol. 44, 4025–4031. doi: 10.1128/JCM.00767-06
Morgan, R., Preidis, G., Kashyap, P., Weizman, A., Sadeghirad, B., McMaster Probiotic, P., et al. (2020). Probiotics reduce mortality and morbidity in preterm, low-birth-weight infants: A systematic review and network meta-analysis of randomized trials. Gastroenterology 159, 467–480. doi: 10.1053/j.gastro.2020.05.096
Morrow, A., Lagomarcino, A., Schibler, K., Taft, D., Yu, Z., Wang, B., et al. (2013). Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome 1:13. doi: 10.1186/2049-2618-1-13
Moschopoulos, C., Kratimenos, P., Koutroulis, I., Shah, B., Mowes, A., and Bhandari, V. (2018). The neurodevelopmental perspective of surgical necrotizing enterocolitis: The role of the gut-brain axis. Mediators Inflamm. 2018:7456857. doi: 10.1155/2018/7456857
Neu, J., and Pammi, M. (2018). Necrotizing enterocolitis: The intestinal microbiome, metabolome and inflammatory mediators. Semin. Fetal Neonatal Med. 23, 400–405. doi: 10.1016/j.siny.2018.08.001
Neu, J., and Walker, W. (2011). Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264. doi: 10.1056/NEJMra1005408
Niemarkt, H., de Meij, T., van de Velde, M., van der Schee, M., van Goudoever, J., Kramer, B., et al. (2015). Necrotizing enterocolitis: A clinical review on diagnostic biomarkers and the role of the intestinal microbiota. Inflamm. Bowel Dis. 21, 436–444.
Niemarkt, H., De Meij, T., van Ganzewinkel, C., de Boer, N., Andriessen, P., Hutten, M., et al. (2019). Necrotizing enterocolitis, gut microbiota, and brain development: Role of the brain-gut axis. Neonatology 115, 423–431. doi: 10.1159/000497420
Nino, D., Zhou, Q., Yamaguchi, Y., Martin, L., Wang, S., Fulton, W., et al. (2018). Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci. Transl. Med. 10:eaan0237. doi: 10.1126/scitranslmed.aan0237
Olm, M., Bhattacharya, N., Crits-Christoph, A., Firek, B., Baker, R., Song, Y., et al. (2019). Necrotizing enterocolitis is preceded by increased gut bacterial replication, Klebsiella, and fimbriae-encoding bacteria. Sci Adv. 5:eaax5727. doi: 10.1126/sciadv.aax5727
Oshiro, T., Nagata, S., Wang, C., Takahashi, T., Tsuji, H., Asahara, T., et al. (2019). Bifidobacterium supplementation of colostrum and breast milk enhances weight gain and metabolic responses associated with microbiota establishment in very-preterm infants. Biomed. Hub. 4, 1–10. doi: 10.1159/000502935
Pammi, M., Cope, J., Tarr, P., Warner, B., Morrow, A., Mai, V., et al. (2017). Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 5:31. doi: 10.1186/s40168-017-0248-8
Patel, R., and Denning, P. (2015). Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr. Res. 78, 232–238. doi: 10.1038/pr.2015.97
Penders, J., Thijs, C., Vink, C., Stelma, F., Snijders, B., Kummeling, I., et al. (2006). Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118, 511–521. doi: 10.1542/peds.2005-2824
Pfister, K., Gray, H., Miller, N., Demerath, E., Georgieff, M., and Ramel, S. (2013). Exploratory study of the relationship of fat-free mass to speed of brain processing in preterm infants. Pediatr. Res. 74, 576–583. doi: 10.1038/pr.2013.138
Pike, K., Brocklehurst, P., Jones, D., Kenyon, S., Salt, A., Taylor, D., et al. (2012). Outcomes at 7 years for babies who developed neonatal necrotising enterocolitis: The ORACLE children study. Arch. Dis. Child. Fetal Neonatal Ed. 97, F318–F322. doi: 10.1136/fetalneonatal-2011-300244
Raba, A., O’Sullivan, A., and Miletin, J. (2021). Pathogenesis of necrotising enterocolitis: The impact of the altered gut microbiota and antibiotic exposure in preterm infants. Acta Paediatr. 110, 433–440. doi: 10.1111/apa.15559
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S., and Medzhitov, R. (2004). Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241. doi: 10.1016/j.cell.2004.07.002
Ramel, S., Brown, L., and Georgieff, M. (2014). The Impact of neonatal illness on nutritional requirements-one size does not fit all. Curr. Pediatr. Rep. 2, 248–254. doi: 10.1007/s40124-014-0059-3
Rees, C., Pierro, A., and Eaton, S. (2007). Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 92, F193–F198.
Regev, R., Arnon, S., Litmanovitz, I., Bauer-Rusek, S., Boyko, V., Lerner-Geva, L., et al. (2016). Association between neonatal morbidities and head growth from birth until discharge in very-low-birthweight infants born preterm: A population-based study. Dev. Med. Child. Neurol. 58, 1159–1166. doi: 10.1111/dmcn.13153
Reuman, P., Duckworth, D., Smith, K., Kagan, R., Bucciarelli, R., and Ayoub, E. (1986). Lack of effect of Lactobacillus on gastrointestinal bacterial colonization in premature infants. Pediatr. Infect. Dis. 5, 663–668. doi: 10.1097/00006454-198611000-00013
Rouge, C., Piloquet, H., Butel, M., Berger, B., Rochat, F., Ferraris, L., et al. (2009). Oral supplementation with probiotics in very-low-birth-weight preterm infants: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 89, 1828–1835.
Roy, A., Chaudhuri, J., Sarkar, D., Ghosh, P., and Chakraborty, S. (2014). Role of enteric supplementation of probiotics on late-onset sepsis by candida species in preterm low birth weight neonates: A randomized, double blind, placebo-controlled trial. N. Am. J. Med. Sci. 6, 50–57. doi: 10.4103/1947-2714.125870
Roze, E., Ta, B., van der Ree, M., Tanis, J., van Braeckel, K., Hulscher, J., et al. (2011). Functional impairments at school age of children with necrotizing enterocolitis or spontaneous intestinal perforation. Pediatr. Res. 70, 619–625.
Saengtawesin, V., Tangpolkaiwalsak, R., and Kanjanapattankul, W. (2014). Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J. Med. Assoc. Thai. 97(Suppl. 6) S20–S25.
Salhab, W., Perlman, J., Silver, L., and Sue Broyles, R. (2004). Necrotizing enterocolitis and neurodevelopmental outcome in extremely low birth weight infants <1000 g. J. Perinatol. 24, 534–540. doi: 10.1038/sj.jp.7211165
Samanta, M., Sarkar, M., Ghosh, P., Ghosh, J., Sinha, M., and Chatterjee, S. (2009). Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J. Trop. Pediatr. 55, 128–131. doi: 10.1093/tropej/fmn091
Sampath, V., Menden, H., Helbling, D., Li, K., Gastonguay, A., Ramchandran, R., et al. (2015). SIGIRR genetic variants in premature infants with necrotizing enterocolitis. Pediatrics 135, e1530–e1534. doi: 10.1542/peds.2014-3386
Sari, F., Dizdar, E., Oguz, S., Erdeve, O., Uras, N., and Dilmen, U. (2011). Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: A randomized, controlled trial. Eur. J. Clin. Nutr. 65, 434–439. doi: 10.1038/ejcn.2010.278
Schiano, T. (2010). Treatment options for hepatic encephalopathy. Pharmacotherapy 30(5 Pt 2), 16S–21S.
Schulzke, S., Deshpande, G., and Patole, S. (2007). Neurodevelopmental outcomes of very low-birth-weight infants with necrotizing enterocolitis: A systematic review of observational studies. Arch. Pediatr. Adolesc. Med. 161, 583–590. doi: 10.1001/archpedi.161.6.583
Selip, D., Jantzie, L., Chang, M., Jackson, M., Fitzgerald, E., Boll, G., et al. (2012). Regional differences in susceptibility to hypoxic-ischemic injury in the preterm brain: Exploring the spectrum from white matter loss to selective grey matter injury in a rat model. Neurol. Res. Int. 2012:725184. doi: 10.1155/2012/725184
Serce, O., Benzer, D., Gursoy, T., Karatekin, G., and Ovali, F. (2013). Efficacy of Saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: A randomised controlled trial. Early Hum Dev. 89, 1033–1036.
Shah, D., Doyle, L., Anderson, P., Bear, M., Daley, A., Hunt, R., et al. (2008). Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J. Pediatr. 153, 170–175.
Shah, T., Meinzen-Derr, J., Gratton, T., Steichen, J., Donovan, E., Yolton, K., et al. (2012). Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. J. Perinatol. 32, 552–558.
Shao, Y., Forster, S., Tsaliki, E., Vervier, K., Strang, A., Simpson, N., et al. (2019). Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 574, 117–121. doi: 10.1038/s41586-019-1560-1
Sharif, S., Meader, N., Oddie, S., Rojas-Reyes, M., and McGuire, W. (2020). Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst. Rev. 10:CD005496.
Shashidhar, A., Suman Rao, P., Nesargi, S., Bhat, S., and Chandrakala, B. (2017). Probiotics for promoting feed tolerance in very low birth weight neonates – a randomized controlled trial. Indian Pediatr. 54, 363–367. doi: 10.1007/s13312-017-1106-2
Shin, S., Kim, E., Yoo, H., Choi, Y., Kim, S., Lee, B., et al. (2016). Surgical necrotizing enterocolitis versus spontaneous intestinal perforation in white matter injury on brain magnetic resonance imaging. Neonatology 110, 148–154. doi: 10.1159/000444387
Sim, K., Shaw, A., Randell, P., Cox, M., McClure, Z., Li, M., et al. (2015). Dysbiosis anticipating necrotizing enterocolitis in very premature infants. Clin. Infect. Dis. 60, 389–397. doi: 10.1093/cid/ciu822
Simon, N., Brady, N., Stafford, R., and Powell, R. (1993). The effect of abdominal incisions on early motor development of infants with necrotizing enterocolitis. Dev. Med. Child. Neurol. 35, 49–53. doi: 10.1111/j.1469-8749.1993.tb11551.x
Smith, P., Hagberg, H., Naylor, A., and Mallard, C. (2014). Neonatal peripheral immune challenge activates microglia and inhibits neurogenesis in the developing murine hippocampus. Dev. Neurosci. 36, 119–131. doi: 10.1159/000359950
Sochocka, M., Diniz, B., and Leszek, J. (2017). Inflammatory response in the CNS: Friend or foe? Mol. Neurobiol. 54, 8071–8089. doi: 10.1007/s12035-016-0297-1
Sodhi, C., Shi, X., Richardson, W., Grant, Z., Shapiro, R., and Prindle, T. Jr., et al. (2010). Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology 138, 185–196. doi: 10.1053/j.gastro.2009.09.045
Sonntag, J., Grimmer, I., Scholz, T., Metze, B., Wit, J., and Obladen, M. (2000). Growth and neurodevelopmental outcome of very low birthweight infants with necrotizing enterocolitis. Acta Paediatr. 89, 528–532. doi: 10.1111/j.1651-2227.2000.tb00332.x
Soraisham, A., Amin, H., Al-Hindi, M., Singhal, N., and Sauve, R. (2006). Does necrotising enterocolitis impact the neurodevelopmental and growth outcomes in preterm infants with birthweight < or = 1250 g? J. Paediatr. Child Health 42, 499–504.
Stratiki, Z., Costalos, C., Sevastiadou, S., Kastanidou, O., Skouroliakou, M., Giakoumatou, A., et al. (2007). The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum. Dev. 83, 575–579. doi: 10.1016/j.earlhumdev.2006.12.002
Strunk, T., Inder, T., Wang, X., Burgner, D., Mallard, C., and Levy, O. (2014). Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect. Dis. 14, 751–762.
Strus, M., Helwich, E., Lauterbach, R., Rzepecka-Weglarz, B., Nowicka, K., Wilinska, M., et al. (2018). Effects of oral probiotic supplementation on gut Lactobacillus and Bifidobacterium populations and the clinical status of low-birth-weight preterm neonates: A multicenter randomized, double-blind, placebo-controlled trial. Infect. Drug Resist. 11, 1557–1571. doi: 10.2147/IDR.S166348
Sudo, N., Chida, Y., Aiba, Y., Sonoda, J., Oyama, N., Yu, X., et al. (2004). Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 558(Pt 1), 263–275. doi: 10.1113/jphysiol.2004.063388
Sun, J., Pan, X., Christiansen, L., Yuan, X., Skovgaard, K., Chatterton, D., et al. (2018). Necrotizing enterocolitis is associated with acute brain responses in preterm pigs. J. Neuroinflammation 15:180. doi: 10.1186/s12974-018-1201-x
Tahraoui, S., Marret, S., Bodenant, C., Leroux, P., Dommergues, M., Evrard, P., et al. (2001). Central role of microglia in neonatal excitotoxic lesions of the murine periventricular white matter. Brain Pathol. 11, 56–71. doi: 10.1111/j.1750-3639.2001.tb00381.x
Tana, C., Umesaki, Y., Imaoka, A., Handa, T., Kanazawa, M., and Fukudo, S. (2010). Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. 22, 512–519. doi: 10.1111/j.1365-2982.2009.01427.x
Tewari, V., Dubey, S., and Gupta, G. (2015). Bacillus clausii for prevention of late-onset sepsis in preterm infants: A randomized controlled trial. J. Trop. Pediatr. 61, 377–385. doi: 10.1093/tropej/fmv050
Tobiansky, R., Lui, K., Roberts, S., and Veddovi, M. (1995). Neurodevelopmental outcome in very low birthweight infants with necrotizing enterocolitis requiring surgery. J. Paediatr. Child Health 31, 233–236. doi: 10.1111/j.1440-1754.1995.tb00792.x
Tokarz-Deptula, B., Sliwa-Dominiak, J., Adamiak, M., Bak, K., and Deptula, W. (2016). Commensal bacteria and immunity of the gastrointestinal, respiratory and genitourinary tracts. Postepy Hig. Med. Dosw. 70, 599–609.
Totsu, S., Terahara, M., and Kusuda, S. (2018). Probiotics and the development of very low birthweight infants: Follow-up study of a randomised trial. BMJ Paediatr. Open 2:e000256.
Treszl, A., Heninger, E., Kalman, A., Schuler, A., Tulassay, T., and Vasarhelyi, B. (2003). Lower prevalence of IL-4 receptor alpha-chain gene G variant in very-low-birth-weight infants with necrotizing enterocolitis. J. Pediatr. Surg. 38, 1374–1378. doi: 10.1016/s0022-3468(03)00399-3
Uribe, A., Alam, M., Johansson, O., Midtvedt, T., and Theodorsson, E. (1994). Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat. Gastroenterology 107, 1259–1269.
Van Niekerk, E., Kirsten, G., Nel, D., and Blaauw, R. (2014). Probiotics, feeding tolerance, and growth: A comparison between HIV-exposed and unexposed very low birth weight infants. Nutrition 30, 645–653. doi: 10.1016/j.nut.2013.10.024
van Vliet, E., de Kieviet, J., Oosterlaan, J., and van Elburg, R. (2013). Perinatal infections and neurodevelopmental outcome in very preterm and very low-birth-weight infants: A meta-analysis. JAMA Pediatr. 167, 662–668. doi: 10.1001/jamapediatrics.2013.1199
Volpe, J. (2011). Systemic inflammation, oligodendroglial maturation, and the encephalopathy of prematurity. Ann Neurol. 70, 525–529. doi: 10.1002/ana.22533
Vongbhavit, K., and Underwood, M. (2016). Prevention of necrotizing enterocolitis through manipulation of the intestinal microbiota of the premature infant. Clin. Ther. 38, 716–732. doi: 10.1016/j.clinthera.2016.01.006
Wadhawan, R., Oh, W., Hintz, S., Blakely, M., Das, A., Bell, E., et al. (2014). Neurodevelopmental outcomes of extremely low birth weight infants with spontaneous intestinal perforation or surgical necrotizing enterocolitis. J. Perinatol. 34, 64–70. doi: 10.1038/jp.2013.128
Wandro, S., Osborne, S., Enriquez, C., Bixby, C., Arrieta, A., and Whiteson, K. (2018). The microbiome and metabolome of preterm infant stool are personalized and not driven by health outcomes, including necrotizing enterocolitis and late-onset sepsis. mSphere 3, e00104–18. doi: 10.1128/mSphere.00104-18
Wang, C., Shoji, H., Sato, H., Nagata, S., Ohtsuka, Y., Shimizu, T., et al. (2007). Effects of oral administration of Bifidobacterium breve on fecal lactic acid and short-chain fatty acids in low birth weight infants. J. Pediatr. Gastroenterol. Nutr. 44, 252–257. doi: 10.1097/01.mpg.0000252184.89922.5f
Wang, K., Fan, L., Kaizaki, A., Pang, Y., Cai, Z., and Tien, L. (2013). Neonatal lipopolysaccharide exposure induces long-lasting learning impairment, less anxiety-like response and hippocampal injury in adult rats. Neuroscience 234, 146–157. doi: 10.1016/j.neuroscience.2012.12.049
Waugh, J., O’Callaghan, M., Tudehope, D., Mohay, H., Burns, Y., Gray, P., et al. (1996). Prevalence and aetiology of neurological impairment in extremely low birthweight infants. J. Paediatr. Child Health 32, 120–124. doi: 10.1111/j.1440-1754.1996.tb00907.x
Wejryd, E., Marchini, G., Frimmel, V., Jonsson, B., and Abrahamsson, T. (2019). Probiotics promoted head growth in extremely low birthweight infants in a double-blind placebo-controlled trial. Acta Paediatr. 108, 62–69. doi: 10.1111/apa.14497
Wikoff, W., Anfora, A., Liu, J., Schultz, P., Lesley, S., Peters, E., et al. (2009). Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U.S.A. 106, 3698–3703.
Williamson, L., Sholar, P., Mistry, R., Smith, S., and Bilbo, S. (2011). Microglia and memory: Modulation by early-life infection. J Neurosci. 31, 15511–15521.
Zani, A., and Pierro, A. (2015). Necrotizing enterocolitis: Controversies and challenges. F1000Res. 4:1373. doi: 10.12688/f1000research.6888.1
Zeber-Lubecka, N., Kulecka, M., Ambrozkiewicz, F., Paziewska, A., Lechowicz, M., Konopka, E., et al. (2016). Effect of Saccharomyces boulardii and mode of delivery on the early development of the gut microbial community in preterm infants. PLoS One 11:e0150306. doi: 10.1371/journal.pone.0150306
Zhou, Q., Nino, D., Yamaguchi, Y., Wang, S., Fulton, W., Jia, H., et al. (2021). Necrotizing enterocolitis induces T lymphocyte-mediated injury in the developing mammalian brain. Sci. Transl. Med. 13:eaay6621. doi: 10.1126/scitranslmed.aay6621
Zhou, W., Yuan, W., Huang, L., Wang, P., Rong, X., and Tang, J. (2015). Association of neonatal necrotizing enterocolitis with myeloid differentiation-2 and GM2 activator protein genetic polymorphisms. Mol. Med. Rep. 12, 974–980. doi: 10.3892/mmr.2015.3499
Zhou, Y., Shan, G., Sodergren, E., Weinstock, G., Walker, W., and Gregory, K. (2015). Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis: A case-control study. PLoS One 10:e0118632. doi: 10.1371/journal.pone.0118632
Keywords: necrotizing enterocolitis, neurodevelopmental impairment, gut-brain axis, microbiota, neurodevelopmental outcomes
Citation: Wang Y, Hang C, Hu J, Li C, Zhan C, Pan J and Yuan T (2023) Role of gut-brain axis in neurodevelopmental impairment of necrotizing enterocolitis. Front. Neurosci. 17:1059552. doi: 10.3389/fnins.2023.1059552
Received: 01 October 2022; Accepted: 02 January 2023;
Published: 19 January 2023.
Edited by:
Silvia Turroni, University of Bologna, ItalyReviewed by:
Kiran Veer Sandhu, University College Cork, IrelandCopyright © 2023 Wang, Hang, Hu, Li, Zhan, Pan and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianming Yuan,  eXVhbnRpYW5taW5nQHpqdS5lZHUuY24=
eXVhbnRpYW5taW5nQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.