
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurosci., 20 September 2022
Sec. Neurogenesis
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.955598
This article is part of the Research TopicMolecular and cellular players of axonal regeneration in injured CNSView all 5 articles
Understanding the regulation of axon growth after injury to the adult central nervous system (CNS) is crucial to improve neural repair. Following acute focal CNS injury, astrocytes are one cellular component of the scar tissue at the primary lesion that is traditionally associated with inhibition of axon regeneration. Advances in genetic models and experimental approaches have broadened knowledge of the capacity of astrocytes to facilitate injury-induced axon growth. This review summarizes findings that support a positive role of astrocytes in axon regeneration and axon sprouting in the mature mammalian CNS, along with potential underlying mechanisms. It is important to recognize that astrocytic functions, including modulation of axon growth, are context-dependent. Evidence suggests that the local injury environment, neuron-intrinsic regenerative potential, and astrocytes’ reactive states determine the astrocytic capacity to support axon growth. An integrated understanding of these factors will optimize therapeutic potential of astrocyte-targeted strategies for neural repair.
Failure of axons to regenerate in the adult mammalian CNS results in persisting functional deficits following CNS insults (Chen and Zheng, 2014). Overcoming barriers to axon regrowth is critical to restoration of neural functions. Besides the limited growth potential of mature CNS neurons, a major impediment to axon growth is the scar tissue—or widely referred to as the glial scar—at the site of acute focal damage. Regeneration-inhibitory activity of the glial scar has been excellently reviewed (Silver and Miller, 2004; Silver et al., 2014; Bradbury and Burnside, 2019). While astrocytes at the lesion border have been widely reputed to be a barrier to axon growth by association with the multicellular scar tissue, cell type-specific interrogation of gene expression and functions suggest that astrocytes are not principally responsible for regenerative failure at the lesion. Accumulating evidence suggests that the capacity of astrocytes to support axon growth in an injured CNS, at and remote from the lesion, may be greater than previously appreciated. In forming an understanding of astrocyte-mediated effects on axon growth, it is important to recognize that they are contextual—influenced at least in part by the local injury environment, neuron-intrinsic regenerative capacity, and astrocytes’ reactive states. This review will compare the astrocytic response to CNS injury between humans and mice, discuss evidence for a positive role of astrocytes in supporting axon regeneration at the lesion and axon sprouting away from the lesion, present potential underlying mechanisms, discuss the diversity of astrocytic injury responses with respect to their innate heterogeneity, and consider the role of the local injury environment in determining astrocytes’ capacity to facilitate axon growth, with the goal to broaden understanding of astrocytes’ potential for neural repair.
Astrocytes react to CNS injury with a range of phenotypic and functional changes that is broadly referred to as reactive astrogliosis (Sofroniew, 2014; Escartin et al., 2021). Following acute focal injury to the CNS (traumatic and ischemic injuries to the brain and spinal cord), scar-forming astrogliosis occurs at the lesion site to isolate tissue damage, where astrocytes upregulate glial fibrillary acidic protein (GFAP), hypertrophy, proliferate, and overlap their cellular processes to form a dense astrocytic border that encloses a lesion core of non-neural cells (Burda and Sofroniew, 2014; Silver et al., 2014). It is important to distinguish the astrocytic scar from the glial scar (Escartin et al., 2021). Although these two terms have been historically synonymous, use of “glial scar” has evolved to broadly describe the entire scar tissue that is a multicellular structure with a GFAP+ astrocytic lesion border and a GFAP– lesion core (Wanner et al., 2013; Zhu et al., 2015; Chen et al., 2018). Therefore, to avoid confusion, and as the field continues to gain cell type-specific understanding of the origin and function of scar components, it would be most informative to specify the cell types rather than using the umbrella term of “glial scar” in discussion of the scar tissue. The astrocytic scar, in this review, specifically refers to the astrocytic component that lines the lesion border. With increasing distance from the lesion, the level of astrocyte reactivity decreases and gradually transitions from proliferating and overlapping scar-forming astrocytes at the primary lesion, to hypertrophic stellate astrocytes that retain their tiling property in reactive tissue, then to non-reactive astrocytes as found in healthy tissue (Wanner et al., 2013). This is generally true for astrogliosis emanating from acute focal lesion in the gray matter. However, damage to white matter tracts or to neurons from which they originate results in non-scar forming astrogliosis in areas of Wallerian degeneration that can extend over a long distance without tapering off in astrocyte reactivity (Wang et al., 2009; Chen et al., 2022).
Astrocytes respond rapidly to blood-brain barrier (BBB) damage. It is postulated that BBB damage at the lesion site creates a gradient of blood-borne immune cells and damage associated molecules that elicits a tapering astrocytic response in the injured spinal cord (Burda and Sofroniew, 2014). Gradation of astrogliosis is conserved between humans and mice (Sofroniew and Vinters, 2010), with some differences in timing and severity of astrogliosis that is likely due to the lack of direct comparison in the type and phase of injury examined. Within days after spinal cord injury (SCI) in humans, or 1–2 days after injury in mice, reactive astrocytes display nuclear enlargement, elongated cellular processes, and cytoskeletal hypertrophy (Buss et al., 2004; Norenberg et al., 2004; Burda and Sofroniew, 2014). Astrocytic encapsulation of the injury site is observed weeks after injury in humans, or 2–3 weeks after injury in mice (Herrmann et al., 2008; Wanner et al., 2013; Burda and Sofroniew, 2014; Chen et al., 2018). In both humans and mice, the astrocytic scar persists chronically once established, present even 30 years after injury in humans (Buss et al., 2007).
Studies of reactive astrocytes in the context of acute focal CNS injury have primarily focused on scar-forming astrocytes at the lesion. Scar-forming astrocytes originate from in situ proliferation of adult astrocytes (Bush et al., 1999; Faulkner et al., 2004; Okada et al., 2006; Barnabe-Heider et al., 2010; Wanner et al., 2013), differentiation of NG2+ oligodendrocyte progenitor cells (Dimou et al., 2008; Barnabe-Heider et al., 2010; Komitova et al., 2011; Hackett et al., 2016, 2018), and minimally, differentiation from ependymal cells (Meletis et al., 2008; Barnabe-Heider et al., 2010; Sabelstrom et al., 2013; Zukor et al., 2013; Ren et al., 2017). Molecular regulators of scar-forming astrogliosis have been comprehensively reviewed (Bradbury and Burnside, 2019; Sofroniew, 2020). The astrocytic scar is neuroprotective in the early phase of acute focal injury, and essential to tissue integrity in both acute and chronic phases of traumatic injury (Faulkner et al., 2004; Okada et al., 2006; Herrmann et al., 2008; Anderson et al., 2016). Disruption of astrocytic scar formation, by genetic ablation of proliferating astrocytes or attenuation of scar formation, results in enlarged lesion volume, widespread inflammatory cell infiltration, extensive neural tissue degeneration, and exacerbated functional outcome (Pekny et al., 1999; Faulkner et al., 2004; Brambilla et al., 2005; Okada et al., 2006; Herrmann et al., 2008; Sahni et al., 2010; Chen et al., 2018). Historically synonymous with the glial scar, the astrocytic component has been reputed as physical and chemical barriers to axon growth in the chronic phase of injury (Silver and Miller, 2004; Bradbury and Burnside, 2019). The extent of scar-forming astrogliosis in human SCI varies from “an impenetrable barrier is practically never seen” (Norenberg et al., 2004) to “dense GFAP-positive matrix can be seen after long survival times” (Buss et al., 2004). This divergence of observations seems to stem from difference in injury severity, with the formation of a dense astrocytic scar in human cases defined as “complete lesions without remaining nerve fibers traversing the lesion center” (Buss et al., 2004). This raises an intriguing possibility that the astrocytic scar may not be as much of a physical impediment to cellular regeneration in human survivors with anatomically incomplete SCI. The chemical barrier presented by the astrocytic scar has been mainly attributed to the production of chondroitin sulfate proteoglycans (CSPGs), which are extracellular matrix molecules generally regarded as inhibitory to axon growth (Davies et al., 1999; Silver and Miller, 2004). As methodological advances continue to improve cellular and molecular resolution of scar tissue composition, it is now known that scar-forming astrocytes are not the sole producers of CSPGs (Silver et al., 2014; Anderson et al., 2016). Supporting this are recent findings that oligodendrocyte progenitor cells (OPCs) at the lesion abundantly and preferentially express axon growth inhibitory CSPGs compared to astrocytes, as revealed by single-cell RNA sequencing (Milich et al., 2021; Wahane et al., 2021). Furthermore, scar-forming astrocytes produce axon growth-permissive extracellular matrix (ECM) molecules (Liesi et al., 1984; Frisen et al., 1995; Anderson et al., 2016), suggesting a potential to support axon growth (discussed in section “Evidence for astrocytes supporting axon regeneration”). As the levels of CSPGs within the scar tissue change temporally following injury, the relative abundance and interaction of growth inhibitory vs. permissive ECM molecules within the scar tissue likely contribute to determine the permissive window for axon regeneration at the lesion (McKeon et al., 1995).
In contrast, the role of moderately reactive astrocytes, which have also been referred to as stellate or diffuse reactive astrocytes, in neural repair remains elusive. This type of reactive astrocytes so far has only been recognized as morphologically distinct from scar-forming astrocytes (Anderson et al., 2014). Remote from the lesion site that is hostile to axon growth, stellate reactive astrocytes may have higher capacity to facilitate axon growth (see section “Evidence for astrocytes supporting axon sprouting”). In a mouse model that genetically stimulates stellate reactive astrocytes, their ability to promote axon sprouting distal to the primary lesion was revealed (see section “Evidence for astrocytes supporting axon sprouting”).
Axon regeneration is defined here as the regrowth of injured axons. In the mature mammalian CNS, damaged axons cannot spontaneously regenerate. The supportive role of astrocytes in adult CNS axon regeneration was revealed under experimental conditions that enhanced axons’ intrinsic capacity to grow. Phosphatase and tensin homolog (Pten) gene deletion or knockdown in corticospinal neurons promotes spontaneous regeneration of severed corticospinal axons after spinal cord injury (Liu et al., 2010; Zukor et al., 2013; Du et al., 2015). Under these conditions, while only a small subset of corticospinal axons regenerated into the primary lesion, a majority of these Pten-deleted regenerating axons grew along thin GFAP+ tissue bridges that developed across the lesion epicenter (Liu et al., 2010; Zukor et al., 2013; Du et al., 2015). Association of the GFAP+ matrix with regenerating axons has been consistently observed following dorsal hemisection, complete crush, or complete transection of the spinal cord at thoracic level T8 in adult mice (Lee et al., 2010; Liu et al., 2010; Zukor et al., 2013; Du et al., 2015). In contrast, regenerating axons did not contact the GFAP– lesion core (Lee et al., 2010; Liu et al., 2010; Zukor et al., 2013). These findings together suggest that the GFAP+ matrix can be axon growth-permissive, a property that may belong to a subtype of scar-forming astrocytes, or may be induced by axons stimulated to grow. It was reported that “although only 42% of the lesion is GFAP+, 80% of the axons in the lesion are in the GFAP+ portion” (Zukor et al., 2013). Notably, extension of GFAP+ bridge and accompanying axon regeneration into the lesion were observed in the chronic phase of injury (Lee et al., 2010; Liu et al., 2010; Zukor et al., 2013; Du et al., 2015), following initial establishment of the astrocytic scar with a well-demarcated GFAP+ and GFAP– lesion boundary in the subacute phase of injury (Okada et al., 2006; Herrmann et al., 2008; Wanner et al., 2013; Zhu et al., 2015; Hara et al., 2017; Chen et al., 2018). These observations suggest injury phase-dependent regulation of astrocytic functions: isolation of tissue damage in the early phase and potential facilitation of axon growth at a later stage.
Lineage tracing showed that GFAP+ bridge-forming cells are likely derived from resident mature astrocytes, with minimal contribution from progeny of ependymal cells in the adult spinal cord (Zukor et al., 2013). Axons regenerated along astroglial bridges 8–12 weeks after injury, but not earlier (Liu et al., 2010; Zukor et al., 2013). In fact, even when neuronal PTEN was deleted 1 year after injury, association of regenerating axons with astroglial bridges was still observed at 19 months after injury (Du et al., 2015), when the astrocytic scar had been chronically established. These findings show that in an environment conducive to the formation of astroglial bridges, their axon growth-supportive potential is unaffected by tissue maturation at the lesion. This contrasts the expectation that a more mature, thus more dense, astrocytic scar would be more obstructive to axon growth. It remains possible, however, that given the long survival period of this study, a more mature astrocytic scar may have diminished capacity to promote axon growth. Elucidating injury stage-specific permissibility of astrocytes to axon growth would be of interest for future investigation. Small lesions seem to favor astroglial bridge formation (Liu et al., 2010; Zukor et al., 2013; Du et al., 2015), as this process likely involves astroglial migration into the lesion from either lesion edge (White et al., 2008; Liu et al., 2010; Du et al., 2015). In the 129X1/SvJ strain of mice, substantial astrocyte migration into the lesion core is associated with robust regeneration of serotonergic and sensory axons into the lesion after midthoracic spinal cord contusion (Ma et al., 2004). Axon regrowth along astroglial bridges under experimental stimulation of neuronal regenerative potential also raises the possibility of bidirectional interaction between regenerating axons and astrocytes at the lesion, such that the growing axon may in turn induce a growth supportive phenotype of astrocytes (Silver, 2016).
Are astrocytes at the lesion required for axon regeneration? This was tested on sensory axons stimulated to regrow, via a peripheral conditioning lesion and provision of neurotrophic factors at the injury site, in a spinal cord injury model of complete crush at T10 (Anderson et al., 2016). Astrocytic scar formation was attenuated either by genetic ablation of proliferating astrocytes or gene deletion of signal transducer and activator of transcription (STAT3), a key regulator of astrocyte proliferation and reactivity (Herrmann et al., 2008; Wanner et al., 2013). Either approach of preventing astrocytic scar formation significantly reduced sensory axon regeneration, even when the neuron-intrinsic growth program was robustly stimulated (Anderson et al., 2016). These results indicate a positive role of reactive astrocytes on axon regeneration at the lesion. It should be taken into consideration, however, that testing the effects of scar-forming astrocytes on axon regeneration by a loss-of-function approach is inherently challenging because the astrocytic scar is required for tissue integrity in both the acute and chronic phases of injury (Bush et al., 1999; Faulkner et al., 2004), making it difficult to distinguish its direct effects on axon regeneration per se from indirect effects via tissue protection. Whether the astrocytic scar examined in this study had matured to a clinically relevant density, and how scar-forming astrocytes can form a cellular barrier to non-neural cells without restraining axon growth are key questions that have been raised (Silver, 2016). One possible answer to the latter question is that the activities of scar-forming astrocytes in corralling inflammatory cells and promoting axon growth are temporally separate over the course of injury. Alternatively, the molecular interface between spared tissue and astrocytes may be axon growth-permissive, whereas that between astrocytes and the non-neural lesion core may be growth inhibitory. Nevertheless, gene expression analysis revealed that scar-forming astrocytes upregulate extracellular matrix molecules that support axon growth, including laminins and beneficial subtypes of CSPGs (Anderson et al., 2016), in addition to growth-inhibitory CSPGs (Davies et al., 1999; Silver et al., 2014; Anderson et al., 2016) and type I collagen (Hara et al., 2017). Converging evidence of injury-dependent production of pro-regenerative molecules by reactive astrocytes at the lesion suggests their potential to support axon growth (Liesi et al., 1984; Frisen et al., 1995; Goss et al., 1998; Lee et al., 1998; Anderson et al., 2016).
Consistent with these findings, stimulation of astrocyte migration at the lesion site by TGFα administration enhanced axon growth into the lesion core following spinal cord contusion at T9 (White et al., 2008, 2011). Axons within the lesion were found to associate with astrocytes that expressed high levels of both growth-supportive laminin and the growth-inhibitory CSPG neurocan (White et al., 2008). Transplantation of astrocytes derived in vitro from rat glial-restricted precursors (GRPs) into cervical spinal cord lesion resulted in robust regeneration of sensory and rubrospinal axons at the lesion, concomitant with improved locomotor recovery following spinal cord injury (Davies et al., 2006). Similarly, transplantation of human GRPs, which differentiated into astrocytes in vivo following transplantation into cervical spinal cord lesion, stimulated regeneration and sprouting of rostral ventral respiratory group axons and improved diaphragm activity (Goulao et al., 2019). Immature rat cortical astrocytes, when co-transplanted with chondroitinase ABC that digests CSPGs, were also able to promote regeneration of basal forebrain axons following microlesion of the cingulum (Filous et al., 2010). These findings show that astrocytes, when stimulated to invade into or placed at the lesion site, have the capacity to facilitate axon regeneration.
Following injury to the adult mammalian CNS, damaged axons fail to regenerate, but spared intact axons can spontaneously grow or sprout (Chen and Zheng, 2014). Injury-induced axon sprouting is a compensatory response that can occur away from the injury site to establish short local or relay connections with denervated neuronal targets (Chen and Zheng, 2014). Importantly, axon sprouting is a form of neural plasticity that contributes to neural recovery in human and animal models of CNS injury (Cafferty and Strittmatter, 2006; Fawcett et al., 2007; Ueno et al., 2012; Chen and Zheng, 2014; Wahl et al., 2014; Jin et al., 2015; Carmichael et al., 2017; Courtine and Sofroniew, 2019).
Intriguingly, astrocytes have been implicated to promote axon sprouting that occurs far from the primary injury site. Following unilateral photothrombotic stroke targeted to the forelimb sensorimotor cortex of one cerebral hemisphere, corticospinal axons that originate from the uninjured hemisphere sprout across the midline in the cervical spinal cord to project into the denervated side (Lindau et al., 2014; Liu et al., 2014; Wahl et al., 2014). Effects of reactive astrocytes on axon sprouting in this model were tested using mice with constitutive whole-body gene deletions of two structural proteins that are highly upregulated in reactive astrocytes: GFAP and vimentin (GFAP–/–Vim–/–) (Liu et al., 2014). GFAP–/–Vim–/– mice exhibit impaired astrocytic reactivity, including attenuated cytoskeletal hypertrophy and enlarged lesion size in response to injury (Pekny et al., 1999; Wilhelmsson et al., 2004; Li et al., 2008). Following unilateral cortical photothrombosis, intra-spinal sprouting of corticospinal axons in GFAP–/–Vim–/– mice was reduced, accompanied by impaired motor recovery (Liu et al., 2014). While contribution from other cell types cannot be ruled out due to global deletion of GFAP and vimentin, these findings suggest that astrocytes support axon sprouting. Interestingly, using the same GFAP–/–Vim–/– mice, but a different CNS injury model of spinal cord injury at low thoracic level T12, sprouting of serotonergic and corticospinal axons was found to decrease at the lumbar spinal cord (Menet et al., 2003). The opposite sprouting response observed may be attributed to difference in injury type, as astrocytes reacting to ischemic stroke have been shown to display molecular signatures beneficial to repair (Zamanian et al., 2012).
The above studies raise the questions of what kind of astrocytes regulate axon sprouting and how. Given that axon sprouting examined occurs distal to the lesion, it is conceivable that non-scar forming reactive astrocytes located away from the lesion play a role. In line with this, reactive astrocytes before scar formation (Hara et al., 2017) or located in spared tissue away from the injury site (Davies et al., 1999) have been shown to possess greater potential to support axon growth than scar-forming astrocytes at the lesion (Davies et al., 1999; Hara et al., 2017). If moderately reactive astrocytes are indeed more capable of supporting axon growth, can this type of reactive astrocytes be amplified to promote axon plasticity after CNS injury?
A mouse model was developed to modulate astrocyte reactivity through gene expression manipulation of leucine zipper-bearing kinase (LZK) in astrocytes, which was identified as an activator of STAT3 signaling (Chen et al., 2018). In the absence of CNS injury, LZK overexpression in adult astrocytes activates STAT3 signaling and stimulates moderate astrocyte reactivity throughout the CNS gray matter, as assessed by the hallmarks of astrogliosis (including upregulation of GFAP, vimentin, cytoskeletal hypertrophy, cell proliferation, and STAT3 activation), while preserving individual astrocyte domains (Chen et al., 2018). These characteristics render them similar to stellate reactive astrocytes, defined as hypertrophic GFAP+ reactive astrocytes that can proliferate, but maintain their tiling property and stellate appearance that resembles mature astrocytes in healthy tissue (Wanner et al., 2013; Anderson et al., 2014). Genetic stimulation of stellate reactive astrocytes via LZK induction in adult astrocytes enhanced corticospinal axon sprouting in the spinal cord following unilateral photothrombotic stroke of the primary motor cortex (Chen et al., 2022). Furthermore, this phenomenon is injury-dependent (Chen et al., 2022). LZK-stimulated stellate reactive astrocytes also produce the cytokine ciliary neurotrophic factor (CNTF) (Chen et al., 2022), which is known to be a sufficient and potent promoter of CNS axon sprouting (Jin et al., 2015). While it remains to be determined the extent to which CNTF accounts for the axon sprouting stimulatory effects of LZK and how these stellate reactive astrocytes impact neuronal functions, these findings nevertheless uncovered an exciting positive role of stellate astrogliosis in axon sprouting, with important implications for shaping neural plasticity in an injured CNS. As further discussed in section “Astrocyte diversity: an astrocyte subtype that promotes axon growth?,” astrocytes’ capacity to support axon regeneration vs. axon sprouting may differ depending on the local injury environment, neuron-intrinsic regenerative potential, and innate heterogeneity of astrocytes.
Potential mechanisms that underlie axon growth-supportive effects of astrocytes include the production of neurotrophic factors, remodeling of the extracellular matrix, clearance of myelin debris, and provision of bioenergetic support for axon growth, discussed below (Figure 1).
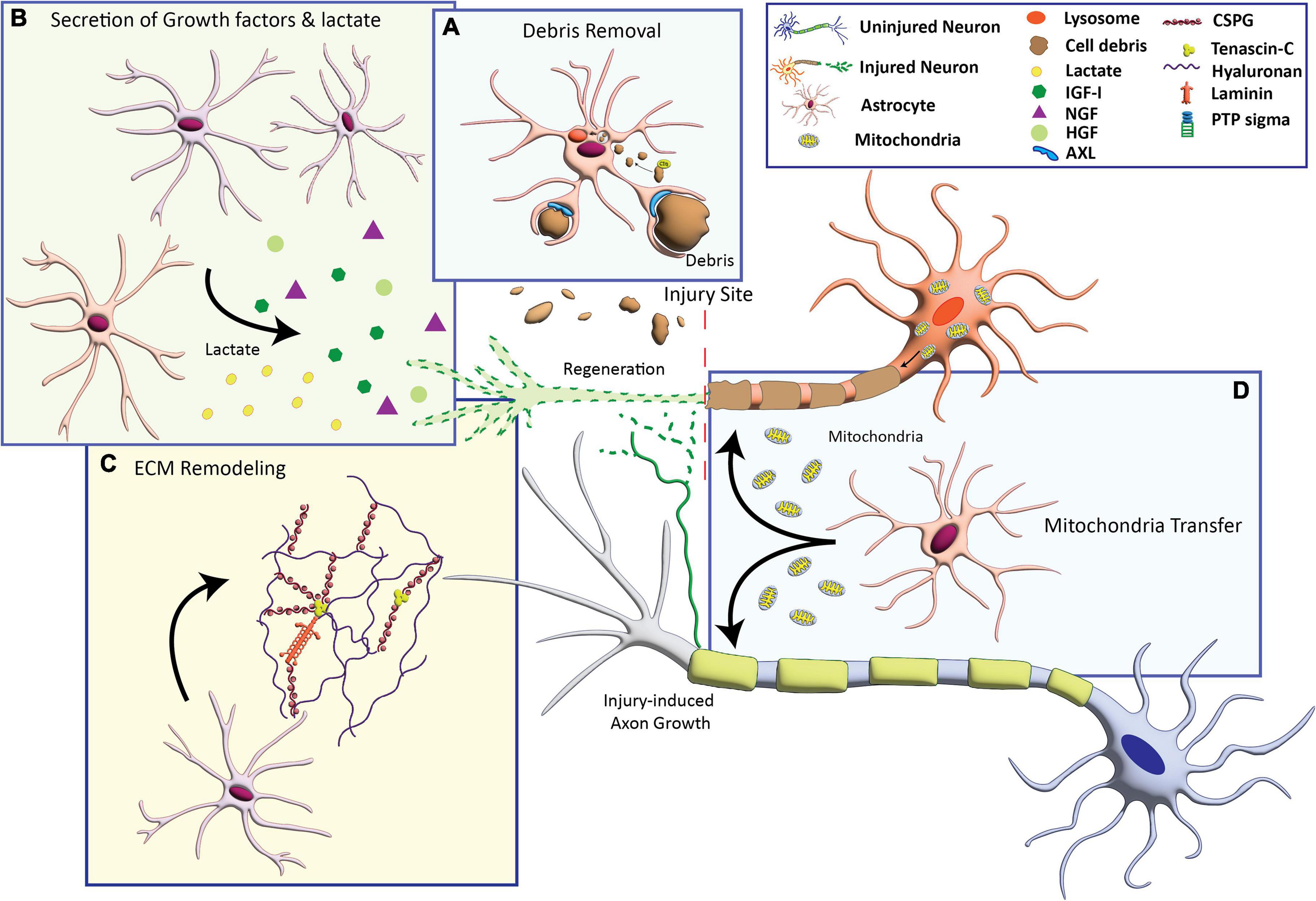
Figure 1. Astrocyte-based mechanisms that can promote axon growth. (A) Activation of phagocytosis to engulf cellular debris that inhibit axon growth. (B) Secretion of growth factors including insulin-like growth factor (IGF-I), nerve growth factor (NGF), and hepatocyte growth factor (HFG), and the metabolite lactate. (C) Production of extracellular matrix (ECM) molecules including laminin and axon growth-permissive chondroitin sulfate proteoglycans (CSPGs). (D) Transfer of healthy mitochondria to compromised neurons to provide bioenergetic support for axon growth.
Reactive astrocytes produce a number of neurotrophic factors upon spinal cord and brain injuries. These include brain-derived neurotrophic factor (BDNF) known to promote neuronal survival and axon growth (Dougherty et al., 2000; Ikeda et al., 2001); ciliary neurotrophic factor (CNTF) (Lee et al., 1998; Tripathi and McTigue, 2008; Chen et al., 2022) shown to robustly stimulate axon regeneration and sprouting of CNS axons (Muller et al., 2007; Jin et al., 2015); nerve growth factor (NGF) (Goss et al., 1998; Krenz and Weaver, 2000) that enhances axon regrowth (Tuszynski et al., 2002; Hannila and Kawaja, 2005; Mesentier-Louro et al., 2019) using reactive astrocytes as a substrate (Kawaja and Gage, 1991); fibroblast growth factor 2 (FGF2) (do Carmo Cunha et al., 2007) shown to promote CNS axon branching (Szebenyi et al., 2001) and sensory axon regeneration (Lee et al., 2017); hepatocyte growth factor (HGF) (Shimamura et al., 2007) capable of increasing CNS axon regrowth (Kitamura et al., 2007; Yamane et al., 2018); and insulin-like growth factor 1 (IGF1) (Yao et al., 1995) with potent regeneration-stimulatory activity on corticospinal axons (Hollis et al., 2009; Liu et al., 2017). Human spinal cord astrocytes, when stimulated with IL1β to undergo astrogliosis in vitro, also express the growth factors such as FGF2, BDNF, and NGF, adapting an overall axon growth-permissive phenotype (Teh et al., 2017).
Scar-forming reactive astrocytes are one of numerous cell types at the lesion that produce CSPGs (Morgenstern et al., 2002; Silver et al., 2014; Anderson et al., 2016; Milich et al., 2021; Wahane et al., 2021), which may be more inhibitory to axon regeneration than degenerating CNS myelin (Davies et al., 1999). It is proposed that CSPGs interfere with axon growth by stabilizing dystrophic growth cones (Filous et al., 2014). Some CSPGs, however, are permissive to axon growth (Busch et al., 2010; Miller and Hsieh-Wilson, 2015) and are also expressed by scar-forming astrocytes (Anderson et al., 2016). Interestingly, while the CSPG4 (also known as NG2) molecule is inhibitory (Dou and Levine, 1994; Tan et al., 2005; Filous et al., 2014), NG2+ oligodendrocyte progenitor cells can support axon growth (Yang et al., 2006). A substantial number of NG2 cells differentiate into scar-forming astrocytes after spinal cord contusion (Hackett et al., 2016, 2018), raising the possibility that NG2 cell-derived astrocytes at the lesion may have a higher capacity to facilitate axon regeneration (Hackett et al., 2018). Finally, after spinal cord injury, axons that grow into the lesion often associate with astrocytes that express laminin, an extracellular matrix molecule known to promote axon growth (Frisen et al., 1995; Ma et al., 2004; White et al., 2008, 2011). Fibronectin produced by astrocytes can also enhance axon regeneration in mature white matter (Tom et al., 2004). Injury-dependent re-expression of axon growth-favorable extracellular matrix proteins along with developmental markers in reactive astrocytes suggests a shift to a more immature cellular state that is conducive to axon regeneration (Silver et al., 1993; Filous et al., 2010).
Myelin debris persists chronically after CNS injury and is a source of myelin-associated inhibitors of axonal growth (Yiu and He, 2006; Geoffroy and Zheng, 2014). CNS myelin debris also stimulates inflammation to cause secondary tissue damage (Chen et al., 2000; Jeon et al., 2008; Tanaka et al., 2009; Sun et al., 2010; Allodi et al., 2012; Abiega et al., 2016; Zhou et al., 2021). Clearance of cellular debris including myelin-derived inhibitors after CNS injury, therefore, can facilitate axon regeneration. Microglia promote axon sprouting, through the proposed mechanism of phagocytosing myelin debris (Jiang et al., 2019). While microglia and monocyte-derived macrophages are the principal phagocytic cells in the injured CNS, reactive astrocytes can also become highly phagocytic by expressing phagocytic receptors and activation of phagocytic pathways early after spinal cord injury, traumatic brain injury, and brain ischemia (Morizawa et al., 2017; Konishi et al., 2020; Wang J. et al., 2020; Jiang et al., 2021; Zhou et al., 2021; Wan et al., 2022). Astrocytes can respond to neighboring damaged neurons through sensing and removal of cell debris in injured tissue, in a process that involves engulfment of debris in acidic endocytic vesicles directed toward lysosome for degradation (Wakida et al., 2018; Wang S. et al., 2020; Wan et al., 2022). Damaged neuron at the single-cell level is sufficient to trigger a “clean up” signal in nearby astrocytes. Astrocytes–microglia cooperation in cell corpse removal has been demonstrated to involve elimination of the cell body by microglia and axon-derived diffuse debris by astrocytes in the maintenance of brain homeostasis (Damisah et al., 2020). After ischemic injury, astrocyte-mediated phagocytosis temporally follows that by microglia and persists well into the subacute phase of injury (Morizawa et al., 2017). These observations suggest astrocytic contribution to cellular debris clearance that facilitates axon growth in the injured CNS.
Mitochondria supply ATP that is essential to neuronal survival and regeneration. Inter-cellular transfer of mitochondria contributes to provide metabolic requirements for axonal regeneration. Astrocytes are a source of functional extracellular mitochondria that support neuronal viability after ischemic stroke. Inhibition of extracellular mitochondria transfer through actin-dependent endocytosis results in failure of axonal growth (Hayakawa et al., 2016). Furthermore, astrocytes secret metabolites to support neuronal functions following CNS insults (Valenza et al., 2015; Chao et al., 2019). Lactate is one key metabolite secreted by astrocytes that acts as a signaling molecule to regulate neuronal functions that include synaptic plasticity and axonal integrity (Jha and Morrison, 2020). It was also demonstrated that lactate-treated astrocyte can induce axon outgrowth upon co-culture (Xu et al., 2020). Importantly, lactate has an essential role in axon regeneration after injury, and substitution of glucose by lactate supports axonal function and survival (Brown et al., 2012; Morrison et al., 2015). Local application of L-lactate, produced by glia including astrocytes under physiological conditions, enhances regeneration of corticospinal axons after spinal cord injury (Li et al., 2020).
The spectrum of astrocytic responses to injury—from isolation of tissue damage, inhibition or facilitation of axon growth, to immunomodulation among other functions—is highly contextual and needs not be conceived as contradictory. They depend on the injury type, phase of injury, location from the lesion, and cellular and molecular makeup of the local environment. Additionally, innate heterogeneity of astrocytes compounds the complexity of their injury response.
Regulation of astrocyte diversity in development by positional cues and neuronal activity has been comprehensively reviewed (Khakh and Deneen, 2019). Physiological adaptation of astrocytes, in form and function, to neural circuits has been well demonstrated (Chai et al., 2017). Evolution of astrocyte complexity was proposed to enable the integration and expansion of processing power of the human brain (Oberheim et al., 2006). scRNA-seq revealed regional astrocyte diversity, defined transcriptomically, in the healthy brain and spinal cord of mice and humans (Saunders et al., 2018; Hasel et al., 2021; Li et al., 2022; Sadick et al., 2022).
Following CNS insult, astrocytes from different brain regions mount region-specific transcriptional responses to the same stimulus, while also sharing common alterations in gene expression (Diaz-Castro et al., 2021). Interestingly, regional identity of astrocytes, as defined by gene expression signatures, is preserved even in a reactive state (Diaz-Castro et al., 2021). scRNA-seq confirmed that distinct astrocyte subtypes mount different transcriptional responses with temporal variation to the same CNS insult (Hasel et al., 2021; Li et al., 2022). Disease-specific interaction of transcriptional regulators together with engagement of core reactivity-promoting transcription factors in reactive astrocytes collectively contributes to differential gene expressions that determine disease-specific astrocytic phenotype (Burda et al., 2022).
Transcriptomic profiling has undoubtedly advanced understanding of astrocyte diversity. Molecular differences, however, do not always reflect functional alterations, knowledge of which remains rudimentary. For example, despite acute neuroinflammation inducing extensive gene expression changes in cortical astrocytes, modest effects on their electrophysiology, intracellular Ca2+ signaling, and gap junction coupling suggest retention of homeostatic functions (Diaz-Castro et al., 2021). Upregulation of genes associated with a neurotoxic astrocyte phenotype (Liddelow et al., 2017) did not correspond with neuronal loss or dysfunction (Diaz-Castro et al., 2021; Burda et al., 2022). Conversely, astrocyte dysfunction can manifest before or without upregulation of reactivity markers (Tong et al., 2014; Shandra et al., 2019). These findings highlight that while molecular signatures are one important identifier of astrocyte subtypes in disease, additional cellular properties and functional outcomes of reactive astrocytes are crucial to constructing a multidimensional understanding of astrocyte diversity in disease.
Astrocytes’ capacity to support axon growth, and therefore neural plasticity, in an injured CNS is a functional outcome important to CNS repair. While the extent to which this capacity is intrinsically determined remains to be explored, the local injury environment and the regenerative capacity of neurons likely influence astrocytes’ ability to promote axon growth. In acute focal injury, it is conceivable that the capacity of astrocytes to support regeneration of damaged axons at the injury site is low, whereas their capacity to support sprouting of spared axons away from the lesion is high. This is because the injury site contains tissue damage that results in a highly growth-inhibitory lesion core and CSPG accumulation (Davies et al., 1999; Lee et al., 2010; Zukor et al., 2013; Du et al., 2015; Anderson et al., 2016; Milich et al., 2021; Stern et al., 2021; Wahane et al., 2021). This regeneration-adverse environment, together with the inability of adult mammalian CNS neurons to regenerate damaged axons (Chen and Zheng, 2014), potentially creates a high barrier for astrocytes to effect axon growth at the primary lesion. In contrast, intact tissue remote from the injury site not only presents a growth-conducive environment, distal sprouting of intact axons remote from the lesion is also a spontaneous neuronal response to injury (Chen and Zheng, 2014). These conditions together conceivably lower the barrier for astrocytes to facilitate axon growth in spared tissue. Environment-dependent plasticity of reactive astrocytes has been demonstrated by conversion into scar-forming or naive phenotype following transplantation into injured or healthy spinal cords, respectively (Hara et al., 2017). The extent to which astrocytic capacity to support axon growth in an injured CNS is spatially determined, and how it can be harnessed therapeutically, would be of great interest for future investigation.
Reactive astrocytes, especially those located at the lesion border, have been widely regarded as inhibitory to regeneration in the adult mammalian CNS. Evidence is presented here to support a positive role of reactive astrocytes in axon growth in the injured mammalian CNS. Their beneficial effects on axon growth are consistent with the observations of exacerbated functional deficits following disruption of astrogliosis in rodent models (Faulkner et al., 2004; Okada et al., 2006; Herrmann et al., 2008). Recent gene expression data, by bulk or single-cell RNA sequencing, of reactive astrocytes collected at the lesion from acute to chronic phases of spinal cord injury will aid unbiased discovery of astrocyte-derived factors that can facilitate axon regeneration in the mature mammalian CNS (Anderson et al., 2016; Milich et al., 2021; Wahane et al., 2021; Wei et al., 2021; Brennan et al., 2022; Burda et al., 2022; Li et al., 2022). Given the long-recognized diversity of astrocytes, based on origin, location, injury type, and molecular signatures (Zhang and Barres, 2010; Khakh and Sofroniew, 2015), a future challenge will be to define context-dependent astrocyte functions, including their regulation of axon plasticity. Are there subtypes of reactive astrocytes with higher capacity to support axon growth after injury? Are pro-regenerative mechanisms differentially engaged by reactive astrocytes in a context-dependent manner to facilitate axon growth? While scar-forming astrocytes have the potential to facilitate axon growth, axon regeneration in the injured CNS is limited by the lack of neuron-intrinsic regenerative ability and an overwhelmingly growth-inhibitory lesion core. We propose that in contrast, stellate reactive astrocytes located in preserved tissue away from the lesion have greater potential to effect axon plasticity in the injured CNS and therefore a more attractive subtype of reactive astrocytes to target for neural repair. Molecular and functional profiling of astrocyte subtypes, and importantly, an integration of this information, will meaningfully advance understanding of astrocyte diversity and open avenues to effectively harness the beneficial functions of reactive astrocytes.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Research in the authors’ laboratory was supported by grants from NIH/NINDS (R01NS121193), American Heart Association (18CDA34060059), Kentucky Spinal Cord and Head Injury Research Trust training fund, and University of Kentucky CCTS pilot award from NIH/NCATS (UL1TR001998).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abiega, O., Beccari, S., Diaz-Aparicio, I., Nadjar, A., Layé, S., Leyrolle, Q., et al. (2016). Neuronal hyperactivity disturbs ATP microgradients, impairs microglial motility, and reduces phagocytic receptor expression triggering apoptosis/microglial phagocytosis uncoupling. PLoS Biol. 14:e1002466. doi: 10.1371/journal.pbio.1002466
Allodi, I., Udina, E., and Navarro, X. (2012). Specificity of peripheral nerve regeneration: Interactions at the axon level. Prog. Neurobiol. 98, 16–37. doi: 10.1016/j.pneurobio.2012.05.005
Anderson, M. A., Ao, Y., and Sofroniew, M. V. (2014). Heterogeneity of reactive astrocytes. Neurosci. Lett. 565, 23–29. doi: 10.1016/j.neulet.2013.12.030
Anderson, M. A., Burda, J. E., Ren, Y., Ao, Y., O’Shea, T. M., Kawaguchi, R., et al. (2016). Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200. doi: 10.1038/nature17623
Barnabe-Heider, F., Goritz, C., Sabelstrom, H., Takebayashi, H., Pfrieger, F. W., Meletis, K., et al. (2010). Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7, 470–482. doi: 10.1016/j.stem.2010.07.014
Bradbury, E. J., and Burnside, E. R. (2019). Moving beyond the glial scar for spinal cord repair. Nat. Commun. 10:3879. doi: 10.1038/s41467-019-11707-7
Brambilla, R., Bracchi-Ricard, V., Hu, W. H., Frydel, B., Bramwell, A., Karmally, S., et al. (2005). Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J. Exp. Med. 202, 145–156. doi: 10.1084/jem.20041918
Brennan, F. H., Li, Y., Wang, C., Ma, A., Guo, Q., Li, Y., et al. (2022). Microglia coordinate cellular interactions during spinal cord repair in mice. Nat. Commun. 13:4096. doi: 10.1038/s41467-022-31797-0
Brown, A. M., Evans, R. D., Black, J., and Ransom, B. R. (2012). Schwann cell glycogen selectively supports myelinated axon function. Ann. Neurol. 72, 406–418. doi: 10.1002/ana.23607
Burda, J. E., and Sofroniew, M. V. (2014). Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81, 229–248. doi: 10.1016/j.neuron.2013.12.034
Burda, J. E., O’Shea, T. M., Ao, Y., Suresh, K. B., Wang, S., Bernstein, A. M., et al. (2022). Divergent transcriptional regulation of astrocyte reactivity across disorders. Nature 606, 557–564. doi: 10.1038/s41586-022-04739-5
Busch, S. A., Horn, K. P., Cuascut, F. X., Hawthorne, A. L., Bai, L., Miller, R. H., et al. (2010). Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J. Neurosci. 30, 255–265. doi: 10.1523/JNEUROSCI.3705-09.2010
Bush, T. G., Puvanachandra, N., Horner, C. H., Polito, A., Ostenfeld, T., Svendsen, C. N., et al. (1999). Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23, 297–308. doi: 10.1016/S0896-6273(00)80781-3
Buss, A., Brook, G. A., Kakulas, B., Martin, D., Franzen, R., Schoenen, J., et al. (2004). Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain 127(Pt 1), 34–44. doi: 10.1093/brain/awh001
Buss, A., Pech, K., Kakulas, B. A., Martin, D., Schoenen, J., Noth, J., et al. (2007). Matrix metalloproteinases and their inhibitors in human traumatic spinal cord injury. BMC Neurol. 7:17. doi: 10.1186/1471-2377-7-17
Cafferty, W. B., and Strittmatter, S. M. (2006). The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J. Neurosci. 26, 12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006
Carmichael, S. T., Kathirvelu, B., Schweppe, C. A., and Nie, E. H. (2017). Molecular, cellular and functional events in axonal sprouting after stroke. Exp. Neurol. 287(Pt 3), 384–394. doi: 10.1016/j.expneurol.2016.02.007
Chai, H., Diaz-Castro, B., Shigetomi, E., Monte, E., Octeau, J. C., Yu, X., et al. (2017). Neural circuit-specialized astrocytes: Transcriptomic, proteomic, morphological, and functional evidence. Neuron 95, 531–549.e9. doi: 10.1016/j.neuron.2017.06.029
Chao, C.-C., Gutiérrez-Vázquez, C., Rothhammer, V., Mayo, L., Wheeler, M. A., Tjon, E. C., et al. (2019). Metabolic control of astrocyte pathogenic activity via cPLA2-MAVS. Cell 179, 1483–1498.e22. doi: 10.1016/j.cell.2019.11.016
Chen, M., Ingle, L., Plautz, E. J., Kong, X., Tang, R., Ghosh, N, et al. (2022). Leucine zipper-bearing kinasedependent stimulation of astrocyte reactivity promotes corticospinal axon sprouting. Front. Cell. Neurosci. 16:969261. doi: 10.1016/j.celrep.2018.02.102
Chen, M. S., Huber, A. B., Van Der Haar, M. E., Frank, M., Schnell, L., Spillmann, A. A., et al. (2000). Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403, 434–439. doi: 10.1038/35000219
Chen, M., and Zheng, B. (2014). Axon plasticity in the mammalian central nervous system after injury. Trends Neurosci. 37, 583–593. doi: 10.1016/j.tins.2014.08.008
Chen, M., Geoffroy, C. G., Meves, J. M., Narang, A., Li, Y., Nguyen, M. T., et al. (2018). Leucine zipper-bearing kinase is a critical regulator of astrocyte reactivity in the adult mammalian CNS. Cell Rep. 22, 3587–3597. doi: 10.1016/j.celrep.2018.02.102
Courtine, G., and Sofroniew, M. V. (2019). Spinal cord repair: Advances in biology and technology. Nat. Med. 25, 898–908. doi: 10.1038/s41591-019-0475-6
Damisah, E. C., Hill, R. A., Rai, A., Chen, F., Rothlin, C. V., Ghosh, S., et al. (2020). Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci. Adv. 6:eaba3239. doi: 10.1126/sciadv.aba3239
Davies, J. E., Huang, C., Proschel, C., Noble, M., Mayer-Proschel, M., and Davies, S. J. (2006). Astrocytes derived from glial-restricted precursors promote spinal cord repair. J. Biol. 5:7. doi: 10.1186/jbiol35
Davies, S. J., Goucher, D. R., Doller, C., and Silver, J. (1999). Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J. Neurosci. 19, 5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999
Diaz-Castro, B., Bernstein, A. M., Coppola, G., Sofroniew, M. V., and Khakh, B. S. (2021). Molecular and functional properties of cortical astrocytes during peripherally induced neuroinflammation. Cell Rep. 36:109508. doi: 10.1016/j.celrep.2021.109508
Dimou, L., Simon, C., Kirchhoff, F., Takebayashi, H., and Gotz, M. (2008). Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 28, 10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008
do Carmo Cunha, J., de Freitas Azevedo Levy, B., de Luca, B. A., de Andrade, M. S., Gomide, V. C., and Chadi, G. (2007). Responses of reactive astrocytes containing S100beta protein and fibroblast growth factor-2 in the border and in the adjacent preserved tissue after a contusion injury of the spinal cord in rats: Implications for wound repair and neuroregeneration. Wound Repair. Regen. 15, 134–146. doi: 10.1111/j.1524-475X.2006.00194.x
Dou, C. L., and Levine, J. M. (1994). Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J. Neurosci. 14, 7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994
Dougherty, K. D., Dreyfus, C. F., and Black, I. B. (2000). Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol. Dis. 7(6 Pt B), 574–585. doi: 10.1006/nbdi.2000.0318
Du, K., Zheng, S., Zhang, Q., Li, S., Gao, X., Wang, J., et al. (2015). Pten deletion promotes regrowth of corticospinal tract axons 1 year after spinal cord injury. J. Neurosci. 35, 9754–9763. doi: 10.1523/JNEUROSCI.3637-14.2015
Escartin, C., Galea, E., Lakatos, A., O’Callaghan, J. P., Petzold, G. C., Serrano-Pozo, A., et al. (2021). Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24, 312–325. doi: 10.1038/s41593-020-00783-4
Faulkner, J. R., Herrmann, J. E., Woo, M. J., Tansey, K. E., Doan, N. B., and Sofroniew, M. V. (2004). Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 24, 2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004
Fawcett, J. W., Curt, A., Steeves, J. D., Coleman, W. P., Tuszynski, M. H., Lammertse, D., et al. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 45, 190–205. doi: 10.1038/sj.sc.3102007
Filous, A. R., Miller, J. H., Coulson-Thomas, Y. M., Horn, K. P., Alilain, W. J., and Silver, J. (2010). Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC. Dev. Neurobiol. 70, 826–841. doi: 10.1002/dneu.20820
Filous, A. R., Tran, A., Howell, C. J., Busch, S. A., Evans, T. A., Stallcup, W. B., et al. (2014). Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J. Neurosci. 34, 16369–16384. doi: 10.1523/JNEUROSCI.1309-14.2014
Frisen, J., Haegerstrand, A., Risling, M., Fried, K., Johansson, C. B., Hammarberg, H., et al. (1995). Spinal axons in central nervous system scar tissue are closely related to laminin-immunoreactive astrocytes. Neuroscience 65, 293–304. doi: 10.1016/0306-4522(94)00467-J
Geoffroy, C. G., and Zheng, B. (2014). Myelin-associated inhibitors in axonal growth after CNS injury. Curr. Opin. Neurobiol. 27, 31–38. doi: 10.1016/j.conb.2014.02.012
Goss, J. R., O’Malley, M. E., Zou, L., Styren, S. D., Kochanek, P. M., and DeKosky, S. T. (1998). Astrocytes are the major source of nerve growth factor upregulation following traumatic brain injury in the rat. Exp. Neurol. 149, 301–309. doi: 10.1006/exnr.1997.6712
Goulao, M., Ghosh, B., Urban, M. W., Sahu, M., Mercogliano, C., Charsar, B. A., et al. (2019). Astrocyte progenitor transplantation promotes regeneration of bulbospinal respiratory axons, recovery of diaphragm function, and a reduced macrophage response following cervical spinal cord injury. Glia 67, 452–466. doi: 10.1002/glia.23555
Hackett, A. R., Lee, D. H., Dawood, A., Rodriguez, M., Funk, L., Tsoulfas, P., et al. (2016). STAT3 and SOCS3 regulate NG2 cell proliferation and differentiation after contusive spinal cord injury. Neurobiol. Dis. 89, 10–22. doi: 10.1016/j.nbd.2016.01.017
Hackett, A. R., Yahn, S. L., Lyapichev, K., Dajnoki, A., Lee, D. H., Rodriguez, M., et al. (2018). Injury type-dependent differentiation of NG2 glia into heterogeneous astrocytes. Exp. Neurol. 308, 72–79. doi: 10.1016/j.expneurol.2018.07.001
Hannila, S. S., and Kawaja, M. D. (2005). Nerve growth factor-mediated collateral sprouting of central sensory axons into deafferentated regions of the dorsal horn is enhanced in the absence of the p75 neurotrophin receptor. J. Comp. Neurol. 486, 331–343. doi: 10.1002/cne.20537
Hara, M., Kobayakawa, K., Ohkawa, Y., Kumamaru, H., Yokota, K., Saito, T., et al. (2017). Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat. Med. 23, 818–828. doi: 10.1038/nm.4354
Hasel, P., Rose, I. V. L., Sadick, J. S., Kim, R. D., and Liddelow, S. A. (2021). Neuroinflammatory astrocyte subtypes in the mouse brain. Nat. Neurosci. 24, 1475–1487. doi: 10.1038/s41593-021-00905-6
Hayakawa, K., Esposito, E., Wang, X., Terasaki, Y., Liu, Y., Xing, C., et al. (2016). Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555. doi: 10.1038/nature18928
Herrmann, J. E., Imura, T., Song, B., Qi, J., Ao, Y., Nguyen, T. K., et al. (2008). STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 28, 7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008
Hollis, E. R. II, Lu, P., Blesch, A., and Tuszynski, M. H. (2009). IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury. Exp. Neurol. 215, 53–59. doi: 10.1016/j.expneurol.2008.09.014
Ikeda, O., Murakami, M., Ino, H., Yamazaki, M., Nemoto, T., Koda, M., et al. (2001). Acute up-regulation of brain-derived neurotrophic factor expression resulting from experimentally induced injury in the rat spinal cord. Acta Neuropathol. 102, 239–245. doi: 10.1007/s004010000357
Jeon, S.-B., Yoon, H. J., Park, S.-H., Kim, I.-H., and Park, E. J. (2008). Sulfatide, a major lipid component of myelin sheath, activates inflammatory responses as an endogenous stimulator in brain-resident immune cells. J. Immunol. 181, 8077–8087. doi: 10.4049/jimmunol.181.11.8077
Jha, M. K., and Morrison, B. M. (2020). Lactate transporters mediate glia-neuron metabolic crosstalk in homeostasis and disease. Front. Cell Neurosci. 14:589582. doi: 10.3389/fncel.2020.589582
Jiang, Y. Q., Armada, K., and Martin, J. H. (2019). Neuronal activity and microglial activation support corticospinal tract and proprioceptive afferent sprouting in spinal circuits after a corticospinal system lesion. Exp. Neurol. 321:113015. doi: 10.1016/j.expneurol.2019.113015
Jiang, Y., Liang, J., Li, R., Peng, Y., Huang, J., and Huang, L. (2021). Basic fibroblast growth factor accelerates myelin debris clearance through activating autophagy to facilitate early peripheral nerve regeneration. J. Cell. Mol. Med. 25, 2596–2608. doi: 10.1111/jcmm.16274
Jin, D., Liu, Y., Sun, F., Wang, X., Liu, X., and He, Z. (2015). Restoration of skilled locomotion by sprouting corticospinal axons induced by co-deletion of PTEN and SOCS3. Nat. Commun. 6:8074. doi: 10.1038/ncomms9074
Kawaja, M. D., and Gage, F. H. (1991). Reactive astrocytes are substrates for the growth of adult CNS axons in the presence of elevated levels of nerve growth factor. Neuron 7, 1019–1030. doi: 10.1016/0896-6273(91)90346-2
Khakh, B. S., and Deneen, B. (2019). The emerging nature of astrocyte diversity. Annu. Rev. Neurosci. 42, 187–207. doi: 10.1146/annurev-neuro-070918-050443
Khakh, B. S., and Sofroniew, M. V. (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952. doi: 10.1038/nn.4043
Kitamura, K., Iwanami, A., Nakamura, M., Yamane, J., Watanabe, K., Suzuki, Y., et al. (2007). Hepatocyte growth factor promotes endogenous repair and functional recovery after spinal cord injury. J. Neurosci. Res. 85, 2332–2342. doi: 10.1002/jnr.21372
Komitova, M., Serwanski, D. R., Lu, Q. R., and Nishiyama, A. (2011). NG2 cells are not a major source of reactive astrocytes after neocortical stab wound injury. Glia 59, 800–809. doi: 10.1002/glia.21152
Konishi, H., Okamoto, T., Hara, Y., Komine, O., Tamada, H., Maeda, M., et al. (2020). Astrocytic phagocytosis is a compensatory mechanism for microglial dysfunction. EMBO J. 39:e104464. doi: 10.15252/embj.2020104464
Krenz, N. R., and Weaver, L. C. (2000). Nerve growth factor in glia and inflammatory cells of the injured rat spinal cord. J. Neurochem. 74, 730–739. doi: 10.1046/j.1471-4159.2000.740730.x
Lee, J. K., Chow, R., Xie, F., Chow, S. Y., Tolentino, K. E., and Zheng, B. (2010). Combined genetic attenuation of myelin and semaphorin-mediated growth inhibition is insufficient to promote serotonergic axon regeneration. J. Neurosci. 30, 10899–10904. doi: 10.1523/JNEUROSCI.2269-10.2010
Lee, M. Y., Kim, C. J., Shin, S. L., Moon, S. H., and Chun, M. H. (1998). Increased ciliary neurotrophic factor expression in reactive astrocytes following spinal cord injury in the rat. Neurosci. Lett. 255, 79–82. doi: 10.1016/S0304-3940(98)00710-1
Lee, S. H., Jin, W. P., Seo, N. R., Pang, K. M., Kim, B., Kim, S. M., et al. (2017). Recombinant human fibroblast growth factor-2 promotes nerve regeneration and functional recovery after mental nerve crush injury. Neural Regen. Res. 12, 629–636. doi: 10.4103/1673-5374.205104
Li, C., Wu, Z., Zhou, L., Shao, J., Hu, X., Xu, W., et al. (2022). Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Signal. Transduct. Target. Ther. 7:65. doi: 10.1038/s41392-022-00885-4
Li, F., Sami, A., Noristani, H. N., Slattery, K., Qiu, J., Groves, T., et al. (2020). Glial metabolic rewiring promotes axon regeneration and functional recovery in the central nervous system. Cell Metab. 32, 767–785.e7. doi: 10.1016/j.cmet.2020.08.015
Li, L., Lundkvist, A., Andersson, D., Wilhelmsson, U., Nagai, N., Pardo, A. C., et al. (2008). Protective role of reactive astrocytes in brain ischemia. J. Cereb. Blood Flow Metab. 28, 468–481. doi: 10.1038/sj.jcbfm.9600546
Liddelow, S. A., Guttenplan, K. A., Clarke, L. E., Bennett, F. C., Bohlen, C. J., Schirmer, L., et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. doi: 10.1038/nature21029
Liesi, P., Kaakkola, S., Dahl, D., and Vaheri, A. (1984). Laminin is induced in astrocytes of adult brain by injury. EMBO J. 3, 683–686. doi: 10.1002/j.1460-2075.1984.tb01867.x
Lindau, N. T., Banninger, B. J., Gullo, M., Good, N. A., Bachmann, L. C., Starkey, M. L., et al. (2014). Rewiring of the corticospinal tract in the adult rat after unilateral stroke and anti-Nogo-A therapy. Brain 137(Pt 3), 739–756. doi: 10.1093/brain/awt336
Liu, K., Lu, Y., Lee, J. K., Samara, R., Willenberg, R., Sears-Kraxberger, I., et al. (2010). PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 13, 1075–1081. doi: 10.1038/nn.2603
Liu, Y., Wang, X., Li, W., Zhang, Q., Li, Y., Zhang, Z., et al. (2017). A sensitized IGF1 treatment restores corticospinal axon-dependent functions. Neuron 95, 817–833.e4. doi: 10.1016/j.neuron.2017.07.037
Liu, Z., Li, Y., Cui, Y., Roberts, C., Lu, M., Wilhelmsson, U., et al. (2014). Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia 62, 2022–2033. doi: 10.1002/glia.22723
Ma, M., Wei, P., Wei, T., Ransohoff, R. M., and Jakeman, L. B. (2004). Enhanced axonal growth into a spinal cord contusion injury site in a strain of mouse (129X1/SvJ) with a diminished inflammatory response. J. Comp. Neurol. 474, 469–486. doi: 10.1002/cne.20149
McKeon, R. J., Hoke, A., and Silver, J. (1995). Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp. Neurol. 136, 32–43. doi: 10.1006/exnr.1995.1081
Meletis, K., Barnabe-Heider, F., Carlen, M., Evergren, E., Tomilin, N., Shupliakov, O., et al. (2008). Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 6:e182. doi: 10.1371/journal.pbio.0060182
Menet, V., Prieto, M., Privat, A., and Gimenez y Ribotta, M. (2003). Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc. Natl. Acad. Sci. U.S.A. 100, 8999–9004. doi: 10.1073/pnas.1533187100
Mesentier-Louro, L. A., Rosso, P., Carito, V., Mendez-Otero, R., Santiago, M. F., Rama, P., et al. (2019). Nerve growth factor role on retinal ganglion cell survival and axon regrowth: Effects of ocular administration in experimental model of optic nerve injury. Mol. Neurobiol. 56, 1056–1069. doi: 10.1007/s12035-018-1154-1
Milich, L. M., Choi, J. S., Ryan, C., Cerqueira, S. R., Benavides, S., Yahn, S. L., et al. (2021). Single-cell analysis of the cellular heterogeneity and interactions in the injured mouse spinal cord. J. Exp. Med. 218:e20210040. doi: 10.1084/jem.20210040
Miller, G. M., and Hsieh-Wilson, L. C. (2015). Sugar-dependent modulation of neuronal development, regeneration, and plasticity by chondroitin sulfate proteoglycans. Exp. Neurol. 274(Pt B), 115–125. doi: 10.1016/j.expneurol.2015.08.015
Morgenstern, D. A., Asher, R. A., and Fawcett, J. W. (2002). Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 137, 313–332. doi: 10.1016/S0079-6123(02)37024-9
Morizawa, Y. M., Hirayama, Y., Ohno, N., Shibata, S., Shigetomi, E., Sui, Y., et al. (2017). Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat. Commun. 8:28. doi: 10.1038/s41467-017-00037-1
Morrison, B. M., Tsingalia, A., Vidensky, S., Lee, Y., Jin, L., Farah, M. H., et al. (2015). Deficiency in monocarboxylate transporter 1 (MCT1) in mice delays regeneration of peripheral nerves following sciatic nerve crush. Exp. Neurol. 263, 325–338. doi: 10.1016/j.expneurol.2014.10.018
Muller, A., Hauk, T. G., and Fischer, D. (2007). Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain 130(Pt 12), 3308–3320. doi: 10.1093/brain/awm257
Norenberg, M. D., Smith, J., and Marcillo, A. (2004). The pathology of human spinal cord injury: Defining the problems. J. Neurotrauma 21, 429–440. doi: 10.1089/089771504323004575
Oberheim, N. A., Wang, X., Goldman, S., and Nedergaard, M. (2006). Astrocytic complexity distinguishes the human brain. Trends Neurosci. 29, 547–553. doi: 10.1016/j.tins.2006.08.004
Okada, S., Nakamura, M., Katoh, H., Miyao, T., Shimazaki, T., Ishii, K., et al. (2006). Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 12, 829–834. doi: 10.1038/nm1425
Pekny, M., Johansson, C. B., Eliasson, C., Stakeberg, J., Wallen, A., Perlmann, T., et al. (1999). Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J. Cell Biol. 145, 503–514. doi: 10.1083/jcb.145.3.503
Ren, Y., Ao, Y., O’Shea, T. M., Burda, J. E., Bernstein, A. M., Brumm, A. J., et al. (2017). Ependymal cell contribution to scar formation after spinal cord injury is minimal, local and dependent on direct ependymal injury. Sci. Rep. 7:41122. doi: 10.1038/srep41122
Sabelstrom, H., Stenudd, M., Reu, P., Dias, D. O., Elfineh, M., Zdunek, S., et al. (2013). Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science 342, 637–640. doi: 10.1126/science.1242576
Sadick, J. S., O’Dea, M. R., Hasel, P., Dykstra, T., Faustin, A., and Liddelow, S. A. (2022). Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer’s disease. Neuron 110, 1788–1805.e10. doi: 10.1016/j.neuron.2022.03.008
Sahni, V., Mukhopadhyay, A., Tysseling, V., Hebert, A., Birch, D., McGuire, T. L., et al. (2010). BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J. Neurosci. 30, 1839–1855. doi: 10.1523/JNEUROSCI.4459-09.2010
Saunders, A., Macosko, E. Z., Wysoker, A., Goldman, M., Krienen, F. M., de Rivera, H., et al. (2018). Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174, 1015–1030.e16. doi: 10.1016/j.cell.2018.07.028
Shandra, O., Winemiller, A. R., Heithoff, B. P., Munoz-Ballester, C., George, K. K., Benko, M. J., et al. (2019). Repetitive diffuse mild traumatic brain injury causes an atypical astrocyte response and spontaneous recurrent seizures. J. Neurosci. 39, 1944–1963. doi: 10.1523/JNEUROSCI.1067-18.2018
Shimamura, M., Sato, N., Sata, M., Wakayama, K., Ogihara, T., and Morishita, R. (2007). Expression of hepatocyte growth factor and c-Met after spinal cord injury in rats. Brain Res. 1151, 188–194. doi: 10.1016/j.brainres.2007.03.022
Silver, J. (2016). The glial scar is more than just astrocytes. Exp. Neurol. 286, 147–149. doi: 10.1016/j.expneurol.2016.06.018
Silver, J., and Miller, J. H. (2004). Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156. doi: 10.1038/nrn1326
Silver, J., Edwards, M. A., and Levitt, P. (1993). Immunocytochemical demonstration of early appearing astroglial structures that form boundaries and pathways along axon tracts in the fetal brain. J. Comp. Neurol. 328, 415–436. doi: 10.1002/cne.903280308
Silver, J., Schwab, M. E., and Popovich, P. G. (2014). Central nervous system regenerative failure: Role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb. Perspect. Biol. 7:a020602. doi: 10.1101/cshperspect.a020602
Sofroniew, M. V. (2014). Astrogliosis. Cold Spring Harb. Perspect. Biol. 7:a020420. doi: 10.1101/cshperspect.a020420
Sofroniew, M. V. (2020). Astrocyte reactivity: Subtypes, states, and functions in CNS innate immunity. Trends Immunol. 41, 758–770. doi: 10.1016/j.it.2020.07.004
Sofroniew, M. V., and Vinters, H. V. (2010). Astrocytes: Biology and pathology. Acta Neuropathol. 119, 7–35. doi: 10.1007/s00401-009-0619-8
Stern, S., Hilton, B. J., Burnside, E. R., Dupraz, S., Handley, E. E., Gonyer, J. M., et al. (2021). RhoA drives actin compaction to restrict axon regeneration and astrocyte reactivity after CNS injury. Neuron 109, 3436–3455.e9. doi: 10.1016/j.neuron.2021.08.014
Sun, X., Wang, X., Chen, T., Li, T., Cao, K., Lu, A., et al. (2010). Myelin activates FAK/Akt/NF-κB pathways and provokes CR3-dependent inflammatory response in murine system. PLoS One 5:e9380. doi: 10.1371/journal.pone.0009380
Szebenyi, G., Dent, E. W., Callaway, J. L., Seys, C., Lueth, H., and Kalil, K. (2001). Fibroblast growth factor-2 promotes axon branching of cortical neurons by influencing morphology and behavior of the primary growth cone. J. Neurosci. 21, 3932–3941. doi: 10.1523/JNEUROSCI.21-11-03932.2001
Tan, A. M., Zhang, W., and Levine, J. M. (2005). NG2: A component of the glial scar that inhibits axon growth. J. Anat. 207, 717–725. doi: 10.1111/j.1469-7580.2005.00452.x
Tanaka, T., Ueno, M., and Yamashita, T. (2009). Engulfment of axon debris by microglia requires p38 MAPK activity. J. Biol. Chem. 284, 21626–21636. doi: 10.1074/jbc.M109.005603
Teh, D. B. L., Prasad, A., Jiang, W., Ariffin, M. Z., Khanna, S., Belorkar, A., et al. (2017). Transcriptome analysis reveals neuroprotective aspects of human reactive astrocytes induced by interleukin 1beta. Sci. Rep. 7:13988. doi: 10.1038/s41598-017-13174-w
Tom, V. J., Doller, C. M., Malouf, A. T., and Silver, J. (2004). Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J. Neurosci. 24, 9282–9290. doi: 10.1523/JNEUROSCI.2120-04.2004
Tong, X., Ao, Y., Faas, G. C., Nwaobi, S. E., Xu, J., Haustein, M. D., et al. (2014). Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci. 17, 694–703. doi: 10.1038/nn.3691
Tripathi, R. B., and McTigue, D. M. (2008). Chronically increased ciliary neurotrophic factor and fibroblast growth factor-2 expression after spinal contusion in rats. J. Comp. Neurol. 510, 129–144. doi: 10.1002/cne.21787
Tuszynski, M. H., Grill, R., Jones, L. L., McKay, H. M., and Blesch, A. (2002). Spontaneous and augmented growth of axons in the primate spinal cord: Effects of local injury and nerve growth factor-secreting cell grafts. J. Comp. Neurol. 449, 88–101. doi: 10.1002/cne.10266
Ueno, M., Hayano, Y., Nakagawa, H., and Yamashita, T. (2012). Intraspinal rewiring of the corticospinal tract requires target-derived brain-derived neurotrophic factor and compensates lost function after brain injury. Brain. 135(Pt 4), 1253–1267. doi: 10.1093/brain/aws053
Valenza, M., Marullo, M., Di Paolo, E., Cesana, E., Zuccato, C., Biella, G., et al. (2015). Disruption of astrocyte-neuron cholesterol cross talk affects neuronal function in Huntington’s disease. Cell Death Differ. 22, 690–702. doi: 10.1038/cdd.2014.162
Wahane, S., Zhou, X., Zhou, X., Guo, L., Friedl, M. S., Kluge, M., et al. (2021). Diversified transcriptional responses of myeloid and glial cells in spinal cord injury shaped by HDAC3 activity. Sci. Adv. 7:eabd8811. doi: 10.1126/sciadv.abd8811
Wahl, A. S., Omlor, W., Rubio, J. C., Chen, J. L., Zheng, H., Schroter, A., et al. (2014). Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science 344, 1250–1255. doi: 10.1126/science.1253050
Wakida, N. M., Cruz, G. M. S., Ro, C. C., Moncada, E. G., Khatibzadeh, N., Flanagan, L. A., et al. (2018). Phagocytic response of astrocytes to damaged neighboring cells. PLoS One 13:e0196153. doi: 10.1371/journal.pone.0196153
Wan, T., Zhu, W., Zhao, Y., Zhang, X., Ye, R., Zuo, M., et al. (2022). Astrocytic phagocytosis contributes to demyelination after focal cortical ischemia in mice. Nat. Commun. 13:1134. doi: 10.1038/s41467-022-28777-9
Wang, J., Sareddy, G. R., Lu, Y., Pratap, U. P., Tang, F., Greene, K. M., et al. (2020). Astrocyte-derived estrogen regulates reactive astrogliosis and is neuroprotective following ischemic brain injury. J. Neurosci. 40, 9751–9771. doi: 10.1523/JNEUROSCI.0888-20.2020
Wang, L., Hu, B., Wong, W. M., Lu, P., Wu, W., and Xu, X. M. (2009). Glial and axonal responses in areas of Wallerian degeneration of the corticospinal and dorsal ascending tracts after spinal cord dorsal funiculotomy. Neuropathology 29, 230–241. doi: 10.1111/j.1440-1789.2008.00969.x
Wang, S., Deng, J., Fu, H., Guo, Z., Zhang, L., and Tang, P. (2020). Astrocytes directly clear myelin debris through endocytosis pathways and followed by excessive gliosis after spinal cord injury. Biochem. Biophys. Res. Commun. 525, 20–26. doi: 10.1016/j.bbrc.2020.02.069
Wanner, I. B., Anderson, M. A., Song, B., Levine, J., Fernandez, A., Gray-Thompson, Z., et al. (2013). Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J. Neurosci. 33, 12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013
Wei, H., Wu, X., You, Y., Duran, R. C., Zheng, Y., Narayanan, K. L., et al. (2021). Systematic analysis of purified astrocytes after SCI unveils Zeb2os function during astrogliosis. Cell Rep. 34:108721. doi: 10.1016/j.celrep.2021.108721
White, R. E., Rao, M., Gensel, J. C., McTigue, D. M., Kaspar, B. K., and Jakeman, L. B. (2011). Transforming growth factor alpha transforms astrocytes to a growth-supportive phenotype after spinal cord injury. J. Neurosci. 31, 15173–15187. doi: 10.1523/JNEUROSCI.3441-11.2011
White, R. E., Yin, F. Q., and Jakeman, L. B. (2008). TGF-alpha increases astrocyte invasion and promotes axonal growth into the lesion following spinal cord injury in mice. Exp. Neurol. 214, 10–24. doi: 10.1016/j.expneurol.2008.06.012
Wilhelmsson, U., Li, L., Pekna, M., Berthold, C. H., Blom, S., Eliasson, C., et al. (2004). Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J. Neurosci. 24, 5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004
Xu, J., Zheng, Y., Lv, S., Kang, J., Yu, Y., Hou, K., et al. (2020). Lactate promotes reactive astrogliosis and confers axon guidance potential to astrocytes under oxygen-glucose deprivation. Neuroscience 442, 54–68. doi: 10.1016/j.neuroscience.2020.06.041
Yamane, K., Mazaki, T., Shiozaki, Y., Yoshida, A., Shinohara, K., Nakamura, M., et al. (2018). Collagen-binding hepatocyte growth factor (HGF) alone or with a gelatin- furfurylamine hydrogel enhances functional recovery in mice after spinal cord injury. Sci. Rep. 8:917. doi: 10.1038/s41598-018-19316-y
Yang, Z., Suzuki, R., Daniels, S. B., Brunquell, C. B., Sala, C. J., and Nishiyama, A. (2006). NG2 glial cells provide a favorable substrate for growing axons. J. Neurosci. 26, 3829–3839. doi: 10.1523/JNEUROSCI.4247-05.2006
Yao, D. L., West, N. R., Bondy, C. A., Brenner, M., Hudson, L. D., Zhou, J., et al. (1995). Cryogenic spinal cord injury induces astrocytic gene expression of insulin-like growth factor I and insulin-like growth factor binding protein 2 during myelin regeneration. J. Neurosci. Res. 40, 647–659. doi: 10.1002/jnr.490400510
Yiu, G., and He, Z. (2006). Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 7, 617–627. doi: 10.1038/nrn1956
Zamanian, J. L., Xu, L., Foo, L. C., Nouri, N., Zhou, L., Giffard, R. G., et al. (2012). Genomic analysis of reactive astrogliosis. J Neurosci. 32, 6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012
Zhang, Y., and Barres, B. A. (2010). Astrocyte heterogeneity: An underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 20, 588–594. doi: 10.1016/j.conb.2010.06.005
Zhou, H., Hu, L., Li, J., Ruan, W., Cao, Y., Zhuang, J., et al. (2021). AXL kinase-mediated astrocytic phagocytosis modulates outcomes of traumatic brain injury. J. Neuroinflamm. 18:154. doi: 10.1186/s12974-021-02201-3
Zhu, Y., Soderblom, C., Krishnan, V., Ashbaugh, J., Bethea, J. R., and Lee, J. K. (2015). Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury. Neurobiol. Dis. 74, 114–125. doi: 10.1016/j.nbd.2014.10.024
Keywords: reactive astrocytes, axon regeneration, axon sprouting, CNS injury, glial scar, spinal cord injury, neural repair, astrogliosis
Citation: Hemati-Gourabi M, Cao T, Romprey MK and Chen M (2022) Capacity of astrocytes to promote axon growth in the injured mammalian central nervous system. Front. Neurosci. 16:955598. doi: 10.3389/fnins.2022.955598
Received: 30 May 2022; Accepted: 15 August 2022;
Published: 20 September 2022.
Edited by:
Jan Kaslin, Australian Regenerative Medicine Institute (ARMI), AustraliaReviewed by:
Margherita Zamboni, Karolinska Institutet (KI), SwedenCopyright © 2022 Hemati-Gourabi, Cao, Romprey and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meifan Chen, bWVpZmFuLmNoZW5AdWt5LmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.