- Intensive Care Unit, Shidong Hospital, Shanghai, China
Cerebral small vessel disease (CSVD) is often referred to as “collaterals disease” in traditional Chinese medicine (TCM), and commonly includes ischemic and hemorrhagic CSVD. TCM has a long history of treating CSVD and has demonstrated unique efficacy. Buyang Huanwu Decoction (BHD) is a classical TCM formula that has been used for the prevention and treatment of stroke for hundreds of years. BHD exerts its therapeutic effects on CSVD through a variety of mechanisms. In this review, the clinical and animal studies on BHD and CSVD were systematically introduced. In addition, the pharmacological mechanisms, active components, and clinical applications of BHD in the treatment of CSVD were reviewed. We believe that an in-depth understanding of BHD, its pharmacological mechanism, disease-drug interaction, and other aspects will help in laying the foundation for its development as a new therapeutic strategy for the treatment of CSVD.
Introduction
Cerebral small vascular disease (CSVD) is a common neurological disease that has serious impact on the patient’s health. Due to the insidious onset of CSVD, it is easy to be ignored by patients and even clinicians (Liu D. et al., 2019). In recent years, with the advancement of research, rapid progress has been made with respect to the risk factors, pathogenesis, clinical manifestations, and evaluation of CSVD (Shi and Wardlaw, 2016). CSVD refers to a series of clinical, imaging, and pathological syndromes caused by various etiologies affecting the small arteries and their distal branches, arterioles, capillaries, and venules in the brain (Li et al., 2018). Based on the imaging characteristics, CSVD includes recent small subcortical infarction, vascularity-induced lacunar infarction and white matter hyperintensity, perivascular space, cerebral microbleed, and cerebral atrophy (Wardlaw et al., 2013). The pathogenesis of CSVD is complex and is considered to be a dynamic disorder of the whole brain. Abnormal neurovascular unit (NVU) function plays an important role in its pathogenesis (Caruso et al., 2019). The NVU is composed of neurons, astrocytes, vascular endothelial cells, pericycles, and vascular smooth muscle cells that interact to regulate fluid and nutrient entry into the stroma, regulate cerebral blood flow, maintain and repair myelin sheath, and clear metabolites to achieve normal cell function (Iadecola, 2017). Changes in the structure or function of NVU for any reason can lead to CSVD (Bailey et al., 2014). The common mechanisms include chronic cerebral ischemia and hypoperfusion, endothelial dysfunction, blood-brain barrier (BBB) damage, inter-tissue fluid reflux disorder, inflammatory response, and genetic factors (Hase et al., 2018). The treatment strategies for CSVD in Western medicine are still limited to the treatment of acute ischemic stroke (AIS), including the control of anti-platelet aggregation, statins, and risk factors. Thus, Western medicine lacks multi-target and comprehensive treatment for CSVD.
Traditional Chinese medicine (TCM) has been developed for thousands of years in China. A TCM formula is developed based on experience through repeated clinical practice. A TCM formula usually consists of multiple drugs which act on the body organs through multiple targets to exert a therapeutic role. This multichannel approach is consistent with the multifactorial pathological mechanism of CSVD (Guo et al., 2020). Emerging studies have suggested that a variety of TCM formulas have therapeutic effects on CSVD. In vivo animal experiments and clinical case-control studies have confirmed the effectiveness of TCM formulas in the treatment of CSVD (Li et al., 2019), and it is believed that TCM formulas could be used as an effective supplement with Western medicine for CSVD therapy.
Cerebral Small Vessel Disease and Collaterals Disease
According to TCM, the pathogenesis of CSVD lies in the collaterals. The collaterals are widely distributed in the human body and have the functions of nurturing and perfusion. They are narrow in shape and are located at the end of the branches of the blood vessels. This also determines the physiological characteristics of the slow movement of qi and blood and the pathological characteristics of qi deficiency and stasis, which can cause collateral dystrophy and stasis that affect the circulation of qi and blood, and in turn lead to abnormal collateral qi and blood infiltration, resulting in collateral disease (Huang D. et al., 2020). Collateral disease can cause qi and blood dysfunction and even structural damage (Eker et al., 2019). Moreover, the theory of collateral disease in TCM states that long-term illness enters the collaterals. Thus, collateral disease is a disease of essential empty and out solid, qi-deficiency is based, blood stasis is symptoms, qi deficiency and blood stasis are important pathogenesis of CSVD. The course of CSVD is dynamic (Nighoghossian and Mechtouff, 2020), with hemorrhage or ischemia constantly appearing. Hemorrhage and ischemia can exist at the same time, leading to the complexity of CSVD stagnation and stasis with old and new lesions, multiple deficiency and excess, and uneven distribution of multiple disease locations of the disease state (Winship, 2015).
According to the principle of “dredging collaterals,” combined with the basic pathogenesis of CSVD of qi deficiency and blood stasis, the main treatment of this disease is to invigorate qi, promote blood circulation, and remove collaterals. Buyang Huanwu Decoction (BHD) was first formulated by Wang Qingren, a doctor of TCM in the Qing Dynasty in “Yilin Cuogai.” The main functions of BHD are promotion of blood circulation and removal of blood stasis, dredging collateral, and supplementing qi, which are suitable for CSVD treatment (Zhang et al., 2018). Since the 1980s, BHD has been widely used in the clinical treatment of ischemic/hemorrhagic stroke (Cui et al., 2015; Dou et al., 2018). Its main components include Astragalus, Ligusticum, Peach kernel, Radix Paeoniae Rubra, Geosaurus, Carthami Flos, and Angelica sinensis. Astragalus tonifies qi; Ligusticum warms meridians and unblocks collaterals, activates blood, and relieves pain; Peach kernel activates blood circulation and removes stasis; Radix Paeoniae Rubra clears heat and cools blood; Geosaurus activates collaterals; Carthami Flos dredges the meridians, qi, and blood; and Angelica sinensis restores vital energy and invigorates the blood. The composition of a single daily dose of BHD is as follows: Astragalus 120 g, Angelica sinensis 6 g, Radix Paeoniae Rubra 4.5 g, Geosaurus 3 g, Ligusticum 3 g, Peach kernel 3 g, and Carthami Flos 3 g. The combination of these seven drugs in BHD exerts the maximum therapeutic effect on “Collaterals disease” in biomedicine.
Traditional Chinese Medicine Prescriptions in Treatment of Cerebral Small Vessel Disease Based on Syndrome Differentiation
There is no record of “CSVD” in ancient Chinese medicine books. In view of the relationship between kidney and brain fully described in TCM theories and ancient TCM books, many modern TCM experts believe that the occurrence of CSVD is inseparable from kidney. Meng and Huo (2016b) believed that kidney deficiency was the basic pathogenesis of CSVD because of the physiological interaction between kidney and brain, the mutual function of ups and down, and the inseparable structure of marrow and brain collateral (Meng and Huo, 2016b). In addition, it has been reported that the cognitive dysfunction of CSVD belongs to the syndrome of deficiency of origin and symptom of reality, which is based on deficiency of kidney and marrow and marked by blood stasis. Therefore, the treatment of CSVD should be filled with essence, nourishing kidney, blood, and Yin, removing stasis and dredging collaterals (Cao et al., 2018). Supported by the biochemical theory of kidney essence and brain marrow in TCM, Zhang et al. (2018) observed the positive intervention effect of kidney nourishing therapy on the cognitive dysfunction caused by CSVD and improved the cognitive level of CSVD patients. Liang et al. (2019) tried to treat CSVD by filling kidney essence and nourishing kidney qi. A total of 252 CSVD subjects were randomly assigned to the intervention group and the control group. Changes in memory and executive screening (MES) scores were observed 24 weeks after treatment. The results showed that the strategy of tonifying kidney and removing stasis could improve MES score of subjects and delay the evolution of CSVD patients from mild cognitive impairment (MCI) to severe cognitive impairment and eventually to dementia (Liang et al., 2019). Meng and Huo (2016a) treated CSVD with kidney-tonifying combined with nootropic drugs, and the results suggested that this method could improve the Montreal cognitive assessment scale (MoCA), microbleed an atomical rating scale, and age-related white matter changes scores of CSVD patients (Meng and Huo, 2016a).
Qi is the governor of blood, qi deficiency leads to slow blood movement, over time, resulting in blood stasis in the collaterals. Phlegm turbid blood stasis is also an important pathogenic factor of CSVD. Therefore, supplementing qi and promoting blood circulation, removing stasis and dredging collaterals are important strategies of treating CSVD. Zhao et al. (2015) treated CSVD with traditional formula “Tongshen Funao Pill.” This formula has the effects of replenishing qi and promoting blood circulation, and it was found that the formula can significantly reduce serum inflammatory factors and improve their cognitive ability in CSVD patients (Zhao et al., 2015). Chen and Li (2019) used Huayu Tongluo Decoction to treat MCI caused by CSVD. The results showed that this formula can significantly improve the executive ability, attention and orientation of patients. The mechanism may be that this formula alleviated the stenosis and occlusion of cerebral small blood vessels, protects the integrity of nerve myelin sheath and cerebral small blood vessel wall, promoted angiogenesis and improved cerebral perfusion after ischemia (Chen and Li, 2019). Zhou H. et al. (2019) reached a similar conclusion, believing that the method of removing stasis and clearing collaterals could improve MoCA and activity of daily living score of CSVD patients, and played a role in improving cognitive function of CSVD patients.
BHD also exerted a therapeutic role in treating CSVD for qi deficiency and blood stasis syndrome (Cai et al., 2007). Xie (2018) selected BHD to treat CSVD through the method of invigorating qi, promoting blood circulation, removing blood stasis and dredging collaterals. The results suggested that BHD could inhibit the chronic inflammatory response and protect the intima of blood vessels, moreover, BHD improved blood rheology, increased the microcirculation, and cerebral perfusion of brain tissue and the syndrome of qi deficiency and blood stasis in patients with CSVD (Xie, 2018). Donepezil has been shown to improve cognitive impairment and delay disease progression in patients with CSVD (Battle et al., 2021). Xue et al. (2019) found in a controlled study that BHD combined with donepezil could improve the cognitive function of CSVD patients better than donepezil alone.
Therefore, BHD has a potential therapeutic effect on CSVD, which can improve cognitive impairment and daily living behavior ability of patients. However, BHD is currently mainly used for the treatment of cerebrovascular diseases (CVD), and clinical studies on the treatment of CSVD are limited. The mechanism of BHD treatment for patients with CSVD needs to be further elaborated. Next, we will further introduce the active ingredients and pharmacological effects of major Chinese herbs in BHD, and further elaborate the potential mechanism of BHD treatment of CSVD through network pharmacology prediction.
Pharmacological Effects on the Active Ingredients of Buyang Huanwu Decoction
Astragalus
Astragalus is the root of the leguminous plant Astragalus mongolicus. It invigorates qi, and thus can be used to treat qi deficiency. Previous studies have shown that Astragalus exerts antitumor and immunoregulatory effects (Yu J. et al., 2021), and treats chronic heart failure (Xu et al., 2021), diabetic nephropathy (Zhao et al., 2021) and diabetes mellitus with depression (Wang W. K. et al., 2021). In addition, Astragalus has been used for the treatment of CVD as it reduces brain tissues ischemia/reperfusion (I/R) injury (Guo L. Y. et al., 2021; Lo et al., 2021) and post cerebral ischemic inflammatory activation (Dou et al., 2021), inhibits brain microvascular endothelial cell injury (Tang X. et al., 2021), protects against thrombolysis-induced hemorrhagic transformation in cerebral ischemia (Pan et al., 2020), prevents Aβ oligomers-induced memory impairment and hippocampal cell apoptosis (Wang X. et al., 2020), and promotes hippocampal neurogenesis (Ni et al., 2020).
Modern pharmacological studies suggest that the active ingredients of Astragalus include astragalus polysaccharide, astragalus saponins, flavonoids calycosin, 3-hydroxy-9,10-dimethoxy pterostane, and astragaloside I, III, V. Astragalus polysaccharide is the main pharmacologically active ingredient in Astragalus. Studies have reported that it can suppress mitochondrial damage-mediated apoptosis (Gao et al., 2021b), modulate gut microbiota (Zhuang et al., 2021), enhance immune response (Zhou et al., 2021), alleviate cognitive impairment and β-amyloid accumulation via Nrf2 pathway (Qin et al., 2020), enhance remyelination (Ye et al., 2021), inhibit the formation of cerebral thrombosis (Sun et al., 2020), protect neurons, and stabilize mitochondrial in a Parkinson disease (PD) mouse model (Liu et al., 2018), and thus exhibit mitochondrial and anti-aging activity (Li et al., 2012).
Ligusticum
Ligusticum is a TCM plant with a wide range of pharmacological functions, and is often used to promote blood circulation and qi, dispel wind, and relieve pain. It is suitable for improving blood stasis, and has been used to treat rheumatic arthralgia. Ligusticum is a qi medicine in blood, with functions of relieving depression and accessing and relieving pain. In recent years, studies have suggested the Ligusticum exerts therapeutic effect on ischemic stroke by neurogenesis and maintaining the BBB (Yu B. et al., 2021). In addition, it demonstrates neuroprotective effects by promoting adult neurogenesis and inhibiting inflammation in the hippocampus of cerebral ischemia rats (Wang M. et al., 2020). Ligusticum attenuates hyperhomocysteinemia-induced Alzheimer-like pathologies in rats (Zhang et al., 2021) and acts against focal cerebral ischemia (Gu et al., 2020). Moreover, Ligusticum alleviates acute lung injury (Jiang et al., 2021), prevents liver fibrosis (Wu et al., 2021), and has an anti-tumor effect (Zhong et al., 2021).
The pharmacological effect of Ligusticum is attributed to many active ingredients. These include trimethylpyridine, butylphthalide, senkyunolide, ferulic acid, chrysophanic acid, and a variety of volatile oils. Ligusticum lactone may be the main pharmacological active component of Ligusticum and is one of the most widely studied compounds of Ligusticum. It ameliorates cognition deficits by regulating docosahexaenoic acid metabolism in APP/PS1 mice (Huang J. et al., 2020), protects vascular endothelial cells from oxidative stress and rescues atherosclerosis by activating multiple NRF2 downstream genes (Zhu et al., 2019), alleviates myocardial ischemia injury through inhibiting autophagy (Wang G. et al., 2018), and reduces atherosclerotic lesions by inhibiting over expression of nuclear factor-κB (NF-κB)-dependent adhesion molecules (Xiao et al., 2014).
Peach Kernel
Peach kernel are dried mature seeds of Prunus persica (L.) Batsch or Prunus Davidiana (Carr.). It can activate blood, remove blood stasis, moisten bowel and relieve constipation, and can be used for the treatment of amenorrhea, lung carbuncle, bowel lump, fall injury, dryness constipation, cough, and asthma. Recent studies have also found that peach kernel can be used to treat diabetic nephropathy (Han J. et al., 2021), colon cancer (Cassiem and De Kock, 2019), glucocorticoid-induced ischemic necrosis of femoral head (Qi and Chen, 2009), and cerebral I/R injury rats by regulating ADORA2A degradation (Li L. L. et al., 2020).
Peach kernel oil is the main active ingredient of peach kernel. Studies have shown that peach kernel oil could significantly decrease the low-density lipoprotein cholesterol levels in serum and reduce the area of the aortic atherosclerotic lesions in ApoE knockout mice. In addition, peach kernel oil could down regulate the tissue factors protein levels to inhibit the formation of atherosclerotic plaque (Hao et al., 2019).
Radix Paeoniae Rubra
Radix Paeoniae Rubra is the dry root of herbaceous peony, and has the effect of clearing heat and cooling blood, promoting blood circulation, and removing blood stasis. Studies have shown that Radix Paeoniae Rubra exerts anti-tumor effect (Xu et al., 2013), improves chronic inflammation disease (Li X. H. et al., 2020), ameliorates lupus nephritis and lupus nephritis (Wang W. et al., 2020), protects against myocardial ischemic injury (Ke et al., 2017), exerts neuroprotective effects on ischemia stroke mice (Luo et al., 2020), has anti-thrombotic effect (Xie et al., 2017), ameliorates focal cerebral ischemic in rats (Gu et al., 2016), and promotes recovery of neurological function of stroke convalescent patients (Zhang et al., 2020).
Pharmacological analysis suggests that Radix Paeoniae Rubra contains Paeoniflorin, new Paeoniflorin, Paeoniae a, Paeoniae B, and palmitic acid. Amongst these compounds, paeoniflorin is the most widely studied and is considered to be the most important pharmacological active component of Radix Paeoniae Rubra. Paeoniflorin is a monoterpene glycoside with neuroprotective effect, and exerts antidepressant effects through enhancement of neuronal Fibroblast growth factor-2 (FGF-2) by microglial inactivation (Cheng J. et al., 2021; Wang X. L. et al., 2021c). It is also effective in preventing prenatal stress-induced learning and memory impairment (Wang X. et al., 2021). In a rat stroke model, paeoniflorin repressed neuroinflammation and facilitated neurogenesis (Tang H. et al., 2021), protected against ischemic brain injury, inhibited apoptosis (Liu et al., 2021), reduced cerebral oxidative stress, improved white matter integrity in hypoxic brain injury (Yang et al., 2021), and attenuated early brain injury through reducing oxidative stress and neuronal apoptosis (Wang T. et al., 2020). In a mouse model of Alzheimer’s disease (AD), paeoniflorin exerted neuroprotective effects via activation of adenosine A receptor (Kong et al., 2020). In addition, it could attenuate impairment of spatial learning and hippocampal long-term potentiation (Liu S. C. et al., 2019) and improve vascular dementia in rats via modulation of cannabinoid receptor 2 (Jinglong et al., 2013; Luo et al., 2018).
Geosaurus
Geosaurus was first proposed in Shennong Classic of Materia Medica. It has been used in combination with a variety of TCMs. Li Shizhen proposed in Compendium of Materia Medica that Geosaurus has the function of activating collaterals, promoting blood circulation, removing blood stasis, and preventing and treating CVD. Clinically, Geosaurus is mainly used for treating myocardial damage (Han et al., 2014), asthma (Li et al., 2009), AD (Ren et al., 2006), and AIS induced by middle-cerebral artery occlusion (Liu et al., 2012). In addition, Geosaurus has the function of protecting cerebral microvascular endothelial cells against oxygen-glucose deprivation reperfusion (Sun et al., 2019) and promoting peripheral nerve regeneration (Chang et al., 2011).
The study of the chemical composition of the Geosaurus helps to understand its pharmacological effects. Modern chemical analysis has found that it contains a variety of chemical components, including lumbrokinase, palmitic acid, linoleic acid, alanine, lysine, and hypoxanthine. The therapeutic effect of lumbrokinase is mainly focused on preventing ischemic stroke and secondary ischemic stroke (Cao et al., 2013). In addition, it has been reported that it exerts anti-thrombosis effect by inhibiting the expression of intercellular adhesion molecule 1 and decreasing the immunoreactions of P-selectin and E-selectin in ischemic lesion (Zhang et al., 2003; Ji et al., 2008), inhibits intrinsic coagulation pathway, and activates fibrinolysis via an increase of t-PA activity (Jin et al., 2000). Lumbrokinase also has protective effects on hippocampus apoptosis and hippocampal function (Huang et al., 2013).
Carthami Flos
Carthami Flos is an annual composite plant. It has the effect of dredging the meridians, qi and blood, dispersing blood stasis, and relieving pain. It is used for the treatment of amenorrhea, dysmenorrhea, chest pain, stagnation and abdominal pain, and prickly pain in chest and flank. Currently, Carthami Flos is believed to have a variety of pharmacological effects, including the antioxidant effect related to the potential anti-aging properties (Satoh et al., 2004), suppression of neutrophilic lung inflammation (Kim et al., 2014), inhibitory effect on cancer cells (Wu et al., 2013), regulation of gastrointestinal motility functions (Kim et al., 2017), treatment of traumatic intracranial hematoma (Sun et al., 2009), protection of hippocampal neurons induced by hypoxia injury (Yu et al., 2018), and protective effects on cerebral I/R injury (Wan et al., 2021).
A number of chemical components have been isolated from Carthami Flos, including carthamin, precarthamin, safflow yellow A and B, safflomin A, chlorogenic acid and caffeic acid may be the main pharmacological component of Carthami Flos. It has been reported in the literature that carthamin improves cerebral ischemia-recycling investment by attenuating information and ferroptosis (Guo H. et al., 2021), ameliorates diabetes mellitus and its cardiovascular composites (Orgah et al., 2020), protects the heart against ischemia/recycling investment (Lu et al., 2019), exerts neuroprotective activities (Hiramatsu et al., 2009).
Angelica Sinensis
Angelica sinensis is a perennial herb [Angelica sinensis (Oliv) Diels] belonging to the umbelliferae family. It consists of the dried roots of the plant. Its pharmacological effects include enriching blood, promoting blood circulation, regulation of painful menstruation, and relieving skin numbness. Angelica sinensis has been used for treating AIS (Han Y. et al., 2021), preventing neural injury and promoting neuronal survival after ischemic stroke (Cheng C. Y. et al., 2021; Luo et al., 2021), protecting against I/R injury in the hippocampus (Cheng C. Y. et al., 2020), reducing Aβ-induced memory impairment (Duan et al., 2016), promoting synaptic plasticity during cognitive recovery (Deng et al., 2015), treating depression (Shen et al., 2016), and inhibiting malignant brain tumor growth effect (Tsai et al., 2006).
Amongst the various components, the neutral oil content in Angelica sinensis was the highest, including ligustilide, ligustryllactone, butyl phthalide, angelica ketone, neoangelica lactone, and coniferol ferulate, which may play an important role in the therapeutic effect of Angelica sinensis. Ligustilide is one of the main active ingredients of Angelica sinensis. Pharmacological studies on ligustilide have focused on its therapeutic effects on ischemic stroke and cognitive impairment. Ligustilide improves aging-induced memory deficit by regulating mitochondrial related inflammation (Zhu et al., 2020). It also contributes to the neuroprotective effect on AD by inhibiting the insulin-like growth factor 1 pathway (Kuang et al., 2014) and alleviates neurotoxicity induced by Aβ25-35 (Gao et al., 2021a). With respect to its therapeutic effects of ischemic stroke, ligustilide protects neurons through a variety of mechanisms, including anti-inflammatory and anti-oxidant signaling pathways, inhibiting ischemia-re-perfusion damage to the ischemic brain, and ameliorating the permeability of the BBB after stroke (Wu et al., 2019).
In summary, BHD may reduce neuronal apoptosis by inhibiting I/R injury, promote angiogenesis, reduce the release of immune-inflammatory factors and NK cell aggregation, improve synaptic plasticity and other mechanisms to protect neurons. In the next section, we describe our attempt to apply network pharmacology to further explore the pharmacological mechanism of BHD therapy for CSVD.
The Active Compounds of Buyang Huanwu Decoction in the Treatment of Cerebral Small Vessel Disease
The anti-CSVD effect of BHD is a synergistic effect of multiple active ingredients, and involves the characteristics of multiple components, multiple targets, and multiple pathways. Network Pharmacology interprets the occurrence and development process of diseases from the perspective of system biology and biological network balance, understands the interaction between drugs and body from the holistic perspective of improving or restoring biological network balance, and guides the discovery of new drugs. With the increase in the research related to network pharmacology, it has been found that its characteristics of integrity and systematization are consistent with the holistic view of TCM and the principle of syndrome differentiation and treatment, and it has been widely used in the study of TCM. By searching the pharmacology database1 of the Traditional Chinese Medicine System Platform, the drug’s distribution, absorption, metabolism, excretion, and other parameters were initially screened, and we finally identified five most effective ingredients in BHD, including: astragalus polysaccharide, paeoniflorin, carthamin, and ligustilide. The Canonical SMILES of five effective components in BHD were obtained from PubChem;2 the 2-D structure was exhibited from the Swiss Target Prediction database (Figure 1). The Lab of Systems Pharmacology(see text footnote 1) and Swiss Target Prediction database3 were utilized to predict the target genes of the five effective components in BHD. The targets of CSVD and vascular dementia (VaD) were obtained from the MalaCards4, which is an integrated database of human maladies and their annotations, modeled on the architecture and richness of the popular GeneCards database of human genes(see text footnote 4) (Rappaport et al., 2017).
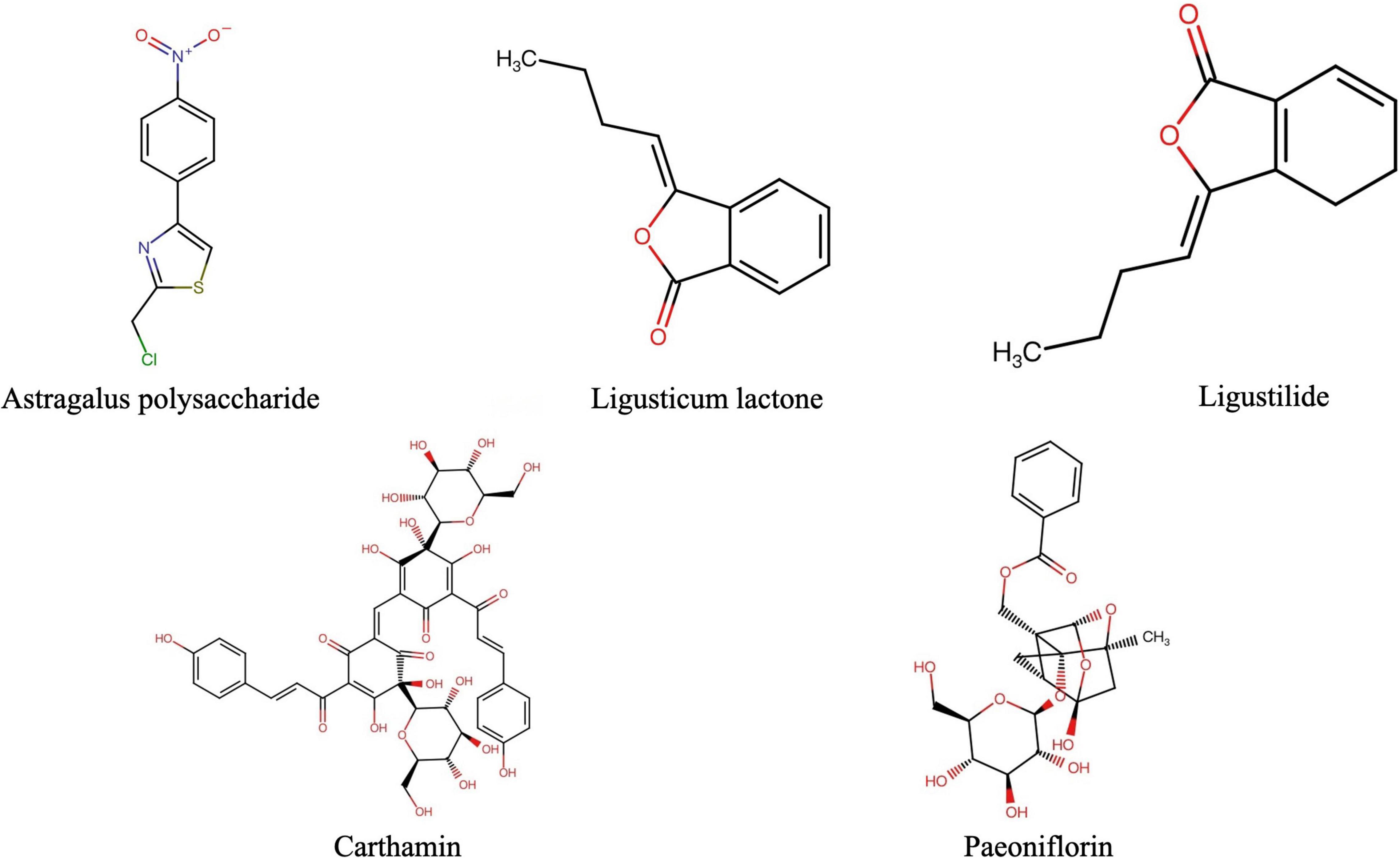
Figure 1. The 2D structures of major pharmacological active ingredients in BHD (BHD, Buyang Huanwu Decoction).
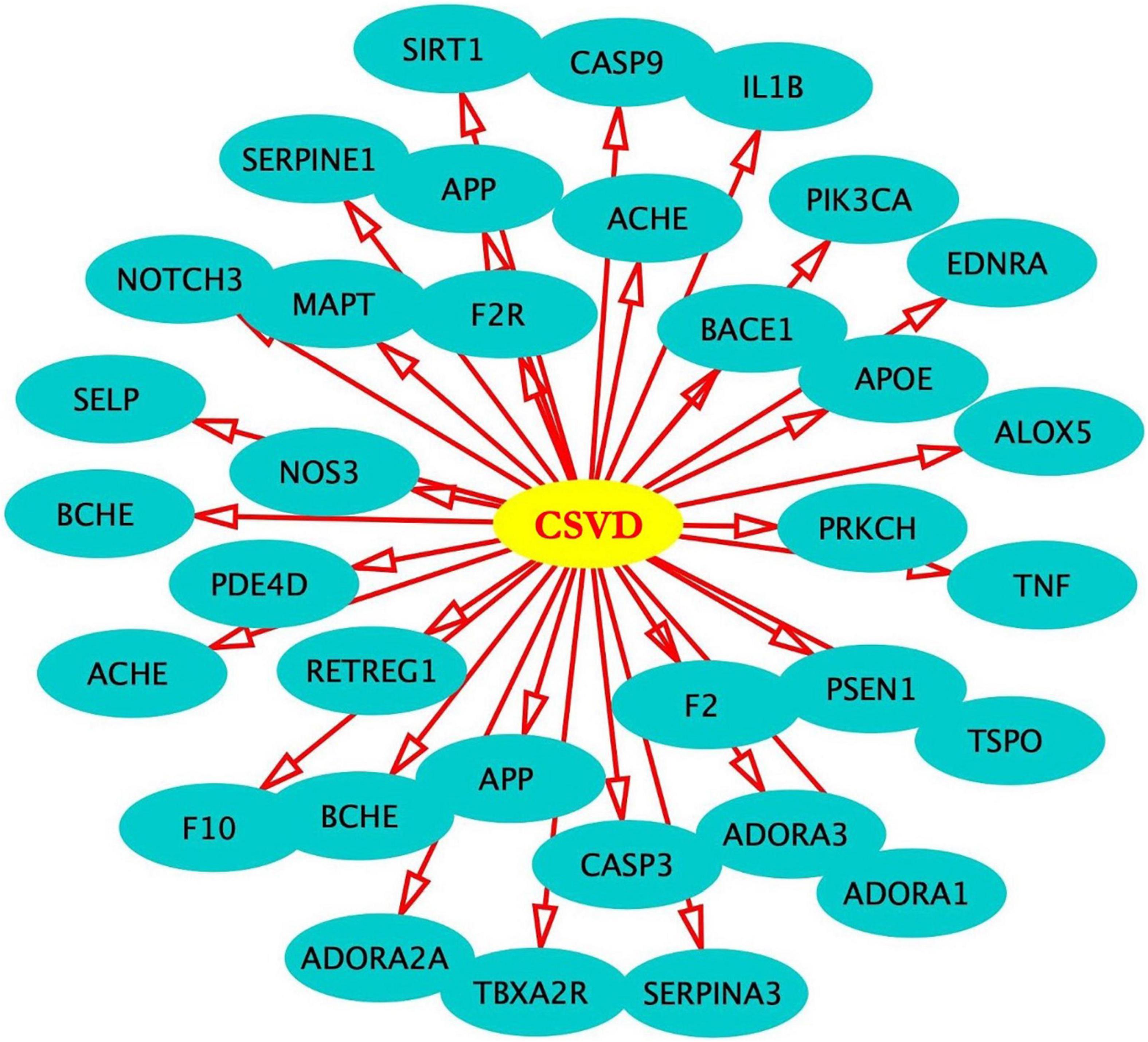
After screening, 324 target genes of BHD, 174 target genes of CSVD and 197 target genes of VaD were identified. Venn diagrams was used to screen two groups of overlapping target genes, 35 collective targets of CSVD, VaD and BHD were obtained (Figure 2). Using Metascape5, we further performed protein-protein interaction (PPI) analysis and interactive visualization of Gene Ontology (GO) networks for collective target genes (Zhou Y. et al., 2019). The PPI was constructed by Cytoscape (Figure 3), and the network of enriched terms was analyzed by Metascape (Figure 4). The top 10 clusters with representative enriched terms of BHD effects on CSVD are shown in Table 1. Therefore, BHD may have therapeutic effects on CSVD through these enriched pathways.
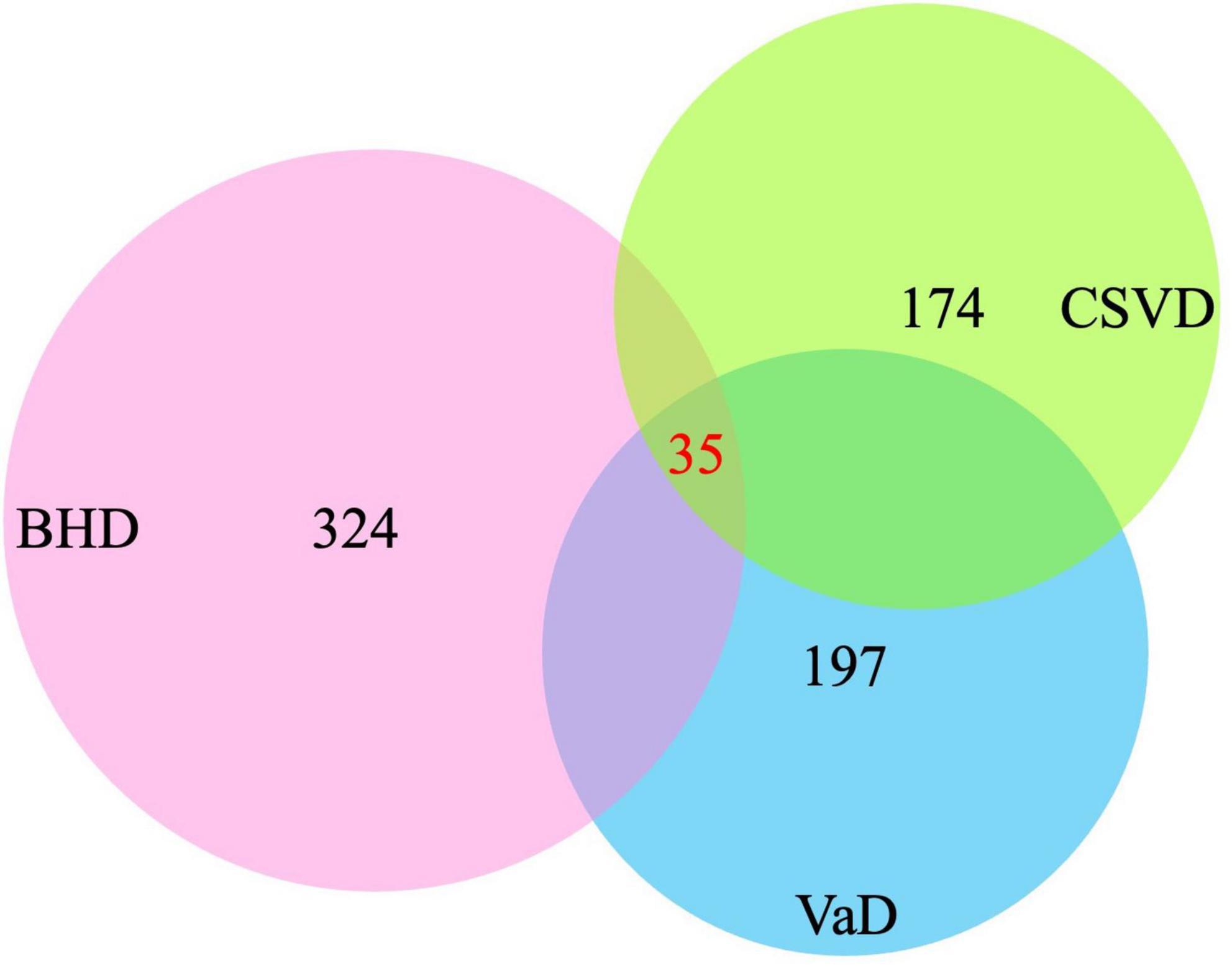
Figure 2. Venn diagrams was used to screen three groups of overlapping target genes, 35 collective targets of CSVD, VaD, and BHD were obtained (BHD, Buyang Huanwu Decoction; CSVD, Cerebral Small Vessel Disease; VaD, vascular dementia).
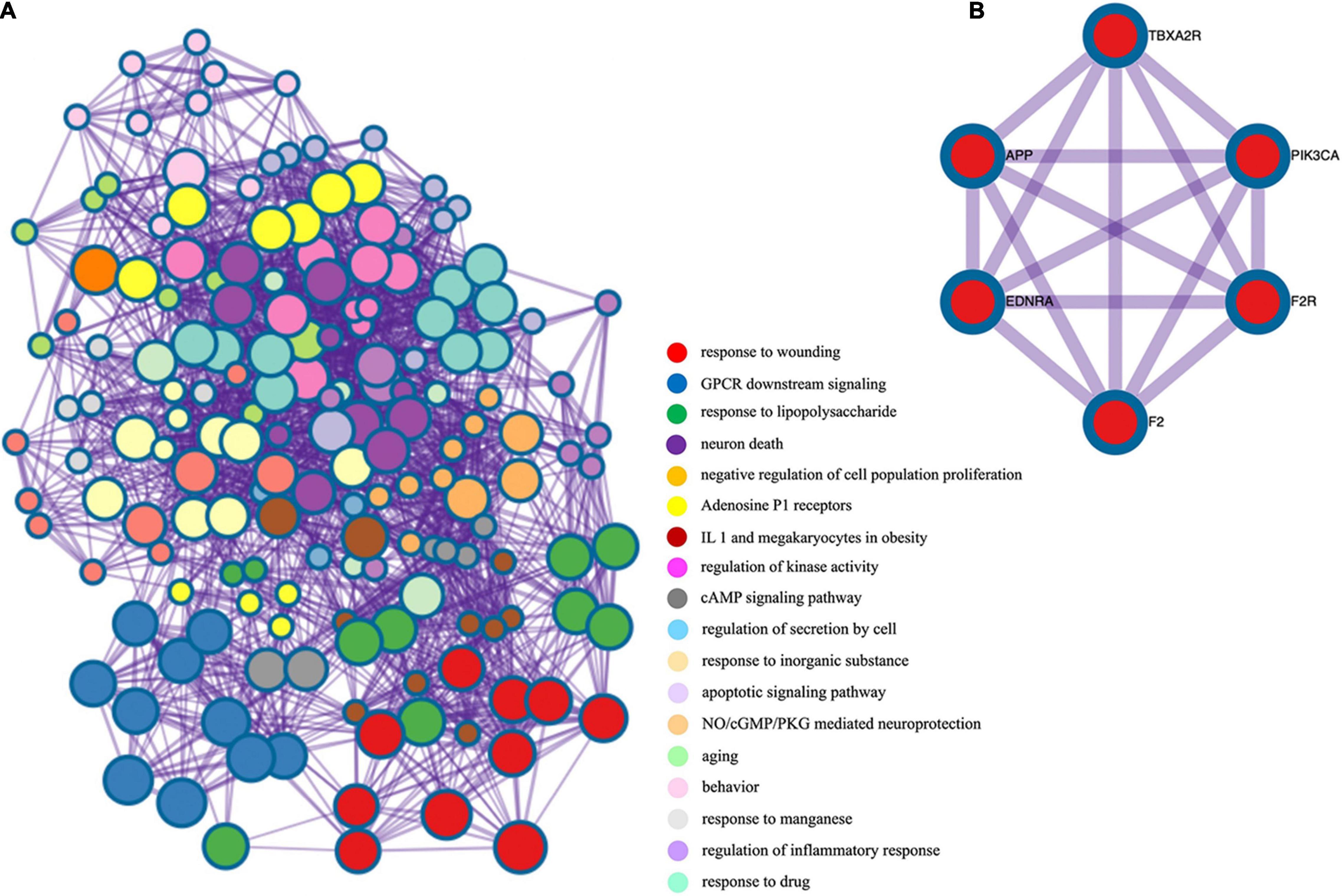
Figure 4. (A) Gene ontology enrichment analysis of overlapping target genes. Cross-examination of the relationship between these genes and GO biological process terms suggested that a substantial number of genes related to immune response were also enriched for other biological process such as defense response, response to cytokine stimulus and the inflammatory response. (B) Protein-protein interaction network and MCODE components identified in the gene list.
Conclusion and Prospect
CSVD is a clinical imaging syndrome that involves small cerebral vessels and is one of the main causes of vascular dementia. It includes hemorrhagic/ischemic and acute/chronic changes. The current treatment strategies lack multi-target and multi-mechanism approaches, which is one of the important factors for the unsatisfactory treatment effect of CSVD in recent years. However, with increased research on the pharmacological mechanisms of TCM, the effective ingredients of TCM have attracted increased attention. BHD is a famous TCM formula, which has the function of promoting blood circulation and removing blood stasis, dredging the meridians and collaterals. It can be used to treat various diseases caused by qi stagnation and blood stasis. At present, it is mainly used to treat various CVD, including ischemic/hemorrhagic diseases. Here, we systemically reviewed the effects of BHD on CVD in terms of its various components, pharmacological mechanisms, clinical studies, and effective components. We further performed PPI and GO pathway analyses to elaborate the possible pharmacological effects of BHD on CSVD.
Currently, BHD is widely used in treating CVD and exerts therapeutic effects with fewer side-effects. However, the active ingredients in BHD still need to be further explored, and the pharmacological mechanism of its treatment for CSVD needs to be clarified. In addition, although clinical studies on the combined application of BHD and aspirin have confirmed that BHD can improve the therapeutic effect and reduce the incidence of complications, it still needs to be further confirmed by well-designed large-scale clinical controlled studies. Network pharmacology provides a new platform to study the effective components and pharmacological effects of TCM. We believe that it will play an important role in promoting the discovery of new effective components in TCM formulas and in the modern application of TCM.
Author Contributions
LS, XY, LW, JY, YW, and MW contributed to the literature search, data extraction, and data analysis. LD contributed to the project design and manuscript writing. All authors have read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ http://www.tcmspw.com/
- ^ https://pubchem.ncbi.nlm.nih.gov/
- ^ http://www.swisstargetprediction.ch/
- ^ https://www.malacards.org/
- ^ http://metascape.org
References
Bailey, E. L., Mcbride, M. W., Beattie, W., Mcclure, J. D., Graham, D., and Dominiczak, A. F. (2014). Differential gene expression in multiple neurological, inflammatory and connective tissue pathways in a spontaneous model of human small vessel stroke. Neuropathol. Appl. Neurobiol. 40, 855–872. doi: 10.1111/nan.12116
Battle, C. E., Abdul-Rahim, A. H., Shenkin, S. D., Hewitt, J., and Quinn, T. J. (2021). Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis. Cochrane Database Syst. Rev. 2:CD013306. doi: 10.1002/14651858.CD013306
Cai, G., Liu, B., Liu, W., Tan, X., Rong, J., and Chen, X. (2007). Buyang Huanwu Decoction can improve recovery of neurological function, reduce infarction volume, stimulate neural proliferation and modulate VEGF and Flk1 expressions in transient focal cerebral ischaemic rat brains. J. Ethnopharmacol. 113, 292–299. doi: 10.1016/j.jep.2007.06.007
Cao, Y. F., Zhong, J. G., and Guo, H. M. (2018). The application of Yangyin and Yu Tongqiao Decoction in the treatment of vascular cognitive dysfunction in small vascular diseases without dementia. Chin. Med. Inform. 35, 89–92.
Cao, Y. J., Zhang, X., Wang, W. H., Zhai, W. Q., Qian, J. F., and Wang, J. S. (2013). Oral fibrinogen-depleting agent lumbrokinase for secondary ischemic stroke prevention: results from a multicenter, randomized, parallel-group and controlled clinical trial. Chin. Med. J. 126, 4060–4065.
Caruso, P., Signori, R., and Moretti, R. (2019). Small vessel disease to subcortical dementia: a dynamic model, which interfaces aging, cholinergic dysregulation and the neurovascular unit. Vasc. Health Risk. Manag. 15, 259–281. doi: 10.2147/VHRM.S190470
Cassiem, W., and De Kock, M. (2019). The anti-proliferative effect of apricot and peach kernel extracts on human colon cancer cells in vitro. BMC Complement Altern. Med. 19:32. doi: 10.1186/s12906-019-2437-4
Chang, Y. M., Chi, W. Y., Lai, T. Y., Chen, Y. S., Tsai, F. J., and Tsai, C. H. (2011). Dilong: role in peripheral nerve regeneration. Evid. Based Complement Alternat. Med. 2011:380809. doi: 10.1093/ecam/neq079
Chen, S. N., and Li, J. T. (2019). Small blood know luo decoction on brain vascular disease caused by the impact of executive function in patients with cognitive dysfunction. J. guangzhou Univers. Traditional Chin. Med. 36, 466–470.
Chen, X., Chen, H., He, Y., Fu, S., Liu, H., and Wang, Q. (2020). Proteomics-Guided Study on Buyang Huanwu Decoction for Its Neuroprotective and Neurogenic Mechanisms for Transient Ischemic Stroke: involvements of EGFR/PI3K/Akt/Bad/14-3-3 and Jak2/Stat3/Cyclin D1 Signaling Cascades. Mol. Neurobiol. 57, 4305–4321. doi: 10.1007/s12035-020-02016-y
Cheng, C. Y., Huang, H. C., Kao, S. T., and Lee, Y. C. (2021). Angelica sinensis extract promotes neuronal survival by enhancing p38 MAPK-mediated hippocampal neurogenesis and dendritic growth in the chronic phase of transient global cerebral ischemia in rats. J. Ethnopharmacol. 278, 114301. doi: 10.1016/j.jep.2021.114301
Cheng, J., Chen, M., Wan, H. Q., Chen, X. Q., Li, C. F., and Zhu, J. X. (2021). Paeoniflorin exerts antidepressant-like effects through enhancing neuronal FGF-2 by microglial inactivation. J. Ethnopharmacol. 274:114046. doi: 10.1016/j.jep.2021.114046
Cheng, C. Y., Kao, S. T., and Lee, Y. C. (2020). Angelica sinensis extract protects against ischemia-reperfusion injury in the hippocampus by activating p38 MAPK-mediated p90RSK/p-Bad and p90RSK/CREB/BDNF signaling after transient global cerebral ischemia in rats. J. Ethnopharmacol. 252:112612. doi: 10.1016/j.jep.2020.112612
Cui, H. J., Yang, A. L., Zhou, H. J., Wang, C., Luo, J. K., and Lin, Y. (2015). Buyang huanwu decoction promotes angiogenesis via vascular endothelial growth factor receptor-2 activation through the PI3K/Akt pathway in a mouse model of intracerebral hemorrhage. BMC Complement Altern. Med. 15:91. doi: 10.1186/s12906-015-0605-8
Deng, M., Sun, H., Shen, J., Fan, Y., Zhang, L., and Zhang, J. (2015). Radix Angelica Sinensis Promotes Synaptic Plasticity During Cognitive Recovery in Chronically Stressed Rats. Curr. Neurovasc. Res. 12, 232–239. doi: 10.2174/1567202612666150603125710
Dou, B., Li, S., Wei, L., Wang, L., Zhu, S., and Wang, Z. (2021). Astragaloside IV suppresses post-ischemic natural killer cell infiltration and activation in the brain: involvement of histone deacetylase inhibition. Front. Med. 15:79–90. doi: 10.1007/s11684-020-0783-8
Dou, B., Zhou, W., Li, S., Wang, L., Wu, X., and Li, Y. (2018). Buyang Huanwu Decoction Attenuates Infiltration of Natural Killer Cells and Protects Against Ischemic Brain Injury. Cell Physiol. Biochem. 50, 1286–1300. doi: 10.1159/000494587
Duan, M. H., Wang, L. N., Jiang, Y. H., Pei, Y. Y., Guan, D. D., Qiu, Z. D., et al. (2016). Angelica sinensis reduced Aβ-induced memory impairment in rats. J. Drug Target 24, 340–347.
Eker, O. F., Rascle, L., Cho, T. H., Mechtouff, L., Derex, L., and Ong, E. (2019). Does Small Vessel Disease Burden Impact Collateral Circulation in Ischemic Stroke Treated by Mechanical Thrombectomy? Stroke 50, 1582–1585. doi: 10.1161/STROKEAHA.119.025608
Gao, L. J., Li, P., Ma, T., Zhong, Z. Q., and Xu, S. J. (2021a). Ligustilide alleviates neurotoxicity in SH-SY5Y cells induced by Aβ(25-35) via regulating endoplasmic reticulum stress and autophagy. Phytother. Res. 35, 1572–1584. doi: 10.1002/ptr.6925
Gao, L. M., Fu, S., Liu, F., Wu, H. B., and Li, W. J. (2021b). Astragalus Polysaccharide Regulates miR-182/Bcl-2 Axis to Relieve Metabolic Memory through Suppressing Mitochondrial Damage-Mediated Apoptosis in Retinal Pigment Epithelial Cells. Pharmacology 106, 520–533. doi: 10.1159/000515901
Gu, J., Chen, J., Yang, N., Hou, X., Wang, J., Tan, X., et al. (2016). Combination of Ligusticum chuanxiong and Radix Paeoniae ameliorate focal cerebral ischemic in MCAO rats via endoplasmic reticulum stress-dependent apoptotic signaling pathway. J. Ethnopharmacol. 187, 313–324. doi: 10.1016/j.jep.2016.04.024
Gu, J., Feng, L., Song, J., Cui, L., Liu, D., Ma, L., et al. (2020). The effect and mechanism of combination of total paeony glycosides and total ligustici phenolic acids against focal cerebral ischemia. Sci. Rep. 10:3689. doi: 10.1038/s41598-020-60357-z
Guo, H., Zhu, L., Tang, P., Chen, D., Li, Y., Li, J., et al. (2021). Carthamin yellow improves cerebral ischemia-reperfusion injury by attenuating inflammation and ferroptosis in rats. Int. J. Mol. Med. 47:52. doi: 10.3892/ijmm.2021.4885
Guo, L. Y., Shi, F. L., Li, M., Sun, J. H., Li, C. G., and Liu, Z. X. (2021). Astragalus protects PC12 cells from 6-hydroxydopamine-induced neuronal damage: a serum pharmacological study. Chin. J. Physiol. 64, 24–31. doi: 10.4103/CJP.CJP_50_20
Guo, M. F., Dai, Y. J., Gao, J. R., and Chen, P. J. (2020). Uncovering the Mechanism of Astragalus membranaceus in the Treatment of Diabetic Nephropathy Based on Network Pharmacology. J. Diabetes Res. 2020:5947304. doi: 10.1155/2020/5947304
Han, C. K., Kuo, W. W., Shen, C. Y., Chen, T. S., Pai, P., Tsai, C. H., et al. (2014). Dilong prevents the high-KCl cardioplegic solution administration-induced apoptosis in H9c2 cardiomyoblast cells mediated by MEK. Am. J. Chin. Med. 42, 1507–1519. doi: 10.1142/S0192415X14500943
Han, J., Wang, X., Hou, J., Liu, Y., Liu, P., and Zhao, T. (2021). Using Network Pharmacology to Explore the Mechanism of Peach Kernel-Safflower in the Treatment of Diabetic Nephropathy. Biomed. Res. Int. 2021:6642584. doi: 10.1155/2021/6642584
Han, Y., Chen, Y., Zhang, Q., Liu, B. W., Yang, L., Xu, Y. H., et al. (2021). Overview of therapeutic potentiality of Angelica sinensis for ischemic stroke. Phytomedicine 90:153652. doi: 10.1016/j.phymed.2021.153652
Hao, E., Pang, G., Du, Z., Lai, Y. H., Chen, J. R., Xie, J., et al. (2019). Peach Kernel Oil Downregulates Expression of Tissue Factor and Reduces Atherosclerosis in ApoE knockout Mice. Int. J. Mol. Sci. 20:405. doi: 10.3390/ijms20020405
Hase, Y., Horsburgh, K., Ihara, M., and Kalaria, R. N. (2018). White matter degeneration in vascular and other ageing-related dementias. J. Neurochem. 144, 617–633. doi: 10.1111/jnc.14271
Hiramatsu, M., Takahashi, T., Komatsu, M., Kido, T., and Kasahara, Y. (2009). Antioxidant and neuroprotective activities of Mogami-benibana (safflower. Carthamus tinctorius Linne). Neurochem. Res. 34, 795–805. doi: 10.1007/s11064-008-9884-5
Huang, C. Y., Kuo, W. W., Liao, H. E., Lin, Y. M., Kuo, C. H., Tsai, F. J., et al. (2013). Lumbrokinase attenuates side-stream-smoke-induced apoptosis and autophagy in young hamster hippocampus: correlated with eNOS induction and NFκB/iNOS/COX-2 signaling suppression. Chem. Res. Toxicol. 26, 654–661. doi: 10.1021/tx300429s
Huang, D., Jia, Z., Chang, L., Zhou, H., Wu, X., and Wei, C. (2020). The role of collateral disease theory in the prevention and treatment of atherosclerosis in post-menopausal women: a narrative review. Ann. Palliat. Med. 9, 2314–2322. doi: 10.21037/apm-20-1257
Huang, J., Wang, X., Xie, L., Wu, M., Zhao, W., Zhang, Y., et al. (2020). Extract of Danggui-Shaoyao-San ameliorates cognition deficits by regulating DHA metabolism in APP/PS1 mice. J. Ethnopharmacol. 253:112673. doi: 10.1016/j.jep.2020.112673
Iadecola, C. (2017). The Neurovascular Unit Coming of Age: a Journey through Neurovascular Coupling in Health and Disease. Neuron 96, 17–42. doi: 10.1016/j.neuron.2017.07.030
Ji, H., Wang, L., Bi, H., Sun, L., Cai, B., Wang, Y., et al. (2008). Mechanisms of lumbrokinase in protection of cerebral ischemia. Eur. J. Pharmacol. 590, 281–289. doi: 10.1016/j.ejphar.2008.05.037
Jiang, R., Xu, J., Zhang, Y., Zhu, X., Liu, J., and Tan, Y. (2021). Ligustrazine Alleviate Acute Lung Injury Through Suppressing Pyroptosis and Apoptosis of Alveolar Macrophages. Front. Pharmacol. 12:680512. doi: 10.3389/fphar.2021.680512
Jin, L., Jin, H., Zhang, G., and Xu, G. (2000). Changes in coagulation and tissue plasminogen activator after the treatment of cerebral infarction with lumbrokinase. Clin. Hemorheol. Microcirc. 23, 213–218.
Jinglong, T., Weijuan, G., Jun, L., Tao, Q., Hongbo, Z., and Shasha, L. (2013). The molecular and electrophysiological mechanism of buyanghuanwu decoction in learning and memory ability of vascular dementia rats. Brain Res. Bull. 99, 13–18. doi: 10.1016/j.brainresbull.2013.09.002
Ke, Z., Wang, G., Yang, L., Qiu, H., Wu, H., Du, M., et al. (2017). Crude terpene glycoside component from Radix paeoniae rubra protects against isoproterenol-induced myocardial ischemic injury via activation of the PI3K/AKT/mTOR signaling pathway. J. Ethnopharmacol. 206, 160–169. doi: 10.1016/j.jep.2017.05.028
Kim, I., Bae, J., and Kim, B. J. (2017). Carthami flos regulates gastrointestinal motility functions. Integr. Med. Res. 6, 404–408. doi: 10.1016/j.imr.2017.08.005
Kim, J., Woo, J., Lyu, J. H., Song, H. H., Jeong, H. S., Ha, K. T., et al. (2014). Carthami Flos suppresses neutrophilic lung inflammation in mice, for which nuclear factor-erythroid 2-related factor-1 is required. Phytomedicine 21, 470–478. doi: 10.1016/j.phymed.2013.10.005
Kong, Y., Peng, Q., Lv, N., Yuan, J., Deng, Z., Liang, X., et al. (2020). Paeoniflorin exerts neuroprotective effects in a transgenic mouse model of Alzheimer’s disease via activation of adenosine A(1) receptor. Neurosci. Lett. 730:135016. doi: 10.1016/j.neulet.2020.135016
Kuang, X., Chen, Y. S., Wang, L. F., Li, Y. J., Liu, K., Zhang, M. X., et al. (2014). Klotho upregulation contributes to the neuroprotection of ligustilide in an Alzheimer’s disease mouse model. Neurobiol. Aging 35, 169–178. doi: 10.1016/j.neurobiolaging.2013.07.019
Li, L. L., Liu, Y. R., Sun, C., Yan, Y. G., Tang, Z. S., Sun, J., et al. (2020). Taoren-dahuang herb pair reduces eicosanoid metabolite shifts by regulating ADORA2A degradation activity in ischaemia/reperfusion injury rats. J. Ethnopharmacol. 260:113014. doi: 10.1016/j.jep.2020.113014
Li, X. H., Liu, Y. R., Jiang, D. H., Tang, Z. S., Qian, D. W., Song, Z. X., et al. (2020). Research on the mechanism of Chinese herbal medicine Radix Paeoniae Rubra in improving chronic pelvic inflammation disease by regulating PTGS2 in the arachidonic acid pathway. Biomed. Pharmacother. 129:110052. doi: 10.1016/j.biopha.2020.110052
Li, Q., Yang, Y., Reis, C., Tao, T., Li, W., Li, X., et al. (2018). Cerebral Small Vessel Disease. Cell Transplant 27, 1711–1722.
Li, S., Cao, G., Deng, Q., Zhu, D., and Yan, F. (2019). Effect of Pushen capsule for treating vascular mild cognitive impairment: a pilot observational study. J. Int. Med. Res. 47, 5483–5496. doi: 10.1177/0300060519859766
Li, X. H., Tu, X. Y., Zhang, D. X., Xu, J. F., Wang, W. Y., Zhang, Y., et al. (2009). Effects of wuwei dilong decoction on inflammatory cells and cytokines in asthma model guinea pigs. J. Tradit. Chin. Med. 29, 220–223. doi: 10.1016/s0254-6272(09)60070-4
Li, X. T., Zhang, Y. K., Kuang, H. X., Jin, F. X., Liu, D. W., Gao, M. B., et al. (2012). Mitochondrial protection and anti-aging activity of Astragalus polysaccharides and their potential mechanism. Int. J. Mol. Sci. 13, 1747–1761. doi: 10.3390/ijms13021747
Liang, F., Huo, Q. P., and Chen, X. (2019). Effects of TCM and WM intervention on Montreal Cognitive Scale and age-related white matter change score of small vascular disease. J. Modern Integr. Chin. Western Med. 28, 1602–1605.
Liu, C. H., Lin, Y. W., Tang, N. Y., Liu, H. J., Huang, C. Y., and Hsieh, C. L. (2012). Effect of oral administration of Pheretima aspergillum (earthworm) in rats with cerebral infarction induced by middle-cerebral artery occlusion. Afr. J. Tradit. Complement Altern. Med. 10, 66–82.
Liu, D., Li, K., Ma, X., Li, Y., Bu, Q., Pan, Z., et al. (2019). Correlations Between the Microstructural Changes of the Medial Temporal Cortex and Mild Cognitive Impairment in Patients With Cerebral Small Vascular Disease (cSVD): a Diffusion Kurtosis Imaging Study. Front. Neurol. 10:1378. doi: 10.3389/fneur.2019.01378
Liu, S. C., Hu, W. Y., Zhang, W. Y., Yang, L., Li, Y., Xiao, Z. C., et al. (2019). Paeoniflorin attenuates impairment of spatial learning and hippocampal long-term potentiation in mice subjected to chronic unpredictable mild stress. Psychopharmacology 236, 2823–2834. doi: 10.1007/s00213-019-05257-5
Liu, H., Chen, S., Guo, C., Tang, W., Liu, W., and Liu, Y. (2018). Astragalus Polysaccharide Protects Neurons and Stabilizes Mitochondrial in a Mouse Model of Parkinson Disease. Med. Sci. Monit. 24, 5192–5199.
Liu, Y. F., Zhang, L., Wu, Q., and Feng, L. Y. (2021). Paeoniflorin ameliorates ischemic injury in rat brain via inhibiting cytochrome c/caspase3/HDAC4 pathway. Acta Pharmacol. Sin. 43, 273–284. doi: 10.1038/s41401-021-00671-y
Lo, W. Y., Liu, C. H., Chen, C. H., and Hsieh, C. L. (2021). Proteomics analysis of protein biomarkers in Astragalus membranaceus- and Astragaloside IV-treated brain tissues in ischemia-reperfusion injured rats. J. Tradit. Complement Med. 11, 369–374. doi: 10.1016/j.jtcme.2021.04.002
Lu, Q. Y., Ma, J. Q., Duan, Y. Y., Sun, Y., Yu, S., Li, B., et al. (2019). Carthamin Yellow Protects the Heart Against Ischemia/Reperfusion Injury With Reduced Reactive Oxygen Species Release and Inflammatory Response. J. Cardiovasc. Pharmacol. 74, 228–234. doi: 10.1097/FJC.0000000000000710
Luo, C., Chen, Q., Liu, B., Wang, S., Yu, H., Guan, X., et al. (2021). The Extracts of Angelica sinensis and Cinnamomum cassia from Oriental Medicinal Foods Regulate Inflammatory and Autophagic Pathways against Neural Injury after Ischemic Stroke. Oxid. Med. Cell Longev. 2021:9663208. doi: 10.1155/2021/9663208
Luo, L., Wu, S., Chen, R., Rao, H., Peng, W., and Su, W. (2020). The study of neuroprotective effects and underlying mechanism of Naoshuantong capsule on ischemia stroke mice. Chin. Med. 15:119. doi: 10.1186/s13020-020-00399-7
Luo, X. Q., Li, A., Yang, X., Xiao, X., Hu, R., Wang, T. W., et al. (2018). Paeoniflorin exerts neuroprotective effects by modulating the M1/M2 subset polarization of microglia/macrophages in the hippocampal CA1 region of vascular dementia rats via cannabinoid receptor 2. Chin. Med. 13:14. doi: 10.1186/s13020-018-0173-1
Meng, S. X., and Huo, Q. P. (2016b). Treatment of cerebral vascular disease from the perspective of kidney. Chin. J. Integr. Tradit. Western Med. Cardio-Cerebrovasc. Dis. 14, 1242–1246.
Meng, S. X., and Huo, Q. P. (2016a). Experience of wenxiao III prescription in the treatment of cerebral vascular disease. Chin. J. Integr. Tradit. Western Med. Cardio-Cerebrovasc. Dis. 14, 1692–1694.
Ni, G. X., Liang, C., Wang, J., Duan, C. Q., Wang, P., and Wang, Y. L. (2020). Astragaloside IV improves neurobehavior and promotes hippocampal neurogenesis in MCAO rats though BDNF-TrkB signaling pathway. Biomed. Pharmacother. 130:110353. doi: 10.1016/j.biopha.2020.110353
Nighoghossian, N., and Mechtouff, L. (2020). Letter by Nighoghossian and Mechtouff Regarding Article, “Collateral Recruitment Is Impaired by Cerebral Small Vessel Disease”. Stroke 51:e165. doi: 10.1161/STROKEAHA.120.030322
Orgah, J. O., He, S., Wang, Y., Jiang, M., Wang, Y., Orgah, E. A., et al. (2020). Pharmacological potential of the combination of Salvia miltiorrhiza (Danshen) and Carthamus tinctorius (Honghua) for diabetes mellitus and its cardiovascular complications. Pharmacol. Res. 153:104654. doi: 10.1016/j.phrs.2020.104654
Pan, R., Tang, X., Wang, H., Huang, Y., Huang, K., Ling, S., et al. (2020). The Combination of Astragalus membranaceus and Ligustrazine Protects Against Thrombolysis-Induced Hemorrhagic Transformation Through PKCδ/Marcks Pathway in Cerebral Ischemia Rats. Cell Transplant 29:963689720946020. doi: 10.1177/0963689720946020
Qi, Z. X., and Chen, L. (2009). Effect of Chinese drugs for promoting blood circulation and eliminating blood stasis on vascular endothelial growth factor expression in rabbits with glucocorticoid-induced ischemic necrosis of femoral head. J. Tradit. Chin. Med. 29, 137–140. doi: 10.1016/s0254-6272(09)60050-9
Qin, X., Hua, J., Lin, S. J., Zheng, H. T., Wang, J. J., Li, W., et al. (2020). Astragalus polysaccharide alleviates cognitive impairment and β-amyloid accumulation in APP/PS1 mice via Nrf2 pathway. Biochem. Biophys. Res. Commun. 531, 431–437. doi: 10.1016/j.bbrc.2020.07.122
Rappaport, N., Twik, M., Plaschkes, I., Nudel, R., Iny Stein, T., Levitt, J., et al. (2017). MalaCards: an amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res. 45, D877–D887. doi: 10.1093/nar/gkw1012
Ren, Y., Houghton, P., and Hider, R. C. (2006). Relevant activities of extracts and constituents of animals used in traditional Chinese medicine for central nervous system effects associated with Alzheimer’s disease. J. Pharm. Pharmacol. 58, 989–996. doi: 10.1211/jpp.58.7.0015
Satoh, A., Yokozawa, T., Cho, E. J., Okamoto, T., and Sei, Y. (2004). Antioxidative effects related to the potential anti-aging properties of the Chinese prescription Kangen-karyu and Carthami Flos in senescence-accelerated mice. Arch. Gerontol. Geriatr. 39, 69–82. doi: 10.1016/j.archger.2004.01.001
Shen, J., Zhang, J., Deng, M., Liu, Y., Hu, Y., and Zhang, L. (2016). The Antidepressant Effect of Angelica sinensis Extracts on Chronic Unpredictable Mild Stress-Induced Depression Is Mediated via the Upregulation of the BDNF Signaling Pathway in Rats. Evid. Based Complement Alternat. Med. 2016:7434692. doi: 10.1155/2016/7434692
Shi, Y., and Wardlaw, J. M. (2016). Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc. Neurol. 1, 83–92. doi: 10.1136/svn-2016-000035
Sun, M., Zhang, J. J., Shan, J. Z., Zhang, H., Jin, C. Y., Xu, S., et al. (2009). Clinical observation of Danhong Injection (herbal TCM product from Radix Salviae miltiorrhizae and Flos Carthami tinctorii) in the treatment of traumatic intracranial hematoma. Phytomedicine 16, 683–689. doi: 10.1016/j.phymed.2009.03.020
Sun, Q., Shi, P., Lin, C., and Ma, J. (2020). Effects of Astragalus Polysaccharides Nanoparticles on Cerebral Thrombosis in SD Rats. Front. Bioeng. Biotechnol. 8:616759. doi: 10.3389/fbioe.2020.616759.
Sun, Z. Y., Wang, F. J., Guo, H., Chen, L., Chai, L. J., Li, R. L., et al. (2019). Shuxuetong injection protects cerebral microvascular endothelial cells against oxygen-glucose deprivation reperfusion. Neural Regen. Res. 14, 783–793. doi: 10.4103/1673-5374.249226
Tang, H., Wu, L., Chen, X., Li, H., Huang, B., Huang, Z., et al. (2021). Paeoniflorin improves functional recovery through repressing neuroinflammation and facilitating neurogenesis in rat stroke model. Peer. J. 9:e10921. doi: 10.7717/peerj.10921
Tang, X., Wang, H., Chen, H., Sun, S., Chen, H., and Pan, R. (2021). Protective Effects of Astragalus Membranaceus and Ligustrazine on Rat Brain Microvascular Endothelial Cell Injury after Oxygen-Glucose Deprivation/Reoxygenation by Suppressing the PKCδ/MARCKS Pathway. Comb Chem. High Throughput Screen 24, 947–956. doi: 10.2174/1386207323999200818170415
Tsai, N. M., Chen, Y. L., Lee, C. C., Lin, P. C., Cheng, Y. L., Chang, W. L., et al. (2006). The natural compound n-butylidenephthalide derived from Angelica sinensis inhibits malignant brain tumor growth in vitro and in vivo. J. Neurochem. 99, 1251–1262. doi: 10.1111/j.1471-4159.2006.04151.x
Wan, H., Yang, Y., Li, Z., Cheng, L., Ding, Z., Wan, H., et al. (2021). Compatibility of ingredients of Danshen (Radix Salviae Miltiorrhizae) and Honghua (Flos Carthami) and their protective effects on cerebral ischemia-reperfusion injury in rats. Exp. Ther. Med. 22:849. doi: 10.3892/etm.2021.10281
Wang, G., Dai, G., Song, J., Zhu, M., Liu, Y., Hou, X., et al. (2018). Lactone Component From Ligusticum chuanxiong Alleviates Myocardial Ischemia Injury Through Inhibiting Autophagy. Front. Pharmacol. 9:301. doi: 10.3389/fphar.2018.00301
Wang, M., Yao, M., Liu, J., Takagi, N., Yang, B., Zhang, M., et al. (2020). Ligusticum chuanxiong exerts neuroprotection by promoting adult neurogenesis and inhibiting inflammation in the hippocampus of ME cerebral ischemia rats. J. Ethnopharmacol. 249:112385. doi: 10.1016/j.jep.2019.112385
Wang, T., Xu, L., Gao, L., Zhao, L., Liu, X. H., Chang, Y. Y., et al. (2020). Paeoniflorin attenuates early brain injury through reducing oxidative stress and neuronal apoptosis after subarachnoid hemorrhage in rats. Metab. Brain Dis. 35, 959–970. doi: 10.1007/s11011-020-00571-w
Wang, W., Cao, L., Wang, X., and Fan, Y. (2020). Radix Paeoniae Rubra Ameliorates Lupus Nephritis in Lupus-Like Symptoms of Mrl Mice by Reducing Intercellular Cell Adhesion Molecule-1, Vascular Cell Adhesion Molecule-1, and Platelet Endothelial Cell Adhesion Molecule-1 Expression. Comb Chem. High Throughput Screen 23, 675–683. doi: 10.2174/1386207323666200517114802
Wang, X., Xu, W., Chen, H., Li, W., Li, W., and Zhu, G. (2020). Astragaloside IV prevents Aβ(1-42) oligomers-induced memory impairment and hippocampal cell apoptosis by promoting PPARγ/BDNF signaling pathway. Brain Res. 1747:147041.
Wang, W. K., Zhou, Y., Fan, L., Sun, Y., Ge, F., and Xue, M. (2021). The antidepressant-like effects of Danggui Buxue Decoction in GK rats by activating CREB/BDNF/TrkB signaling pathway. Phytomedicine 89:153600. doi: 10.1016/j.phymed.2021.153600
Wang, X., Hao, J. C., Shang, B., Yang, K. L., He, X. Z., Wang, Z. L., et al. (2021). Paeoniflorin ameliorates oxidase stress in Glutamate-stimulated SY5Y and prenatally stressed female offspring through Nrf2/HO-1 signaling pathway. J. Affect. Disord. 294, 189–199. doi: 10.1016/j.jad.2021.07.054
Wang, X. L., Feng, S. T., Wang, Y. T., Chen, N. H., Wang, Z. Z., and Zhang, Y. (2021c). Paeoniflorin: a neuroprotective monoterpenoid glycoside with promising anti-depressive properties. Phytomedicine 90:153669. doi: 10.1016/j.phymed.2021.153669
Wardlaw, J. M., Smith, E. E., Biessels, G. J., Cordonnier, C., Fazekas, F., Frayne, R., et al. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12, 822–838. doi: 10.1016/S1474-4422(13)70124-8
Winship, I. R. (2015). Cerebral collaterals and collateral therapeutics for acute ischemic stroke. Microcirculation 22, 228–236. doi: 10.1111/micc.12177
Wu, J. Y., Yu, Z. L., Fong, W. F., and Shi, Y. Q. (2013). Chemotherapeutic activities of Carthami Flos and its reversal effect on multidrug resistance in cancer cells. Afr. J. Tradit. Complement Altern. Med. 10, 36–40.
Wu, J. Z., Li, Y. J., Huang, G. R., Xu, B., Zhou, F., Liu, R. P., et al. (2021). Mechanisms exploration of Angelicae Sinensis Radix and Ligusticum Chuanxiong Rhizoma herb-pair for liver fibrosis prevention based on network pharmacology and experimental pharmacologylogy. Chin. J. Nat. Med. 19, 241–254. doi: 10.1016/S1875-5364(21)60026-2
Wu, S., Wang, N., Li, J., Wang, G., Seto, S. W., Chang, D., et al. (2019). Ligustilide Ameliorates the Permeability of the Blood-Brain Barrier Model In Vitro During Oxygen-Glucose Deprivation Injury Through HIF/VEGF Pathway. J. Cardiovasc. Pharmacol. 73, 316–325. doi: 10.1097/FJC.0000000000000664
Xiao, Y., Wang, Y. C., Li, L. L., Jin, Y. C., Sironi, L., Wang, Y., et al. (2014). Lactones from Ligusticum chuanxiong Hort. reduces atherosclerotic lesions in apoE-deficient mice via inhibiting over expression of NF-kB-dependent adhesion molecules. Fitoterapia 95, 240–246. doi: 10.1016/j.fitote.2014.02.012
Xie, K. H. (2018). Effect of Buyanghuanwu Decoction with Different Doses of Astragalus Membranaceus on hsCRP and HCY of Small Cerebral Vascular Disease. Guangzhou: Guangzhou University of Traditional Chinese Medicine.
Xie, P., Cui, L., Shan, Y., and Kang, W. Y. (2017). Antithrombotic Effect and Mechanism of Radix Paeoniae Rubra. Biomed. Res. Int. 2017:9475074. doi: 10.1155/2017/9475074
Xu, J., Zhang, Z., Zhou, K., Li, Y., Wan, J., Mao, T., et al. (2021). Integration of network pharmacology and molecular docking technology reveals the mechanism of the herbal pairing of Codonopsis Pilosula (Franch.) Nannf and Astragalus Membranaceus (Fisch.) Bge on chronic heart failure. Ann. Palliat. Med. 10, 7942–7959. doi: 10.21037/apm-21-1469
Xu, W., Zhong, W., Liu, J., Liu, H., and Zhu, B. (2013). Study on anti-tumor effect of total glycosides from Radix paeoniae rubra in S180 tumor-bearing mice. Afr. J. Tradit. Complement Altern. Med. 10, 580–585. doi: 10.4314/ajtcam.v10i3.29
Xue, S. L., Yan, Z. H., and Yang, S. L. (2019). Based on “preserving the main gods together” observation for Yang also five soup combined clinical curative effect of three for cognitive impairment after stroke. Chin. J. Clin. Med. 31, 713–716.
Yang, F., Li, Y., Sheng, X., and Liu, Y. (2021). Paeoniflorin treatment regulates TLR4/NF-κB signaling, reduces cerebral oxidative stress and improves white matter integrity in neonatal hypoxic brain injury. Kor. J. Physiol. Pharmacol. 25, 97–109. doi: 10.4196/kjpp.2021.25.2.97
Ye, N., Cruz, J., Peng, X., Ma, J., Zhang, A., and Cheng, X. (2021). Remyelination is enhanced by Astragalus polysaccharides through inducing the differentiation of oligodendrocytes from neural stem cells in cuprizone model of demyelination. Brain Res. 1763:147459. doi: 10.1016/j.brainres.2021.147459
Yu, B., Yao, Y., Zhang, X., Ruan, M., Zhang, Z., Xu, L., et al. (2021). Synergic Neuroprotection Between Ligusticum Chuanxiong Hort and Borneol Against Ischemic Stroke by Neurogenesis via Modulating Reactive Astrogliosis and Maintaining the Blood-Brain Barrier. Front. Pharmacol. 12:666790. doi: 10.3389/fphar.2021.666790
Yu, J., Dong, X. D., Jiao, J. S., Ji, H. Y., and Liu, A. J. (2021). Antitumor and immunoregulatory activities of a novel polysaccharide from Astragalus membranaceus on S180 tumor-bearing mice. Int. J. Biol. Macromol. 189, 930–993. doi: 10.1016/j.ijbiomac.2021.08.099
Yu, L., Wan, H., Jin, W., Yang, J., Li, C., Dai, L., et al. (2018). Protective effects of effective ingredients of Danshen (Radix Salviae Miltiorrhizae) and Honghua (Flos Carthami) compatibility after rat hippocampal neurons induced by hypoxia injury. J. Tradit. Chin. Med. 38, 685–697.
Zhang, Q., Wang, J., Zhu, L., Jiang, S. J., Liu, J., Wang, L. X., et al. (2021). Ligustrazine Attenuates Hyperhomocysteinemia-induced Alzheimer-like Pathologies in Rats. Curr. Med. Sci. 41, 548–554. doi: 10.1007/s11596-021-2379-1
Zhang, S. X., Xu, H. Y., and Bi, J. X. (2018). Study on cognitive dysfunction induced by small vascular disease. Hum. J. Tradit. Chin. Med. 34, 107–109.
Zhang, X., Zhang, J., Kuang, P., Lang, S., Wu, W., Yuan, Y., et al. (2003). The effect of lumbrokinase on P-selectin and E-selectin in cerebral ischemia model of rat. J. Tradit. Chin. Med. 23, 141–146.
Zhang, X., Zhang, X. F., Wang, L., Guo, D. Y., Zhang, J. M., Chen, Y. G., et al. (2020). Analysis of Clinical Efficacy of Traditional Chinese Medicine in Recovery Stage of Stroke: a Systematic Review and Meta-Analysis. Cardiovasc. Ther. 2020:7172052. doi: 10.1155/2020/7172052
Zhao, J., Mo, C., Shi, W., Meng, L., and Ai, J. (2021). Network Pharmacology Combined with Bioinformatics to Investigate the Mechanisms and Molecular Targets of Astragalus Radix-Panax notoginseng Herb Pair on Treating Diabetic Nephropathy. Evid. Based Complement Alternat. Med. 2021:9980981.
Zhao, X. L., Fan, J. P., and Li, J. C. (2015). Through god after brain pills on the impact of cognitive dysfunction small brain vascular disease. J. Chin. Modern Distance Educ. Chin. Med. 13, 41–42.
Zhong, C., Liu, Z., Zhang, X., Pu, Y., Yang, Z., and Bao, Y. (2021). Physicochemical properties of polysaccharides from Ligusticum chuanxiong and analysis of their anti-tumor potential through immunoregulation. Food Funct. 12, 1719–1731. doi: 10.1039/d0fo02978e
Zhou, H., Ma, J. J., and Luo, G. Y. (2019). Tongxinluo combined therapy with butyl phthalide recognition of dysfunction caused by cerebral small vascular disease clinical effect. Clin. Med. Res. Prac. 4, 65–66.
Zhou, Y., Zhou, B., Pache, L., Chang, M., Khodabakhshi, A. H., Tanaseichuk, O., et al. (2019). Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10:1523. doi: 10.1038/s41467-019-09234-6
Zhou, Y., Zong, Y., Liu, Z., Zhao, H., Zhao, X., and Wang, J. (2021). Astragalus Polysaccharides Enhance the Immune Response to OVA Antigen in BALB/c Mice. Biomed. Res. Int. 2021:9976079. doi: 10.1155/2021/9976079
Zhu, W. L., Zheng, J. Y., Cai, W. W., Dai, Z., Li, B. Y., Xu, T. T., et al. (2020). Ligustilide improves aging-induced memory deficit by regulating mitochondrial related inflammation in SAMP8 mice. Aging 12, 3175–3189.
Zhu, Y., Zhang, Y., Huang, X., Xie, Y., Qu, Y., Long, H., et al. (2019). Z-Ligustilide protects vascular endothelial cells from oxidative stress and rescues high fat diet-induced atherosclerosis by activating multiple NRF2 downstream genes. Atherosclerosis 284, 110–120. doi: 10.1016/j.atherosclerosis.2019.02.010
Keywords: Buyang Huanwu Decoction, cerebral small vessel disease, collaterals disease, pharmacodynamics, active component
Citation: Sun L, Ye X, Wang L, Yu J, Wu Y, Wang M and Dai L (2022) A Review of Traditional Chinese Medicine, Buyang Huanwu Decoction for the Treatment of Cerebral Small Vessel Disease. Front. Neurosci. 16:942188. doi: 10.3389/fnins.2022.942188
Received: 12 May 2022; Accepted: 02 June 2022;
Published: 29 June 2022.
Edited by:
Zhengwu Peng, Fourth Military Medical University, ChinaReviewed by:
Tao Tang, Central South University, ChinaCuihong Zhou, Fourth Military Medical University, China
Copyright © 2022 Sun, Ye, Wang, Yu, Wu, Wang and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihua Dai, MTM4MTgzMzIwNTJAMTM5LmNvbQ==
 Liying Sun
Liying Sun Lihua Dai
Lihua Dai