
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci. , 23 June 2022
Sec. Autonomic Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.917197
This article is part of the Research Topic Gut Microbiota and the Nervous System, Volume II View all 5 articles
Inflammatory bowel disease (IBD), comprising Crohn’s disease and Ulcerative colitis, is a relapsing and remitting disease of the gastrointestinal tract, presenting with chronic inflammation, ulceration, gastrointestinal bleeding, and abdominal pain. Up to 80% of patients suffering from IBD experience acute pain, which dissipates when the underlying inflammation and tissue damage resolves. However, despite achieving endoscopic remission with no signs of ongoing intestinal inflammation or damage, 30–50% of IBD patients in remission experience chronic abdominal pain, suggesting altered sensory neuronal processing in this disorder. Furthermore, effective treatment for chronic pain is limited such that 5–25% of IBD outpatients are treated with narcotics, with associated morbidity and mortality. IBD patients commonly present with substantial alterations to the microbial community structure within the gastrointestinal tract, known as dysbiosis. The same is also true in irritable bowel syndrome (IBS), a chronic disorder characterized by altered bowel habits and abdominal pain, in the absence of inflammation. An emerging body of literature suggests that the gut microbiome plays an important role in visceral hypersensitivity. Specific microbial metabolites have an intimate relationship with host receptors that are highly expressed on host cell and neurons, suggesting that microbial metabolites play a key role in visceral hypersensitivity. In this review, we will discuss the techniques used to analysis the metabolome, current potential metabolite targets for visceral hypersensitivity, and discuss the current literature that evaluates the role of the post-inflammatory microbiota and metabolites in visceral hypersensitivity.
Inflammatory bowel diseases (IBD), including Crohn’s disease and ulcerative colitis (UC), as well as irritable bowel syndrome (IBS) are some of the most commonly diagnosed gastrointestinal disorders (Rakoff-Nahoum and Medzhitov, 2006). IBD are chronic debilitating illnesses, with increasing global incidence (Kaplan and Windsor, 2021). IBS is characterized by chronic abdominal pain associated with a change in bowel habits, affecting 11% of the population worldwide (Lacy et al., 2016). Both disorders have an associated high socioeconomic burden, poor quality of life and are associated with chronic abdominal pain (Piovani et al., 2020). The gut microbiome is known to affect a wide variety of gastrointestinal processes (Thursby and Juge, 2017) and plays a role in the pathogenesis of several gastrointestinal disorders, including IBD and IBS (Morgan et al., 2012; Pittayanon et al., 2019). Dysbiosis, or a change in the abundance and composition of bacteria, is characteristic of several gastrointestinal disorders, including IBD (Khan et al., 2019) and IBS (Pittayanon et al., 2019), although it is unknown whether these changes are causal to the disease or a consequence of changes in gastrointestinal motility, diet and gut inflammation. In humans with IBD and in animal models of colitis, sequencing of the intestinal microbiota (metagenomic or amplicon) has characterized phyla level shifts in the proportion of microbial species (Peterson et al., 2008; Tong et al., 2013). Whereas a healthy microbiota consists of the four major phyla, Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, this is often shifted in patients with IBD to a composition that is more abundant in Gram-negative species, such as Proteobacteria and Bacteroidetes (Peterson et al., 2008). As a result of these phyla level shifts, a decrease in overall species diversity within the colonic microbiome is commonly associated with IBD (Peterson et al., 2008). While these shifts in the composition of the gut microbiota have been extensively characterized in IBD, our understanding of the impact that these changes have on the intestinal metabolome is still developing. An emerging body of literature suggests that microbial metabolism plays a role in the pathogenesis of visceral hypersensitivity, through the production of neuroactive molecules such as neurotransmitters (Tsavkelova et al., 2000; Mawe and Hoffman, 2013; Pokusaeva et al., 2017) and microbial products of metabolism such as SCFA (Esquerre et al., 2020) (see Figure 1).
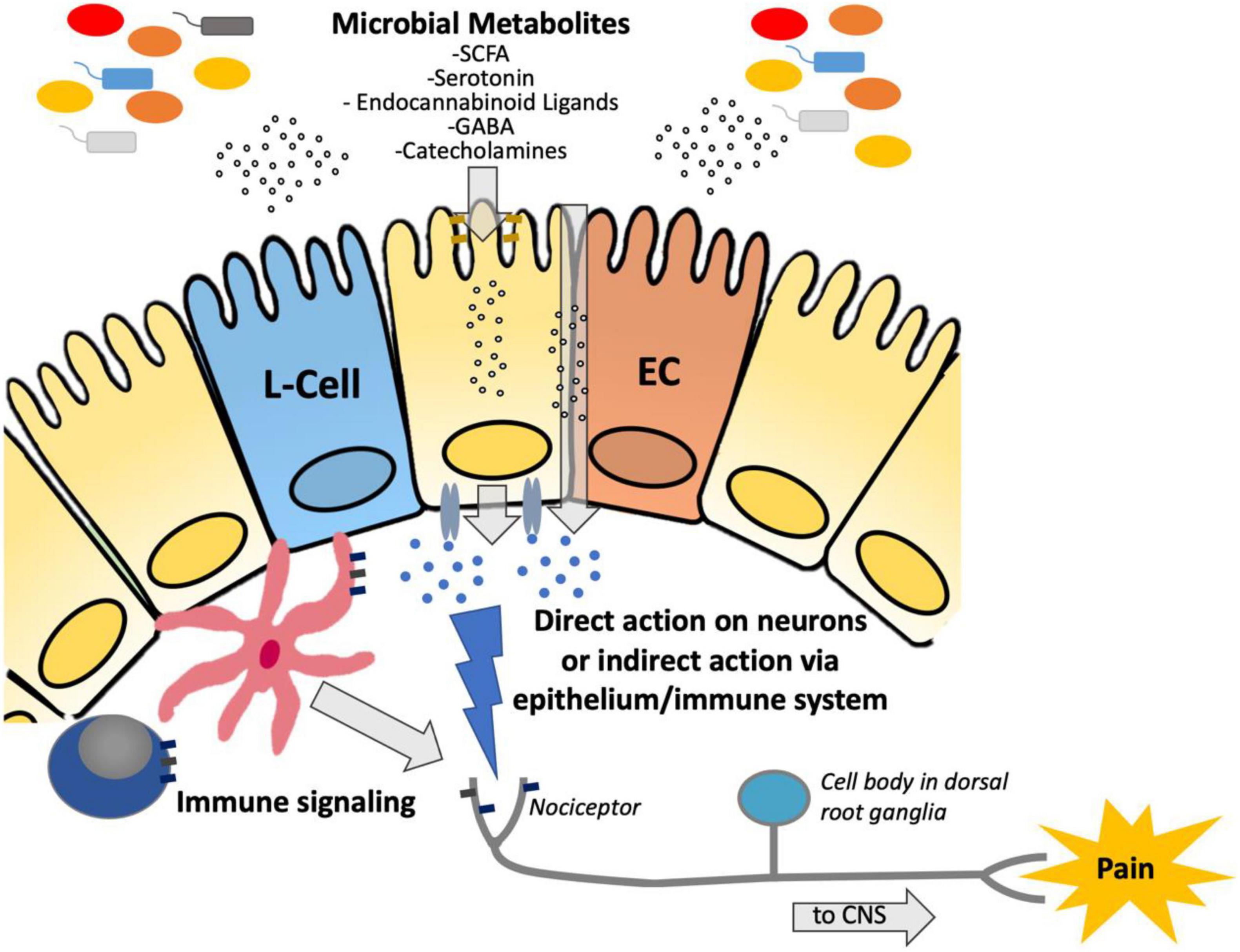
Figure 1. Diagram demonstrating the suggested pathway by which microbiota-derived metabolites are transferred across the epithelium to either (a) directly interact with nociceptors to modulate hypersensitivity or (b) indirectly act via immune stimulation to modulate hypersensitivity. SCFA, short chain fatty acids; GABA, Gamma-aminobutyric acid; ECC, enterochromaffin cell; CNS, central nervous system.
The metabolome refers to a collection of roughly 5,000 low molecular weight (<1 kD) molecules that are produced by microbes and host cells as a result of cellular metabolism (Bowling and Thomas, 2014). Metabolic processes play a fundamental role in all biological processes and an emerging body of literature suggests that host/microbiome dynamics can directly affect immune function (Mager et al., 2020; Yang and Cong, 2021), modulate the clinical presentation of diseases (Chu et al., 2019; Schirmer et al., 2019; Lavelle and Sokol, 2020), and may play a direct role in visceral pain. Although an emerging body of literature suggest that metabolism affects epithelial, neuronal, and immune function, the molecular mechanisms underlying these associations remain unclear. However, systematic interrogation of host/microbial dynamics using metabolomics approaches is proving new insights into how gut microbiota can modulate gastrointestinal diseases via metabolism.
One of the most common symptoms experienced by patients with IBD and IBS is abdominal pain (Brierley and Linden, 2014). Pain can be sub-divided into two sub-categories; visceral pain, which refers to the pain response originating within internal organs such as the intestine, while somatic pain refers to pain originating from muscle, bone, and soft tissue. In the context of IBD, 80% of patients report acute abdominal pain, which is associated with disease flares and increased intestinal inflammation and/or obstruction (Hurtado-Lorenzo et al., 2021). However, 30–50% of IBD patients experience chronic abdominal pain which can persist despite achieving endoscopic remission (Hurtado-Lorenzo et al., 2021). Individuals with IBD can also present with widespread somatic pain in the absence of inflammation (Regueiro et al., 2017), indicating altered sensory neural processing in this disorder. Most importantly, chronic abdominal pain in the absence of inflammation is a severe burden to patients, with significant associated anxiety, depression, and decreased in quality of life and increased healthcare utilization (Gracie et al., 2018). Studies investigating the pathophysiological mechanisms underlying chronic pain in IBD patients in remission are lacking, and effective treatments are just as limited (Hurtado-Lorenzo et al., 2021).
Chronic pain is a disorder of the brain gut axis, and both central and peripheral mechanisms contribute to its pathogenesis (Regueiro et al., 2017). Painful sensation from the gut is relayed to the central nervous system by nociceptors or pain-sensitive neurons with peripheral nerve terminals in the wall of the intestine (Brookes et al., 2013). Nociceptors have their cell bodies located in dorsal root ganglia and central terminal that synapse in the dorsal horn of the spinal cord; the colon is innervated by thoracolumbar and sacral afferents (Hockley et al., 2019), as well as vagal afferents (Borgmann et al., 2021; Jia et al., 2021). Nociceptors can be “sensitized,” defined as a decrease in the threshold for stimulation and an increase in the magnitude of the response, by neuropeptides and inflammatory mediators released by tissue damage (Gold and Gebhart, 2010). Nociceptor sensitization can lead to hyperalgesia, or an exaggerated pain response, as well as allodynia, or pain caused by what would be innocuous stimuli under normal conditions (Gold and Gebhart, 2010).
In both the clinical setting and in animal models, visceral pain can be assessed through the response to colorectal distention, where balloon distention of the colon is performed using a barostat to measure pain tolerance (Keszthelyi et al., 2012). In animal models, pain is measured quantitatively by either measuring the heart rate or the visceromotor response, which uses electrodes to measure abdominal contractions (Keszthelyi et al., 2012). Visceral hypersensitivity to colorectal distention is a hallmark pathophysiologic characteristic of chronic abdominal pain in IBD and IBS (Keszthelyi et al., 2012).
The current literature (Tsavkelova et al., 2000; Mawe and Hoffman, 2013; Pokusaeva et al., 2017; Esquerre et al., 2020) strongly implicates a role for the intestinal microbiota in the development of visceral hypersensitivity in the absence of active inflammation, suggesting that crosstalk between the host and the microbiota via microbial metabolites can result in visceral pain. Herein, we review the current state of the literature linking visceral pain to microbial metabolism and systematically review the proposed molecular mechanisms linking pain in the absence of active inflammation in IBD and IBS to microbial metabolism.
One of the primary challenges in studying the role that the microbiota plays in visceral pain is the significant logistical and biological complexities inherent to microbiome research. Microbes can have diverse metabolic capacities and the species-to-species differences in their ability to consume specific nutrients can have a profound impact on the metabolic composition of the gastrointestinal tract. Consequently, perturbations in the microbiome community can dramatically reshape the molecules that are ultimately passed along to the host. Although host- microbe interactions are known to play an important role in pain and many other physiological functions, unraveling this complexity is challenging in the context of microbiota studies.
Studying these metabolic phenomena is analytically challenging due to the broad chemical diversity of molecules that can potentiate host-microbe interactions. Short-chain fatty acids (SCFA) (Brestoff and Artis, 2013; Neis et al., 2015), amino acids (Wu et al., 2021), bile acids (Ridlon et al., 2014; Wahlström et al., 2016), hormones (Martin et al., 2019), secondary metabolites (Wang et al., 2019), and complex carbohydrates (Flint et al., 2012) are just a few examples of microbial metabolites that modulate host-metabolism. These broad chemical classes cannot all be captured on a single analytical platform and thus the selection of instrumentation can have a direct impact on biological mechanisms that can be investigated (see metabolomics platforms below).
Another factor which affects the complexity of microbiota profiling is that microbial community composition and its metabolome varies along the gastrointestinal tract (Kozik et al., 2019; Zhu et al., 2021). Thus, the microbial community composition is different between ileal luminal samples from colonic and fecal samples (Crespo-Piazuelo et al., 2018), as well along the length of the colon itself (Flynn et al., 2018). Within the colon, studies have demonstrated that fecal sampling does not fully capitulate the luminal microbial community of the proximal colon (Gu et al., 2013; Zhu et al., 2021). Furthermore, studies have demonstrated striking differences between luminal and mucosal samples within the colon itself, specifically regarding mucosa-associated bacteria such as Bifidobacterium, Lactobacillus, and Akkermansia (Heinsen et al., 2015; Kozik et al., 2019). In a study by Miyauchi et al. (2022) lavage samples were collected from 20 healthy donors, where the microbial community composition was compared to respective fecal samples. Substantial difference in microbial community composition within patients was observed between the two sample-types, with the lavage samples containing significantly more Bifidobacteriaceae and Coriobacteriaceae (Miyauchi et al., 2022). Given the substantial differences in the microbial community structure along the gastrointestinal tract, it can be expected the metabolome would also be different, thus adding to the challenges of mapping changes to the luminal metabolome. Furthermore, epithelial uptake of such metabolites along the intestinal tract would further impact the metabolomic composition within the fecal sample.
Another major challenge in studying microbial phenotypes is that the metabolic composition of the gastrointestinal tract and other sites results from the cumulative metabolic activity of the entire microbial community. Although individual microbes can produce unique molecules, most metabolites present in the gut can be consumed by a range of species. Moreover, the waste products generated through microbial metabolism can frequently be further metabolized by other gut microflora, thus creating complex chains of cross-feeding interactions that can obscure individual contributions to a host phenotype (Henriques et al., 2020).
An additional layer of complexity inherent to studying microbiome impacts on host physiology is that the metabolites produced by the gut microflora can have distal effects on a wide variety of host tissues. As has been shown in studies by Claus et al. (2008) and Wikoff et al. (2009), where the gut microflora has direct impact on the metabolic composition of peripheral tissues. Microbial metabolites are diffused or transported across the epithelium and can be detected throughout the body, such as in feces/intestinal biopsies (Rivera-Chávez et al., 2016), urine (Khamis et al., 2017), blood (Bi et al., 2020), liver (Raja et al., 2021), brain (Quintero et al., 2021), cerebral spinal fluid (Quintero et al., 2021), saliva (Chen and Yu, 2019), spleen (Jang et al., 2018), lymph fluid/nodes (Lee et al., 2019), muscle tissue (McGarrah et al., 2018), and lungs (Roque and Romero, 2021). Thus, unraveling pain phenotypes requires analysis of both the local and distal effects that could potentially arise from microbial metabolites.
Over the last decade, analytical technology for tracking host-pathogen dynamics at the genomic, transcriptomic, proteomic, and metabolomics level has advanced considerably, and these tools now serve as the foundation for understanding complex phenotypes such as visceral pain (Moloney et al., 2016; Lucarini et al., 2022). Although whole genome sequencing of individual microbes remains an important element in understanding complex disease (Hollister et al., 2019; Minerbi et al., 2019), there has been an increasing shift toward using genomics to map microbial phylogenetic changes in the microbiome (Liu et al., 2021) via amplicon sequencing (16S rRNA) (Liu et al., 2021) or metagenomic (shotgun) sequencing (Peterson et al., 2008). Both of these approaches allow complex microbial communities to be mapped phylogenetically and can provide insights into how these populations change in response to complex human diseases, such as IBD (Amos et al., 2021). Though powerful, metagenomics is frequently limited in its ability to identify individual species and cannot distinguish between live and dead microbes. Consequently, this tool provides a definitive view of the overall phylogenetic composition of samples, but a limited view of biological activity (e.g., what proteins or metabolites are being secreted, and the host’s response to these molecules).
Limitations in genomics have been increasingly addressed via transcriptomic approaches, which can be used to map the mRNA, non-coding RNA, and micro-RNA present in the gut microenvironment. These studies are largely done through RNA sequencing (RNA-seq) strategies, which borrow heavily from metagenomics and are advantageous because they can capture transcripts from both host and microbe and thus can be used to map a comprehensive collection of genes that are activated or deactivated in response to IBD and other complex diseases (Lloyd-Price et al., 2019).
Proteomics approaches are also emerging as an important strategy for studying microbial environments (Aakko et al., 2020). Modern proteomics methods can quantify thousands of proteins in samples and recent advances in data independent acquisition (Aakko et al., 2020) and metaproteomics strategies (Kleiner, 2019) have dramatically improved the utility of this strategy for complex investigations of host/microbiome interactions. Proteomics strategies are particularly relevant in the context of researching metabolic mechanisms, as secreted enzymes (both from the host and the microbes) play a major role in catabolizing complex carbohydrates, lipids, proteins, and other nutritional sources from the gut and therefore have a direct impact on the composition of each microenvironment.
Metabolomics has emerged as a mainstream strategy for investigating metabolism on a systems-biology scale. Recent advances in mass spectrometry and informatics have made metabolomics much more accessible in recent years and these tools have been applied with increasing frequency to unravel complex host/microbiome metabolic interactions. A rapidly growing body of literature has shown that these intraspecies dynamics play a direct role in modulating the availability of nutrients (Claus et al., 2008; Wikoff et al., 2009; Koh et al., 2016; Putnam and Goodman, 2020), the pharmacokinetics of certain drugs (Taylor et al., 2019; Klünemann et al., 2021), and can modulate the clinical presentation of a wide range of diseases (Han et al., 2015; Blacher et al., 2019; Canfora et al., 2019; Mager et al., 2020). Importantly, several studies (Cai et al., 2022) have shown host/microbiome/metabolic connections may play a role in Parkinson’s disease (Mulak and Bonaz, 2015), depression (Painsipp et al., 2009), autism (Sharon et al., 2019), Alzheimer’s disease (Govindarajan et al., 2011), dementia (Liu et al., 2015), and a variety of other disorders that affect neuronal function (De Vadder et al., 2014; Strandwitz, 2018; Dalile et al., 2019). Although these microbial/host interactions appear to influence a wide spectrum of metabolic functions (e.g., amino acid, nucleotide, lipid, carbohydrate, vitamin/cofactors, and energy metabolism), much of the literature has centered on the role that SCFA and bile acids play in these complex diseases. However, this relatively narrow metabolic focus is beginning to broaden as a greater diversity of molecules are found to play a role in immunity (e.g., inosine Mager et al., 2020), dysbiosis (e.g., H2S; Sultan et al., 2021), and chronic disease (e.g., trimethylamine N-oxide; TMAO; Morgell et al., 2021).
Modern metabolomic studies are typically conducted using nuclear magnetic resonance (NMR) spectroscopy (Jacobs et al., 2008), gas chromatography mass spectrometry (GC-MS) (Hoving et al., 2018), and liquid chromatography mass spectrometry (LC-MS) (Chen et al., 2019). The relative merits, shortcomings, and pitfalls of each analytical platform has been extensively reviewed elsewhere (Lu et al., 2017). Briefly, GC-MS is most effective for analyzing volatile compounds and lipids, LC-MS is most effective for analyzing water-soluble compounds, and NMR is most appropriate when absolute quantification (Lewis et al., 2007; Markley et al., 2017) for unbiased detection of diverse molecular classes is paramount (Lewis et al., 2012).
Beyond these generalized characteristics, there are range of factors that affect the utility of each platform in the context of microbiome studies. Most importantly, the broad diversity of chemical classes involved in microbiome projects can significantly complicate the analytical strategy. SCFAs, for example, which are the subject of intense research in microbiome research, are most amenable to analysis by GC-MS (Zhang et al., 2019). Carbohydrates and alcohols, on the other hand, are most easily analyzed by NMR (Karu et al., 2018), while polar compound are most easily resolved by LC-MS using hydrophilic interaction liquid chromatography (HILIC) (Iturrospe et al., 2021). Consequently, no one technique can serve as a generalized platform for microbiome metabolomics. To address this, researchers either use a combination of techniques or will employ specialized analytical methods, generally involving chemical derivatization of the metabolites to improve their analytical properties for a particular platform. For example, aniline derivatization can be used to enable SCFA analysis by reverse phase (C18) LC-MS (Bihan et al., 2022), benzyl chloroformate can be used to improve the retention of amino acids via reverse phase chromatography (Peoples et al., 2018) and silylation can be used to improve the volatility of water-soluble analytes for analysis by GC-MS (Villas-Bôas et al., 2006). In summary, no single analytical platform is ideally suited to microbiome metabolomics and researchers must use combinations of techniques to capture the full breadth of chemical diversity inherent to this field. Despite these challenges, most project can be satisfactorily completed on either LC-MS or GC-MS platforms.
Although NMR is playing an increasingly smaller role in metabolomics due to its limited sensitivity (Markley et al., 2017; Karu et al., 2018), it still has important merits for microbiome analyses. One consideration is that NMR performance does not degrade over long cohorts. All MS-based platforms (especially LC-MS) suffer from progressive fouling of the electrospray source and ion optics that degrade instrument performance over time (Kang et al., 2017). In contrast, NMR samples are external to the electronics and thus are not subject to carry over or progressive fouling. This can help improve the reproducibility (Reade et al., 2014) of projects, especially in studies involving dirty samples (e.g., feces) which can lead to problems in MS analyses (Reade et al., 2014).
As expected, it is evident that alterations to microbial community diversity results in parallel changes in the metabolomic milieu of the intestinal microenvironment. Alterations to the intestinal microbiota resulting in changes to the metabolome occurs for a variety of reasons, such as dietary changes (Tang et al., 2019), antibiotic use (Theriot et al., 2014), disease states [i.e., IBD (Heinken et al., 2021), IBS (Mars et al., 2020), obesity (Cirulli et al., 2019), type 2 diabetes (Arneth et al., 2019)], host-genetics (Imhann et al., 2018), age (Houtkooper et al., 2011), breast feeding (Henrick et al., 2021), and mode of birth (Carter et al., 2019). With the rapid increase of sequencing technology over the last decade, studies highlighting the impact that these factors have on the intestinal microbiota is extensive, however, analysis on the impact that these alterations have on the metabolome are limited.
In the first 3-years of life, the host’s microbiota is developing rapidly to achieve a permanent state of homeostasis (Milani et al., 2017). During these first 3-years, alterations to the developing microbiota can result in life-long impacts on both the microbiome and metabolome (Milani et al., 2017). It is well known that microbes have diverse metabolic preferences. These differences have profound impacts on the complement of molecules that are taken up or secreted by the microbiota, which in turn changes both the community composition of the microbiota and host-metabolism (Le Chatelier et al., 2013; David et al., 2014). Studies have also demonstrated that the birth route, either vaginal or c-section, results in dramatic differences in the resident microbiota of the nasopharyngeal, skin, gut, and oral cavities (Shao et al., 2019). Babies that were delivered by c-section were found to have a microbiota that was dominated by Staphylococcus and Streptococcus, whereas vaginal delivery resulted in an increased abundance of Lactobacillus species (Marchioro et al., 2019). The differences in delivery methods were later shown to have profound changes to the infant metabolome. For example, cesarean-delivered babies had substantially lower glucose, inulin, non-esterified fatty acids, and acylcarnitine levels when compared to vaginal-delivered babies (Marchioro et al., 2019). Community differences within the intestinal microbiota is also observed between babies that were breast-fed and formula-fed, where breast-fed babies were found to have a greater abundance of species belonging to Lactobacillus and Bifidobacterium when compared to formula-fed infants (Phan et al., 2019). Combining the results of 12 studies that compared breast-fed and formula-fed infants, found a total of 261 fecal metabolites of which 151 were considered significantly different between the two feeding modalities. However, only 10 (acetate, alanine, creatine, glutamine, lactate, urea, citrate, formate, threonine, and glycine) of the 151 metabolites were consistently altered between the two feeding modalities (Phan et al., 2019). The majority of intestinal microbiome studies have been performed to evaluate the impact that diet has on both the microbiota and metabolome. A study by Schnorr et al. (2014), demonstrated that individuals who consumed a diet which was high in plant-derived fibers had a substantial increase in microbial community abundance in the stool, while in a later study, individuals who consumed a low-fiber diet were found to have lower intestinal microbial diversity (Sonnenburg et al., 2016). An increase in gut microbial diversity, via a high-fiber diet, also results in substantial increases in fermented metabolite products, such as SCFAs (Sonnenburg et al., 2016).
Antibiotics have a large impact on the intestinal microbiota, thus leading to substantial decreases in metabolites that are essential energy sources within the colon for colonocytes (Antunes et al., 2011). Several studies have demonstrated that treatment with antibiotics abolishes the overall community diversity within the intestinal tract (Antunes et al., 2011; Korpela and de Vos, 2016; Haak et al., 2019). The immediate and long-term impact that a 2-day treatment of broad-spectrum (ciprofloxacin, vancomycin, and metronidazole) antibiotics have on the intestinal microbiota is a decrease in species richness paired with a marked change in the community composition (Haak et al., 2019). Significant increases in Gram-negative bacteria, particularly proteobacteria, and decreases in obligate anaerobes were also seen after antibiotic treatment (Haak et al., 2019). Thirty one-months after broad-spectrum antibiotic treatment, there was partial but not full recovery in the diversity index (Haak et al., 2019). Thus, aggressive broad spectrum antibiotic treatment can have a long-lasting impact on the microbiota.
As expected, antibiotic treatment leads to equally long-term changes in the metabolome. Several studies have demonstrated the impact that antibiotic treatment has on the metabolome, where SCFAs and bile acids are commonly depleted in human and animal models (Theriot et al., 2014; Zhang Y. et al., 2014; Kuno et al., 2018; Zarrinpar et al., 2018). Clostridia represents one of the largest classes of anaerobic bacteria in the mammalian intestinal microbiota and are highly sensitive to antibiotics. Families within this class, particularly Lachnospiraceae, are well known butyrate-producing commensals (Theriot et al., 2014). This demonstrates the impact that aggressive antibiotic use has on not only the microbiota but on gut microbiota-derived metabolites.
As discussed earlier, environmental factors such as diet and antibiotic use have profound influences on the composition of the gut microbiota. Host genetics also has a significant impact on microbial composition. Genome-wide studies of both the host and the microbiota have identified variants in several human genes that are responsible for signaling, immunity, and epithelial-function which can in turn have a significant impact on overall gut microbial composition (Seregin et al., 2017; Imhann et al., 2018; Hu et al., 2021). Recent studies have also identified several genetic variants within individuals that can predispose a host to the onset of disease, such as IBS and IBD, and which are furthermore linked to increased visceral pain (Bonfiglio et al., 2021; Ledergerber et al., 2021; Vollert et al., 2022). A full discussion of these genetic alterations are beyond the scope of this review, however, a full discussion on host genetics and the microbiota in IBD was recently published by Qiu et al. (2022).
One of the most explored gastrointestinal diseases when it comes to changes in microbial and metabolome composition in the last decade has been IBD. As described earlier, dysbiosis is common in patients suffering from IBD, where extensive community profiling studies describe phylum level shifts within the intestinal microenvironment of patients suffering from this disease. These studies, followed up with extensive targeted and untargeted metabolomics, found that patients with ulcerative colitis and Crohn’s lack microbiota-derived metabolites that are essential to maintaining proper gut health (Heinken et al., 2021). It remains unclear whether these changes to both the microbiota and metabolome are a cause or a result of intestinal inflammation. However, several studies demonstrate that these metabolomic changes are at the very least, preventing the host from achieving homeostasis within the intestinal tract (Lloyd-Price et al., 2019). A multi-omics study from the Human Microbiome Project which followed 132 individuals [consisting of patients with ulcerative colitis (UC), Crohn’s disease (CD), and healthy participants] for 1 year demonstrated that the fecal microbial community of UC patients with active disease was significantly different when compared to the control cohort (Lloyd-Price et al., 2019). UC patients demonstrated a significant reduction in community diversity and had a lower abundance of obligate anaerobes, specifically those belonging to the genus Roseburia and Clostridium clusters IV and XIVA. The effect of reduced anaerobes was evident in their metabolomic profiling, as UC patients had reduced levels of butyrate within their stool (Lloyd-Price et al., 2019).
Given the data that IBD-induced dysbiosis has a deleterious effect on the intestinal microenvironment, there have been several efforts to re-establish a “healthy” intestinal microenvironment in this disease. Although commonly used for the treatment of recurrent C. difficile infection, fecal microbiota transplant (FMT) is currently being explored as a preferred treatment option for active inflammation in UC and CD (Kelly C. R. et al., 2015). To date, studies have not demonstrated a significant benefit of FMT in either UC or CD, as only 28% of patients were able to achieve remission (compared to 9% in the placebo group), with 49% achieving a clinical response with treatment (compared to 28% in the placebo group) (Levy and Allegretti, 2019). In order to improve upon these lackluster findings, newer studies have incorporated prebiotic supplementation prior to FMT with some encouraging results (Scaldaferri et al., 2013). Additional studies with larger number of patients need to be performed to confirm these findings.
Another approach to restore the microbial microenvironment within the intestinal tract is through the use of pre- and/or probiotics. A prebiotic, such as high fiber supplementation, is a dietary nutrient that will enhance the growth of specific commensal bacteria and their metabolites in an effort to achieve intestinal homeostasis (Scaldaferri et al., 2013). In contrast, a probiotic is a single species or consortium of live strain specific bacteria that are cultured in vitro and are ingested by an individual to colonize the gastrointestinal tract (Scaldaferri et al., 2013). Probiotic treatment for active UC has been ongoing for several years, with the most commonly utilized strains being species within Clostridia, Lactobacillus, and Bifidobacterium (Jakubczyk et al., 2020). Various combinations of these microbes are currently in clinical trials, where their specific goal is to increase butyrate production within the colon in UC (Lavelle and Sokol, 2020). It is possible that these microbial-directed therapies will be used as adjuncts alongside biologic therapies in the induction and maintenance of remission in UC, although further studies are needed.
The positive impact that SCFAs have within the colon have been heavily characterized throughout the literature (Hinnebusch et al., 2002; Kim et al., 2014; Bachem et al., 2019). Butyrate is the primary energy source for colonocytes, but SCFAs have further positive effects beyond energy metabolism, such has decreasing luminal pH to enhance nutrient absorption (Lupton and Kurtz, 1993); maintaining microbiota composition via stimulating phagocytic activity (Wu et al., 2020); activating G-protein coupled receptors on neurons (Nøhr et al., 2015), epithelial (Al Mahri et al., 2020), enteroendocrine (Reimann and Gribble, 2016) and innate immune cells (Smith et al., 2013); inhibiting intracellular histone deacetylase activity (Hinnebusch et al., 2002); enhancing barrier function via tight junction protein stimulation (ZO-1 and Occludin) (Wang et al., 2018); stabilizing hypoxia-inducible factor (Kelly C. J. et al., 2015), and increasing Muc-2 mucin production (Gaudier et al., 2004). All these functions are pivotal in achieving homeostasis within the intestinal tract, primarily the colon.
It is clear that microbial metabolites play a significant contribution in limiting intestinal inflammation; in addition, the beneficial effects of metabolites, in particular SCFAs, can be observed systemically (Zaiss et al., 2015). Over 90% of SCFAs are absorbed by the colonic epithelium as a primary energy source for host cells, however, 10% are taken up by capillaries and transported via the portal vein to the liver prior to entering the systemic circulation (Topping and Clifton, 2001). Several systemic immune cells, epithelial cells and neurons express G protein coupled receptors for SCFAs including the free fatty acid receptor 2 (FFAR2), FFAR3, and G protein-like receptor 109A. Through these receptors, SCFAs are able to enhance metabolic activity and immune regulatory effects throughout the body (den Besten et al., 2013). The immune regulatory effects of SCFAs is an emerging field, and a full discussion of these findings can be found in a review by Parada Venegas et al. (2019).
IBS remains the most common reason for referral to gastroenterology and is associated with poor quality of life, anxiety, depression, and considerable economic burden (Ma et al., 2021; Shah et al., 2021). The pathogenesis of IBS is complex, with aberrant brain-gut interactions being at its center (Black and Ford, 2020). Several observations suggest that dysbiosis may play a key role in the pathophysiology of IBS. Patients can develop IBS after an episode of infectious enteritis, termed post-infectious IBS, with a range of bacterial pathogens including campylobacter, salmonella and shigella, being implicated. Both host and pathogen factors play a role in this process, with the severity of the illness and presence of elongating toxin being those associated with considerable relative risk (Barbara et al., 2016). Antibiotic therapy for non-enteric infections is also associated with an increased risk of developing IBS (Paula et al., 2015), suggesting that dysbiosis is an important risk factor in IBS pathogenesis. A recent systematic review demonstrated that there may be a “microbiome signature” in IBS, with an overall decrease in uncultured Clostridiales, in particular Bifidobacterium and Faecalibacterium genus, and an increase in Lactobacillaceae, Bacteroides, and Enterobacteriaceae when compared to controls, although there was considerable heterogeneity in the studies examined (Pittayanon et al., 2019). Faecalibacterium, in particular F. prausnitzii, are known to be anti-inflammatory, as well as major butyrate producers strongly associated with gut health (Lopez-Siles et al., 2017). Interestingly, F. prausnitzii was identified as a source of an anti-nociceptive serine protease that was able to decrease the excitability of mouse dorsal root ganglia neurons through a protease activating receptor -4 (PAR-4) dependent pathway (Sessenwein et al., 2017). It is tempting to speculate that a decrease in baseline F. prausnitzii may play a role in abdominal pain in IBS. However, given the correlative nature of these studies, it is difficult to know whether these microbial changes are causative to the disorder, or are secondary to changes in GI motility, diet, use of medications etc.
Given the link between symptom severity and the gut microbiome, it is not surprising that therapies targeting dysbiosis are being used to treat IBS. A recent systematic review and meta-analysis identified that probiotics, in particular multi-strain formulations, have a modest effect on IBS symptom severity (Ford et al., 2018) but the mechanism of action for the most part remains unclear. There is some speculation that probiotics and bacterial metabolites can signal directly to the brain to improve central symptoms associated with IBS (termed “psychobiotics,” reviewed in Sarkar et al., 2016). A recent randomized controlled trial of 44 adults with IBS with diarrhea-predominance or mixed bowel habits and mild to moderate anxiety and/or depression compared treatment with the probiotic Bifidobacterium longum NCC3001 to placebo (Pinto-Sanchez et al., 2017). Patients treated with B. longum showed a significant reduction in depression scores and an associated improvement in quality of life, with functional MRI studies showing reduced responses in regions of the brain that process negative emotion. Evaluation of urine metabolomics demonstrated an increase in methylamines and aromatic amino acids, including the host-bacterial co-metabolite 4-cresol sulfate, decreased levels of which are associated with depression. Interestingly, no change in IBS symptom severity or fecal microbiota profiles were seen in B. longum-treated patients, suggesting direct signaling of B. longum metabolites to the central nervous system (Pinto-Sanchez et al., 2017). These data suggest that probiotics may play a complex role in the treatment of IBS.
Other strategies to normalize the intestinal microenvironment including antibiotics, FMT and prebiotics/dietary modification have also been used to treat IBS. The non-absorbable antibiotic rifaximin is approved for the treatment of diarrhea-predominant IBS patients and has modest effects on IBS symptom severity (Pimentel et al., 2011; Lembo et al., 2016). However, the mechanism of action of rifaximin is unclear, with only minimal changes in the composition of gut microbiota being observed (Ford et al., 2018; Pimentel and Lembo, 2020). FMT has been evaluated in randomized controlled trials in IBS, but a recent systematic review and meta-analysis did not demonstrate significant improvements in symptom severity when compared to placebo, although there was some heterogeneity depending on the modality of FMT delivery (Ianiro et al., 2019).
Perhaps the best studied microbial strategy to treat IBS is dietary modification. Patients often associate gut symptoms with food consumption and show a preference toward dietary treatment (Sturkenboom et al., 2022). The low FODMAP (fermentable oligo- di- mono-saccharide and polyol) diet has gained considerable traction to treat IBS and is superior to other dietary interventions (Black et al., 2021). FODMAPs are fermentable prebiotics, which are thought to increase colonic gas production, causing visceral hypersensitivity to colonic distention in IBS patients (Major et al., 2017). Interestingly, the low FODMAPs diet has also been shown to be effective for abdominal pain in IBD patients in remission (Prince et al., 2016; Cox et al., 2020), suggesting that “saccharolytic-rich dysbiosis,” may be a common microbial cause that contributes to the pathogenesis of visceral hypersensitivity in both IBD and IBS patients (Chumpitazi et al., 2015; Rossi et al., 2018).
Metabolomics can be used to predict the response to the low FODMAPs diet. The presence of certain volatile organic compounds in fecal samples was able to discriminate responders from non-responders in a randomized cross-over trial of IBS patients (Rossi et al., 2018). Another study that randomized IBS patients to either a low or high FODMAPs diet found significant differences in urine metabolite profiles after the 3-week study. Amongst the altered metabolites was histamine, which was elevated at baseline and was significantly decreased after the low FODMAPs diet (McIntosh et al., 2017). The source of histamine may be either host- or microbial-derived; histamine is known to participate in the pathogenesis of visceral hypersensitivity via sensitization of nociceptors through the histamine-1 receptor (De Palma and Bercik, 2022). These data suggest that metabolomics may allow the identification of patients who will benefit from this type of treatment strategy.
There are concerns, however, regarding the long-term use of this restrictive diet in IBS, as the low FODMAPs diet leads to a decrease in SCFA-producing species, such as Bifidobacteria (Halmos et al., 2015). Even after dietician guidance and careful reintroduction, fecal SCFA content remains decreased despite a more “personalized” FODMAPs restriction, the long-term consequences of which are not known. However, this “personalized” approach results in long term symptom improvement and patient satisfaction in IBS (Staudacher et al., 2022). Interestingly, there are data that suggest SCFAs may be involved in the pathogenesis of visceral hypersensitivity, as discussed below.
Although several studies have demonstrated the beneficial impact that SCFAs have on host metabolic function and immune regulation, studies investigating the effect that these and other microbial metabolites have on the nervous system, specifically with regards to pain, are still emerging. Microbes can produce neuroactive molecules, such as toxins (Chiu et al., 2013; Blake et al., 2018; Yang et al., 2021), neurotransmitters (Tsavkelova et al., 2000; Mawe and Hoffman, 2013; Pokusaeva et al., 2017), proteases [which stimulate neuronal protease-activated receptors (PAR)] (Sessenwein et al., 2017), and metabolites including SCFAs (Baj et al., 2019; Lomax et al., 2019). Bacterial products can signal directly to nerves, or can act indirectly through the immune system, epithelial cells, or enteroendocrine cells to activate nociceptors (Lagomarsino et al., 2021) (see Figure 1). Both vagal (Borgmann et al., 2021; Jia et al., 2021) and spinal afferents are involved in nociception.
The gut-brain axis is a bidirectional signaling pathway between the central nervous system and the gut (Lomax et al., 2019). Indeed, several studies have shown that neurological diseases can alter the gut microbiota (Quigley, 2017), while a dysbiotic microbiota has also been shown to change behavior (Pinto-Sanchez et al., 2017). This data highlights a key role of the microbiota in the gut-brain axis, in which bacterial metabolites play a crucial role in this bidirectional communication. As described below, there are several studies that have shown a role for the microbiota in the pathogenesis of visceral hypersensitivity, however, studies exploring the role of the metabolome are limited. Thus far, there have been a handful of studies characterizing metabolite interactions with the nervous system.
Previous studies using germ-free models (Luczynski et al., 2016) and antibiotic-treated (Verdú et al., 2006; Hoban et al., 2016) models have shown that the microbiota plays a role in visceral pain. Germ-free mice are mice that were breed under sterile conditions and remain sterile their entire life; their intestinal tract lacks a microbiota (Bhattarai and Kashyap, 2016). Antibiotic treatment depletes and alters the intestinal microbiota. It is important to note that antibiotic-treated and germ-free models each have their own advantages and disadvantages. Germ-free mice are considered immunocompromised with distinct physiological and metabolic deficits (Bhattarai and Kashyap, 2016); these animals also demonstrate an altered enteric nervous system (Collins et al., 2014; Filipe et al., 2018). Antibiotic-treated mice have a competent immune system and GI physiology that is unaltered from naïve mice, however, antibiotics can have off-target effects, including causing low-grade intestinal inflammation, as well as increasing visceral hypersensitivity alone (O’Mahony et al., 2014). A study by Vicentini et al. (2021) demonstrated that broad spectrum antibiotic treatment in mice affected the structure and function of the GI tract, resulting in a loss of enteric neurons in both the submucosal and myenteric plexuses. However, supplementation with SCFAs post-antibiotic treatment restored enteric neuronal loss (Vicentini et al., 2021).
Using a germ-free model, Luczynski et al. (2016) demonstrated that male mice had increased visceral hypersensitivity to colorectal distention when compared to their specific pathogen free (SPF) littermates. Furthermore, recolonization could reverse this visceral hypersensitivity. Interestingly, germ-free females did not have increased visceral hypersensitivity when compared to SPF counterparts; estrous-cycle induced changes in visceral pain were abolished in the germ-free animals (Tramullas et al., 2021). Visceral hypersensitivity to colorectal distention was also observed in mice treated with broad-spectrum antibiotics; this change was accompanied by increases in substance P immunoreactivity (Verdú et al., 2006). In another study, the fecal microbiota transplant model was used to evaluate the role of the microbiota in visceral hypersensitivity. Crouzet et al. (2013) colonized gnotobiotic rats with a microbiota that replicated IBS dysbiosis (consisting of more sulfate-reducing bacteria and Enterobacteriaceae and less Bifidobacteria). It was found that the rats who received IBS-like microbiota had increased visceral hypersensitivity when compared to gnotobiotic rats that received a healthy microbiota, suggesting that IBS-associated hypersensitivity is in part caused by changes to the intestinal microbiota (Crouzet et al., 2013; De Palma et al., 2017).
See Table 1 for a simplified description of studies demonstrating the role and mechanism of metabolites in visceral pain.
The post-inflammatory dextran sodium sulfate (DSS) colitis model is an established model of chronic visceral pain, mimicking chronic pain in the post-inflammatory state in IBD. Mice are allowed to recover for 5 weeks after exposure to chemically induced colitis, and then develop visceral hypersensitivity to colorectal distention (Esquerre et al., 2020). Esquerre et al. (2020) demonstrated that FMT of post-inflammatory DSS stool into antibiotic-treated mice resulted in visceral hyperalgesia compared to antibiotic treatment alone; FMT of control stool dampened visceral hypersensitivity (Esquerre et al., 2020). Post-inflammatory mice exhibited changes in the microbiome, with significant increases in SCFA-producing species, such as Lachnospiraceae and Ruminococcus, and stool SCFA content when compared to control mice. Importantly, SCFAs were able to sensitize transient receptor potential vanilloid-1 (TRPV1) expressing nociceptors, suggesting that microbial-derived metabolites play a role in post-inflammatory pain (Esquerre et al., 2020).
Esquerre et al. (2020) showed a pro-nociceptive effect of SCFA, which is at odds with the ability of SCFAs to reduce colonic inflammation and immune activation. Interestingly, another study investigated the ability of SCFA enemas to improve visceral hypersensitivity in a haptenizing model of colitis, 2,4,6-trinitrobenzenesulfonic acid solution (TNBS), in rats (Tarrerias et al., 2002). Although visceral hypersensitivity was reduced in control rats that received butyrate enemas, visceral pain remained unchanged in rats that were exposed to TNBS and treated with butyrate enemas (Tarrerias et al., 2002). In healthy patients and mice, butyrate enemas caused a reduction in abdominal pain to colorectal distention (Vanhoutvin et al., 2009; Russo et al., 2016). In contrast, butyrate enemas increased visceral hypersensitivity through a MAP kinase dependent pathway in rats (Xu et al., 2013).
SCFAs can also modulate visceral hypersensitivity through an indirect mechanism. The SCFA receptors, FFAR2 and FFAR3 are highly expressed on intestinal L cells which contain GLP-1 (Chimerel et al., 2014). When stimulated by SCFAs, L-cells release glucagon like-peptide-1 (GLP-1) (Psichas et al., 2015). This increased secretion was not observed in mice lacking FFAR2 or FFAR3 (Psichas et al., 2015). Activation of the GLP-1 receptor on neurons can reduce visceral hypersensitivity (Gong et al., 2014). Taken together, these data show that SCFAs play a role in visceral hypersensitivity, but further studies are needed to understand the mechanism behind this and to reconcile data showing both pro- vs. anti-nociceptive roles.
Bile acids are thought to play a role in the pathogenesis of IBS as a subset of patients with diarrhea-predominant IBS have an increase in colonic bile acids and can be treated with bile acid sequestrants (Wadhwa et al., 2015). Bile acids are traditionally associated with their role in lipid absorption. Primary bile acids are synthesized by the liver and undergo deconjugation by colonic bacteria to form multiple secondary bile acids, including deoxycholic acid (DCA; recently reviewed; Ní Dhonnabháín et al., 2021). DCA may play a role in visceral hypersensitivity. In a mouse model, DCA was able to increase afferent nerve firing by stimulating 5HT release from EC cells. In the proximal colon, the effect of DCA was inhibited by a 5HT3 receptor antagonist. However, DCA was also able to increase the excitability of nociceptors directly, in a 5HT-independent manner (Yu et al., 2019). In a separate study, colonic DCA enemas increased visceral pain to colonic distention in a rat model, an effect which involved the release of mast cell-derived nerve growth factor (NGF) (Li et al., 2019). NGF was able to increase the expression of neuronal TRPV1, a key receptor involved in nociception. Mast cells are known to form close contacts with nerve terminals in seminal biopsy studies of IBS patients and participate in the pathogenesis of visceral hypersensitivity (Barbara et al., 2007, 2004; Hasler et al., 2022). Interestingly, studies report an increase in secondary bile acids in diarrhea-predominant IBS, due to an excess of Clostridia-rich microbiota in this disease (recently reviewed; Gu et al., 2022). Thus, it is possible that secondary-bile acid induced visceral hypersensitivity contributes to the pathogenesis of abdominal pain in vivo.
Serotonin is a major neurotransmitter within the gastrointestinal tract, that plays an essential role in GI motility. Indeed, drugs targeting the serotonin receptor 5HT3, which is expressed on nociceptors, have been extensively studied for the treatment of visceral hypersensitivity (Mawe and Hoffman, 2013). Serotonin also plays a key role in microbial sensing via enterochromaffin (EC) cells, which are specialized neuroendocrine cells lining the intestinal epithelium that are responsible for GI motility and enzyme secretion (Mawe and Hoffman, 2013; Legan et al., 2022). A study by Reigstad et al. (2015) demonstrated that the rate limiting enzyme for serotonin synthesis, tryptophan hydroxylase (TH) was increased in germ-free mice colonized with human stool compared to germ-free mice alone. In vitro treatment of a human EC cell line with SCFA increased TH production (Reigstad et al., 2015). This data demonstrates that bacterial-derived luminal SCFAs can be detected by EC cells, which in turn secrete basolateral serotonin when activated. A study performed by El-Ayache and Galligan (2019), determined that disrupting the serotonin reuptake transporter (SERT) in female rats increased visceral hypersensitivity, through increased serotonin signaling at dorsal spinal 5HT3 receptors. However, the same phenomenon was not seen in male rats (El-Ayache and Galligan, 2019), suggesting a sex-dependent pain pathway which has been previously reported before in the CNS (Kogler et al., 2016; Mapplebeck et al., 2017). Although these studies do not show that the microbiota directly cause visceral hypersensitivity, there is clear evidence to suggest that communication between the microbiota and the host facilitates visceral hypersensitivity.
Dietary tryptophan is metabolized to 5HT in EC cells but is a substrate for the kynurenine pathway in the epithelium and immune cells, and the indole pathway in gut microbes. Indole derivatives bind to the aryl hydrocarbon (AhR) receptor (Agus et al., 2018). Dysbiosis and a subsequent alteration in tryptophan metabolism is thought to contribute to the pathogenesis of several GI diseases, including IBD and IBS (Kennedy et al., 2017; Agus et al., 2018). Peripheral kynurenine activity was shown to be correlated with the severity of IBS symptoms (Fitzgerald et al., 2008). In an animal model of IBS, decreased activity of the indole pathway and AhR-dependent IL-22 production, was correlated with anxiety-like behaviors; visceral pain was not evaluated in this report (Maëva et al., 2022). However, dysregulated tryptophan metabolism may contribute to the pathogenesis of visceral pain through altered central serotonergic functioning, and subsequent changes in central pain perception (Labus et al., 2011). Thus, microbial tryptophan metabolism may modulate 5HT-dependent visceral pain through both central and peripheral pathways.
The microbiota has the capacity to synthesize and secrete functional neurotransmitters. Gamma-aminobutyric acid (GABA), it is the main neurotransmitter within the central cortex and spinal cord (Pokusaeva et al., 2017). Species belonging to Lactobacillus and Bifidobacterium have been identified as a source of intestinal GABA production (Yunes et al., 2016). In rats, agonists of GABA receptors have demonstrated the ability to inhibit colorectal distention induced visceral pain (Hara et al., 1999). In a study by Pokusaeva et al. (2017), Bifidobacterium dentium was shown to produce GABA via enzymatic decarboxylation of glutamine. Probiotic supplementation of Bifidobacterium suppressed neuronal activity resulting in reduced visceral hypersensitivity in a rat fecal retention model (Pokusaeva et al., 2017). Thus, signaling through GABA receptors via microbial-derived GABA can prevent visceral hypersensitivity. Interestingly, analysis of the fecal metabolome in individuals suffering from IBD as well as IBS demonstrated a depletion in GABA levels (Aggarwal et al., 2018; Heinken et al., 2021), suggesting that this may represent a key mechanism whereby the dysbiotic microbiota can modulate visceral pain.
Catecholamines are monoamine neurotransmitters or hormones used to induce stimulation and response within the mammalian body. There are three main catecholamines: epinephrine, norepinephrine, and dopamine. Dopamine is the major neurotransmitter involved in reward-motivation behavior and is a precursor for the other catecholamines: norepinephrine and epinephrine. Norepinephrine and epinephrine are responsible for the “fight or flight” response. The release of norepinephrine in response to heterotypic chronic stress in rats demonstrated a direct role for this neurotransmitter in increasing visceral hypersensitivity to colorectal distention. Norepinephrine was able to increase the expression of nerve growth factor along the colonic epithelium, which was then able to sensitize nociceptive nerves in the absence of inflammation (Winston et al., 2010). Commensal bacteria that reside within the microbiota have demonstrated the ability to respond to and produce these catecholamines. A study by Freestone et al. (2002) demonstrated that the growth rate of pathogenic enterohemorrhagic E. coli was increased in the presence of norepinephrine and dopamine. This effect was commonly observed in other pathogenic bacteria as well (O’Donnell et al., 2006). Several commensal bacteria, particularly Bacillus sp., have demonstrated the ability to produce norepinephrine and dopamine (Tsavkelova et al., 2000). Thus, it is possible that microbial-produced neurotransmitters may play a role in the pathogenesis of visceral pain.
Cannabinoids have long been used to treat abdominal pain and disorders of GI motility (Izzo and Sharkey, 2010; Goyal et al., 2017) and are extensively utilized by patients with IBS and IBD (Adejumo et al., 2019; Nasser et al., 2020; Bogale et al., 2021; Hryhorowicz et al., 2021). There is evidence that the body’s endogenous cannabinoid system, the endocannabinoid system, which is involved in the control of gastrointestinal motility, sensation and visceral pain, is altered in both IBS (Camilleri et al., 2008, 2013; Fichna et al., 2013; Zhang S.-C. et al., 2014) and IBD (Storr et al., 2009, 2010; Alhouayek and Muccioli, 2012; Strisciuglio et al., 2018). Interestingly, the gut microbiome interacts with the endocannabinoid system (Hosseinkhani et al., 2021; Iannotti and Di Marzo, 2021), while endocannabinoids have been shown to modulate microbiota-driven changes in pain neurotransmission (Rousseaux et al., 2007; Aguilera et al., 2013; Cani et al., 2016; Rea et al., 2021). For example, the probiotic Lactobacillus acidophilus was able to induce the expression of the cannabinoid CB2 receptor as well as the μ-opiate receptor in epithelial cells both in vitro as well as in vivo in rodent models, which in turn led to a decrease in visceral sensitivity (Rousseaux et al., 2007). Commensal bacteria can produce endocannabinoid-like molecules, such as the anandamide-like N-acyl amides (Cohen et al., 2017) and the linoleic acid metabolite 10-oxo-12(Z)-octadecenoic acid (Kim et al., 2017). 5HT3 receptor-dependent release of anandamide in the duodenum is known to be anti-nociceptive (Feng et al., 2014) in a rat model, while linoleic acid metabolites have been reported to sensitize TRPV1, and increase both mechanical and thermal hypersensitivity (Sisignano et al., 2016). It remains to be determined whether a microbial source of endocannabinoid-like molecules plays a role in visceral hypersensitivity.
Recently, there has been exciting data indicating that vagal afferents may be involved in nociception. Vagal afferents are known to modify central pain processing in the spinal cord and brain (Bonaz et al., 2016). Vagal afferents express TRPV1 (Dworsky-Fried et al., 2020), SCFA receptors FFAR3 (Nøhr et al., 2015), as well as TLR4 (Jia et al., 2021), suggesting that microbial metabolites released within the gastrointestinal tract can modulate visceral pain within the host. In a recent study by Jia et al. (2021), it was demonstrated that lipopolysaccharide (LPS) was able to activate TLR4 on vagal afferents, which stimulated the release of calcitonin gene-related peptide (CGRP) release from vagal ganglia. They found that Tlr4 mRNA was enriched in vagal afferents expressing the sodium channel Nav1.8, which is well known to play a role in pain neurotransmission (Nguyen and Yarov-Yarovoy, 2022). These afferents also co-expressed CGRP (Jia et al., 2021). Although this particular study did not evaluate visceral pain, it is well known that CGRP signaling may be involved in afferent nerve sensitization and visceral organ hypersensitivity (Plourde et al., 1997; Delafoy et al., 2006; Noor-Mohammadi et al., 2021). Patients with IBD and IBS are reported to have a decrease in vagal tone (Pellissier et al., 2010). Subdiaphragmatic vagotomy as well as the application of lidocaine to abdominal vagal nerves was shown to blunt the response to colorectal distention, suggesting a potential anti-nociceptive role of vagal afferent stimulation (Chen et al., 2008). For a full discussion on this topic, the reader is referred to an excellent recent review on vagal/microbial interactions that was recently published by Bonaz et al. (2018).
The staggering increase in IBD diagnoses each year across the developed regions of the world has been a large focus of research and drug development, as there is a dire need for new therapies with limited side effects. With this rapid increase in cases, patients that achieve endoscopic remission have persistent abdominal pain and visceral hypersensitivity. Studies to date have shown that the microbiota are involved in the pathogenesis of visceral hypersensitivity. However, the majority of these are strictly observational, where germ-free or antibiotic models are paired with amplicon sequencing to characterize a role for the microbiota in visceral hypersensitivity. Few studies have evaluated changes to the human metabolome in patients with visceral hypersensitivity, with even fewer studies taking these observed metabolomic changes and evaluating the interaction that these metabolites have on the host pain response in both the periphery and the central nervous system. In the last decade, the field of metabolomics has made great advancements, and the current techniques of targeted and untargeted analysis of a heterogenous samples (such as the feces or biopsies) can be utilized to identify specific metabolites unique to patients suffering from visceral hypersensitivity. These can in turn be tested in animal models and in vitro systems to evaluate putative mechanisms underlying visceral pain and hypersensitivity. This may in turn lead to future targeted treatments for visceral pain, either through the use of FMT, pro/prebiotics, dietary therapies, targeted antibiotics, or metabolite receptor- agonists/antagonists. Future studies need to move away from current observational based community profiling experiments and investigate direct and indirect mechanisms whereby microbial metabolites sensitize nociceptors.
AS primarily drafted the manuscript. DB, IL, and YN drafted portions of the manuscript and critically revised the manuscript for important intellectual content. All authors approved the final version for submission.
This work was supported by the Canadian Institutes of Health Research (CIHR) and the Weston Family Microbiome Initiative (to YN). IL was supported by an Alberta Innovates-Health Solutions (AIHS, Translational Health Chair), The Natural Sciences and Engineering Research Council (NSERC-DG 04547). DB was supported by IMPACTT, the CIHR Canadian Microbiome Core.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aakko, J., Pietilä, S., Suomi, T., Mahmoudian, M., Toivonen, R., Kouvonen, P., et al. (2020). Data-independent acquisition mass spectrometry in metaproteomics of gut microbiota—implementation and computational analysis. J. Proteome Res. 19, 432–436. doi: 10.1021/acs.jproteome.9b00606
Adejumo, A. C., Ajayi, T. O., Adegbala, O. M., and Bukong, T. N. (2019). Higher odds of irritable bowel syndrome among hospitalized patients using cannabis: a propensity-matched analysis. Eur. J. Gastroenterol. Hepatol. 31, 756–765. doi: 10.1097/MEG.0000000000001382
Aggarwal, S., Ahuja, V., and Paul, J. (2018). Dysregulation of GABAergic signalling contributes in the pathogenesis of Diarrhea-predominant irritable bowel syndrome. J. Neurogastroenterol. Motil. 24, 422–430. doi: 10.5056/jnm17100
Aguilera, M., Vergara, P., and Martínez, V. (2013). Stress and antibiotics alter luminal and wall-adhered microbiota and enhance the local expression of visceral sensory-related systems in mice. Neurogastroenterol. Motil. 25, e515–e529. doi: 10.1111/nmo.12154
Agus, A., Planchais, J., and Sokol, H. (2018). Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724. doi: 10.1016/j.chom.2018.05.003
Al Mahri, S., Al Ghamdi, A., Akiel, M., Al Aujan, M., Mohammad, S., and Aziz, M. A. (2020). Free fatty acids receptors 2 and 3 control cell proliferation by regulating cellular glucose uptake. World J. Gastrointest. Oncol. 12, 514–525. doi: 10.4251/wjgo.v12.i5.514
Alhouayek, M., and Muccioli, G. G. (2012). The endocannabinoid system in inflammatory bowel diseases: from pathophysiology to therapeutic opportunity. Trends Mol. Med. 18, 615–625.
Amos, G. C. A., Sergaki, C., Logan, A., Iriarte, R., Bannaga, A., Chandrapalan, S., et al. (2021). Exploring how microbiome signatures change across inflammatory bowel disease conditions and disease locations. Sci. Rep. 11:18699. doi: 10.1038/s41598-021-96942-z
Antunes, L. C. M., Han, J., Ferreira, R. B. R., Loliæ, P., Borchers, C. H., and Finlay, B. B. (2011). Effect of antibiotic treatment on the intestinal metabolome. Antimicrob. Agents Chemother. 55, 1494–1503. doi: 10.1128/AAC.01664-10
Arneth, B., Arneth, R., and Shams, M. (2019). Metabolomics of type 1 and type 2 diabetes. Int. J. Mol. Sci. 20:2467.
Bachem, A., Makhlouf, C., Binger, K. J., de Souza, D. P., Tull, D., Hochheiser, K., et al. (2019). Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8(+) T Cells. Immunity 51, 285–297.e5. doi: 10.1016/j.immuni.2019.06.002
Baj, A., Moro, E., Bistoletti, M., Orlandi, V., Crema, F., and Giaroni, C. (2019). Glutamatergic signaling along the microbiota-gut-brain axis. Int. J. Mol. Sci. 20:1482. doi: 10.3390/ijms20061482
Barbara, G., Feinle-Bisset, C., Ghoshal, U. C., Quigley, E. M., Santos, J., Vanner, S., et al. (2016). The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 150, 1305–1318. doi: 10.1053/j.gastro.2016.02.028
Barbara, G., Stanghellini, V., De Giorgio, R., Cremon, C., Cottrell, G. S., Santini, D., et al. (2004). Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126, 693–702. doi: 10.1053/j.gastro.2003.11.055
Barbara, G., Wang, B., Stanghellini, V., de Giorgio, R., Cremon, C., Di Nardo, G., et al. (2007). Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132, 26–37. doi: 10.1053/j.gastro.2006.11.039
Bhattarai, Y., and Kashyap, P. C. (2016). Germ-free mice model for studying host-microbial interactions. Methods Mol. Biol. 1438, 123–135. doi: 10.1007/978-1-4939-3661-8_8
Bi, H., Guo, Z., Jia, X., Liu, H., Ma, L., and Xue, L. (2020). The key points in the pre-analytical procedures of blood and urine samples in metabolomics studies. Metabolomics 16:68. doi: 10.1007/s11306-020-01666-2
Bihan, D. G., Rydzak, T., Wyss, M., Pittman, K., McCoy, K. D., and Lewis, I. A. (2022). Method for absolute quantification of short chain fatty acids via reverse phase chromatography mass spectrometry. PLoS One 17:e0267093. doi: 10.1371/journal.pone.0267093
Blacher, E., Bashiardes, S., Shapiro, H., Rothschild, D., Mor, U., Dori-Bachash, M., et al. (2019). Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480. doi: 10.1038/s41586-019-1443-5
Black, C. J., and Ford, A. C. (2020). Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 17, 473–486. doi: 10.1038/s41575-020-0286-8
Black, C. J., Staudacher, H. M., and Ford, A. C. (2021). Efficacy of a Low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut 71, 1117–1126. doi: 10.1136/gutjnl-2021-325214
Blake, K. J., Baral, P., Voisin, T., Lubkin, A., Pinho-Ribeiro, F. A., Adams, K. L., et al. (2018). Staphylococcus aureus produces pain through pore-forming toxins and neuronal TRPV1 That Is Silenced by QX-314. Nat. Commun. 9:37. doi: 10.1038/s41467-017-02448-6
Bogale, K., Raup-Konsavage, W., Dalessio, S., Vrana, K., and Coates, M. D. (2021). Cannabis and cannabis derivatives for abdominal pain management in inflammatory bowel disease. Med. Cannabis Cannabinoids 4, 97–106. doi: 10.1159/000517425
Bonaz, B., Bazin, T., and Pellissier, S. (2018). The Vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 12:49. doi: 10.3389/fnins.2018.00049
Bonaz, B., Sinniger, V., and Pellissier, S. (2016). Vagal Tone: effects on sensitivity, motility, and inflammation. Neurogastroenterol. Motil. 28, 455–462. doi: 10.1111/nmo.12817
Bonfiglio, F., Liu, X., Smillie, C., Pandit, A., Kurilshikov, A., Bacigalupe, R., et al. (2021). GWAS of stool frequency provides insights into gastrointestinal motility and irritable bowel syndrome. Cell Genom. 1:None. doi: 10.1016/j.xgen.2021.100069
Borgmann, D., Ciglieri, E., Biglari, N., Brandt, C., Cremer, A. L., Backes, H., et al. (2021). Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism. Cell Metab. 33, 1466–1482.e7. doi: 10.1016/j.cmet.2021.05.002
Brestoff, J. R., and Artis, D. (2013). Commensal bacteria at the interface of host metabolism and the immune system. Nat. Immunol. 14, 676–684. doi: 10.1038/ni.2640
Brierley, S. M., and Linden, D. R. (2014). Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 11, 611–627. doi: 10.1038/nrgastro.2014.103
Brookes, S. J. H., Spencer, N. J., Costa, M., and Zagorodnyuk, V. P. (2013). Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastroenterol. Hepatol. 10, 286–296. doi: 10.1038/nrgastro.2013.29
Cai, J., Sun, L., and Gonzalez, F. J. (2022). Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 30, 289–300. doi: 10.1016/j.chom.2022.02.004
Camilleri, M., Carlson, P., McKinzie, S., Grudell, A., Busciglio, I., Burton, D., et al. (2008). Genetic variation in endocannabinoid metabolism, gastrointestinal motility, and sensation. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G13–G19. doi: 10.1152/ajpgi.00371.2007
Camilleri, M., Kolar, G. J., Vazquez-Roque, M. I., Carlson, P., Burton, D. D., and Zinsmeister, A. R. (2013). Cannabinoid receptor 1 gene and irritable bowel syndrome: phenotype and quantitative traits. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G553–G560. doi: 10.1152/ajpgi.00376.2012
Canfora, E. E., Meex, R. C. R., Venema, K., and Blaak, E. E. (2019). Gut Microbial Metabolites in Obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 15, 261–273. doi: 10.1038/s41574-019-0156-z
Cani, P. D., Plovier, H., Van Hul, M., Geurts, L., Delzenne, N. M., Druart, C., et al. (2016). Endocannabinoids–at the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 12, 133–143. doi: 10.1038/nrendo.2015.211
Carter, R. A., Pan, K., Harville, E. W., McRitchie, S., and Sumner, S. (2019). Metabolomics to reveal biomarkers and pathways of preterm birth: a systematic review and epidemiologic perspective. Metabolomics 15:124. doi: 10.1007/s11306-019-1587-1
Chen, M. X., Wang, S.-Y., Kuo, C.-H., and Tsai, I.-L. (2019). Metabolome analysis for investigating host-gut microbiota interactions. J. Formos. Med. Assoc. 118(Suppl.), S10–S22. doi: 10.1016/j.jfma.2018.09.007
Chen, S. L., Wu, X. Y., Cao, Z. J., Fan, J., Wang, M., Owyang, C., et al. (2008). Subdiaphragmatic vagal afferent nerves modulate visceral pain. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1441–G1449. doi: 10.1152/ajpgi.00588.2007
Chen, X., and Yu, D. (2019). Metabolomics study of oral cancers. Metabolomics 15:22. doi: 10.1007/s11306-019-1483-8
Chimerel, C., Emery, E., Summers, D. K., Keyser, U., Gribble, F. M., and Reimann, F. (2014). Bacterial Metabolite Indole Modulates Incretin Secretion from Intestinal Enteroendocrine L Cells. Cell Rep. 9, 1202–1208. doi: 10.1016/j.celrep.2014.10.032
Chiu, I. M., Heesters, B. A., Ghasemlou, N., Von Hehn, C. A., Zhao, F., Tran, J., et al. (2013). Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501, 52–57. doi: 10.1038/nature12479
Chu, C., Murdock, M. H., Jing, D., Won, T. H., Chung, H., Kressel, A. M., et al. (2019). The microbiota regulate neuronal function and fear extinction learning. Nature 574, 543–548. doi: 10.1038/s41586-019-1644-y
Chumpitazi, B. P., Cope, J. L., Hollister, E. B., Tsai, C. M., McMeans, A. R., Luna, R. A., et al. (2015). Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment. Pharmacol. Ther. 42, 418–427. doi: 10.1111/apt.13286
Cirulli, E. T., Guo, L., Leon Swisher, C., Shah, N., Huang, L., Napier, L. A., et al. (2019). Profound perturbation of the metabolome in obesity is associated with health risk. Cell Metab. 29, 488–500.e2. doi: 10.1016/j.cmet.2018.09.022
Claus, S. P., Tsang, T. M., Wang, Y., Cloarec, O., Skordi, E., Martin, F.-P., et al. (2008). Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol. Syst. Biol. 4:219. doi: 10.1038/msb.2008.56
Cohen, L. J., Esterhazy, D., Kim, S.-H., Lemetre, C., Aguilar, R. R., Gordon, E. A., et al. (2017). Commensal Bacteria Make GPCR ligands that mimic human signalling molecules. Nature 549, 48–53.
Collins, J., Borojevic, R., Verdu, E. F., Huizinga, J. D., and Ratcliffe, E. M. (2014). Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol. Motil. 26, 98–107. doi: 10.1111/nmo.12236
Cox, S. R., Lindsay, J. O., Fromentin, S., Stagg, A. J., McCarthy, N. E., Galleron, N., et al. (2020). Effects of Low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology 158, 176–188.e7. doi: 10.1053/j.gastro.2019.09.024
Crespo-Piazuelo, D., Estellé, J., Revilla, M., Criado-Mesas, L., Ramayo-Caldas, Y., Óvilo, C., et al. (2018). Characterization of bacterial microbiota compositions along the intestinal tract in pigs and their interactions and functions. Sci. Rep. 8:12727. doi: 10.1038/s41598-018-30932-6
Crouzet, L., Gaultier, E., Del’Homme, C., Cartier, C., Delmas, E., Dapoigny, M., et al. (2013). The Hypersensitivity to Colonic Distension of IBS Patients Can Be Transferred to Rats through Their Fecal Microbiota. Neurogastroenterol. Motil. 25, e272–e282. doi: 10.1111/nmo.12103
Dalile, B., Van Oudenhove, L., Vervliet, B., and Verbeke, K. (2019). The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478. doi: 10.1038/s41575-019-0157-3
David, L. A., Maurice, C. F., Carmody, R. N., Gootenberg, D. B., Button, J. E., Wolfe, B. E., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. doi: 10.1038/nature12820
De Palma, G., and Bercik, P. (2022). Long-Term Personalized Low FODMAP Diet in IBS. Neurogastroenterol. Motil. 34:e14356. doi: 10.1111/nmo.14356
De Palma, G., Lynch, M. D. J., Lu, J., Dang, V. T., Deng, Y., Jury, J., et al. (2017). Transplantation of Fecal Microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci. Transl. Med. 9:eaaf6397. doi: 10.1126/scitranslmed.aaf6397
De Vadder, F., Kovatcheva-Datchary, P., Goncalves, D., Vinera, J., Zitoun, C., Duchampt, A., et al. (2014). Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156, 84–96. doi: 10.1016/j.cell.2013.12.016
Delafoy, L., Gelot, A., Ardid, D., Eschalier, A., Bertrand, C., Doherty, A. M., et al. (2006). Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut 55, 940–945. doi: 10.1136/gut.2005.064063
den Besten, G., van Eunen, K., Groen, A. K., Venema, K., Reijngoud, D.-J., and Bakker, B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340. doi: 10.1194/jlr.R036012
Dworsky-Fried, Z., Kerr, B. J., and Taylor, A. M. W. (2020). Microbes, Microglia, and Pain. Neurobiol. Pain 7:100045. doi: 10.1016/j.ynpai.2020.100045
El-Ayache, N., and Galligan, J. J. (2019). 5-HT(3) receptor signaling in serotonin transporter-knockout rats: a female sex-specific animal model of visceral hypersensitivity. Am. J. Physiol. Gastrointest. Liver Physiol. 316, G132–G143. doi: 10.1152/ajpgi.00131.2018
Esquerre, N., Basso, L., Defaye, M., Vicentini, F. A., Cluny, N., Bihan, D., et al. (2020). Colitis-induced microbial perturbation promotes postinflammatory visceral hypersensitivity. Cell. Mol. Gastroenterol. Hepatol. 10, 225–244. doi: 10.1016/j.jcmgh.2020.04.003
Feng, C.-C., Yan, X.-J., Chen, X., Wang, E.-M., Liu, Q., Zhang, L.-Y., et al. (2014). Vagal Anandamide Signaling via cannabinoid receptor 1 contributes to luminal 5-HT modulation of visceral nociception in rats. Pain 155, 1591–1604. doi: 10.1016/j.pain.2014.05.005
Fichna, J., Wood, J. T., Papanastasiou, M., Vadivel, S. K., Oprocha, P., Sałaga, M., et al. (2013). Endocannabinoid and cannabinoid-like fatty acid amide levels correlate with pain-related symptoms in patients with IBS-D and IBS-C: a Pilot Study. PLoS One 8:e85073. doi: 10.1371/journal.pone.0085073
Filipe, D. V., Estelle, G., Louise, M. H., Gérard, K. J., M.A., E. O. L., and Fredrik, B. (2018). Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. U.S.A. 115, 6458–6463. doi: 10.1073/pnas.1720017115
Fitzgerald, P., Cassidy Eugene, M., Clarke, G., Scully, P., Barry, S., Quigley Eamonn, M. M., et al. (2008). Tryptophan catabolism in females with irritable bowel syndrome: relationship to interferon-gamma, severity of symptoms and psychiatric co-morbidity. Neurogastroenterol. Motil. 20, 1291–1297. doi: 10.1111/j.1365-2982.2008.01195.x
Flint, H. J., Scott, K. P., Duncan, S. H., Louis, P., and Forano, E. (2012). Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3, 289–306. doi: 10.4161/gmic.19897
Flynn, K. J., Ruffin, M. T. IV, Turgeon, D. K., and Schloss, P. D. (2018). Spatial variation of the native colon microbiota in healthy adults. Cancer Prev. Res. 11, 393–402. doi: 10.1158/1940-6207.CAPR-17-0370
Ford, A. C., Harris, L. A., Lacy, B. E., Quigley, E. M. M., and Moayyedi, P. (2018). Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 48, 1044–1060. doi: 10.1111/apt.15001
Freestone, P. P., Williams, P. H., Haigh, R. D., Maggs, A. F., Neal, C. P., and Lyte, M. (2002). Growth stimulation of intestinal commensal Escherichia coli by Catecholamines: a possible contributory factor in trauma-induced sepsis. Shock 18, 465–470. doi: 10.1097/00024382-200211000-00014
Gaudier, E., Jarry, A., Blottière, H. M., de Coppet, P., Buisine, M. P., Aubert, J. P., et al. (2004). Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G1168–G1174. doi: 10.1152/ajpgi.00219.2004
Gold, M. S., and Gebhart, G. F. (2010). Nociceptor sensitization in pain pathogenesis. Nat. Med. 16, 1248–1257. doi: 10.1038/nm.2235
Gong, N., Xiao, Q., Zhu, B., Zhang, C.-Y., Wang, Y.-C., Fan, H., et al. (2014). Activation of spinal glucagon-like peptide-1 receptors specifically suppresses pain hypersensitivity. J. Neurosci. 34, 5322–5334. doi: 10.1523/JNEUROSCI.4703-13.2014
Govindarajan, N., Agis-Balboa, R. C., Walter, J., Sananbenesi, F., and Fischer, A. (2011). Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J. Alzheimers Dis. 26, 187–197. doi: 10.3233/JAD-2011-110080
Goyal, H., Singla, U., Gupta, U., and May, E. (2017). Role of cannabis in digestive disorders. Eur. J. Gastroenterol. Hepatol. 29, 135–143. doi: 10.1097/MEG.0000000000000779
Gracie, D. J., Hamlin, J. P., and Ford, A. C. (2018). Longitudinal impact of IBS-type symptoms on disease activity, healthcare utilization, psychological health, and quality of life in inflammatory bowel disease. Am. J. Gastroenterol. 113, 702–712. doi: 10.1038/s41395-018-0021-z
Gu, S., Chen, D., Zhang, J.-N., Lv, X., Wang, K., Duan, L.-P., et al. (2013). Bacterial community mapping of the mouse gastrointestinal tract. PLoS One 8:e74957. doi: 10.1371/journal.pone.0074957
Gu, Y., Li, L., Yang, M., Liu, T., Song, X., Qin, X., et al. (2022). Bile Acid-Gut Microbiota Crosstalk in Irritable Bowel Syndrome. Crit. Rev. Microbiol. [Epub ahead of print]. doi: 10.1080/1040841X.2022.2058353
Haak, B. W., Lankelma, J. M., Hugenholtz, F., Belzer, C., de Vos, W. M., and Wiersinga, W. J. (2019). Long-term impact of oral vancomycin, ciprofloxacin and metronidazole on the gut microbiota in healthy humans. J. Antimicrob. Chemother. 74, 782–786. doi: 10.1093/jac/dky471
Halmos, E. P., Christophersen, C. T., Bird, A. R., Shepherd, S. J., Gibson, P. R., and Muir, J. G. (2015). Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 64, 93–100. doi: 10.1136/gutjnl-2014-307264
Han, J., Lin, K., Sequeira, C., and Borchers, C. H. (2015). An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 854, 86–94. doi: 10.1016/j.aca.2014.11.015
Hara, K., Saito, Y., Kirihara, Y., Yamada, Y., Sakura, S., and Kosaka, Y. (1999). The interaction of antinociceptive effects of morphine and GABA receptor agonists within the rat spinal cord. Anesth. Analg. 89, 422–427. doi: 10.1097/00000539-199908000-00032
Hasler, W. L., Grabauskas, G., Singh, P., and Owyang, C. (2022). Mast cell mediation of visceral sensation and permeability in irritable bowel syndrome. Neurogastroenterol. Motil. 10.1111/nmo.14339 [Epub ahead of print]. doi: 10.1111/nmo.14339
Heinken, A., Hertel, J., and Thiele, I. (2021). Metabolic modelling reveals broad changes in gut microbial metabolism in inflammatory bowel disease patients with dysbiosis. NPJ Syst. Biol. Appl. 7:19. doi: 10.1038/s41540-021-00178-6
Heinsen, F.-A., Knecht, H., Neulinger, S. C., Schmitz, R. A., Knecht, C., Kühbacher, T., et al. (2015). Dynamic changes of the luminal and mucosa-associated gut microbiota during and after antibiotic therapy with paromomycin. Gut Microbes 6, 243–254. doi: 10.1080/19490976.2015.1062959
Henrick, B. M., Rodriguez, L., Lakshmikanth, T., Pou, C., Henckel, E., Arzoomand, A., et al. (2021). Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898.e11. doi: 10.1016/j.cell.2021.05.030
Henriques, S. F., Dhakan, D. B., Serra, L., Francisco, A. P., Carvalho-Santos, Z., Baltazar, C., et al. (2020). Metabolic cross-feeding in imbalanced diets allows gut microbes to improve reproduction and alter host behaviour. Nat. Commun. 11:4236. doi: 10.1038/s41467-020-18049-9
Hinnebusch, B. F., Meng, S., Wu, J. T., Archer, S. Y., and Hodin, R. A. (2002). The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 132, 1012–1017. doi: 10.1093/jn/132.5.1012
Hoban, A. E., Moloney, R. D., Golubeva, A. V., McVey Neufeld, K. A., O’Sullivan, O., Patterson, E., et al. (2016). Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience 339, 463–477.
Hockley, J. R. F., Taylor, T. S., Callejo, G., Wilbrey, A. L., Gutteridge, A., Bach, K., et al. (2019). Single-Cell RNAseq reveals seven classes of colonic sensory neuron. Gut 68, 633–644. doi: 10.1136/gutjnl-2017-315631
Hollister, E. B., Oezguen, N., Chumpitazi, B. P., Luna, R. A., Weidler, E. M., Rubio-Gonzales, M., et al. (2019). Leveraging human microbiome features to diagnose and stratify children with irritable bowel syndrome. J. Mol. Diagn. 21, 449–461. doi: 10.1016/j.jmoldx.2019.01.006
Hosseinkhani, F., Heinken, A., Thiele, I., Lindenburg, P. W., Harms, A. C., and Hankemeier, T. (2021). The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes 13, 1–22. doi: 10.1080/19490976.2021.1882927
Houtkooper, R. H., Argmann, C., Houten, S. M., Cantó, C., Jeninga, E. H., Andreux, P. A., et al. (2011). The metabolic footprint of aging in mice. Sci. Rep. 1:134. doi: 10.1038/srep00134
Hoving, L. R., Heijink, M., van Harmelen, V., van Dijk, K. W., and Giera, M. (2018). GC-MS analysis of short-chain fatty acids in Feces, Cecum Content, and Blood Samples. Methods Mol. Biol. 1730, 247–256. doi: 10.1007/978-1-4939-7592-1_17
Hryhorowicz, S., Kaczmarek-Ryś, M., Zieliñska, A., Scott, R. J., Słomski, R., and Pławski, A. (2021). Endocannabinoid system as a promising therapeutic target in inflammatory bowel disease - a systematic review. Front. Immunol. 12:790803. doi: 10.3389/fimmu.2021.790803
Hu, S., Vich Vila, A., Gacesa, R., Collij, V., Stevens, C., Fu, J. M., et al. (2021). Whole exome sequencing analyses reveal gene-microbiota interactions in the context of IBD. Gut 70, 285–296. doi: 10.1136/gutjnl-2019-319706
Hurtado-Lorenzo, A., Honig, G., Weaver, S. A., Larkin, P. B., and Heller, C. (2021). Chronic abdominal pain in IBD research initiative: unraveling biological mechanisms and patient heterogeneity to personalize treatment and improve clinical outcomes. Crohns Colitis 3:otab034.
Ianiro, G., Eusebi, L. H., Black, C. J., Gasbarrini, A., Cammarota, G., and Ford, A. C. (2019). Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 50, 240–248. doi: 10.1111/apt.15330
Iannotti, F. A., and Di Marzo, V. (2021). The gut microbiome, endocannabinoids and metabolic disorders. J. Endocrinol. 248, R83–R97. doi: 10.1530/JOE-20-0444
Imhann, F., Vich Vila, A., Bonder, M. J., Fu, J., Gevers, D., Visschedijk, M. C., et al. (2018). Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 67, 108–119. doi: 10.1136/gutjnl-2016-312135
Iturrospe, E., Da Silva, K. M., Talavera Andújar, B., Cuykx, M., Boeckmans, J., Vanhaecke, T., et al. (2021). An exploratory approach for an oriented development of an untargeted hydrophilic interaction liquid chromatography-mass spectrometry platform for polar metabolites in biological matrices. J. Chromatogr. A 1637:461807. doi: 10.1016/j.chroma.2020.461807
Izzo, A. A., and Sharkey, K. A. (2010). Cannabinoids and the gut: new developments and emerging concepts. Pharmacol. Ther. 126, 21–38. doi: 10.1016/j.pharmthera.2009.12.005
Jacobs, D. M., Deltimple, N., van Velzen, E., van Dorsten, F. A., Bingham, M., Vaughan, E. E., et al. (2008). (1)H NMR metabolite profiling of Feces as a tool to assess the impact of nutrition on the human microbiome. NMR Biomed. 21, 615–626. doi: 10.1002/nbm.1233
Jakubczyk, D., Leszczyñska, K., and Górska, S. (2020). The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)—A Critical Review. Nutrients 12:1973. doi: 10.3390/nu12071973
Jang, C., Chen, L., and Rabinowitz, J. D. (2018). Metabolomics and Isotope Tracing. Cell 173, 822–837. doi: 10.1021/acs.analchem.1c04430
Jia, L., Lee, S., Tierney, J. A., Elmquist, J. K., Burton, M. D., and Gautron, L. (2021). TLR4 signaling selectively and directly promotes cgrp release from vagal afferents in the mouse. eNeuro 8:ENEURO.0254-20.2020. doi: 10.1523/ENEURO.0254-20.2020
Kang, Y., Schneider, B. B., and Covey, T. R. (2017). On the nature of mass spectrometer analyzer contamination. J. Am. Soc. Mass Spectrom. 28, 2384–2392. doi: 10.1007/s13361-017-1747-3
Kaplan, G. G., and Windsor, J. W. (2021). The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 18, 56–66. doi: 10.1038/s41575-020-00360-x
Karu, N., Deng, L., Slae, M., Guo, A. C., Sajed, T., Huynh, H., et al. (2018). A review on human Fecal metabolomics: methods, applications and the human fecal metabolome database. Anal. Chim. Acta 1030, 1–24. doi: 10.1016/j.aca.2018.05.031
Kelly, C. J., Zheng, L., Campbell, E. L., Saeedi, B., Scholz, C. C., Bayless, A. J., et al. (2015). Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17, 662–671. doi: 10.1016/j.chom.2015.03.005
Kelly, C. R., Kahn, S., Kashyap, P., Laine, L., Rubin, D., Atreja, A., et al. (2015). Update on Fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 149, 223–237. doi: 10.1053/j.gastro.2015.05.008
Kennedy, P. J., Cryan, J. F., Dinan, T. G., and Clarke, G. (2017). Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112, 399–412. doi: 10.1016/j.neuropharm.2016.07.002
Keszthelyi, D., Troost, F. J., and Masclee, A. A. (2012). Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G141–G154. doi: 10.1152/ajpgi.00060.2012
Khamis, M. M., Adamko, D. J., and El-Aneed, A. (2017). Mass spectrometric based approaches in urine metabolomics and biomarker discovery. Mass Spectrom. Rev. 36, 115–134. doi: 10.1002/mas.21455
Khan, I., Ullah, N., Zha, L., Bai, Y., Khan, A., Zhao, T., et al. (2019). Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 8:126. doi: 10.3390/pathogens8030126
Kim, C. H., Park, J., and Kim, M. (2014). Gut microbiota-derived short-chain fatty acids, t cells, and inflammation. Immune Netw. 14, 277–288. doi: 10.4110/in.2014.14.6.277
Kim, M., Furuzono, T., Yamakuni, K., Li, Y., Kim, Y.-I., Takahashi, H., et al. (2017). 10-Oxo-12(Z)-Octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, enhances energy metabolism by activation of TRPV1. FASEB J. 31, 5036–5048. doi: 10.1096/fj.201700151R
Kleiner, M. (2019). Metaproteomics: much more than measuring gene expression in microbial communities. mSystems 4:e00115-19. doi: 10.1128/mSystems.00115-19
Klünemann, M., Andrejev, S., Blasche, S., Mateus, A., Phapale, P., Devendran, S., et al. (2021). Bioaccumulation of therapeutic drugs by human gut bacteria. Nature 597, 533–538. doi: 10.1038/s41586-021-03891-8
Kogler, L., Müller, V. I., Seidel, E.-M., Boubela, R., Kalcher, K., Moser, E., et al. (2016). Sex differences in the functional connectivity of the amygdalae in association with cortisol. Neuroimage 134, 410–423. doi: 10.1016/j.neuroimage.2016.03.064
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., and Bäckhed, F. (2016). From Dietary Fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345. doi: 10.1016/j.cell.2016.05.041
Korpela, K., and de Vos, W. M. (2016). Antibiotic use in childhood alters the gut microbiota and predisposes to overweight. Microb. Cell 3, 296–298. doi: 10.15698/mic2016.07.514
Kozik, A. J., Nakatsu, C. H., Chun, H., and Jones-Hall, Y. L. (2019). Comparison of the Fecal, Cecal, and Mucus Microbiome in Male and Female Mice after TNBS-Induced Colitis. PLoS One 14:e0225079. doi: 10.1371/journal.pone.0225079
Kuno, T., Hirayama-Kurogi, M., Ito, S., and Ohtsuki, S. (2018). Reduction in hepatic secondary bile acids caused by short-term antibiotic-induced dysbiosis decreases mouse serum glucose and triglyceride levels. Sci. Rep. 8:1253. doi: 10.1038/s41598-018-19545-1
Labus, J. S., Mayer, E. A., Jarcho, J., Kilpatrick, L. A., Kilkens, T. O. C., Evers, E. A. T., et al. (2011). Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut 60, 1196–1203. doi: 10.1136/gut.2010.213447
Lacy, B. E., Mearin, F., Chang, L., Chey, W. D., Lembo, A. J., Simren, M., et al. (2016). Bowel Disorders. Gastroenterology 150, 1393–1407.e5.
Lagomarsino, V. N., Kostic, A. D., and Chiu, I. M. (2021). Mechanisms of microbial-neuronal interactions in pain and nociception. Neurobiol. Pain 9:100056. doi: 10.1016/j.ynpai.2020.100056
Lavelle, A., and Sokol, H. (2020). Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17, 223–237. doi: 10.1038/s41575-019-0258-z
Le Chatelier, E., Nielsen, T., Qin, J., Prifti, E., Hildebrand, F., Falony, G., et al. (2013). Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546. doi: 10.1038/nature12506
Ledergerber, M., Lang, B. M., Heinrich, H., Biedermann, L., Begré, S., Zeitz, J., et al. (2021). Abdominal pain in patients with inflammatory bowel disease: association with single-nucleotide polymorphisms prevalent in irritable bowel syndrome and clinical management. BMC Gastroenterol. 21:53. doi: 10.1186/s12876-021-01622-x
Lee, C.-K., Jeong, S.-H., Jang, C., Bae, H., Kim, Y. H., Park, I., et al. (2019). Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science 363, 644–649. doi: 10.1126/science.aav0173
Legan, T. B., Lavoie, B., and Mawe, G. M. (2022). Direct and indirect mechanisms by which the gut microbiota influence host serotonin systems. Neurogastroenterol. Motil. [Epub ahead of print]. doi: 10.1111/nmo.14346
Lembo, A., Pimentel, M., Rao, S. S., Schoenfeld, P., Cash, B., Weinstock, L. B., et al. (2016). Repeat treatment with rifaximin is safe and effective in patients with Diarrhea-predominant irritable bowel syndrome. Gastroenterology 151, 1113–1121. doi: 10.1053/j.gastro.2016.08.003
Levy, A. N., and Allegretti, J. R. (2019). Insights into the role of fecal microbiota transplantation for the treatment of inflammatory bowel disease. Ther. Adv. Gastroenterol. 12:1756284819836893. doi: 10.1177/1756284819836893
Lewis, I. A., Schommer, S. C., Hodis, B., Robb, K. A., Tonelli, M., Westler, W. M., et al. (2007). Method for determining molar concentrations of metabolites in complex solutions from two-Dimensional 1H-13C NMR Spectra. Anal. Chem. 79, 9385–9390. doi: 10.1021/ac071583z
Lewis, I. A., Shortreed, M. R., Hegeman, A. D., and Markley, J. L. (2012). “Novel NMR and MS approaches to metabolomics,” in The Handbook of Metabolomics, eds T.W.-M. Fan, A. N. Lane, and R. M. Higashi (Totowa, NJ: Humana Press), 199–230.
Li, W.-T., Luo, Q.-Q., Wang, B., Chen, X., Yan, X.-J., Qiu, H.-Y., et al. (2019). Bile acids induce visceral hypersensitivity via mucosal mast cell-to-Nociceptor Signaling That Involves the Farnesoid X receptor/nerve growth factor/transient receptor potential vanilloid 1 Axis. FASEB J. 33, 2435–2450. doi: 10.1096/fj.201800935RR
Liu, J., Sun, J., Wang, F., Yu, X., Ling, Z., Li, H., et al. (2015). Neuroprotective effects of Clostridium butyricum against Vascular Dementia in Mice via Metabolic Butyrate. Biomed Res. Int. 2015, 412946. doi: 10.1155/2015/412946
Liu, Y., Li, W., Yang, H., Zhang, X., Wang, W., Jia, S., et al. (2021). Leveraging 16S RRNA microbiome sequencing data to identify bacterial signatures for irritable bowel syndrome. Front. Cell. Infect. Microbiol. 11:645951. doi: 10.3389/fcimb.2021.645951
Lloyd-Price, J., Arze, C., Ananthakrishnan, A. N., Schirmer, M., Avila-Pacheco, J., Poon, T. W., et al. (2019). Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662. doi: 10.1038/s41586-019-1237-9
Lomax, A. E., Pradhananga, S., Sessenwein, J. L., and O’Malley, D. (2019). Bacterial modulation of visceral sensation: mediators and mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G363–G372. doi: 10.1152/ajpgi.00052.2019
Lopez-Siles, M., Duncan, S. H., Garcia-Gil, L. J., and Martinez-Medina, M. (2017). Faecalibacterium Prausnitzii: from microbiology to diagnostics and prognostics. ISME J. 11, 841–852. doi: 10.1038/ismej.2016.176
Lu, W., Su, X., Klein, M. S., Lewis, I. A., Fiehn, O., and Rabinowitz, J. D. (2017). Metabolite measurement: pitfalls to avoid and practices to follow. Annu. Rev. Biochem. 86, 277–304. doi: 10.1146/annurev-biochem-061516-044952
Lucarini, E., Di Pilato, V., Parisio, C., Micheli, L., Toti, A., Pacini, A., et al. (2022). Visceral sensitivity modulation by faecal microbiota transplantation: the active role of gut bacteria in pain persistence. Pain 163, 861–877. doi: 10.1097/j.pain.0000000000002438
Luczynski, P., McVey Neufeld, K.-A., Oriach, C. S., Clarke, G., Dinan, T. G., and Cryan, J. F. (2016). Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int. J. Neuropsychopharmacol. 19:pyw020. doi: 10.1093/ijnp/pyw020
Lupton, J. R., and Kurtz, P. P. (1993). Relationship of colonic luminal short-chain fatty acids and PH to in vivo cell proliferation in rats. J. Nutr. 123, 1522–1530. doi: 10.1093/jn/123.9.1522
Ma, C., Congly, S. E., Novak, K. L., Belletrutti, P. J., Raman, M., Woo, M., et al. (2021). Epidemiologic burden and treatment of chronic symptomatic functional bowel disorders in the united states: a nationwide analysis. Gastroenterology 160, 88–98.e4. doi: 10.1053/j.gastro.2020.09.041
Maëva, M., Elodie, B., Nathalie, R., Manon, D., Marjolène, S., Valentine, D., et al. (2022). AhR/IL-22 pathway as new target for the treatment of post-infectious irritable bowel syndrome symptoms. Gut Microbes 14:2022997. doi: 10.1080/19490976.2021.2022997
Mager, L. F., Burkhard, R., Pett, N., Cooke, N. C. A., Brown, K., Ramay, H., et al. (2020). Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369, 1481–1489. doi: 10.1126/science.abc3421
Major, G., Pritchard, S., Murray, K., Alappadan, J. P., Hoad, C. L., Marciani, L., et al. (2017). Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology 152, 124–133.e2. doi: 10.1053/j.gastro.2016.09.062
Mapplebeck, J. C. S., Beggs, S., and Salter, M. W. (2017). Molecules in pain and sex: a developing story. Mol. Brain 10:9. doi: 10.1186/s13041-017-0289-8
Marchioro, L., Shokry, E., Geraghty, A. A., O’Brien, E. C., Uhl, O., Koletzko, B., et al. (2019). Caesarean section, but not induction of labour, is associated with major changes in cord blood metabolome. Sci. Rep. 9:17562. doi: 10.1038/s41598-019-53810-1
Markley, J. L., Brüschweiler, R., Edison, A. S., Eghbalnia, H. R., Powers, R., Raftery, D., et al. (2017). The Future of NMR-Based Metabolomics. Curr. Opin. Biotechnol. 43, 34–40.
Mars, R. A. T., Yang, Y., Ward, T., Houtti, M., Priya, S., Lekatz, H. R., et al. (2020). Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell 182, 1460–1473.e7.
Martin, A. M., Sun, E. W., Rogers, G. B., and Keating, D. J. (2019). The influence of the gut microbiome on host metabolism through the regulation of gut hormone release. Front. Physiol. 10:428. doi: 10.3389/fphys.2019.00428
Mawe, G. M., and Hoffman, J. M. (2013). Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 10, 473–486. doi: 10.1038/nrgastro.2013.105
McGarrah, R. W., Crown, S. B., Zhang, G.-F., Shah, S. H., and Newgard, C. B. (2018). Cardiovascular metabolomics. Circ. Res. 122, 1238–1258. doi: 10.2217/rme-2018-0104
McIntosh, K., Reed, D. E., Schneider, T., Dang, F., Keshteli, A. H., De Palma, G., et al. (2017). FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut 66, 1241–1251.
Milani, C., Duranti, S., Bottacini, F., Casey, E., Turroni, F., Mahony, J., et al. (2017). The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 81:e00036-17. doi: 10.1128/MMBR.00036-17
Minerbi, A., Gonzalez, E., Brereton, N. J. B., Anjarkouchian, A., Dewar, K., Fitzcharles, M.-A., et al. (2019). Altered microbiome composition in individuals with fibromyalgia. Pain 160, 2589–2602. doi: 10.1097/j.pain.0000000000001640
Miyauchi, E., Taida, T., Kawasumi, M., Ohkusa, T., Sato, N., and Ohno, H. (2022). Analysis of colonic mucosa-associated microbiota using endoscopically collected lavage. Sci. Rep. 12:1758. doi: 10.1038/s41598-022-05936-y
Moloney, R. D., Johnson, A. C., O’Mahony, S. M., Dinan, T. G., Greenwood-Van Meerveld, B., and Cryan, J. F. (2016). Stress and the microbiota-gut-brain axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci. Ther. 22, 102–117. doi: 10.1111/cns.12490
Morgan, X. C., Tickle, T. L., Sokol, H., Gevers, D., Devaney, K. L., Ward, D. V., et al. (2012). Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 13:R79. doi: 10.1186/gb-2012-13-9-r79
Morgell, A., Reisz, J. A., Ateeb, Z., Davanian, H., Reinsbach, S. E., Halimi, A., et al. (2021). Metabolic characterization of plasma and cyst fluid from cystic precursors to pancreatic cancer patients reveal metabolic signatures of bacterial infection. J. Proteome Res. 20, 2725–2738.
Mulak, A., and Bonaz, B. (2015). Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 21, 10609–10620.
Nasser, Y., Woo, M., and Andrews, C. N. (2020). Cannabis in gastroenterology: watch your head! a review of use in inflammatory bowel disease, functional gut disorders, and gut-related adverse effects. Curr. Treat. Options Gastroenterol. 18, 519–530. doi: 10.1007/s11938-020-00323-w
Neis, E. P. J. G., Dejong, C. H. C., and Rensen, S. S. (2015). The role of microbial amino acid metabolism in host metabolism. Nutrients 7, 2930–2946. doi: 10.3390/nu7042930
Nguyen, P. T., and Yarov-Yarovoy, V. (2022). Towards structure-guided development of pain therapeutics targeting voltage-gated sodium channels. Front. Pharmacol. 13:842032. doi: 10.3389/fphar.2022.842032
Ní Dhonnabháín, R., Xiao, Q., and O’Malley, D. (2021). Aberrant gut-to-brain signaling in irritable bowel syndrome - the role of bile acids. Front. Endocrinol. 12:745190. doi: 10.3389/fendo.2021.745190
Nøhr, M. K., Egerod, K. L., Christiansen, S. H., Gille, A., Offermanns, S., Schwartz, T. W., et al. (2015). Expression of the short chain fatty acid receptor gpr41/ffar3 in autonomic and somatic sensory ganglia. Neuroscience 290, 126–137. doi: 10.1016/j.neuroscience.2015.01.040
Noor-Mohammadi, E., Ligon, C. O., Mackenzie, K., Stratton, J., Shnider, S., and Greenwood-Van Meerveld, B. (2021). A monoclonal anti–calcitonin gene-related peptide antibody decreases stress-induced colonic hypersensitivity. J. Pharmacol. Exp. Ther. 379, 270–279. doi: 10.1124/jpet.121.000731
O’Donnell, P. M., Aviles, H., Lyte, M., and Sonnenfeld, G. (2006). Enhancement of in vitro growth of pathogenic bacteria by norepinephrine: importance of inoculum density and role of transferrin. Appl. Environ. Microbiol. 72, 5097–5099. doi: 10.1128/AEM.00075-06
O’Mahony, S. M., Felice, V. D., Nally, K., Savignac, H. M., Claesson, M. J., Scully, P., et al. (2014). Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 277, 885–901. doi: 10.1016/j.neuroscience.2014.07.054
Painsipp, E., Herzog, H., and Holzer, P. (2009). The gut-mood axis: a novel role of the gut hormone peptide YY on emotional-affective behaviour in mice. BMC Pharmacol. 9, (Suppl. 2):A13.
Parada Venegas, D., De la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., et al. (2019). Short Chain Fatty Acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10:277. doi: 10.3389/fimmu.2019.00277
Paula, H., Grover, M., Halder, S. L., Locke, G. R. III, Schleck, C. D., Zinsmeister, A. R., et al. (2015). Non-enteric infections, antibiotic use, and risk of development of functional gastrointestinal disorders. Neurogastroenterol. Motil. 27, 1580–1586. doi: 10.1111/nmo.12655
Pellissier, S., Dantzer, C., Canini, F., Mathieu, N., and Bonaz, B. (2010). Psychological adjustment and autonomic disturbances in inflammatory bowel diseases and irritable bowel syndrome. Psychoneuroendocrinology 35, 653–662. doi: 10.1016/j.psyneuen.2009.10.004
Peoples, J. N. R., Maxmillian, T., Le, Q., Nadtochiy, S. M., Brookes, P. S., Porter, G. A., et al. (2018). Metabolomics reveals critical adrenergic regulatory checkpoints in glycolysis and pentose–phosphate pathways in embryonic heart. J. Biol. Chem. 293, 6925–6941. doi: 10.1074/jbc.RA118.002566
Peterson, D. A., Frank, D. N., Pace, N. R., and Gordon, J. I. (2008). Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe 3, 417–427. doi: 10.1016/j.chom.2008.05.001
Phan, M., Momin, S. R., Senn, M. K., and Wood, A. C. (2019). Metabolomic insights into the effects of breast milk versus formula milk feeding in infants. Curr. Nutr. Rep. 8, 295–306. doi: 10.1007/s13668-019-00284-2
Pimentel, M., and Lembo, A. (2020). Microbiome and its role in irritable bowel syndrome. Dig. Dis. Sci. 65, 829–839.
Pimentel, M., Lembo, A., Chey, W. D., Zakko, S., Ringel, Y., Yu, J., et al. (2011). Rifaximin therapy for patients with irritable bowel syndrome without constipation. N. Engl. J. Med. 364, 22–32.
Pinto-Sanchez, M. I., Hall, G. B., Ghajar, K., Nardelli, A., Bolino, C., Lau, J. T., et al. (2017). Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 153, 448–459.e8. doi: 10.1053/j.gastro.2017.05.003
Piovani, D., Danese, S., Peyrin-Biroulet, L., and Bonovas, S. (2020). Environmental, nutritional, and socioeconomic determinants of IBD incidence: a global ecological study. J. Crohns Colitis 14, 323–331. doi: 10.1093/ecco-jcc/jjz150
Pittayanon, R., Lau, J. T., Yuan, Y., Leontiadis, G. I., Tse, F., Surette, M., et al. (2019). Gut microbiota in patients with irritable bowel syndrome-A systematic review. Gastroenterology 157, 97–108.
Plourde, V., St-Pierre, S., and Quirion, R. (1997). Calcitonin gene-related peptide in viscerosensitive response to colorectal distension in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 273, G191–G196. doi: 10.1152/ajpgi.1997.273.1.G191
Pokusaeva, K., Johnson, C., Luk, B., Uribe, G., Fu, Y., Oezguen, N., et al. (2017). GABA-Producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 29:e12904. doi: 10.1111/nmo.12904
Prince, A. C., Myers, C. E., Joyce, T., Irving, P., Lomer, M., and Whelan, K. (2016). Fermentable carbohydrate restriction (Low FODMAP Diet) in clinical practice improves functional gastrointestinal symptoms in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 22, 1129–1136. doi: 10.1097/MIB.0000000000000708
Psichas, A., Sleeth, M. L., Murphy, K. G., Brooks, L., Bewick, G. A., Hanyaloglu, A. C., et al. (2015). The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in Rodents. Int. J. Obes. 39, 424–429. doi: 10.1038/ijo.2014.153
Putnam, E. E., and Goodman, A. L. (2020). B vitamin acquisition by gut commensal Bacteria. PLoS Pathog. 16:e1008208. doi: 10.1371/journal.ppat.1008208
Qiu, P., Ishimoto, T., Fu, L., Zhang, J., Zhang, Z., and Liu, Y. (2022). The gut microbiota in inflammatory bowel disease. Front. Cell. Infect. Microbiol. 12:733992. doi: 10.3389/fcimb.2022.733992
Quigley, E. M. M. (2017). Microbiota-brain-gut axis and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 17:94. doi: 10.1007/s11910-017-0802-6
Quintero, M. E., Pontes, J. G. M., and Tasic, L. (2021). Metabolomics in degenerative brain diseases. Brain Res. 1773:147704. doi: 10.1016/j.brainres.2021.147704
Raja, G., Gupta, H., Gebru, Y. A., Youn, G. S., Choi, Y. R., Kim, H. S., et al. (2021). Recent advances of microbiome-associated metabolomics profiling in liver disease: principles, mechanisms, and applications. Int. J. Mol. Sci. 22:1160. doi: 10.3390/ijms22031160
Rakoff-Nahoum, S., and Medzhitov, R. (2006). Role of the innate immune system and host-commensal mutualism. Curr. Top. Microbiol. Immunol. 308, 1–18. doi: 10.1007/3-540-30657-9_1
Rea, K., O’ Mahony, S. M., and Cryan, J. F. (2021). High and Mighty? Cannabinoids and the microbiome in pain. Neurobiol. Pain 9:100061. doi: 10.1016/j.ynpai.2021.100061
Reade, S., Mayor, A., Aggio, R., Khalid, T., Pritchard, D. M., Ewer, A. K., et al. (2014). Optimisation of sample preparation for direct SPME-GC-MS analysis of murine and human faecal volatile organic compounds for metabolomic Studies. J. Anal. Bioanal. Tech. 5:184.
Regueiro, M., Greer, J. B., and Szigethy, E. (2017). Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology 152, 430–439.e4. doi: 10.1053/j.gastro.2016.10.036
Reigstad, C. S., Salmonson, C. E., Rainey, J. F. III, Szurszewski, J. H., Linden, D. R., Sonnenburg, J. L., et al. (2015). Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on Enterochromaffin cells. FASEB J. 29, 1395–1403. doi: 10.1096/fj.14-259598
Reimann, F., and Gribble, F. M. (2016). Mechanisms underlying glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 Secretion. J. Diabetes Investig. 7, (Suppl. 1), 13–19. doi: 10.1111/jdi.12478
Ridlon, J. M., Kang, D. J., Hylemon, P. B., and Bajaj, J. S. (2014). Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 30, 332–338.
Rivera-Chávez, F., Zhang, L. F., Faber, F., Lopez, C. A., Byndloss, M. X., Olsan, E. E., et al. (2016). Depletion of butyrate-producing clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host Microbe 19, 443–454. doi: 10.1016/j.chom.2016.03.004
Roque, W., and Romero, F. (2021). Cellular metabolomics of pulmonary fibrosis, from amino acids to lipids. Am. J. Physiol. Cell Physiol. 320, C689–C695. doi: 10.1152/ajpcell.00586.2020
Rossi, M., Aggio, R., Staudacher, H. M., Lomer, M. C., Lindsay, J. O., Irving, P., et al. (2018). Volatile organic compounds in Feces associate with response to dietary intervention in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 16, 385–391.e1. doi: 10.1016/j.cgh.2017.09.055
Rousseaux, C., Thuru, X., Gelot, A., Barnich, N., Neut, C., Dubuquoy, L., et al. (2007). Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 13, 35–37. doi: 10.1038/nm1521
Russo, R., De Caro, C., Avagliano, C., Cristiano, C., La Rana, G., Mattace Raso, G., et al. (2016). Sodium butyrate and its synthetic amide derivative modulate nociceptive behaviors in mice. Pharmacol. Res. 103, 279–291. doi: 10.1016/j.phrs.2015.11.026
Sarkar, A., Lehto, S. M., Harty, S., Dinan, T. G., Cryan, J. F., and Burnet, P. W. J. (2016). Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 39, 763–781. doi: 10.1016/j.tins.2016.09.002
Scaldaferri, F., Gerardi, V., Lopetuso, L. R., Del Zompo, F., Mangiola, F., Boškoski, I., et al. (2013). Gut microbial flora, prebiotics, and probiotics in IBD: their current usage and utility. Biomed Res. Int. 2013:435268. doi: 10.1155/2013/435268
Schirmer, M., Garner, A., Vlamakis, H., and Xavier, R. J. (2019). Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 17, 497–511. doi: 10.1038/s41579-019-0213-6
Schnorr, S. L., Candela, M., Rampelli, S., Centanni, M., Consolandi, C., Basaglia, G., et al. (2014). Gut Microbiome of the Hadza Hunter-Gatherers. Nat. Commun. 5:3654.
Seregin, S. S., Golovchenko, N., Schaf, B., Chen, J., Pudlo, N. A., Mitchell, J., et al. (2017). NLRP6 Protects Il10(-/-) Mice from Colitis by Limiting Colonization of Akkermansia muciniphila. Cell Rep. 19:2174.
Sessenwein, J. L., Baker, C. C., Pradhananga, S., Maitland, M. E., Petrof, E. O., Allen-Vercoe, E., et al. (2017). Protease-mediated suppression of DRG neuron excitability by commensal bacteria. J. Neurosci. 37, 11758–11768. doi: 10.1523/JNEUROSCI.1672-17.2017
Shah, E. D., Chang, L., Salwen-Deremer, J. K., Gibson, P. R., Keefer, L., Muir, J. G., et al. (2021). Contrasting clinician and insurer perspectives to managing irritable bowel syndrome: multilevel modeling analysis. Am. J. Gastroenterol. 116, 748–757. doi: 10.14309/ajg.0000000000000989
Shao, Y., Forster, S. C., Tsaliki, E., Vervier, K., Strang, A., Simpson, N., et al. (2019). Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 574, 117–121. doi: 10.1038/s41586-019-1560-1
Sharon, G., Cruz, N. J., Kang, D.-W., Gandal, M. J., Wang, B., Kim, Y.-M., et al. (2019). Human Gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 177, 1600–1618.e17. doi: 10.1016/j.cell.2019.05.004
Sisignano, M., Angioni, C., Park, C.-K., Meyer Dos Santos, S., Jordan, H., Kuzikov, M., et al. (2016). Targeting CYP2J to reduce paclitaxel-induced peripheral neuropathic pain. Proc. Natl. Acad. Sci. U.S.A. 113, 12544–12549. doi: 10.1073/pnas.1613246113
Smith, P. M., Howitt, M. R., Panikov, N., Michaud, M., Gallini, C. A., Bohlooly-Y, M., et al. (2013). The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science 341, 569–573. doi: 10.1126/science.1241165
Sonnenburg, E. D., Smits, S. A., Tikhonov, M., Higginbottom, S. K., Wingreen, N. S., and Sonnenburg, J. L. (2016). Diet-induced extinctions in the gut microbiota compound over generations. Nature 529, 212–215. doi: 10.1038/nature16504
Staudacher, H. M., Rossi, M., Kaminski, T., Dimidi, E., Ralph, F. S. E., Wilson, B., et al. (2022). Long-Term Personalized Low FODMAP Diet Improves Symptoms and Maintains Luminal Bifidobacteria Abundance in Irritable Bowel Syndrome. Neurogastroenterol. Motil. 34:e14241. doi: 10.1111/nmo.14241
Storr, M., Emmerdinger, D., Diegelmann, J., Pfennig, S., Ochsenkühn, T., Göke, B., et al. (2010). The Cannabinoid 1 Receptor (CNR1) 1359 G/A polymorphism modulates susceptibility to ulcerative colitis and the phenotype in Crohn’s Disease. PLoS One 5:e9453. doi: 10.1371/journal.pone.0009453
Storr, M., Emmerdinger, D., Diegelmann, J., Yüce, B., Pfennig, S., Ochsenkühn, T., et al. (2009). The role of fatty acid hydrolase gene variants in inflammatory bowel disease. Aliment. Pharmacol. Ther. 29, 542–551. doi: 10.1111/j.1365-2036.2008.03910.x
Strisciuglio, C., Bellini, G., Miele, E., Martinelli, M., Cenni, S., Tortora, C., et al. (2018). Cannabinoid Receptor 2 functional variant contributes to the risk for pediatric inflammatory bowel disease. J. Clin. Gastroenterol. 52, e37–e43. doi: 10.1097/MCG.0000000000000755
Sturkenboom, R., Keszthelyi, D., Masclee, A. A. M., and Essers, B. A. B. (2022). Discrete choice experiment reveals strong preference for dietary treatment among patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. [Epub ahead of print]. doi: 10.1016/j.cgh.2022.02.016
Sultan, S., El-Mowafy, M., Elgaml, A., Ahmed, T. A. E., Hassan, H., and Mottawea, W. (2021). Metabolic influences of gut microbiota dysbiosis on inflammatory bowel disease. Front. Physiol. 12:715506. doi: 10.3389/fphys.2021.715506
Tang, Z.-Z., Chen, G., Hong, Q., Huang, S., Smith, H. M., Shah, R. D., et al. (2019). Multi-Omic analysis of the microbiome and metabolome in healthy subjects reveals microbiome-dependent relationships between diet and metabolites. Front. Genet. 10:454. doi: 10.3389/fgene.2019.00454
Tarrerias, A. L., Millecamps, M., Alloui, A., Beaughard, C., Kemeny, J. L., Bourdu, S., et al. (2002). Short-chain fatty acid enemas fail to decrease colonic hypersensitivity and inflammation in TNBS-induced colonic inflammation in rats. Pain 100, 91–97. doi: 10.1016/s0304-3959(02)00234-8
Taylor, M. R., Flannigan, K. L., Rahim, H., Mohamud, A., Lewis, I. A., Hirota, S. A., et al. (2019). Vancomycin relieves mycophenolate mofetil-induced gastrointestinal toxicity by eliminating gut bacterial β-glucuronidase activity. Sci. Adv. 5:eaax2358. doi: 10.1126/sciadv.aax2358
Theriot, C. M., Koenigsknecht, M. J., Carlson, P. E. J., Hatton, G. E., Nelson, A. M., Li, B., et al. (2014). Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile Infection. Nat. Commun. 5:3114. doi: 10.1038/ncomms4114
Thursby, E., and Juge, N. (2017). Introduction to the human gut microbiota. Biochem. J. 474, 1823–1836.
Tong, M., Li, X., Wegener Parfrey, L., Roth, B., Ippoliti, A., Wei, B., et al. (2013). A Modular organization of the human intestinal mucosal microbiota and its association with inflammatory bowel disease. PLoS One 8:e80702. doi: 10.1371/journal.pone.0080702
Topping, D. L., and Clifton, P. M. (2001). Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81, 1031–1064. doi: 10.1152/physrev.2001.81.3.1031
Tramullas, M., Collins, J. M., Fitzgerald, P., Dinan, T. G., O’ Mahony, S. M., and Cryan, J. F. (2021). Estrous cycle and ovariectomy-induced changes in visceral pain are microbiota-dependent. iScience 24:102850. doi: 10.1016/j.isci.2021.102850
Tsavkelova, E. A., Botvinko, I. V., Kudrin, V. S., and Oleskin, A. V. (2000). Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl. Biochem. 372, 115–117.
Vanhoutvin, S. A. L. W., Troost, F. J., Kilkens, T. O. C., Lindsey, P. J., Hamer, H. M., Jonkers, D. M. A. E., et al. (2009). The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol. Motil. 21:952-e76. doi: 10.1111/j.1365-2982.2009.01324.x
Verdú, E. F., Bercik, P., Verma-Gandhu, M., Huang, X.-X., Blennerhassett, P., Jackson, W., et al. (2006). Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 55, 182–190. doi: 10.1136/gut.2005.066100
Vicentini, F. A., Keenan, C. M., Wallace, L. E., Woods, C., Cavin, J.-B., Flockton, A. R., et al. (2021). Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome 9:210. doi: 10.1186/s40168-021-01165-z
Villas-Bôas, S. G., Noel, S., Lane, G. A., Attwood, G., and Cookson, A. (2006). Extracellular metabolomics: a metabolic footprinting approach to assess fiber degradation in complex media. Anal. Biochem. 349, 297–305. doi: 10.1016/j.ab.2005.11.019
Vollert, J., Wang, R., Regis, S., Yetman, H., Lembo, A. J., Kaptchuk, T. J., et al. (2022). Genotypes of pain and analgesia in a randomized trial of irritable bowel syndrome. Front. Psychiatry 13:842030. doi: 10.3389/fpsyt.2022.842030
Wadhwa, A., Camilleri, M., and Grover, M. (2015). New and investigational agents for irritable bowel syndrome. Curr. Gastroenterol. Rep. 17:46. doi: 10.1007/s11894-015-0473-x
Wahlström, A., Sayin, S. I., Marschall, H.-U., and Bäckhed, F. (2016). Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50. doi: 10.1016/j.cmet.2016.05.005
Wang, J., Ji, H., Wang, S., Liu, H., Zhang, W., Zhang, D., et al. (2018). Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 9:1953. doi: 10.3389/fmicb.2018.01953
Wang, K., Liao, M., Zhou, N., Bao, L., Ma, K., Zheng, Z., et al. (2019). Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 26, 222–235.e5. doi: 10.1016/j.celrep.2018.12.028
Wikoff, W. R., Anfora, A. T., Liu, J., Schultz, P. G., Lesley, S. A., Peters, E. C., et al. (2009). Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U.S.A. 106, 3698–3703. doi: 10.1073/pnas.0812874106
Winston, J. H., Xu, G.-Y., and Sarna, S. K. (2010). Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology 138, 294–304.e3. doi: 10.1053/j.gastro.2009.09.054
Wu, L., Tang, Z., Chen, H., Ren, Z., Ding, Q., Liang, K., et al. (2021). Mutual interaction between gut microbiota and protein/amino acid metabolism for host mucosal immunity and health. Anim. Nutr. 7, 11–16. doi: 10.1016/j.aninu.2020.11.003
Wu, T., Li, H., Su, C., Xu, F., Yang, G., Sun, K., et al. (2020). Microbiota-derived short-chain fatty acids promote LAMTOR2-mediated immune responses in macrophages. mSystems 5:e00587-20. doi: 10.1128/mSystems.00587-20
Xu, D., Wu, X., Grabauskas, G., and Owyang, C. (2013). Butyrate-induced colonic hypersensitivity is mediated by mitogen-activated protein kinase activation in rat dorsal root Ganglia. Gut 62, 1466–1474. doi: 10.1136/gutjnl-2012-302260
Yang, N. J., Neel, D. V., Deng, L., Heyang, M., Kennedy-Curran, A., Tong, V. S., et al. (2021). Nociceptive Sensory Neurons Mediate Inflammation Induced by Bacillus Anthracis Edema Toxin. Front. Immunol. 12:642373. doi: 10.3389/fimmu.2021.642373
Yang, W., and Cong, Y. (2021). Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 18, 866–877. doi: 10.1038/s41423-021-00661-4
Yu, Y., Villalobos-Hernandez, E. C., Pradhananga, S., Baker, C. C., Keating, C., Grundy, D., et al. (2019). Deoxycholic acid activates colonic afferent nerves via 5-HT(3) receptor-dependent and -independent mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G275–G284. doi: 10.1152/ajpgi.00016.2019
Yunes, R. A., Poluektova, E. U., Dyachkova, M. S., Klimina, K. M., Kovtun, A. S., Averina, O. V., et al. (2016). GABA production and structure of GadB/GadC Genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 42, 197–204. doi: 10.1016/j.anaerobe.2016.10.011
Zaiss, M. M., Rapin, A., Lebon, L., Dubey, L. K., Mosconi, I., Sarter, K., et al. (2015). The intestinal microbiota contributes to the ability of helminths to modulate allergic inflammation. Immunity 43, 998–1010. doi: 10.1016/j.immuni.2015.09.012
Zarrinpar, A., Chaix, A., Xu, Z. Z., Chang, M. W., Marotz, C. A., Saghatelian, A., et al. (2018). Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat. Commun. 9:2872. doi: 10.1038/s41467-018-05336-9
Zhang, S., Wang, H., and Zhu, M.-J. (2019). A Sensitive GC/MS Detection Method for Analyzing microbial metabolites short chain fatty acids in Fecal and Serum Samples. Talanta 196, 249–254. doi: 10.1016/j.talanta.2018.12.049
Zhang, S.-C., Wang, W.-L., Su, P.-J., Jiang, K.-L., and Yuan, Z.-W. (2014). Decreased enteric fatty acid amide hydrolase activity is associated with colonic inertia in slow transit constipation. J. Gastroenterol. Hepatol. 29, 276–283. doi: 10.1111/jgh.12346
Zhang, Y., Limaye, P. B., Renaud, H. J., and Klaassen, C. D. (2014). Effect of various antibiotics on modulation of intestinal microbiota and bile acid profile in mice. Toxicol. Appl. Pharmacol. 277, 138–145. doi: 10.1016/j.taap.2014.03.009
Keywords: visceral pain, inflammatory bowel disease, irritable bowel syndrome, microbiome, metabolomics
Citation: Shute A, Bihan DG, Lewis IA and Nasser Y (2022) Metabolomics: The Key to Unraveling the Role of the Microbiome in Visceral Pain Neurotransmission. Front. Neurosci. 16:917197. doi: 10.3389/fnins.2022.917197
Received: 10 April 2022; Accepted: 30 May 2022;
Published: 23 June 2022.
Edited by:
Karen-Anne McVey Neufeld, McMaster University, CanadaReviewed by:
Roberta Imperatore, University of Sannio, ItalyCopyright © 2022 Shute, Bihan, Lewis and Nasser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasmin Nasser, eW5hc3NlckB1Y2FsZ2FyeS5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.