
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci. , 30 June 2022
Sec. Neurodevelopment
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.894955
This article is part of the Research Topic Changes in Metabolic Processes Affecting Brain Development View all 5 articles
The mechanisms by which various pathogens cause congenital infections have been studied extensively, aiding in the understanding of the detrimental effects these infections can have on fetal/neonatal neurological development. Recent studies have focused on the gut-brain axis as pivotal in neurodevelopment, with congenital infections causing substantial disruptions. There remains controversy surrounding the purported sterility of the placenta as well as concerns regarding the effects of exposure to antibiotics used during pregnancy on neonatal microbiome development and how early exposure to microbes or antibiotics can shape the gut-brain axis. Long-term neurodevelopmental consequences, such as autism spectrum disorder, attention deficit hyperactivity disorder, and cerebral palsy, may be attributable, in part, to early life infection and changes in the immature gut microbiome. The goal of this review is thus to critically evaluate the current evidence related to early life infection affecting neurodevelopment through the gut-brain axis.
Congenital infections are a group of diseases that are present from the fetal into the early postnatal stage of life, which have been found to have adverse effects on metabolic, microbiome, and neurological development in the neonate (Surtees et al., 1990; Mechtler and Kinkel, 1993; Terrazzan Nutricionist et al., 2020). TORCH is a common acronym used to refer to a set of these infections: (T)oxoplasmosis, (O)ther agents such as syphilis and parvovirus B19, Rubella, (C)ytomegalovirus infection (CMV), and (H)erpes simplex virus (HSV) (Macedo-da-Silva et al., 2020; Jaan and Rajnik, 2022). In addition, recent studies have been conducted to understand the effects of other viruses that cause congenital infection—such as Zika virus (ZIKV) and the novel SARS-CoV-2 coronavirus (virus causing COVID-19)—on fetal development (Hoffman et al., 2021; Nunez et al., 2021). While the pathophysiology of the congenital infections has been investigated at length, recent developments in metabolomics and cellular physiology have begun to elucidate metabolic adaptations and derangements in the fetus that accompany these infections.
Another form of fetal infection, which usually occurs around the time of delivery or in the setting of premature, pre-labor rupture of membranes (PPROM) is called chorioamnionitis, also known as intraamniotic infection or inflammation (III) (Cappelletti et al., 2020). Bacteria ascend the female genital tract and infect the membranes surrounding the fetus as well as amniotic fluid, resulting in a pro-inflammatory state in the fetus (Kemp, 2014). III is a risk factor for adverse long-term neurodevelopmental outcomes including cerebral palsy, although the mechanisms leading to neurocognitive dysfunction remain incompletely understood (Huleihel et al., 2004; Elovitz et al., 2011; Brown et al., 2017).
Recently, the role of the gut-brain axis has come to light as affecting myriad functions important for normal neonatal health and development, including cognitive development (Griffiths and Mazmanian, 2018). Furthermore, although not frequently thought about in clinical practice, antibiotic exposure in utero likely has effects on neonatal microbiome development, which in turn could influence brain development (Grech et al., 2021). There is controversy surrounding the sterility vs. colonization of the placenta and fetus, with the classic teaching that the fetal gut and placenta are sterile, but some emerging evidence refuting this (Aagaard et al., 2014; Briana et al., 2021; Kennedy et al., 2021). Such colonization could have far-reaching implications for fetal development, as the developing gut microbiome has been found to be important for the manifestation of chronic diseases including obesity, metabolic dysfunction, diabetes in addition to neurological disorders such as autism spectrum disorder (ASD) and multiple sclerosis (MS) (Tilg and Kaser, 2011; Barlow et al., 2015; Cryan et al., 2020). In this review article, we focus on development of the gut-brain axis in the fetus and neonate, with attention to how these become disrupted in the setting of congenital infection/inflammation or exposure to antibiotics. In the following sections, we critically appraise the influence of different case examples of infectious diseases on fetal gut-brain development.
Microglia, the resident immune cells of the brain, derive from erythromyeloid progenitors in the extraembryonic yolk sac and establish their place in the fetal brain early in development, prior to neurogenesis or blood brain barrier (BBB) formation in human (Kracht et al., 2020). They are important not only for immune function and response in the brain, but also for synaptic pruning and modulating neurogenesis based on experiments in rodents (Sierra et al., 2010; Abdel-Haq et al., 2019). A genetic fate mapping study in rodents identified microglia as a highly dynamic, self-renewing population, and in the absence of systemic or local inflammation compromising BBB function, remain separate from peripheral bone marrow-derived monocyte/macrophage populations (Tay et al., 2017). Human microglia gain immune surveillance and phagocytic function early in fetal life (from 9 to 18 weeks of gestation) (Kracht et al., 2020).
The gut-brain axis actively shapes microglial behavior and development. Globally, the gut microbiome influences the development of the host’s innate and adaptive immune responses (Abdel-Haq et al., 2019). This holds not only for the host’s peripheral immune cells, but also centrally within the brain. For instance, Erny et al. (2015) discovered that the host microbiome contributes greatly to microglial homeostasis. Germ-free mice exhibit severe impairments in microglial function, for instance defects in genetic regulation of immune activation and signal transduction via the interferon and Jak3/STAT1 pathway (Erny et al., 2015). It is likely that these effects occur via alterations in short-chain fatty acid (SCFA) signaling, as gut bacteria are key metabolizers of nutrition-derived fatty acids important for global and central nervous system (CNS)-specific immune function, as restoration of SCFA in germ-free mice rescued microglial function (Erny et al., 2015; Venegas et al., 2019; Figure 1).
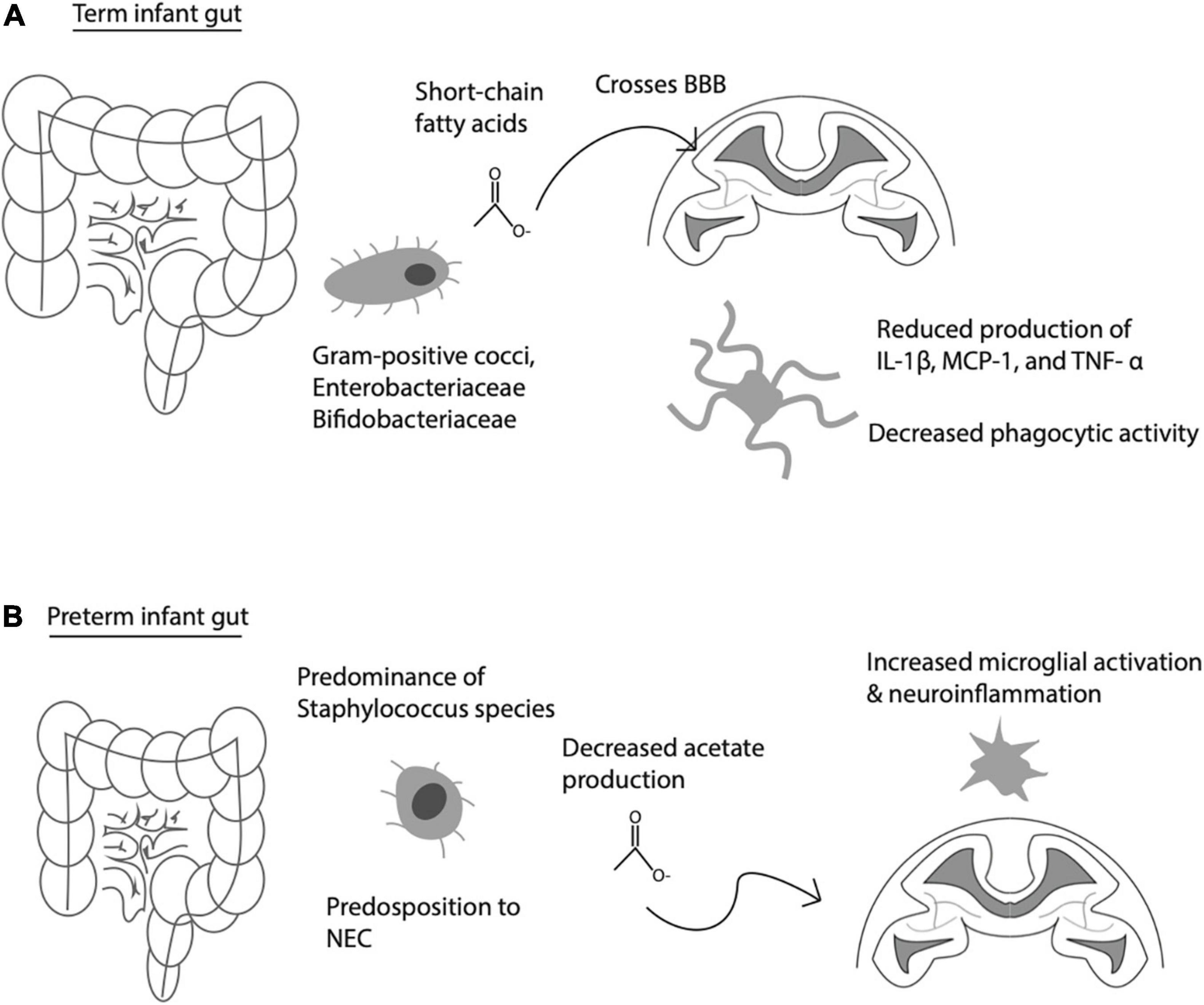
Figure 1. Communication between gut-derived short chain fatty acids (SCFA) and brain immune activity. (A) Normal interaction between gut-derived SCFA, which cross the blood brain barrier (BBB) to influence microglia activity under physiological conditions. (B) Premature infant gut with altered gut flora, predisposition to necrotizing enterocolitis (NEC), decreased acetate production, leading to increased microglial activation, and increased risk of neuroinflammation.
When infection is present during pregnancy, inflammatory products—such as those associated with congenital infections and III—trigger microglial activation, resulting in neuroinflammation marked by the presence of IL-1β, TNF-α, and IL-6 (Lu and Claud, 2019). Microglial activation is also evidenced by white matter damage, which was found in animal studies modeling maternal infection utilizing LPS-induced mice to understand the role of microbiota inflammation on neurodevelopment (Burd et al., 2010; Elovitz et al., 2011). These same animal models have also linked the regulation of neural activity to the microbiome’s activation of microglia, further providing direct evidence of a reciprocally communicating gut-brain axis (Cryan et al., 2020). Antibiotic treatment in pregnant women can influence gut microbiome composition, specifically preterm neonates exposed to antibiotics demonstrating fewer Frimicutes and more Proteobacteria, accompanied by altered short chain fatty acid (SCFA) profiles, vs. those not exposed (Dahl et al., 2018). In turn, SCFAs produced by gut bacteria can cross the blood brain barrier and alter microglia cytokine release and dampen phagocytic activity in vitro (see Figure 1; Wenzel et al., 2020). While term infants have predominantly gram positive cocci, Enterobacteriaceae and Bifidobacteriaceae species, preterm infants have altered speciation, including higher abundance of Strephyloccaceae and delayed Bifidobacteriaceae colonization, associated with lower acetate levels, which may in turn lead to higher levels of microglial activation and neuroinflammation among preterm infants (Tauchi et al., 2019; Figure 1). This is corroborated by the finding of increased numbers of activated microglia in the brains of the preterm human brain, especially in the developing white matter (Supramaniam et al., 2013). These findings highlight the potential influence of the microbiota have on neuro-immune development.
It is also important to mention the role of the vagus nerve at the interface of the gut and brain interaction. Vagal nerve afferents innervate the gastrointestinal tract along its length, most notably in the form of the myenteric plexus, a network of neurons and glia between the smoothe and longitudinal muscle layers of the intestinal wall (Phillips and Powley, 2007). Testing the effects of the vagus nerve on the connection between brain and gut has historically been achieved by performing vagotomy, the surgical severing of the vagus nerve. A well-known phenomenon related to inflammation in general is the associated anorexia or lack of appetite. In mice, the mechanism underlying this effect has been experimentally determined to require vagal innervation. For instance, mice treated with lipopolysaccharide (LPS), a potent pro-inflammatory mediator, exhibit decreased food-motivated behavior, an effect that is inhibited by subdiaphragmatic vagotomy (8,590,821). Vagotomy does not, however, inhibit the body’s pyogenic response to proinflammatory cytokines, suggesting that the vagus nerve is responsible for some, but not all of the physiological responses to pathogens (Luheshi et al., 2000). Furthermore, SCFAs, produced by gut bacterial fermentation and known to cross the blood brain barrier (BBB), have been shown to improve survival in a mouse model of sepsis, an effect that is actually inhibited by surgical vagotomy, suggesting the vagus nerve’s importance in mediating communication between gut and brain in the context of severe infection (Okumura et al., 2021). Apart from the vagus nerve’s effect on food intake and food-related behavior during infection, it has also been hypothesized that diseases affecting both gut and brain may stem from abnormal connectivity or pathology involving the vagus nerve. For instance, Parkinson disease, which involves not only the well-known neurological signs and symptoms of bradykinesia, resting tremor, muscle rigidity, and postural instability, is accompanied by changes in function of the gastrointestinal tract, including colonic dysmotility and constipation. It has been posited that alpha-synuclein, a pathological misfolded protein implicated in Parkinson disease pathogenesis, actually spreads via the vagus nerve, potentially explaining an underlying common pathogenesis involving the CNS and GI tract (Breen et al., 2019).
In the following sections, we first explore the roles of congenitally acquired viral pathogens in triggering abnormal communication between the brain and gut.
We will examine how several case examples of RNA virus infections shape the fetal/neonatal gastrointestinal and neurological systems and attempt to draw connections between the two. The emergence of novel infections such as (Zika virus) ZIKV and COVID-19 has sparked investigation to understand the extent to which they alter the fetal microbiome, and thus, fetal neurodevelopment through the gut-brain axis. We group these two viruses together in this section as they share several commonalities, including that they are both positive-sense RNA viruses that have potential for vertical transmission (more common with ZIKV), and have demonstrated adverse perinatal and neonatal outcomes and characteristic placental pathologies in infected individuals, and limited therapeutic options at present (McBroom, 2021).
It has been established that congenital ZIKV has significant impacts on fetal brain development, resulting in microcephaly in 0.15–2% of cases, neural tube defects (0.1% of cases), ocular abnormalities (0.3% of cases), and high levels of proinflammatory mediators (Coelho and Crovella, 2017; Cragan et al., 2017; Lum et al., 2017; Nguyen et al., 2017). We have previously shown that ZIKV inflicts neuroinflammation by evoking an IL-1β response in the placenta (Lei et al., 2019). Current studies relating ZIKV to the gut microbiome focus largely on adult rodent models. For instance, non-pregnant adult mice infected with ZIKV exhibit significant decrease in bacteria belonging to the Actinobacteria and Firmicutes phyla while there was an increase in Deferribacteres and Spirochaetes phyla when compared to non-infected mice, as determined by next-generation sequencing (Correa et al., 2021). What impact does this ZIKV-induced gut dysbiosis have on the developing fetus? Although direct evidence is sparse for how ZIKV may alter the host gut microbiome to influence brain development, this may occur via changes in host immune response. For instance, ZIKV-infected mice depleted of Stat2 exhibit more severe infection, as measured by survival, mobility/paralysis, and body weight as compared to Stat2-intact mice, suggesting a role of the interferon-STAT2 pathway in ZIKV pathogenesis (Tripathi et al., 2017). Alterations in gut flora including Salmonella infection and abundance of butyrate-producing organisms can lead to changes in STAT2-signaling, suggesting one pathway by which ZIKV may exert its pathogenesis (Wilson et al., 2019; Chemudupati et al., 2020).
Aside from direct effects of ZIKV on the developing gut and brain, there may be underlying genetic susceptibility to ZIKV-related congenital infection. For instance, Santos et al. (2019) found that a single nucleotide polymorphism (SNP) in the Toll-like receptor 3 (TLR3) exhibited increased odds of congenital ZIKV infection vs. controls lacking this SNP. The investigators further found that a SNP in the tumor necrosis factor alpha (TNF-α) gene conferred a heightened risk of microcephaly compared to those babies lacking this SNP. Likewise, alterations in epigenetic programming may influence the natural course of ZIKV infection. Decreased methylation of plasminogen activator inhibitor-1 (PAI-1, a regulator of innate immunity) was associated with a higher risk of microcephaly, for example (Nwanaji-Enwerem et al., 2021). These results suggest that genetic and epigenetic regulatory changes related to inflammatory cascades and immune activation may predispose to ZIKV contraction and more severe disease.
Due to the evolving and novel nature of the COVID-19 infection and the ongoing studies associated with it, a comprehensive understanding of its effect on the development of the fetal microbiome and neurological development has yet to be exhaustively investigated. Thus far, COVID-19 has been associated with adverse perinatal outcomes including increased risk of preterm birth, intrauterine growth restriction, and stillborn (McBroom, 2021; Dubucs et al., 2022). One review explored the emerging pediatric inflammatory syndrome accompanying COVID-19 infections in children, citing differences in the pediatric gut microbiome as responsible for the varying responses to infection (Mondal et al., 2021). The investigators analyzed stool samples 30 days after resolution of COVID-19 infection and found that there was a significant lower representation of gut microbiota in infected children compared to the healthy controls (Mondal et al., 2021; Yeoh et al., 2021). This is indicative of the infection eliciting an inflammatory response that ultimately altered the pediatric microbiome. These alterations in gut microflora may be associated with the increasingly reported neuropsychiatric phenomena that have been associated with COVID-19 infection, although this remains to be definitively demonstrated especially in the pediatric population (Mondal et al., 2021). Do genetic or epigenetic differences in the population predispose to congenital COVID acquisition and severity of infection? It has been reported that polymorphisms in the ABO gene as well as genes related to viral entry (e.g., angiotensin-converting enzyme 2, ACE2) have been associated with increased propensity to developing COVID-19 infection in adults, although these findings remain to be replicated in the setting of congenital infections (Benetti et al., 2020; Vargas-Alarcon et al., 2022). Epigenetic factors that have been associated with COVID-19 infection include methylation of the gene encoding ACE2, which also interestingly differs by sex, with females showing less DNA methylation as compared to males (Cardenas et al., 2021). Again, whether this translates into greater transmissibility across the placenta or confers more severe fetal infection remains to be seen.
Based on the above emerging evidence, it seems plausible that ZIKV and COVID-19 may lead to alterations in perinatal and early life gut-brain interactions. Longitudinal studies following those infected will be required to draw definitive conclusions regarding long-term effects on the gut microbiome and health outcomes across the lifespan.
We next focus on several DNA viruses that have also been implicated in development of neural-gut interactions. Parvovirus B19 is a single-stranded DNA virus that can cross the human placenta and lead to severe fetal anemia and hydrops fetalis via inhibition of fetal erythropoiesis (de Jong et al., 2006). Experimental studies in animals have shown that parvovirus not only affects the hematopoietic system, but also the developing gut. Parvovirus-infected Wister rats exhibit intestinal dysbiosis, with reduction in probiotic bacteria of Parabacteroides and Butyricicoccus genera, along with increased Methanobrevibacter and Syntrophococcus along with enteritis and inflammatory cells noted in the ileum (Xiang et al., 2019). Up to 16% of neonates afflicted with congenital parvovirus infection exhibit neurodevelopmental delay, which is likely a product of multiple synergistic mechanisms, including compromised fetal brain oxygenation from anemia, abnormal gut-brain development, and possibly direct neurological insult (Ornoy and Ergaz, 2017).
By the same token, CMV is a DNA virus of the herpesvirus family and a cause of congenital TORCH infection. In adults, CMV is well-known to cause enteritis, especially in immunocompromised hosts, those in shock states, those with concurrent pneumonia or chronic kidney disease, or with concurrent antibiotic exposure (Yeh et al., 2021). Does CMV have potential to inflict similar injury in the context of congenital infection? In the neonatal population, one study investigated neonatal intensive care unit admissions with patients infected with CMV at or close to birth, and found clinical signs ranging from abdominal distention, feeding intolerance, to diarrhea, protein-losing enteropathy, necrotizing enterocolitis, and associated hypernatremic hypovolemia (Cheong et al., 2004). Prenatally, congenital CMV may be detected on anatomy ultrasound with the presence of echogenic bowel, demonstrating that the early fetal gut may indeed be colonized or infected with these microbes well before birth (Nigro et al., 2012). Congenital CMV causes a robust innate immune response in the fetus, with monocytes, macrophages, and dendritic cells upregulating pro-inflammatory cytokines via toll-like receptors (TLRs) (Brizic et al., 2018). A recent study by Baasch et al. (2021) found that CMV infects macrophages and induces a stem-like state in these cells, impairs their antigen-presenting and phagocytic properties, and ramps up CMV replication in a mouse model of CMV. Mechanistically, they identified that these changes occur via upregulation of the transcription factor ZEB1 by Wnt signaling. It is possible that a similar process occurs in the context of embryonic/fetal CMV infection, although this remains to be shown.
We still lack evidence as to whether the cerebral effects of CMV (ventriculomegaly, sensorineural hearing loss, chorioretinitis, learning impairment) occur via direct insult from CMV particles entering the brain and causing an inflammatory response, or whether global disruptions in immune cells in the gut and circulation may also play a role, although we suspect that both are likely at play (Weisblum et al., 2014; Davis et al., 2017). Interestingly, polymorphisms in TNF-α and β-cytokine receptors can confer an increased risk of congential CMV infection, suggesting that some fetuses may be particularly susceptible to infection, and potentially explaining how many neonates are unaffected by maternal CMV infection (Arav-Boger et al., 2002). A future study to address this question could investigate whether neurocognitive disorders occur in neonates born with only CMV infecting the gut without any evidence of direct CNS involvement [for instance, infection occurring after formation of the blood brain barrier (BBB), or lacking the ultrasonographic hallmarks of periventricular plaques/calcifications].
One other member of the herpesvirus family and cause of congenital TORCH infection is herpes simplex virus (HSV), which upregulates inflammatory pathways and leads to neurological and gut inflammation. HSV enters fetal circulation through infection of Hofbauer cells, placental monocytes, and induces expression of IL-1β, a pro-inflammatory cytokine (Hendrix et al., 2020). The pro-inflammatory environment induced by HSV fosters multisystem organ dysfunction, as manifested by abnormal electroencephalogram (EEG) findings including seizure activity and potentially neonatal encephalopathy, as well as gastrointestinal/metabolic effects such as hepatic inflammation manifested by elevations in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) with subsequent impaired glucose metabolism and hypoglycemia, correlating positively with degree of neonatal encephalopathy (O’Dea et al., 2020). From experimental studies in mice, it has been determined that HSV can infect the enteric nervous system, specifically the myenteric ganglia, leading to colonic dysmotility and toxic megacolon, a potential cause of severe neonatal morbidity and mortality in the context of this infection (Khoury-Hanold et al., 2016).
Aside from viruses, live pathogens also interfere with proper gut microbiome establishment with potential ramifications on proper CNS development. Toxoplasma gondii (T. gondii) is a cause of neonatal encephalitis and enteritis (Shao et al., 2020). In a mouse model of congenital toxoplasmosis, the cecal microbiome of mice infected with T. gondii is significantly altered, with increases in the relative abundance of Proteobacteria as well as harmful bacteria, such as Bilopha and Desulfovibrio species, and a decrease In Lactobacillus species (Shao et al., 2020). This bacterial dysbiosis appears to be mediated by macrophage induced nitrate production. Nitrate from activated macrophages serves as an energy source for enterobacter species (Wang et al., 2019). Mechanistically, infection with T. gondii causes intestinal dysbiosis by activating intestinal macrophages and upregulating nitrate production, leading to overgrowth of Enterobacteriaceae (Wang et al., 2019). Depletion of butyrate-producing organisms leads the colonic epithelium to increase expression of Nos2 by surface epithelial cells via PPAR-γ signaling, thus increasing production of nitrate (Byndloss et al., 2017). Such changes in immune function could have far-reaching implications for proper brain homeostasis. For instance, microglia in the brains of T. gondii-infected fetuses cause focal nodules, likely through activation of complement signaling (Munday and Mason, 1979; Shinjyo et al., 2020). How the organisms gain access to the CNS is multifactorial: (1) BBB permeability is immature in the fetal brain, with tight junctions first expressed in the cerebral vasculature beginning at approximately 12 weeks of fetal life in humans and approximately E18 in mice; (2) chronic inflammation such as that caused by T. gondii infection, can promote increased BBB permeability and leukocyte infiltration through elevated interleukin-1β (IL-1β) signaling (Shaftel et al., 2007; Gov et al., 2013; Goasdoue et al., 2017; Kirschen et al., 2018). Thus, parasites may also gain access to the fetal brain and gut, leading to disruptive alterations in their maturation and creating an inflammatory environment with consequences on neurodevelopment.
While some congenital infections may have a protracted, insidious course, affecting development across gestation, others present acutely with signs and symptoms of inflammation and a pronounced host response. One such example is III, also known as chorioamnionitis, an acute bacterial infection of the fetal membranes and placenta.
III is a common gestational complication associated with adverse infant outcomes such as preterm birth (PTB), neonatal sepsis, cardiopulmonary complications, and neurological injuries (Tita and Andrews, 2010). III can be defined as inflammation of the membranes and chorion of the placenta caused by the transmission of infectious organisms via retrograde infection from the lower genital tract (Tita and Andrews, 2010; Redline, 2012; Xiao et al., 2018). It can further be subdivided into three categories: histologic, microbiologic, and clinical. Histologic III is defined by pathological findings from placental histology such as funisitis or vasculitis; microbiological III involves the extraction of organisms from amniotic fluid or placental cultures; clinical III is characterized by maternal fever, maternal leukocytosis, and maternal and/or fetal tachycardia, which may be accompanied by uterine fundal tenderness or purulent vaginal discharge (Tita and Andrews, 2010; Xiao et al., 2018). III is responsible for a pro-inflammatory response by the fetus, known as Fetal Inflammatory Response Syndrome (FIRS), which can be characterized by an increase in the expression of the cytokines and chemokines IL-1, IL-6, CCL2, and TNF-α in the placenta and increased expression of IL-1β and TNF-α in the fetal brain (Tita and Andrews, 2010; Redline, 2012; Makinson et al., 2017; Hester et al., 2018; Stojanovska et al., 2018; Xiao et al., 2018; Chen et al., 2020; Briana et al., 2021). These biological markers are also indicative of alterations in neurological development.
Experimental models of III have utilized model organisms exposed to intrauterine lipopolysaccharide (LPS), allowing for the assessment of placental and fetal immune responses to a pro-inflammatory trigger (Redline, 2012; Makinson et al., 2017; Hester et al., 2018; Stojanovska et al., 2018; Xiao et al., 2018; Chen et al., 2020; Briana et al., 2021). In a study using mice, cytokines were measured 48 h after exposure to LPS. This study found that IL-1β, TNF-α, and CCL2 levels were increased in exposed mice, suggestive of recapitulation of human findings in III (Redline, 2012). Next looking at the brains of these pseudo-infected fetal mice, the investigators found white matter damage, increased numbers of microglia, and postnatal neurobehavioral changes, in particular with increased anxiety-related behaviors on elevated zero maze and light-dark box test, albeit with an anxiolytic-like phenotype on the open field test (Redline, 2012; Makinson et al., 2017). In another LPS-exposed mouse model, III significantly reduced hippocampal neurogenesis and granule cell density and resulted in improper ectopic granule cell migration (Hester et al., 2018). A separate experiment utilizing lambs exposed to LPS found reduced neuronal cell density, edema of the cortical gray matter, and blood vessel extravasation in the brain as a result of III (Stojanovska et al., 2018).
It is evident across a variety of studies that III has a significant impact on fetal brain development in ways that may be connected to alterations in the neonatal gut microbiome. In connection with long-term consequences, these physiological changes have been established as causes of neurological disorders such as cerebral palsy and autism and mental health conditions such as schizophrenia, anxiety, and depression (Makinson et al., 2017; Hester et al., 2018; Stojanovska et al., 2018; Chen et al., 2020; Briana and Malamitsi-Puchner, 2021). Changes to the fetal brain also harbor repercussions related to the fetal microbiome due to responses to III, with compromised placental or gut sterility, for example. Recently, studies have found that alterations to the fetal gut microbiome correlated with neurodevelopment and neuropsychiatric disorders in those previously afflicted with intrauterine infection (Ma et al., 2019; Warner, 2019).
A closer look at specific congenital infections resulting from III establishes the fetal response and adverse effects these infections can have on the fetal microbiome. For instance, Group B streptococcus (GBS) infection during labor or birth can lead to invasive GBS disease, encompassing a set of far-reaching sequelae on the neonatal organ systems, including alterations in neonatal intestinal composition, with reduction in Bifidobacteria or an increase of Firmicutes species (Aloisio et al., 2014; Stearns et al., 2017; Freudenhammer et al., 2021). As described in the next section, exposure to antibiotics to treat infections such as GBS may create their own set of issues related to disruption of the developing fetal gut, which may have complex effects on early development.
Colonization of the gut is an essential step in the developing immune system. Initial gut microbiota is acquired during the neonatal phase via transmission of maternal microbes to the offspring both prenatally and during childbirth, marking it as a critical phase for sterility and immunity (Walker et al., 2017). External factors, such as antibiotics or dietary patterns, can affect the development of a healthy gut microbiome. Often to treat III and reduce the effects of congenital infections on both the mother and fetus, antibiotics are administered. A thorough investigation of the mechanisms and consequences antibiotics may have on the maternal and/or fetal gut microbiome is yet to occur, but there appears to be a clear trend in the studies that have been conducted thus far: early antibiotic exposure negatively influences the fetal gut microbiota, resulting in lasting effects on neurological development (Cantey et al., 2018; Eck et al., 2020; Zhou et al., 2020). Studies have found probiotics to have a positive outcome with regards to mitigating the harmful effects of antibiotic exposure (Rutten et al., 2015; Gong et al., 2021; Zhong et al., 2021).
While antibiotics are viewed as essential to alleviating infection, their administration has been found to induce microbiota dysbiosis and create antibiotic resistant bacteria, resulting in increased fetal health risks. When mothers receive antibiotic treatment for infection, the vaginal microbiota that is passed to the offspring becomes unbalanced, which disrupts the development of the fetal microbiome (Zhou et al., 2020). One specific study analyzed Lactobacillus species from maternal vaginal swabs and neonatal meconium of mothers and neonates that did or did not receive antibiotics and found that groups who experienced antibiotic exposure had significantly lower loads of the bacteria (Zhou et al., 2020). The study suggested that exposed infants had elevated early onset sepsis due to this exposure as well (Zhou et al., 2020). In a different study investigating the development of intestinal microbiota post-antibiotic exposure in term infants, administration resulted in lower Bacteroidetes colonization and a differential development of the overall microbiome (Eck et al., 2020). A third study found that antibiotic use produced a less diverse microbiome, resulting in increased risks for early to late-onset sepsis, necrotizing enterocolitis (NEC), and in some cases, death (Cantey et al., 2018; Figure 2). NEC is the most common surgical emergency among preterm infants, and is more likely among infants weighing less than 1,500 g. Risk factors include prematurity, factors that predispose to gut dysbiosis, and lack of breastfeeding (Coggins et al., 2015). A metagenomic analysis of fecal samples performed by Olm et al. (2019) identified Klebsiella species, fimbriated bacteria, and bacteria that use quarom sensing as being significantly more common among NEC samples vs. control samples.
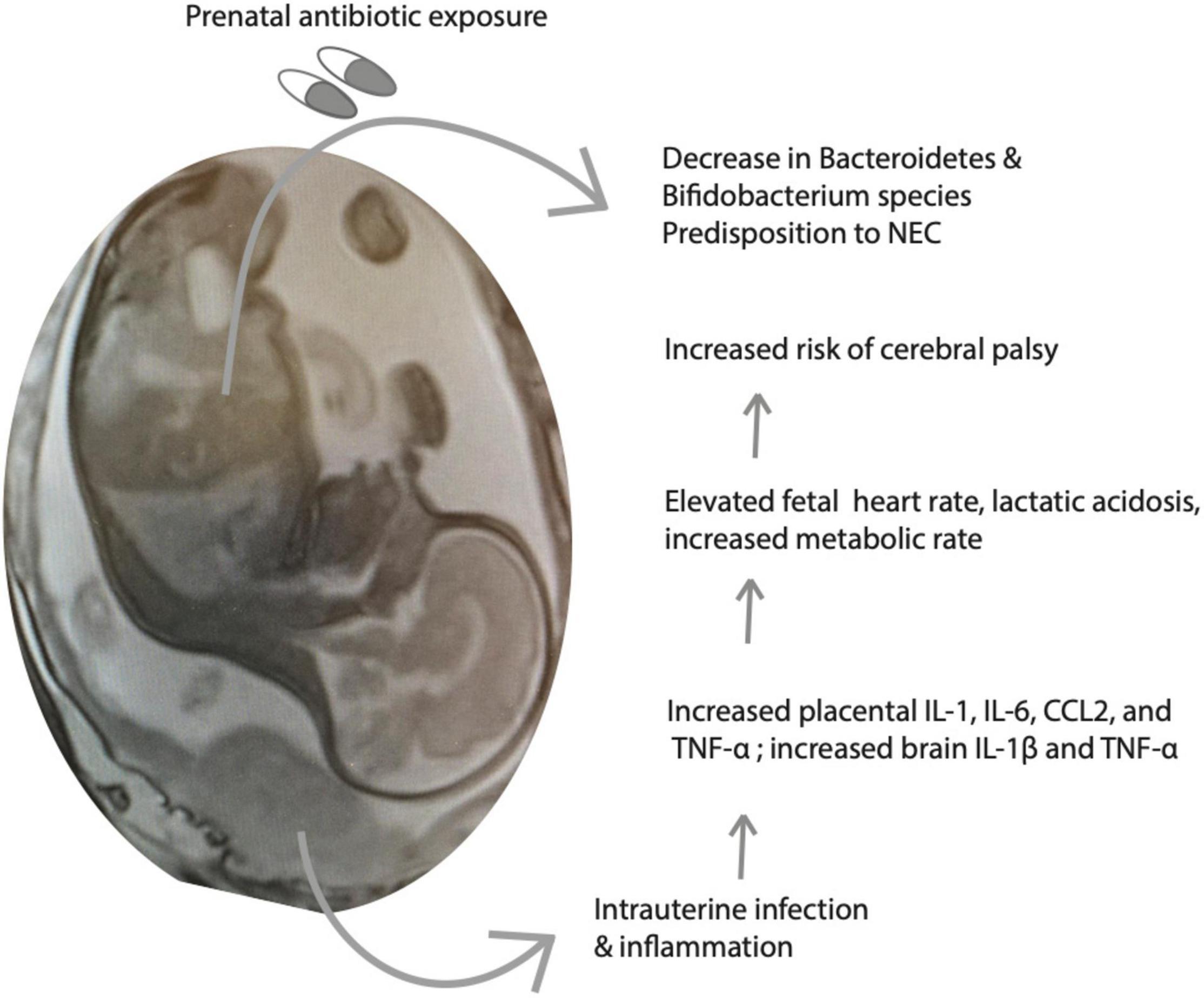
Figure 2. Intrauterine infection and inflammation (III) and treatment with antibiotics alter fetal metabolism. Shown is a schematic of the consequences of III on fetal inflammatory mediators and metabolic parameters, predisposing to increased risk of neurological injury and cerebral palsy. Antibiotics are important to treat the infection, however, they may have untoward consequences on neonatal gut flora maturation.
In addition to antibiotics, antiviral medications may play some role in metabolic changes in the neonatal microbiome establishment. For instance, the infants of pregnant women infected with HIV have been found to have altered 1-carbon metabolism, potentially affecting the CNS (Surtees et al., 1990; Figure 3). It is unclear whether these changes are attributable to the virus per se or to highly active anti-retroviral therapy (HAART), well-known for causing adverse metabolic effects on lipid profiles, adiposity accumulation/redistribution, and cardiovascular disease (Levy et al., 2017). Although effects of HIV and/or HART on fetal development are generally considered minimal, they do include small for gestational age (SGA), low birth weight (LBW), and tenofovir may be associated with slower growth at 1 year of life (Jao et al., 2015). Additionally, experiments in rats have identified that hypoglycemic episodes (which are common in premature neonates) may be more common and may lead to exacerbated neurotoxicity in the hippocampus in the presence of the HIV protein GP120 acting on chemokine receptors (Barks et al., 1995; Figure 3).
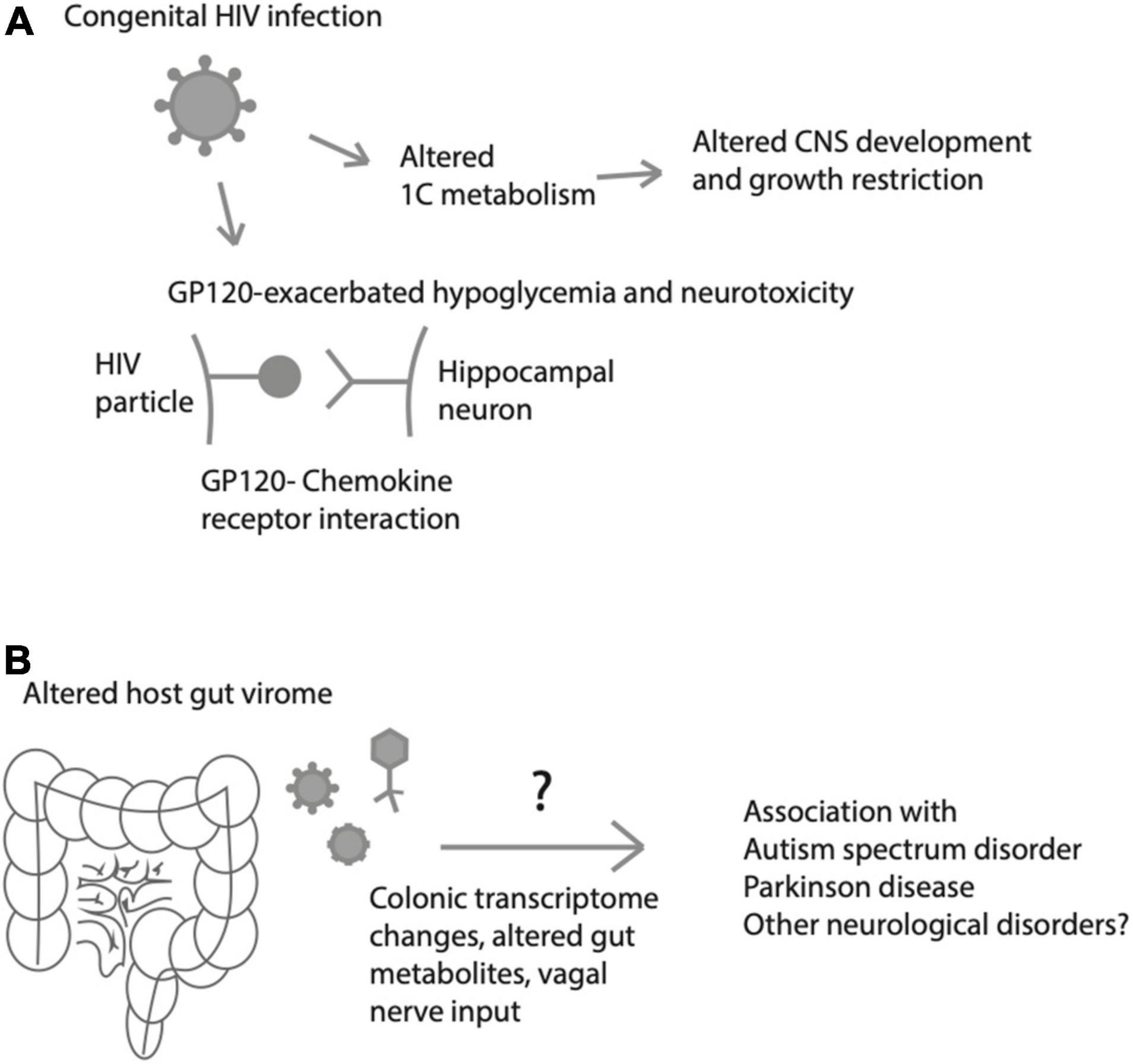
Figure 3. Effects of viruses on fetal/neonatal metabolism and neurological disease or injury. (A) Metabolic effects of HIV that may lead to altered fetal growth, central nervous system (CNS) development), glucose processing and excitotoxic brain injury. (B) Neonatal gut virome influences colonic transcriptome and gut metabolites, which may predispose to a variety of neurological disorders later in life.
It is evident that prenatal and postnatal antibiotic exposure affects the composition of the fetal and neonate gut microbiome, and further research has aimed to find a solution to diminish these effects. A promising potential remedy appears to be probiotic supplementation, as this can promote the growth of bacteria conducive to a healthy microbiome and restore the diversity of gut microbiota. In a randomized, controlled study, probiotics were administered simultaneously with antibiotics along with post-antibiotic treatment, resulting in the remodeling of the gut microbiota (Zhong et al., 2021). This suggests that probiotics may promote recovery or restoration of physiological neonatal gut microbiome formation following antibiotic treatment. Another study investigating term born infants exposed or not exposed to antibiotics focused on fecal samples obtained during the first year of life and found that the microbiota could be altered by probiotic administration to restore a healthy microbiome (Rutten et al., 2015). Similar work has been performed in preterm infants, with a smaller study investigating 40 premature infants finding that antibiotics increased pathogenic bacteria and reduced replication and repair functions as well as purine metabolism pathways (Gong et al., 2021). On the other hand, probiotic administration in these neonates increased the number of commensal bacteria, alleviating the negative effects of the antibiotics (Gong et al., 2021). Thus, while antibiotic exposure may lead to detrimental effects on the neonatal gut microbiome, further research is required to understand how probiotics or other early life interventions may be utilized to mitigate the effects of such antibiotics to protect the immunity of neonates.
Figure 2 depicts a summary of the interactions between perinatal infection and antibiotics, immune activation, and the developing gut-brain axis.
Congenital infections have demonstrated profound effects on fetal and neonatal neurological development. The gut-brain axis has proven to be a pivotal participant in neurodevelopment, and congenital infections may contribute to abnormal maturation of the forming neonatal gut and developing brain. While the TORCH infections have been studied extensively, novel pathogens including COVID-19 and ZIKV will require further investigation to understand their full impact on the developing gut-brain axis. By the same token, antibiotics are often used prenatally and in the early postnatal period for treatment of conditions such as intraamniotic infection, maternal pulmonary or urinary tract infections, or neonatal bacterial infections. These treatments are often employed without consideration of potential effects on the developing gut, yet, this early exposure to antibiotics likely affects the developing gut microbiome in ways that will impact gastrointestinal and systemic health for the lifespan of the individual.
We are beginning to understand that the CNS communicates with systemic circulation likely via microglia, the brain immune cells that sense blood-borne cues that may be gut-derived to influence activation and inflammatory responses. We look forward to future investigations of the relationship between the developing gut-brain axis and early life exposures to pathogens and antibiotics, which may hold the key to still incompletely understood diseases such as schizophrenia, mood disorders, and cerebral palsy.
Here, we have focused largely on the neonatal gut microbiome, with a bias toward bacterial organisms. However, this discussion neglects an increasingly recognized large number of viruses that colonize the neonatal gut and may have important roles in metabolic homeostasis and gut maturation. While under physiological circumstances, the gut is entirely devoid of viruses at birth, recent work from Liang et al. (2020) has shown that following bacterial colonization of the neonatal gut, bacteriophages come to inhabit the gut by 1–4 months of life, as measured by quantitative polymerase chain reaction (qPCR) of meconium and stool samples of neonates. Importantly, whether the infant is breastfed vs. formula-fed influences the bacterial as well as the viral landscapes of the developing infant gut, suggesting a role of immune activity (either from passive immunity from breastmilk-derived antibodies or from maternal skin flora passing into the fetal gut) on both viral and bacterial colonization of the developing gut (Liang et al., 2020). The gut virome is also an increasingly recognized contributor to proper neurological health, with altered host virome-brain interactions being implicated in disorders including autism spectrum disorder and Parkinson disease, perhaps through perturbations in the colonic transcriptome (Kang et al., 2017; Mihindukulasuriya et al., 2021; Stockdale et al., 2021; Figure 3). How these interactions between immune system, gut microbiome and virome and neurological systems interact in normal and pathological ways remains an open question that will require more basic and clinical investigation.
GK and SP wrote the original draft. IB edited the draft. All authors proofread and edited the final draft and agreed with the final version.
This work was funded through the National Institutes of Health (NIH) award 5R01HD097608-04 to IB and through internal departmental funding (Kelly Society Award) to GK.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aagaard, K., Ma, J., Antony, K. M., Ganu, R., Petrosino, J., and Versalovic, J. (2014). The placenta harbors a unique microbiome. Sci. Transl. Med. 6:237ra265.
Abdel-Haq, R., Schlachetzki, J. C. M., Glass, C. K., and Mazmanian, S. K. (2019). Microbiome-microglia connections via the gut-brain axis. J. Exp. Med. 216, 41–59. doi: 10.1084/jem.20180794
Aloisio, I., Mazzola, G., Corvaglia, L. T., Tonti, G., Faldella, G., Biavati, B., et al. (2014). Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl. Microbiol. Biotechnol. 98, 6051–6060. doi: 10.1007/s00253-014-5712-9
Arav-Boger, R., Willoughby, R. E., Pass, R. F., Zong, J. C., Jang, W. J., Alcendor, D., et al. (2002). Polymorphisms of the cytomegalovirus (CMV)-encoded tumor necrosis factor-alpha and beta-chemokine receptors in congenital CMV disease. J. Infect. Dis. 186, 1057–1064. doi: 10.1086/344238
Baasch, S., Giansanti, P., Kolter, J., Riedl, A., Forde, A. J., Runge, S., et al. (2021). Cytomegalovirus subverts macrophage identity. Cell 184:e3725. doi: 10.1016/j.cell.2021.05.009
Barks, J. D., Sun, R., Malinak, C., and Silverstein, F. S. (1995). gp120, an HIV-1 protein, increases susceptibility to hypoglycemic and ischemic brain injury in perinatal rats. Exp. Neurol. 132, 123–133. doi: 10.1016/0014-4886(95)90066-7
Barlow, G. M., Yu, A., and Mathur, R. (2015). Role of the Gut Microbiome in Obesity and Diabetes Mellitus. Nutr. Clin. Pract. 30, 787–797. doi: 10.1177/0884533615609896
Benetti, E., Tita, R., Spiga, O., Ciolfi, A., Birolo, G., Bruselles, A., et al. (2020). ACE2 gene variants may underlie interindividual variability and susceptibility to COVID-19 in the Italian population. Eur. J. Hum. Genet. 28, 1602–1614. doi: 10.1038/s41431-020-0691-z
Breen, D. P., Halliday, G. M., and Lang, A. E. (2019). Gut-brain axis and the spread of alpha-synuclein pathology: vagal highway or dead end? Mov. Disord. 34, 307–316. doi: 10.1002/mds.27556
Briana, D. D., and Malamitsi-Puchner, A. (2021). Chorioamnionitis in utero, schizophrenia in adulthood: limited current evidence-future research focus? J. Matern. Fetal Neonatal. Med. 1–6. [Epub ahead of print].
Briana, D. D., Papaevangelou, V., and Malamitsi-Puchner, A. (2021). The jury is still out on the existence of a placental microbiome. Acta Paediatr. 110, 2958–2963. doi: 10.1111/apa.16048
Brizic, I., Hirsl, L., Britt, W. J., Krmpotic, A., and Jonjic, S. (2018). Immune responses to congenital cytomegalovirus infection. Microbes Infect. 20, 543–551.
Brown, A. G., Tulina, N. M., Barila, G. O., Hester, M. S., and Elovitz, M. A. (2017). Exposure to intrauterine inflammation alters metabolomic profiles in the amniotic fluid, fetal and neonatal brain in the mouse. PLoS One 12:e0186656. doi: 10.1371/journal.pone.0186656
Burd, I., Bentz, A. I., Chai, J., Gonzalez, J., Monnerie, H., Le Roux, P. D., et al. (2010). Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J. Neurosci. Res. 88, 1872–1881. doi: 10.1002/jnr.22368
Byndloss, M. X., Olsan, E. E., Rivera-Chavez, F., Tiffany, C. R., Cevallos, S. A., Lokken, K. L., et al. (2017). Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575. doi: 10.1126/science.aam9949
Cantey, J. B., Pyle, A. K., Wozniak, P. S., Hynan, L. S., and Sanchez, P. J. (2018). Early Antibiotic Exposure and Adverse Outcomes in Preterm, Very Low Birth Weight Infants. J. Pediatr. 203, 62–67. doi: 10.1016/j.jpeds.2018.07.036
Cappelletti, M., Presicce, P., and Kallapur, S. G. (2020). Immunobiology of Acute Chorioamnionitis. Front. Immunol. 11:649. doi: 10.3389/fimmu.2020.00649
Cardenas, A., Rifas-Shiman, S. L., Sordillo, J. E., DeMeo, D. L., Baccarelli, A. A., Hivert, M. F., et al. (2021). DNA methylation architecture of the ACE2 gene in nasal cells of children. Sci. Rep. 11:7107. doi: 10.1038/s41598-021-86494-7
Chemudupati, M., Kenney, A. D., Smith, A. C., Fillinger, R. J., Zhang, L., Zani, A., et al. (2020). Butyrate Reprograms Expression of Specific Interferon-Stimulated Genes. J. Virol. 94, e00326–20. doi: 10.1128/JVI.00326-20
Chen, H. J., Antonson, A. M., Rajasekera, T. A., Patterson, J. M., Bailey, M. T., and Gur, T. L. (2020). Prenatal stress causes intrauterine inflammation and serotonergic dysfunction, and long-term behavioral deficits through microbe- and CCL2-dependent mechanisms. Transl. Psychiatry 10:191. doi: 10.1038/s41398-020-00876-5
Cheong, J. L., Cowan, F. M., and Modi, N. (2004). Gastrointestinal manifestations of postnatal cytomegalovirus infection in infants admitted to a neonatal intensive care unit over a five year period. Arch. Dis. Child. Fetal. Neonatal. Ed. 89, F367–9. doi: 10.1136/adc.2003.032821
Coelho, A. V. C., and Crovella, S. (2017). Microcephaly Prevalence in Infants Born to Zika Virus-Infected Women: a Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 18:1714. doi: 10.3390/ijms18081714
Coggins, S. A., Wynn, J. L., and Weitkamp, J. H. (2015). Infectious causes of necrotizing enterocolitis. Clin. Perinatol. 42, 133–154. doi: 10.1016/j.clp.2014.10.012
Correa, R., de Oliveira Santos, I., Braz-de-Melo, H. A., de Sant’Ana, L. P., das Neves Almeida, R., Pasquarelli-do-Nascimento, G., et al. (2021). Gut microbiota modulation induced by Zika virus infection in immunocompetent mice. Sci. Rep. 11:1421. doi: 10.1038/s41598-020-80893-y
Cragan, J. D., Mai, C. T., Petersen, E. E., Liberman, R. F., Forestieri, N. E., Stevens, A. C., et al. (2017). Baseline Prevalence of Birth Defects Associated with Congenital Zika Virus Infection - Massachusetts, North Carolina, and Atlanta, Georgia, 2013-2014. MMWR Morb. Mortal. Wkly. Rep. 66, 219–222. doi: 10.15585/mmwr.mm6608a4
Cryan, J. F., O’Riordan, K. J., Sandhu, K., Peterson, V., and Dinan, T. G. (2020). The gut microbiome in neurological disorders. Lancet Neurol. 19, 179–194.
Dahl, C., Stigum, H., Valeur, J., Iszatt, N., Lenters, V., Peddada, S., et al. (2018). Preterm infants have distinct microbiomes not explained by mode of delivery, breastfeeding duration or antibiotic exposure. Int. J. Epidemiol. 47, 1658–1669. doi: 10.1093/ije/dyy064
Davis, N. L., King, C. C., and Kourtis, A. P. (2017). Cytomegalovirus infection in pregnancy. Birth Defects Res. 109, 336–346.
de Jong, E. P., de Haan, T. R., Kroes, A. C., Beersma, M. F., Oepkes, D., and Walther, F. J. (2006). Parvovirus B19 infection in pregnancy. J. Clin. Virol. 36, 63–66.
Dubucs, C., Groussolles, M., Ousselin, J., Sartor, A., Van Acker, N., Vayssiere, C., et al. (2022). Severe placental lesions due to maternal SARS-CoV-2 infection associated to intrauterine fetal death. Hum. Pathol. 121, 46–55. doi: 10.1016/j.humpath.2021.12.012
Eck, A., Rutten, N., Singendonk, M. M. J., Rijkers, G. T., Savelkoul, P. H. M., Meijssen, C. B., et al. (2020). Neonatal microbiota development and the effect of early life antibiotics are determined by two distinct settler types. PLoS One 15:e0228133. doi: 10.1371/journal.pone.0228133
Elovitz, M. A., Brown, A. G., Breen, K., Anton, L., Maubert, M., and Burd, I. (2011). Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int. J. Dev. Neurosci. 29, 663–671. doi: 10.1016/j.ijdevneu.2011.02.011
Erny, D., Hrabe de Angelis, A. L., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., et al. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977. doi: 10.1038/nn.4030
Freudenhammer, M., Karampatsas, K., Le Doare, K., Lander, F., Armann, J., Acero Moreno, D., et al. (2021). Invasive Group B Streptococcus Disease With Recurrence and in Multiples: towards a Better Understanding of GBS Late-Onset Sepsis. Front. Immunol. 12:617925. doi: 10.3389/fimmu.2021.617925
Goasdoue, K., Miller, S. M., Colditz, P. B., and Bjorkman, S. T. (2017). Review: the blood-brain barrier; protecting the developing fetal brain. Placenta 54, 111–116. doi: 10.1016/j.placenta.2016.12.005
Gong, C., Yang, L., Liu, K., Shen, S., Zhang, Q., Li, H., et al. (2021). Effects of Antibiotic Treatment and Probiotics on the Gut Microbiome of 40 Infants Delivered Before Term by Cesarean Section Analysed by Using 16S rRNA Quantitative Polymerase Chain Reaction Sequencing. Med. Sci. Monit. 27:e928467. doi: 10.12659/MSM.928467
Gov, L., Karimzadeh, A., Ueno, N., and Lodoen, M. B. (2013). Human innate immunity to Toxoplasma gondii is mediated by host caspase-1 and ASC and parasite GRA15. mBio 4, e00255–13. doi: 10.1128/mBio.00255-13
Grech, A., Collins, C. E., Holmes, A., Lal, R., Duncanson, K., Taylor, R., et al. (2021). Maternal exposures and the infant gut microbiome: a systematic review with meta-analysis. Gut. Microbes 13, 1–30. doi: 10.1080/19490976.2021.1897210
Griffiths, J. A., and Mazmanian, S. K. (2018). Emerging evidence linking the gut microbiome to neurologic disorders. Genome Med. 10:98. doi: 10.1186/s13073-018-0609-3
Hendrix, P., Tang, Z., Silasi, M., Racicot, K. E., Mor, G., Abrahams, V. M., et al. (2020). Herpesvirus-infected Hofbauer cells activate endothelial cells through an IL-1beta-dependent mechanism. Placenta 91, 59–65. doi: 10.1016/j.placenta.2020.01.010
Hester, M. S., Tulina, N., Brown, A., Barila, G., and Elovitz, M. A. (2018). Intrauterine inflammation reduces postnatal neurogenesis in the hippocampal subgranular zone and leads to accumulation of hilar ectopic granule cells. Brain Res. 1685, 51–59. doi: 10.1016/j.brainres.2018.02.005
Hoffman, M. C., Freedman, R., Law, A. J., Clark, A. M., and Hunter, S. K. (2021). Maternal nutrients and effects of gestational COVID-19 infection on fetal brain development. Clin. Nutr. ESPEN 43, 1–8. doi: 10.1016/j.clnesp.2021.04.019
Huleihel, M., Golan, H., and Hallak, M. (2004). Intrauterine infection/inflammation during pregnancy and offspring brain damages: possible mechanisms involved. Rep. Biol. Endocrinol. 2:17. doi: 10.1186/1477-7827-2-17
Jao, J., Agwu, A., Mhango, G., Kim, A., Park, K., Posada, R., et al. (2015). Growth patterns in the first year of life differ in infants born to perinatally vs. nonperinatally HIV-infected women. AIDS 29, 111–116. doi: 10.1097/QAD.0000000000000501
Kang, D. W., Adams, J. B., Gregory, A. C., Borody, T., Chittick, L., Fasano, A., et al. (2017). Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5:10. doi: 10.1186/s40168-016-0225-7
Kemp, M. W. (2014). Preterm birth, intrauterine infection, and fetal inflammation. Front. Immunol. 5:574. doi: 10.3389/fimmu.2014.00574
Kennedy, K. M., Gerlach, M. J., Adam, T., Heimesaat, M. M., Rossi, L., Surette, M. G., et al. (2021). Fetal meconium does not have a detectable microbiota before birth. Nat. Microbiol. 6, 865–873. doi: 10.1038/s41564-021-00904-0
Khoury-Hanold, W., Yordy, B., Kong, P., Kong, Y., Ge, W., Szigeti-Buck, K., et al. (2016). Viral Spread to Enteric Neurons Links Genital HSV-1 Infection to Toxic Megacolon and Lethality. Cell Host Microbe 19, 788–799. doi: 10.1016/j.chom.2016.05.008
Kirschen, G. W., Kery, R., and Ge, S. (2018). The Hippocampal Neuro-Glio-Vascular Network: metabolic Vulnerability and Potential Neurogenic Regeneration in Disease. Brain Plast 3, 129–144. doi: 10.3233/BPL-170055
Kracht, L., Borggrewe, M., Eskandar, S., Brouwer, N., Chuva de Sousa Lopes, S. M., Laman, J. D., et al. (2020). Human fetal microglia acquire homeostatic immune-sensing properties early in development. Science 369, 530–537. doi: 10.1126/science.aba5906
Lei, J., Vermillion, M. S., Jia, B., Xie, H., Xie, L., McLane, M. W., et al. (2019). IL-1 receptor antagonist therapy mitigates placental dysfunction and perinatal injury following Zika virus infection. JCI Insight 4:e122678. doi: 10.1172/jci.insight.122678
Levy, M. E., Greenberg, A. E., Hart, R., Powers Happ, L., Hadigan, C., Castel, A., et al. (2017). High burden of metabolic comorbidities in a citywide cohort of HIV outpatients: evolving health care needs of people aging with HIV in Washington. DC. HIV Med. 18, 724–735. doi: 10.1111/hiv.12516
Liang, G., Zhao, C., Zhang, H., Mattei, L., Sherrill-Mix, S., Bittinger, K., et al. (2020). The stepwise assembly of the neonatal virome is modulated by breastfeeding. Nature 581, 470–474. doi: 10.1038/s41586-020-2192-1
Lu, J., and Claud, E. C. (2019). Connection between gut microbiome and brain development in preterm infants. Dev. Psychobiol. 61, 739–751. doi: 10.1002/dev.21806
Luheshi, G. N., Bluthe, R. M., Rushforth, D., Mulcahy, N., Konsman, J. P., Goldbach, M., et al. (2000). Vagotomy attenuates the behavioural but not the pyrogenic effects of interleukin-1 in rats. Auton. Neurosci. 85, 127–132. doi: 10.1016/S1566-0702(00)00231-9
Lum, F. M., Low, D. K., Fan, Y., Tan, J. J., Lee, B., Chan, J. K., et al. (2017). Zika Virus Infects Human Fetal Brain Microglia and Induces Inflammation. Clin. Infect. Dis. 64, 914–920. doi: 10.1093/cid/ciw878
Ma, Q., Xing, C., Long, W., Wang, H. Y., Liu, Q., and Wang, R. F. (2019). Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J. Neuroinflammation 16:53. doi: 10.1186/s12974-019-1434-3
Macedo-da-Silva, J., Marinho, C. R. F., Palmisano, G., and Rosa-Fernandes, L. (2020). Lights and Shadows of TORCH Infection Proteomics. Genes 11:894. doi: 10.3390/genes11080894
Makinson, R., Lloyd, K., Rayasam, A., McKee, S., Brown, A., Barila, G., et al. (2017). Intrauterine inflammation induces sex-specific effects on neuroinflammation, white matter, and behavior. Brain Behav. Immun. 66, 277–288. doi: 10.1016/j.bbi.2017.07.016
McBroom, K. (2021). A Comparison of Zika Virus and COVID-19: clinical Overview and Public Health Messaging. J. Midwifery Womens Health 66, 334–342. doi: 10.1111/jmwh.13230
Mechtler, L. L., and Kinkel, P. R. (1993). Magnetic resonance imaging in demyelinating, infectious, metabolic, and congenital diseases. Curr. Opin. Neurol. 6, 912–918. doi: 10.1097/00019052-199312000-00014
Mihindukulasuriya, K. A., Mars, R. A. T., Johnson, A. J., Ward, T., Priya, S., Lekatz, H. R., et al. (2021). Multi-Omics Analyses Show Disease, Diet, and Transcriptome Interactions With the Virome. Gastroenterology 161, 1194–1207.e8. doi: 10.1053/j.gastro.2021.06.077
Mondal, M. S., Dey, T., Dhar, D., Shah, R., and Parvin, N. (2021). Gut Microbiome: a Hidden Clue to the Complications of COVID-19. Curr. Drug Targets 5, 001–005.
Munday, B. L., and Mason, R. W. (1979). Toxoplasmosis as a cause of perinatal death in goats. Aust. Vet. J. 55, 485–487. doi: 10.1111/j.1751-0813.1979.tb00375.x
Nguyen, S. M., Antony, K. M., Dudley, D. M., Kohn, S., Simmons, H. A., Wolfe, B., et al. (2017). Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 13:e1006378. doi: 10.1371/journal.ppat.1006378
Nigro, G., Adler, S. P., Gatta, E., Mascaretti, G., Megaloikonomou, A., La Torre, R., et al. (2012). Fetal hyperechogenic bowel may indicate congenital cytomegalovirus disease responsive to immunoglobulin therapy. J. Matern. Fetal Neonatal. Med. 25, 2202–2205. doi: 10.3109/14767058.2012.684111
Nunez, N., Saurina, J., and Nunez, O. (2021). Authenticity Assessment and Fraud Quantitation of Coffee Adulterated with Chicory, Barley, and Flours by Untargeted HPLC-UV-FLD Fingerprinting and Chemometrics. Foods 10:840. doi: 10.3390/foods10040840
Nwanaji-Enwerem, J. C., Laan, L. V., Avakame, E. F., Scott, K. A., Burris, H. H., and Cardenas, A. (2021). Associations of DNA Methylation Mortality Risk Markers with Congenital Microcephaly from Zika Virus: a Study of Brazilian Children Less than 4 Years of Age. J. Trop. Pediatr. 67:fmab020. doi: 10.1093/tropej/fmab020
O’Dea, M., Sweetman, D., Bonifacio, S. L., El-Dib, M., Austin, T., and Molloy, E. J. (2020). Management of Multi Organ Dysfunction in Neonatal Encephalopathy. Front. Pediatr. 8:239. doi: 10.3389/fped.2020.00239
Okumura, T., Nozu, T., Ishioh, M., Igarashi, S., Kumei, S., and Ohhira, M. (2021). Centrally administered butyrate improves gut barrier function, visceral sensation and septic lethality in rats. J. Pharmacol. Sci. 146, 183–191. doi: 10.1016/j.jphs.2021.04.005
Olm, M. R., Bhattacharya, N., Crits-Christoph, A., Firek, B. A., Baker, R., Song, Y. S., et al. (2019). Necrotizing enterocolitis is preceded by increased gut bacterial replication, Klebsiella, and fimbriae-encoding bacteria. Sci. Adv. 5:eaax5727. doi: 10.1126/sciadv.aax5727
Ornoy, A., and Ergaz, Z. (2017). Parvovirus B19 infection during pregnancy and risks to the fetus. Birth Defects Res. 109, 311–323. doi: 10.1002/bdra.23588
Phillips, R. J., and Powley, T. L. (2007). Innervation of the gastrointestinal tract: patterns of aging. Auton. Neurosci. 136, 1–19. doi: 10.1016/j.autneu.2007.04.005
Redline, R. W. (2012). Inflammatory response in acute chorioamnionitis. Semin. Fetal Neonatal. Med. 17, 20–25. doi: 10.1016/j.siny.2011.08.003
Rutten, N. B., Rijkers, G. T., Meijssen, C. B., Crijns, C. E., Oudshoorn, J. H., van der Ent, C. K., et al. (2015). Intestinal microbiota composition after antibiotic treatment in early life: the INCA study. BMC Pediatr. 15:204. doi: 10.1186/s12887-015-0519-0
Santos, C. N. O., Ribeiro, D. R., Cardoso Alves, J., Cazzaniga, R. A., Magalhaes, L. S., de Souza, M. S. F., et al. (2019). Association Between Zika Virus Microcephaly in Newborns With the rs3775291 Variant in Toll-Like Receptor 3 and rs1799964 Variant at Tumor Necrosis Factor-alpha Gene. J. Infect. Dis. 220, 1797–1801. doi: 10.1093/infdis/jiz392
Shaftel, S. S., Carlson, T. J., Olschowka, J. A., Kyrkanides, S., Matousek, S. B., and O’Banion, M. K. (2007). Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J. Neurosci. 27, 9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007
Shao, D. Y., Bai, X., Tong, M. W., Zhang, Y. Y., Liu, X. L., Zhou, Y. H., et al. (2020). Changes to the gut microbiota in mice induced by infection with Toxoplasma gondii. Acta Trop. 203:105301. doi: 10.1016/j.actatropica.2019.105301
Shinjyo, N., Hikosaka, K., Kido, Y., Yoshida, H., and Norose, K. (2020). Toxoplasma Infection Induces Sustained Up-Regulation of Complement Factor B and C5a Receptor in the Mouse Brain via Microglial Activation: implication for the Alternative Complement Pathway Activation and Anaphylatoxin Signaling in Cerebral Toxoplasmosis. Front. Immunol. 11:603924. doi: 10.3389/fimmu.2020.603924
Sierra, A., Encinas, J. M., Deudero, J. J., Chancey, J. H., Enikolopov, G., Overstreet-Wadiche, L. S., et al. (2010). Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495. doi: 10.1016/j.stem.2010.08.014
Stearns, J. C., Simioni, J., Gunn, E., McDonald, H., Holloway, A. C., Thabane, L., et al. (2017). Intrapartum antibiotics for GBS prophylaxis alter colonization patterns in the early infant gut microbiome of low risk infants. Sci. Rep. 7:16527. doi: 10.1038/s41598-017-16606-9
Stockdale, S. R., Draper, L. A., O’Donovan, S. M., Barton, W., O’Sullivan, O., Volpicelli-Daley, L. A., et al. (2021). Alpha-synuclein alters the faecal viromes of rats in a gut-initiated model of Parkinson’s disease. Commun. Biol. 4:1140. doi: 10.1038/s42003-021-02666-1
Stojanovska, V., Atik, A., Nitsos, I., Skiold, B., Barton, S. K., Zahra, V. A., et al. (2018). Effects of Intrauterine Inflammation on Cortical Gray Matter of Near-Term Lambs. Front. Pediatr. 6:145. doi: 10.3389/fped.2018.00145
Supramaniam, V., Vontell, R., Srinivasan, L., Wyatt-Ashmead, J., Hagberg, H., and Rutherford, M. (2013). Microglia activation in the extremely preterm human brain. Pediatr. Res. 73, 301–309. doi: 10.1038/pr.2012.186
Surtees, R., Hyland, K., and Smith, I. (1990). Central-nervous-system methyl-group metabolism in children with neurological complications of HIV infection. Lancet 335, 619–621. doi: 10.1016/0140-6736(90)90409-x
Tauchi, H., Yahagi, K., Yamauchi, T., Hara, T., Yamaoka, R., Tsukuda, N., et al. (2019). Gut microbiota development of preterm infants hospitalised in intensive care units. Benef. Microbes 10, 641–651. doi: 10.3920/BM2019.0003
Tay, T. L., Mai, D., Dautzenberg, J., Fernandez-Klett, F., Lin, G., and Sagar, et al. (2017). A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 20, 793–803. doi: 10.1038/nn.4547
Terrazzan Nutricionist, A. C., Procianoy, R. S., Roesch, L. F. W., Corso, A. L., Dobbler, P. T., and Silveira, R. C. (2020). Meconium microbiome and its relation to neonatal growth and head circumference catch-up in preterm infants. PLoS One 15:e0238632. doi: 10.1371/journal.pone.0238632
Tilg, H., and Kaser, A. (2011). Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Invest. 121, 2126–2132.
Tita, A. T., and Andrews, W. W. (2010). Diagnosis and management of clinical chorioamnionitis. Clin. Perinatol. 37, 339–354. doi: 10.1016/j.clp.2010.02.003
Tripathi, S., Balasubramaniam, V. R., Brown, J. A., Mena, I., Grant, A., Bardina, S. V., et al. (2017). A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathog. 13:e1006258. doi: 10.1371/journal.ppat.1006258
Vargas-Alarcon, G., Ramirez-Bello, J., Posadas-Sanchez, R., Rojas-Velasco, G., Lopez-Reyes, A., Martinez-Gomez, L., et al. (2022). The rs8176740 T/A and rs512770 T/C Genetic Variants of the ABO Gene Increased the Risk of COVID-19, as well as the Plasma Concentration Platelets. Biomolecules 12:486. doi: 10.3390/biom12040486
Venegas, D., De la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., et al. (2019). Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 10:277. doi: 10.3389/fimmu.2019.00277
Walker, R. W., Clemente, J. C., Peter, I., and Loos, R. J. F. (2017). The prenatal gut microbiome: are we colonized with bacteria in utero? Pediatr. Obes. 12, 3–17. doi: 10.1111/ijpo.12217
Wang, S., El-Fahmawi, A., Christian, D. A., Fang, Q., Radaelli, E., Chen, L., et al. (2019). Infection-Induced Intestinal Dysbiosis Is Mediated by Macrophage Activation and Nitrate Production. mBio 10, e00935–19. doi: 10.1128/mBio.00935-19
Warner, B. B. (2019). The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr. Res. 85, 216–224. doi: 10.1038/s41390-018-0191-9
Weisblum, Y., Panet, A., Haimov-Kochman, R., and Wolf, D. G. (2014). Models of vertical cytomegalovirus (CMV) transmission and pathogenesis. Semin. Immunopathol. 36, 615–625. doi: 10.1007/s00281-014-0449-1
Wenzel, T. J., Gates, E. J., Ranger, A. L., and Klegeris, A. (2020). Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol. Cell. Neurosci. 105:103493. doi: 10.1016/j.mcn.2020.103493
Wilson, R. P., Tursi, S. A., Rapsinski, G. J., Medeiros, N. J., Le, L. S., Kotredes, K. P., et al. (2019). STAT2 dependent Type I Interferon response promotes dysbiosis and luminal expansion of the enteric pathogen Salmonella Typhimurium. PLoS Pathog. 15:e1007745. doi: 10.1371/journal.ppat.1007745
Xiang, Z., Tong, W., Guo, Z., Xu, Y., Guo, J., Ruan, Y., et al. (2019). Rat H1 parvovirus infection leads to alterations in gut microbiota. Pathog. Dis. 77:ftz058. doi: 10.1093/femspd/ftz058
Xiao, D., Zhu, T., Qu, Y., Gou, X., Huang, Q., Li, X., et al. (2018). Maternal chorioamnionitis and neurodevelopmental outcomes in preterm and very preterm neonates: a meta-analysis. PLoS One 13:e0208302. doi: 10.1371/journal.pone.0208302
Yeh, P. J., Chiu, C. T., Lai, M. W., Wu, R. C., Chen, C. M., Kuo, C. J., et al. (2021). Clinical manifestations, risk factors, and prognostic factors of cytomegalovirus enteritis. Gut Pathog. 13:53. doi: 10.1186/s13099-021-00450-4
Yeoh, Y. K., Zuo, T., Lui, G. C., Zhang, F., Liu, Q., Li, A. Y., et al. (2021). Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70, 698–706. doi: 10.1136/gutjnl-2020-323020
Zhong, H., Wang, X. G., Wang, J., Chen, Y. J., Qin, H. L., and Yang, R. (2021). Impact of probiotics supplement on the gut microbiota in neonates with antibiotic exposure: an open-label single-center randomized parallel controlled study. World J. Pediatr. 17, 385–393. doi: 10.1007/s12519-021-00443-y
Keywords: microbiome, virome, TORCH infections, neuroinflammation, neurodevelopmental disorders
Citation: Kirschen GW, Panda S and Burd I (2022) Congenital Infection Influence on Early Brain Development Through the Gut-Brain Axis. Front. Neurosci. 16:894955. doi: 10.3389/fnins.2022.894955
Received: 12 March 2022; Accepted: 23 May 2022;
Published: 30 June 2022.
Edited by:
Gregor Eichele, Independent Researcher, Munich, GermanyReviewed by:
Daniel Erny, University of Freiburg Medical Center, GermanyCopyright © 2022 Kirschen, Panda and Burd. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gregory W. Kirschen, R2tpcnNjaDdAamguZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.