
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci. , 24 May 2022
Sec. Autonomic Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.877937
This article is part of the Research Topic Gut Microbiota and the Nervous System, Volume II View all 5 articles
Ischemic stroke (IS) is among the top prevalent neurologic disorders globally today. Risk factors such as hypertension, diabetes, and aging, contribute to the development of IS, and patients with these risk factors face heavier therapeutic burden and worse prognosis. Microbiota–gut–brain axis describes the crosstalk between the gut flora, intestine, and center nervous system, which conduct homeostatic effects through the bacterial metabolites, the regulation of immune activity, also the contact with enteric nerve ends and vagus nerve. Nowadays, more studies have paid attention to the important roles that microbiota–gut–brain axis played in the risk factors of IS. In the current article, we will review the recent works focusing on the bi-directional impacts of gut dysbiosis and the pathogenic process of IS-related risk factors, for the purpose to summarize some novel findings in this area, and try to understand how probiotics could limit the development of IS via different strategies.
Ischemic stroke (IS) is one of the most common neurologic disorders in industrialized and developing countries (Favate and Younger, 2016; Pan et al., 2016; Takashima et al., 2017; Khan et al., 2019). According to the recent epidemiologic studies by the American Heart Association, the age adjusted death rate for stroke as an underlying cause of death was 37.1 per 100,000 in the USA, and a potential increase about 65% of stroke death is forecasted for the year 2030 (Virani et al., 2021). IS brings huge economic burden on the society and families, due to its severe symptoms at the onset, high expense during the treatment, and prolonged physical disability (Demaerschalk et al., 2010; Ma et al., 2021). During the past several decades, the recanalization therapy against AIS, i.e., thrombolysis and thrombectomy, were considered as efficient management to save the penumbral zone, thus to avoid the enlargement of the infarcted area (Jovin et al., 2015; Balodis et al., 2019). However, either the thrombolysis or the thrombectomy has limitations, which make it unsuitable for every patient. The time window of intervention is short, which must within the range of about 4.5–8 h directly after the onset of stroke. A significant proportion, 30–40% of patients, who underwent the therapy on-time, still suffered from so called “failed recanalization,” i.e., unsuccessful reconstruction of cerebral blood flow, and usually indicates a worse prognosis (Flottmann et al., 2018; Leischner et al., 2019). Thus, it is important to address the underlying mechanisms causing the persistent neuronal injury during the IS other than just removal of vessel occlusion.
The pathologic process of IS is complicated. The brain is not only suffered from the loss of oxygen and glucose, but also suffered from the secretion of damage associated molecular patterns (DAMPs) from de-oxygenized cells. DAMPs lead to direct death of neurons, injury of blood–brain barrier, alteration of endocrine system, and overreacted inflammation (Bustamante et al., 2016). Different types of cytokines and chemokines, released by injured tissue, could activate the resident microglia and recruit peripheral immune cells into the ischemic region, causing secondary damage on the penumbra (Gauberti et al., 2016). Adoptive immune components like T cells, migrated to the infarcted area in a later phase, and aid to change the local immune imbalance. Regulatory T cells (Tregs), to be specific, are the major immunosuppressive cell type that release anti-inflammatory cytokines like interleukin-10 (IL-10) and tumor growth factor β (TGF-β) (Xie et al., 2019; Santamaría-Cadavid et al., 2020). Overall, the neuroinflammation plays an important role either in the acute or chronic phase of IS, while clinically, there is still far from clear how the alteration of inflammatory statues would affect the general prognosis of IS patients. However, recent studies about microbiota–gut–brain axis and its correlation to chronic disorders do shed some lights on the future of IS management.
The microbiota–gut–brain axis describes the bi-directional communication between the brain and gastrointestinal tract. It is estimated that the human gut microbiota consists around 100 times of genes as that of human genome (Gill et al., 2006), and studies did find solid evidence that gut flora is connected with brain functions (Heijtz et al., 2011; Mohajeri et al., 2018; van Staaveren et al., 2021). The vagus nerve, for example, binds the brain directly with intestinal walls. The metabolites of gut bacteria, like short-chain fatty acid (SCFAs), could trigger the nerve plexuses in the intestinal wall, then sending signals through the ends of vagus nerve, to the solitary nucleus and finally reaches the diencephalon. On the other hand, the excitation of the vagus nerve could change the biological characters of gut flora in an efferent way, thus build a modulatory reflex (Forsythe et al., 2014). The peripheral immune system is also partly controlled by the microbiota–gut–brain axis, and various types of probiotics showed beneficial effects by restriction of overreacted immune responses (Fung et al., 2017). Furthermore, based on the existence of hierarchical structure of endocrine system and the feedback mechanism, various types of hormones, such as serotonin and corticosteroids, are demonstrated to be able to maintain the gut–brain homeostasis after neural injury (Stasi et al., 2019). Dysbiosis of gut flora has significant consequences for the host, especially affecting the nervous system during IS. Singh et al. (2016) identified reduced species diversity and bacterial overgrowth in middle cerebral artery occlusion (MCAO) model, and re-colonization by dysbiotic flora to germ-free mice led to increased ischemic volume after experimental stroke, suggesting an important role of a healthy bacterial composition in the normal progress of IS. Instead the direct linkages between IS and gut microbiota found in animal models of IS, it might be more interesting to explore how the microbiota could, in some cases, induce the onset of IS.
Various risk factors have been discovered of IS, including hypertension, diabetes/obesity, cardiac disorder and aging, etc. Many hints were unveiled either in clinic or laboratory about the relationships between these risk factors and the failed modulatory effects by microbiota-gut-brain axis, or, to be specific, the bi-directional effects of carrying the risk factors and gut dysbiosis. In this review, we will summarize the recent research articles related to the crosstalk between gut flora and brain infarction, with the focus mainly on the risk factors of IS, and possible pathogenic mechanisms that might lead to the onset of stroke. Moreover, we will also introduce some novel therapeutic opportunities toward the risk factors of IS based on the current understanding of microbiota–gut–brain axis.
High blood pressure brings extra shear stress on the cerebral artery, and usually results in vessel injury and atherosclerosis, which are major pathologic process leading to IS. By comparison of microbiome in feces between people with normal blood pressure and hypertensive patients via 16S amplicon sequencing, Dan et al. (2019) found 54 differential genera between the hypertension group and control group. Parabacteroides, Desulfovibrio, and Christensenella showed higher abundance in either the hypertension group, or the patients with just systolic hypertension, probably due to the reduction of hydrogen sulfide (H2S), and following dampened inhibition of oxidation of butyrate and reduce energy supply of epithelium (Calderón-Pérez et al., 2021).
Direct linkage of metabolites from gut flora, to the hypertension and increased risk of stroke was demonstrated by Nie et al. Trimethylamine N-oxide (TMAO) is derived from the dietary choline and L-carnitine with assistance of gut microbiota. In this nested case–control study, hypertensive patients with higher level of TMAO had a 34% higher risk of stroke onset, compared to patients with lower level of TMAO (Nie et al., 2018). Moreover, spontaneous hypertensive stroke-prone (SHRSP) rats, which showed high incidence of stroke by 16 weeks of age, were found to have lower Bacteroidetes/Firmicutes ratio, if fed by high fat diet, indicating a tight correlation between diet, microbiota, and genetic background susceptible to high blood pressure and stroke (Singh et al., 2019).
An unhealthy lifestyle like the overconsumption of high-salt food is among the top several causes of hypertension. The 4-week continuous feeding of high-sodium diet in mice led to increase in the abundance of Alistipes and Ruminococcaceae, as well as reduction in the abundance of Lactobacillus (Zhang et al., 2019). The reduction of some bacteria species like Bacteroides fragilis by high-salt diet also led to higher secretion of intestinal-derived corticosterone, and the transplantation of feces from hypertensive mice fed by high-salt diet could lift up the receiver mice's blood pressure above normal, indicating the gut flora itself is able to modulate the blood pressure by modulation of hormone level (Yan et al., 2020). Furthermore, the changes of several types of SCFAs were found in the feces of rats fed by a high-salt diet; and the SCFAs also contributed to the development of hypertension (Bier et al., 2018; Chang et al., 2020). In a clinical study focusing on the stool metabolism changes in hypertensive patients, levels of acetate, butyrate propionate increased gradually from patients with normotension, borderline blood pressure, and hypertension, accompanied by taxa alterations of gut flora (Huart et al., 2019). By chronic oral treatment with butyrate-producing bacteria on spontaneously hypertensive rats, the increase in blood pressure and Firmicutes/Bacteroidetes ratio was prevented, and these effects might be mediated by the reduction of lipopolysaccharide (LPS)-Toll-like receptor 4 (TLR-4) pathway, and the infiltration of Tregs in the vasculature (Robles-Vera et al., 2020b). The same group also demonstrated that rats with deoxycorticosterone acetate (DOCA)-salt-induced hypertension had lower blood pressure after feeding with Bifidobacterium breve, and possible mechanisms beneath include improved colonic integrity, restored Th17 and regulatory T cells, and increased nitric oxide-dependent vasorelaxation (Robles-Vera et al., 2020a).
The involvement of neuroinflammation is discovered in patients with hypertension. Loss of normal neural microenvironment could facilitate the infarcted area to enlarge, and tight linkages were found between bowel inflammation and brain inflammation. Signals of gut dysbiosis are transmitted to the cardiovascular brain centers, i.e., paraventricular nucleus (PVN), rostral ventrolateral medulla (RVLM) and nucleus tractus solitaries (NTS), via the afferent fibers of the vagus nerve and circulating immune cells and cytokines (Wang et al., 2021). Stool samples collected from patients with inflammatory bowel disorders contained microbiota favoring gamma-aminobutyric acid (GABA) degradation. This microbiome change was consistent with a deficiency of GABAergic crosstalk between the mood center and PVN in the hypothalamus (Stevens et al., 2021). Kefir, a fermented milk drink, showed anti-hypertensive effects by changing the microbial composition in mouse by oral gavage (5% kefir grains in whole milk, 0.3 ml/100 g body weight), which was also correlated to reduced levels of IL-6 and tumor necrosis factor α (TNF-α), as well as attenuated microglial activation in the PVN and RVLM (de Almeida Silva et al., 2020). These evidences suggest that the gut microbiota could help to control the vessel constriction and inhibit the hypertension via its metabolites and the nervous system.
Another mechanism that gut microbiota helps to control hypertension is by changing the pharmacokinetics of antihypertensive drugs. Amlodipine, a member of calcium channel blocker, decreased and its metabolites increased when incubated with human and rat feces, and systemic exposure to amlodipine was elevated in rats treated with antibiotics, suggesting the reduced drug metabolic rate after the damage to normal flora (Yoo et al., 2016).
The intrauterine environment is demonstrated to affect the offspring for hypertension development in their adulthood (Edwards et al., 1993; Ojeda et al., 2008). In the rat model of systolic hypertension during pregnancy, Li et al. (2020) found maternal intake of captopril, a member of angiotensin-converting enzyme inhibitors (ACEIs), could modulate the constitution of the gut microbiome of both the dams and the pups by significant increases in Corprococcus and Oscillospira, and persistently lower systolic blood pressure. Interestingly, it was found that the maternal captopril feeding attenuated the microglia activation and neuroinflammation of the male offspring, suggesting the gender of the offspring might play a role in the sensitivity to the mother's intrauterine contact with ACEI (Gülke et al., 2018). On the other hand, the administration of the probiotic, Lactobacillus casei, in the pregnant dams was found successfully in attenuating the development of hypertension in offspring induced by high-fructose consumption (Hsu et al., 2018).
Diabetes and obesity are well-recognized risk factors for IS in both the elder (Meschia et al., 2014) and young populations (Mitchell et al., 2015). IS Patients with diabetes and/or obesity had higher odds for functional disability and overall worse prognosis (Park et al., 2019; Bailey et al., 2020). Similar finding was addressed by animal study, for MCAO rats with diet-induced obesity had larger infarcted area, smaller arterial lumen, and thicker vessel walls (Deutsch et al., 2009).
In diabetic mice with cerebral infarction/reperfusion injury, changing of microbiota by adding Clostridium butyricum showed positive effects, such as reduction of blood glucose level, amelioration of cognitive impairment, and attenuation of histopathologic changes in hippocampus (Sun et al., 2016). This study suggested that the specific strain of probiotics had benefits on neurologic behavior, as well as brain pathologic changes after stroke, with a possible target against diabetes, one of its major risk factors.
Due to the digestion and absorption mainly happen in the gastrointestinal tract, the gut microbiota is at the priority for sensing and responding to the nutrient components. Extra food intake would change the structure of gut flora, and the shifts of bacterial taxa might lead to obesity. Turnbaugh et al. demonstrated that mice with obesity had greater abundance of Firmicutes and increased ratio of it to Bacteroidetes, which were correlated with higher levels of acetate and butyrate, and lower calories contained in the caeca of obese mice. Interestingly, this kind of obesity could be adopted by fecal transplantation to WT mice (Turnbaugh et al., 2006). On the other hand, antibiotic treatment also affects the maintenance of energy balance. A 10% weight gain was found on patients with vancomycin treatment (Million et al., 2013). Chen et al., by adding engineered E. coli that highly express N-acylphosphatidylethanolamines (NAPEs) in the drinking water, successfully reduced the daily food intake of mice. They also found higher expression of genes encoding fatty acid oxidation in the gut microbiome, and reduced infiltration of monocytes/macrophages into liver (Chen et al., 2014). Another example is that the administration of Lactobacillus plantarum HACo1, isolated from fermented Korean kimchi, could reduce the mesenteric adipose depot and upregulate the lipid oxidative genes like leptin (Park et al., 2017). All the above studies suggest that a shifted microbiome, together with its metabolome, no matter triggered by diet or probiotics, could cause changes of the host's maintenance of energy balance.
The 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA-reductase) inhibitors like statins are widely used for the treatment of hyperlipidemia. Statin treatment was found associated with lower prevalence of gut dysbiosis identified as lower Bact2 enterotype (Bacteroides2, an intestinal microbiota configuration with high Bacteroides/Faecalibacterium ratio and low microbial cell density), thus helps to maintain a normal gut–brain axis (Vieira-Silva et al., 2020). Statin sensitivity is also correlated with the gut flora biodiversity (Sun et al., 2018).
Similar findings were revealed in the research area of diabetes. Increased monocyte/macrophages, together with the expression of inflammatory cytokines were detected via the biopsy analysis of duodenal mucosa from patients with diabetes, and an increase of Firmicutes/Bacteroidetes ratio was found by 16S rRNA sequencing (Pellegrini et al., 2017). Treatment of prebiotics like non-digestible polysaccharide brought a faster 2-h glucose clearance in mice with high-fat diet, accompanied by a faster decline glucose level induced by exogenous insulin injection, and these evidences were correlated with a preserved bacterial diversity in the gut (Ahmadi et al., 2019). Another mechanism of increased glucose metabolism is through decreasing duodenal contraction. Fournel et al. (2017), by measuring the electrical duodenal activity in vivo, found that a bioactive peptide, apelin, could modify acetylcholine and nitric oxide release from neurons of enteric nervous system (ENS), led to raised insulin release, and upregulated the gene expression of type-4 glucose transporter. Interestingly, according to some studies, the microbiota composition of the offspring of patients with gestational diabetes was also changed, and early stage oligosaccharide consumption was positively correlated with the abundance of infant Ruminococcus, indicating the existence of a maternal microbial imprinting along glucose intake (Su et al., 2018; Wang et al., 2018; Ponzo et al., 2019).
Intestinal inflammation is known as a common complication of diabetes. Increase of pro-inflammatory cytokines and lesioned intestinal tissues are discovered in animals with diabetes. The ENS by this way could be affected and lost the reflex ability to modulate bowel constriction, thus building a malignant loop that facilitates the diabetes to develop. Feeding of probiotics could reverse this process by correction of the intestinal injury and maintenance of a normal ENS loop (Bessac et al., 2018). Besides of this, another beneficial neurological mechanism of probiotic on diabetic individuals is by the attenuation of synaptic injury. By feeding diabetic rats with a probiotic complex containing two types of Lactobacillus and one type of Bifidobacterium, Davari et al. (2013) found enhanced activation of superoxide dismutase and increased insulin in their circulation, and the electrophysiological results indicated the recovered basic synaptic transmission and restored hippocampal long-term potential (LTP).
For elder populations, the incidence of IS could significantly rise up (Kapral et al., 2017; Khan et al., 2021). According to a recent epidemiologic study, stroke happened to 7.6% of adults aged 60 and above (Teh et al., 2018). Increased age is associated with a greater prevalence of vascular risk factors, as well as alterations of gut microbiota. The presences of Bifidobacterium, Faecalibacterium, and Bacteroides were found reduced in people above age 66, which were correlated with reduced fecal concentrations of SCFAs (Salazar et al., 2019). According to a study by Tan et al. (2021), the lack of SCFAs was linked to acute IS patients, and the level of SCFAs was negatively related to stroke severity and prognosis. This study might partly explain why elder patients with IS usually bear worse outcome. Also, the cognitive deficits in the elder IS patients were demonstrated to be a result of SCFAs' deficiency (Liu et al., 2020).
By using the experimental stroke model in aged C57BL/6 mice (18–20 months), Spychala et al. demonstrated increased Firmicutes/Bacteroidetes ratio in aged group. The fecal transplantation of young microbiota to elder mice leads to correction of gut microbiome, also positive changes in neurologic performances and survival rates, while the opposite transplantation, i.e., from aged mice to young mice, induced negative prognosis (Spychala et al., 2018). The SCFAs' effects on IS, which previously addressed by clinical studies, were also confirmed in animal works. Lee et al. (2020) demonstrated that not only the full young microbiome transplantation could attenuate symptoms in old MCAO mice, just a selected combination of SCFA producing bacteria strains, Bifidobacterium longum, Clostridium symbiosum, Faecalibacterium prausnitzii, and Lactobacillus fermentum, could alleviate poststroke neurological deficits. Possible explanation for SCFA's benefits on elder patients is the inhibition on the overproduction of reactive oxygen species (ROS) and nitric oxide (NO), and the prevention of the reduction of mitochondrial fusion gene expression (Hu et al., 2020).
Gut permeability was increased in the aged individuals, as what Qi et al. (2017) demonstrated that the serum concentration of zonulin, a marker of leaky gut, was 22% higher in the older adults as compared to young adults. Higher circulating zonulin was found to be a potential predictor of increased systolic blood pressure (Kim et al., 2017), as well as obesity and hyperlipidemia (Ohlsson et al., 2017). Probiotic administration showed protective effects on normal structures of intestinal epithelium. A human-origin probiotic cocktail containing 5 Lactobacillus and 5 Enterococcus strains was able to prevent leaky gut and inflammation in older mice (Ahmadi et al., 2020). Some probiotics like Lactobacillus could release polyphenol (Subrota et al., 2013), and polyphenol-rich diet on older people with leaky gut showed pronounced increase of the serum zonublin, together with reduction in blood pressure (Guglielmetti et al., 2020).
Thrombus formation in the atrium brings a major source of embolus for cerebral artery and leads to IS. Patients with atrial fibrillation (AF) had global alterations of gut microbiome, including the overgrowth of Ruminococcus and Streptococcus, and the reduction of Faecalibacterium and Alistipes, etc., which were correlated with changed metabolic patterns in fecal and serum samples (Zuo et al., 2019). TMAO was also demonstrated to be able to facilitate the shortening effects on the effective refractory period of myocardium, and widening its window of vulnerability on mice with AF, possibly due to its function on cardiac autonomic nervous system (Yu et al., 2018).
In a hospital-based case/control study, patients with cardioembolic (CE) stroke had higher level of TMAO than patients with large artery atherosclerotic (LAA) stroke and healthy controls, suggesting a different metabolism pattern for stroke patients with cardiac origin (Xu et al., 2021). Moreover, distinct microbial composition was found between patients with CE and LAA stroke, for higher abundance of Streptococcus was demonstrated in the CE group, while the similar change was not seen in LAA group, compared to normal control. The functional and metabolic changes in patients with CE stroke were studied, for the microbial genes related to membrane transport and glycolipid metabolism showed increase, while the genes related to vitamin and glycan synthesis, as well as amino sugar and nucleotide sugar metabolism (Xu et al., 2020). All the evidence implied CE stroke did not share the same microbial characteristics with LAA stroke, and some bacteria like Streptococcus might conduct the pathogenic effects in CE stroke.
Cardiomyopathy predisposes to thrombus formation, and patients with cardiomyopathy had much higher incidences for IS occurrence (Crawford et al., 2004). However, few studies were found to focus on the direct crosstalk of cardiomyopathy and gut flora. The pathologic changes in myocardium might either be a direct or a subsequent change following the gut dysbiosis. Moreover, since viral infection to the myocardium could induce cardiomyopathy; works focusing on gut flora changes in patients with viral cardiomyopathy at early phases might be able to answer whether the gut flora management could slow down the anatomical change of the cardiac structure, thus reducing the risk of IS in the long-range.
Gut microbiota also contributes to the metabolic process of some cardiac drugs, thus indirectly affecting the process of cardiac disorders and the formation of thrombosis. Erythromycin and tetracycline treatment before and concurrent with digoxin could lead to reduced excretion of digoxin reduction products in urine and stool, followed by a two-fold increase of serum digoxin, which might induce cardiac arrhythmia (Lindenbaum et al., 1981).
Tobacco use contributes to the negative change of the immune system through damage of gut microbiota. Shanahan et al. (2018) discovered that either the previous or the current smokers had significantly reduced the bacterial diversity in the upper small intestinal mucosa, which was accompanied by more Firmicutes, Rothia and less Prevotella, and Neisseria. Maternal nicotine exposure via lactating showed decreased Bacteroidetes, increased Firmicultes and Actinobacteria in the feces of the offspring, indicating long-lasting effects of nicotine to change offspring's microbiota and eating behavior, which might predict extra calories intake and diabetes/obesity development (Rodrigues et al., 2021). Moreover, a recent study by Hosseini et al. (2021) demonstrated that the smoking-associated changes in microbiome were absent if the smoker quit smoke for more than 10 years, suggesting the necessity for smoking cessation for the purpose of maintaining healthy gut flora.
Nicotine not only causes tissue damage of lung itself, but also further breaks the integrity of intestine. Mis-homing of dendritic cells and T cells from injured lung to the intestinal epithelium, together with extra circulating IL-6, IL-13, and TNF-α, drives cross-organ inflammation (Gui et al., 2021). It is worth to mention that the nicotine's impacts on gut microbiota also follow a sex-specific manner. The oxidative stress and DNA repair genes were only enriched in nicotine-treated male mice. Multiple types of neurotransmitters, such as serine, aspartic acid, and glycine, were also only increased in the feces of male mice (Chi et al., 2017). However, not many articles nowadays focused on the direct connection between the nicotine exposure and the gut flora-induced neuroinflammatory alterations in IS patients, partly due to that smoking also behaves as a risk factor for cardiac disorders and hypertension, which might make the ‘smoking-gut dysbiosis-stroke' a rather difficult topic to study.
In the current review, we summarized some recent findings relating to the crosstalk between the gut flora, the brain, and the risk factors of IS. Microbiota–gut–brain axis conducts multisystemic modulatory functions to attenuate the negative impacts of these risk factors had on the body. Through the degradation of extra oxidative substances, protection of intestinal epithelium, releasing SCFAs, metabolism of TMAO, inhibition of overwhelming immune injury, and transmission of signals via ENS and the vagus nerve, a healthy gut flora plays essential roles in the homeostatic maintenance in various risk factors of IS. Chronic metabolic diseases like hypertension and diabetes are tightly linked to gut dysbiosis by the reasons of nutrition and lifestyle. Probiotic treatment not only just change the microenvironment that limit the pathologic progress of these risk factors, but also conduct supplementary effect by facilitating the pharmaceutical treatment like calcium channel blocker and statin (all above mechanisms are briefly illustrated in Figure 1). The considerations imply further exploration on the gut microbiota management before and after the onset of IS, with possible routine monitoring of gut flora and lifestyle alterations, and specified clinical cares for IS patients with these gut dysbiosis related risk factors.
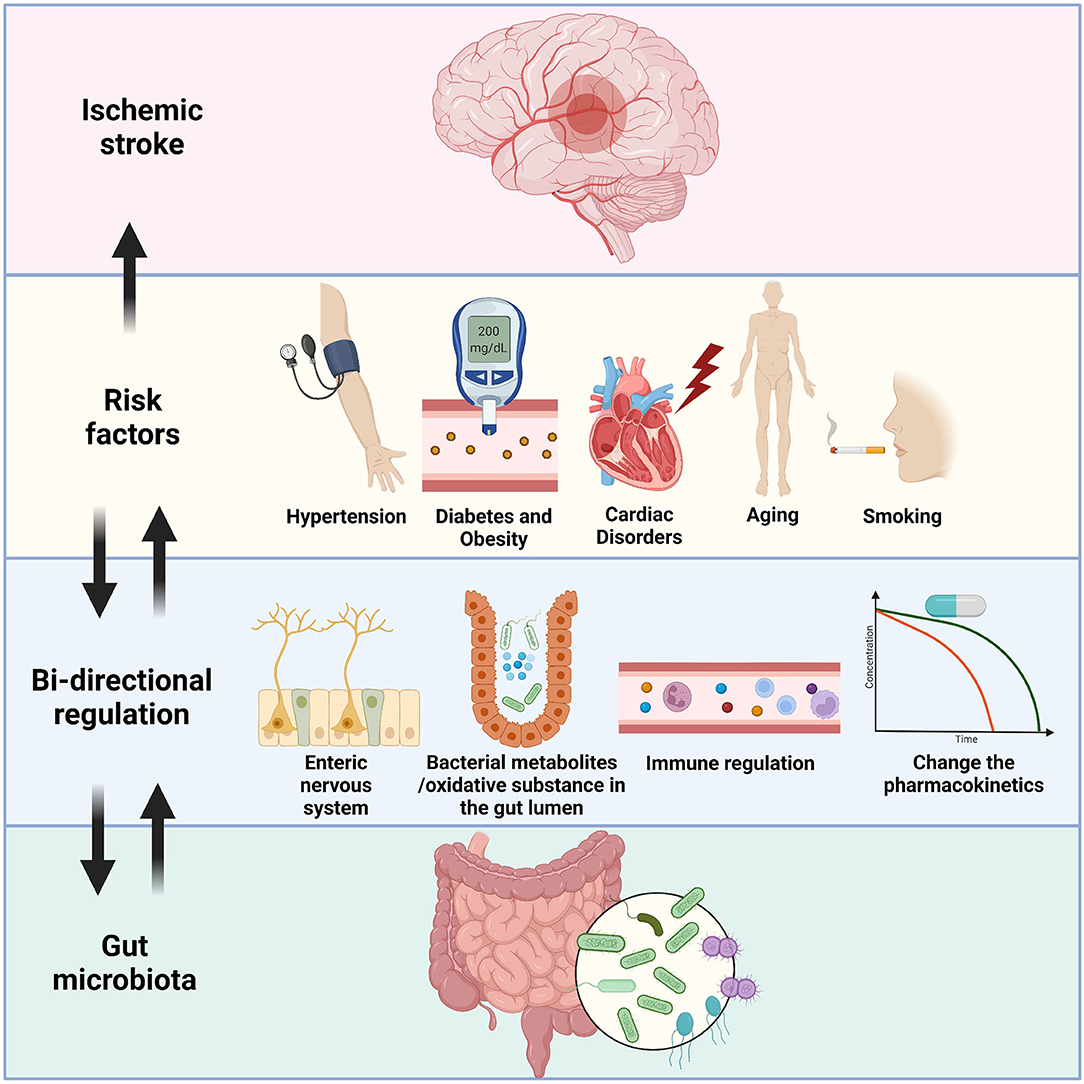
Figure 1. An illustration of the bi-directional relationship between gut microbiota and the risk factors of IS. Hypertension, diabetes/obesity, cardiac disorders, aging, and smoking are well-known risk factors of IS, and IS patients with these factors usually have unsatisfied prognosis. Gut flora could send signals through the enteric nervous system and reach specific brain areas like hypothalamus to control blood pressure and eating habit of the host. Some probiotics showed ability to lowering down the severity of these pre-stroke disorders by reduction of oxidative substances or secretion of short-chain fatty acids. Overreacted immune cells, including T cells, monocytes, and microglia were restricted by the administration of probiotics. Furthermore, the bacteria could also affect the pharmacokinetics of drugs, thus indirectly inhibit the development of diseases like hypertension. On the other hand, the gut dysbiosis is detected in the people who had these risk factors, which could fasten the general pathologic processes, and facilitate the onset of IS by similar mechanisms mentioned above.
YuL, JD, and YW participated in idea conceptualization. YuL, ZZ, and YiL participated in the preparation of the manuscript. YuL participated in the editing of the manuscripts. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmadi, S., Nagpal, R., Wang, S., Gagliano, J., Kitzman, D. W., Soleimanian-Zad, S., et al. (2019). Prebiotics from acorn and sago prevent high-fat-diet-induced insulin resistance via microbiome-gut-brain axis modulation. J. Nutr. Biochem. 67, 1–13. doi: 10.1016/j.jnutbio.2019.01.011
Ahmadi, S., Wang, S., Nagpal, R., Wang, B., Jain, S., Razazan, A., et al. (2020). A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 5, 132055. doi: 10.1172/jci.insight.132055
Bailey, R. R., Serra, M. C., and McGrath, R. P. (2020). Obesity and diabetes are jointly associated with functional disability in stroke survivors. Disabil. Health J. 13, 100914. doi: 10.1016/j.dhjo.2020.100914
Balodis, A., Radzina, M., Miglane, E., Rudd, A., Millers, A., Savlovskis, J., et al. (2019). Endovascular thrombectomy in anterior circulation stroke and clinical value of bridging with intravenous thrombolysis. Acta Radiol. 60, 308–314. doi: 10.1177/0284185118780897
Bessac, A., Cani, P. D., Meunier, E., Dietrich, G., and Knauf, C. (2018). Inflammation and gut-brain axis during type 2 diabetes: focus on the crosstalk between intestinal immune cells and enteric nervous system. Front. Neurosci. 12, 725. doi: 10.3389/fnins.2018.00725
Bier, A., Braun, T., Khasbab, R., Di Segni, A., Grossman, E., Haberman, Y., et al. (2018). A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients 10, 1154. doi: 10.3390/nu10091154
Bustamante, A., Simats, A., Vilar-Bergua, A., García-Berrocoso, T., and Montaner, J. (2016). Blood/brain biomarkers of inflammation after stroke and their association with outcome: from C-reactive protein to damage-associated molecular patterns. Neurotherapeutics 13, 671–684. doi: 10.1007/s13311-016-0470-2
Calderón-Pérez, L., Llauradó, E., Companys, J., Pla-Pagà, L., Pedret, A., Rubió, L., et al. (2021). Interplay between dietary phenolic compound intake and the human gut microbiome in hypertension: a cross-sectional study. Food Chem. 344, 128567. doi: 10.1016/j.foodchem.2020.128567
Chang, Y., Chen, Y., Zhou, Q., Wang, C., Chen, L., Di, W., et al. (2020). Short-chain fatty acids accompanying changes in the gut microbiome contribute to the development of hypertension in patients with preeclampsia. Clin. Sci. 134, 289–302. doi: 10.1042/CS20191253
Chen, Z., Guo, L., Zhang, Y., Walzem, R. L., Pendergast, J. S., Printz, R. L., et al. (2014). Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J. Clin. Investig. 124, 3391–3406. doi: 10.1172/JCI72517
Chi, L., Mahbub, R., Gao, B., Bian, X., Tu, P., Ru, H., et al. (2017). Nicotine alters the gut microbiome and metabolites of gut-brain interactions in a sex-specific manner. Chem. Res. Toxicol. 30, 2110–2119. doi: 10.1021/acs.chemrestox.7b00162
Crawford, T. C., Smith, I. V. W. T, Velazquez, E. J., Taylor, S. M., Jollis, J. G., et al. (2004). Prognostic usefulness of left ventricular thrombus by echocardiography in dilated cardiomyopathy in predicting stroke, transient ischemic attack, and death. Am. J. Cardiol. 93, 500–503. doi: 10.1016/j.amjcard.2003.10.056
Dan, X., Mushi, Z., Baili, W., Han, L., Enqi, W., Huanhu, Z., et al. (2019). Differential analysis of hypertension-associated intestinal microbiota. Int. J. Med. Sci. 16, 872. doi: 10.7150/ijms.29322
Davari, S., Talaei, S. A., and Alaei, H. (2013). Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience 240, 287–296. doi: 10.1016/j.neuroscience.2013.02.055
de Almeida Silva, M., Mowry, F. E., Peaden, S. C., Andrade, T. U., and Biancardi, V. C. (2020). Kefir ameliorates hypertension via gut-brain mechanisms in spontaneously hypertensive rats. J. Nutr. Biochem. 77, 108318. doi: 10.1016/j.jnutbio.2019.108318
Demaerschalk, B. M., Hwang, H.-M., and Leung, G. (2010). US cost burden of ischemic stroke: a systematic literature review. Am. J. Manag. Care 16, 525–533.
Deutsch, C., Portik-Dobos, V., Smith, A. D., Ergul, A., and Dorrance, A. M. (2009). Diet-induced obesity causes cerebral vessel remodeling and increases the damage caused by ischemic stroke. Microvasc. Res. 78, 100–106. doi: 10.1016/j.mvr.2009.04.004
Edwards, C. R., Benediktsson, R., Lindsay, R. S., and Seckl, J. R. (1993). Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet 341, 355–357. doi: 10.1016/0140-6736(93)90148-A
Favate, A. S., and Younger, D. S. (2016). Epidemiology of ischemic stroke. Neurologic clinics 34, 967–980. doi: 10.1016/j.ncl.2016.06.013
Flottmann, F., Leischner, H., Broocks, G., Nawabi, J., Bernhardt, M., Faizy, T. D., et al. (2018). Recanalization rate per retrieval attempt in mechanical thrombectomy for acute ischemic stroke. Stroke 49, 2523–2525. doi: 10.1161/STROKEAHA.118.022737
Forsythe, P., Bienenstock, J., and Kunze, W. A. (2014). Vagal pathways for microbiome-brain-gut axis communication. Microb. Endocrinol. 817, 115–133. doi: 10.1007/978-1-4939-0897-4_5
Fournel, A., Drougard, A., Duparc, T., Marlin, A., Brierley, S. M., Castro, J., et al. (2017). Apelin targets gut contraction to control glucose metabolism via the brain. Gut 66, 258–269. doi: 10.1136/gutjnl-2015-310230
Fung, T. C., Olson, C. A., and Hsiao, E. Y. (2017). Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155. doi: 10.1038/nn.4476
Gauberti, M., De Lizarrondo, S. M., and Vivien, D. (2016). The “inflammatory penumbra” in ischemic stroke: from clinical data to experimental evidence. Eur. Stroke J. 1, 20–27. doi: 10.1177/2396987316630249
Gill, S. R., Pop, M., DeBoy, R. T., Eckburg, P. B., Turnbaugh, P. J., Samuel, B. S., et al. (2006). Metagenomic analysis of the human distal gut microbiome. Science 312, 1355–1359. doi: 10.1126/science.1124234
Guglielmetti, S., Bernardi, S., Del Bo, C., Cherubini, A., Porrini, M., Gargari, G., et al. (2020). Effect of a polyphenol-rich dietary pattern on intestinal permeability and gut and blood microbiomics in older subjects: study protocol of the MaPLE randomised controlled trial. BMC Geriatr. 20, 1–10. doi: 10.1186/s12877-020-1472-9
Gui, X., Yang, Z., and Li, M. D. (2021). Effect of cigarette smoke on gut microbiota: state of knowledge. Front. Physiol. 12, 816. doi: 10.3389/fphys.2021.673341
Gülke, E., Gelderblom, M., and Magnus, T. (2018). Danger signals in stroke and their role on microglia activation after ischemia. Therap. Adv. Neurol. Disord. 11, 1756286418774254. doi: 10.1177/1756286418774254
Heijtz, R. D., Wang, S., Anuar, F., Qian, Y., Björkholm, B., Samuelsson, A., et al. (2011). Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. 108, 3047–3052. doi: 10.1073/pnas.1010529108
Hosseini, A., Chang, C., Leite, G., Wang, J., Chatterjee, C., Barlow, G., et al. (2021). “Smoking changes the small bowel microbiome but appears reversible with smoking cessation compared to control subjects: results from the reimagine study. Gastroenterology 160, S734–S734. doi: 10.1016/S0016-5085(21)02466-5
Hsu, C.-N., Lin, Y.-J., Hou, C.-Y., and Tain, Y.-L. (2018). Maternal administration of probiotic or prebiotic prevents male adult rat offspring against developmental programming of hypertension induced by high fructose consumption in pregnancy and lactation. Nutrients 10, 1229. doi: 10.3390/nu10091229
Hu, S., Kuwabara, R., de Haan, B. J., Smink, A. M., and de Vos, P. (2020). Acetate and butyrate improve β-cell metabolism and mitochondrial respiration under oxidative stress. Int. J. Mol. Sci. 21, 1542. doi: 10.3390/ijms21041542
Huart, J., Leenders, J., Taminiau, B., Descy, J., Saint-Remy, A., Daube, G., et al. (2019). Gut microbiota and fecal levels of short-chain fatty acids differ upon 24-hour blood pressure levels in men. Hypertension 74, 1005–1013. doi: 10.1161/HYPERTENSIONAHA.118.12588
Jovin, T. G., Chamorro, A., Cobo, E., de Miquel, M. A., Molina, C. A., Rovira, A., et al. (2015). Thrombectomy within 8 hours after symptom onset in ischemic stroke. New Engl. J. Med. 372, 2296–2306. doi: 10.1056/NEJMoa1503780
Kapral, M. K., Fang, J., Alibhai, S. M., Cram, P., Cheung, A. M., Casaubon, L. K., et al. (2017). Risk of fractures after stroke: results from the Ontario Stroke Registry. Neurology 88, 57–64. doi: 10.1212/WNL.0000000000003457
Khan, M. I., Khan, J. I., Ahmed, S. I., and Ali, S. (2019). The epidemiology of stroke in a developing country (Pakistan). Pak. J. Neurol. Sci. (PJNS) 13, 30–44. doi: 10.1016/j.jns.2019.10.476
Khan, S. U., Khan, M. Z., Khan, M. U., Khan, M. S., Mamas, M. A., Rashid, M., et al. (2021). Clinical and economic burden of stroke among young, midlife, and older adults in the United States, 2002-2017. Mayo Clin. Proc. Innov. Qual. Outcomes 5, 431–441. doi: 10.1016/j.mayocpiqo.2021.01.015
Kim, S., Goel, R., Qi, Y., Richards, E. M., Mohammed, M., Handberg, E. M., et al. (2017). Plasma zonulin, along with a unique gut microbiome profile, are potential predictors of systolic blood pressure in humans. Hypertension 70(suppl_1), A004. doi: 10.1161/hyp.70.suppl_1.004
Lee, J., d'Aigle, J., Atadja, L., Quaicoe, V., Honarpisheh, P., Ganesh, B. P., et al. (2020). Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circul. Res. 127, 453–465. doi: 10.1161/CIRCRESAHA.119.316448
Leischner, H., Flottmann, F., Hanning, U., Broocks, G., Faizy, T. D., Deb-Chatterji, M., et al. (2019). Reasons for failed endovascular recanalization attempts in stroke patients. J. Neurointerv. Surg. 11, 439–442. doi: 10.1136/neurintsurg-2018-014060
Li, H.-B., Yang, T., Richards, E. M., Pepine, C. J., and Raizada, M. K. (2020). Maternal treatment with captopril persistently alters gut-brain communication and attenuates hypertension of male offspring. Hypertension 75, 1315–1324. doi: 10.1161/HYPERTENSIONAHA.120.14736
Lindenbaum, J., Rund, D. G., Butler Jr, V. P., Tse-Eng, D., and Saha, J. R. (1981). Inactivation of digoxin by the gut flora: reversal by antibiotic therapy. New Engl. J. Med. 305, 789–794. doi: 10.1056/NEJM198110013051403
Liu, Y., Kong, C., Gong, L., Zhang, X., Zhu, Y., Wang, H., et al. (2020). The association of post-stroke cognitive impairment and gut microbiota and its corresponding metabolites. J. Alzheimer's Dis. 73, 1455–1466. doi: 10.3233/JAD-191066
Ma, Z., Deng, G., Meng, Z., and Wu, H. (2021). Hospitalization expenditures and out-of-pocket expenses in patients with stroke in Northeast China, 2015-2017: a pooled cross-sectional study. Front. Pharmacol. 2455. doi: 10.3389/fphar.2020.596183
Meschia, J. F., Bushnell, C., Boden-Albala, B., Braun, L. T., Bravata, D. M., Chaturvedi, S., et al. (2014). Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45, 3754–3832. doi: 10.1161/STR.0000000000000046
Million, M., Thuny, F., Angelakis, E., Casalta, J., Giorgi, R., Habib, G., et al. (2013). Lactobacillus reuteri and Escherichia coli in the human gut microbiota may predict weight gain associated with vancomycin treatment. Nutr. Diab. 3, e87–e87. doi: 10.1038/nutd.2013.28
Mitchell, A. B., Cole, J. W., McArdle, P. F., Cheng, Y.-C., Ryan, K. A., Sparks, M. J., et al. (2015). Obesity increases risk of ischemic stroke in young adults. Stroke 46, 1690–1692. doi: 10.1161/STROKEAHA.115.008940
Mohajeri, M. H., La Fata, G., Steinert, R. E., and Weber, P. (2018). Relationship between the gut microbiome and brain function. Nutr. Rev. 76, 481–496. doi: 10.1093/nutrit/nuy009
Nie, J., Xie, L., Zhao, B.-x., Li, Y., Qiu, B., Zhu, F., et al. (2018). Serum trimethylamine N-oxide concentration is positively associated with first stroke in hypertensive patients. Stroke 49, 2021–2028. doi: 10.1161/STROKEAHA.118.021997
Ohlsson, B., Orho-Melander, M., and Nilsson, P. M. (2017). Higher levels of serum zonulin may rather be associated with increased risk of obesity and hyperlipidemia, than with gastrointestinal symptoms or disease manifestations. Int. J. Mol. Sci. 18, 582. doi: 10.3390/ijms18030582
Ojeda, N. B., Grigore, D., and Alexander, B. T. (2008). Intrauterine growth restriction: fetal programming of hypertension and kidney disease. Adv. Chronic Kidney Dis. 15, 101–106. doi: 10.1053/j.ackd.2008.01.001
Pan, Y., Song, T., Chen, R., Li, H., Zhao, X., Liu, L., et al. (2016). Socioeconomic deprivation and mortality in people after ischemic stroke: The China National Stroke Registry. Int. J. Stroke 11, 557–564. doi: 10.1177/1747493016641121
Park, H., Lee, H. W., Yoo, J., Lee, H. S., Nam, H. S., Kim, Y. D., et al. (2019). Body mass index and prognosis in ischemic stroke patients with type 2 diabetes mellitus. Front. Neurol. 10, 563. doi: 10.3389/fneur.2019.00563
Park, S., Ji, Y., Jung, H.-Y., Park, H., Kang, J., Choi, S.-H., et al. (2017). Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissue accumulation in a diet-induced obesity murine model. Appl.. Microbiol. Biotechnol. 101, 1605–1614. doi: 10.1007/s00253-016-7953-2
Pellegrini, S., Sordi, V., Bolla, A. M., Saita, D., Ferrarese, R., Canducci, F., et al. (2017). Duodenal mucosa of patients with type 1 diabetes shows distinctive inflammatory profile and microbiota. J. Clin. Endocrinol. Metab. 102, 1468–1477. doi: 10.1210/jc.2016-3222
Ponzo, V., Ferrocino, I., Zarovska, A., Amenta, M. B., Leone, F., Monzeglio, C., et al. (2019). The microbiota composition of the offspring of patients with gestational diabetes mellitus (GDM). PLoS ONE 14, e0226545. doi: 10.1371/journal.pone.0226545
Qi, Y., Goel, R., Kim, S., Richards, E. M., Carter, C. S., Pepine, C. J., et al. (2017). Intestinal permeability biomarker zonulin is elevated in healthy aging. J. Am. Med. Direct. Assoc. 18, 810e811–810.e814. doi: 10.1016/j.jamda.2017.05.018
Robles-Vera, I., de la Visitación, N., Toral, M., Sánchez, M., Romero, M., Gómez-Guzmán, M., et al. (2020a). Probiotic Bifidobacterium breve prevents DOCA-salt hypertension. FASEB J. 34, 13626–13640. doi: 10.1096/fj.202001532R
Robles-Vera, I., Toral, M., de la Visitación, N., Sánchez, M., Gómez-Guzmán, M., Romero, M., et al. (2020b). Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: Role of short-chain fatty acids. Mol. Nutr. Food Res. 64, 1900616. doi: 10.1002/mnfr.201900616
Rodrigues, V., Moura, E., Peixoto, T., Soares, P., Lopes, B., Oliveira, E., et al. (2021). Changes in gut-brain axis parameters in adult rats of both sexes with different feeding pattern that were early nicotine-exposed. Food Chem. Toxicol. 158, 112656. doi: 10.1016/j.fct.2021.112656
Salazar, N., Arboleya, S., Fernández-Navarro, T., de Los Reyes-Gavilán, C. G., Gonzalez, S., and Gueimonde, M. (2019). Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: a cross-sectional study. Nutrients 11, 1765. doi: 10.3390/nu11081765
Santamaría-Cadavid, M., Rodríguez-Castro, E., Rodríguez-Yáñez, M., Arias-Rivas, S., López-Dequidt, I., Pérez-Mato, M., et al. (2020). Regulatory T cells participate in the recovery of ischemic stroke patients. BMC Neurol. 20, 1–10. doi: 10.1186/s12883-020-01648-w
Shanahan, E. R., Shah, A., Koloski, N., Walker, M. M., Talley, N. J., Morrison, M., et al. (2018). Influence of cigarette smoking on the human duodenal mucosa-associated microbiota. Microbiome 6, 1–12. doi: 10.1186/s40168-018-0531-3
Singh, A., Zapata, R. C., Pezeshki, A., Workentine, M. L., and Chelikani, P. K. (2019). Host genetics and diet composition interact to modulate gut microbiota and predisposition to metabolic syndrome in spontaneously hypertensive stroke-prone rats. FASEB J. 33, 6748–6766. doi: 10.1096/fj.201801627RRR
Singh, V., Roth, S., Llovera, G., Sadler, R., Garzetti, D., Stecher, B., et al. (2016). Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 36, 7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016
Spychala, M. S., Venna, V. R., Jandzinski, M., Doran, S. J., Durgan, D. J., Ganesh, B. P., et al. (2018). Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 84, 23–36. doi: 10.1002/ana.25250
Stasi, C., Sadalla, S., and Milani, S. (2019). The relationship between the serotonin metabolism, gut-microbiota and the gut-brain axis. Curr. Drug Metab. 20, 646–655. doi: 10.2174/1389200220666190725115503
Stevens, B. R., Pepine, C. J., Richards, E. M., Kim, S., and Raizada, M. K. (2021). Depressive hypertension: a proposed human endotype of brain/gut microbiome dysbiosis. Am. Heart J. 239, 27–37. doi: 10.1016/j.ahj.2021.05.002
Su, M., Nie, Y., Shao, R., Duan, S., Jiang, Y., Wang, M., et al. (2018). Diversified gut microbiota in newborns of mothers with gestational diabetes mellitus. PLoS ONE 13, e0205695. doi: 10.1371/journal.pone.0205695
Subrota, H., Shilpa, V., Brij, S., Vandna, K., and Surajit, M. (2013). Antioxidative activity and polyphenol content in fermented soy milk supplemented with WPC-70 by probiotic Lactobacilli. Int. Food Res. J. 20, 2125.
Sun, B., Li, L., and Zhou, X. (2018). Comparative analysis of the gut microbiota in distinct statin response patients in East China. J. Microbiol. 56, 886–892. doi: 10.1007/s12275-018-8152-x
Sun, J., Wang, F., Ling, Z., Yu, X., Chen, W., Li, H., et al. (2016). Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 1642, 180–188. doi: 10.1016/j.brainres.2016.03.042
Takashima, N., Arima, H., Kita, Y., Fujii, T., Miyamatsu, N., Komori, M., et al. (2017). Incidence, management and short-term outcome of stroke in a general population of 1.4 million japanese-shiga stroke registry. Circul. J. 2017, CJ-17–0177. doi: 10.1253/circj.CJ-17-0177
Tan, C., Wu, Q., Wang, H., Gao, X., Xu, R., Cui, Z., et al. (2021). Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. J. Parent. Enteral Nutr. 45, 518–529. doi: 10.1002/jpen.1861
Teh, W. L., Abdin, E., Vaingankar, J. A., Seow, E., Sagayadevan, V., Shafie, S., et al. (2018). Prevalence of stroke, risk factors, disability and care needs in older adults in Singapore: results from the WiSE study. BMJ Open 8, e020285. doi: 10.1136/bmjopen-2017-020285
Turnbaugh, P. J., Ley, R. E., Mahowald, M. A., Magrini, V., Mardis, E. R., and Gordon, J. I. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. doi: 10.1038/nature05414
van Staaveren, N., Forsythe, P., van der Eijk, J. A., Fuchs, D., Gostner, J. M., Mindus, C., et al. (2021). “The microbiota-gut-brain axis in determining social behaviours of animals,” in Bridging Research Disciplines to Advance Animal Welfare Science: a Practical Guide (Wallingford: CABI), 172–189.
Vieira-Silva, S., Falony, G., Belda, E., Nielsen, T., Aron-Wisnewsky, J., Chakaroun, R., et al. (2020). Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature 581, 310–315. doi: 10.1038/s41586-020-2269-x
Virani, S. S., Alonso, A., Aparicio, H. J., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., et al. (2021). Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation 143, e254–e743. doi: 10.1161/CIR.0000000000000950
Wang, J., Zheng, J., Shi, W., Du, N., Xu, X., Zhang, Y., et al. (2018). Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 67, 1614–1625. doi: 10.1136/gutjnl-2018-315988
Wang, X., Chen, Z., Geng, B., and Cai, J. (2021). The bidirectional signal communication of microbiota-gut-brain axis in hypertension. Int. J. Hypertens. 2021, 8174789. doi: 10.1155/2021/8174789
Xie, L., Li, W., Hersh, J., Liu, R., and Yang, S.-H. (2019). Experimental ischemic stroke induces long-term T cell activation in the brain. J. Cereb. Blood Flow Metab. 39, 2268–2276. doi: 10.1177/0271678X18792372
Xu, D., Wang, K., Yuan, L., Lin, Q., Li, H., Xu, Y., et al. (2020). Dysbiosis of gut microbiota with increased trimethylamine N-oxide level in patients with large artery atherosclerotic and cardioembolic strokes. Research Square. doi: 10.21203/rs.3.rs-22813/v1
Xu, D.-J., Wang, K.-C., Yuan, L.-B., Li, H.-F., Xu, Y.-Y., Wei, L.-Y., et al. (2021). Compositional and functional alterations of gut microbiota in patients with stroke. Nutr. Metab. Cardiovasc. Dis. 31, 3434–3448. doi: 10.1016/j.numecd.2021.08.045
Yan, X., Jin, J., Su, X., Yin, X., Gao, J., Wang, X., et al. (2020). Intestinal flora modulates blood pressure by regulating the synthesis of intestinal-derived corticosterone in high salt-induced hypertension. Circul. Res. 126, 839–853. doi: 10.1161/CIRCRESAHA.119.316394
Yoo, H. H., Kim, I. S., Yoo, D.-H., and Kim, D.-H. (2016). Effects of orally administered antibiotics on the bioavailability of amlodipine: gut microbiota-mediated drug interaction. J. Hypertens. 34, 156–162. doi: 10.1097/HJH.0000000000000773
Yu, L., Meng, G., Huang, B., Zhou, X., Stavrakis, S., Wang, M., et al. (2018). A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int. J. Cardiol. 255, 92–98. doi: 10.1016/j.ijcard.2017.11.071
Zhang, Z., Zhao, J., Tian, C., Chen, X., Li, H., Wei, X., et al. (2019). Targeting the gut microbiota to investigate the mechanism of lactulose in negating the effects of a high-salt diet on hypertension. Mol. Nutr. Food Res. 63, 1800941. doi: 10.1002/mnfr.201800941
Keywords: microbiota–gut–brain axis, ischemic stroke, risk factors, dysbiosis, probiotics
Citation: Liu Y, Dong J, Zhang Z, Liu Y and Wang Y (2022) How Brain Infarction Links With the Microbiota–Gut–Brain Axis: Hints From Studies Focusing on the Risk Factors for Ischemic Stroke. Front. Neurosci. 16:877937. doi: 10.3389/fnins.2022.877937
Received: 17 February 2022; Accepted: 14 April 2022;
Published: 24 May 2022.
Edited by:
Karen-Anne McVey Neufeld, McMaster University, CanadaReviewed by:
Jesus Miguel Pradillo, Complutense University of Madrid, SpainCopyright © 2022 Liu, Dong, Zhang, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunpeng Liu, bGl1eXVucGVuZzY2NDRAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.