
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurosci., 27 April 2022
Sec. Neuropharmacology
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.867034
This article is part of the Research TopicEmerging Inner Ear TherapiesView all 5 articles
Cisplatin is a known ototoxic chemotherapy drug, causing irreversible hearing loss. Evidence has shown that cisplatin causes inner ear damage as a result of adduct formation, a proinflammatory environment and the generation of reactive oxygen species within the inner ear. The main cochlear targets for cisplatin are commonly known to be the outer hair cells, the stria vascularis and the spiral ganglion neurons. Further evidence has shown that certain transporters can mediate cisplatin influx into the inner ear cells including organic cation transporter 2 (OCT2) and the copper transporter Ctr1. However, the expression profiles for these transporters within inner ear cells are not consistent in the literature, and expression of OCT2 and Ctr1 has also been observed in supporting cells. Organ of Corti supporting cells are essential for hair cell activity and survival. Special interest has been devoted to gap junction expression by these cells as certain mutations have been linked to hearing loss. Interestingly, cisplatin appears to affect connexin expression in the inner ear. While investigations regarding cisplatin-induced hearing loss have been focused mainly on the known targets previously mentioned, the role of supporting cells for cisplatin-induced ototoxicity has been overlooked. In this mini review, we discuss the implications of supporting cells expressing OCT2 and Ctr1 as well as the potential role of gap junctions in cisplatin-induced cytotoxicity.
The cochlear sensory epithelium, also known as the organ of Corti, contains both hair cells (HCs) and supporting cells. The HCs, or sensory cells, are specialized and translate the mechanical energy of sound into neurophysiological signals. The supporting cells, on the other hand, are involved in the maintenance of the epithelium during hearing and development. Although they are not the actual transducers, they are essential, and without them, hearing would not be possible (Wan et al., 2013). In fact, some mutations linked to genetic deafness affect genes expressed in supporting cells but not in HCs, such as connexin (Cx) genes for gap junction formation (Forge et al., 1999; Martínez et al., 2009). There are approximately 15 supporting cells per each inner hair cell (IHC) and there are different types of supporting cells including: border, inner phalangeal, pillar, Deiters’ (outer phalangeal cells), and Hensen’s cells (Merchant et al., 2010). They span the whole thickness of the epithelium providing a rigid but vibratile backbone for the organ of Corti. In their luminal domain, they form tight unions with HCs, electrochemically isolating the endolymph, and enabling the endocochlear potential. However, at the basolateral domain, they are widely connected to HCs with important communicative, nutritive, and homeostatic functions (Wangemann, 2006; Zdebik et al., 2009). They can mediate recycling of neurotransmitters and ions, and also perform immunologic and housekeeping functions (Abrashkin et al., 2006; Bird et al., 2010). They can phagocyte or eject fragments of cells, debris, and even whole HCs from the epithelium that have been terminally injured, and produce a phalangeal scar to keep the integrity and electrochemical properties of the epithelium (Kelley et al., 1995; Żak et al., 2016). During organ development, supporting cells participate in cell patterning, polarity and synaptogenesis. There is evidence from other species that supporting cells can perform regenerative functions (Cotanche, 1987; Corwin and Cotanche, 1988). In mammals, although there is no spontaneous cochlear regeneration, it is possible to induce trans-differentiation or proliferation in supporting cells in vitro and in vivo (Chen and Segil, 1999; Löwenheim et al., 1999; Izumikawa et al., 2005; Sage et al., 2005; White et al., 2006; Yu et al., 2010; Yang et al., 2012; Mizutari et al., 2013; Atkinson et al., 2014; Cox et al., 2014; Silva and Maass, 2019). Additionally, supporting cells are more resistant than HCs and usually remain in the epithelium upon damage (McFadden et al., 2002, 2004; Raphael et al., 2007; deTorres et al., 2019). Thus, they are potential targets for regenerative therapies. Despite their importance, they have drawn much less attention and have not been studied as much as HCs (Wan et al., 2013; Waldhaus et al., 2015; Maass et al., 2016). Moreover, it is known that persistent or very severe damage to the sensory epithelium may destroy supporting cells and result in a flat epithelium, in which the normal columnar specialized epithelium is replaced by a non-specialized monolayer epithelium (McFadden et al., 2002, 2004; Raphael et al., 2007; Izumikawa et al., 2008). Several drugs can induce damage to the organ of Corti, however, their effects have been mostly characterized for HCs, and not for supporting cells (Anniko and Sobin, 1986; Slattery and Warchol, 2010).
In this article, we review the potential roles supporting cells may play in cisplatin-induced ototoxicity.
Cisplatin is a commonly used chemotherapeutic agent worldwide. It is a platinating agent that is used to treat various types of cancers (Gold and Raja, 2021). While it is quite efficient as a chemotherapeutic agent, it is known to cause various dose-limiting side effects which include nephrotoxicity and ototoxicity (Wensing and Ciarimboli, 2013). Cisplatin cytotoxicity derives mainly from its capacity to form irreversible DNA adducts, disrupting replication and transcription, and leading to cell death as the cell fails to repair itself (Jamieson and Lippard, 1999).
Cisplatin is a small, and highly reactive molecule and it is believed to enter the inner ear cells through passive diffusion and various transporters, mainly organic cation transporter 2 (OCT2)/solute carrier (SLC) 22A2, and copper transporter Ctr1 (Ciarimboli et al., 2010; More et al., 2010). Once inside the cell, it undergoes an aquation reaction and can bind irreversibly to DNA, RNA and proteins, which leads to cell death, mainly by apoptosis (Johnstone et al., 2016). Ototoxicity is believed to occur as a result of these adducts, a proinflammatory environment and the generation of reactive oxygen species (ROS) within the inner ear. Cisplatin increases the release of proinflammatory cytokines TNF-α, IL-1β and IL-6, and activates MAPKs and factor NF-κB which in turn, promote the expression of pro-inflammatory genes (So et al., 2008). It also activates signal transducer and activator of transcription family proteins, STAT1 and STAT6, which also promote an inflammatory response (Gentilin et al., 2019).
Reactive oxygen species play a major role in cisplatin-induced ototoxicity. They can activate NOX3, a NADPH oxidase highly expressed in the cochlea (Bánfi et al., 2004), which leads to lipid peroxidation and the accumulation of ROS. Cisplatin also depletes glutathione and antioxidant enzymes, which further increases lipid peroxidation (Rybak et al., 2007). As a result of the overwhelming oxidative stress, the cells undergo apoptosis (Sheth et al., 2017; Gentilin et al., 2019). Interestingly, a recent study has shown that following cisplatin exposure in a murine model, Nox3 expression is increased in supporting cells and outer hair cells (OHCs), especially at the basal turn of the cochlea, yet, OHCs but not supporting cells exhibited ROS-induced apoptosis from endogenously produced ROS and/or that of surrounding supporting cells (Mohri et al., 2021) indicating that somehow supporting cells are resistant to ROS-induced cell damage.
On the other hand, morphological analysis following cisplatin application shows that supporting cells exhibit signs of structural damage even before HC loss (Ramírez-Camacho et al., 2004). Moreover, phagocytosis of dead HCs by supporting cells seems to be impaired after cisplatin treatment (Monzack et al., 2015), indicating that supporting cells can be a direct target of cisplatin damage. The association between supporting cells and HC survival can be further supported by the finding that constitutive activation of PI3K-dependent survival signals in some supporting cells, by means of specific genetic ablation of Phosphatase and Tensin Homolog (PTEN), protects HCs from cisplatin damage (Jadali et al., 2017). This protection only occurs in the nearest neighboring HC to the supporting cell presenting greater activity for PI3K, probably through the activation of Checkpoint Kinase 1 (CHK1), which allows supporting cells to repair cisplatin-induced DNA damage (Jadali et al., 2017). Hence, the protection of supporting cells could indirectly protect HCs from cisplatin damage through the secretion of unknown extracellular signaling molecules or other cell-cell signaling molecules that activate HC survival programs.
Clinically, cisplatin-induced ototoxicity presents as a high frequency sensorineural hearing loss that progresses toward the low frequencies (Sheth et al., 2017). Hearing loss is progressive and can also appear months to years after the end of the chemotherapy treatment (Einarsson et al., 2010; Waissbluth et al., 2018). Platinum has been shown to be retained in the inner ear after cisplatin chemotherapy and this has been suggested as one of the explanations for progressive hearing loss (Breglio et al., 2017). Patients often have tinnitus (Sakamoto et al., 2000; Dille et al., 2010; Frisina et al., 2016; Brooks and Knight, 2018) and in some cases, bilateral vestibulopathy (Prayuenyong et al., 2018).
An important subset of patients are pediatric patients because hearing loss can have debilitating long term effects including difficulties in learning, speech delays, and psychosocial impairment (Sheth et al., 2017). Another subset of high-risk patients are patients with cancers requiring concomitant head and neck radiotherapy, as this is an independent risk factor for developing hearing loss. Other risk factors include age (<5 years, >65 years), type of administration, renal function, cumulative cisplatin dose, concomitant use of other ototoxic medications and genetic predisposition (Paken et al., 2019). To date, there are no protective strategies or treatments for cisplatin-induced hearing loss that are FDA-approved. Despite its clinical relevance, the mechanisms by which cisplatin induces hearing loss are still under scrutiny, without clear evidence supporting a principal venue.
van Ruijven et al. (2004) described that cisplatin causes HC loss which is accompanied by protrusion of supporting cells into Nuel’s space and the tunnel of Corti, resulting in a disturbed microarchitecture of the organ of Corti. Thereafter, the same group proposed that the main inner ear targets for cisplatin toxicity are the OHCs, stria vascularis and the spiral ganglion neurons (SGN) (van Ruijven et al., 2005). The latter indicates that cisplatin may induce generalized damage to cochlear tissue more than a cell-type specific effect. Evidence shows that transporters that may mediate cisplatin influx include OCT2 and Ctr1, and they are expressed in cochlear tissue (Figure 1). However, the expression profiles for these transporters are not consistent in the literature. More et al. (2010) observed the presence of Ctr1 in the IHCs, OHCs, stria vascularis and SGN; and OCT2 in the stria vascularis and SGNs but absent from HCs, in 3–4 weeks mouse cochleae. Ding et al. (2011) found Ctr1 in HCs, epithelium of the stria vascularis, and SGN in postnatal day 3 rat pups while Fransson et al. (2017) observed expression in the IHC synapse area, Deiters’ cells and stria vascularis in guinea pigs.
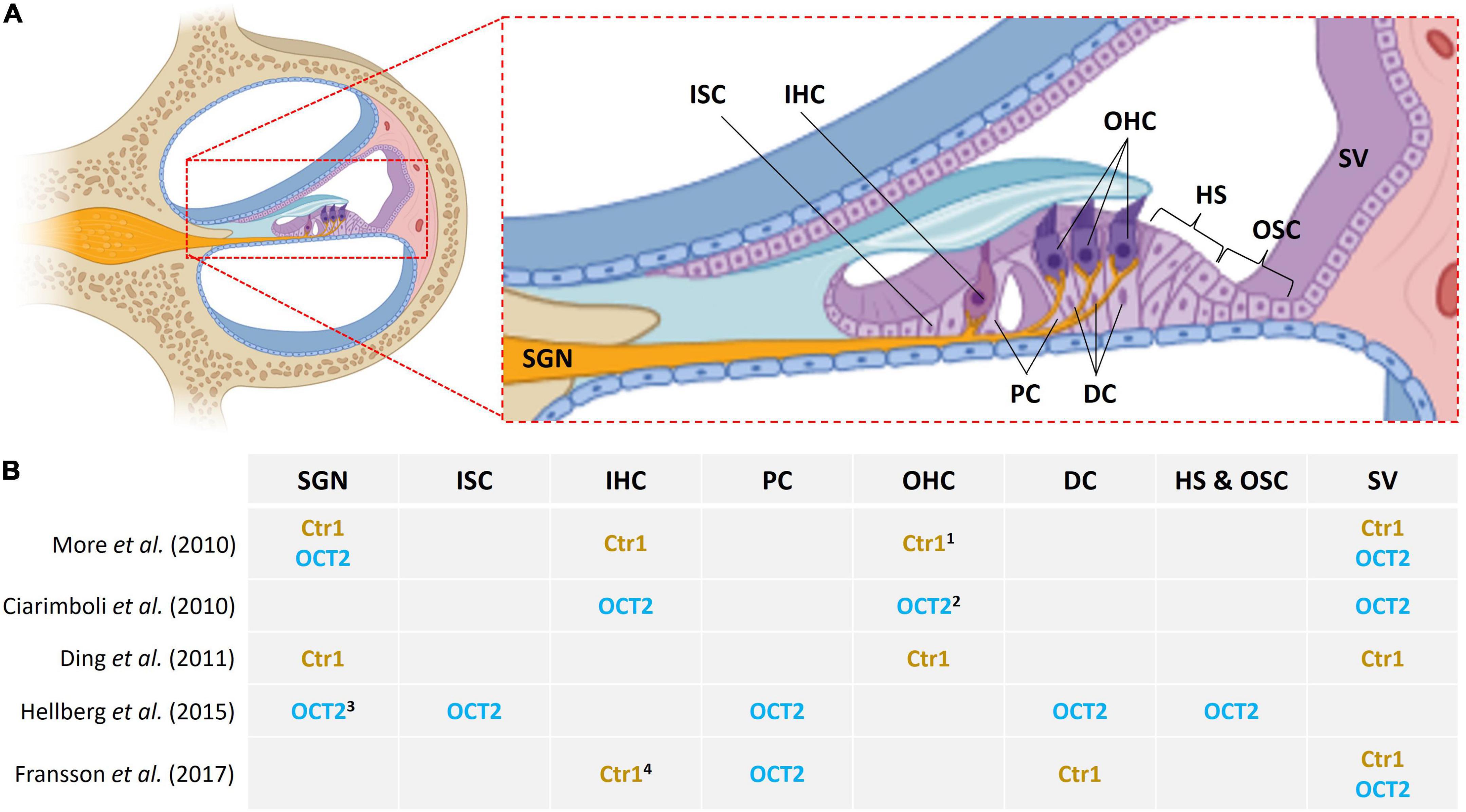
Figure 1. Location of Ctr1 and OCT2 in inner ear cells. (A) Midmodiolar cross section of the cochlea with emphasis on the organ of Corti. Different cell types are indicated as follows: SGN, spiral ganglion neurons; ISC, inner sulcus cells; IHC, inner hair cells; PC, pillar cells; OHC, outer hair cells; DC, Deiters’ cells; HS, Hensen’s cells; OSC, outer sulcus cells; SV, stria vascularis. (B) Variable and inconsistent localization of Ctr1 and OCT2 in the inner ear in various studies; 1 base of OHC, 2 apical pole of OHC, 3 SGN type 1, 4 IHC synapse area.
On the other hand, Ciarimboli et al. (2010) observed OCT2 distributed throughout IHCs, at the apical pole of OHCs, and the stria vascularis but not in the SGN; they do, however, mention some expression in the inner spiral bundle fibers. They also looked into Ctr1; it was present in mouse cochleae but in lower expression as compared to OCT2, hence they decided not to perform further testing for Ctr1; they focused on OCT2 seen as it had greater expression by RT-PCR. Later on, Hellberg et al. (2015) found OCT2 in supporting cells and SGN but not HCs or the stria. They report OCT2 in Deiters’ cells, Hensen’s cells, outer and inner sulcus cells, and outer and inner pillar cells (Hellberg et al., 2015). Consistently, Fransson et al. (2017) also showed strong immunoreactivity for OCT2 in inner and outer pillar cells, and also found Ctr1 in Deiters’ cells and in the stria but not in OHCs.
Another interesting finding is the presence of ATP7A in the pillar cells (Ding et al., 2011). P-type ATPases, copper-transporting ATP7A and ATP7B, are believed to be involved in cisplatin sequestration and efflux (Safaei, 2006), and overexpression has been linked to cisplatin resistance in cancer cells (Samimi et al., 2004). Remarkably, although pillar cells express OCT2, they also express ATP7A and only limited uptake of cisplatin has been reported in supporting cells; while ATP7B was localized in the OHCs of postnatal day 3 rat pups (Ding et al., 2011).
Connexins (Cxs) are transmembrane proteins pivotal for cell communication as they assemble in hemichannels and gap junction channels (GJCs). Hemichannels connect the cell with its external milieu either in a paracrine or autocrine manner (Sáez and Leybaert, 2014). GJCs are aqueous pores through appositional plasma membranes in neighboring cells that connect their cytoplasm. Both hemichannels and GJCs allow the passage of molecules and ions that are essential to maintain cell homeostasis (Sáez et al., 2003) such as ATP, miRNA, glucose, NAD, second messengers and other signaling molecules (Kang et al., 2008; Zong et al., 2016). In physiological conditions, extracellular Ca2+ and the resting membrane potential keep hemichannels closed, and depending on the Cx isoform, hemichannels can be opened by diverse signaling mechanisms including phosphorylation, calcium-calmodulin, nitrosylation, etc. (Sáez and Leybaert, 2014).
Several Cx genes have been identified in the cochlea, including Cx26, Cx30, Cx31, Cx29, Cx43, and Cx45 (Forge et al., 2003a; Martínez et al., 2009). Although HCs do not express Cxs, supporting cells are highly interconnected through GJCs formed mainly by Cx26 and Cx30, forming functional heteromeric hemichannels and GJCs (Ahmad et al., 2003; Forge et al., 2003b; Sun et al., 2005; Verselis, 2019), with some reports indicating expression of Cx43 in rodent prenatal stages (Cohen-Salmon et al., 2004; Jagger and Forge, 2015). Mutations in Cx26 gene (GJB2) are responsible for 50% of genetic deafness causing sensorineural hearing loss due to cochlear malfunction (Kelsell et al., 1997; Martínez et al., 2009; García et al., 2015, 2016; Verselis, 2019). Cxs are involved in the regulation and recycling of K+ and pH maintenance, along with the passage of molecules such as ATP, IP3 and others between supporting cells (Zhao et al., 2005; Jagger and Forge, 2015; Verselis, 2019), maintaining the sensitivity and viability of HCs (Ramírez-Camacho et al., 2006; Zhu et al., 2013) and general homeostasis of the auditory sensory epithelium.
In certain types of cancer cells, communication through GJCs allows the spread of toxic signals to adjoining cells in response to cisplatin treatment, potentiating cell death, a process named “Bystander effect” (Jensen and Glazer, 2004; Arora et al., 2018). A similar mechanism has been proposed for cisplatin-induced toxicity in the organ of Corti. A report showed that inhibition of GJCs with 18α-GA, a non-selective blocker, reduced cisplatin-induced apoptosis of auditory HCs (Kim et al., 2014). They suggest Cx43 may play a proapoptotic role (Kim et al., 2016) as treatment of HEI-OC1 cells, an undifferentiated organ of Corti progenitor cell line, with Cx43 siRNA, present greater cell viability compared to control cells during in vitro cisplatin treatment (Kim et al., 2014). However, a recent study contradicts this “Bystander effect” and the necessity of Cx43 function in cisplatin-induced propagation of death signals in the organ of Corti (Abitbol et al., 2020). In this report, researchers found that in organotypic cochlear cultures from two Cx43-mutant mouse strains expressing Cx43 mutations characterized by moderate (Cx43I130T/+) or severe (Cx43G60S/+) reduction of Cx43 GJC function, cisplatin-induced HC apoptosis was similar to wild type cochlear cultures. In addition, the inhibition of GJC with carbenoxolone did not modify cisplatin-induced HC death (Abitbol et al., 2020). However, as mentioned early, in the mature inner ear, Cx43 is not expressed in supporting cells (Jagger and Forge, 2015).
On the other hand, in some conditions, GJCs can dilute toxic signals induced by cisplatin, which can lead to protection (Hong et al., 2012). In fact, it has been proposed that gap junctions protect non-cancer cells from cisplatin toxicity while enhancing it in tumor cells (Hong et al., 2012; Zhang et al., 2015). Protective signals such as cAMP can pass through gap junctions spreading the transcellular activation of cAMP/PKA/CREB signaling, thereby reducing cisplatin toxicity in organ of Corti cells (Kim et al., 2021). Supporting previous findings, gap junction enhancers, all-trans retinoic acid and quinoline, potentiate the effects of forskolin induced-cAMP production on cell survival via activation of cAMP/PKA/CREB (Kim et al., 2021). Moreover, mice treated with cisplatin exhibited damage to the stria vascularis and reduced endocochlear potentials. This was associated with decreasing expressions of Cx26 and Cx43 in marginal and basal cells of the stria vascularis (Zhang et al., 2020). The latter result is consistent with findings showing that the deletion or mutation of the Cx26 gene negatively impacts the endocochlear potential (Mei et al., 2017). In addition, Wang et al. (2010) found that high concentrations of cisplatin or oxaliplatin inhibit the activity of GJCs formed by Cx26 and Cx32 in HeLa cells. Furthermore, using reconstituted connexin-containing liposomes (immunopurified Cx26/Cx32 hemichannels), the authors found that cisplatin reduces the activity of the purified hemichannels, suggesting that cisplatin may interfere directly with channels made by Cx26 and Cx32. Interestingly, the concentration range for cisplatin used in this study (0.5–7.5 mg/ml) is similar to the cisplatin plasma concentration found in patients during cancer treatment. Moreover, prolonged treatment with cisplatin (48 h) reduces the expression of Cx26 in transfected HeLa cells. Therefore, the inhibition of GJCs by cisplatin and oxaliplatin decreases the cytotoxicity of these compounds, thereby generating a form of resistance to these antitumor agents (Wang et al., 2010). However, in the cochlea, the possible inhibition of gap junctions induced by cisplatin may contribute to HC death as gap junctions are critical for homeostasis and cochlear function.
The precise pathophysiology of cisplatin-induced ototoxicity remains unknown. What we do know is that oxidative stress, inflammation and DNA adducts lead to cell death in the cochlea. It appears OCT2 and Ctr1, which would allow cisplatin influx, are expressed in supporting cells, yet the OHCs die initially; and that the distribution of potential transporters is variable depending on experiment/cells/species studied. Most interestingly, if OHCs are highly susceptible to cisplatin, a logical question would be: are these cells accumulating more cisplatin than other structures in the cochlea? Well, it appears they don’t. Immunohistochemical detection of cisplatin-DNA adducts has been observed in the nuclei of most cells in the organ of Corti and the lateral wall after cisplatin administration (van Ruijven et al., 2005). Using laser ablation coupled to ICP-MS, concentrations of platinum within the organ of Corti were indistinguishable from neighboring tissue, suggesting no specific accumulation of cisplatin within HCs (Breglio et al., 2017). Cisplatin does, however, accumulate consistently in the stria vascularis (van Ruijven et al., 2005; Thomas et al., 2006; Breglio et al., 2017), and it has been proposed that disruption of cochlear fluid homeostasis could lead to HC apoptosis (Prayuenyong et al., 2021).
Another possibility is that HCs are somehow exposed to cell death signals that may be from surrounding cells. We know Cxs are present in all types of non-sensory cells in the cochlea and that they are essential for hearing (Wu et al., 2019). There is also evidence that they can spread either toxic or protective signals generated by cisplatin to adjoining cells (Figure 2). However, contradictory evidence on the exact role of gap junctional intercellular communication in cisplatin-induced ototoxicity impedes a mechanistic model. There is variable expression of Cxs in the organ of Corti and it has been demonstrated that cisplatin can decrease the activity and expression of Cxs. Hence, a decreased expression in the supporting cells, or lateral wall, could perhaps be involved in OHC death.
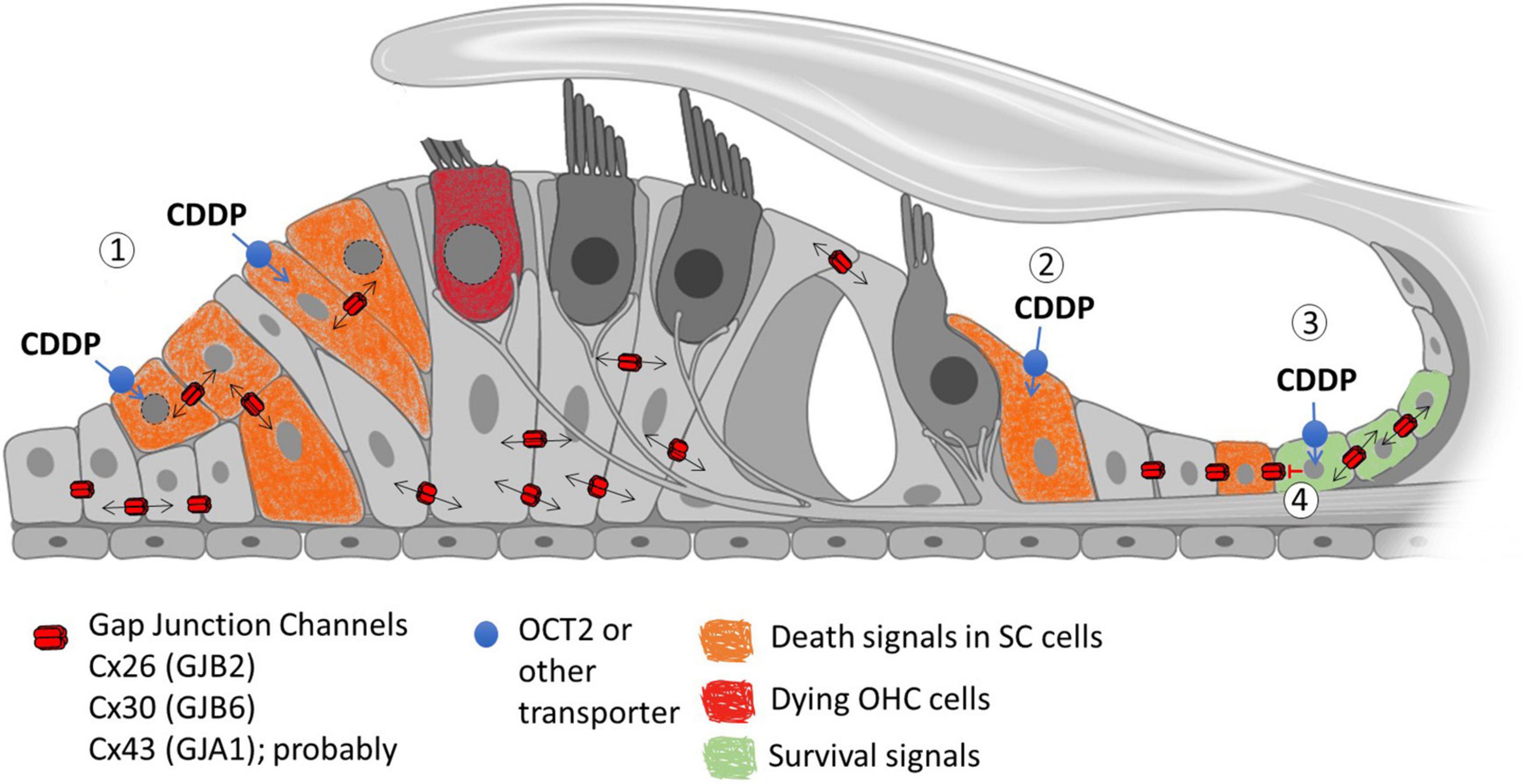
Figure 2. Possible role of Gap Junction Channels (GJC) in the propagation of cisplatin-induced cellular signals. (1) Cisplatin (CDDP) can potentially enter supporting cells (SCs) through non-selective transporters such as OCT2. Inside the cells, cisplatin induces toxicity by damaging DNA and mitochondria, producing ROS and other death signals that can propagate through GJC, spreading cell death signals through the sensory epithelium (Bystander effect). (2) Cisplatin can potentially enter SCs that lack intercellular communication through gap junctions, which restrict toxicity only to these cells. (3) Alternatively, GJC can potentially allow the transmission of protective signals that can reduce cell death between the coupled cells affected by cisplatin. (4) The loss of gap junction communication induced by cisplatin could also induce loss of cell viability in SCs.
While the commonly known areas of the cochlea that are affected by cisplatin are the OHCs, stria vascularis and SGN, greater research efforts should be focused on the supporting cells as they are essential for HC activity and survival. Further research is needed to better understand the pathophysiology of cisplatin-induced ototoxicity, cisplatin inner ear trafficking, as well as the functions of gap junctions in the cochlea.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding was provided by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT-ANID) grants 11201142 for SW, and 1171240 for ADM; Fondo Nacional de Investigación en Salud (FONIS-FONDEF SA18I0194) for HS; the Chilean Millennium Centro Interdisciplinario de Neurociencia de Valparaíso grant P09-022F for ADM and HS, and Fundacion Guillermo Puelma for JCM.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abitbol, J., Beach, R., Barr, K., Esseltine, J., Allman, B., and Laird, D. (2020). Cisplatin-induced ototoxicity in organotypic cochlear cultures occurs independent of gap junctional intercellular communication. Cell Death Dis. 11:342. doi: 10.1038/s41419-020-2551-8
Abrashkin, K. A., Izumikawa, M., Miyazawa, T., Wang, C.-H., Crumling, M. A., Swiderski, D. L., et al. (2006). The fate of outer hair cells after acoustic or ototoxic insults. Hear. Res. 2018, 20–29. doi: 10.1016/j.heares.2006.04.001
Ahmad, S., Chen, S., Sun, J., and Lin, X. (2003). Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Biochem. Biophys. Res. Commun. 307, 362–368. doi: 10.1016/s0006-291x(03)01166-5
Anniko, M., and Sobin, A. (1986). Cisplatin: evaluation of its ototoxic potential. Am. J. Otolaryngol. 7, 276–293. doi: 10.1016/s0196-0709(86)80050-3
Arora, S., Heyza, J. R., Chalfin, E. C., Ruch, R. J., and Patrick, S. M. (2018). Gap junction intercellular communication positively regulates cisplatin toxicity by inducing DNA damage through bystander signaling. Cancers 10:368. doi: 10.3390/cancers10100368
Atkinson, P. J., Wise, A. K., Flynn, B. O., Nayagam, B. A., and Richardson, R. T. (2014). Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PLoS One 9:e102077. doi: 10.1371/journal.pone.0102077
Bánfi, B., Malgrange, B., Knisz, J., Steger, K., Dubois-Dauphin, M., and Krause, K. H. (2004). NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 29, 46065–46072. doi: 10.1074/jbc.M403046200
Bird, J. E., Daudet, N., Warchol, M. E., and Gale, J. E. (2010). Supporting cells eliminate dying sensory hair cells to maintain epithelial integrity in the avian inner ear. J. Neurosci. 30, 12545–12556. doi: 10.1523/JNEUROSCI.3042-10.2010
Breglio, A. M., Rusheen, A. E., Shide, E. D., Fernandez, K. A., Spielbauer, K. K., McLachlin, K. M., et al. (2017). Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat. Commun. 8:1654. doi: 10.1038/s41467-017-01837-1
Brooks, B., and Knight, K. (2018). Ototoxicity monitoring in children treated with platinum chemotherapy. Int. J. Audiol. 57, S34–S40. doi: 10.1080/14992027.2017.1355570
Chen, P., and Segil, N. (1999). p27 (Kip1) links cell proliferation to morphogenesis in the developing organ of corti. Development 126, 1581–1590. doi: 10.1242/dev.126.8.1581
Ciarimboli, G., Deuster, D., Knief, A., Sperling, M., Holtkamp, M., Edemir, B., et al. (2010). Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 176, 1169–1180. doi: 10.2353/ajpath.2010.090610
Cohen-Salmon, M., Maxeiner, S., Kruger, O., Theis, M., Willecke, K., and Petit, C. (2004). Expression of the connexin43- and connexin45-encoding genes in the developing and mature mouse inner ear. Cell Tissue Res. 316, 15–22. doi: 10.1007/s00441-004-0861-2
Corwin, J. T., and Cotanche, D. A. (1988). Regeneration of sensory hair cells after acoustic trauma. Science 240, 1772–1774. doi: 10.1126/science.3381100
Cotanche, D. A. (1987). Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear. Res. 30, 181–195. doi: 10.1016/0378-5955(87)90135-3
Cox, B. C., Chai, R., Lenoir, A., Liu, Z., Zhang, L., Nguyen, D.-H., et al. (2014). Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development 141, 1599–1599. doi: 10.1242/dev.103036
deTorres, A., Olszewski, R. T., Lopez, I. A., Ishiyama, A., Linthicum, F. H., and Hoa, M. (2019). Supporting cell survival after cochlear implant surgery. Laryngoscope 129, E36–E40. doi: 10.1002/lary.27539
Dille, M. F., Konrad-Martin, D., Gallun, F., Helt, W. J., Gordon, J. S., Reavis, K. M., et al. (2010). Tinnitus onset rates from chemotherapeutic agents and ototoxic antibiotics: results of a large prospective study. J. Am. Acad. Audiol. 21, 409–417. doi: 10.3766/jaaa.21.6.6
Ding, D., He, J., Allman, B. L., Yu, D., Jiang, H., Seigel, G. M., et al. (2011). Cisplatin ototoxicity in rat cochlear organotypic cultures. Hear. Res. 282, 196–203. doi: 10.1016/j.heares.2011.08.002
Einarsson, E. J., Petersen, H., Wiebe, T., Fransson, P. A., Grenner, J., Magnusson, M., et al. (2010). Long term hearing degeneration after platinum-based chemotherapy in childhood. Int. J. Audiol. 49, 765–771. doi: 10.3109/14992027.2010.485595
Forge, A., Becker, D., Casalotti, S., Edwards, J., Evans, W. H., Lench, N., et al. (1999). Gap junctions and connexin expression in the inner ear. Novartis Found. Symp. 219, 34–50. doi: 10.1002/9780470515587.ch9
Forge, A., Becker, D., Casalotti, S., Edwards, J., Marziano, N., and Nevill, G. (2003a). Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessment of connexin composition in mammals. J. Comp. Neurol. 467, 207–231. doi: 10.1002/cne.10916
Forge, A., Marziano, N. K., Casalotti, S. O., Becker, D. L., and Jagger, D. (2003b). The inner ear contains heteromeric channels composed of cx26 and cx30 and deafness-related mutations in cx26 have a dominant negative effect on cx30. Cell Commun. Adhes. 10, 341–346. doi: 10.1080/cac.10.4-6.341.346
Fransson, A. E., Kisiel, M., Pirttilä, K., Pettersson, C., Videhult Pierre, P., and Laurell, G. F. E. (2017). Hydrogen inhalation protects against ototoxicity induced by intravenous cisplatin in the guinea pig. Front. Cell. Neurosci. 11:280. doi: 10.3389/fncel.2017.00280
Frisina, R. D., Wheeler, H. E., Fossa, S. D., Kerns, S. L., Fung, C., Sesso, H. D., et al. (2016). Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J. Clin. Oncol. 34, 2712–2720. doi: 10.1200/JCO.2016.66.8822
García, I. E., Bosen, F., Mujica, P., Pupo, A., Flores-Muñoz, C., Jara, O., et al. (2016). From hyperactive connexin26 hemichannels to impairments in epidermal calcium gradient and permeability barrier in the keratitis-ichthyosis-deafness syndrome. J. Invest. Dermatol. 136, 574–583. doi: 10.1016/j.jid.2015.11.017
García, I. E., Maripillan, J., Jara, O., Ceriani, R., Palacios-Munoz, A., Ramachandran, J., et al. (2015). Keratitis-ichthyosis-deafness syndrome-associated Cx26 mutants produce nonfunctional gap junctions but hyperactive hemichannels when co-expressed with wild type Cx43. J. Invest. Dermatol. 135, 1338–1347. doi: 10.1038/jid.2015.20
Gentilin, E., Simoni, E., Candito, M., Cazzador, D., and Astolfi, L. (2019). Cisplatin-induced ototoxicity: updates on molecular targets. Trends Mol. Med. 25, 1123–1132. doi: 10.1016/j.molmed.2019.08.002
Gold, J. M., and Raja, A. (2021). Cisplatin In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing.
Hellberg, V., Gahm, C., Liu, W., Ehrsson, H., Rask-Andersen, H., and Laurell, G. (2015). Immunohistochemical localization of OCT2 in the cochlea of various species. Laryngoscope 125, E320–E325. doi: 10.1002/lary.25304
Hong, X., Wang, Q., Yang, Y., Zheng, S., Tong, X., Zhang, S., et al. (2012). Gap junctions propagate opposite effects in normal and tumor testicular cells in response to cisplatin. Cancer Lett. 317, 165–171. doi: 10.1016/j.canlet.2011.11.019
Izumikawa, M., Batts, S. A., Miyazawa, T., Swiderski, D. L., and Raphael, Y. (2008). Response of the flat cochlear epithelium to forced expression of Atoh1. Hear. Res. 240, 52–56. doi: 10.1016/j.heares.2008.02.007
Izumikawa, M., Minoda, R., Kawamoto, K., Abrashkin, K. A., Swiderski, D. L., Dolan, D. F., et al. (2005). Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med. 11, 271–276. doi: 10.1038/nm1193
Jadali, A., Ying, Y. M., and Kwan, K. Y. (2017). Activation of CHK1 in supporting cells indirectly promotes hair cell survival. Front. Cell. Neurosci. 11:137. doi: 10.3389/fncel.2017.00137
Jagger, D. J., and Forge, A. (2015). Connexins and gap junctions in the inner ear–it’s not just about K+ recycling. Cell Tissue Res. 360, 633–644. doi: 10.1007/s00441-014-2029-z
Jamieson, E. R., and Lippard, S. J. (1999). Structure, recognition, and processing of cisplatin-DNA adducts. Chem. Rev. 8, 2467–2498. doi: 10.1021/cr980421n
Jensen, R., and Glazer, P. M. (2004). Cell-interdependent cisplatin killing by Ku/DNA-dependent protein kinase signaling transduced through gap junctions. Proc. Natl. Acad. Sci. U.S.A 101, 6134–6139. doi: 10.1073/pnas.0400051101
Johnstone, T. C., Suntharalingam, K., and Lippard, S. J. (2016). The next generation of platinum drugs: targeted Pt(II) agents. nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 116, 3436–3486. doi: 10.1021/acs.chemrev.5b00597
Kang, J., Kang, N., Lovatt, D., Torres, A., Zhao, Z., Lin, J., et al. (2008). Connexin 43 hemichannels are permeable to ATP. J. Neurosci. 28, 4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008
Kelley, M. W., Talreja, D. R., and Corwin, J. T. (1995). Replacement of hair cells after laser microbeam irradiation in cultured organs of corti from embryonic and neonatal mice. J. Neurosci. 15, 3013–3026. doi: 10.1523/JNEUROSCI.15-04-03013.1995
Kelsell, D. P., Dunlop, J., Stevens, H. P., Lench, N. J., Liang, J. N., Parry, G., et al. (1997). Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387, 80–83. doi: 10.1038/387080a0
Kim, Y. J., Kim, J., Kim, Y. S., Shin, B., Choo, O. S., Lee, J. J., et al. (2016). Connexin 43 acts as a proapoptotic modulator in cisplatin-induced auditory cell death. Antioxid. Redox Signal. 25, 623–636. doi: 10.1089/ars.2015.6412
Kim, Y. J., Kim, J., Tian, C., Lim, H. J., Kim, Y. S., Chung, J. H., et al. (2014). Prevention of cisplatin-induced ototoxicity by the inhibition of gap junctional intercellular communication in auditory cells. Cell. Mol. Life Sci. 71, 3859–3871. doi: 10.1007/s00018-014-1594-3
Kim, Y. J., Lee, J. S., Kim, H., Jang, J. H., and Choung, Y. H. (2021). Gap junction-mediated intercellular communication of cAMP prevents CDDP-Induced ototoxicity via cAMP/PKA/CREB pathway. Int. J. Mol. Sci. 22:6327. doi: 10.3390/ijms22126327
Löwenheim, H., Furness, D. N., Kil, J., Zinn, C., Gültig, K., Fero, M. L., et al. (1999). Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc. Natl. Acad. Sci. U.S.A. 96, 4084–4088. doi: 10.1073/pnas.96.7.4084
Maass, J. C., Gu, R., Cai, T., Wan, Y.-W., Cantellano, S. C., Asprer, J. S. T., et al. (2016). Transcriptomic analysis of mouse cochlear supporting cell maturation reveals large-scale changes in notch responsiveness prior to the onset of hearing. PLoS One 11:e0167286. doi: 10.1371/journal.pone.0167286
Martínez, A. D., Acuña, R., Figueroa, V., Maripillan, J., and Nicholson, B. (2009). Gap-junction channels dysfunction in deafness and hearing loss. Antioxid. Redox Signal. 11, 309–322. doi: 10.1089/ars.2008.2138
McFadden, S. L., Ding, D., Jiang, H., and Salvi, R. J. (2004). Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 997, 40–51. doi: 10.1016/j.brainres.2003.10.031
McFadden, S. L., Ding, D., Jiang, H., Woo, J. M., and Salvi, R. J. (2002). Chinchilla models of selective cochlear hair cell loss. Hear. Res. 174, 230–238. doi: 10.1016/s0378-5955(02)00697-4
Mei, L., Chen, J., Zong, L., Zhu, Y., Liang, L., Jones, R. O., et al. (2017). A deafness mechanism of digenic Cx26 (GJB2) and Cx30 (GJB6) mutations: reduction of endocochlear potential by impairment of heterogeneous gap junctional function in the cochlear lateral wall. Neurobiol. Dis. 108, 195–203. doi: 10.1016/j.nbd.2017.08.002
Merchant, S. N., Nadol, J. B., and Schuknecht, H. F. (2010). Schuknecht’s Pathology of the Ear. Shelton, CT: PMPH-USA.
Mizutari, K., Fujioka, M., Hosoya, M., Bramhall, N., Okano, H. J., Okano, H., et al. (2013). Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 77, 58–69. doi: 10.1016/j.neuron.2012.10.032
Mohri, H., Ninoyu, Y., Sakaguchi, H., Hirano, S., Saito, N., and Ueyama, T. (2021). Nox3-derived superoxide in cochleae induces sensorineural hearing loss. J. Neurosci. 26, 4716–4731. doi: 10.1523/JNEUROSCI.2672-20.2021
Monzack, E. L., May, L. A., Roy, S., Gale, J. E., and Cunningham, L. L. (2015). Live imaging the phagocytic activity of inner ear supporting cells in response to hair cell death. Cell Death Different. 22, 1995–2005. doi: 10.1038/cdd.2015.48
More, S. S., Akil, O., Ianculescu, A. G., Geier, E. G., Lustig, L. R., and Giacomini, K. M. (2010). Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J. Neurosci. 30, 9500–9509. doi: 10.1523/JNEUROSCI.1544-10.2010
Paken, J., Govender, C. D., Pillay, M., and Sewram, V. (2019). A review of cisplatin-associated ototoxicity. Semin. Hear. 40, 108–121. doi: 10.1055/s-0039-1684041
Prayuenyong, P., Baguley, D.M., Kros, C.J., and Steyger, P.S. (2021). Preferential cochleotoxicity of cisplatin. Front. Neurosci. 15:6–5268. doi: 10.3389/fnins.2021.695268
Prayuenyong, P., Taylor, J., Pearson, S. E., Gomez, R., Patel, P. M., Hall, D. A., et al. (2018). Vestibulotoxicity associated with platinum-based chemotherapy in survivors of cancer: a scoping review. Front. Oncol. 25:363. doi: 10.3389/fonc.2018.00363
Ramírez-Camacho, R., García-Berrocal, J. R., Buján, J., Martín-Marero, A., and Trinidad, A. (2004). Supporting cells as a target of cisplatin-induced inner ear damage: therapeutic implications. Laryngoscope 114, 533–537. doi: 10.1097/00005537-200403000-00027
Ramírez-Camacho, R., García-Berrocal, J. R., Trinidad, A., González-García, J. A., Verdaguer, J. M., Ibáñez, A., et al. (2006). Central role of supporting cells in cochlear homeostasis and pathology. Med. Hypotheses 67, 550–555. doi: 10.1016/j.mehy.2006.02.044
Raphael, Y., Kim, Y.-H., Osumi, Y., and Izumikawa, M. (2007). Non-sensory cells in the deafened organ of corti: approaches for repair. Int. J. Dev. Biol. 51, 649–654. doi: 10.1387/ijdb.072370yr
Rybak, L. P., Whitworth, C. A., Mukherjea, D., and Ramkumar, V. (2007). Mechanisms of cisplatin-induced ototoxicity and prevention. Hear. Res. 226, 57–67. doi: 10.1016/j.heares.2006.09.015
Sáez, J. C., Berthoud, V. M., Branes, M. C., Martinez, A. D., and Beyer, E. C. (2003). Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 83, 1359–1400. doi: 10.1152/physrev.00007.2003
Sáez, J. C., and Leybaert, L. (2014). Hunting for connexin hemichannels. FEBS Lett. 588, 1205–1211. doi: 10.1016/j.febslet.2014.03.004
Safaei, R. (2006). Role of copper transporters in the uptake and efflux of platinum containing drugs. Cancer Lett. 234, 34–39. doi: 10.1016/j.canlet.2005.07.046
Sage, C., Huang, M., Karimi, K., Gutierrez, G., Vollrath, M. A., Zhang, D.-S., et al. (2005). Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science 307, 1114–1118. doi: 10.1126/science.1106642
Sakamoto, M., Kaga, K., and Kamio, T. (2000). Extended high-frequency ototoxicity induced by the first administration of cisplatin. Otolaryngol. Head Neck Surg. 122, 828–833. doi: 10.1016/s0194-5998(00)70009-x
Samimi, G., Safaei, R., Katano, K., Holzer, A.K., Rochdi, M., Tomioka, M., et al. (2004). Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin. Cancer Res. 10:4661–4669. doi: 10.1158/1078-0432.CCR-04-0137
Sheth, S., Mukherjea, D., Rybak, L. P., and Ramkumar, V. (2017). Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front. Cell. Neurosci. 11:338. doi: 10.3389/fncel.2017.00338
Silva, S. A., and Maass, J. C. (2019). p27Kip1 down-regulation as achieved by two clinically feasible means did not induce proliferation of supporting cells in the rat neonatal cochlea in vivo. Hear. Res. 373, 10–22. doi: 10.1016/j.heares.2018.12.002
Slattery, E. L., and Warchol, M. E. (2010). Cisplatin ototoxicity blocks sensory regeneration in the avian inner ear. J. Neurosci. 30, 3473–3481. doi: 10.1523/JNEUROSCI.4316-09.2010
So, H., Kim, H., Kim, Y., Kim, E., Pae, H. O., Chung, H. T., et al. (2008). Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J. Assoc. Res. Otolaryngol. 9, 290–306. doi: 10.1007/s10162-008-0126-y
Sun, J., Ahmad, S., Chen, S., Tang, W., Zhang, Y., Chen, P., et al. (2005). Cochlear gap junctions coassembled from Cx26 and 30 show faster intercellular Ca2+ signaling than homomeric counterparts. Am. J. Physiol. Cell Physiol. 288, C613–C623. doi: 10.1152/ajpcell.00341.2004
Thomas, J. P., Lautermann, J., Liedert, B., Seiler, F., and Thomale, J. (2006). High accumulation of platinum-DNA adducts in strial marginal cells of the cochlea is an early event in cisplatin but not carboplatin ototoxicity. Mol. Pharmacol. 70, 23–29. doi: 10.1124/mol.106.022244
van Ruijven, M. W., de Groot, J. C., Klis, S. F., and Smoorenburg, G. F. (2005). The cochlear targets of cisplatin: an electrophysiological and morphological time-sequence study. Hear. Res. 205, 241–248. doi: 10.1016/j.heares.2005.03.023
van Ruijven, M. W., de Groot, J. C., and Smoorenburg, G. F. (2004). Time sequence of degeneration pattern in the guinea pig cochlea during cisplatin administration. a quantitative histological study. Hear. Res. 197, 44–54. doi: 10.1016/j.heares.2004.07.014
Verselis, V. K. (2019). Connexin hemichannels and cochlear function. Neurosci. Lett. 695, 40–45. doi: 10.1016/j.neulet.2017.09.020
Waissbluth, S., Chuang, A., Del Valle, Á, and Cordova, M. (2018). Long term platinum-induced ototoxicity in pediatric patients. Int. J. Pediatr. Otorhinolaryngol. 107, 75–79. doi: 10.1016/j.ijporl.2018.01.028
Waldhaus, J., Durruthy-Durruthy, R., and Heller, S. (2015). Quantitative high-resolution cellular map of the organ of corti. Cell Rep. 11, 1385–1399. doi: 10.1016/j.celrep.2015.04.062
Wan, G., Corfas, G., and Stone, J. S. (2013). Inner ear supporting cells: rethinking the silent majority. Semin. Cell Dev. Biol. 24, 448–459. doi: 10.1016/j.semcdb.2013.03.009
Wang, Q., You, T., Yuan, D., Han, X., Hong, X., He, B., et al. (2010). Cisplatin and oxaliplatin inhibit gap junctional communication by direct action and by reduction of connexin expression, thereby counteracting cytotoxic efficacy. J. Pharmacol. Exp. Ther. 333, 903–911. doi: 10.1124/jpet.109.165274
Wangemann, P. (2006). Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J. Physiol. 576, 11–21. doi: 10.1113/jphysiol.2006.112888
Wensing, K. U., and Ciarimboli, G. (2013). Saving ears and kidneys from cisplatin. Anticancer Res. 33, 4183–4188.
White, P. M., Doetzlhofer, A., Lee, Y. S., Groves, A. K., and Segil, N. (2006). Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature 441, 984–987. doi: 10.1038/nature04849
Wu, X., Zhang, W., Li, Y., and Lin, X. (2019). Structure and function of cochlear gap junctions and implications for the translation of cochlear gene therapies. Front. Cell. Neurosci. 13:529. doi: 10.3389/fncel.2019.00529
Yang, S. M., Chen, W., Guo, W.-W., Jia, S., Sun, J.-H., Liu, H.-Z., et al. (2012). Regeneration of stereocilia of hair cells by forced atoh1 expression in the adult mammalian cochlea. PLoS One 7:e46355. doi: 10.1371/journal.pone.0046355
Yu, Y., Weber, T., Yamashita, T., Liu, Z., Valentine, M. B., Cox, B. C., et al. (2010). In vivo proliferation of postmitotic cochlear supporting cells by acute ablation of the retinoblastoma protein in neonatal mice. J. Neurosci. 30, 5927–5936. doi: 10.1523/JNEUROSCI.5989-09.2010
Żak, M., van der Linden, C. A., Bezdjian, A., Hendriksen, F. G., Klis, S. F. L., and Grolman, W. (2016). Scar formation in mice deafened with kanamycin and furosemide. Microsc. Res. Tech. 79, 766–772. doi: 10.1002/jemt.22695
Zdebik, A. A., Wangemann, P., and Jentsch, T. J. (2009). Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology 24, 307–316. doi: 10.1152/physiol.00018.2009
Zhang, N., Cai, J., Xu, L., Wang, H., and Liu, W. (2020). Cisplatin-induced stria vascularis damage is associated with inflammation and fibrosis. Neural. Plast. 2020:8851525. doi: 10.1155/2020/8851525
Zhang, Y., Tao, L., Fan, L., Peng, Y., Yang, K., Zhao, Y., et al. (2015). Different gap junction-propagated effects on cisplatin transfer result in opposite responses to cisplatin in normal cells versus tumor cells. Sci. Rep. 5:12563. doi: 10.1038/srep12563
Zhao, H. B., Yu, N., and Fleming, C. R. (2005). Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc. Natl. Acad. Sci. U.S.A. 20, 18724–18729. doi: 10.1073/pnas.0506481102
Zhu, Y., Liang, C., Chen, J., Zong, L., Chen, G. D., and Zhao, H. B. (2013). Active cochlear amplification is dependent on supporting cell gap junctions. Nat. Commun. 4:1786. doi: 10.1038/ncomms2806
Keywords: cisplatin, ototoxicity, supporting cells, gap junction, connexin, hemichannels
Citation: Waissbluth S, Maass JC, Sanchez HA and Martínez AD (2022) Supporting Cells and Their Potential Roles in Cisplatin-Induced Ototoxicity. Front. Neurosci. 16:867034. doi: 10.3389/fnins.2022.867034
Received: 31 January 2022; Accepted: 06 April 2022;
Published: 27 April 2022.
Edited by:
Andrei N. Lukashkin, University of Brighton, United KingdomReviewed by:
Jun Yang, Shanghai Jiao Tong University, ChinaCopyright © 2022 Waissbluth, Maass, Sanchez and Martínez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sofia Waissbluth, c29maWEud2Fpc3NibHV0aEBnbWFpbC5jb20=; c29maWEud2Fpc3NibHV0aEBtYWlsLm1jZ2lsbC5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.